Summary
Taxonomic status: Bacteria; Phylum Proteobacteria; Class Gammaproteobacteria; Order Xanthomonadales; Family Xanthomonadaceae; Genus Xanthomonas; Species Xanthomonas citri ssp. citri (Xcc).
Host range: Compatible hosts vary in their susceptibility to citrus canker (CC), with grapefruit, lime and lemon being the most susceptible, sweet orange being moderately susceptible, and kumquat and calamondin being amongst the least susceptible.
Microbiological properties: Xcc is a rod‐shaped (1.5–2.0 × 0.5–0.75 µm), Gram‐negative, aerobic bacterium with a single polar flagellum. The bacterium forms yellow colonies on culture media as a result of the production of xanthomonadin.
Distribution: Present in South America, the British Virgin Islands, Africa, the Middle East, India, Asia and the South Pacific islands. Localized incidence in the USA, Argentina, Brazil, Bolivia, Uruguay, Senegal, Mali, Burkina Faso, Tanzania, Iran, Saudi Arabia, Yemen and Bangladesh. Widespread throughout Paraguay, Comoros, China, Japan, Malaysia and Vietnam. Eradicated from South Africa, Australia and New Zealand. Absent from Europe.
Keywords: Asiatic citrus canker, Xanthomonas axonopodis pv. citri
Introduction
Xanthomonas citri ssp. citri is a member of the family Xanthomonadaceae, one of the largest and most important groups of bacterial phytopathogens, and has been used as a model organism for the study of pathogenesis and phylogeny. Investigations of the pathogenome of X. citri ssp. citri have provided insights into bacterial phytopathogen host specificities, plant host recognition of pathogens, the means by which a plant host's cellular machinery is altered by the pathogen to facilitate infection, and pathogen propagation and dissemination. Analysis of the chromosomal and plasmid DNA of X. citri ssp. citri has helped to establish the evolutionary relationship between xanthomonad pathovars.
Xanthomonas citri ssp. citri is the causative agent of citrus canker (CC) disease and, as a pathogen of a globally important fruit crop, Citrus, has been the subject of extensive study with respect to epidemiology and disease management. Practices to exclude or quarantine X. citri ssp. citri continue to be refined wherever citrus is cultivated. Methodologies and products to manage and eradicate CC continue to be developed around the world.
In this review, we present the most recent findings and developments in the study of X. citri ssp. citri and CC, including in the areas of taxonomy, detection, the pathogenome, host–pathogen interaction, epidemiology, biofilm formation and management.
Taxonomy of CC Xanthomonads
CC, also referred to as Asiatic CC, was first observed in the USA during an outbreak in several southeastern states in the early 1900s (Stevens, 1914). Hasse received samples from Florida, Texas and Mississippi in 1914 and successfully isolated the bacterium (Hasse, 1915). Following characterization and pathogenicity experiments, Hasse named the bacterium Pseudomonas citri (Hasse, 1915). The bacterium has since been placed in various genera, including Bacterium, Phytomonas and, finally, Xanthomonas in 1939 as Xanthomonas citri (Doidge, 1916; Dowson, 1939; Jehle, 1916; Society of American Bacteriologists et al., 1923). Strains within this species have been referred to as A strains to indicate that they are associated with Asiatic CC. In the 1970s, two additional CC‐producing xanthomonads were identified and initially designated as group C strains, which cause canker lesions only in Key lime (Citrus aurantifolia), and group B strains, which have a wider host range (Namekata and Oliveira, 1972; Rosetti, 1977).
The bacterium remained in X. citri until 1978, when it was placed in X. campestris pv. citri by Dye in order to preserve citri at the infrasubspecific level (Young et al., 1978). The bacterium was then transferred to X. citri by Gabriel et al. (1989), and the B and C strains were placed in X. campestris pv. aurantifolii. Young et al. argued that further work was needed relating to the placement of the CC strains in X. citri and therefore recommended that the A, B and C strains continue to be designated as X. campestris pv. citri (Young et al., 1991). Using DNA–DNA hybridization (DDH) based on renaturation rates with a diverse array of Xanthomonas strains, Vauterin et al. (1995) transferred the A strains to X. axonopodis pv. citri and the B and C strains to X. axonopodis pv. aurantifolii.
Schaad et al. (2005) used the S1 nuclease technique for DNA–DNA relatedness to place the X. axonopodis pv. citri strains in X. smithii ssp. smithii and the X. axonopodis pv. aurantifolii strains in X. fuscans ssp. aurantifolii. The placement of strains in X. smithii was later deemed illegitimate because of the preceding legitimate proposal by Gabriel et al. (1989). Schaad et al. (2006) then emended their original recommendation, placing these strains in X. citri. In 2007, X. citri ssp. citri was formally validated (Euzeby, 2007).
Recently, Constantin et al. (2016) have proposed significant changes to xanthomonad taxonomy, including within X. citri. Using a polyphasic approach which included multilocus sequence analysis (MLSA), DDH calculation of whole‐genome average nucleotide identity values and phenotypic analyses, they recommended the addition of a number of pathovars within X. axonopodis, as well as placement of members of X. fuscans, into X. citri. As such, X. fuscans ssp. aurantifolii has been recommended to be transferred to X. citri as X. citri pv. aurantifolii (Constantin et al., 2016). The authors have submitted their recommendations to the International Journal of Systematic and Evolutionary Microbiology for these changes, and they have been approved (M.‐A. Jaques, personal communication). For now, X. citri ssp. citri (Xcc) and X. fuscans ssp. aurantifolii (Xfa) are the most recent prokaryotic names with standing in nomenclature for those bacteria that cause CC.
Detection and Identification of Xanthomonads Causing Citrus Bacterial Canker
There are several techniques used for the diagnosis of CC. However, in most cases, when no official confirmation is needed, the disease can be identified by the recognition of typical symptoms (Behlau and Belasque, 2014; Gottwald et al., 2002; Schubert et al., 2001). Isolation of Xcc from lesions on solid medium, followed by the observation of xanthomonad‐like colonies, i.e. yellow, convex, circular, semi‐translucent and with regular margin, may also be used to confirm the causal agent (Schaad et al., 2001). Pathogenicity tests on susceptible citrus species may be performed by the infiltration of a bacterial suspension adjusted to 108 colony‐forming units (CFU)/mL into the leaf mesophyll, followed by the observation of water soaking and raised margins in the infiltrated portion of the leaf 2–4 days after inoculation (Behlau and Belasque, 2014; Schubert et al., 2001).
When symptoms are atypical or an official confirmation is necessary for quarantine purposes, DNA‐based assays and serological tests are commonly used methods for CC diagnosis. Although molecular methods are able to detect the presence of Xcc in infected plant tissue, even before the appearance of canker lesions, serology‐based tests are usually sufficient for Xcc detection from symptomatic tissue. Several sets of primers have been designed for polymerase chain reaction (PCR) detection of Xcc based on rDNA sequences, plasmid‐borne genes and pathogenicity regulatory factors (Coletta‐Filho et al., 2006; Cubero and Graham, 2002, 2004, 2005; Hartung, 1992; Mavrodieva et al., 2004; Sun et al., 2004). More recently, the development of real‐time PCR and loop‐mediated isothermal amplification protocols has increased the accuracy of diagnostic tests for the detection of Xcc (Cubero and Graham, 2005; Golmohammadi et al., 2007; Mavrodieva et al., 2004; Park et al., 2006; Rigano et al., 2010). Rep‐PCR with BOX and ERIC primers has also been used to differentiate strains within the same Xcc pathotype (Cubero and Graham, 2002; Jaciani et al., 2012; Louws et al., 1999). The serology‐based test, also known as enzyme‐linked immunosorbent assay (ELISA), is based on the ability of an antibody to recognize and bind to a specific antigen, in this case a substance associated with Xcc. ELISA has been demonstrated to be useful for the rapid diagnosis of CC (Alvarez et al., 1991; Bouzar et al., 1994; Civerolo and Fan, 1982; Gottwald et al., 1991; Sun et al., 2004). This test is usually performed in the laboratory, but is also available as a strip‐based kit easy to use in the field, where disease is suspected. These kits do not require special equipment or training to use and the results are obtained within a few minutes (Al‐Saleh et al., 2014).
Other older techniques for the detection of Xcc have been developed. These include physiological characterization, fatty acid profile analyses, protein profiling, hybridization, restriction fragment length polymorphism analysis and comparison of plasmid DNA patterns (Bouzar et al., 1994; Egel et al., 1991; Gottwald et al., 1991; Hartung, 1992; Pruvost et al., 1992; Sun et al., 2004; Vauterin et al., 1991a, 1991b, 1996a, 1996b; Verniere et al., 1991, 1998).
Xanthomonad Pathogenome
A major landmark in the characterization of Xcc‐A was the complete genome sequencing of strain Xcc‐A306 in 2002 (da Silva et al., 2002). The genome of this strain consists of a 5 175 554‐base pair (bp) chromosome encoding approximately 4500 genes, and two plasmids (da Silva et al., 2002). The single circular chromosome has a 64.7% G + C content, and 4314 predicted open reading frames (ORFs) (Van Sluys et al., 2002). The two plasmids, pXAC64 (64 920 bp) and pXAC33 (33 699 bp), have 61.4% and 61.9% G + C content, respectively (da Silva et al., 2002; Van Sluys et al., 2002). Approximately 7% of Xcc genes are involved in pathogenicity, virulence and ecological adaptation (Van Sluys et al., 2002).
Some features discovered in the Xcc‐A genome were the large number of cell wall‐degrading enzymes (CWDEs), proteases, iron receptors, genes related to energy metabolism pathways, the type 2 secretion system (T2SS) and type 3 secretion system (T3SS), genes for flagella structural units and chemotactic protein genes, and the xanthomonadin and xanthan gum synthesis gene cluster (gumB to gumM), which are important in the epiphytic phase of the life cycle (Dunger et al., 2007). There are genes for the production of biofilms, which facilitate the formation of bacterial aggregates in the apoplast, proliferation of the bacterium and subsequent symptom development (Rigano et al., 2007).
The 23‐kb hrp (for hypersensitive reaction and pathogenicity) region has six operons, designated as hrpA to hrpF (Rossier et al., 2000). The hrp cluster is part of a pathogenicity island in the Xcc genome and encodes the T3SS. This cluster also possesses transposases and has a different G + C content from the rest of the genome, providing evidence for the acquisition of the hrp cluster via horizontal gene transfer (Hacker and Kaper, 2000). The left border of the core region (hrpA to hrpE) carries different species‐dependent effector genes, which contribute to pathogenicity on different hosts. The hrpB operon, which encodes eight proteins, has been demonstrated to be very stable at the species level, and its sequence can be used for phylogenetic analysis and species determination in xanthomonads (Leite et al., 1994; Obradovic et al., 2004). The right border, called the HrpF peninsula, is the more variable subregion among xanthomonads in terms of overall structure and gene content (Tampakaki et al., 2010). The Xcc hrpG and hrpX genes collectively regulate all 24 T3SS genes, 23 T3SS effector genes and 29 T2SS substrate genes, as well as XacPNP (Guo et al., 2011). T2SS substrate products include proteases, lipases and CWDEs. The genes hrpG and hrpX are involved in the regulation of amino acid biosynthesis, oxidative phosphorylation, the pentose‐phosphate pathway, phenolic catabolism and the transport of sugar, iron and potassium in response to exposure to the host environment (Guo et al., 2011). An additional 124 and 90 unknown genes are regulated by hrpG and hrpX, respectively (Guo et al., 2011). HrpG induces the expression of 11 proteins secreted by the T2SS, as well as being a regulator of the T3SS (Yamazaki et al., 2008a). Together with HrpG, another T3SS regulator, HrpXct, is involved in the in planta multiplication of Xcc (Yamazaki et al., 2008b).
After sequencing the Xcc‐A306 genome, the São Paulo State Science Foundation (FAPESP) Genome Program sequenced the genomes of Xfa‐B (strain B69) and Xfa‐C (strain Xc70) (Moreira et al., 2010). The characterization of the sequenced genomes revealed genes for T3SS effectors, type 4 secretion system (T4SS) proteins, biofilm formation, quorum sensing (QS), sugar acquisition, flagellum construction and lipopolysaccharide (LPS) synthesis. The genomes of the Xfa‐B and Xfa‐C strains were compared with the Xcc‐A306 genome as a reference. In general terms, the Xfa‐B strain genome had more similarities with the Xcc‐A306 genome (87%) than with the Xfa‐C strain (84%). The Xfa‐B strain genome contained more T3SS effectors than the Xfa‐C strain, and some specific T3SS effectors were shared between Xcc‐A and Xfa‐B, but were not found in Xfa‐C. A few T3SS effectors were found only in Xfa‐C, showing a divergence in the pathogenic mechanisms developed for each strain. Compared with Xcc‐A306, both Xfa‐B and Xfa‐C shared 46% of the sequence of the Xcc plasmid pXAC33, the Xfa‐B strain shared 61% of the Xcc plasmid pXAC64 sequence, whereas the Xfa‐C strain shared only 55% of pXAC64.
A comparative genomic analysis of strains Xcc‐A306 and Xcc‐Aw12879, an A strain isolated in Florida and pathogenic on Key lime and alemow (Sun et al., 2004), indicated that the xopAG‐avrGf1 gene in Xcc‐Aw12879 contributed to the host range specificity in Xcc‐Aw (Jalan et al., 2013b). Other effectors present in Xcc‐Aw, such as XopAF (which is also found in Xfa‐B and Xfa‐C), contribute to the virulence of Xcc‐Aw (Jalan et al., 2013a). Although the complete genome of Xcc‐Aw12789 shows a close relationship with Xcc‐A306, numerous inversions and translocations between both genomes were found. Xcc‐Aw also possesses two plasmids: pXacw19, which does not have any similarity with Xcc‐A306, and pXacw58, which carries two transcription activator‐like effector (TALE) genes, denoted pthAw1 and pthAw2.
In 2010, several Xcc‐A, Xcc‐A* (produces canker lesions in Mexican lime but not grapefruit; Verniere et al., 1998), Xcc‐Aw and X. citri pv. bilvae (which causes CC‐like symptoms in Key lime) strains were characterized by amplified fragment length polymorphism (AFLP) and MLSA based on four partial housekeeping gene sequences (atpD, dnaK, efp and gyrB). Based on the high genetic relatedness amongst the strains, the authors suggested new nomenclature for one of the strains of X. citri pv. bilvae, which is now considered as a junior synonym of Xcc‐A* strains. The authors also demonstrated a close relationship between Xcc‐Aw and Xcc‐A* strains, which were proposed as a highly variable subgroup of Xcc‐A strains (Ngoc et al., 2010). Recently, a complete genome comparison (pan‐genome analysis) of 25 sequenced strains of Xcc‐A, Xcc‐Aw and Xcc‐A* has revealed 85% similarity in the genome between these three groups (Jalan et al., 2013a, 2013b). Most of the variation between groups was observed between Xcc‐Aw and Xcc‐A* versus typical Xcc‐A strains. The variation was associated principally with chromosome deletions, transposition events and plasmid insertions.
