Summary
Inducible plant defences against pathogens are stimulated by infections and comprise several classes of pathogenesis‐related (PR) proteins. Endo‐β‐1,3‐glucanases (EGases) belong to the PR‐2 class and their expression is induced by many pathogenic fungi and oomycetes, suggesting that EGases play a role in the hydrolysis of pathogen cell walls. However, reports of a direct effect of EGases on cell walls of plant pathogens are scarce. Here, we characterized three EGases from Vitis vinifera whose expression is induced during infection by Plasmopara viticola, the causal agent of downy mildew. Recombinant proteins were expressed in Escherichia coli. The enzymatic characteristics of these three enzymes were measured in vitro and in planta. A functional assay performed in vitro on germinated P. viticola spores revealed a strong anti‐P. viticola activity for EGase3, which strikingly was that with the lowest in vitro catalytic efficiency. To our knowledge, this work shows, for the first time, the direct effect against downy mildew of EGases of the PR‐2 family from Vitis.
Keywords: endo‐β‐1,3‐glucanase; grapevine; Plasmopara viticola; PR‐2; Vitis vinifera
Introduction
Plants possess defences against pathogens involving an array of molecular mechanisms (Danilova, 2006; Hatsugai and Hara‐Nishimura, 2010; Jones and Dangl, 2006). The first line of defence is a physical and chemical barrier, comprising the cuticle (Hückelhoven, 2007; Reina‐Pinto and Yephremov, 2009), lignin deposits on cell walls, phytoalexins (Ahuja et al., 2012; Jeandet et al., 2002) and antimicrobial proteins (Cândido et al., 2011). However, these compounds do not protect against all pathogens. A second line of defence comprises receptor‐mediated defences that are induced following pathogen recognition, such as cell wall modifications (Hückelhoven, 2007), the release of reactive oxygen species (ROS) (Desikan et al., 1998; Mittler et al., 2011), the accumulation of secondary metabolites and the synthesis of proteins known as pathogenesis‐related (PR) proteins (van Loon et al., 2006; Stintzi et al., 1993). The last line of defence is the hypersensitive response, leading to cell death (Hara‐Nishimura and Hatsugai, 2011; Hofius et al., 2011).
PR proteins are synthesized by plants in response to pathogen attack and their synthesis is induced by signalling compounds, such as jasmonate, salicylate or ethylene (van Loon et al., 2006). They comprise an array of proteins with different functions, such as oxidases, chitinases and protease inhibitors. Based on their function, PR proteins are classified into 17 families, from PR‐1 to PR‐17 (van Loon et al., 2006; van Loon and van Strien, 1999). The PR‐2 class is composed of endo‐β‐1,3‐glucanases (EGases) (Balasubramanian et al., 2012), which are widespread enzymes in plants. They are not only involved in defence responses, but also in many physiological functions, such as pollen development (Worrall et al., 1992), germination (Morohashi and Matsushima, 2000), cell division and elongation (Ebrahim et al., 2011), and fruit ripening (Leubner‐Metzger, 2005).
Plant EGases are grouped into several classes, according to their amino acid sequence, structural properties and subcellular localization (Davies and Henrissat, 1995). Class I EGases have been described as basic proteins, localized in the vacuole (Bulcke et al., 1989). They generally harbour an N‐terminal signal peptide and a short glycosylated C‐terminal sequence for vacuolar targeting. Class II, III and IV EGases are usually acidic, deprived of the C‐terminal sequence (Bulcke et al., 1989; Kauffmann et al., 1987) and are generally secreted by default in the apoplasm. Several EGase transcripts are induced on infection (Jin et al., 2007; Neuhaus et al., 1992; Ward et al., 1991), but their expression level can also change during plant development (Balasubramanian et al., 2012). EGases hydrolyse β‐1,3‐glucosidic bonds in β‐1,3‐glucans, which are structural components of the cell walls of pathogenic fungi and oomycetes (Adams, 2004; Wessels, 1994). EGases are thus believed to directly hydrolyse pathogen cell walls, causing the lysis of pathogen cells (Britto et al., 2013). Accordingly, enzymes belonging to Class I have been reported to inhibit the growth of fungi in vitro (Arlorio et al., 1992; Ebrahim et al., 2011). EGases also contribute to plant defence by producing oligosaccharide elicitors that trigger the synthesis of other PR‐compounds (Klarzynski et al., 2000), such as phytoalexins (Keen et al., 1983).
Although numerous investigations have described the role of EGases in plant defence, only a few have described a direct effect of EGases on a pathogen. Here, we describe the cloning, protein purification and enzymatic analysis of three EGases from Vitis vinifera whose expression is induced on infection by Plasmopara viticola, the causal agent of grapevine downy mildew. We show that the enzyme displaying the lowest catalytic activity in vitro has the strongest effect on P. viticola germinated spores. Our results provide evidence of the effect of V. vinifera EGase activity on pathogen viability and suggest that care must be taken when interpreting the results of in vitro experiments with artificial substrates.
Results
Selection of V. vinifera EGases and enzyme classification
The protein sequences of two full‐length, mRNA‐derived EGases induced by pathogen infection (accession numbers AAF4467 and CAB91554) were used to search the grapevine genome for similar sequences. We retrieved a total of 31 proteins with strong similarity to either of the two query proteins (blastp, E value ≤ 10e‐40). The alignment of these proteins, the query sequences and sequences of EGases from Arabidopsis, tobacco and soybean allowed the identification of eight potentially misannotated or truncated proteins that were excluded from downstream analysis, which resulted in the phylogenetic tree shown in Fig. 1a. It is worth noting that all the EGases that are involved in responses to biotic stress in species other than grapevine cluster together in a clade of the phylogenetic tree.
Figure 1.
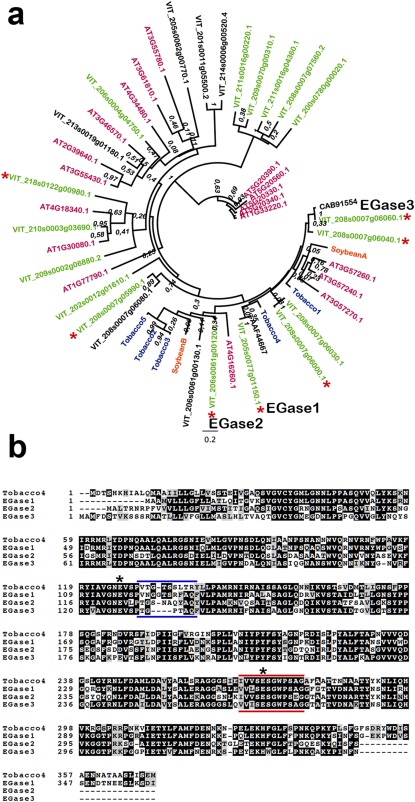
Analysis of grapevine endo‐β‐1,3‐glucanase (EGase) sequences. (a) Phylogenetic tree containing grapevine (black and green), Arabidopsis (magenta), tobacco (blue) and soybean (orange) endoglucanases. Grapevine sequences in green indicate proteins with a predicted signal peptide. Asterisks indicate grapevine genes for which evidence of expression is available. Numbers on the nodes indicate bootstrap values. Sequences selected as EGase1, EGase2 and EGase3 are specified. Grapevine and Arabidopsis endoglucanases are named according to the Grapevine Genome Database (v2) at CRIBI and The Arabidopsis Information Resource (TAIR), respectively (see Experimental procedures). The accession numbers in the tree correspond to the two original sequences used in the blast search. Accession numbers for tobacco and soybean glucanases are as follows: Tobacco1, CAA38324; Tobacco2, AAA34079; Tobacco3, AAD33880; Tobacco4, AAA34078; Tobacco5, AAA34103.1; SoybeanA, AAA33946; SoybeanB, AAR26001. (b) Alignment of the three selected grapevine endoglucanases and a tobacco endoglucanase possessing a vacuolar targeting signal. The black background shows identical amino acid residues. The grey background represents similar amino acids. Asterisks indicate predicted catalytic glutamic acid (Glu) residues. Blue bars show the divergent region in the vicinity of a catalytic Glu. Red bars show the glycosyl hydrolase family 17 signature.
