Summary
The type VI protein secretion system (T6SS) is essential for the virulence of several Gram‐negative bacteria. In this study, we identified a T6SS gene cluster in Acidovorax citrulli, a plant‐pathogenic bacterium that causes bacterial fruit blotch (BFB) of cucurbits. One T6SS cluster, of approximately 25 kb in length and comprising 17 genes, was found in the A. citrulli AAC00‐1 genome. Seventeen A. citrulli mutants were generated, each with a deletion of a single T6SS core gene. There were significant differences in BFB seed‐to‐seedling transmission between wild‐type A. citrulli strain, xjl12, and ΔvasD, ΔimpK, ΔimpJ and ΔimpF mutants (71.71%, 9.83%, 8.41%, 7.15% and 5.99% BFB disease index, respectively). In addition, we observed that these four mutants were reduced in melon seed colonization and biofilm formation; however, they were not affected in virulence when infiltrated into melon seedling tissues. There were no significant differences in BFB seed‐to‐seedling transmission, melon tissue colonization and biofilm formation between xjl12 and the other 13 T6SS mutants. Overall, our results indicate that T6SS plays a role in seed‐to‐seedling transmission of BFB on melon.
Keywords: Acidovorax citrulli, biofilm formation, seed‐to‐seedling transmission, T6SS
Introduction
Acidovorax citrulli is the causal agent of bacterial fruit blotch (BFB) (Schaad et al., 1978, 2008; Willems et al., 1992), a serious threat to cucurbit (mainly watermelon and melon) production worldwide. The bacterium is seed borne (Hopkins and Thompson, 2002), and seed transmission and infested/infected seeds represent the most important primary inoculum sources for BFB outbreaks. Hence, for effective BFB management, it is critical to limit seed‐to‐seedling transmission of A. citrulli, which requires an accurate understanding of the host–pathogen interactions underlying this phenomenon. In an effort to better understand A. citrulli host–pathogen interactions, Bahar et al. (2009) demonstrated that type IV pili and polar flagella play an important role in virulence, twitching motility and biofilm formation. Johnson et al. (2009) showed that the A. citrulli type III secretion system (T3SS) and, in particular, the hrcC gene that encodes the type III pilus protein, are important for pathogenicity, but are not required for the colonization of germinating seeds during the early stages of seed‐to‐seedling transmission of BFB. The role of T3SS in A. citrulli pathogenicity and the ability to induce a hypersensitive response (HR) was also confirmed by the characterization of hrcV mutants (Bahar and Burdman, 2010). In contrast, Johnson et al. (2009) reported that the type II secretion system (T2SS) contributed to watermelon seed colonization during seed‐to‐seedling transmission of BFB.
Secreted effector proteins play a central role in the interaction between bacterial phytopathogens and their host plants (Kostakioti et al., 2005; Mougous et al., 2006). To date, six different secretion systems have been found in Gram‐negative pathogenic bacteria (Mougous et al., 2006; Pukatzki et al., 2006). Currently, the mechanism of the type VI secretion system (T6SS) and the functions of the secreted effectors are poorly understood. In previous studies, T6SS has been shown to enhance the adaptability of bacteria to environmental conditions (Weber et al., 2009), to mediate pathogenicity to host cells (Pukatzki et al., 2006; Suarez et al., 2008; Zheng and Leung, 2007) and to affect other bacterial functions, including biofilm formation (Aschtgen et al., 2010), modulation of quorum sensing and stress response in Vibrio anguillarum (Weber et al., 2009). These functions promote the establishment of commensalistic or mutualistic relationships between bacteria and eukaryotes (Bernard et al., 2010; Chow and Mazmanian, 2010; Jani and Cotter, 2010). In addition, Huddleston (2011) reported that the T6SS effector proteins, Tse1 and Tse3, degrade the peptidoglycan of other bacteria, conferring a competitive advantage to Pseudomonas aeruginosa.
Studies of human and animal pathogens have contributed most to our current understanding of the structure and function of T6SS. However, T6SSs are also prevalent in the genomes of plant pathogens and other plant‐associated bacteria, such as Agrobacterium tumefaciens, Pectobacterium atrosepticum and Pseudomonas syringae (Mattinen et al., 2007; Records and Gross, 2010; Wu et al., 2008). Wang (2008) showed that the deletion of both T6SS gene clusters in P. syringae pv. tomato DC3000 reduced the bacterium's ability to colonize Nicotiana benthamiana leaves and induce bacterial speck symptoms on tomato leaves. When either the T6SS‐II or T6SS‐III gene cluster was deleted separately, the disease severity and bacterial colonization of plant tissues were reduced. In contrast, deletion of the valine–glycine repeat protein G1 (vgrG1) or vgrG2, two core T6SS genes, had no effect on disease development on tomato or N. benthamiana.
In another study, microarray analysis showed that the T6SS of Ag. tumefaciens was induced by mildly acidic conditions, such as those encountered in plant tissues and in the rhizosphere (Yuan et al., 2008). It was shown that deletion of haemolysin co‐regulated protein (hcp), a core T6SS gene, from Ag. tumefaciens resulted in reduced tumorigenesis on potato tuber slices (Wu et al., 2008). Similarly, Mattinen et al. (2007) showed that the T6SS of Pe. atrosepticum was induced by potato tuber extracts. Using transcriptome profiling, Liu et al. (2008) showed that the T6SS of Pe. atrosepticum was regulated by quorum sensing.
