Summary
After invasion into intercellular spaces of tomato plants, the soil‐borne, plant‐pathogenic Ralstonia solanacearum strain OE1‐1 forms mushroom‐shaped biofilms (mushroom‐type biofilms, mBFs) on tomato cells, leading to its virulence. The strain OE1‐1 produces aryl‐furanone secondary metabolites, ralfuranones (A, B, J, K and L), dependent on the quorum sensing (QS) system, with methyl 3‐hydroxymyristate (3‐OH MAME) synthesized by PhcB as a QS signal. Ralfuranones are associated with the feedback loop of the QS system. A ralfuranone productivity‐deficient mutant (ΔralA) exhibited significantly reduced growth in intercellular spaces compared with strain OE1‐1, losing its virulence. To analyse the function of ralfuranones in mBF formation by OE1‐1 cells, we observed cell aggregates of R. solanacearum strains statically incubated in tomato apoplast fluids on filters under a scanning electron microscope. The ΔralA strain formed significantly fewer microcolonies and mBFs than strain OE1‐1. Supplementation of ralfuranones A, B, J and K, but not L, significantly enhanced the development of mBF formation by ΔralA. Furthermore, a phcB‐ and ralA‐deleted mutant (ΔphcB/ralA) exhibited less formation of mBFs than OE1‐1, although a QS‐deficient, phcB‐deleted mutant formed mBFs similar to OE1‐1. Supplementation with 3‐OH MAME significantly reduced the formation of mBFs by ΔphcB/ralA. The application of each ralfuranone significantly increased the formation of mBFs by ΔphcB/ralA supplied with 3‐OH MAME. Together, our findings indicate that ralfuranones are implicated not only in the development of mBFs by strain OE1‐1, but also in the suppression of QS‐mediated negative regulation of mBF formation.
Keywords: mushroom‐type biofilm, ralfuranones, Ralstonia solanacearum, virulence
Introduction
Bacterial wilt caused by Ralstonia solanacearum is one of the most devastating bacterial plant diseases in the tropics, subtropics and warm temperature regions worldwide (Mansfield et al., 2012). Ralstonia solanacearum is a soil‐borne bacterium that normally invades plant roots from the soil through wounds or natural openings where secondary roots emerge (Araud‐Razou et al., 1998), colonizes intercellular spaces of the root cortex and vascular parenchyma, and eventually enters xylem vessels and spreads up into the stems and leaves through the xylem (Hikichi, 2016; Hikichi et al., 2017; Vasse et al., 1995). This colonization in intercellular spaces is required for the virulence of the wild‐type strain of R. solanacearum (OE1–1) (Hikichi, 2016; Hikichi et al., 1999, 2017; Kanda et al., 2003, 2008; Mori et al., 2016; Shinohara et al., 2005). Following the invasion of the intercellular spaces of tomato plants, strain OE1–1 forms mushroom‐shaped biofilms (mushroom‐type biofilms, mBFs) on the surfaces of tomato cells adjacent to intercellular spaces, leading to the colonization of intercellular spaces (Hikichi, 2016; Hikichi et al., 2017; Mori et al., 2016). Therefore, mBF formation on tomato cells after invasion of the intercellular spaces is essential for the virulence of R. solanacearum on tomato plants. Interestingly, the observation of cell aggregates of R. solanacearum strains incubated on filters under a scanning electron microscope (SEM) suggests that the apoplast fluids from tomato plants are better than xylem fluids for mBF formation by strain OE1–1 (Mori et al., 2016).
Cells of many bacteria communicate with each other by releasing, sensing and responding to small diffusible signal molecules, allowing them to regulate their cooperative activities and physiological processes through quorum sensing (QS; Li and Tian, 2012). The ability of bacteria to communicate and behave as a group for social interactions, similar to a multicellular organism, provides significant benefits to bacteria in terms of host colonization, formation of biofilms, defence against competitors and adaptation to changing environments. Bacterial biofilms often consist of mushroom‐shaped complex multicellular structures (Klausen et al., 2003), similar to mBFs produced by R. solanacearum strain OE1–1 (Mori et al., 2016). Bacterial surface components and extracellular compounds, such as flagella, lipopolysaccharides and exopolysaccharides (EPSs), in combination with environmental and QS signals, are crucial for cell aggregation and biofilm development in most bacterial species (Schembri et al., 2001). Furthermore, many pathogenic bacteria use QS‐controlled cell–cell signalling to regulate the expression of factors contributing to virulence (Ham, 2013).
Ralstonia solanacearum strains AW1 and K60 produce methyl 3‐hydroxypalmitate (3‐OH PAME) as a QS signal in phc QS (Flavier et al., 1997; Kai et al., 2015). However, strains OE1–1 and GMI1000 produce methyl 3‐hydroxymyristate (3‐OH MAME), but not 3‐OH PAME, as a QS signal (Kai et al., 2015). Both QS signals are synthesized by PhcB, a putative methyltransferase (Flavier et al., 1997; Kai et al., 2015). When 3‐OH PAME or 3‐OH MAME reaches a threshold level, it induces the ability of the histidine kinase PhcS to phosphorylate the response regulator PhcR, resulting in elevated levels of functional PhcA, a LysR‐type transcriptional regulator, which plays a central role in phc QS as the global virulence regulator (Genin and Denny, 2012). Differences of the deduced amino acid sequences of PhcB and PhcS among R. solanacearum strains correspond to the productivity of these QS signals (Kai et al., 2015).
Ralstonia solanacearum synthesizes aryl‐furanone secondary metabolites, known as ralfuranones A, B, I, J, K and L (Fig. S1, see Supporting Information), which are secreted extracellularly (Kai et al., 2014, 2016; Pauly et al., 2013). Ralfuranone I is a precursor of the other ralfuranones. The expression of the ralfuranone synthase, encoded by ralA, is dependent on activated PhcA, and is involved in the biosynthesis of ralfuranones (Kai et al., 2014, 2016; Schneider et al., 2009; Wackler et al., 2011). Ralfuranones are implicated in the feedback loop of phc QS (Mori et al., 2017). Furthermore, ralfuranones are implicated in the expression of vsrAD and vsrBC encoding the two‐component regulatory system, which is involved in the adaptation of the bacterium to a specific niche or environmental condition during the pathogen life cycle. From this evidence, it has been proposed that the integrated intracellular/intercellular signalling of OE1–1 cells via each of the ralfuranones, coupled with phc QS, contributes to the elaborate and tunable regulation of its virulence. Therefore, ralfuranones are needed for the full virulence of R. solanacearum when directly inoculated into xylem vessels of tomato plants (Kai et al., 2014). Although ralfuranones are implicated in the productivity of the major EPS, EPS I, and cell aggregation by strain OE1–1 (Mori et al., 2017), the involvement of ralfuranones in mBF formation after invasion of intercellular spaces, which is required for R. solanacearum virulence, remains unknown.
To elucidate how ralfuranones influence the colonization of OE1–1 after the invasion of intercellular spaces, we first analysed the colonization of the ralfuranone‐deficient mutant ΔralA in roots and its virulence on tomato plants inoculated using the root dipping method. Because these analyses implicated ralfuranones in the colonization of OE1–1 in intercellular spaces, we then analysed mBF formation by R. solanacearum strains incubated in tomato apoplast fluids on filters using a SEM.
