Summary
Calcineurin is a conserved calcium/calmodulin‐dependent protein phosphatase, consisting of a catalytic subunit A and a regulatory subunit B, which is involved in calcium‐dependent signalling and regulation of various important cellular processes. In this study, we functionally characterized the catalytic subunit A (CnaA) of the endophytic fungus Epichloë festucae which forms a symbiotic association with the grass host Lolium perenne. We deleted the CnaA‐encoding gene cnaA in E. festucae and examined its role in hyphal growth, cell wall integrity and symbiosis. This ΔcnaA strain had a severe growth defect with loss of radial growth and hyper‐branched hyphae. Transmission electron microscopy and confocal microscopy analysis of the mutant revealed cell wall defects, aberrant septation and the formation of intrahyphal hyphae, both in culture and in planta. The mutant strain also showed a reduced infection rate in planta. The fluorescence of mutant hyphae stained with WGA‐AF488 was reduced, indicating reduced chitin accessibility. Together, these results show that E. festucae CnaA is required for fungal growth, maintaining cell wall integrity and host colonization.
Keywords: calcineurin, CnaA, Epichloë, hyphal growth
Introduction
Calcineurin is a eukaryote calcium/calmodulin‐dependent serine/threonine‐specific protein phosphatase, and one of the best‐characterized components of calcium signalling pathways. This enzyme is a heterodimer containing catalytic subunit calcineurin A (CnaA) and regulatory subunit calcineurin B (CnaB). Calmodulin binds to the calcineurin AB heterodimer in response to increased cytosolic calcium levels, releasing the CnaA autoinhibitory domain, thereby activating phosphatase activity, which, in turn, dephosphorylates downstream target proteins (Rumi‐Masante et al., 2012; Rusnak and Mertz, 2000). In fungi, calcineurin signalling is mediated through the calcineurin‐responsive transcription factor CRZ1, which plays a crucial role in the regulation of expression of genes involved in controlling ion stress, cell wall integrity, hyphal growth and a number of other developmental processes (Stie and Fox, 2008; Thewes, 2014). Calcineurin has been widely studied in human fungal pathogens, where it has been shown to regulate events essential for pathogenesis, such as the growth of Cryptococcus neoformans at 37 °C (Cruz et al., 2001). In Candida albicans, calcineurin is involved in multi‐drug resistance (Hameed et al., 2011) and the maintenance of cell wall integrity through the regulation of the chitin synthesis genes chs2 and chs8 (Munro et al., 2007). Studies in Aspergillus fumigatus have demonstrated a crucial role for calcineurin in hyphal elongation and septa formation (Juvvadi et al., 2011, 2016; da Silva Ferreira et al., 2007).
Calcineurin signalling has also been investigated in fungal–plant associations. In Ustilago maydis, the causative agent of corn smut disease, calcineurin is essential for virulence, with mutants unable to form tumours on infected plants (Egan et al., 2009). In Ustilago hordei, which causes barley smut disease, the deletion of both calcineurin subunit genes results in decreased host infection rates (Cervantes‐Chavez et al., 2011). In Magnaporthe oryzae, appressorium formation is blocked when cultures are treated with the calcineurin inhibitor cyclosporin A or when the calcineurin A gene, MCNA, is silenced by RNAi (Choi et al., 2009b). Disruption of M. oryzae CRZ1 confirmed that calcineurin signalling is required for the infection process, with mutants seldom developing infectious hyphae (Choi et al., 2009a), possibly as a result of the reduced turgor pressure of the mutant (Zhang et al., 2009). Botrytis cinerea crz1 mutants are defective in host penetration, but this phenotype can be rescued by osmotic stabilizers, demonstrating the importance of cell wall integrity for host colonization by fungal pathogens (Schumacher et al., 2008). Although these examples highlight the importance of calcineurin for host colonization, the precise role of this protein in each of these processes is not yet fully understood.
The endophytic fungus Epichloë festucae forms a highly regulated mutualistic interaction with perennial ryegrass (Lolium perenne). In this association, fungal hyphae grow in the intercellular spaces of the host plant, a biological niche that provides a source of nutrients, a stable environment and a transmission vector through host seeds (Schardl et al., 2004). Perennial ryegrass plants infected with E. festucae show enhanced tolerance to various biotic and abiotic stresses, including protection from insect herbivory (Tanaka et al., 2005) and enhanced drought tolerance (Hahn et al., 2008). Studies have shown that the association between E. festucae and L. perenne represents a good model system for the study of mutualistic fungal–grass interactions, particularly the signalling pathways required for the maintenance of a stable interaction (Scott, 2001). Disruption of NADPH oxidase‐catalysed reactive oxygen species (ROS) production in the fungus leads to a dramatic breakdown in fungal–host signalling characterized by a switch from restrictive to proliferative fungal growth, the loss of host apical dominance and premature host senescence (Takemoto et al., 2006; Tanaka et al., 2006, 2008). The deletion of two E. festucae genes encoding mitogen‐activated protein (MAP) kinases, sakA (stress‐activated) and mpkA (cell wall integrity, CWI), also impacts severely on the association, resulting in major effects on host growth and development similar to those observed for pathogenic interactions (Becker et al., 2015; Eaton et al., 2010). High‐throughput mRNA sequencing of wild‐type (WT) and ΔsakA mutant associations revealed dramatic changes in both fungal and plant gene expression, showing that the host recognizes the sakA mutant as a pathogen (Eaton et al., 2010). These studies clearly demonstrate the importance of fungal signalling pathways in the regulation of the E. festucae–perennial ryegrass association.
Given the importance of calcineurin signalling in fungal pathogenesis, we set out to determine the role of this pathway in the mutualistic symbiotic interaction between E. festucae and its grass host L. perenne. A reverse genetic approach was used to functionally characterize the role of the E. festucae calcineurin catalytic subunit through studies of ΔcnaA mutants in axenic culture and in planta.
Results
Some Epichloë species contain multiple copies of cnaA
To determine whether E. festucae possesses a gene encoding the calcineurin catalytic subunit CnaA, a tblastn search of the E. festucae Fl1 and E. festucae E2368 genome sequences (Schardl et al., 2013) was carried out using Aspergillus nidulans, Neurospora crassa and Saccharomyces cerevisiae CnaA sequences as queries. This search identified one homologue in E. festucae Fl1 (cnaA; gene model EfM3.035130) and two homologues in E. festucae E2368 (cnaA1 and cnaA2; gene models EfM3.035130 and EfM3.051970). The two cnaA homologues in E. festucae E2368 were located at distinct genomic loci. The cnaA2 locus was rich in repetitive elements compared with the cnaA and cnaA1 loci (Fig. 1a). The nucleotide sequences of E. festucae Fl1 cnaA and E. festucae E2368 cnaA1 were identical, with open reading frames (ORFs) of 1692 bp encoding polypeptides of 563 amino acids. The nucleotide sequence of E. festucae E2368 cnaA2 had a 1830‐bp ORF encoding a 609‐amino‐acid polypeptide. The translated amino acid sequences of E. festucae CnaA1 and CnaA2 shared 67% identity. The E. festucae CnaA1 and CnaA2 sequences were aligned with CnaA sequences from other species, including A. nidulans, N. crassa, S. cerevisiae, A. fumigatus, Fusarium oxysporum and Homo sapiens. This alignment showed that the E. festucae homologues contained all conserved domains characteristic of calcineurin catalytic A proteins, namely the catalytic domain, the calcineurin B‐binding domain, the calmodulin‐binding domain and the autoinhibitory domain (Fig. 1b). This supported the identification of the E. festucae CnaA proteins as calcineurin catalytic A subunits. All domains were highly conserved amongst the species analysed (Fig. 1b). A search of the E. festucae Fl1 and E. festucae E2368 genome sequences for a gene encoding the calcineurin regulatory subunit CnaB identified one homologue (gene model EfM3.027360) in each genome.
Figure 1.
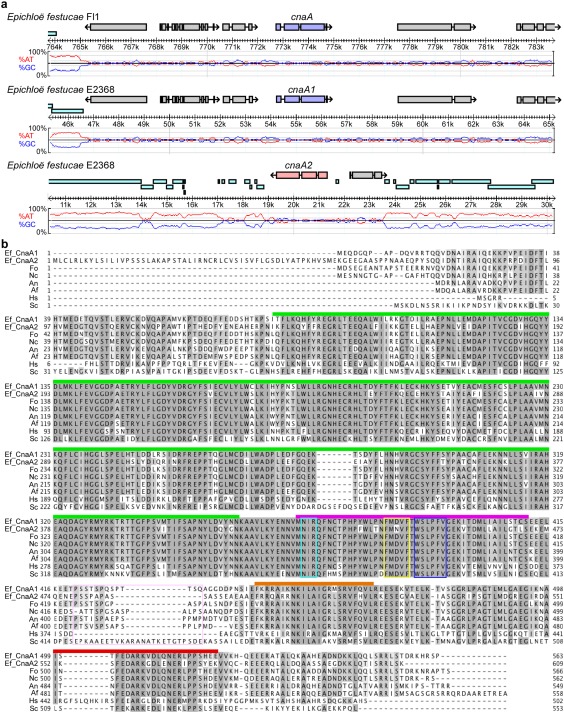
Multiple copies of cnaA in Epichloë festucae. (a) Physical maps of the cnaA loci in E. festucae Fl1 and E2368. The percentage AT/GC content (red/blue traces) and repetitive elements (cyan bars) are shown. Scale bar indicates the position within host contigs. (b) Alignment of the deduced amino acid sequences of cnaA1 and cnaA2 from E. festucae and cnaA from Fusarium oxysporum (Fo, XP018243626), Neurospora crassa (Nc, XP011394598), Aspergillus nidulans (An, P48457), Aspergillus fumigatus (Af, XP753703), Saccharomyces cerevisiae (Sc, NP013537) and human (Hs, AAB23769). Conserved domains are shown with coloured bars: catalytic (green), calcineurin B‐binding (purple), calmodulin‐binding (orange) and autoinhibitory region (red).
