Summary
Mitogen‐activated protein kinase (MAPK) cascades mediate cellular responses to environmental signals. Previous studies in the fungal pathogen Fusarium oxysporum have revealed a crucial role of Fmk1, the MAPK orthologous to Saccharomyces cerevisiae Fus3/Kss1, in vegetative hyphal fusion and plant infection. Here, we genetically dissected the individual and combined contributions of the three MAPKs Fmk1, Mpk1 and Hog1 in the regulation of development, stress response and virulence of F. oxysporum on plant and animal hosts. Mutants lacking Fmk1 or Mpk1 were affected in reactive oxygen species (ROS) homeostasis and impaired in hyphal fusion and aggregation. Loss of Mpk1 also led to increased sensitivity to cell wall and heat stress, which was exacerbated by simultaneous inactivation of Fmk1, suggesting that both MAPKs contribute to cellular adaptation to high temperature, a prerequisite for mammalian pathogens. Deletion of Hog1 caused increased sensitivity to hyperosmotic stress and resulted in partial rescue of the restricted colony growth phenotype of the mpk1Δ mutant. Infection assays on tomato plants and the invertebrate animal host Galleria mellonella revealed distinct and additive contributions of the different MAPKs to virulence. Our results indicate that positive and negative cross‐talk between the three MAPK pathways regulates stress adaptation, development and virulence in the cross‐kingdom pathogen F. oxysporum.
Keywords: cross‐talk, development, Fusarium oxysporum, MAPKs, stress response, virulence
Introduction
Fungal pathogens can sense and respond to a wide variety of stimuli from the environment and their host organisms. Among the key pathways functioning in the molecular perception of these cues are mitogen‐activated protein kinase (MAPK) cascades, a family of evolutionarily conserved three‐tiered protein kinase modules composed of a MAPK kinase kinase (MAPKKK) that phosphorylates the downstream MAPK kinase (MAPKK), which, in turn, activates the MAPK for the downstream transmission of cellular signals (Turrà et al., 2014; Widmann et al., 1999). In the budding yeast Saccharomyces cerevisiae, five MAPKs have been reported, Fus3, Kss1, Mpk1/Slt2, Hog1 and Smk1, which regulate pheromone response, filamentation and invasive growth, cell wall integrity, high‐osmolarity stress response and spore wall assembly, respectively (Chen and Thorner, 2007). By contrast, most ascomycete fungi only possess three MAPKs which are orthologous to Fus3/Kss1, Mpk1 and Hog1 (Turrà et al., 2014).
Components of the Fus3/Kss1 MAPK cascade are broadly conserved regulators of infection‐related morphogenesis and invasive growth in plant‐pathogenic fungi. For example, in the rice pathogen Magnaporthe oryzae, deletion of the orthologous MAPK Pmk1 leads to impaired formation of appressoria and loss of virulence (Xu and Hamer, 1996). Similarly, mutants in the orthologous MAPKs of phylogenetically distant phytopathogens, including both appressoria‐forming and appressoria‐non‐forming species, fail to infect the host even when applied directly on wounded plant tissues (Di Pietro et al., 2001; Jenczmionka et al., 2003; Lev et al., 1999; Mey et al., 2002b; Ruiz‐Roldán et al., 2001; Takano et al., 2000). Thus, loss of virulence in these mutants is caused by a general defect in invasive growth and host penetration.
The MAPK Mpk1 is required for fungal cell wall remodelling and integrity, which are critical during cell cycle progression and in response to cell wall stress. Both processes are highly relevant for fungal pathogens during interaction with their host (Brown et al., 2014; Levin, 2005). Deletion of mpk1 orthologues in different pathogen species results in hypersensitivity to cell wall‐damaging agents, such as Congo red (CR) or calcofluor white (CFW), as well as to plant defence compounds, such as chitinases, glucanases, antimicrobial peptides or phytoalexins (Hou et al., 2002; Joubert et al., 2011; Mehrabi et al., 2006; Mey et al., 2002a; Ramamoorthy et al., 2007; Valiante et al., 2015; Xu et al., 1998). Importantly, mpk1 mutants also show defects in host sensing, penetration and colonization, leading to decreased virulence on plant and animal hosts (Diez‐Orejas et al., 1997; Igbaria et al., 2008; Kojima et al., 2002; Kraus et al., 2003; Mey et al., 2002a; Rui and Hahn, 2007; Turrà et al., 2015; Xu et al., 1998). In addition to pathogenicity, the Mpk1 cascade regulates developmental processes, such as hyphal growth on solid surfaces and vegetative hyphal fusion (Hou et al., 2002; Maerz et al., 2008; Turrà et al., 2015; Xu et al., 1998).
The third fungal MAPK, Hog1 (high‐osmolarity glycerol), plays a pivotal role in the adaptive response to hyperosmotic stress and mediates sensitivity to certain fungicides, such as phenylpyrroles and dicarboximides (Dixon et al., 1999; Igbaria et al., 2008; Lin and Chung, 2010; Park et al., 2004; Segmuller et al., 2007; Van Thuat et al., 2012). Hog1 orthologues contribute to virulence in a number of phytopathogens (Igbaria et al., 2008; Turrà et al., 2014; Van Thuat et al., 2012), but are dispensable in others (Dixon et al., 1999). Importantly, they appear to be universally required for infection in fungal pathogens of mammals and insects, as reported in the dimorphic fungi Cryptococcus neoformans and Candida albicans, in which Hog1 controls morphogenetic differentiation and resistance to phagocytic killing (Alonso‐Monge et al., 1999; Arana et al., 2007; Bahn et al., 2005; Jin et al., 2012). However, the exact role of Hog1 in fungal virulence is largely unknown.
The soil‐inhabiting ascomycete Fusarium oxysporum causes vascular wilt disease in a large number of field and glasshouse crops, leading to economically important losses worldwide (Dean et al., 2012). Fusarium oxysporum has also been reported as an emerging human pathogen that can cause lethal systemic infections in immunocompromised individuals (Nucci and Anaissie, 2007). Previous work has established that Fmk1, the F. oxysporum MAPK orthologous to S. cerevisiae Fus3/Kss1, as well as its downstream transcription factor Ste12, are essential for invasive hyphal growth and plant infection (Di Pietro et al., 2001; Rispail and Di Pietro, 2009). Moreover, Fmk1 regulates a number of virulence‐related processes, such as hyphal adhesion, vegetative hyphal fusion, the production of plant cell wall‐degrading enzymes (CWDEs) and the chemotropic sensing of nutrients (Di Pietro et al., 2001; Prados‐Rosales and Di Pietro, 2008; Turrà and Di Pietro, 2015; Turrà et al., 2015). Interestingly, despite the critical role in plant infection, Fmk1, as well as its orthologues in other fungal pathogens, are largely dispensable for virulence on mammalian and invertebrate hosts (Csank et al., 1998; Davidson et al., 2003; Navarro‐Velasco et al., 2011; Ortoneda et al., 2004).
Previous studies performed in a number of species suggested that fungal MAPK cascades have broadly conserved, but also species‐specific, functions in pathogenicity. The aim of the present study was to systematically dissect the role of the three MAPKs Fmk1, Mpk1 and Hog1 in development, stress response and virulence of F. oxysporum on plant and animal hosts. In order to investigate putative cross‐talk interactions, a set of mutant strains was generated in which the different MAPK genes were deleted either individually or in combination. Our results reveal both unique and shared functions of F. oxysporum Fmk1, Mpk1 and Hog1, and suggest that the three MAPKs contribute in a coordinate manner to the remarkable physiological and pathogenic versatility of this cross‐kingdom fungal pathogen.
Results
The three F. oxysporum MAPKs differentially contribute to cell wall, hyperosmotic, oxidative and heat stress responses
To explore the biological roles of the three MAPKs Fmk1, Mpk1 and Hog1, we obtained single‐ and double‐deletion mutants in all possible combinations. As F. oxysporum Fmk1 and Mpk1 had been identified in previous studies, the corresponding single and double mutants were already available (Di Pietro et al., 2001; Turrà et al., 2015). blastp analysis of the F. oxysporum genome database identified a single hog1 orthologue, FOXG_06318, encoding a predicted 358‐amino‐acid protein showing 77% identity with S. cerevisiae Hog1. The hog1Δ single and double mutants were obtained by replacing the entire coding region with the hygromycin resistance cassette in the wild‐type and fmk1Δ background or with the phleomycin resistance cassette in the mpk1Δ background (Fig. S1A, see Supporting Information). Polymerase chain reaction (PCR) analysis with gene‐specific primers identified several putative transformants producing amplification patterns indicative of homologous gene replacement. Southern blot analysis of these transformants confirmed the replacement of a 10‐kb EcoRI fragment, corresponding to the wild‐type hog1 allele, with a fragment of 7 or 5.5 kb in the wild‐type and fmk1Δ background (Fig. S1B,C) or mpk1Δ background (Fig. S1D), respectively. Complemented strains (hog1Δ+hog1) were obtained by re‐insertion of a 5‐kb DNA fragment encompassing the complete hog1 gene into protoplasts of the hog1Δ strain by co‐transformation with the phleomycin resistance marker. PCR with gene‐specific primers yielded an amplification product identical to that obtained from the wild‐type strain in five independent transformants, but not in the hog1Δ mutant, suggesting that these strains re‐integrated an intact copy of the gene (Fig. S1E).
To test the role of the different MAPK pathways in vegetative hyphal growth, the colony diameter was measured in two different media: nutrient‐poor minimal medium (MM) and nutrient‐rich YPD (3% yeast extract, 1% peptone, 2% glucose and 1.5% agar) medium. The fmk1Δ and mpk1Δ mutants exhibited a significantly slower growth rate at the optimum temperature (28 °C), particularly on MM, and this phenotype was exacerbated in the fmk1Δ mpk1Δ double mutant (Fig. S2A–C, see Supporting Information). At sublethal high temperature (34 °C), hyphal growth was severely restricted in all fungal strains, but loss of Hog1 or Mpk1, either alone or in combination with any of the other MAPKs, exacerbated the growth defect at high temperature (Fig. 1A,B).
Figure 1.
