Summary
Cacao swollen shoot virus (CSSV) is a major pathogen of cacao (Theobroma cacao) in Africa, and long‐standing efforts to limit its spread by the culling of infected trees have had very limited success. CSSV is a particularly difficult virus to study, as it has a very narrow host range, limited to several tropical tree species. Furthermore, the virus is not mechanically transmissible, and its insect vector can only be used with difficulty. Thus, the only efficient means to infect cacao plants that have been experimentally described so far are by particle bombardment or the agroinoculation of cacao plants with an infectious clone. We have genetically transformed three non‐host species with an infectious form of the CSSV genome: two experimental hosts widely used in plant virology (Nicotiana tabacum and N. benthamiana) and the model species Arabidopsis thaliana. In transformed plants of all three species, the CSSV genome was able to replicate, and, in tobacco, CSSV particles could be observed by immunosorbent electron microscopy, demonstrating that the complete virus cycle could be completed in a non‐host plant. These results will greatly facilitate the preliminary testing of CSSV control strategies using plants that are easy to raise and to transform genetically.
Keywords: Badnavirus, cacao, Cacao swollen shoot virus, pararetrovirus, virus replication
Cacao swollen shoot virus (CSSV) is a member of the genus Badnavirus, family Caulimoviridae, and infects naturally only cacao (Theobroma cacao) and a few other tree species, such as baobab (Adansonia digitata), kapok (Ceiba pentandra) and cola (Cola gigantea, C. chlamydanta). Its genome is composed of a circular double‐stranded DNA molecule of approximately 7.1 kb enclosed in unenveloped bacilliform particles of variable length (for a review, see Muller, 2008). As with all pararetroviruses, the CSSV genome replicates via an RNA intermediate, which serves as a template for the synthesis of double‐stranded DNA progeny genomes, the same RNA serving as the mRNA encoding the viral proteins. Related badnaviruses infect other important tropical crops, such as banana, sugarcane, yam, pepper and pineapple (Borah et al., 2013). CSSV is a serious problem for the production of chocolate and related products, as it has a major impact on cocoa production in West Africa, and the eradication programmes currently used as control measures have had very limited effectiveness (Dzahini‐Obiatey et al., 2006). Plant breeding approaches to address the problem have not proved to be promising so far, as, to the best of our knowledge, there is no resistant cacao germplasm that can be used in classical breeding programmes. Cacao genotypes with a varying level of tolerance exist, but the tolerance is difficult to study, as it appears to be polygenic, and its expression is strongly influenced by environmental conditions (Adomako et al., 2006). These factors make natural genetic tolerance problematic to integrate into cacao molecular breeding programmes.
Furthermore, CSSV has proven to be a difficult virus to study. Viral particles are difficult to purify from infected cacao tissues and, as a result, the CSSV genome was only cloned and sequenced in 1993 (Hagen et al., 1993). The development of more efficient full‐length cloning strategies subsequently led to the cloning and sequencing of the genome of five additional CSSV strains (Muller and Sackley, 2005). In addition, artificial infection of cacao plants is relatively difficult (Hagen et al., 1994; Jacquot et al., 1999), as is transmission by natural vector mealybugs (Roivainen, 1976), and there are no known easily infected herbaceous hosts.
A recombinant plasmid vector bearing 1.2 copies of the CSSV genome has been used previously to infect cacao seeds by particle bombardment (Hagen et al., 1994), and the same 1.2‐copy construct in a vector for Agrobacterium‐mediated plant transformation, pBCPX2, has been used to infect cacao seedlings by inoculating the bacterium into the stems of young seedlings (Jacquot et al., 1999). To the best of our knowledge, these techniques have not been attempted on non‐host species. We have used the same vector (Fig. 1A) to infect cacao by rub inoculating seeds with a suspension of Agrobacterium bearing pBCPX2. The seed coat was removed from fresh cacao seeds immediately after their removal from the pod, and the exposed cotyledons were rubbed briefly with carborundum. The rubbed seeds were then dipped in a suspension of Agrobacterium, prepared as described by Jacquot et al. (1999), and the plants were grown in the glasshouse. As shown in Fig. 1C, the agro‐inoculated cacao plants displayed typical disease symptoms. These plants were used as positive controls in further experiments.
