Summary
Plants possess an innate immune system capable of restricting invasion by most potential pathogens. At the cell surface, the recognition of microbe‐associated molecular patterns (MAMPs) and/or damage‐associated molecular patterns (DAMPs) by pattern recognition receptors (PRRs) represents the first event for the prompt mounting of an effective immune response. Pathogens have evolved effectors that block MAMP‐triggered immunity. The Pseudomonas syringae effector AvrPto abolishes immunity triggered by the peptide MAMPs flg22 and elf18, derived from the bacterial flagellin and elongation factor Tu, respectively, by inhibiting the kinase function of the corresponding receptors FLS2 and EFR, as well as their co‐receptors BAK1 and BKK1. Oligogalacturonides (OGs), a well‐known class of DAMPs, are oligomers of α‐1,4‐linked galacturonosyl residues, released on partial degradation of the plant cell wall homogalacturonan. We show here that AvrPto affects only a subset of the OG‐triggered immune responses and that, among these responses, only a subset is affected by the concomitant loss of BAK1 and BKK1. However, the antagonistic effect on auxin‐related responses is not affected by either AvrPto or the loss of BAK1/BKK1. These observations reveal an unprecedented complexity among the MAMP/DAMP response cascades. We also show that the signalling system mediated by Peps, another class of DAMPs, and their receptors PEPRs, contributes to OG‐activated immunity. We hypothesize that OGs are sensed through multiple and partially redundant perception/transduction complexes, some targeted by AvrPto, but not necessarily comprising BAK1 and BKK1.
Keywords: Arabidopsis thaliana, Botrytis cinerea, oligogalacturonides, plant immunity
Introduction
Plants, as sessile organisms, are continually exposed to adverse conditions, both biotic and abiotic. As a result of the absence of an adaptive immune system, plants rely on an innate immune system that depends on efficient pathogen sensing and the rapid establishment of defence responses (Bohm et al., 2014; Gomez‐Gomez, 2004). Pathogen detection occurs through the direct binding of broadly conserved pathogen molecules (pathogen‐/microbe‐associated molecular patterns, PAMPs/MAMPs) by a large arsenal of transmembrane pattern recognition receptors (PRRs). Specific PRRs also detect host‐derived damage‐associated molecular patterns (DAMPs), including cell wall fragments or peptides produced by mechanical injuries or lytic activities of microbe‐secreted enzymes (Benedetti et al., 2015; Savatin et al., 2014b). Activated PRRs trigger immune responses by switching on different and parallel signal transduction pathways. This represents the first layer of the plant immune system and is known as PAMP (or pattern)‐triggered immunity (PTI) (Zipfel, 2014). Successful pathogens must overcome PTI in order to cause disease, either by evading or suppressing this important first layer of plant innate immunity. To do this, pathogens have evolved effectors, which are delivered both to the plant apoplast and inside the host cells. In an evolutionary arms race, plants have developed the ability to detect such effectors through other types of receptor, called resistance (R) proteins, and to counteract the invasion by activating effector‐triggered immunity (ETI) (Jones and Dangl, 2006; Macho and Zipfel, 2015).
Studies on plant defence‐related signalling pathways have focused on MAMP perception and transduction. So far, the best studied PRRs are FLAGELLIN‐SENSITIVE 2 (FLS2) and ELONGATION FACTOR‐Tu RECEPTOR (EFR), which bind flg22, an epitope derived from the bacterial flagellin, and elf18, an epitope derived from the elongation factor thermo‐unstable, respectively (Gomez‐Gomez and Boller, 2000; Zipfel et al., 2006). However, a well‐known class of DAMPs is represented by the oligogalacturonides (OGs), which are pectin‐derived oligosaccharides released from plant cell walls on partial degradation of homogalacturonan (Ferrari et al., 2013). In Arabidopsis thaliana WALL‐ASSOCIATED KINASE 1 (WAK1), a transmembrane receptor kinase containing epidermal growth factor‐like repeats, has been identified as an OG receptor (Brutus et al., 2010). A second well‐characterized class of DAMP is represented by the Arabidopsis peptides Pep1–8, which originate from the cleavage of the corresponding precursor proteins, named PROPEPs (Huffaker et al., 2006). The expression of PROPEP genes is induced in response to both biotic and abiotic stimuli, as well as during development (Bartels et al., 2013; Huffaker et al., 2006). Peps are differentially perceived by PEP1 RECEPTOR 1 (PEPR1), which binds Pep1–6, and PEPR2, which, instead, binds only Pep1 and Pep2 (Krol et al., 2010; Yamaguchi et al., 2010).
Reverse genetic approaches have elucidated elements involved in MAMP signalling, for example the important role of the co‐receptor BRI1‐ASSOCIATED RECEPTOR KINASE1 (BAK1/SERK3) and its closest paralogue BAK1‐LIKE1/SERK4 (BKK1/SERK4), both members of the SOMATIC EMBRYOGENESIS RECEPTOR‐LIKE KINASE (SERK) gene family (Albrecht et al., 2008), in the response to flg22 and elf18 (Kim et al., 2013). BAK1 is also required for response to other MAMPs, such as HrpZ, peptidoglycan and lipopolysaccharide, but not chitin or necrosis‐inducing Phytophthora protein 1 (NPP1) (Shan et al., 2008) and, together with BKK1, mediates responses to Pep1 (Roux et al., 2011).
The current vision is that, after flg22 or elf18 perception, BAK1 and BKK1 form tight complexes with both FLS2 and EFR to initiate downstream signalling (Chinchilla et al., 2007; Roux et al., 2011). BAK1 and BKK1 also provide partially overlapping activity in the brassinosteroid (BR)‐dependent signalling pathway (He et al., 2007).
AvrPto is a type III kinase inhibitor effector of the hemibiotrophic bacterium Pseudomonas syringae pv. tomato DC3000 (Pst) that triggers gene‐for‐gene resistance and the hypersensitive response (HR) in tomato plants carrying the corresponding R gene Pto, encoding a serine/threonine kinase (Pedley and Martin, 2003). In Arabidopsis, AvrPto suppresses the plant defence responses elicited by MAMPs (Cui et al., 2005; Hauck et al., 2003; Li et al., 2005). For example, all flg22‐induced responses tested so far, i.e. the accumulation of H2O2 (Xiang et al., 2008), mitogen‐activated protein kinase (MAPK) activation and induction of defence‐related genes (He et al., 2006), are strongly reduced by AvrPto. Moreover, constitutive in planta expression of AvrPto affects plant development, leading to a phenotype similar to that of weak mutants insensitive to BRs (Shan et al., 2008). Suppression of MAMP‐induced responses by AvrPto occurs through direct binding and inhibition of elements in the perception complexes. A dispute is open as to whether this effector targets BAK1 or FLS2 (Shan et al., 2008; Xiang et al., 2008), and evidence in support of both possibilities has been provided (Cheng et al., 2011; Goehre et al., 2008; Xiang et al., 2011).
Because there is a large overlap between responses elicited by OGs and MAMPs (Asai et al., 2002; Denoux et al., 2008; Galletti et al., 2008, 2011), it is conceivable that MAMPs and OGs share some elements in their signalling pathways.
In this work, we have investigated whether, in Arabidopsis, AvrPto inhibits OG‐induced immunity, and whether BAK1 and BKK1 are involved in OG signalling, using plants that express AvrPto in an oestradiol‐inducible manner and single and double bak1‐5 and bkk1‐1 mutants. bak1‐5 is a semi‐dominant allele carrying a single amino acid substitution in the kinase domain of BAK1 that reduces its activity (Schwessinger et al., 2011). The mutant exhibits normal growth and is strongly impaired in flg22‐ and elf18‐induced responses (Roux et al., 2011; Schwessinger et al., 2011). However, the bak1‐5 bkk1‐1 double mutant, which also has a normal phenotype, shows more marked defects in the early and late responses to flg22 and elf18 compared with the bak1‐5 mutant, and is more susceptible to Pst and the obligate biotrophic oomycete Hyaloperonospora arabidopsidis (Roux et al., 2011).
We show here that, unlike the response to flg22, only a subset of defence responses induced by OGs is suppressed in the presence of AvrPto and, in turn, only a subset of these is affected by the loss of both BAK1 and BKK1. Moreover, we show that the Pep–PEPR signalling system contributes to OG‐induced resistance to Botrytis cinerea and full induction of PATHOGENESIS‐RELATED GENE1 (PR1). The induction of PHOSPHATE‐INDUCED 1 (PHI‐1) and the inhibition of the auxin‐regulated gene appear, instead, to be independent of all of these elements. Our observations reveal a complexity in the OG signalling pathways that is unique among the characterized MAMPs and DAMPs, and suggest that OGs are sensed and transduced by multiple and redundant complexes, only some of which involve BAK1 and BKK1 and are targeted by AvrPto.