A molecular database generated by AFLP analysis of 55 previously characterized Xcc‐A strains was used to elucidate which factors determined the differential host pathogenicity in Xcc‐A* strains (Escalon et al., 2013; Ngoc et al., 2009). In this work, 66 T3SS effectors were identified, where 28 were common to Xcc‐A, Xcc‐A* and Xcc‐Aw. Two of the effectors, XopAG and XopC1, appeared to be limited to Xcc‐A* and Xcc‐Aw strains. Interestingly, the T3SS effector XopAG‐avrGf1 was found in all strains of Xcc‐Aw and Xcc‐A* which were only pathogenic in key lime, whereas XopC1 was found in only four of the Xcc‐A* strains that showed limited host range. The effector XopC1 does not elicit a hypersensitive response (HR), nor does it affect the pathogenicity of the strains after its deletion. Different combinations of T3SS effectors present in the strains were used to create phylogenetic trees to explain the limited host range of Xcc‐A* strains, but, except for the presence of the gene avrGf1, were not able to strictly correlate the presence of a specific combination of effectors with the reduced host range. The Xcc‐A* strains appear to be the most highly diverse group, with greater diversity than has been observed in Xcc‐A strains. It has been suggested that the presence of homologous T3SS effectors in X. citri pv. bilvae is the result of fluid horizontal genetic exchange between different Xanthomonas species (Escalon et al., 2013).
Several Xcc strains (14 Xcc‐A, three Xcc‐A* and four Xcc‐Aw) collected from North America and Asia were sequenced, and comparative genomic and evolutionary analyses were conducted (Zhang et al., 2015). The authors calculated the pan‐genome of these 21 Xcc strains using the OrthoMCL method. The core genome comprised 3912 orthologous clusters, whereas the pan‐genome contained 5147 orthologous clusters. Based on the genome analysis, Xcc‐A strains formed a separate clade from the Xcc‐Aw and Xcc‐A* strains, as revealed by the phylogenetic tree reconstructed on the basis of the core genome, the phyletic distribution of accessory orthologous clusters of the strains analysed, and the different origin of genomic islands of the two groups. This finding is consistent with a previous report based on multilocus variable number of tandem repeat analysis (Pruvost et al., 2015). However, these results differ from other results, which suggest that the strains from each pathotype form monophyletic clades, with a short branch shared by the Xcc‐Aw and Xcc‐A pathotypes (Gordon et al., 2015). Such differences in the investigations of Xcc phylogeny call for further studies to understand the evolution of Xcc strains (Gordon et al., 2015; Zhang et al., 2015). It is presumed that the acquisition of beneficial genes and the loss of detrimental genes has most likely allowed Xcc‐A to infect a broader range of hosts relative to Xcc‐A* and Xcc‐Aw. These studies imply that the Xcc population is clonal in structure. Many genes related to virulence, especially genes involved in the T3SS and effectors, are affected by positive selection, further highlighting the contribution of positive selection to the evolution of Xcc (Zhang et al., 2015).
A detailed study was performed on 157 Xcc strains from Brazil, which were compared for their T3SS effector profiles using a qualitative PCR‐Southern blot technique (Jaciani et al., 2012). Low genetic variability was observed for strains isolated in the northern part of the country, but more diversity was present in the strains isolated in the southern part, where the disease was more prevalent (Jaciani et al., 2012). In China, several Xcc strains from nine citrus‐growing regions were characterized and compared for the variability of TALEs (Ye et al., 2013). The result of the analysis of 105 strains with differential pathogenicity on a set of citrus hosts showed that the strains varied in the number of TALEs, ranging from three to five pthA genes (Ye et al., 2013). A comparison of the strains through hybridization with a probe based on the Xcc‐A3213 pthA gene allowed the separation of the strains into 14 genotypes, with more than 80% of the strains being placed in two major groups (Ye et al., 2013). The lack of hybridization observed in some strains was correlated with a lower virulence of these specific strains and could be correlated with variation in the sequences of the pthA genes (Lin et al., 2005, 2011; Ye et al., 2013). A slight modification in the number of repeats of the pthA4 gene produced changes in the pathogenicity of the bacterium, specifically in the induction of CC symptoms (Lin et al., 2013).
The host plant genes, whose products recognize pathogen effectors, are known as R genes, and the pathogen pathogenicity (pth) genes, which encode these recognizable effectors, are also referred to as avirulence (avr) genes. The products of R genes, either directly or indirectly, interact with the products of avr genes (Mysore and Ryu, 2004). The interaction between the products of R and avr genes is termed gene‐for‐gene resistance. No R genes corresponding to Xcc, and therefore imparting resistance to Xcc to the host plant, have been identified in citrus or citrus related species to date (Brunings and Gabriel, 2003; Khalaf et al., 2007).
Host–Pathogen Interaction and Infection Process
Xcc inoculum is deposited on host tissue via rain splash and is capable of swimming short distances with its flagellum. It then enters the host directly through wounds or through stomatal openings (Graham et al., 1992). Once within the apoplastic space, the bacterium adheres to a host cell wall surface with either an hrp or T4SS pilus (Brunings and Gabriel, 2003; He, 1998). Once populations of Xcc achieve sufficient density, bacteria shed their flagella, transitioning away from a planktonic lifestyle and aggregating into a biofilm composed of the polysaccharide xanthan and other components (Costerton et al., 1995; Graham et al., 2004). Within days of infecting a compatible host, Xcc elicits the characteristic hypertrophy and hyperplasia symptoms of CC (Schubert et al., 2001).
PthA is a member of the avrBs3/pthA gene family (Brunings and Gabriel, 2003). PthA is a homologous protein to AvrBs3, which possesses a nuclear localization signal (NLS) and is translocated into the host cell via the T3SS and targets the nucleus, where it activates the expression of the host genome's upa20 following binding to that gene's promoter (Kay et al., 2007). Upa20 is a regulator of cell size and avrBs3, like pthA, reprograms infected cells by mimicking host transcription factors by binding to the conserved promoter region of upa20 (Kay et al., 2007). The activation of upa20 by avrBs3 is believed to be one of the mechanisms by which Xcc induces hypertrophy in susceptible hosts (Kay et al., 2007). The hypertrophic and hyperplastic growth of host tissues is accomplished through the injection into the host cell of PthA (Brunings and Gabriel, 2003; Duan et al., 1999; Swarup et al., 1991; Yang and Gabriel, 1995). The expression of this single gene of Xcc, pthA, in citrus cells is sufficient to cause the division, enlargement and death of host cells (Duan et al., 1999). The specific pthA gene present in strain Xcc306, referred to as PthA4, facilitated by its NLS, is conveyed to the host cell nucleus by the host cell's own machinery, where it mimics the host's own transcription factors activating the susceptibility gene, LOB1 (lateral organ boundaries 1) (Hu et al., 2014; Yang and Gabriel, 1995). This specific TALE sequence (pthA4) is responsible for the elicitation of CC symptoms through the activation of specific genes in the host (Shantharaj et al., 2013). Mutations of pthA4 result in a loss of hypertrophy, but have shown variation in the effect on bacterial growth in host leaf tissue (Figueiredo et al., 2011a; Hu et al., 2014; Yan and Wang, 2012).
PthA4 up‐regulates the host gene CsLOB‐1, a member of the LOB transcription factor family, and CsSWEET1, a homologue of the SWEET sugar transporter and rice disease susceptibility gene family (Fig. 1) (Hu et al., 2013). PthA4 interacts in planta with the C‐terminal domain (CTD) of RNA polymerase II, inhibiting the activity of a CTD‐associated cyclophilin (Cyp), and with the protein CsMAF1, which represses tRNA synthesis and cell growth through its interaction with the RNA polymerase III (Domingues et al., 2012; Soprano et al., 2013). The importance of PthaA induction of LOB1 was further confirmed when genome editing of CsLOB1 rendered citrus resistant to CC (Jia et al., 2017).
Figure 1.
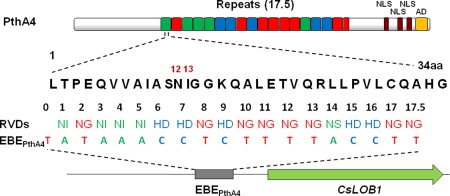
The transcription activator‐like effector (TALE), PthA4, contains 17.5 repeats in which the repeat‐variable diresidues (RVDs) bind to the effector‐binding element (EBE) upstream of the susceptibility gene, CsLob1, and turn on CsLob1. The arrangement of the RVDs dictates the target of TALE, which, in this case, is EBEPthA4. aa, amino acid; NLS, nuclear localization signal.
PthA2 and PthA4 target auxin and gibberellin synthesis in citrus, and recruit RNA polymerase II to initiate their targeted transcription (Pereira et al., 2014). Whether through their regulation of auxin or gibberellin, or some other mechanism, host plants also increase ethylene production in response to PthA2 and PthA4 (Pereira et al., 2014). Transiently expressed PthA2 individually regulates genes involved in DNA metabolism, replication and integration, whereas PthA4 individually regulates microtubule‐based processes and movement, and, together, they regulate sugar metabolism, lipid transport and cell wall organization (Pereira et al., 2014).
In addition to pthA, Xcc possesses three additional pthA (PthA4) alleles, two of which weakly elicit cankers and one that is non‐functional (Brunings and Gabriel, 2003; Swarup et al., 1992). The pthA homologue, hssB3.0, was found to be involved in the reduced virulence of Xcc in Citrus grandis (pomelo), and hssB3.0 was subsequently discovered in all weakly aggressive strains of Xcc (Shiotani et al., 2007). The pthA homologues, pthB, pthC and pthAW, are found in the Xaa‐B, Xaa‐C and Xcc‐Aw strains, respectively, and are required for those strains to induce canker symptoms (Brunings and Gabriel, 2003; Al‐Saadi et al., 2007). The gene pthA and its homologues, when introduced into non‐hosts, are suspected to induce HR in an R gene‐based gene‐for‐gene interaction, suggesting a role for pthA in host specificity (Swarup et al., 1992).
Experiments involving Xcc with mutations of pthA, however, differentiated pthA from other hrp genes in that, although Xcc mutants lost their ability to elicit an HR on one non‐host, bean (Phaseolus vulgaris L. cv ‘California Light Red’), the mutant bacterium retained its ability to elicit an HR on another non‐host, cotton (Gossypium hirsutum L.) (Swarup et al., 1992). PthB is found in Xaa‐B on a plasmid, pXcB, which also possesses ORFs that encode a T4SS, for conjugation and horizontal exchange of plasmid DNA (El Yacoubi et al., 2007).
As Xcc‐infected host cells divide and enlarge, they come into closer contact with one another, reducing the free apoplastic space which is filled by hygroscopic xanthan produced by Xcc in response to QS by a small‐molecule diffusible signal factor (DSF) (Brunings and Gabriel, 2003; Tang et al., 1991). This causes the characteristic water‐soaking symptoms around expanding lesions as capillary action brings in water which hydrates the xanthan (Popham et al., 1993). PthA is also suspected of inducing limited host cell death to assist in the rupture of the epidermis, to provide additional nutrition for bacterial growth, or both. In addition to the increased production of xanthan, QS and DSF are believed to be involved in the up‐regulation of additional avr genes after a certain population threshold, including those which increase the production of cellulases, proteases and pectinases (da Silva et al., 2002). Although no R genes have been identified in citrus, the avr gene, avrGf1, a member of the XopAG effector family in Xcc‐Aw, was shown to elicit an HR in grapefruit (Fig. 2B,C) (Rybak et al., 2009). When the hrpG gene from Xcc‐Aw was expressed in X. perforans, infection of grapefruit with the transconjugant resulted in an HR, whereas wild‐type X. perforans normally elicits no response, indicating a yet unidentified HR‐inducing factor (Rybak et al., 2009). Transconjugants possessing avrGf1, but lacking a functional T3SS, failed to elicit HR in grapefruit (Rybak et al., 2009). Likewise, avrGf1 encodes a chloroplast localization signal (CLS) required for targeting the host cell's chloroplasts, and CLS knockout mutants failed to elicit an HR, indicating that both a functional T3SS and an intact CLS are required for avrGf1 to act as an effector in host cells (Fig. 2A,D) (Figueiredo et al., 2011b; Rybak et al., 2009). The XopAG homologue, avrGf2, which also elicits an HR in grapefruit, has been isolated from Xcc‐C (Gochez et al., 2015). A Cyp‐binding site (GPLL) present in avrGf2 (and in every member of the XopAG effector family) was essential for the expression of an HR (Gochez et al., 2016). Mutagenesis of the GPLL site in avrGf2 resulted in a loss of the ability to elicit an HR (Gochez et al., 2016). Molecular modelling and in silico docking studies for the Cyp–avrGf2 interaction predicted the binding of citrus Cyp, which accelerates protein folding through a peptidyl–prolyl isomerase activity that catalyses a cis–trans isomerization of proline (Pro) peptide bonds (Gochez et al., 2016). The proposed model for the mode of action of avrGf2 in citrus suggests an important role for Cyp as a catalyst. AvrGf2 enters the plant cell, using the T3SS, as an inactive form; once inside the cell, it is recognized by Cyp and cis–trans modified (Gochez et al., 2016). This conformational change in avrGf2 thereby triggers the resistance response (Gochez et al., 2016). In addition, the CTD of XopAG family effectors contains a highly conserved motif, CLNAxYD, which was identified to be crucial for the induction of HR based on site‐directed mutagenesis (Gochez et al., 2016).
Figure 2.
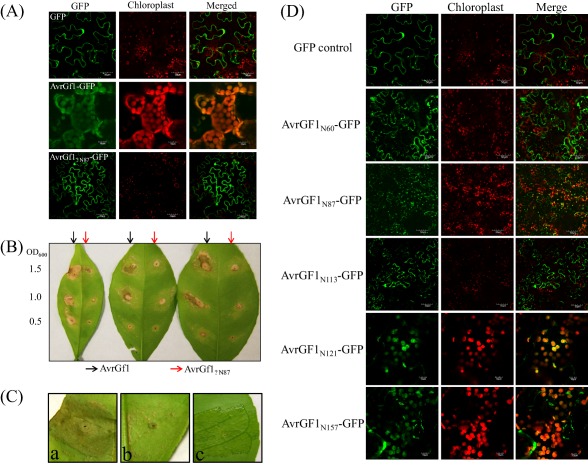
Characterization of the transit peptide located at the N‐terminal moiety of the AvrGF1 effector. (A) AvrGF1‐GFP, AvrGf1ΔN87‐GFP and green fluorescent protein (GFP) empty vector were transiently expressed in Nicotiana benthamiana leaves through Agrobacterium‐mediated infiltration. Images were taken at 3 days post‐inoculation (dpi) using confocal microscopy. (B) Agrobacterium strains harbouring AvrGF1 and AvrGf1ΔN87 were needlelessly infiltrated into sweet orange leaves with different optical densities (ODs) as presented. Hypersensitive response (HR) induction was observed and photographed at 3 dpi. (C) Xanthomonas citri strains harbouring AvrGF1, HpaA‐AvrGf1 and HpaA‐AvrGf1ΔN87 were inoculated into grapefruit leaves to test the HR. Images (a) and (b) were photographed at 2 dpi, and (c) was photographed at 5 dpi. (a) 306/p53‐AvrGf1; (b) 306/p53‐HpaA‐AvrGf1; (c) 306/p53‐HpaA‐AvrGf1ΔN87. (D) Different lengths of amino acid residues from AvrGF1 N‐terminus were fused with GFP. Chimeric proteins were transiently expressed in N. benthamiana leaves through Agrobacterium‐mediated infiltration. Images were photographed at 3 dpi using confocal microscopy.