Bingle et al. (2008) identified the putative cluster of T6SS genes in the A. citrulli genome. In their report, the A. citrulli T6SS comprised one gene cluster, but lacked a vgrG homologue, which raised doubts about the functionality of the system. To date, no experimental evidence has been provided to support the role of T6SS in A. citrulli pathogenicity or in seed‐to‐seedling transmission of the BFB pathogen. In this study, a 17‐gene cluster encoding signature T6SS proteins in A. citrulli was identified in the AAC00‐1 genome sequence. Seventeen core T6SS genes were deleted individually to generate mutants in the A. citrulli xjl12 background. Using these mutants, the objective of this work was to investigate the role of the A. citrulli T6SS in the seed‐to‐seedling transmission of BFB in melon.
Results
Acidovorax citrulli has a T6SS cluster
Based on bioinformatics analysis of genome sequences for P. aeruginosa, Vibrio cholerae, Edwardsiella tarda, Salmonella typhimurium and Rhizobium leguminosarum, one T6SS cluster was found in the AAC00‐1 genome. This 25‐kb cluster comprised 17 core genes (GenBank Accession Number: NC_008752) (Fig. 1) including homologues of hcp (Aave_1465) to clpB (Aave_1482) (Table 1).
Figure 1.
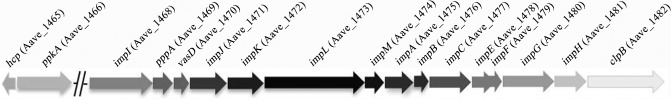
Arrangement of Acidovorax citrulli AAC00‐1 type VI secretion system gene cluster indicating the relative position of gene homologues.
Table 1.
Type VI secretion system genes in the Acidovorax citrulli AAC00‐1 genome and homologues in P seudomonas aeruginosa, V ibrio cholerae, E dwarsilella tarda and R hizobium leguminosarum
| Protein name | AAC00‐1 locus tag | Pseudomonas aeruginosa | Identity/homology (%) | Vibrio cholerae | Identity/homology (%) | Edwardsiella tarda | Identity/homology (%) | Salmonella typhimurium | Identity/homology (%) | Rhizobium leguminosarum | Identity/homology (%) | Conserved domain |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hcp | Aave_1465 | PA0085/Hcp1 | 67/162 | VCA0117/VasH | 29/140 | EvpC | 47/162 | SciK | 64/160 | Hcp | 47/162 | DUF796 |
| SciM | 64/160 | |||||||||||
| PpkA | Aave_1466 | PA0074/PpkA | — | — | — | — | PKc | |||||
| ImpI | Aave_1468 | PA0081/Fha1 | 90/306 | VCA0112/VasC | 27/84 | — | — | ImpI | 0 | FHA | ||
| PppA | Aave_1469 | PA0075/PppA | 70/208 | — | — | — | — | PP2Cc | ||||
| VasD | Aave_1470 | PA0080/Lip1 | 48/113 | VCA0113/VasD | 34/128 | EvpL | 0 | SciN | 43/111 | — | COG3521 | |
| ImpJ | Aave_1471 | PA0079/HsiJ1 | 208/443 | VCA0114/VasE | 178/444 | EvpM | 116/477 | SciO | 166/446 | ImpJ | 192/447 | DUF876 |
| ImpK | Aave_1472 | PA0078/DotU1 | 174/382 | VCA0115/VasF | 72/224 | EvpN | 35/108 | SciP | 123/346 | ImpK | 141/412 | DUF2077 |
| ImpL | Aave_1473 | PA0077/IcmF1 | 445/1142 | VCA020/VasK | 312/1218 | EvpO | 106/366 | SciS | 408/1241 | ImpL | 382/1193 | ImcF,DUF1215 |
| ImpM | Aave_1474 | PA0076/PppB | 44/139 | — | — | — | ImpM | 41/91 | DUF2094 | |||
| ImpA | Aave_1475 | PA0082/HsiA1 | 120/359 | VCA0119/VasJ | 15/60 | EvpK | 17/51 | SciA | 104/341 | ImpA | 80/362 | ImpA family |
| ImpB | Aave_1476 | PA0083/HsiB1 | 119/158 | VCA0107 | 0 | EvpA | 66/157 | SciH | 117/170 | ImpB | 64/152 | DUF770 |
| ImpC | Aave_1477 | PA0084/HsiC1 | 382/498 | VCA0108 | 184/430 | EvpB | 232/441 | SciI | 378/503 | ImpC | 243/490 | COG3517/DUF877 |
| ImpE | Aave_1478 | PA0086/HsiE1 | 113/249 | — | — | SciE | 93/241 | ImpE | 65/236 | ImpE | ||
| ImpF | Aave_1479 | PA0087/HsiF1 | 71/161 | VCA0109 | 0 | EvpE | 43/152 | SciD | 62/153 | ImpF | 21/54 | DUF1316 |
| ImpG | Aave_1480 | PA0088/HsiG1 | 306/625 | VCA0110/VasA | 179/638 | EvpF | 219/629 | SciV | 273/628 | ImpG | 214/624 | DUF879 |
| ImpH | Aave_1481 | PA0089/HsiH1 | 146/301 | VCA0111/VasB | 81/277 | EvpG | 120/329 | SciB | 114/290 | ImpH | 97/322 | DUF1305 |
| ClpB | Aave_1482 | PA0090/ClpV1 | 186/463 | VCA0116/VasG | 277/684 | EvpH | 290/659 | SciG | 270/503 | ClpB | 305/725 | ClpN, AAA ATPase |
Effect of T6SS on seed‐to‐seedling transmission of BFB
Melon seeds (n = 1000) were inoculated with ∼1 × 106 colony‐forming units (CFU)/mL of A. citrulli xjl12 and individual T6SS protein mutants and planted in sterile test cups (three seeds per cup). BFB seed‐to‐seedling transmission percentage and disease severity were observed daily for 12 days after planting. Wild‐type (WT) strain xjl12 caused a mean BFB index of 71.71% by 12 days after planting, whereas mutants ΔvasD, ΔimpJ, ΔimpK and ΔimpF caused disease indices of 9.83%, 8.41%, 7.15% and 5.99%, respectively (Fig. 2). In all cases, the differences between the WT strain xjl12 and strains ΔvasD, ΔimpJ, ΔimpK and ΔimpF were statistically significant (P < 0.05). The complemented strains ΔvasDcomp, ΔimpJcomp, ΔimpKcomp and ΔimpFcomp induced mean BFB indices of 66.39%, 64.47%, 67.19% and 59.19%, respectively, by 12 days after planting. There were no significant (P > 0.05) differences in the BFB index between WT strain xjl12 and the complemented strains, including ΔvasDcomp, ΔimpJcomp and ΔimpKcomp. The disease index of the complemented strain ΔimpFcomp was only partially rescued. The differences in disease indices between the WT strain and the other 13 T6SS mutants were not significant (P > 0.05).
