Summary
Taxonomy and History
Hemileia vastatrix Berk. and Broome (Basidiomycota, Pucciniales) was described in 1869 in eastern Africa and Ceylon as the agent of coffee leaf rust and has spread to all coffee cultivation areas worldwide. Major disease outbreaks in Asia, Africa and America caused and continue to cause severe yield losses, making this the most important disease of Arabica coffee, a cash crop for many tropical and sub‐tropical countries.
Life cycle and Disease symptoms
Hemileia vastatrix is a hemicyclic fungus with the urediniosporic life cycle as its most important (if not only) source of inoculum. Chlorotic spots are the first macroscopic symptoms, preceding the differentiation of suprastomatal, bouquet‐shaped, orange‐coloured uredinia. The disease can cause yield losses of up to 35% and have a polyetic epidemiological impact on subsequent years.
Disease control
Although the use of fungicides is one of the preferred immediate control measures, the use of resistant cultivars is considered to be the most effective and durable disease control strategy. The discovery of ‘Híbrido de Timor’ provided sources of resistance that have been used in several breeding programmes and that have been proven to be effective and durable, as some have been in use for more than 30 years.
Genetic diversity and Molecular pathogenicity
Although exhibiting limited genetic polymorphism, the very large genome of H. vastatrix (c. 797 Mbp) conceals great pathological diversity, with more than 50 physiological races. Gene expression studies have revealed a very precocious activation of signalling pathways and production of putative effectors, suggesting that the plant–fungus dialogue starts as early as at the germ tube stage, and have provided clues for the identification of avr genes.
Keywords: coffea, coffee leaf rust, Hemileia vastatrix, obligate biotrophy, Pucciniales
Introduction
Coffee is the most important agricultural commodity, with an estimated retail value of 70 billion US dollars. It is crucial for the economy of more than 60 countries and is the main source of income for more than 100 million people (Hoffmann, 2014; ICO, 2016). Coffee leaf rust (CLR) causes losses of one to two billion US dollars annually (McCook, 2006) and is one of the main limiting factors of Arabica coffee (Coffea arabica) production worldwide. CLR was first recorded by an English explorer in 1861 near Lake Victoria (East Africa) on wild Coffea species. The disease symptoms and signs include large orange spore masses on the lower leaf surface, leading to premature leaf fall (Fig. 1). The causal agent was described as Hemileia vastatrix Berkeley and Broome (1869). Soon after its first report, the disease wiped out coffee cultivation from Ceylon (Sri Lanka), with devastating social and economic consequences (Morris, 1880). Since this sudden and devastating outbreak, CLR has become one of the most well‐known diseases in the history of plant pathology.
Figure 1.

Coffee leaf rust symptoms and signs. (A) Chlorotic spots and urediniosporic sori on the lower leaf surface. (B) Severe defoliation in plants at the front as a result of disease, contrasting with resistant plants elsewhere in the field.
The two main cultivated coffee species, C. canephora (Robusta coffee) and C. arabica, account, on average, for 40% and 60%, respectively, of the world's coffee production (ICO, 2016). Coffea arabica is native to the relatively dry and high‐altitude areas of Ethiopia and northern Kenya and its genetic pool is considered to have low diversity (Steiger et al., 2002). Arabica coffee was domesticated in Yemen, and its cultivation subsequently spread to Asia, America and other parts of Africa. Severe genetic bottlenecks during its domestication have narrowed even further the genetic diversity of the crop: it is believed that a single coffee plant from the Botanical Garden of Amsterdam was one of the progenitors of most of the current coffee cultivars. These genetic bottlenecks were particularly relevant for rust response traits, as its domestication in Yemen, the driest coffee cultivation area in the world, led to the absence of selection pressure towards rust resistance (Rodrigues et al., 1975). Coffee germplasm disseminated from Yemen was most probably free of rust. Further selection and adaptation to other regions and climates in different parts of Asia and America during the 17th and 18th centuries occurred in the absence of the pathogen, but nonetheless under disease‐favourable conditions. The 19th century epidemic in Ceylon was the outcome of such genetic, biological and agronomic circumstances (McCook, 2006; McCook and Vandermeer, 2015). Since then, rust has spread to most coffee‐growing countries worldwide, first from 1870 to 1920 through the coffee zones of the Indian Ocean Basin and the Pacific, second reaching the Africa Atlantic countries in the 1950s and 1960s, and, finally, crossing the Atlantic Ocean (Muller, 1971), presumably carried by wind currents (Bowden et al., 1971), spreading throughout South and Central America during the 1970s and 1980s. In the second half of the 20th century, the identification and characterization of ‘Híbrido de Timor’ (HDT) populations provided the basis for a breeding programme that enabled the release of rust‐resistant cultivars in different coffee‐growing countries, including the Americas (Rodrigues et al., 1975; Silva et al., 2006). Recently CLR has regained notoriety because of a severe and widespread epidemic throughout Central America, Colombia, Peru and Ecuador, as a result of the convergence of several agronomic, climatic and economic factors (Avelino et al., 2015; Cressey, 2013; Rozo et al., 2012). Yield losses were up to 35%, with a direct impact on the income and livelihood of hundreds of thousands of farmers and labourers.
Taxonomy and Phylogeny
The genus Hemileia is a member of the phylum Basidiomycota, class Pucciniomycetes, order Pucciniales (rust fungi). It comprises 42 species occurring mainly in tropical to sub‐tropical regions of Africa and Asia, mostly on uncultivated Rubiaceae and Apocynaceae plants (Ritschel, 2005). Hemileia vastatrix is the type species of the genus. As described by Berkeley and Broome (1869) and Ward (1889), H. vastatrix urediniospores are reniform, 28–36 × 18–28 µm; the urediniospore wall is hyaline, strongly warted on the convex face, smooth on the straight or concave face, and 1 µm thick; teliospores are spherical, subglobose to napiform, 20–28 µm in diameter; the teliospore wall is hyaline, smooth and 1 µm thick. Hemileia vastatrix can be distinguished from H. coffeicola, as the latter produces sori scattered throughout the leaf and presents urediniospores with fewer but larger spines. Both rusts have C. arabica and other Coffea species as hosts, but H. coffeicola is of low economic importance and is geographically confined (Ritschel, 2005).
The genus Hemileia is distinguished from other rust genera by the unique combination of three morphological features: suprastomatal bouquet‐shaped sori; ovoid to reniform urediniospores with a smooth ventral side and a delicately to coarsely echinulated convex dorsal side; and angular‐globose to very irregular teliospores (Ritschel, 2005). Molecular evidence consistently places the genus Hemileia among the more basal phylogenetic group of the Pucciniales (Aime, 2006; Grasso et al., 2006; McTaggart et al., 2016; Silva et al., 2015a; Wingfield et al., 2004). Hemileia is presently included in the Mikronegeriaceae (Aime, 2006; McTaggart et al., 2016), a family which probably represents the most ancient rust lineages and diverged at c. 91–96 million years ago. These findings challenge the old notion that ancestral rusts are harboured by phylogenetically ancestral hosts and support the view that the ancestors to the extant rusts may have been tropical short‐cycled species that evolved on angiosperms (McTaggart et al., 2016).
Life Cycle and Infection Process
Hemileia vastatrix is a hemicyclic fungus producing urediniospores, teliospores and basidiospores, whereas pycniospores and aeciospores are not known. Urediniospores and teliospores are produced in the same sorus, but at different times. Urediniospores are dikaryotic and represent the asexual cycle, re‐infecting the leaves whenever environmental conditions are favourable. Teliospores occur rarely and germinate in situ, producing a promycelium from which four basidiospores are formed (Chinnappa and Sreenivasan, 1965; Coutinho et al., 1995; Fernandes RC et al., 2009; Rodrigues et al., 1980). Basidiospores cannot infect coffee, but no other host plant has been identified (Kushalappa and Eskes, 1989; Rodrigues et al., 1980). However, several reports have proposed that H. vastatrix could be depicted as a primitive autoecious rust lacking pycnial and aecial stages (Hennen and Figueiredo, 1984), with urediniospores functionally acting as teliospores (Carvalho et al., 2011; Rajendren, 1967; Rodrigues et al., 1980). On the other hand, H. vastatrix may have lost the ability to produce sexual spores during evolution since adaptation to survival would have mostly been achieved in the uredinial stage. The prevalence of uredinial stages in short‐cycled rust species is considered an adaptation to the short growing seasons and heterogeneous vegetation landscapes in the tropics (Berndt, 2012).
The initiation of H. vastatrix infection on coffee leaves, like other rust fungi, involves specific events, including adhesion to the host surface, urediniospore germination, appressorium formation over stomata, penetration and inter‐ and intracellular colonization (Fig. 2).
Figure 2.

Hemileia vastatrix infection process. (A) Urediniospore (u), scanning electron microscopy (SEM). (B) Germinated urediniospore (u) with germ tube (gt) and appressorium (ap) over stomata on the lower surface of the coffee leaf, 17 h after inoculation (hai), SEM. (C) Appressorium (ap) over stomata and penetration hypha (arrow), 24 hai, light microscopy (LM). (D) Appressorium (ap) over stomata and intercellular hypha with an haustorium (h) within a subsidiary cell, 48 hai, LM. (E) Intercellular hyphae (arrows) and haustoria (h) within epidermal and mesophyll cells, 20 days after inoculation (dai), LM. (F) Haustorium (h) within a spongy parenchyma cell, 20 dai, SEM. (G) Intercellular hyphae (arrows) in the spongy parenchyma, 20 dai, SEM. (H) Urediniosporic sorum protruding through the stomata in a bouquet shape, 21 dai, LM. (I) Urediniosporic sorum, 21 dai, SEM.
Adhesion to the host, an essential step in the successful establishment of pathogenesis, prevents fungal displacement and is critical for the correct sensing of topographic signals involved in thigmotropic responses and for the differentiation and function of the appressoria (Braun and Howard, 1994). In H. vastatrix, the involvement of esterases has been demonstrated in urediniospore adhesion and appressoria differentiation, both in planta and in vitro (Azinheira et al., 2007).