Glucanases involved in defence are expected to be either vacuolar or apoplastic. We thus searched for proteins having a predicted signal peptide and whose expression was confirmed by the existence of the corresponding cDNA, yielding seven proteins (Fig. 1a, green and asterisk). Investigation of the RNA‐Seq data revealed that two of the seven genes (VIT_208s0007g06040, VIT_18s0122g00980) were expressed in berries, two genes (VIT_08s0007g05990, VIT_08s0007g06000) were non‐responsive to treatments and three genes (see below) were induced to different degrees in response to P. viticola infection (Table S1, see Supporting Information).
The three predicted proteins whose expression was induced on P. viticola infection were selected for detailed biochemical analysis and are referred to hereafter as EGase1 (VIT_205s0077g01150), EGase2 (VIT_206s0061g00120) and EGase3 (VIT_208s0007g06060). All three proteins belong to the clade containing the biotic stress‐induced proteins in Fig. 1a. An alignment of the three proteins is presented in Fig. 1b. Pairwise identity between EGases varies from 57% to 60%, with amino acid similarity ranging from 72% to 76%. Enzyme characteristics deduced from databanks are shown in Table 1.
Table 1.
Biochemical characteristics of the three endo‐β‐1,3‐glucanases (EGases) from Vitis.
| EGase1 | EGase2 | EGase3 | |
|---|---|---|---|
| Gene | VIT_205s0077g01150 | VIT_206s0061g00120 | VIT_208s0007g06060 |
| Accession no. | Q9M563 | A7PQW3 | F6HLL8 |
| GH family number | GH17 | GH17 | GH17 |
| Clan | GH‐A | GH‐A | GH‐A |
| Three‐dimensional structure status | (β/α)8 | (β/α)8 | (β/α)8 |
| E.C. | 3.2.1.39 | 3.2.1.39 | 3.2.1.39 |
| Residues | 376 | 344 | 345 |
| MW (Da) | 41286.04 | 37092.64 | 37501.89 |
| pI | 9.5 | 4.93 | 9.39 |
| Catalytic Glu | 132; 278 | 123; 268 | 127; 269 |
| Predicted mature protein | 39–278 | 27–268 | 27–269 |
Accession number is from UniprotKB. GH family number, clan, three‐dimensional structure status and E.C. are from the CAZy (carbohydrate‐active enzymes database) site (http://www.cazy.org). Molecular weights (MW) and pI were determined from the enzyme sequences. Catalytic glutamic acid (Glu) residues were predicted using PROSITE.
The three EGases from Vitis belong to the GH17 family and to the GH‐A clan (Lombard et al., 2014). They have the same EC number (EC 3.2.1.39) and thus are likely to display similar enzymatic properties. They have close theoretical molecular weights, but whereas EGase1 and EGase3 have theoretical basic pI values of 9.5 and 9.39, respectively, EGase2 has an acidic theoretical pI value of 4.93. According to the pI values and the data from the literature (Bulcke et al., 1989; Ebrahim et al., 2011), EGase1 and EGase3 are considered to be Class I endoglucanases and are predicted to be localized in the vacuole. However, it should be noted that only EGase1, but not EGase3, exhibits a C‐terminal vacuolar targeting sequence (Fig. 1b). EGase2 is predicted to be a Class II glucanase with an apoplastic localization.
Analysis of gene expression
To verify that all three EGases are expressed, we studied their expression in grapevine leaves during P. viticola infection by means of semi‐quantitative reverse transcription‐polymerase chain reaction (RT‐PCR). As presented in Fig. 2a, the expression of EGase2 and EGase3 was induced at 24 h post‐infection (hpi) by P. viticola, simultaneous with the detectable increase in pathogen growth, as shown by the increase in pathogen actin expression. Meanwhile, EGase1 expression remained low and was weakly induced by infection. All three EGases were weakly expressed in grapevine leaves mock inoculated with water, with a transient induction observed for EGase2 and EGase3 at 72 hpi (Fig. S1, see Supporting Information).
Figure 2.
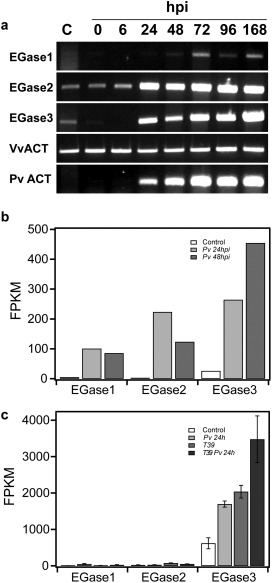
Expression of grapevine endoglucanases on Plasmopara viticola infection. (a) Semi‐quantitative reverse transcription‐polymerase chain reaction (RT‐PCR) showing the expression of grapevine endo‐β‐1,3‐glucanases (EGases) at 0, 6, 24, 48, 72, 96 and 168 h post‐inoculation (hpi). Non‐inoculated leaves were used as control (C). PvACT, Plasmopara viticola actin; VvACT, Vitis vinifera actin. The results are representative of two independent experiments. (b) Expression of EGases on grapevine leaves based on RNA‐Seq data from Vannozzi et al. (2012). Pv 24hpi, P. viticola‐infected leaves at 24 hpi; Pv 48hpi, P. viticola‐infected leaves at 48 hpi; FPKM, fragments per kilobase of exon per million fragments mapped. (c) Expression of EGases on grapevine leaves based on RNA‐Seq data from Perazzolli et al. (2012). Pv 24h, P. viticola‐infected leaves at 24 hpi; T39, leaves treated with Trichoderma harzianum T39, which induces resistance to downy mildew; T39 Pv 24h, P. viticola‐infected leaves following treatment with T39 at 24 hpi; FPKM, fragments per kilobase of exon per million fragments mapped. Data shown are the means and standard errors of three biological replicates.
In parallel, we compared the expression of all three EGases in silico in two independent RNA‐Seq datasets involving P. viticola infection. EGase3 expression was strongly induced by infection in both datasets and showed the highest basal expression level, EGase1 was weakly induced by P. viticola infection in both datasets and, finally, the EGase2 induction level was dependent on the dataset (Fig. 2b,c; Table S1).
Enzyme purification, activity and kinetic parameters
The results of protein purification are shown in Fig. 3. All EGases were obtained with purity higher than 95%. The molecular weights measured on sodium dodecylsulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) for EGase1, EGase2 and EGase3 were 39, 34 and 36 kDa, respectively, close to the predicted molecular weights of the mature proteins (38.5, 35 and 35 kDa, respectively), showing that no observable proteolysis occurred during purification.
Figure 3.
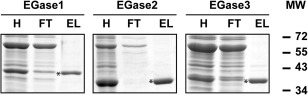
Sodium dodecylsulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) of purified grapevine endoglucanases. H, soluble lysate containing recombinant endo‐β‐1,3‐glucanase (EGase); FT, flow‐through of affinity Ni‐TED column; EL, purified recombinant EGase, indicated by an asterisk. Two micrograms of each enzyme were run on a 12% acrylamide gel and stained with colloidal Coomassie blue. MW, molecular weight.
The specific activities of the EGases were measured at 37°C, using laminarin as a substrate (Table 2). EGase2 had the highest specific activity (35.2 ± 1.3 nkat/nmol); the specific activities of EGase1 and EGase3 (17.2 ± 1.6 and 3.4 ± 0.2 nkat/nmol, respectively) were approximately one‐half and one‐tenth of that of EGase1, respectively.
Table 2.
Kinetic parameters of the three endo‐β‐1,3‐glucanases (EGases) from Vitis vinifera.
| Activity (nkat/nmol) | K M (µm) | k cat (s−1) | K cat/K M (µm −1 s−1) | |
|---|---|---|---|---|
| EGase1 | 17.2 ± 1.6 | 147 ± 15.0 | 34 ± 3.0 | 0.23 |
| EGase2 | 35.2 ± 1.3 | 228 ± 25.0 | 92 ± 7.0 | 0.4 |
| EGase3 | 3.4 ± 0.2 | 81 ± 2.5 | 3 ± 0.2 | 0.04 |
The kinetic parameters of the three enzymes were also measured using laminarin as a substrate and the results are shown in Table 2. The Michaelis constant K M approximates the affinity of the substrate for the enzyme (and is considered to be an apparent dissociation constant); the turnover constant k cat defines the maximum number of substrate molecules converted to product per unit of time; the specific constant k cat/K M combines the effectiveness of reaction with the effectiveness of substrate binding.
