Summary
Botryosphaeria dothidea is the type species of Botryosphaeria (Botryosphaeriaceae, Botryosphaeriales). Fungi residing in this order are amongst the most widespread and important canker and dieback pathogens of trees worldwide, with B. dothidea one of the most common species on a large number of hosts. Its taxonomic circumscription has undergone substantial change in the past decade, making it difficult to interpret the large volume of literature linked to the name B. dothidea. This pathogen profile synthesizes the current understanding of B. dothidea pertaining to its distribution, host associations and role as a pathogen in managed and natural woody environments. The prolonged latent infection or endophytic phase is of particular importance, as it implies that the fungus can easily pass undetected by quarantine systems in traded living plants, fruits and other plant parts. Infections typically become obvious only under conditions of host stress, when disease symptoms develop. This study also considers the knowledge emerging from the recently sequenced B. dothidea genome, elucidating previously unknown aspects of the species, including mating and host infection strategies. Despite more than 150 years of research on B. dothidea, there is clearly much to be learned regarding this global tree pathogen. This is increasingly important given the stresses imposed on various woody hosts as a result of climate change.
Taxonomy
Botryosphaeria dothidea (Moug. ex Fr) Ces. & De Not, 1863. Kingdom Fungi, Phylum Ascomycota, Class Dothideomycetes, Order Botryosphaeriales, Family Botryosphaeriaceae, Genus Botryosphaeria, Species dothidea.
Host range
Confirmed on more than 24 host genera, including woody plants, such as Acacia (= Vachellia), Eucalyptus, Vitis and Pistachio.
Disease symptoms
Associated with twig, branch and stem cankers, tip and branch dieback, fruit rot, blue stain and plant death.
Useful websites
The Botryosphaeria site for detailed morphological descriptions (http://www.crem.fct.unl.pt/botryosphaeria_site/);
Systematic Mycology and Microbiology Laboratory Fungal Database for all literature and associated hosts (https://nt.ars-grin.gov/fungaldatabases/);
TreeBASE link for the combined ITS and TEF‐1α tree (http://purl.org/phylo/treebase/phylows/study/TB2:S18906);
DOE Joint Genome Institute, JGI Mycocosm for the Botryosphaeria dothidea genome (http://genome.jgi.doe.gov/Botdo1_1/Botdo1_1.home.html).
Keywords: Botryosphaeria dothidea, climate change, endophyte, global pathogen, latent pathogen, quarantine
Introduction
The ascomycete fungus Botryosphaeria dothidea is the type species of Botryosphaeria (Botryosphaeriaceae, Botryosphaeriales) residing in the Dothideomycetes. Since the original description of B. dothidea in 1853 (Cesati and De Notaris, 1853), numerous morphologically similar specimens have been reported as representing this pathogen. In retrospect, however, many of these specimens have been shown to belong to other species. The advent of DNA‐based identification has largely resolved this important problem as a result of the epitypification of this species (Slippers et al., 2004a). As a consequence of previous taxonomic confusion, reports of this fungus on a wide range of hosts and in numerous countries prior to the application of DNA sequencing techniques are either incorrect, or must at least be viewed with circumspection.
Botryosphaeria dothidea and other species in the Botryosphaeriaceae were considered as wound‐infecting pathogens for many years. During the course of the last few decades, however, these fungi have been recognized primarily as endophytes that infect healthy tissue of woody plants and remain dormant until the onset of stress conditions (Fisher et al., 1993; Johnson et al., 1992; Maresi et al., 2007; Perez et al., 2010; Petrini and Fisher, 1988; Sakalidis et al., 2011a; Smith et al., 1996a, 1996b; Stanosz et al., 2005). In the case of B. dothidea, the distinction between endophyte and latent pathogen is of little value and simply reflects a snapshot in time of the complex lifestyle of this plant‐infecting fungus. This is true for many pathogens that have a latent phase during a part of their lifecycle (Hyde and Soytong, 2008; Rai and Agarkar, 2014; Schulz and Boyle, 2005).
Climate change is expected to increase the stress on many plant communities, including trees in natural woody ecosystems, managed forests and agriculture (Kirilenko and Sedjo, 2007; Lavalle et al., 2009; Sturrock et al., 2011). Consequently, the potential impact of the Botryosphaeriaceae in general, but specifically B. dothidea, which is a widespread pathogen already present as an endophyte in numerous plant communities in various parts of the world, might be exacerbated (Desprez‐Loustau et al., 2006). Already, the impact of the pathogen in relation to climatic conditions and stress has been documented in plant communities in parts of southern Europe (Piškur et al., 2011), Australia (Dakin et al., 2010), South Africa (Van Der Linde et al., 2011, 2012) and other countries (Brown and Hendrix, 1981; Ma et al., 2001a, 2001b; Sturrock et al., 2011).
Reports prior to the application of DNA sequence data and phylogenetic inference treated B. dothidea as a conglomerate of taxa. Therefore, the scientific literature regarding this fungus must be interpreted with care. In this review, we address this issue by considering the extensive knowledge regarding B. dothidea, and the family in which it resides, as it has been updated during the course of the past decade through accurate DNA‐based identification. We also utilize currently available public data to characterize its confirmed host associations and geographical distribution. Available knowledge regarding the epidemiology of this latent pathogen, its role in the environment and how this might be influenced by climate change are considered. Furthermore, key insights emerging from the recently sequenced genome of B. dothidea are highlighted. This is carried out with the specific intention of laying a foundation for future studies aimed at understanding the ability of the fungus to avoid host defence systems and to establish continuing endophytic infections.
Taxonomy and Identification
Prior to its epitypification by Slippers et al. (2004a), there was confusion pertaining to the taxonomy and identification of B. dothidea. Cesati and De Notaris (1863) described the genus Botryosphaeria Ces. & De Not., with the addition of B. berengeriana De Not., by De Notaris (1863). No type species was selected for the genus at the time, but Barr (1972) designated B. dothidea (Moug.: Fr.) Ces. & De Not. (= Sphaeria dothidea Moug. ex Fr.) as the lectotype species for the genus. Subsequently, many additional Botryosphaeria species have been described, often based on the fact that they were isolated from different hosts and despite morphological similarities between specimens. von Arx and Müller (1954) reduced many of these species to synonymy with B. quercuum and B. dothidea based on the morphology of their sexual morphs. von Arx and Müller (1975) and von Arx (1987) later restricted B. dothidea to isolates that were pathogenic on roses, and considered B. berengeriana (including B. ribis) to be polyphagous and widespread. Pennycook and Samuels (1985), however, recognized that B. dothidea was a complex of species that could be distinguished based on morphological differences of the asexual Fusicoccum states, and separated F. aesculi Corda, F. parvum Pennycook & Samuels and F. luteum Pennycook & Samuels from B. dothidea (Pennycook and Samuels, 1985).
Jacobs and Rehner (1998) produced the first DNA‐based phylogeny for Botryosphaeria and related asexual genera, based on sequences for the internal transcribed spacer (ITS) rDNA locus. The Botryosphaeria spp. included in their study formed clades corresponding to species having dark, Diplodia‐like conidia and species having hyaline, Fusicoccum‐like conidia (Jacobs and Rehner, 1998). Subsequent DNA‐based studies supported these clades and suggested that Botryosphaeria spp. with hyaline, fusoid conidia should reside in Fusicoccum and those with dark to opaque and ellipsoid mature conidia should be placed in Diplodia, Lasiodiplodia or in the sections Hyala and Brunnea (Denman et al., 2000; Zhou and Stanosz, 2001). These developments provided the basis for the further resolution of the species complex grouped under the name B. dothidea. Neofusicoccum luteum was the first species to be separated from B. dothidea based on colony morphology and DNA sequence data (Phillips et al., 2002). The taxonomic confusion surrounding B. dothidea was finally resolved by the epitypification of the species by Slippers et al. (2004a). Based on a collection from the same region (Crocifisso, Switzerland) and host (Prunus sp.) in which the original type specimen had been collected, the epitype specimen was linked to a culture and DNA barcode. This made it possible to connect subsequent studies to a reliable name (Slippers et al., 2004a).
