Summary
Wheat is one of the primary staple foods throughout the planet. Significant yield gains in wheat production over the past 40 years have resulted in a steady balance of supply versus demand. However, predicted global population growth rates and dietary changes mean that substantial yield gains over the next several decades will be needed to meet this escalating demand. A key component to meeting this challenge is better management of fungal incited diseases, which can be responsible for 15%–20% yield losses per annum. Prominent diseases of wheat that currently contribute to these losses include the rusts, blotches and head blight/scab. Other recently emerged or relatively unnoticed diseases, such as wheat blast and spot blotch, respectively, also threaten grain production. This review seeks to provide an overview of the impact, distribution and management strategies of these diseases. In addition, the biology of the pathogens and the molecular basis of their interaction with wheat are discussed.
Keywords: blotch, fungal pathogens, Fusarium head blight/scab, Helminthosporium, Magnaporthe, rusts, wheat diseases
Introduction
Wheat is one of the world's most important staple grains and is the leading source of calories and plant‐derived protein in human food (Curtis et al., 2002). In 2015/2016, 735 million tonnes of wheat were produced globally, worth approximately US$ 145 billion. A recent assessment of wheat production by the Food and Agricultural Organization of the United Nations indicates that current wheat supply is ample for global demand (http://www.fao.org/worldfoodsituation/csdb/en/). Nevertheless, future production must increase as the global population is estimated to exceed nine billion people by 2050. As such, it is predicted that annual cereal production must grow by almost one billion tonnes. Furthermore, increased consumption of wheat products throughout many countries in Asia and changes to the grain quality requirements to meet the ‘hidden hunger’ objectives demand additional crop production (Anonymous, 2017; Shewry et al., 2016).
The continual drive to match yield and quality increases is not without its challenges. Decreasing availability of suitable farm land, climate change and a variety of unpredictable abiotic and biotic stresses continually pose threats to wheat production locally and globally. The decline in the genetic diversity of wheat, in the pursuit of elite high‐performing cultivars, has contributed to a perfect storm in pathogen emergence to the point at which diseases threaten global wheat supplies. Pathogenic fungi represent a significant constraint to wheat production. This review considers the key diseases and causal pathogenic fungi affecting crop production, as well as those emerging as threats. In each case, we consider the geographical distribution, impact (if information available), disease management strategies and briefly address the current status of the molecular understanding of each interaction.
The Wheat Rusts
Rust pathogens have hindered global wheat production since the domestication of the crop and continue to threaten the world's wheat supply (Roelfs et al., 1992). It is estimated that global annual losses to wheat rust pathogens range between US$ 4.3 to 5.0 billion (P. Pardey, University of Minnesota, unpublished).
Rust fungi are obligate biotrophic organisms that are completely dependent on nutritional resources obtained from living host cells for growth and reproduction (Cummins & Hiratsuka, 2004; Duplessis et al., 2012). Rust species vary in their ability to infect certain hosts and this differential biology is reflected in the classification of formae speciales (ff. spp.) (Eriksson, 1894). There are three wheat rust diseases, namely stem, stripe and leaf rust, all caused by members of the Basidiomycete family, genus Puccinia, named P. graminis f. sp. tritici (Pgt), P. striiformis f. sp. tritici (Pst) and P. triticina (Pt), respectively (Fig. 1) (McIntosh et al., 1995) (Fig. 1).
Figure 1.

Symptoms of wheat rust diseases caused by Puccinia graminis f. sp. tritici (A), Puccinia striiformis f. sp. tritici (B) and Puccinia triticina (C). Photographs courtesy of Rohit Mago.
Wheat stem rust
Puccinia graminis f. sp. tritici Ericks and Henn. (Pgt), the causal agent of wheat stem (black) rust, is widely distributed around the world, although less common than the other two wheat rusts (Leonard & Szabo, 2005; Singh et al., 2015). Pgt is usually found in regions in which warm and moist conditions prevail, and symptoms of infection are typically manifested as masses of red‐brick urediniospores on leaf sheaths, stems, glumes and awns of susceptible plants (Kolmer, 2005). Yield losses caused by stem rust are associated with a reduction in grain size and lodging of the plant (Leonard & Szabo, 2005).
Historically, stem rust epidemics have occurred throughout major wheat‐producing areas, and the need to control this disease served as a cornerstone to the Green Revolution which led to the introduction of semi‐dwarf, stem rust‐resistant wheat varieties (Figueroa et al., 2016). Although stem rust has been well controlled in many parts of the world, forecasting models assuming the absence of durable resistance estimate that global losses would average 6.2 million metric tons per year or higher under severe epidemics (Pardey et al., 2013).
In recent years, stem rust has gained significance as new virulence traits have evolved in Pgt populations, demonstrating the vulnerability of broadly used wheat cultivars across the globe (Pretorius et al., 2000; Singh et al., 2015). The emergence of the Ug99 race in Uganda in 1998, its subsequent geographical expansion within Africa, to the Middle East, and the appearance of Ug99 variants illustrate the imminent threat to wheat production (Singh et al., 2015). Estimates suggest that 90% of wheat varieties in the world are susceptible to Ug99, justifying elevated concerns about food security (Singh et al., 2011). Likewise, other Ug99 unrelated races have appeared in various parts of the world, reducing the efficacy of the newly identified and deployed sources of resistance. The ‘Digalu’ race caused a devastating epidemic in Ethiopia in 2014 and a similar race has been reported in Germany (Olivera Firpo et al., 2015, 2017). In 2016, another ‘broadly’ virulent race was detected in an outbreak in Sicily (Bhattacharya, 2017).
Wheat stripe rust
The disease wheat stripe (yellow) rust is caused by P. striiformis Westend. f. sp. tritici (Pst), a pathogen highly prevalent in temperate regions with cool and wet weather conditions (Chen et al., 2014). Stripe rust is currently the most economically important wheat rust disease with yield losses reaching 100% in susceptible cultivars (Chen, 2005). Approximately 88% of the world's wheat varieties are susceptible to Pst and global losses inflicted by the disease are nearly US$ 1 billion annually (Beddow et al., 2015; Wellings, 2011). In Australia, losses caused by stripe rust are estimated at AU$ 127 million (Murray & Brennan, 2009).
Wheat stripe rust has been reported in more than 60 countries and evidence suggests a significant global geographical expansion of Pst in the last 50 years (Beddow et al., 2015; Chen, 2005). Since 2000, aggressive races of Pst adapted to higher temperature climates have spread to parts of the world that were previously less affected by this disease (Ali et al., 2014). Although populations of Pst appear to be clonal in Europe, Australia and North America, there are significant levels of genotypic diversity within some pathogen populations (Chen et al., 2014). Such polymorphic populations are evident in western China and Central Asia, consistent with the Himalayan and nearby regions as the centre of pathogen diversity where sexual recombination appears to be common (Ali et al., 2014; Hovmøller et al., 2011). More recently, new race groups have emerged and swept through Europe in 2011, 2012/13 and 2015, and genetic analysis places their origin in Himalayan regions, indicating the role of incursions in altering the population structure of the pathogen (Hovmøller et al., 2015; Hubbard et al., 2015).
Wheat leaf rust
Puccinia triticina Eriks. (Pt) is the causal agent of leaf rust (Anikster et al., 1997; Bolton et al., 2008), the most common and widely distributed of the three wheat rust diseases (Bolton et al., 2008; Huerta‐Espino et al., 2011). The pathogen is prevalent in areas with mild temperatures and moist conditions. Yield losses associated with infection are attributed to a reduction in kernel weight and numbers of grain per head. Although grain losses caused by leaf rust display temporal and geographical variation, the economic significance of the disease is substantial (Huerta‐Espino et al., 2011; Kolmer, 2005). From 2000 to 2004 in the USA, estimated losses caused by Pt reached over US$ 350 million (Huerta‐Espino et al., 2011). In Australia, losses ascribed to this disease are calculated at AU$ 12 million (Murray & Brennan, 2009).
Leaf rust is a problematic disease because the pathogen displays high diversity, there is a constant emergence of new virulence profiles and the pathogen exhibits high adaptability to a wide range of climates (Huerta‐Espino et al., 2011; Kolmer, 2005; McCallum et al., 2016). The centre of origin of Pt is in the Fertile Crescent region of the Middle East, where both primary and alternative hosts exist; however, in most parts of the world, the population of Pt is clonal (Bolton et al., 2008; Kolmer, 2005).
General overview of the life cycle of wheat rust fungi
The full life cycle of wheat rust fungi involves the production of five types of spores associated with either asexual or sexual reproduction, which occurs in wheat or another unrelated non‐cereal host, respectively (Jin et al., 2010; McIntosh, 2009). The devastating asexual reproductive phase is driven by urediniospores, which mediate infection through multiple developmental stages, such as haustoria formation (Harder & Chong, 1984; Staples & Macko, 2004). Haustoria are structures essential not only to acquire nutrients, but to deliver effectors into the plant cell, allowing the suppression of plant defences and cell reprogramming to accommodate fungal growth (Garnica et al., 2014; Panstruga & Dodds, 2009; Ramachandran et al., 2016). Several Mahonia and Berberis spp. (barberry) serve as alternative hosts for Pgt (Leonard & Szabo, 2005; Roelfs, 1985) and Pst (Jin et al., 2010; Wang & Chen, 2017). The alternative hosts for Pt include species of Thalictrum, Anchusa, Clematis and Isopyrum (Bolton et al., 2008; Huerta‐Espino et al., 2011).
Molecular understanding of the rust–wheat interactions
The molecular and genetic basis underpinning wheat rust pathogenicity is not well characterized. The lack of efficient genetic transformation methods and the inability to generate in vitro fungal cultures have limited progress to define the underlying molecular factors governing rust resistance or susceptibility. Nevertheless, genetic resistance studies have provided a strong foundation to understand the basic components of these interactions.
Genetic resistance to rust infection has been identified as either race‐specific (also known as seedling or qualitative resistance) or non‐race‐specific resistance (Periyannan et al., 2017). More than 150 wheat rust resistance genes have been genetically defined in wheat or wild relatives, most conferring race‐specific resistance (McIntosh et al., 1995, 2013). At least 50 of these genes are designated Stem rust (Sr) resistance genes that are responsible for reactions to Pgt (McIntosh et al., 1995, 2013). Sr31 is one of the most widely utilized race‐specific genes against Pgt (Singh et al., 2006). Unfortunately, the evolution of virulence to Sr31 led to the emergence and spread of Ug99. Other important genes, such as Sr21, Sr24, Sr36, Sr38 and SrTmp, have also been overcome by the Ug99 lineage or Digalu races (Jin et al., 2008, 2009; Olivera Firpo et al., 2015; Pretorius et al., 2010). To date, the genes Sr2, Sr25, Sr23, Sr33, Sr35, Sr45 and Sr50 are considered to be the most valuable to protect against the newly evolved races (Singh et al., 2015). More than 70 genes are designated Yellow rust (Yr) resistance genes and these have been shown to condition reactions to Pst (Chen, 2005; McIntosh et al., 2013). However, in many parts of the world, Pst virulence has been reported for the majority of these genes. Resistance to Pt is conditioned by 68 Leaf rust (Lr) genes, with Lr1, Lr3, Lr10 and Lr20 being commonly used in global wheat cultivars (Dakouri et al., 2013; McIntosh et al., 1995). In general, the constant emergence of new rust races thwarts the maintenance of effective sources of genetic resistance in the field and highlights the challenges to control these diseases solely with genetic resistance (Ellis et al., 2014).