Results
AvrPto inhibits OG‐triggered reactive oxygen species (ROS) production and callose deposition, but partially affects the activation of MPK3 and MPK6
Whether or not AvrPto blocks the signal transduction triggered by OGs was investigated by generating β‐oestradiol‐inducible AvrPto plants and analysing responses that are typical of OG action. Seven T1 independent plants showing the presence of AvrPto transcripts in excised leaves treated with β‐oestradiol (1 µm) for 48 h were initially selected; all were allowed to self‐pollinate to produce T2 seeds. For further analysis, two lines (#4 and #5) were chosen which showed a 3 : 1 segregation of resistance to the antibiotic hygromycin in the T2 progeny, and homozygous T3 seeds (#4.1 and #5.1) were obtained. Transcript levels, examined in T3 seedlings treated for 48 h with 1 µm β‐oestradiol, were higher in line 4.1 than in line 5.1; as expected, AvrPto transcripts were absent in wild‐type plants (Fig. S1a, see Supporting Information). The AvrPto 4.1 line, germinated and grown for 10 days in the presence of the inducer (1 µm), displayed an overall inhibition of shoot and root development and a large number of root hairs. The AvrPto 5.1 line, grown in the same conditions, showed a similar, but less pronounced, phenotype (Fig. S1b). In these lines, responses to OGs and flg22 were analysed in seedlings grown in the presence of β‐oestradiol (1 µm), or dimethyl sulfoxide (DMSO) as a control, for the last 2 days before elicitor treatment, and in adult plants, sprayed with β‐oestradiol (10 µm) or DMSO, three times within a week, before elicitor treatment or infection.
Both OGs and MAMPs rapidly and transiently activate, through phosphorylation, MAPK cascades, including the single kinases MPK3 and MPK6 (Galletti et al., 2011; Savatin et al., 2014a; Zipfel et al., 2008). We examined the levels of phosphorylated MPK3 and MPK6 in transgenic and wild‐type seedlings 5, 10 and 15 min after elicitor treatment by western blot analysis, using a commercial antibody generated against the human homologues of these MAPKs (α‐p44/p42). In response to OGs, the activation of MPK3 and MPK6 was partially reduced only in the high‐expressing AvrPto 4.1 line compared with the wild‐type (Fig. 1), whereas it was completely abolished and strongly reduced in lines 4.1 and 5.1, respectively, in response to flg22, as expected (He et al., 2006).
Figure 1.
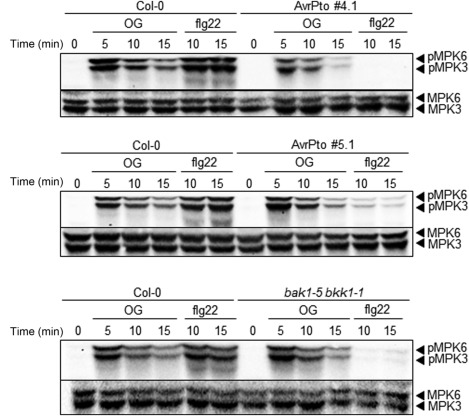
Mitogen‐activated protein kinase (MAPK) activation induced by oligogalacturonides (OGs) is partially affected only in AvrPto‐expressing line 4.1, but not in line 5.1 and the bak1‐5 bkk1‐1 double mutant. Levels of phosphorylated MPK3 and MPK6 (pMPK3 and pMPK6) in seedlings of wild‐type (Col‐0), AvrPto‐expressing lines (#4.1 and #5.1) and bak1‐5 bkk1‐1 after elicitation with OGs (40 µg/mL) or flg22 at the indicated time points were determined by immunoblot analysis using an anti‐p44/42‐ERK antibody (top panels). Levels of MPK3 and MPK6 total proteins were determined using specific antibodies (bottom panels). The identity of individual MAPKs, as determined by size, is indicated by arrows. Experiments were repeated three times with similar results.
Extracellular ROS production is another well‐known response to OGs as well as to MAMPs which, in Arabidopsis, is mediated by the NAD(P)H oxidase respiratory burst oxidase homologue D (RBOHD) (Galletti et al., 2008). OG‐induced extracellular production of hydrogen peroxide by adult leaf discs of AvrPto line 4.1 was found to be severely affected compared with that of the wild‐type; the defect was confirmed, although less pronounced, in the second independent line, i.e. #5.1 (Fig. 2a). Callose deposition, another typical response to OGs and flg22, which is mediated by RBOHD‐dependent hydrogen peroxide production (Galletti et al., 2008; Zhang et al., 2007), was also impaired in both AvrPto‐expressing lines compared with the wild‐type (Fig. 2b). ROS production and callose accumulation were defective in response to flg22 in both AvrPto lines (Fig. 2a,b), as expected (Xiang et al., 2008).
Figure 2.
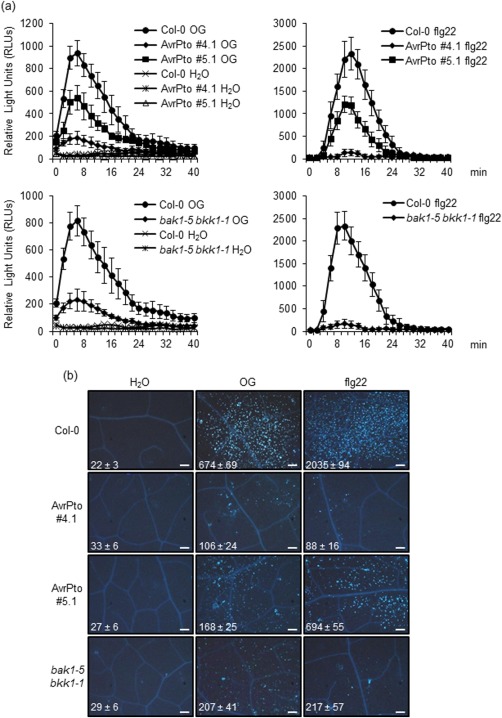
Reactive oxygen species (ROS) production and callose deposition in response to oligogalacturonides (OGs) are impaired in AvrPto‐expressing and bak1‐5 bkk1‐1 double mutant plants. (a) ROS production, expressed in relative light units (RLUs), was measured in leaf discs of wild‐type (Col‐0), AvrPto‐expressing lines (#4.1 and #5.1) and bak1‐5 bkk1‐1 mutant plants during elicitation with water, OGs or flg22. Results are the average ± standard error (SE) (n = 12). (b) Callose deposition visualized by aniline blue staining in leaves of Col‐0, AvrPto‐expressing lines (#4.1 and #5.1) and bak1‐5 bkk1‐1 mutant plants 24 h after syringe infiltration with water, OGs or flg22. All images are at the same scale (scale bar, 0.2 mm; 10× magnification). The average number of callose deposits (± SE) of 10 different leaf samples for each treatment is indicated in the images. Experiments in (a) and (b) were repeated three times with similar results.
AvrPto affects OG‐regulated expression of a subset of defence response genes
Because transcriptional reprogramming is another response to MAMPs and OGs (Denoux et al., 2008), the effect of AvrPto on OG‐triggered defence gene induction was analysed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). PHI‐1, FLG22‐INDUCED RECEPTOR‐LIKE KINASE 1 (FRK1), CYTOCHROME P450 (CYP81F2) and RETICULINE‐OXIDASE HOMOLOGUE (RET‐OX) genes, here indicated as early elicitor‐induced genes because their expression reaches a maximum within 1 h (Denoux et al., 2008; Gravino et al., 2015; Savatin et al., 2014a), were analysed in a time course up to 3 h. In addition, the expression of late genes which, after elicitor treatment, reach maximal induction at about 3 h [POLYGALACTURONASE‐INHIBITING PROTEIN 1 (PGIP1) and PHYTOALEXIN DEFICIENT 3 (PAD3)] or at least 8 h [PLANT DEFENSIN 1.2 (PDF1.2) and PR1], here indicated as late 1 (L1) and late 2 (L2) genes, respectively, was analysed. In both AvrPto lines, among the early genes, the expression of FRK1, CYP81F2 and RET‐OX, but not that of PHI‐1, was reduced in response to OGs, compared with the wild‐type, at all time points analysed (Fig. 3a). However, the expression of all four genes was severely affected in response to flg22 (Fig. S2a, see Supporting Information), as expected (He et al., 2006). Reduction of FRK1, CYP81F2 and RET‐OX transcripts in AvrPto #5.1 seedlings was more moderate than in #4.1 (Figs 3a and S2a).
Figure 3.