Subsequent to the designation of the epitype, a number of species and even families have been separated from B. dothidea (Crous et al., 2006; Damm et al., 2007; Phillips et al., 2008, 2013; Slippers et al., 2013). Despite the narrower species delimitation after epitypification, reports of B. dothidea during the last decade have confirmed that it is one of the most widespread and important endophytes or latent pathogens occurring on a large number of plants important in agriculture, forestry and natural forest ecosystems (Jami et al., 2013a; Pavlic et al., 2007; Phillips et al., 2005; Piškur et al., 2011; Qiu et al., 2008; Slippers and Wingfield, 2007; Tang et al., 2012; Xu et al., 2015).
Morphological characters have been important in the identification of fungi in the past. The ascospores of B. dothidea are unicellular, hyaline, 17–22 µm in length and fusoid to ovoid with tapered ends. Conidia are unicellular, narrowly or irregularly fusiform with rounded ends. Conidia are also 17–22 µm in length, hyaline and rarely form a septum before germination (Phillips et al., 2013; Slippers et al., 2004a). In culture, colonies are olivaceous, becoming dark grey and black in reverse as the colony ages. The mycelial mat is moderately dense with smooth margins (Phillips et al., 2013; Slippers et al., 2004a). Pycnidia produced on water agar and sterilized host twigs or pine needles are solitary, globose and covered by mycelium. Pycnidia contain a single ostiole with white to creamy contents. Conidia are similar to those produced in nature, except that they are 20–30 µm in length and are more narrowly fusiform (Slippers et al., 2004a). These morphological characteristics are no longer routinely used in the identification of B. dothidea, given the variation amongst isolates and the fact that they overlap with other species in the Botryosphaeriaceae.
DNA‐based identification of B. dothidea typically relies on the sequences linked to the ex‐type isolate (CMW8000/CBS115476) for β‐tubulin (BT, AY236927), rDNA ITS (AY236949) and translation elongation factor‐1α (TEF‐1α, AY236898) (Slippers et al., 2004a). As of November 2016, more than 2600 sequences had been generated for B. dothidea and deposited in GenBank [National Center for Biotechnology Information (NCBI); www.ncbi.nlm.hih.gov], most for the three above‐mentioned loci. The majority of the sequences in GenBank are of the ITS rDNA locus, which has been shown to clearly distinguish this species from its closest known relatives (Phillips et al., 2013). Given that cryptic species have regularly been discovered in this family during the course of the last decade, it is recommended that sequence data of ITS be combined with those of TEF‐1α and at least one other phylogenetic marker, where possible.
Distribution and Host Associations
Botryosphaeria dothidea has been reported from hundreds of plant species with a broad global distribution. For example, there are 959 fungal host reports and 282 literature records of B. dothidea and its synonyms listed in the Systematic Mycology and Microbiology Laboratory Fungus‐Host Distributions Database (Farr and Rossman, 2015; November 2016). Many of these reports are of uncertain validity because of the previously confusing taxonomy for the species. In order to gain a more realistic perspective of the host range of B. dothidea as it is currently defined, GenBank was mined for sequences of Botryosphaeria for the ITS, TEF‐1α and BT regions. Sequences for these loci were downloaded for the genus Botryosphaeria (ITS, 1434; TEF‐1α, 398; BT, 272) in November 2015. A multigene phylogeny was inferred for isolates (424) that were represented by the ITS and TEF‐1α DNA regions because the combination would give more robust haplotypes than ITS alone. There was also no overlap between the BT and TEF‐1α datasets (see Methods S1).
The sequences for this analysis represented specimens that originated from 24 host genera (representing 17 families) and 18 different countries spread over six continents (Table S1, see Supporting Information). The 240 isolates representing B. dothidea from GenBank clustered in 46 haplotypes, whereby two haplotypes contained the vast majority of sequences (Fig. 1). However, with the exception of a group of haplotypes representing Rosaceae (i.e. Malus and Pyrus spp.) in China (Fig. 1), the minimum spanning tree did not separate specimens according to host genera or country of origin.
Figure 1.
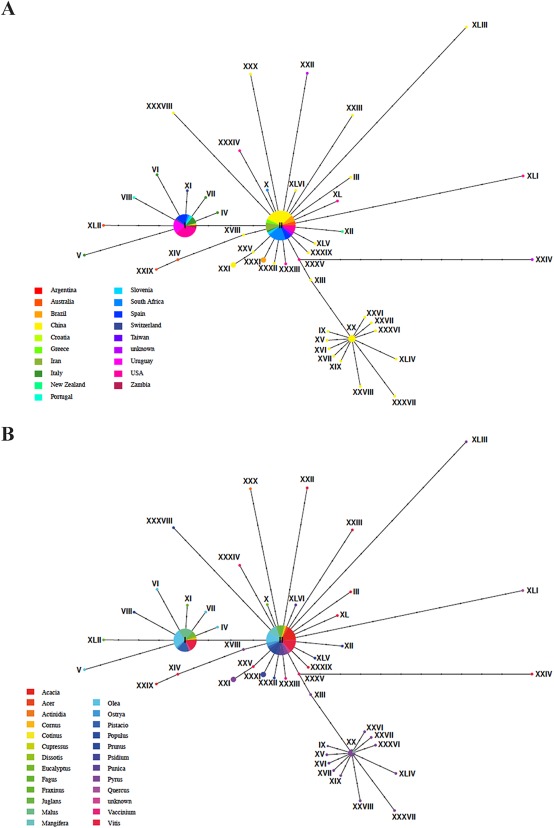
(A) Haplotype network representing 240 Botryosphaeria dothidea isolates from 18 different countries divided into 46 haplotypes. (B) Haplotype network representing the 24 host genera from which B. dothidea was isolated. Isolates could not be separated by country of origin or host genera.
The low host specificity and broad geographical distribution of haplotypes for B. dothidea confirmed previous studies. This is surprising, as most studies that scrutinize pathogen species with low host specificity often recover many host‐specific cryptic species (Filipe et al., 2012; Stergiopoulos and Gordon, 2014). Our data indicate that B. dothidea does not show any host preference. This was confirmed through single haplotypes that exist on many different hosts, although their wide distribution was somewhat surprising. Conidia and ascospores of B. dothidea, as for other Botryosphaeriaceae, are considered to be dispersed by wind and rain over relatively short distances (Ahimera et al., 2004; Amponsah et al., 2009; van Niekerk et al., 2010; Pusey, 1989; Sutton, 1981; Swart and Wingfield, 1991; Úrbez‐Torres et al., 2010). Such limited dispersal must be assumed to result in a population structure that is shaped by geographical distance. However, the low host specificity could, in part, contribute to the low genetic structure between geographical locations by allowing the fungus to colonize many hosts in a given area. Many of the hosts in our analysis are agriculture‐, forestry‐ or horticulture‐related plants. The current widespread distribution of a few haplotypes of B. dothidea globally has most probably resulted from anthropogenic long‐distance dispersal via the global trade of plants and plant products, illustrating the phytosanitary implications of undiscovered plant pathogens.
Epidemiology and Host Infection
Species in the Botryosphaeriaceae are considered to be stress‐associated pathogens (Czemmel et al., 2015; Desprez‐Loustau et al., 2006; Ma et al., 2001b; Mehl et al., 2013; van Niekerk et al., 2011; Piškur et al., 2011; Slippers and Wingfield, 2007; Smith et al., 1994; Stanosz et al., 2001; Zhang et al., 2013). Disease expression is more often associated with abiotic stresses, such as drought, physical damage, waterlogging, frost and unsuitable growing environments (Bostock et al., 2014; Desprez‐Loustau et al., 2006; Ma et al., 2001b; van Niekerk et al., 2011; Ragazzi et al., 1999; Sakalidis et al., 2011b; Slippers and Wingfield, 2007; Stanosz et al., 2001; Zhang et al., 2013). When these stressful conditions occur, there are often a variety of Botryosphaeriaceae isolated from different plant tissues, potentially all contributing to the observed symptoms, albeit to varying degrees (e.g. Jami et al., 2015).