The cloning of 10 race‐specific genes in wheat (Sr22, Sr33, Sr35, Sr45, Sr50, Yr10, Lr1, Lr21, Lr10, Lr22) has demonstrated that, as in other plants, these genes encode nucleotide‐binding site leucine‐rich repeat (NBS‐LRR) proteins (Ellis et al., 2014; Mago et al., 2015; Steuernagel et al., 2016; Thind et al., 2017), and hence resistance responses must be governed by the direct or indirect recognition of cognate Avr factors. So far, no Avr factor has been characterized in the wheat rust fungi; however, genome sequencing and transcript predictions indicate the presence of rich effector repertoires (Bruce et al., 2013; Cantu et al., 2013; Duplessis et al., 2011; Garnica et al., 2013; Upadhyaya et al., 2015). In addition, limited numbers of sexual crosses showing genetic segregation for virulence and avirulence phenotypes support the hypothesis that rust–wheat interactions conform to the gene‐for‐gene model (Samborski & Dyck, 1968; Zambino et al., 2000). The evolution of physiological races is also consistent with this model. Physiological races in wheat rust fungi are defined by standard classification systems which establish relationships between disease phenotypes and specific race‐specific genes present in fixed sets of differential wheat cultivars (Chen et al., 2002; Jin et al., 2008; Long & Kolmer, 1989; McIntosh et al., 1995). A general feature in populations of wheat rust fungi is the appearance of virulence traits which overcome deployed race‐specific resistance in the field, a phenomenon known as ‘boom‐and‐bust cycles’ (Hulbert & Pumphrey, 2014). These pathogenicity changes can be the result of genetic recombination by sexual reproduction or somatic hybridization and serial mutations in the absence of alternative hosts (Lei et al., 2016; Park & Wellings, 2012; Wang & McCallum, 2009).
Non‐race‐specific resistance, often referred to as adult plant resistance (APR), confers quantitative resistance against wheat rusts (Periyannan et al., 2017). In these cases, the characteristic partial resistance phenotypes limit inoculum build‐up and the likelihood of the occurrence of epidemics. Examples of this type of resistance include Sr2, Lr34, Lr46, Lr67, Lr68 and Yr36 (Ellis et al., 2014). The mechanism by which these genes exert their function is not well understood. Cloning of Yr36 (Fu et al., 2009) revealed the role of a cytoplasmic protein kinase in mediating resistance. In contrast, Lr34 and Lr67 encode an ATP‐binding cassette transporter and hexose transporter, respectively (Dodds & Lagudah, 2016; Krattinger et al., 2009; Moore et al., 2015).
Management strategies for wheat rust diseases
Management strategies to mitigate the effect of wheat rust diseases include cultural control practices, in addition to chemical and genetic control (Ellis et al., 2014). The removal of inter‐crop ‘green bridges’ with tillage and the eradication of alternative hosts are some of the cultural practices to help manage wheat rust diseases (Kolmer et al., 2007; Zadoks & Bouwman, 1985). The use of genetic resistance has traditionally been the method of choice because fungicide treatments can be costly, weather dependent and raise environmental and health concerns. Nevertheless, the recent emergence of new rust races for which genetic resistance is unavailable has led to a more widespread use of fungicides. There are several chemical formulations approved to control wheat rusts. In general, quinone outside inhibitors (QoIs), 14α‐demethylation inhibitors (DMIs) and the recent use of succinate dehydrogenase inhibitors (SDHI) are effective (Oliver, 2014). In Australia, during the period 2003–2005, approximately AU$ 40–90 million per year was spent on chemical control to prevent stripe rust epidemics (Wellings, 2007). These regular chemical applications pose a potential risk of the reduction or loss of fungicide sensitivity (Arduim et al., 2012; Oliver, 2014).
Both race‐specific and non‐race‐specific resistances are utilized to manage all three wheat rust diseases (Ellis et al., 2014). The value of non‐race‐specific resistance genes is highly recognized because of their durability and protection against multiple pathogens or races. To date, breeding programmes have favoured the use of either gene stacking or pyramiding in order to achieve resistance durability, and often generate combinations of race‐specific and non‐race‐specific resistance genes with additive effects to optimize protection.
The airborne nature of rust pathogens, in combination with the local evolution of new races and the documented consequences of exotic rust incursions, means that coordinated international surveillance programmes are crucial to guide management strategies (Park et al., 2011). The information obtained via pathogen surveys informs policies, research and development investments, as well as crop protection and breeding approaches. In response to the emergence of Ug99, a global cereal rust monitoring system was created to collect geospatial and time‐sensitive data on rust prevalence and race structure (Hodson et al., 2009; Park et al., 2011). Such a resource has proven to be extremely valuable and will serve as a model to monitor other important pathogens.
The Blotch Diseases
The Ascomycete fungi Zymoseptoria tritici, Parastagonospora nodorum and Pyrenophora tritici‐repentis are the causal agents of Septoria tritici blotch (STB), Septoria nodorum blotch (SNB) and tan spot (TS), respectively (Fig. 2). Collectively, these diseases are described as the blotch diseases. Although these diseases are known to form complexes, each will be summarized independently.
Figure 2.

Symptoms of foliar blotch diseases. (A) Septoria tritici blotch. (B) Tan spot. (C) Septoria nodorum blotch. (D) Spot blotch. Photographs courtesy of Dr Megan McDonald (A, B) and Erin Hill (C, D).
Septoria tritici blotch
Zymoseptoria tritici (formerly known as Mycosphaerella graminicola or Septoria tritici) (Zt) is the causal agent of STB, the primary leaf disease of wheat in temperate growing regions. In Europe, STB is currently regarded as the primary threat to wheat production, and is estimated to cost growers throughout the EU €280–1200 million per annum, including direct losses and control costs (Fones & Gurr, 2015). Actual losses associated with STB are less clear in other growing regions. In Australia, STB is reported to be responsible for only AU$ 20 million in losses per year (Murray & Brennan, 2009). However, the prevalence of this disease has increased in recent years (Milgate et al., 2014).
Molecular understanding of the Zt–wheat interaction
The colonization pattern of Zt is unique amongst the blotch pathogens because the sparse apoplastically dwelling hyphae undergo a protracted asymptomatic growth phase (7–11 days post‐infection). During this latent phase, the pathogen grows slowly and does not appear to actively acquire nutrients (Keon et al., 2005; Sánchez‐Vallet et al., 2015). This phase is followed by the onset of necrotic symptoms as the host cells lyse, which increases nutrient availability for the pathogen to sporulate and complete its infection cycle.
The molecular basis of the infection cycle described above is poorly understood. A myriad of approaches, including forward genetics, reverse genetics, functional genomics and next‐generation sequencing, have attempted to identify the key molecules facilitating pathogen establishment and host specificity. The study by Marshall et al. (2011) has demonstrated that Zt actively subverts host defence mechanisms during the latent phase of the infection through the secretion of the chitin‐binding effector protein Mg3LysM. The inactivation of Mg3LysM resulted in the abolishment of apoplast colonization of susceptible wheat leaves.
A seminal advance in our understanding of this interaction has been reported recently through the cloning of the first avirulence gene from Zt. Zhong et al. (2017) used a combination of genome‐wide association studies and traditional map‐based cloning approaches to identify AvrStb6. AvrStb6 is a small secreted protein which elicits a resistance phenotype in Stb6 wheat lines. AvrStb6 has no similarity to other known proteins and displays evidence of undergoing strong diversifying selection, typical of genes involved in gene‐for‐gene interaction. The contribution though of AvrStb6 to virulence remains elusive.
Management strategies for STB
STB is primarily managed through two classes of fungicide: SDHIs and DMIs. Multi‐site inhibitor fungicides (e.g. chlorothalonil) are also used, although these lack the efficacy of the SDHIs and DMIs and are only useful as protectants. Other fungicide chemistries, previously efficacious against STB (e.g. QoIs), are no longer used because of the evolution of resistance (Torriani et al., 2015). The efficacy of existing chemistries is also now being re‐considered because of the performance reduction of DMIs in the field (Dooley et al., 2016; Heick et al., 2017). Furthermore, the future availability of some DMIs and SHDIs remains in doubt because of the impending EU Regulations and the emergence of new SHDI semi‐resistant strains (Jess et al., 2014). Despite this, chemical control, often involving two or three sprays per season, remains the primary mechanism for STB control in Europe. Renewed emphasis has now been placed on the use of alternative management strategies (Arraiano & Brown, 2017; Torriani et al., 2015).
Another major component of STB management is through genetic resistance. Twenty major genes have been mapped that contribute to qualitative resistance to STB (Brown et al., 2015). Sources of quantitative resistance have also been identified. Although only accounting for a moderate percentage of disease resistance, quantitatively inherited resistance is more durable under field conditions and often confers a broad‐spectrum resistance that is effective against multiple Zt genotypes. Brown et al. (2015), who provide a comprehensive background to the genetics of STB resistance, reported that 167 genomic regions harbouring quantitative trait loci (QTLs) have been identified. Phenotyping of these QTLs has demonstrated their involvement in different stages of disease progression, including sporulation, necrosis and latency. Unlike many other pathogens, numerous genetically different Zt genotypes can be routinely found infecting a single wheat leaf or a single field. In a comprehensive comparative study involving individual lesions, fields and regions, Zt genotype diversity in Switzerland, Texas and Israel was found to be high; for example, variation within a single field ranged from 79% to 100% of maximum possible values (Linde et al., 2002). These findings indicate a significant potential risk for the spread of Zt mutant alleles that would enable the breakdown of single major resistance genes.
Septoria nodorum blotch
Parastagonospora nodorum (Pn) is the causal agent of SNB. The disease is prominent throughout Australia, costing the industry approximately AU$ 100 million per annum (Murray & Brennan, 2009). Outside of Australia, the significance of SNB is less conclusive with documented evidence of direct losses difficult to source. Anecdotally, reports have emerged that the disease is prevalent throughout parts of France and the Scandinavian countries. In the UK, SNB was fully replaced by STB in the 1980s (Bearchell et al., 2005).
Molecular understanding of the Pn–wheat interaction
In contrast with Zt, symptom development as a result of Pn infection on susceptible cultivars develops rapidly, and the pathogen can complete its infection cycle in a week if conditions are suitable (Solomon et al., 2006). Recent studies have demonstrated that Pn secretes host‐specific effector proteins that facilitate in planta growth (Oliver et al., 2012). Although it is well documented that each of these effectors induces host cell death in a susceptible genetic background, evidence also suggests that each protein has a role in the suppression of host defence responses. For example, the Tox1 protein, which displays homology to chitin‐binding proteins, protects Pn from wheat chitinases induced as part of the host defence response (Liu et al., 2016). Similarly, Tox3 and ToxA interact with wheat PR‐1 proteins (Breen et al., 2016; Lu et al., 2014). Although evidence suggests that these interactions may serve independent functions, there is support for the hypothesis that the Tox3–PR‐1 interaction mediates host defence and facilitates disease (Breen et al., 2016, 2017).
The Pn effectors induce cell death and necrosis as an outcome of their interaction with a cognate dominant susceptibility gene (ToxA–Tsn1, Tox1–Snn1 and Tox3–Snn3). Tsn1 encodes a protein of the typical NBS‐LRR resistance gene structure that does not directly interact with ToxA, but is required for ToxA‐induced necrosis (Faris et al., 2010). Snn1 encodes a membrane‐bound wall‐associated kinase which interacts directly with Tox1 to induce cell death (Shi et al., 2016). Collectively, the cloning and characterization of these effectors and corresponding host‐interacting proteins have fundamentally advanced our understanding of host‐specific necrotrophic diseases.
Management strategies for SNB
SNB is effectively controlled by a combination of host genetics, chemical control and cultural practices, such as crop rotations (Francki, 2013). Most commercially available fungicides successfully manage SNB and, despite intensive use, fungicide resistance has been detected rarely. However, like many other foliar pathogens, resistance to the QoI fungicide azoxystrobin has been reported in Scandinavia (Blixt et al., 2009).