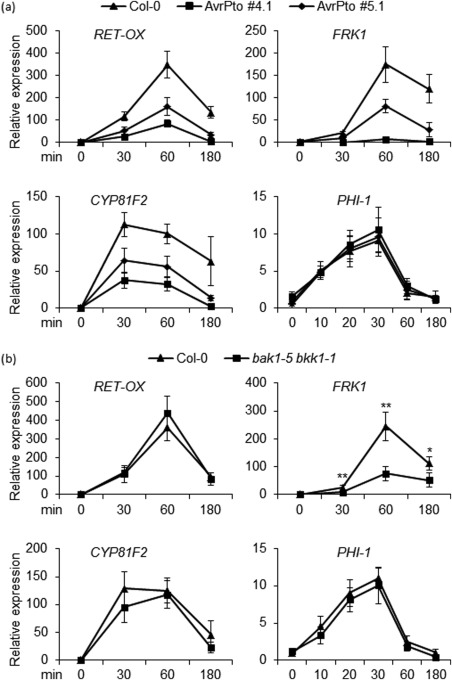
Oligogalacturonide (OG)‐triggered induction of only a subset of early defence response genes is affected in AvrPto‐expressing and bak1‐5 bkk1‐1 mutant seedlings. Expression of the indicated early‐induced defence marker genes was analysed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) in seedlings of wild‐type (Col‐0) and AvrPto‐expressing lines (#4.1 and #5.1) (a) or bak1‐5 bkk1‐1 mutant (b) after treatment with OGs (40 µg/mL) at the indicated time points. Transcript levels are shown as the mean of at least three independent experiments [± standard error (SE); n = 20 in each experiment] normalized to UBQ5 expression and plotted relative to expression in water‐treated Col‐0. In (a), the differences in OG‐triggered induction of RET‐OX, FRK1 and CYP81F2, at 30, 60 and 180 min, between Col‐0 and both 4.1 and 5.1 lines, were statistically significant (Student's t‐test; P < 0.01); in (b), the asterisks indicate statistically significant differences between transgenic/mutant and wild‐type samples at the indicated time points, according to Student's t‐test (*P < 0.05; **P < 0.01). Please see text for expansion of gene abbreviations.
The L1 genes PAD3 and PGIP1 showed a basal expression two‐ to three‐fold higher than that of the wild‐type and accumulated at a significantly lower level in response to both OGs and flg22 in both AvrPto lines (Fig. 4a). The L2 genes PDF1.2 and PR1 showed an increased basal expression (more than 150‐fold and seven‐fold higher, respectively) relative to the wild‐type, higher than (for PDF1.2) or comparable with (PR‐1) the expression normally induced by OGs and flg22 in the wild‐type. Both genes were not further up‐regulated by either elicitor (Fig. 4a).
Figure 4.
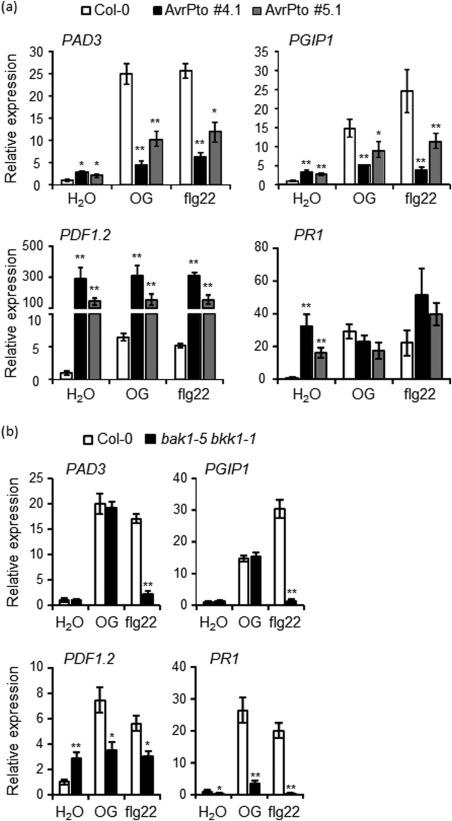
Loss of BAK1 and BKK1 affects only a subset of the oligogalacturonide (OG)‐induced late defence response genes that are affected by AvrPto. Expression of the indicated late‐induced defence marker genes, analysed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR), in seedlings of the wild‐type (Col‐0) and AvrPto‐expressing lines (#4.1 and #5.1) (a) or bak1‐5 bkk1‐1 mutant (b) after 3 h (for PAD3 and PGIP1) and 8 h (for PDF1.2 and PR1) of treatment with water, OGs (50 µg/mL) and flg22. Transcript levels are shown as the mean of at least three independent experiments [± standard error (SE); n = 20 in each experiment] normalized to UBQ5 expression and plotted relative to expression in water‐treated Col‐0. In (a) and (b), asterisks indicate statistically significant differences between mutant and wild‐type samples, according to Student's t‐test (*P < 0.05; **P < 0.01). Please see text for expansion of gene abbreviations.
AvrPto affects basal resistance to B. cinerea and protection induced by both OGs and flg22
The effect of AvrPto on the OG‐induced protection against pathogens was examined. Leaves of β‐oestradiol‐pretreated plants were drop inoculated with B. cinerea conidia 24 h after water, OG or flg22 spray pretreatment; disease lesions were measured at 48 h post‐inoculation (hpi). In the absence of pretreatment with OGs or flg22, AvrPto #4.1 plants showed disease lesions that were statistically larger (40%) than in wild‐type plants; moreover, no protection was observed after treatment with both elicitors (Fig. 5a). The AvrPto 5.1 line displayed no protection against B. cinerea after OGs or flg22 pretreatment, as observed in line 4.1; however, basal resistance to the fungus was less affected than in AvrPto #4.1 plants (Fig. 5a), indicating that the presence of AvrPto affects basal resistance to B. cinerea and abolishes protection induced by both OGs and flg22.
Figure 5.
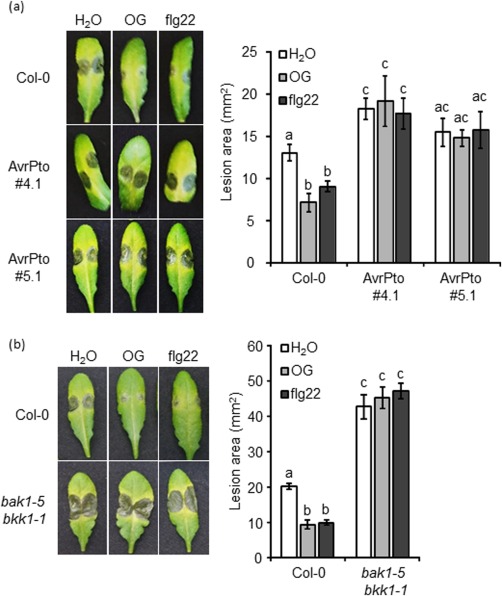
Basal resistance and elicitor‐induced protection against Botrytis cinerea are affected in AvrPto‐expressing and bak1‐5 bkk1‐1 mutant plants. Wild‐type (Col‐0) plants and AvrPto‐expressing lines (#4.1 and #5.1) (a) or bak1‐5 bkk1‐1 mutant plants (b) were sprayed with water, oligogalacturonides (OGs) or flg22 and, after 24 h, leaves were inoculated with B. cinerea spores. Lesion areas were measured at 48 h post‐inoculation (hpi). Results are average ± standard error (SE) (n = 20 lesions). Different letters above the bars indicate statistically significant differences between samples, as determined by analysis of variance (ANOVA) with Tukey's honestly significant difference (HSD) test [P < 0.05 in (a); P < 0.01 in (b)]. The experiments were repeated three times with similar results.
AvrPto does not affect OG‐induced inhibition of auxin‐related gene expression
Both OGs and MAMPs also regulate developmental responses, probably because of their ability to antagonize auxin through mechanisms that are still unknown (Savatin et al., 2011). Whether the auxin antagonistic activity of OGs and MAMPs is affected in AvrPto seedlings was investigated by analysing the expression of the auxin‐regulated genes, INDOLE‐3‐ACETIC ACID‐INDUCED PROTEIN5 (IAA5), SMALL AUXIN UP RNA (SAUR) AC1 (SAUR‐AC1) and SAUR16, on 1 h of treatment with indole‐3‐acetic acid (IAA), IAA + OGs or IAA + flg22. The expression of the three genes induced by IAA was comparable in the AvrPto lines and similar to that of the wild‐type, although basal expression in transgenic lines was lower. On IAA + OG co‐treatment, both AvrPto lines showed a normal inhibition of IAA‐induced expression of all three genes (Fig. S3, see Supporting Information), whereas inhibition did not take place on flg22 + IAA co‐treatment, in agreement with the notion that all flg22 responses are inhibited by AvrPto (Fig. S3).
Loss of BAK1 and BKK1 affects only a subset of the OG‐induced defence responses that are affected by AvrPto
The contribution of BAK1 and BKK1 to the OG‐induced responses described above was further analysed using the bak1‐5 and bkk1‐1 single mutants (see molecular characterization of the mutants in Fig. S1c) and the bak1‐5 bkk1‐1 double mutant. Both single mutants showed no significant alteration of the OG‐induced responses, and only bak1‐5 showed defects in the response to flg22, namely MAPK activation (Fig. S4, see Supporting Information), the oxidative burst (Fig. S5a, see Supporting Information) and up‐regulation of all defence response marker genes (Fig. S6, see Supporting Information), in agreement with literature data (Roux et al., 2011; Schwessinger et al., 2011); flg22‐induced callose deposition and protection against B. cinerea were also defective in this mutant (Figs S5b and S7, see Supporting Information).