Figure 1.
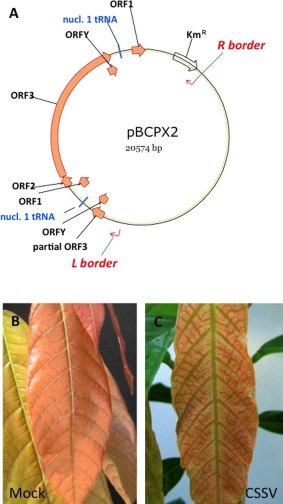
Infection of cacao plants with Cacao swollen shoot virus (CSSV). (A) Structure of the plasmid pBCPX2 which was used for Agrobacterium‐mediated infection of cacao and stable transformation of non‐host plant species. The open reading frames (ORFs) of the longer than unit‐length copy of the CSSV genome are shown in orange. Not shown to scale. Young leaves of mock‐inoculated (B) and infected (C) cacao plants, 7 weeks after inoculation.
The same Agrobacterium strain bearing pBCPX2 was used to stably transform tobacco (Nicotiana tabacum Xanthi XHFD8) according to standard protocols (Horsch et al., 1985). As pBCPX2 also includes an nptII kanamycin resistance gene, transformed plants were selected on kanamycin‐containing medium. DNA blot hybridization was used in a manner that made it possible to distinguish the longer than unit‐length copy of the CSSV genome stably integrated in the plant genome from unit‐length episomal circular copies of the CSSV genome produced by replication from the integrated copies. Figure 2A shows the structure of the T‐DNA that was expected to be integrated in the transformed tobacco genomes, and the cleavage sites of three restriction enzymes that cut only once in the CSSV genome. Digoxigenin (DIG)‐labelled DNA probes corresponding to an 881‐nucleotide fragment of the CSSV genome were synthesized using the PCR DIG Probe Synthesis Kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer's instructions, using pBCPX2 as template and primers CSSV4068+ (TGTGCCCCAATATACTGCAA) and CSSV4949– (ACAATCTGCTGGTGGGTTTC). When transformed plant DNA is digested with NdeI, hybridization with the probe, which anneals at the position indicated in Fig. 2A, will reveal the circular CSSV molecules as linearized 7.1‐kb fragments, and also right‐border fragments of various sizes composed of both the remaining portion of the T‐DNA and DNA of the flanking sequences in the plant genome. Of the 11 transformed tobacco lines tested (Fig. 2B), X5 and X8 displayed a fragment that migrated at the same position as the positive control‐infected cacao DNA sample. This suggests the presence of episomal CSSV DNA, but does not formally exclude the possibility that the 7.1‐kb bands could also be border fragments including integrated and plant DNA. In order to clarify this point, DNA of lines X5 and X8 was also digested with NsiI and SphI, which both cut the CSSV genome once, but also cut the inserted T‐DNA between the CSSV sequence and the nptII gene (Fig. 2A). When NsiI‐ or SphI‐digested DNA of X5 and X8 was probed (Fig. 2C), the 7.1‐kb band was still observed, as well as the expected 4.0‐ and 5.2‐kb bands digested from the T‐DNA. The additional smaller bands in X8 suggest the presence of a second T‐DNA copy. These results confirm that the CSSV genome replicated in tobacco lines X5 and X8 to produce episomal circular viral genomes.
Figure 2.
