Summary
Septins are a highly conserved family of GTP‐binding proteins that contribute to many cellular and metabolic functions, including cell polarity, cytokinesis, cell morphogenesis and pathogenesis. In this study, we characterized the septins FaCdc3 and FaCdc12 in the filamentous fungus Fusarium asiaticum. The functions of FaCdc3 and FaCdc12 were evaluated by constructing deletion mutants of FaCdc3 and FaCdc12, designated ΔFaCdc3‐5 and ΔFaCdc12‐71, respectively. The deletion mutants exhibited a reduced rate of mycelial growth, increased aerial hyphae formation, irregularly shaped hyphae, reduced conidiation and a lack of sexual reproduction in wheat kernels. Histochemical analysis revealed that the conidia and hyphae of ΔFaCdc3‐5 and ΔFaCdc12‐71 formed large lipid droplets (LDs). ΔFaCdc3‐5 and ΔFaCdc12‐71 also exhibited increased resistance to agents that induce osmotic stress and damage the cell membrane and cell wall. In addition, the hyphae and conidia of the two mutants formed fewer septa than those of the wild‐type and exhibited aberrant nuclear distribution. Pathogenicity assays showed that ΔFaCdc3‐5 and ΔFaCdc12‐71 exhibited reduced virulence on wheat spikelets, which was indirectly correlated with a reduced level of deoxynivalenol accumulation. All of these defects were restored by genetic complementation of the two mutants with the parental FaCdc3 and FaCdc12. These results indicate that FaCdc3 and FaCdc12 play a critical role in various cellular processes in F. asiaticum.
Keywords: asexual and sexual development, cytokinesis, deoxynivalenol, Fusarium asiaticum, lipid metabolism, pathogenicity, septins
Introduction
Fusarium head blight (FHB) severely decreases wheat yields, leading to economic losses worldwide (Goswami and Kistler, 2004; Leslie and Summerell, 2006; Starkey et al., 2007). In North America in the 1990s, for example, the total economic losses caused by FHB were estimated to be $3 billion (Windels, 2000). In addition to reducing grain yield and quality, the fungus produces harmful mycotoxins in infected grain. These mycotoxins, which include deoxynivalenol (DON), nivalenol and zearalenones, are a threat to human and animal health (Desjardins, 2006; Sutton, 1982). Despite the economic impact of FHB, efficient strategies to control FHB are not available. An understanding of the functions of genes in FHB could contribute to the control of this pathogen.
Although a number of Fusarium spp. can cause FHB, the primary aetiological agents of this disease belong to the Fusarium graminearum complex, which contains at least 11 phylogenetic species, including F. acaciae‐mearnsii, F. asiaticum, F. austroamericanum, F. boothii, F. brasilicum, F. cortaderiae, F. gerlachii, F.graminearum, F. meridionale, F. mesoamericanum and F. vorosii (Donnell et al., 2004; Starkey et al., 2007; Tóth et al., 2005). Different Fusarium spp. may be associated with FHB in differentregions of the world because of variations in cropping systems and climate (Nisessen, 2007; Xu et al., 2008). Zhang et al. (2007) analysed 299 isolates collected from China, and found that 231 isolates (77.3%) belonged to F. asiaticum and the remaining 68 isolates to F. graminearum. Fusarium asiaticum can produce several trichothecene mycotoxins, including DON, 3‐acetyldeoxynivalenol, 15‐acetyldeoxynivalenol (15‐Ac‐DON), nivalenol and 4‐acetylnivalenol, whereas F. graminearum produces DON and 15‐Ac‐DON.
Septins are GTPases that form filaments in fungi and animals. The combination of GTPase activity and filament formation has led to comparisons between the septins and the cytoskeletal elements actin and tubulin (Field and Kellogg, 1999; Kinoshita, 2006). Seven members of the septin family in budding yeast have been identified to date. Five (Shs1p/Sep7p, Cdc3p, Cdc10p, Cdc11p and Cdc12p) are expressed during mitosis and are involved in cytokinesis, and two (Spr3p and Spr28p) are expressed exclusively during sporulation (Versele and Thorner, 2005). Of these, only four are important for viability, namely Cdc3, Cdc10, Cdc11 and Cdc12, which were first identified as temperature‐sensitive cell division cycle (cdc) mutants isolated by Hartwell (1971). Mutation of any of the major septins Cdc3, Cdc10, Cdc11 or Cdc12 prevents the development of the septin ring, leading to mitotic delay and elongated buds (Longtine et al., 1996). During the sexual stage, Spr28 and Spr3 are distributed with Cdc12, Cdc3 and Cdc11 at the leading margin of the prospore membrane as it encapsulates the nucleus (De Virgilio et al., 1996; Fares et al., 1996; Ozsarac et al., 1995). Cdc10, which binds to Cdc3 and Cdc11, localizes at the centre of the core heteropolymeric septin complex (Bertin et al., 2008).
The functions of septins have also been well investigated in many filamentous fungi. Aspergillus nidulans contains five septin genes, including aspA (an orthologue of Saccharomyces cerevisiae cdc11), aspB (an orthologue of S. cerevisiae cdc3), aspC (an orthologue of S. cerevisiae cdc12), aspD (an orthologue of S. cerevisiae cdc10) and aspE (an orthologue exclusively from filamentous fungi) (Momany et al., 2001; Pan et al., 2007). Both AspA and AspC are important for normal development, especially for the prevention of the abnormal emergence of germ tubes and mycelia branches (Lindsey et al., 2010). In the rice blast fungus Magnaporthe oryzae, septation is required for appressorium development, and thus the septins in M. oryzae mainly affect the initiation of infection (Saunders et al., 2010). However, in the basidiomycete Ustilago maydis, septins are also required for correct morphogenesis, but are not required for virulence (Alvarez‐Tabarés and Pérez‐Martín, 2010). In addition, septins can assemble into more than three different structures that coexist in a cell: bud neck collars, band‐like structures at the growing tip and long septin fibres near the cell cortex that link the two poles in U. maydis. When lacking septins, U. maydis cells become wider at the central region and lose their elongated shape and polarity. These results indicate that both the number and function of septins differ among fungi.
Previous research has shown that S. cerevisiae and Candida albicans cannot survive when the orthologous genes cdc3 and cdc12 are deleted (Frazier et al. 1998; Warenda and Konopka, 2002). We were thus interested in the role of Cdc3 and Cdc12 in F. asiaticum. Based on these studies, we hypothesized that FaCdc3 and FaCdc12 might play important roles in the regulation of fungal development and secondary metabolism in F. asiaticum. To address this hypothesis, the main objective of this study was to analyse the functions of FaCdc3 and FaCdc12 in F. asiaticum.
Results
Sequence analysis of FaCdc3 and FaCdc12 in F. asiaticum
Based on amino acid similarity to F. graminearum FgCdc3 and FgCdc12, FaCdc3 and FaCdc12 were identified from the genome of F. asiaticum. After sequencing the full‐length genomic DNA and cDNA for FaCdc3 and FaCdc12, we determined that the full FaCdc3 is 1969 bp, contains four introns and encodes a protein with 563 amino acids. The nucleotide and amino acid sequences of FaCdc3 share 99.44% and 99.64% identity with the FgCdc3 sequences of F. graminearum (Figs S1A and S2A, see Supporting Information). The full FaCdc12 is 1362 bp, contains three introns and encodes a protein with 454 amino acids. The nucleotide and amino acid sequences of FaCdc12 share 99.49% and 100% identity with the FgCdc12 sequences of F. graminearum (Figs S1B and S2B).
Deletion and complementation of FaCdc3 and FaCdc12 in F. asiaticum
To investigate the function of FaCdc3 and FaCdc12, we generated targeted deletion mutants by transformation of the gene replacement cassette HPH‐HSV‐tk in the parental zj‐1 strain (Figs S3A and S4A, see Supporting Information). Following purification by single‐spore isolation, FaCdc3 and FaCdc12 transformants were obtained. To identify putative deletion strains, polymerase chain reaction (PCR) amplification with different combinations of primers was used to detect the integration of the left and right portion of the deletion cassette and replacement of the coding sequence by the HPH‐HSV‐tk cassette (Figs S3B and S4B). Southern blotting analysis using genomic DNA of the parental strain and mutants showed a profile consistent with the insertion of the HPH‐HSV‐tk cassette in the target locus by a double recombination event (Figs S3C and S4C). The ΔFaCdc3‐5 and ΔFaCdc12‐71 mutants were complemented with the wild‐type genes FaCdc3 and FaCdc12 to obtain ΔFaCdc3‐5C and ΔFaCdc12‐71C, respectively. The complementation was confirmed by Southern blotting and reverse transcription‐polymerase chain reaction (RT‐PCR) analysis (Figs S3C and S4C).