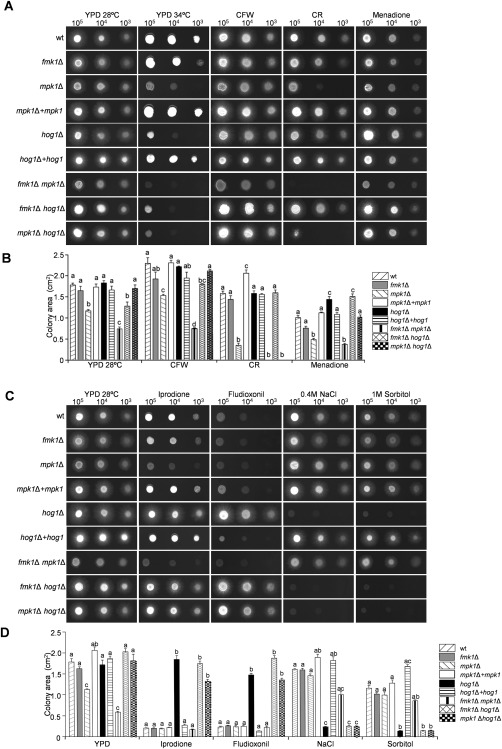
Stress response phenotypes of Fusarium oxysporum mitogen‐activated protein kinase (MAPK) mutants. (A, C) Colony phenotypes of the indicated strains grown on YPD (3% yeast extract, 1% peptone, 2% glucose and 1.5% agar) medium at different temperatures (28 or 34 ºC) or at 28 ºC in the presence of the indicated compounds. Plates were spot inoculated with the indicated number of microconidia, incubated for 2 days at 28 ºC or 4 days at 34 ºC, and scanned. Bar, 1 cm. (B, D) Quantitative analysis of the colony area (cm2) performed using MultiGauge software. Data were analysed and plotted with the program GraphPad Prism version 5. Error bars represent standard deviations of colony areas calculated from four independent replicates. Values with the same letter are not significantly different according to Dunnett's multiple comparison test (P ≤ 0.05). CFW, calcofluor white; CR, Congo red; wt, wild‐type.
In an earlier study, increased sensitivity of the F. oxysporum mpk1Δ mutant towards the cell wall‐targeting compounds CFW and CR was reported (Turrà et al., 2015). Here, we confirmed this phenotype which clearly distinguished mpk1Δ from fmk1Δ and hog1Δ (Fig. 1A,B). Interestingly, double deletion of mpk1 and fmk1 genes resulted in an additional growth reduction compared with either of the single mutants, suggesting that both MAPKs contribute independently to the cell wall stress response. Furthermore, the fmk1Δ hog1Δ and mpk1Δ hog1Δ double mutants were more sensitive to cell wall stress compounds and showed increased phosphorylation of Mpk1 and Fmk1 MAPKs, respectively, compared with the single mutants (Figs 1A,B and 2A–C). However, this phenotype was only visible on CFW for the fmk1Δ hog1Δ and on CR for the mpk1Δ hog1Δ strain. Interestingly, loss of hog1 in the mpk1Δ background largely rescued the colony growth defect on YPD, both in the absence and presence of CFW (Fig. 1A,B).
Figure 2.
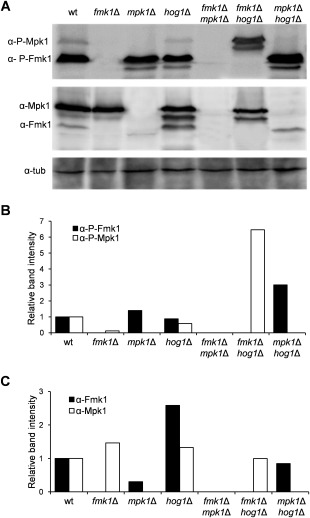
Phosphorylation of Fmk1 and Mpk1 is altered in mitogen‐activated protein kinase (MAPK) deletion mutants of Fusarium oxysporum. (A) Protein extracts from fungal mycelium of the indicated strains were subjected to immunoblot analysis with anti‐phospho‐p44/42 MAPK antibody (α‐P‐Fmk1 and α‐P‐Mpk1) or anti‐Fus3 and anti‐Mpk1 antibodies (α‐Fmk1 and α‐Mpk1). Anti‐α‐tubulin antibody (α‐tub) was used as loading control. (B, C) The graph shows the ratio of P‐Fmk1 and P‐Mpk1 (B) or Fmk1 and Mpk1 (C) band intensities normalized to the tubulin control and expressed relative to wild‐type levels.
In response to oxidative stress caused by menadione, contrasting effects on colony growth were observed in the different mutants (Fig. 1A,B). Whereas loss of Mpk1 resulted in significant growth reduction, the mutants lacking Hog1 showed enhanced growth under these conditions. In line with this, growth of the mpk1Δ hog1Δ double mutant was comparable with that of the wild‐type strain, suggesting a compensatory effect of the two mutations.
As reported previously in a number of fungal pathogens (Dixon et al., 1999; Igbaria et al., 2008; Lin and Chung, 2010; Park et al., 2004; Segmuller et al., 2007; Van Thuat et al., 2012), single and double mutants lacking Hog1 displayed increased resistance to the dicarboximide and phenylpyrrole fungicides iprodione and fludioxonil, and were highly sensitive to hyperosmotic stress (Fig. 1C,D). Unexpectedly, the fmk1Δ mpk1Δ double mutant showed an opposite phenotype, namely decreased resistance to the fungicides and increased resistance to hyperosmotic stress (Fig. 1C,D). These results suggest that Fmk1 and Mpk1 may act antagonistically to Hog1. This idea was further supported by the finding that hyperosmotic stress, which activates the Hog1 cascade, reversed the restricted colony growth phenotype of the mpk1Δ and fmk1Δ mpk1Δ mutants (Fig. 1C,D).
Reactive oxygen species (ROS) production in the different fungal strains was examined by staining the fungal colony with 4‐nitroblue tetrazolium chloride (NBT). In the wild‐type strain, formazan precipitates, which are indicative of the production of superoxide, were typically more intense at the colony periphery and more abundant on nutrient‐rich medium (Fig. S2B). By contrast, the fmk1Δ and mpk1Δ single and double mutants showed a different pattern, with higher superoxide production at the colony centre. The same phenotype was found in fmk1Δ hog1Δ and mpk1Δ hog1Δ double mutants. Detailed observation of the colony margin confirmed the presence of high ROS levels, particularly on rich medium, in fmk1Δ and mpk1Δ, but not in the hog1Δ mutant (Fig. S2B). These results suggest that the different MAPK pathways play distinct roles in the regulation of ROS generation during hyphal development and colony establishment.
Fmk1 and Mpk1 are required for vegetative hyphal fusion and formation of mycelial aggregates
A previous study has revealed that Fmk1 is required for vegetative hyphal fusion of F. oxysporum (Prados‐Rosales and Di Pietro, 2008). In line with this, we found that fmk1Δ strains were impaired in hyphal fusion (Table 1). In addition, mpk1Δ mutants also failed to undergo hyphal fusion, as did all the double mutants lacking either one or both of these MAPKs. By contrast, hog1Δ mutants performed hyphal fusion as efficiently as the wild‐type strain. Moreover, the addition of specific Hog1 activators or inhibitors to the wild‐type strain had no significant effect on the hyphal fusion rate (Fig. S3A–C, see Supporting Information). Taken together, these results indicate that Hog1 is not involved in the regulation of vegetative hyphal fusion.
Table 1.
Fmk1 and Mpk1, but not Hog1, are required for vegetative hyphal fusion (VHF) in Fusarium oxysporum.
| Strain | VHF |
|---|---|
| wt | + |
| fmk1Δ | − |
| fmk1Δ + fmk1 | + |
| mpk1Δ | − |
| mpk1Δ + mpk1 | + |
| hog1Δ#14 | + |
| hog1Δ#15 | + |
| fmk1Δ mpk1Δ | − |
| fmk1Δ hog1Δ | − |
| mpk1Δ hog1Δ | − |
Presence (+) or absence (−) of VHF was determined by spreading microconidia on top of a plate containing 4 mL of solid minimal medium. After 14 h at 28° C, VHF events were observed microscopically. Three hundred germlings were examined for each isolate and each experiment was repeated at least three times.
During the growth of F. oxysporum in liquid medium, hyphal adhesion and fusion lead to the formation of macroscopically visible hyphal networks (Di Pietro et al., 2001; Lopez‐Berges et al., 2010; Prados‐Rosales and Di Pietro, 2008). Although the wild‐type strain produced dense hyphal aggregates on sodium nitrate, the fmk1Δ, mpk1Δ, fmk1Δ mpk1Δ and fmk1Δ hog1Δ mutants failed to produce such networks, most likely as a consequence of impaired hyphal fusion (Fig. 3A). Interestingly, aggregate formation was partly restored in the hyphal fusion‐defective mpk1Δ hog1Δ strain. Moreover, Fmk1 phosphorylation was increased in the mpk1Δ hog1Δ mutant in comparison with the wild‐type (Fig. 2A,B), indicating a possible negative role of Hog1 in Fmk1‐mediated hyphal adhesion. As reported previously (Lopez‐Berges et al., 2010), the wild‐type strain failed to produce aggregates in the presence of ammonium. Moreover, none of the tested mutants formed hyphal aggregates under these conditions.
Figure 3.
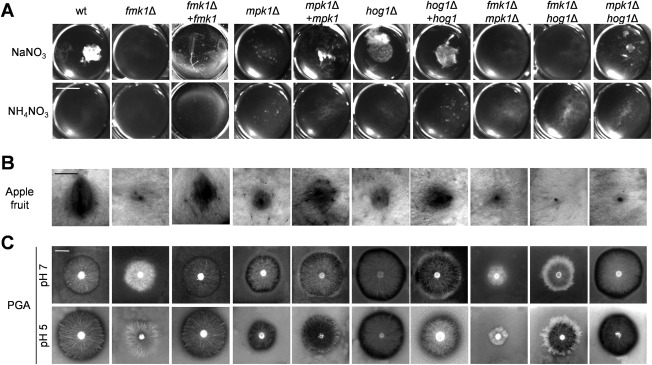
The mitogen‐activated protein kinases (MAPKs) Fmk1, Mpk1 and Hog1 regulate different virulence‐associated traits. (A) Hyphal aggregates forming after 36 h of growth in potato dextrose broth (PDB) diluted 1 : 50 with water and supplemented with 25 mm of the indicated nitrogen source. Fungal cultures were vortexed to dissociate weakly adhered hyphae, transferred to a multiwell plate and observed using a binocular microscope. (B) Invasive growth on apple fruit inoculated with microconidia of the indicated strains and incubated at 28 °C for 4 days. (C) The production of extracellular polygalacturonase activity was determined by the inoculating plates containing polygalacturonic acid (PGA) with microconidia of the indicated strains. Enzymatic activity was visualized as a contrasting halo underneath the fungal colony after 3 days of growth at 28 °C.
