Summary
Mucins are highly glycosylated polypeptides involved in many host–parasite interactions, but their function in plant‐parasitic nematodes is still unknown. In this study, a mucin‐like gene was cloned from Meloidogyne incognita (Mi‐muc‐1, 1125 bp) and characterized. The protein was found to be rich in serine and threonine with numerous O‐glycosylation sites in the sequence. Quantitative real‐time polymerase chain reaction (qRT‐PCR) showed the highest expression in the adult female and in situ hybridization revealed the localization of Mi‐muc‐1 mRNA expression in the tail area in the region of the phasmid. Knockdown of Mi‐muc‐1 revealed a dual role: (1) immunologically, there was a significant decrease in attachment of Pasteuria penetrans endospores and a reduction in binding assays with human red blood cells (RBCs), suggesting that Mi‐MUC‐1 is a glycoprotein present on the surface coat of infective second‐stage juveniles (J2s) and is involved in cellular adhesion to the cuticle of infective J2s; pretreatment of J2s with different carbohydrates indicated that the RBCs bind to J2 cuticle receptors different from those involved in the interaction of Pasteuria endospores with Mi‐MUC‐1; (2) the long‐term effect of RNA interference (RNAi)‐mediated knockdown of Mi‐muc‐1 led to a significant reduction in nematode fecundity, suggesting a possible function for this mucin as a mediator in the interaction between the nematode and the host plant.
Keywords: attachment, fecundity, glycocalyx, immunity, M. incognita, mucin, P. penetrans
INTRODUCTION
The cuticle is a multi‐layered flexible structure in nematodes that acts as a hydrostatic exoskeleton and provides protection against the abiotic and biotic environment, including interactions with pathogenic microorganisms and, if parasitic, its hosts (Page, 2013). It is part of the extracellular matrix which maintains the nematode’s anatomical integrity and plays a crucial role in movement and growth (Lee, 2002). The cuticle, together with its surface coat, has been most extensively studied in Caenorhabditis elegans and, over the last several years, the nematode has become a model for the study of microbial pathogens (Ewbank, 2002; Sifri et al., 2005). Early research suggested that the cuticle and its surface coat were products of the hypodermis from which they were secreted (Wright, 1987); however, this view is now questionable as there is growing evidence that genes that play a role in the building of complex structures present in the cuticle’s surface coat are expressed in seam cells, and mutations to these genes are known to affect bacterial adhesion (Gravato‐Nobre et al., 2011).
The surface coat of animal‐parasitic nematodes contains glycans, which are thought to be influential in modulating the interaction with their hosts (Blaxter et al., 1992; Maizels et al., 2001; Politz and Philipp, 1992). Carbohydrate moieties on the surface of parasites are highly variable (Hicks et al., 2000; Theodoropoulos et al., 2001), and there are even variations among different populations of a particular species of parasite, having altered glycans as surface antigens or receptors (Appleton and Romaris, 2001; Haslam et al., 2000; Maass et al., 2007; Skelly and Wilson, 2006). The outermost cuticular layer appears to possess a number of specialized features and has been found to contain cuticulins, lipids, surface‐associated proteins, carbohydrates and probably glycoproteins (Blaxter et al., 1992; Page et al., 1992). Several biochemical and serological approaches used to characterize the surface coat properties of animal‐parasitic nematodes have revealed that rapid changes in the structure of the surface coat can occur between pre‐ and post‐parasitic forms (Proudfoot et al., 1993). Human red blood corpuscles have also been found to be useful in the study and characterization of the carbohydrate moieties present on the surface coat of plant‐parasitic nematodes (Spiegel and McClure, 1995; Spiegel et al., 1991).
Root‐knot nematodes (RKNs: Meloidogyne spp.) are economically one of the most damaging root pests and are responsible for extensive losses of staple food crops (Chitwood, 2003; Jones et al., 2013). These microscopic parasitic animals can manipulate plant defences and are thought to reprogram the cell cycle during the formation of specialized feeding cells (giant cells) in the roots for their continued nutrient supply (Gheysen and Mitchum, 2011). Owing to the lack of availability of effective management tools, and the global ban/restriction of many nematicides, alternative management strategies are being sought (Seid et al., 2015). Biological control offers the prospect to provide an alternative in an environmentally benign and self‐sustaining manner (Collange et al., 2011).
Pasteuria penetrans (Thorne) Sayre and Starr, a Gram‐positive, endospore‐forming soil bacterium of the Bacillus–Clostridium clade (Charles et al., 2005; Preston et al., 2003) has the potential to be developed into a commercial biological control agent for the management of RKN species [preferably the southern RKN Meloidogyne incognita (Kofoid and White) Chitwood] by turning the adult female cadaver into an ‘endospore sac’ (Davies, 2009; Stirling, 2014). When the cadaver eventually disintegrates, the bacterial endospores are released and lie dormant in the soil until they attach to the cuticle surface of the migrating pre‐parasitic second‐stage juveniles (J2s). They then germinate, by a process that is not understood, to form rhizoids which penetrate the developing female and eventually fragment to produce bacterial rods that proliferate and destroy the nematode’s reproductive capacity, ultimately resulting in the death of the adult female (Davies et al., 2011). The attachment of endospores to the cuticle of RKN J2s is likely to be governed by several factors and the cuticle surface coat plays a pivotal role in the attachment process (Davies and Danks, 1992; Spiegel and McClure, 1995).
Genome survey sequences of P. penetrans have been found to contain genes that encode collagen‐like proteins (Davies and Opperman, 2006), similar to those of P. ramosa (Moulton et al., 2009) and other endospore‐forming, animal‐parasitic Bacillus spp. (Sylvestre et al., 2003, 2005 ). It has been hypothesized that these collagen‐like proteins, which produce a hair‐like nap on the surface of the Pasteuria endospores, facilitate the attachment of the endospores to the nematode cuticle in a Velcro‐like fashion (Davies, 2009). Several investigations have shown that the nature of the attachment process is highly host specific, but is not linked to the phylogeny of the nematode (Davies et al., 2001). The nature of the cuticle receptor and the mechanism by which Pasteuria endospores bind are a matter of speculation; however, pretreatment of infective juveniles with a range of glycolytic enzymes and proteases suggests that surface coat glycans may help to explain the host specificity observed in the attachment of Pasteuria endospores to RKN cuticles (Davies and Danks, 1992; Davies and Redden, 1997; Davies et al., 1994).
Mucins are a family of high‐molecular‐weight proteins produced by the epithelial tissues in most organisms of the Kingdom Animalia (Marin et al., 2007). These proteins contain tandem repeats of amino acids rich in serine and threonine in their backbone which serve as sites for O‐glycosylation and have been suggested to play a role in immune defence (Hall and Altun, 2008). They are associated with both the innate and adapted immune systems and can be secreted or membrane bound to form a protective barrier over the epithelium (Loukas et al., 2000; Magalhães et al., 2010; Schulenburg et al., 2004; Strous and Dekker, 1992; Theodoropoulos et al., 2001). Membrane‐associated mucins are closely involved in the adhesion status of cells through electrostatic charge and steric effects of long chains protruding from the surface. The overabundance of certain membrane‐associated mucins suggests the role of the surface coat in the immune evasion of parasitic nematodes by changing the nematode surface cuticle adherence to defence cells (Gems and Maizels, 1996). Investigations using RNA interference (RNAi)‐mediated knockdown of mucin‐like proteins in C. elegans showed altered lectin binding to the surface coat (K. G. Davies et al., unpublished data), suggesting that it contains mucin‐like proteins amongst other glycosylated protein secretions (Gems and Maizels, 1996; Hemmer et al., 1991).
Although the roles of the majority of mucins in C. elegans are unknown, it is clear that bah, bus and srf mutants, which show altered bacterial adhesion, are glycosyltransferase mutants; glycosyltransferases are involved in the building of complex glycans on the surface coat of the cuticle (Gravato‐Nobre and Hodgkin, 2011). One of these mutations in C. elegans, bus‐4, which confers resistance to Microbacterium nematophilum, Yersinia pestis and Y. pseudotuberculosis, also shows altered mucin expression (Parsons et al., 2014). It has been hypothesized that mucin‐like peptides may also play a role in the attachment of P. penetrans endospores to the cuticle of RKNs (Davies, 2009; Davies and Curtis, 2011).
In this study, we identified and characterized a full‐length mucin‐like gene from the RKN M. incognita. In the absence of any tractable transformation tool for forward genetics screening, RNAi was used as a reverse genetics approach to assess the function of this gene (Dutta et al., 2015; Phani et al., 2017). Quantitative real‐time polymerase chain reaction (qRT‐PCR) was used to establish the transcriptional levels in different developmental stages of the nematode species and in situ hybridization was performed for the localization of mRNA expression in the body of the nematode. Knockdown of the mucin‐like gene by RNAi was employed and its effect on the attachment of Pasteuria endospores was studied; further characterization was investigated using red blood cells (RBCs) and inhibitory carbohydrates. Lastly, the role of the mucin‐like gene was also investigated for its effect on post‐infection nematode development and reproduction.
RESULTS
Molecular analysis, in situ hybridization and differential stage‐specific expression of Mi‐muc‐1
Based on the expressed sequence tag (EST) sequence (947 bp) of the mucin‐like gene from the cDNA library of M. incognita, the 1125‐bp full‐length cDNA sequence was amplified and cloned. The plasmid was named Mi‐muc‐1, encoding for a deduced 374‐bp amino acid sequence. The full‐length cDNA sequence was submitted to GenBank (Accession Number MG579969). Based on the analysis, the protein sequence was found to be rich in serine and threonine (26.2%) and contained numerous O‐glycosylation sites (Table S1, see Supporting Information).
The localization of Mi‐muc‐1 mRNA expression was determined by in situ hybridization in the pre‐parasitic J2s of M. incognita. The hybridization signal with digoxigenin (DIG)‐labelled antisense probe was detected in the tail region of the nematode, an area in which the phasmid is located (Fig. 1A); DIG‐labelled sense probes of the control group showed no such signal (Fig. 1B) and, similarly, no signal was detected in the anterior body part of the nematode with the DIG‐labelled antisense probe (Fig. S1, see Supporting Information). In addition, the localization of the mRNA expression of the other two homologues of Mi‐muc‐1, namely MucX and MucY, was also restricted to the tail region as for Mi‐muc‐1 (Figs S2 and S3, see Supporting Information).
Figure 1.