Figure 2.
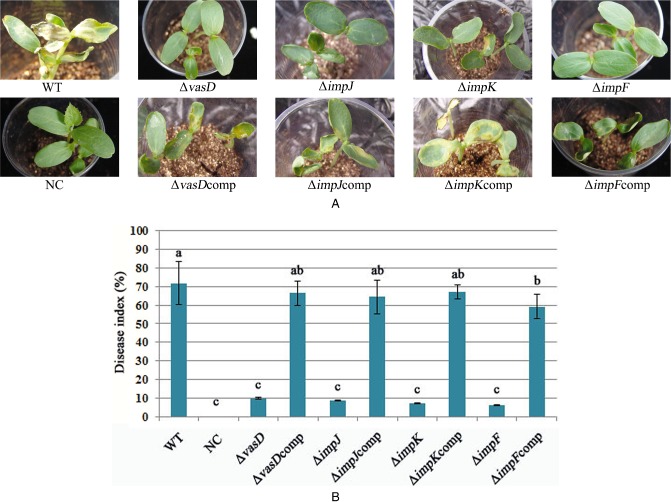
Effects of type VI secretion system (T6SS) on seed‐to‐seedling transmission of Acidovorax citrulli. Melon seeds (cv. Huanghou, n = 1000) were inoculated with ∼1 × 106 colony‐forming units (CFU)/mL of wild‐type or T6SS mutants, and three seeds were planted per sterile test cup. Seedlings were evaluated 12 days after planting and disease severity was evaluated based on a 0–5 scale. The experiment was repeated three times. WT, wild‐type strain of A. citrulli, xjl12; NC, negative control, double‐distilled H2O (ddH2O); ΔvasD, vasD gene deletion mutant of A. citrulli; ΔvasDcomp, complemented strain of ΔvasD; ΔimpJ, impJ gene deletion mutant of A. citrulli; ΔimpJcomp, complemented strain of ΔimpJ; ΔimpK, impK gene deletion mutant of A. citrulli; ΔimpKcomp, complemented strain of ΔimpK; ΔimpF, impF gene deletion mutant of A. citrulli; ΔimpFcomp, complemented strain of ΔimpF. Vertical bars represent standard errors of the means. Different letters above the data bars indicate a significant difference between the wild‐type strain and T6SS mutants, complemented strains or negative control (P < 0.05, t‐test).
Effect of T6SS on A. citrulli colonization of melon seeds during germination
The reduction in melon seed‐to‐seedling transmission of BFB by the A. citrulli single T6SS gene mutants ΔvasD, ΔimpJ, ΔimpK and ΔimpF prompted the investigation of the role of T6SS in A. citrulli colonization of melon seeds during germination. The role of T6SS in A. citrulli colonization of melon seeds during the early stages of germination was determined by measuring the bacterial populations on artificially inoculated seeds during the initial 96 h of seed germination. Individual seeds were infiltrated with A. citrulli xjl12 (WT) or individual T6SS mutants, and bacterial populations per seed were estimated at 24‐h intervals for 96 h. The populations of all strains increased on germinating melon seeds by 96 h after planting (Fig. 3). By 48 h after planting, the mean populations of xjl12, ΔvasD, ΔimpJ, ΔimpK and ΔimpF were ∼8.91 × 106, ∼3.80 × 104, ∼1.35 × 104, ∼5.25 × 105 and ∼4.57 × 104 CFU/g of seed, respectively. By 96 h after planting, the mean populations of xjl12, ΔvasD, ΔimpJ, ΔimpK and ΔimpF were ∼4.27 × 109, ∼4.17 × 105, ∼1.29 × 105, ∼3.24 × 106 and ∼1.05 × 105 CFU/g of seed, respectively. Based on the analysis of AUPDC (area under population dynamics curve) data, there were significant differences between the abilities of ΔvasD, ΔimpJ, ΔimpK and ΔimpF to colonize seed relative to the WT strain (P < 0.05). There was no significant difference in the colonization of melon seed between xjl12, ΔvasDcomp, ΔimpJcomp and ΔimpKcomp (P > 0.05). However, populations of ΔimpFcomp remained at ∼2.51 × 106 CFU/g of seed for 48 h and ∼1.74 × 108 CFU/g of seed for 96 h. This suggested that the colonization of melon seed by ΔimpFcomp was only partially rescued.