The three F. oxysporum MAPKs contribute to different extents to infectious growth and virulence on plant and animal hosts
Fmk1 has been shown previously to control infectious growth functions in F. oxysporum, including the ability to invade and colonize living fruit tissue (Di Pietro et al., 2001; Prados‐Rosales and Di Pietro, 2008). Here, we found that both mpk1Δ and hog1Δ, as well as the double mutants, also exhibited significantly reduced invasive growth on apple slices, although some degree of fruit tissue colonization around the inoculation site was still visible (Figs 3B and S4A, see Supporting Information).
Concomitant with the defects in infectious growth, the fmk1Δ mutants also exhibited decreased extracellular pectinolytic activity, an important trait associated with the virulence of F. oxysporum on tomato plants (Bravo Ruiz et al., 2016; Delgado‐Jarana et al., 2005). In contrast with the fmk1Δ mutant, which was impaired in clear halo production on polygalacturonic acid (PGA) medium, the mpk1Δ and hog1Δ mutants produced a larger clear halo surrounding the colony (Figs 3C and S4B). This phenotype was most pronounced in the hog1Δ and mpk1Δ hog1Δ strains grown at pH 5. Loss of Fmk1 in all genetic backgrounds was invariably associated with a decrease in pectinolytic activity. Thus, while Fmk1 acts as a positive regulator of pectinolytic activity, Mpk1 and Hog1 appear to negatively regulate this process.
We next tested the role of the different MAPKs in the ability of F. oxysporum to cause disease on tomato plants. Plants inoculated with the wild‐type strain showed a progressive increase in wilt symptoms, and most were dead by 18 days after inoculation (Fig. 4; Table S3, see Supporting Information). By contrast, plants inoculated with single or double mutants lacking the fmk1 gene failed to develop disease symptoms and remained alive even 30 days after inoculation (P < 0.0001 versus wild‐type). These results confirm the previous finding that Fmk1 is absolutely essential for the virulence of F. oxysporum on tomato (Di Pietro et al., 2001). The mpk1Δ and hog1Δ mutants showed a partial, but significant, decrease in virulence, which was more pronounced in mpk1Δ (mpk1Δ, P < 0.0001; hog1Δ, P = 0.0214), and was fully restored on complementation with the corresponding wild‐type allele. The progression of disease symptoms in the plants inoculated with these mutants was largely arrested after 15 days, leading to survival of a higher number of plants. Importantly, simultaneous loss of Mpk1 and Hog1 caused an even stronger attenuation of mortality compared with each single mutant (P < 0.0002 versus hog1Δ; not significant versus mpk1Δ). Thus, these two MAPKs appear to have distinct and complementary functions during plant infection.
Figure 4.
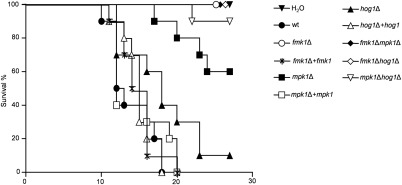
Fmk1, Mpk1 and Hog1 mitogen‐activated protein kinases (MAPKs) differentially contribute to the virulence of Fusarium oxysporum on tomato plants. Kaplan–Meier plots showing survival of tomato plants (cv. Monica) inoculated by dipping roots into a suspension of 5 × 106 microconidia/mL of the indicated fungal strains. Experiments were performed at least three times with similar results. Percentage survival of tomato plants was plotted for 30 days. Data shown are from one representative experiment.
Previous work has established that the tomato‐pathogenic F. oxysporum isolate used in this study can kill immunodepressed mice (Ortoneda et al., 2004) as well as larvae of the wax moth Galleria mellonella, a non‐vertebrate infection model which has been widely used to study fungal pathogens of humans (Mylonakis et al., 2007; Navarro‐Velasco et al., 2011). The injection of microconidia of the wild‐type or the fmk1Δ mutant into the haemocoel of G. mellonella resulted in rapid killing of the larvae (Fig. 5A–C; Table S4, see Supporting Information). By contrast, the mpk1Δ and hog1Δ mutants showed a significant decrease in virulence (mpk1Δ, P < 0.0001; hog1Δ, P = 0.0031 versus wild‐type), which was fully reversed in the complemented strains. The severity of killing was further reduced in the different double MAPK mutants, with more than 50% of the larvae surviving 7 days after inoculation, although the additive effect was only significant in the mpk1Δ hog1Δ strain (P = 0.0078 versus mpk1Δ; P = 0.0258 versus hog1Δ). These results suggest that Mpk1 and Hog1 have distinct and additive virulence functions in this invertebrate animal infection model.
Figure 5.
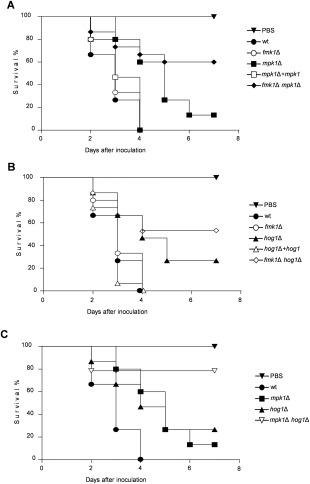
Fmk1, Mpk1 and Hog1 mitogen‐activated protein kinases (MAPKs) differentially contribute to the virulence of Fusarium oxysporum on the animal host Galleria mellonella. A–C. Kaplan–Meier plots showing survival of G. mellonella larvae after injection of 1.6 × 103 microconidia of the indicated F. oxysporum strains into the haemocoel and incubation at 30 ºC. Percentage survival was plotted for 7 days. All experiments were performed at least four times with similar results. Data shown are from one representative experiment.
Discussion
Three conserved MAPK modules regulate key physiological and developmental processes in fungi (Hamel et al., 2012; Rispail et al., 2009; Turrà et al., 2014; Zhao et al., 2007). Although each MAPK pathway has distinct cellular functions, increasing evidence suggests that cross‐talk between different MAPKs is essential for the generation of an optimally orchestrated response (Fey et al., 2012; Frei dit Frey et al., 2014; Igbaria et al., 2008; Niba et al., 2013; Saito, 2010). Here, we have genetically dissected the contribution of each of the three MAPKs, Fmk1, Mpk1, and Hog1, in the economically important fungal pathogen F. oxysporum. Our study reveals both unique and combined functions in the regulation of developmental processes, such as hyphal growth, fusion and aggregation, as well as in the response to various stresses and during infection of plant and animal hosts (Fig. 6).
Figure 6.
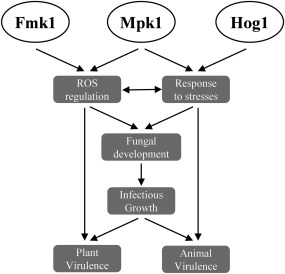
Schematic diagram showing the roles of Fmk1, Mpk1 and Hog1 mitogen‐activated protein kinases (MAPKs) in the stress response, development and virulence of Fusarium oxysporum. Mpk1 and Hog1 regulate cellular adaptation to different types of stress, whereas Fmk1 and Mpk1 jointly contribute to reactive oxygen species (ROS) homeostasis. Both processes impact on fungal development and infectious growth, and either directly or indirectly affect virulence on plant and animal hosts.
Function of Fmk1, Mpk1 and Hog1 MAPKs in stress responses
The ability of fungi to occupy a wide range of ecological niches exposes these organisms to a variety of challenges, including high temperature, cell wall, oxidative and hyperosmotic stresses. The severity of these external insults is likely to be further exacerbated during host infection (Brown et al., 2014; Egan et al., 2007; Yang et al., 2015). For instance, the release of ROS has been reported as a first line of defence against fungal invasion in plants and animals (Brown et al., 2014; Mellersh et al., 2002; Nicola et al., 2008). Accordingly, transcriptomic and proteomic surveys in a number of fungal pathogen species have revealed host‐induced activation of stress‐related genes inside the plant or animal tissue (Fradin et al., 2005; Irmer et al., 2015; Lorenz et al., 2004; Rubin‐Bejerano et al., 2003; Yang et al., 2015). Hog1 has been implicated in cellular adaptation to hyperosmotic, heat, oxidative and citric acid stresses (Alonso‐Monge et al., 2003; Bilsland et al., 2004; Lawrence et al., 2004; Saito and Posas, 2012; Winkler et al., 2002) Here, we found that deletion of the hog1 gene in F. oxysporum caused increased sensitivity to hyperosmotic stress and enhanced resistance to the fungicides iprodione and fludioxonil. Both phenotypes are Hog1 specific, as they were neither alleviated nor exacerbated by the additional deletion of other MAPKs. The hog1Δ mutant also showed increased resistance towards menadione, whereas the mpk1Δ mutant exhibited higher sensitivity. These phenotypes were reversed in the double mutant, suggesting that Hog1 and Mpk1 might have opposite functions in the menadione‐triggered oxidative stress response. Importantly, hog1Δ mutants in the closely related species Fusarium graminearum also exhibited increased resistance to oxidants (Van Thuat et al., 2012). However, in other fungal pathogens, such as Cochliobolus heterostrophus and C. albicans, loss of Hog1 led to increased sensitivity to oxidative stress (Alonso‐Monge et al., 2003; Igbaria et al., 2008), suggesting that oxidant and hyperosmotic stress response signalling may have evolutionarily diverged in different fungal species.