Presence of linearized episomal Cacao swollen shoot virus (CSSV) genomic DNA in transgenic tobacco (Nicotiana tabacum) lines. (A) Schematic diagram of the T‐DNA of pBCPX2, which can be transferred by Agrobacterium to plant genomes, showing the longer than unit‐length copy of the CSSV genome (orange arrow) and the kanamycin resistance marker gene (white arrow). (B) DNA from transgenic tobacco lines X1–X11 was digested with NdeI, which cleaves the CSSV genome once; hybridization with the CSSV‐specific probe revealed 7.1‐kb DNA corresponding to episomal CSSV DNA and right border fragments of different sizes. (C) DNA of lines X5 and X8 was digested with NdeI, NsiI or SphI, which cleave the CSSV genome once. Hybridization revealed episomal CSSV DNA and fragments of other sizes corresponding to border or internal fragments. Cacao +, CSSV‐infected cacao plant; wt, wild‐type non‐transformed plant.
Infection was further demonstrated by immunosorbent electron microscopy (ISEM) detection of CSSV particles, using the method described by Jacquot et al. (1999). As shown in Fig. 3, scattered particles were detected in extracts of tobacco X8 plants, which were indistinguishable from authentic CSSV particles observed in cacao plants infected with the same 1.2‐copy clone, either by bombardment or agro‐inoculation (Hagen et al., 1994; Jacquot et al., 1999). Although this evidence shows that CSSV can complete its replication cycle, including virion formation, none of the tobacco plants expressed symptoms of infection.
Figure 3.
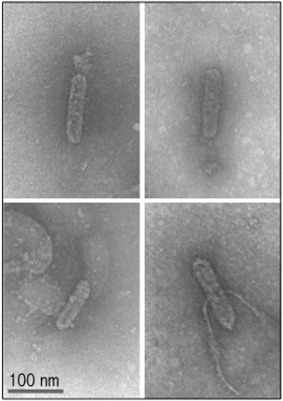
Observation of Cacao swollen shoot virus (CSSV) particles in transgenic tobacco (Nicotiana tabacum) line X8. Immunosorbent electron microscopy (ISEM) observation of CSSV particles in an extract of transgenic tobacco line X8.
In order to determine whether the replication of CSSV in tobacco plants was specific to that species, we used standard protocols to transform two additional species: Nicotiana benthamiana, widely used as a model species in plant virology, and Arabidopsis thaliana Col0, the dominant model for studies in plant molecular genetics. As shown in Fig. 4A, N. benthamiana lines B24, B25 and B28 clearly accumulated CSSV episomal DNA, similar to tobacco lines X5 and X8, and traces were observed in line B29. Similar levels of accumulation of 7.1‐kb CSSV DNA were observed in Arabidopsis lines A1, A3 and A4 (Fig. 4B).
Figure 4.
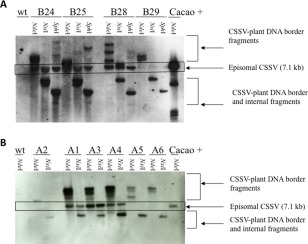
Presence of linearized episomal Cacao swollen shoot virus (CSSV) genomic DNA in transgenic Nicotiana benthamiana and Arabidopsis thaliana lines. (A) DNA from transgenic N. benthamiana lines B24, B25, B28 and B29 was digested with NdeI, NsiI or SphI, which cleave the CSSV genome once. Hybridization with the CSSV‐specific probe revealed 7.1‐kb DNA corresponding to linearized episomal CSSV DNA and fragments of other sizes corresponding to border or internal fragments. (B) DNA from transgenic A. thaliana lines A1 to A6 was digested with NdeI or NsiI, which cleave the CSSV genome once. Hybridization was as in (A). Cacao+, CSSV‐infected cacao plant; wt, wild‐type non‐transformed plant.