Figure 3.
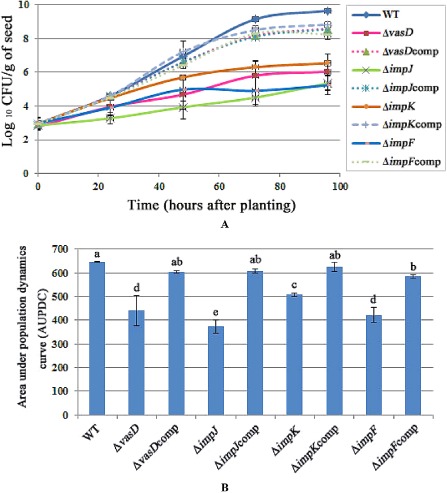
(A) Effect of type VI secretion system (T6SS) on colonization of germinating melon seeds by Acidovorax citrulli. (B) Bar chart of area under population dynamics curve (AUPDC) calculated for seed infiltrated with 103 colony‐forming units (CFU) of wild‐type or T6SS mutants. WT, wild‐type strain of A. citrulli, xjl12; ΔvasD, vasD gene deletion mutant of A. citrulli; ΔvasDcomp, complemented strain of ΔvasD; ΔimpJ, impJ gene deletion mutant of A. citrulli; ΔimpJcomp, complemented strain of ΔimpJ; ΔimpK, impK gene deletion mutant of A. citrulli; ΔimpKcomp, complemented strain of ΔimpK; ΔimpF, impF gene deletion mutant of A. citrulli; ΔimpFcomp, complemented strain of ΔimpF. Vertical bars represent standard errors of the means. Different letters above the data bars indicate a significant difference between the wild‐type strain and mutants, complemented strains or negative control (P < 0.05, t‐test).
T6SS is involved in A. citrulli biofilm formation
The quantification of the biofilm confirmed that A. citrulli xjl12 (WT) produced significantly (P < 0.05) more biofilm than the T6SS mutants ΔvasD, ΔimpJ, ΔimpK and ΔimpF. Optical density (OD) values for stained biofilms for xjl12 (WT), ΔvasD, ΔimpJ, ΔimpK and ΔimpF were 2.20, 0.29, 0.10, 1.07 and 0.12, respectively (Fig. 4). In addition, biofilm formation by ΔimpK was significantly (P < 0.05) higher than that of ΔimpJ and ΔimpF (Fig. 4). There was no significant difference in biofilm formation between the WT strain xjl12, ΔvasDcomp, ΔimpJcomp and ΔimpKcomp (P > 0.05). In contrast, complementation of strain ΔimpF only partially rescued WT biofilm formation. There were no significant (P > 0.05) differences in biofilm formation between the other single T6SS gene mutants and the WT strain.
Figure 4.
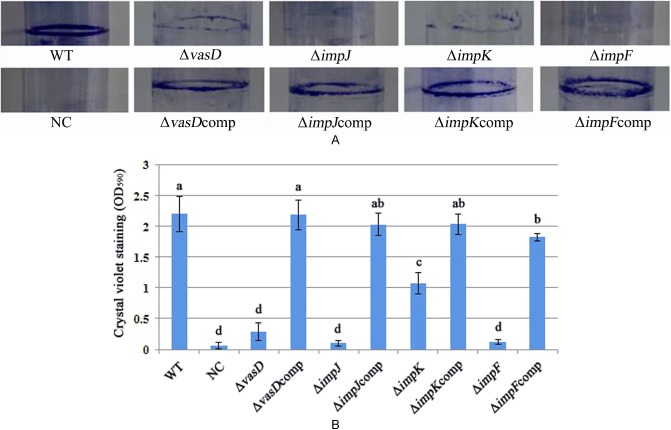
Effect of type VI secretion system (T6SS) on Acidovorax citrulli biofilm formation. (A) Images of biofilms formed in glass tubes after staining with methyl violet. (B) Biofilm was quantified after washing the methyl violet stain with ethanol and measuring the optical density at 590 nm. The experiment was repeated three times. WT, wild‐type strain of A. citrulli, xjl12; NC, negative control, double‐distilled H2O (ddH2O); ΔvasD, vasD gene deletion mutant of A. citrulli; ΔvasDcomp, complemented strain of ΔvasD; ΔimpJ, impJ gene deletion mutant of A. citrulli; ΔimpJcomp, complemented strain of ΔimpJ; ΔimpK, impK gene deletion mutant of A. citrulli; ΔimpKcomp, complemented strain of ΔimpK; ΔimpF, impF gene deletion mutant of A. citrulli; ΔimpFcomp, complemented strain of ΔimpF. Vertical bars represent standard errors of the means. Different letters above the data bars indicate a significant difference between the wild‐type strain and T6SS mutants, complemented T6SS mutant strains or negative control (P < 0.05, t‐test).