Disease symptoms (Fig. 2) include twig, branch and stem cankers, tip and branch dieback, fruit rots, blue stain or, in extreme cases, the death of the host plant (Michailides, 1991; Slippers and Wingfield, 2007; Smith et al., 1994; Swart and Wingfield, 1991). Although the association of B. dothidea with such symptoms before 2004 must remain uncertain, recent studies have linked B. dothidea to similar symptoms on a variety of hosts. These include apple ring rot (Kim et al., 2004; Tang et al., 2012; Xu et al., 2015), fruit rot of olives (Phillips et al., 2005), grapevine trunk disease (Li et al., 2010; van Niekerk et al., 2006; Qiu et al., 2008), leaf spots and lesions on horticultural plants (Cunnington et al., 2007), as well as dieback and stem cankers on acacia (Gezahgne et al., 2004), eucalypt (Burgess et al., 2005; Gezahgne et al., 2004; Mohali et al., 2007), European hop hornbeam (Jurc et al., 2006), pine (Gezahgne et al., 2004), mango (Slippers et al., 2005), poplar (Grasso and Granata, 2010; Slippers et al., 2004b) and stone fruit (Inderbitzin et al., 2010; Slippers et al., 2004b; Wang et al., 2011). Many more host associations and specific symptoms have been reported, but not confirmed using sequence data (Farr and Rossman, 2015).
Figure 2.
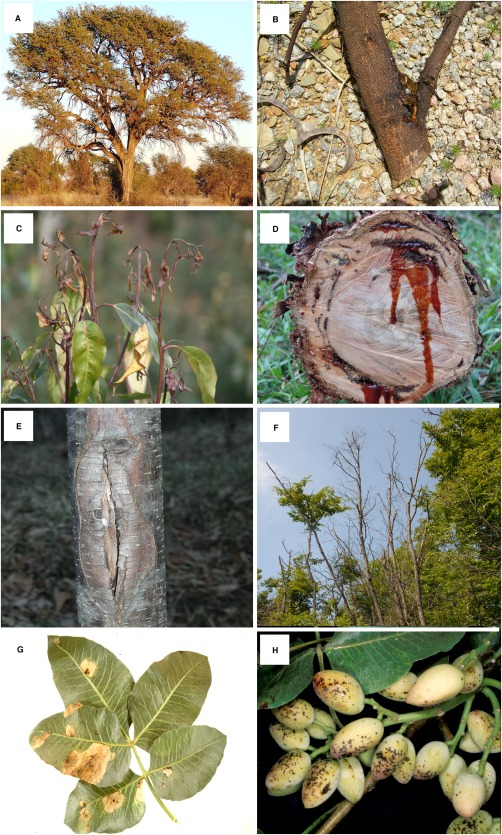
Different disease symptoms caused by Botryosphaeria dothidea, representing four different hosts spanning four different continents. (A) Healthy Acacia erioloba (= Vachellia), a native host in Africa. (B) Kino secretions from the trunk of Acacia. (C) Dieback symptoms on Eucalyptus, a non‐native host in South America. (D) Kino secretions from the trunk of Eucalyptus. (E) Canker on the trunk of Ostrya carpinifolia, a native host in Europe. (F) Dieback symptoms on Ostrya carpinifolia. (G) Leaf lesion on the leaves of Pistachio, a non‐native host in North America. (H) Early disease symptoms on the fruit of Pistachio.
Prior to its epitypification in 2004, it was assumed that B. dothidea could infect its hosts through either wounds (Michailides, 1991) or endophytically through natural openings (Kim et al., 1999; Michailides, 1991; Smith, 2001). Direct penetration was also possible on apple fruits through the formation of appressoria (Jurick et al., 2013; Kim et al., 1999). Much is still unknown regarding the infection biology of B. dothidea, especially how it penetrates without causing a host response. Preliminary data, for example, indicate that the fungus is capable of infecting Eucalyptus grandis leaves by direct penetration via the formation of appressorium‐like structures at the germ tube tips, without causing a host response, and persisting endophytically (Marsberg et al., unpublished data; University of Pretoria). Future infection studies on hosts from which B. dothidea has been isolated as an endophyte are required to characterize the various stages of this process. Questions that remain to be answered include the location of the endophytic hyphae within infected plant tissues, as well as the level of activity within this tissue.
Latent Pathogens and Quarantine
Botryosphaeria dothidea is known to be a serious plant pathogen and is listed in many countries as a quarantine organism. The fact that it frequently exists as an endophyte in asymptomatic tissue for prolonged periods of time poses a significant quarantine challenge (Slippers and Wingfield, 2007). Neither B. dothidea nor its synonyms currently appear on the European and Mediterranean Plant Protection Organization (EPPO) A1 and A2 lists of pests recommended for regulation as quarantine pests (http://www.eppo.int/QUARANTINE/quarantine.htm), but they do occur in the EPPO global database (https://gd.eppo.int/taxon/BOTSDO). Infected plant tissues and seed can easily be moved between countries and regions without any visible indications of infection. Inspection‐based quarantine procedures that target only symptomatic material are therefore not able to detect it.
The low host specificity of B. dothidea increases the likelihood that it will spread to new hosts once it is introduced into a new area. It is thus not surprising that various studies have provided evidence that B. dothidea infects both native and non‐native trees in areas in which it is found (Jami et al., 2013a, 2013b; Mohali et al., 2007; Pavlic et al., 2007; Slippers et al., 2009). In some cases, native and non‐native hosts grow in close proximity, e.g. Pistachio and native hosts in California (Inderbitzin et al., 2010; Ma et al., 2001a). In other cases, B. dothidea is common both on native and non‐native hosts that are not in close proximity, such as the isolated native stands of the widely distributed Acacia (= Vachellia) trees in the dry northern regions of South Africa (Jami et al., 2015). Therefore, B. dothidea could be equally threatening to both commercial enterprises and native ecosystems. The same situation is most probably also true for other Botryosphaeriaceae, such as the important conifer pathogen Diplodia sapinea (Burgess et al., 2001; Slippers and Wingfield, 2007; Smith, 2001; Smith et al., 1996a). Figure 3 broadly encompasses the dispersal of B. dothidea between trees, species and regions with the potential threat to quarantine systems.
Figure 3.
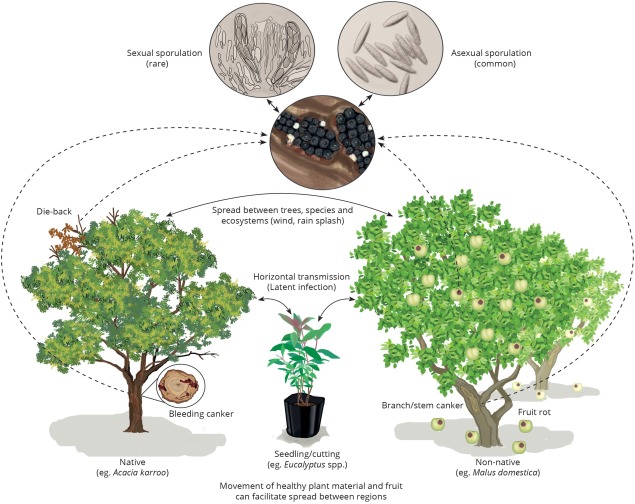
An illustrative example of typical disease symptoms, host associations, sporulation and potential spread of Botryosphaeria dothidea. The illustration depicts infection of the fungus on three hosts, as observed in South Africa, including native (Acacia karroo) and non‐native (Eucalyptus and Malus domestica) hosts. Typical disease symptoms include dieback, cankers and fruit rot. Fruiting structures containing sexual and/or asexual spores are often found associated with the disease symptoms and these are dispersed through wind or rain splash. Movement of latent or endophytically infected plant material to new regions poses a threat to quarantine systems. Given the broad host range and geographical distribution of B. dothidea, all three hosts could be replaced by various others, as could the geographical setting.
Our haplotype analysis (Fig. 1) suggests that the same B. dothidea genotypes occur in different regions of the world, implying that quarantine measures, as they are currently applied, are often futile. However, there is little understanding of the distribution and diversity of important traits, such as aggressiveness, on specific hosts. Efforts to reduce the spread of this potentially important pathogen, as well as other latent pathogens, consequently represent a sensible goal.