A blastn search of 17 other sequenced Epichloë genomes (Schardl et al., 2013) using cnaA, cnaA1 and cnaA2 as the query sequences identified only one homologue of cnaA/cnaA1 in each genome, except that of Epichloë uncinata, which contained two cnaA/cnaA1 homologues (Table S1, see Supporting Information). Given that E. uncinata is an interspecific hybrid of Epichloë bromicola and Epichloë typhina ssp. poae (Leuchtmann et al., 2014), the two cnaA/cnaA1 copies probably derive from these parental strains. No cnaA2 homologues were identified. Using Southern analysis, we further analysed 20 other Epichloë strains whose genomes have not been sequenced for duplication of cnaA. This analysis identified three E. festucae strains (E. festucae E189, E. festucae Fr1 and E. festucae 1035.33) and three strains from other Epichloë species (Epichloë elymi WWG1, E. elymi WWG3 and Epichloë baconii E424) which appeared to contain cnaA2 (Fig. S1, see Supporting Information). However, E. festucae strain E189 is the parent of both E2368 and E1035.33.
Deletion of E. festucae Fl1 cnaA causes severe growth defects in culture
To study the role of calcineurin signalling in E. festucae hyphal growth and the interaction of this endophyte with its host L. perenne, we generated an E. festucae Fl1 cnaA deletion strain by transforming protoplasts of E. festucae Fl1 with a restriction enzyme‐generated fragment from pMMI4 containing a hygromycin resistance gene (see Experimental procedures). Polymerase chain reaction (PCR) screening of hygromycin‐resistant (HygR) transformants identified two strains (ΔcnaA#12 and ΔcnaA#27) with banding patterns characteristic of targeted replacement events. Southern analysis of genomic DNA digests from these transformants confirmed that ΔcnaA#12 contained a single insertion of the replacement cassette into the cnaA locus (Fig. S2, see Supporting Information). This cnaA deletion strain (henceforth ΔcnaA) was selected for further experiments.
The culture growth phenotype of ΔcnaA on potato dextrose agar (PDA) plates was defective compared with WT. The radial growth of ΔcnaA was severely reduced and lacked aerial hyphae, resulting in a compact colony (Fig. 2a). In addition, a yellow pigment was observed around the periphery of the ΔcnaA mycelia which was not observed for WT (Fig. 2a). Differential interference contrast (DIC) microscopic analysis revealed that the ΔcnaA strain showed increased and irregularly branched hyphae that were swollen and convoluted (Fig. 2b). Hyphal debris was observed within the area of yellow pigment around the colony. All abnormal phenotypes were fully rescued when WT cnaA was reintroduced into the ΔcnaA mutant strain (Figs 2,b and S2). Given that Mg2+ restores growth defects of calcineurin mutants (Schumacher et al., 2008), we investigated the growth of ΔcnaA on PDA supplemented with MgCl2. In the presence of Mg2+, growth defects of ΔcnaA were partially remediated with increased radial growth and more aerial hyphae, and the lack of a yellow pigment in the medium (Fig. S3, see Supporting Information). Surprisingly, remediation of this phenotype was also observed when compact and dense mycelia of ΔcnaA colonies were coarsely ground and spread over PDA (Fig. S3). On account of these observations, we used both non‐remediated ΔcnaA (henceforth ΔcnaA NR) and remediated ΔcnaA (henceforth ΔcnaA R) colonies for in planta experiments (described below).
Figure 2.
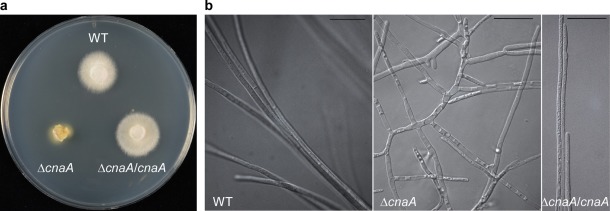
Deletion of Epichloë festucae Fl1 cnaA causes severe growth defects in culture. (a) Colony morphology of E. festucae Fl1 wild‐type (WT), cnaA deletion strain (ΔcnaA) and ΔcnaA strain complemented with WT copy of E. festucae Fl1 cnaA (ΔcnaA/cnaA) grown on potato dextrose agar for 6 days. (b) Comparison of differential interference contrast image of ΔcnaA culture showing highly deregulated growth pattern with increased branching and altered hyphal morphology compared with the wild‐type (WT) and complementation strain (ΔcnaA/cnaA). Bar, 20 μm.
Epichloë festucae CnaA contributes to cell wall organization
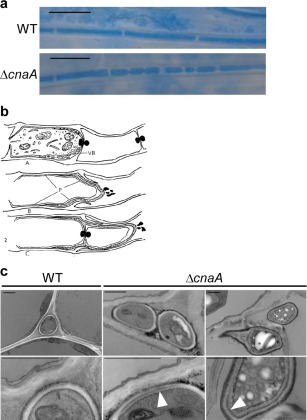
Given that the calcineurin signalling pathway contributes to the regulation of cell wall biosynthesis (Fortwendel et al., 2009), we investigated the role of E. festucae Fl1 CnaA in chitin synthesis. We specifically tested the ΔcnaA mutant strain for chitin distribution in the cell wall by calcofluor white staining. The WT strain showed a distinctive pattern of strongly stained hyphal tips and septa, whereas the ΔcnaA mutant strain exhibited an altered staining pattern (Fig. 3). The mutant strain showed a reduced zone of staining at the hyphal tips and a scattered localization of stained aggregations along the apical hyphal compartment (Fig. 3). The mutant strain also exhibited weakly stained irregular septa with shorter hyphal compartments compared with the WT strain, but hyphal anastomosis was not affected. The reintroduction of WT cnaA into ΔcnaA restored the WT calcofluor white staining pattern (Fig. 3).
Figure 3.

Deletion of Epichloë festucae Fl1 cnaA causes defects in septation and the distribution of cell wall components. Confocal images of E. festucae Fl1 wild‐type (WT), ΔcnaA and ΔcnaA/cnaA cultures stained with calcofluor white, which binds to chitin, showing a loss of the WT staining pattern in the ΔcnaA strain. Bar, 20 μm.
As the calcineurin mutant strain showed a defective distribution of cell wall components, we performed transmission electron microscopy (TEM) to examine cell wall organization in the mutant strain grown on PDA plates. This analysis revealed peculiar formations within the calcineurin mutant hyphae that resembled sections of cytoplasm surrounded by a secondary cell wall in the parent cell (Fig. 4a; indicated by yellow arrowheads). In transverse sections, mutant hyphae appeared to have two or three closely packed layers of cell wall (Fig. 4a; lower panel). In addition, the mutant hyphae showed disrupted outer cell walls and incomplete septa (Fig. 4a, lower panel; indicated by red and white arrowheads). This hypha within hypha morphology strongly resembles the phenomenon of intrahyphal hyphae, which is described in filamentous fungi (Becker et al., 2015; Calonge, 1968). This phenomenon was studied in detail in Sclerotinia fructigena (Calonge, 1968) and drawings produced in that study showed structures very similar to those seen in the ΔcnaA strain (Fig. 4b). Together, these results suggest that the deletion of cnaA in E. festucae Fl1 leads to the disruption of cell wall organization, abnormal septation and the formation of intrahyphal hyphae.
Figure 4.

Deletion of Epichloë festucae Fl1 cnaA causes the formation of intrahyphal hyphae. (a) Transmission electron micrographs of E. festucae Fl1 wild‐type (WT) and ΔcnaA strains showing extensive formation of intrahyphal cell walls in the mutant hyphae (indicated by yellow arrowheads) and abnormal septa (indicated by white arrowheads). Red arrowheads indicate disrupted outer cell wall. Bar, 2 μm. (b) Reproduction of a drawing from Calonge (1968) showing intrahyphal hyphae breaking out from the mother hypha of Sclerotinia fructigena. This schematic diagram captures the transmission electron micrographs of the hyphal structures observed in the ΔcnaA strain.
The duplicate cnaA genes in E. festucae E2368 are functionally redundant
We constructed targeted deletions of cnaA1 and cnaA2 to functionally characterize these duplicate genes in E. festucae E2368. Restriction enzyme‐generated fragments from pMMI4 and pMMI13, containing replacement cassettes for cnaA1 and cnaA2, respectively, were transformed into E. festucae E2368 protoplasts and the resulting transformants were screened for true replacements (Fig. S4, see Supporting Information). This screening identified two deletion strains (ΔcnaA1#10 and ΔcnaA1#17) for cnaA1 and two deletion strains (ΔcnaA2#24 and ΔcnaA2#27) for cnaA2. The mutant strains ΔcnaA1#17 (henceforth ΔcnaA1) and ΔcnaA2#27 (henceforth ΔcnaA2) were used for further experiments.