Host genetics play a significant role in controlling SNB, and multiple QTLs have been reported to confer both quantitative and qualitative resistance (Francki, 2013). However, the discovery of the genotype‐specific effector proteins ToxA, Tox1 and Tox3 has impacted significantly on cultivar development and deployment (Friesen et al., 2006; Liu et al., 2009, 2012). The purified effector proteins have been exploited commercially (through leaf infiltrations) to rapidly identify and disregard lines sensitive to specific effectors, and therefore susceptible to the pathogen (Vleeshouwers & Oliver, 2014). This application illustrates how breakthroughs in understanding molecular plant–pathogen interactions can expedite disease management practices.
Tan spot
Tan spot (TS) is caused by the ascomycete fungal pathogen Pyrenophora tritici‐repentis (Ptr) and results in decreased kernel weight and numbers of grains per head (Shabeer & Bockus, 1988). TS is found in most parts of the wheat‐growing world, including Europe, North America and Australia. In Asia, this pathogen is a component of the Helminthosporium leaf blight complex (Duveiller et al., 2007). The rise of TS as a significant disease in affected areas has been attributed to the use of minimum or zero tillage practices (Bockus & Claasen, 1992; Rees & Platz, 1979). However, the worldwide impact of the disease is difficult to assess because of a lack of available data. For example, there is anecdotal evidence that TS is prevalent within the UK, but there are no data publically available to support this. Disease surveys conducted in Australia in 2009 concluded that TS was the primary cause of yield loss, costing the local wheat industry in excess of AU$ 200 million in losses per annum (Murray & Brennan, 2009)
Molecular understanding of the Ptr–wheat interaction
The pathogenicity of Ptr is largely attributed to three necrotrophic effectors: ToxA, ToxB and ToxC. The products of each of these genes interact specifically in an inverse gene‐for‐gene manner with the host susceptibility genes Tsn1, Tsc2 and Tsc1, respectively, to facilitate disease (Ciuffetti et al., 2010). It has been hypothesized that ToxA was acquired by Ptr through a horizontal gene transfer event from Pn, and that this acquisition provided a significant fitness advantage to Ptr (Friesen et al., 2006). ToxB also encodes a small secreted protein that induces a chlorotic response in the presence of the host susceptibility locus Tsc2 (Ciuffetti et al., 2010). Unlike ToxA, ToxB is a multi‐copy gene within the Ptr genome and virulence has been reported to correlate with copy number. Potential ToxB homologues have been detected in a variety of other pathogens, including within Bipolaris, Alternaria and species in the Pyrenophora (Ciuffetti et al., 2010). The product of ToxC has not yet been identified, but it is thought to interact with Tsc1 in wheat resulting in a chlorotic phenotype (Effertz et al., 2002).
Management strategies for TS
Given the relatively recent emergence of the disease (or, at least, its recognition), the attention to TS management appears to be minimal compared with the diseases described above. Host genetics and the understanding of the Ptr race structure are important management tools. There are currently eight races of pathogen that have been identified, which are defined by the presence/absence of ToxA, ToxB and ToxC. ToxA is harboured in races 1, 2, 7 and 8, whereas the ToxB gene is present in races 5, 6, 7 and 8 (Strelkov & Lamari, 2003). Infiltration studies using culture filtrates have shown that ToxC is produced in races 1, 3, 6 and 8. The recognition of this race structure has implications for resistance breeding. For example, ToxB is lacking from all races of the pathogen in Australia. Consequently, breeders can focus on counter‐selection for Tsn1 (i.e. removal of ToxA sensitivity) to improve TS resistance, rather than divert resources towards Tsc2 (Antoni et al., 2010; Liu et al., 2017).
Chemical control also has a significant role in TS management. Fungicide applications result in yield increases ranging from 0.8 to 4.4 tonnes/ha depending on the level of tillage (Jørgensen & Olsen, 2007). Efficacy tests on different fungicides have demonstrated that the QoI chemicals (e.g. pyraclostrobin) are more effective than the DMIs (e.g. propiconazole).
Fusarium Head Blight/Scab Disease
Fusarium head blight (FHB) disease (also known as wheat scab or ear blight) leads to premature senescence of the wheat head and is caused primarily by the Ascomycete fungus Fusarium graminearum (Fg) (Fig. 3). In combination with other cereal‐infecting Fusarium species, many regionally unique species complexes exist to cause severe FHB epidemics [reviewed in Brown & Proctor (2013); http://scabusa.org/]. Globally, FHB is the most serious and hazardous floral disease of wheat. In the USA, China, the EU, UK, Africa, Brazil and elsewhere, severe FHB epidemics occur at a minimum of every fourth or fifth year. In the USA, yield losses as a result of FHB were estimated to be US$ 3 billion between the early 1990s and 2008 (Schumann & D'Arcy, 2009). Wheat crops are particularly prone to FHB if rain prevails just prior to and during crop anthesis. The main consequences of FHB disease are three‐fold: grain yield and quality are reduced, which compromises the overall harvest and subsequent marketability; moreover, the accumulation of various sesquiterpenoid trichothecene mycotoxins [such as the type B toxin deoxynivalenol (DON)] in the grain presents a major food safety risk and health hazard to humans, animals and natural ecosystems. In many countries, legal limits are in place on the permitted mycotoxin levels for the various end uses. For example, in the EU and USA, for human consumption, the respective permitted levels are 1250 and 2000 ppb for unprocessed products, and between 200–750 and 1000 ppb for finished products (http://scabusa.org). In North America, new highly virulent Fg strains have recently emerged that produce two novel type A trichothecene mycotoxins, namely NX‐2 and NX‐3, which present additional health risks (Varga et al., 2015).
Figure 3.
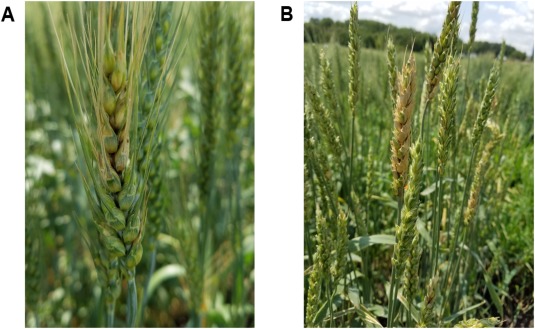
Symptoms of Fusarium head blight/scab. (A) Early infection signs manifested as a partially bleached wheat head. (B) Advanced infection of Fusarium graminearum manifested as an almost completely bleached wheat head.
Molecular understanding of the Fg–wheat interaction
The process by which Fg infects wheat heads is unique when compared with other fungal–plant interactions. Infections start with direct entry into open florets, followed by penetration of the various floral tissues with or without the formation of infection cushions (Boenisch & Schäfer, 2011). Fg infections continue with extracellular hyphae advancing between live host cells without causing visible disease symptoms, reminiscent of an apoplastic biotroph (Brown et al., 2010). This symptomless infection phase continues and extends for over a centimetre beyond any visibly bleached wheat tissues. The symptomatic phase occurs when surrounding fungal hyphae enter the wheat cells and is accompanied by host death. This intracellular Fg colonization of dead wheat cells has all the hallmarks of a necrotrophic pathogen (Brown et al., 2010). Complete wheat head colonization and bleaching take between 10 and 14 days.
During the symptomless phase, extensive Fg transcriptional changes occur, which are distinct from those observed during the symptomatic phase (Brown et al., 2017; Lysøe et al., 2011). These include the rapid activation of various Tri genes, responsible for trichothecene and DON production, other co‐regulated gene clusters and non‐clustered genes of known and unknown functions. DON production by Fg is known to be essential for disease formation in wheat spikes (Cuzick et al., 2008). DON inhibits protein translation in eukaryotes (reviewed in Varga et al., 2015). In planta, the presence of DON may reduce early induced plant defence responses. At higher concentrations, DON induces plant programmed cell death (PCD) and activates specific plant defences (Desmond et al., 2008). Plant PCD could assist nutrient acquisition by Fg.
Several other small secreted enzymes and secondary metabolites are required for disease formation. These are the iron‐scavenging secreted siderophore triacetyl fusarinine C (TAFC) (Oide et al., 2006), the secreted lipase Fgl1 (Blümke et al., 2014) and several carbohydrate‐active enzymes (CAZymes) that can modify and deconstruct plant cell walls and induce host cell death (Brown et al., 2012; Sperschneider et al., 2016). At least 390 Fg genes with a wide range of evolutionarily conserved functions in intracellular signalling, enzyme reactions and transcription are known to be required for pathogenicity or to contribute to virulence. Full details of each of these genes are available in the Pathogen–Host Interactions database (PHI‐base) (Urban et al., 2017).
So far, only a small region on wheat chromosome 3BS, where a major QTL conferring FHB resistance, known as Fhb1, is located, has been molecularly and functionally characterized in detail. Early studies revealed that the Fhb1 locus potentially either encodes or regulates the expression of a DON‐glucosyltransferase involved in DON detoxification (Lemmens et al., 2005). Recent findings have shown that a single gene in the Fhb1 locus, encoding a pore‐forming toxin‐like protein (PFT), plays a major role in FHB resistance that is unrelated to DON detoxification (Rawat et al., 2016). However, at least two reported, but as yet unpublished, studies have indicated that other gene types residing within the QTL containing Fhb1 may also contribute to host resistance.
Management strategies for FHB
The use of genetic‐based resistance to FHB represents the most cost‐effective control strategy (Wegulo et al., 2015), but is proving to be slow and complex to achieve in elite commercial wheats. To date, only a few unrelated moderately resistant sources have been identified: for example, the cultivars Sumai‐3 from China and Frontana from Brazil. FHB resistance is controlled by multiple major and minor QTLs that are often associated with a fitness cost or yield penalty (Gilbert & Haber, 2013). The two commercially important types of FHB resistance are classified as type I (resistance to initial infection) and type II (resistance to the spread of FHB in the host) (Cuthbert et al., 2006; Kubo et al., 2010; Niwa et al., 2014; Schroeder & Christensen, 1963). An alternative breeding approach is to select against known susceptibility targets in wheat, for example, a locus residing near one of the ‘Green revolution’ semi‐dwarfing Rht genes (Srinivasachary et al., 2009).
Fungicides are an integral part of the FHB disease management strategy in Europe. However, triazole (DMIs) mixtures applied during the optimal crop flowering period can only provide approximately 30%–60% FHB control (McMullen et al., 2012) as a result of Fg's intrinsic resistance to triazoles (Fan et al., 2013). Fungicide efficacy is reduced further when adverse weather conditions delay applications. In addition, technical challenges exist in the effective treatment of both the early‐ and late‐flowering tillers with a single application.
Cultural practices include tillage to bury infected crop residues, thereby preventing the development of the next generation of ascospores. Crop rotation with non‐host species is used to reduce FHB intensity and DON accumulation (Dill‐Macky & Jones, 2000). For example, the DON content of harvest grain in soybean–wheat rotations was 25% lower than in wheat–wheat rotations and 49% lower than in maize–wheat rotations. The ripe crop can also be left in the field to stand to wash out the mycotoxins prior to harvest (R. Dill‐Macky, unpublished). Post‐harvest operations to reduce mycotoxin contamination in feed wheats include grain cleaning to remove damaged, pink‐coloured or light grains which harbour the highest mycotoxin levels.
Host‐induced gene silencing (HIGS) has emerged recently as a novel transgenic approach to control FHB (Koch & Kogel, 2014). In this approach, transgenic plants express long double‐stranded or hairpin RNAs, with sequences identical to parts of the coding regions of Fg essential genes, which induce the plant's RNA silencing machinery to generate small interfering RNAs (siRNAs). The latter are mobile and can enter fungal cells [using unknown mechanism(s)] where they trigger the sequence‐specific degradation of fungal mRNA targets. Selected Fg HIGS targets include the Tri5 gene and specific members of the chitin synthetase gene family. In particular, the study by Cheng et al. (2015) indicated the high potential of HIGS under field conditions to reduce FHB disease and mycotoxin contamination to minimal levels.