Urediniospore germination requires water and is optimal at about 24°C. After appressorium formation, the fungus penetrates the host through the stomata, forming a penetration hypha that grows into the substomatal chamber (Fig. 2A–C). This hypha produces two thick lateral branches, resembling an anchor, a unique trait of H. vastatrix. Each lateral branch of the anchor differentiates into a haustorial mother cell (HMC) that gives rise to a haustorium, which primarily infects the stomatal subsidiary cells (Fig. 2D), another unique feature of H. vastatrix. The fungus continues to grow, forming more intercellular hyphae, including HMCs, and a large number of haustoria in the cells of the spongy and palisade parenchyma and even of the upper epidermis (Fig. 2E–G). At this stage, chloroses are macroscopically visible (McCain and Hennen, 1984). Hyphae invade substomatal cavities and interweave, differentiating protosori. Approximately 3 weeks after infection, urediniosporic sori protrude through the stomata in a bouquet shape. These appear as orange‐coloured pustules, the typical sign of this disease (Figs 1A, 2H, I). The formation of suprastomatal sori is a trait that seems to be restricted to tropical rusts and has apparently evolved convergently in a number of rust genera (Berndt, 2012).
Ultrastructural studies have revealed that the germination of H. vastatrix urediniospores is accomplished through one or two germ pores (Rijo and Sargent, 1983). The germ tube wall is composed of an electron‐lucent band apparently derived from the germ pore matrix, which is subjacent to the innermost urediniospore wall layer. The cytoplasm content, including the two nuclei and other organelles, passes through the germ pore (Fig. 3A). During this phase, the spore exhibits a high metabolic activity characterized by an increase in the number of mitochondria and the generation of endoplasmic reticulum, ribosomes, lomasomes and small vesicles (Fig. 3B).
Figure 3.
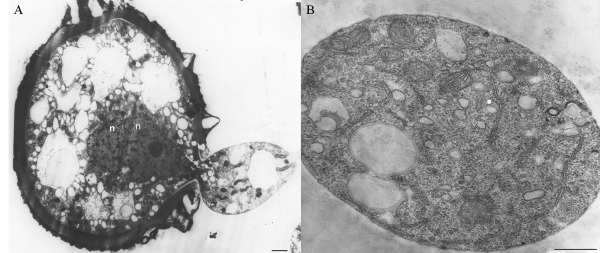
Hemileia vastatrix urediniospore germination (Rijo and Sargent, 1983). (A) Beginning of germination, with cytoplasm passing through the germ pore with two nuclei (n) and one evident nucleolus (bar, 1 µm). (B) Transverse section of the germ tube showing different organelles (bar, 0.5 µm).
β‐1,3‐Glucans and chitin are the major components of the cell wall of H. vastatrix urediniospores and infection structures (Maxemiuc‐Naccache and Dietrich, 1981; Silva et al., 1999). Both polymers are regularly distributed over the walls of pre‐penetration fungal structures. In the intercellular hyphae and haustoria, β‐1,3‐glucans are also regularly distributed, whereas chitin accumulates preferentially over the internal parts of fungal cell walls, being less exposed to the eventual action of host chitinases, a general rule in rust fungi (Deising et al., 1996; Silva et al., 1999).
Ultrastructural studies have revealed that H. vastatrix intercellular hyphae and haustoria are similar to those of other rust fungi (as revised by Silva et al., 1999). The cytoplasm contains abundant endoplasmic reticulum, occasional tubular complexes, mitochondria, ribosomes and lipid droplets.
Intercellular hyphae begin host cell penetration from HMCs, which have a thick multi‐layered wall that attaches firmly to the host cell wall. Silva et al. (1999) observed that, during H. vastatrix haustoria formation, plant cell wall degradation was restricted to the site of host cell penetration with minimal damage to other cells. This is a common goal of biotrophic fungi, as they depend on the living host cells (Mendgen and Hahn, 2002; Schulze‐Lefert and Panstruga, 2003).
The haustorium is composed of a neck, which presents a ring with two electron‐opaque bands, and a body. Reaching far into the plant cell, the entire structure becomes surrounded by newly synthesized and highly modified plant plasmalemma, designated as the extrahaustorial membrane. Haustoria are thus not truly intracellular. Indeed, a zone of separation between host plasma membranes and the pathogen is established and comprises the haustorial cell wall and the extrahaustorial matrix (Harder and Chong, 1991; Mendgen and Voegele, 2005).
Similar to other biotrophs, H. vastatrix interacts intimately through such interfaces with the host cells, modifying the metabolic processes to serve its needs. This mode of interaction involves an effective suppression of the host immune system by effector proteins, known as effector‐triggered susceptibility (Doehlemann and Hemetsberger, 2013; Hok et al., 2010). In coffee plants susceptible to H. vastatrix, during the early stages of the infection process, a decrease in the abundance of hydrolases (sugar and peptides) and oxidases constitutively present in the apoplastic fluid of coffee leaves has been observed (Guerra‐Guimarães et al., 2014, 2015). However, during later stages, an increase in the abundance of defence‐related proteins, such as phenylalanine ammonia lyase, peroxidase, superoxide dismutase, chitinase, Pathogenesis‐Related gene 1 (PR1), thaumatin‐like, NtPRp27 protein and β‐1,3‐glucanase, has been detected (Guerra‐Guimarães et al., 2009a, 2009b; Silva et al., 2002, 2008). Furthermore, the hypersensitive cell death (hypersensitive response, HR) of guard and subsidiary cells and the encasement of some haustoria have also been observed, but in a low percentage of infection sites. Nevertheless, these plant defence responses occur too late to efficiently prevent fungal growth and sporulation (Silva et al., 1999, 2002, 2006).
Epidemiology
CLR infections seldom kill the host plant, although severe infections affect the yield in subsequent years because they hamper vegetative development and can generate polyetic epidemics over successive seasons. Climate (including the altitude effect), shade, soil fertility and canopy architecture influence disease severity. Although a wealth of knowledge has been accumulated on epidemics and modelling, disease management strategies are still frequently ineffective. This gap may be explained by the very large number of environmental variables, their interaction at a given moment and their cumulative effect over time (Avelino et al., 2004). Fluctuations in the price of coffee influence producer decisions regarding crop management, which, in turn, condition rust incidence and severity. This process seems to have become more acute in recent decades with the liberalization of prices and of cultivation quotas, and a sharp decrease in support of research and agricultural extension (McCook and Vandermeer, 2015).
A direct link between agricultural intensification and disease severity was reported during the Ceylon epidemic (Ward, 1882). Since then, epidemiology has gained several analytical tools, and multivariate studies at local, national and regional levels have contributed to a better understanding of the role of the relevant variables in the disease outcome. Usually, the peak of CLR epidemics occurs during fruit harvest; therefore, primary yield losses are frequently of low importance. Secondary losses, arising from low yield as a result of reduced vegetative growth caused by the previous epidemic, tend to be more important than primary losses (Avelino et al., 1991). Modelling of rust epidemics according to agroecological variables has revealed the relevance of local agronomic factors, including shade, canopy density and soil fertility, interacting with regional environmental factors, such as rainfall (Avelino et al., 2006; Boudrot et al., 2016). At the global level, climate change scenarios have also been analysed in the context of CLR, with shorter incubation periods being forecasted (Ghini et al., 2011) and disease‐favouring scenarios mapped (Alves et al., 2011). Indeed, this disease has been increasingly reported at higher altitudes in recent years (Boudrot et al., 2016; Rozo et al., 2012).
Disease Control
Disease resistance breeding
The breeding of coffee plants for resistance to rust is considered to be the best disease management strategy, both environmentally and economically (Silva et al., 2006). The first effective effort to select resistant germplasm was conducted in India in 1911, giving rise to the release of the cultivar ‘Kent's’, which replaced the susceptible cultivar ‘Coorg’ (revised by Rodrigues et al., 1975). Several missions to Ethiopia were subsequently conducted, but no effective resistance sources were identified (Rodrigues et al., 1975). In the 1950s, concern for the potential introduction of rust into the American continent (Wellman, 1953) led F. Wellman and W. Cowgill to conduct field missions in the Eastern Hemisphere, collecting more than 100 coffee types new to the Americas. In collaboration with Branquinho d'Oliveira, the work of these researchers led the USA and Portuguese governments to provide financial support for the creation of the Coffee Rusts Research Center (CIFC, Centro de Investigação das Ferrugens do Cafeeiro) in Portugal, located far from coffee‐growing regions and thus centralizing research on CLR at the international level. Since 1955, CIFC has received and characterized coffee and rust germplasm and supplied breeding programmes at coffee research institutions with characterized resistance sources, together with scientific and technical information and training. One of the first practical results of the research carried out at CIFC was the demonstration that all cultivars grown at that time in America (including ‘Typica’, ‘Caturra’, ‘Mundo Novo’ and ‘Bourbon’) were susceptible to this disease (Rodrigues et al., 1975). The investigation of CLR away from coffee‐growing areas enabled CIFC to receive plant and fungal material from collaborating institutions around the world, which, in turn, allowed breeders in coffee‐growing countries to have their genotypes characterized for resistance to races that were not present in such countries.
HDT (Híbrido de Timor) populations were derived from a plant discovered on the island of Timor in 1927 exhibiting resistance to rust among ‘Typica’ coffee crops (Bettencourt, 1981). In the 1950s, these populations were shown to be natural hybrids between C. arabica and C. canephora, most offering resistance to all rust races known at that time (Rodrigues et al., 1975). In 1960, CIFC started a breeding programme aiming to transfer resistance from HDT to the main Arabica cultivars. Some selected F1 and F2 plants with resistance to all known races were supplied free of charge to all institutions in coffee‐growing countries that requested these materials. The hybrids Caturra × HDT CIFC832/1 and Villa Sarchi × HDT CIFC832/2 synthesized at CIFC gave rise to the Catimor and Sarchimor populations, respectively. These populations, as well as others developed in Colombia (Caturra × HDT CIFC1343) and Brazil (Catuaí × HDT CIFC2570), are the sources of the majority of currently grown rust‐resistant varieties. These populations combine the resistance of HDT and the good agronomic traits of commercial varieties (Bettencourt and Rodrigues, 1988; Rodrigues et al., 1975), attaining similar levels of beverage quality to those obtained from pure Arabica genotypes (Bertrand et al., 2003; van der Vossen, 2003). The selection of HDT‐derived genotypes adapted to local agroecological conditions and the subsequent release of cultivars have been conducted by several institutions, namely the Universidade Federal de Viçosa (UFV), the Empresa de Pesquisa Agropecuária de Minas Gerais (EPAMIG), the Instituto Agronômico de Campinas (IAC), the Instituto Agronômico do Paraná (IAPAR) and the Fundação Procafé/Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) in Brazil; the Centro Nacional de Investigaciones de Café (CENICAFÉ) in Colombia; the Centro Agronómico Tropical de Investigación y Enseñanza (CATIE) and the Instituto del Café de Costa Rica (ICAFE) in Costa Rica; the Asociación Nacional del Café (ANACAFÉ) in Guatemala; the Instituto Salvadoreño de Investigaciones del Café (ISIC) in El Salvador; the Instituto Hondureño del Café (IHCAFE) in Honduras; the Instituto Mexicano del Café in Mexico; the Hawaii Agriculture Research Center (HARC) in Hawaii, USA; the Coffee Research Foundation (CRF) in Kenya; the Tanzania Coffee Research Institute (TaCRI) in Tanzania; the Central Coffee Research Institute (CCRI) in India; and the Dehong Tropical Agriculture Research Institute of Yunnan (DTARI) in China.