Deletion of either cnaA1 or cnaA2 did not result in the severe growth phenotype observed for E. festucae Fl1 ΔcnaA (Figs 5a and 2a). Although the colony morphology and growth rate of the ΔcnaA2 strain were similar to those of E. festucae E2368 WT, the ΔcnaA1 strain had a slightly slower growth rate relative to WT (Fig. 5a). Given that the E. festucae Fl1 cnaA and E. festucae E2368 cnaA1 nucleotide sequences were identical, we reasoned that the reintroduction of cnaA1 into ΔcnaA was unnecessary, given that cnaA complemented the ΔcnaA strain. Hence, we only used E. festucae E2368 cnaA2 to attempt complementation of the E. festucae Fl1 ΔcnaA strain. The resulting ΔcnaA/cnaA2 strain restored the growth defects of ΔcnaA, indicating that cnaA1 and cnaA2 are functionally redundant in culture (Fig. 5b).
Figure 5.

The duplicate cnaA genes of Epichloë festucae E2368 are functionally redundant. (a) Colony morphology of E. festucae E2368 wild‐type (WT), ΔcnaA1#17 (ΔcnaA1) and ΔcnaA2#27 (ΔcnaA2) cultures grown on potato dextrose agar for 6 days. (b) Colony morphology of E. festucae Fl1 ΔcnaA complemented with the WT copy of E. festucae E2368 cnaA2 (ΔcnaA/cnaA2) grown on potato dextrose agar for 6 days.
Disruption of cnaA induces a necrotic response in planta
To test the role of calcineurin signalling in the establishment of a symbiotic interaction, L. perenne seedlings were inoculated with both non‐remediated and remediated ΔcnaA strains (ΔcnaA NR and ΔcnaA R, respectively). Interestingly, seedlings inoculated with ΔcnaA NR colonies developed a strong host defence response at 5–9 days post‐inoculation that was visible as a brown coloration around the inoculation region (Fig. 6a). The response in some seedlings was strong enough to cause host death. However, a host defence response was absent when seedlings were inoculated with ΔcnaA R or WT colonies (Fig. 6a).
Figure 6.

Deletion of Epichloë festucae Fl1 cnaA elicits a host defence response in Lolium perenne seedlings. (a) Light micrographs of L. perenne seedlings at 10 days post‐inoculation with non‐remediated (ΔcnaA NR) and remediated (ΔcnaA R) cultures of Fl1 ΔcnaA and wild‐type (WT). (b) Epichloë festucae grow outs from L. perenne plants infected with non‐remediated ΔcnaA (ΔcnaA NR), remediated ΔcnaA (ΔcnaA R) and wild‐type (WT) Fl1.
Given that inoculum from a ΔcnaA NR colony, but not a ΔcnaA R colony, caused necrosis of seedling tissue (Fig. 6a), we investigated whether the remediation of mutant colonies induced irreversible changes in the mutant strain. To test this, fungal colonies were grown from tissue of 12‐week‐old infected plants on PDA plates. Both ΔcnaA NR and ΔcnaA R colonies showed a similar growth phenotype and grew as compact, dense masses of hyphae, unlike the WT strain (Fig. 6b). This suggests that remediation of mutant colonies does not induce an irreversible change in ΔcnaA.
Deletion of cnaA in E. festucae Fl1 reduces host colonization and induces the formation of intrahyphal hyphae in planta
To determine whether CnaA is involved in host colonization, L. perenne seedlings were inoculated with E. festucae Fl1 WT, ΔcnaA (both NR and R colonies) and ΔcnaA complementation strains (ΔcnaA/cnaA and ΔcnaA/cnaA2), and tested for infection at 8 weeks post‐inoculation. Both ΔcnaA NR and ΔcnaA R colonies showed a significantly decreased infection rate in planta (Fig. 7a, upper panel). The infection rate in planta was restored to WT levels by the introduction of either cnaA or cnaA2 into the ΔcnaA strain (Fig. 7a, upper panel). Further, plants infected with ΔcnaA NR colonies showed a lower number of hyphae per 100 μm of epidermal peel relative to plants infected with WT. However, the significance of this result was marginal (t 3 = 3.198, P = 0.0498).
Figure 7.
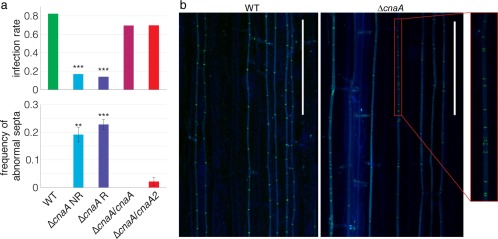
Epichloë festucae Fl1 ΔcnaA shows a reduced infection rate and abnormal septal morphology and patterning in planta. (a) Deletion of cnaA in E. festucae reduces the plant infection rate (upper panel). Deletion of cnaA in E. festucae increases the number of abnormal septa (lower panel). (b) Confocal micrographs of aniline blue‐ and wheat germ agglutinin‐conjugated Alexa Fluor®488 (WGA‐AF488)‐stained hyphae of wild‐type (WT) and ΔcnaA mutant in planta showing irregular septa formation. Bar, 100 μm.
We also investigated the effect of deletion of cnaA on septa formation in planta. For this analysis, we compared the number of hyphae with abnormal septa between plants infected with WT, ΔcnaA and complemented strains. The hyphae from both ΔcnaA NR‐ and ΔcnaA R‐infected plants showed a significant increase in frequency of abnormal septa (Fig. 7a, lower panel). Although the reintroduction of cnaA into ΔcnaA completely restored the septal morphology to WT, with no abnormal septa observed in ΔcnaA/cnaA, the introduction of cnaA2 into ΔcnaA only partially restored the septal morphology, as abnormal septa were observed for ΔcnaA/cnaA2.
We further investigated septa formation in planta by confocal microscopy with aniline blue and wheat germ agglutinin‐conjugated Alexa Fluor®488 (WGA‐AF488) stains, which target β‐glucan (blue pseudocolour) and chitin (green pseudocolour), respectively. The WT hyphae showed characteristic blue‐stained hyphal cell walls with evenly spaced bright green septa (Fig. 7b). In contrast, septa in ΔcnaA were not evenly distributed and were also not as brightly stained as in WT hyphae (Fig. 7b). Hyphae in ΔcnaA contained regions in which multiple septa were formed in very close proximity, suggesting defects in hyphal compartmentalization. Although ΔcnaA hyphae were defective in septum formation, the parallel restrictive growth of ΔcnaA hyphae in planta was similar to that of WT. We further investigated the disruption in the formation of hyphal compartments using light microscopy and TEM. The epidermal peels of infected plants were stained with aniline blue and analysed by light microscopy. This analysis revealed a distinctive constricted pattern in ΔcnaA hyphae (Fig. 8a). As with the structures seen in cultures grown on PDA plates, this pattern resembled the intrahyphal hyphae structures described previously in S. fructigena (Fig. 8b) (Calonge, 1968). The presence of intrahyphal hyphae in planta was confirmed using TEM. In transverse sections, ΔcnaA hyphae showed multiple cell wall layers within a single hypha (Fig. 8c; indicated by white arrowheads).
Figure 8.
Deletion of cnaA in Epichloë festucae Fl1 causes the formation of intrahyphal hyphae in planta. (a) Light micrographs of aniline blue‐stained hyphae of ΔcnaA and wild‐type (WT) showing constricted hyphae and increased septa formation in ΔcnaA relative to WT. Bar, 10 μm. (b) Reproduction of a drawing from Calonge (1968) showing intrahyphal hyphae breaking out from the mother hypha of Sclerotinia fructigena. This schematic diagram captures the light micrograph image of the hyphal structures observed in planta for E. festucae ΔcnaA hyphae. (c) Transmission electron micrographs of wild‐type (WT) and ΔcnaA mutant in planta showing duplication of the cell wall in mutant hyphae, suggesting the presence of intrahyphal hyphae. White arrowheads point to a double cell wall. Bar, 1 μm.
Discussion
Calcineurin plays a very important role in coordinating the cellular responses to Ca2+ signalling from lower eukaryotes to humans, and is essential for pathogenic fungal adaptation to environmental stress, survival, virulence and pathogenicity. It also regulates hyphal growth, cell wall integrity, appressoria development and mating in other fungi. In this study, we investigated the function of the calcineurin catalytic subunit A (cnaA) in the endophytic fungus E. festucae, a mutualistic symbiont of grasses. We generated E. festucae cnaA deletion strains and showed that this gene is very important for fungal growth and in the colonization of the grass host L. perenne.
Calcineurin is a highly conserved protein and consists of two subunits: the catalytic subunit A (CnaA) and the regulatory subunit B (CnaB). Although the genomes of most fungi contain a single cnaA copy, we found two cnaA paralogues in E. festucae E2368 and several other Epichloë strains. The presence of multiple cnaA copies has also been reported for fungi of the Clavicipitaceae family, Beauveria bassiana and Cordyceps militaris, which contain two paralogues each (Li et al., 2015), whereas the genomes of fungi in the Zygomycetes family, Mucor circinelloides and Rhizopus delemar, contain three paralogues each (Lee et al., 2013). These cnaA genes are often flanked with the same repetitive sequences, which might explain the occurrence of multiple cnaA genes through individual gene duplication events (Lee et al., 2013).