Mutations in hrpG, hrpX or hrpA genes resulted in a complete loss of pathogenicity (Figueiredo et al., 2011a). These mutants continued to elicit HRs in non‐host tomato, indicating the presence of an hrp‐independent elicitor of HR (Figueiredo et al., 2011a). Other components of the Xcc Hrp cluster, hrpB, hrpD and hrpF, are essential for canker production in hosts and induce HR in non‐hosts (Dunger et al., 2005). Various protein–protein interactions have been discovered amongst the various sequenced genes of the Xcc hrp pathogenicity island, including between HrpG and XAC0095, between HpaA, HpaB and HrcV, between HrpB1, HrpD6 and HrpW, and between HrpB2 and HrcU (Alegria et al., 2004). The two‐component system of ColR/ColS in Xcc positively regulates the expression of hrpD6, hpaF, the LPS synthesis gene rfbC and katE, a catalase gene. ColS, a transmembrane sensor kinase, responds to an environmental stimulus and autophosphorylates; it then transfers the phosphoryl group to the cognate response regulator, ColR, which affects the regulatory effect (Yan and Wang, 2011).
The TonB‐dependent receptor (TBDR), which comprises a receptor protein family, assembles in the outer membrane of Gram‐negative bacteria, acts as an iron transporter and takes up iron‐siderophore complexes and vitamin B12 (Aini et al., 2010). The TBDR, XAC4131, does not appear to play an important role in pathogenicity; however, mutation of this gene in Xcc resulted in a delay in HR in tobacco (Aini et al., 2010). This TBDR, XAC4131, controls the expression of hrpG, an hrp regulatory gene (Aini et al., 2010). Transcription of XAC4131 is regulated by Fur protein (Aini et al., 2010).
Flagellin, a highly conserved component of Xcc flagella, is a common pathogen‐associated molecular pattern (PAMP) and an elicitor of the plant immune response (Gómez‐Gómez and Boller, 2002). Within 6 h of exposure to Xflg22, the specific PAMP protein of Xcc encoded by flg22, multiple defence‐related genes in kumquat are induced, whereas the same PAMP elicits no response in highly susceptible grapefruit (Shi et al., 2015). Xcc LPSs are also recognized as PAMPs, and activate basal defence in host and non‐host plants (Petrocelli et al., 2012). A citrus mitogen‐activated protein kinase (MAPK), CsMAPK1, appears to be another factor in the host response to infection, leading to reduced susceptibility to disease. CsMAPK1 is induced by X. axonopodis pv. aurantifolii pathotype C (Xaa‐C) in sweet orange, a non‐host, but not by Xcc (de Oliveira et al., 2013). Artificially increased expression of CsMAPK1 in sweet orange infected with Xcc caused reduced canker symptoms and decreased bacterial growth (de Oliveira et al., 2013).
Xcc infection results in an increase in the synthesis and mobilization of auxin and gibberellic acid (Cernadas and Benedetti, 2009). These increases lead to the up‐regulation of genes associated with the host production of cellulases, pectinesterases and expansins, and the down‐regulation of genes associated with the production of auxins, pectinacetyltransferases and xyloglucan galactosyltransferases (Cernadas and Benedetti, 2009). Correspondingly, auxin inhibition reduces canker severity, whereas gibberellic acid inhibition reduces auxin‐dependent transcription otherwise induced by Xcc infection (Cernadas and Benedetti, 2009). Xcc uses a plant natriuretic peptide, XacPNP, to modify host homeostasis and growth (Gottig et al., 2008). PNPs are a class of extracellular, systemically mobile molecules, which elicit multiple plant responses involved in homeostasis and growth, including stomatal opening, water uptake and tissue‐specific ion movement (Ludidi et al., 2004; Maryani et al., 2001; Pharmawati et al., 2001). XacPNP has no known homologue in other bacteria (Gottig et al., 2008). The production of XacPNP is induced by the nutrient‐poor apoplast, and this production is believed to be a mechanism by which Xcc modifies its host to create a more favourable environment (Gottig et al., 2008). Instead of restricting nutrition to the site of infection, the plant is induced via XacPNP to continue to send resources to the affected area, which are then used as food for the multiplying Xcc population. XacPNP deletion mutants elicit larger areas of necrotic tissue in their host, suggesting that, in the absence of XacPNP, the host plant is better able to properly respond to infection by restricting the flow of nutrients and water to the infected area (Gottig et al., 2008).
Epidemiology
Xcc is disseminated via wind‐driven rain, and the incidence and severity of CC are exacerbated by the larval feeding of the Asian citrus leafminer (CLM; Phyllocnistis citrella) (Belasque et al., 2005; Bock et al., 2005; Gottwald et al., 2007; Graham et al., 2004). Xcc is at the greatest concentration of inoculum immediately following wetting of viable lesions (Bock et al., 2005). Inoculum concentration in rain water is lower after about an hour of rain and wind; however, bacteria reproduce quickly enough between storms for large initial concentrations to be available for each wind‐driven rain event (Bock et al., 2005). Temperatures between 20 and 30 °C support adequate growth of Xcc and, as such, tropical and subtropical environments can support large populations year round (Bock et al., 2005). In addition to favourable temperatures, tropical and subtropical rain events tend to be frequent, short and produce strong gusty winds, a combination highly favourable for the spread of Xcc (Bock et al., 2005). Higher speed winds lead to greater plant wounding, which results in more extreme infections in terms of both severity and incidence (Bock et al., 2010a). Hurricanes and tornados are capable of widely dispersing inoculum (Bock et al., 2010a; Gottwald and Irey, 2007). Under optimal conditions of temperature, humidity and inoculum, symptoms can manifest around 7 days after infection (Schubert et al., 2001). When temperatures or inoculum levels are low, symptoms can take 2 months or more to manifest (Schubert et al., 2001). When temperatures exceed 40 °C, symptoms typically do not develop on sweet orange, but develop on Tahiti lime, given sufficient leaf wetness duration (Christiano et al., 2009; Dalla Pria et al., 2006). Prolonged leaf wetness increases the severity of CC in sweet orange and lesion development in Tahiti lime (Christiano et al., 2009; Dalla Pria et al., 2006).
CC produces local erumpent lesions on young expanding leaf, stem and green fruit tissue (Schubert et al., 2001). Lesions on leaves and fruit often have a water‐soaked margin, surrounded by a chlorotic halo, but stem lesions do not have chlorotic haloes (Schubert et al., 2001). With age, CC lesions grow in diameter and turn brown with a corky appearance (Schubert et al., 2001). Eventually, leaf lesions may fall out and heavy infestation can lead to defoliation (Schubert et al., 2001). Xcc infection is directly related to ethylene production and subsequent defoliation, which occurs more rapidly with higher inoculum concentration and with infections closer to the stipules (Dutta and Biggs, 1991; Goto et al., 1980). Young canker lesions on host leaves, stems and fruit have a higher initial inoculum concentration than older lesions (Bock et al., 2005).
Depending on temperature, host citrus type and initial inoculum concentration, within 1–3 weeks post‐infection the host epidermis ruptures, releasing bacteria to the surface, where they can spread to initiate new infection cycles (Brunings and Gabriel, 2003). Bacterial exudates from citrus lesions contain high concentrations of biofilm‐coated aggregates of Xcc inoculum, which serve to spread the bacteria to young exposed tissue on the same plant or on new plants (Timmer et al., 1991). Xcc infiltrates the host via wounds and natural stomatal and hydathode openings. Tissues of citrus plants susceptible to CC show an increased tolerance to the disease with maturity (Lee, 1921). The decrease in water congestion of fully expanded, mature leaves is a major factor in their resistance to bacterial infection (Verniere et al., 2003). The correlation between maturity and CC susceptibility seems to be similar for stems, fruits and leaves, with stomatal infection of these tissues being restricted to the period of expansion (Graham et al., 2004; Verniere et al., 2003). Although mature bark‐covered stems may resist infection, those stems which are infected whilst green can maintain populations of Xcc for years, producing inoculum from the raised corky lesions when wetted (Schubert et al., 2001). Xcc survives primarily and seasonally within the confines of the lesions. Outside of lesions, Xcc survives only 1–3 days on inanimate surfaces, such as clothing and agricultural equipment, and no more than 2 months in soil as a result of competition with saprophytes (Graham et al., 1989; Schubert et al., 2001). In tropical environments with mild winter temperatures, viable Xcc cells persist in the margins of older lesions on leaves, fruit and twigs (Pruvost et al., 2002).
Biofilm Formation
Among the different strains of Xcc, those with restricted host ranges produce more biofilm on leaves and fruits of their host than on non‐hosts, indicating the importance of biofilm production as a virulence factor (Sena‐Vélez et al., 2015). The regulation of pathogenicity factors (rpf) gene cluster is responsible for the synthesis of extracellular enzymes, such as proteases, endoglucanases and polygalacturonases, which inhabit the extracellular polymeric substance (EPS) (Dunger et al., 2007). The rpf gene cluster also regulates the production of xanthan, a secreted exopolysaccharide (Dunger et al., 2007). The Xcc gene, galU, is necessary for growth in grapefruit, and is involved in the biosynthesis of xanthan, capsular polysaccharide and biofilm formation (Guo et al., 2010). The rpfG gene specifically regulates genes involved in the synthesis of extracellular enzymes and EPS, and the formation of biofilms (Slater et al., 2000). Various genes primarily involved in activities such as amino acid synthesis, energy metabolism, DNA replication and transcription, membrane transport and signal transduction, also seem to be involved in biofilm formation (Malamud et al., 2013). Mutation of the T3SS resulted in changes in the expression of proteins involved in EPS production, impairment of leaf‐associated growth and elimination of the ability to form a biofilm (Zimaro et al., 2014).
The rpf gene cluster also encodes the cell–cell signalling system (Rigano et al., 2007). Xanthan production in X. campestris is regulated by a cell–cell signalling system encoded by rpf genes and DSF, and evidence suggests a similar mechanism (rpf/DSF) within Xcc (Crossman and Dow, 2004; Rigano et al., 2007; Siciliano et al., 2006). Structurally, biofilm development is initiated by bacterial attachment to a surface, followed by the formation of bacterial aggregates called microcolonies (Rigano et al., 2007). These microcolonies are tightly packed bacteria, sometimes in regular hexagonal formation, separated by channels of water (Rigano et al., 2007). Xanthan and the presence of extracellular DNA are important during the early stages of biofilm formation (Rigano et al., 2007; Sena‐Vélez et al., 2016). The xanthan in the biofilm provides protection from both desiccation and plant antimicrobial compounds (Dow et al., 2003). In terms of pathogenicity, xanthan can contribute to plant susceptibility by suppressing callose deposition (Yun et al., 2006). Xcc grown on nutrient‐rich medium shows retarded development of microcolonies, suggesting that cues for biofilm formation are dependent on the relatively nutrient‐poor environment of the intercellular spaces of leaf mesophyll tissue (Rigano et al., 2007). Proteome changes in mature Xcc colonies in a biofilm versus planktonic cells include an up‐regulation in the production of proteins involved in the outer membrane and receptor/transport proteins, indicating how the external biofilm structure is maintained and how molecules and signals are disseminated (Zimaro et al., 2013).
Within the rpf gene cluster is the gum cluster, which is composed of 12 ORFs and is 98% conserved across the plant‐pathogenic xanthomonads (Dunger et al., 2007). The Xcc glycotransferase gene, gumD, is involved in the synthesis of the first xanthan lipid intermediate and is an essential component of biofilm formation (Rigano et al., 2007). Deletion mutants of gumD in Xcc remove the ability to produce xanthan (Dunger et al., 2007). However, gumD is not necessary for virulence, as gumD mutants of Xcc still multiply at the same rate as the wild‐type, still elicit HR in non‐hosts and remain pathogenic (Dunger et al., 2007). However, impairment of biofilm formation in Xcc reduces its survival rate whilst under oxidative stress in the stationary growth phase (Xcc is naturally susceptible to oxidative stress during the exponential growth phase when no EPS is produced), as well as reducing its epiphytic growth and survival on leaves (Dunger et al., 2007; Rigano et al., 2007). Another gene in the gum cluster is gumB, which encodes a protein that polymerizes a pentasaccharide intermediate into mature xanthan (Vojnov et al., 1998). Experiments on gumB mutants revealed that, although Xcc remained pathogenic, it manifested reduced symptoms within its host (Rigano et al., 2007). Various mutants which show deficiency in biofilm formation, including gumB mutants, also show an impairment in motility, chemotaxis and virulence (Malamud et al., 2013).
Eventually during disease development it is necessary for the biofilm to be detached and disaggregated in order to facilitate the dispersal of Xcc. Nutrient deprivation, the transition to stationary phase growth or population density, or a combination of some or all of these factors, plus possibly as yet undiscovered factors, might drive detachment (Lamed and Bayer, 1986; O'Toole et al., 2000; Stoodley et al., 2002). The DSF endo‐β‐1,4‐mannanase promotes the transition from aggregate to planktonic lifestyle (Dow et al., 2003).
Management of CC
In regions in which CC has not yet become endemic, measures of control are focused on the isolation and eradication of the pathogen, minimization of dissemination, reduction of sources of inoculum and protection of susceptible tissues from infection (Behlau et al., 2016; Civerolo, 1981; Leite and Mohan, 1990). In locations in which CC has become endemic and eradication is no longer feasible, measures for the management of the disease are adopted to avoid or reduce crop loss. A CC integrated management programme involves the planting of CC‐free nursery stock, choice of less susceptible citrus cultivars, deployment of arboreal windbreaks, spraying with copper‐based bactericides, control of CLM and application of systemic acquired resistance (SAR) inducers (Behlau and Belasque, 2014; Behlau et al., 2008, 2010b; Gottwald and Timmer, 1995; Gottwald et al., 2002; Graham and Myers, 2009, 2013; Graham et al., 2010, 2011; Leite and Mohan, 1990; Stein et al., 2007).
In addition to avoiding long‐distance Xcc dissemination, the establishment of new citrus plantings with CC‐free trees is important to postpone and reduce the severity of outbreaks. After planting in the field, infected nursery trees serve as sources of inoculum, which is released from the pre‐existing lesions in the presence of water and transported to the surrounding trees by wind during rainstorms (Bock et al., 2010a; Danos et al., 1984; Gottwald et al., 1992). However, because immature plant tissue of citrus trees is more susceptible to CC, delaying the establishment of the disease in orchards within endemic areas may significantly reduce its impact (Gottwald and Graham, 1992). Newly planted trees are more susceptible because leaf flush production is more frequent and represents a higher proportion of the canopy volume relative to more mature trees (Behlau et al., 2010a; Leite and Mohan, 1990). Moreover, CLM feeds on young leaf tissues, and therefore contributes to increased Xcc severity in young groves (Christiano et al., 2007; Hall et al., 2010; Jesus et al., 2006).
Together with copper sprays, windbreaks are highly effective for the management of CC (Gottwald and Timmer, 1995; Leite and Mohan, 1990; Moschini et al., 2014). This control measure reduces the direct interaction of wind with trees, providing less favourable conditions for the spread and penetration of bacteria into the host. The arboreal barrier also lessens the damage caused by high‐speed winds, reducing entry wounds for the bacterium. In Argentina, measured levels of CC on trees protected by windbreaks were lower than those on trees treated with copper sprays (Gottwald and Timmer, 1995).