Discussion
Bacterial pathogens use a range of different secretion systems to deliver virulence factors into host cells. Recently, a novel secretion system was discovered in several Gram‐negative bacterial pathogens and was designated as T6SS (Das and Chaudhuri, 2003). T6SSs have been shown to play a role in the virulence of a range of animal and plant pathogens, including V. cholerae, P. aeruginosa, Ed. tarda, Salmonella enterica serovar gallinarum, avian pathogenic Escherichia coli, Ag. tumefaciens, Pe. atrosepticum, Xanthomanas oryzae and P. syringae (Aschtgen et al., 2010; Blondel et al., 2010; Filloux et al., 2008; Hsu et al., 2009; Mattinen et al., 2007; Records and Gross, 2010; Shrivastava and Mande, 2008; Tseng et al., 2009; Wu et al., 2008; Zheng and Leung, 2007). However, the exact mechanism of T6SS and the contribution of T6SS effectors to virulence remain to be elucidated. T6SSs are typically encoded by clusters of 12 to more than 20 genes; however, the minimal number of genes needed to produce a functional apparatus is 13 (Boyer et al., 2009). In V. cholerae and P. aeruginosa, the T6SS exports haemolysin co‐regulated proteins (Hcp) and valine–glycine repeat (Vgr) proteins (Mougous et al., 2006; Pukatzki et al., 2006). These proteins have been proposed to act as effectors associated with cytotoxicity in some in vitro models (Mougous et al., 2006; Pukatzki et al., 2006). Hcp1, which forms hexameric rings that assemble into nanotubes in vitro, plays an important role in the pathogenicity of P. aeruginosa (Mougous et al., 2006). In the current study, however, deletion of hcp did not reduce A. citrulli virulence or the seed‐to‐seedling transmission of BFB on melon. The A. citrulli mutant xjl12Δhcp colonized melon cotyledons and induced BFB symptoms at WT levels. It is possible that the Hcp protein requires an inducible signal for it to be active, as observed with T6SS in Ag. tumefaciens (Wu et al., 2012). More detailed studies are needed to prove that hcp is secreted by A. citrulli.
In the current study, a 17‐gene cluster encoding signature T6SS proteins in A. citrulli was identified in the AAC00‐1 genome. Seventeen core T6SS genes were deleted individually to generate mutants in an A. citrulli xjl12 background. Of these mutants, four (ΔvasD, ΔimpK, ΔimpJ and ΔimpF) were impaired in seed‐to‐seedling transmission of BFB in melon and in biofilm formation. Our results showed that the sciN‐like vasD (Aave_1470) gene is required for biofilm formation of A. citrulli on glass surfaces. Aschtgen et al. (2008) showed that SciN is an outer membrane lipoprotein that is exposed to the periplasmic space of enteroaggregative E. coli, as revealed by the inhibition of its processing by globomycin and in vivo labelling with [3H]palmitic acid. Lipoproteins have been identified in some secretion systems, including T2SS, T3SS and T4SS, as having a large number of subunits (Allaoui et al., 1992; D'Enfert and Pugsley, 1989; Fernandez et al., 1996; Schuch and Maurelli, 2001; Shevchik and Condemine, 1998). In all of these secretion systems, lipoproteins are essential components and have been shown to be involved in secretion machine assembly. Aschtgen et al. (2008) also demonstrated that SciN is critical for biofilm formation. In addition, mutations in the T6SS gene cluster have been found to be involved in biofilm formation for V. parahaemolyticus and P. aeruginosa (Enos‐Berlage et al., 2005; Sauer et al., 2002; Southey‐Pillig et al., 2005).
Another A. citrulli T6SS protein, ImpJ (Aave_1471), is homologous (40%) to the V. cholera TssK‐like VCA0114 protein, which has been identified as a trimeric cytoplasmic protein that interacts with components of phage‐like and membrane anchoring complexes of the T6SS (Zoued et al., 2013). VCA0114 has also been shown to be critical for a functional T6SS (Zheng et al., 2011). The current study suggests that ImpJ is important for A. citrulli biofilm formation and seed‐to‐seedling transmission, even though its specific functions are unknown.
A V. cholerae T6SS protein identified as VCA0109 has sequence homology to T4 gp25, which forms part of bacteriophage tail baseplates (Leiman et al., 2009). In addition, the VCA0109‐like protein, HsiF1, has been shown recently to localize to the cytoplasm of P. aeruginosa (Lossi et al., 2011). Furthermore, VCA0109 has been shown to be essential for sheath biogenesis in V. cholerae (Basler et al., 2012). Although detailed characterization studies must be conducted, our results show that the VCA0109‐like ImpF (Aave_1479) is required for A. citrulli biofilm formation on glass surfaces and for seed‐to‐seedling transmission. Interestingly, to our knowledge, this is the first report to associate T6SS with seed‐to‐seedling transmission of bacterial plant pathogens. In this study, complementation of strain ΔimpF only partially rescued the WT strain, which might be due to the expression vector contains impF extrachromosomally and impF forms part of bacteriophage tail baseplates. Future detailed characterization of A. citrulli T6SS should provide important information to understand how it is assembled and how it functions, particularly with regard to how it contributes to seed‐to‐seedling transmission of BFB.