Results
ΔralA loses its virulence on tomato plants
We inoculated 5‐week‐old tomato plants with R. solanacearum strain OE1–1, the ralfuranone productivity‐deficient ΔralA mutant (Kai et al., 2014) and the native ralA‐complemented ΔralA strain ralA‐comp (Kai et al., 2014) by root dipping. The number of ΔralA mutants in roots at 1 day post‐inoculation was not significantly different from that of OE1–1 (P > 0.05, Fig. 1a). However, the population of ΔralA at days 2 and 3 was significantly smaller than that of OE1–1 (P < 0.05). The population of ralA‐comp was not significantly different from that of OE1–1 (P > 0.05).
Figure 1.
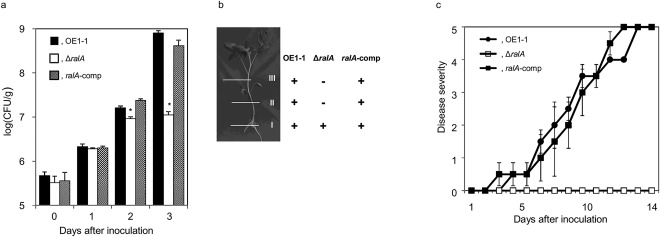
Behaviour and virulence of Ralstonia solanacearum strain OE1–1, the ralfuranone‐deficient mutant ΔralA and the complemented ΔralA mutant ralA‐comp in tomato plants. (a) The population of R. solanacearum strains in the roots of tomato plants inoculated by root dipping was analysed using Hara–Ono medium. CFU, colony‐forming unit. (b) Ralstonia solanacearum strains in roots (I) and stems (II and III) of tomato plants at 10 days after inoculation by root dipping were detected using the plate‐printing assay. (c) Bacterial wilt on tomato plants inoculated with R. solanacearum strains by root dipping was assayed. Plants were rated on a 0–5 disease index scale: 0, no wilting; 1, 1%–25% wilting; 2, 26%–50% wilting; 3, 51%–75% wilting; 4, 76%–99% wilting; 5, dead. Bars indicate standard errors. Asterisks indicate a significant difference from the wild‐type (P < 0.05, t‐test).
In a plate‐printing assay, OE1–1 and ralA‐comp were detected in both inoculated roots and stems of tomato plants, whereas ΔralA was not detected beyond the inoculated roots (Fig. 1b).
ΔralA lost its virulence on tomato plants inoculated by root dipping, whereas the complemented mutant ralA‐comp showed virulence levels similar to those of OE1–1 (Fig. 1c).
In vitro mBF formation by R. solanacearum strain OE1–1
Previously, we have observed mBFs produced by R. solanacearum strain OE1–1 incubated in tomato apoplast fluids on filters under a SEM (Mori et al., 2016). Using this system, we first analysed cell aggregates produced by R. solanacearum strain OE1–1 incubated for 24 h (Fig. S2a, see Supporting Information). Ralstonia solanacearum strain OE1–1 produced microcolonies (Fig. S2b), immature mBFs (Fig. S2c) and mature mBFs (Fig. S2d). We then compared the numbers of microcolonies, immature mBFs and mature mBFs of OE1–1 cells incubated in tomato apoplast fluid on filters for 24 or 32 h. The diameters of the cell aggregates of R. solanacearum strains were assayed using Nodame (Tanabata et al., 2014). The number of microcolonies of OE1–1 cells incubated for 32 h was increased significantly compared with that for 24 h (P < 0.05, Fig. 2). Although the numbers of immature mBFs and mature mBFs of OE1–1 cells incubated for 32 h were increased slightly compared with those for 24 h, there was no significant difference between them (P > 0.05). Although we observed collapsing mBFs when incubated for 42 h, no collapsing mBFs were observed when incubated for both 24 and 32 h (data not shown). As a result of this work, we chose to investigate mBF formation in apoplast fluids on filters after 24 h.
Figure 2.
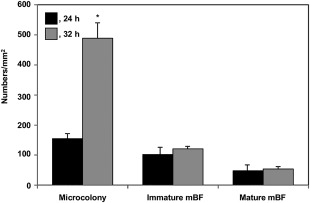
The numbers of microcolonies of 1–5 μm in diameter, immature mushroom‐type biofilms (mBFs) of 5–10 μm in diameter and mature mBFs of >10 μm in diameter produced by cells of Ralstonia solanacearum strain OE1–1 incubated in tomato apoplast fluid on nano‐percolators for 24 or 32 h. Bars indicate the standard errors. Asterisks indicate a significant difference from OE1–1 incubated for 24 h (P < 0.05, t‐test).
ΔralA exhibits attachment ability, similar to OE1–1
During biofilm formation, cells of strain OE1–1 first attach to the surface and then produce flat microcolonies followed by mBFs (Mori et al., 2016). We observed the attachment ability of strains incubated in tomato apoplast fluids under a phase contrast microscope. OE1–1 cells attached to glass slides (Fig. S3a, see Supporting Information). The attachment of the ΔralA mutant to glass slides (Fig. S3b) was similar to that of OE1–1 and ralA‐comp (Fig. S3c).
Ralfuranones are involved in mBF formation by R. solanacearum strain OE1–1 incubated in tomato apoplast fluids
The ΔralA mutant formed significantly fewer microcolonies (Fig. 3a) and immature mBFs (Fig. 3b) than OE1–1 and ralA‐comp (P < 0.05; Table S1, see Supporting Information). The ΔralA mutant formed few mature mBFs (Fig. 3c). However, the numbers of mature mBFs produced by the ralA‐comp strain did not differ significantly from those produced by OE1–1 (P > 0.05, Fig. 3a, Table S1).
Figure 3.
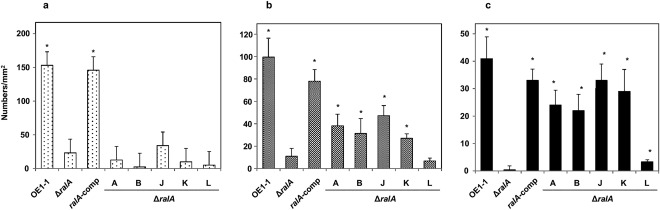
Influence of ralfuranones on the formation of microcolonies of 1–5 μm in diameter (a), immature mushroom‐type biofilms (mBFs) of 5–10 μm in diameter (b) and mature mBFs of >10 μm in diameter (c) by Ralstonia solanacearum strains. Cells of R. solanacearum strain OE1–1, the ralfuranone‐deficient mutant (ΔralA), with or without application of ralfuranones A, B, J, K or L at concentrations of 20 μm, and the complemented ΔralA mutant (ralA‐comp) incubated in tomato apoplast fluid on nano‐percolators were observed under a scanning electron microscope. Bars indicate the standard errors. Asterisks indicate a significant difference from ΔralA (P < 0.05, t‐test).
Ralfuranones A, B, J, K and L at a concentration of 20 μm lead to the recovery of cell aggregation by the ΔralA mutant (Mori et al., 2017). We analysed mBF formation by ΔralA supplemented with ralfuranones A, B, J, K or L at a concentration of 20 μm. The application of ralfuranones A, B, J, K or L did not lead to significantly increased microcolony formation by ΔralA (P > 0.05, Fig. 3a, Table S1). However, the application of ralfuranones A, B, J or K resulted in significantly increased formation of both immature mBFs (P < 0.05, Fig. 3b, Table S1) and mature mBFs (P < 0.05, Fig. 3c, Table S1) by the ΔralA strain, whereas 20 μm of ralfuranone L increased slightly the formation of mature mBFs by the ΔralA strain (Fig. 3c, Table S1).