Rust resistance in HDT populations is conferred by Robusta‐derived genes, such as SH6, SH7, SH8, SH9 and others not yet identified, in addition to Arabica resistance genes (SH1, SH2, SH4 and SH5). These genes, together with SH3 (derived from C. liberica), condition coffee response to rust according to Flor's gene‐to‐gene theory (Noronha‐Wagner and Bettencourt, 1967), enabling the classification of genotypes into physiological groups, ranging from resistant to all known rust races to susceptible to almost all known races (Bettencourt and Rodrigues, 1988; Várzea et al., 1989; CIFC records). The usefulness of HDT populations as resistance donors led to several studies seeking to identify markers linked to resistance genes (Brito et al., 2010; Diola et al., 2011; Romero et al., 2014), targeting downstream marker‐assisted selection approaches.
The importance of HDT populations as resistance sources relies on the long durability of some of these resistance factors, which, in some cases, have been in use for more than 30 years. For instance, the genotype HDT CIFC832/2 carries additional genome introgressions compared with other genotypes (Herrera et al., 2014) and presents a pre‐haustorial (non‐host‐like) resistance (Diniz et al., 2012). Indeed, the post‐haustorial resistance response is typically found in most coffee–H. vastatrix interactions (Silva et al. 2002, 2006). The cytological and biochemical aspects of coffee resistance to CLR have been revised by Silva et al. (2006) and addressed by Diniz et al. (2012). In brief, both pre‐ and post‐haustorial resistances are associated with the HR and with the activation of several genes, including receptor‐like kinases, WRKY transcription factors, glycosyltransferases, lipoxygenases and PRs (Cacas et al., 2011; Diniz et al., 2012; Diola et al., 2013; Fernandez et al., 2004; Ganesh et al., 2006; Guzzo et al., 2009; Ramiro et al., 2010; Silva et al., 2006).
As the resistance in several HDT‐derived genotypes is being lost (Diniz et al., 2012), new sources of resistance are being investigated. Given the ample resistance found in C. canephora, together with the successful history of HDT, one tempting approach for the identification of new sources of resistance for Arabica coffee is to perform C. arabica × C. canephora crosses. Such studies have promised new resistance sources (Caicedo et al., 2013; Herrera et al., 2009; Mahé et al., 2007; Romero et al., 2010). To breed one of India's most popular Arabica genotypes (S.795), Prakash et al. (2011) developed two SCAR markers closely linked to the SH3 gene. This is a highly effective rust resistance gene naturally introgressed into C. arabica from C. liberica (Prakash et al., 2004). Partial and non‐specific polygenic resistances have been evidenced in C. canephora, in some C. arabica genotypes and in some interspecific hybrids (as revised by Silva et al., 2006). This corroborates previous reports suggesting that, in addition to SH genes, other major and minor genes might condition coffee–rust interactions (Bettencourt and Rodrigues, 1988). Such studies, however, are hampered by the need for a laborious and time‐consuming downstream breeding effort in order to introduce resistance factors into elite lines with adequate agronomic and quality traits.
The identification of resistance in wild C. arabica populations would be of interest as it avoids breeding to eliminate undesired traits from other Coffea species. However, the analysis of wild C. arabica germplasm has so far provided little support for the identification of resistance sources, as rust occurs frequently among plants in forests across the native range of C. arabica in Ethiopia (Samnegard et al., 2014). Information regarding the susceptibility of wild germplasm to the different rust races is scarce (Rodrigues et al., 1975), and the very low genetic diversity among wild populations suggests little promise of success in finding new sources of resistance (Davis et al., 2012; Steiger et al., 2002).
Chemical control
Chemical control of CLR is the obvious choice in the absence of a resistance genotype and of other effective disease management strategies. Chemical control represents an environmental hazard and a social concern (organic coffee is increasingly valued; Ibañez and Blackman, 2016), as well as an economic burden. For instance, in Tanzania, 50% of the coffee cultivation production costs can be attributed to the chemical control of the two main fungal diseases, CLR and coffee berry disease (Kilambo et al., 2013). Preventative treatments are typically carried out with copper‐based fungicides, whereas curative treatments are conducted with systemic fungicides (e.g. epoxiconazole, pyraclostrobin). The combined or alternate use of copper‐based and systemic fungicides is advised to avoid the risk of selecting fungicide‐resistant rust populations (Zambolim, 2016). Research regarding the best practices for the application of such products is developed locally according to agroecological variables and epidemiology (e.g. Souza et al., 2011).
Additional control strategies
The reduction in the availability of effective approved fungicides because of health and environmental concerns has made it necessary to intensify research for the development of novel, effective and sustainable disease control solutions. The effects of potassium silicate and essential oils have been tested recently, but with limited success (Lopes et al., 2013 and Pereira et al., 2012, respectively). However, promising results have been obtained with a resistance inducer of the benzothiadiazole (BTH) group, such as acibenzolar‐S‐methyl (Fernandes et al., 2013; Guzzo et al., 2001; Marchi et al., 2002). BTH‐treated coffee leaves overexpress genes involved in pathogenesis‐related protein synthesis, the oxidative burst and cell wall strengthening, suggesting a general shift in metabolism from housekeeping to defence (Nardi et al., 2006). The effects of phosphites and plant formulations based on the by‐products of the coffee and citrus industries for the control of CLR have been evaluated in the glasshouse and in the field. Some of the formulations have shown an intermediate to good efficiency compared with standard fungicides, proving to be effective alternatives for the management of coffee rust and other diseases (Carvalho et al., 2012; Fernandes LHM et al., 2009; Monteiro et al., 2013; Santos et al., 2007).
Hemileia vastatrix spores are hyperparasitized by the Ascomycete fungus Lecanicillium lecanii. Although unable to effectively control CLR, this hyperparasite is capable of reducing spore viability and disease severity (Vandermeer et al., 2010). Lecanicillium lecanii is primarily an entomopathogen of the green coffee scale Coccus viridis, which, in turn, has a mutualistic association with the arboreal nesting ant Azteca instabilis. The relationships between these organisms suggest that complex ecological interactions may play an important role in disease incidence and severity, potentially explaining why CLR is sometimes a severe epidemic and other times a troublesome but not devastating problem (Vandermeer et al., 2014).
Bacteria and fungi present in the coffee ecosystem have been investigated for their use as potential biocontrol agents against H. vastatrix. Specific strains of the bacteria Pseudomonas putida, Bacillus megaterium and B. thuringiensis, together with two Fusarium sp. isolates, provide promising levels of antagonism (Haddad et al., 2009, 2013, 2014; Silva et al., 2012).
Genetic and Physiological Diversity
Evidence of high pathogenic variability in CLR was recognized at an early stage and associated with the breakdown of resistance. Physiological specialization was first described by Mayne (1932, 1942), who identified four rust races. Since then, world surveys of coffee rust races have been historically pursued and amplified by CIFC from 1952 with d'Oliveira until today based on H. vastatrix spore samples from the most diverse coffee‐producing regions of the world. Distinct races or pathotypes have been regularly identified through the differentiation of isolates on a set of coffee host materials bearing different resistance gene combinations (differentials) under prescribed testing conditions (d'Oliveira, 1954−57). Currently, more than 50 rust physiological races and 23 coffee differentials have been identified (Rodrigues et al., 1975; Várzea et al., 2009; and CIFC records).
Races are attributed to isolates with distinct and unique combinations of virulence genes as inferred by Flor's gene‐to‐gene theory and described as sequential roman numerals in order of detection (Noronha‐Wagner and Bettencourt, 1967). Thus, as no further genetic confirmation has been possible so far, inferred rust race genotypes comprise virulence genes ranging from v1 to v9 in isolates derived from C. arabica and tetraploid interspecific hybrids, whereas those of the races that attack diploid coffee species are not known. Given this direct correlation with the coffee host resistance genotypes, virulence genes v1 to v5 can be traced back to an Arabica‐type origin (v1/SH1 from ‘Geisha’; v2/SH2 from ‘Kent's’; v3/SH3 from a C. liberica introgression; v4/SH4 from ‘Kaffa’; v5/SH5 from nearly all cultivars), whereas v6 to v9 reflect the additional Robusta heritage of SH6 to SH9 genes present in HDT and other interspecific hybrids. However, virulence profile characterization, particularly of isolates infecting HDT derivatives, can only go as far as the available collection of coffee differential genotypes allows, leaving many genotypes incompletely or entirely unidentified. As presumed from the dynamic host–pathogen co‐evolutionary arms race, in which short‐term selection of pathogen strains with fitness advantages is promoted, new pathotypes with increased virulence have been continuously appearing.
Within the scale of coffee differentials, race II (v5) presents the most restricted infection spectrum and is considered to be the most common and widespread rust race in the world, acquiring a generalized occurrence, probably as a consequence of the uniform genetic background of most C. arabica cultivars worldwide (Bettencourt, 1981). However, the geographical distribution of rust races seems to be entirely dependent on the coffee genotypes planted locally, and the prevalence of certain races in a given area thus seems to occur accordingly (Bettencourt, 1981). Evidence of the selection pressure exerted by coffee resistance genes on the origin and distribution of pathotypes is easy to find (Várzea and Marques, 2005), but it is in India that the highest number of rust races is registered. This country holds the most ancient breeding programme for CLR resistance in the world, involving regular and massive introductions of new or experimental resistant coffee materials into the field, to which usually follows the appearance of new pathotypes with enlarged virulence spectra (Várzea and Marques, 2005). This process recently resulted in the identification of CLR in India in genotypes resistant to all known rust races (Prakash et al., 2015). Currently, the range of coffee rust virulence profiles most probably goes far beyond those of the races characterized so far.