Heat shock responses are important for the virulence of bacterial and fungal pathogens of humans (Klancnik et al., 2014; Shapiro and Cowen, 2012). We hypothesized that resistance to high temperature should be a prerequisite for the ability of a cross‐kingdom pathogen, such as F. oxysporum, to infect both plant and mammalian hosts. In S. cerevisiae, heat stress triggers the coordinated activation of Mpk1 and Hog1, which includes the regulation of specific tyrosine protein phosphatases that block inappropriate cross‐talk between both pathways (Kamada et al., 1995; Winkler et al., 2002). Similarly, our results revealed a cooperative role of Mpk1 and Hog1 in the heat stress response of F. oxysporum, as the mpk1Δ hog1Δ double mutant was significantly more affected by high temperature than either single mutant. In addition, we found evidence for a minor contribution of Fmk1 to the heat stress response, given that the fmk1Δ mpk1Δ double mutant was more sensitive than the mpk1Δ strain. Thus, all three MAPKs appear to contribute to the efficient cellular adaptation of F. oxysporum to high temperatures. Recently, changes in the phosphorylation pattern of the three MAPKs on heat shock were observed in the human pathogen C. albicans as part of a long‐term thermal adaptation programme associated with cell wall remodelling (Leach et al., 2012).
In S. cerevisiae and in a number of plant‐pathogenic fungi, Mpk1 is required for the adaptive response to cell wall stress (Levin, 2005; Turrà et al., 2014). Fusarium oxysporum mutants lacking Mpk1 or its upstream components Rho1, Bck1 or Mkk2 exhibit increased sensitivity to CR and CFW, and display dramatically restricted colony growth on solid surfaces (Martínez‐Rocha et al., 2008; Turrà et al., 2015). Here, we noted that the restricted growth phenotype of the mpk1Δ mutant was reversed by additional deletion of fmk1 and, in particular, hog1. Moreover, partial reversal of the CFW sensitivity phenotype of mpk1Δ was observed in the mpk1Δ hog1Δ double mutant. Taken together, our results demonstrate that each of the three F. oxysporum MAPKs makes important contributions to cellular stress adaptation, and suggest that cross‐talk between different MAPK pathways is required for the orchestration of cellular responses to hostile environments.
Evidence for ROS‐mediated developmental roles of Fmk1 and Mpk1 MAPKs
Recent findings have suggested that the precise spatio‐temporal control of ROS production in fungal cells drives a number of developmental processes, such as germination, vegetative hyphal fusion and differentiation of sexual fruiting bodies or penetration structures (Dirschnabel et al., 2014; Egan et al., 2007; Lara‐Ortiz et al., 2003; Roca et al., 2012; Takemoto et al., 2007). Here, we observed a correlation between the defect of different MAPK mutants to fine tune ROS production and their failure to perform vegetative hyphal fusion or to produce hyphal aggregates. Thus, fmk1Δ and mpk1Δ, but not hog1Δ, mutants showed abnormally increased ROS levels and were impaired in vegetative hyphal fusion and aggregation. Both processes are of relevance, as they occur during the early infection stages of F. oxysporum (Prados‐Rosales and Di Pietro, 2008). Our findings are in line with previous studies proposing that the Fmk1 and Mpk1 pathways regulate ROS production by controlling the expression of NADPH oxidase (Nox) genes (Dirschnabel et al., 2014; Lalucque et al., 2012; Segmuller et al., 2008), and that deletion of Nox1 or its regulator NoxR leads to increased ROS production and impaired hyphal fusion (Dirschnabel et al., 2014; Kayano et al., 2013; Roca et al., 2012). It is interesting to note that Fmk1 and Mpk1 are essential for hyphal fusion (Hou et al., 2002; Maerz et al., 2008; Pandey et al., 2004; Prados‐Rosales and Di Pietro, 2008), which is repressed on rich media (Ishikawa et al., 2010; Lopez‐Berges et al., 2010; Roca et al., 2012), and that ROS generation in fungal mycelium is also regulated by nutrients, being more abundant in nutrient‐rich conditions. Thus, Fmk1 and Mpk1 may play a role in both nutrient sensing and in the regulation of developmental processes promoted by nutrient deprivation.
In contrast with Neurospora crassa, in which mutants in components of the Hog1 pathway are impaired in hyphal fusion (Maerz et al., 2008), hog1 deletion in F. oxysporum did not affect ROS levels, hyphal fusion and aggregation. This further supports the idea that the Hog1 MAPK might have undergone evolutionary specialization in different fungal species, as further evidenced by the finding that this MAPK is dispensable for the oxidative stress response of F. oxysporum.
The formation of hyphal aggregates was abolished in the fmk1Δ and mpk1Δ mutants, and partly restored in the mpk1Δ hog1Δ, but not fmk1Δ hog1Δ, double mutants. These results suggest that aggregation of F. oxysporum hyphae may involve both fusion‐dependent and fusion‐independent mechanisms. The latter could be related to cell surface adhesiveness, as shown in C. albicans, where aggregation of yeast cells during the formation of biofilms is mediated by agglutinins and glycosylphosphatidylinositol‐anchored glycoproteins (Chandra et al., 2001; Granger et al., 2005). Analysis of agglutinin gene orthologues and their role in the cell adhesiveness of F. oxysporum should provide further insights into this process.
Fmk1, Mpk1 and Hog1 MAPKs regulate distinct virulence functions
In F. oxysporum, the MAPK Fmk1 is essential for multiple virulence‐related traits on plants, such as root adhesion, penetration of cellophane membranes, infectious growth on fruit tissue and secretion of CWDEs, as well as for successful colonization of the host plant tomato (Di Pietro et al., 2001; Perez‐Nadales and Di Pietro, 2011; Prados‐Rosales and Di Pietro, 2008; Rispail and Di Pietro, 2009). Here, we found that some of the Fmk1‐controlled functions linked to invasive growth are also altered in the mpk1Δ and hog1Δ mutants. For example, although the mpk1Δ and hog1Δ mutants showed increased secretion of polygalacturonase activity in vitro, their ability to colonize apple fruit tissue and to infect tomato plants was reduced significantly. Thus, although Fmk1 regulates a wide subset of virulence‐related functions, Mpk1 and Hog1 contribute to plant infection through additional Fmk1‐independent functions. Strikingly, despite its fundamental contribution to virulence on plants, Fmk1 is dispensable for the pathogenicity of F. oxysporum on invertebrate and mammalian animal hosts (Navarro‐Velasco et al., 2011; Ortoneda et al., 2004). By contrast, we showed here that both Mpk1 and Hog1 MAPKs are required for full virulence of F. oxysporum on G. mellonella. These findings are in line with studies in Cr. neoformans, C. albicans and C. glabrata, showing that mutants lacking Mpk1 display attenuated virulence in mouse models, whereas mpk1‐overexpressing strains exhibit an increased survival rate in mice (Diez‐Orejas et al., 1997; Kraus et al., 2003; Miyazaki et al., 2010). However, deletion of Hog1 or its upstream MAPKK Pbs2 in Cr. neoformans, C. albicans or Metarhizium acridum caused attenuated virulence in mouse and insect models, respectively (Bahn et al., 2005; Cheetham et al., 2011; Jin et al., 2012).
Interestingly, simultaneous deletion of fmk1 in the mpk1Δ and hog1Δ mutant backgrounds led to a further loss in virulence on Galleria compared with the single mutants. The most likely explanation for this outcome is that the virulence of F. oxysporum on animal hosts is controlled by multiple MAPK pathways with distinct and additive inputs. Previous studies have revealed that simultaneous deletion of components of the filamentation/invasive growth MAPK cascade and the cyclic adenosine monophosphate (cAMP)–protein kinase A pathway in C. albicans or F. oxysporum impaired virulence on immunodepressed mice to a much greater extent than individual mutation of each component, suggesting complementary functions of the two cell signalling cascades in fungal virulence (Lo et al., 1997; Prados‐Rosales et al., 2006).
In summary, our study provides new evidence for distinct and cooperative roles of Fmk1, Mpk1 and Hog1 MAPKs in the virulence of F. oxysporum on tomato plants and on G. mellonella larvae (Fig. 6). This finding may have practical applications, as it suggests that combinations of compounds that simultaneously target different MAPK signalling pathways should represent a novel and promising strategy for the treatment of fungal infections in different pathosystems. Further studies are required to understand how environmental cues trigger changes in the phosphorylation or subcellular localization of the three MAPKs, and how cross‐talk between these and other cell signalling pathways generates the appropriate outputs, to allow successful adaptation of this cross‐kingdom fungal pathogen to phylogenetically distant and biologically diverse host organisms.
Experimental Procedures
Fungal isolates and culture conditions
Fusarium oxysporum f. sp. lycopersici wild‐type isolate 4287 (FGSC 9935) was used in all experiments (Table S1, see Supporting Information). The generation and molecular characterization of F. oxysporum fmk1Δ, mpk1Δ and fmk1Δ mpk1Δ mutants have been described previously (Di Pietro et al., 2001; Turrà et al., 2015). All fungal strains were stored as microconidial suspensions at −80 °C with 30% glycerol. For the extraction of genomic DNA and microconidia production, cultures were grown in potato dextrose broth (PDB; Difco, Madrid, Spain) at 28 °C with shaking at 170 rpm (Di Pietro et al., 1998).
Quantification of vegetative hyphal fusion and hyphal aggregation events
To observe germling fusion events, microconidia from 5‐day‐old cultures were suspended in water, and their concentration was adjusted to 7 × 107 mL−1. Fifty microliters of conidial suspension were spread with the help of 10 glass beads (0.5 cm) on top of a plate containing 4 mL of solid MM (Di Pietro et al., 1998) supplemented with 25 mm NaNO3. To test the effect of Hog1 activators/inhibitors on vegetative hyphal fusion, a 5‐µL drop of NaCl (0, 0.4, 0.8, 1.2 m), the inhibitor SB202190 [30 µm in dimethylsulfoxide (DMSO); Sigma‐Aldrich, Madrid, Spain] or DMSO as a control was added on top of the medium. Plates were incubated for 14 h at 28 °C and vegetative hyphal fusion events were counted in an Olympus BH‐2 microscope (Olympus Iberia, Barcelona, Spain) using differential interference contrast imaging (400× magnification). Three hundred germlings were examined for each isolate and each experiment was repeated at least three times. The number of germ tubes involved in vegetative hyphal fusion was scored and expressed as a percentage of fusing hyphae over the total number of counted hyphae. For macroscopic analysis of hyphal aggregates, fungal strains were grown for 36 h in PDB diluted 1 : 50 with water and supplemented with 25 mm of the indicated nitrogen source, and observed using a Leica binocular microscope (Leica Microsistemas S.L.U., Barcelona, Spain). Photographs were recorded with a Leica DC 300F digital camera.