Population Genetics and Mating
Surprisingly, for a pathogen of such wide distribution and importance, few population genetic studies have been conducted on B. dothidea. Where these have been undertaken, they have mostly focused on disease outbreaks. In one Californian study, a low level of diversity was observed in isolates from Pistachio (Ma et al., 2001a). A subset of isolates collected from the same location in different years was identical, suggesting that the B. dothidea population in California is highly clonal (Ma et al., 2001a). The B. dothidea isolates from the non‐Pistachio hosts had a greater diversity than those collected from Pistachio trees in the same area. This suggests that the pathogen had most probably become established in the area long before Pistachio was commercialized in California, and that it is possibly native to the area (Ma et al., 2001a). An alternative explanation could be that the fungus was introduced into the area multiple times and/or from various sources. This latter scenario is possible in the light of the apparently extensive human‐mediated movement of the fungus on crops and other plants globally, but does not explain the lack of diversity on Pistachio (see the section on ‘Distribution and host associations’). Furthermore, B. dothidea has been found to occur on almond, olive and blackberry in California (Inderbitzin et al., 2010). Because the fungus is known to occur on almond, the almond orchards were thought to serve as sources of inoculum to the neighbouring vegetation, but the opposite situation could also have applied.
Piškur et al. (2011) studied the population diversity of an outbreak of B. dothidea on European hop hornbeam (Ostrya carpinifolia) in Slovenia and northern Italy, using amplified fragment length polymorphism (AFLP) markers. The population linked to the outbreak was highly diverse and did not appear to be the result of a recent introduction or a particularly pathogenic clone. Rather, the population resembled what would be expected from an established, possibly native, latent pathogen that had emerged to cause widespread dieback when extreme weather conditions (drought and heat) imposed undue stress on the plant communities in these regions. The diversity structure did not correlate with specific tissues or geographical location across Slovenia and Italy (Piškur et al., 2011). The possibility of B. dothidea being native to Europe is supported by its first isolation there in 1863 by Cesati and De Notaris, although this should be seen in the light of the majority of fungal taxonomy work at the time being performed in Europe.
Both sexual and asexual stages have been reported for B. dothidea (Slippers et al., 2004a), but it is unknown whether the species is homo‐ or heterothallic. The sexual stage is rarely encountered in nature, sometimes co‐occurring with the asexual stage, and it has never been recovered from culture. The more common asexual stage of B. dothidea is thus believed to play the most prominent role in dispersal and in structuring diversity in populations. An understanding of these dynamics is important in order to inform future studies on the population genetics, spread and adaptive ability of the pathogen.
Genomics research is beginning to shed light on questions relating to the reproductive strategies in the Botryosphaeriales. The first MAT gene sequence for a species in the Botryosphaeriales was described for Diplodia sapinea, a well‐known pathogen of Pinus spp. (Bihon et al., 2012, 2014; Swart and Wingfield, 1991). Because a sexual stage has never been observed in D. sapinea (Bihon et al., 2012, 2014), it was surprising to find the complete set of MAT genes necessary for heterothallic reproduction in complementary isolates. This finding, as well as the diversity of alleles at these loci, suggests a rarely observed, heterothallic sexual cycle (Bihon et al., 2014).
In the present study, the MAT genes of B. dothidea were compared with those of D. sapinea. As in D. sapinea, the MAT1‐1 genes in B. dothidea consisted of the MAT1‐1‐1 and MAT1‐1‐4 genes, whereas the MAT1‐2 genes consisted of the MAT 1‐2‐1 and MAT1‐2‐5 genes. However, unlike D. sapinea, both MAT1‐1 and MAT1‐2 genes were present in a single individual. Indeed, all four characterized MAT genes grouped together in the genome at a single locus (Fig. 4), indicating that B. dothidea has a homothallic mating system. The genes adjacent to the MAT genes on this locus are inverted when comparing B. dothidea with D. sapinea (Fig. 4).
Figure 4.
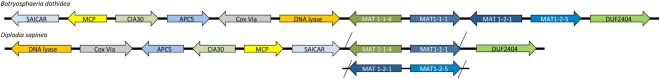
Comparison of the genomic architecture of the MAT locus and surrounding genes between Botryosphaeria dothidea and Diplodia sapinea. All four characterized MAT genes group together at a single locus, suggesting a homothallic mating‐type system in B. dothidea. Arrows represent gene order and orientation, but genes and intergenic regions are not to scale. Abbreviations: CIA30, complex I intermediate‐associated protein 30; Cox VIa, cytochrome C oxidase subunit VIa; DUF2404, putative integral membrane protein containing DUF2404 domain; MCP, mitochondrial carrier protein; SAICAR, SAICAR (phosphoribosylaminoimidazolesuccinocarboxamide) synthase.
Genomics of Host Infection and Future Research
The B. dothidea genome has been sequenced as part of the Assembling the Fungal Tree of Life Project and curated as part of the 1000 fungal genomes initiative of the Fungal Genomics Program (FGP) of the Department of Energy (DoE) Joint Genomics Institute (JGI; http://1000.fungalgenomes.org). It establishes an opportunity to investigate one of the most intriguing questions relating to the infection biology of B. dothidea, namely understanding the mechanisms of host infection without symptom development for a prolonged period.
In order to provide a foundation for future studies, we analysed potential pathogenic factors of the B. dothidea genome in a simple comparison with that of the most closely related genome that has been annotated and analysed, namely Zymoseptoria tritici (synonym: Mycosphaerella graminicola; Mycosphaerellaceae) (Goodwin et al., 2011). These pathogens both have a latent infection phase, although, for Z. tritici, it is typically much shorter and transitory in nature compared with B. dothidea. The genomes have similar sizes of ∼38 Mb (Z. tritici) and ∼34 Mb (B. dothidea). However, the genome structure of the two fungi appears to be different in key aspects related to host–pathogen interactions. For example, the carbohydrate active enzyme (CAZyme) repertoire of B. dothidea (n = 623) is expanded relative to that of Z. tritici (n = 440) (Table S2, see Supporting Information). For pathogens, CAZymes are key in plant interactions, as they are responsible for the breakdown of plant cell wall components, namely cellulose, hemicellulose, xylan, xyloglucan, mannan and pectin (Gan et al., 2013; Knogge, 1996).
The mechanism of penetration and avoidance of host reactions by endophytic fungi, such as B. dothidea, are poorly understood. It has been suggested that the reduced number of genes coding for cell wall‐degrading enzymes in Z. tritici could be an evolutionary adaptation linked to ‘stealth’ penetration during the latent, biotrophic phase of infection (Goodwin et al., 2011; Kema et al., 2008). The CAZyme repertoire of B. dothidea suggests that it uses a different mechanism for avoidance of host defences. The expanded CAZyme repertoire of B. dothidea can possibly be explained by the tissue it infects. In this regard, it is quite different from Z. tritici, which infects the leaves of its cereal hosts (Poaceae), whereas B. dothidea is mainly known to be a canker pathogen and a colonizer of woody tissue, although it has been observed in leaves.
Analysis of the genome showed that B. dothidea has three distinctive glycoside hydrolase families (GH27, GH33 and GH75), of which GH33 is not present in the Z. tritici genome. The GH33 hydrolase family consists of sialidases which hydrolyse the glycosidic linkages of terminal sialic residues in oligosaccharides. Sialidases can act as pathogenicity factors, which can assist in host adaptation by avoiding host recognition or by inhibiting host defence responses (Alviano et al., 2004). This is an example of a potential mechanism that could be investigated using these genomic data to understand how B. dothidea infects without resulting in symptoms and exists as an endophyte.
It is clear from this simple comparison of B. dothidea and Z. tritici that it is unreasonable to assume that latent pathogens with an endophytic life stage share a similar repertoire of genes. The comparison of processes across different host–pathogen systems will be needed to clarify whether there are common patterns to this process. As with other pathogen–host interactions (Teixeira et al., 2014), genomics and dual RNA sequencing approaches of both B. dothidea and its host(s) are required to provide insight into the mechanisms launched by B. dothidea that are countered by the host, and vice versa. In the case of B. dothidea, the genome sequence of its host E. grandis has recently become available (Myburg et al., 2014). This offers an opportunity to examine induced defence responses to B. dothidea (Naidoo et al., 2014) to further qualify its endophytic and latent lifestyle.