Involvement of FaCdc3 and FaCdc12 in the regulation of colony morphology and pigment formation in F. asiaticum
ΔFaCdc3‐5 and ΔFaCdc12‐71 grew significantly more slowly than the wild‐type strain (Table 1) and exhibited increased aerial hyphal growth and increased yellow pigment production on potato sucrose agar (PSA) plates (Fig. 1A). However, on complete medium (CM) plates, the deletion mutants exhibited increased production of a pink–red pigment (Fig. 1A). The hyphae of ΔFaCdc3‐5 and ΔFaCdc12‐71 mutants were more curved and branched than those of the wild‐type strain (Fig. 1B). The phenotypic defects of ΔFaCdc3‐5 and ΔFaCdc12‐71 on PSA and CM media were restored by genetic complementation with the wild‐type FaCdc3 and FaCdc12 in the complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C (Fig. 1A,B). These results indicate that FaCdc3 and FaCdc12 play an important role in hyphal growth and pigment formation in F. asiaticum.
Table 1.
Phenotypes of the parental strain zj‐1, deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71, and complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C of Fusarium asiaticum in terms of growth, conidiation, deoxynivalenol (DON) production, perithecia production and virulence.
| Strain | Growth rate on two media (mm/day)* | Conidiation (× 105 mL) | DON production (μg/g) † | Perithecium covered in each seed ‡ | Percentage of diseased spikelets § | |
|---|---|---|---|---|---|---|
| PSA | CM | |||||
| zj‐1 | 26.44 ± 0.2a | 23.4 ± 0.2a | 4.43 ± 0.58a | 2.24 ± 0.13a | +++ | 23.14 ± 2.19a |
| ΔFaCdc3‐5 | 9.3 ± 0.1b | 8.7 ± 0.1b | 0.12 ± 0.05b | 0.91 ± 0.04b | + | 5.37 ± 1.29b |
| ΔFaCdc3‐5C | 25.7 ± 0.4a | 23.2 ± 0.7a | 4 ± 0.36a | 2.13 ± 0.13a | +++ | 25.83 ± 5.15a |
| ΔFaCdc12‐71 | 8.3 ± 0.4c | 7.2 ± 0.3c | 0.15 ± 0.09b | 0.69 ± 0.07b | + | 4.42 ± 1.55b |
| ΔFaCdc12‐71C | 25.9 ± 0.7a | 22.8 ± 0.8a | 4.13 ± 0.40a | 2.09 ± 0.13a | +++ | 23.97 ± 3.55a |
CM, complete medium; PSA, potato sucrose agar.
*Growth rate and conidiation were measured after incubation for 3 and 7 days, respectively. The mean and standard deviation were calculated from three replicates. The same letter indicates that there is no significant difference. Different letters are used to mark statistically significant differences (P = 0.05).
†DON production in glucose–yeast extract–peptone (GYEP) inoculated with 1 × 105 spores and cultured for 7 days. Data were collected from three replicates.
‡Perithecium covered in each seed is scored as ‘+++’, ‘++’ or ‘+’ when the perithecium covered more than two‐thirds, between one‐third and two‐thirds, or less than one‐third of each seed.
§Average percentage of diseased spikelets per spike at 15 days after inoculation. Thirty spikes were inoculated for each strain.
Figure 1.
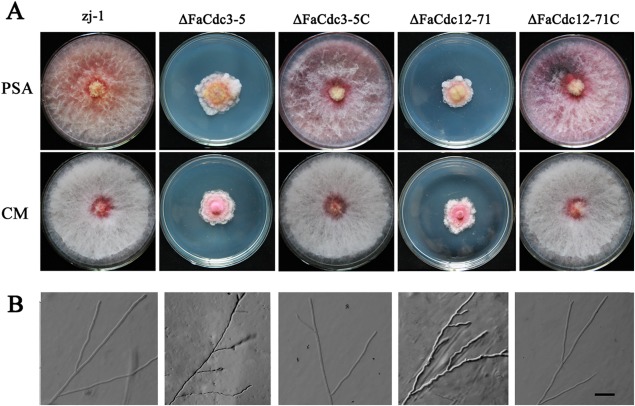
Effect of FaCdc3 and FaCdc12 on the morphology of Fusarium asiaticum colonies and hyphae. (A) The parental strain zj‐1, deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71, and complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C were grown on solid media [potato sucrose agar (PSA) and complete medium (CM)] for 5 days at 25 °C. (B) Hyphal tip morphology and branching patterns of zj‐1, ΔFaCdc3‐5, ΔFaCdc12‐71, ΔFaCdc3‐5C and ΔFaCdc12‐71C on water agar. Bar, 20 μm.
Sensitivity of ΔFaCdc3‐5 and ΔFaCdc12‐71 to various environmental stresses
We investigated the effect of ΔFaCdc3‐5 and ΔFaCdc12‐71 on the responses of F. asiaticum to various stresses. Compared with the wild‐type strain and complemented strains, ΔFaCdc3‐5 and ΔFaCdc12‐71 reduced the inhibition of mycelial growth responding to the osmotic stress caused by 1.2 m NaCl and 1.2 m KCl (Figs 2A and S5, see Supporting Information). Moreover, ΔFaCdc3‐5 and ΔFaCdc12‐71 also displayed decreased sensitivity to the cell membrane‐damaging agent sodium dodecylsulfate (SDS) and the cell wall‐damaging agent Congo red compared with the wild‐type strain and the complemented strains (Figs 2B and S5). To further confirm the involvement of FaCdc3 and FaCdc12 in the regulation of the cell wall integrity (CWI) pathway, we determined the expression of FaMkk1 and FaSlt2, which are homologous to the S. cerevisiae CWI core element genes Mkk1 and Slt2, respectively (Rodriguez et al., 1985). Based on a previous study (Zheng et al., 2013), as expected, we found that the expression levels of FaMkk1 and FaSlt2 were up‐regulated in ΔFaCdc3‐5 and ΔFaCdc12‐71 compared with the wild‐type (Fig. 2C). These results indicate that FaCdc3 and FaCdc12 are involved in the sensitivity of F. asiaticum to various stresses.
Figure 2.
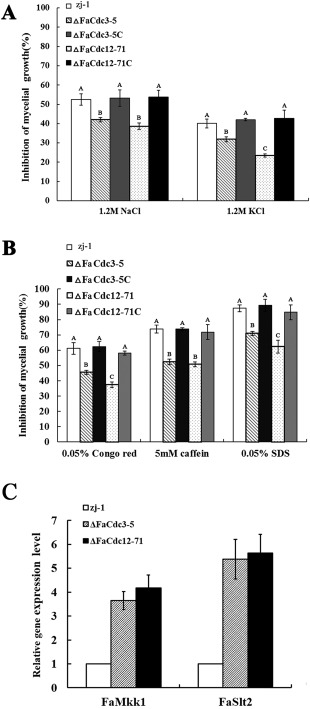
Sensitivity of the parental strain zj‐1, deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71, and complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C of Fusarium asiaticum to agents that generate osmotic stress (NaCl and KCl) (A) or damage cell membranes (sodium dodecylsulfate, SDS) and cell walls (Congo red and caffeine) (B). (C) Expression of two genes involved in cell wall integrity (FaMkk1 and FaSlt2) in ΔFaCdc3‐5, ΔFaCdc12‐71 and the parental strain zj‐1. Values are the means and standard errors of three experiments.
Requirement of FaCdc3 and FaCdc12 for conidiation, perithecia formation and lipid metabolism in F. asiaticum
Because septins have been shown to regulate asexual development in several fungi (Hernández‐Rodríguez et al., 2012), we assessed conidiation in FaCdc3 and FaCdc12 deletion mutants. ΔFaCdc3‐5 and ΔFaCdc12‐71 produced fewer conidia than the wild‐type strain and the complemented strains in mung bean liquid (MBL) medium (Fig. 3A; Table 1). We also assessed the possibility that FaCdc3 and FaCdc12 influence sexual reproduction. The wild‐type and complemented mutants produced abundant perithecia on autoclaved wheat seeds, whereas ΔFaCdc3‐5 and ΔFaCdc12‐71 produced very few perithecia on autoclaved wheat seeds (Fig. 3B). These results show that FaCdc3 and FaCdc12 play a significant role in sexual and asexual reproduction in F. asiaticum.