Emerging and ‘Under the Radar’ Diseases
Spot blotch and Helminthosporium leaf blight
Bipolaris sorokiniana (syn. Helminthosporium sativum, teleomorph Cochliobolus sativus) (Bs) is a devastating pathogen causing both foliar and root diseases (Fig. 2). The foliar diseases have the most impact on wheat production and these can be delineated into spot blotch (SB) and also Helminthosporium leaf blight (HLB), a disease complex comprising both SB and TS (Duveiller et al., 2005). These diseases are the major biotic constraint to wheat production in the Eastern Gangetic Plains encompassing the main growing regions in India, Bangladesh and Nepal (Duveiller & Sharma, 2009; Saari, 1985; Singh et al., 2004). Losses to these diseases can reach up to 50% under conditions conducive to infection (Sharma & Duveiller, 2004; Singh et al., 2004). Significant losses have also been recorded in the warmer humid growing climates in South America (Duveiller & Sharma, 2012). Notably, the distribution of the disease appears to be comparable to wheat blast through South America and Asia (described below).
Molecular understanding of the Bs–wheat interaction
The molecular basis of the Bs–wheat interaction is poorly understood. Currently, only a handful of genes have been characterized from the pathogen and no Avr proteins or small molecules have yet been identified.
Nevertheless, recent genome sequencing efforts have made progress towards an understanding of the basis of the disease. McDonald et al. (2017) sequenced three Bs isolates from eastern Australia and identified that one of these isolates contained a gene nearly identical to ToxA as described in Pn and Ptr. Further analysis revealed that ToxA was present in approximately 30% of Australian Bs isolates and the existence of two haplotypes differing by only 1 bp. The gene was harboured within a larger 12‐kb region that is also shared between these blotch pathogens, suggesting that this mobile genetic element plays a key role in facilitating pathogenicity in these related fungi. ToxA has also been reported in North American isolates of the pathogen (T. Friesen, personal communication); however, it is unknown whether the gene is present in isolates from wheat‐growing areas affected by the disease. If ToxA is dominant within pathogen populations in affected areas, concerted efforts could be made to eliminate the Tsn1 susceptibility gene from local wheat varieties.
Management strategies for SB
The control of these diseases is problematic and the integration of complementary management approaches is often applied. Resistance breeding, chemical management and agronomic practices play key roles in the management of SB and HLB. As with most diseases, breeding for resistance is preferred. Given that most wheat growers in the affected areas are often small holders, fungicides are not always available, leaving growers reliant on resistance breeding (Duveiller, 2004). The genetics of SB and HLB resistance in wheat is quantitative (Joshi et al., 2004; Singh et al., 2016). Sources of resistance to both HLB and SB have included wheat germplasm from Brazil, Zambia and China, together with progenies from wide cross derivatives, including the use of synthetic hexaploids and classical hexaploid wheat and Chinese materials. The exploitation of these lines and others via QTL and association mapping has confirmed that many genes play a role in the resistance to these diseases (Singh et al., 2016). A detailed list of SB‐resistant genotypes is described by Duveiller & Sharma (2012).
Fungicides contribute to the control of SB and HLB, with the treatment of seeds and foliar symptoms shown to be effective in the management of disease. In a recent study by Sharma‐Poudyal et al. (2016), a combination of seed treatment, together with a single foliar spray, was found to be particularly effective. Initial treatment of wheat seed with carboxin + thiram resulted in 9% and 8% grain yield increases in successive years compared with control treatment. A subsequent single foliar post‐flowering spray further increased grain yields by up to 15%, demonstrating the potential of this complementary fungicide approach (Sharma‐Poudyal et al., 2016).
Planting time is also crucial in minimizing the losses caused by SB and HLB, presumably because of the increased growth rate of Bs at higher temperatures and also the increased likelihood of temperatures exceeding the threshold level of 28 °C when host resistance is known to break down (Duveiller et al., 2005; Nema & Joshi, 1973). Nutrient and water stresses also contribute to disease severity, with yield losses almost doubling when wheat is grown under suboptimal conditions (Sharma & Duveiller, 2006).
Wheat blast
Wheat blast (WB) is a devastating disease caused by the pathogenic fungus Magnaporthe oryzae Triticum pathotype (MoT) (synonym Pyricularia oryzae Triticum pathotype and Pyricularia graminis‐tritici) (Castroagudín et al., 2016; Cruz & Valent, 2017). WB was first identified in Paraná State in Brazil in 1985, and was subsequently disseminated to Bolivia, Argentina and Paraguay (Igarashi et al., 1986). The disease was confined to South America until its discovery in Bangladesh in 2016 (Islam et al., 2016), and more recently in India (Bhattacharya & Pal, 2017). A phylogenomics and populations genetics study indicated that the disease did not evolve independently in South Asia, but was probably caused by an incursion of a South American lineage of MoT (Islam et al., 2016).
WB is primarily a head disease. The typical symptoms range from small elliptical lesions to complete bleaching and empty spikes (Igarashi et al., 1986, 1990). Yield losses of 40%–100% have been reported (Igarashi, 1990). Foliar lesions caused by MoT have also been described; however, their role and significance in grain yield losses remain unknown (Cruz et al., 2016a; Igarashi, 1990). Warm and humid conditions are required for WB development (i.e. temperatures at 25 °C with at least a 10‐h wetting period) (Cardoso et al., 2008). Accordingly, a recent study in the USA listed Louisiana, Mississippi and Florida as the most high‐risk states to a WB outbreak (Cruz et al., 2016a).
Management strategies for WB
Given the recent emergence of WB in developing countries, disease control has been a priority. The efficacy of chemicals to control WB remains questionable. Reported studies have indicated that the performance of foliar fungicides only reduces disease incidence by 50% (compared with non‐fungicide treatments), and that this efficiency is typically far lower in the weather conditions, described above, that favour disease development (Maciel, 2011). Little information is available on the chemistries used in the field, although QoI fungicides, either as the sole active ingredient or in mixtures, have been documented for WB control in Brazil (Castroagudín et al., 2015). However, resistance to QoIs in South America has increased dramatically over the last 10 years to the point at which 90% of tested isolates carry a resistant allele. As such, there are no currently documented effective fungicide strategies in Brazil (Castroagudín et al., 2015)
Seed treatment with fungicides has also been tested to control the initial establishment of the disease. However, the length of time from seed germination to heading has led to questions with regard to the efficacy of seed treatments. Nonetheless, seed treatment trials in Brazil and also the USA have proven to be successful, at least in the management of seed‐borne infections (Bockus et al., 2015; Goulart & Paiva 1991; Igarashi, 1990).
A more desirable approach to control WB is through the use of host genetics. Unfortunately, these approaches have been only partially effective because of the lack of identifiable genetic resistance. Trials to screen for genetic resistance have also been hampered by the need to undertake field testing (i.e. no correlation between seedling resistance and yield losses) and the localized nature of the disease.
Recently, Cruz et al. (2016b) have assessed the effect of the 2NS translocation from Aegilops ventricosa (Zhuk.) Chennav on WB (head and leaf) resistance. This translocation carries a 25–38‐cM distal segment of chromosome arm 2NS from A. ventricosa to the distal region of chromosome arm 2AS in wheat (Helguera et al., 2003). Using near‐isogenic lines with and without 2NS, phenotyping in the field revealed that 2NS conferred a significant decrease in WB symptoms on heads, but, interestingly, not foliar symptoms. However, a small percentage of MoT isolates tested appeared to overcome the 2NS‐conferred resistance. However, these data are promising and provide the only robust evidence to date of a natural source of resistance to WB.
Several other wheat genes have been identified to play a role in resistance to MoT, including Rmg2, Rmg3, Rmg7 and Rmg8 (Anh et al., 2015; Tagle et al., 2015; Zhan et al., 2008). Although these may be promising candidates for resistance breeding, any potential impact is difficult to determine as disease screening was undertaken on either seedlings or detached heads, and field confirmation would be needed prior to any conclusion that these genes may have an effective role in WB management.
Concluding Remarks
It is, without question, the case that fungal diseases provide a significant challenge to the maximization of wheat yields, now and into the future. In this review, we have attempted to briefly summarize the key aspects of some of the most significant foliar and floral wheat diseases currently threatening production. We acknowledge that there are many other diseases that also threaten production (e.g. powdery mildew, take‐all, eyespot, bare patch, crown rot); however, space limitations restricted our selection of diseases to those currently considered to have the greatest impact on yield. Although clearly not exhaustive, this review provides a reference point for colleagues in molecular plant pathology to appreciate the complexity of these diseases and to consider them in a more holistic manner.
Acknowledgements
We acknowledge support by the University of Minnesota Experimental Station USDA‐NIFA Hatch/Figueroa project MIN‐22–058. Rothamsted Research receives grant‐aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) UK as part of the Institute Strategic Programme grants 20:20® wheat [BB/J/00426X/1] and Designing Future Wheat [BB/P016855/1]. PHI‐base receives support from the BBSRC as a National Capability [BB/J/004383/1] and the PhytoPath1 and Phytopath2 projects [BB/I000488/1, BB/K020056/1]. The BBSRC funds open access publication. PSS would like to acknowledge the support of the Australian Grains Research and Development Corporation (ANU00026). We thank Professor Ruth Dill‐Macky for allowing us access to the Fusarium Head Blight nursery at the University of Minnesota. The author(s) declare that there are no conflicting interests. We apologize to colleagues whose research could not be cited because of space restrictions.