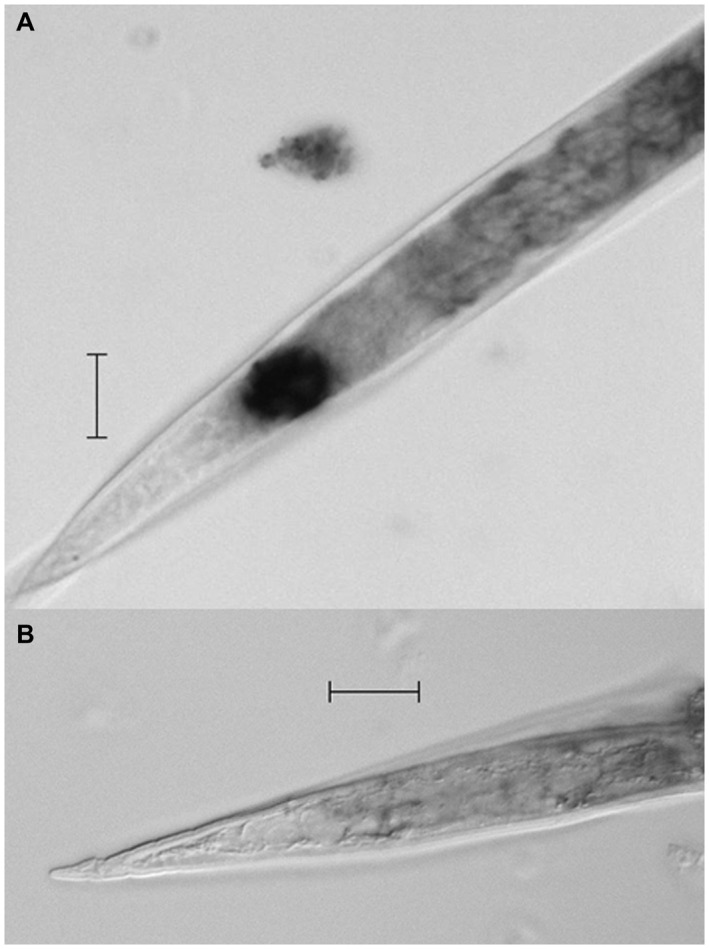
Localization of Mi‐muc‐1 mRNA in Meloidogyne incognita second‐stage juveniles (J2s) by in situ hybridization. (A) Hybridization of antisense digoxigenin (DIG)‐labelled cDNA probe shows the location in the phasmid area of the tail region. (B) Hybridization with DIG‐labelled sense cDNA probe was used as a control. Scale bar, 20 µm.
To determine the expression of Mi‐muc‐1 in different life stages of M. incognita, qRT‐PCR was performed. Using the expression level in eggs as a reference, Mi‐muc‐1 was found to be significantly (P < 0.05) upregulated in the pre‐parasitic J2s, parasitic J2s and pre‐egg‐laying females (Fig. 2). The highest level of expression was observed in the pre‐egg‐laying females, followed by the pre‐parasitic J2s, compared with insignificant expression in the third‐/fourth‐stage juveniles (J3s/J4s).
Figure 2.
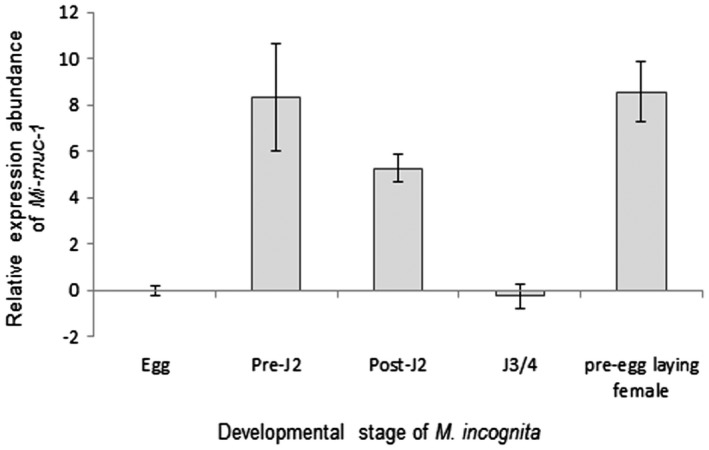
Relative expression abundance of Mi‐muc‐1 in different developmental stages of Meloidogyne incognita. Using the 2–ΔΔCT method, the relative expression level was quantified. Using the transcript level in eggs as a reference, the expression of Mi‐muc‐1 was found to be highest in pre‐egg‐laying females, followed by pre‐parasitic second‐stage juveniles (J2s). Each bar represents the mean ± standard error (SE).
Knockdown of Mi‐muc‐1 and its effect on endospore and RBC attachment
The designed double‐stranded RNA (dsRNA) fragment resulted in the specific knockdown of Mi‐muc‐1. On BLAST search against the M. incognita database, two other mucin‐like sequences were retrieved which were amplified and cloned successfully (MucX and MucY). Alignment of the sequences revealed the percentage similarity of MucX and MucY to be 47.43% and 58.89%, respectively (at the nucleotide level), with Mi‐muc‐1. The result of qRT‐PCR showed no transcriptional alteration of MucX and MucY on knockdown of Mi‐muc‐1 (Fig. S4, see Supporting Information).
The in vitro RNAi‐mediated knockdown of Mi‐muc‐1 significantly (P < 0.05) reduced the adherence of endospores to the cuticle surface of M. incognita J2s. Approximately, 6 ± 2 endospores could attach to the target dsRNA‐treated worms, which was almost five times lower than that observed in the control groups. In comparison, the freshly hatched J2s (31 ± 6 endospores) and double‐stranded green fluorescent protein (dsGFP)‐treated J2s (30 ± 4 endospores) showed no significant difference in endospore adhesion (Fig. 3).
Figure 3.
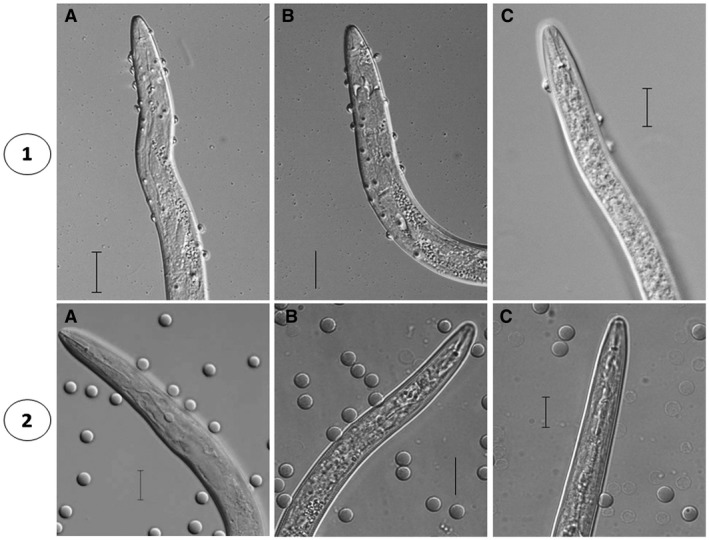
Effect of Mi‐muc‐1 silencing on the attachment of endospores and red blood cells (RBCs) to Meloidogyne incognita second‐stage juveniles (J2s). (1) Decreased endospore attachment to cuticle surface on silencing of Mi‐muc‐1. (2) Decreased RBC adhesion to cuticle surface on silencing of Mi‐muc‐1. A, freshly hatched J2s; B, double‐stranded green fluorescent protein (dsGFP)‐treated J2s; C, Mi‐muc‐1‐silenced J2s. Scale bar, 20 µm.
The knockdown of Mi‐muc‐1 by dsRNA treatment was also found to cause significant (P < 0.05) reduction in adherence of RBCs to the cuticle surface. The average number of RBCs adhered to dsRNA‐treated juveniles was found to be 4 ± 3 vs. 12 ± 5 in freshly hatched J2s and 10 ± 6 in dsGFP‐treated J2s (Fig. 3), showing no significant difference in the last two cases.
Assessment of endospore/RBC attachment on pre‐incubation in carbohydrate
The pre‐incubation of J2s in different carbohydrates affected the attachment of P. penetrans endospores to the juvenile cuticle surface of M. incognita. The average number of endospores attached to J2s is shown in Fig. 4. This resulted in a reduction of 51%, 75% and 79% in the presence of d‐glucose, d‐galactose and d‐xylose, respectively (Table 1), when compared with an average of 29 ± 7 endospores for the untreated control.
Figure 4.
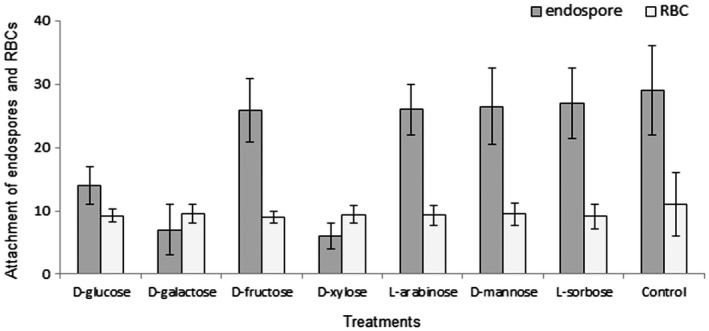
Attachment of endospores and red blood cells (RBCs) to second‐stage juvenile (J2) cuticle after incubation in different carbohydrates. d‐Glucose, d‐galactose and d‐xylose showed significantly (P < 0.05) less endospore attachment relative to d‐fructose, l‐arabinose, d‐mannose, l‐sorbose and control, whereas no statistically significant difference (P < 0.05) was observed for RBC attachment between different sugars.
Table 1.
Percentage decrease in endospore and RBC attachment over control after incubation in different carbohydrates (ANOVA endospore and RBC attachment; P < 0.05).
| Sugars | Endospore attachment | RBC attachment | ||||
|---|---|---|---|---|---|---|
| Treated | % change | Treated | % change | |||
| D‐glucose | 14±3 | 51 | 9.2±1 | 8 | ||
| D‐galactose | 7±4 | 75 | 9.5±1.5 | 5 | ||
| D‐fructose | 25.9±5 | 11 | 9±1 | 10 | ||
| D‐xylose | 6±2 | 79 | 9.4±1.4 | 6 | ||
| L‐arabinose | 26±4 | 10 | 9.3±1.6 | 7 | ||
| D‐mannose | 26.5±6.1 | 9 | 9.5±1.8 | 5 | ||
| L‐sorbose | 27±5.5 | 7 | 9.1±1.9 | 9 | ||
| Control | 29±7 | NA | 11±5 | NA | ||
The binding of RBCs was also found to be affected by the incubation of J2s in carbohydrate solutions (Fig. 4); however, there was no statistically significant difference between any of the different sugars (Table 1).