A phc QS‐deficient mutant (phcB‐deleted mutant ΔphcB) retains its ability to produce mBFs
Ralfuranones are implicated in the feedback loop of phc QS (Mori et al., 2017). We therefore analysed the formation of mBFs by a phc QS‐deficient mutant (phcB‐deleted mutant ΔphcB) (Kai et al., 2015). The numbers of microcolonies (Fig. 4a), immature mBFs (Fig. 4b) and mature mBFs (Fig. 4c) produced by the ΔphcB mutant did not differ significantly from those produced by OE1–1 (P > 0.05; Table S2, see Supporting Information). However, a phcB‐ and ralA‐deleted mutant (ΔphcB/ralA) produced significantly fewer microcolonies and mature mBFs than strains OE1–1 and ΔphcB (P < 0.05).
Figure 4.
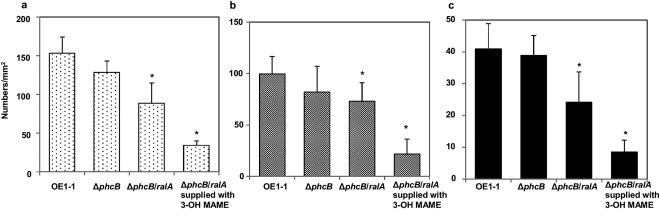
Influence of phc quorum sensing (QS) on the formation of microcolonies of 1–5 μm in diameter (a), immature mushroom‐type biofilms (mBFs) of 5–10 μm in diameter (b) and mature mBFs of >10 μm in diameter (c) by Ralstonia solanacearum strains. Cells of R. solanacearum strain OE1–1, the phc QS‐deficient mutant (ΔphcB), and the phcB‐ and ralA‐deficient mutant (ΔphcB/ralA), with or without application of methyl 3‐hydroxymyristate (3‐OH MAME) at a concentration of 100 nm, incubated in tomato apoplast fluid on nano‐percolators were observed under a scanning electron microscope. Bars indicate the standard errors. Asterisks indicate a significant difference from ΔralA (P < 0.05, t‐test).
The application of 3‐OH MAME at a concentration of 100 nm leads to the recovery of the activity of phc QS by ΔphcB (Kai et al., 2015). We analysed the formation of mBFs by the ΔphcB/ralA mutant supplied with 3‐OH MAME at a concentration of 100 nm. The application of 3‐OH MAME resulted in a significantly reduced formation of microcolonies (Fig. 4a), immature mBFs (Fig. 4b) and mature mBFs (Fig. 4c) by ΔphcB/ralA (P < 0.05, Table S2).
We then analysed the formation of mBFs by the ΔphcB/ralA mutant supplied with 3‐OH MAME at a concentration of 100 nm and ralfuranones A, B, J, K or L at a concentration of 20 μm. The additional application of each ralfuranone significantly enhanced the formation of microcolonies (Fig. 5a), immature mBFs (Fig. 5b) and mature mBFs (Fig. 5c) by ΔphcB/ralA supplied with 3‐OH MAME (P < 0.05; Table S3, see Supporting Information). In particular, ΔphcB/ralA supplied with both 3‐OH MAME and ralfuranones J or K formed significantly more mBFs than strain OE1–1 (P < 0.05).
Figure 5.
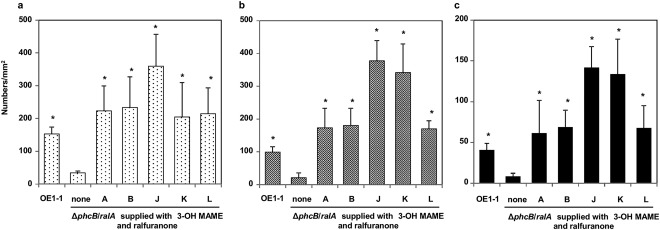
Effect of phc quorum sensing and ralfuranones on the formation of microcolonies of 1–5 μm in diameter (a), immature mushroom‐type biofilms (mBFs) of 5–10 μm in diameter (b) and mature mBFs of >10 μm in diameter (c) by Ralstonia solanacearum strains. Cells of R. solanacearum strain OE1–1 and the phcB‐ and ralA‐deficient mutant ΔphcB/ralA, with application of methyl 3‐hydroxymyristate (3‐OH MAME) at a concentration of 100 nm and with or without application of ralfuranones A, B, J, K or L at a concentration of 20 μm, incubated in apoplast fluids from tomato plants on nano‐percolators were observed under a scanning electron microscope. Bars indicate the standard errors. Asterisks indicate a significant difference from ΔphcB/ralA supplemented with 3‐OH MAME (P < 0.05, t‐test).
EPS I productivity‐deficient mutant retains its ability to produce mBFs, but not cell aggregation
It has been proposed that EPS I is involved in biofilm formation by R. solanacearum (Genin and Denny, 2012). We assayed cell aggregation by the ΔphcB mutant and an EPS I productivity‐deficient mutant (ΔepsB), which exhibited significantly less EPS I productivity (P < 0.05, Fig. 6a). Both ΔepsB and ΔphcB exhibited significantly reduced cell aggregation compared with strain OE1–1 (P < 0.05, Fig. 6b). The means of the optical density at 600 nm (OD600) of strain OE1–1, ΔepsB and ΔphcB cultures were 0.75, 0.69 and 0.72, respectively, and there was no significant difference between them (P > 0.05).
Figure 6.
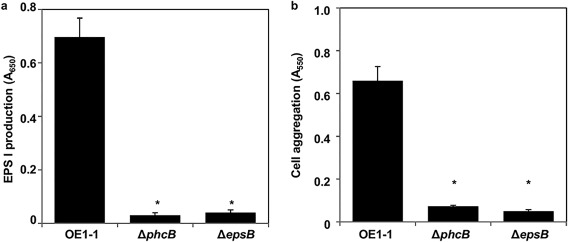
Production of exopolysaccharide I (EPS I) and cell aggregation by the epsB‐deleted ΔepsB mutant of Ralstonia solanacearum. (a) Immunological quantification of EPS I in the supernatants of the phc quorum sensing (QS)‐deficient mutant ΔphcB and ΔepsB of R. solanacearum was analysed using enzyme‐linked immunosorbent assay (ELISA) with anti‐R. solanacearum EPS I antibodies. EPS I productivity was quantified by the absorbance at 650 nm (A 650). (b) Cell aggregation by R. solanacearum strains incubated in 1/4 × M63 medium in polyvinylchloride (PVC) plate wells was stained with crystal violet and its absorbance at 550 nm (A 550) was assayed. Bars indicate the standard errors. Asterisks indicate significant difference from wild‐type strain OE1–1 (P < 0.05, t‐test).
We then analysed the formation of mBFs by ΔepsB. ΔepsB produced significantly more microcolonies (Fig. 7a) and immature mBFs (Fig. 7b) than strain OE1–1 (P < 0.05). However, ΔepsB produced mature mBFs at a similar level to strain OE1–1 (Fig. 7c).