The spray application of copper‐based bactericides is a key measure for CC management. Copper is usually applied during the spring and summer months, when immature plant tissue is abundant and climatic conditions are most favourable to the pathogen. This measure is strictly preventative with no curative or systemic activity, as copper acts by reducing inoculum buildup on new leaf flush and protecting expanding fruit and leaf surfaces from infection (Behlau et al., 2008, 2010b; Graham et al., 2010, 2011, 2016a). Fixed or insoluble forms of copper, such as copper hydroxide, copper oxychloride and copper oxide, are the formulations most widely used. Because copper ions are slowly released, fixed copper is less phytotoxic to plants and provides better residual activity against Xcc than can be achieved with non‐fixed copper. After being sprayed, an insoluble copper film is formed on the treated surface, which prevents new infections by reducing the viability of Xcc accessing the susceptible plant tissue. Copper ions gradually released from the fixed forms confer chemical protection by killing the bacterium on arrival on the treated surface (Graham et al., 2010; Menkissoglu and Lindow, 1991). The rate of metallic copper used and the frequency of spray applications per season depend on the weather conditions and the desired period of protection (Behlau et al., 2010b, 2017b). About 0.54–1.12 kg of metallic copper per hectare every 21 days is recommended to protect spring flush growth or fruit surfaces (Behlau et al., 2017b; Dewdney et al., 2016; Scapin et al., 2015).
CC management on susceptible citrus cultivars is challenging when exclusively using copper bactericides, because wind‐blown rain introduces Xcc directly into stomata, bypassing the protective copper film on the plant surface (Behlau et al., 2010b; Bock et al., 2010b; Graham and Myers, 2013; Leite and Mohan, 1990; Stein et al., 2007). Therefore, when conditions are conducive to CC outbreaks, the combined use of copper sprays and windbreaks is mandatory to successfully control the disease. Even with windbreaks, frequent re‐applications of copper are required to protect fruits that are continuously expanding over a 90–120‐day timeframe, depending on the citrus cultivar (Behlau et al., 2010b; Stall et al., 1982; Stein et al., 2007). Although copper sprays greatly contribute to CC control, they should be used with caution. Sprayed copper accumulates in the soil, where it may negatively affect root growth and nutrient uptake by the citrus trees (Alva et al., 1995; Fan et al., 2011; Graham et al., 1986). In addition, high copper concentrations may lead to fruit blemishing as a result of phytotoxicity, and the frequent use of copper in citrus orchards favours the development of copper‐resistant strains in xanthomonad populations, which may impair future disease management with these bactericides (Albrigo et al., 1997; Behlau et al., 2011, 2013; Canteros et al., 2010; Graham et al., 2007).
CC severity and incidence increase in the presence of CLM, and lesions coincident with CLM‐induced leaf damage are larger, denser and produce more inoculum than stomatal infections (Gottwald et al., 2007; Graham et al., 2004). CLMs feed and rapidly reproduce on vigorous flushes of young citrus trees with the potential for explosive increases in Xcc inoculum (Gottwald, 2010; Stein et al., 2007). Mated females oviposit on expanding leaves, and emerging larvae tunnel under the leaf cuticle to feed. CLM leaf damage by tunnelling increases the host's vulnerability to CC by creating wounds which expose the inner mesophyll tissue to infection (Graham et al., 2004). CLM infests all types of citrus and other members of the Rutaceae family, but its preferred hosts on which to feed and oviposit are grapefruit, tangerine and pumelo (Stelinski et al., 2010). Control of the mining larvae is mainly performed by the application of abamectin and neonicotinoid insecticides (Powell et al., 2007; Stein et al., 2007). In previous studies, soil applications of neonicotinoids were highly effective for reducing foliar infection and CC‐induced defoliation on non‐bearing grapefruit trees (Graham and Myers, 2009, 2013). Systemic neonicotinoid insecticides, such as imidacloprid, thiamethoxam and clothianidin, can be applied year‐round as soil drenches to non‐bearing citrus trees for the control of CLM and the associated infection of leafminer galleries by Xcc (Rogers et al., 2015). Soil‐applied neonicotinoid insecticides are used in young orchards (up to 3 years), whereas abamectin may be sprayed on the foliage of trees of all ages. Foliar‐applied insecticides provide a shorter period of protection relative to soil applications and are performed during summer and autumn to protect developing leaves when the first CLM mined leaf galleries are observed, usually at the feather leaf stage. Neonicotinoids take longer to reach the canopy, and these insecticides should be applied about 2 weeks prior to leaf expansion in order to be available in the flushes when the CLM larvae begin to feed (Rogers, 2012; Rogers et al., 2015).
Biological control of CLM has limited efficiency. The best‐known and studied natural enemy of CLM is the wasp Ageniaspis citricola and, although this predator may show high parasitism rates, its deployment has not been demonstrated to be an equivalently effective alternative to the use of chemical sprays, especially when there are high infestations of CLM (Hoy and Jessey, 2004; Johnson and Henne, 2003). CLM is parasitized by numerous others organisms; however, these parasites are often eradicated by pesticides applied to combat CLM itself (Elekcioglu, 2013; Mafi and Ohbayashi, 2010; Pena et al., 2000).
There have been successful attempts to control CLM by disruption of mating with artificially synthesized pheromones which behave either as a competitive disruptor (an attractant) or as a non‐competitive disruptor, which includes substances that either desensitize the insect to its natural pheromone or confuse the insect by overloading the environment with the pheromone, a component of the pheromone or a synthetic mimic (Miller et al., 2006; Stelinski et al., 2008, 2010). Disruption of CLM flush infestation was significantly reduced by the application of only 1.5 g of the active ingredient (a 3 : 1 blend of (Z,Z,E)‐7,11,13‐hexadecatrienal–(Z,Z)‐7,11‐hexadecadienal) per hectare of citrus grove twice in a 221‐day investigation (Stelinski et al., 2008). The same blend mixed with the insecticide permethrin killed 100% of all CLM which came into contact with it (Stelinski and Czokajlo, 2010).
SAR is a natural plant defence that provides long‐lasting protection against a broad spectrum of microorganisms (An and Mou, 2011; Fu and Dong, 2013). SAR requires the signal molecule salicylic acid (SA) and is associated with the accumulation of pathogenesis‐related (PR) proteins, which are thought to contribute to resistance (Zhang YX et al., 2010). SAR may be activated in the absence of pathogens by treating plants with chemical inducers (Gorlach et al., 1996). Acibenzolar‐S‐methyl (ASM), a functional homologue of SA, is the most widely known commercially produced inducer of SAR (Tally et al., 1999). In glasshouse trials, soil drenches of ASM, as well as neonicotinoids, induced a high and persistent up‐regulation of PR gene expression that was correlated with a reduction in CC lesions for up to 24 weeks after treatment (Francis et al., 2009). Furthermore, SAR inducers demonstrated the potential to augment CC control with copper sprays (Graham and Myers, 2013). ASM provides a non‐insecticidal option for sustaining SAR activity in trees greater than 3 m in height with low risk of movement through the root zone into the soil profile. Thus, soil‐applied ASM provides an option for delivering the SAR compound to fruiting trees and has been shown to increase the efficacy of copper spray programmes when used in tandem (Graham and Myers, 2013). Integration of ASM may also enable a reduction in the rate and frequency of copper sprays and potentially mitigate the risk of copper resistance development. Integration of ASM with copper has been demonstrated to protect crops in which copper‐resistant pathogen strains predominate, as was reported for the management of bacterial speck and bacterial spot of tomato (Louws et al., 2001). Other chemicals that have been successfully tested but, for different reasons, are not widely used for CC control are streptomycin and zinc oxide (Behlau et al., 2012b; Graham et al., 2010, 2007, 2016b).
Although copper‐based bactericides have proven to be effective for the control of CC, these chemicals are unable to completely suppress the inoculum, and frequent applications throughout the spring and summer are required to minimize losses (Behlau et al., 2008, 2010b, 2017b; Graham et al., 2010, 2011, 2016a; Scapin et al., 2015). The pressure imposed by the continuous application of copper drives the selection of resistant strains and favours a gradual increase in the frequency of resistant pathogens within the bacterial population, which jeopardizes the continued effectiveness of the sprays (Sundin et al., 1989). Xcc strains resistant to copper (CuR) were detected in Argentina in the mid‐1990s (Canteros et al., 2010). Genetic characterization of copper resistance determinants in these strains revealed that the resistance is mainly caused by the plasmid‐borne gene cluster copLAB, which is also present in strains of CuR Xanthomonas alfalfae ssp. citrumelonis (Xac) (Behlau et al., 2011).
Horizontal transfer of copper resistance determinants by conjugation is the primary mechanism for the acquisition of copper resistance by bacteria, including Xcc and Xac (Behlau et al., 2012a). Copper resistance in bacteria is conferred by several genes normally organized in operons; therefore, a natural spontaneous mutation conferring copper resistance is unlikely to occur within bacterial populations (Mellano and Cooksey, 1988). Previous findings have indicated that copper sequestration and accumulation are the primary copper resistance mechanisms in Xanthomonas, and copL regulates the expression of copA and copB, which encode for copper‐binding proteins (Cooksey, 1990; Voloudakis et al., 2005).
Comparison of the partial nucleotide sequences of copLAB from CuR Xcc with other species of CuR Xanthomonas revealed that the copLAB cluster is present in many species of Xanthomonas from different parts of the world, and multiple independent introductions of resistance genes have occurred in resistant populations of Xcc in Argentina and Xac in Florida, USA (Behlau et al., 2013). The alignment of partial sequences of resistance genes among strains of CuR Xanthomonas revealed homology of ≥92%, ≥96% and ≥91% for copL, copA and copB, respectively. The presence of copLAB homologues was also observed in epiphytic bacteria, such as non‐pathogenic xanthomonads and a Stenotrophomonas maltophilia strain isolated from citrus (Behlau et al., 2012a). cop genes have been mistakenly annotated and studied as putative copper resistance genes for several xanthomonads (Hsiao et al., 2011; da Silva et al., 2002; Teixeira et al., 2008). This misattribution is mainly because cohLAB genes, homologues of cop genes, are present on the chromosomes of both copper‐sensitive (CuS) and CuR xanthomonads (Behlau et al., 2011, 2013). Both gene groups have been shown to be responsive to copper; however, coh genes are not responsible for copper resistance, but are probably necessary for homeostasis or copper metabolism (Behlau et al., 2011; Hsiao et al., 2011; Teixeira et al., 2008). Although CuR Xanthomonas strains grow on MGY agar amended with copper sulfate at 400 mg/L, CuS strains can continue to grow only up to copper concentrations of 75–150 mg/L (Behlau et al., 2012a, 2013, 2017a). More recently, copper tolerant (CuT) strains of Xanthomonas have been identified. These strains can grow at intermediate concentrations of copper sulfate and are differentiated from the CuR strains by their lack of the copper resistance cluster copLAB, and from the CuS strains by a greater expression of cohLAB in the presence of copper (F. Behlau, 2016).
In addition to breeding commercial citrus varieties with less CC‐susceptible citrus types and relatives, other attempts have been made to defend against Xcc with varying degrees of success. Copper biocides are a standard method of control; however, the ability of Xcc to acquire plasmid‐borne genes conferring resistance to copper sprays greatly limits their long‐term use. d‐Leucine and 3‐indolylacetonitrile (IAN) were found to inhibit Xcc from producing a biofilm and increased the bacterium's susceptibility to copper (CuSO4) treatments; furthermore, IAN repressed the expression of genes involved in chemotaxis and motility (Li and Wang, 2014). Phage treatment of Xcc has some degree of efficacy for moderately sensitive Valencia oranges (about 41% disease reduction), but has proven to be ineffective on grapefruit, a more susceptible host (Balogh et al., 2008).
Transient expression of Bs2 (an R gene from pepper, which provides resistance to X. campestris pv. vesicatoria) in citrus has been reported to lead to decreased susceptibility to CC (Sendin et al., 2012). Likewise, transgenic citrus with the rice Xa21 R gene showed decreased susceptibility to CC (Mendes et al., 2010). In addition, transgenic grapefruit and sweet orange expressing the Arabidopsis NPR1 gene (AtNPR1), a positive regulator of SAR, were less susceptible to CC (Zhang XD et al., 2010). The application of ASM for the induction of SAR has also shown effectiveness in reducing the incidence and severity of CC (Graham and Myers, 2013). Control of Xcc via foliar applications of chemicals which trigger the host plant's induced systemic resistance do not seem particularly effective on their own (Graham and Leite, 2004). SA foliar treatments increase endogenous SA and reduce lesion incidence and size (Wang and Liu, 2012).
Clustered Regularly Interspace Short Palindromic Repeats technology using the Cas9 enzyme and guide RNA has shown promise in generating canker‐resistant citrus varieties by modifying the PthA4 effector‐binding elements (EBEs) in the promoter or coding region of the CsLOB1 gene (Jia et al., 2017, 2016). Genome editing of the disease susceptibility gene CsLOB1 in Duncan grapefruit confers resistance to CC, which might provide a long‐term solution to control CC if non‐transgenic EBEPthA4‐ or CsLOB1‐modified plants are produced successfully without negative effects on other horticultural traits (Jia et al., 2017).