No direct link between such high phenotypic diversity and molecular diversity has been found. Molecular makers for the characterization of variation in H. vastatrix populations have been elusive. Simple sequence repeat (SSR) markers, which are ideal for population genetics, failed to provide polymorphic loci with sufficient analytical resolution (Cristancho and Escobar, 2008; Rozo et al., 2012). Sequencing of the rDNA‐ITS region was also inadequate for sequence data analysis because of the presence of multiple copies, whereas other nuclear loci showed no variation or very few polymorphic sites (Batista et al., 2010). Random amplification of polymorphic DNAs (RAPDs) and amplified fragment length polymorphisms (AFLPs) are the only informative markers documented so far, suggesting that genetic variation may be unevenly distributed along the genome.
Using RAPD markers, Gouveia et al. (2005) found a moderate genetic diversity and a high genetic differentiation in a global geographical range of H. vastatrix populations, with indications of clonal multiplication, but no evidence of population structure with regard to physiological race, host or geographical origin. Subsequent genetic diversity studies have predominantly been focused in Brazil (Cabral et al., 2016; Maia et al., 2013; Nunes et al., 2009) and Colombia (Rozo et al., 2012), addressing local populations. These populations represent the isolates with the lowest level of genetic differentiation in the study of Gouveia et al. (2005) compared with African and Asian populations, suggesting a possibly more recent origin. Indeed, South America was the last continent to be reached by H. vastatrix, and it is feasible that American isolates are derived from a few introduced since 1970 in Brazil, where it was first reported (Muller, 1971). All studies consistently report low differentiation and unstructured variability in H. vastatrix populations considering host and geographical origin, supported by high gene flow, probably as a consequence of effective long‐distance dispersal and host germplasm exchange. Furthermore, large continuous areas of coffee plantations facilitate the stepwise movement of rust epidemics and genotype distribution (Nunes et al., 2009). This pattern of evenly partitioned genetic variation found so far in CLR is consistent with the long‐standing recognition of this fungus as an asexual species. However, evidence of random mating was found from an estimation of locus linkage disequilibrium (Cabral et al., 2016; Maia et al., 2013; Nunes et al., 2009), suggesting some sort of recombination event. For instance, parasexual phenomena, including somatic hybridization, have been documented in rusts (Park and Wellings, 2012). Thus, despite the data provided by these recent studies, the mechanisms and dynamics of population genetic variation in H. vastatrix remain unknown. Such an endeavour is close to being accomplished by increasing the resolution of the molecular population analyses at the genome scale using next‐generation sequencing of a more comprehensive rust sampling (Silva et al., 2015b).
Cytogenomics and Molecular Pathogenicity
Rusts were recently noted as the order with the largest average genome size among fungi (Talhinhas et al., 2015; Tavares et al., 2014). Nevertheless, the c. 800‐Mbp genome of H. vastatrix (Carvalho et al., 2014; Tavares et al., 2014) stands out as one of the largest among rusts (Ramos et al., 2015; Tavares et al., 2014) and the largest genome of fungal pathogens of economic relevance. The genome of H. vastatrix appears to also vary in size: in a preliminary study including 11 isolates representing distinct races, the genome size ranged from 765 to 839 Mbp, with a general average (± standard deviation) of 797 ± 27 Mbp (CIFC team, unpublished data). In addition, studies concerning the cytological characterization of the H. vastatrix karyotype were performed on metaphase nuclei obtained during urediniospore germination and revealed 10 chromosomes (Tavares et al., 2013).
A link between biotrophic specialization and genome size expansion has been established (Spanu, 2012). Rust genomes are known to be vastly populated with non‐coding regions (e.g. Duplessis et al., 2011), suggesting that such non‐coding regions and hypothetical polyploidy could explain the vast genome size of H. vastatrix. Indeed, Cristancho et al. (2014) suggested that 74% of the H. vastatrix assembled genome contains repetitive regions. A draft assembly obtained from our ongoing genome sequencing work on H. vastatrix accounts for less than 10% of the predicted genome size, yet a set of genes representing approximately 80% of the total number of genes in the Melampsora larici‐populina genome was identified (CIFC team, unpublished data).
The unusually large genome size of H. vastatrix and the abundance of non‐coding regions, including repetitive regions, could explain the difficulties in obtaining a saturated genomic sequencing. With the recent advances in genome sequencing technology, the sequencing of rusts has become feasible, and sequencing initiatives of rusts with large genomes of economic and scientific importance (e.g. Phakopsora pachyrhizi and Uromyces fabae; Loehrer et al., 2014 and Link et al., 2014, respectively) are ongoing, suggesting that the conditions are being met for a high‐quality genome sequence of H. vastatrix to be obtained in the near future.
Although the genome of H. vastatrix has not been fully unveiled, transcriptomic approaches have already helped to gain insight into the functional genome. To this end, several technical strategies have been developed to circumvent the difficulties caused by the biotrophic nature of this pathogen, which implies that fungal samples (other than urediniospores) are physically mixed with the host in infected leaf samples. These include:
—an in silico approach using a bioinformatic pipeline based on homology scores and codon usage bias, applied to distinguish between fungal and plant transcripts (Fernandez et al., 2012);
—a protocol developed to isolate good‐quality fungal RNA from appressoria differentiated in planta (Loureiro et al., 2015);
—a method for the selection and validation of reverse transcription‐quantitative polymerase chain reaction (RT‐qPCR) reference genes for fungal gene expression studies, considering the fungal biomass variation in planta throughout infection (Vieira et al., 2011).
A transcriptomic analysis of H. vastatrix germinating urediniospores, appressoria and an in planta haustoria‐rich sample (Fernandez et al., 2012; Talhinhas et al., 2014) led to the identification and annotation of 9234 transcripts (i.e. >50% of the genes predicted in sequenced rust genomes). Only 784 sequences were shared by the three conditions, and 75% were unique to a single library. Database comparisons further indicated that half of these H. vastatrix transcripts present no significant homology to genomic or transcriptomic data from other rusts, potentially representing novel or very divergent genes.
The annotation of H. vastatrix transcripts and a comparison of their relative abundance in each of the three sampling stages suggest a particularly active metabolism, translational activity and the production of new structures in appressoria, and intense signalling, transport and secretory activity and cellular multiplication in germinating urediniospores. In the haustoria‐rich phase, results suggest intense signalling and nutrient uptake from the host to the fungus (Talhinhas et al., 2014).
One hundred and forty‐eight transcripts encoding putative carbohydrate‐active enzymes (CAZymes) were identified, representing c. 45% of the CAZymes in the genomes of the poplar and the wheat stem rust fungi. These CAZyme transcripts are more frequently expressed during the early stages of infection, suggesting their involvement in the appressoria‐mediated penetration of coffee leaf stomata, probably combining lytic and physical mechanisms (Talhinhas et al., 2014). Among the CAZymes, chitin deacetylases (CD‐As), with a potential role in avoiding recognition by plant chitin receptors and hydrolases present in the extracellular space in the leaf apoplast (Gueddari et al., 2002), were analysed in H. vastatrix. Seven different CD‐A‐like genes were identified, and a phylogenetic analysis divided CD‐As into two groups: one specific to H. vastatrix and another very similar to CD‐As from different basidiomycetes, suggesting distinct biological roles (Azevedo et al., 2013). Expression studies throughout the infection process showed different profiles in compatible (susceptibility) and incompatible (resistance) interactions (Azevedo et al., 2013), with a peak of expression in compatible interactions coinciding with spore germination and appressoria formation (Vieira et al., 2012), as noted previously in U. fabae (Deising and Siegrist, 1995).
Five hundred and sixteen H. vastatrix genes were identified as putatively encoding secreted proteins (Talhinhas et al., 2014). At least 50% of these predicted secreted proteins have no homology to proteins in other rusts and may therefore be specific to H. vastatrix. Although less numerous, genes encoding putative secreted proteins in germinating urediniospores and appressoria RNA libraries are more abundantly expressed than those in the late infection library. The analysis of PFAM domains on the H. vastatrix secretome enabled the identification of 121 different PFAM domains. Transcripts with no PFAM domains and no homologies were further analysed for the presence of new conserved motifs (e.g. particular protein structure at particular positions, which suggests some common functions; Alfano, 2009; Saunders et al., 2012), aiming to identify new avirulence (Avr) proteins (Gonçalves et al., 2013). Three positional motifs were identified in four H. vastatrix transcripts, pointing to a conserved organization that resembles the structure found in the RXLR class of effector genes from oomycetes. These H. vastatrix genes were up‐regulated in incompatible versus compatible interactions, suggesting a role in the induction of plant immunity (Gonçalves et al., 2013).
Conclusions and Future Perspectives
In spite of its destructiveness, worldwide distribution and economic impact on the production of such an important cash crop as coffee, H. vastatrix has not been as widely studied as other rust fungi. As a pathosystem, the H. vastatrix–coffee interaction has some biological peculiarities and can be used as a historical, economic and epidemiological case study. The complex developmentally regulated infection process of H. vastatrix includes unique colonization features, such as haustorial invasion of stomata subsidiary cells before further tissue colonization and the induction of an HR as early as the appressorial stage. However, at the molecular level, much remains to be learned about the specific mechanisms underlying pathogenicity and virulence. The 516 putative H. vastatrix effector proteins identified may prompt the identification of coffee R genes encoding target proteins, providing additional tools to accelerate and improve disease resistance breeding.
The discovery and characterization of resistance in HDT populations and its deployment into commercial cultivars through breeding programmes still represent the major breakthroughs in the history of CLR disease resistance breeding. Nevertheless, the appearance of new rust races capable of overcoming such resistances raises the need to understand H. vastatrix virulence evolution and to better characterize known sources of resistance and/or to discover new ones.
So far, high phenotypic diversity has not been reflected within the molecular diversity studied, and no population genetic structure has been found. Through large‐scale population and evolutionary genomic studies, the efforts to unveil the c. 800‐Mbp genome of H. vastatrix populations will certainly shed light not only on the reasons for such a huge genome size, but also on signatures of diversity creation. With the increasing feasibility of the generation of genomic data and sophistication of statistical methods, our understanding of H. vastatrix adaptive evolution will probably be boosted in the near future. This will provide deep insight into the diversity‐generating mechanisms that render this a very successful and adaptable pathogen capable of overcoming resistance factors with relative ease. An improved annotated genome sequence would also greatly assist molecular research in H. vastatrix. Exploitation of these data, however, will still require the development or adaptation of methods for functional analysis, as well as the combination of different tools, from biochemistry to transcriptomics, to address the remaining unanswered questions about this important pathogen.