Figure 7.
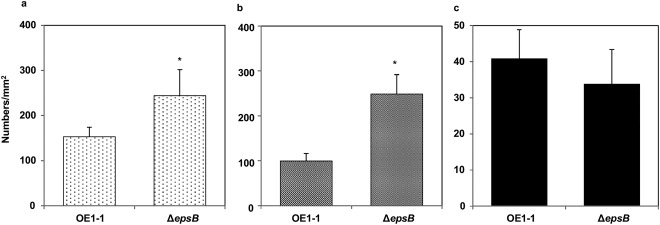
Influence of exopolysaccharide I (EPS I) on the formation of microcolonies of 1–5 μm in diameter (a), immature mushroom‐type biofilms (mBFs) of 5–10 μm in diameter (b) and mature mBFs at >10 μm in diameter (c) by Ralstonia solanacearum strains. Cells of R. solanacearum strains OE1–1 and the epsB‐deleted ΔepsB mutant incubated in apoplast fluids from tomato plants on nano‐percolators were observed under a scanning electron microscope. Bars indicate the standard errors. Asterisks indicate a significant difference from ΔphcB/ralA supplemented with methyl 3‐hydroxymyristate (3‐OH MAME) (P < 0.05, t‐test).
Discussion
After colonization of the intercellular spaces of roots, R. solanacearum enters xylem vessels and spreads up into the stems and leaves through the xylem (Hikichi, 2016; Hikichi et al., 2017; Vasse et al., 1995), where its high level of multiplication leads to wilting symptoms, as a result of the reduced sap flow caused by the presence of a large number of bacterial cells and EPS slime produced by the bacteria in some xylem vessels (Genin and Denny, 2012). Therefore, it has been proposed that EPS is an important virulence factor. The production of the major EPS, EPS I, is positively regulated by phc QS. Furthermore, ralfuranones are positively regulated by phc QS and are implicated in the feedback loop of phc QS (Mori et al., 2017), positively regulating EPS I production. Therefore, when directly inoculated into xylem vessels of tomato plants, ralfuranones contribute to the full virulence of OE1–1 (Kai et al., 2014). In addition, the current study has shown the involvement of extracellularly produced ralfuranones in the colonization of the intercellular spaces of tomato plants by OE1–1 (Fig. 1a), leading to systemic infection (Fig. 1b). Therefore, ralfuranones are required for OE1–1 virulence on tomato plants inoculated through roots (Fig. 1c). Furthermore, the observation of mBF formation by R. solanacearum strains using the in vitro mBF formation system showed the involvement of ralfuranones in the development of mBFs (Fig. 3), but not in the attachment of strain OE1–1 cells (Fig. S3). Therefore, the many functions of ralfuranones in the infection route of OE1–1 may lead to the formation of mature mBFs on host cells after invasion into intercellular spaces, systemic infectivity and bacterial cell aggregation in xylem vessels, contributing to OE1–1 virulence.
Ralstonia solanacearum produces a consortium of plant cell wall‐degrading enzymes, e.g. β‐1,4‐endoglucanase, a β‐1,4‐cellobiohydrolase and a pectin methyl esterase, the production of which is positively regulated through phc QS (González and Allen, 2003; Huang and Allen, 1997; Tans‐Kersten et al., 1998). The production and extracellular secretion of these enzymes through the type II secretion system are required for invasion into xylem vessels, leading to virulence (Liu et al., 2005; Tsujimoto et al., 2008). The results in this study showed the involvement of ralfuranones in mBF formation (Fig. 3). Furthermore, ΔralA lost its systemic infectivity (Fig. 1b), suggesting that ralfuranone production may be involved in OE1–1 invasion into xylem vessels. Mature mBFs produced by strain OE1–1 can dissolve, releasing cells from the mBF (Mori et al., 2016). It is thus thought that OE1–1 cells released from mature mBFs can invade xylem vessels.
EPSs are required for autoaggregation and biofilm development in most bacterial species (Bogino et al., 2013). The expression of the eps operon, including epsB, is positively regulated by phc QS, producing the major EPS, EPS I, dependent on phc QS by R. solanacearum (Genin and Denny, 2012). Assays of cell aggregation by R. solanacearum strains showed that EPS I produced dependently on phc QS is required for cell aggregation by strain OE1–1 (Fig. 6). Interestingly, deficiency of EPS I productivity by epsB deletion led to the enhanced formation of microcolonies and immature mBFs, but did not affect significantly the formation of mature mBFs (Fig. 7). It is thus thought that EPS I may be directly involved in cell aggregation but not the development of mature mBFs by strain OE1–1 (Fig. S4, see Supporting Information).
The application of 3‐OH MAME led to the recovery of the phc QS‐dependent phenotypes, such as EPS production and ralfuranone productivity, by ΔphcB (Kai et al., 2015). The reduced formation of mBFs by ΔphcB/ralA relative to strains OE1–1 and ΔphcB, and the significantly reduced formation of mBFs by ΔphcB/ralA with application of 3‐OH MAME, suggest that phc QS may negatively regulate mBF formation in the absence of ralfuranones (Fig. 4; Table S1). The formation of immature and mature mBFs by ΔralA supplemented with ralfuranone A, B, J or K (Fig. 5b,c) suggests the involvement of these ralfuranones in the development of mBF formation (Fig. S3; Table S2). Furthermore, the co‐application of ralfuranone A, B, J, K or L increased the formation of not only mBFs, but also microcolonies, by ΔphcB/ralA supplemented with 3‐OH MAME (Fig. 5; Table S2), suggesting that ralfuranones A, B, J, K and L (especially J and K) may suppress the phc QS‐mediated negative regulation of mBF formation. Ralfuranone I is non‐enzymatically converted into ralfuranone B (Fig. S1; Kai et al., 2016). The non‐enzymatic elimination of benzaldehyde from ralfuranone B produces ralfuranone A, and ralfuranones J and K are the products of the enzymatic oxidation of ralfuranone B. It is thought that a change in production of ralfuranones J and K from ralfuranone B by OE1–1 may especially contribute to all stages during mBF formation.
QS is the regulation system for gene expression in response to changes in cell population density (Miller and Bassler, 2001). Bacteria produce and release QS signals that increase in concentration as a function of cell density. The detection of a minimal threshold stimulatory concentration of a QS signal leads to a change in gene expression. Ralfuranones, whose production is induced through phc QS by strain OE1–1 (Kai et al., 2014), affect the phc QS feedback loop (Mori et al., 2017). Biofilm formation is a temporal process for Gram‐negative proteobacteria, in which a transition through distinct stages of multicellular organization is involved. During biofilm formation, planktonic bacteria first attach to surfaces and grow there (Monds and O'Toole, 2009). The clonal growth of attached cells or the active translocation of cells across the surface leads to microcolony formation. Microcolonies grow in size and coalesce, forming biofilms. Mature biofilms are then dissolved, releasing planktonic cells. QS functioning during microcolony formation is required for the formation of mature biofilms (Bogino et al., 2013; Rinaudi and Giordano, 2010). In R. solanacearum, immediately after invasion into the intercellular spaces, the expression of lecM encoding a lectin, RS‐IIL, is positively regulated by HrpG (Mori et al., 2016; Sudakevitz et al., 2004; Valls et al., 2006). RS‐IIL is required for the attachment ability of strain OE1–1 to the surface of plant cells, leading to the formation of mBFs (Mori et al., 2016). In this study, ralfuranones A, B, J and K, whose production is induced through phc QS, contributed to both the formation of microcolonies and the development of mBFs (Fig. 3). Furthermore, strain OE1–1 formed mature mBFs during incubation for 24 h (Fig. 2). Therefore, integrated signalling via not only RS‐IIL, but also ralfuranones, whose production is dependent on phc QS, is implicated in mBF formation by R. solanacearum strain OE1–1 (Fig. S4).