The K M values showed that EGase3 had the highest affinity (81 ± 2.5 µm) for the substrate, followed by EGase1 (147 ± 15 µm) and EGase2 (228 ± 25 µm). Based on the turnover constant (k cat) values, EGase2 was the most efficient enzyme to transform substrate into product (92 ± 7.0 s−1), followed by EGase1 (34 ± 3.0 s−1) and the least efficient enzyme EGase3 (3 ± 0.2 s−1).
The specificity constant (k cat/K M) of EGase2 had the highest value, followed by EGase1 and EGase3. Overall, the specific activities and specificity constants for the three EGases revealed the same relative efficiency for the three enzymes: EGase1 exhibited around one‐half of the EGase2 activity and EGase3 showed 10% of the activity of EGase2.
The differences in activity are surprising because all three enzymes show a high level of sequence identity. It should be noted that, apart from the vacuolar targeting signal, the most significant differences between enzyme sequences are located at the N‐terminus and in the vicinity of one of the predicted catalytic residues (Fig. 1b).
Substrate specificity and effect of pH and temperature on glucanase activity
In order to establish enzyme specificity, the activity of EGases was assessed on several substrates (see Experimental Procedures). The production of reducing sugars could not be detected using these substrates, suggesting a narrow substrate specificity of the three enzymes for β‐1,3‐branched sugars.
To evaluate the effect of pH, the activity of the three EGases was measured at 37°C, at pH values ranging from 3.0 to 7.5, with 0.5 increments. The results are presented in Fig. 4. EGase1 had a maximum activity at pH 5.5, whereas the maximum activity for both EGase2 and EGase3 was achieved at pH 5.0. Taken together, the three enzymes are active in the same pH range, with a wider pH range for EGase2.
Figure 4.
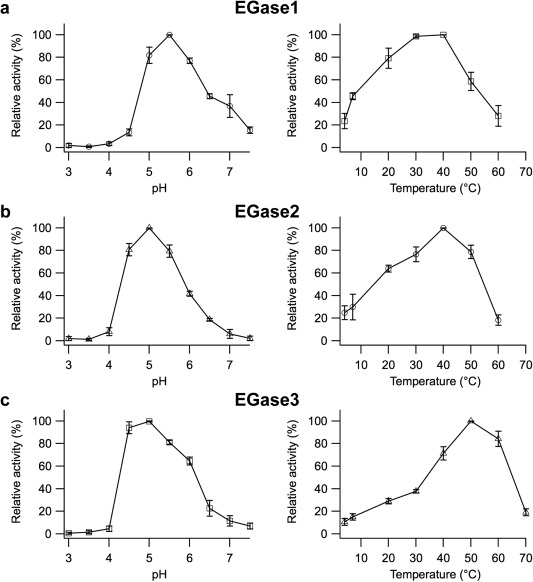
Effect of pH and temperature on grapevine glucanase enzymatic activity. Effect of pH and temperature on the activity of EGase1 (a), EGase2 (b) and EGase3 (c). Effect of pH (left panels) was measured at a constant temperature of 37°C. Effect of temperature (right panels) was measured at a constant pH of 5.4. The pH of the buffer was not affected by temperature. In both cases, the activity is expressed relative to the maximum activity of 100%. Data are the means of three independent experiments with no technical repetitions, and the error bars represent the standard deviation.
The effect of temperature on activity was also assessed. As shown in Fig. 4, the optimum temperature was around 40°C for EGase1 and EGase2, and higher, around 50°C, for EGase3. It is worth noting that the relative activity of EGase3 at 60°C is 80% of its maximum activity, compared with only 20% for EGase1 and EGase2.
Enzymatic activity of in planta‐expressed glucanases
To evaluate the enzyme activity when expressed in planta, the three EGases were transiently expressed in Nicotiana benthamiana leaves by agroinfiltration. Glucanase activity was measured in leaf protein extracts at 2 days post‐infiltration using laminarin as a substrate. The results of enzyme activity in planta recapitulated the results obtained in vitro (Fig. 5).
Figure 5.
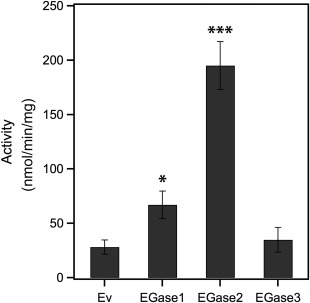
Activity of grapevine endoglucanases produced in planta. Glucanase activity in crude extracts of Nicotiana benthamiana leaves transiently expressing grapevine endoglucanases. Plants agroinfiltrated with an empty vector (Ev) were used as controls. Mean value and standard error were obtained from three biological repetitions, each including two technical repetitions. Asterisks indicate samples significantly different from Ev in a Dunett's test at *P = 0.1 and ***P = 0.01.
Effect of glucanases on germinated P. viticola spores
In order to evaluate the potential role of EGase1, EGase2 and EGase3 in the defence against pathogens, we studied their effect on in vitro‐germinated spores of P. viticola. To do so, germinated spores were incubated with each of the three enzymes, and the viability and integrity of zoospores were evaluated over time.
Observations were performed at 60‐s intervals for 12 min following enzyme addition. The results of spore counting under transmitted or fluorescent light at 0 and 12 min are shown in Fig. 6 and Table S2 (see Supporting Information). Suspension buffer had little effect on the viability of germinated spores. EGase2 did not show any important effects on the survival and integrity of germinated spores. The presence of EGase1 resulted in a decrease in living spores, without a noticeable change in spore structural integrity (Fig. 6b,c). Finally, the addition of EGase3 resulted in an important decrease in both the number of living spores and structural integrity (Fig. 6b,c).
Figure 6.
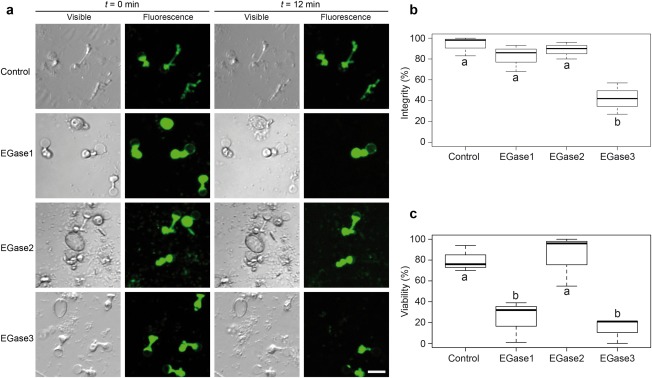
Effect of endoglucanases on germinated Plasmopara viticola spores. (a) Glucanase assay on P. viticola germinated spores. Each line represents a different treatment, where purified endo‐β‐1,3‐glucanases (EGases) or buffer as control were added to the spore suspension. The results of each treatment are illustrated by a photograph of the same microscopic field taken at different times (t = 0 min and t = 12 min) under two different light sources. Fluorescent light reveals fluorescein diacetate (FDA) fluorescence and indicates live zoospores, whereas visible light allows the counting of structurally intact zoospores. The effect on spore viability can be observed for all three EGases (fluorescence panels). The effect of treatment on spore integrity is visible for EGase3. Bar represents 15 µm. (b) Percentage of spores showing structural integrity after 12 min of treatment. Percentages were calculated by counting spores at t = 0 min and t = 12 min in visible light. (c) Percentage of spore viability after 12 min of treatment. Percentages were calculated by counting spores at t = 0 min and t = 12 min in fluorescent light. In (b) and (c), boxplots show the results of three independent experiments without technical repetitions. Boxes with the same letter are not significantly different in a Tukey's honestly significant difference (HSD) test at P = 0.05. Raw data for spore counting are detailed in Table S2.
Discussion
Here, we report the expression analysis, cloning and biochemical characterization of three EGases from V. vinifera that we named EGase1, EGase2 and EGase3.