We observed that the A. citrulli AAC00‐1 T6SS cluster lacks a vgrG homologue. However, by bioinformatics analysis, 12 genes encoding putative VgrG proteins (Aave_0481, Aave_3347, Aave_0497, Aave_2840, Aave_3486, Aave_2127, Aave_4009, Aave_2047, Aave_2735, Aave_0241, Aave_3752 and Aave_3783) were observed (Table S2, see Supporting Information) throughout the genome. VgrG proteins are structurally similar to T4 bacteriophage tail spike proteins, gp27 and gp5 (Pukatzki et al., 2007). The existence of multiple copies of putative vgrG genes in the AAC00‐1 genome is interesting, as it is unclear why a bacterium might have so many copies of this gene. Many bacteria have several copies of vgrG (Hachani et al., 2011; Smits et al., 2010). However, the presence of these putative vgrG homologues may explain why A. citrulli has a functional T6SS, even though the T6SS cluster does not include vgrG. The functionality of these homologues will be explored in future studies.
In many pathogens, biofilm formation is crucial for disease development (Merz et al., 1999). Type IV pili and polar flagella have been shown to be involved in biofilm formation of A. citrulli using microfluidic flow chambers (Bahar et al., 2009). Preliminary observations indicating that A. citrulli grows as an epiphytic, non‐pathogen on seeds during the initial stages of seed germination prompted us to investigate the role of T6SS in the putative switch to pathogenic growth on emerging melon seedlings. Based on our observations that BFB seed‐to‐seedling transmission and biofilm formation are reduced for A. citrulli T6SS mutants ΔvasD, ΔimpK, ΔimpJ and ΔimpF, it is possible that biofilm formation might be critical for A. citrulli colonization of melon seed tissues during the early stages of germination. A similar phenomenon has been reported for the avian pathogenic E. coli (De Pace et al., 2011) based on the observation that an icmF mutant displayed decreased adherence to and invasion of epithelial cells, as well as decreased intra‐macrophage survival. In addition, the icmF mutant was defective in biofilm formation on abiotic surfaces (De Pace et al., 2011). It is possible that this reduced ability to form a biofilm leads to a decrease in the disease index in the seed‐to‐seedling BFB transmission assay. Overall, our results provide evidence that A. citrulli has a functional T6SS for biofilm formation and seed‐to‐seedling transmission of BFB on melon. To our knowledge, this is the first report of the effect of T6SS on seed‐to‐seedling transmission of a phytopathogenic bacterium.
Experimental Procedures
Bacterial cultures, media and inoculum preparation
The bacterial strains and plasmids used in this study are described in Table 2. Acidovorax citrulli was grown at 28 °C on Luria–Bertani (LB) agar or broth (Sambrook et al., 1989) and E. coli was grown on LB agar or broth at 37 °C. Minimal medium (MMX) (Daniels et al., 1984) was routinely used as minimal medium and, when required, the culture media were supplemented with the following antibiotics: kanamycin (Km), 50 mg/mL; rifampicin (Rif), 100 mg/mL; gentamicin (Gm), 50 mg/mL.
Table 2.
Strains and plasmids used in this study. All complemented strains were generated in the A cidovorax citrulli strain xjl12 background
| Bacteria, plasmids | Relevant characteristics* | Source |
|---|---|---|
| Acidovorax citrulli | ||
| xjl12 | Wild‐type, RifR | Laboratory collection |
| Δhcp | hcp knockout mutant | This study |
| ΔppkA | ppkA knockout mutant | This study |
| ΔimpI | impI knockout mutant | This study |
| ΔpppA | pppA knockout mutant | This study |
| ΔvasD | vasD knockout mutant | This study |
| ΔvasDcomp | ΔvasD complemented with intact vasD gene, KmR | This study |
| ΔimpJ | impJ knockout mutant | This study |
| ΔimpJcomp | ΔimpJ complemented with intact impJ gene, KmR | This study |
| ΔimpK | impK knockout mutant | This study |
| ΔimpKcomp | ΔimpK complemented with intact impK gene, KmR | This study |
| ΔimpL | impL knockout mutant | This study |
| ΔimpM | impM knockout mutant | This study |
| ΔimpA | impA knockout mutant | This study |
| ΔimpB | impB knockout mutant | This study |
| ΔimpC | impC knockout mutant | This study |
| ΔimpE | impE knockout mutant | This study |
| ΔimpF | impF knockout mutant | This study |
| ΔimpFcomp | ΔimpF complemented with intact impF gene, KmR | This study |
| ΔimpG | impG knockout mutant | This study |
| ΔimpH | impH knockout mutant | This study |
| ΔclpB | clpB knockout mutant | This study |
| Escherichia coli | ||
| DH5α(λpir) | Φ80 lacZΔM15,Δ(lacZYA‐argF)U169. recA1, endA1.thi‐1 | TaKaRa, Dalian, China |
| S17‐1(λpir) | Λpir pro hsdR, recA | Simon et al. (1983) |
| Plasmids | ||
| pMD19‐T | ‘TA’ cloning vector | TaKaRa |
| pEX18GM | Suicide vector with a sacB gene, GmR | Hoang et al. (1998) |
| pUFR034 | Broad‐host‐range cloning vector, IncW, KmR, Mob +, Mob(p), lacZalpha, | DeFeyter et al. (1990) |
*RifR, GmR and KmR indicate resistance to rifamycin, gentamicin and kanamycin, respectively.