Apoplast fluids are preferential for mBF formation by strain OE1–1 (Mori et al., 2016). This mBF formation is independent of phc QS‐dependent aggregation by OE1–1 cells (Fig. 4; Table S1). Interestingly, RS‐IIL, whose production is induced by PhcA functioning at an OE1–1 cell density of more than 107 colony forming units (CFU)/mL, is also implicated in EPS I production, leading to aggregation by OE1–1 cells (Mori et al., 2016). Furthermore, the lecM‐deleted mutant exhibits reduced virulence when inoculated directly into xylem. RS‐IIL is reportedly involved in cell aggregation by R. solanacearum strain UW551 in the xylem vessels of tomato plants, leading to its full virulence (Meng et al., 2015). Taken together, these results indicate that the integrated intracellular/intercellular signalling of R. solanacearum cells via each ralfuranone and RS‐IIL, coupled with phc QS, is implicated in the regulation of mBF formation after invasion into intercellular spaces and aggregation in xylem vessels by R. solanacearum cells (Fig. S4).
Experimental Procedures
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. Ralstonia solanacearum strains were routinely grown in 1/4 × M63 medium (Cohen and Rickenberg, 1956) at 30 °C, and Escherichia coli strains were grown in Luria–Bertani (LB) medium (Hanahan, 1983) at 37 °C. The following antibiotics were used in selective media in the amounts indicated (μg/mL): ampicillin (Amp), 75; gentamycin, 25; kanamycin (Km), 50.
Table 1.
Strains and plasmids used in this study.
| Relevant characteristics | Source | |
|---|---|---|
| Plasmids used for cloning | ||
| pMD20 | pUC19 derivative, Ampr | Takara Bio |
| pMD20epsB | pMD20 derivative carrying a 1441‐bp DNA fragment for epsB deletion, Ampr | This study |
| pK18mobsacB | Kmr, oriT (RP4), sacB, lacZα | Kvitko and Collmer (2011) |
| pΔralA | pK18mobsacB derivative carrying 1759‐bp DNA fragment for ralA deletion, Kmr | Kai et al. (2014) |
| pDepsB | pK18mobsacB derivative carrying a 1441‐bp DNA fragment for epsB deletion | This study |
| Escherichia coli strain | ||
| DH5α | recA1 endA1 gyrA96 thi‐1 hsdR17supE44 Δ(lac)U169(ϕ80lacΔM15) | Takara Bio |
| Ralstonia solanacearum strains | ||
| OE1–1 | Wild‐type strain, phylotype I, race 1, biovar 4 | Hikichi et al. (1999) |
| ΔralA | ralA‐deleted mutant of OE1–1 | Kai et al. (2014) |
| ralA‐comp | A transformant of ΔralA with pCralA containing native ralA | Kai et al. (2014) |
| ΔphcB | phcB‐deleted mutant of OE1–1 | Kai et al. (2015) |
| ΔphcB/ΔralA | ralA‐deleted mutant of ΔphcB | This study |
| ΔepsB | epsB‐deleted mutant of OE1–1 | This study |
General DNA manipulations
The isolation of genomic DNA, plasmid DNA manipulations and polymerase chain reaction (PCR) were performed using standard techniques (Sambrook et al., 1989). Ralstonia solanacearum was transformed by electroporation as described previously (Mori et al., 2016). Double‐stranded DNA sequencing templates were prepared with Fast Gene™ Plasmid miniprep kits (NIPPON Genetics, Tokyo, Japan) and sequences were determined using an Automated DNA Sequencer Model 373 (Applied Biosystems, Foster City, CA, USA) with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). DNA sequence data were analysed using DNASYS‐Mac software (Hitachi Software Engineering, Yokohama, Japan). Enzymes, including restriction endonucleases (Takara Bio, Ohtsu, Japan), were used according to the manufacturer's instructions.
Creation of an epsB‐deleted mutant ΔepsB
A 716‐bp DNA fragment (epsB‐1) was PCR amplified from the genomic DNA of OE1–1 with primers epsB‐1‐FW (5′‐TCCGATCCGCGCAATGTC‐3′) and epsB‐1‐RV (5′‐GCAGCCGCTACATCAGGGTCGTATTCCGTG‐3′). A 745‐bp DNA fragment (epsB‐2) was PCR amplified from the genomic DNA of OE1–1 with primers epsB‐2‐FW (5′‐GACCCTGATGTAGCGGCTGCAGCGCGGC‐3′) and epsB‐2‐RV (5′‐GCCGGCACGCCTTCATTG‐3′). Using epsB‐1 and epsB‐2 as templates, a 1441‐bp DNA fragment was PCR amplified with primers epsB‐1‐FW and epsB‐2‐RV, and then cloned into pMD20 (Takara Bio) to create pMD20epsB. The pMD20epsB construct was digested with BamHI and HindIII to release a 1.4‐kbp fragment, which was ligated into the BamHI and HindIII sites of pK18mobsacB (Kvitko and Collmer, 2011) to create pDepsB. This plasmid was electroporated into OE1–1 cells, and kanamycin‐resistant and sucrose‐sensitive recombinants were selected. Recombinants were incubated in PY medium [polypeptone (0.5%), yeast extract (0.2%)] for 6 h, and a kanamycin‐sensitive and sucrose‐resistant recombinant, ΔepsB, was selected. DNA for sequencing was PCR amplified with primers epsB‐SQ‐FW3 (5′‐CAGCAAGGTCTTCGTGACCG‐3′) and epsB‐SQ‐RV3 (5′‐CCGATGCGATACGGGATCAG‐3′) to verify the correct substitution of the deleted epsB in OE1–1 (data not shown).
Creation of a phcB‐ and ralA‐deleted ΔphcB/ralA mutant
The plasmid pΔralA (Kai et al., 2014) was electroporated into the phcB‐deleted ΔphcB mutant (Kai et al., 2015), and kanamycin‐resistant and sucrose‐sensitive recombinants were selected. Recombinants were incubated in PY medium for 6 h, and a kanamycin‐sensitive and sucrose‐resistant recombinant, ΔphcB/ralA, was selected. DNA for sequencing was PCR amplified with primers ral‐SQ‐FW3 (5′‐TGCGTTAGGCATGGTTTGG‐3′) and ral‐SQ‐RV3 (5′‐TTGTAGGCGTTCATGGTCC‐3′) to verify the correct substitution of the deleted ralA in ΔphcB (data not shown).
Synthesis of ralfuranones and 3‐OH MAME
Ralfuranones A, B, J, K and L (Kai et al., 2014, 2016) and 3‐OH MAME (Kai et al., 2015) were synthesized in previous studies.