Nucleic acid manipulations, targeted gene deletion and gene complementation
Targeted gene replacement of the hog1 gene in the wild‐type and different mutant backgrounds was performed as reported previously (Lopez‐Berges et al., 2010) using the split‐marker method with the hygromycin or phleomycin resistance cassettes and complementation of the mutants by co‐transformation with the phleomycin resistance cassette. The strategy for gene deletion and the oligonucleotides used to generate PCR fragments for gene replacement, complementation or identification of the mutants are shown in Fig. S1 and Table S2 (see Supporting Information). PCRs were routinely performed with the High Fidelity Template PCR system (Roche Diagnostics, Barcelona, Spain) using an MJ Mini personal thermal cycler (Bio‐Rad, Alcobendas, Spain). The amplified flanking sequences were PCR fused with partially overlapping truncated versions of the hygromycin B (Hygr) or phleomycin (Phleor) resistance cassettes. Transformants were purified by monoconidial isolation and analysed by PCR and Southern blot to verify homologous insertion of the construct. For complementation of the osmosensitive hog1 deletion mutant, transformed protoplasts were incubated overnight in osmotic medium (1.2 m MgSO4, 100 mm Na2HPO4, pH 5.8) before applying the selective agent phleomycin. Transformants showing wild‐type colony phenotypes in the presence of 0.4 m NaCl or iprodione were analysed by PCR to confirm the presence of the wild‐type hog1 allele.
Western blot analysis
Microconidia were germinated for 15 h in PDB. Protein extraction and western blot analysis from mycelial samples were performed as described previously (Masachis et al., 2016) using Phospho‐p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E) XP® Rabbit monoclonal antibody (mAb) (no. 4370), horseradish peroxidase (HRP)‐conjugated anti‐rabbit (no. 7074) and LumiGLO Reagent (no. 7003), all from Cell Signaling Technology (Danvers, MA, USA), to detect both phosphorylated Fmk1 and Mpk1 MAPKs. Unphosphorylated Fmk1 and Mpk1 were detected using anti‐Fus3 (γN‐19) (sc6772) and anti‐Mpk1 (yN‐19) (sc‐6802), respectively, and donkey anti‐goat IgG‐HRP (sc2020) as secondary antibody (Santa Cruz Biotechnology, Heidelberg, Germany). Anti‐mouse‐α‐tubulin antibody (sc69971) and goat anti‐rat IgGHRP secondary antibody (sc2006) (Santa Cruz Biotechnology) were used to detect the loading control α‐tubulin. Hybridizing bands were visualized using the ECL Select western blotting detection reagent (GE Healthcare, Madrid, Spain). Band intensity was quantified using MultiGauge V3.0 software (Fuji, Tokyo, Japan).
Colony growth assays
For phenotypic analysis of colony growth, drops containing serial dilutions (2 × 105, 2 × 104 and 2 × 103 mL−1) of freshly obtained microconidia were spotted onto agar plates with complete rich medium (YPD). For cell wall and oxidative stress assays, 5 μg/mL CR prepared in water, 5 μg/mL CFW prepared in 0.5% KOH, 83% glycerol (both from Sigma‐Aldrich) and 10 µg/mL menadione in DMSO were added to 50 mm 2‐(N‐morpholino)ethanesulfonic acid (MES)‐buffered YPD plates, pH 6.5 (Ram and Klis, 2006). For fungicide resistance assays, 1 mg/mL stock solutions of fludioxonil and iprodione in DMSO or ethanol, respectively, were added to YPD medium to a final concentration of 10 µg/mL (Rispail and Di Pietro, 2010). For hyperosmotic stress assays, YPD plates were supplemented with 0.4 m NaCl or 1 m sorbitol. Plates were incubated for 2 days at 28 °C and imaged, except for heat stress assays in which they were incubated for 4 days at 34 °C. The colony area was measured using MultiGauge software (Fuji, Tokyo, Japan). Data were analysed and plotted with the program GraphPad Prism version 5 (GraphPad Software, La Jolla, CA, USA).
For superoxide detection, freshly obtained microconidia were spotted onto YPD or MM. Plates were incubated at 28 °C for 5 days and then stained with 3 mL of an aqueous solution of 2.5 mm NBT (Roche Life Sciences, Barcelona, Spain) for 30 min. The reaction was stopped by the addition of ethanol, and the pattern of formazan staining was observed on the whole colony or on individual hyphae by cutting out a small square (about 1 × 1 cm2) of an agar block at the edge of the colony and imaging in a Zeiss Axio Imager M2 microscope (Zeiss, Barcelona, Spain). Images were captured with an Evolve Photometrics digital camera (Photometrics, Tucson, AZ, USA) using Axiovision 4.8 software (Zeiss).
All experiments were performed at least three times with similar results. The data presented are from one representative experiment.
Assays for virulence‐related phenotypes
Assays for polygalacturonase production were carried out as described previously (Rispail and Di Pietro, 2009) on PGA medium (0.5% PGA, 2% glucose, 0.2% ammonium sulfate, 1.5% agar) buffered with 100 mm MES to pH 5 or 7. The quantification of extracellular pectinolytic activity was performed by subtracting the area of the clear halo surrounding the colony margin from the area of the colony. Invasive growth on apple fruit slices (variety Golden Delicious) was performed as described by López‐Berges et al. (2009). Halo, colony and necrotic areas were quantified using MultiGauge V3.0 software.
Galleria mellonella infection assays
Galleria mellonella infection assays were carried out as described by Navarro‐Velasco et al. (2011). Briefly, larvae were inoculated with 0.8 µL of a microconidial suspension (1.6 × 105 conidia/larva). Twenty larvae per strain were inoculated, and survival was checked daily. A Kaplan–Meier test was used to assess the statistical significance of differences in survival among groups. Data were plotted using the software GraphPad Prism version 5. P < 0.05 was considered to be significant. Experiments were performed four times with similar results. The data presented are from one representative experiment.
Tomato plant infection assays
Tomato root infection assays were performed as described by Di Pietro et al. (2001) using the susceptible cultivar Monika (kindly provided by Syngenta Seeds, Almeria, Spain). Briefly, 2‐week‐old tomato seedlings were inoculated by submerging roots for 30 min in a suspension of 5 × 106 microconidia/mL of the different F. oxysporum strains, planted in vermiculite and maintained in a growth chamber (28 °C; photoperiod 14 h light/10 h dark). Fifteen plants were used for each treatment. Plant survival was recorded for 30 days. A Kaplan–Meier test was used to assess the statistical significance of the differences in survival among groups. Data were plotted using the software GraphPad Prism version 5. P < 0.05 was considered to be significant. Experiments were performed three times with similar results. The data presented are from one representative experiment.
Bioinformatic prediction of Hog1 and accession numbers
The predicted F. oxysporum Hog1 protein was identified in the Fusarium Comparative Database at the Broad Institute (http://www.broadinstitute.org/annotation/genome/fusarium_group/MultiHome.html) using a blastp search with the S. cerevisiae Hog1 protein sequence. Sequence data are found in the Fusarium Comparative Database under accession number FOXG_06318.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Targeted deletion of the Fusarium oxysporum hog1 gene. (A) Physical maps of the F. oxysporum hog1 locus and the split‐marker gene replacement constructs obtained by fusion polymerase chain reaction (PCR). The relative positions of the primers used for PCR are indicated. Hygr, hygromycin resistance gene; Phleor, phleomycin resistance gene; PgpdA, gpdA promoter; TtrpC, TrpC terminator (both from Aspergillus nidulans). Discontinuous lines represent the fragments used for co‐transformation. (B–D) Southern blot analysis of putative hog1Δ (B), fmk1Δ hog1Δ (C) and mpk1Δ hog1Δ (D) transformants. Genomic DNA of the wild‐type (wt) strain and five individual transformants was treated with the indicated restriction enzyme, separated on a 0.7% agarose gel, transferred to a nylon membrane and hybridized with the DNA probe indicated. (E) PCR amplification of genomic DNA of the wt strain, the hog1Δ mutant and four independent hog1Δ+hog1 complemented strains, using primers Hog1F2 and Hog1stop. M, molecular size markers.
Fig. S2 Superoxide production phenotypes caused by single and double deletion of mitogen‐activated protein kinase (MAPK) genes. (A, B) Growth of the indicated strains on minimal medium (MM) (A) or YPD (3% yeast extract, 1% peptone, 2% glucose and 1.5% agar) plates (B), before and after staining with 4‐nitroblue tetrazolium chloride (NBT). Images below the plates show enlargements of the colony margins. Bar, 1 cm. (C) Quantitative analysis of the colony area (cm2) after 5 days of growth at 28 ºC was performed using MultiGauge software. Data were analysed and plotted with the program GraphPad Prism version 5. Error bars represent standard deviations of colony areas calculated from four independent replicates. Values with the same letter are not significantly different according to Dunnett's multiple comparison test (P ≤ 0.05).
Fig. S3 Hog1 activity is dispensable for vegetative hyphal fusion in F. oxysporum. (A–C) Fifty microlitres of a 7 × 107 mL–1 conidial suspension were spread on top of a plate containing 4 mL of solid minimal medium (MM) supplemented with 25 mm NaNO3. Where indicated, a 5‐µL drop of NaCl (0, 0.4, 0.8, 1.2 M), the specific p38 mitogen‐activated protein kinase (MAPK) inhibitor SB202190 (30 µM) or dimethylsulfoxide (DMSO) was added on top of the medium, and the plates were incubated for 14 h at 28 °C. The number of germ tubes involved in vegetative hyphal fusion was scored and expressed as a percentage of fusing hyphae over the total number of counted hyphae. (A) Percentage of vegetative hyphal fusion of the indicated strains. (B, C) Percentage of vegetative hyphal fusion in the wild‐type (wt) strain was scored in the area in which NaCl (B), SB202190 or DMSO (control) (C) had been previously added. Experiments were performed three times with similar results. Statistical analysis was conducted using t‐test; values with the same letter are not significantly different.