Conclusions
Considerable taxonomic confusion has characterized the studies of B. dothidea in the past. Although the epitypification of B. dothidea has aided in the clarification of the confusion and misperceptions, many still prevail, despite the number of confirmed host and geographical reports of the pathogen. The importance of B. dothidea as a pathogen has been underestimated and this is largely a result of its endophytic nature. Under adverse environmental conditions, the fungus is capable of making the switch from ‘friend’ to ‘foe’ with potentially serious consequences for the hosts that it infects. As a group, the Botryosphaeriaceae provide an apt illustration that the distinction between endophytes and pathogens is artificial. This is mainly because these definitions are based on different stages in a lifecycle rather than on distinct lifecycles.
The latent stages of fungal plant infection must be considered in terms of quarantine, and this is clearly not the case at present. This is particularly important because new species, genotypes or mating types can be introduced as endophytes. As quarantine systems generally rely on visual inspection, these potential pathogens are nearly impossible to detect. Human actions have clearly and unwittingly introduced new genotypes of tree pathogens into new areas and hosts on a massive scale (Wingfield et al., 2015), a problem vividly illustrated by the distribution of shared haplotypes of B. dothidea across the globe. Because many of the hosts of B. dothidea have not co‐evolved with the fungus, it can be speculated that at least some of these plants have resistance mechanisms or recognition systems that are not adapted to the fungus. For the same reasons, native populations of B. dothidea also pose a risk to introduced (non‐native) trees propagated for commercial purposes.
Climate change could affect stress‐associated canker‐forming and dieback pathogens of plants. The Botryosphaeriaceae have been highlighted as one of the possible threats in this regard (Desprez‐Loustau et al., 2006). As climate change affects environmental conditions, it equally affects both pathogens and their hosts (Ahanger et al., 2013). This could have an effect on the expansion of the geographical distribution and host range of some pathogens, because conditions may become favourable in areas in which the pathogen was not previously detected (Chakraborty, 2013; Sturrock et al., 2011). Because B. dothidea has a wide host range, any potential expansion in its distribution as a result of environmental changes could result in previously uninfected hosts being affected. Adverse conditions may also cause the host plant to become stressed, which, in turn, would afford latent pathogens, such as B. dothidea, the opportunity to cause disease (Sturrock et al., 2011).
The available genomic sequence for B. dothidea provides an important resource to gain an understanding of its endophytic and latent pathogenic nature. There is a particularly important opportunity for transcriptome analysis during infection to identify secreted molecules that induce the plant defence response. Such studies have the potential to inform general mechanisms of endophytic infections, which, despite their common occurrence in all plants, are very poorly understood.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Methods S1 Haplotype methodology.
Table S1 Country and host range of the 240 Botryosphaeria dothidea isolates used in the haplotype analysis.
Table S2 Carbohydrate active enzyme (CAZyme) comparisons of Botryosphaeria dothidea and Zymoseptoria tritici.
Acknowledgements
We thank the National Research Foundation (NRF) of South Africa and members of the Tree Protection Co‐operative Programme (TPCP) for financial assistance. This material is based on work supported by the National Science Foundation (DEB‐0732993 to J.W.S.). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. We acknowledge the following people for the photographs used in Fig. 2: Jolanda Roux (a), Fahimeh Jami (b), Carlos Rodas (c, d), Dusan Jurc and Barabara Piškur (e, f) and Themis Michailides (g, h). We further thank Glenda Brits for her assistance in the production of Fig. 3.
References
- Ahanger, R.A. , Bhat, H.A. , Bhat, T.A. , Ganie, S.A. , Lone, A.A. , Wani, I.A. , Ganai, S.A. , Haq, S. , Khan, O.A. , Junaid, J.M. and Bhat, T.A. (2013) Impact of climate change on plant diseases. Int. J. Mod. Plant Anim. Sci. 1, 105–115. [Google Scholar]
- Ahimera, N. , Gisler, S. , Morgan, D.P. and Michailides, T.J. (2004) Effects of single‐drop impactions and natural and simulated rains on the dispersal of Botryosphaeria dothidea conidia. Phytopathology, 94, 1189–1197. [DOI] [PubMed] [Google Scholar]
- Alviano, D.S. , Rodrigues, M.L. , Almeida, C.A. , Santos, A.L.S. , Couceiro, J.N.S.S. , Soares, R.M.A. , Travassos, L.R. and Alviano, C.S. (2004) Differential expression of sialylglycoconjugates and sialidase activity in distinct morphological stages of Fonsecaea pedrosoi . Arch. Microbiol. 181, 278–286. [DOI] [PubMed] [Google Scholar]
- Amponsah, N.T. , Jones, E.E. , Ridgway, H.J. and Jaspers, M.V. (2009) Rainwater dispersal of Botryosphaeria conidia from infected grapevines. N. Z. Plant Protect. 62, 228–233. [Google Scholar]
- von Arx, J.A. (1987) Plant pathogenic fungi. Beih. Nov. Hedwig. 87, 288. [Google Scholar]
- von Arx, J.A. and Müller, E. (1954) Die Gattungen der amerosporen Pyrenomyceten. Beitr. Kryptogamenfl. Schweiz. 11, 1–434. [Google Scholar]
- von Arx, J.A. and Müller, E. (1975) A re‐evaluation of the bitunicate Ascomycetes with keys to families and genera. Stud. Mycol. 9, 1–159. [Google Scholar]
- Barr, M.E. (1972) Preliminary studies on the Dothideales in temperate North America. Contr. Univ. Mich. Herb. 9, 523–638. [Google Scholar]
- Bihon, W. , Burgess, T. , Slippers, B. , Wingfield, M.J. and Wingfield, B.D. (2012) High level of genetic diversity and cryptic recombination is widespread in introduced Diplodia pinea populations. Australas. Plant Pathol. 41, 41–46. [Google Scholar]
- Bihon, W. , Wingfield, M.J. , Slippers, B. , Duong, T.A. and Wingfield, B.D. (2014) MAT gene idiomorphs suggest a heterothallic sexual cycle in a predominantly asexual and important pine pathogen. Fungal Genet. Biol. 62, 55–61. [DOI] [PubMed] [Google Scholar]
- Bostock, R.M. , Pye, M.F. and Roubtsova, T.V. (2014) Exploiting the nexus in abiotic and biotic stress perception and response. Annu. Rev. Phytopathol. 52, 517–549. [DOI] [PubMed] [Google Scholar]
- Brown, E.A. and Hendrix, F.F. (1981) Pathogenicity and histopathology of Botryosphaeria dothidea on apple stems. Phytopathology, 71, 375–379. [Google Scholar]
- Burgess, T.I. , Wingfield, B.D. and Wingfield, M.J. (2001) Comparison of genotypic diversity in native and introduced populations of Sphaeropsis sapinea isolated from Pinus radiata . Mycol. Res. 105, 1331–1339. [Google Scholar]
- Burgess, T.I. , Barber, P.A. and Hardy, G.E.S.J. (2005) Botryosphaeria spp. associated with eucalypts in Western Australia, including the description of Fusicoccum macroclavatum sp. nov. Australas. Plant Pathol. 34, 557–567. [Google Scholar]
- Cesati, V. and De Notaris, G. (1863) Schema di classificazione degli sferiacei italici aschigeri oiu’ o meno appartenenti al genera Sphaeria nell'antico significato attribuitoglide. Persoon. Comment Soc. Crittog. Ital. 1, 177–240. [Google Scholar]
- Chakraborty, S. (2013) Migrate or evolve: options for plant pathogens under climate change. Global Change Biol. 19, 1985–2000. [DOI] [PubMed] [Google Scholar]
- Crous, P.W. , Slippers, B. , Wingfield, M.J. , Rheeder, J. , Marasas, W.F.O. and Alan, J.L. (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 55, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington, J.H. , Priest, M.J. , Powney, R.A. and Cother, N.J. (2007) Diversity of Botryosphaeria species on horticultural plants in Victoria and New South Wales. Australas. Plant Pathol. 36, 157–159. [Google Scholar]
- Czemmel, S. , Galarneau, E.R. , Travadon, R. , McElrone, A.J. , Cramer, G.R. and Baumgartner, K. (2015) Genes expressed in grapevine leaves reveal latent wood infection by the fungal pathogen Neofusicoccum parvum . PLoS One, 10, e0121828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin, N. , White, D. , Hardy, G.E.St.J. and Burgess, T.I. (2010) The opportunistic pathogen, Neofusicoccum australe, is responsible for crown dieback of peppermint (Agonis flexuosa) in Western Australia. Australas. Plant Pathol. 39, 202–206. [Google Scholar]
- Damm, U. , Fourie, P.H. and Crous, P.W. (2007) Aplosporella prunicola, a novel species of anamorphic Botryosphaeriaceae. Fungal Divers. 27, 35–43. [Google Scholar]
- Denman, S. , Crous, P.W. , Taylor, J.E. , Kang, J.C. , Pascoe, I. and Wingfield, M.J. (2000) An overview of the taxonomic history of Botryosphaeria, and a re‐evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Stud. Mycol. 45, 129–140. [Google Scholar]
- De Notaris, G. (1863) Sferiacei Italici, pp. 82–84. Genova: Tipi del R.I. de’ Sordo‐Muti. [Google Scholar]
- Desprez‐Loustau, M.L. , Marcais, B. , Nageleisen, L.M. , Piou, D. and Vannini, A. (2006) Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 63, 597–612. [Google Scholar]
- Farr, D.F. and Rossman, A.Y. (2015) Systematic Mycology and Microbiology Laboratory Fungus‐Host Distributions Database. http://nt.ars-grin.gov/fungaldatabases/. Accessed 5 November 2016.