Calcineurin has both general and specific roles in different organisms. In some fungi, the calcineurin catalytic subunit is an essential gene, including in A. nidulans, where it plays a role in growth arrest at an early stage of the cell cycle (Alshahni et al., 2016; Prokisch et al., 1997; Rasmussen et al., 1994; Viaud et al., 2003). However, in most fungi, the deletion or knockdown of the calcineurin catalytic subunit results in defective hyphal morphology. Such strains with an impaired calcineurin signalling pathway show severely restricted growth phenotypes, with compact colonies and highly branched and stunted hyphae (Choi et al., 2009b; Egan et al., 2009; Harren et al., 2012; Odom et al., 1997; Steinbach et al., 2006). Although E. festucae cnaA is not an essential gene, the deletion of cnaA caused a severe growth defect in E. festucae. The mutant strain showed markedly reduced radial growth, with irregular, highly branched hyphae and loss of aerial hyphae. In addition, the ΔcnaA mutant showed abnormal septation, with unevenly distributed and irregularly spaced septa that were often in close proximity, resulting in high variability in hyphal compartment length. These phenotypes are similar to the ΔcnaA mutant phenotypes of B. cinerea and A. fumigatus (Harren et al., 2012; Juvvadi et al., 2011). Interestingly, although individual A. fumigatus ΔcnaA and ΔcnaB strains showed abnormal septa that were curved and wavy, incomplete septation was only observed in A. fumigatus ΔcnaA/ΔcnaB double‐deletion strains (Juvvadi et al., 2011). In contrast, we observed incomplete septation in the single‐deletion ΔcnaA strain. Abnormal septation was also observed for chitin synthase deletion strains in A. nidulans and A. fumigatus (Fukuda et al., 2009; Ichinomiya et al., 2005; Muszkieta et al., 2014). These defective hyphal morphology and abnormal septation features of ΔcnaA mutants indicate changes in cell wall composition. In filamentous fungi, chitin is a major component of the cell wall that is progressively deposited at the hyphal tips and septa during hyphal growth. In our ΔcnaA mutant strain, we observed disrupted distribution of chitin along the hyphae. In other fungi, a link between remodelling of chitin and glucan cell wall components and the calcineurin signalling pathway has been shown. For example, in A. fumigatus, calcineurin has been shown to control β‐glucan and chitin levels through the down‐regulation of β‐1,3‐glucan synthase catalytic subunit and chitin synthase‐encoding genes (Cramer et al., 2008; Fortwendel et al., 2009; Juvvadi et al., 2011). Similar down‐regulation of glucan synthase‐encoding genes as a result of calcineurin pathway impairment has also been observed in M. oryzae, C. albicans and Beauveria bassiana (Choi et al., 2009a; Li et al., 2015; Sanglard et al., 2003). In contrast, the expression of U. hordei and C. neoformans glucan synthase‐encoding genes was increased in a calcineurin‐dependent manner (Cervantes‐Chavez et al., 2011; Kraus et al., 2003). These observations suggest that components of the calcineurin pathway act via distinct mechanisms for the remodelling of cell wall components in ascomycetes versus basidiomycetes. Interactions between the components of protein kinase C, high‐osmolarity glycerol (HOG) and calcineurin pathways have been observed to regulate chitin and glucan synthesis (Cervantes‐Chavez et al., 2011; Juvvadi and Steinbach, 2015; Munro et al., 2007). Interestingly, calcineurin also interacts with the components of the HOG pathway to regulate the synthesis of secondary metabolites, as shown in Arthroderma vanbreuseghemii, where calcineurin A affects the production of a yellow pigment under hyperosmotic conditions (Alshahni et al., 2016). In our study, the ΔcnaA strain secreted a yellow pigment into the agar medium that was not observed for the WT strain. Yellow pigment production, together with the hyphal growth defects observed in the mutant strain, were remediated in the presence of osmotic stabilizers.
The localization of calcineurin subunits at the hyphal tip and septum, and calcineurin complex activity at the hyphal septum, is essential for normal hyphal elongation and proper septation (Juvvadi et al., 2011). Various domains in calcineurin have been implicated for localization and function at the septum. The FMDVF motif that forms a bridge between two known substrate‐binding motifs, PxIxIT and LxVP, is required for septal localization and calcineurin function (Juvvadi et al., 2016). Epichloë festucae CnaA also possesses this motif, together with the serine–proline‐rich region (SPRR) proposed to be important for hyphal growth and virulence through phosphorylation of the SPRR domain (Juvvadi et al., 2013).
In addition to aberrant hyphal morphology and septation, the ΔcnaA strain also formed intrahyphal hyphae, both in culture and in planta. The formation of intrahyphal hyphae is widespread in fungi, occurring in response to hyphal and/or cellular damage (Bowman et al., 2006; Calonge, 1968; Takeshita et al., 2006). Such structures have been observed previously in CWI MAP kinase, membrane‐associated protein and component of striatin‐interacting phosphatase and kinase complex mutants of E. festucae; chitin synthase mutants of A. nidulans, Fusarium graminearum and Colletotrichum graminicola; and glycosylphosphatidylinositol synthesis gene mutants of N. crassa and C. graminicola (Becker et al., 2015; Bowman et al., 2006; Green et al., 2016, 2017; Horiuchi et al., 1999; Kim et al., 2009; Oliveira‐Garcia and Deising, 2016; Werner et al., 2007). In A. fumigatus, intrahyphal hyphae formation was promoted by treatment with caspofungin, which inhibits β‐1,3‐glucan synthase (Walker et al., 2015). These observations suggest that the formation of intrahyphal hyphae derives from defects in CWI, and it has been proposed to promote fungal survival under stress conditions (Kim and Hyun, 2007). Although the mechanisms governing the formation of intrahyphal hyphae are not clear, Woronin body sealing of septal pores has been proposed to play an important role (Becker et al., 2016).
Calcineurin is also required for virulence and pathogenesis in diverse pathogenic fungi, including C. neoformans, C. albicans, A. fumigatus, Sclerotinia sclerotiorum, M. oryzae, U. maydis, U. hordei and B. cinerea, but acts through distinct molecular mechanisms (Blankenship et al., 2003; Cervantes‐Chavez et al., 2011; Choi et al., 2009b; Cruz et al., 2001; Egan et al., 2009; Harel et al., 2006; Odom et al., 1997; Schumacher et al., 2008; da Silva Ferreira et al., 2007; Steinbach et al., 2006). Although E. festucae forms a symbiotic interaction with L. perenne, the NADPH oxidase nox mutants have been shown to cause a defence response in ryegrass plants, inducing cell wall thickening and increased callose deposition (Becker et al., 2016). Inoculation of L. perenne seedlings with ΔcnaA induced a necrosis response. One hypothesis is that the yellow pigment produced by ΔcnaA NR acts as an elicitor. This hypothesis is supported by observations that the ΔcnaA R strain, which did not produce the yellow pigment and was similar to the WT strain in terms of hyphal morphology, failed to induce a necrosis response. However, it remains to be investigated whether this yellow pigment is also produced by the mutant strain in planta. The hyphal morphology of ΔcnaA in planta was comparable with that in culture, except for the lack of hyper‐branching, with defects in cell wall organization, abnormal septation and intrahyphal hyphae formation. The ΔcnaA strain showed significantly reduced host colonization. In filamentous plant‐pathogenic fungi, the calcineurin pathway has been shown to be important for virulence by affecting infection‐related morphogenesis (Choi et al., 2009b; Egan et al., 2009). In B. cinerea, defects in the calcineurin pathway result in defective mycelium‐derived infection (Schumacher et al., 2008). This is improved by Mg2+ supplementation, suggesting that cell wall stability contributes to infection and subsequent hyphal growth. Although E. festucae forms expressoria (appressorium‐like structures that allow endophytic hyphae to exit the host through the cuticle), appressoria‐like structures that allow entry to the host have not been observed (Becker et al., 2016). Based on these observations and our study, we suggest that the reduced colonization of plants by the ΔcnaA strain is caused by defects in cell wall stability.
In summary, we have identified and functionally characterized the calcineurin catalytic subunit A gene (cnaA) in E. festucae. We have shown that E. festucae cnaA plays an important role in hyphal growth, cell wall organization and intrahyphal hyphae formation, both in culture and in planta. Calcineurin A also plays a significant role in the colonization of the host plant.
Experimental Procedures
Strains and growth conditions
Escherichia coli cultures were routinely grown in lysogeny broth (LB) or on LB agar containing ampicillin (100 μg/mL), as described previously (Miller, 1972).
Epichloë festucae cultures were routinely grown on 2.4% PDA or in potato dextrose broth (PDB), as described previously (Moon et al., 2000, 1999). For genomic DNA extraction for PCR screening, E. festucae cultures were grown from mycelia (approximately 1‐cm2 section) in 150 μL of 2.4% PDB for 3 days. A description of all biological materials used in this study is given in Table S2 (see Supporting Information).
Plant growth and endophyte inoculation conditions
Endophyte‐free seedlings of perennial ryegrass L. perenne cv. Samson were inoculated with E. festucae, as described previously (Latch and Christensen, 1985). Plants were grown in root trainers in an environmentally controlled growth room at 22 °C with a photoperiod of 16 h light (approximately 100 μE/m2/s). At 8 weeks post‐inoculation, plants were tested for endophyte infection by immunoblotting (Tanaka et al., 2005).