Thus, it is evident that, although the attachment profile of endospores and RBCs is reduced by the use of different carbohydrate molecules, only in the case of Pasteuria endospores does this vary significantly (P < 0.05) between the different sugars tested, with d‐glucose, d‐galactose and d‐xylose exhibiting the greatest effects.
Evaluation of M. incognita development and P. penetrans establishment
The long‐term effect of RNAi‐mediated knockdown of Mi‐muc‐1 on the development and reproduction potential of M. incognita was assessed by infection bioassay using CYG germination pouches (Mega International, St Paul, MN, USA). At 30 days post‐inoculation (dpi), when the nematodes had completed their life cycle, dsRNA‐treated worms showed lower reproduction potential relative to control worms. A significant (P < 0.05) reduction was observed with respect to the number of galls, adult females, egg mass, eggs per egg mass and multiplication factor (MF) (Fig. 5). Approximately 29 ± 2.92, 28 ± 2.86 and 20 ± 2.25 galls developed per plant inoculated with freshly hatched, dsGFP‐treated and target dsRNA‐treated J2s, respectively. The average number of females developed per plant on infection with target dsRNA‐treated worms was 25 ± 2.84, whereas, for inoculation with freshly hatched and dsGFP‐treated worms, the numbers were 34 ± 3.93 and 33 ± 4.01, respectively. The average numbers of egg masses per plant produced by control worms (freshly hatched, 33 ± 3.46; dsGFP‐treated, 32 ± 2.73) were found to be significantly (P < 0.05) higher than that for the target dsRNA‐treated nematodes (23 ± 3.58). The approximate numbers of eggs per egg mass produced by freshly hatched, dsGFP‐treated and target dsRNA‐treated worms were found to be 582 ± 53, 579 ± 49 and 495 ± 56, respectively. MF (indicative of reproductive fitness and parasitic success) was reduced by approximately 40% in plants infected with Mi‐muc‐1 dsRNA‐treated J2s, as compared with infection with freshly hatched and dsGFP‐treated J2s. Hence, silencing of Mi‐muc‐1 in infective J2s retarded the development and reproduction potential of M. incognita in the host plant.
Figure 5.
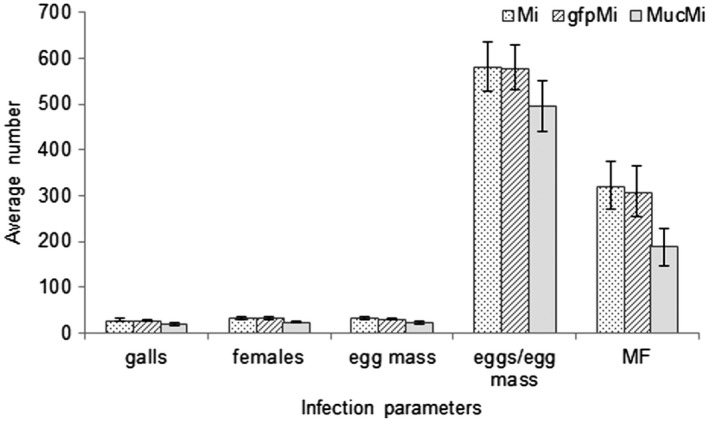
Assessment of parasitism on silencing of Mi‐muc‐1. The number of galls, adult females and egg mass per plant, eggs per egg mass and multiplication factor (MF) were reduced on silencing of Mi‐muc‐1 in second‐stage juveniles (J2s) over controls. Mi, freshly hatched J2s; gfpMi, double‐stranded green fluorescent protein (dsGFP)‐treated J2s; MucMi, Mi‐muc‐1‐silenced J2s.
Further, the silencing of Mi‐muc‐1 was found to exert a measurable effect on the development and establishment of P. penetrans inside adult M. incognita females. On infection with endospore‐encumbered target dsRNA‐treated J2s, the percentage of infected females was recorded as 13.66% ± 1.50%, which was significantly (P < 0.05) lower than that observed for the controls (Fig. 6). Inoculation with endospore‐encumbered freshly hatched (40.33% ± 2.33%) and dsGFP‐treated (39.01%±4.55%) J2s showed no significant difference.
Figure 6.
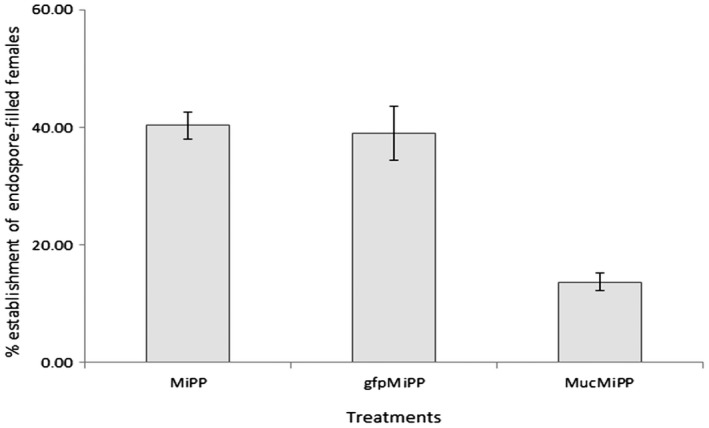
Percentage establishment of endospore‐filled females. The number of endospore‐filled females was reduced on Mi‐muc‐1 silencing relative to controls. MiPP, endospore‐encumbered freshly hatched second‐stage juveniles (J2s); gfpMiPP, endospore‐encumbered double‐stranded green fluorescent protein (dsGFP)‐treated J2s; MucMiPP, endospore‐encumbered Mi‐muc‐1‐silenced J2s.
DISCUSSION
The results reported here demonstrate that the mucin‐like protein (Mi–MUC‐1) in M. incognita plays a dual role: (1) it facilitates the attachment of P. penetrans endospores to the cuticle of infective juveniles; and (2) it interferes with nematode development and fecundity. The present investigation is the first to identify and molecularly characterize a mucin‐like protein from a plant‐parasitic nematode with two functions. The attachment profiles of endospores and RBCs differed in the bioassays in which Mi‐MUC‐1 was involved and J2s were pretreated with a combination of different sugars. These results indicate the presence of more than one cellular attachment mechanism to the juvenile cuticle of M. incognita.
The reduction in endospore attachment on pre‐incubation in d‐glucose, d‐galactose and d‐xylose indicates that the ligands present on the nematode cuticle involved in attachment can recognize these sugars. Decreased attachment after incubation of the juveniles in carbohydrates, followed by washing, suggests that the sugar molecules chemically bind in a stable manner and block the receptors. The observation that the change in adhesion profile in response to different sugars and the interaction with knockdown of Mi‐muc‐1 is different between endospores and RBCs indicates that Mi‐MUC‐1 is not the only cuticle receptor by which cells (endospore/RBC) can bind to the J2 cuticle, and suggests the availability of other biochemical ligands. This result updates the previous observations of Bird et al. (1989) and Davies and Danks (1992), in which the effect of the pre‐incubation of juveniles in different carbohydrates was tested for endospore attachment, and d‐xylose was found to reduce the attachment; however, in the later study, d‐glucose and d‐galactose did not show any effect.
The presence of carbohydrate residues on the surface of RKNs has been documented previously (Davies et al., 1988; Ibrahim, 1991; McClure and Zuckerman, 1982; Robertson et al., 1989; Spiegel and McClure, 1991) and carbohydrate recognition domains on the cuticle surface have also been predicted to interact with the N‐acetylglucosamine moieties on the spore surface (Davies and Danks, 1992). Interestingly, d‐glucose and d‐galactose were not found to exert any significant effect on endospore attachment in a previous study (Davies and Danks, 1992), whereas pre‐incubation in these two carbohydrates significantly reduced endospore attachment in the present investigation. This variation may be a result of differences in the biochemical composition of the endospore surface (Davies and Redden, 1997; Davies et al., 1994) or, indeed, the surface coat of J2s, which is also known to be dynamic and exhibits interspecific variation (Davies et al., 2001, 2008).
Compared with Pasteuria endospores, pretreatment of J2s with carbohydrates only had a marginal effect on the adhesion of RBCs, suggesting that different biochemical ligands are involved. This may be a result of the coevolutionary arms race between P. penetrans and its host nematode M. incognita, resulting in the high specificity observed in their attachment (Davies, 2009). This contrasts with the RBC binding assay, in which the interaction is likely to be much less specific, as RBCs and plant‐parasitic nematodes have not undergone prolonged coevolution. The reduced adherence of RBCs to the body surface on silencing of Mi‐muc‐1 further strengthens the hypothesis of the occurrence of mucin‐like proteins on the cuticle. A dense coating of glycans stabilizes the RBCs (Evans and Graham, 1991; Eylar et al., 1962; Fernandes et al., 2011; Schauer, 2009) and aids in their attachment to other surfaces (Heidrich and Leutner, 1974; Winzler et al., 1967); the RBC glycoproteins may therefore aid in the stabilization of Mi‐MUC‐1 of M. incognita helping in their adhesion.
The results of in situ hybridization revealed that Mi‐muc‐1 expression was localized in the tail region of M. incognita in an area similar to that identified by Premachandran et al. (1988) and Bellafiore et al. (2008) as the phasmid. Many non‐structural surface‐associated proteins on the nematode body surface are thought to be secreted from gland cells, namely amphids, pharyngeal glands, excretory pores, phasmids, hypodermis and seam cells (Gravato‐Nobre et al., 2011), and are subjected to continuous turnover (Blaxter and Robertson, 1998; Davies and Curtis, 2011). The constant sloughing off and renewal of these surface antigens protects the nematodes during movement in the soil environment as well as inside the host plant (Blaxter and Robertson, 1998; Curtis et al., 2011). The localization of Mi‐muc‐1 expression around the phasmid is an indication that Mi‐MUC‐1 is secreted through the phasmidial pore and spreads over the cuticle surface. Interestingly, in C. elegans, in which seam cells are important in producing glycans to build complex surface carbohydrates (Gravato‐Nobre et al., 2011), a gene has been identified (bus‐4) that produces an altered mucin responsible for bacterial adhesion (Parsons et al., 2014). Some of these complex molecules present in the surface coat have also been documented in the subventral oesophageal glands in RKNs (Haegeman et al, 2013; Roze et al, 2008) and the internal excretory glands of the animal‐parasitic nematode Toxocara canis (Gems and Maizels, 1996; Hayes et al, 1990), and are therefore likely to have more than one role. Reduction in endospore attachment on silencing of Mi‐muc‐1 suggests that the mucin‐like protein is involved in the facilitation of microbial adhesion on the surface of the nematode cuticle. Similar results have been observed previously (Derrien et al., 2010; Martin‐Sosa et al., 2002; Parsons et al., 2014; Ruiz‐Palacios et al., 2003; Sanchez et al., 2009), where mucins have been implicated to play a decisive role in the adhesion of microbial cells in C. elegans and humans.