Expression of EGase2 and EGase3 mRNAs was clearly induced on P. viticola infection, and the induction paralleled the expression of P. viticola actin, suggesting a link between pathogen presence and the induction of EGase2 and EGase3. EGase1 expression was weaker, but its induction was confirmed by inspection of RNA‐Seq data. Based on the accession numbers provided by the authors, EGase1 has been reported to be induced by Botrytis cinerea (Bézier et al., 2002), laminarin (Aziz et al., 2003) and UV light (Bonomelli et al., 2004). The results of induction of EGase3 on infection by P. viticola were also confirmed by the analysis of two independent sets of RNA‐Seq data (Fig. 2b,c, Table S1). Based on the accession numbers provided by the authors, EGase3 has been reported to be induced by ethephon (Jacobs et al., 1999), by infection with Erysiphe necator, the causal agent of powdery mildew (Jacobs et al., 1999), by the non‐host oomycete Pseudoperonospora cubensis (Kortekamp, 2006) and by P. viticola (Kortekamp, 2006), although induction in the latter was observed in a resistant species, but not in a susceptible cultivar. Finally, inspection of available RNA‐Seq data revealed different levels of induction for EGase2 on P. viticola infection depending on the dataset. Our results are thus in agreement with previous reports.
Basic EGases, such as EGase1 and EGase3, have been commonly classified as Class I and considered to be localized to the vacuole. However, EGase3 lacks a C‐terminal vacuolar sorting signal. There are other examples of basic EGases lacking a vacuolar sorting C‐terminal extension (Liu et al., 2010; Xie et al., 2015) suggesting that they are not necessarily vacuolar. Indeed, Xie et al. (2015) reported ZmGns, a maize basic EGase that lacks a vacuolar targeting signal and shows antimicrobial activity. Intriguingly, the closest relatives to ZmGns in grapevine are EGase1 (53% identity, 93% alignment cover) and EGase3 (48% identity, 98% alignment cover).
In vitro enzymatic assays revealed that the enzymatic activity and kinetic constants showed important differences between all three EGases, results that were confirmed when using EGases expressed in planta (Fig. 5). Values obtained for K M were similar to those measured for three basic EGases from barley (Hrmova and Fincher, 1993), but the k cat values calculated in this study were lower. One reason for the low catalytic efficiency and differences in the catalytic parameters could be differences in substrate specificity. However, enzymatic assays performed with several substrates with various proportions of β‐1,3 links did not shed any light on this matter. Concerning pH and temperature dependence, the values found here were in agreement with the optimal temperatures for endoglucanases (Hrmova and Fincher, 1993; Kikuchi et al., 2005). The optimum pH values for EGase1, EGase2 and EGase3 were pH 5.5, pH 4.5 and pH 5, respectively. Interestingly, the pH and temperature dependence of EGase3 were very similar to those for ZmGns (Xie et al., 2015), suggesting possible functional similarity. It is tempting to speculate that the large range in pH and temperature dependence of the three EGases may provide adaptive function(s) of GH‐17 EGases to different environmental, biotic or abiotic conditions.
Despite showing the highest activity in vitro, EGase2 did not show any antimicrobial activity against P. viticola germinated spores. A possible function for EGase2 could be callose degradation. Callose is a high‐molecular‐weight β‐1,3‐glucan that is commonly induced following pathogen challenge. Although mainly considered as a way to reinforce the cell wall and thus prevent pathogen penetration, callose also acts as a negative regulator of salicylic acid (SA)‐mediated responses (Nishimura et al., 2003; Oide et al., 2013). Accordingly, plant EGases have been reported to possess callose‐degrading activity and to influence the outcome of different plant–pathogen interactions (Beffa and Meins, 1996; Oide et al., 2013). In particular, Oide et al. (2013) reported a pathogen‐induced acidic glucanase with callose‐degrading activity, which led us to speculate that EGase2 could be involved in callose degradation and, consequently, activate SA‐mediated responses. Alternatively, EGase2 could be involved in the degradation of endogenous molecules whose products could act as elicitors of defence responses (Klarzynski et al., 2000), which have been proven to be efficient against P. viticola infection in grapevine (Aziz et al., 2003).
EGase1 and EGase3 showed a behaviour opposite to EGase2. EGase1 and, especially, EGase3 showed antimicrobial activity against P. viticola germinated spores, despite presenting the lowest activity in vitro against laminarin. We can rule out the possibility that this observation is caused by a contaminating activity in the protein extracts (e.g. chitinase or protease), as all EGases were produced in the same bacterial strain and purified using identical procedures. The differences in activity could be explained if branching surrounding the β‐1,3 bonds is more favourable to EGase3 activity in the context of a cell wall than inside laminarin. Alternatively, the antimicrobial activity could be independent of glucanase activity; rather than hydrolysing the cell walls, EGase3 could interfere with cell wall synthesis and lead to pathogen lysis. A better knowledge of the composition of zoospores, and experiments to determine whether these structures are lysed by EGases, would shed light on this matter. Whatever the mechanism behind the antimicrobial activity of EGase3, the fact that it was the grapevine EGase showing the strongest increase in expression following P. viticola infection (Fig. 2b,c; Perazzolli et al., 2012; Vannozzi et al., 2012) supports its direct role in defence against P. viticola. Furthermore, EGase3 is strongly induced by treatment with the ascomycete Trichoderma harzianum strain T39 (T39), a biocontrol agent that induces resistance to P. viticola (Perazzolli et al., 2012). T39‐induced resistance to P. viticola could thus be explained, in part, by the antimicrobial activity associated with a higher level of EGase3 expression.
The most obvious function for plant EGases is the degradation of cell walls from fungal and oomycete pathogens, which are composed of different amounts of β‐1,3‐glucans. Accordingly, the expression of EGases is induced on pathogen attack (Jin et al., 2007; Ward et al., 1991). Efforts to achieve disease resistance by the overexpression of EGases have produced variable results (Balasubramanian et al., 2012; van Loon et al., 2006) and, in most cases, they showed little or no effect of glucanases on pathogenicity. Altogether, reports of a direct lytic effect of glucanases on plant pathogens are scarce; other than this report, Xie et al. (2015) reported an effect of ZmGns on the germination of Aspergillus flavus, whereas Arlorio et al. (1992) showed changes in fungal cell wall morphology associated with the presence of a combination of chitinases and glucanases, although the main effect was caused by chitinases. At this point it is worth noting the multiple similarities between maize ZmGns and grapevine EGase3, which prompted us to propose that the two enzymes are functional homologues.
In summary, we have reported the cloning and characterization of three grapevine EGases of the GH‐17 family. Their enzymatic characteristics suggest a wide and complementary range of action. The expression of EGase2 and EGase3 was induced on P. viticola infection, but only EGase3 displayed strong antimicrobial activity with only poor in vitro activity. To the best of our knowledge, this is the first report of the lytic effect of a plant endoglucanase on a plant pathogenic oomycete.
Oomycetes use glucanase‐inhibiting proteins (GIPs) to block the action of plant EGases and to colonize plants (Rose et al., 2002). The identification of the P. viticola GIP inhibiting EGase3 will allow the performance of structural research to understand the molecular basis of the interactions between the two proteins. This knowledge will, in turn, open up the way to the use of gene‐editing technologies to modify the structure of EGase3 in order to avoid interaction with GIP, whilst maintaining enzymatic activity, which should result in increased resistance to P. viticola.
Experimental Procedures
Ampicillin, laminarin, tris(hydroxymethyl)aminomethane (Tris), 3(N‐morpholino)propanesulfonic acid (MOPS) and fluorescein diacetate (FDA) were obtained from Sigma‐Aldrich (St Louis, MO, USA). Tris‐glycine‐SDS buffer (10×), acrylamide (37.5/1) and pre‐stained molecular weight markers were obtained from Euromedex (Souffelweyersheim, France). The Protino Ni2+ tris‐carboxymethyl ethylene diamine (Ni‐TED) 150 kit was obtained from Macherey‐Nagel (Düren, Germany). Ethylenediaminetetraacetic acid (EDTA)‐free protease inhibitor cocktail was purchased from Roche (Basel, Switzerland). LR and BP clonases were obtained from Life Technologies‐Thermo Fisher Scientific (Waltham, MA, USA). Escherichia coli Arctic Express strain was obtained from Agilent Technologies (Santa Clara, CA, USA). The MiniPrep kit was purchased from QIAGEN (Hilden, Germany).
Plant material
Seedlings of V. vinifera cv. Muscat Ottonel, which is susceptible to downy mildew, were grown on stone wool in a glasshouse at 22°C/19°C (day/night) with a photoperiod of 16 h of light. Nicotiana benthamiana plants were grown in a glasshouse at 22°C/18°C (day/night) with a photoperiod of 14 h of light.