References
- Aini, L.Q. , Hirata, H. and Tsuyumu, S. (2010) A TonB‐dependent transducer is responsible for regulation of pathogenicity‐related genes in Xanthomonas axonopodis pv. citri . J. Gen. Plant Pathol. 76, 132–142. [Google Scholar]
- Albrigo, L.G. , Timmer, L.W. , Townsend, K. and Beck, H.W. (1997) Copper fungicides‐residues for disease control and potential for spray burn. Proc. Florida State Hortic. Soc. 110, 67–70. [Google Scholar]
- Alegria, M.C. , Docena, C. , Khater, L. , Ramos, C.H.I. , da Silva, A.C.R. and Farah, C.S. (2004) New protein–protein interactions identified for the regulatory and structural components and substrates of the type III secretion system of the phytopathogen Xanthomonas axonopodis pathovar citri . J. Bacteriol. 186, 6186–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Saadi, A. , Reddy, J.D. , Duan, Y.P. , Brunings, A.M. , Yuan, Q.P. and Gabriel, D.W. (2007) All five host‐range variants of Xanthomonas citri carry one pthA homolog with 17.5 repeats on citrus, but none determine host‐range pathogenicity variation. Mol. Plant–Microbe Interact. 20, 934–943. [DOI] [PubMed] [Google Scholar]
- Al‐Saleh, M.A. , Widyawan, A. , Saleh, A.A. and Ibrahim, Y.E. (2014) Distribution and pathotype identification of Xanthomonas citri subsp. citri recovered from southwestern region of Saudi Arabia. Afr. J. Microbiol. Res. 8, 673–679. [Google Scholar]
- Alva, A.K. , Graham, J.H. and Anderson, C.A. (1995) Soil‐pH and copper effects on young Hamlin orange trees. Soil. Sci. Soc. Am. J. 59, 481–487. [Google Scholar]
- Alvarez, A.M. , Benedict, A.A. , Mizumoto, C.Y. , Pollard, L.W. and Civerolo, E.L. (1991) Analysis of Xanthomonas campestris pv citri and X. c. citrumelo with monoclonal‐antibodies. Phytopathology, 81, 857–865. [Google Scholar]
- An, C.F. and Mou, Z.L. (2011) Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 53, 412–428. [DOI] [PubMed] [Google Scholar]
- Balogh, B. , Canteros, B.I. , Stall, K.E. and Jones, J.B. (2008) Control of citrus canker and citrus bacterial spot with bacteriophages. Plant Dis. 92, 1048–1052. [DOI] [PubMed] [Google Scholar]
- Behlau, F. and Belasque, J. (2014) Cancro Citrico – A Doenca e Seu Controle. Araraquara, Brazil: Fundecitrus. [Google Scholar]
- Behlau, F. , Belasque, J. , Bergamin, A. , Graham, J.H. , Leite, R.P. and Gottwald, T.R. (2008) Copper sprays and windbreaks for control of citrus canker on young orange trees in southern Brazil. Crop Prot. 27, 807–813. [Google Scholar]
- Behlau, F. , Amorim, L. , Belasque, J. , Bergamin, A. , Leite, R.P. and Graham, J.H. and Gottwald, T.R. (2010a) Annual and polyetic progression of citrus canker on trees protected with copper sprays. Plant Pathol. 59, 1031–1036. [Google Scholar]
- Behlau, F. , Belasque, J. , Graham, J.H. and Leite, R.P. (2010b) Effect of frequency of copper applications on control of citrus canker and the yield of young bearing sweet orange trees. Crop Prot. 29, 300–305. [Google Scholar]
- Behlau, F. , Canteros, B.I. , Minsavage, G.V. , Jones, J.B. and Graham, J.H. (2011) Molecular characterization of copper resistance genes from Xanthomonas citri subsp citri and Xanthomonas alfalfae subsp citrumelonis . Appl. Environ. Microb. 77, 4089–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behlau, F. , Canteros, B.I. , Jones, J.B. and Graham, J.H. (2012a) Copper resistance genes from different xanthomonads and citrus epiphytic bacteria confer resistance to Xanthomonas citri subsp citri . Eur. J. Plant Pathol. 133, 949–963. [Google Scholar]
- Behlau, F. , Jones, J.B. , Myers, M.E. and Graham, J.H. (2012b) Monitoring for resistant populations of Xanthomonas citri subsp citri and epiphytic bacteria on citrus trees treated with copper or streptomycin using a new semi‐selective medium. Eur. J. Plant Pathol. 132, 259–270. [Google Scholar]
- Behlau, F. , Hong, J.C. , Jones, J.B. and Graham, J.H. (2013) Evidence for acquisition of copper reistance genes from different sources in citrus‐associated xanthomonads. Phytopathology, 103, 409–418. [DOI] [PubMed] [Google Scholar]
- Behlau, F. , Fonesca, A.E. and Belasque, J. Jr . (2016) A comprehensive analysis of the citrus eradication program in Sao Paulo State from 1999 to 2009. Plant Pathol. 65, 1390–1399. [Google Scholar]
- Behlau, F. , Gochez, A. , Lugo, A. , Elibox, W. , Minsavage, G. , Potnis, N. , White, F. , Ebrahim, M. , Jones, J. and Ramsubhag, A. (2017a) Characterization of a unique copper resistance gene cluster in Xanthomonas campestris pv. campestris isolated in Trinidad, West Indies. Eur. J. Plant Pathol. 147, 671–681. [Google Scholar]
- Behlau, F. , Scandelai, L. , Junior, G. and Lanza, F. (2017b) Soluble and insoluble copper formulations and metallic copper rate for control of citrus canker on sweet orange trees. Crop Prot. 94, 185–191. [Google Scholar]
- Belasque, J. , Parra‐Pedrazzoli, A.L. , Rodrigues Neto, J. , Yamamoto, P.T. , Chagas, M.C.M. , Parra, J.R.P. , Vinyard, B.T. and Hartung, J.S. (2005) Adult citrus leadminers (Phyllocnistis citrella) are not efficient vectors for Xanthomonas axonopodis pv. citri . Plant Dis. 89, 590–594. [DOI] [PubMed] [Google Scholar]
- Bock, C.H. , Parker, P.E. and Gottwald, T.R. (2005) Effect of simulated wind‐driven rain on duration and distance of dispersal of Xanthomonas axonopodis pv. citri from canker‐infected citrus trees. Plant Dis. 89, 71–80. [DOI] [PubMed] [Google Scholar]
- Bock, C.H. , Graham, J.H. , Gottwald, T.R. , Cook, A.Z. and Parker, P.E. (2010a) Wind speed and wind‐associated leaf injury affect severity of citrus canker on Swingle citrumelo. Eur. J. Plant Pathol. 128, 21–38. [Google Scholar]
- Bock, C.H. , Graham, J.H. , Gottwald, T.R. , Cook, A.Z. and Parker, P.E. (2010b) Wind speed effects on the quantity of Xanthomonas citri subsp. citri dispersed downwind from canopies of grapefruit trees infected with citrus canker. Plant Dis. 94, 725–736. [DOI] [PubMed] [Google Scholar]
- Bouzar, H. , Jones, J.B. , Stall, R.E. , Hodge, N.C. , Minsavage, G.V. , Benedict, A.A and Alvarez, A.M. (1994) Physiological, chemical, serological, and pathogenic analyses of a worldwide collection of Xanthomonas campestris pv vesicatoria strains. Phytopathology, 84, 663–671. [Google Scholar]
- Brunings, A.M. and Gabriel, D.W. (2003) Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 4, 141–157. [DOI] [PubMed] [Google Scholar]
- Canteros, B.I. , Gochez, A.M. , Rybak, M.A. , Minsavage, G.V. , Jones, J.B. and Stall, R.E. (2010) Management and characterization of plasmid‐encoded copper resistance in Xanthomonas axonopodis pv. citri p. 145. International Conference on Plant Pathogenic Bacteria. 12. ICPPB. 2010 06 07–11, June 7–11, 2010. Saint‐Denis, France. FR.
- Cernadas, R.A. and Benedetti, C.E. (2009) Role of auxin and gibberellin in citrus canker development and in the transcriptional control of cell‐wall remodeling genes modulated by Xanthomonas axonopodis pv. citri . Plant Sci. 177, 190–195. [Google Scholar]
- Christiano, R.S.C. , Dalla Pria, M. , Jesus, W.C. , Parra, J.R.P. , Amorim, L. and Bergamin, A. (2007) Effect of citrus leaf‐miner damage, mechanical damage and inoculum concentration on severity of symptoms of Asiatic citrus canker in Tahiti lime. Crop Prot. 26, 59–65. [Google Scholar]
- Christiano, R.S.C. , Dalla Pria, M. , Jesus, W.C. , Amorim, L. and Bergamin, A. (2009) Modelling the progress of Asiatic citrus canker on Tahiti lime in relation to temperature and leaf wetness. Eur. J. Plant Pathol. 124, 1–7. [Google Scholar]
- Civerolo, E.L. (1981) Citrus bacterial canker disease: An overview. Proc. Intn. Soc. Citric. 1, 390–394. [Google Scholar]
- Civerolo, E.L. and Fan, F. (1982) Xanthomonas campestris pv citri detection and identification by enzyme‐linked immunosorbent‐assay. Plant Dis. 66, 231–236. [Google Scholar]
- Coletta‐Filho, H.D. , Takita, M.A. , Souza, A.A. , Neto, J.R. , Destefano, S.A.L. , Hartung, J.S. and Machado, M.A. (2006) Primers based on the rpf gene region provide improved detection of Xanthomonas axonopodis pv. citri in naturally and artificially infected citrus plants. J. Appl. Microbiol. 100, 279–285. [DOI] [PubMed] [Google Scholar]
- Constantin, E.C. , Cleenwerck, I. , Maes, M. , Baeyen, S. , Van Malderghem, C. , De Vos, P. and Cottyn, B. (2016) Genetic characterization of strains named as Xanthomonas axonopodis pv. dieffenbachiae leads to a taxonomic revision of the X. axonopodis species complex. Plant Pathol. 65, 792–806. [Google Scholar]
- Cooksey, D.A. (1990) Genetics of bactericide resistance in plant pathogenic bacteria. Annu. Rev. Phytopathol. 28, 201–219. [Google Scholar]
- Costerton, J.W. , Lewandowski, Z. , Caldwell, D.E. , Korber, D.R. and Lappin‐Scott, H.M. (1995) Microbial biofilms. Annu. Rev. Microbiol. 49, 711–745. [DOI] [PubMed] [Google Scholar]
- Crossman, L. and Dow, J.M. (2004) Biofilm formation and dispersal in Xanthomonas campestris . Microbes Infect. 6, 623–629. [DOI] [PubMed] [Google Scholar]
- Cubero, J. and Graham, J.H. (2002) Genetic relationship among worldwide strains of Xanthomonas causing canker in citrus species and design of new primers for their identification by PCR. Appl. Environ. Microbiol. 68, 1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubero, J. and Graham, J.H. (2004) The leucine‐responsive regulatory protein (lrp) gene for characterization of the relationship among Xanthomonas species. Int. J. Syst. Evol. Microbiol. 54, 429–437. [DOI] [PubMed] [Google Scholar]
- Cubero, J. and Graham, J.H. (2005) Quantitative real‐time polymerase chain reaction for bacterial enumeration and allelic discrimination to differentiate Xanthomonas strains on citrus. Phytopathology, 95, 1333–1340. [DOI] [PubMed] [Google Scholar]
- Dalla Pria, M. , Christiano, R.C.S. , Furtado, E.L. , Amorim, L. and Bergamin, A. (2006) Effect of temperature and leaf wetness duration on infection of sweet oranges by Asiatic citrus canker. Plant Pathol. 55, 657–663. [Google Scholar]
- Danos, E. , Berger, R.D. and Stall, R.E. (1984) Temporal and spatial spread of citrus canker within groves. Phytopathology, 74, 904–908. [Google Scholar]
- Dewdney, M. , Graham, J.H. and Rogers, M.E. (2016) Citrus Canker. Florida Citrus Pest Management Guide, SP‐43, 93–96. Gainesville, FL: University of Florida. [Google Scholar]
- Doidge, E.M. (1916) The Origin and Cause of Citrus Canker in South Africa. Union So. Africa Dept. Agr. Sei. Bul. 8, 20. [Google Scholar]
- Domingues, M.N. , de Campos, B.M. , de Oliveira, M.L.P. , de Mello, U.Q. and Benedetti, C.E. (2012) TAL effectors target the c‐terminal domain of RNA polymerase II (CTD) by inhibiting the prolyl‐isomerase activity of a CTD‐associated cyclophilin. PLoS One, 7, e41553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow, J.M. , Crossman, L. , Findlay, K. , He, Y.Q. , Feng, J.X. and Tang, J.L. (2003) Biofilm dispersal in Xanthomonas campestris is controlled by cell–cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA, 100, 10 995–11 000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson, W.J. (1939) On the systematic position and generic names of the gram negative bacterial plant pathogens. Zentr. Bakteriol. Parasitenk. Abt. II. 100, 177–193. [Google Scholar]
- Duan, Y.P. , Castaneda, A. , Zhao, G. , Erdos, G. and Gabriel, D.W. (1999) Expression of a single, host‐specific, bacterial pathogenicity gene in plant cells elicits division, enlargement, and cell death. Mol. Plant–Microbe Interact. 12, 556–560. [Google Scholar]
- Dunger, G. , Arabolaza, A.L. , Gottig, N. , Orellano, E.G. and Ottado, J. (2005) Participation of Xanthomonas axonopodis pv. citri hrp cluster in citrus canker and nonhost plant responses. Plant Pathol. 54, 781–788. [Google Scholar]
- Dunger, G. , Relling, V.M. , Tondo, M.L. , Barreras, M. , Ielpi, L. , Orellano, E.G. and Ottado, J. (2007) Xanthan is not essential for pathogenicity in citrus canker but contributes to Xanthomonas epiphytic survival. Arch. Microbiol. 188, 127–135. [DOI] [PubMed] [Google Scholar]
- Dutta, S. and Biggs, R.H. (1991) Regulation of ethylene biosynthesis in citrus leaves infected with Xanthomonas campestris pv citri . Physiol. Plant. 82, 225–230. [Google Scholar]
- Egel, D.S. , Graham, J.H. and Stall, R.E. (1991) Genomic relatedness of Xanthomonas campestris strains causing diseases of citrus. Appl. Environ. Microbiol. 57, 2724–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elekcioglu, N.Z. (2013) Host–parasitoid relations between citrus leafminer, Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) and its parasitoid Citrostichus phyllocnistoides Narayanan (Hymenoptera: Eulophidae). Turk. Entomol. Derg. Tu. 37, 503–512. [Google Scholar]
- El Yacoubi, B. , Brunings, A.M. , Yuan, Q. , Shankar, S. and Gabriel, D.W. (2007) In planta horizontal transfer of a major pathogenicity effector gene. Appl. Environ. Microbiol. 73, 1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalon, A. , Javegny, S. , Vernière, C. , Noël, L.D. , Vital, K. and Poussier, S. (2013) Variations in type III effector repertoires, pathological phenotypes and host range of Xanthomonas citri pv. citri pathotypes. Mol. Plant Pathol. 14, 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euzeby, J. (2007) List of new names and new combinations previously effectively, but no validly, published, list. Int. J. Syst. Evol. Microbiol. 57, 893–897. [DOI] [PubMed] [Google Scholar]
- Fan, J.H. , He, Z.L. , Ma, L.N.Q. and Stoffella, P.J. (2011) Accumulation and availability of copper in citrus grove soils as affected by fungicide application. J. Soil Sediment. 11, 639–648. [Google Scholar]