Defective responses were instead observed in the bak1‐5 bkk1‐1 double mutant treated with OGs, but only in a subset of that emerged as affected by the expression of AvrPto (Table 1). As in AvrPto lines, H2O2 accumulation, callose deposition, the induced protection (Figs 2a,b and 5b) and OG‐induced expression of FRK1, PDF1.2 and PR1 (Figs 3b and 4b) were all impaired relative to the wild‐type. Basal expression of PDF1.2 was also significantly higher, although not as high as that normally observed on elicitation with OGs in wild‐type seedlings or in AvrPto lines (Fig. 4b). However, unlike in AvrPto plants, the OG‐induced expression of CYP81F2, RET‐OX, PAD3 and PGIP1 was not affected, nor was their basal expression (Figs 3b and 4b). MAPK phosphorylation and inhibition of the auxin response induced by OGs were normal (Figs 1 and S3).
Table 1.
Behaviour* of the immune responses induced by oligogalacturonides (OGs) and flg22 in the mutant/transgenic plants.
| Response | Timing† | OG | flg22 | ||||
|---|---|---|---|---|---|---|---|
| AvrPto | bak1‐5 bkk1 | pepr1 pepr2 | AvrPto | bak1‐5 bkk1 | pepr1 pepr2 | ||
| Protection against Botrytis cinerea | Late 2 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| PR1 up‐regulation | Late 2 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| PDF1.2 up‐regulation | Late 2 | ↓ | ↓ | = | ↓ | ↓ | ↓ |
| Callose deposition | Late 2 | ↓ | ↓ | = | ↓ | ↓ | = |
| ROS production | Early | ↓ | ↓ | = | ↓ | ↓ | = |
| FRK1 up‐regulation | Early | ↓ | ↓ | = | ↓ | ↓ | = |
| CYP81F2 up‐regulation | Early | ↓ | = | = | ↓ | ↓ | = |
| RET‐OX up‐regulation | Early | ↓ | = | = | ↓ | ↓ | = |
| PAD3 up‐regulation | Late 1 | ↓ | = | = | ↓ | ↓ | = |
| PGIP1 up‐regulation | Late 1 | ↓ | = | = | ↓ | ↓ | = |
| MAPK activation | Early | ↓ | = | = | ↓ | ↓ | = |
| PHI‐1 up‐regulation | Early | = | = | = | ↓ | ↓ | = |
| Inhibition of auxin responses | Early | = | = | = | ↓ | = | = |
MAPK, mitogen‐activated protein kinase; ROS, reactive oxygen species. Please see text for expansion of gene abbreviations.
*↓, affected (reduced); =, not affected.
†Phase in which each response reaches maximal expression. Early, maximal response up to around 1 h; late 1 and late 2, maximal response around 3 h and at least 8 h, respectively.
All responses to flg22 were strongly reduced (Figs 1, 2a,b, 4b, 5b and S2b), with the unexpected exception of the inhibition of the auxin‐induced gene expression (Fig. S3).
OGs strongly up‐regulate PROPEP2 and PROPEP3 and loss of PEPR1 and PEPR2 affects the OG‐induced expression of PR1 and protection against B. cinerea
Microarray data indicate that OGs, like MAMPs, strongly activate the expression of PROPEP2 and PROPEP3 (Denoux et al., 2008). We confirmed these results by analyzing, via qRT‐PCR, the induction of PROPEP2 and PROPEP3 in response to OGs and, in parallel, to flg22 and elf18. The expression of PROPEP2 was induced as early as 30 min in wild‐type seedlings by all three elicitors and in a similar manner, whereas the expression of PROPEP3 was more highly induced by flg22 and elf18 relative to OGs (Fig. 6a). Ethylene signalling has been shown to mediate elf18‐triggered expression of PROPEP2, but not that of PROPEP3 (Tintor et al., 2013). The induction of the two genes in response to OGs was therefore analysed in the ein2‐5 mutant, which is strongly impaired in ethylene signalling (Alonso et al., 1999). As this mutant shows a low responsiveness to flg22 because of a lower expression of FLS2 (Boutrot et al., 2010; Mersmann et al., 2010), but normally senses elf18 and OGs (Gravino et al., 2015; Tintor et al., 2013), only the latter MAMP was used in our analysis. At 30 min, expression of PROPEP2 induced by both OGs and elf18 was reduced, whereas that of PROPEP3 was even enhanced, compared with the wild‐type (Fig. 6b). The OG‐ as well as flg22‐ and elf18‐mediated induction of these two genes was also evaluated in the cpk5 cpk6 cpk11 triple mutant (Boudsocq et al., 2010), which is defective in OG‐triggered ethylene production (Gravino et al., 2015). The induction of both genes by all three elicitors was reduced significantly in the cpk5 cpk6 cpk11 mutant (Fig. 6a). These data suggest that CPK5, CPK6 and CPK11 are required for elicitor‐induced expression of both PROPEP2 and PROPEP3, whereas EIN2 is required only for the induction of PROPEP2.
Figure 6.
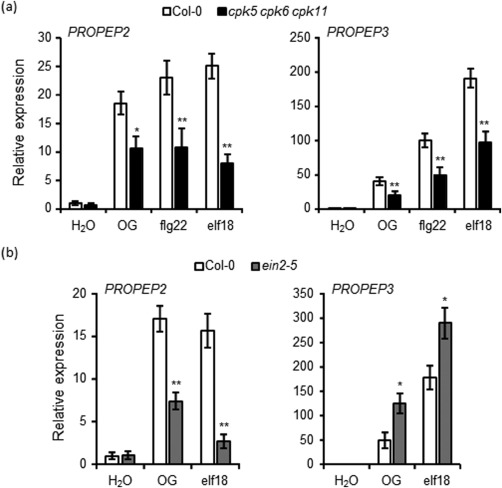
Oligogalacturonide (OG)‐triggered induction of the expression of PROPEP2, but not of PROPEP3, is dependent on ethylene. The expression of PROPEP2 and PROPEP3, analysed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR), in seedlings of wild‐type (Col‐0) and cpk5 cpk6 cpk11 mutant (a) or ein2‐5 mutant (b) after 30 min of treatment with water, OGs (50 µg/mL), flg22 and elf18 as indicated. Transcript levels are shown as the mean of at least three independent experiments [± standard error (SE); n = 20 in each experiment] normalized to UBQ5 expression and plotted relative to expression in water‐treated Col‐0. In (a) and (b), asterisks indicate statistically significant differences between mutant and wild‐type samples, according to Student's t‐test (*P < 0.05; **P < 0.01).
The possible role of Peps in OG signalling was investigated using a pepr1 pepr2 double mutant. Among the responses analysed in AvrPto plants and in the bak1 bkk1 mutant, only OG‐induced expression of PR1 and protection against B. cinerea were affected in the pepr1 pepr2 double mutant. Both responses were also defective in response to flg22, together with the expression of PDF1.2 (Fig. 7a,b). Moreover, our experiments confirm that PEPR1 and PEPR2 are involved in the basal resistance against B. cinerea (Liu et al., 2013), as lesions caused by this fungus were significantly larger in water‐sprayed leaves of the mutant compared with the wild‐type (Fig. 7b). Responses to OGs and flg22 that were not affected in the pepr1 pepr2 mutant are shown in Figs S4, S5a,b and S8a,b (see Supporting Information). All the results obtained with the AvrPto plants, and the bak1 bkk1 and pepr1 pepr2 mutants, are summarized in Table 1.
Figure 7.