Figure 3.

Impact of FaCdc3 and FaCdc12 on the production of conidia (A) and perithecia (B) by the parental strain zj‐1, deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71, and complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C of Fusarium asiaticum. (A) Conidia were quantified after incubation with shaking in 10 mL of mung bean liquid medium for 7 days. Values are means and standard errors of three experiments. (B) Fungal‐colonized wheat kernels were placed on sterile wet sand at 25 ºC with 80% relative humidity and a 12‐h photoperiod for 15 days to induce the formation of perithecia, which appear dark.
Histochemical staining of conidia and hyphae with Nile red revealed that lipid droplets (LDs) were larger in ΔFaCdc3‐5 and ΔFaCdc12‐71 than in the wild‐type strain (Fig. 4A–J). In addition, quantitative real‐time PCR analysis showed that the expression of five genes associated with fatty acid biosynthesis were up‐regulated in ΔFaCdc3‐5, and nine were up‐regulated in ΔFaCdc12‐71 (Table 2). These results suggest that FaCdc3 and FaCdc12 are involved in the regulation of lipid metabolism in F. asiaticum.
Figure 4.
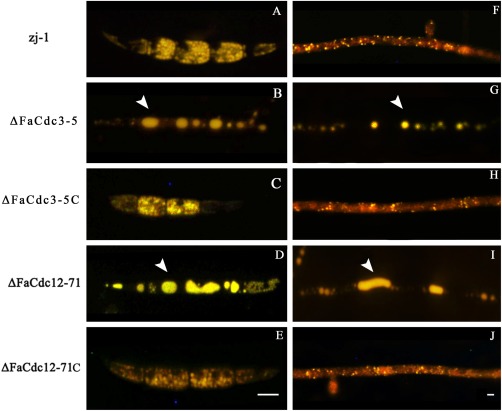
Histochemical analysis of lipid droplets in conidia and hyphae. Lipid droplets in conidia (A–E) and hyphae (F–J) of the parental strain zj‐1, deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71, and complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C were stained with Nile red and examined using inverted fluorescence microscopy. Bar, 10 μm.
Table 2.
Expression changes of the genes involved in fatty acid biosynthesis in deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71 detected by serial analysis of the gene expression method.
| Pathway | Accession number | Putative function | Fold change in gene expression* | |
|---|---|---|---|---|
| ΔFaCdc3‐5 | ΔFaCdc12‐71 | |||
| Fatty acid biosynthesis | FGSG_07223 † | Hypothetical protein similar to short‐chain dehydrogenase family protein | 1.13 ± 0.19 | 4.05 ± 0.75 |
| FGSG_01857 † | Hypothetical protein similar to 3‐oxoacyl‐acyl‐carrier‐protein reductase | 3.45 ± 0.69 | 3.03 ± 0.30 | |
| FGSG_01419 † | Hypothetical protein similar to AMP‐binding protein | 2.65 ± 0.87 | 3.08 ± 0.36 | |
| FGSG_08843 † | Hypothetical protein similar to AMP‐dependent CoA ligase | 0.83 ± 0.31 | 2.59 ± 0.83 | |
| FGSG_09424 † | Hypothetical protein similar to fadD35 | 3.12 ± 0.53 | 2.55 ± 0.22 | |
| FGSG_05140 † | Hypothetical protein similar to acyl‐CoA dehydrogenase family protein | 1.10 ± 0.27 | 3.79 ± 0.41 | |
| FGSG_05551 † | Hypothetical protein similar to peroxisomal D3,D2‐enoyl‐CoA isomerase | 1.26 ± 0.21 | 3.41 ± 0.43 | |
| FGSG_09979 † | Hypothetical protein similar to enoyl‐CoA hydratase/isomerase family protein | 2.59 ± 0.35 | 1.89 ± 0.12 | |
*Fold change value represents the change in expression in FaCdc3 and FaCdc12 deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71 relative to that in the wild‐type strain zj‐1.
†Primers for each gene of the Fusarium asiaticum strain zj‐1 were designed on the basis of the whole nucleotide sequence of FGSG_XXXXX.3 from the genome database of Fusarium graminearum PH‐1.
FaCdc3 and FaCdc12 are involved in septum formation and nuclear distribution in F. asiaticum
Previous studies have shown that septins are indispensable for normal septum formation and cytokinesis in the budding yeast S. cerevisiae (Adams and Pringle, 1984; Hartwell, 1971). To investigate the roles of FaCdc3 and FaCdc12 in septum development and nuclear distribution in F. asiaticum, we stained the conidia and hyphae of the wild‐type, two deletion mutants and two complemented mutants with calcofluor white. In contrast with the conidia of the wild‐type and the complemented mutant, the conidia of ΔFaCdc3‐5 and ΔFaCdc12‐71 formed fewer septa (Fig. 5A,B). In addition, the hyphae of both ΔFaCdc3‐5 and ΔFaCdc12‐71 mutants formed fewer septa than those of the wild‐type and the complemented mutants (Fig. 5C,D). Moreover, the nuclei were regularly distributed in the conidia and hyphae of the wild‐type and complemented strains, but unevenly distributed and clustered in ΔFaCdc3‐5 and ΔFaCdc12‐71 (Fig. 6A–C). These results indicate that FaCdc3 and FaCdc12 affect septum formation and nuclear division in F. asiaticum.
Figure 5.
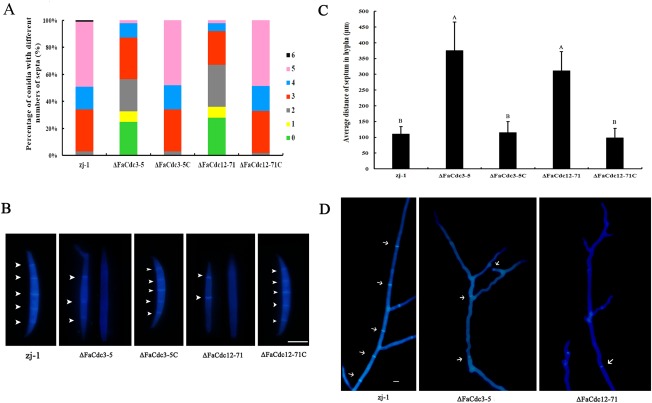
Septa in conidia and hyphae of the parental strain zj‐1, deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71, and complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C. (A) Percentage of conidia with different numbers of septa. (B) Variation in septum number per conidia exhibited by each strain. (C) Average distance of septum in hypha. (D) Septa of hyphae in each strain. Bar, 10 μm. Septa were stained with calcofluor white and examined with episcopic fluorescence microscopy.
Figure 6.
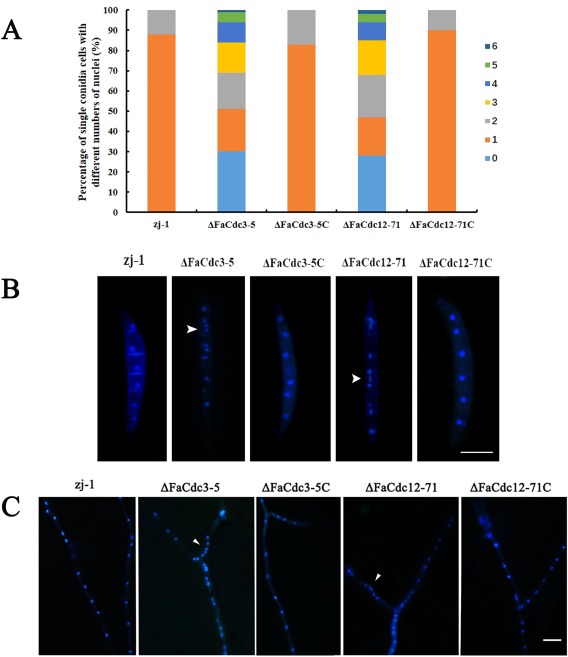
(A) Percentage of single conidial cells with different numbers of nuclei. Nuclear distribution in conidia (B) and hyphae (C) of the parental strain zj‐1, deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71, and complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C. Nuclei were labelled with 4',6‐diamidino‐2‐phenylindole (DAPI). Bar, 20 μm.