The ability to replicate the CSSV genome was inherited in progeny plants (Table 1). With a few exceptions, in all tobacco and N. benthamiana lines, the segregation of kanamycin resistance was compatible with a single site of insertion into the plant genome. There was clearly more than one copy in tobacco line X10 and, as N. benthamiana lines B25, B28 and B29 were sterile, their progeny could not be evaluated. Line B6 was of particular interest, as only 8% of the progeny were kanamycin resistant. This probably suggests transcriptional silencing of the transgene (Vermeersch et al., 2013), which is also compatible with there being a large number of copies inserted, as evidenced by large numbers of border fragments observed in DNA blot hybridizations (not shown).
Table 1.
Genetic and molecular characterization of Nicotiana tabacum and N. benthamiana lines transformed with potentially infectious Cacao swollen shoot virus (CSSV) DNA.
| Line number | KmR T1 progeny (%) | Number of integration sites | Relative level of accumulation of CSSV DNA | |
|---|---|---|---|---|
| N. tabacum Xanthi | X1 | 72 | 1 | + |
| X2 | 77 | 1 | – | |
| X3 | 78 | 1 | – | |
| X4 | 70 | 1 | + | |
| X5 | 74 | 1 | ++ | |
| X6 | 72 | 1 | – | |
| X7 | 75 | 1 | – | |
| X8 | 74 | 1 | ++/+++ | |
| X9 | 73 | 1 | – | |
| X10 | 94 | 2 | – | |
| X11 | 73 | 1 | – | |
| N. benthamiana | B1 | 77 | 1 | – |
| B2 | 74 | 1 | – | |
| B5 | 79 | 1 | – | |
| B6 | 8 | >1 | – | |
| B7 | 63 | 1 | – | |
| B8 | 77 | 1 | – | |
| B9 | 80 | 1 | – | |
| B10 | 72 | 1 | – | |
| B16 | 75 | 1 | – | |
| B20 | 75 | 1 | – | |
| B21 | 78 | 1 | – | |
| B24 | 66 | 1 | ++ | |
| B25 | ND | ND | ++ | |
| B28 | ND | ND | ++ | |
| B29 | ND | ND | +/− |
KmR, kanamycin resistant; ND, not determined, T1 plants sterile.
The possibility that the nptII gene was transcriptionally silenced in line B6 raises the interesting question of why a relatively small proportion of the tobacco and N. benthamiana lines accumulated detectable levels of CSSV episomal DNA. Only further study will show whether this is a result of silencing at the transcriptional or post‐transcriptional level.
We have shown that CSSV can complete its cycle in non‐host plants of the Brassicaceae and Solanaceae families when a longer than unit‐length copy of the viral genome is stably inserted into the non‐host genome. This suggests that non‐host cells contain all of the factors necessary for the CSSV replication cycle and, by elimination, indicates that one of the major determinants of the CSSV host range is the ability of the virus to move within its host. As none of the plant lines expressing the CSSV genome displayed any physical abnormalities, the species tested may lack factors related to symptom expression, or perhaps the level of activity of the virus was insufficient to cause them.
The plants described here should prove to be useful for several approaches to aid in our further understanding of CSSV. They could be used as a platform to test the ability of various possible virus resistance transgene strategies (Prins et al., 2008; Tenllado et al., 2004; Tyagi et al., 2008) to confer protection against CSSV. Another interesting possibility would be to test whether protection against CSSV can be conferred by the inoculation of plants with DNA or RNA, as demonstrated by Pooggin et al. (2003) for single‐stranded DNA plant viruses.
Acknowledgements
We thank Isabelle Bornard for the immunosorbent electron microscopy of Cacao swollen shoot virus particles and P.‐Y. Teycheney for providing cacao pods. This research was supported in part by the Fondazione Cassamarca. The authors declare no conflicts of interest.
References
- Adomako, B. , Adu‐Ampomah, Y. and Ollennu, L.L.A. (2006) Evaluation of resistance to Cacao swollen shoot virus (CSSV): methods, problems and selections In: Global Approaches to Cocoa Germplasm Utilization and Conservation (Eskes A.B. and Efron Y., eds), pp. 208–216. Amsterdam: Common Fund for Commodities (CFC); London: International Cocoa Organization (ICCO); Rome: International Plant Genetic Resources Institute (IPGRI).