References
- Ali, S. , Gladieux, P. , Leconte, M. , Gautier, A. , Justesen, A.F. , Hovmøller, M.S. , Enjalbert, J. and de Vallavieille‐Pope, C. (2014) Origin, migration routes and worldwide population genetic structure of the wheat yellow rust pathogen Puccinia striiformis f. sp. tritici . PLoS Pathog. 10, e1003903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anh, V.L. , Anh, N.T. , Tagle, A.G. , Vy, T.T.P. , Inoue, Y. , Takumi, S. , Chuma, I. and Tosa, Y. (2015) Rmg8, a new gene for resistance to Triticum isolates of Pyricularia oryzae in hexaploid wheat. Phytopathology, 105, 1568–1572. [DOI] [PubMed] [Google Scholar]
- Anikster, Y. , Bushnell, W. , Roelfs, A. , Eilam, T. and Manisterski, J. (1997) Puccinia recondita causing leaf rust on cultivated wheats, wild wheats, and rye. Can. J. Bot. 75, 2082–2096. [Google Scholar]
- Anonymous . (2017) Of rice and men. A circular tale of changing food preferences The Economist, Print Edition, International. [Google Scholar]
- Antoni, E.A. , Rybak, K. , Tucker, M.P. , Hane, J.K. , Solomon, P.S. , Drenth, A. , Shankar, M. and Oliver, R.P. (2010) Ubiquity of ToxA and absence of ToxB in Australian populations of Pyrenophora tritici‐repentis . Austral. Plant Pathol. 39, 63–68. [Google Scholar]
- Arduim, G.D.S. , Reis, E.M. , Barcellos, A.L. and Turra, C. (2012) In vivo sensitivity reduction of Puccinia triticina races, causal agent of wheat leaf rust, to DMI and QoI fungicides. Summa. Phytopathol. 38, 306–311. [Google Scholar]
- Arraiano, L.S. and Brown, J.K.M. (2017) Sources of resistance and susceptibility to Septoria tritici blotch of wheat. Mol. Plant Pathol. 18, 276–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearchell, S.J. , Fraaije, B.A. , Shaw, M.W. and Fitt, B.D.L. (2005) Wheat archive links long‐term fungal pathogen population dynamics to air pollution. Proc. Natl. Acad. Sci. USA, 102, 5438–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddow, J.M. , Pardey, P.G. , Chai, Y. , Hurley, T.M. , Kriticos, D.J. , Braun, H.‐J. , Park, R.F. , Cuddy, W.S. and Yonow, T. (2015) Research investment implications of shifts in the global geography of wheat stripe rust. Nat. Plants, 1, 15 132. [DOI] [PubMed] [Google Scholar]
- Bhattacharya, R. and Pal, S. (2017) Deadly wheat blast symptoms enter India through the Bangladesh border, Bengal govt burning crops on war footing. Hindustan Times. [Google Scholar]
- Bhattacharya, S. (2017) Deadly new wheat disease threatens Europe's crops. Nature, 542, 145–146. [DOI] [PubMed] [Google Scholar]
- Blixt, E. , Djurle, A. , Yuen, J. and Olson, Å. (2009) Fungicide sensitivity in Swedish isolates of Phaeosphaeria nodorum . Plant Pathol. 58, 655–664. [Google Scholar]
- Blümke, A. , Falter, C. , Herrfurth, C. , Sode, B. , Bode, R. , Schäfer, W. , Feussner, I. and Voigt, C.A. (2014) Secreted fungal effector lipase releases free fatty acids to inhibit innate immunity‐related callose formation during wheat head infection. Plant Physiol. 165, 346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockus, W.W. and Claasen, M.M. (1992) Effects of crop rotation and residue management practices on severity of tan spot of winter wheat. Plant Dis. 76, 633–636. [Google Scholar]
- Bockus, W.W. , Cruz, C.C. , Stack, J.P. and Valent, B. (2015) Effect of seed‐treatment fungicides on sporulation of Magnaporthe oryzae from wheat seed, 2014. Plant Dis. Manag. Rep. 9, ST004. [Google Scholar]
- Boenisch, M.J. and Schäfer, W. (2011) Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 11, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton, M.D. , Kolmer, J.A. and Garvin, D.F. (2008) Wheat leaf rust caused by Puccinia triticina . Mol. Plant Pathol. 9, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen, S. , Williams, S.J. , Winterberg, B. , Kobe, B. and Solomon, P.S. (2016) Wheat PR‐1 proteins are targeted by necrotrophic pathogen effector proteins. Plant J. 88, 13–25. [DOI] [PubMed] [Google Scholar]
- Breen, S. , Williams, S.J. , Outram, M. , Kobe, B. and Solomon, P.S. (2017) Emerging insights into the functions of pathogenesis‐related protein 1. Trends Plant Sci. 22, 871–879. [DOI] [PubMed] [Google Scholar]
- Brown, D.W. and Proctor, R.H. (2013) Fusarium: Genomics, Molecular and Cellular Biology. Norfolk, United Kingdom: Caister Academic Press. [Google Scholar]
- Brown, J.K.M. , Chartrain, L. , Lasserre‐Zuber, P. and Saintenac, C. (2015) Genetics of resistance to Zymoseptoria tritici and applications to wheat breeding. Fungal Genet. Biol. 79, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, N.A. , Urban, M. , van de Meene, A.M.L. and Hammond‐Kosack, K.E. (2010) The infection biology of Fusarium graminearum: defining the pathways of spikelet to spikelet colonisation in wheat ears. Fungal Biol. 114, 555–571. [DOI] [PubMed] [Google Scholar]
- Brown, N.A. , Antoniw, J. and Hammond‐Kosack, K.E. (2012) The predicted secretome of the plant pathogenic fungus Fusarium graminearum: a refined comparative analysis. PLoS One, 7, e33731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, N.A. , Evans, J. , Mead, A. and Hammond‐Kosack, K.E. (2017) A spatial temporal analysis of the Fusarium graminearum transcriptome during symptomless and symptomatic wheat infection. Mol. Plant Pathol. in press. doi: 10.1111/mpp.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, M. , Neugebauer, K.A. , Joly, D.L. , Migeon, P. , Cuomo, C.A. , Wang, S. , Akhunov, E. , Bakkeren, G. , Kolmer, J.A. and Fellers, J.P. (2013) Using transcription of six Puccinia triticina races to identify the effective secretome during infection of wheat. Front. Plant Sci. 4, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu, D. , Segovia, V. , MacLean, D. , Bayles, R. , Chen, X. , Kamoun, S. , Dubcovsky, J. , Saunders, D.G. and Uauy, C. (2013) Genome analyses of the wheat yellow (stripe) rust pathogen Puccinia striiformis f. sp. tritici reveal polymorphic and haustorial expressed secreted proteins as candidate effectors. BMC Genomics, 14, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso, C.A.D.A. , Reis, E.M. and Moreira, E.N. (2008) Development of a warning system for wheat blast caused by Pyricularia grisea . Summa Phytopathol. 34, 216–221. [Google Scholar]
- Castroagudín, V.L. , Ceresini, P.C. , De Oliveira, S.C. , Reges, J.T.A. , Maciel, J.L.N. , Bonato, A.L.V. , Dorigan, A.F. and McDonald, B.A. (2015) Resistance to QoI fungicides is widespread in Brazilian populations of the wheat blast pathogen Magnaporthe oryzae . Phytopathology, 105, 284–294. [DOI] [PubMed] [Google Scholar]
- Castroagudín, V.L. , Moreira, S.I. , Pereira, D.A.S. , Moreira, S.S. , Brunner, P.C. , Maciel, J.L.N. , Crous, P.W. , McDonald, B.A. , Alves, E. and Ceresini, P.C. (2016) Pyricularia graminis‐tritici sp. nov., a new Pyricularia species causing wheat blast. Persoonia, 37, 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Wellings, C. , Chen, X. , Kang, Z. and Liu, T. (2014) Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici . Mol. Plant Pathol. 15, 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. (2005) Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can. J. Plant Pathol. 27, 314–337. [Google Scholar]
- Chen, X. , Moore, M. , Milus, E.A. , Long, D.L. , Line, R.F. , Marshall, D. and Jackson, L. (2002) Wheat stripe rust epidemics and races of Puccinia striiformis f. sp. tritici in the United States in 2000. Plant Dis. 86, 39–46. [DOI] [PubMed] [Google Scholar]
- Cheng, W. , Song, X.S. , Li, H.P. , Cao, L.H. , Sun, K. , Qiu, X.L. , Xu, Y.B. , Yang, P. , Huang, T. , Zhang, J.B. , Qu, B. and Liao, Y.C. (2015) Host‐induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 13, 1335–1345. [DOI] [PubMed] [Google Scholar]
- Ciuffetti, L.M. , Manning, V.A. , Pandelova, I. , Betts, M.F. and Martinez, J.P. (2010) Host‐selective toxins, Ptr ToxA and Ptr ToxB, as necrotrophic effectors in the Pyrenophora tritici‐repentis–wheat interaction. New Phytol. 187, 911–919. [DOI] [PubMed] [Google Scholar]
- Cruz, C.D. and Valent, B. (2017) Wheat blast disease: danger on the move. Trop. Plant Pathol. 42, 210–222. doi: 10.1007/s40858-40017-40159-z. [DOI] [Google Scholar]
- Cruz, C.D. , Magarey, R.D. , Christie, D.N. , Fowler, G.A. , Fernandes, J.M. , Bockus, W.W. , Valent, B. and Stack, J.P. (2016a) Climate suitability for Magnaporthe oryzae Triticum pathotype in the United States. Plant Dis. 100, 1979–1987. [DOI] [PubMed] [Google Scholar]
- Cruz, C.D. , Peterson, G.L. , Bockus, W.W. , Kankanala, P. , Dubcovsky, J. , Jordan, K.W. , Akhunov, E. , Chumley, F. , Baldelomar, F.D. and Valent, B. (2016b) The 2NS translocation from Aegilops ventricosa confers resistance to the Triticum pathotype of Magnaporthe oryzae . Crop Sci. 56, 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins, G.B. and Hiratsuka, Y. (2004) Illustrated Genera of Rust Fungi. St. Paul, MN: APS Press. [Google Scholar]
- Curtis, B.C. , Rajaram, S. and Gómez Macpherson, H. (2002) Bread Wheat; Improvement and Production. FAO Plant Production and Protection Series No. 30. FAO, Rome.
- Cuthbert, P.A. , Somers, D.J. , Thomas, J. , Cloutier, S. and Brulé‐Babel, A. (2006) Fine mapping Fhb1, a major gene controlling fusarium head blight resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 112, 1465–1472. [DOI] [PubMed] [Google Scholar]
- Cuzick, A. , Urban, M. and Hammond‐Kosack, K. (2008) Fusarium graminearum gene deletion mutants map1 and tri5 reveal similarities and differences in the pathogenicity requirements to cause disease on Arabidopsis and wheat floral tissue. New Phytol. 177, 990–1000. [DOI] [PubMed] [Google Scholar]
- Dakouri, A. , McCallum, B.D. , Radovanovic, N. and Cloutier, S. (2013) Molecular and phenotypic characterization of seedling and adult plant leaf rust resistance in a world wheat collection. Mol. Breed. 32, 663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond, O.J. , Manners, J.M. , Stephens, A.E. , Maclean, D.J. , Schenk, P.M. , Gardiner, D.M. , Munn, A.L. and Kazan, K. (2008) The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol. Plant Pathol. 9, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill‐Macky, R. and Jones, R.K. (2000) The effect of previous crop residues and tillage on fusarium head blight of wheat. Plant Dis. 84, 71–76. [DOI] [PubMed] [Google Scholar]
- Dodds, P.N. and Lagudah, E.S. (2016) Starving the enemy. Science, 354, 1377–1378. [DOI] [PubMed] [Google Scholar]
- Dooley, H. , Shaw, M.W. , Mehenni‐Ciz, J. , Spink, J. and Kildea, S. (2016) Detection of Zymoseptoria tritici SDHI‐insensitive field isolates carrying the SdhC‐H152R and SdhD‐R47W substitutions. Pest Manag. Sci. 72, 2203–2207. [DOI] [PubMed] [Google Scholar]
- Duplessis, S. , Cuomo, C.A. , Lin, Y.C. , Aerts, A. , Tisserant, E. , Veneault‐Fourrey, C. , Joly, D.L. , Hacquard, S. , Amselem, J. , Cantarel, B.L. , Chiu, R. , Coutinho, P.M. , Feau, N. , Field, M. , Frey, P. , Gelhaye, E. , Goldberg, J. , Grabherr, M.G. , Kodira, C.D. , Kohler, A. , Kues, U. , Lindquist, E.A. , Lucas, S.M. , Mago, R. , Mauceli, E. , Morin, E. , Murat, C. , Pangilinan, J.L. , Park, R. , Pearson, M. , Quesneville, H. , Rouhier, N. , Sakthikumar, S. , Salamov, A.A. , Schmutz, J. , Selles, B. , Shapiro, H. , Tanguay, P. , Tuskan, G.A. , Henrissat, B. , Van de Peer, Y. , Rouze, P. , Ellis, J.G. , Dodds, P.N. , Schein, J.E. , Zhong, S. , Hamelin, R.C. , Grigoriev, I.V. , Szabo, L.J. and Martin, F. (2011) Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proc. Natl. Acad. Sci. USA, 108, 9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplessis, S. , Joly, D.L. and Dodds, P.N. (2012) Rust effectors In: Effectors in Plant–Microbe Interactions. Wiley‐Blackwell, pp. 155–193. [Google Scholar]
- Duveiller, E. (2004) Controlling foliar blights of wheat in the rice–wheat systems of Asia. Plant Dis. 88, 552–556. [DOI] [PubMed] [Google Scholar]
- Duveiller, E. and Sharma, R.C. (2009) Genetic improvement and crop management strategies to minimize yield losses in warm non‐traditional wheat growing areas due to spot blotch pathogen Cochliobolus sativus . J. Phytopathol. 157, 521–534. [Google Scholar]
- Duveiller, E. and Sharma, R.C. (2012) Wheat resistance to spot blotch or foliar blight In: Disease Resistance in Wheat. (Sharma, I., ed.), pp. 120–135. Wallingford, UK: CABI. [Google Scholar]
- Duveiller, E. , Kandel, Y.R. , Sharma, R.C. and Shrestha, S.M. (2005) Epidemiology of foliar blights (spot blotch and tan spot) of wheat in the plains bordering the Himalayas. Phytopathology, 95, 248–256. [DOI] [PubMed] [Google Scholar]
- Duveiller, E. , Singh, R.P. and Nicol, J.M. (2007) The challenges of maintaining wheat productivity: pests, diseases, and potential epidemics. Euphytica, 157, 417–430. [Google Scholar]
- Duveiller, E. , Hodson, D. and Tiedmann, A. (2010) Wheat blast caused by Magnaporthe grisea: a reality and new challenge for wheat research. 8th Int Wheat Conf. 2010; St. Petersburg. Vavilov Research Institute of Plant Industry.