DNA extraction, PCR and sequencing
Plasmid DNA was extracted using a High Pure Plasmid Isolation Kit (Roche, Basel, Switzerland) according to the manufacturer's instructions. Fungal genomic DNA for PCR screening to identify gene replacement mutants was extracted from mycelia. These mycelia were grown in 150 μL of 2.4% PDB, collected by centrifugation and ground in 150 μL of lysis buffer [100 mm tris(hydroxymethyl)aminomethane (Tris), 100 mm sodium ethylenediaminetetraacetate (Na2‐EDTA), 1% sodium dodecylsulfate (SDS), pH 8.0]. The mixture was incubated at 70 °C for 30 min, followed by the addition of 150 μL of 5 m potassium acetate and a further 10 min of incubation on ice. Samples were then centrifuged at 17,000 g for 10 min. The supernatant was transferred into a fresh Eppendorf tube and 0.6 vol of isopropanol was added. DNA was pelleted by centrifugation at 17,000 g for 15 min. The pelleted DNA was washed in 70% ethanol, air dried and resuspended in 20 μL of sterile milli‐Q water. A 2‐μL sample was used as template for PCR. Fungal genomic DNA for Southern analysis and for the generation of fragments used in plasmid construction was extracted from freeze‐dried mycelia, as described previously (Byrd et al., 1990).
Standard PCR and PCR screening were performed with Taq DNA polymerase (Roche) according to the manufacturer's instructions in a 50‐μL reaction volume. When proofreading activity was required, PCR was performed with Pfx50™ DNA polymerase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions.
DNA fragments were sequenced using the dideoxynucleotide chain termination method with the Big‐Dye Terminator Version 3.1 Ready Reaction Cycle Sequencing Kit (Applied BioSystems, Carlsbad, CA, USA) and separated using an ABI3730 genetic analyser (Applied BioSystems, MacVector Inc., Apex, NC, USA). Sequence data were assembled and analysed using MacVector sequence assembly software, version 12.0.5.
Preparation of deletion and complementation constructs
The lists of all plasmids and primers used in the preparation of the constructs are given in Tables S2 and S3 (see Supporting Information).
The E. festucae Fl1 cnaA and E. festucae E2368 cnaA1 replacement construct pMMI4 was generated by cloning restriction enzyme‐digested fragments from E. festucae Fl1 cosmid 2C5. This cosmid was isolated from an E. festucae Fl1 cosmid library (Tanaka et al., 2005) by colony hybridization using a probe specific for Fl1 cnaA, which was amplified with primers MI13 and MI14 using E. festucae Fl1 genomic DNA as template. A 1.4‐kb PstI/BamHI fragment 5′ of cnaA was cloned 3′ of the hph cassette and a 2.1‐kb EcoRI/EcoRV fragment 3′ of cnaA was cloned 5′ of the hph cassette in the plasmid pSF15.15, generating the cnaA/cnaA1 replacement construct pMMI4. The E. festucae E2368 cnaA2 replacement construct pMMI13 was generated using PCR‐amplified fragments. A 1.95‐kb PCR fragment 5′ of cnaA2 was amplified with primers 1372‐2F and cna1372R and cloned into the vector pCR4‐TOPO. From the resultant plasmid, a 1.7‐kb EcoRI fragment containing the 5′ cnaA2 flanking sequence was obtained and cloned into plasmid pSF17.8. A 1.28‐kb PCR fragment 3′ of cnaA2 was amplified with primers cna2F and 1372‐5R, and cloned into the vector pCR4‐TOPO. From the resultant plasmid, a 1.5‐kb EcoRI fragment containing the 3′ cnaA2 flanking sequence was obtained and cloned into plasmid pSF13.5. From this plasmid, a 1.5‐kb XbaI fragment containing the 3′ cnaA2 flanking sequence was obtained and cloned into plasmid pSF17.8 containing the 5′ cnaA2 flanking sequence, giving the cnaA2 replacement construct pMMI13. The cnaA complementation construct pMMI11 was prepared by cloning a 6‐kb EcoRV fragment from cosmid 2C5 into plasmid pSF17.8. The cnaA2 complementation construct pMMI12 was prepared by cloning a 4.5‐kb PCR fragment amplified with primers 1372‐2F and 1372‐5R into the vector pII99.
Epichloë festucae transformation and screening for mutants
Epichloë festucae protoplasts were prepared as described previously (Young et al., 2005). However, because of its slow growth, mycelia for the preparation of E. festucae Fl1 ΔcnaA protoplasts were grown for 10 days instead of 7 days. Protoplasts were transformed with 5 μg of linear PCR‐amplified, restriction enzyme‐digested or circular plasmid DNA using the method described previously (Itoh et al., 1994). Transformants were selected on regeneration medium (PDA medium supplemented with 0.8 m sucrose) containing either hygromycin (150 μg/mL) or geneticin (200 μg/mL), and nuclear‐purified by three rounds of subculture on selection medium.
To generate E. festucae Fl1 cnaA and E. festucae E2368 cnaA1 deletion strains, protoplasts of these strains were transformed with a linear 5‐kb HindIII/EcoRV fragment from pMMI4, and transformants were selected on medium containing hygromycin. Transformants were screened with primers MI13 and MI14 to distinguish WT (1‐kb) from replacement sequences (no PCR product). To generate E. festucae E2368 cnaA2 deletion strain, E2368 protoplasts were transformed with a linear 5‐kb XhoI/HpaI fragment from pMMI13, and transformants were selected on medium containing geneticin. Transformants were screened with primers MI15 and MI16 to distinguish WT (1‐kb) from replacement sequences (no PCR product). For complementation of the E. festucae Fl1 ΔcnaA strain, ΔcnaA protoplasts were transformed with either pMMI11 (containing E. festucae Fl1 cnaA) or pMMI12 (containing E. festucae E2368 cnaA2), and transformants were selected on medium containing geneticin. Reintroduction of cnaA or cnaA2 was confirmed by PCR using primers MI13 and MI14 (for cnaA) or MI15 and MI16 (for cnaA2).
DNA hybridization
Epichloë festucae genomic DNA digests were separated by agarose gel electrophoresis and transferred to positively charged nylon membranes (Roche). DNA was then fixed to membrane by UV light cross‐linking in a Cex‐800 UV light cross‐linker (Ultra‐Lum, Claremont, CA, USA) at 254 nm for 2 min. DNA probes were labelled with [α‐32P]‐dCTP (3000 Ci/mmol; Amersham Biosciences, Buckinghamshire, UK) using a High Prime DNA labelling kit (Roche), and hybridizations were performed according to the manufacturer's instructions. Hybridized bands were visualized by autoradiography.
Microscopy
Epichloë festucae cultures for examination by microscopy were taken directly from PDA plates by excising a 0.2‐cm2 piece of mycelium from the plate, placed on a microscope slide and covered with a cover slip. Bright field microscopy was performed using a Zeiss (Oberkochen, Germany) Axiophot light microscope and images were recorded using a Leica (Wetzlar, Germany) DCF320 digital camera. Light microscopy was used to examine the growth of 6‐day‐old E. festucae strains in culture by DIC, using a ×100 immersion objective. Calcofluor white staining was performed by immersing mycelia in 3 mg/mL calcofluor solution (Sigma, St. Louis, MO, USA), incubation in the dark for 30 min and observation under an Olympus IX71 inverted fluorescence microscope using a ×60 immersion objective and the filter setting for the capture of calcofluor white.
Epichloë festucae hyphae in planta were visualized by taking epidermal peels from the inner surfaces of the outer leaf sheaths and mounting them in aniline blue stain [88% lactic acid, 50% glycerol, 0.1% (w/v) aniline blue], which stains hyphal cytoplasm. The leaf was immersed in aniline blue stain, covered with a cover slip, heated briefly to aid penetration of the stain and examined by light microscopy using ×20 and ×100 objectives. Confocal microscopy was performed using a Leica SP5 DM6000B confocal microscope. Confocal microscopy was used to visualize chitin distribution and hyphae and septa in the in planta sample employing WGA‐AF488 staining (Molecular Probes/Invitrogen, Eugene, OR, USA). For WGA‐AF488 staining, infected plant tissue was treated overnight in 95% ethanol at 4 °C, and then with 10% potassium hydroxide for 3 h. Tissue was then washed three times with phosphate buffered saline (PBS) (pH 7.4) and incubated in staining solution (0.2% aniline blue, 0.2% Tween‐20, 0.01 mg/mL WGA‐AF488 in PBS, pH 7.4). Confocal images were taken using a ×40 objective with excitation at 405 nm (aniline blue) and 488 nm (WGA‐AF488). Images were taken as a stack of six focal planes with 2‐μm z‐steps, giving a total thickness of 10 μm. TEM was performed using a Philips (Amsterdam, The Netherlands) CM10 transmission electron microscope and images were recorded using an SIS Morada digital camera. Samples for TEM were taken from either 6‐day‐old cultures or 8‐week‐old plants. Plant samples were prepared by cutting transversely through the pseudostem. Tissues were then fixed in 3% glutaraldehyde and 2% formaldehyde in 0.1 m phosphate buffer, pH 7.2, for 1 h, and transverse sections were prepared for TEM, as described previously (Spiers and Hopcroft, 1993).
Abnormal hyphal morphology in planta was analysed using ×100 aniline blue images. Twenty images were taken per plant, four plants per strain, except for ΔcnaA/cnaA where three plants were used. Images were taken at sequential lateral locations that contained hyphae (relative to the leaf axis) with no overlap. Images were analysed by counting the total number of hyphae and the number of hyphae showing abnormal morphology. Hyphae were considered to be ‘abnormal’ if they had swollen regions, were not straight or showed irregular septa. The fraction of abnormal hyphae was then calculated for each plant and the results were compared using a two‐sided Student's t‐test.