Effect of FaCdc3 and FaCdc12 on the pathogenicity of F. asiaticum
Previous studies have shown that septins are not required for virulence in the plant‐pathogenic fungus U. maydis (Alvarez‐Tabarés and Pérez‐Martín, 2010). To determine whether the septins FaCdc3 and FaCdc12 are associated with pathogenicity in F. asiaticum, we assessed the ability of ΔFaCdc3‐5 and ΔFaCdc12‐71 to infect wheat spikes via point inoculation of flowering wheat heads with conidial suspensions (Zheng et al., 2013). Fifteen days after inoculation, the wild‐type and complemented strains had colonized the inoculated spikelet, and >50% of the spikelets in inoculated wheat heads showed blight symptoms (Fig. 7). However, under the same conditions, the FaCdc3 and FaCdc12 deletion mutants caused infection only at the point of inoculation. These results demonstrate that FaCdc3 and FaCdc12 are involved in the full virulence of F. asiaticum.
Figure 7.

Virulence of the parental strain zj‐1, deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71, and complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C on wheat heads. Each floret of wheat cultivar Zhenmai 5 was injected with 20 μL of a conidial suspension (5 × 105/mL) and maintained in a humid glasshouse. Wheat heads were photographed 15 days after inoculation.
FaCdc3 and FaCdc12 regulate DON biosynthesis and the expression of TRI5 and TRI6
Trichothecene mycotoxins are sesquiterpenes and secondary metabolites of F. asiaticum strains, and the trichothecene mycotoxin DON is an important virulence factor in F. graminearum (Desjardins et al., 1996; Proctor et al., 1995; Seong et al., 2009). Because the deletion of FaCdc3 and FaCdc12 reduced virulence in F. asiaticum, the ability of ΔFaCdc3 and ΔFaCdc12 to produce DON was assessed. After each strain had been cultured in glucose–yeast extract–peptone (GYEP) medium at 28 ºC in the dark for 7 days, ΔFaCdc3‐5 produced about one‐half and ΔFaCdc12‐71 produced about one‐third of the quantity of DON relative to the wild‐type strain zj‐1 or the complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C, respectively (Fig. 8A; Table 1). The expression levels of TRI5 and TRI6 were significantly lower in ΔFaCdc3‐5 and ΔFaCdc12‐71 than in the wild‐type and complemented strains (Fig. 8B). These results indicate that FaCdc3 and FaCdc12 are important for the biosynthesis of DON in F. asiaticum.
Figure 8.
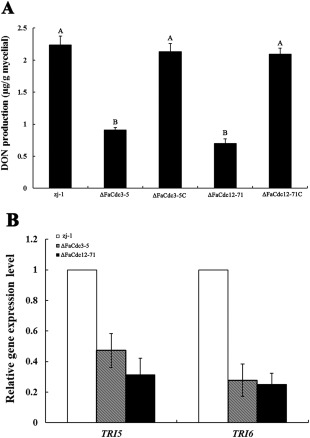
Deoxynivalenol (DON) production (A) and expression of TRI5 and TRI6 (B) in the parental strain zj‐1, deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71, and complemented strains ΔFaCdc3‐5C and ΔFaCdc12‐71C. Production and expression were assessed after 7 days in glucose–yeast extract–peptone (GYEP) medium. Expression levels of TRI5 and TRI6 in deletion mutants ΔFaCdc3‐5 and ΔFaCdc12‐71 are relative to the level in zj‐1. Values are the means and standard errors of three experiments.
Discussion
Septins are highly conserved in fungi and participate in diverse but critical processes, including morphogenesis, vegetative development and host infection. Unlike S. cerevisiae and C. albicans, we found that FaCdc3 and FaCdc12 are not essential for cell viability in F. asiaticum. This finding is in agreement with data from other filamentous fungi, such as A. nidulans, Ashbya gossypii and the fission yeast Saccharomyces pombe, for which none of the homologous genes were essential (Berlin et al., 2003; DeMay et al., 2009; Helfer and Gladfelter, 2006; Hernández‐Rodríguez et al., 2012; Lindsey et al., 2010; Tasto et al., 2003; Warenda et al., 2003; Wu et al., 2010). These reports also indicate that Cdc3 and Cdc12 perform species‐specific roles in various fungi.
The phenotypes of ΔFaCdc3 and ΔFaCdc12 mutants include a reduced mycelial growth rate, irregularly shaped hyphae with an abnormal branching pattern, and malformed conidia, which are straight instead of falciform, indicating a disruption of cell polarity. Aberrant branching patterns have also been observed in the homologous Cdc3 and Cdc12 deletion mutants of A. nidulans, Ashbya gossypii and Neurospora crassa (Berepiki and Read, 2013; Helfer and Gladfelter, 2006; Lindsey et al., 2010). In addition, ΔFaCdc3 and ΔFaCdc12 mutants exhibit reduced conidiation and sexual reproduction. The disruption of FaCdc3 and FaCdc12 interferes with both asexual growth and sexual reproduction in F. asiaticum, which is consistent with the important role of Cdc3 and Cdc12 in proliferation and cytokinesis in S. cerevisiae and other filamentous fungi (Berepiki and Read, 2013; Fares et al., 1996; Hernández‐Rodríguez et al., 2012; Lindsey et al., 2010; Momany et al., 2001). These results demonstrate a critical role for Cdc3 and Cdc12 in cell polar growth and sexual and asexual development in diverse species of fungi.
In S. cerevisiae, septins are required for the localized deposition of chitin for the construction of cell walls and are closely associated with plasma membrane integrity (DeMarini et al., 1997). During hyphal growth, septins assemble and disassemble, forming bands at the base of emerging germ tubes and septation sites, collars at hyphal tips and filaments in mature chlamydospores, concomitant with thickening of the cell wall (Gladfelter et al., 2005; Longtine et al., 1996). In this study, ΔFaCdc3 and ΔFaCdc12 mutants displayed decreased sensitivity to cell wall‐damaging agents, which is in agreement with the up‐regulation of genes related to cell wall integrity.
Efficient neck localization of Ssk2p is critical for adaptation to osmotic stress in the high‐osmolarity growth (HOG) pathway and requires an intact septin cytoskeleton in S. cerevisiae (Yuzyuk et al., 2002). In this study, the septin mutants ΔFaCdc3 and ΔFaCdc12 exhibited reduced sensitivity to 1.2 m NaCl and 1.2 m KCl, which can trigger similar adaptive osmoregulation responses in mitogen‐activated protein kinase (MAPK) pathway in eukaryotic cells (Duan et al., 2013; Jiang et al., 2011a, 2011b). In addition, the actin cytoskeleton, for which septins are required for the correct disassembly and assembly of actin bundles, rapidly disassembles in response to osmotic stress and is induced to reassemble only after osmotic balance with the environment is re‐established in S. cerevisiae (Kinoshita et al., 2002; Yuzyuk et al., 2002). These results suggest that the septins Cdc3 and Cdc12 are involved in the responses to osmotic stress in S. cerevisiae and the filamentous fungus F. asiaticum.
The S. cerevisiae septins Cdc3 and Cdc12 are critical major components of bud neck filaments during cell septation, septum formation and complete cytokinesis, whereas, in A. nidulans, Ashbya gossypii, C. albicans and Saccharomyces pombe homologous Cdc3 and Cdc12 mutants, septa are still formed but, in some instances, appear abnormal (Berlin et al., 2003; Helfer and Gladfelter, 2006; Lindsey et al., 2010; Mostowy and Cossart, 2012; Tasto et al., 2003; Warenda and Konopka, 2002). In this study, calcofluor white staining and statistics demonstrated that ΔFaCdc3 and ΔFaCdc12 formed aberrant septa in conidia and hypha, which is not surprising given the known involvement of core septins in septation in other fungi (Hartwell, 1971; Wu et al., 2010). In addition, the hyphae and conidia of septin mutants ΔFaCdc3 and ΔFaCdc12 exhibited significant defects in nuclear distribution. Previous reports have shown that septins mediate nuclear movement during cell division in budding yeast (Kusch et al., 2002). Similarly, AspB and AspC mutants, homologous to Cdc3 and Cdc12, also show abnormally spaced clusters of nuclei in A. nidulans, whereas Cdc12 deletion mutants exhibit regular nuclear distribution in Cryptococcus neoformans (Hernández‐Rodríguez et al., 2012; Kozubowski and Heitman, 2010; Lindsey et al., 2010). These data suggest that the septins Cdc3 and Cdc12 are necessary for normal nuclear distribution and septa formation, play a key role in cytokinesis in F. asiaticum and play species‐specific roles in diverse fungal species.