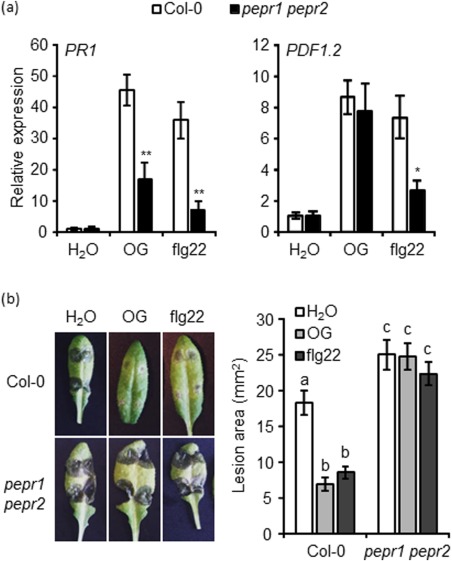
Oligogalacturonide (OG)‐ and flg22‐triggered induction of PR1 expression and protection against Botrytis cinerea are affected in the pepr1 pepr2 double mutant. (a) Expression of PR1 and PDF1.2, analysed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR), in seedlings of the wild‐type (Col‐0) and pepr1 pepr2 mutant 8 h after treatment with water, OGs (50 µg/mL) and flg22. Transcript levels are shown as the mean of at least three independent experiments [± standard error (SE); n = 20 in each experiment] normalized to UBQ5 expression and plotted relative to expression in water‐treated Col‐0. Asterisks indicate statistically significant differences between mutant and wild‐type samples, according to Student's t‐test (*P < 0.05; **P < 0.01). (b) Col‐0 and pepr1 pepr2 mutant plants were sprayed with water, OGs or flg22 and, after 24 h, leaves were inoculated with B. cinerea spores. Lesion areas were measured at 48 h post‐inoculation (hpi). Results are the average ± SE (n = 20 lesions). Different letters above the bars indicate statistically significant differences between samples, as determined by analysis of variance (ANOVA) with Tukey's honestly significant difference (HSD) test (P < 0.05). The experiment was repeated three times with similar results. Please see text for expansion of gene abbreviations.
Discussion
This work deepens our understanding of OG signalling by uncovering that AvrPto affects OG‐triggered immunity, in addition to MAMP‐triggered immunity, and demonstrating an important role of the pattern recognition co‐receptors BAK1 and BKK1 as well as the receptors PEPR1 and PEPR2. Our results also show that perception and early transduction of the OG signal are much more complex than those of flg22. Whereas all the flg22‐induced defence responses analysed here, with one exception that is discussed below, are affected by AvrPto and the lack of BAK1 and BKK1, only a subset of those induced by OGs is affected by AvrPto, and, among these, only a subset is affected by the lack of BAK1 and BKK1 (see Table 1).
OG‐induced ROS production, callose deposition, up‐regulation of FRK1, PDF1.2 and PR1 as well as protection against B. cinerea are all affected by both AvrPto and the lack of BAK1/BKK1, demonstrating that both co‐receptors play an important role in OG signalling. These responses are not affected in the corresponding single mutants, indicating a redundant and equal contribution of these leucine‐rich repeat receptor‐like protein kinases (LRR‐RLKs).
Notably, AvrPto affects some OG‐induced responses that are BAK1/BKK1 independent, i.e. the up‐regulation of the expression of the pairs CYP81F2/RET‐OX and PAD3/PGIP1 (partially and completely, respectively, inhibited in the high‐expressing AvrPto line), as well as the activation of MPK3 and MPK6. The latter effect was not observed in the low‐expressing AvrPto line, which nevertheless shows partial inhibition of the expression of all four genes, indicating that inhibition of OG‐induced MPK3 and MPK6 phosphorylation requires levels of AvrPto higher than those affecting the expression of these genes. Our observations provide further evidence that a defective elicitor‐induced defence response gene expression may occur in the presence of an apparently unaffected phosphorylation of MPK3 and MPK6 (Savatin et al., 2014a; Schwessinger et al., 2011). Thus, MPK3 and MPK6 phosphorylation may not be sufficient for full activation of the immune gene expression response by elicitors.
Conversely, the induction of the expression of PHI‐1 and the antagonism with auxin emerged as responses totally independent from AvrPto, BAK1 and BKK1. An intriguing result is the independence from BAK1/BKK1 of the flg22‐induced inhibition of auxin‐regulated gene expression, a response related to the growth–defence trade off that is, instead, suppressed by AvrPto. At present, this is the only flg22 response with this feature.
Both the presence of AvrPto and the lack of BAK1 and BKK1 lead to a higher susceptibility to B. cinerea, in agreement with previous results (Zhang et al., 2013), and to higher basal expression of PDF1.2 (much more conspicuous in AvrPto plants). The latter observation suggests that, in normal conditions, this gene is under a negative action of BAK1 and BKK1, as well as of other elements that are also targets of AvrPto, and that overexpression of PDF1.2 alone is not sufficient for resistance against B. cinerea.
The existence of subsets of OG responses that are differentially affected in the mutants analysed strongly suggests that different types of perception/transduction complexes mediate OG signalling. Responses that are both AvrPto and BAK1/BKK1 dependent point to the existence of perception/transduction complex(es) that function mainly through BAK1 and BKK1. AvrPto‐dependent BAK1/BKK1‐independent responses, instead, point to different types of complex(es), which may comprise co‐receptors other than BAK1 and BKK1 and/or receptors of a different class, targeted, however, by AvrPto. The behaviour of the low‐expressing AvrPto line, which shows inhibition of the OG‐induced up‐regulation of RET‐OX, CYP81F2, PAD3 and PGIP1, albeit at a partial extent, but not of MPK3 and MPK6 activation, may reflect a further heterogeneity in the OG‐triggered BAK1/BKK1‐independent perception/transduction pathways and therefore different genetic requirements. For example, up‐regulation of PGIP1 and PAD3 appears to be downstream of receptor/transduction complex(es) that can be completely blocked by AvrPto, whereas that of CYP81F2 and RET‐OX may also involve elements with low or no affinity for AvrPto. Similarly, phosphorylation of MAPKs may be mediated by receptor/co‐receptor kinases or other types of kinases also characterized by a low affinity for AvrPto.
Finally, a third type of complex, not requiring the function of BAK1 and BKK1 and capable of eluding the suppression action of the effector, may be involved in the OG responses that are independent of AvrPto, BAK1 and BKK1. The possibility exists that a combinatorial interaction between different receptors and intracellular, extracellular or plasma membrane co‐receptors may lead to the observed complexity. Moreover, each type of receptor complex may be redundant.
Members of the SERK family, other than BAK1 and BKK1, i.e. SERK1 and SERK2 [SERK5 is described to be defective in the Col‐0 ecotype used in this work (Wu et al., 2015)], are good candidates for playing a role in OG‐ and flg22‐triggered BAK1/BKK1‐independent responses. The role of these proteins in OG‐induced responses has never been investigated. As FLS2 is considered to be the only receptor of flg22, and SERK1 and SERK2 have been demonstrated to be recruited to the FLS2 perception complex (Roux et al., 2011), inhibition by flg22 of auxin‐regulated gene expression, an AvrPto‐dependent response, may be mediated specifically by these co‐receptors. Our results are consistent with the hypotheses that direct targets of AvrPto are the co‐receptors SERKs, or FLS2 alone, as proposed previously (Xiang et al., 2008). However, antagonism with auxin is independent not only of BAK1/BKK1, but also of AvrPto, in the case of OGs, suggesting that it may be downstream of receptor/co‐receptors unique to the OG perception/signalling pathway.
Whether WAK1, the only OG receptor identified so far, interacts with AvrPto or BAK1, BKK1 or other SERKs is not yet known and, in general, none of the five WAK family members has been identified to date among the interactors of either AvrPto or BAK1 (Bogdanove and Martin, 2000; Halter et al., 2014). AvrPto has been shown to interact with receptor kinases of different classes, e.g. the LRR‐type receptors FLS2, EFR and BAK1, and the LysM‐type CERK1, in addition to interacting directly with the protein kinase Pto in tomato cells (Gimenez‐Ibanez et al., 2009; Zipfel and Rathjen, 2008); thus, an interaction of AVrPto with WAKs cannot be ruled out, considering the difficulties in the extraction and purification of these proteins (Decreux and Messiaen, 2005; He et al., 1996; Wagner and Kohorn, 2001).
Among the danger signals so far characterized, the complexity of OG signalling is unprecedented. Such a complexity has long been envisioned, as no mutants have been identified to date that are completely insensitive to these elicitors. Moreover, a redundant role of the different WAK members in OG perception is likely, but not yet demonstrated, although WAK2, in addition to WAK1, can bind in vitro OGs and pectin (Decreux and Messiaen, 2005; Decreux et al., 2006; Kohorn et al., 2012).
The multiplicity of perception/transduction complexes for OGs, as suggested by this work, may recall the complexity of the homogalacturonan/OG vertebrate analogue signaling system (Cervone et al., 2015). This involves the extracellular matrix component hyaluronan (HA) and its fragments, which are produced after tissue injury or on enzymatic activity of hyaluronidases, either synthesized by animal cells or secreted by pathogens (Kreil, 1995), and induce different inflammatory‐related responses in different cell types and tissues, so that HA is now thought to be an immune regulator in human diseases (Jiang et al., 2011). Both HA and HA fragments are bound by different and numerous proteins, both membrane linked and extracellular, such as the PRRs, Toll‐like receptors TLR2 and TLR4, CD44, a polymorphic type I transmembrane glycoprotein, known as the major cell‐surface HA‐binding protein, RHAMM (receptor for HA‐mediated motility expressed protein) and BREVICAN (also called BEHAB, for brain enriched hyaluronan binding) (Jiang et al., 2011; Lee‐Sayer et al., 2015). In this case, signalling results from the specific combination of different elements depending on the tissue and cell type, and from the situation that needs to be perceived, e.g. infectious versus non‐infectious tissue injury. Signalling mediated by animal PRRs, including the different TLRs, is highly complex (Tan et al., 2014) and, in most cases, involves combinatorial and/or sequential stimulation of different perception/transduction complexes, allowing the activation of different immune responses in a synergistic manner, thus amplifying the signal, and/or in a compensatory manner, providing robustness to the signalling system. The combined action of different elements also allows a fine tuning of the immune response, in order to discriminate between pathogenic and non‐pathogenic microbes and, in general, to efficiently respond to different types of pathogen (Kim et al., 2014). A similar complexity may exist in signalling mediated by homogalacturonan/OGs.