The number of hyphae in planta was analysed using ×20 aniline blue images. The entire lateral width and depth of each peel were imaged at a single longitudinal location using ×20 bright field images. Imaging through the entire depth of each peel was achieved by taking five images at 6‐µm z‐step distances apart, starting just below the peel and finishing just above the slide, resulting in a total image depth of approximately 24 μm for each peel. The image analysis program Corel (Ottawa, Ontario, Canada) Draw was used to draw and measure a line through the centre of each peel perpendicular to the leaf axis and to count the number of hyphae that crossed this line. By pooling the data from all peels of each plant, the number of hyphae per 100 μm can be generated and compared between different plants and symbioses. Results were compared using a two‐sided Student's t‐test.
Infection rate analysis
The infection rates of WT, mutant and complementation strains were analysed by inoculating 216 L. perenne seedlings per strain over a 3‐week period. The significance of infection rate differences between strains was analysed using Fisher's exact test (two‐tailed). Infection statistics were performed on surviving plants only, but no statistically significant differences in host death frequency were observed between different strains.
Bioinformatics analysis
Epichloë festucae genomic sequences were obtained from the E. festucae Genome Projects at the University of Kentucky (Schardl et al., 2013), and other gene and protein sequences were obtained from the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/). Multiple sequence alignments and identity scores were generated by clustal W in MacVector version 12.0.5 using the default settings.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 cnaA copy number varies amongst strains of Epichloë species. (a) Autoradiograph of DNA blot of BamHI genomic digests of E. festucae strains. (b) Autoradiograph of DNA blot of BamHI genomic digests of Epichloë species, including Epichloë elymi (WWG1, WWG3, E184), Epichloë brachyelytri (E1040, E1124), Epichloë bromicola (E501, E799), Epichloë baconii (E424), Epichloë typhina (E425 and E505) and Epichloë typhina ssp. poae (E1022). Both DNA blots were probed with a [32P]‐labelled cnaA2‐containing polymerase chain reaction (PCR) fragment that was generated with primers 1372‐2F and 1372‐5R using E. festucae E2368 genomic DNA as the template. This probe was expected to hybridize to BamHI fragments of 8.8, 5.9 and 1.3 kb in E. festucae E2368. Four strains of E. festucae (E189, E2368, Fr1, 1035.33) showed the expected hybridization pattern (*). However, three strains of other Epichloë spp. (WWG1, WWG3, E424) showed a similar, but not identical, pattern of hybridization (#). A difference in the size of the largest hybridization band present in E. elymi strains, as well as the absence of the two largest bands in E. baconii, suggest deletions in these genomes.
Fig. S2 Deletion of Epichloë festucae Fl1 cnaA. (a) Physical map of the cnaA wild‐type (WT) genomic region, linear insert of cnaA deletion construct pMMI4 and cnaA complementation construct pMMI11, showing restriction enzyme sites for PstI (P), BamHI (B), EcoRI (E), EcoRV (EV), HindIII (H) and NdeI (N). (b) Autoradiograph of a Southern blot of NdeI genomic DNA digests of E. festucae Fl1 wild‐type (WT) and cnaA deletion strains (ΔcnaA), probed with [32P]‐labelled pMMI4. The expected sizes of the fragments are shown.
Fig. S3 Remediation of Epichloë festucae Fl1 ΔcnaA culture phenotype. Remediation of the ΔcnaA culture phenotype (secretion of yellow pigment) observed when a plug of the mutant was grown on potato dextrose agar (PDA) by spreading ground mycelia on PDA or by subculture of a plug of the mutant on PDA supplemented with MgCl2.
Fig. S4 Deletion of Epichloë festucae E2368 cnaA1 and cnaA2. (a) Physical map of the cnaA1 wild‐type (WT) genomic region, linear insert of cnaA replacement construct pMMI4, showing restriction enzyme sites for PstI (P), BamHI (B), EcoRI (E), EcoRV (EV) and HindIII (H). The arrowheads indicate the primers used for screening of the replacement event. (b) Polymerase chain reaction (PCR) products of the expected size generated with the primer pair MI13 and MI14, specific for cnaA1. (c) Physical map of the cnaA2 wild‐type (WT) genomic region, linear insert of cnaA2 deletion construct pMMI13 and cnaA2 complementation construct pMMI12, used to complement the E. festucae Fl1 ΔcnaA strain. The arrowheads indicate the primers used for preparation of the deletion and complementation constructs and for screening of the replacement event. (d) PCR products of the expected size generated with primer pair MI15 and MI16, specific for cnaA2.
Table S1 Calcineurin A‐encoding genes in sequenced Epichloë genomes.
Table S2 Biological material.
Table S3 Primers used in this study.
Acknowledgements
The authors thank Matthew Savoian, Jordan Taylor and Niki Murray (Manawatu Microscopy and Imaging Centre, Massey University, Palmerston North, New Zealand), and Arvina Ram (Massey University), for technical assistance.
References
- Alshahni, M.M. , Shimizu, K. , Yoshimoto, M. , Yamada, T. , Nishiyama, Y. , Arai, T. and Makimura, K. (2016) Genetic and phenotypic analyses of calcineurin A subunit in Arthroderma vanbreuseghemii . Med. Mycol. 54, 207–218. [DOI] [PubMed] [Google Scholar]
- Becker, M. , Becker, Y. , Green, K. and Scott, B. (2016) The endophytic symbiont Epichloë festucae establishes an epiphyllous net on the surface of Lolium perenne leaves by development of an expressorium, an appressorium‐like leaf exit structure. New Phytol. 211, 240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, Y. , Eaton, C.J. , Brasell, E. , May, K.J. , Becker, M. , Hassing, B. , Cartwright, G.M. , Reinhold, L. and Scott, B. (2015) The fungal cell‐wall integrity MAPK cascade is crucial for hyphal network formation and maintenance of restrictive growth of Epichloë festucae in symbiosis with Lolium perenne . Mol. Plant–Microbe Interact. 28, 69–85. [DOI] [PubMed] [Google Scholar]
- Blankenship, J.R. , Wormley, F.L. , Boyce, M.K. , Schell, W.A. , Filler, S.G. , Perfect, J.R. and Heitman, J. (2003) Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell, 2, 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, S.M. , Piwowar, A. , Al Dabbous, M. , Vierula, J. and Free, S.J. (2006) Mutational analysis of the glycosylphosphatidylinositol (GPI) anchor pathway demonstrates that GPI‐anchored proteins are required for cell wall biogenesis and normal hyphal growth in Neurospora crassa . Eukaryot. Cell, 5, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, A.D. , Schardl, C.L. , Songlin, P.J. , Mogen, K.L. and Siegel, M.R. (1990) The beta‐tubulin gene of Epichloë typhina from perennial ryegrass (Lolium perenne). Curr. Genet. 18, 347–354. [DOI] [PubMed] [Google Scholar]
- Calonge, F.D. (1968) Origin and development of intrahyphal hyphae in Sclerotinia fructigena . Mycologia, 60, 932–942. [Google Scholar]
- Cervantes‐Chavez, J.A. , Ali, S. and Bakkeren, G. (2011) Response to environmental stresses, cell‐wall integrity, and virulence are orchestrated through the calcineurin pathway in Ustilago hordei . Mol. Plant–Microbe Interact. 24, 219–232. [DOI] [PubMed] [Google Scholar]
- Choi, J. , Kim, Y. , Kim, S. , Park, J. and Lee, Y.H. (2009a) MoCRZ1, a gene encoding a calcineurin‐responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae . Fungal Genet. Biol. 46, 243–254. [DOI] [PubMed] [Google Scholar]
- Choi, J.H. , Kim, Y. and Lee, Y.H. (2009b) Functional analysis of MCNA, a gene encoding a catalytic subunit of calcineurin, in the rice blast fungus Magnaporthe oryzae . J. Microbiol. Biotechnol. 19, 11–16. [PubMed] [Google Scholar]
- Cramer, R.A. Jr. , Perfect, B.Z. , Pinchai, N. , Park, S. , Perlin, D.S. , Asfaw, Y.G. , Heitman, J. , Perfect, J.R. and Steinbach, W.J. (2008) Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus . Eukaryot. Cell, 7, 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, M.C. , Fox, D.S. and Heitman, J. (2001) Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans . EMBO J. 20, 1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Ferreira, M.E.D.S. , Heinekamp, T. , Härtl, A. , Brakhage, A.A. , Semighini, C.P. , Harris, S.D. , Savoldi, M. , de Gouvêa, P.F. , Goldman, M.H.D.S. and Goldman, G.H. (2007) Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 44, 219–230. [DOI] [PubMed] [Google Scholar]