Attachment ability assay
Glass slides were soaked in suspensions of R. solanacearum strains at 1.0 × 108 CFU/mL in Petri dishes (diameter, 9 cm). After incubation for 24 h at 30 °C, the glass slides were washed twice with deionized water and observed under a phase contrast microscope (FSX‐100, Olympus, Tokyo, Japan) (Mori et al., 2016).
Observation of R. solanacearum cells incubated on nano‐percolators under a SEM
We dropped overnight cultures of R. solanacearum strains adjusted to OD600 = 0.005 (10 μL) onto 190 μL of medium on nano‐percolator filters (JEOL, Tokyo, Japan) and incubated them without shaking at 30 °C (Mori et al., 2016). After removal of the supernatant, we added 100 µL of ice‐cold 4% paraformaldehyde in phosphate‐buffered saline (PBS) to the bacteria on the nano‐percolator filters and fixed the bacteria at room temperature for 1 h. The fixed bacteria were washed twice with deionized water and then dried at room temperature. The bacteria on the nano‐percolator filters were vacuum deposited with gold using an ion sputter coater (Hitachi E‐1010, Hitachi High‐Technologies, Tokyo, Japan) according to the manufacturer's instructions. The sample on the filter was mounted directly on the specimen holder and examined using a Miniscope® TM‐3000 SEM (Hitachi High‐Technologies Corporation) with a magnification of 250. The diameter of cell aggregates produced by R. solanacearum strains in an area of 0.3267 mm2 was then assayed using Nodame (Nodule Area Measuring Software, http://phenotyping.image.cooan.jp/; Tanabata et al., 2014). Each assay was repeated in 15 successive trials. The numbers of microcolonies (Fig. S2b) of 1–5 μm in diameter, immature mBFs (Fig. S2c) of 5–10 μm in diameter and mature mBFs (Fig. S2d) of >10 μm in diameter were assessed using Nodame, and were statistically analysed using the Tukey–Kramer honestly significant difference (HSD) test (n = 15) following an analysis of variance (ANOVA) with Easy R software (Saitama Medical Center, Jichi Medical University, Saitama, Japan; Kanda, 2013) (Tables S1–S3).
EPS I productivity
Quantitative analyses of EPS I production were conducted using an enzyme‐linked immunosorbent assay (ELISA) (Mori et al., 2016). The overnight culture of R. solanacearum strains was rinsed, and diluted to a cell density of 1.0 × 102 CFU/mL; 100 μL of this cell suspension were spread on plates of 1/4 × M63 agar medium and incubated for 2 days at 30 °C. The cells were then re‐suspended to 1.0 × 105 CFU/mL. Cell density was confirmed through dilution plating. EPS I was quantified using anti‐R. solanacearum EPS I antibodies with ELISA (Agdia Inc., Elkhart, IN, USA) according to the manufacturer's instructions per 100 μL volume (1.0 × 104 CFU) of cell suspension (three technical replicates were assessed). EPS I productivity was quantified by the absorbance at 650 nm.
Bacterial cell aggregation assay
We inoculated 5 μL of overnight culture of R. solanacearum adjusted to OD600 = 0.005 onto 95 μL of medium in wells of a polyvinylchloride (PVC) microtitre plate (NUNC MICRO WELL PLATE, Thermo Fisher Scientific Inc., Waltham, MA, USA). After incubation without shaking for 24 h at 30 °C, 25 μL of 1.0% crystal violet solution were added to the wells. After 15 min of incubation at room temperature, the unbound crystal violet stain was gently removed with a pipette. The wells were washed with distilled water, 70% ethanol and distilled water in turn. The crystal violet in each well was solubilized by adding 100 μL of 100% ethanol, and was quantified by the absorbance at 550 nm (Mori et al., 2016).
Virulence assays
The near‐isogenic line GCR26 of tomato (Solanum lycopersicum cv. Craigella) was kindly provided by NIAS GENEbank (http://www.gene.affrc.go.jp/index_en.php). Tomato plants were grown in pots containing a mixture of vermiculite and peat moss (3 : 1) and watered with fivefold‐diluted Hoagland's solution in a growth room at 25 °C under 10 000 lx for 16 h per day (Hikichi et al., 1999). The roots of 5‐week‐old tomato plants were soaked in bacterial suspension at 1.0 × 108 CFU/mL for 30 min and then washed in running water. The inoculated plants were grown in water‐culture pots (Yamato Water Culture Pot No. 1, Yamato Plastic Co. Ltd, Yamatotakada, Japan) with fivefold‐diluted Hoagland's solution in a growth room at 25 °C under 10 000 lx for 16 h per day. Inoculum concentrations were determined spectrophotometrically and confirmed by dilution plating. Within each trial, 12 plants of each strain were treated, yielding 60 plants per strain. Plants were coded and inspected for wilting symptoms daily after inoculation. Plants were rated on a 0–5 disease index scale: 0, no wilting; 1, 1%–25% wilting; 2, 26%–50% wilting; 3, 51%–75% wilting; 4, 76%–99% wilting; 5, dead. Each assay was repeated in five successive trials.
Bacterial populations and behaviour in tomato plants
Roots were excised at 0, 1, 2 and 3 days post‐inoculation from five plants that had been inoculated with bacteria through the roots. Each sample was immersed in 70% ethanol solution for 1 min, followed by twice washing with distilled water, and ground in 1 mL of distilled water using a mortar and pestle. A 0.1‐mL sample of the original suspension or a 10‐fold serial dilution was spread onto three Hara–Ono medium (Hara and Ono, 1983) plates. For ralA‐comp, the medium contained gentamycin at 25 μg/mL. The colonies were counted after 2 days of incubation at 30 °C.
A plate‐printing assay was performed as follows (Kanda et al., 2008). At 10 days post‐inoculation by root dipping, the roots and stems of five tomato plants were cut into three pieces each using razor blades. The cut site of each piece (I, roots; II and III, stems; Fig. 1b) was pressed onto Hara–Ono medium for OE1–1 and ΔralA, and onto Hara–Ono medium containing gentamycin at 25 μg/mL for ralA‐comp, and then incubated at 30 °C for 3 days.
Apoplast extractions
To extract the apoplast fluid from the leaves of tomato plants, the third leaf down from the apex of 5‐week‐old tomato plants was cut, washed with distilled water and dried with a paper towel (Mori et al., 2016). One to three leaves were then introduced into a 1‐mL syringe with 15 mL of distilled water, and pressure–vacuum cycles were applied until the leaves were completely infiltrated. Following infiltration, the leaves were carefully removed from the syringe and blotted with a paper towel. Each leaf was then introduced into a 5‐mL syringe placed inside a 50‐mL conical tube containing a 1.5‐mL collection tube. Apoplast extract was collected by centrifuging the tubes at 4000 g for 5 min at room temperature. The fraction collected in the 1.5‐mL tube was centrifuged again for 5 min at 4000 g at room temperature. The supernatant was filtered through a 0.2‐μm pore size membrane filter (Minisart CE; Sartorius, Gottingen, Germany) and stored at −20 °C until use.
Accession number of nucleotide sequence
The nucleotide sequence of epsB of strain OE1–1 has been deposited in DDBJ/GenBank/EMBL under accession number LC102463.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Schematic diagram of the quorum sensing and conversion of ralfuranone I to various ralfuranones in Ralstonia solanacearum strain OE1‐1.