Lipids are essential components for life. They are synthesized and modified in the cell for use in metabolism, membranes and signalling. To avoid toxicity, cells convert excess lipids into inert neutral lipids, which are stored in membrane‐bound organelles as LDs (Pol et al., 2014; Walther and Farese, 2012). In budding yeast, the quadruple mutant Δare1Δare2Δdga1Δlro1, which cannot synthesize neutral lipids and therefore lacks LDs, exhibits abnormal septa, unstable septin assembly during cytokinesis and prolonged exocytosis at the division site at the end of cytokinesis (Yang et al., 2016). In this study, we also observed that the conidia and hyphae of ΔFaCdc3 and ΔFaCdc12 mutants contained abnormal LDs compared with those of the wild‐type strain. This could be because ΔFaCdc3 and ΔFaCdc12 mutants formed fewer septa and underwent abnormal cytokinesis, leading to excess lipids stored in LDs. Further biological experiments are needed to confirm this hypothesis. To our knowledge, this is the first report indicating that the septins Cdc3 and Cdc12 affect lipid biosynthesis in filamentous fungi.
The roles of Cdc3 and Cdc12 in virulence are obviously diverse among various fungal pathogens. In agreement with a previous report on septins involved in virulence in Cryptococcus neoformans, a pathogenic basidiomycete fungus (Kozubowski and Heitman, 2010), we found that deletion of FaCdc3 and FaCdc12 substantially affected virulence in F. asiaticum. These results are consistent with the characterization of septins in U. maydis (Alvarez‐Tabarés and Pérez‐Martín, 2010), which describes severely affected virulence in the sep3 deletion mutant. Furthermore, reactive oxygen species (ROS) and osmotic stress generated by the host plant, as well as the integrity of fungal cell walls, play important roles in the interaction of fungal and host plant tissue (Bowman and Free, 2006). In the current study, we found that ΔFaCdc3 and ΔFaCdc12 displayed decreased sensitivity to cell wall‐damaging agents, which may contribute to the deleterious effect of ΔFaCdc3 and ΔFaCdc12 on virulence, and should be studied in future research. Previous studies have shown that the trichothecene toxin DON, although not required for infection, is an important virulence factor for spread within the rachis tissue of F. graminearum (Bai et al., 2002; Desjardins et al., 1996; Jansen et al., 2005; Maier et al., 2006; Proctor et al., 1995; Seong et al., 2009). In this study, ΔFaCdc3 and ΔFaCdc12 mutants produced significantly less DON than the wild‐type strain. This result is consistent with the observation that ΔFaCdc3 and ΔFaCdc12 mutants cause scab symptoms in inoculated or nearby spikelets, but cannot spread within the rachis.
In conclusion, we found that FaCdc3 and FaCdc12 play a significant role in the growth, sexual and asexual development of F. asiaticum. We also found that FaCdc3 and FaCdc12 are important for septation, nuclear distribution, cytokinesis, virulence and response to various stresses. Based on phenotypic analysis, FaCdc3 and FaCdc12 appear to have very similar roles in most of the examined processes. Our results provide the foundation for the further investigation of the mechanisms of control of septation and cytokinesis in F. asiaticum, and may facilitate the development of septin‐targeted fungicides to control FHB and reduce mycotoxins in cereal crops.
Experimental Procedures
Strains and culture conditions
Fusarium asiaticum strain zj‐1, which was isolated in Zhejiang Province, China, in 2000, was used as the wild‐type. The wild‐type strain and transformants generated in this study were maintained on PSA (200 g potato, 20 g sucrose, 20 g agar and 1 L water). CM (1% glucose, 0.2% peptone, 0.1% yeast extract, 0.1% casamino acids, nitrate salts, trace elements, 0.01% vitamins and 1 L water, pH 6.5) was used for mycelial growth assays. MBL medium (30 g of mung beans boiled in 1 L water for 20 min and filtered through cheesecloth) was used for conidiation assays (Bai and Shaner, 1996). GYEP liquid medium (5% glucose, 0.1% yeast extract and 0.1% peptone) was used for DON assays.
Sequence analysis of FaCdc3 and FaCdc12 in F. asiaticum
FgCdc3 and FgCdc12 (F. graminearum genome accession numbers FGSG_05315.3 and FGSG_07380) were originally identified through homology searches of the F. graminearum amino acid sequence (available at http://www.broadinstitute.org/annotation/genome/fusarium_group/MultiHome.html) using blast with AspB and AspC from A. nidulans as query. Based on the whole nucleotide sequences of FGSG_05315 and FGSG_07380, each orthologue of FaCdc3 and FaCdc12 was sequenced from F. asiaticum strain zj‐1. The sequences of FaCdc3 and FaCdc12 were compared with those of FGSG_05315 and FGSG_07380.
Generation of FaCdc3 and FaCdc12 deletion mutants
To investigate the functions of FaCdc3 and FaCdc12 in F. asiaticum, we constructed deletion mutants. We first constructed the gene replacement cassette ΔFaCdc3, which carries the hygromycin resistance gene and herpes simplex virus thymidine kinase gene flanked by DNA sequences corresponding to the sequences located at the 5′ (left junction) and 3′ (right junction) ends of the FaCdc3 gene. This cassette was constructed with double‐joint PCR as described previously (Yu et al., 2004). In the first PCR round, fragments 1.5 kb upstream and 1.4 kb downstream of FaCdc3 were amplified from the genomic DNA of strain zj‐1 using the primer pairs P1/P2 and P3/P4, respectively. The primers HTF/HTR were used to amplify a 3.5‐kb fragment encoding the HPH‐HSV‐tk cassette containing the hygromycin resistance gene, the herpes simplex virus thymidine kinase gene and the A. nidulans trpC promoter. This cassette was initially amplified from the PtrpChptA‐PItk plasmid (Yu et al., 2004). The three amplicons (left junction, HPH‐HSV‐tk cassette and right junction) were gel purified using an OMEGA BIO‐TEK (Shanghai, China) gel purification kit, mixed at a molar ratio of 1 : 3 : 1 and used as a template for the fusion PCR round, which was performed using La‐Taq Polymerase (TaKaRa, Dalian, China) without primers. In the third PCR round, 1 μL of product from the second PCR round was used as DNA template to amplify a 6.4‐kb DNA fragment using the primers P1/P4. The DNA fragment generated from the third PCR round, which carried the HPH‐HSV‐tk cassette fused to the FaCdc3 flanking region, was gel purified and used to transform protoplasts of F. asiaticum strain zj‐1. The same procedures were used to construct deletion mutants of FaCdc12.
Complementation of FaCdc3 and FaCdc12 deletion mutants
One FaCdc3 deletion mutant (ΔFaCdc3‐5) was complemented with the full‐length FaCdc3 gene to confirm that the phenotypic changes of the FaCdc3 deletion mutant were caused by the disruption of the gene. The vector for the complementation of ΔFaCdc3‐5 was amplified from the genomic DNA of strain zj‐1 using primers P7/P8 (Figs S3A and S4A; Table S1, see Supporting Information). The complemented strain was designated ΔFaCdc3‐5C. The same procedure was used to generate ΔFaCdc12‐71C from the FaCdc12 deletion mutant ΔFaCdc12‐71.
Protoplast preparation and transformation of F. asiaticum
For the preparation of protoplasts, conidia of the zj‐1 strain were harvested from 7‐day‐old cultures grown in MBL medium and added to YEPD liquid medium (w/v, 1% peptone, 0.3% yeast extract, 2% glucose). After 12 h at 25 °C, the young mycelia in YEPD liquid medium were filtered, washed with 0.7 m NaCl and treated with lysing enzyme (5 mg/mL in 0.7 m NaCl, Sigma), driselase (5 mg/mL in 0.7 m NaCl, Sigma, St. Louis, MO, USA) and chitinase (0.05 mg/mL in 0.7 m NaCl, Sigma). After 2 h at 30 °C, the enzyme solution was filtered through three layers of lens paper to eliminate mycelial residues. The protoplasts in the filtrate were then washed twice with 0.7 m NaCl, twice with STC (0.8 m sorbitol, 0.05 m Tris, pH 8.0, 50 mm CaCl2) and resuspended in SPTC (STC with 40% w/v PEG 6000) buffer (STC : SPTC = 4 : 1).