- Eaton, C.J. , Cox, M.P. , Ambrose, B. , Becker, M. , Hesse, U. , Schardl, C.L. and Scott, B. (2010) Disruption of signaling in a fungal–grass symbiosis leads to pathogenesis. Plant Physiol. 153, 1780–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan, J.D. , Garcia‐Pedrajas, M.D. , Andrews, D.L. and Gold, S.E. (2009) Calcineurin is an antagonist to PKA protein phosphorylation required for postmating filamentation and virulence, while PP2A is required for viability in Ustilago maydis . Mol. Plant–Microbe Interact. 22, 1293–1301. [DOI] [PubMed] [Google Scholar]
- Fortwendel, J.R. , Juvvadi, P.R. , Pinchai, N. , Perfect, B.Z. , Alspaugh, J.A. , Perfect, J.R. and Steinbach, W.J. (2009) Differential effects of inhibiting chitin and 1,3‐β‐D‐glucan synthesis in Ras and calcineurin mutants of Aspergillus fumigatus . Antimicrob. Agents Chemother. 53, 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, K. , Yamada, K. , Deoka, K. , Yamashita, S. , Ohta, A. and Horiuchi, H. (2009) Class III chitin synthase ChsB of Aspergillus nidulans localizes at the sites of polarized cell wall synthesis and is required for conidial development. Eukaryot. Cell, 8, 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, K.A. , Becker, Y. , Fitzsimons, H.L. and Scott, B. (2016) An Epichloë festucae homologue of MOB3, a component of the STRIPAK complex, is required for the establishment of a mutualistic symbiotic interaction with Lolium perenne . Mol. Plant Pathol. 17, 1480–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, K.A. , Becker, Y. , Tanaka, A. , Takemoto, D. , Fitzsimons, H.L. , Seiler, S. , Lalucque, H. , Silar, P. and Scott, B. (2017) SymB and SymC, two membrane associated proteins, are required for Epichloë festucae hyphal cell–cell fusion and maintenance of a mutualistic interaction with Lolium perenne . Mol. Microbiol. 103, 657–677. [DOI] [PubMed] [Google Scholar]
- Hahn, H. , McManus, M.T. , Warnstorff, K. , Monahan, B.J. , Young, C.A. , Davies, E. , Tapper, B.A. and Scott, B. (2008) Neotyphodium fungal endophytes confer physiological protection to perennial ryegrass (Lolium perenne L.) subjected to a water deficit. Environ. Exp. Bot. 63, 183–199. [Google Scholar]
- Hameed, S. , Dhamgaye, S. , Singh, A. , Goswami, S.K. and Prasad, R. (2011) Calcineurin signaling and membrane lipid homeostasis regulates iron mediated multidrug resistance mechanisms in Candida albicans . PLoS One, 6, e18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel, A. , Bercovich, S. and Yarden, O. (2006) Calcineurin is required for sclerotial development and pathogenicity of Sclerotinia sclerotiorum in an oxalic acid‐independent manner. Mol. Plant–Microbe Interact. 19, 682–693. [DOI] [PubMed] [Google Scholar]
- Harren, K. , Schumacher, J. and Tudzynski, B. (2012) The Ca2+/calcineurin‐dependent signaling pathway in the gray mold Botrytis cinerea: the role of calcipressin in modulating calcineurin activity. PLoS One, 7, e41761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi, H. , Fujiwara, M. , Yamashita, S. , Ohta, A. and Takagi, M. (1999) Proliferation of intrahyphal hyphae caused by disruption of csmA, which encodes a class V chitin synthase with a myosin motor‐like domain in Aspergillus nidulans . J. Bacteriol. 181, 3721–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinomiya, M. , Yamada, E. , Yamashita, S. , Ohta, A. and Horiuchi, H. (2005) Class I and class II chitin synthases are involved in septum formation in the filamentous fungus Aspergillus nidulans . Eukaryot. Cell, 4, 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, Y. , Johnson, R. and Scott, B. (1994) Integrative transformation of the mycotoxin‐producing fungus, Penicillium paxilli . Curr. Genet. 25, 508–513. [DOI] [PubMed] [Google Scholar]
- Juvvadi, P.R. , Fortwendel, J.R. , Rogg, L.E. , Burns, K.A. , Randell, S.H. and Steinbach, W.J. (2011) Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus . Mol. Microbiol. 82, 1235–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi, P.R. , Gehrke, C. , Fortwendel, J.R. , Lamoth, F. , Soderblom, E.J. , Cook, E.C. , Hast, M.A. , Asfaw, Y.G. , Moseley, M.A. , Creamer, T.P. and Steinbach, W.J. (2013) Phosphorylation of calcineurin at a novel serine–proline rich region orchestrates hyphal growth and virulence in Aspergillus fumigatus . PLoS Pathog. 9, e1003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi, P.R. , Pemble, C.W. , Ma, Y. and Steinbach, W.J. (2016) Novel motif in calcineurin catalytic subunit is required for septal localization of calcineurin in Aspergillus fumigatus . FEBS Lett. 590, 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi, P.R. and Steinbach, W.J. (2015) Calcineurin orchestrates hyphal growth, septation, drug resistance and pathogenesis of Aspergillus fumigatus: where do we go from here? Pathogens, 4, 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.W. and Hyun, J.W. (2007) Nonhost‐associated proliferation of intrahyphal hyphae of citrus scab fungus Elsinoe fawcettii: refining the perception of cell‐within‐a‐cell organization. Micron, 38, 565–571. [DOI] [PubMed] [Google Scholar]
- Kim, J.E. , Lee, H.J. , Lee, J. , Kim, K.W. , Yun, S.H. , Shim, W.B. and Lee, Y.W. (2009) Gibberella zeae chitin synthase genes, GzCHS5 and GzCHS7, are required for hyphal growth, perithecia formation, and pathogenicity. Curr. Genet. 55, 449–459. [DOI] [PubMed] [Google Scholar]
- Kraus, P.R. , Fox, D.S. , Cox, G.M. and Heitman, J. (2003) The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48, 1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latch, G.C.M. and Christensen, M.J. (1985) Artificial infection of grasses with endophytes. Ann. Appl. Biol. 107, 17–24. [Google Scholar]
- Lee, S.C. , Li, A. , Calo, S. and Heitman, J. (2013) Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides . PLoS Pathog. 9, e1003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchtmann, A. , Bacon, C.W. , Schardl, C.L. , White, J.F. Jr. and Tadych, M. (2014) Nomenclatural realignment of Neotyphodium species with genus Epichloë . Mycologia, 106, 202–215. [DOI] [PubMed] [Google Scholar]
- Li, F. , Wang, Z.L. , Zhang, L.B. , Ying, S.H. and Feng, M.G. (2015) The role of three calcineurin subunits and a related transcription factor (Crz1) in conidiation, multistress tolerance and virulence in Beauveria bassiana . Appl. Microbiol. Biotechnol. 99, 827–840. [DOI] [PubMed] [Google Scholar]
- Miller, J.H. (1972) Experiments in Molecular Genetics. New York: Cold Spring Harbour Laboratory Press. [Google Scholar]
- Moon, C.D. , Scott, B. , Schardl, C.L. and Christensen, M.J. (2000) The evolutionary origins of Epichloë endophytes from annual ryegrasses. Mycologia, 92, 1103–1118. [Google Scholar]
- Moon, C.D. , Tapper, B.A. and Scott, B. (1999) Identification of Epichloë endophytes in planta by a microsatellite‐based PCR fingerprinting assay with automated analysis. Appl. Environ. Microbiol. 65, 1268–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, C.A. , Selvaggini, S. , de Bruijn, I. , Walker, L. , Lenardon, M.D. , Gerssen, B. , Milne, S. , Brown, A.J. and Gow, N.A. (2007) The PKC, HOG and Ca2+ signalling pathways co‐ordinately regulate chitin synthesis in Candida albicans . Mol. Microbiol. 63, 1399–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszkieta, L. , Aimanianda, V. , Mellado, E. , Gribaldo, S. , Alcàzar‐Fuoli, L. , Szewczyk, E. , Prevost, M.‐C. and Latgé, J.‐P. (2014) Deciphering the role of the chitin synthase families 1 and 2 in the in vivo and in vitro growth of Aspergillus fumigatus by multiple gene targeting deletion. Cell Microbiol. 16, 1784–1805. [DOI] [PubMed] [Google Scholar]
- Odom, A. , Muir, S. , Lim, E. , Toffaletti, D.L. , Perfect, J. and Heitman, J. (1997) Calcineurin is required for virulence of Cryptococcus neoformans . EMBO J. 16, 2576–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira‐Garcia, E. and Deising, H.B. (2016) The glycosylphosphatidylinositol anchor biosynthesis genes GPI12, GAA1, and GPI8 are essential for cell‐wall integrity and pathogenicity of the maize anthracnose fungus Colletotrichum graminicola . Mol. Plant–Microbe Interact. 29, 889–901. [DOI] [PubMed] [Google Scholar]
- Prokisch, H. , Yarden, O. , Dieminger, M. , Tropschug, M. and Barthelmess, I.B. (1997) Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol. Gen. Genet. 256, 104–114. [DOI] [PubMed] [Google Scholar]
- Rasmussen, C. , Garen, C. , Brining, S. , Kincaid, R.L. , Means, R.L. and Means, A.R. (1994) The calmodulin‐dependent protein phosphatase catalytic subunit (calcineurin A) is an essential gene in Aspergillus nidulans . EMBO J. 13, 3917–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumi‐Masante, J. , Rusinga, F.I. , Lester, T.E. , Dunlap, T.B. , Williams, T.D. , Dunker, A.K. , Weis, D.D. and Creamer, T.P. (2012) Structural basis for activation of calcineurin by calmodulin. J. Mol. Biol. 415, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak, F. and Mertz, P. (2000) Calcineurin: form and function. Physiol. Rev. 80, 1483–1521. [DOI] [PubMed] [Google Scholar]