- Filipe, J.A.N. , Cobb, R.C. , Meentemeyer, R.K. , Lee, C.A. , Valachovic, Y.S. , Cook, A.R. , Rizzo, D.M. and Gilligan, C.A. (2012) Landscape epidemiology and control of pathogens with cryptic and long‐distance dispersal: sudden oak death in northern Californian forests. PLoS Comput. Biol. 8, e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, P. , Petrini, O. and Sutton, B. (1993) A comparative study of fungal endophytes in leaves, xylem and bark of Eucalyptus in Australia and England. Sydowia, 45, 338–345. [Google Scholar]
- Gan, P. , Ikeda, K. , Irieda, H. , Narusaka, M. , O'connell, R.J. , Narusaka, Y. , Takano, Y. , Kubo, Y. and Shirasu, K. (2013) Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 197, 1236–1249. [DOI] [PubMed] [Google Scholar]
- Gezahgne, A. , Roux, J. , Slippers, B. and Wingfield, M.J. (2004) Identification of the causal agent of Botryosphaeria stem canker in Ethiopian Eucalyptus plantations. S. Afr. J. Bot. 70, 241–248. [Google Scholar]
- Goodwin, S.B. , M'barek, S.B. , Dhillon, B. , Wittenberg, A.H.J. , Crane, C.F. , Hane, J.K. , Foster, A.J. , Van der Lee, T.A. , Grimwood, J. , Aerts, A. , Antoniw, J. , Bailey, A. , Bluhm, B. , Bowler, J. , Bristow, J. , van der Burgt, A. , Canto‐Canché, B. , Churchill, A.C. , Conde‐Ferràez, L. , Cools, H.J. , Coutinho, P.M. , Csukai, M. , Dehal, P. , De Wit, P. , Donzelli, B. , van de Geest, H.C. , van Ham, R.C. , Hammond‐Kosack, K.E. , Henrissat, B. , Kilian, A. , Kobayashi, A.K. , Koopmann, E. , Kourmpetis, Y. , Kuzniar, A. , Lindquist, E. , Lombard, V. , Maliepaard, C. , Martins, N. , Mehrabi, R. , Nap, J.P. , Ponomarenko, A. , Rudd, J.J. , Salamov, A. , Schmutz, J. , Schouten, H.J. , Shapiro, H. , Stergiopoulos, I. , Torriani, S.F. , Tu, H. , de Vries, R.P. , Waalwijk, C. , Ware, S.B. , Wiebenga, A. , Zwiers, L.H. , Oliver, R.P. , Grigoriev, I.V. and Kema, G.H. (2011) Finished genome of the fungal wheat pathogen Mycosphaerella graminicola reveals dispensome structure, chromosoma plasticity, and stealth pathogenesis. PLoS Genet. 7, e1002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso, F.M. and Granata, G. (2010) First report of Botryosphaeria dothidea associated with dieback of aspen (Populus tremula) in Italy. Plant Pathol. 59, 807. [Google Scholar]
- Hyde, K.D. and Soytong, K. (2008) The fungal endophyte dilemma. Fungal Divers. 33, 163–173. [Google Scholar]
- Inderbitzin, P. , Bostock, R.M. , Trouillas, F.P. and Michailides, T.J. (2010) A six locus phylogeny reveals high species diversity in Botryosphaeriaceae from California almond. Mycologia, 102, 1350–1368. [DOI] [PubMed] [Google Scholar]
- Jacobs, K.A. and Rehner, S.A. (1998) Comparison of cultural and morphological characters and ITS sequences in anamorphs of Botryosphaeria and related taxa. Mycologia, 90, 601–610. [Google Scholar]
- Jami, F. , Slippers, B. , Wingfield, M.J. and Gryzenhout, M. (2013a) Botryosphaeriaceae species overlap on four unrelated, native South African hosts. Fungal Biol. 118, 168–179. [DOI] [PubMed] [Google Scholar]
- Jami, F. , Slippers, B. , Wingfield, M.J. and Gryzenhout, M. (2013b) Greater Botryosphaeriaceae diversity in healthy than associated diseased Acacia karroo tree tissues. Australas. Plant Pathol. 42, 421–430. [Google Scholar]
- Jami, F. , Slippers, B. , Wingfield, M.J. , Loots, M.T. and Gryzenhout, M. (2015) Temporal and spatial variation of Botryosphaeriaceae associated with Acacia karroo in South Africa. Fungal Ecol. 15, 51–62. [Google Scholar]
- Johnson, G.I. , Mead, A.J. , Cooke, A.W. and Dean, J.R. (1992) Mango stem end rot pathogens – fruit infection by endophytic colonisation of the inflorescence and pedicel. Ann. Appl. Biol. 120, 225–234. [Google Scholar]
- Jurc, D. , Ogris, N. , Grebenc, T. and Kraigher, H. (2006) First report of Botryosphaeria dothidea causing bark dieback of European hop hornbeam in Slovenia. Plant Pathol. 55, 299. [Google Scholar]
- Jurick, W.M. II , Vico, I. , Gaskins, V.L. , Janisiewicz, W.J. and Peter, K.A. (2013) First report of Botryosphaeria dothidea causing white rot on apple fruit in Maryland. Plant Dis. 97, 999. [DOI] [PubMed] [Google Scholar]
- Kema, G.H.J. , van der Lee, T.A.J. , Mendes, O. , Verstappen, E.C.P. , Lankhorst, R.K. , Sandbrink, H. , van der Burgt, A. , Zwiers, L.H. , Csukai, M. and Waalwijk, C. (2008) Large‐scale gene discovery in the Septoria tritici blotch fungus Mycosphaerella graminicola with a focus on in planta expression. Mol. Plant–Microbe Interact. 21, 1249–1260. [DOI] [PubMed] [Google Scholar]
- Kim, K.W. , Park, E.W. and Ahn, K.K. (1999) Pre‐penetration behaviour of Botryosphaeria dothidea on apple fruits. Plant Pathol. J. 15, 223–227. [Google Scholar]
- Kim, K.W. , Park, E.W. and Kim, K.S. (2004) Glyoxysomal nature of microbodies complexed with lipid globules in Botryosphaeria dothidea . Biochem. Cell Biol. 94, 970–977. [DOI] [PubMed] [Google Scholar]
- Kirilenko, A.P. and Sedjo, R.A. (2007) Climate change impacts on forestry. PNAS, 104, 19 697–19 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knogge, W. (1996) Fungal infection of plants. Plant Cell, 8, 1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalle, C. , Micale, F. , Houston, T.D. , Camia, A. , Hiederer, R. , Lazar, C. , Conte, C. , Amatulli, G. and Genovese, G. (2009) Climate change in Europe. 3. Impact on agriculture and forestry. Agron. Sustain. Dev. 29, 433–446. [Google Scholar]
- Li, X. , Yan, J. , Kong, F. , Qiao, G. , Zhang, Z. and Wang, Z. (2010) Botryosphaeria dothidea causing canker of grapevine newly reported in China. Plant Pathol. 59, 1170. [Google Scholar]
- Ma, Z. , Boehm, E.W.A. , Luo, Y. and Michailides, T.J. (2001a) Population structure of Botryosphaeria dothidea from Pistachio and other hosts in California. Phytopathology, 91, 665–672. [DOI] [PubMed] [Google Scholar]
- Ma, Z. , Morgan, D.P. and Michailides, T.J. (2001b) Effects of water stress on Botryosphaeria blight of Pistachio caused by Botryosphaeria dothidea . Plant Dis. 85, 745–749. [DOI] [PubMed] [Google Scholar]
- Maresi, G. , Luchi, N. , Pinzani, P. , Pazzagli, M. and Capretti, P. (2007) Detection of Diplodia pinea in asymptomatic pine shoots and its relation to the normalized isolation index. For. Pathol. 37, 272–280. [Google Scholar]