Generation of T6SS mutants of A. citrulli
Strain descriptions are given in Table 2. Deletion mutants of T6SS genes were generated by the sacB‐based allele replacement method, as described previously (Johnson et al., 2011; Zou et al., 2011). Deletion mutants were confirmed by polymerase chain reaction (PCR) amplification using primers flanking the genes of interest. Primer sequences used for mutant construction are listed in Table S1 (see Supporting Information). The resulting constructs were introduced into E. coli S17‐1(λpir) for use in biparental mating with A. citrulli strain xjl12. For complementation, the coding regions of these genes were amplified by PCR using the primers listed in Table S1, and cloned into the expression vector pUFR034. The resulting constructs were transferred into the corresponding mutants by biparental mating. blastn, blastx, blastp sequence homologies and conserved protein domain analyses were performed using the National Center for Biotechnology Information (NCBI) blast. Protein sequences were analysed using the NCBI database.
Effect of T6SS on seed‐to‐seedling transmission of BFB
To determine the effect of T6SS on seed‐to‐seedling transmission of BFB, melon seeds (cv. Huanghou, n = 1000 seeds) were inoculated by immersion in 200 mL of a cell suspension containing ∼1 × 106 CFU/mL of A. citrulli strain xjl12 and each T6SS deletion mutant for 2 h. Seeds treated in a similar manner with sterile double‐distilled H2O served as a negative control. After inoculation, seeds were air dried at room temperature for 24 h and then planted, three seeds per sterile test cup (Wuhao, Nanjing, China), in soil‐less potting medium (Xingnong, Zhenjiang, China) saturated with deionized water. Seeds were incubated at 100% relative humidity and 28 °C for 12 days. The proportion of seedlings displaying BFB symptoms was recorded daily and the experiment was repeated three times. Each seedling was also evaluated for BFB severity daily based on the disease index, as described previously (Araujo et al., 2005), with the following modifications. The disease severity scale ranged from ‘0’ to ‘5’: 0, no symptoms; 1, necrotic lesions on approximately 25% of the cotyledons; 2, necrotic lesions on approximately 50% of the cotyledons; 3, necrotic lesions on approximately 75% of the cotyledons; 4, necrotic lesions on approximately 100% of the cotyledons; and 5, total death of the seedling. The disease index (DI) was calculated each day based on the following formula:
where A is the disease class (0, 1, 2, 3, 4 or 5) and B is the number of plants showing that disease class per treatment.
Effect of T6SS on A. citrulli colonization of germinating melon seeds
The role of T6SS in A. citrulli seed colonization during the early stages of seed germination was determined by infiltrating melon seeds (cv. Huanghou) with ∼1 × 103 CFU of each T6SS mutant strain or double‐distilled H2O as a negative control, as described previously (Walcott et al., 2006). After inoculation, melon seeds (n = 25) were incubated in transparent plastic boxes (Wuhao) on moist blotter paper (Whatman, England, UK) at 28 °C with 100% relative humidity. Three seeds were collected at 0, 24, 48, 72 and 96 h after planting, and bacterial populations were estimated by macerating each seed separately in 900 μL of double‐distilled H2O in a sterile microcentrifuge tube; 100 μL aliquots of appropriate 10‐fold serial dilutions of each seed homogenate were spread onto LB agar plates with appropriate antibiotics and incubated for 48–96 h at 28 °C. Subsequently, the A. citrulli colonies were counted. The experiment was repeated five times and plots of log10 bacterial CFU/seed against time were used to calculate AUPDC values (Bjarko and Line, 1988) as follows:
where Yi is the log10 bacterial population at the ith observation, Xi is the time in hours at the ith observation and n is the total number of observations. These data were then used to determine the significance of the effects of T6SS on A. citrulli colonization of germinating melon seeds.
Effect of T6SS on A. citrulli biofilm formation
Biofilm assays were performed on T6SS mutants of A. citrulli using glass tubes, as described by Aschtgen et al. (2010), with the following modifications. Briefly, cell suspensions (overnight broth cultures adjusted to OD590 = 1.0) of A. citrulli strain xjl12 and each T6SS mutant were diluted 1:99 in LB broth in glass tubes and incubated at 28 °C for 48 h without agitation. Cultures were then poured out and tubes were rinsed three times with sterile double‐distilled H2O. Following fixation at 80 °C for 20 min, biofilms were stained with 1% crystal violet for 45 min and then washed three times with double‐distilled H2O. For quantification, biofilms were dissolved in 3 mL of 95% ethanol for 2 h, and OD590 of the stained suspension was measured using a spectrophotometer (Biophotometer, Eppendorf, Hambug, Germany).
Statistical analyses
All analyses were conducted using SPSS 14.0 (SPSS Inc., Chicago, IL, USA). The t‐test (P = 0.05) was used to determine significant differences in disease severity, bacterial growth (AUPDC) and biofilm formation among WT and T6SS mutants of A. citrulli.
Supporting information
Table S1 Oligonucleotide primers used in this study.
Table S2 Valine–glycine repeat G (VgrG) proteins in the Acidovorax citrulli AAC00‐1 genome and homologues in Vibrio cholerae, Edwardsiella tarda and Rhizobium leguminosarum.
Acknowledgements
This research was supported by the Research and Development Special Fund for Public Welfare Industry (No. 201003066) and Science and Technology Support Xinjiang Project (No. 201191133).