Fig. S2 (a) Observation of cells of Ralstonia solanacearum strain OE1‐1 incubated in tomato apoplast fluid on nano‐percolators for 24 h under a scanning electron microscope. OE1‐1 cells produced microcolonies of 1–5 μm in diameter (b), immature mushroom‐type biofilms (mBFs) of 5–10 μm in diameter (c) and mature mBFs of >10 μm in diameter (d).
Fig. S3 Attachment ability of Ralstonia solanacearum strains OE1‐1 (a), ralfuranone‐deficient mutant ΔralA (b) and the complemented ΔralA mutant ralA‐comp (c). Glass slides were soaked in suspensions of R. solanacearum strains.
Fig. S4 Model of the regulation of mushroom‐type biofilm (mBF) formation, the production of major exopolysaccharide EPS I and cell aggregation by Ralstonia solanacearum strain OE1‐1 through phc quorum sensing. 3‐OH MAME, methyl 3‐hydroxymyristate.
Table S1 Tukey–Kramer analysis of the involvement of ralfuranones in the formation of microcolonies, immature mushroom‐type biofilms and mature mushroom‐type biofilms by Ralstonia solanacearum strains.
Table S2 Tukey–Kramer analysis of the involvement of phc quorum sensing in the formation of microcolonies, immature mushroom‐type biofilms and mature mushroom‐type biofilms by Ralstonia solanacearum strains.
Table S3 Tukey–Kramer analysis of the relationship between phc quorum sensing and ralfuranones in the formation of microcolonies, immature mushroom‐type biofilms and mature mushroom‐type biofilms by Ralstonia solanacearum strains.
Acknowledgements
We thank Dr Takanari Tanabata for valuable suggestions on the mBF formation assay. This work was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (Nos. 24580066, 25292029, 26660036, 17H03773 and 17K19271), a research grant from the Towa Foundation for Food Science & Research and a research grant from Sumitomo Chemical Co. Ltd. to Y.H., and a Sasakawa Scientific Research Grant from The Japan Science Society (No. 28–540) to Y.M.
References
- Araud‐Razou, I. , Vasse, J. , Montrozier, H. , Etchebar, C. and Trigalet, A. (1998) Detection and visualization of the major acidic exopolysaccharide of Ralstonia solanacearum and its role in tomato root infection and vascular colonization. Eur. J. Plant Pathol. 104, 795–809. [Google Scholar]
- Bogino, P.C. , Oliva, M.M. , Sorroche, F.G. and Giordano, W. (2013) The role of bacterial biofilms and surface components in plant–bacterial associations. Int. J. Mol. Sci. 14, 15 838–15 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, G.N. and Rickenberg, H.V. (1956) La galactoside‐perméase d’Escherichia coli . Ann. Inst. Pasteur (Paris), 91, 693–720. [PubMed] [Google Scholar]
- Flavier, A.B. , Clough, S.J. , Schell, M.A. and Denny, T.P. (1997) Identification of 3‐hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum . Mol. Microbiol. 26, 251–259. [DOI] [PubMed] [Google Scholar]
- Genin, S. and Denny, T.P. (2012) Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 50, 67–89. [DOI] [PubMed] [Google Scholar]
- González, E.T. and Allen, C. (2003) Characterization of a Ralstonia solanacearum operon required for polygalacturonate degradation and uptake of galacturonic acid. Mol. Plant–Microbe Interact. 16, 536–544. [DOI] [PubMed] [Google Scholar]
- Ham, J.H. (2013) Intercellular and intracellular signalling systems that globally control the expression of virulence genes in plant pathogenic bacteria. Mol. Plant Pathol. 14, 308–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D. (1983) Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580. [DOI] [PubMed] [Google Scholar]
- Hara, H. and Ono, K. (1983) Ecological studies on the bacterial wilt of tobacco, caused by Pseudomonas solanacearum E. F. Smith. I. A selective medium for isolation and detection of P. solanacearum . Bull. Okayama Tob. Exp. Stn. 42, 127–138. [Google Scholar]
- Hikichi, Y. (2016) Interactions between plant pathogenic bacteria and host plants during the establishment of susceptibility. J. Gen. Plant Pathol. 82, 326–331. [Google Scholar]
- Hikichi, Y. , Nakazawa‐Nasu, Y. , Kitanosono, S. , Suzuki, K. and Okuno, T. (1999) The behavior of genetically lux‐marked Ralstonia solanacearum in grafted tomato cultivars resistant or susceptible to bacterial wilt. Ann. Phytopathol. Soc. Jpn. 65, 597–603. [Google Scholar]
- Hikichi, Y. , Mori, Y. , Ishikwa, S. , Hayashi, K. , Ohnishi, K. , Kiba, A. and Kai, K. (2017) Regulation involved in colonization of intercellular spaces of host plants in Ralstonia solanacearum . Front. Plant Sci. 8, 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Q. and Allen, C. (1997) An exo‐poly‐alpha‐D‐galacturonosidase, PehB, is required for wild‐type virulence of Ralstonia solanacearum . J. Bacteriol. 179, 7369–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai, K. , Ohnishi, H. , Mori, Y. , Kiba, A. , Ohnishi, K. and Hikichi, Y. (2014) Involvement of ralfuranone production in the virulence of Ralstonia solanacearum OE1–1. Chembiochem. 15, 2590–2597. [DOI] [PubMed] [Google Scholar]
- Kai, K. , Ohnishi, H. , Shimatani, M. , Ishikawa, S. , Mori, Y. , Kiba, A. , Ohnishi, K. , Tabuchi, M. and Hikichi, Y. (2015) Methyl 3‐hydroxymyristate, a diffusible signal mediating phc quorum sensing in Ralstonia solanacearum . Chembiochem. 16, 2309–2318. [DOI] [PubMed] [Google Scholar]
- Kai, K. , Ohnishi, H. , Kiba, A. , Ohnishi, K. and Hikichi, Y. (2016) Studies on the biosynthesis of ralfuranones in Ralstonia solanacearum . Biosci. Biotechnol. Biochem. 80, 440–444. [DOI] [PubMed] [Google Scholar]
- Kanda, A. , Yasukohchi, M. , Ohnishi, K. , Kiba, A. , Okuno, T. and Hikichi, Y. (2003) Ectopic expression of Ralstonia solanacearum effector protein PopA early in invasion results in loss of virulence. Mol. Plant–Microbe Interact. 16, 447–455. [DOI] [PubMed] [Google Scholar]
- Kanda, A. , Tsuneishi, K. , Mori, A. , Ohnishi, K. , Kiba, A. and Hikichi, Y. (2008) An amino acid substitution at position 740 in σ70 of Ralstonia solanacearum strain OE1–1 affects its in planta growth. Appl. Environ. Microbiol. 74, 5841–5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda, Y. (2013) Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen, M. , Aaes‐Jogenen, A. , Molin, S. and Tolker‐Nielsen, T. (2003) Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50, 61–68. [DOI] [PubMed] [Google Scholar]
- Kvitko, B.H. and Collmer, A. (2011) Construction of Pseudomonas syringae pv. tomato DC3000 mutant and polymutant strains. Methods Mol. Biol. 712, 109–128. [DOI] [PubMed] [Google Scholar]