- Figueiredo, J.F.L. , Minsavage, G.V. , Graham, J.H. , White, F.F. and Jones, J.B. (2011a) Mutational analysis of type III effector genes from Xanthomonas citri subsp citri . Eur. J. Plant Pathol. 130, 339–347. [Google Scholar]
- Figueiredo, J.F.L. , Romer, P. , Lahaye, T. , Graham, J.H. , White, F.F. and Jones, J.B. (2011b) Agrobacterium‐mediated transient expression in citrus leaves: a rapid tool for gene expression and functional gene assay. Plant Cell Rep. 30, 1339–1345. [DOI] [PubMed] [Google Scholar]
- Francis, M.I. , Redondo, A. , Burns, J.K. and Graham, J.H. (2009) Soil application of imidacloprid and related SAR‐inducing compounds produces effective and persistent control of citrus canker. Eur. J. Plant Pathol. 124, 283–292. [Google Scholar]
- Fu, Z.Q. and Dong, X.N. (2013) Systemic acquired resistance: turning local infection into global defense. Annu. Rev. Plant Biol. 64, 839–863. [DOI] [PubMed] [Google Scholar]
- Gabriel, D.W. , Kingsley, M.T. , Hunter, J.E. and Gottwald, T. (1989) Reinstatement of Xanthomonas citri (ex Hasse) and Xanthomonas phaseoli (ex Smith) to species and reclassification of all Xanthomonas campestris pv citri strains. Int. J. Syst. Bacteriol. 39, 14–22. [Google Scholar]
- Gochez, A. , Minsavage, G.V. , Potnis, N. , Canteros, B.I. , Stall, R.E. and Jones, J. (2015) A functional xopAG homologue in Xanthomonas fuscans pv. aurantifolii strain C limits host range. Plant Pathol. 64, 1–8. [Google Scholar]
- Gochez, A.M. , Shantharaj, D. , Potnis, N. , Zhou, X. , Minsavage, G.V. , White, F.F , Wang, N. , Hurlbert, J.C. and Jones, J.B. (2016) Molecular characterization of xopAG effector‐avrGf2 from Xanthomonas fuscans subsp. aurantifolii in grapefruit. Mol. Plant Pathol. 18, 405–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golmohammadi, M. , Cubero, J. , Penalver, J. , Quesada, J.M. , Lopez, M.M. and Llop, P. (2007) Diagnosis of Xanthomonas axonopodis pv. citri, causal agent of citrus canker, in commercial fruits by isolation and PCR‐based methods. J. Appl. Microbiol. 103, 2309–2315. [DOI] [PubMed] [Google Scholar]
- Gómez‐Gómez, L. and Boller, T. (2002) Flagellin perception: a paradigm for innate immunity. Trends Plant Sci. 7, 251–256. [DOI] [PubMed] [Google Scholar]
- Gordon, J.L. , Lefeuvre, P. , Escalon, A. , Barbe, V. , Cruveiller, S. , Gagnevin, L. and Pruvost, O. (2015) Comparative genomics of 43 strains of Xanthomonas citri pv. citri reveals the evolutionary events giving rise to pathotypes with different host ranges. BMC Genomics, 16, 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach, J. , Volrath, S. , Knauf‐Beiter, G. , Hengy, G. , Beckhove, U. , Kogel, K.‐H. , Oostendorp, M. , Staub, T. , Ward, E. , Kessmann, H. and Ryals, J. (1996) Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell, 8, 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, M. , Yaguchi, Y. and Hyodo, H. (1980) Ethylene production in citrus leaves infected with Xanthomonas citri and its relation to defoliation. Physiol. Plant Pathol. 16, 343–350. [Google Scholar]
- Gottig, N. , Garavaglia, B.S. , Daurelio, L.D. , Valentine, A. , Gehring, C. , Orellano, E.G. and Ottado, J. (2008) Xanthomonas axonopodis pv. citri uses a plant natriuretic peptide‐like protein to modify host homeostasis. Proc. Natl. Acad. Sci. USA, 105, 18 631–18 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald, T.R. (2010) Current epidemiological understanding of citrus huanglongbing. Annu. Rev. Phytopathol. 48, 119–139. [DOI] [PubMed] [Google Scholar]
- Gottwald, T.R. and Graham, J.H. (1992) A device for precise and nondisruptive stomatal inoculation of leaf tissue with bacterial pathogens. Phytopathology, 82, 930–935. [Google Scholar]
- Gottwald, T.R and Irey, M. (2007) Post‐hurricane analysis of citrus canker II: predictive model estimation of disease spread and area potentially impacted by various eradication protocols following catastrophic weather events. Plant Health Progr. 1, http://www.apsnet.org/publications/apsnetfeatures/Pages/CitrusCankerII.aspx. doi: 10.1094/PHP-2007-0405-1001-RS. [DOI] [Google Scholar]
- Gottwald, T.R. and Timmer, L.W. (1995) The efficacy of windbreaks in reducing the spread of citrus canker caused by Xanthomonas campestris pv citri . Trop. Agric. 72, 194–201. [Google Scholar]
- Gottwald, T.R. , Alvarez, A.M. , Hartung, J.S. and Benedict, A.A. (1991). Diversity of Xanthomonas campestris pv citrumelo strains associated with epidemics of citrus bacterial spot in Florida citrus nurseries – correlation of detached leaf, monoclonal‐antibody, and restriction‐fragment‐length‐polymorphism assays. Phytopathology, 81, 749–753. [Google Scholar]
- Gottwald, T.R. , Reynolds, K.M. , Campbell, C.L. and Timmer, L.W. (1992) Spatial and spatiotemporal autocorrelation analysis of citrus canker epidemics in citrus nurseries and groves in Argentina. Phytopathology, 82, 843–851. [Google Scholar]
- Gottwald, T.R. , Graham, J.H. and Schubert, T.S. (2002) Citrus canker. The pathogen and its impact. Plant Health Progr. doi: 10.1094/PHP-2002-0812-01-R. [DOI] [Google Scholar]
- Gottwald, T.R. , Bassanezi, R.B. , Amorim, L. and Bergamin‐Filho, A. (2007) Spatial pattern analysis of citrus canker‐infected plantings in Sao Paulo, Brazil, and augmentation of infection elicited by the Asian leafminer. Phytopathology, 97, 674–683. [DOI] [PubMed] [Google Scholar]
- Graham, J. and Myers, M. (2009) Soil drenches of imidacloprid, thiamethoxam and acibenzolar‐S‐methyl for induction of SAR to control citrus canker in young citrus trees. Phytopathology, 99, S46–S46. [DOI] [PubMed] [Google Scholar]
- Graham, J. , Dewdney, M. and Myers, M. (2010) Streptomycin and copper formulations for control of citrus canker on grapefruit. Proc. Florida State Hortic. Soc. 123, 92–99. [Google Scholar]
- Graham, J. , Dewdney, M. and Yonce, H. (2011) Comparison of copper formulations for control of citrus canker on ‘Hamlin’ orange. Proc. Florida State Hortic. Soc. 124, 79–84. [Google Scholar]
- Graham, J.H. and Leite, R.P. (2004) Lack of control of citrus canker by induced systemic resistance compounds. Plant Dis. 88, 745–750. [DOI] [PubMed] [Google Scholar]
- Graham, J.H. and Myers, M.E. (2013) Integration of soil applied neonicotinoid insecticides and acibenzolar‐S‐methyl for systemic acquired resistance (SAR) control of citrus canker on young citrus trees. Crop Prot. 54, 239–243. [Google Scholar]
- Graham, J.H. , Timmer, L.W. and Fardelmann, D. (1986) Toxicity of fungicidal copper in soil to citrus seedlings and vesicular–arbuscular mycorrhizal fungi. Phytopathology, 76, 66–70. [Google Scholar]
- Graham, J.H. , Gottwald, T.R. , Civerolo, E.L. and Mcguire, R.G. (1989) Population‐dynamics and survival of Xanthomonas campestris in soil in citrus nurseries in Maryland and Argentina. Plant Dis. 73, 423–427. [Google Scholar]
- Graham, J.H. , Gottwald, T.R. , Riley, T.D. and Achor, D. (1992) Penetration through leaf stomata and growth of strains of Xanthomonas campestris in citrus cultivars varying in susceptibility to bacterial diseases. Phytopathology, 82, 1319–1325. [Google Scholar]
- Graham, J.H. , Gottwald, T.R. , Cubero, J. and Achor, D.S. (2004) Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 5, 1–15. [DOI] [PubMed] [Google Scholar]
- Graham, J.H. , Leite, R.P. and Yonce, H.D. (2007) Streptomycin controls citrus canker in Brazil and Florida and reduces risk of copper phytotoxicity on grapefruit. Phytopathology, 97, S42. [Google Scholar]
- Graham, J.H. , Brooks, C. and Yonce, H.D. (2016a) Importance of early season copper sprays for protection of Hamlin orange fruit against citrus canker infection and premature fruit drop. Proc. Florida State Hortic. Soc. 129, 74–78. [Google Scholar]
- Graham, J.H. , Johnson, E.G. , Myers, M.E. , Young, M. , Rajasekaran, P. , Das, S. and Santra, S. (2016b) Potential of nano‐formulated zinc oxide for control of citrus canker on grapefruit trees. Plant Dis. 100, 2442–2447. [DOI] [PubMed] [Google Scholar]
- Guo, Y.P. , Sagaram, U.S. , Kim, J.S. and Wang, N. (2010) Requirement of the galU gene for polysaccharide production by and pathogenicity and growth in planta of Xanthomonas citri subsp citri . Appl. Environ. Microbiol. 76, 2234–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y.P. , Figueiredo, F. , Jones, J. and Wang, N. (2011) HrpG and hrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis pv. citri . Mol. Plant–Microbe Interact. 24, 649–661. [DOI] [PubMed] [Google Scholar]
- Hacker, J. and Kaper, J.B. (2000) Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54, 641–679. [DOI] [PubMed] [Google Scholar]
- Hall, D.G. , Gottwald, T.R. and Bock, C.H. (2010) Exacerbation of citrus canker by citrus leafminer Phyllocnistis citrella in Florida. Fla. Entomol. 93, 558–566. [Google Scholar]
- Hartung, J.S. (1992) Plasmid‐based hybridization probes for detection and identification of Xanthomonas campestris pv citri . Plant Dis. 76, 889–893. [Google Scholar]
- Hasse, C.H. (1915) Pseudomonas citri, the cause of citrus canker – a preliminary report. J. Agric. Res. 4, 97–100. [Google Scholar]
- He, S.Y. (1998) Type III protein secretion systems in plant and animal pathogenic bacteria. Annu. Rev. Phytopathol. 36, 363–392. [DOI] [PubMed] [Google Scholar]
- Hoy, M.A. and Jessey, C. (2004) Ageniaspis citricola (Hymenoptera: Encyrtidae) established in Bermuda. Fla. Entomol. 87, 229–230. [Google Scholar]
- Hsiao, Y.M. , Liu, Y.F. , Lee, P.Y. , Hsu, P.C. , Tseng, S.Y. and Pan, Y.C. (2011) Functional characterization of copA gene encoding multicopper oxidase in Xanthomonas campestris pv. campestris . J. Agric. Food Chem. 59, 9290–9302. [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Zhang, J. , Jia, H. , White, F. , Wang, N. , Yang, B. and Jones, J.B. (2013) Diverse TAL effectors converge on a single host susceptibility gene in citrus canker. Phytopathology, 103, 62. [Google Scholar]
- Hu, Y. , Zhang, J. , Jia, H. , Sosso, D. , Li, T. , Frommer, W.B. , Yang, B. , White, F.F. , Wang, N. and Jones, J.B. (2014) Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. USA, 111, E521–E529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaciani, F.J. , Ferro, J.A. , Ferro, M.I.T. , Verniere, C. , Pruvost, O. and Belasque, J. (2012) Genetic diversity of a Brazilian strain collection of Xanthomonas citri subsp citri based on the type III effector protein genes. Plant Dis. 96, 193–203. [DOI] [PubMed] [Google Scholar]
- Jalan, N. , Kumar, D. , Andrade, M.O. , Yu, F. , Jones, J.B. , Graham, J.H. , White, F.F. , Setubal, J.C. and Wang, N. (2013a) Comparative genomic and transcriptome analyses of pathotypes of Xanthomonas citri subsp. citri provide insights into mechanisms of bacterial virulence and host range. BMC Genomics, 14, 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalan, N. , Kumar, D. , Yu, F. , Jones, J.B. , Graham, J.H. and Wang, N. (2013b) Complete genome sequence of Xanthomonas citri subsp. citri Strain aw12879, a restricted‐host‐range citrus canker‐causing bacterium. Genome Announc. 1(3), e00235–13. doi: 10.1128/genomeA.00235-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle, R.A. (1916) Characteristics of citrus canker and of the causal organism. Q. Bull. 1, 2–12. [Google Scholar]
- Jesus, W.C. , Belasque, J. , Amorim, L. , Christiano, R.S.C. , Parra, J.R.P. and Bergamin Filho, A. (2006) Injuries caused by citrus leafminer (Phyllocnistis citrella) exacerbate citrus canker (Xanthomonas axonopodis pv. citri) infections. Fitopatol. Bras. 31, 277–283. [Google Scholar]
- Jia, H. , Zhang, Y. , Orbović, V. , Xu, J. , White, F.F. , Jones, J.B. and Wang, N. (2017) Genome editing of the disease susceptibility gene CsLob1 in citrus confers resistance to citrus canker. Plant Biotechnol. J. 15, 817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, H.G. , Orbovic, V. , Jones, J.B. and Wang, N. (2016) Modification of the PthA4 effector binding elements in Type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccΔpthA4:dCsLOB1.3 infection. Plant Biotechnol. J. 14, 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S.J. and Henne, D.C. (2003) Biological control of the citrus leafminer with Ageniaspis citricola (Hymenoptera: Encyrtidae) in Lousiana. Proc. Florida State Hortic. Soc. 116, 224–226. [Google Scholar]
- Kay, S. , Hahn, S. , Marois, E. , Hause, G. and Bonas, U. (2007) A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science, 318, 648–651. [DOI] [PubMed] [Google Scholar]
- Khalaf, A. , Moore, G.A. , Jones, J.B. and Gmitter, F.G. (2007) New insights into the resistance of Nagami kumquat to canker disease. Physiol. Mol. Plant. 71, 240–250. [Google Scholar]
- Lamed, R. and Bayer, E.A. (1986) Contact and cellulolysis in Clostridium thermocellum via extensile surface organelles. Experientia, 42, 72–73. [Google Scholar]
- Lee, H.A. (1921) The increase in resistance to citrus canker with the advance in maturity of citrus trees. Phytopathology, 11, 70–73. [Google Scholar]
- Leite, R. , Egel, D. and Stall, R. (1994) Genetic analysis of hrp‐related DNA sequences of Xanthomonas campestris strains causing diseases of citrus. Appl. Environ. Microbiol. 60, 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite, R.P. and Mohan, S.K. (1990) Integrated management of the citrus bacterial canker disease caused by Xanthomonas campestris pv citri in the state of Parana, Brazil. Crop Prot. 9, 3–7. [Google Scholar]
- Li, J.Y. and Wang, N. (2014) Foliar application of biofilm formation‐inhibiting compounds enhances control of citrus canker caused by Xanthomonas citri subsp citri . Phytopathology, 104, 134–142. [DOI] [PubMed] [Google Scholar]
- Lin, H. , Hsu, S. , Hwang, A. and Tzeng, K. (2005) Phenotypic and genetic characterization of novel strains of Xanthomonas axonopodis pv. citri which induce atypical symptoms on citrus leaves in Taiwan. Plant Pathol. Bull. 14, 227–238. [Google Scholar]