During the 150‐year history of CLR, much knowledge has been gained regarding its biology, epidemiology and control, but evolving agronomic and ecological conditions, together with the evolving pathogen itself, make this a challenging pathosystem both to economy and to science. Further investment in CLR research is strongly needed, bridging biology and agronomy, in order to provide farmers with effective control strategies and durable resistance sources.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
The Fundação para a Ciência e a Tecnologia (FCT, Portugal) is acknowledged for grants SFRH/BD/893972012, SFRH/BD/86736/2012, SFRH/BPD/104629/2014, SFRH/BD/84188/2012 and SFRH/BPD/88994/2012, attributed to A.V., D.N.S., D.B., I.D. and P.T., respectively, for support to projects PTDC/AGR‐GPL/114949/2009, PTDC/AGR‐GPL/119943/2010 and PTDC/BIA‐MIC/1716/2014, and for support to the research units LEAF (UID/AGR/04129/2013) and cE3c (UID/BIA/00329/2013). Wiley Editing Services is acknowledged for English language usage review and Dr Muthumeenaski Sreenivasaprasad for critical reading of the manuscript and valuable suggestions.
References
- Aime, M.C. (2006) Toward resolving family‐level relationships in rust fungi (Uredinales). Mycoscience, 47, 112–122. [Google Scholar]
- Alfano, J.R. (2009) Roadmap for future research on plant pathogen effectors. Mol. Plant Pathol. 10, 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, M.C. , Carvalho, L.G. , Pozza, E.A. , Sanches, L. and Maia, J.C.S. (2011) Ecological zoning of soybean rust, coffee rust and banana black sigatoka based on Brazilian climate changes. Procedia Environ. Sci. 6, 35–49. [Google Scholar]
- Avelino, J. , Muller, R.A. , Cilas, C. and Velasco‐Pascual, H. (1991) Development and behaviour of coffee orange rust (Hemileia vastatrix Berk. and Br.) in plantations undergoing modernization, planted with dwarf varieties in South‐East Mexico. Café Cacao Thé, 35, 21–37. [Google Scholar]
- Avelino, J. , Willocquet, L. and Savary, S. (2004) Effects of crop management patterns on coffee rust epidemics. Plant Pathol. 53, 541–547. [Google Scholar]
- Avelino, J. , Zelaya, H. , Merlo, A. , Pineda, A. , Ordoñez, M. and Savary, S. (2006) The intensity of a coffee rust epidemic is dependent on production situations. Ecol. Model. 197, 431–447. [Google Scholar]
- Avelino, J. , Cristancho, M. , Georgiou, S. , Imbach, P. , Aguilar, L. , Bornemann, G. , Läderach, P. , Anzueto, F. , Hruska, A.J. and Morales, C. (2015) The coffee rust crises in Colombia and Central America (2008–2013): impacts, plausible causes and proposed solutions. Food Sec. 7, 303–321. [Google Scholar]
- Azevedo, L. , Talhinhas, P. , Azinheira, H.G. , Silva, M.C. and Tavares, S. (2013) Chitin deacetylase genes from Hemileia vastatrix (causal agent of coffee leaf rust), identification and gene expression during the infection cycle. In: 10th International Congress of Plant Pathology, 25–30 August 2013, Beijing, China. International Society for Plant Pathology, P23.072, p. 335. Acta Phytopathol Sin. 43 (Suppl), 335. [Google Scholar]
- Azinheira, H.G. , Guerra‐Guimarães, L. , Silva, M.C. , Várzea, V.M.P. and Ricardo, C.P. (2007) Esterase activity and adhesion during the early stages of Hemileia vastatrix differentiation. In: Proceedings of the 21st International Conference on Coffee Science (ASIC), 11–15 September 2007, Montpellier, France, Montpellier: Association for Science and Information on Coffee ‐ASIC. pp. 1325–1329.
- Batista, D. , Guerra‐Guimarães, L. , Talhinhas, P. , Loureiro, A. , Silva, D.N. , Gonzalez, L. , Pereira, A.P. , Vieira, A. , Azinheira, H.G. , Struck, C. , Silva, M.C. , Paulo, O.S. and Várzea, V. (2010) Analysis of population genetic diversity and differentiation in Hemileia vastatrix by molecular markers. In: Proceedings of the 23rd International Conference on Coffee Science (ASIC), 3–8 October 2010, Bali, pp. 738–742. Paris: Association for Science and Information on Coffee ‐ASIC.
- Berkeley, M.J. and Broome, C.E. (1869) Hemileia vastatrix. Gardeners’ Chronicle, 6, 1157. [Google Scholar]
- Berndt, R. (2012) Species richness, taxonomy and peculiarities of the neotropical rust fungi: are they more diverse in the Neotropics? Biodivers. Conserv. 21, 2299–2322. [Google Scholar]
- Bertrand, B. , Guyot, B. , Anthony, F. and Lashermes, P. (2003) Impact of the Coffea canephora gene introgression on beverage quality of C. arabica . Theor. Appl. Genet. 107, 387–394. [DOI] [PubMed] [Google Scholar]
- Bettencourt, A.J. (1981) Melhoramento genético do cafeeiro. Transferência de factores de resistência à H. vastatrix Berk & Br. para as principais cultivares de Coffea arabica L. Lisbon: Centro de Investigação das Ferrugens do Cafeeiro (CIFC/IICT).
- Bettencourt, A.J. and Rodrigues, C.J. Jr. (1988) Principles and practice of coffee breeding for resistance to rust and other diseases In: Coffee Agronomy, Vol. 4 (Clarke R.J. and Macrae R., eds), pp. 199–234. London and New York: Elsevier. [Google Scholar]
- Boudrot, A. , Pico, J. , Merle, I. , Granados, E. , Vílchez, S. , Tixier, P. , Virgínio Filho, E.M. , Casanoves, F. , Tapia, A. , Allinne, C. , Rice, R.A. and Avelino, J. (2016) Shade effects on the dispersal of airborne Hemileia vastatrix uredospores. Phytopathology, 106, 572–580. [DOI] [PubMed] [Google Scholar]
- Bowden, J. , Gregory, P.H. and Johnson, C.G. (1971) Possible wind transport of coffee leaf rust across the Atlantic Ocean. Nature, 229, 500–501. [DOI] [PubMed] [Google Scholar]
- Braun, E.J. and Howard, R.J. (1994) Adhesion of fungal spores and germlings to host plant surfaces In: The Protistan Cell Surface (Wetherbee R., Pickett‐Heaps J.D. and Anderson R.A., eds), pp. 202–212. Vienna: Springer. [Google Scholar]
- Brito, G.G. , Caixeta, E.T. , Gallina, A.P. , Zambolim, E.M. , Zambolim, L. , Diola, V. and Loureiro, M.E. (2010) Inheritance of coffee leaf rust resistance and identification of AFLP markers linked to the resistance gene. Euphytica, 173, 255–264. [Google Scholar]
- Cabral, P.G.C. , Maciel‐Zambolim, E. , Oliveira, S.A.S. , Caixeta, E.T. and Zambolim, L. (2016) Genetic diversity and structure of Hemileia vastatrix populations on Coffea spp. Plant Pathol. 65, 196–204. [Google Scholar]
- Cacas, J.L. , Petitot, A.S. , Bernier, L. , Estevan, J. , Conejero, G. , Mongrand, S. and Fernandez, D. (2011) Identification and characterization of the Nonrace specific Disease Resistance 1 (NDR1) orthologous protein in coffee. BMC Plant Biol. 11, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo, B.L.C. , Guerrero, H.A.C. , Roux, J. and Wingfield, M.J. (2013) New coffee (Coffea arabica) genotypes derived from Coffea canephora exhibiting high levels of resistance to leaf rust and Ceratocystis canker. Trop. Plant Pathol. 38, 485–494. [Google Scholar]
- Carvalho, C.R. , Fernandes, R.C. , Carvalho, G.M.A. , Barreto, R.W. and Evans, H.C. (2011) Cryptosexuality and the genetic diversity paradox in Coffee Rust, Hemileia vastatrix . PloS One, 6, e26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, G.M.A. , Carvalho, C.R. , Barreto, R.W. and Evans, H.C. (2014) Coffee rust genome measured using flow cytometry: does size matter? Plant Pathol. 63, 1022–1026. [Google Scholar]
- Carvalho, V. , Rodrigo, L. , Nathan, R. and Silva, N. (2012) Alternativas de controle de doenças do cafeeiro. Coffee Sci. 7, 42–49. [Google Scholar]
- Chinnappa, C.C. and Sreenivasan, M.S. (1965) Cytological studies on germinating teliospores of Hemileia vastatrix . Caryologia, 18, 625–631. [Google Scholar]
- Coutinho, T.A. , Rijkenberg, F.H.J. and Vanasch, M.A.J. (1995) Teliospores of Hemileia vastatrix . Mycol. Res. 99, 932–934. [Google Scholar]
- Cressey, D. (2013) Coffee rust regains foothold. Nature, 493, 587. [DOI] [PubMed] [Google Scholar]
- Cristancho, M. and Escobar, C. (2008) Transferability of SSR markers from related Uredinales species to the coffee rust Hemileia vastatrix . Genet. Mol. Res. 7, 1186–1192. [DOI] [PubMed] [Google Scholar]
- Cristancho, M.A. , Botero‐Rozo, D.O. , Giraldo, W. , Tabima, J. , Riaño‐Pachón, D.M. , Escobar, C. , Rozo, Y. , Rivera, L.F. , Durán, A. , Restrepo, S. , Eilam, T. , Anikster, Y. and Gaitán, A.L. (2014) Annotation of a hybrid partial genome of the coffee rust (Hemileia vastatrix) contributes to the gene repertoire catalog of the Pucciniales. Front. Plant Sci. 5, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, A.P. , Gole, T.W. , Baena, S. and Moat, J. (2012) The impact of climate change on natural populations of Arabica coffee: predicting future trends and identifying priorities. PloS One, 7, e47981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deising, H. and Siegrist, J. (1995) Chitin deacetylase activity of the rust Uromyces viciae‐fabae is controlled by fungal morphogenesis. FEMS Microbiol. Lett. 127, 207–211. [Google Scholar]
- Deising, H. , Heiller, S. , Rauscher, M. , Xu, H. and Mendgen, K. (1996) Cellular aspects of rust infection structure differentiation In: Histology, Ultrastructure and Molecular Cytology of Plant–Microorganism Interaction (Nicole M. and Gianinazzi‐Pearson V., eds), pp. 135–156. Amsterdam: Kluwer Academic Publishers. [Google Scholar]
- Diola, V. , Brito, G.G. , Caixeta, E.T. , Maciel‐Zambolim, E. , Sakiyama, N.S. and Loureiro, M.E. (2011) High‐density genetic mapping for coffee leaf rust resistance. Tree Genet. Genom. 7, 1199–1208. [Google Scholar]
- Diola, V. , Brito, G.G. , Caixeta, E.T. , Pereira, L.F.P. and Loureiro, M.E. (2013) A new set of differentially expressed signaling genes is early expressed in coffee leaf rust race II incompatible interaction. Funct. Integr. Genom. 13, 379–389. [DOI] [PubMed] [Google Scholar]
- Diniz, I. , Talhinhas, P. , Azinheira, H.G. , Várzea, V. , Medeira, C. , Maia, I. , Petitot, A.S. , Nicole, M. , Fernandez, D. and Silva, M.C. (2012) Cellular and molecular analyses of coffee resistance to Hemileia vastatrix and nonhost resistance to Uromyces vignae in the resistance‐donor genotype HDT832/2. Eur. J. Plant Pathol. 133, 141–157. [Google Scholar]
- Doehlemann, G. and Hemetsberger, C. (2013) Apoplastic immunity and its suppression by filamentous plant pathogens. New Phytol. 198, 1001–1016. [DOI] [PubMed] [Google Scholar]
- Duplessis, S. , Cuomo, C.A. , Lin, Y.C. , Aerts, A. , Tisserant, E. , Veneault‐Fourrey, C. , Joly, D.L. , Hacquard, S. , Amselem, J. , Cantarel, B.L. , Chiu, R. , Coutinho, P.M. , Feau, N. , Field, M. , Frey, P. , Gelhaye, E. , Goldberg, J. , Grabherr, M.G. , Kodira, C.D. , Kohler, A. , Kües, U. , Lindquist, E.A. , Lucas, S.M. , Mago, R. , Mauceli, E. , Morin, E. , Murat, C. , Pangilinan, J.L. , Park, R. , Pearson, M. , Quesneville, H. , Rouhier, N. , Sakthikumar, S. , Salamov, A.A. , Schmutz, J. , Selles, B. , Shapiro, H. , Tanguay, P. , Tuskan, G.A. , Henrissat, B. , Van de Peer, Y. , Rouzé, P. , Ellis, J.G. , Dodds, P.N. , Schein, J.E. , Zhong, S. , Hamelin, R.C. , Grigoriev, I.V. , Szabo, L.J. and Martin, F. (2011) Obligate biotrophy features unravelled by the genomic analysis of rust fungi. Proc. Natl. Acad. Sci. USA, 108, 9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes, L.H.M. , Resende, M.L.V. , Costa, B.G. , Dias, H.C.B. and Villela, G.M. (2009) Ativador de resistência ASM (Bion®) no controle da ferrugem (Hemileia vastatrix Berk & Br.) na cultura do cafeeiro (Coffea arabica L.) em campo. In: VI Simpósio de Pesquisa dos Cafés do Brasil, Vitória, Espirito Santo, Brazil. Brasília: EMBRAPA Café.