For transformation, 107 protoplasts in 200 μL of SPTC buffer and 40 μL (100 μg/μL) of target DNA in 10 μL of heparin sodium were mixed and incubated on ice for 30 min; 1 mL of SPTC was then mixed with the suspension and incubated at room temperature for 20 min. Protoplasts were mixed into 200 mL of regeneration medium (0.1% yeast extract, 0.1% casein hydrolysate, 1.0 m sucrose, 1.6% granulated agar) at 43 °C, and the mixture was poured into 9‐cm‐diameter Petri plates (20 mL per plate) and incubated at 25 °C. After 12–24 h, the plates were overlaid with 10 mL of selective agar (0.1% yeast extract, 0.1% casein hydrolysate, 1.2 m sucrose, 1.2% granulated agar containing 100 μg/mL hygromycin B) and further incubated. Transformants were obtained at 3 days post‐transformation. They were transferred to fresh PSA plates containing 100 μg/mL hygromycin B (which supports the growth of the transformants, but not the complemented mutants) and 0.2 μm floxuridine (which supports the growth of the complemented mutants, but not the transformants). The putative transformants were purified by single‐spore isolation. Transformation of ΔFgCdc3‐5 with the full‐length FgCdc3 gene, or of ΔFgCdc12‐71 with the full‐length FgCdc12 gene, was conducted as described above, except that floxuridine was used as a selection agent.
Sensitivity of mycelial growth to stresses and the production of conidia and perithecia
We determined the sensitivity of the deletion mutants to osmotic stress generated by NaCl and KCl, and cell membrane damage generated by SDS. Mycelial growth was measured after the mutants and controls had been incubated at 25 °C for 38 days in the dark on PSA plates containing 1.2 m NaCl, 1.2 m KCl, 0.05% (w/v) Congo red, 5 mm caffeine or 0.05% SDS (w/v). The percentage of mycelial growth inhibition (RGI) was calculated using the formula RGI = [(A – B)/(A – 5)] × 100, where A is the colony diameter of the control and B is the colony diameter of the treated sample (Zheng et al., 2013). The experiment was performed three times.
For the conidiation assay, eight mycelial plugs were placed in 100 mL of MBL medium and shaken (175 rpm) at 25 ºC in the light. After 7 days, the spores were counted with a haemocytometer (Qiu et al., 2011). Each strain was represented by three replicate flasks, and the experiment was repeated twice.
The perithecium production assay was conducted as described by Qiu et al. (2011) with some modifications. In brief, the deletion mutants and controls were cultured on autoclaved wheat seeds for 10 days at 25 °C. The colonized seeds were then placed on sterile wet sand in a 9‐mm‐diameter Petri dish and kept at 25 °C with 80% relative humidity and a 12‐h photoperiod. Each strain was represented by three Petri plates. After 15 days, the seeds were scored as ‘+++’, ‘++’ or ‘+’ when perithecia covered more than two‐thirds, between one‐third and two‐thirds, or less than one‐third of each seed, respectively. Each strain was represented by three replicate plates, and the experiment was repeated twice.
Quantitative RT‐PCR
RNA samples were isolated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). First‐strand cDNA was synthesized with the PrimeScript® RT reagent kit (TaKaRa). All quantitative RT‐PCRs were performed with an ABI 7500 real‐time detection system (Applied Biosystems, Foster City, CA, USA). The primers used for quantitative RT‐PCR analysis are listed in Table S1. All data were normalized to actin gene expression, and relative changes in gene expression levels were analysed with ABI 7500 SDS software (Applied Biosystems), which automatically sets the baseline. Data from three biological replicates were used to calculate the means and standard deviations.
Pathogenicity assay on flowering wheat heads
After incubation in MBL medium for 7 days, conidia of each strain (wild‐type, deletion mutants and complemented deletion mutants) were collected by filtration through three layers of lens paper and subsequently resuspended in sterile distilled water to a concentration of 106 conidia/mL. Wheat heads of the Zhenmai cultivar (which is susceptible to F. asiaticum) were inoculated with 10 μL of a conidial suspension as described by Gale et al. (2002). The plants were moistened for 2 days, and the wheat plants were maintained in a glasshouse. Each strain was represented by 20 replicate wheat heads. Fifteen days after inoculation, the number of infected spikelets in each inoculated wheat head was determined, and the data were assessed by Fisher's least significant difference test. The experiment was repeated twice.
Analysis of DON production in vitro
In an assay for DON production in vitro, conidia of the wild‐type strain, the two deletion mutants and the complemented deletion mutants were added to GYEP liquid medium (1 × 105 conidia/mL). After 7 days at 28 °C, the filtrate was collected and the mycelia were dried and weighed. DON was extracted from the filtrate with ethyl acetate, derivatized, mixed with iso‐octane and analysed by gas chromatography‐mass spectrometry (GC‐MS) (Zheng et al., 2013). DON production was expressed per gram of mycelium. The expression levels of two trichothecene biosynthesis genes, TRI5 and TRI6, were analysed by quantitative real‐time PCR using RNA samples from mycelia grown in GYEP medium. Three biological replicates were tested for each strain.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 The alignment of the nucleotide sequences of FaCdc3 (A) and FaCdc12 (B) from Fusarium asiaticum with FgCdc3 and FgCdc12 from Fusarium graminearum.
Fig. S2 The alignment of the amino acid sequences of FaCdc3 (A) and FaCdc12 (B) from Fusarium asiaticum with FgCdc3 and FgCdc12 from Fusarium graminearum. Red boxes show differences in amino acid sites.
Fig. S3 Generation and identification of FaCdc3 gene deletion mutants. (A) Gene replacement and complementation strategy for the FaCdc3 gene. The gene replacement cassette HPH‐HSV‐tk contains the hygromycin resistance gene and the herpes simplex virus thymidine kinase gene. Primer binding sites are indicated by arrows (see Table S1 for primer sequences). (B) Polymerase chain reaction (PCR) strategy to screen ΔFaCdc3 transformants: (a) PCR performed with primer pair P7/P8; a 2.3‐kb amplified fragment indicates ΔFaCdc3 integration at the left junction; (b) PCR performed with primer pair P9/P10; a 2.2‐kb amplified fragment indicates ΔFaCdc3 integration at the right junction; (c) PCR performed with primer pair P7/P10; a 1.3‐kb amplified fragment indicates a wild‐type gene locus. (C) Southern blot hybridization analysis of zj‐1, ΔFaCdc3‐5 and ΔFaCdc3‐5C using a 518‐bp right homologous arm fragment as a probe with genomic DNA digested with Eco81Ι.
Fig. S4 Generation and identification of FaCdc12 gene deletion mutants. (A) Gene replacement and complementation strategy for the FaCdc12 gene. The gene replacement cassette HPH‐HSV‐tk contains the hygromycin resistance gene and the herpes simplex virus thymidine kinase gene. Primer binding sites are indicated by arrows (see Table S1 for primer sequences). (B) Polymerase chain reaction (PCR) strategy to screen ΔFaCdc12 transformants: (a) PCR performed with primer pair F7/F8; a 2.0‐kb amplified fragment indicates ΔFaCdc12 integration at the left junction; (b) PCR performed with primer pair F9/F10; a 2.2‐kb amplified fragment indicates ΔFaCdc12 integration at the right junction; (c) PCR performed with primer pair F7/F10; a 0.5‐kb amplified fragment indicates a wild‐type gene locus. (C) Southern blot hybridization analysis of zj‐1, ΔFaCdc12‐71 and ΔFaCdc12‐71C using a 599‐bp right homologous arm fragment as a probe with genomic DNA digested with EcoRI.
Fig. S5 Assays for defects in stress responses. Cultures of the wild type (zj‐1), FaCdc3 and FaCdc12 deletion mutants (ΔFaCdc3‐5 and ΔFaCdc3‐12) and complemented strains (ΔFaCdc3‐5C and ΔFaCdc3‐12C) on potato sucrose agar (PSA) with or without 1.2 m NaCl or KCl, 0.05% Congo red, 5 mm caffeine or 0.05% sodium dodecylsulfate (SDS). Cultures were photographed after 3–10 days at 25 °C.
Table S1 Oligonucleotide primers used in this study.
ACKNOWLEDGEMENTS
This work was supported by the National Science Foundation of China (Nos. 31171880 and 31672065), the Chinese ‘973’ Program (No. 2012CB114000), the ‘863’ Program (No. 2012AA101502) and the Special Fund for Agro‐scientific Research in the Public Interest (Nos. 201303023 and 201303025).