Mi‐muc‐1 was found to be expressed in pre‐parasitic (infective) J2s, parasitic J2s and females of M. incognita, indicating its presence in different phases of the nematode life cycle. The highest levels of expression were observed in pre‐parasitic and post‐parasitic J2s and pre‐egg‐laying females, with very little expression in eggs, J3 and J4 stages. The high expression of Mi‐muc‐1 in pre‐egg‐laying females is indicative of its possible involvement in nematode reproduction and fecundity. Interestingly, this result is consistent with the observations of Ganji et al. (2014), where C‐type lectin (CTL) expression was found to be highest in sedentary females of Rotylenchulus reniformis, a semi‐endoparasitic nematode species. CTLs are one of the most prominent proteins, having a carbohydrate recognition domain which includes multiple calcium binding sites (Harcus et al., 2009), and have also been found to interfere with host immunity (Loukas and Maizels, 2000). The presence of CTLs on the surface of the marine nematode species, Laxus oneistus, is responsible for binding with the symbiotic bacteria necessary for nematode metabolism (Bulgheresi et al., 2006), and they have also been implicated in the bacterial infection of C. elegans (O’Rourke et al., 2006) and, more recently, in the recognition of bacterial, viral and fungal pathogens in other parasitic nematodes (Hoving et al., 2014). Therefore, the expression of Mi‐muc‐1 in both the adult females and infective J2s suggests a dual function: (1) outside the plant host to help overcome the effects of pathogens and parasites taking part in the innate immune response (Hasnain et al., 2012; Loukas et al., 2000; Schulenburg et al., 2004; Theodoropoulos et al., 2001); and (2) inside the plant, where it may also play a role in the nematode–plant interaction, directly affecting its fecundity.
The reduced number of females and decreased egg production on Mi‐muc‐1 dsRNA feeding suggests an additional role for the Mi‐MUC‐1 protein in affecting the fecundity of M. incognita. The reduction in the number of females on the root may be the result of either direct or indirect interactions: direct interactions may be because Mi‐MUC‐1 knockdown, which affects endospore and RBC adhesion, also has an effect on J2 mobility, thereby influencing root invasion; indirect effects may be via changes in the ability of the plant to recognize that it is undergoing parasitism by the nematode. The latter indirect effect provides evidence supporting the hypothesis of Kaplan and Davis (1987), who suggested that the nematode surface coat plays a role in plant–nematode interactions. The surface coat has been an active area of research for several decades and is thought to play a role in nematode–plant interactions, as it is known to be readily shed and replaced both outside and within the roots (Curtis et al., 2011; Davies and Curtis, 2011; Gravato‐Nobre et al., 1999; Lin and McClure, 1996; Spiegel and McClure, 1995). Interestingly, the most dominant model for the description of plant–pathogen interactions is the zig‐zag model of coevolution between microbial‐associated molecular patterns (MAMPs), secreted/excreted by the pathogen, and pattern recognition receptors (PRRs), which, in turn, ultimately leads to either a susceptibility or resistance response of the host (Jones and Dangl, 2006). The nematode surface coat could be a source of nematode effectors (Rosso and Grenier, 2011; Smant and Jones, 2011), which hitherto has provided a too narrowly defined bias (Cook et al., 2015) focusing on the identification and characterization of genes and proteins. A whole range of molecules have been ascribed the function of MAMPs; many of these include glycosides and have sugar‐based moieties, e.g. chitin, peptidoglycan and lipopolysaccharide (Iriti and Faoro, 2009). It has long been recognized that a component of the surface coat, nemin, is an elicitor of trapping devices in nematode predatory fungi. Recent research has characterized nemin as a member of a family of small lipophilic signalling molecules, ascarosides, which also appear to play a role in nematode–plant interactions (Manosalva et al., 2015). The ascarosides, as a group, consist of a glycoside with a lipophilic fatty acid side chain, and are only found in nematodes; it is interesting that fatty acid and retinol‐binding (FAR) proteins, which are also only found in nematodes and are associated with the nematode cuticle, appear to affect microbial adhesion to the cuticle and nematode interactions with the plant host (Phani et al., 2017).
Thus, in summary, we have demonstrated the presence of a mucin‐like protein in the cuticle surface coat of M. incognita, where it has two functions: (1) it plays a pivotal role in the attachment and interaction of P. penetrans endospores; and (2) it also appears to be indirectly involved in the nematode–plant interaction.
EXPERIMENTAL PROCEDURES
Nematode population
The single egg mass culture of an Indian isolate of M. incognita (Kofoid and White) Chitwood race 1 was multiplied on a susceptible tomato plant (Solanum lycopersicum L. cv. Pusa ruby) in a glasshouse at the ICAR‐Indian Agricultural Research Institute, New Delhi, India. Egg masses were manually picked from the infected roots and hatched via a modified Baermann’s assembly (Whitehead and Hemming, 1965). Freshly hatched J2s were used for experimental purposes.
Bioinformatics
Fifteen genes encoding mucin‐like proteins in C. elegans were retrieved from the WormBase (version: WS260) database together with their translated products (Table S2, see Supporting Information). The protein sequences were analysed for their amino acid compositions and percentage serine and threonine content. The sequences were then subjected to BLAST search in WormBase Parasite (https://parasite.wormbase.org/Tools/Blast) and Meloidogyne genomic resources, INRA (https://www6.inra.fr/meloidogyne_incognita/Genomic-resources2/Blast) to retrieve the homologous sequences in M. incognita. The identities of the exact putative mucin‐like gene hits in M. incognita were obtained from the top scoring reciprocal BLAST hits (sequences with the smallest expected value and a large bit score). The return protein sequences were then stringently analysed for their percentage serine and threonine content using the ExPasy ProtParam tool (https://web.expasy.org/protparam/) and O‐glycosylation sites were predicted using the NetOGlyc 4.0 (https://www.cbs.dtu.dk/services/NetOGlyc/) server. Gene‐specific primers were designed (https://eu.idtdna.com/Primerquest/Home/Index) using customized parameters to amplify the partial sequence of the putatively assigned mucin‐like gene in M. incognita.
Cloning, sequencing and characterization of mucin‐like gene in M. incognita
Total RNA was extracted from the freshly hatched J2s of M. incognita with a NucleoSpin® RNA kit (Macherey‐Nagel, Düren, Germany) according to the manufacturer’s protocol, and cDNA was synthesized as described previously (Phani et al., 2017). A partial sequence of the putative mucin‐like gene (termed Mi‐muc‐1) was amplified from the cDNA using specific primers (Mi_muc_F and Mi_muc_R), cloned into the pGEM‐T Easy vector and sequenced via the Sanger sequencing method. To clone the full‐length Mi‐muc‐1, the partial sequence was subjected to BLAST search in WormBase Parasite to retrieve the corresponding coding sequence (CDS) transcript from the M. incognita genome (Abad et al., 2008). Accordingly, primers (InM2 and MR) were designed, PCR amplified, cloned and sequenced as described previously. Finally, the partial sequence and newly obtained sequence were aligned to obtain the complete Mi‐muc‐1 sequence. Additional primers (MF and MR) were designed from ATG to TAA of the full‐length sequence, amplified from cDNA, cloned and sequenced for further confirmation. The primer details are given in Table 2.
Table 2.
List of primers used in this study.
| Primer names | Primer sequence (5′ ‐ 3′) | Product length (bp) | Tm (°C) |
|---|---|---|---|
| Mi_muc_F | GTAATCCTTACAGGCCCTTCTC | 947 | 62 |
| Mi_muc _R | TGACCCGTTGCACAATTACCGC | ||
| InM2 | CTACAACAACAACCTTACCAACT | 428 | 62 |
| MR | TTATTTTTTTGTCCCGAAATAACCGTTAGC | ||
| MF | ATGTATAACGCCACATCTGGG | 1125 | 62 |
| MR | TTATTTTTTTGTCCCGAAATAACCGTTAGC | ||
| M2_F | AGAGGATGGAAACACGTATGG | 216 | 62 |
| M2_R | CCTACCCACAGTGAACTGAAA | ||
| Mucq_F | GCTCGGAGCTGAAGTTGTATTA | 96 | 60 |
| Mucq_R | GTGTTTGTTTGACACGCAGTTA | ||
| dM2_F | GAGAGGATGGAAACACGTATGG | 512 | 62 |
| dM2_R | TGGCTGCGTTGTAGTTGTAG | ||
| MucX_F | GGGAAGAGAAGAGCGTTATGG | 799 | 62 |
| MucX_R | AACGTGGACTCATGTGGAATAG | ||
| MucY_F | CTACCGCTGAACCAACTACAA | 655 | 62 |
| MucY_R | GATTGACCCGTTGCACAATTAC | ||
| qMucX_F | CTACGACAGAGGAACCAACTAAA | 101 | 60 |
| qMucX_R | ACAGAATGGAGGAGGCTTTG | ||
| qMucY_F | TCTACCACGACGACCTTTAATTG | 118 | 60 |
| qMucY_R | GACATTCGCACACTCCTTGA | ||
| gfp F | AGCGGCACGACTTCTTCA | 750 | 60 |
| gfp R | GTGTGGACAGGTAATGGTTGT | ||
| 18S_Mi_RT F | TCAACGTGCTTGTCCTACCCTGAA | 115 | 60 |
| 18S_Mi_RT R | TGTGTACAAAGGGCAGGGACGTAA | ||
| qMi‐actin F | TGACTCTGGAGATGGTGTTACG | 142 | 60 |
| qMi‐actin R | GTGATGACTTGACCGTCAGGC | ||
| X_ISH_F | CCCGCTACTACAACAGAAGAAC | 233 | 62 |
| X_ISH_R | CAGAATGGAGGAGGCTTTGTAG | ||
| Y_ISH_F | CTACCACCCTTCCAACAACAA | 232 | 62 |
| Y_ISH_R | GATTGACCCGTTGCACAATTAC |
Sequence homology was compared with the non‐redundant protein (nr) and nucleotide (nt) databases using BLASTX and BLASTN (https://blast.ncbi.nlm.nih.gov/Blast.cgi) allowing the low complexity regions in the algorithm parameter. The CDSs were predicted by the National Center for Biotechnology Information (NCBI) ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) and conserved domains were analysed by the Conserved Domain Database in NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The ExPasy ProtParam tool (https://web.expasy.org/protparam/) and NetOGlyc 4.0 (https://www.cbs.dtu.dk/services/NetOGlyc/) server were used to analyse the protein sequence and the presence of O‐glycosylation sites.