Phylogenetic and sequence analyses
Grapevine EGase sequences were identified by blast against the proteome deduced from the V. vinifera line 40024 genome sequence (Jaillon et al., 2007) using two known grapevine EGases (accession numbers AAF4667 and CAB91554). Proteins were retrieved from the Grape Genome database (v2) at CRIBI (Vitulo et al., 2014). Arabidopsis thaliana EGases were obtained by performing a search on The Arabidopsis Information Resource (TAIR, http://www.arabidopsis.org/) using the term ‘family 17’, which produced 57 entries. Visual inspection of the output revealed 47 EGases. The 18 EGases predicted to be apoplastic were included in the analysis. Arabidopsis accessions AT3G57240, AT3G57260, AT3G57270 and AT4G16260 have been reported to be induced by biotic stresses (Dong et al., 1991; Doxey et al., 2007). Five EGases from tobacco (accession numbers CAA38324, AAA34079, AAD33880, AAA34078 and AAA34103.1) and two from soybean (accession numbers AAA33946 and AAR26001), reported as PR (Rose et al., 2002; Takeuchi et al., 1990), were included in the analysis.
The putative expression of grapevine EGases was determined by the presence of associated cDNA clones in the grapevine Genome Browser at Genoscope (http://www.genoscope.cns.fr/externe/GenomeBrowser/Vitis/) and by the analysis of publicly available RNA‐Seq data (see In silico expression analysis section).
Alignments were performed with Muscle and curated with GBlocks. Phylogenetic trees were constructed using PhyML with LG model and 100 bootstrap repetitions. Analyses were performed at http://www.phylogeny.fr (Dereeper et al., 2008). Alignments in Fig. 1b were performed with ClustalW and identities were highlighted using BoxShade at Swiss EMBnet (http://www.ch.embnet.org/).
Semi‐quantitative RT‐PCR
Plasmopara viticola‐infected material for RT‐PCR experiments was obtained by inoculating leaf discs as described by Peressotti et al. (2010). Leaf discs were prepared from the third and fourth leaf counting from the apex from V. vinifera seedlings. Discs were bulked and distributed in Petri dishes. Leaf discs were inoculated by spraying a solution of 50 000 sporangia/mL. Mock‐inoculated material was obtained by replacing the P. viticola suspension with water. At each time point, 10 leaf discs were snap frozen in liquid nitrogen and conserved at −80°C. RNA extraction, first‐strand cDNA synthesis and PCR amplification were performed as described previously (Santos‐Rosa et al., 2008). Amplifications consisted of 25 cycles of 20 s at 94°C, 20 s at 60°C and 60 s at 72°C, followed by a final extension step of 10 min at 72°C. PCR products were separated on 1% agarose gels in 1 × Tris‐Acetate‐EDTA (TAE) and stained using ethidium bromide. Primer sequences are listed in Table S3 (see Supporting Information).
In silico expression analysis
Sixty‐five grapevine RNA‐Seq raw data sequence files generated within the framework of seven different experiments (Da Silva et al., 2013; Jones et al., 2014; Perazzolli et al., 2012; Ramos et al., 2014; Sweetman et al., 2012; Vannozzi et al., 2012; Venturini et al., 2013) were retrieved from the National Center for Biotechnology Information (NCBI) SRA public database (http://www.ncbi.nlm.nih.gov/sra). Data were formatted in the fastq format using the fastq‐dump command from the SRA Toolkit package version 2.3.4 (http://www.ncbi.nlm.nih.gov/books/NBK158900).
RNA‐Seq reads were mapped against the V1 annotation of the grapevine line 40024 12x genome sequence, stored in the Grape Genome Database hosted at CRIBI. Alignments were performed using GSNAP version 2013‐11‐27 (Wu and Nacu, 2010) with the parameters: ‐B 4, ‐N 1, ‐n 3, ‐nofails and the quality protocol according to the experiment. These files were parsed to keep the best, unique and paired (if paired‐end reads) alignments using an in‐house‐developed script.
The number of fragments aligned was counted using the command htseq‐count from the HTSeq framework version 0.6.0 (Anders et al., 2015) with the parameters: ‐m intersection‐nonempty and ‐s no. FPKMs (fragments per kilobase of exon per million fragments mapped) were calculated for every gene using an in‐house‐developed script.
Gene cloning, protein expression and purification
The three grapevine EGases were cloned and expressed as described previously (Belval et al., 2015). Briefly, for transient expression in plants, sequences were PCR amplified using high‐fidelity polymerases and cloned into binary plasmid pBIN61 using restriction endonucleases. For protein expression, sequences were amplified from binary clones, cloned into suitable plasmid vectors (pHGWA, Busso et al., 2005) by recombination using BP clonase, and transformed into E. coli Arctic Express. The integrity of genes was verified by sequencing. The primers are listed in Table S3.
For bacterial expression of proteins, overnight culture (2 mL) was transferred into 200 mL of Lysogeny broth (LB) containing ampicillin (100 µg/mL) and chloramphenicol (50 µg/mL), and was grown for 3 h at 28°C. It was placed at 10°C for 1 h and protein production was induced by the addition of 1 mm isopropyl‐β‐D‐1‐thiogalactopyranoside (IPTG) for 24 h at 10°C. Bacteria were collected by centrifugation (6000 g, 10 min), washed with cold phosphate‐buffered saline (PBS), centrifuged again and suspended in 1 : 100 culture volume of PBS with protease inhibitors. Bacteria were then disrupted using a Branson sonifier 250 (Emerson, St Louis, MO, USA) until clarification, typically 3 × 30 s. Cell fragments were pelleted by a 10‐min centrifugation at 12 000 g. Supernatant was collected, aliquoted and frozen at −20°C.
Enzymes were purified using Protino Ni‐TED columns according to the manufacturer's instructions. To eliminate Cpn60 chaperone (GroEL) contamination, a supplementary wash after sample loading was performed according to Belval et al. (2015) for EGase1 and EGase3. Briefly, resin was washed with 5 mL of 2 m urea/20 mm Tris, pH 6.8. This urea wash was not necessary for EGase2 purification. After washing with the kit buffer (Lysis‐Equilibration‐Wash, LEW, 1×), enzymes were eluted with elution buffer in fractions of 250 µL. Protein concentration was determined by measuring the absorbance at 280 nm using a Nanodrop ND‐1000 spectrophotometer (NanoDrop, Wilmington, DE, USA). Enzyme‐containing fractions were pooled and dialysed/concentrated using Amicon Ultra 10k devices (Millipore, Molsheim, France), according to the manufacturer's instructions, against 50 mm NaCl/20 mm N−2‐hydroxyethylpiperazine‐N'‐2‐ethanesulfonic acid (Hepes), pH 7, containing EDTA‐free protease inhibitors. Protein concentration was measured as described above. Blank was performed with buffer containing protease inhibitor.
Protein electrophoresis
SDS‐PAGE (12% acrylamide) was performed according to Laemmli (1970). Gels were stained using colloidal Coomassie blue (Neuhoff et al., 1988).
In vitro enzymatic assays
Enzyme activities were determined by measuring the increase in reducing sugars released in a solution of laminarin in 0.1 m of sodium acetate or MOPS buffer, according to the methods of Karvonen and Somersalo (1952) and Somogyi (1952). The katal is defined as the enzyme activity catalysing the formation of 1 mole of glucose equivalent per second.
To determine the effect of pH on enzymatic activity, the specific activity of each EGase was measured in the pH range 3–7.5 in pH 0.5 increments, using sodium acetate buffer (0.1 m) for pH 3–6.5 and MOPS buffer for pH 6.5–7.5, keeping the temperature constant at 37°C.
To assess the effect of temperature, specific activities of EGases were measured at temperatures ranging from 0–4°C up to 50°C for EGase1 and EGase2, and 0–4°C up to 70°C for EGase3, by 10°C increments, in 0.1 m sodium acetate buffer, pH 5.4. The relative activities were then expressed as the percentage of the maximum specific activity. Three biological repeats were performed for each pH and temperature.
Measurements of kinetic parameters were performed at 37°C in 0.1 m sodium acetate buffer (pH 5.4) containing various concentrations of laminarin (0–2 mg/mL; 0–0.34 mm) for 5 min. K M (µm) and k cat (s−1) were calculated using non‐linear regression analysis with IgorPro (WaveMetrics, Inc., Lake Oswego, OR, USA). The enzyme quantity in the assays was varied between 1 × 10−5 and 8 × 10−5 µm.