- Effertz, R.J. , Meinhardt, S.W. , Anderson, J.A. , Jordahl, J.G. and Francl, L.J. (2002) Identification of a chlorosis‐inducing toxin from Pyrenophora tritici‐repentis and the chromosomal location of an insensitivity locus in wheat. Phytopathology, 92, 527–533. [DOI] [PubMed] [Google Scholar]
- Ellis, J.G. , Lagudah, E.S. , Spielmeyer, W. and Dodds, P.N. (2014) The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 5, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, J. (1894) Ueber die Specialisirung des Parasitismus bei den Getreiderostpilzen. Ber. Dtsch. Bot. Ges. 12, 292–331. [Google Scholar]
- Fan, J. , Urban, M. , Parker, J.E. , Brewer, H.C. , Kelly, S.L. , Hammond‐Kosack, K.E. , Fraaije, B.A. , Liu, X. and Cools, H.J. (2013) Characterization of the sterol 14α‐demethylases of Fusarium graminearum identifies a novel genus‐specific CYP51 function. New Phytol. 198, 821–835. [DOI] [PubMed] [Google Scholar]
- Faris, J.D. , Zhang, Z. , Lu, H. , Lu, S. , Reddy, L. , Cloutier, S. , Fellers, J.P. , Meinhardt, S.W. , Rasmussen, J.B. , Xu, S.S. , Oliver, R.P. , Simons, K.J. and Friesen, T.L. (2010) A unique wheat disease resistance‐like gene governs effector‐triggered susceptibility to necrotrophic pathogens. Proc. Natl. Acad. Sci. USA, 107, 13 544–13 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa, M. , Upadhyaya, N.M. , Sperschneider, J. , Park, R.F. , Szabo, L.J. , Steffenson, B. , Ellis, J.G. and Dodds, P.N. (2016) Changing the game: using integrative genomics to probe virulence mechanisms of the stem rust pathogen Puccinia graminis f. sp. tritici . Front. Plant Sci. 7, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fones, H. and Gurr, S. (2015) The impact of Septoria tritici Blotch disease on wheat: an EU perspective. Fungal Genet. Biol. 79, 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francki, M.G. (2013) Improving Stagonospora nodorum resistance in wheat: a review. Crop Sci. 53, 355–365. [Google Scholar]
- Friesen, T.L. , Stukenbrock, E.H. , Liu, Z. , Meinhardt, S. , Ling, H. , Faris, J.D. , Rasmussen, J.B. , Solomon, P.S. , McDonald, B.A. and Oliver, R.P. (2006) Emergence of a new disease as a result of interspecific virulence gene transfer. Nat. Genet. 38, 953–956. [DOI] [PubMed] [Google Scholar]
- Fu, D. , Uauy, C. , Distelfeld, A. , Blechl, A. , Epstein, L. , Chen, X. , Sela, H. , Fahima, T. and Dubcovsky, J. (2009) A kinase‐START gene confers temperature‐dependent resistance to wheat stripe rust. Science, 323, 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnica, D.P. , Upadhyaya, N.M. , Dodds, P.N. and Rathjen, J.P. (2013) Strategies for wheat stripe rust pathogenicity identified by transcriptome sequencing. PLoS One, 8, e67150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnica, D.P. , Nemri, A. , Upadhyaya, N.M. , Rathjen, J.P. and Dodds, P.N. (2014) The ins and outs of rust haustoria. PLoS Pathog. 10, e1004329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, J. and Haber, S. (2013) Overview of some recent research developments in fusarium head blight of wheat. Can. J. Plant Pathol. 35, 149–174. [Google Scholar]
- Goulart, A. and Paiva, F. (1991) Controle de Pyricularia oryzae e Helminthosporium sativum pelo tratamento de sementes de trigo com fungicidas. Pesqui. Agropecu. Bras. 26, 11–12. [Google Scholar]
- Harder, D.E. and Chong, J. (1984) Structure and physiology of haustoria In: The Cereal Rusts, Vol. I (Bushnell W.R. and Roelfs A.P., eds), pp. 416–460. Academic Press, Inc; Available at http://www.sciencedirect.com/science/book/9780121484019. [Google Scholar]
- Heick, T.M. , Justesen, A.F. and Jørgensen, L.N. (2017) Resistance of wheat pathogen Zymoseptoria tritici to DMI and QoI fungicides in the Nordic‐Baltic region – a status. Eur. J. Plant Pathol. 102, 1–14. [Google Scholar]
- Helguera, M. , Khan, I.A. , Kolmer, J. , Lijavetzky, D. , Zhong‐Qi, L. and Dubcovsky, J. (2003) PCR assays for the Lr37‐Yr17‐Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Sci. 43, 1839–1847. [Google Scholar]
- Hodson, D. , Cressman, K. , Nazari, K. , Park, R. and Yahyaoui, A. (2009) The global cereal rust monitoring system In: BGRI Technical Workshop. (McIntosh, R., ed.), pp. 35–46. Obregon, Mexcio. [Google Scholar]
- Hovmøller, M.S. , Sørensen, C.K. , Walter, S. and Justesen, A.F. (2011) Diversity of Puccinia striiformis on cereals and grasses. Annu. Rev. Phytopathol. 49, 197–217. [DOI] [PubMed] [Google Scholar]
- Hovmøller, M.S. , Walter, S. , Bayles, R.A. , Hubbard, A. , Flath, K. , Sommerfeldt, N. , Leconte, M. , Czembor, P. , Rodriguez‐Algaba, J. , Thach, T. , Hansen, J.G. , Lassen, P. , Justesen, A.F. , Ali, S. and de Vallavieille‐Pope, C. (2015) Replacement of the European wheat yellow rust population by new races from the centre of diversity in the near‐Himalayan region. Plant Pathol. 65, 402–411. [Google Scholar]
- Hubbard, A. , Lewis, C.M. , Yoshida, K. , Ramirez‐Gonzalez, R.H. , de Vallavieille‐Pope, C. , Thomas, J. , Kamoun, S. , Bayles, R. , Uauy, C. and Saunders, D.G.O. (2015) Field pathogenomics reveals the emergence of a diverse wheat yellow rust population. Genome Biol. 16, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta‐Espino, J. , Singh, R. , German, S. , McCallum, B. , Park, R. , Chen, W.Q. , Bhardwaj, S. and Goyeau, H. (2011) Global status of wheat leaf rust caused by Puccinia triticina . Euphytica, 179, 143–160. [Google Scholar]
- Hulbert, S. and Pumphrey, M. (2014) A time for more booms and fewer busts? Unraveling cereal–rust interactions. Mol. Plant–Microbe Interact. 27, 207–214. [DOI] [PubMed] [Google Scholar]
- Igarashi, S. (1990) Update on wheat blast (Pyricularia oryzae) in Brazil In: Update on wheat blast (Pyricularia oryzae) in Brazil. (Saunders, D., ed.). Mexico. [Google Scholar]
- Igarashi, S. , Utimada, C. , Igarashi, L. , Kazuma, A. and Lopes, R. (1986) Pyricularia sp. em trigo. I. Ocorreňcia de Pyricularia sp. no Estado do Paranà. Fitopatol. Bras. 11, 351–352. [Google Scholar]
- Islam, M.T. , Croll, D. , Gladieux, P. , Soanes, D.M. , Persoons, A. , Bhattacharjee, P. , Hossain, M.S. , Gupta, D.R. , Rahman, M.M. , Mahboob, M.G. , Cook, N. , Salam, M.U. , Surovy, M.Z. , Sancho, V.B. , Maciel, J.L.N. , NhaniJúnior, A. , Castroagudín, V.L. , Reges, J.T.A. , Ceresini, P.C. , Ravel, S. , Kellner, R. , Fournier, E. , Tharreau, D. , Lebrun, M.H. , McDonald, B.A. , Stitt, T. , Swan, D. , Talbot, N.J. , Saunders, D.G.O. , Win, J. and Kamoun, S. (2016) Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae . BMC Biol. 14, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jess, S. , Kildea, S. , Moody, A. , Rennick, G. , Murchie, A.K. and Cooke, L.R. (2014) European Union policy on pesticides: implications for agriculture in Ireland. Pest Manag. Sci. 70, 1646–1654. [DOI] [PubMed] [Google Scholar]
- Jin, Y. , Szabo, L.J. , Pretorius, Z.A. , Singh, R.P. , Ward, R. and Fetch, T Jr . (2008) Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici . Plant Dis. 92, 923–926. [DOI] [PubMed] [Google Scholar]
- Jin, Y. , Szabo, L. , Rouse, M. , Fetch, T. Jr , Pretorius, Z. , Wanyera, R. and Njau, P. (2009) Detection of virulence to resistance gene Sr36 within the TTKS race lineage of Puccinia graminis f. sp. tritici . Plant Dis. 93, 367–370. [DOI] [PubMed] [Google Scholar]
- Jin, Y. , Szabo, L.J. and Carson, M. (2010) Century‐old mystery of Puccinia striiformis life history solved with the identification of Berberis as an alternate host. Phytopathology, 100, 432–435. [DOI] [PubMed] [Google Scholar]
- Jørgensen, L.N. and Olsen, L.V. (2007) Control of tan spot (Drechslera tritici‐repentis) using cultivar resistance, tillage methods and fungicides. Crop Protect. 26, 1606–1616. [Google Scholar]
- Joshi, A.K. , Kumar, S. , Chand, R. and Ortiz‐Ferrara, G. (2004) Inheritance of resistance to spot blotch caused by Bipolaris sorokiniana in spring wheat. Plant Breed. 123, 213–219. [Google Scholar]
- Keon, J. , Rudd, J.J. , Antoniw, J. , Skinner, W. , Hargreaves, J. and Hammond‐Kosack, K. (2005) Metabolic and stress adaptation by Mycosphaerella graminicola during sporulation in its host revealed through microarray transcription profiling. Mol. Plant Pathol. 6, 527–540. [DOI] [PubMed] [Google Scholar]
- Koch, A. and Kogel, K.H. (2014) New wind in the sails: improving the agronomic value of crop plants through RNAi‐mediated gene silencing. Plant Biotechnol. J. 12, 821–831. [DOI] [PubMed] [Google Scholar]
- Kolmer, J.A. (2005) Tracking wheat rust on a continental scale. Curr. Opin. Plant Biol. 8, 441–449. [DOI] [PubMed] [Google Scholar]
- Kolmer, J. , Jin, Y. and Long, D. (2007) Wheat leaf and stem rust in the United States. Crop Pasture Sci. 58, 631–638. [Google Scholar]
- Krattinger, S.G. , Lagudah, E.S. , Spielmeyer, W. , Singh, R.P. , Huerta‐Espino, J. , McFadden, H. , Bossolini, E. , Selter, L.L. and Keller, B. (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science, 323, 1360–1363. [DOI] [PubMed] [Google Scholar]
- Kubo, K. , Kawada, N. , Fujita, M. , Hatta, K. , Oda, S. and Nakajima, T. (2010) Effect of cleistogamy on Fusarium head blight resistance in wheat. Breed. Sci. 60, 405–411. [Google Scholar]
- Lei, Y. , Wang, M. , Wan, A. , Xia, C. , See, D.R. , Zhang, M. and Chen, X. (2016) Virulence and molecular characterization of experimental isolates of the stripe rust pathogen (Puccinia striiformis) indicate somatic recombination. Phytopathology, 107, 329–344. [DOI] [PubMed] [Google Scholar]
- Lemmens, M. , Scholz, U. , Berthiller, F. , Dall'Asta, C. , Koutnik, A. , Schuhmacher, R. , Adam, G. , Buerstmayr, H. , Mesterházy, Á. , Krska, R. and Ruckenbauer, P. (2005) The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for fusarium head blight resistance in wheat. Mol. Plant–Microbe Interact. 18, 1318–1324. [DOI] [PubMed] [Google Scholar]