Fig. S4 Quantification of fruit tissue necrotic area and halo production on polygalacturonic acid medium. (A) The graph shows the mean relative intensity of the necrotic area produced by mutant strains normalized to that of the wild‐type (wt) strain. The average pixel intensity of necrotic areas was subtracted from the average pixel intensity of the control (water‐inoculated area) from the same apple slice. Error bars indicate standard deviation; n = 4 biological replicates. (B) Extracellular pectinolytic activity was quantified by subtracting the area of the colony from the area of the clear halo. The graph shows the mean area of clear halo produced by mutants normalized to that of wt. Data are from one representative experiment. Experiments were performed three times with similar results. Data were analysed and plotted with the program GraphPad Prism version 5. Values with the same letter are not significantly different according to Dunnett's multiple comparison test (P ≤ 0.05).
Table S1 Fusarium oxysporum strains used in this study.
Table S2 List of primers used in this study.
Table S3 Statistical significance (P values) of Solanum lycopersicum survival curves after inoculation with the Fusarium oxysporum wild‐type strain or different gene knockout mutants. NS, not statistically significant.
Table S4 Statistical significance (P values) of Galleria mellonella survival curves after inoculation with the Fusarium oxysporum wild‐type strain or different gene knockout mutants. NS, not statistically significant.
Acknowledgements
The authors are grateful to E. Martínez Aguilera for technical assistance. This work was supported by grants BIO2010‐15505 and BIO2013‐47870‐R from the Spanish Ministerio de Innovación y Competitividad (MINECO), BIO‐296 from Junta de Andalucia and BIO2008‐04479 from MINECO/ERA‐NET PathoGenoMics to A.D.P.
References
- Alonso‐Monge, R. , Navarro‐Garcia, F. , Molero, G. , Diez‐Orejas, R. , Gustin, M. , Pla, J. , Sánchez, M. and Nombela, C. (1999) Role of the mitogen‐activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans . J. Bacteriol. 181, 3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso‐Monge, R. , Navarro‐Garcia, F. , Roman, E. , Negredo, A.I. , Eisman, B. , Nombela, C. and Pla, J. (2003) The Hog1 mitogen‐activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans . Eukaryot. Cell, 2, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana, D.M. , Alonso‐Monge, R. , Du, C. , Calderone, R. and Pla, J. (2007) Differential susceptibility of mitogen‐activated protein kinase pathway mutants to oxidative‐mediated killing by phagocytes in the fungal pathogen Candida albicans . Cell. Microbiol. 9, 1647–1659. [DOI] [PubMed] [Google Scholar]
- Bahn, Y.S. , Kojima, K. , Cox, G.M. and Heitman, J. (2005) Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans . Mol. Biol. Cell. 16, 2285–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland, E. , Molin, C. , Swaminathan, S. , Ramne, A. and Sunnerhagen, P. (2004) Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol. Microbiol. 53, 1743–1756. [DOI] [PubMed] [Google Scholar]
- Bravo Ruiz, G. , Di Pietro, A. and Roncero, M.I. (2016) Combined action of the major secreted exo‐ and endopolygalacturonases is required for full virulence of Fusarium oxysporum . Mol. Plant Pathol. 17, 339–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A. , Budge, S. , Kaloriti, D. , Tillmann, A. , Jacobsen, M. and Yin, Z. (2014) Stress adaptation in a pathogenic fungus. J. Exp. Biol. 217, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra, J. , Kuhn, D.M. , Mukherjee, P.K. , Hoyer, L.L. , McCormick, T. and Ghannoum, M.A. (2001) Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183, 5385–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham, J. , MacCallum, D.M. , Doris, K.S. , da Silva Dantas, A. , Scorfield, S. , Odds, F. , Smith, D.A. and Quinn, J. (2011) MAPKKK‐independent regulation of the Hog1 stress‐activated protein kinase in Candida albicans . J. Biol. Chem. 286, 42 002–42 016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R.E. and Thorner, J. (2007) Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae Biochim. Biophys. Acta, 1773, 1311–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csank, C. , Schräppel, K. , Leberer, E. , Harcus, D. , Mohamed, O. , Meloche, S. , Thomas, D.Y. and Whiteway, M. (1998) Roles of the Candida albicans mitogen‐activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 66, 2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, R.C. , Nichols, C.B. , Cox, G.M. , Perfect, J.R. and Heitman, J. (2003) A MAP kinase cascade composed of cell type specific and non‐specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans . Mol. Microbiol. 49, 469–485. [DOI] [PubMed] [Google Scholar]
- Dean, R. , Van Kan, J.A. , Pretorius, Z.A. , Hammond‐Kosack, K.E. , Di Pietro, A. , Spanu, P.D. , Rudd, J.J. , Dickman, M. , Kahmann, R. , Ellis, J. and Foster, G.D. (2012) The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado‐Jarana, J. , Martínez‐Rocha, A.L. , Roldán‐Rodriguez, R. , Roncero, M. I. G. and Di Pietro, A. (2005) Fusarium oxysporum G‐protein beta subunit Fgb1 regulates hyphal growth, development, and virulence through multiple signalling pathways. Fungal. Genet. Biol. 42, 61–72. [DOI] [PubMed] [Google Scholar]
- Diez‐Orejas, R. , Molero, G. , Navarro‐Garcia, F. , Pla, J. , Nombela, C. and Sanchez‐Perez, M. (1997) Reduced virulence of Candida albicans MKC1 mutants: a role for mitogen‐activated protein kinase in pathogenesis. Infect. Immun. 65, 833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro, A. , Garcia‐Maceira, F.I. , Huertas‐Gonzalez, M.D. , Ruiz‐Roldan, M.C. , Caracuel, Z. , Barbieri, A.S. and Roncero, M.I.G. (1998) Endopolygalacturonase PG1 in different formae speciales of Fusarium oxysporum . Appl. Environ. Microbiol. 64, 1967–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro, A. , Garcia‐Maceira, F.I. , Meglecz, E. and Roncero, M.I. (2001) A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 39, 1140–1152. [PubMed] [Google Scholar]
- Dirschnabel, D.E. , Nowrousian, M. , Cano‐Dominguez, N. , Aguirre, J. , Teichert, I. and Kuck, U. (2014) New insights into the roles of NADPH oxidases in sexual development and ascospore germination in Sordaria macrospora . Genetics, 196, 729–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, K.P. , Xu, J.R. , Smirnoff, N. and Talbot, N.J. (1999) Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium‐mediated plant infection by Magnaporthe grisea . Plant Cell, 11, 2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan, M.J. , Wang, Z.Y. , Jones, M.A. , Smirnoff, N. and Talbot, N.J. (2007) Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. USA, 104, 11 772–11 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey, D. , Croucher, D.R. , Kolch, W. and Kholodenko, B.N. (2012) Crosstalk and signalling switches in mitogen‐activated protein kinase cascades. Front. Physiol. 3, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin, C. , De Groot, P. , MacCallum, D. , Schaller, M. , Klis, F. , Odds, F.C. and Hube, B. (2005) Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol. Microbiol. 56, 397–415. [DOI] [PubMed] [Google Scholar]
- Frei dit Frey, N. , Garcia, A.V. , Bigeard, J. , Zaag, R. , Bueso, E. , Garmier, M. , Pateyron, S. , de Tauzia‐Moreau, M.L. , Brunaud, V. , Balzergue, S. , Colcombet, J. , Aubourg, S. , Martin‐Magniette, M.L. and Hirt, H. (2014) Functional analysis of Arabidopsis immune‐related MAPKs uncovers a role for MPK3 as negative regulator of inducible defences. Genome Biol. 15, R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger, B.L. , Flenniken, M.L. , Davis, D.A. , Mitchell, A.P. and Cutler, J.E. (2005) Yeast wall protein 1 of Candida albicans . Microbiology, 151, 1631–1644. [DOI] [PubMed] [Google Scholar]
- Hamel, L.P. , Nicole, M.C. , Duplessis, S. and Ellis, B.E. (2012) Mitogen‐activated protein kinase signaling in plant‐interacting fungi: distinct messages from conserved messengers. Plant Cell, 24, 1327–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, Z. , Xue, C. , Peng, Y. , Katan, T. , Kistler, H.C. and Xu, J.R. (2002) A mitogen‐activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant–Microbe Interact. 15, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Igbaria, A. , Lev, S. , Rose, M.S. , Lee, B.N. , Hadar, R. , Degani, O. and Horwitz, B.A. (2008) Distinct and combined roles of the MAP kinases of Cochliobolus heterostrophus in virulence and stress responses. Mol. Plant–Microbe Interact. 21, 769–780. [DOI] [PubMed] [Google Scholar]
- Irmer, H. , Tarazona, S. , Sasse, C. , Olbermann, P. , Loeffler, J. , Krappmann, S. , Conesa, A. and Braus, G.H. (2015) RNAseq analysis of Aspergillus fumigatus in blood reveals a just wait and see resting stage behavior. BMC Genomics, 16, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, F.H. , Souza, E.A. , Read, N.D. and Roca, M.G. (2010) Live‐cell imaging of conidial fusion in the bean pathogen, Colletotrichum lindemuthianum . Fungal Biol. 114, 2–9. [DOI] [PubMed] [Google Scholar]
- Jenczmionka, N.J. , Maier, F.J. , Lösch, A.P. and Schäfer, W. (2003) Mating, conidiation and pathogenicity of Fusarium graminearum, the main causal agent of the head‐blight disease of wheat, are regulated by the MAP kinase gpmk1. Curr. Genet. 43, 87–95. [DOI] [PubMed] [Google Scholar]
- Jin, K. , Ming, Y. and Xia, Y.X. (2012) MaHog1, a Hog1‐type mitogen‐activated protein kinase gene, contributes to stress tolerance and virulence of the entomopathogenic fungus Metarhizium acridum . Microbiology, 158, 2987–2996. [DOI] [PubMed] [Google Scholar]