- Lin, H.‐C. , Chu, M.‐K. , Lin, Y.‐C. , Deng, W.‐L. , Chang, H. , Hsu, S.‐T. and Tzeng, K.‐C. (2011) A single amino acid substitution in pthA of Xanthomonas axonopodis pv. citri altering canker formation on grapefruit leaves. Eur. J. Plant Pathol. 130, 143–154. [Google Scholar]
- Lin, H.‐C. , Chang, Y.‐A. and Chang, H. (2013) A pthA homolog from a variant of Xanthomonas axonopodis pv. citri enhances virulence without inducing canker symptom. Eur. J. Plant Pathol. 137, 677–688. [Google Scholar]
- Louws, F.J. , Rademaker, J.L.W. and de Bruijn, F.J. (1999) The three Ds of PCR‐based genomic analysis of phytobacteria: diversity, detection, and disease diagnosis. Annu. Rev. Phytopathol. 37, 81–125. [DOI] [PubMed] [Google Scholar]
- Louws, F.J. , Wilson, M. , Campbell, H.L. , Cuppels, D.A. , Jones, J.B. , Shoemaker, P.B. , Sahin, F. and Miller, S.A. (2001) Field control of bacterial spot and bacterial speck of tomato using a plant activator. Plant Dis. 85, 481–488. [DOI] [PubMed] [Google Scholar]
- Ludidi, N. , Morse, M. , Sayed, M. , Wherrett, T. , Shabala, S. and Gehring, C. (2004) A recombinant plant natriuretic peptide causes rapid and spatially differentiated K+, Na+ and H+ flux changes in Arabidopsis thaliana roots. Plant Cell Physiol. 45, 1093–1098. [DOI] [PubMed] [Google Scholar]
- Mafi, S. and Ohbayashi, N. (2010) Biology of Chrysocharis pentheus, an endoparasitoid wasp of the citrus leafminer Phyllocnistis citrella Stainton. J. Agric. Sci. Technol. 12, 145–154. [Google Scholar]
- Malamud, F. , Homem, R.A. , Conforte, V.P. , Yaryura, P.M. , Castagnaro, A.P. , Marano, M.R. , do Amaral, A.M. and Vojnov, A.A. (2013) Identification and characterization of biofilm formation‐defective mutants of Xanthomonas citri subsp. citri . Microbiology, 159, 1911–1919. [DOI] [PubMed] [Google Scholar]
- Maryani, M.M. , Bradley, G. , Cahill, D.M. and Gehring, C.A. (2001) Natriuretic peptides and immunoreactants modify osmoticum‐dependent volume changes in Solanum tuberosum L. mesophyll cell protoplasts. Plant Sci. 161, 443–452. [Google Scholar]
- Mavrodieva, V. , Levy, L. and Gabriel, D.W. (2004) Improved sampling methods for real‐time polymerase chain reaction diagnosis of citrus canker from field samples. Phytopathology, 94, 61–68. [DOI] [PubMed] [Google Scholar]
- Mellano, M.A. and Cooksey, D.A. (1988) Nucleotide‐sequence and organization of copper resistance genes from Pseudomonas syringae pv tomato . J. Bacteriol. 170, 2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes, B.M.J. , Cardoso, S.C. , Boscariol‐Camargo, R.L. , Cruz, R.B. , Mourão Filho, F.A.A. and Bergamin Filho, A. (2010) Reduction in susceptibility to Xanthomonas axonopodis pv. citri in transgenic Citrus sinensis expressing the rice xa21 gene. Plant Pathol. 59, 68–75. [Google Scholar]
- Menkissoglu, O. and Lindow, S.E. (1991) Chemical forms of copper on leaves in relation to the bactericidal activity of cupric hydroxide deposits on plants. Phytopathology, 81, 1263–1270. [Google Scholar]
- Miller, J.R. , Gut, L.J. , de Lame, F.M. and Stelinski, L.L. (2006) Differentiation of competitive vs. non‐competitive mechanisms mediating disruption of moth sexual communication by point sources of sex pheromone (Part I): theory. J. Chem. Ecol. 32, 2089–2114. [DOI] [PubMed] [Google Scholar]
- Moreira, L.M. , Almeida, N.F. , Potnis, N. , Digiampietri, L.A. , Adi, S.S. , Bortolossi, J.C. , da Silva, A.C. , da Silva, A.M. , de Moraes, F.E. , de Oliveira, J.C. , de Souza, R.F. , Fancincani, A.P. , Ferraz, A.L. , Ferro, M.I. , Furlan, L.R. , Gimenez, D.F. , Jones, J.B. , Kitajima, E.W. , Laia, M.L. , Leite, R.P. , Nishiyama, M.Y. , Rodrigues Neto, J. , Nociti, L.A. , Norman, D.J. , Ostroski, E.H. , Pereira, H.A. , Staskawicz, B.J. , Tezza, R.I. , Ferro, J.A. , Vinatzer, B.A. and Setubal, J.C. (2010) Novel insights into the genomic basis of citrus canker based on the genome sequences of two strains of Xanthomonas fuscans subsp. aurantifolii . BMC Genomics, 11, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschini, R.C. , Canteros, B.I. , Martinez, M.I. and De Ruyver, R. (2014) Quantification of the environmental effect on citrus canker intensity at increasing distances from a natural windbreak in northeastern Argentina. Australas Plant Pathol. 43, 653–662. [Google Scholar]
- Mysore, K.S. and Ryu, C.M. (2004) Nonhost resistance: how much do we know? Trends Plant Sci. 9, 97–104. [DOI] [PubMed] [Google Scholar]
- Namekata, T. and Oliveira, A.D. (1972) Comparative serological studies between Xanthomonas citri and a bacterium causing canker on Mexican lime In: Proceedings of the Third International Conference on Plant Pathogenic Bacteria (Maas Geesteranus H.P. eds), pp. 151–2. Wageningen, the Netherlands: Centre of the Agricultural Publication and Documentation. [Google Scholar]
- Ngoc, L.B.T. , Vernière, C. , Boyer, C. , Vital, K. , Pruvost, O. , Le Mai, N. and Le Thi Thu, H. (2009) Pathotype identification of Xanthomonas citri pv. citri strains causing citrus canker in Vietnam. Plant Dis. 93, 671. [DOI] [PubMed] [Google Scholar]
- Ngoc, L.B.T. , Verniere, C. , Jouen, E. , Ah‐You, N. , Lefeuvre, P. , Chiroleu, F. , Gagnevin, L. and Pruvost, O. (2010) Amplified fragment length polymorphism and multilocus sequence analysis‐based genotypic relatedness among pathogenic variants of Xanthomonas citri pv. citri and Xanthomonas campestris pv. bilvae . Int. J. Syst. Evol. Microbiol. 60, 515–525. [DOI] [PubMed] [Google Scholar]
- Obradovic, A. , Mavridis, A. , Rudolph, K. , Janse, J.D. , Arsenijevic, M. , Jones, J.B. , Minsavage, G.V. and Wang, J.‐F. (2004) Characterization and PCR‐based typing of Xanthomonas campestris pv. vesicatoria from peppers and tomatoes in Serbia. Eur. J. Plant Pathol. 110, 285–292. [Google Scholar]
- de Oliveira, M.L.P. , Silva, C.C.D. , Abe, V.Y. , Costa, M.G.C. , Cernadas, R.A. and Benedetti, C.E. (2013) Increased resistance against citrus canker mediated by a citrus mitogen‐activated protein kinase. Mol. Plant–Microbe Interact. 26, 1190–1199. [DOI] [PubMed] [Google Scholar]
- O'Toole, G. , Kaplan, H.B. and Kolter, R. (2000) Biofilm formation as microbial development. Annu. Rev. Microbiol. 54, 49–79. [DOI] [PubMed] [Google Scholar]
- Park, D.S. , Wook Hyun, J. , Jin Park, Y. , Sun Kim, J. , Wan Kang, H. , Ho Hahn, J. and Joo Go, S. (2006) Sensitive and specific detection of Xanthomonas axonopodis pv. citri by PCR using pathovar specific primers based on hrpW gene sequences. Microbiol. Res. 161, 145–149. [DOI] [PubMed] [Google Scholar]
- Pena, J.E. , Hunsberger, A. and Schaffer, B. (2000) Citrus leafminer (Lepidoptera: Gracillariidae) density: effect on yield of ‘Tahiti’ lime. J. Econ. Entomol. 93, 374–379. [DOI] [PubMed] [Google Scholar]
- Pereira, A.L.A. , Carazzolle, M.F. , Abe, V.Y. , de Oliveira, M.L.P. , Domingues, M.N. , Silva, J.C. , Cernadas, R.A. and Benedetti, C.E. (2014) Identification of putative TAL effector targets of the citrus canker pathogens shows functional convergence underlying disease development and defense response. BMC Genomics, 15, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocelli, S. , Tondo, M.L. , Daurelio, L.D. and Orellano, E.G. (2012) Modifications of Xanthomonas axonopodis pv. citri lipopolysaccharide affect the basal response and the virulence process during citrus canker. PLoS One, 7, e40051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmawati, M. , Maryani, M.M. , Nikolakopoulos, T. , Gehring, C.A. and Irving, H.R. (2001) Cyclic GMP modulates stomatal opening induced by natriuretic peptides and immunoreactive analogues. Plant Physiol. Biochem. 39, 385–394. [Google Scholar]
- Popham, P.L. , Pike, S.M. , Novacky, A. and Pallardy, S.G. (1993) Water relation alterations observed during hypersensitive reaction induced by bacteria. Plant Physiol. 103, 1243–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, C.A. , Burton, M.S. , Pelosi, R. , Ritenour, M.A. and Bullock, R.C. (2007) Seasonal abundance and insecticidal control of citrus leafminer in a citrus orchard. Hortscience, 42, 1636–1638. [Google Scholar]
- Pruvost, O. , Hartung, J.S. , Civerolo, E.L. , Dubois, C. and Perrier, X. (1992) Plasmid DNA fingerprints distinguish pathotypes of Xanthomonas campestris pv citri, the causal agent of citrus bacterial canker disease. Phytopathology, 82, 485–490. [Google Scholar]
- Pruvost, O. , Boher, B. , Brocherieux, C. , Nicole, M. and Chiroleu, F. (2002) Survival of Xanthomonas axonopodis pv. citri in leaf lesions under tropical environmental conditions and simulated splash dispersal of inoculum. Phytopathology, 92, 336–346. [DOI] [PubMed] [Google Scholar]
- Pruvost, O. , et al., (2015) Genetic structure analysis of strains causing citrus canker in Iran reveals the presence of two different lineages of Xanthomonas citri pv citri pathotype A*. Plant Pathology, 64(4), 776–784, ISSN 0032‐0862, doi: 10.1111/ppa.12324. [Google Scholar]
- Rigano, L.A. , Siciliano, F. , Enrique, R. , Sendín, L. , Filippone, P. , Torres, P.S. , Qüesta, J. , Dow, J.M. , Castagnaro, A.P. , Vojnov, A.A. and Marano, M.R. (2007) Biofilm formation, epiphytic fitness, and canker development in Xanthomonas axonopodis pv citri . Mol. Plant–Microbe Interact. 20, 1222–1230. [DOI] [PubMed] [Google Scholar]
- Rigano, L.A. , Marano, M.R. , Castagnaro, A.P. , Do Amaral, A.M. and Vojnov, A.A. (2010) Rapid and sensitive detection of citrus bacterial canker by loop‐mediated isothermal amplification combined with simple visual evaluation methods. BMC Microbiol. 10, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, M.E. (2012) Protection of young citrus trees from Asian citrus psyllid and HLB. Physiol. Plant Pathol. 93, 10–15. [Google Scholar]
- Rogers, M.E. , Stansly, P.A. and Stelinski, L.L. (2015) Asian citrus psyllid and citrus leafminer In: Florida Citrus Pest Management Guide (Roger M.E. and Dewdney M.M., eds), pp. 89–91. Gainesville, FL: University of Florida, IFAS. [Google Scholar]
- Rossier, O. , Van den Ackerveken, G. and Bonas, U. (2000) HrpB2 and hrpF from Xanthomonas are type III‐secreted proteins and essential for pathogenicity and recognition by the host plant. Mol. Microbiol. 38, 828–838. [DOI] [PubMed] [Google Scholar]
- Rosetti, V. (1977) Citrus canker in Latin America: a review In: International Citrus Congress 2nd: 1977: Orlando, Florida. Orlando, Florida: International Society of Citriculture, p. 3. [Google Scholar]
- Rybak, M. , Minsavage, G.V. , Stall, R.E. and Jones, J.B. (2009) Identification of Xanthomonas citri ssp citri host specificity genes in a heterologous expression host. Mol. Plant Pathol. 10, 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapin, M.D. , Behlau, F. , Scandelai, L.H.M. , Fernandes, R.S. , Silva, G.J. and Ramos, H.H. (2015) Tree‐row‐volume‐based sprays of copper bactericide for control of citrus canker. Crop Prot. 77, 119–126. [Google Scholar]
- Schaad, N.W. , Jones, J.B. and Chun, W. (2001) Laboratory Guide for Identification of Plant Pathogenic Bacteria. St. Paul, MN: American Phytopathological Society. [Google Scholar]
- Schaad, N.W. , Postnikova, E. , Lacy, G. , Sechler, A. , Agarkova, I. , Stromberg, P.E. , Stromberg, V.K. and Vidaver, A.K. (2005) Reclassification of Xanthomonas campestris pv. citri (ex Hasse 1915) Dye 1978 forms A, B/C/D, and E as X. smithii subsp. citri (ex Hasse) sp. nov. nom. rev. comb. nov., X. fuscans subsp. aurantifolii (ex Gabriel 1989) sp. nov. nom. rev. comb. nov., and X. alfalfae subsp. citrumelo (ex Riker and Jones) Gabriel et al., 1989 sp. nov. nom. rev. comb. nov.; X. campestris pv. malvacearum (ex Smith 1901) Dye 1978 as X. smithii subsp. smithii nov. comb. nov. nom. nov.; X. campestris pv. alfalfae (ex Riker and Jones, 1935) Dye 1978 as X. alfalfae subsp. alfalfae (ex Riker et al., 1935) sp. nov. nom. rev.; and ‘‘var. fuscans’’ of X. campestris pv. phaseoli (ex Smith, 1987) Dye 1978 as X. fuscans subsp. fuscans sp. nov. Syst. Appl. Microbiol. 28, 494–518. [DOI] [PubMed] [Google Scholar]
- Schaad, N.W. , Postnikova, E. , Lacy, G. , Sechler, A. , Agarkova, I. , Stromberg, P.E. , Stromberg, V.K. and Vidaver, A.K. (2006) Emended classification of Xanthomonad pathogens on citrus. Syst. Appl. Microbiol. 29, 690–695. [DOI] [PubMed] [Google Scholar]
- Schubert, T.S. , Rizvi, S.A. , Sun, X. , Gottwald, T.R. , Graham, J.H. and Dixon, W.N. (2001) Meeting the challenge of eradicating citrus canker in Florida – again. Am. Phytopathol. Soc. 85, 340–356. [DOI] [PubMed] [Google Scholar]
- Sena‐Vélez, M. , Redondo, C. , Gell, I. , Ferragud, E. , Johnson, E. , Graham, J.H. and Cubero, J. (2015) Biofilm formation and motility of Xanthomonas strains with different citrus host range. Plant Pathol. 64, 767–775. [Google Scholar]
- Sena‐Vélez, M. , Redondo, C. , Graham, J.H. and Cubero, J. (2016) Presence of extracellular DNA during biofilm formation by Xanthomonas citri subsp citri strains with different host range. PLoS One, 11, e0156695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendin, L.N. , Filippone, M.P. , Orce, I.G. , Rigano, L. , Enrique, R. , Peña, L. , Vojnov, A.A. , Marano, M.R. and Castagnaro, A.P. (2012) Transient expression of pepper bs2 gene in Citrus limon as an approach to evaluate its utility for management of citrus canker disease. Plant Pathol. 61, 648–657. [Google Scholar]
- Shantharaj, D. , Minsavage, J. , Stall, R. , Lahaye, T. , Strauss, A. , Hu, Y. and Horvath, D .(2013) Deciphering specificities of TAL effectors in Xanthomonas citri and prospects in citrus. Phytopathology, 103, S2. [Google Scholar]