Substrate specificity
The specific activities of the three enzymes were measured with laminarin and also with several substrates solubilized in 0.1 m sodium acetate, pH 5.4, in the conditions described in the previous section: dextran from Leuconostoc [linear α(1,6)‐linked glucose units and α(1,3)‐linked branches], carboxymethyl cellulose (β‐1,4‐d‐glucopyranose polymer of cellulose), β‐d‐glucan from barley (a polymer of β‐d‐glucose with 70% of glucose residues 1,4‐linked and 30% 1,3‐linked; Kim et al., 2011) and xylan (a polysaccharide composed of 1,4‐xylose).
In planta glucanase activity assays
Transient expression of EGases in N. benthamiana leaves was performed by agroinfiltration. Agrobacterium containing the binary constructs was grown for 2 days at 28°C in 5 mL of medium containing 50 µg/mL kanamycin and 2.5 µg/mL tetracycline. After centrifugation, bacteria were resuspended in a solution containing 10 mm 2‐(N‐morpholino)ethanesulfonic acid (MES), 10 mm MgCl2 and 150 µm acetosyringone, incubated at room temperature for 2–3 h, and infiltrated at an optical density at 600 nm (OD600) of 0.2 using a needleless syringe, as described in Scofield et al. (1996).
Two leaves of 4‐week‐old N. benthamiana seedlings were infiltrated with each EGase. Leaves were sampled at 2 days post‐infiltration and protein crude extracts were prepared by grinding the leaves in extraction buffer (0.1 m sodium acetate, 14 mm β‐mercaptoethanol, 1% polyvinylpyrrolidone‐40, pH 5.2) using 6 mL of buffer per 1 g of fresh leaf. Glucanase activity was measured using laminarin as substrate (Derckel et al., 1998).
Functional assays on downy mildew
Plasmopara viticola strain Resdur was collected from V. vinifera Chardonnay in the experimental field of INRA in Colmar (France) and maintained on seedlings of V. vinifera cultivar Muscat Ottonel grown at 21°C and 100% relative humidity. Five days after inoculation, sporangia were harvested from infected leaves by shaking in sterile distilled water and the suspension was diluted to 20 × 103 sporangia/mL. Aliquots of 1 mL of this suspension were placed in wells on 24‐well plates and incubated for 2–3 h at 21°C to obtain germinated spores. Three biological repetitions were performed. In each experiment, a microscopic field (640 × 640 µm2 for two experiments and 1280 × 1280 µm2 for one experiment) containing zoospores was selected to perform time‐lapse observation. Then, 3 µL of a 1% FDA solution were added to each well to reveal live zoospores (Widholm, 1972). Imaging was started 2 min after FDA addition (t = 0 min). EGases were added (t = 1 min) and time‐lapse experiments were performed for 12 min at 1‐min intervals between frames. Samples in which the enzymes were replaced by buffer were used as negative controls. Zoospores were then manually counted on photographs at t = 0 min and t = 12 min in visible and fluorescent light. Fluorescent light reveals FDA fluorescence and indicates live zoospores, whereas visible light allows the counting of structurally intact zoospores. Counting data are presented in Table S2.
Observations were made using a confocal laser scanning microscope (Zeiss LSM510, Zeiss, Oberkochen, Germany) with an EC Plan‐Neofluar (10X/0.30 M27) objective lens under multitrack mode. Excitation/emission wavelengths were 488 nm/505–550 nm for FDA‐stained germinated spores. Images were analysed using ImageJ (Schneider et al., 2012) and figures were mounted using the plugin FigureJ (Mutterer and Zinck, 2013).
Statistical analysis was performed using R version 2.13.1 (R Core Team (2013)).
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Expression of grapevine endoglucanases on spraying with water.
Table S1 RNA‐Seq data from the literature summarizing the mRNA expression of grapevine endo‐β‐1,3‐glucanases (EGases).
Table S2 Raw data for spore counting used to prepare Fig. 6b,c.
Table S3 Primers used in this study.
Acknowledgements
We warmly thank Marie‐Annick Dorne and Marie‐Céline Lacombe for preparation of the leaves infected by Plasmopara viticola. We are grateful to Jérôme Mutterer for assistance with confocal microscopy, to Eric Duchêne for help with statistical analyses and to Philippe Hugueney for critical reading of the manuscript. We also thank the experimental unit of INRA‐Colmar for their valuable technical support in the production of plants in the glasshouse. The authors declare no conflicts of interest. This work was supported by the INRA Department ‘Santé des Plantes et Environnement’.
Contributor Information
Pere Mestre, Email: pere.mestre@colmar.inra.fr.
Jean‐François Chich, Email: jfchich@colmar.inra.fr.
References
- Adams, D.J. (2004) Fungal cell wall chitinases and glucanases. Microbiology, 150, 2029–2035. [DOI] [PubMed] [Google Scholar]
- Ahuja, I. , Kissen, R. and Bones, A.M. (2012) Phytoalexins in defense against pathogens. Trends Plant Sci. 17, 73–90. [DOI] [PubMed] [Google Scholar]
- Anders, S. , Pyl, P.T. and Huber, W. (2015) HTSeq–a Python framework to work with high‐throughput sequencing data. Bioinformatics, 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlorio, M. , Ludwig, A. , Boller, T. and Bonfante, P. (1992) Inhibition of fungal growth by plant chitinases and β‐1,3‐glucanases. Protoplasma, 171, 34–43. [Google Scholar]
- Aziz, A. , Poinssot, B. , Daire, X. , Adrian, M. , Bézier, A. , Lambert, B. , Joubert, J.M. and Pugin, A. (2003) Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola . Mol. Plant–Microbe Interact. 16, 1118–1128. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, V. , Vashisht, D. , Cletus, J. and Sakthivel, N. (2012) Plant β‐1,3‐glucanases: their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 34, 1983–1990. [DOI] [PubMed] [Google Scholar]
- Beffa, R. and Meins, F. (1996) Pathogenesis‐related functions of plant beta‐1,3‐glucanases investigated by antisense transformation–a review. Gene, 179, 97–103. [DOI] [PubMed] [Google Scholar]
- Belval, L. , Marquette, A. , Mestre, P. , Piron, M.C. , Demangeat, G. , Merdinoglu, D. and Chich, J.F. (2015) A fast and simple method to eliminate Cpn60 from functional recombinant proteins produced by E. coli Arctic Express. Protein Expr. Purif. 109, 29–34. [DOI] [PubMed] [Google Scholar]
- Bézier, A. , Lambert, B. and Baillieul, F. (2002) Cloning of a grapevine Botrytis‐responsive gene that has homology to the tobacco hypersensitivity‐related hsr203J. J. Exp. Bot. 53, 2279–2280. [DOI] [PubMed] [Google Scholar]
- Bonomelli, A. , Mercier, L. , Franchel, J. , Baillieul, F. , Benizri, E. and Mauro, M.C. (2004) Response of grapevine defenses to UV‐C exposure. Am. J. Enol. Vitic. 55, 51–59. [Google Scholar]
- Britto, D.S. , Pirovani, C.P. , Andrade, B.S. , Dos Santos, T.P. , Pungartnik, C. , Cascardo, J.C.M. , Micheli, F. and Gesteira, A.S. (2013) Recombinant β‐1,3‐1,4‐glucanase from Theobroma cacao impairs Moniliophthora perniciosa mycelial growth. Mol. Biol. Rep. 40, 5417–5427. [DOI] [PubMed] [Google Scholar]
- Bulcke, M.V. , Bauw, G. , Castresana, C. , Van Montagu, M. and Vandekerckhove, J. (1989) Characterization of vacuolar and extracellular beta(1,3)‐glucanases of tobacco: evidence for a strictly compartmentalized plant defense system. Proc. Natl. Acad. Sci. USA, 86, 2673–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso, D. , Delagoutte‐Busso, B. and Moras, D. (2005) Construction of a set of Gateway‐based destination vectors for high‐throughput cloning and expression screening in Escherichia coli . Anal. Biochem. 343, 313–321. [DOI] [PubMed] [Google Scholar]