Finally, we have shown here that PEPR1 and PEPR2 are also shared elements between OG and MAMP signalling, and are required for PR1 up‐regulation and protection against B. cinerea induced by OGs or flg22. Both responses are dependent on the ethylene signalling pathway (Gravino et al., 2015; Tintor et al., 2013). Because PEPR1 and PEPR2 are required for an appropriate response to ethylene, as 1‐aminocyclopropane‐1‐carboxylic acid (ACC)‐induced seedling growth inhibition, gene expression and protection against B. cinerea are affected in the pepr1 pepr2 mutant (Liu et al., 2013), the requirement of these receptors is probably a result of their role in the ethylene response. Moreover, OG‐ and elf18‐induced up‐regulation of PROPEP2, but not of PROPEP3, is ethylene dependent. Thus, as in the case of elf18 (Tintor et al., 2013), Pep2 is likely to be a player in the cascade that links OG perception to ethylene and the downstream responses, i.e. PR1 induction and acquired resistance against B. cinerea (Gravino et al., 2015). Because PROPEP1 is induced only to a limited extent (about two‐fold) by OGs, whereas PROPEP2 and PROPEP3 are strongly induced (Denoux et al., 2008), Pep1 is unlikely to play a major role in OG‐induced protection against B. cinerea, although this peptide has been shown to enhance resistance to this fungus (Liu et al., 2013).
PEPR1 and PEPR2 are instead dispensable for MAPK activation and ROS production by both OGs and flg22 (this work; Bartels et al., 2013; Krol et al., 2010), and for all the other responses induced by these elicitors (Table 1), except for the up‐regulation of PDF1.2, which occurs at a similar extent in response to the two elicitors in the wild‐type, but appears to require PEPR1 and PEPR2 only in the case of flg22. This defect is unlikely to be a result of the reduced responsiveness to ethylene of the pepr1 pepr2 mutant (Liu et al., 2013), as OG‐induced PDF1.2 expression is also strongly dependent on the ethylene signalling pathway (Gravino et al., 2015). The basis of this different behaviour is therefore not obvious, unless OGs activate an additional ethylene‐dependent, but PEPR1/PEPR2‐independent, pathway that leads to the up‐regulation of this gene. This pathway may be activated by at least one of the different perception/transduction complexes proposed here to mediate the response to OGs. Our identification of the distinct subsets of OG responses provides specific markers that may help to elucidate the complexity of OG signalling.
Experimental Procedures
Plant growth and treatment
Arabidopsis (Arabidopsis thaliana) Columbia‐0 (Col‐0) wild‐type seeds were purchased from Lehle Seeds (Round Rock, TX, USA). The bkk1‐1 (salk_057955), pepr1‐1 (salk_059281) and pepr2‐1 (salk_098161) single mutants (in the Col‐0 background) were purchased from The European Arabidopsis Stock Centre (Nottingham, UK). The bak1‐5 and bak1‐5 bkk1‐1 mutants (in the Col‐0 background) were kindly provided by Cyril Zipfel (The Sainsbury Laboratory, Norwich Research Park, Norwich, UK). The pepr1‐1 pepr2‐1 double mutant was generated by crossing the pepr single mutants. Conditional AvrPto‐expressing plants were generated by expressing the AvrPto coding sequence in Col‐0 under an oestradiol‐inducible promoter. The DNA sequence encoding AvrPto was amplified from the pET29a plasmid kindly provided by Jen Sheen (Department of Molecular Biology, Massachusetts General Hospital, Department of Genetics, Harvard Medical School, Boston, MA, USA). The cassette containing Avrpto and the β‐oestradiol inducible system were obtained using Gateway Recombination Cloning Technology (Life Technologies, Carlsbad, CA, USA) and the gateway‐compatible pMDC7 binary vector (Karimi et al., 2002), obtained from Plant System Biology (Ghent University; http://gateway.psb.ugent.be/). T1 independent plants were screened by antibiotic (hygromycin) resistance and analysed by PCR analysis (primer sequences in Table S1, see Supporting Information) for the presence of AvrPto transcripts in excised leaves treated with β‐oestradiol (1 μm) for 48 h. Among the homozygous T3 plants, two lines (#4.1 and #5.1) were chosen for the analyses.
For treatments of seedlings, seeds were surface sterilized and grown in multi‐well plates (approximately 10 seeds/well) containing 2 mL per well of Murashige and Skoog (MS) medium (Sigma‐Aldrich, Saint Louis, MO, USA; Murashige and Skoog, 1962), supplemented with 0.5% sucrose, at 22 °C and 70% relative humidity under a 16‐h/8‐h light/dark cycle (approximately 120 μmol/m2/s). AvrPto transgenic seedlings were grown in the presence of 1 μm β‐oestradiol or DMSO, as a control, 48 h before analyses. After 9 days, the medium was adjusted to a final volume of 1 mL and treatments with water, OGs (40 or 50 μg/mL), flg22 (10 nm) or elf18 (10 nm) were performed after an additional day. Analysis of the auxin antagonistic action of OGs or flg22 was performed as described previously (Savatin et al., 2011, 2014a). Root length analysis was performed in Col‐0 and AvrPto transgenic seedlings grown for 10 days in MS agar (0.8%) medium supplemented with 1% sucrose and 1 μm β‐oestradiol or DMSO.
For B. cinerea protection assay, 4‐week‐old plants were sprayed with water, OGs (200 μg/mL) or flg22 (1 µm). For ROS and callose analyses, 4‐week‐old plants were treated with OGs (200 μg/mL) or flg22 (100 nm). AvrPto transgenic plants were sprayed with 10 μm β‐oestradiol or DMSO, three times within a week, before elicitor treatment. Plants were grown at 22 °C and 70% relative humidity under a 12‐h/12‐h light/dark cycle (approximately 120 μmol/m2/s).
OGs with an average degree of polymerization (DP) of 10–16, as assessed by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry, were prepared as described previously (Bellincampi et al., 2000). The flg22 and elf18 peptides were synthesized by EZBiolab (Carmel, IN, USA).
Gene expression analysis
Gene expression analyses were performed as described previously (Savatin et al., 2014a). Primer sequences are shown in Table S1.
Immunoblot assay
Immunoblot assays were performed as described previously (Savatin et al., 2014a).
Botrytis cinerea protection assay
Protection assays against B. cinerea were performed as described previously (Savatin et al., 2014a).
Measurement of ROS
ROS measurements were performed as described previously (Gigli et al., 2015).
Callose deposition
Callose deposition was performed as described previously (Galletti et al., 2008). Callose quantification was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Characterization of mutants and AvrPto‐expressing plants.
Fig. S2 Flg22‐triggered induction of early defence response genes is affected in AvrPto‐expressing and bak1‐5 bkk1‐1 mutant seedlings.
Fig. S3 Inhibition of auxin‐regulated gene expression by oligogalacturonides (OGs) is not affected in AvrPto‐expressing and bak1‐5 bkk1‐1 mutant seedlings.
Fig. S4 Mitogen‐activated protein kinase (MAPK) activation induced by oligogalacturonides (OGs) is not affected in bak1‐5, bkk1‐1 and pepr1 pepr2 mutants.
Fig. S5 Reactive oxygen species (ROS) production and callose deposition in response to oligogalacturonides (OGs) are not impaired in the single mutants bak1‐5 and bkk1‐1 and in the double mutant pepr1 pepr2.
Fig. S6 Oligogalacturonide (OG)‐triggered induction of early and late defence response genes is not affected in the bak1‐5 and bkk1‐1 single mutants.
Fig. S7 Oligogalacturonide (OG)‐induced protection against Botrytis cinerea is not affected in the bak1‐5 and bkk1‐1 single mutants.
Fig. S8 Oligogalacturonide (OG)‐triggered induction of early and late defence response genes and inhibition of auxin‐regulated gene expression are not affected in the pepr1 pepr2 double mutant.
Table S1 Primers used in this work.
Acknowledgements
This work was supported by Università di Roma Sapienza, Award Grant 2014‐C26H14CEJA. The authors have no conflicts of interest to declare.