- Leonard, K.J. and Szabo, L.J. (2005) Stem rust of small grains and grasses caused by Puccinia graminis . Mol. Plant Pathol. 6, 99–111. [DOI] [PubMed] [Google Scholar]
- Linde, C.C. , Zhan, J. and McDonald, B.A. (2002) Population structure of Mycosphaerella graminicola: from lesions to continents. Phytopathology, 92, 946–955. [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Faris, J.D. , Oliver, R.P. , Tan, K.‐C. , Solomon, P.S. , McDonald, M.C. , McDonald, B.A. , Nunez, A. , Lu, S. , Rasmussen, J.B. and Friesen, T.L. (2009) SnTox3 acts in effector triggered susceptibility to induce disease on wheat carrying the Snn3 gene. PLoS Pathog. 5, e1000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Zhang, Z. , Faris, J.D. , Oliver, R.P. , Syme, R. , McDonald, M.C. , McDonald, B.A. , Solomon, P.S. , Lu, S. , Shelver, W.L. , Xu, S. and Friesen, T.L. (2012) The cysteine rich necrotrophic effector SnTox1 produced by Stagonospora nodorum triggers susceptibility of wheat lines harboring Snn1 . PLoS Pathog. 8, e1002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Gao, Y. , Kim, Y.M. , Faris, J.D. , Shelver, W.L. , de Wit, P.J.G.M. , Xu, S.S. and Friesen, T.L. (2016) SnTox1, a Parastagonospora nodorum necrotrophic effector, is a dual‐function protein that facilitates infection while protecting from wheat‐produced chitinases. New Phytol. 211, 1052–1064. [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Zurn, J.D. , Kariyawasam, G. , Faris, J.D. , Shi, G. , Hansen, J. , Rasmussen, J.B. and Acevedo, M. (2017) Inverse gene‐for‐gene interactions contribute additively to tan spot susceptibility in wheat. Theor. Appl. Genet. 130, 1267–1276. [DOI] [PubMed] [Google Scholar]
- Long, D. and Kolmer, J. (1989) A North American system of nomenclature for Puccinia recondita f. sp. tritici . Phytopathology, 79, 525–529. [Google Scholar]
- Lu, S. , Faris, J.D. , Sherwood, R. , Friesen, T.L. and Edwards, M.C. (2014) A dimeric PR‐1‐type pathogenesis‐related protein interacts with ToxA and potentially mediates ToxA‐induced necrosis in sensitive wheat. Mol. Plant Pathol. 15, 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysøe, E. , Seong, K.Y. and Kistler, H.C. (2011) The transcriptome of Fusarium graminearum during the infection of wheat. Mol. Plant–Microbe Interact. 24, 995–1000. [DOI] [PubMed] [Google Scholar]
- Maciel, J.L.N. (2011) Magnaporthe oryzae, the blast pathogen: current status and options for its control. CAB Rev. 6, 1–8. [Google Scholar]
- Mago, R. , Zhang, P. , Vautrin, S. , Šimková, H. , Bansal, U. , Luo, M.‐C. , Rouse, M. , Karaoglu, H. , Periyannan, S. , Kolmer, J. , Jin, Y. , Ayliffe, M.A. , Bariana, H. , Park, R.F. , McIntosh, R. , Doležel, J. , Bergès, H. , Spielmeyer, W. , Lagudah, E.S. , Ellis, J.G. and Dodds, P.N. (2015) The wheat Sr50 reveals a rich diversity at a cereal disease resistance locus. Nat. Plants, 1, 15 186. [DOI] [PubMed] [Google Scholar]
- Marshall, R. , Kombrink, A. , Motteram, J. , Loza‐Reyes, E. , Lucas, J. , Hammond‐Kosack, K.E. , Thomma, B.P.H.J. and Rudd, J.J. (2011) Analysis of two in planta expressed LysM effector homologs from the fungus Mycosphaerella graminicola reveals novel functional properties and varying contributions to virulence on wheat. Plant Physiol. 156, 756–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum, B.D. , Hiebert, C.W. , Cloutier, S. , Bakkeren, G. , Rosa, S.B. , Humphreys, D.G. , Marais, G.F. , McCartney, C.A. , Panwar, V. , Rampitsch, C. , Saville, B.J. and Wang, X. (2016) A review of wheat leaf rust research and the development of resistant cultivars in Canada. Can. J. Plant Pathol. 38, 1–18. [Google Scholar]
- McDonald, M.C. , Ahren, D. , Simpfendorfer, S. , Milgate, A. and Solomon, P.S. (2017) The discovery of the virulence gene ToxA in the wheat and barley pathogen Bipolaris sorokiniana . Mol. Plant Pathol. in press. doi: 10.1111/mpp.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, R. (2009) The history and status of the wheat rusts In: NGRI Technical Workshop. (McIntosh R.A., ed.), pp. 11–24. Obregon, Mexcio. [Google Scholar]
- McIntosh, R.A. , Wellings, C.R. and Park, R.F. (1995) Wheat Rusts: An Atlas of Resistance Genes. East Melbourne, Australia: CSIRO. [Google Scholar]
- McIntosh, R. , Yamazaki, Y. , Dubcovsky, J. , Rogers, W. , Morris, C. , Somers, D. , Appels, R. and Devos, K. (2013) Catalogue of gene symbols for wheat Supplement. https://shigen.nig.ac.jp/wheat/komugi/genes/macgene/supplement2012.pdf [Google Scholar]
- McMullen, M. , Bergstrom, G. , De Wolf, E. , Dill‐Macky, R. , Hershman, D. , Shaner, G. and Van Sanford, D. (2012) A unified effort to fight an enemy of wheat and barley: fusarium head blight. Plant Dis. 96, 1712–1728. [DOI] [PubMed] [Google Scholar]
- Milgate, A. , Hollaway, G. , Wallwork, H. and Thomas, G. (2014. ) Septoria Tritici Blotch: GRDC Fact Sheet. Canberra, Australia: GRDC.
- Moore, J.W. , Herrera‐Foessel, S. , Lan, C. , Schnippenkoetter, W. , Ayliffe, M. , Huerta‐Espino, J. , Lillemo, M. , Viccars, L. , Milne, R. , Periyannan, S. , Kong, X. , Spielmeyer, W. , Talbot, M. , Bariana, H. , Patrick, J.W. , Dodds, P. , Singh, R. and Lagudah, E. (2015) A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat. Genet. 47, 1494–1498. [DOI] [PubMed] [Google Scholar]
- Murray, G.M. and Brennan, J.P. (2009) Estimating disease losses to the Australian wheat industry. Australas. Plant Pathol. 38, 558–570. [Google Scholar]
- Nema, K.G. and Joshi, L.H. (1973) Spot blotch disease of wheat in relation to host age, temperature and moisture. Indian Phytopathol. 26, 41–48. [Google Scholar]
- Niwa, S. , Kubo, K. , Lewis, J. , Kikuchi, R. , Alagu, M. and Ban, T. (2014) Variations for Fusarium head blight resistance associated with genomic diversity in different sources of the resistant wheat cultivar 'Sumai 3'. Breed. Sci. 64, 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oide, S. , Moeder, W. , Krasnoff, S. , Gibson, D. , Haas, H. , Yoshioka, K. and Turgeon, B.G. (2006) NPS6, encoding a nonribosomal peptide synthetase involved in siderophore‐mediated iron metabolism, is a conserved virulence determinant of plant pathogenic ascomycetes. Plant Cell, 18, 2836–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, R.P. (2014) A reassessment of the risk of rust fungi developing resistance to fungicides. Pest Manag. Sci. 70, 1641–1645. [DOI] [PubMed] [Google Scholar]
- Oliver, R.P. , Friesen, T.L. , Faris, J.D. and Solomon, P.S. (2012) Stagonospora nodorum: from pathology to genomics and host resistance. Annu. Rev. Phytopathol. 50, 23–43. [DOI] [PubMed] [Google Scholar]
- Olivera Firpo, P. , Newcomb, M. , Szabo, L. , Rouse, M.N. , Johnson, J.L. , Gale, S.W. , Luster, D. , Hodson, D. , Cox, J.A. and Burgin, L. (2015) Phenotypic and genotypic characterization of race TKTTF of Puccinia graminis f. sp. tritici that caused a wheat stem rust epidemic in southern Ethiopia in 2013/14. Phytopathology, 105, 917–928. [DOI] [PubMed] [Google Scholar]
- Olivera Firpo, P. , Newcomb, M. , Flath, K. , Sommerfeldt‐Impe, N. , Szabo, L. , Carter, M. , Luster, D. and Jin, Y. (2017) Characterization of Puccinia graminis f. sp. tritici isolates derived from an unusual wheat stem rust outbreak in Germany in 2013. Plant Pathol. 66, 1258–1266. doi: 10.1111/ppa.12674. [DOI] [Google Scholar]
- Panstruga, R. and Dodds, P.N. (2009) Terrific protein traffic: the mystery of effector protein delivery by filamentous plant pathogens. Science, 324, 748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardey, P. , Beddow, J. , Kriticos, D. , Hurley, T. , Park, R. , Duveiller, E. , Sutherst, R. , Burdon, J. and Hodson, D. (2013) Right‐sizing stem‐rust research. Science, 340, 147–148. [DOI] [PubMed] [Google Scholar]
- Park, R. , Fetch, T. , Hodson, D. , Jin, Y. , Nazari, K. , Prashar, M. and Pretorius, Z. (2011) International surveillance of wheat rust pathogens: progress and challenges. Euphytica, 179, 109–117. [Google Scholar]
- Park, R.F. and Wellings, C.R. (2012) Somatic hybridization in the Uredinales. Annu. Rev. Phytopathol. 50, 219–239. [DOI] [PubMed] [Google Scholar]
- Periyannan, S. , Milne, R.J. , Figueroa, M. , Lagudah, E.S. and Dodds, P.N. (2017) An overview of genetic rust resistance: from broad to specific mechanisms. PLoS Pathog. 13, e1006380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius, Z.A. , Singh, R.P. , Wagoire, W.W. and Payne, T.S. (2000) Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis. f. sp. tritici in Uganda. Plant Dis. 84, 203. [DOI] [PubMed] [Google Scholar]
- Pretorius, Z. , Bender, C. , Visser, B. and Terefe, T. (2010) First report of a Puccinia graminis f. sp. tritici race virulent to the Sr24 and Sr31 wheat stem rust resistance genes in South Africa. Plant Dis. 94, 784. [DOI] [PubMed] [Google Scholar]
- Ramachandran, S.R. , Yin, C. , Kud, J. , Tanaka, K. , Mahoney, A.K. , Xiao, F. and Hulbert, S.H. (2016) Effectors from wheat rust fungi suppress multiple plant defense responses. Phytopathology, 107, 75–83. [DOI] [PubMed] [Google Scholar]
- Rawat, N. , Pumphrey, M.O. , Liu, S. , Zhang, X. , Tiwari, V.K. , Ando, K. , Trick, H.N. , Bockus, W.W. , Akhunov, E. , Anderson, J.A. and Gill, B.S. (2016) Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore‐forming toxin‐like domain conferring resistance to Fusarium head blight. Nat. Genet. 48, 1576–1580. [DOI] [PubMed] [Google Scholar]
- Rees, R.G. and Platz, G.J. (1979) The occurrence and control of yellow spot of wheat in north‐eastern australia. Aust. J. Exp. Agric. 19, 362–372. [Google Scholar]