- Cândido, E. de S., Pinto, M.F.S. , Pelegrini, P.B. , Lima, T.B. , Silva, O.N. , Pogue, R. , Grossi‐de, S.M.F. and Franco, O.L. , (2011) Plant storage proteins with antimicrobial activity: novel insights into plant defense mechanisms. FASEB J. 25, 3290–3305. [DOI] [PubMed] [Google Scholar]
- Danilova, N. (2006) The evolution of immune mechanisms. J. Exp. Zool. B: Mol. Dev. Evol. 306, 496–520. [DOI] [PubMed] [Google Scholar]
- Da Silva, C. , Zamperin, G. , Ferrarini, A. , Minio, A. , Dal Molin, A. , Venturini, L. , Buson, G. , Tononi, P. , Avanzato, C. , Zago, E. , Boido, E. , Dellacassa, E. , Gaggero, C. , Pezzotti, M. , Carrau, F. and Delledonne, M. (2013) The high polyphenol content of grapevine cultivar tannat berries is conferred primarily by genes that are not shared with the reference genome. Plant Cell, 25, 4777–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, G. and Henrissat, B. (1995) Structures and mechanisms of glycosyl hydrolases. Structure, 3, 853–859. [DOI] [PubMed] [Google Scholar]
- Derckel, J.P. , Audran, J.C. , Haye, B. , Lambert, B. and Legendre, L. (1998) Characterization, induction by wounding and salicylic acid, and activity against Botrytis cinerea of chitinases and β‐1,3‐glucanases of ripening grape berries. Physiol. Plant. 104, 56–64. [Google Scholar]
- Dereeper, A. , Guignon, V. , Blanc, G. , Audic, S. , Buffet, S. , Chevenet, F. , Dufayard, J.F. , Guindon, S. , Lefort, V. , Lescot, M. , Claverie, J.M. and Gascuel, O. (2008) Phylogeny.fr: robust phylogenetic analysis for the non‐specialist. Nucleic Acids Res. 36, W465–W469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R. , Reynolds, A. , Hancock, J.T. and Neill, S.J. (1998) Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem. J. 330, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, X. , Mindrinos, M. , Davis, K.R. and Ausubel, F.M. (1991) lnduction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell, 3, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey, A.C. , Yaish, M.W. , Moffatt, B.A. , Griffith, M. and McConkey, B.J. (2007) Functional divergence in the Arabidopsis beta‐1,3‐glucanase gene family inferred by phylogenetic reconstruction of expression states. Mol. Biol. Evol. 24, 1045–1055. [DOI] [PubMed] [Google Scholar]
- Ebrahim, S. , Usha, K. and Singh, B. (2011) Science against microbial pathogens: communicating current research and technological advances In: Microbiology Book Series (Méndez‐Vilas A., ed.), Badajoz, Spain, pp. 1043–1054. [Google Scholar]
- Hara‐Nishimura, I. and Hatsugai, N. (2011) The role of vacuole in plant cell death. Cell Death Differ. 18, 1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsugai, N. and Hara‐Nishimura, I. (2010) Two vacuole‐mediated defense strategies in plants. Plant Signal. Behav. 5, 1568–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofius, D. , Munch, D. , Bressendorff, S. , Mundy, J. and Petersen, M. (2011) Role of autophagy in disease resistance and hypersensitive response‐associated cell death. Cell Death Differ. 18, 1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrmova, M. and Fincher, G. (1993) Purification and properties of three (1–>3)‐beta‐D‐glucanase isoenzymes from young leaves of barley (Hordeum vulgare). Biochem. J. 289, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hückelhoven, R. (2007) Cell wall‐associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 45, 101–127. [DOI] [PubMed] [Google Scholar]
- Jacobs, A.K. , Dry, I.B. and Robinson, S.P. (1999) Induction of different pathogenesis‐related cDNAs in grapevine infected with powdery mildew and treated with ethephon. Plant Pathol. 48, 325–336. [Google Scholar]
- Jaillon, O. , Aury, J.M. , Noel, B. , Policriti, A. , Clepet, C. , Casagrande, A. , Choisne, N. , Aubourg, S. , Vitulo, N. , Jubin, C. , Vezzi, A. , Legeai, F. , Hugueney, P. , Dasilva, C. , Horner, D. , Mica, E. , Jublot, D. , Poulain, J. , Bruyère, C. , Billault, A. , Segurens, B. , Gouyvenoux, M. , Ugarte, E. , Cattonaro, F. , Anthouard, V. , Vico, V. , Del Fabbro, C. , Alaux, M. , Di Gaspero, G. , Dumas, V. , Felice, N. , Paillard, S. , Juman, I. , Moroldo, M. , Scalabrin, S. , Canaguier, A. , Le Clainche, I. , Malacrida, G. , Durand, E. , Pesole, G. , Laucou, V. , Chatelet, P. , Merdinoglu, D. , Delledonne, M. , Pezzotti, M. , Lecharny, A. , Scarpelli, C. , Artiguenave, F. , Pè, M.E. , Valle, G. , Morgante, M. , Caboche, M. , Adam‐Blondon, A.F. , Weissenbach, J. , Quétier, F. , Wincker, P. , French–Italian Public Consortium for Grapevine Genome Characterization . (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature, 449, 463–467. [DOI] [PubMed] [Google Scholar]
- Jeandet, P. , Douillet‐Breuil, A.C. , Bessis, R. , Debord, S. , Sbaghi, M. and Adrian, M. (2002) Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J. Agric. Food Chem. 50, 2731–2741. [DOI] [PubMed] [Google Scholar]
- Jin, X. , Feng, D. , Wang, H. and Wang, J. (2007) A novel tissue‐specific plantain beta‐1,3‐glucanase gene that is regulated in response to infection by Fusarium oxysporum fsp. cubense . Biotechnol. Lett. 29, 1431–1437. [DOI] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Jones, L. , Riaz, S. , Morales‐Cruz, A. , Amrine, K.C.H. , McGuire, B. , Gubler, W.D. , Walker, M.A. and Cantu, D. (2014) Adaptive genomic structural variation in the grape powdery mildew pathogen, Erysiphe necator . BMC Genomics, 15, 1081–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvonen, M.J. and Somersalo, O. (1952) A note on the determination of fructose and glucose by the Somogyi method. Ann. Med. Exp. Biol. Fenn. 30, 31–34. [PubMed] [Google Scholar]
- Kauffmann, S. , Legrand, M. , Geoffroy, P. and Fritig, B. (1987) Biological function of 'pathogenesis‐related' proteins: four PR proteins of tobacco have 1,3‐beta‐glucanase activity. EMBO J. 6, 3209–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen, N.T. , Yoshikawa, M. and Wang, M.C. (1983) Phytoalexin elicitor activity of carbohydrates from Phytophthora megasperma f.sp. glycinea and other sources. Plant Physiol. 71, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, T. , Shibuya, H. and Jones, J.T. (2005) Molecular and biochemical characterization of an endo‐beta‐1,3‐glucanase from the pinewood nematode Bursaphelenchus xylophilus acquired by horizontal gene transfer from bacteria. Biochem. J. 389, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.S. , Park, K.G. , Baek, S.B. and Kim, J.G. (2011) Inheritance of (1–3)(1–4)‐beta‐D‐glucan content in barley (Hordeum vulgare L.). J. Crop Sci. Biotechnol. 14, 239–245. [Google Scholar]
- Klarzynski, O. , Plesse, B. , Joubert, J.M. , Yvin, J.C. , Kopp, M. , Kloareg, B. and Fritig, B. (2000) Linear beta‐1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 124, 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortekamp, A. (2006) Expression analysis of defence‐related genes in grapevine leaves after inoculation with a host and a non‐host pathogen. Plant Physiol. Biochem. 44, 58–67. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Leubner‐Metzger, G. (2005) beta‐1,3‐Glucanase gene expression in low‐hydrated seeds as a mechanism for dormancy release during tobacco after‐ripening. Plant J. 41, 133–145. [DOI] [PubMed] [Google Scholar]
- Liu, B. , Xue, X. , Cui, S. , Zhang, X. , Han, Q. , Zhu, L. , Liang, X. , Wang, X. , Huang, L. , Chen, X. and Kang, Z. (2010) Cloning and characterization of a wheat beta‐1,3‐glucanase gene induced by the stripe rust pathogen Puccinia striiformis f. sp. tritici . Mol. Biol. Rep. 37, 1045–1052. [DOI] [PubMed] [Google Scholar]