- Joubert, A. , Bataille‐Simoneau, N. , Campion, C. , Guillemette, T. , Hudhomme, P. , Iacomi‐Vasilescu, B. , Leroy, T. , Pochon, S. , Poupard, P. and Simoneau, P. (2011) Cell wall integrity and high osmolarity glycerol pathways are required for adaptation of Alternaria brassicicola to cell wall stress caused by brassicaceous indolic phytoalexins. Cell. Microbiol. 13, 62–80. [DOI] [PubMed] [Google Scholar]
- Kamada, Y. , Jung, U.S. , Piotrowski, J. and Levin, D.E. (1995) The protein kinase C‐activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9, 1559–1571. [DOI] [PubMed] [Google Scholar]
- Kayano, Y. , Tanaka, A. , Akano, F. , Scott, B. and Takemoto, D. (2013) Differential roles of NADPH oxidases and associated regulators in polarized growth, conidiation and hyphal fusion in the symbiotic fungus Epichloe festucae . Fungal Genet. Biol. 56, 87–97. [DOI] [PubMed] [Google Scholar]
- Klancnik, A. , Vuckovic, D. , Jamnik, P. , Abram, M. and Mozina, S.S. (2014) Stress response and virulence of heat‐stressed Campylobacter jejuni . Microbes Environ. 29, 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, K. , Kikuchi, T. , Takano, Y. , Oshiro, E. and Okuno, T. (2002) The mitogen‐activated protein kinase gene MAF1 is essential for the early differentiation phase of appressorium formation in Colletotrichum lagenarium . Mol. Plant–Microbe Interact. 15, 1268–1276. [DOI] [PubMed] [Google Scholar]
- Kraus, P.R. , Fox, D.S. , Cox, G.M. and Heitman, J. (2003) The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48, 1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalucque, H. , Malagnac, F. , Brun, S. , Kicka, S. and Silar, P. (2012) A non‐Mendelian MAPK‐generated hereditary unit controlled by a second MAPK pathway in Podospora anserine . Genetics, 191, 419–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara‐Ortiz, T. , Riveros‐Rosas, H. and Aguirre, J. (2003) Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans . Mol. Microbiol. 50, 1241–1255. [DOI] [PubMed] [Google Scholar]
- Lawrence, C.L. , Botting, C.H. , Antrobus, R. and Coote, P.J. (2004) Evidence of a new role for the high‐osmolarity glycerol mitogen‐activated protein kinase pathway in yeast: regulating adaptation to citric acid stress. Mol. Cell Biol. 24, 3307–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach, M.D. , Budge, S. , Walker, L. , Munro, C. , Cowen, L.E. and Brown, A.J. (2012) Hsp90 orchestrates transcriptional regulation by Hsf1 and cell wall remodelling by MAPK signalling during thermal adaptation in a pathogenic yeast. PLoS Pathog. 8, e1003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev, S. , Sharon, A. , Hadar, R. , Ma, H. and Horwitz, B.A. (1999) A mitogen‐activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: diverse roles for mitogen‐activated protein kinase homologs in foliar pathogens. Proc . Natl. Acad. Sci. USA, 96, 13 542–13 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, D.E. (2005) Cell wall integrity signaling in Saccharomyces cerevisiae . Microbiol. Mol. Biol. Rev. 69, 262–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C.H. and Chung, K.R. (2010) Specialized and shared functions of the histidine kinase‐ and HOG1 MAP kinase‐mediated signaling pathways in Alternaria alternata, a filamentous fungal pathogen of citrus. Fungal. Genet. Biol. 47, 818–827. [DOI] [PubMed] [Google Scholar]
- Lo, H.J. , Kohler, J.R. , DiDomenico, B. , Loebenberg, D. , Cacciapuoti, A. and Fink, G.R. (1997) Nonfilamentous C. albicans mutants are avirulent. Cell, 90, 939–949. [DOI] [PubMed] [Google Scholar]
- López‐Berges, M.S. , Di Pietro, A. , Daboussi, M.J. , Wahab, H.A. , Vasnier, C. , Roncero, M.I.G. , Dufresne, M. and Hera, C. (2009) Identification of virulence genes in Fusarium oxysporum f. sp. lycopersici by large‐scale transposon tagging. Mol. Plant Pathol. 10, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Berges, M.S. , Rispail, N. , Prados‐Rosales, R.C. and Di Pietro, A. (2010) A nitrogen response pathway regulates virulence in plant pathogenic fungi: role of TOR and the bZIP protein MeaB. Plant Signal. Behav. 5, 1623–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, M.C. , Bender, J.A. and Fink, G.R. (2004) Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell, 3, 1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerz, S. , Ziv, C. , Vogt, N. , Helmstaedt, K. , Cohen, N. , Gorovits, R. , Yarden, O. and Seiler, S. (2008) The nuclear Dbf2‐related kinase COT1 and the mitogen‐activated protein kinases MAK1 and MAK2 genetically interact to regulate filamentous growth, hyphal fusion and sexual development in Neurospora crassa . Genetics, 179, 1313–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Rocha, A.L. , Roncero, M.I.G. , López‐Ramirez, A. , Mariné, M. , Guarro, J. , Martínez‐Cadena, G. and Di Pietro, A. (2008) Rho1 has distinct functions in morphogenesis, cell wall biosynthesis and virulence of Fusarium oxysporum . Cell. Microbiol. 10, 1339–1351. [DOI] [PubMed] [Google Scholar]
- Masachis, S. , Segorbe, D. , Turrà, D. , Leon‐Ruiz, M. , Fürst, U. , El Ghalid, M. , Leonard, G. , López‐Berges, M.S. , Richards, T.A. , Felix, G. and Di Pietro, A. (2016) A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 1, 16 043. [DOI] [PubMed] [Google Scholar]
- Mehrabi, R. , Van der Lee, T. , Waalwijk, C. and Gert, H.J. (2006) MgSlt2, a cellular integrity MAP kinase gene of the fungal wheat pathogen Mycosphaerella graminicola, is dispensable for penetration but essential for invasive growth. Mol. Plant–Microbe Interact. 19, 389–398. [DOI] [PubMed] [Google Scholar]
- Mellersh, D.G. , Foulds, I.V. , Higgins, V.J. and Heath, M.C. (2002) H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. Plant J. 29, 257–268. [DOI] [PubMed] [Google Scholar]
- Mey, G. , Held, K. , Scheffer, J. , Tenberge, K.B. and Tudzynski, P. (2002a) CPMK2, an SLT2‐homologous mitogen‐activated protein (MAP) kinase, is essential for pathogenesis of Claviceps purpurea on rye: evidence for a second conserved pathogenesis‐related MAP kinase cascade in phytopathogenic fungi. Mol. Microbiol. 46, 305–318. [DOI] [PubMed] [Google Scholar]
- Mey, G. , Oeser, B. , Lebrun, M.H. and Tudzynski, P. (2002b) The biotrophic, non‐appressorium‐forming grass pathogen Claviceps purpurea needs a Fus3/Pmk1 homologous mitogen‐activated protein kinase for colonization of rye ovarian tissue. Mol. Plant–Microbe Interact. 15, 303–312. [DOI] [PubMed] [Google Scholar]
- Miyazaki, T. , Inamine, T. , Yamauchi, S. , Nagayoshi, Y. , Saijo, T. , Izumikawa, K. , Seki, M. , Kakeya, H. , Yamamoto, Y. , Yanagihara, K. , Miyazaki, Y. and Kohno, S. (2010) Role of the Slt2 mitogen‐activated protein kinase pathway in cell wall integrity and virulence in Candida glabrata . FEMS Yeast Res. 10, 343–352. [DOI] [PubMed] [Google Scholar]
- Mylonakis, E. , Casadevall, A. and Ausubel, F.M. (2007) Exploiting amoeboid and non‐vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog. 3, e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro‐Velasco, G.Y. , Prados‐Rosales, R.C. , Ortiz‐Urquiza, A. , Quesada‐Moraga, E. and Di Pietro, A. (2011) Galleria mellonella as model host for the trans‐kingdom pathogen Fusarium oxysporum . Fungal Genet. Biol. 48, 1124–1129. [DOI] [PubMed] [Google Scholar]
- Niba, E.T. , Nagaya, H. , Kanno, T. , Tsuchiya, A. , Gotoh, A. , Tabata, C. , Kuribayashi, K. , Nakano, T. and Nishizaki, T. (2013) Crosstalk between PI3 kinase/PDK1/Akt/Rac1 and Ras/Raf/MEK/ERK pathways downstream PDGF receptor. Cell Physiol. Biochem. 31, 905–913. [DOI] [PubMed] [Google Scholar]
- Nicola, A.M. , Casadevall, A. and Goldman, D.L. (2008) Fungal killing by mammalian phagocytic cells. Curr. Opin. Microbiol. 11, 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci, M. and Anaissie, E. (2007) Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 20, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortoneda, M. , Guarro, J. , Madrid, M.P. , Caracuel, Z. , Roncero, M.I.G. , Mayayo, E. and Di Pietro, A. (2004) Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect. Immun. 72, 1760–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, A. , Roca, M.G. , Read, N.D. and Glass, N.L. (2004) Role of a mitogen‐activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa . Eukaryot. Cell, 3, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.M. , Choi, E.S. , Kim, M.J. , Cha, B.J. , Yang, M.S. and Kim, D.H. (2004) Characterization of HOG1 homologue, CpMK1, from Cryphonectria parasitica and evidence for hypovirus‐mediated perturbation of its phosphorylation in response to hypertonic stress. Mol. Microbiol. 51, 1267–1277. [DOI] [PubMed] [Google Scholar]
- Perez‐Nadales, E. and Di Pietro, A. (2011) The membrane mucin Msb2 regulates invasive growth and plant infection in Fusarium oxysporum. Plant Cell, 23, 1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados‐Rosales, R.C. and Di Pietro, A. (2008) Vegetative hyphal fusion is not essential for plant infection by Fusarium oxysporum . Eukaryot. Cell, 7, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prados‐Rosales, R.C. , Serena, C. , Delgado‐Jarana, J. , Guarro, J. and Di Pietro, A. (2006) Distinct signalling pathways coordinately contribute to virulence of Fusarium oxysporum on mammalian hosts. Microb. Infect. 8, 2825–2831. [DOI] [PubMed] [Google Scholar]
- Ram, A.F. and Klis, F.M. (2006) Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat. Protoc. 1, 2253–2256. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy, V. , Zhao, X. , Snyder, A.K. , Xu, J.R. and Shah, D.M. (2007) Two mitogen‐activated protein kinase signalling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum . Cell. Microbiol. 9, 1491–1506. [DOI] [PubMed] [Google Scholar]
- Rispail, N. and Di Pietro, A. (2009) Fusarium oxysporum Ste12 controls invasive growth and virulence downstream of the Fmk1 MAPK cascade. Mol. Plant–Microbe Interact. 22, 830–839. [DOI] [PubMed] [Google Scholar]