References
- Albrecht, C. , Russinova, E. , Kemmerling, B. , Kwaaitaal, M. and de Vries, S.C. (2008) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid‐dependent and ‐independent signaling pathways. Plant Physiol. 148, 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, J.M. , Hirayama, T. , Roman, G. , Nourizadeh, S. and Ecker, J.R. (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science, 284, 2148–2152. [DOI] [PubMed] [Google Scholar]
- Asai, T. , Tena, G. , Plotnikova, J. , Willmann, M.R. , Chiu, W.L. , Gomez‐Gomez, L. , Boller, T. , Ausubel, F.M. and Sheen, J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature, 415, 977–983. [DOI] [PubMed] [Google Scholar]
- Bartels, S. , Lori, M. , Mbengue, M. , van Verk, M. , Klauser, D. , Hander, T. , Boni, R. , Robatzek, S. and Boller, T. (2013) The family of Peps and their precursors in Arabidopsis: differential expression and localization but similar induction of pattern‐triggered immune responses. J. Exp. Bot. 64, 5309–5321. [DOI] [PubMed] [Google Scholar]
- Bellincampi, D. , Dipierro, N. , Salvi, G. , Cervone, F. and De Lorenzo, G. (2000) Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin‐regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 122, 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti, M. , Pontiggia, D. , Raggi, S. , Cheng, Z.Y. , Scaloni, F. , Ferrari, S. , Ausubel, F.M. , Cervone, F. and De Lorenzo, G. (2015) Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage‐associated molecular patterns. Proc. Natl. Acad. Sci. USA, 112, 5533–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove, A.J. and Martin, G.B. (2000) AvrPto‐dependent Pto‐interacting proteins and AvrPto‐interacting proteins in tomato. Proc. Natl. Acad. Sci. USA, 97, 8836–8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm, H. , Albert, I. , Oome, S. , Raaymakers, T.M. , Van den Ackerveken, G. and Nurnberger, T. (2014) A conserved peptide pattern from a widespread microbial virulence factor triggers pattern‐induced immunity in Arabidopsis. Plos Pathog. 10, e1004491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq, M. , Willmann, M.R. , McCormack, M. , Lee, H. , Shan, L. , He, P. , Bush, J. , Cheng, S.H. and Sheen, J. (2010) Differential innate immune signalling via Ca2+ sensor protein kinases. Nature, 464, 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrot, F. , Segonzac, C. , Chang, K.N. , Qiao, H. , Ecker, J.R. , Zipfel, C. and Rathjen, J.P. (2010) Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene‐dependent transcription factors EIN3 and EIL1. Proc. Natl. Acad. Sci. USA, 107, 14 502–14 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutus, A. , Sicilia, F. , Macone, A. , Cervone, F. and De Lorenzo, G. (2010) A domain swap approach reveals a role of the plant wall‐associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. USA, 107, 9452–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervone, F. , Ausubel, F.M. and De Lorenzo, G. (2015) Enhancing immunity by engineering DAMPs. Oncotarget, 6, 28 523–28 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, W. , Munkvold, K.R. , Gao, H. , Mathieu, J. , Schwizer, S. , Wang, S. , Yan, Y.B. , Wang, J. , Martin, G.B. and Chai, J. (2011) Structural analysis of Pseudomonas syringae AvrPtoB bound to host BAK1 reveals two similar kinase‐interacting domains in a type III effector. Cell Host Microbe, 10, 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla, D. , Zipfel, C. , Robatzek, S. , Kemmerling, B. , Nurnberger, T. , Jones, J.D. , Felix, G. and Boller, T. (2007) A flagellin‐induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature, 448, 497–500. [DOI] [PubMed] [Google Scholar]
- Cui, J. , Bahrami, A.K. , Pringle, E.G. , Hernandez‐Guzman, G. , Bender, C.L. , Pierce, N.E. and Ausubel, F.M. (2005) Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc. Natl. Acad. Sci. USA, 102, 1791–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decreux, A. and Messiaen, J. (2005) Wall‐associated kinase WAK1 interacts with cell wall pectins in a calcium‐induced conformation. Plant Cell Physiol. 46, 268–278. [DOI] [PubMed] [Google Scholar]
- Decreux, A. , Thomas, A. , Spies, B. , Brasseur, R. , Van Cutsem, P. and Messiaen, J. (2006) In vitro characterization of the homogalacturonan‐binding domain of the wall‐associated kinase WAK1 using site‐directed mutagenesis. Phytochemistry, 67, 1068–1079. [DOI] [PubMed] [Google Scholar]
- Denoux, C. , Galletti, R. , Mammarella, N. , Gopalan, S. , Werck, D. , De Lorenzo, G. , Ferrari, S. , Ausubel, F.M. and Dewdney, J. (2008) Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant. 1, 423–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, S. , Savatin, D.V. , Sicilia, F. , Gramegna, G. , Cervone, F. and De Lorenzo, G. (2013) Oligogalacturonides: plant damage‐associated molecular patterns and regulators of growth and development. Front. Plant Sci. 4, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti, R. , Denoux, C. , Gambetta, S. , Dewdney, J. , Ausubel, F.M. , De Lorenzo, G. and Ferrari, S. (2008) The AtrbohD‐mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea . Plant Physiol. 148, 1695–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletti, R. , Ferrari, S. and De Lorenzo, G. (2011) Arabidopsis MPK3 and MPK6 play different roles in basal and oligogalacturonide‐ or flagellin‐induced resistance against Botrytis cinerea . Plant Physiol. 157, 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli, B.N. , Gravino, M. and Savatin, D.V. (2015) Luminol‐based assay for detection of immunity elicitor‐induced hydrogen peroxide production in Arabidopsis thaliana leaves. Bio‐protocol, 5, e1685 http://www.bio-protocol.org/e1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez‐Ibanez, S. , Hann, D.R. , Ntoukakis, V. , Petutschnig, E. , Lipka, V. and Rathjen, J.P. (2009) AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr. Biol. 19, 423–429. [DOI] [PubMed] [Google Scholar]
- Goehre, V. , Spallek, T. , Haeweker, H. , Mersmann, S. , Mentzel, T. , Boller, T. , de Torres, M. , Mansfield, J.W. and Robatzek, S. (2008) Plant pattern‐recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr. Biol. 18, 1824–1832. [DOI] [PubMed] [Google Scholar]
- Gomez‐Gomez, L. (2004) Plant perception systems for pathogen recognition and defence. Mol. Immunol. 41, 1055–1062. [DOI] [PubMed] [Google Scholar]
- Gomez‐Gomez, L. and Boller, T. (2000) FLS2: an LRR receptor‐like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell, 5, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Gravino, M. , Savatin, D.V. , Macone, A. and De Lorenzo, G. (2015) Ethylene production in Botrytis cinerea‐ and oligogalacturonide‐induced immunity requires calcium‐dependent protein kinases. Plant J. 84, 1073–1086. [DOI] [PubMed] [Google Scholar]
- Halter, T. , Imkampe, J. , Mazzotta, S. , Wierzba, M. , Postel, S. , Bucherl, C. , Kiefer, C. , Stahl, M. , Chinchilla, D. , Wang, X. , Nurnberger, T. , Zipfel, C. , Clouse, S. , Borst, J.W. , Boeren, S. , de Vries, S.C. , Tax, F. and Kemmerling, B. (2014) The leucine‐rich repeat receptor kinase BIR2 is a negative regulator of BAK1 in plant immunity. Curr. Biol. 24, 134–143. [DOI] [PubMed] [Google Scholar]
- Hauck, P. , Thilmony, R. and He, S.Y. (2003) A Pseudomonas syringae type III effector suppresses cell wall‐based extracellular defense in susceptible Arabidopsis plants. Proc. Natl. Acad. Sci. USA, 100, 8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Z.H. , Fujiki, M. and Kohorn, B.D. (1996) A cell wall‐associated, receptor‐like protein kinase. J. Biol. Chem. 271, 19 789–19 793. [DOI] [PubMed] [Google Scholar]
- He, P. , Shan, L. , Lin, N.C. , Martin, G.B. , Kemmerling, B. , Nurnberger, T. and Sheen, J. (2006) Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell, 125, 563–575. [DOI] [PubMed] [Google Scholar]
- He, K. , Gou, X. , Yuan, T. , Lin, H. , Asami, T. , Yoshida, S. , Russell, S.D. and Li, J. (2007) BAK1 and BKK1 regulate brassinosteroid‐dependent growth and brassinosteroid‐independent cell‐death pathways. Curr. Biol. 17, 1109–1115. [DOI] [PubMed] [Google Scholar]
- Huffaker, A. , Pearce, G. and Ryan, C.A. (2006) An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. USA, 103, 10 098–10 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, D. , Liang, J. and Noble, P.W. (2011) Hyaluronan as an immune regulator in human diseases. Physiol Rev. 91, 221–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J.D. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Karimi, M. , Inze, D. and Depicker, A. (2002) GATEWAY vectors for Agrobacterium‐mediated plant transformation. Trends Plant Sci. 7, 193–195. [DOI] [PubMed] [Google Scholar]