In vitro RNAi of mucin‐like gene in M. incognita J2s
A 512‐bp fragment, amplified from cDNA using specific primers (dM2_F and dM2_R), was used to synthesize the dsRNA of Mi‐muc‐1 following the methodology described previously (Phani et al., 2017). Approximately 1500 freshly hatched M. incognita J2s were soaked in a solution with 0.1 mg/mL target dsRNA for in vitro RNAi, as described previously (Urwin et al., 2002). Soaking was continued for 15 h in the dark on a slowly moving rotator at 28 °C. Post‐incubation, the J2s were washed with molecular‐grade nuclease‐free water to remove any dsRNA contamination and total RNA was extracted from the dsRNA‐treated J2s using a NucleoSpin® RNA kit (Macherey‐Nagel) following the manufacturer’s instructions. The RNA was reverse transcribed to cDNA and the level of transcript suppression after dsRNA feeding was quantified and analysed by qRT‐PCR. All soaking experiments were repeated thrice. dsRNA from a non‐endogenous gene (gfp, HF675000) was used as a non‐native negative control.
To confirm the specificity of dsRNA‐mediated knockdown of the target gene, the translated sequence of the full‐length Mi‐muc‐1 was subjected to BLAST search against the M. incognita genome database using WormBase Parasite. The retrieved sequences with considerable homology (<1e‐10) were analysed for high serine and threonine content, and specific primers were designed for the amplification and cloning of the targets from cDNA. qRT‐PCR was performed to confirm the RNAi‐mediated silencing specificity of Mi‐muc‐1. dsGFP (HF675000) was used as a non‐native negative control. The primer details are provided in Table 2.
Post‐RNAi phenotyping for P. penetrans endospore attachment
The endospores of P. penetrans (strain AII‐329, Pasteuria collection, ICAR‐IARI, New Delhi, India) were produced on adzuki bean [Vigna angularis (Willd.) Ohwi and Ohashi] with a single egg mass culture of M. incognita, as described previously (Rao et al., 2012). To check the effect of Mi‐muc‐1 dsRNA treatment on endospore attachment to the J2 cuticle surface, approximately 200 dsRNA‐treated J2s were removed from the soaking solution, mixed with 100 μL of spore suspension (2.5 × 103 mL–1) and centrifuged at 5000 g for 5 min (Hewlett and Dickson, 1993). The endospore adhesion was quantified by removing 30 J2s from the suspension; spore attachment was observed and photographed using a Zeiss, Jena, Germany Axiocam M2m compound microscope equipped with differential interference contrast (DIC) optics. Freshly hatched J2s and J2s soaked in dsGFP were used as controls. All assays were performed in triplicate.
Post‐RNAi phenotyping for RBC attachment
To test the effect of Mi‐muc‐1 dsRNA treatment on the attachment of human RBCs (kindly provided by Dr Sujaya Raghavendra for experimental purposes only) to the J2 cuticle surface, approximately 200 dsRNA‐treated J2s were removed from the soaking solution and placed in a 1.5‐mL microfuge tube. The solution was centrifuged at 5000 g for 5 min, the supernatant was removed and J2s were washed twice with Ringer’s solution. Following this, the J2s were topped with 50 μL of RBC suspension (in Ringer’s solution) (2.5 × 103 mL–1) and centrifuged at 8000 g for 8 min. The adhesion of RBCs was quantified by removing around 30 J2s from the suspension; attachment was observed using a Zeiss Axiocam M2m compound microscope and photographed. Freshly hatched J2s and J2s soaked in dsGFP were used as controls. All assays were performed in triplicate.
Pre‐incubation of M. incognita in carbohydrates and endospore/RBC attachment assay
To test the effect of incubation in different carbohydrates on the attachment of endospores and RBCs to the J2 cuticle surface, approximately 200 freshly hatched J2s were taken in a 1.5‐mL microfuge tube, centrifuged at 5000 g for 5 min and the supernatant was removed. The J2s were then resuspended in 200 µL of treatment solution and incubated at room temperature (28 °C) for 2 h on a slowly moving rotator. d‐Glucose, d‐galactose, d‐fructose, d‐xylose, l‐arabinose, d‐mannose and l‐sorbose were used for assay purposes, at 0.4 m concentration each, to prevent bursting of the nematode body. The treated J2s were then washed with double‐distilled water thrice and subjected for attachment study. Around 30 J2s were taken from each of the treatment solutions, and endospore and RBC attachments were measured. Photographs were taken using a Zeiss Axiocam M2m compound microscope and freshly hatched J2s were used as a control. All assays were performed in triplicate.
In situ hybridization
Gene‐specific probes were used to localize the mRNA expression site of Mi‐muc‐1 by in situ hybridization, as described in Kimber et al. (2002). Approximately 20 000 J2s were fixed in 2% paraformaldehyde at 4 °C for 18 h, followed by a 4‐h incubation at room temperature (28 °C). Specific primers (M2_F and M2_R; Table 2) were used to amplify the DIG‐labelled sense and antisense probes (Roche, Mannheim, Germany) from the cDNA of M. incognita pre‐parasitic J2s. DIG‐labelled sense or antisense probes were added separately to the hybridization solution containing the nematode sections, and then incubated at 50 °C for 12 h in a hybridization chamber. Following hybridization, the nematodes were stained and photomicrographs were taken with a Zeiss Axiocam M2m compound microscope. The experiment was repeated three times. In addition, in situ hybridization was also carried out for the Mi‐muc‐1 homologues (MucX and MucY) to localize their mRNA expression site with specially designed primers (Table 2).
Analysis of mRNA levels at different life stages of M. incognita
The expression patterns of Mi‐muc‐1 in different developmental stages of M. incognita were analysed by qRT‐PCR with specially designed primers (Mucq_F and Mucq_R). For the extraction of the developmental stages of M. incognita, infection was performed on adzuki bean [Vigna angularis (Willd.) Ohwi and Ohashi] using CYG growth pouches (Mega International). The pouches were maintained in a growth chamber as described previously (Phani et al., 2017; Umarao et al., 2013) and various developmental stages were hand dissected at different intervals. Total RNA was extracted from approximately 1500 pre‐parasitic J2s, parasitic J2s (extracted between 4 and 6 dpi), J3s/J4s (extracted between 8 and 14 dpi) and pre‐egg‐laying females (extracted between 20 and 25 dpi), treated with RQ1 RNase‐free DNase (Promega, Madison, WI, USA) to remove genomic DNA contamination and finally reverse transcribed into cDNA. qRT‐PCR was performed in a realplex2 thermal cycler (Eppendorf, Hamburg, Germany) using a SYBR Green Supermix Kit (Eurogentec, Liege, Belgium), as described previously (Phani et al., 2017). 18S rRNA (HE667742) and actin (BE225475), two constitutively expressed genes, were used as references to normalize the gene expression level. Three biological and three technical replicates were maintained for each sample. The data were analysed by the ΔΔCt method (Livak and Schmittgen, 2001); the results were expressed as log2‐transformed fold change values and Student’s t‐test was performed. Primer details are provided in Table 2.
Study of nematode development and P. penetrans establishment
To test the effect of Mi‐muc‐1 knockdown on the reproduction potential of M. incognita and establishment of P. penetrans, infection was analysed in CYG growth pouches (Mega International) using adzuki bean as a susceptible host. The transfer of germinated seeds in pouches, maintenance for root proliferation, setting up of root infection and post‐infection maintenance of plants were conducted as described previously (Phani et al., 2017; Umarao et al., 2013). Mi‐muc‐1 dsRNA‐treated J2s and endospore‐encumbered Mi‐muc‐1 dsRNA‐treated J2s were used for inoculation purposes with five replications for each treatment. Freshly hatched J2s, endospore‐encumbered freshly hatched J2s, J2s treated with dsGFP and endospore‐encumbered dsGFP treated J2s were used as controls. The numbers of galls, adult females and egg masses produced per plant, number of eggs per egg mass and MF were considered as infection parameters to examine the reproduction potential of M. incognita. The percentage establishment of P. penetrans was calculated as [number of infected females/(number of infected females + number of uninfected females)] × 100, considering each pouch as a replicate.
Statistical analyses
One‐way analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) test (significance level at P < 0.05) were used to analyse the bioassay and expression data employing SAS version 9.3 for Windows (SAS Software, Inc. Cary, North Carolina, USA).
Supporting information
Fig. S1 The hybridization of digoxigenin (DIG)‐labelled sense (A) and antisense (B) cDNA probe of Mi‐muc‐1 showed no signalling in the anterior body part of Meloidogyne incognita (scale bar, 20 µm).
Fig. S2 The in situ hybridization result for MucX. The result showed no signalling in the anterior body part of Meloidogyne incognita with digoxigenin (DIG)‐labelled sense (A) and antisense (B) probes. The hybridization of antisense DIG‐labelled cDNA probe showed the location of mRNA expression in the phasmid area of the tail region (D) as compared with the control (C) (scale bar, 20 µm).
Fig. S3 The in situ hybridization result for MucY. The result showed no signalling in the anterior body part of Meloidogyne incognita with digoxigenin (DIG)‐labelled sense (A) and antisense (B) probes. The hybridization of antisense DIG‐labelled cDNA probe showed the location of mRNA expression in the phasmid area of the tail region (D) as compared with the control (C). However, some non‐specific signals were also detected in the posterior body part (D) (scale bar, 20 µm).
Fig. S4 (a) Sequence information of putative Mi‐muc‐1 homologues. (b) CLUSTAL 2.1 multiple sequence alignment. (c) Percent identity matrix (created by Clustal 2.1). (d) Quantitative real‐time polymerase chain reaction (qRT‐PCR) analysis shows no transcriptional alteration of MucX and MucY on downregulation of Mi‐muc‐1 (figure below) [double‐stranded green fluorescent protein (dsGFP) was used as a non‐native negative control].