- Borah, B.K. , Sharma, S. , Kant, R. , Johnson, A.A.M. , Saigopal, D.V.R. and Dasgupta, I. (2013) Bacilliform DNA‐containing plant viruses in the tropics: commonalities within a genetically diverse group. Mol. Plant Pathol. 14, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzahini‐Obiatey, H. , Akumfi Ameyaw, G. and Ollennu, L.L.A. (2006) Control of cocoa swollen shoot disease by eradicating infected trees in Ghana: a survey of treated and replanted areas. Crop Prot. 25, 647–652. [Google Scholar]
- Hagen, L. , Jacquemond, M. , Lépingle, A. , Lot, H. and Tepfer, M. (1993) Nucleotide sequence and genomic organization of cacao swollen shoot virus. Virology, 196, 619–628. [DOI] [PubMed] [Google Scholar]
- Hagen, L. , Lot, H. , Godon, C. , Tepfer, M. and Jacquemond, M. (1994) Infection of Theobroma cacao using cloned DNA of cacao swollen shoot virus and particle bombardment. Phytopathology, 84, 1239–1243. [Google Scholar]
- Horsch, R.B. , Fry, J.E. , Hoffmann, N.L. , Eicholtz, D. , Rogers, S.G. and Fraley, R.T. (1985) A simple and general method for transferring genes into plants. Science, 227, 1229–1231. [DOI] [PubMed] [Google Scholar]
- Jacquot, E. , Hagen, L.S. , Michler, P. , Rohfritsch, O. , Stussi‐Garaud, C. , Keller, M. , Jacquemond, M. and Yot, P. (1999) In situ localization of cacao swollen shoot virus in agroinfected Theobroma cacao . Arch. Virol. 144, 259–271. [DOI] [PubMed] [Google Scholar]
- Muller, E. (2008) Cacao swollen shoot virus In: Characterization, Diagnosis and Management of Plant Viruses, Vol. 1 (Rao G.P., Khurana S.M.P. and Lenardon S.L., eds), pp. 423–444. Houston, TX: Stadium Press. [Google Scholar]
- Muller, E. and Sackley, S. (2005) Molecular variability analysis of five new complete cacao swollen shoot virus genomic sequences. Arch. Virol. 150, 53–66. [DOI] [PubMed] [Google Scholar]
- Pooggin, M. , Shivaprasad, P.V. , Veluthambi, K. and Hohn, T. (2003) RNAi targeting of DNA virus in plants. Nat. Biotechnol. 21, 131–132. [DOI] [PubMed] [Google Scholar]
- Prins, M. , Laimler, M. , Noris, E. , Schubert, J. , Wassenegger, M. and Tepfer, M. (2008) Strategies for antiviral resistance in transgenic plants. Mol. Plant Pathol. 9, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roivainen, O. (1976) Transmission of cacao viruses by mealybugs (Homoptera: Pseudococcidae). J. Sci. Agric. Soc. Finland (Helsinki), 48, 203–304. [Google Scholar]
- Tenllado, F. , Llave, C. and Díaz‐Ruíz, J.R. (2004) RNA interference as a new biotechnological tool for the control of virus diseases in plants. Virus Res. 102, 85–96. [DOI] [PubMed] [Google Scholar]
- Tyagi, H. , Rajasubramaniam, S. , Rajam, M.V. and Dasgupta, I. (2008) RNA‐interference in rice against Rice tungro bacilliform virus results in its decreased accumulation in inoculated rice plants. Transgenic Res. 17, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeersch, L. , De Winne, N. , Holf, J. , Bleys, A. , Kovarik, A. and Depicker, A. (2013) Transitive RNA silencing signals induce cytosine methylation of a transgenic but not an endogenous target. Plant J. 74, 867–879. [DOI] [PubMed] [Google Scholar]