- Fernandes, L.H.M. , Resende, M.L.V. , Costa, B.G. , Pereira, R.B. , Costa, B.H.G. , Monteiro, A.C.A. and Ribeiro Junior, P.M. (2013) Acibenzolar‐S‐Methyl no controle da ferrugem e da Cercosporiose do cafeeiro em condições de campo. Coffee Sci. 8, 24–32. [Google Scholar]
- Fernandes, R.C. , Evans, H.C. and Barreto, R.W. (2009) Confirmation of the occurrence of teliospores of Hemileia vastatrix in Brazil with observations on their mode of germination. Trop. Plant Pathol. 34, 108–113. [Google Scholar]
- Fernandez, D. , Santos, P. , Agostini, C. , Bon, M.C. , Petitot, A.S. , Silva, M.C. , Guerra‐Guimarães, L. , Ribeiro, A. , Argout, X. and Nicole, M. (2004) Coffee (Coffea arabica L.) genes early expressed during infection by the rust fungus (Hemileia vastatrix). Mol. Plant Pathol. 5, 527–536. [DOI] [PubMed] [Google Scholar]
- Fernandez, D. , Tisserant, E. , Talhinhas, P. , Azinheira, H. , Vieira, A. , Petitot, A.S. , Loureiro, A. , Poulain, J. , Da Silva, C. , Silva, M.C. and Duplessis, S. (2012) 454‐pyrosequencing of Coffea arabica leaves infected by the rust fungus Hemileia vastatrix reveals in planta expressed pathogen‐secreted proteins and plant functions triggered in a late compatible plant–rust interaction. Mol. Plant Pathol. 13, 17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh, D. , Petitot, A. , Silva, M.C. , Alary, R. , Lecouls, A.C. and Fernandez, D. (2006) Monitoring of the early molecular resistance responses of coffee (Coffea arabica L.) to the rust fungus (Hemileia vastatrix) using real‐time quantitative RT‐PCR. Plant Sci. 170, 1045–1051. [Google Scholar]
- Ghini, R. , Hamada, E. , Pedro Júnior, M.J. and Gonçalves, R.R.V. (2011) Incubation period of Hemileia vastatrix in coffee plants in Brazil simulated under climate change. Summa Phytopathol. 37, 85–93. [Google Scholar]
- Gonçalves, C. , Tavares, S. , Azinheira, H.G. , Oliveira, H. , Silva, M.C. and Talhinhas, P. (2013) Identification of small secreted proteins from Hemileia vastatrix, candidate effectors of coffee leaf rust disease. In: 10th International Congress of Plant Pathology, 25–30 August 2013, Beijing, China. International Society for Plant Pathology, P23.071, p. 335. Acta Phytopathol. Sin. 43 (Suppl), 335. [Google Scholar]
- Gouveia, M.M.C. , Ribeiro, A. , Várzea, V.M.P. and Rodrigues, C.J. Jr. (2005) Genetic diversity in Hemileia vastatrix based on RAPD markers. Mycologia, 97, 396–404. [DOI] [PubMed] [Google Scholar]
- Grasso, V. , Sierotzki, H. , Garibaldi, A. and Gisi, U. (2006) Relatedness among agronomically important rusts based on mitochondrial cytochrome b gene and ribosomal ITS sequences. J. Phytopathol. 154, 110–118. [Google Scholar]
- Gueddari, N.E.E. , Rauchhaus, U. , Moerschbacher, B.M. and Deising, H.B. (2002) Developmentally regulated conversion of surface‐exposed chitin to chitosan in cell walls of plant pathogenic fungi. New Phytol. 156, 103–112. [Google Scholar]
- Guerra‐Guimarães, L. , Cardoso, S. , Martins, I. , Loureiro, A. , Bernardes da Silva, A. , Várzea, V.M.P. and Silva, M.C. (2009a) Differential induction of superoxide dismutase in Coffea arabica–Hemileia vastatrix interactions In: Proceedings of the 22nd International Conference on Coffee Science, 14–19 September 2008, Campinas, Brazil, pp. 1036–1039. Paris: Association for Science and Information on Coffee ‐ASIC.
- Guerra‐Guimarães, L. , Silva, M.C. , Struck, C. , Loureiro, A. , Nicole, M. , Rodrigues, C.J. and Ricardo, C.P. (2009b) Chitinases of Coffea arabica genotypes resistant to orange rust Hemileia vastatrix . Biol. Plant. 53, 702–706. [Google Scholar]
- Guerra‐Guimarães, L. , Vieira, A. , Chaves, I. , Pinheiro, C. , Queiroz, W. , Renaut, J. and Ricardo, C.P. (2014) Effect of greenhouse conditions on the leaf apoplast proteome of Coffea arabica plants. J. Proteomics, 104, 128–139. [DOI] [PubMed] [Google Scholar]
- Guerra‐Guimarães, L. , Tenente, R. , Pinheiro, C. , Chaves, I. , Silva, M.C. , Cardoso, F.M.H. , Planchon, S. , Barros, D.R. , Renaut, J. and Ricardo, C.P. (2015) Proteomic analysis of apoplastic fluid of Coffea arabica leaves highlights novel biomarkers for resistance against Hemileia vastatrix . Front. Plant Sci. 6, 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo, S.D. , Castro, R.M. , de Kida, K. and Martins, E.M.F. (2001) Ação protetora do acibenzolar‐S‐methyl em plantas de cafeeiro contra ferrugem. Arq. Inst. Biol. 68, 89–94. [Google Scholar]
- Guzzo, S.D. , Harakava, R. and Tsai, S.M. (2009) Identification of coffee genes expressed during systemic acquired resistance and incompatible interaction with Hemileia vastatrix . J. Phytopathol. 157, 625–638. [Google Scholar]
- Haddad, F. , Maffia, L.A. , Mizubuti, E.S.G. and Teixeira, H. (2009) Biological control of coffee rust by antagonistic bacteria under field conditions in Brazil. Biol. Control. 49, 114–119. [Google Scholar]
- Haddad, F. , Saraiva, R.M. , Mizubuti, E.S.G. , Romeiro, R.S. and Maffia, L.A. (2013) Antifungal compounds as a mechanism to control Hemileia vastatrix by antagonistic bacteria. Trop. Plant Pathol. 38, 398–405. [Google Scholar]
- Haddad, F. , Saraiva, R.M. , Mizubuti, E.S.G. , Romeiro, R.S. and Maffia, L.A. (2014) Isolation and selection of Hemileia vastatrix antagonists. Eur. J. Plant Pathol. 139, 763–772. [Google Scholar]
- Harder, D.E. and Chong, J. (1991) Rust haustoria In: Electron Microscopy of Plant Pathogens (Mendgen K. and Lesemann D.‐E., eds), pp. 235–250. Berlin: Springer. [Google Scholar]
- Hennen, J.F. and Figueiredo, M.B. (1984) The life cycle of Hemileia vastatrix In: Simpósio sobre Ferrugem do Cafeeiro, pp. 47–56. Oeiras: Centro de Investigação das Ferrugens do Cafeeiro, Instituto de Investigação Científica Tropical. [Google Scholar]
- Herrera, J.C. , Alvarado, G.A. , Cortina, G.H.A. , Combes, M.C. , Romero, G.G. and Lashermes, P. (2009) Genetic analysis of partial resistance to coffee leaf rust (Hemileia vastatrix Berk & Br.) introgressed into the cultivated Coffea arabica L. from the diploid C. canephora species. Euphytica 167, 57–67. [Google Scholar]
- Herrera, J.C. , Villegas, A.M. , Garcia, F.A. , Dereeper, A. , Combes, M.C. , Posada, H.E. and Lashermes, P. (2014) Genomic relationships among different Timor hybrid (Coffea L.) accessions as revealed by SNP identification and RNA‐Seq analysis In: Advances in Computational Biology (Castillo L.F, Cristancho M, Isaza G, Pinzón A. and Corchado Rodríguez J.M, eds), pp. 161–168. Berlin: Springer. [Google Scholar]
- Hoffmann, J. (2014) The World Atlas of Coffee: From Beans to Brewing – Coffees Explored, Explained and Enjoyed. London: James Hoffman Octopus Publishing Group. [Google Scholar]
- Hok, S. , Attard, A. and Keller, H. (2010) Getting the most from the host: how pathogens force plants to cooperate in disease. Mol. Plant–Microbe Interact. 23, 1253–1259. [DOI] [PubMed] [Google Scholar]
- Ibañez, M. and Blackman, A. (2016) Is eco‐certification a win–win for developing country agriculture? Organic coffee certification in Colombia. World Dev. 82, 14–27. [Google Scholar]
- ICO (2016) World Coffee Production. International Coffee Organization. http://www.ico.org/prices/po-production.pdf [accessed 16 May 2016].