Table S1 The NetOGlyc prediction result. The output conforms to the GFF version 2 format (https://www.cbs.dtu.dk/services/NetOGlyc/). For the input sequence Mi‐MUC‐1, the server has provided a list of potential glycosylation sites, showing their positions in the sequence and the prediction confidence scores. Only the sites with scores higher than 0.5 are predicted as glycosylated and marked with the string ‘#POSITIVE’ in the comment field.
Table S2 List of mucin‐like proteins of Caenorhabditis elegans.
ACKNOWLEDGEMENTS
The authors deeply acknowledge the British Council UKIERI project grant DST 59FX34 2014 for funding the research (fund recipient: K.G.D.). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
PhD student V.P. acknowledges the PG School ICAR‐IARI for necessary support during the research work. The authors are thankful to Dr Gautam Chawla (Division of Nematology, ICAR‐IARI) for providing microscope facilities and Dr Supradip Saha (Division of Agricultural Chemicals, ICAR‐IARI) for providing the carbohydrates.
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest with any third party.
Contributor Information
Keith G Davies, Email: k.davies@herts.ac.uk.
Uma Rao, Email: umarao@iari.res.in.
References
- Abad, P. , Gouzy, J. , Aury, J.M. , Sereno, P.C. , Danchin, E.G. , Deleury, E. , Barbeoch, L.P. , Anthouard, V. , Artiguenave, F. , Blok, V.C. , Caillaud, M.C. , Coutinho, P.M. , Dasilva, C. , Luca, F.D. , Deau, F. , Esquibet, M. , Flutre, T. , Goldstone, J.V. , Hamamouch, N. , Hewezi, T. , Jaillon, O. , Jubin, C. , Leonetti, P. , Magliano, M. , Maier, T.R. , Markov, G.V. , McVeigh, P. , Pesole, G. , Poulain, J. , Rechavi, M.R. , Sallet, E. , Steinbach, D. , Ugarte, E. , Abad Veronico, P. , Baum, T.J. , Blaxter, M. , Davis, E.L. , Ewbank, J.J. , Henrissat, B. , Jones, J.T. , Laudet, V. , Maule, A.G. , Quesneville, H. , Rosso, M. , Schiex, T. , Smant, G. , Weissenbach, J. and Wincker, P. (2008) Genome sequence of the metazoan plant‐parasitic nematode Meloidogyne incognita . Nat. Biotechnol. 8, 909–915. 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- Appleton, J.A. and Romaris, F. (2001) A pivotal role for glycans at the interface between Trichinella spiralis and its host. Vet. Parasitol. 101, 249–260. PMID: 11707300. [DOI] [PubMed] [Google Scholar]
- Bellafiore, S. , Shen, Z. , Rosso, M.N. , Abad, P. , Shih, P. and Briggs, S.P. (2008) Direct identification of the Meloidogyne incognita secretome reveals proteins with host cell reprogramming potential. PLoS Pathog. 4, e1000192 10.1371/journal.ppat.1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, A.F. , Bonig, I. and Bacic, A. (1989) Factors affecting the adhesion of micro‐organisms to the surfaces of plant‐parasitic nematodes. Parasitology, 15, 301–308. 10.1017/S0031182000059801. [DOI] [Google Scholar]
- Blaxter, M.L. , Page, A.P. , Rudin, W. and Maizels, R.M. (1992) Nematode surface coats: actively evading immunity. Parasitol. Today, 8, 243–246. PMID: 15463630. [DOI] [PubMed] [Google Scholar]
- Blaxter, M.L. and Robertson, W.M. (1998) The cuticle In: The Physiology and Biochemistry of Free‐Living and Plant‐Parasitic Nematodes (Perry R.N. and Wright D.J., eds.), pp. 25–48. Wallingford, Oxfordshire: CABI. [Google Scholar]
- Bulgheresi, S. , Schabussova, I. , Chen, T. , Mullin, N.P. , Maizels, R.M. and Ott, J.A. (2006) A new C‐type lectin similar to the human immunoreceptor DC‐SIGN mediates symbiont acquisition by a marine nematode. Appl. Environ. Microbiol. 72, 2950–2956. 10.1128/AEM.72.4.2950-2956.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles, L. , Carbonne, I. , Davies, K.G. , Bird, D. , Burke, M. , Kerry, B.R. and Opperman, C.H. (2005) Phylogenetic analysis of Pasteuria penetrans using multiple genetic loci. J. Bacteriol. 187, 5700–5708. 10.1128/JB.187.16.5700-5708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood, D.J. (2003) Research on plant‐parasitic nematode biology conducted by the United States Department of Agriculture‐Agricultural Research Service. Pest Manag. Sci. 59, 748–753. PMID: 12846325. [DOI] [PubMed] [Google Scholar]
- Collange, B. , Navarrete, M. , Peyre, G. , Mateille, T. and Tchamitchian, M. (2011) Root‐knot nematode (Meloidogyne) management in vegetable crop production: the challenge of an agronomic system analysis. Crop Prot. 30, 1251–1262. 10.1016/j.cropro.2011.04.016. [DOI] [Google Scholar]
- Cook, D.E. , Mesarich, C.H. and Thomma, B.P.H.J. (2015) Understanding plant immunity as a surveillance system to detect invasion. Annu. Rev. Phytopathol. 53, 541–563. 10.1146/annurev-phyto-080614-120114. [DOI] [PubMed] [Google Scholar]
- Curtis, R.H.C. , Jones, J.T. , Davies, K.G. , Sharon, E. and Spiegel, Y. (2011) Plant nematode surfaces In: Biological Control of Plant‐Parasitic Nematodes: Building Coherence Between Microbial Ecology and Molecular Mechanisms Davies K.G. and Spiegel Y. eds.), pp. 115–144. Berlin: Springer‐Verlag. [Google Scholar]
- Davies, K.G. (2009) Understanding the interaction between an obligate hyperparasitic bacterium, Pasteuria penetrans and its obligate plant parasitic nematode host, Meloidogyne spp. Adv. Parasitol. 68, 211–245. 10.1016/S0065-308X(08)00609-X. [DOI] [PubMed] [Google Scholar]
- Davies, K.G. and Curtis, R.H. (2011) Cuticle surface coat of plant‐parasitic nematodes. Annu. Rev. Phytopathol. 49, 135–156. 10.1146/annurev-phyto-121310-111406. [DOI] [PubMed] [Google Scholar]
- Davies, K.G. and Danks, C. (1992) Interspecific differences in the nematode surface coat between Meloidogyne incognita and M. arenaria related to the adhesion of the bacterium Pasteuria penetrans . Parasitology, 105, 475–480. 10.1017/S0031182000074655. [DOI] [Google Scholar]
- Davies, K.G. , Kerry, B.R. and Flynn, C.A. (1988) Observations on the pathogenicity of Pasteuria penetrans, a parasite of root‐knot nematodes. Ann. Appl. Biol. 112, 1491–1501. 10.1111/j.1744-7348.1988.tb02086.x. [DOI] [Google Scholar]
- Davies, K.G. , Fargette, M. , Balla, G. , Daudi, A. , Duponnois, R. , Gowen, S.R. , Mateille, T. , Phillips, M.S. , Sawadogo, A. , Trivino, C. , Vouyoukalou, E. and Trudgill, D.L. (2001) Cuticle heterogeneity as exhibited by Pasteuria spore attachment is not linked to the phylogeny of the parthenogenetic root‐knot nematodes (Meloidogyne spp.). Parasitology, 122, 111–120. PMID: 11197759. [DOI] [PubMed] [Google Scholar]
- Davies, K.G. and Opperman, C.H. (2006) A potential role for collagen in the attachment of Pasteuria penetrans to nematode cuticle. IOBC/WPRS Bull. 29, 11–15. [Google Scholar]
- Davies, K.G. and Redden, M. (1997) Diversity and partial characterisation of putative virulence determinants in Pasteuria penetrans, the hyperparasite of root‐knot nematodes. J. Appl. Microbiol. 83, 227–235. PMID: 9281826. [DOI] [PubMed] [Google Scholar]
- Davies, K.G. , Redden, M. and Pearson, T.K. (1994) Endospore heterogeneity in Pasteuria penetrans related to attachment to plant‐parasitic nematodes. Lett. Appl. Microbiol. 19, 370–373. 10.1111/j.1472-765X.1994.tb00478.x. [DOI] [Google Scholar]
- Davies, K.G. , Rowe, J.A. and Williamson, V.M. (2008) Inter‐ and intra‐specific cuticle variation between amphimictic and parthenogenetic species of root‐knot nematode (Meloidogyne spp.) as revealed by a bacterial parasite (Pasteuria penetrans). Int. J. Parasitol. 38, 851–859. 10.1016/j.ijpara.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Davies, K.G. , Rowe, J.A. , Manzanilla‐Lopez, R. and Opperman, C.H. (2011) Re‐evaluation of the life‐cycle of Pasteuria penetrans in root‐knot nematodes, Meloidogyne spp. Nematology, 13, 825–835. 10.1163/138855410X552670. [DOI] [Google Scholar]
- Derrien, M. , van Passel, M.W.J. , van de Bovenkamp, J.H.B. , Schipper, R.G. , de Vos, W.M. and Dekker, J. (2010) Mucin‐bacterial interactions in the human oral cavity and digestive tract. Gut. Microbes, 1, 254–268. 10.4161/gmic.1.4.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, T.K. , Papolu, P.K. , Banakar, P. , Choudhary, D. , Sirohi, A. and Rao, U. (2015) Tomato transgenic plants expressing hairpin construct of a nematode protease gene conferred enhanced resistance to root‐knot nematodes. Front. Microbiol. 6, 260 10.3389/fmicb.2015.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, W. and Graham, J. (1991) Membrane Structure and Function. New York: Oxford University Press. [Google Scholar]
- Ewbank, J.J. (2002) Tackling both sides of the host pathogen equation with Caenorhabditis elegans . Microbes Infect. 4, 247–256. PMID: 11880058. [DOI] [PubMed] [Google Scholar]
- Eylar, E.H. , Madoff, M.A. , Bordy, O.V. and Oncley, J.L. (1962) The contribution of sialic acid to the surface charge of the erythrocyte. J. Biol. Chem. 237, 1992–2000. PMID: 13891108. [PubMed] [Google Scholar]
- Fernandes, H.P. , Cesar, C.L. and Barjas‐Castro, M.L. (2011) Electrical properties of the red blood cell membrane and immunohematological investigation. Rev. Bras. Hematol. Hemoter. 33, 297–301. 10.5581/1516-8484.20110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganji, S. , Jenkins, J.N. and Wubben, M.J. (2014) Molecular characterization of the reniform nematode C‐type lectin gene family reveals a likely role in mitigating environmental stresses during plant parasitism. Gene, 537, 269–278. 10.1016/j.gene.2013.12.048. [DOI] [PubMed] [Google Scholar]