- Mehl, J.W.M. , Slippers, B. , Roux, J. and Wingfield, M.J. (2013) Cankers and other diseases caused by the Botryosphaeriaceae In: Infectious Forest Diseases (Gonthier P. and Nicolotti G., eds), pp. 298–317. Boston, MA: CAB International. [Google Scholar]
- Michailides, T.J. (1991) Pathogenicity, distribution, sources of inoculum, and infection courts of Botryosphaeria dothidea on Pistachio . Phytopathology, 81, 566–573. [Google Scholar]
- Mohali, S. , Slippers, B. and Wingfield, M.J. (2007) Identification of Botryosphaeriaceae from Eucalyptus, Acacia and Pinus in Venezuela. Fungal Divers. 25, 103–125. [Google Scholar]
- Myburg, A.A. , Grattapaglia, D. , Tuskan, G.A. , Hellsten, U. , Hayes, R.D. , Grimwood, J. , Jenkins, J. , Lindquist, E. , Tice, H. , Bauer, D. , Goodstein, D.M. , Dubchak, I. , Poliakov, A. , Mizrachi, E. , Kullan, A.R. , Hussey, S.G. , Pinard, D. , van der Merwe, K. , Singh, P. , van Jaarsveld, I. , Silva‐Junior, O.B. , Togawa, R.C. , Pappas, M.R. , Faria, D.A. , Sansaloni, C.P. , Petroli, C.D. , Yang, X. , Ranjan, P. , Tschaplinski, T.J. , Ye, C.Y. , Li, T. , Sterck, L. , Vanneste, K. , Murat, F. , Soler, M. , Clemente, H.S. , Saidi, N. , Cassan‐Wang, H. , Dunand, C. , Hefer, C.A. , Bornberg‐Bauer, E. , Kersting, A.R. , Vining, K. , Amarasinghe, V. , Ranik, M. , Naithani, S. , Elser, J. , Boyd, A.E. , Liston, A. , Spatafora, J.W. , Dharmwardhana, P. , Raja, R. , Sullivan, C. , Romanel, E. , Alves‐Ferreira, M. , Külheim, C. , Foley, W. , Carocha, V. , Paiva, J. , Kudrna, D. , Brommonschenkel, S.H. , Pasquali, G. , Byrne, M. , Rigault, P. , Tibbits, J. , Spokevicius, A. , Jones, R.C. , Steane, D.A. , Vaillancourt, R.E. , Potts, B.M. , Joubert, F. , Barry, K. , Pappas, G.J. , Strauss, S.H. , Jaiswal, P. , Grima‐Pettenati, J. , Salse, J. , Van de Peer, Y. , Rokhsar, D.S. and Schmutz, J. (2014) The genome of Eucalyptus grandis . Nature, 510, 356–362. [DOI] [PubMed] [Google Scholar]
- Naidoo, S. , Külheim, C. , Zwart, L. , Mangwanda, R. , Oates, C.N. , Visser, E.A. , Wilken, F.E. , Mamni, T.B. and Myburg, A.A. (2014) Uncovering the defence responses of Eucalyptus to pests and pathogens in the genomics age. Tree Physiol. 34, 931–943. [DOI] [PubMed] [Google Scholar]
- van Niekerk, J.M. , Fourie, P.H. , Halleen, F. and Crous, P.W. (2006) Botryosphaeria spp. as grapevine trunk disease pathogens. Phytopathol. Mediterr. 45, 43–54. [Google Scholar]
- van Niekerk, J.M. , Calitz, F.J. , Halleen, F. and Fourie, P.H. (2010) Temporal spore dispersal patterns of grapevine trunk pathogens in South Africa. Eur. J. Plant Pathol. 127, 375–390. [Google Scholar]
- van Niekerk, J.M. , Strever, A.E. , Du Toit, G. , Halleen, F. and Fourie, P.H. (2011) Influence of water stress on Botryosphaeriaceae disease expression in grapevines. Phytopathol. Mediterr. 50, 151–165. [Google Scholar]
- Pavlic, D. , Slippers, B. , Coutinho, T.A. and Wingfield, M.J. (2007) Botryosphaeriaceae occurring on native Syzygium cordatum in South Africa and their potential threat to Eucalyptus . Plant Pathol. 56, 624–636. [Google Scholar]
- Pennycook, S.R. and Samuels, G.J. (1985) Botryosphaeria and Fusicoccum species associated with ripe fruit rot of Actinidia deliciosa (kiwifruit) in New Zealand. Mycotaxon, 24, 445–458. [Google Scholar]
- Perez, C.A. , Wingfield, M.J. , Slippers, B. , Altier, N.A. and Blanchette, R.A. (2010) Endophytic and canker‐associated Botryosphaeriaceae occurring on non‐native Eucalyptus and native Myrtaceae trees in Uruguay. Fungal Divers. 41, 53–69. [Google Scholar]
- Petrini, O. and Fisher, P.J. (1988) A comparative study of fungal endophytes in xylem and whole stem of Pinus sylvestris and Fagus sylvatica . T. Brit. Mycol. Soc. 91, 233–238. [Google Scholar]
- Phillips, A.J.L. , Fonseca, F. , Povoa, V. , Castilho, R. and Nolasco, G. (2002) A reassessment of the anamorphic fungus Fusicoccum luteum and description of its telemorph. Sydowia, 54, 59–77. [Google Scholar]
- Phillips, A.J.L. , Rumbos, I.C. , Alves, A. and Correia, A. (2005) Morphology and phylogeny of Botryosphaeria dothidea causing fruit rot of olives. Mycopathologia, 159, 433–439. [DOI] [PubMed] [Google Scholar]
- Phillips, A.J.L. , Alves, A. , Pennycook, S.R. , Johnston, P.R. , Ramaley, A. , Akulov, A. and Crous, P.W. (2008) Resolving the phylogenetic and taxonomic status of dark‐spored teleomorph genera in the Botryosphaeriaceae. Persoonia, 21, 29–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, A.J.L. , Alves, A. , Abdollahzadeh, J. , Slippers, B. , Wingfield, M.J. , Groenewald, J.Z. and Crous, P.W. (2013) The Botryosphaeriaceae: genera and species known from culture. Stud. Mycol. 76, 51–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piškur, B. , Pavlic, D. , Slippers, B. , Ogris, N. , Maresi, G. , Wingfield, M.J. and Jurc, J. (2011) Diversity and pathogenicity of Botryosphaeriaceae on declining Ostrya carpinifolia in Slovenia and Italy following extreme weather conditions. Eur. J. Forest Res. 130, 235–249. [Google Scholar]
- Pusey, P.L. (1989) Availability and dispersal of ascospores and conidia of Botryosphaeria in peach orchards. Phytopathology, 79, 635–639. [Google Scholar]
- Qiu, Y. , Savocchia, S. , Steel, C.C. and Ash, G.J. (2008) Botryosphaeria dothidea associated with grapevine trunk disease in south‐eastern Australia. Australas. Plant Pathol. 37, 482–484. [Google Scholar]
- Ragazzi, A. , Moricca, S. and Dellavalle, I. (1999) Water stress and the development of cankers by Diplodia mutila on Quercus robur . J. Phytopathol. 147, 425–428. [Google Scholar]
- Rai, M. and Agarkar, G. (2014) Plant–fungal interactions: what triggers the fungi to switch among lifestyles? Crit. Rev. Microbiol. 42 , 428–438. [DOI] [PubMed] [Google Scholar]
- Sakalidis, M.L. , Hardy, G.E.S.J. and Burgess, T.I. (2011a) Endophytes as potential pathogens of the baobab species Adansonia gregorii: a focus on the Botryosphaeriaceae. Fungal Ecol. 4, 1–14. [Google Scholar]
- Sakalidis, M.L. , Ray, J.D. , Lanoiselet, V. , Hardy, G.E.S.J. and Burgess, T.I. (2011b) Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley Region of Western Australia. Eur. J. Plant Pathol. 130, 379–391. [Google Scholar]
- Schulz, B. and Boyle, C. (2005) The endophytic continuum. Mycol. Res. 109, 661–686. [DOI] [PubMed] [Google Scholar]