- Kilambo, D.L. , Reuben, S.O.W.M. and Mamiro, D.P. (2013) Responses of compact coffee clones against Coffee Berry and Coffee Leaf Rust diseases in Tanzania. J. Plant Studies, 2, 81–94. [Google Scholar]
- Kushalappa, A.C. and Eskes, A.B. (1989) Advances in coffee rust research. Annu. Rev. Phytopathol. 27, 503–531. [Google Scholar]
- Link, T. , Seibel, C. and Voegele, R.T. (2014) Early insights into the genome sequence of Uromyces fabae . Front. Plant Sci. 5, 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehrer, M. , Vogel, A. , Huettel, B. , Reinhardt, R. , Benes, V. , Duplessis, S. , Usadel, B. and Schaffrath, U. (2014) On the current status of Phakopsora pachyrhizi genome sequencing. Front. Plant Sci. 5, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, U.P. , Zambolim, L. , Neto, P.N.S. , Souza, A.F. , Capucho, A.S. and Rodrigues, F.A. (2013) Effect of foliar application of potassium silicate on the progress of coffee leaf rust. Trop. Plant Pathol. 38, 547–551. [Google Scholar]
- Loureiro, A. , Azinheira, H.G. , Silva, M.C. and Talhinhas, P. (2015) A method for obtaining RNA from Hemileia vastatrix appressoria produced in planta, suitable for transcriptomic analyses. Fungal Biol. 119, 1093–1099. [DOI] [PubMed] [Google Scholar]
- Mahé, L. , Várzea, V.M.P. , Le Pierès, D. , Combes, M.C. and Lashermes, P. (2007) A new source of resistance against coffee leaf rust from New‐Caledonian natural interspecific hybrids between Coffea arabica and Coffea canephora . Plant Breed. 126, 638–641. [Google Scholar]
- Maia, T.A. , Zambolim, E.M. , Caixeta, E.T. , Mizubuti, E.S. and Zambolim, L. (2013) The population structure of Hemileia vastatrix in Brazil inferred from AFLP. Austral. Plant Pathol. 42, 533–542. [Google Scholar]
- Marchi, C.E. , Borges, M.D.E.F. and Resende, M.L.V. (2002) Induced protection by benzothiadiazole against rust (Hemileia vastatrix) in coffee plants. Ciênc. Agrotec. 26, 1103–1106. [Google Scholar]
- Maxemiuc‐Naccache, V. and Dietrich, S.M.C. (1981) Cell wall composition of spores of Hemileia vastatrix (coffee rust). Rev. Microbiol. 12, 61–64. [Google Scholar]
- Mayne, W.W. (1932) Physiologic specialization of Hemileia vastatrix B. & Br. Nature, 129, 150. [Google Scholar]
- Mayne, W.W. (1942) Annual Report of the Coffee Scientific Officer 1941–42. Mysore Coffee Experimental Station. Bulletin nº 24.
- McCain, J.W. and Hennen, J.F. (1984) Development of the uredinial thallus and sorus in the orange coffee rust fungus, Hemileia vastatrix . Phytopathology, 74, 714–721. [Google Scholar]
- McCook, S. (2006) Global rust belt: Hemileia vastatrix and the ecological integration of world coffee production since 1850. J. Global Hist. 1, 177–195. [Google Scholar]
- McCook, S. and Vandermeer, J. (2015) The big rust and the Red Queen: long‐term perspectives on coffee rust research. Phytopathology, 105, 1164–1173. [DOI] [PubMed] [Google Scholar]
- McTaggart, A.R. , Shivas, R.G. , van der Nest, M.A. , Roux, J. , Wingfield, B. and Wingfield, M.J. (2016) Host jumps shaped the diversity of extant rust fungi (Pucciniales). New Phytol. 209, 1149–1158. [DOI] [PubMed] [Google Scholar]
- Mendgen, K. and Hahn, M. (2002) Plant infection and the establishment of fungal biotrophy. Trends Plant Sci. 7, 352–356. [DOI] [PubMed] [Google Scholar]
- Mendgen, K. and Voegele, R.T. (2005). Biology of rusts and mechanisms of infection In Durable Resistance to Coffee Leaf Rust (Zambolim L., Zambolim E. and Várzea V.M.P., eds), pp. 233–248. Viçosa: Universidade Federal de Viçosa. [Google Scholar]
- Monteiro, A.C.A. , Resende, M.L.V. , Costa, J.R. , Carvalho, C.A. , Silva, J.A.G. , Rennó, M.H.L. , Silva, P.F.M. , Ribeiro Júnior, P.M. and Mathioni, S.M. (2013) Associação de indutores de resistência para o manejo da ferrugem do cafeeiro e análise bioquímica da resposta de defesa induzida. In: VIII Simpósio de Pesquisa dos Cafés do Brasil, Salvador, Bahia. Brasília: EMBRAPA Café.
- Morris, D. (1880) Note on the structure and habit of Hemileia vastatrix, the coffee‐leaf disease of Ceylon and Southern India 1880. J. Linnean Soc., Bot. 17, 512–517. [Google Scholar]
- Muller, R.A. (1971) La rouille du caféier (Hemileia vastatrix) sur le continent Américain. Café, Cacao, Thé, 15, 25. [Google Scholar]
- Nardi, B. , Dreos, R. , Terra, L. , Martellossi, C. , Asquini, E. , Tornincasa, P. , Gasperini, D. , Pacchioni, B. , Rathinavelu, R. , Pallavicini, A. and Graziosi, G. (2006) Differential responses of Coffea arabica L. leaves and roots to chemically induced systemic acquired resistance. Genome, 49, 1594–1605. [DOI] [PubMed] [Google Scholar]
- Noronha‐Wagner, M. and Bettencourt, A.J. (1967) Genetic study of resistance of Coffea sp. to leaf rust. I. Identification and behaviour of four factors conditioning disease reaction in Coffea arabica to twelve physiologic races of Hemileia vastatrix . Can. J. Bot. 45, 2021–2031. [Google Scholar]
- Nunes, C.C. , Maffia, L.A. , Mizubuti, E.S.G. , Brommonschenkel, S.H. and Silva, J.C. (2009) Genetic diversity of populations of Hemileia vastatrix from organic and conventional coffee plantations in Brazil. Austral. Plant Pathol. 38, 445–452. [Google Scholar]
- d'Oliveira, B. (1954. −57). As ferrugens do cafeeiro. Rev. Café Port. 1(4), 5–13; 2(5), 5–12; 2(6), 5–15; 2(7), 9–187; 2(8), 5–22; 4(16), 5–15.
- Park, R.F. and Wellings, C.R. (2012) Somatic Hybridization in the Uredinales. Annu. Rev. Phytopathol 50, 219–239. [DOI] [PubMed] [Google Scholar]
- Pereira, R.B. , Lucas, G.C. , Perina, F.J. and Alves, E. (2012) Essential oils for rust control on coffee plants. Ciênc. Agrotec. 36, 16–24. [Google Scholar]
- Prakash, N.S. , Marques, D.V. , Várzea, V.M.P. , Silva, M.C. , Combes, M.C. and Lashermes, P. (2004) Introgression molecular analysis of a leaf rust resistance gene from Coffea liberica into C. arabica L. Theor. Appl. Genet. 109, 1311–1317. [DOI] [PubMed] [Google Scholar]
- Prakash, N.S. , Muniswamy, B. , Hanumantha, B.T. , Sreenath, H.L. , Sundaresha, Kumar Deepak, Suresh, N., Santhosh, P., Soumya, P.R., Asha, Bhat, M., Bhat, S.S. and Jayarama, (2011) Marker assisted selection and breeding for leaf rust resistance in coffee (Coffea arabica L.) – some recent leads. Indian J. Genet. Plant Breed. 71, 185–189. [Google Scholar]
- Prakash, N.S. , Devasia, J. , Das Divya, K. , Manjunatha, B.N. , Seetharama, H.G. , Kumar, A. and Jayarama, (2015) Breeding for rust resistance in Arabica – where we are and what next? In: Proceedings of the 25th International Conference on Coffee Science (ASIC), 8–13 September 2014, Armenia, Colombia, B10. Paris: Association for Science and Information on Coffee.
- Rajendren, R.B. (1967) A new type of nuclear life cycle in Hemileia vastatrix . Mycologia, 59, 279–285. [Google Scholar]
- Ramiro, D. , Jalloul, A. , Petitot, A.S. , Maluf, M. and Fernandez, D. (2010) Identification of coffee WRKY transcription factor genes and expression profiling in resistance responses to pathogens. Tree Genet. Gen. 6, 767–781. [Google Scholar]
- Ramos, A.P. , Tavares, S. , Tavares, D. , Silva, M.C. , Loureiro, J. and Talhinhas, P. (2015) Flow cytometry reveals that the rust fungus Uromyces bidentis (Pucciniales) possesses the largest fungal genome reported, 2489 Mbp. Mol. Plant Pathol. 16, 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijo, L. and Sargent, J.A. (1983) The fine structure of the uredospore and germ tube of Hemileia vastatrix In: Simpósio sobre Ferrugens do Cafeeiro, Oeiras, Portugal, pp. 295–319. Oeiras: Centro de Investigação das Ferrugens do Cafeeiro, Instituto de Investigação Científica Tropical.