- Gems, D.H. and Maizels, R.M. (1996) An abundantly expressed mucin‐like protein from Toxocara canis infective larvae: the precursor of the larval surface coat glycoproteins. Proc. Natl. Acad. Sci. USA, 93, 1665–1670. PMID: 8643687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen, G. and Mitchum, L.G. (2011) How nematodes manipulate plant development pathways for infection. Curr. Opin. Plant Biol. 14, 415–421. 10.1016/j.pbi.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Gravato‐Nobre, M.J. and Hodgkin, J. (2011) Microbial interaction with Caenorhabditis elegans: lessons from a model organism In: Biological Control of Plant‐Parasitic Nematodes: Building Coherence Between Microbial Ecology and Molecular Mechanisms (Davies K.G. and Spiegel Y., eds.), pp. 65–90. Berlin: Springer‐Verlag. [Google Scholar]
- Gravato‐Nobre, M.J. , McClure, M.A. , Dolan, L. , Calder, G. , Davies, K.G. , Mulligan, B. , Evans, K. and von Mende, N. (1999) Meloidogyne incognita surface antigen epitopes in infected Arabidopsis roots. J. Nematol. 31, 212–223. PMID: 19270892. [PMC free article] [PubMed] [Google Scholar]
- Gravato‐Nobre, M.J. , Stroud, D. , O'Rourke, D. , Darby, C. and Hodgkin, J. (2011) Glycosylation genes expressed in seam cells determine complex surface properties and bacterial adhesion to the cuticle of Caenorhabditis elegans . Genetics, 187, 141–155. 10.1534/genetics.110.122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman, A. , Bauters, L. , Kyndt, T. , Rahman, M.M. and Gheysen, G. (2013) Identification of candidate effector genes in the transcriptome of the rice root knot nematode Meloidogyne graminicola . Mol. Plant Pathol. 14, 379–390. 10.1111/mpp.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, D.H. and Altun, Z.F. (2008) C. elegans Atlas. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Harcus, Y. , Nicoll, G. , Murray, J. , Filbey, K. , Gomez‐Escobar, N. and Maizels, R.M. (2009) C‐type lectins from the nematode parasites Heligmosomoides polygyrus and Nippostrongylus brasiliensis . Parasitol. Int. 58, 461–470. 10.1016/j.parint.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam, S.M. , Coles, G.C. , Morris, H.R. and Dell, A. (2000) Structural characterisation of the N‐glycans of Dictyocaulus viviparous: discovery of the Lewis(X) structure in a nematode. Glycobiology, 10, 223–229. PMID: 10642614. [DOI] [PubMed] [Google Scholar]
- Hasnain, S.Z. , Gallagher, A.L. , Grencis, R.K. and Thornton, D.J. (2012) A new role for mucins in immunity: insights from gastrointestinal nematode infection. Int. J. Biochem. Cell Biol. 45, 364–374. 10.1016/j.biocel.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Hayes, D.F. , Silberstein, D.S. , Rodrique, S.W. and Kufe, D.W. (1990) DF3 antigen, a human epithelial‐cell mucin, inhibits adhesion of eosinophils to antibody‐coated targets. J. Immunol. 145, 962–970. PMID: 2373864. [PubMed] [Google Scholar]
- Heidrich, H.G. and Leutner, G. (1974) Two types of vesicles from the erythrocyte‐ghost membrane differing in surface charge. Separation and characterization by preparative free‐flow electrophoresis. Eur. J. Biochem. 41, 37–43. PMID: 4816455. [DOI] [PubMed] [Google Scholar]
- Hemmer, R.M. , Donkin, S.G. , Chin, K.J. , Grenache, D.G. , Bhatt, H. and Politz, S.M. (1991) Altered expression of an L1‐specific, O‐linked cuticle surface glycoprotein in mutants of the nematode Caenorhabditis elegans . J. Cell Biol. 115, 1237–1247. PMID: 1955471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett, T.E. and Dickson, D.W. (1993) A centrifugation method for attaching endospores of Pasteuria spp. to nematodes. Suppl. J. Nematol. 25, 785–788. [PMC free article] [PubMed] [Google Scholar]
- Hicks, S.J. , Theodoropoulos, G. , Carrington, S.D. and Corfield, A.P. (2000) The role of mucins in host‐parasite interactions. Part I – protozoan parasites. Parasitol. Today, 16, 476–481. PMID: 11063858. [DOI] [PubMed] [Google Scholar]
- Hoving, J.C. , Wilson, G.J. and Brown, G.D. (2014) Signalling C‐type lectin receptors, microbial recognition and immunity. Cell Microbiol. 16, 185–194. 10.1111/cmi.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, S.K. (1991) Distribution of carbohydrates on the cuticle of several developmental stages of Meloidogyne javanica . Nematologica, 37, 275–284. 10.1163/187529291X00277. [DOI] [Google Scholar]
- Iriti, M. and Faoro, F. (2009) Chitosan as a MAMP, searching for a PRR. Plant Signal. Behav. 4, 66–68. PMID: 19704712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Jones, J.T. , Haegeman, A. , Danchin, E.G.J. , Gaur, H.S. , Helder, J. , Jones, M.G.K. , Kikuchi, T. , Manzanilla‐Lopez, R. , Palomares‐Rius, J.E. , Wesemael, W.M. and Perry, R.N. (2013) Top 10 plant‐parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 14, 946–961. 10.1111/mpp.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, D.T. and Davis, E.L. (1987) Mechanisms of plant incompatibility with nematodes In: Vistas on Nematology Veech J.A. and Dickson D.W., eds.), pp. 267–276. Hyattsville, MC: Society of Nematologists. [Google Scholar]
- Kimber, M.J. , Fleming, C.C. , Prior, A. , Jones, J.T. , Halton, D.W. and Maule, A.G. (2002) Localisation of Globodera pallida FMRFamide‐related peptide encoding genes using in situ hybridization. Int. J. Parasitol. 32, 1095–1105. PMID: 12117492. [DOI] [PubMed] [Google Scholar]
- Lee, D.L. (2002) The Biology of Nematodes. London: CRC Press. [Google Scholar]
- Lin, H.J. and McClure, M.A. (1996) Surface coat of Meloidogyne incognita . J. Nematol. 28, 216–224. PMCID: PMC2619683. [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2ΔΔCt method. Methods, 25, 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loukas, A. , Hintz, M. , Linder, D. , Mullin, N.P. , Parkinson, J. , Tetteh, K.K.A. and Maizels, R.M. (2000) A family of secreted mucins from the parasitic nematode Toxocara canis bears diverse mucin domains but shares similar flanking six‐cysteine repeat motifs. J. Biol. Chem. 275, 39 600–39 607. 10.1074/jbc.M005632200. [DOI] [PubMed] [Google Scholar]
- Loukas, A. and Maizels, R.M. (2000) Helminth C‐type lectins and host–parasite interactions. Parasitol. Today, 16, 333–339. PMID: 10900481. [DOI] [PubMed] [Google Scholar]
- Maass, D.R. , Harrison, G.B.L. , Grant, W.N. and Shoemaker, C.B. (2007) Three surface antigens dominate the mucosal antibody response to gastrointestinal L3‐stage strongylid nematodes in field immune sheep. Int. J. Parasitol. 37, 953–962. 10.1016/j.ijpara.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Magalhães, A. , Ismail, M.N. and Reis, A.C. (2010) Sweet receptors mediate the adhesion of the gastric pathogen Helicobacter pylori: glycoproteomic strategies. Expert Rev. Proteomics, 7, 307–310. 10.1586/epr.10.18. [DOI] [PubMed] [Google Scholar]
- Maizels, R.M. , Gomez‐Escobar, N. , Gregory, W.F. , Murray, J. and Zang, X.X. (2001) Immune evasion genes from filarial nematodes. Int. J. Parasitol. 31, 889–898. PMID: 11406138. [DOI] [PubMed] [Google Scholar]
- Manosalva, P. , Manohar, M. , von Reuss, S.H. , Chen, S. , Koch, A. , Kaplan, F. , Choe, A. , Micikas, R.J. , Wang, X. , Kogel, K. , Sternberg, P.W. , Williamson, V.M. , Schroeder, F.C. and Klessig, D.F. (2015) Conserved nematode signalling molecules elicit plant defenses and pathogen resistance. Nat. Commun. 6, 7795 10.1038/ncomms8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin, F. , Luquet, G. , Marie, B. and Medakovic, D. (2007) Molluscan shell proteins: primary structure, origin, and evolution. Curr. Top. Dev. Biol. 80, 209–276. 10.1016/S0070-2153(07)80006-8. [DOI] [PubMed] [Google Scholar]
- Martin‐Sosa, S. , Martin, M.J. and Hueso, P. (2002) The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichia coli human strains. J. Nutr. 132, 3067–3072. PMID: 12368397. [DOI] [PubMed] [Google Scholar]
- McClure, M.A. and Zuckerman, B.M. (1982) Localization of cuticular binding sites of concanavalin A on Caenorhabditis elegans and Meloidogyne incognita . J. Nematol. 14, 39–44. PMC2618142. [PMC free article] [PubMed] [Google Scholar]
- Moulton, L. , Traunecker, E. , McElroy, K. , Du Pasquier, L. and Ebert, D. (2009) Identification of a polymorphic collagen‐like protein in the crustacean bacteria Pasteuria ramosa . Res. Microbiol. 160, 792–799. 10.1016/j.resmic.2009.08.016. [DOI] [PubMed] [Google Scholar]
- O’Rourke, D. , Baban, D. , Demidova, M. , Mott, R. and Hodgkin, J. (2006) Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum . Genome Res. 16, 1005–1016. 10.1101/gr.50823006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, A.P. (2013) The nematode cuticle: synthesis, modification and turnover In: Parasitic Nematodes: Molecular Biology, Biochemistry, and Immunology, 2nd edn Kennedy M.W. and Harnett W., eds.), pp. 337–350. Wallingford, Oxfordshire: CABI. [Google Scholar]
- Page, A.P. , Hamilton, A.J. and Maizels, R.M. (1992) Toxocara canis: monoclonal antibodies to carbohydrate epitopes of secreted (TES) antigens localize to different secretion‐related structures in infective larvae. Exp. Parasitol. 75, 56–71. PMID: 1379195. [DOI] [PubMed] [Google Scholar]