- Slippers, B. and Wingfield, M.J. (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol. Rev. 21, 90–106. [Google Scholar]
- Slippers, B. , Crous, P.W. , Denman, S. , Coutinho, T.A. , Wingfield, B.D. and Wingfield, M.J. (2004a) Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea . Mycologia, 96, 83–101. [PubMed] [Google Scholar]
- Slippers, B. , Fourie, G. , Crous, P.W. , Coutinho, T.A. , Wingfield, B.D. , Carnegie, J. , and Wingfield, M.J. (2004b) Speciation and distribution of Botryosphaeria spp. on native and introduced Eucalyptus trees in Australia and South Africa. Stud. Mycol. 50, 343–358. [Google Scholar]
- Slippers, B. , Johnson, G.I. , Crous, P.W. , Coutinho, T.A. , Wingfield, B.D. and Wingfield, M.J. (2005) Phylogenetic and morphological re‐evaluation of the Botryosphaeria species causing diseases of Mangifera indica . Mycologia, 97, 99–110. [DOI] [PubMed] [Google Scholar]
- Slippers, B. , Burgess, T. , Pavlic, D. , Ahumada, R. , Maleme, H. , Mohali, S. , Rodas, C. and Wingfield, M.J. (2009) A diverse assemblage of Botryosphaeriaceae infect Eucalyptus in native and non‐native environments. South. For. 71, 101–110. [Google Scholar]
- Slippers, B. , Boissin, E. , Phillips, A.J.L. , Groenewald, J.Z. , Lombard, L. , Wingfield, M.J. , Postma, A. , Burgess, T. and Crous, P.W. (2013) Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Stud. Mycol. 76, 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, H. (2001) Biology of Botryosphaeria dothidea and Sphaeropsis sapinea as endophytes of Eucalypts and Pines in South Africa (Unpublished doctoral thesis). University of the Free State, Bloemfontein, South Africa. [Google Scholar]
- Smith, H. , Kemp, G.H.J. and Wingfield, M.J. (1994) Canker and die‐back of Eucalyptus in South Africa caused by Botryosphaeria dothidea . Plant Pathol. 43, 1031–1034. [Google Scholar]
- Smith, H. , Wingfield, M.J. , Crous, P.W. and Coutinho, T.A. (1996a) Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. S. Afr. J. Bot. 62, 86–88. [Google Scholar]
- Smith, H. , Wingfield, M.J. and Petrini, O. (1996b) Botryosphaeria dothidea endophytic in Eucalyptus grandis and Eucalyptus nitens in South Africa. For. Ecol. Manag. 89, 189–195. [Google Scholar]
- Stanosz, G.R. , Blodgett, J.T. , Smith, D.R. and Krger, E.L. (2001) Water stress and Sphaeropsis sapinea as a latent pathogen of red pine seedlings. New Phytol. 149, 531–538. [DOI] [PubMed] [Google Scholar]
- Stanosz, G.R. , Smith, D.R. and Albers, J.S. (2005) Surveys for asymptomatic persistence of Sphaeropsis sapinea on or in stems of red pine seedlings from seven Great Lakes region nurseries. For. Pathol. 35, 233–244. [Google Scholar]
- Stergiopoulos, I. and Gordon, T.R. (2014) Cryptic fungal infections: the hidden agenda of plant pathogens. Front. Plant Sci. 5, 506–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock, R.N. , Frankel, S.J. , Brown, A.V. , Hennon, P.E. , Kleiejunas, J.T. , Lewis, K.J. , Worrall, J.J. and Woods, A.J. (2011) Climate change and forest diseases. Plant Pathol. 60, 133–149. [Google Scholar]
- Sutton, T.B. (1981) Production and dispersal of ascospores and conidia by Physalospora obtusa and Botryosphaeria dothidea in apple orchards. Phytopathology, 71, 584–589. [Google Scholar]
- Swart, W.J. and Wingfield, M.J. (1991) Biology and control of Sphaeropsis sapinea on Pinus species in South Africa. Plant Dis. 75, 761–766. [Google Scholar]
- Tang, W. , Ding, Z. , Zhou, Z.Q. , Wang, Y.Z. and Guo, L.Y. (2012) Phylogenetic and pathogenic analyses show that the causal agent of apple ring rot in China is Botryosphaeria dothidea . Plant Dis. 96, 486–496. [DOI] [PubMed] [Google Scholar]
- Teixeira, P.J.P.L. , de Toledo Thomazella, D.P. , Reis, O. , do Prado, P.F.V. , do Rio, M.C.S. , Fiorin, G.L. , José, J. , Costa, G.G. , Negri, V.A. , Mondego, J.M. , Mieczkowski, P. and Pereira, G.A. (2014) High‐resolution transcript profiling of the atypical biotrophic interaction between Theobroma cacao and the fungal pathogen Moniliophthora perniciosa . Plant Cell, 26, 4245–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Úrbez‐Torres, J.R. , Battany, M. , Bettiga, L.J. , Gispert, C. , McGourty, G. , Roncoroni, J. , Smith, R.J. , Verdegaal, P. and Gubler, W.D. (2010) Botryosphaeriaceae species spore‐trapping studies in California vineyards. Plant Dis. 94, 717–724. [DOI] [PubMed] [Google Scholar]
- Van Der Linde, J.A. , Six, D.L. , Wingfield, M.J. and Roux, J. (2011) Lasiodiplodia species associated with dying Euphorbia ingens in South Africa. South. For. 73, 165–173. [Google Scholar]
- Van Der Linde, J.A. , Roux, J. , Wingfield, M.J. and Six, D.L. (2012) Die‐off of giant Euphorbia trees in South Africa: symptoms and relationships to climate. S. Afr. J. Bot. 83, 172–185. [Google Scholar]
- Wang, F. , Zhao, L. and Li, G. (2011) Identification and characterisation of Botryosphaeria spp. causing gummosis of peach trees in Hubei Province, central China. Plant Dis. 95, 1378–1384. [DOI] [PubMed] [Google Scholar]
- Wingfield, M.J. , Brockerhoff, E.G. , Wingfield, B.D. and Slippers, B. (2015) Planted forest health: the need for a global strategy. Science, 349, 832–836. [DOI] [PubMed] [Google Scholar]
- Xu, C. , Wang, C. , Ju, L. , Zhang, R. , Biggs, A.R. , Tanaka, E. , Li, B. and Sun, G. (2015) Multiple locus genealogies and phenotypic characters reappraise the causal agents of apple ring rot in China. Fungal Divers. 71, 215–231. [Google Scholar]
- Zhang, Z.X. , Deng, D.F. , Qi, W.J. , Fan, S.S. , Cao, Y. , Huang, J.G. and Liu, Z.Y. (2013) Botryosphaeria dothidea, the causal agent of a new canker disease of Tatarian dogwood (Cornus alba) in China. Australas. Plant Pathol. 42, 113–119. [Google Scholar]
- Zhou, S. and Stanosz, G.R. (2001) Relationships among Botryosphaeria species and associated anamorphic fungi inferred from the analyses of ITS and 5.8S rDNA sequences. Mycologia, 93, 516–527. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Methods S1 Haplotype methodology.
Table S1 Country and host range of the 240 Botryosphaeria dothidea isolates used in the haplotype analysis.
Table S2 Carbohydrate active enzyme (CAZyme) comparisons of Botryosphaeria dothidea and Zymoseptoria tritici.