- Li, Y.H. and Tian, X. (2012) Quorum sensing and bacterial social interactions in biofilms. Sensor (Basel), 12, 2519–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Zhang, S. , Schell, M.A. and Denny, T.P. (2005) Pyramiding unmarked deletions in Ralstonia solanacearum shows that secreted proteins in addition to plant cell‐wall‐degrading enzymes contribute to virulence. Mol. Plant–Microbe Interact. 18, 1296–1305. [DOI] [PubMed] [Google Scholar]
- Mansfield, J. , Genin, S. , Magori, S. , Citovsky, V. , Sriariyanum, M. , Ronald, P. , Dow, M. , Verdier, V. , Beer, S.V. , Machado, M.A. , Toth, I. , Salmond, G. and Foster, G.D. (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13, 614–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, F. , Babujee, L. , Jacobs, J.M. and Allen, C. (2015) Comparative transcriptome analysis reveals cool virulence factors of Ralstonia solanacearum race 3 biovar 2. PLoS One, 10, e0139090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M.B. and Bassler, B.L. (2001) Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199. [DOI] [PubMed] [Google Scholar]
- Monds, R.D. and O'Toole, G.A. (2009) The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol. 17, 73–87. [DOI] [PubMed] [Google Scholar]
- Mori, Y. , Inoue, K. , Ikeda, K. , Nakayashiki, H. , Higashimoto, C. , Ohnishi, K. , Kiba, A. and Hikichi, Y. (2016) The vascular plant pathogenic bacterium Ralstonia solanacearum produces biofilms required for its virulence on the surfaces of tomato cells adjacent to intercellular spaces. Mol. Plant Pathol. 17, 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, Y. , Ohnishi, H. , Shimatani, M. , Morikawa, Y. , Ishikawa, S. , Ohnishi, K. , Kiba, A. , Kai, K. and Hikichi, Y. (2017) Involvement of ralfuranones in the quorum sensing signaling pathway and virulence of Ralstonia solanacearum strain OE1–1. Mol. Plant Pathol. [Epub ahead of print] doi: 10.1111/mpp.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly, J. , Spiteller, D. , Linz, J. , Jacobs, J. , Allen, C. , Nett, M. and Hoffmeister, D. (2013) Ralfuranone thioether production by the plant pathogen Ralstonia solanacearum . Chembiochem. 14, 2169–2178. [DOI] [PubMed] [Google Scholar]
- Rinaudi, L.V. and Giordano, W. (2010) An integrated view of biofilm formation in rhizobia. FEMS Microbiol. Lett. 304, 1–11. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. , and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Schembri, M.A. , Christiansen, G. and Klemm, P. (2001) FimH‐mediated autoaggregation of E. coli . Mol. Microbiol. 41, 1419–1430. [DOI] [PubMed] [Google Scholar]
- Schneider, P. , Jacobs, J.M. , Neres, J. , Aldrich, C.C. , Allen, C. , Nett, M. and Hoffmeister, D. (2009) The global virulence regulators VsrAD and PhcA control secondary metabolism in the plant pathogen Ralstonia solanacearum . Chembiochem. 10, 2730–2732. [DOI] [PubMed] [Google Scholar]
- Shinohara, R. , Kanda, A. , Ohnishi, K. , Kiba, A. and Hikichi, Y. (2005) Contribution of folate biosynthesis to Ralstonia solanacearum proliferation in the intercellular spaces. Appl. Environ. Microbiol. 71, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakevitz, D. , Kostlánová, N. , Blatman‐Jan, G. , Mitchell, E.P. , Lerrer, B. , Wimmerová, M. , Katcoff, D.J. , Imberty, A. and Gilboa‐Garber, N. (2004) A new Ralstonia solanacearum high‐affinity mannose‐binding lectin RS‐IIL structurally resembling the Pseudomonas aeruginosa fucose‐specific lectin PA‐IIL. Mol. Microbiol. 52, 691–700. [DOI] [PubMed] [Google Scholar]
- Tanabata, S. , Tanabata, T. , Saito, A. , Tajima, S. , Watanabe, S. , Ishikawa, K. , Ohtake, N. , Sueyoshi, K. and Ohyama, T. (2014) Computational image analysis method for measuring size of nodule growth in soybean. Jpn. J. Soil Sci. Plant Nutr. 85, 43–47. [Google Scholar]
- Tans‐Kersten, J. , Guan, Y. and Allen, C. (1998) Ralstonia solanacearum pectin methylesterase is required for growth on methylated pectin but not for bacterial wilt virulence. Appl. Environ. Microbiol. 64, 4918–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto, S. , Nakaho, K. , Adachi, M. , Ohnishi, K. , Kiba, A. and Hikichi, Y. (2008) Contribution of the type II secretion system in systemic infectivity of Ralstonia solanacearum through xylem vessels. J. Gen. Plant Pathol. 74, 71–75. [Google Scholar]
- Valls, M. , Genin, S. and Boucher, C. (2006) Integrated regulation of the type III secretion system and other virulence determinants in Ralstonia solanacearum . PLoS Pathog. 2, e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse, J. , Frey, P. and Trigalet, A. (1995) Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by Pseudomonas solanacearum . Mol. Plant–Microbe Interact. 8, 241–251. [Google Scholar]
- Wackler, B. , Schneider, P. , Jacobs, J.M. , Pauly, J. , Allen, C. , Nett, M. and Hoffmeister, D. (2011) Ralfuranone biosynthesis in Ralstonia solanacearum suggests functional divergence in the quinone synthetase family of enzymes. Chem. Biol. 18, 354–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Schematic diagram of the quorum sensing and conversion of ralfuranone I to various ralfuranones in Ralstonia solanacearum strain OE1‐1.
Fig. S2 (a) Observation of cells of Ralstonia solanacearum strain OE1‐1 incubated in tomato apoplast fluid on nano‐percolators for 24 h under a scanning electron microscope. OE1‐1 cells produced microcolonies of 1–5 μm in diameter (b), immature mushroom‐type biofilms (mBFs) of 5–10 μm in diameter (c) and mature mBFs of >10 μm in diameter (d).
Fig. S3 Attachment ability of Ralstonia solanacearum strains OE1‐1 (a), ralfuranone‐deficient mutant ΔralA (b) and the complemented ΔralA mutant ralA‐comp (c). Glass slides were soaked in suspensions of R. solanacearum strains.
Fig. S4 Model of the regulation of mushroom‐type biofilm (mBF) formation, the production of major exopolysaccharide EPS I and cell aggregation by Ralstonia solanacearum strain OE1‐1 through phc quorum sensing. 3‐OH MAME, methyl 3‐hydroxymyristate.
Table S1 Tukey–Kramer analysis of the involvement of ralfuranones in the formation of microcolonies, immature mushroom‐type biofilms and mature mushroom‐type biofilms by Ralstonia solanacearum strains.
Table S2 Tukey–Kramer analysis of the involvement of phc quorum sensing in the formation of microcolonies, immature mushroom‐type biofilms and mature mushroom‐type biofilms by Ralstonia solanacearum strains.
Table S3 Tukey–Kramer analysis of the relationship between phc quorum sensing and ralfuranones in the formation of microcolonies, immature mushroom‐type biofilms and mature mushroom‐type biofilms by Ralstonia solanacearum strains.