- Ritschel, A. (2005) Monograph of the genus Hemileia (Uredinales) In: Bibliotheca Mycologica, vol.200 (Bresinsky A., Butin H. and Tudzinski P., eds), pp. 3–132. Stuttgart: J. Cramer. [Google Scholar]
- Rodrigues, C.J. Jr. , Bettencourt, A.J. and Rijo, L. (1975) Races of the pathogen and resistance to coffee rust. Annu. Rev. Phytopathol. 13, 49–70. [Google Scholar]
- Rodrigues, C.J. Jr ., Rijo, L. and Medeiros, E.F. (1980) Germinação anómala dos uredósporos de Hemileia vastatrix, o agente causal da ferrugem alaranjada do cafeeiro. Garcia de Orta, Série Estudos Agronómicos. 7, 17–20. [Google Scholar]
- Romero, G. , Alvarado, G. , Cortina, H. , Ligarreto, G. , Galeano, N.F. and Herrera, J.C. (2010) Partial resistance to leaf rust (Hemileia vastatrix) in coffee (Coffea arabica L.): genetic analysis and molecular characterization of putative candidate genes. Mol. Breed. 25, 685–697. [Google Scholar]
- Romero, G. , Vásquez, L.M. , Lashermes, P. and Herrera, J.C. (2014) Identification of a major QTL for adult plant resistance to coffee leaf rust (Hemileia vastatrix) in the natural Timor hybrid (Coffea arabica × C. canephora). Plant Breed. 133, 121–129. [Google Scholar]
- Rozo, Y. , Escobar, C. , Gaitán, A. and Cristancho, M. (2012) Aggressiveness and genetic diversity of Hemileia vastatrix during an epidemic in Colombia. J. Phytopathol. 160, 732–740. [Google Scholar]
- Samnegard, U. , Hamback, P.A. , Nemomissa, S. and Hylander, K. (2014) Local and regional variation in local frequency of multiple coffee pests across a mosaic landscape in Coffea Arabica's native range. Biotropica, 46, 276–284. [Google Scholar]
- Santos, F.S. , Souza, P.E. , Resende, M.L.V. , Pozza, E.A. , Miranda, J.C. and Je Pests Acrossand Manerba, F.C. (2007) Effect of vegetal extracts on the progress of foliar diseases in organic coffee. Fitopatol. Bras. 32, 59–63. [Google Scholar]
- Saunders, D.G.O. , Win, J. , Cano, L.M. , Szabo, L.J. , Kamoun, S. and Raffaele, S. (2012) Using hierarchical clustering of secreted protein families to classify and rank candidate effectors of rust fungi. PLoS One, 7, e29847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze‐Lefert, P. and Panstruga, R. (2003) Establishment of biotrophy by parasitic fungi and reprogramming of host cells for disease resistance. Annu. Rev. Phytopathol. 41, 641–667. [DOI] [PubMed] [Google Scholar]
- Silva, D.N. , Duplessis, S. , Talhinhas, P. , Azinheira, H. , Paulo, O.S. and Batista, D. (2015a) Genomic patterns of positive selection at the origin of rust fungi. PLoS One, 10, e0143959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, D.N. , Várzea, V. , Pereira, A.P. , Paulo, O.S. and Silva, M.C. (2015b) Population genomics of Hemileia vastatrix using RAD sequencing. In: Proceedings of the 25th International Conference on Coffee Science (ASIC), 8–13 September 2014, Armenia, Colombia, PB. 248. Paris: Association for Science and Information on Coffee.
- Silva, H.S.A. , Tozzi, J.P.L. , Terrasan, C.R.F. and Bettiol, W. (2012) Endophytic microorganisms from coffee tissues as plant growth promoters and biocontrol agents of coffee leaf rust. Biol. Control, 63, 62–67. [Google Scholar]
- Silva, M.C. , Nicole, M. , Rijo, L. , Geiger, J.P. and Rodrigues Jr, C.J. (1999) Cytochemistry of plant–rust fungus interface during the compatible interaction Coffea arabica (cv. Caturra)–Hemileia vastatrix (race III). Int. J. Plant Sci. 160, 79–91. [Google Scholar]
- Silva, M.C. , Nicole, M. , Guerra‐Guimarães, L. and Rodrigues Jr, C.J. (2002) Hypersensitive cell death and posthaustorial defence responses arrest the orange rust (Hemileia vastatrix) growth in resistant coffee leaves. Physiol. Mol. Plant Pathol. 60, 169–183. [Google Scholar]
- Silva, M.C. , Várzea, V. , Guerra‐Guimarães, L. , Azinheira, H.G. , Fernandez, D. , Petitot, A.S. , Bertrand, B. , Lashermes, P. and Nicole, M. (2006) Coffee resistance to the main diseases: leaf rust and coffee berry disease. Braz. J. Plant Physiol. 18, 119–147. [Google Scholar]
- Silva, M.C. , Guerra‐Guimarães, L. , Loureiro, A. and Nicole, M. (2008) Involvement of peroxidases in the coffee resistance to orange rust (Hemileia vastatrix). Physiol. Mol. Plant Pathol. 72, 29–38. [Google Scholar]
- Souza, A.F. , Zambolim, Z. , Jesus, V.C. and Cecon, P.R. (2011) Chemical approaches to manage coffee leaf rust in drip irrigated trees. Austral. Plant Pathol. 40, 293–300. [Google Scholar]
- Spanu, P.D. (2012) The genomics of obligate (and nonobligate) biotrophs. Annu. Rev. Phytopathol. 50, 91–109. [DOI] [PubMed] [Google Scholar]
- Steiger, L. , Nagai, C. , Moore, H. , Morden, W. , Osgood, V. and Ming, R. (2002) AFLP analysis of genetic diversity within and among Coffea arabica cultivars. Theor. Appl. Genet. 105, 209–215. [DOI] [PubMed] [Google Scholar]
- Talhinhas, P. , Azinheira, H. , Vieira, B. , Loureiro, A. , Tavares, S. , Batista, D. , Morin, E. , Petitot, A.S. , Paulo, O.S. , Poulain, J. , Da Silva, C. , Duplessis, S. , Silva, M.C. and Fernandez, D. (2014) Overview of the functional virulent genome of the coffee leaf rust pathogen Hemileia vastatrix with an emphasis on early stages of infection. Front. Plant Sci. 5, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhinhas, P. , Ramos, A.P. , Tavares, D. , Tavares, S. and Loureiro, J. (2015) Genome size estimates for six rust (Pucciniales) species. Rev. Ciênc. Agr. 38, 178–185. [Google Scholar]
- Tavares, S. , Opinião, A.I. , Loureiro, A. , Azinheira, H.G. , Silva, M.C. , Talhinhas, P. and Abranches, R. (2013) The karyotype of Hemileia vastatrix, the causal agent of coffee leaf rust. In: Proceedings of the 24th International Conference on Coffee Science (ASIC), 11–16 November 2012, San José, pp. 1409–1413. Paris: Association for Science and Information on Coffee.
- Tavares, S. , Ramos, A.P. , Pires, A.S. , Azinheira, H.G. , Caldeirinha, P. , Link, T. , Abranches, R. , Silva, M.C. , Voegele, R.T. , Loureiro, J. and Talhinhas, P. (2014) Genome size analyses of Pucciniales reveal the largest fungal genomes. Front. Plant Sci. 5, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermeer, J. , Perfecto, I. and Philpott, S. (2010) Ecological complexity and pest control in organic coffee production: uncovering an autonomous ecosystem service. BioScience, 60, 527–537. [Google Scholar]
- Vandermeer, J. , Jackson, D. and Perfecto, I. (2014) Qualitative dynamics of the coffee rust epidemic: educating intuition with theoretical ecology. BioScience, 64, 210–218. [Google Scholar]
- van der Vossen, H.A.M. (2003) The cup quality of disease‐resistant cultivars of arabica coffee (Coffea arabica). Exp. Agric. 45, 323–332. [Google Scholar]
- Várzea, V.M.P. and Marques, D.V. (2005) Population variability of Hemileia vastatrix vs coffee durable resistance In: Durable Resistance to Coffee Leaf Rust (Zambolim L., Zambolim E. and Várzea V.M.P., eds), pp. 53–74. Viçosa: Universidade Federal de Viçosa. [Google Scholar]
- Várzea, V.M.P. , Rodrigues, C.J. Jr ., Passo, J.E. and Palma, S. (1989) New rust genotypes and a new coffee genotype in Catimor 45. In: Proceedings of the 13th International Scientific Colloquium on Coffee, 21–25 August 1989, Paipa, Colombia, pp. 745–748. Paris: Association for Science and Information on Coffee (ASIC).
- Várzea, V.M.P. , Marques, V.D. , Pereira, A.P. and Silva, M.C. (2009) The use of Sarchimor derivatives in coffee breeding resistance to leaf rust. In: Proceedings of the 22nd International Conference on Coffee Science, 14–19 September 2008, Campinas, Brazil, pp. 1424–1429. Paris: Association for Science and Information on Coffee (ASIC).
- Vieira, A. , Talhinhas, P. , Loureiro, A. , Duplessis, S. , Fernandez, D. , Silva, M.C. , Paulo, O.S. and Azinheira, H.G. (2011) Validation of RT‐qPCR reference genes for in planta expression studies in the obligate biotrophic pathogen Hemileia vastatrix, the causal agent of coffee leaf rust. Fungal Biol. 115, 891–901. [DOI] [PubMed] [Google Scholar]
- Vieira, A. , Talhinhas, P. , Loureiro, A. , Thürich, J. , Duplessis, S. , Fernandez, D. , Silva, M.C. , Paulo, O.S. and Azinheira, H.G. (2012) Expression profiling of genes involved in the biotrophic colonisation of Coffea arabica leaves by Hemileia vastatrix . Eur. J. Plant Pathol. 133, 261–277. [Google Scholar]
- Ward, H.M. (1882) Researches on the life‐history of Hemileia vastatrix, the fungus of the “coffee‐leaf disease”. J. Linnean Soc. London, Bot. 19, 299–335. [Google Scholar]
- Ward, H.M. (1889) On the morphology of Hemileia vastatrix Berk. and Br. (the fungus of the Coffee disease of Ceylon). Q. J. Microsc. Sci. 22, 1–11. [Google Scholar]
- Wellman, F.L. (1953) The Americas face up to the threat of coffee rust. For. Agric. 17, 1–7. [Google Scholar]
- Wingfield, B.D. , Ericson, L. , Szaro, T. and Burdon, J.J. (2004) Phylogenetic patterns in the Uredinales. Austral. Plant Pathol. 33, 327–335. [Google Scholar]
- Zambolim, L. (2016) Current status and management of coffee leaf rust in Brazil. Trop. Plant Pathol. 41, 1–8. [Google Scholar]