- Parsons, L.M. , Mizanur, R.M. , Jankowska, E. , Hodgkin, J. , O′Rourke, D., Stroud, D. , Ghosh, S. and Cipollo, J.F. (2014) Caenorhabditis elegans bacterial pathogen resistant bus‐4 mutants produce altered mucins. PLoS One, 9, e107250 10.1371/journal.pone.0107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phani, V. , Shivakumara, T.N. , Davies, K.G. and Rao, U. (2017) Meloidogyne incognita fatty acid‐ and retinol‐binding protein (Mi‐FAR‐1) affects nematode infection of plant roots and the attachment of Pasteuria penetrans endospores. Front. Microbiol. 8, 2122 10.3389/fmicb.2017.02122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politz, S.M. and Philipp, M. (1992) Caenorhabditis elegans as a model for parasitic nematodes: a focus on the cuticle. Parasitol. Today, 8, 6–12. PMID: 15463517. [DOI] [PubMed] [Google Scholar]
- Premachandran, D. , VonMende, N. , Hussey, R.S. and McClure, M.A. (1988) A method for staining nematode secretions and structures. J. Nematol. 20, 70–78. PMC2618786. [PMC free article] [PubMed] [Google Scholar]
- Preston, J.F. , Dickson, D.W. , Muruniak, J.E. , Nong, G. , Brito, J.A. , Schmidt, L.M. , & Giblin‐Davis, R.M. (2003). Pasteuria spp.: systematics and phylogeny of these bacterial parasites of phytopathogenic nematodes. J. Nematol. 35, 198–207. PMC2620627. [PMC free article] [PubMed] [Google Scholar]
- Proudfoot, L.J. , Kusel, J.R. , Smith, H.V. , Harnett, W. , Worms, M.J. and Kennedy, M.W. (1993) Rapid changes in the surface of parasitic nematodes during transition from pre‐ to post‐parasitic forms. Parasitology, 107, 107–117. PMID: 8355993. [DOI] [PubMed] [Google Scholar]
- Rao, U. , Mauchline, T.H. and Davies, K.G. (2012) The 16S rRNA gene of Pasteuria penetrans provides an early diagnostic of infection of root‐knot nematodes (Meloidogyne spp.). Nematology, 14, 799–804. 10.1163/156854112X627318. [DOI] [Google Scholar]
- Robertson, W.M. , Spiegel, Y. , Jansson, H.B. , Marban‐Mendoza, N. and Zuckerman, B.M. (1989) Surface carbohydrates of plant parasitic nematodes. Nematologica, 35, 180–186. 10.1163/002825989X00313. [DOI] [Google Scholar]
- Rosso, M.N. and Grenier, E. (2011) Other nematode effectors and evolutionary constraints In: Genomics and Molecular Genetics of Plant‐Nematode Interactions Jones J., Gheysen G. and Fenoll C., eds.), pp. 287–307. Berlin: Springer‐Verlag. [Google Scholar]
- Roze, E. , Hanse, B. , Mitreva, M. , Vanholme, B. , Bakker, J. and Smant, G. (2008) Mining the secretome of the root‐knot nematode Meloidogyne chitwoodi for candidate parasitism genes. Mol. Plant Pathol. 9, 1–10. 10.1111/j.1364-3703.2007.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Palacios, G.M. , Cervantes, L.E. , Ramos, P. , Chavez‐Munguia, B. and Newburg, D.S. (2003) Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha1, 2Gal beta1, 4GlcNAc) and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 278, 14 112–14 120. 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- Sanchez, B. , Arias, S. , Chaignepain, S. , Denayrolles, M. , Schmitter, J.M. , Bressollier, P. and Urdaci, M.C. (2009) Identification of surface proteins involved in the adhesion of a probiotic Bacillus cereus strain to mucin and fibronectin. Microbiology, 155, 1708–1716. 10.1099/mic.0.025288-0. [DOI] [PubMed] [Google Scholar]
- Schauer, R. (2009) Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 19, 507–514. 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg, H. , Kurz, C.L. and Ewbank, J.J. (2004) Evolution of the innate immune system: the worm perspective. Immunol. Rev. 198, 36–58. PMID: 15199953. [DOI] [PubMed] [Google Scholar]
- Seid, A. , Fininsa, C. , Mekete, T. , Decraemer, W. and Wesemael, W.M. (2015) Tomato (Solanum lycopersicum) and root‐knot nematodes (Meloidogyne spp.) – a century old battle. Nematology, 17, 995–1009. doi: 10.1163/15685411-00002935. [DOI] [Google Scholar]
- Sifri, C.D. , Begun, J. and Ausubel, F.M. (2005) The worm has turned—microbial virulence modeled in Caenorhabditis elegans . Trends Microbiol. 13, 119–127. 10.1016/j.tim.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Skelly, P.J. and Wilson, R.A. (2006) Making sense of the schistosome surface. Adv. Parasitol. 63, 185–284. 10.1016/S0065-308X(06)63003-0. [DOI] [PubMed] [Google Scholar]
- Smant, G. and Jones, J. (2011) Suppression of plant defences by nematodes In: Genomics and Molecular Genetics of Plant‐Nematode Interactions Jones J., Gheysen G. and Fenoll C., eds.), pp. 273–286. Berlin: Springer‐Verlag. [Google Scholar]
- Spiegel, Y. and McClure, M.A. (1991) Stage‐specific differences in lectin binding to the surface of Anguina tritici and Meloidogyne incognita . J. Nematol. 23, 259–263. PMC2619147. [PMC free article] [PubMed] [Google Scholar]
- Spiegel, Y. and McClure, M.A. (1995) The surface coat of plant‐parasitic nematodes: chemical composition, origin, and biological role – a review. J. Nematol. 27, 127–134 PMC2619597. [PMC free article] [PubMed] [Google Scholar]
- Spiegel, Y. , Sharon, E. , Kahane, I. and McClure, M.A. (1991) Surface binding of red blood cells to different nematodes. J. Nematol. 23, 551 PMC2619597. [Google Scholar]
- Stirling, G.R. (2014) The Biological Control of Plant‐Parasitic Nematodes: Soil Ecosystem Management and Sustainable Agriculture. Wallingford, Oxfordshire: CABI. [Google Scholar]
- Strous, G.J. and Dekker, J. (1992) Mucin‐type glycoproteins. Crit. Rev. Biochem. Mol. Biol. 27, 57–92. 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Sylvestre, P. , Couture‐Tosi, E. and Mock, M. (2003) Polymorphism in the collagen‐like region of the Bacillus anthracis BclA protein leads to variation in length in the exosporium filament length. J. Bacteriol. 185, 5155–5163. 10.1128/JB.185.5.1555-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre, P. , Couture‐Tosi, E. and Mock, M. (2005) Contribution of ExsFA and ExsFB proteins to the localisation of BclA on the spore surface and to the stability of the Bacillus anthracis exosporium. J. Bacteriol. 187, 5122–5128. 10.1128/JB.187.15.5122-5128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoropoulos, G. , Hicks, S.J. , Corfield, A.P. , Miller, B.G. and Carrington, S.D. (2001) The role of mucins in host–parasite interactions: part II – helminth parasites. Trends Parasitol. 17, 130–135. PMID: 11286796. [DOI] [PubMed] [Google Scholar]
- Umarao, Tyagi, N. , Sharma, A. , Kamaraju, D. , Banakar, P. and Rao, S.B. (2013) Soil less medium for culturing and multiplication of root knot and cyst nematodes for basic and applied studies. Ann. Pl Prot. Sci. 21, 148–153. [Google Scholar]
- Urwin, P.E. , Lilley, C.J. and Atkinson, H.J. (2002) Ingestion of double‐stranded RNA by pre‐parasitic juvenile cyst nematodes leads to RNA interference. Mol. Plant–Microbe Interact. 15, 747–752. 10.1094/MPMI.2002.15.8.747. [DOI] [PubMed] [Google Scholar]
- Whitehead, A.G. and Hemming, J.R. (1965) A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann. Appl. Biol. 55, 25–38. 10.1111/j.1744-7348.1965.tb07864.x. [DOI] [Google Scholar]
- Winzler, R.J. , Harris, E.D. , Pekas, D.J. , Johnson, C.A. and Weber, P. (1967) Studies on glycopeptides released by trypsin from intact human erythrocytes. Biochemistry, 6, 2195–2202. PMID: 6049454. [DOI] [PubMed] [Google Scholar]
- Wright, K.A. (1987) The nematode's cuticle: its surface and the epidermis: function, homology, analogy: a current consensus. J. Parasitol. 73, 1077–1083. PMID: 3325620. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 The hybridization of digoxigenin (DIG)‐labelled sense (A) and antisense (B) cDNA probe of Mi‐muc‐1 showed no signalling in the anterior body part of Meloidogyne incognita (scale bar, 20 µm).
Fig. S2 The in situ hybridization result for MucX. The result showed no signalling in the anterior body part of Meloidogyne incognita with digoxigenin (DIG)‐labelled sense (A) and antisense (B) probes. The hybridization of antisense DIG‐labelled cDNA probe showed the location of mRNA expression in the phasmid area of the tail region (D) as compared with the control (C) (scale bar, 20 µm).
Fig. S3 The in situ hybridization result for MucY. The result showed no signalling in the anterior body part of Meloidogyne incognita with digoxigenin (DIG)‐labelled sense (A) and antisense (B) probes. The hybridization of antisense DIG‐labelled cDNA probe showed the location of mRNA expression in the phasmid area of the tail region (D) as compared with the control (C). However, some non‐specific signals were also detected in the posterior body part (D) (scale bar, 20 µm).
Fig. S4 (a) Sequence information of putative Mi‐muc‐1 homologues. (b) CLUSTAL 2.1 multiple sequence alignment. (c) Percent identity matrix (created by Clustal 2.1). (d) Quantitative real‐time polymerase chain reaction (qRT‐PCR) analysis shows no transcriptional alteration of MucX and MucY on downregulation of Mi‐muc‐1 (figure below) [double‐stranded green fluorescent protein (dsGFP) was used as a non‐native negative control].
Table S1 The NetOGlyc prediction result. The output conforms to the GFF version 2 format (https://www.cbs.dtu.dk/services/NetOGlyc/). For the input sequence Mi‐MUC‐1, the server has provided a list of potential glycosylation sites, showing their positions in the sequence and the prediction confidence scores. Only the sites with scores higher than 0.5 are predicted as glycosylated and marked with the string ‘#POSITIVE’ in the comment field.
Table S2 List of mucin‐like proteins of Caenorhabditis elegans.