- Roelfs, A.P. (1985) Wheat and Rye Stem Rust. Orlando, FL: Academic Press. [Google Scholar]
- Roelfs, A.P. , Singh, R.P. and Saari, E.E. (1992) Rust Diseases of Wheat: Concepts and Methods of Disease Management. Mexico, DF: CIMMYT. [Google Scholar]
- Saari, E.E. (1985) Distribution and importance of root rot diseases of wheat, barley and triticale in South and Southeast Asia Mexico, DF (Mexico), CIMMYT. pp. 37–51. [Google Scholar]
- Samborski, D. and Dyck, P. (1968) Inheritance of virulence in wheat leaf rust on the standard differential wheat varieties. Can. J. Genet. Cytol. 10, 24–32. [Google Scholar]
- Sánchez‐Vallet, A. , McDonald, M.C. , Solomon, P.S. and McDonald, B.A. (2015) Is Zymoseptoria tritici a hemibiotroph? Fungal Genet. Biol. 79, 29–32. [DOI] [PubMed] [Google Scholar]
- Schroeder, H.W. and Christensen, J.J. (1963) Factors affecting resistance of wheat to scab caused by Gibberella zeae . Phytopathology, 53, 831–838. [Google Scholar]
- Schumann, G.L. and D'Arcy, C.J. (2009) Essential Plant Pathology. St. Paul, MN: APS Press. [Google Scholar]
- Shabeer, A. and Bockus, W.W. (1988) Tan spot effects on yield and yield components relative to growth stage in winter wheat. Plant Dis. 72, 599–602. [Google Scholar]
- Sharma, R.C. and Duveiller, E. (2004) Effect of helminthosporium leaf blight on performance of timely and late‐seeded wheat under optimal and stressed levels of soil fertility and moisture. Field Crops Res. 89, 205–218. [Google Scholar]
- Sharma, R.C. and Duveiller, E. (2006) Spot blotch continues to cause substantial grain yield reductions under resource‐limited farming conditions. J. Phytopathol. 154, 482–488. [Google Scholar]
- Sharma‐Poudyal, D. , Sharma, R.C. and Duveiller, E. (2016) Control of Helminthosporium leaf blight of spring wheat using seed treatments and single foliar spray in Indo‐Gangetic Plains of Nepal. Crop Protect. 88, 161–166. [Google Scholar]
- Shewry, P.R. , Pellny, T.K. and Lovegrove, A. (2016) Is modern wheat bad for health? Nat. Plants, 2, 16097. [DOI] [PubMed] [Google Scholar]
- Shi, G. , Zhang, Z. , Friesen, T.L. , Raats, D. , Fahima, T. , Brueggeman, R.S. , Lu, S. , Trick, H.N. , Liu, Z. , Chao, W. , Frenkel, Z. , Xu, S.S. , Rasmussen, J.B. and Faris, J.D. (2016) The hijacking of a receptor kinase‐driven pathway by a wheat fungal pathogen leads to disease. Sci. Adv. 2, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, D.P. , Sharma, A.K. , Tewari, A.N. , Singh, K.P. , Singh, A.K. , Singh, R.N. , Singh, S.P. , Kalappanawar, I.K. , Dodan, D.S. and Singh, V.K. (2004) Assessment of losses due to leaf blight in popular varieties of wheat (Triticum aestivum) under different sowing conditions and agroclimatic zones in India. Indian J. Agric. Sci. 74, 110–113. [Google Scholar]
- Singh, R.P. , Hodson, D.P. , Jin, Y. , Huerta, E.J. , Kinyua, M. , Wanyera, R. , Njau, P. and Ward, R.W. (2006) Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Rev. 1, 54. [Google Scholar]
- Singh, R.P. , Hodson, D.P. , Huerta‐Espino, J. , Jin, Y. , Bhavani, S. , Njau, P. , Herrera‐Foessel, S. , Singh, P.K. , Singh, S. and Govindan, V. (2011) The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu. Rev. Phytopathol. 49, 465–481. [DOI] [PubMed] [Google Scholar]
- Singh, R.P. , Hodson, D.P. , Jin, Y. , Lagudah, E.S. , Ayliffe, M.A. , Bhavani, S. , Rouse, M.N. , Pretorius, Z.A. , Szabo, L.J. , Huerta‐Espino, J. , Basnet, B.R. , Lan, C. and Hovmøller, M.S. (2015) Emergence and spread of new races of wheat stem rust fungus: continued threat to food security and prospects of genetic control. Phytopathology, 105, 872–884. [DOI] [PubMed] [Google Scholar]
- Singh, V. , Singh, G. , Chaudhury, A. , Ojha, A. , Tyagi, B.S. , Chowdhary, A.K. and Sheoran, S. (2016) Phenotyping at hot spots and tagging of QTLs conferring spot blotch resistance in bread wheat. Mol. Biol. Rep. 43, 1–11. [DOI] [PubMed] [Google Scholar]
- Solomon, P.S. , Wilson, T.J.G. , Rybak, K. , Parker, K. , Lowe, R.G.T. and Oliver, R.P. (2006) Structural characterisation of the interaction between Triticum aestivum and the dothideomycete pathogen Stagonospora nodorum . Eur. J. Plant Pathol. 114, 275–282. [Google Scholar]
- Sperschneider, J. , Gardiner, D.M. , Dodds, P.N. , Tini, F. , Covarelli, L. , Singh, K.B. , Manners, J.M. and Taylor, J.M. (2016) EffectorP: predicting fungal effector proteins from secretomes using machine learning. New Phytol. 210, 743–761. [DOI] [PubMed] [Google Scholar]
- Srinivasachary, Gosman, N. , Steed, A. , Hollins, T.W. , Bayles, R. , Jennings, P. and Nicholson, P. (2009) Semi‐dwarfing Rht‐B1 and Rht‐D1 loci of wheat differ significantly in their influence on resistance to Fusarium head blight. Theor. Appl. Genet. 118, 695–702. [DOI] [PubMed] [Google Scholar]
- Staples, R.C. and Macko, V. (2004) Germination of urediospores and differentiation of infection structures In: The Cereal Rusts, Vol. I (Bushell W.R. and Roelfs A.P., eds), pp. 250–282. Orlando, USA; Academic Press, Inc. [Google Scholar]
- Steuernagel, B. , Periyannan, S.K. , Hernández‐Pinzón, I. , Witek, K. , Rouse, M.N. , Yu, G. , Hatta, A. , Ayliffe, M. , Bariana, H. and Jones, J.D. (2016) Rapid cloning of disease‐resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 34, 652–655. [DOI] [PubMed] [Google Scholar]
- Strelkov, S.E. and Lamari, L. (2003) Host–parasite interactions in tan spot Pyrenophora tritici‐repentis of wheat. Can. J. Plant Pathol. 25, 339–349. [Google Scholar]
- Tagle, A.G. , Chuma, I. and Tosa, Y. (2015) Rmg7, a new gene for resistance to Triticum isolates of Pyricularia oryzae identified in tetraploid wheat. Phytopathology, 105, 495–499. [DOI] [PubMed] [Google Scholar]
- Thind, A.K. , Wicker, T. , Simkova, H. , Fossati, D. , Moullet, O. , Brabant, C. , Vrana, J. , Dolezel, J. and Krattinger, S.G. (2017) Rapid cloning of genes in hexaploid wheat using cultivar‐specific long‐range chromosome assembly. Nat. Biotechnol. 35, 793–796. doi: 10.1038/nbt.3877. [DOI] [PubMed] [Google Scholar]
- Torriani, S.F.F. , Melichar, J.P.E. , Mills, C. , Pain, N. , Sierotzki, H. and Courbot, M. (2015) Zymoseptoria tritici: a major threat to wheat production, integrated approaches to control. Fungal Genet. Biol. 79, 8–12. [DOI] [PubMed] [Google Scholar]
- Upadhyaya, N.M. , Garnica, D.P. , Karaoglu, H. , Sperschneider, J. , Nemri, A. , Xu, B. , Mago, R. , Cuomo, C.A. , Rathjen, J.P. , Park, R.F. , Ellis, J.G. and Dodds, P.N. (2015) Comparative genomics of Australian isolates of the wheat stem rust pathogen Puccinia graminis f. sp. tritici reveals extensive polymorphism in candidate effector genes. Front. Plant Sci. 5, 759. Article [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban, M. , Cuzick, A. , Rutherford, K. , Irvine, A. , Pedro, H. , Pant, R. , Sadanadan, V. , Khamari, L. , Billal, S. , Mohanty, S. and Hammond‐Kosack, K.E. (2017) PHI‐base: a new interface and further additions for the multi‐species pathogen–host interactions database. Nucleic Acids Res. 45, D604–D610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga, E. , Wiesenberger, G. , Hametner, C. , Ward, T.J. , Dong, Y. , Schöfbeck, D. , McCormick, S. , Broz, K. , Stückler, R. , Schuhmacher, R. , Krska, R. , Kistler, H.C. , Berthiller, F. and Adam, G. (2015) New tricks of an old enemy: isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. Environ. Microbiol. 17, 2588–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleeshouwers, V.G.A.A. and Oliver, R.P. (2014) Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Mol. Plant–Microbe Interact. 27, 196–206. [DOI] [PubMed] [Google Scholar]
- Wang, M.N. and Chen, X. (2017) First report of Oregon grape (Mahonia aquifolium) as an alternate host for the wheat stripe rust pathogen (Puccinia striiformis f. sp. tritici) under artificial inoculation. Phytopathology, 107, 329–344. [DOI] [PubMed] [Google Scholar]
- Wang, X. and McCallum, B. (2009) Fusion body formation, germ tube anastomosis, and nuclear migration during the germination of urediniospores of the wheat leaf rust fungus, Puccinia triticina . Phytopathology, 99, 1355–1364. [DOI] [PubMed] [Google Scholar]
- Wegulo, S.N. , Baenziger, P.S. , Hernandez Nopsa, J. , Bockus, W.W. and Hallen‐Adams, H. (2015) Management of Fusarium head blight of wheat and barley. Crop Protect. 73, 100–107. [Google Scholar]
- Wellings, C. (2007) Puccinia striiformis in Australia: a review of the incursion, evolution, and adaptation of stripe rust in the period 1979–2006. Crop Pasture Sci. 58, 567–575. [Google Scholar]
- Wellings, C.R. (2011) Global status of stripe rust: a review of historical and current threats. Euphytica, 179, 129–141. [Google Scholar]
- Zadoks, J.C. and Bouwman, J.J. (1985) Epidemiology in Europe In: The Cereal Rusts Volume II. Diseases, distibution, epidemiology and control (Roelfs, A. P. and Bushnell, W. R., eds), pp. 329–369. Orlando, USA: Academic Press. [Google Scholar]
- Zambino, P. , Kubelik, A. and Szabo, L. (2000) Gene action and linkage of avirulence genes to DNA markers in the rust fungus Puccinia graminis . Phytopathology, 90, 819–826. [DOI] [PubMed] [Google Scholar]
- Zhan, S.W. , Mayama, S. and Tosa, Y. (2008) Identification of two genes for resistance to Triticum isolates of Magnaporthe oryzae in wheat. Genome, 51, 216–221. [DOI] [PubMed] [Google Scholar]
- Zhong, Z. , Marcel, T.C. , Hartmann, F.E. , Ma, X. , Plissonneau, C. , Zala, M. , Ducasse, A. , Confais, J. , Compain, J. , Lapalu, N. , Amselem, J. , McDonald, B.A. , Croll, D. and Palma‐Guerrero, J. (2017) A small secreted protein in Zymoseptoria tritici is responsible for avirulence on wheat cultivars carrying the Stb6 resistance gene. New Phytol. 214, 619–631. [DOI] [PubMed] [Google Scholar]