REFERENCES
- Adams, A. and Pringle, J.R. (1984) Relationship of actin and tubulin distribution to bud growth in wild‐type and morphogenetic‐mutant Saccharomyces cerevisiae . J. Cell Biol. 98, 934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez‐Tabarés, I. and Pérez‐Martín, J. (2010) Septins from the phytopathogenic fungus Ustilago maydis are required for proper morphogenesis but dispensable for virulence. PLoS One, 5, e12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, G.H. and Shaner, G. (1996) Variation in Fusarium graminearum and cultivar resistance to wheat scab. Plant Dis. 80, 975–979. [Google Scholar]
- Bai, G.H. , Desjardins, A. and Plattner, R. (2002) Deoxynivalenol‐nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia, 153, 91–98. [DOI] [PubMed] [Google Scholar]
- Berepiki, A. and Read, N.D. (2013) Septins are important for cell polarity, septation and asexual spore formation in Neurospora crassa and show different patterns of localisation at germ tube tips. PLoS One, 8, e63843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin, A. , Paoletti, A. and Chang, F. (2003) Mid2p stabilizes septin rings during cytokinesis in fission yeast. J. Cell Biol. 160, 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin, A. , McMurray, M.A. , Grob, P. , Park, S.S. , Garcia, G. , Patanwala, I. , Ng, H.L. , Alber, T. , Thorner, J. and Nogales, E. (2008) Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc. Natl. Acad. Sci. USA, 105, 8274–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, S.M. and Free, S.J. (2006) The structure and synthesis of the fungal cell wall. Bioessays, 28, 799–808. [DOI] [PubMed] [Google Scholar]
- DeMarini, D.J. , Adams, A.E. , Fares, H. , De Virgilio, C. , Valle, G. , Chuang, J.S. and Pringle, J.R. (1997) A septin‐based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 139, 75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMay, B.S. , Meseroll, R.A. , Occhipinti, P. and Gladfelter, A.S. (2009) Regulation of distinct septin rings in a single cell by Elm1p and Gin4p kinases. Mol. Biol. Cell, 20, 2311–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins, A.E. (2006) Fusarium Mycotoxins: Chemistry, Genetics, and Biology. St Paul, MN: American Phytopathological Society (APS Press). [Google Scholar]
- Desjardins, A.E. , Proctor, R.H. , Bai, G. , McCormick, S.P. , Shaner, G. , Buechley, G. and Hohn, T.M. (1996) Reduced virulence of trichothecene‐nonproducing mutants of Gibberella zeae in wheat field tests. Mol. Plant–Microbe Interact. 9, 775–781. [Google Scholar]
- De Virgilio, C. , De Marini, D.J. and Pringle, J.R. (1996) SPR28, a sixth member of the septin gene family in Saccharomyces cerevisiae that is expressed specifically in sporulating cells. Microbiology, 142, 2897–2905. [DOI] [PubMed] [Google Scholar]
- Donnell, K.O. , Ward, T.J. , Geiser, D.M. , Kistler, H.C. and Aokid, T. (2004) Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet. Biol. 41, 600–623. [DOI] [PubMed] [Google Scholar]
- Duan, Y. , Ge, C. , Liu, S. , Wang, J. and Zhou, M. (2013) A two‐component histidine kinase Shk1 controls stress response, sclerotial formation and fungicide resistance in Sclerotinia sclerotiorum . Mol. Plant Pathol. 14, 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares, H. , Goetsch, L. and Pringle, J.R. (1996) Identification of a developmentally regulated septin and involvement of the septins in spore formation in Saccharomyces cerevisiae . J. Cell Biol. 132, 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, C.M. and Kellogg, D. (1999) Septins: cytoskeletal polymers or signalling GTPases. Trends Cell Biol. 9, 387–394. [DOI] [PubMed] [Google Scholar]
- Frazier, J.A. , Wong, M.L. , Longtine, M.S. , Pringle, J.R. , Mann, M. , Mitchison, T.J. and Field, C. (1998) Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J. Cell Biol. 143, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, L.R. , Chen, L.F. , Hernick, C. , Takamura, K. and Kistler, H. (2002) Population analysis of Fusarium graminearum from wheat fields in eastern China. Phytopathology, 92, 1315–1322. [DOI] [PubMed] [Google Scholar]
- Gladfelter, A.S. , Kozubowski, L. , Zyla, T.R. and Lew, D.J. (2005) Interplay between septin organization, cell cycle and cell shape in yeast. J. Cell Sci. 118, 1617–1628. [DOI] [PubMed] [Google Scholar]
- Goswami, R.S. and Kistler, H.C. (2004) Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 5, 515–525. [DOI] [PubMed] [Google Scholar]
- Hartwell, L. (1971) Genetic control of the cell division cycle in yeast: IV. Genes controlling bud emergence and cytokinesis. Exp. Cell Res. 69, 265–276. [DOI] [PubMed] [Google Scholar]
- Helfer, H. and Gladfelter, A.S. (2006) AgSwe1p regulates mitosis in response to morphogenesis and nutrients in multinucleated Ashbya gossypii cells. Mol. Biol. Cell, 17, 4494–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Rodríguez, Y. , Hastings, S. and Momany, M. (2012) The septin AspB in Aspergillus nidulans forms bars and filaments and plays roles in growth emergence and conidiation. Eukaryot. Cell, 11, 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, C. , von Wettstein, D. , Schafer, W. , Kogel, K.H. , Felk, A. and Maier, F.J. (2005) Infection patterns in barley and wheat spikes inoculated with wild‐type and trichodiene synthase gene disrupted Fusarium graminearum . Proc. Natl. Acad. Sci. USA, 102, 16 892–16 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Liu, X. , Yin, Y. and Ma, Z. (2011a) Involvement of a velvet protein FgVeA in the regulation of asexual development, lipid and secondary metabolisms and virulence in Fusarium graminearum . PLoS One, 6, e28291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J.H. , Yun, Y.Z. , Fu, J. , Shim, W.B. and Ma, Z.H. (2011b) Involvement of a putative response regulator FgRrg‐1 in osmotic stress response, fungicide resistance and virulence in Fusarium graminearum . Mol. Plant Pathol. 12, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, M. (2006) Diversity of septin scaffolds. Curr. Opin. Cell Biol. 18, 54–60. [DOI] [PubMed] [Google Scholar]
- Kinoshita, M. , Coughlin, M.L. , Straight, A.F. and Mitchison, T.J. (2002) Self‐ and actin‐templated assembly of mammalian septins. Dev. Cell, 3, 791–802. [DOI] [PubMed] [Google Scholar]
- Kozubowski, L. and Heitman, J. (2010) Septins enforce morphogenetic events during sexual reproduction and contribute to virulence of Cryptococcus neoformans . Mol. Microbiol. 75, 658–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch, J. , Meyer, A. , Snyder, M.P. and Barral, Y. (2002) Microtubule capture by the cleavage apparatus is required for proper spindle positioning in yeast. Genes Dev. 16, 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie, J.F. and Summerell, B.A. (2006) The Fusarium Laboratory Manual. Blackwell Publishing; Professional, 2121, 274–275. [Google Scholar]
- Lindsey, R. , Cowden, S. , Hernández‐Rodríguez, Y. and Momany, M. (2010) Septins AspA and AspC are important for normal development and limit the emergence of new growth foci in the multicellular fungus Aspergillus nidulans . Eukaryot. Cell, 9, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M.S. , DeMarini, D.J. , Valencik, M.L. , Al‐Awar, O.S. , Fares, H. , De Virgilio, C. and Pringle, J.R. (1996) The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8, 106–119. [DOI] [PubMed] [Google Scholar]
- Maier, F.J. , Miedaner, T. , Hadeler, B. , Felk, A. , Salomon, S. and Lemmens, M. (2006) Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Mol. Plant Pathol. 7, 449–461. [DOI] [PubMed] [Google Scholar]
- Momany, M. , Zhao, J. , Lindsey, R. and Westfall, P.J. (2001) Characterization of the Aspergillus nidulans septin (asp) gene family. Genetics, 157, 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy, S. and Cossart, P. (2012) Septins: the fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 13, 183–194. [DOI] [PubMed] [Google Scholar]
- Nisessen, L. (2007) PCR‐based diagnosis and quantification of mycotoxin producing fungi. Int. J. Food Microbiol. 19, 38–46. [DOI] [PubMed] [Google Scholar]