- Shi, Q.C. , Febres, V.J. , Jones, J.B. and Moore, G.A. (2015) Responsiveness of different citrus genotypes to the Xanthomonas citri ssp citri‐derived pathogen‐associated molecular pattern (PAMP) flg22 correlates with resistance to citrus canker. Mol. Plant Pathol. 16, 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotani, H. , Fujikawa, T. , Ishihara, H. , Tsuyumu, S. and Ozaki, K. (2007) A pthA homolog from Xanthomonas axonopodis pv. citri responsible for host‐specific suppression of virulence. J. Bacteriol. 189, 3271–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano, F. , Torres, P. , Sendin, L. , Bermejo, C. , Filippone, P. , Vellice, G. , Ramallo, J. , Castagnaro, A. , Vojnov, A. and Marano, M.R. (2006) Analysis of the molecular basis of Xanthomonas axonopodis pv. citri pathogenesis in Citrus limon . Electron J. Biotechnol. 9, 199–204. [Google Scholar]
- da Silva, A.C.R. , Ferro, J.A. , Reinach, F.C. , Farah, C.S. , Furlan, L.R. , Quaggio, R.B. , Monteiro‐Vitorello, C.B. , Van Sluys, M.A. , Almeida, N.F. , Alves, L.M.C. , do Amaral, A.M. , Bertolini, M.C. , Camargo, L.E.A. , Camarotte, G. , Cannavan, F. , Cardozo, J. , Chambergo, F. , Ciapina, L.P. , Cicarelli, R.M.B. , Coutinho, L.L. , Cursino‐Santos, J.R. , El‐Dorry, H. , Faria, J.B. , Ferreira, A.J.S. , Ferreira, R.C.C. , Ferro, M.I.T. , Formighieri, E.F. , Franco, M.C. , Greggio, C.C. , Gruber, A. , Katsuyama, A.M. , Kishi, L.T. , Leite, R.P. , Lemos, E.G.M. , Lemos, M.V.F. , Locali, E.C. , Machado, M.A. , Madeira, A.M.B.N. , Martinez‐Rossi, N.M. , Martins, E.C. , Meidanis, J. , Menck, C.F.M. , Miyaki, C.Y. , Moon, D.H. , Moreira, L.M. , Novo, M.T.M. , Okura, V.K. , Oliveira, M.C. , Oliveira, V.R. , Pereira, H.A. , Rossi, A. , Sena, J.A.D. , Silva, C. , de Souza, R.F. , Spinola, L.A.F. , Takita, M.A. , Tamura, R.E. , Teixeira, E.C. , Tezza, R.I.D. , Trindade dos Santos, M. , Truffi, D. , Tsai, S.M. , White, F.F. , Setubal, J.C. and Kitajima, J.P. (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature, 417, 459–463. [DOI] [PubMed] [Google Scholar]
- Slater, H. , Alvarez‐Morales, A. , Barber, C.E. , Daniels, M.J. and Dow, J.M. (2000) A two‐component system involving an HD‐GYP domain protein links cell–cell signalling to pathogenicity gene expression in Xanthomonas campestris . Mol. Microbiol. 38, 986–1003. [DOI] [PubMed] [Google Scholar]
- Society of American Bacteriologists ., Bergey, D.H. and Katherine Golden Bitting Collection on Gastronomy (Library of Congress). (1923) Bergey's Manual of Determinative Bacteriology; a Key for the Identification of Organisms of the Class Schizomycetes. Baltimore, MA: Williams & Wilkins Company. [Google Scholar]
- Soprano, A.S. , Abe, V.Y. , Smetana, J.H. and Benedetti, C.E. (2013) Citrus maf1, a repressor of RNA pol III, binds the Xanthomonas citri canker elicitor pthA4 and suppresses citrus canker development. Plant Physiol. 163, 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stall, R. , Miller, J. , Marco, G. and Canteros de Echenique, B. (1982) Timing of sprays to control cancrosis of grapefruit in Argentina In: Proceedings of the International Society of Citriculture/[International Citrus Congress, November 9–12, 1981, Tokyo, Japan (Matsumoto K., ed.), pp. 414–417. Shimizu, Japan: International Society of Citriculture. [Google Scholar]
- Stein, B. , Ramallo, J. , Foguet, L. and Graham, J.H. (2007) Citrus leafminer control and copper sprays for management of citrus canker on lemon in Tucuman, Argentina. Proc. Florida State Hortic. Soc. 120, 127–131. [Google Scholar]
- Stelinski, L.L. and Czokajlo, D. (2010) Suppression of citrus leafminer, Phyllocnistis citrella, with an attract‐and‐kill formulation. Entomol. Exp. Appl. 134, 69–77. [Google Scholar]
- Stelinski, L.L. , Miller, J.R. and Rogers, M.E. (2008) Mating disruption of citrus leafminer mediated by a noncompetitive mechanism at a remarkably low pheromone release rate. J. Chem. Ecol. 34, 1107–1113. [DOI] [PubMed] [Google Scholar]
- Stelinski, L.L. , Lapointe, S.L. and Meyer, W.L. (2010) Season‐long mating disruption of citrus leafminer, Phyllocnistis citrella Stainton, with an emulsified wax formulation of pheromone. J. Appl. Entomol. 134, 512–520. [Google Scholar]
- Stevens, H.E. (1914) Citrus canker. A preliminary bulletin. Florida Agric. Expt. Sta. Bull. 122, 113–118. [Google Scholar]
- Stoodley, P. , Sauer, K. , Davies, D.G. and Costerton, J.W. (2002) Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56, 187–209. [DOI] [PubMed] [Google Scholar]
- Sun, X. , Stall, R.E. , Jones, J.B. , Cubero, J. , Gottwald, T.R. , Graham, J.H. , Dixon, W.N. , Schubert, T.S. , Chaloux, P.H. , Stromberg, V.K. , Lacy, G.H. and Sutton, B.D. (2004) Detection and characterization of a new strain of citrus canker bacteria from key Mexican lime and alemow in South Florida. Plant Dis. 88, 1179–1188. [DOI] [PubMed] [Google Scholar]
- Sundin, G.W. , Jones, A.L. and Fulbright, D.W. (1989) Copper resistance in Pseudomonas syringae pv syringae from cherry orchards and its associated transfer in vitro and in planta with a plasmid. Phytopathology, 79, 861–865. [Google Scholar]
- Swarup, S. , Defeyter, R. , Brlansky, R.H. and Gabriel, D.W. (1991) A pathogenicity locus from Xanthomonas citri enables strains from several pathovars of Xanthomonas campestris to elicit cankerlike lesions on citrus. Phytopathology, 81, 802–809. [Google Scholar]
- Swarup, S. , Yang, Y.N. , Kingsley, M.T. and Gabriel, D.W. (1992) An Xanthomonas citri pathogenicity gene, ptha, pleiotropically encodes gratuitous avirulence on nonhosts. Mol. Plant–Microbe Interact. 5, 204–213. [DOI] [PubMed] [Google Scholar]
- Tally, A. , Oostendorp, M. , Lawton, K. , Staub, T. and Bassi, B. (1999) Commercial development of elicitors of induced resistance to pathogens In: Induced Plant Defenses against Pathogens and Herbivores: Biochemistry, Ecology, and Agriculture (Agrawal A.A., Tuzun S. and Bent E., eds), pp. 357–369. St. Paul, MN: American Phytopathological Society Press. [Google Scholar]
- Tampakaki, A.P. , Skandalis, N. , Gazi, A.D. , Bastaki, M.N. , Sarris, P.F. , Charova, S.N. , Kokkinidis, M. and Panopoulos, N.J. (2010) Playing the “harp”: evolution of our understanding of hrp/hrc genes 1. Annu. Rev. Phytopathol. 48, 347–370. [DOI] [PubMed] [Google Scholar]
- Tang, J.L. , Liu, Y.N. , Barber, C.E. , Dow, J.M. , Wootton, J.C. and Daniels, M.J. (1991) Genetic and molecular analysis of a cluster of rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris . Mol. Gen. Genet. 226, 409–417. [DOI] [PubMed] [Google Scholar]
- Teixeira, E.C. , de Oliveira, J.C.F. , Novo, M.T.M. and Bertolini, M.C. (2008) The copper resistance operon copAB from Xanthomonas axonopodis pathovar citri: gene inactivation results in copper sensitivity. Microbiology, 154, 402–412. [DOI] [PubMed] [Google Scholar]
- Timmer, L.W. , Gottwald, T.R. and Zitko, S.E. (1991) Bacterial exudation from lesions of Asiatic citrus canker and citrus bacterial spot. Plant Dis. 75, 192–195. [Google Scholar]
- Van Sluys, M.A. , Monteiro‐Vitorello, C.B. , Camargo, L.E.A. , Menck, C.F.M. , da Silva, A.C.R. , Ferro, J.A. , Oliveira, M.C. , Setubal, J.C. , Kitajima, J.P. and Simpson, A.J. (2002) Comparative genomic analysis of plant‐associated bacteria. Annu. Rev. Phytopathol. 40, 169–189. [DOI] [PubMed] [Google Scholar]
- Vauterin, L. , Swings, J. and Kersters, K. (1991a) Grouping of Xanthomonas campestris pathovars by SDS‐PAGE of proteins. J. Gen. Microbiol. 137, 1677–1687. [Google Scholar]
- Vauterin, L. , Yang, P. , Hoste, B. , Vancanneyt, M. , Civerolo, E.L. and Swings, J and Kersters, K. (1991b) Differentiation of Xanthomonas campestris pv citri strains by sodium dodecyl sulfate‐polyacrylamide gel‐electrophoresis of proteins, fatty‐acid analysis, and DNA–DNA hybridization. Int. J. Syst. Bacteriol. 41, 535–542. [Google Scholar]
- Vauterin, L. , Hoste, B. , Kersters, K. and Swings, J. (1995) Reclassification of Xanthomonas . Int. J. Syst. Bacteriol. 45, 472–489. [Google Scholar]
- Vauterin, L. , Yang, P. , Alvarez, A. , Takikawa, Y. , Roth, D.A. , Vidaver, A.K. , Stall, R.E. , Kersters, K. and Swings, J. (1996a) Identification of non‐pathogenic Xanthomonas strains associated with plants. Syst. Appl. Microbiol. 19, 96–105. [Google Scholar]
- Vauterin, L. , Yang, P. and Swings, J. (1996b) Utilization of fatty acid methyl esters for the differentiation of new Xanthomonas species. Int. J. Syst. Bacteriol. 46, 298–304. [Google Scholar]
- Verniere, C. , Devaux, M. , Pruvost, O. , Couteau, A. and Luisetti, J. (1991) Studies on the biochemical and physiological variations among strains of Xanthomonas campestris pv. citri, the causal agent of citrus bacterial canker disease. Fruits, 46, 162–170. [Google Scholar]
- Verniere, C. , Hartung, J.S. , Pruvost, O.P. , Civerolo, E.L. , Alvarez, A.M. , Maestri, P. and Luisetti, J. (1998) Characterization of phenotypically distinct strains of Xanthomonas axonopodis pv. citri from Southwest Asia. Eur. J. Plant Pathol. 104, 477–487. [Google Scholar]
- Verniere, C.J. , Gottwald, T.R. and Pruvost, O. (2003) Disease development and symptom expression of Xanthomonas axonopodis pv. citri in various citrus plant tissues. Phytopathology, 93, 832–843. [DOI] [PubMed] [Google Scholar]
- Vojnov, A.A. , Zorreguieta, A. , Dow, J.M. , Daniels, M.J. and Dankert, M.A. (1998) Evidence for a role for the gumB and gumC gene products in the formation of xanthan from its pentasaccharide repeating unit by Xanthomonas campestris . Microbiology, 144, 1487–1493. [DOI] [PubMed] [Google Scholar]
- Voloudakis, A.E. , Reignier, T.M. and Cooksey, D.A. (2005) Regulation of resistance to copper in Xanthomonas axonopodis pv. vesicatoria . Appl. Environ. Microbiol. 71, 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. and Liu, J.H. (2012) Exogenous treatment with salicylic acid attenuates occurrence of citrus canker in susceptible navel orange (Citrus sinensis Osbeck). J. Plant Physiol. 169, 1143–1149. [DOI] [PubMed] [Google Scholar]
- Yamazaki, A. , Hirata, H. and Tsuyumu, S. (2008a) HrpG regulates type II secretory proteins in Xanthomonas axonopodis pv. citri . J. Gen. Plant Pathol. 74, 138–150. [Google Scholar]
- Yamazaki, A. , Hirata, H. and Tsuyumu, S. (2008b) Type III regulators hrpG and hrpXct control synthesis of alpha‐amylase, which is involved in in planta multiplication of Xanthomonas axonopodis pv. citri . J. Gen. Plant Pathol. 74, 254–257. [Google Scholar]
- Yan, Q. and Wang, N.A. (2011) The colR/colS two‐component system plays multiple roles in the pathogenicity of the citrus canker pathogen Xanthomonas citri subsp citri . J. Bacteriol. 193, 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Q. and Wang, N. (2012) High‐throughput screening and analysis of genes of Xanthomonas citri subsp citri involved in citrus canker symptom development. Mol. Plant–Microbe Interact. 25, 69–84. [DOI] [PubMed] [Google Scholar]
- Yang, Y.N. and Gabriel, D.W. (1995) Xanthomonas avirulence/pathogenicity gene family encodes functional‐plant nuclear targeting signals. Mol. Plant–Microbe Interact. 8, 627–631. [DOI] [PubMed] [Google Scholar]
- Ye, G. , Hong, N. , Zou, L.‐F. , Zou, H.‐S. , Zakria, M. , Wang, G.‐P. and Chen, G.‐Y. (2013) TALE‐based genetic diversity of Chinese isolates of the citrus canker pathogen Xanthomonas citri subsp. citri . Plant Dis. 97, 1187–1194. [DOI] [PubMed] [Google Scholar]
- Young, J.M. , Dye, D.W. , Bradbury, J.F. , Panagopoulos, C.G. and Robbs, C.F. (1978) Proposed nomenclature and classification for plant pathogenic bacteria. N. Z. J. Agric. Res. 21, 153–177. [Google Scholar]
- Young, J.M. , Bradbury, J.F. , Gardan, L. , Gvozdyak, R.I. , Stead, D.E. , Takikawa, Y. and Vidaver, A.K. (1991) Comment on the reinstatement of Xanthomonas citri (Ex Hasse 1915) Gabriel et al 1989 and X. phaseoli (Ex Smith 1897) Gabriel et al 1989 – indication of the need for minimal standards for the genus Xanthomonas . Int. J. Syst. Bacteriol. 41, 172–177. [Google Scholar]
- Yun, M.H. , Torres, P.S. , El Oirdi, M ., Rigano, L.A. , Gonzalez‐Lamothe, R. , Marano, M.R. , Castagnaro, A.P. , Dankert, M.A. , Bouarab, K. and Vojnov, A.A. (2006) Xanthan induces plant susceptibility by suppressing callose deposition. Plant Physiol. 141, 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X.D. , Francis, M.I. , Dawson, W.O. , Graham, J.H. , Orbovic, V. , Triplett, E.W. and Mou, Z. (2010) Over‐expression of the Arabidopsis NPR1 gene in citrus increases resistance to citrus canker. Eur. J. Plant Pathol. 128, 91–100. [Google Scholar]
- Zhang, Y. , Jalan, N. , Zhou, X. , Goss, E. , Jones, J.B. , Setubal, J.C. , Deng, X. and Wang, N. (2015) Positive selection is the main driving force for evolution of citrus canker‐causing Xanthomonas . ISME J. 9, 2128–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.X. , Xu, S.H. , Ding, P.T. , Wang, D.M. , Cheng, Y.T. , He, J. , Gao, M. , Xu, F. , Li, Y. , Zhu, Z. , Li, X. and Zhang, Y. (2010) Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant‐specific family of transcription factors. Proc. Natl. Acad. Sci. USA, 107, 18 220–18 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimaro, T. , Thomas, L. , Marondedze, C. , Garavaglia, B.S. , Gehring, C. , Ottado, J. and Gottig, N. (2013) Insights into Xanthomonas axonopodis pv. citri biofilm through proteomics. BMC Microbiol. 13, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimaro, T. , Thomas, L. , Marondedze, C. , Sgro, G.G. , Garofalo, C.G. , Ficarra, F.A. , Gehring, C. , Ottado, J. and Gottig, N. (2014) The type III protein secretion system contributes to Xanthomonas citri subsp citri biofilm formation. BMC Microbiol. 14, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