- Lombard, V. , Golaconda Ramulu, H. , Drula, E. , Coutinho, P.M. and Henrissat, B. (2014) The carbohydrate‐active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–D495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon, L.C. and van Strien, E.A. (1999) The families of pathogenesis‐related proteins, their activities, and comparative analysis of PR‐1 type proteins. Physiol. Mol. Plant Pathol. 55, 85–97. [Google Scholar]
- van Loon, L.C. , Rep, M. and Pieterse, C.M.J. (2006) Significance of inducible defense‐related proteins in infected plants. Annu. Rev. Phytopathol. 44, 135–162. [DOI] [PubMed] [Google Scholar]
- Mittler, R. , Vanderauwera, S. , Suzuki, N. , Miller, G. , Tognetti, V.B. , Vandepoele, K. , Gollery, M. , Shulaev, V. and Van Breusegem, F. (2011) ROS signaling: the new wave? Trends Plant Sci. 16, 300–309. [DOI] [PubMed] [Google Scholar]
- Morohashi, Y. and Matsushima, H. (2000) Development of beta‐1,3‐glucanase activity in germinated tomato seeds. J. Exp. Bot. 51, 1381–1387. [PubMed] [Google Scholar]
- Mutterer, J. and Zinck, E. (2013) Quick‐and‐clean article figures with FigureJ. J. Microsc. 252, 89–91. [DOI] [PubMed] [Google Scholar]
- Neuhaus, J.M. , Flores, S. , Keefe, D. , Ahl‐Goy, P. and Meins, F., Jr . (1992) The function of vacuolar beta‐1,3‐glucanase investigated by antisense transformation. Susceptibility of transgenic Nicotiana sylvestris plants to Cercospora nicotianae infection. Plant Mol. Biol. 19, 803–813. [DOI] [PubMed] [Google Scholar]
- Neuhoff, V. , Arold, N. , Taube, D. and Ehrhardt, W. (1988) Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G‐250 and R‐250. Electrophoresis, 9, 255–262. [DOI] [PubMed] [Google Scholar]
- Nishimura, M.T. , Stein, M. , Hou, B.H. , Vogel, J.P. , Edwards, H. and Somerville, S.C. (2003) Loss of a callose synthase results in salicylic acid‐dependent disease resistance. Science, 301, 969–972. [DOI] [PubMed] [Google Scholar]
- Oide, S. , Bejai, S. , Staal, J. , Guan, N. , Kaliff, M. and Dixelius, C. (2013) A novel role of PR2 in abscisic acid (ABA) mediated, pathogen‐induced callose deposition in Arabidopsis thaliana . New Phytol. 200, 1187–1199. [DOI] [PubMed] [Google Scholar]
- Perazzolli, M. , Moretto, M. , Fontana, P. , Ferrarini, A. , Velasco, R. , Moser, C. , Delledonne, M. and Pertot, I. (2012) Downy mildew resistance induced by Trichoderma harzianum T39 in susceptible grapevines partially mimics transcriptional changes of resistant genotypes. BMC Genomics, 13, 660–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peressotti, E. , Wiedemann‐Merdinoglu, S. , Delmotte, F. , Bellin, D. , Di Gaspero, G. , Testolin, R. , Merdinoglu, D. and Mestre, P. (2010) Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol. 10, 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, M.J. , Coito, J. , Silva, H. , Cunha, J. , Costa, M.M. and Rocheta, M. (2014) Flower development and sex specification in wild grapevine. BMC Genomics, 15, 1095–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Reina‐Pinto, J.J. and Yephremov, A. (2009) Surface lipids and plant defenses. Plant Physiol. Biochem. 47, 540–549. [DOI] [PubMed] [Google Scholar]
- Rose, J.K.C. , Ham, K.S. , Darvill, A.G. and Albersheim, P. (2002) Molecular cloning and characterization of glucanase inhibitor proteins: coevolution of a counterdefense mechanism by plant pathogens. Plant Cell, 14, 1329–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐Rosa, M. , Poutaraud, A. , Merdinoglu, D. and Mestre, P. (2008) Development of a transient expression system in grapevine via agro‐infiltration. Plant Cell Rep. 27, 1053–1063. [DOI] [PubMed] [Google Scholar]
- Schneider, C.A. , Rasband, W.S. and Eliceiri, K.W. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat. Methods, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield, S.R. , Tobias, C.M. , Rathjen, J.P. , Chang, J.H. , Lavelle, D.T. , Michelmore, R.W. and Staskawicz, B.J. (1996) Molecular basis of gene‐for‐gene specificity in bacterial speck disease of tomato. Science, 274, 2063–2065. [DOI] [PubMed] [Google Scholar]
- Somogyi, M. (1952) Notes on sugar determination. J. Biol. Chem. 195, 19–23. [PubMed] [Google Scholar]
- Stintzi, A. , Heitz, T. , Prasad, V. , Wiedemann‐Merdinoglu, S. , Kauffmann, S. , Geoffroy, P. , Legrand, M. and Fritig, B. (1993) Plant “pathogenesis‐related” proteins and their role in defense against pathogens. Biochimie, 75, 687–706. [DOI] [PubMed] [Google Scholar]
- Sweetman, C. , Wong, D.C. , Ford, C.M. and Drew, D.P. (2012) Transcriptome analysis at four developmental stages of grape berry (Vitis vinifera cv. Shiraz) provides insights into regulated and coordinated gene expression. BMC Genomics, 13, 691–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, Y. , Yoshikawa, M. , Takeba, G. , Tanaka, K. , Shibata, D. and Horino, O. (1990) Molecular cloning and ethylene induction of mRNA encoding a phytoalexin elicitor‐releasing factor, beta‐1,3‐endoglucanase, in soybean. Plant Physiol. 93, 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannozzi, A. , Dry, I.B. , Fasoli, M. , Zenoni, S. and Lucchin, M. (2012) Genome‐wide analysis of the grapevine stilbene synthase multigenic family: genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 12, 130–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturini, L. , Ferrarini, A. , Zenoni, S. , Tornielli, G.B. , Fasoli, M. , Dal Santo, S. , Minio, A. , Buson, G. , Tononi, P. , Zago, E.D. , Zamperin, G. , Bellin, D. , Pezzotti, M. and Delledonne, M. (2013) De novo transcriptome characterization of Vitis vinifera cv. Corvina unveils varietal diversity. BMC Genomics, 14, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitulo, N. , Forcato, C. , Carpinelli, E.C. , Telatin, A. , Campagna, D. , D'Angelo, M. , Zimbello, R. , Corso, M. , Vannozzi, A. , Bonghi, C. , Lucchin, M. and Valle, G. (2014) A deep survey of alternative splicing in grape reveals changes in the splicing machinery related to tissue, stress condition and genotype. BMC Plant Biol. 14, 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, E.R. , Payne, G.B. , Moyer, M.B. , Williams, S.C. , Dincher, S.S. , Sharkey, K.C. , Beck, J.J. , Taylor, H.T. , Ahl‐Goy, P. , Meins, F. and Ryals, J.A. (1991) Differential regulation of beta‐1,3‐glucanase messenger RNAs in response to pathogen infection. Plant Physiol. 96, 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels, J.G.H. (1994) Developmental regulation of fungal cell wall formation. Annu. Rev. Phytopathol. 32, 413–437. [Google Scholar]
- Widholm, J.M. (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 47, 189–194. [DOI] [PubMed] [Google Scholar]
- Worrall, D. , Hird, D.L. , Hodge, R. , Paul, W. , Draper, J. and Scott, R. (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell, 4, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, T.D. and Nacu, S. (2010) Fast and SNP‐tolerant detection of complex variants and splicing in short reads. Bioinformatics, 26, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y.R. , Raruang, Y. , Chen, Z.Y. , Brown, R.L. and Cleveland, T.E. (2015) ZmGns, a maize class I β‐1,3‐glucanase, is induced by biotic stresses and possesses strong antimicrobial activity. J. Integr. Plant Biol. 57, 271–283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Expression of grapevine endoglucanases on spraying with water.
Table S1 RNA‐Seq data from the literature summarizing the mRNA expression of grapevine endo‐β‐1,3‐glucanases (EGases).
Table S2 Raw data for spore counting used to prepare Fig. 6b,c.
Table S3 Primers used in this study.