- Sanglard, D. , Ischer, F. , Marchetti, O. , Entenza, J. and Bille, J. (2003) Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48, 959–976. [DOI] [PubMed] [Google Scholar]
- Schardl, C.L. , Leuchtmann, A. and Spiering, M.J. (2004) Symbioses of grasses with seedborne fungal endophytes. Annu. Rev. Plant Biol. 55, 315–340. [DOI] [PubMed] [Google Scholar]
- Schardl, C.L. , Young, C.A. , Hesse, U. , Amyotte, S.G. , Andreeva, K. , Calie, P.J. , Fleetwood, D.J. , Haws, D.C. , Moore, N. , Oeser, B. , Panaccione, D.G. , Schweri, K.K. , Voisey, C.R. , Farman, M.L. , Jaromczyk, J.W. , Roe, B.A. , O'Sullivan, D.M. , Scott, B. , Tudzynski, P. , An, Z. , Arnaoudova, E.G. , Bullock, C.T. , Charlton, N.D. , Chen, L. , Cox, M. , Dinkins, R.D. , Florea, S. , Glenn, A.E. , Gordon, A. , Güldener, U. , Harris, D.R. , Hollin, W. , Jaromczyk, J. , Johnson, R.D. , Khan, A.K. , Leistner, E. , Leuchtmann, A. , Li, C. , Liu, JGe. , Liu, J. , Liu, M. , Mace, W. , Machado, C. , Nagabhyru, P. , Pan, J. , Schmid, J. , Sugawara, K. , Steiner, U. , Takach, J.E. , Tanaka, E. , Webb, J.S. , Wilson, E.V. , Wiseman, J.L. , Yoshida, R. and Zeng, Z. (2013) Plant‐symbiotic fungi as chemical engineers: multi‐genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 9, e1003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, J. , de Larrinoa, I.F. and Tudzynski, B. (2008) Calcineurin‐responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot. Cell, 7, 584–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, B. (2001) Epichloë endophytes: fungal symbionts of grasses. Curr. Opin. Microbiol. 4, 393–398. [DOI] [PubMed] [Google Scholar]
- Spiers, A.G. and Hopcroft, D.H. (1993) Black canker and leaf spot of Salix in New Zealand caused by Glomerella miyabeana (Colletotrichum gloeosporioides). Eur. J. Forest Pathol. 23, 92–102. [Google Scholar]
- Steinbach, W.J. , Cramer, R.A. Jr. , Perfect, B.Z. , Asfaw, Y.G. , Sauer, T.C. , Najvar, L.K. , Kirkpatrick, W.R. , Patterson, T.F. , Benjamin, D.K. Jr. , Heitman, J. and Perfect, J.R. (2006) Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus . Eukaryot. Cell, 5, 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stie, J. and Fox, D. (2008) Calcineurin regulation in fungi and beyond. Eukaryot. Cell, 7, 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto, D. , Tanaka, A. and Scott, B. (2006) A p67Phox‐like regulator is recruited to control hyphal branching in a fungal–grass mutualistic symbiosis. Plant Cell, 18, 2807–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita, N. , Yamashita, S. , Ohta, A. and Horiuchi, H. (2006) Aspergillus nidulans class V and VI chitin synthases CsmA and CsmB, each with a myosin motor‐like domain, perform compensatory functions that are essential for hyphal tip growth. Mol. Microbiol. 59, 1380–1394. [DOI] [PubMed] [Google Scholar]
- Tanaka, A. , Christensen, M.J. , Takemoto, D. , Park, P. and Scott, B. (2006) Reactive oxygen species play a role in regulating a fungus–perennial ryegrass mutualistic interaction. Plant Cell, 18, 1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, A. , Takemoto, D. , Hyon, G.S. , Park, P. and Scott, B. (2008) NoxA activation by the small GTPase RacA is required to maintain a mutualistic symbiotic association between Epichloë festucae and perennial ryegrass. Mol. Microbiol. 68, 1165–1178. [DOI] [PubMed] [Google Scholar]
- Tanaka, A. , Tapper, B.A. , Popay, A. , Parker, E.J. and Scott, B. (2005) A symbiosis expressed non‐ribosomal peptide synthetase from a mutualistic fungal endophyte of perennial ryegrass confers protection to the symbiotum from insect herbivory. Mol. Microbiol. 57, 1036–1050. [DOI] [PubMed] [Google Scholar]
- Thewes, S. (2014) Calcineurin‐Crz1 signaling in lower eukaryotes. Eukaryot. Cell, 13, 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud, M. , Brunet‐Simon, A. , Brygoo, Y. , Pradier, J.M. and Levis, C. (2003) Cyclophilin A and calcineurin functions investigated by gene inactivation, cyclosporin A inhibition and cDNA arrays approaches in the phytopathogenic fungus Botrytis cinerea . Mol. Microbiol. 50, 1451–1465. [DOI] [PubMed] [Google Scholar]
- Walker, L.A. , Lee, K.K. , Munro, C.A. and Gow, N.A. (2015) Caspofungin treatment of Aspergillus fumigatus results in ChsG‐dependent upregulation of chitin synthesis and the formation of chitin‐rich microcolonies. Antimicrob. Agents Chemother. 59, 5932–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, S. , Sugui, J.A. , Steinberg, G. and Deising, H.B. (2007) A chitin synthase with a myosin‐like motor domain is essential for hyphal growth, appressorium differentiation, and pathogenicity of the maize anthracnose fungus Colletotrichum graminicola . Mol. Plant–Microbe Interact. 20, 1555–1567. [DOI] [PubMed] [Google Scholar]
- Young, C.A. , Bryant, M.K. , Christensen, M.J. , Tapper, B.A. , Bryan, G.T. and Scott, B. (2005) Molecular cloning and genetic analysis of a symbiosis‐expressed gene cluster for lolitrem biosynthesis from a mutualistic endophyte of perennial ryegrass. Mol . Genet. Genomics, 274, 13–29. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Zhao, Q. , Liu, K. , Zhang, Z. , Wang, Y. and Zheng, X. (2009) MgCRZ1, a transcription factor of Magnaporthe grisea, controls growth, development and is involved in full virulence. FEMS Microbiol. Lett. 293, 160–169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 cnaA copy number varies amongst strains of Epichloë species. (a) Autoradiograph of DNA blot of BamHI genomic digests of E. festucae strains. (b) Autoradiograph of DNA blot of BamHI genomic digests of Epichloë species, including Epichloë elymi (WWG1, WWG3, E184), Epichloë brachyelytri (E1040, E1124), Epichloë bromicola (E501, E799), Epichloë baconii (E424), Epichloë typhina (E425 and E505) and Epichloë typhina ssp. poae (E1022). Both DNA blots were probed with a [32P]‐labelled cnaA2‐containing polymerase chain reaction (PCR) fragment that was generated with primers 1372‐2F and 1372‐5R using E. festucae E2368 genomic DNA as the template. This probe was expected to hybridize to BamHI fragments of 8.8, 5.9 and 1.3 kb in E. festucae E2368. Four strains of E. festucae (E189, E2368, Fr1, 1035.33) showed the expected hybridization pattern (*). However, three strains of other Epichloë spp. (WWG1, WWG3, E424) showed a similar, but not identical, pattern of hybridization (#). A difference in the size of the largest hybridization band present in E. elymi strains, as well as the absence of the two largest bands in E. baconii, suggest deletions in these genomes.
Fig. S2 Deletion of Epichloë festucae Fl1 cnaA. (a) Physical map of the cnaA wild‐type (WT) genomic region, linear insert of cnaA deletion construct pMMI4 and cnaA complementation construct pMMI11, showing restriction enzyme sites for PstI (P), BamHI (B), EcoRI (E), EcoRV (EV), HindIII (H) and NdeI (N). (b) Autoradiograph of a Southern blot of NdeI genomic DNA digests of E. festucae Fl1 wild‐type (WT) and cnaA deletion strains (ΔcnaA), probed with [32P]‐labelled pMMI4. The expected sizes of the fragments are shown.
Fig. S3 Remediation of Epichloë festucae Fl1 ΔcnaA culture phenotype. Remediation of the ΔcnaA culture phenotype (secretion of yellow pigment) observed when a plug of the mutant was grown on potato dextrose agar (PDA) by spreading ground mycelia on PDA or by subculture of a plug of the mutant on PDA supplemented with MgCl2.
Fig. S4 Deletion of Epichloë festucae E2368 cnaA1 and cnaA2. (a) Physical map of the cnaA1 wild‐type (WT) genomic region, linear insert of cnaA replacement construct pMMI4, showing restriction enzyme sites for PstI (P), BamHI (B), EcoRI (E), EcoRV (EV) and HindIII (H). The arrowheads indicate the primers used for screening of the replacement event. (b) Polymerase chain reaction (PCR) products of the expected size generated with the primer pair MI13 and MI14, specific for cnaA1. (c) Physical map of the cnaA2 wild‐type (WT) genomic region, linear insert of cnaA2 deletion construct pMMI13 and cnaA2 complementation construct pMMI12, used to complement the E. festucae Fl1 ΔcnaA strain. The arrowheads indicate the primers used for preparation of the deletion and complementation constructs and for screening of the replacement event. (d) PCR products of the expected size generated with primer pair MI15 and MI16, specific for cnaA2.
Table S1 Calcineurin A‐encoding genes in sequenced Epichloë genomes.
Table S2 Biological material.
Table S3 Primers used in this study.