- Kim, B.H. , Kim, S.Y. and Nam, K.H. (2013) Assessing the diverse functions of BAK1 and its homologs in Arabidopsis, beyond BR signaling and PTI responses. Mol. Cell, 35, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. , Tsuda, K. , Igarashi, D. , Hillmer, R.A. , Sakakibara, H. , Myers, C.L. and Katagiri, F. (2014) Mechanisms underlying robustness and tunability in a plant immune signaling network. Cell Host Microbe, 15, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn, B.D. , Kohorn, S.L. , Todorova, T. , Baptiste, G. , Stansky, K. and McCullough, M. (2012) A dominant allele of Arabidopsis pectin‐binding wall‐associated kinase induces a stress response suppressed by MPK6 but not MPK3 mutations. Mol. Plant. 5, 841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil, G. (1995) Hyaluronidases—a group of neglected enzymes. Protein Sci. 4, 1666–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol, E. , Mentzel, T. , Chinchilla, D. , Boller, T. , Felix, G. , Kemmerling, B. , Postel, S. , Arents, M. , Jeworutzki, E. , Al Rasheid, K.A. , Becker, D. and Hedrich, R. (2010) Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J. Biol. Chem. 285, 13 471–13 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee‐Sayer, S.S. , Dong, Y. , Arif, A.A. , Olsson, M. , Brown, K.L. and Johnson, P. (2015) The where, when, how, and why of hyaluronan binding by immune cells. Front Immunol. 6, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Lin, H. , Zhang, W. , Zou, Y. , Zhang, J. , Tang, X. and Zhou, J.M. (2005) Flagellin induces innate immunity in nonhost interactions that is suppressed by Pseudomonas syringae effectors. Proc. Natl. Acad. Sci. USA, 102, 12 990–12 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z.X. , Wu, Y. , Yang, F. , Zhang, Y.Y. , Chen, S. , Xie, Q. , Tian, X.J. and Zhou, J.M. (2013) BIK1 interacts with PEPRs to mediate ethylene‐induced immunity. Proc. Natl. Acad. Sci. USA, 110, 6205–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho, A.P. and Zipfel, C. (2015) Targeting of plant pattern recognition receptor‐triggered immunity by bacterial type‐III secretion system effectors. Curr. Opin. Microbiol. 23, 14–22. [DOI] [PubMed] [Google Scholar]
- Mersmann, S. , Bourdais, G. , Rietz, S. and Robatzek, S. (2010) Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 154, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T. and Skoog, F. (1962) Revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 15, 437–479. [Google Scholar]
- Pedley, K.F. and Martin, G.B. (2003) Molecular basis of Pto‐mediated resistance to bacterial speck disease in tomato. Annu. Rev. Phytopathol. 41, 215–243. [DOI] [PubMed] [Google Scholar]
- Roux, M. , Schwessinger, B. , Albrecht, C. , Chinchilla, D. , Jones, A. , Holton, N. , Malinovsky, F.G. , Tor, M. , De Vries, S. and Zipfel, C. (2011) The Arabidopsis leucine‐rich repeat receptor‐like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell, 23, 2440–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatin, D.V. , Ferrari, S. , Sicilia, F. and De Lorenzo, G. (2011) Oligogalacturonide–auxin antagonism does not require posttranscriptional gene silencing or stabilization of auxin response repressors in Arabidopsis. Plant Physiol. 157, 1163–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatin, D.V. , Gigli, B.N. , Marti, L. , Fabbri, C. , Cervone, F. and De Lorenzo, G. (2014a) The Arabidopsis NPK1‐related protein kinases ANPs are required for elicitor‐induced oxidative burst and immunity. Plant Physiol. 165, 1188–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatin, D.V. , Gramegna, G. , Modesti, V. and Cervone, F. (2014b) Wounding in the plant tissue: the defense of a dangerous passage. Front Plant Sci. 5, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger, B. , Roux, M. , Kadota, Y. , Ntoukakis, V. , Sklenar, J. , Jones, A. and Zipfel, C. (2011) Phosphorylation‐dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor‐like kinase BAK1. Plos Genet. 7, e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan, L. , He, P. , Li, J. , Heese, A. , Peck, S.C. , Nurnberger, T. , Martin, G.B. and Sheen, J. (2008) Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor‐signaling complexes and impede plant immunity. Cell Host Microbe, 4, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, R.S. , Ho, B. , Leung, B.P. and Ding, J.L. (2014) TLR cross‐talk confers specificity to innate immunity. Int. Rev. Immunol. 33, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintor, N. , Ross, A. , Kanehara, K. , Yamada, K. , Fan, L. , Kemmerling, B. , Nurnberger, T. , Tsuda, K. and Saijo, Y. (2013) Layered pattern receptor signaling via ethylene and endogenous elicitor peptides during Arabidopsis immunity to bacterial infection. Proc. Natl. Acad. Sci. USA, 110, 6211–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, T.A. and Kohorn, B.D. (2001) Wall‐associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell, 13, 303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, W. , Wu, Y. , Gao, Y. , Li, M. , Yin, H. , Lv, M. , Zhao, J. , Li, J. and He, K. (2015) Somatic embryogenesis receptor‐like kinase 5 in the ecotype Landsberg erecta of Arabidopsis is a functional RD LRR‐RLK in regulating brassinosteroid signaling and cell death control. Front Plant Sci. 6, 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, T. , Zong, N. , Zou, Y. , Wu, Y. , Zhang, J. , Xing, W. , Li, Y. , Tang, X. , Zhu, L. , Chai, J. and Zhou, J.M. (2008) Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr. Biol. 18, 74–80. [DOI] [PubMed] [Google Scholar]
- Xiang, T. , Zong, N. , Zhang, J. , Chen, J. , Chen, M. and Zhou, J.M. (2011) BAK1 is not a target of the Pseudomonas syringae effector AvrPto. Mol. Plant–Microbe Interact. 24, 100–107. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Y. , Huffaker, A. , Bryan, A.C. , Tax, F.E. and Ryan, C.A. (2010) PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell, 22, 508–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Shao, F. , Li, Y. , Cui, H. , Chen, L. , Li, H. , Zou, Y. , Long, C. , Lan, L. , Chai, J. , Chen, S. , Tang, X. and Zhou, J.M. (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP‐induced immunity in plants. Cell Host Microbe, 1, 175–185. [DOI] [PubMed] [Google Scholar]
- Zhang, W.G. , Fraiture, M. , Kolb, D. , Loffelhardt, B. , Desaki, Y. , Boutrot, F.F.G. , Tor, M. , Zipfel, C. , Gust, A.A. and Brunner, F. (2013) Arabidopsis receptor‐like protein30 and receptor‐like kinase suppressor of BIR1‐1/EVERSHED mediate innate immunity to necrotrophic fungi. Plant Cell, 25, 4227–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel, C. (2014) Plant pattern‐recognition receptors. Trends Immunol. 35, 345–351. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. and Rathjen, J.P. (2008) Plant immunity: AvrPto targets the frontline. Curr. Biol. 18, R218–R220. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. , Kunze, G. , Chinchilla, D. , Caniard, A. , Jones, J.D.G. , Boller, T. and Felix, G. (2006) Perception of the bacterial PAMP EF‐Tu by the receptor EFR restricts Agrobacterium‐mediated transformation. Cell, 125, 749–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Characterization of mutants and AvrPto‐expressing plants.
Fig. S2 Flg22‐triggered induction of early defence response genes is affected in AvrPto‐expressing and bak1‐5 bkk1‐1 mutant seedlings.
Fig. S3 Inhibition of auxin‐regulated gene expression by oligogalacturonides (OGs) is not affected in AvrPto‐expressing and bak1‐5 bkk1‐1 mutant seedlings.
Fig. S4 Mitogen‐activated protein kinase (MAPK) activation induced by oligogalacturonides (OGs) is not affected in bak1‐5, bkk1‐1 and pepr1 pepr2 mutants.
Fig. S5 Reactive oxygen species (ROS) production and callose deposition in response to oligogalacturonides (OGs) are not impaired in the single mutants bak1‐5 and bkk1‐1 and in the double mutant pepr1 pepr2.
Fig. S6 Oligogalacturonide (OG)‐triggered induction of early and late defence response genes is not affected in the bak1‐5 and bkk1‐1 single mutants.
Fig. S7 Oligogalacturonide (OG)‐induced protection against Botrytis cinerea is not affected in the bak1‐5 and bkk1‐1 single mutants.
Fig. S8 Oligogalacturonide (OG)‐triggered induction of early and late defence response genes and inhibition of auxin‐regulated gene expression are not affected in the pepr1 pepr2 double mutant.
Table S1 Primers used in this work.