- Rispail, N. and Di Pietro, A. (2010) The two‐component histidine kinase Fhk1 controls stress adaptation and virulence of Fusarium oxysporum . Mol. Plant Pathol. 11, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispail, N. , Soanes, D.M. , Ant, C. , Czajkowski, R. , Grünler, A. , Huguet, R. , Perez‐Nadales, E. , Poli, A. , Sartorel, E. , Valiante, V. , Yang, M. , Beffa, R. , Brakhage, A.A , . Gow, N.A. , Kahmann, R. , Lebrun, M.H. , Lenasi, H. , Perez‐Martin, J. , Talbot, N.J. , Wendland, J. and Di Pietro, A. (2009) Comparative genomics of MAP kinase and calcium‐calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet. Biol. 46, 287–298. [DOI] [PubMed] [Google Scholar]
- Roca, M.G. , Weichert, M. , Siegmund, U. , Tudzynski, P. and Fleißner, A. (2012) Germling fusion via conidial anastomosis tubes in the grey mould Botrytis cinerea requires NADPH oxidase activity. Fungal Biol. 116, 379–387. [DOI] [PubMed] [Google Scholar]
- Rubin‐Bejerano, I. , Fraser, I. , Grisafi, P. and Fink, G.R. (2003) Phagocytosis by neutrophils induces an amino acid deprivation response in Saccharomyces cerevisiae and Candida albicans. Proc . Natl. Acad. Sci. USA, 100, 11 007–11 012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui, O. and Hahn, M. (2007) The Slt2‐type MAP kinase Bmp3 of Botrytis cinerea is required for normal saprotrophic growth, conidiation, plant surface sensing and host tissue colonization. Mol. Plant Pathol. 8, 173–184. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Roldán, M.C. , Maier, F.J. and Schäfer, W. (2001) PTK1, a mitogen‐activated‐protein kinase gene, is required for conidiation, appressorium formation, and pathogenicity of Pyrenophora teres on barley. Mol. Plant–Microbe Interact. 14, 116–125. [DOI] [PubMed] [Google Scholar]
- Saito, H. (2010) Regulation of cross‐talk in yeast MAPK signaling pathways. Curr. Opin. Microbiol. 13, 677–683. [DOI] [PubMed] [Google Scholar]
- Saito, H. and Posas, F. (2012) Response to hyperosmotic stress. Genetics, 192, 289–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segmuller, N. , Ellendorf, U. , Tudzynski, B. and Tudzynski, P. (2007) BcSAK1, a stress‐activated mitogen‐activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea . Eukaryot. Cell, 6, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segmuller, N. , Kokkelink, L. , Giesbert, S. , Odinius, D. , van Kan, J. and Tudzynski, P. (2008) NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea . Mol. Plant–Microbe Interact. 21, 808–819. [DOI] [PubMed] [Google Scholar]
- Shapiro, R.S. and Cowen, L.E. (2012) Thermal control of microbial development and virulence: molecular mechanisms of microbial temperature sensing. MBio, 3, e00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, Y. , Kikuchi, T. , Kubo, Y. , Hamer, J.E. , Mise, K. and Furusawa, I. (2000) The Colletotrichum lagenarium MAP kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol. Plant–Microbe Interact. 13, 374–383. [DOI] [PubMed] [Google Scholar]
- Takemoto, D. , Tanaka, A. and Scott, B. (2007) NADPH oxidases in fungi: diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet. Biol. 44, 1065–1076. [DOI] [PubMed] [Google Scholar]
- Turrà, D. and Di Pietro, A. (2015) Chemotropic sensing in fungus–plant interactions. Curr. Opin. Plant Biol. 26, 135–140. [DOI] [PubMed] [Google Scholar]
- Turrà, D. , Segorbe, D. and Di Pietro, A. (2014) Protein kinases in plant pathogenic fungi: conserved regulators of infection. Annu. Rev. Phytopathol. 52, 267–288. [DOI] [PubMed] [Google Scholar]
- Turrà, D. , El Ghalid, M. , Rossi, F. and Di Pietro, A. (2015) Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature, 527, 521–524. [DOI] [PubMed] [Google Scholar]
- Valiante, V. , Macheleidt, J. , Foge, M. and Brakhage, A.A. (2015) The Aspergillus fumigatus cell wall integrity signaling pathway: drug target, compensatory pathways, and virulence. Front. Microbiol. 6, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Thuat, N. , Schafer, W. and Bormann, J. (2012) The stress‐activated protein kinase FgOS‐2 is a key regulator in the life cycle of the cereal pathogen Fusarium graminearum . Mol. Plant–Microbe Interact. 25, 1142–1156. [DOI] [PubMed] [Google Scholar]
- Widmann, C. , Gibson, S. , Jarpe, M.B. and Johnson, G.L. (1999) Mitogen‐activated protein kinase: conservation of a three‐kinase module from yeast to human. Physiol. Rev. 79, 143–180. [DOI] [PubMed] [Google Scholar]
- Winkler, A. , Arkind, C. , Mattison, C.P. , Burkholder, A. , Knoche, K. and Ota, I. (2002) Heat stress activates the yeast high‐osmolarity glycerol mitogen‐activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryot. Cell, 1, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J.R. and Hamer, J.E. (1996) MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea . Genes Dev. 10, 2696–2706. [DOI] [PubMed] [Google Scholar]
- Xu, J.R. , Staiger, C.J. and Hamer, J.E. (1998) Inactivation of the mitogen‐activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc . Natl. Acad. Sci. USA, 95, 12 713–12 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F. , Li, W. , Derbyshire, M. , Larsen, M. , Rudd, J. and Palmisano, G. (2015) Unraveling incompatibility between wheat and the fungal pathogen Zymoseptoria tritici through apoplastic proteomics. BMC Genomics, 16, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Mehrabi, R. and Xu, J.R. (2007) Mitogen‐activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell, 6, 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Targeted deletion of the Fusarium oxysporum hog1 gene. (A) Physical maps of the F. oxysporum hog1 locus and the split‐marker gene replacement constructs obtained by fusion polymerase chain reaction (PCR). The relative positions of the primers used for PCR are indicated. Hygr, hygromycin resistance gene; Phleor, phleomycin resistance gene; PgpdA, gpdA promoter; TtrpC, TrpC terminator (both from Aspergillus nidulans). Discontinuous lines represent the fragments used for co‐transformation. (B–D) Southern blot analysis of putative hog1Δ (B), fmk1Δ hog1Δ (C) and mpk1Δ hog1Δ (D) transformants. Genomic DNA of the wild‐type (wt) strain and five individual transformants was treated with the indicated restriction enzyme, separated on a 0.7% agarose gel, transferred to a nylon membrane and hybridized with the DNA probe indicated. (E) PCR amplification of genomic DNA of the wt strain, the hog1Δ mutant and four independent hog1Δ+hog1 complemented strains, using primers Hog1F2 and Hog1stop. M, molecular size markers.
Fig. S2 Superoxide production phenotypes caused by single and double deletion of mitogen‐activated protein kinase (MAPK) genes. (A, B) Growth of the indicated strains on minimal medium (MM) (A) or YPD (3% yeast extract, 1% peptone, 2% glucose and 1.5% agar) plates (B), before and after staining with 4‐nitroblue tetrazolium chloride (NBT). Images below the plates show enlargements of the colony margins. Bar, 1 cm. (C) Quantitative analysis of the colony area (cm2) after 5 days of growth at 28 ºC was performed using MultiGauge software. Data were analysed and plotted with the program GraphPad Prism version 5. Error bars represent standard deviations of colony areas calculated from four independent replicates. Values with the same letter are not significantly different according to Dunnett's multiple comparison test (P ≤ 0.05).
Fig. S3 Hog1 activity is dispensable for vegetative hyphal fusion in F. oxysporum. (A–C) Fifty microlitres of a 7 × 107 mL–1 conidial suspension were spread on top of a plate containing 4 mL of solid minimal medium (MM) supplemented with 25 mm NaNO3. Where indicated, a 5‐µL drop of NaCl (0, 0.4, 0.8, 1.2 M), the specific p38 mitogen‐activated protein kinase (MAPK) inhibitor SB202190 (30 µM) or dimethylsulfoxide (DMSO) was added on top of the medium, and the plates were incubated for 14 h at 28 °C. The number of germ tubes involved in vegetative hyphal fusion was scored and expressed as a percentage of fusing hyphae over the total number of counted hyphae. (A) Percentage of vegetative hyphal fusion of the indicated strains. (B, C) Percentage of vegetative hyphal fusion in the wild‐type (wt) strain was scored in the area in which NaCl (B), SB202190 or DMSO (control) (C) had been previously added. Experiments were performed three times with similar results. Statistical analysis was conducted using t‐test; values with the same letter are not significantly different.
Fig. S4 Quantification of fruit tissue necrotic area and halo production on polygalacturonic acid medium. (A) The graph shows the mean relative intensity of the necrotic area produced by mutant strains normalized to that of the wild‐type (wt) strain. The average pixel intensity of necrotic areas was subtracted from the average pixel intensity of the control (water‐inoculated area) from the same apple slice. Error bars indicate standard deviation; n = 4 biological replicates. (B) Extracellular pectinolytic activity was quantified by subtracting the area of the colony from the area of the clear halo. The graph shows the mean area of clear halo produced by mutants normalized to that of wt. Data are from one representative experiment. Experiments were performed three times with similar results. Data were analysed and plotted with the program GraphPad Prism version 5. Values with the same letter are not significantly different according to Dunnett's multiple comparison test (P ≤ 0.05).
Table S1 Fusarium oxysporum strains used in this study.
Table S2 List of primers used in this study.
Table S3 Statistical significance (P values) of Solanum lycopersicum survival curves after inoculation with the Fusarium oxysporum wild‐type strain or different gene knockout mutants. NS, not statistically significant.
Table S4 Statistical significance (P values) of Galleria mellonella survival curves after inoculation with the Fusarium oxysporum wild‐type strain or different gene knockout mutants. NS, not statistically significant.