- Ozsarac, N. , Bhattacharyya, M. , Dawes, I.W. and Clancy, M.J. (1995) The SPR3 gene encodes a sporulation‐specific homologue of the yeast CDC3/10/11/12 family of bud neck microfilaments and is regulated by ABFI. Gene, 164, 157–162. [DOI] [PubMed] [Google Scholar]
- Pan, F. , Malmberg, R.L. and Momany, M. (2007) Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol. Biol. 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol, A. , Gross, S.P. and Parton, R.G. (2014) Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J. Cell Biol. 204, 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor, R.H. , Hohn, T.M. and McCormick, S.P. (1995) Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant–Microbe Interact. 8, 1995–1908. [DOI] [PubMed] [Google Scholar]
- Qiu, J. , Xu, J. , Yu, J. , Bi, C. , Chen, C. and Zhou, M. (2011) Localisation of the benzimidazole fungicide binding site of Gibberella zeae β2‐tubulin studied by site‐directed mutagenesis. Pest Manag. Sci. 67, 191–198. [DOI] [PubMed] [Google Scholar]
- Rodriguez, R.J. , Low, C. , Bottema, C.D.K. and Parks, L.W. (1985) Multiple functions for sterols in Saccharomyces cerevisiae . Biochim. Biophys. Acta, 837, 336–343. [DOI] [PubMed] [Google Scholar]
- Saunders, D.G. , Dagdas, Y.F. and Talbot, N.J. (2010) Spatial uncoupling of mitosis and cytokinesis during appressorium‐mediated plant infection by the rice blast fungus Magnaporthe oryzae. Plant Cell, 22, 2417–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong, K.Y. , Pasquali, M. , Zhou, X. , Song, J. , Hilburn, K. , McCormick, S. , Dong, Y. , Xu, J.R. and Kistler, H.C. (2009) Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol. Microbiol. 72, 354–367. [DOI] [PubMed] [Google Scholar]
- Starkey, D.E. , Ward, T.J. , Aoki, T. , Gale, L.R. , Kistler, H.C. , Geiser, D.M. , Suga, H. , Toth, B. , Varga, J. and O'Donnell, K. (2007) Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genet. Biol. 44, 1191–1204. [DOI] [PubMed] [Google Scholar]
- Sutton, J.C. (1982) Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum . Can. J. Plant Pathol. 4, 195–209. [Google Scholar]
- Tasto, J.J. , Morrell, J.L. and Gould, K.L. (2003) An anillin homologue, Mid2p, acts during fission yeast cytokinesis to organize the septin ring and promote cell separation. J. Cell Biol. 160, 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth, B. , Mesterházy, Á. , Horváth, Z. , Bartók, T. , Varga, M. , and Varga, J. (2005) Genetic variability of central European isolates of the Fusarium graminearum species complex. Eur . J. Plant Pathol. 113, 35–45. [Google Scholar]
- Versele, M. and Thorner, J. (2005) Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol. 15, 414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther, T.C. and Farese, R.V. (2012) Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 81, 687–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenda, A.J. and Konopka, J.B. (2002) Septin function in Candida albicans morphogenesis. Mol. Biol. Cell, 13, 2732–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warenda, A.J. , Kauffman, S. , Sherrill, T.P. , Becker, J.A. and Konopka, J.B. (2003) Candida albicans septin mutants are defective for invasive growth and virulence. Infect. Immun. 71, 4045–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windels, C.E. (2000) Economic and social impacts of Fusarium head blight, changing farms and rural communities in the northern great plains. Phytopathology, 90, 17–21. [DOI] [PubMed] [Google Scholar]
- Wu, J.Q. , Ye, Y. , Wang, N. , Pollard, T.D. and Pringle, J.R. (2010) Cooperation between the septins and the actomyosin ring and role of a cell‐integrity pathway during cell division in fission yeast. Genetics, 186, 897–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X.M. , Parry, D.W. , Nicholson, P. , Thomsett, M.A. , Simpson, D. , Edwards, S.G. , Cooke, B.M. , Doohan, F.M. , Monaghan, S. , Moretti, A. , Tocco, G. , Mule, G. , Hornok, L. , Béki, E. , Tatnell, J. and Ritieni, A. (2008) Within‐field variability of Fusarium head blight pathogens and their associated mycotoxins. Eur. J. Plant Pathol. 120, 21–34. [Google Scholar]
- Yang, P.‐L. , Hsu, T.‐H. , Wang, C.‐W. and Chen, R.‐H. (2016) Lipid droplets maintain lipid homeostasis during anaphase for efficient cell separation in budding yeast. Mol. Biol. Cell, 27, 2368–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J.H. , Hamari, Z. , Han, K.H. , Seo, J.A. , Reyes‐Domínguez, Y. and Scazzocchio, C. (2004) Double‐joint PCR: a PCR‐based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41, 973–981. [DOI] [PubMed] [Google Scholar]
- Yuzyuk, T. , Foehr, M. and Amberg, D.C. (2002) The MEK kinase Ssk2p promotes actin cytoskeleton recovery after osmotic stress. Mol. Biol. Cell, 13, 2869–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J.B. , Li, H.P. , Dang, F.J. , Qu, B. , Xu, Y.B. , Zhao, C.S. and Liao, Y.C. (2007) Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycol. Res. 111, 967–975. [DOI] [PubMed] [Google Scholar]
- Zheng, Z. , Gao, T. , Hou, Y. and Zhou, M. (2013) Involvement of the anucleate primary sterigmata protein FgApsB in vegetative differentiation, asexual development, nuclear migration, and virulence in Fusarium graminearum . FEMS Microbiol, Lett. 349, 88–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 The alignment of the nucleotide sequences of FaCdc3 (A) and FaCdc12 (B) from Fusarium asiaticum with FgCdc3 and FgCdc12 from Fusarium graminearum.
Fig. S2 The alignment of the amino acid sequences of FaCdc3 (A) and FaCdc12 (B) from Fusarium asiaticum with FgCdc3 and FgCdc12 from Fusarium graminearum. Red boxes show differences in amino acid sites.
Fig. S3 Generation and identification of FaCdc3 gene deletion mutants. (A) Gene replacement and complementation strategy for the FaCdc3 gene. The gene replacement cassette HPH‐HSV‐tk contains the hygromycin resistance gene and the herpes simplex virus thymidine kinase gene. Primer binding sites are indicated by arrows (see Table S1 for primer sequences). (B) Polymerase chain reaction (PCR) strategy to screen ΔFaCdc3 transformants: (a) PCR performed with primer pair P7/P8; a 2.3‐kb amplified fragment indicates ΔFaCdc3 integration at the left junction; (b) PCR performed with primer pair P9/P10; a 2.2‐kb amplified fragment indicates ΔFaCdc3 integration at the right junction; (c) PCR performed with primer pair P7/P10; a 1.3‐kb amplified fragment indicates a wild‐type gene locus. (C) Southern blot hybridization analysis of zj‐1, ΔFaCdc3‐5 and ΔFaCdc3‐5C using a 518‐bp right homologous arm fragment as a probe with genomic DNA digested with Eco81Ι.
Fig. S4 Generation and identification of FaCdc12 gene deletion mutants. (A) Gene replacement and complementation strategy for the FaCdc12 gene. The gene replacement cassette HPH‐HSV‐tk contains the hygromycin resistance gene and the herpes simplex virus thymidine kinase gene. Primer binding sites are indicated by arrows (see Table S1 for primer sequences). (B) Polymerase chain reaction (PCR) strategy to screen ΔFaCdc12 transformants: (a) PCR performed with primer pair F7/F8; a 2.0‐kb amplified fragment indicates ΔFaCdc12 integration at the left junction; (b) PCR performed with primer pair F9/F10; a 2.2‐kb amplified fragment indicates ΔFaCdc12 integration at the right junction; (c) PCR performed with primer pair F7/F10; a 0.5‐kb amplified fragment indicates a wild‐type gene locus. (C) Southern blot hybridization analysis of zj‐1, ΔFaCdc12‐71 and ΔFaCdc12‐71C using a 599‐bp right homologous arm fragment as a probe with genomic DNA digested with EcoRI.
Fig. S5 Assays for defects in stress responses. Cultures of the wild type (zj‐1), FaCdc3 and FaCdc12 deletion mutants (ΔFaCdc3‐5 and ΔFaCdc3‐12) and complemented strains (ΔFaCdc3‐5C and ΔFaCdc3‐12C) on potato sucrose agar (PSA) with or without 1.2 m NaCl or KCl, 0.05% Congo red, 5 mm caffeine or 0.05% sodium dodecylsulfate (SDS). Cultures were photographed after 3–10 days at 25 °C.
Table S1 Oligonucleotide primers used in this study.