References
- Allaoui, A. , Sansonetti, P.J. and Parsot, C. (1992) MxiJ, a lipoprotein involved in secretion of Shigella Ipa invasins, is homologous to YscJ, a secretion factor of the Yersinia Yop proteins. J. Bacteriol. 174, 7661–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo, D.V. , Mariano, R.L.R. and Michereff, S.J. (2005) Metodos de inoculacao de Acidovorax avenae subsp. citrulli em melao. Summ. Phytopathol. 31, 69–73. [Google Scholar]
- Aschtgen, M.S. , Bernard, C.S. , De Bentzmann, S. , Lloubes, R. and Cascales, E. (2008) SciN is an outer membrane lipoprotein required for type VI secretion in enteroaggregative Escherichia coli . J. Bacteriol. 190, 7523–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschtgen, M.S. , Gavioli, M. , Dessen, A. , Lloubès, R. and Cascales, E. (2010) The SciZ protein anchors the enteroaggregative Escherichia coli Type VI secretion system to the cell wall. Mol. Microbiol. 75, 886–899. [DOI] [PubMed] [Google Scholar]
- Bahar, O. and Burdman, S. (2010) Bacterial fruit blotch: a threat to the cucurbit industry. Israel J. Plant Sci. 58, 19–31. [Google Scholar]
- Bahar, O. , Goffer, T. and Burdman, S. (2009) Type IV pili are required for virulence, twitching motility, and biofilm formation of Acidovorax avenae subsp. citrulli . MPMI, 22, 909–920. [DOI] [PubMed] [Google Scholar]
- Basler, M. , Pilhofer, M. , Henderson, G.P. , Jensen, G.J. and Mekalanos, J.J. (2012) Type VI secretion requires a dynamic contractile phage tail‐like structure. Nature, 483, 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, C.S. , Brunet, Y.R. , Gueguen, E. and Cascales, E. (2010) Nooks and crannies in type VI secretion regulation. J. Bacteriol. 192, 3850–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle, L.E. , Bailey, C.M. and Pallen, M.J. (2008) Type VI secretion: a beginner's guide. Curr. Opin. Microbiol. 11, 3–8. [DOI] [PubMed] [Google Scholar]
- Bjarko, M.E. and Line, R.F. (1988) Heritability and number of genes controlling leaf rust resistance in four cultivars of wheat. Phytopathology, 78, 457–461. [Google Scholar]
- Blondel, C.J. , Yang, H.J. , Castro, B. , Chiang, S. , Toro, C.S. , Zaldívar, M. , Contreras, I. , Andrews‐Polymenis, H.L. and Santiviago, C.A. (2010) Contribution of the type VI secretion system encoded in SPI‐19 to chicken colonization by Salmonella enterica serotypes Gallinarum and Enteritidis. PLoS ONE, 5, e11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, F. , Fichant, G. , Berthod, J. , Vandenbrouck, Y. and Attree, I. (2009) Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics, 10, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, J. and Mazmanian, S.K. (2010) A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe, 7, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Enfert, C. and Pugsley, A. (1989) Klebsiella pneumoniae pulS gene encodes an outer membrane lipoprotein required for pullulanase secretion. J. Bacteriol. 171, 3673–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, M.J. , Barber, C.E. , Turner, P.C. , Sawczyc, M.K. , Byrde, R.J. and Fielding, A.H. (1984) Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J. 3, 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, S. and Chaudhuri, K. (2003) Identification of a unique IAHP (IcmF associated homologous proteins) cluster in V. cholerae and other proteobacteria through in silico analysis. In Silico Biol. 3, 287–300. [PubMed] [Google Scholar]
- De Pace, F. , Boldrin de Paiva, J. , Nakazato, G. , Lancellotti, M. , Sircili, M.P. , Guedes Stehling, E. , Dias da Silveira, W. and Sperandio, V. (2011) Characterization of IcmF of the type VI secretion system in an avian pathogenic Escherichia coli (APEC) strain. Microbiology, 157, 2954–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeyter, R. , Kado, C.I. and Gabriel, D.W. (1990) Small, stable shuttle vectors for use in Xanthomonas . Gene, 88, 65–72. [DOI] [PubMed] [Google Scholar]
- Enos‐Berlage, J. , Guvener, Z. , Keenan, C. and McCarter, L.L. (2005) Genetic determinants of biofilm development of opaque and translucent V. parahaemolyticus . Mol. Microbiol. 55, 1160–1182. [DOI] [PubMed] [Google Scholar]
- Fernandez, D. , Dang, T.A.T. , Spudich, G.M. , Zhou, X.R. , Berger, B.B. and Christie, P.J. (1996) The Agrobacterium tumefaciens virB7 gene product, a proposed component of the T‐complex transport apparatus, is a membrane associated lipoprotein exposed at the periplasmic surface. J. Bacteriol. 178, 3156–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux, A. , Hachani, A. and Bleves, S. (2008) The bacterial type VI secretion machine: yet another player for protein transport across membranes. Microbiology, 154, 1570–1583. [DOI] [PubMed] [Google Scholar]
- Hachani, A. , Lossi, N.S. , Hamilton, A. , Jones, C. , Bleves, S. , Albesa‐Jove, D. and Filloux, A. (2011) Type VI secretion system in Pseudomonas aeruginosa: secretion and multimerization of VgrG proteins. J. Biol. Chem. 286, 12 317–12 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang, T.T. , Karkhoff‐Schweizer, R.R. , Kutchma, A.J. and Schweizer, H.P. (1998) A broad‐host‐range Flp‐FRT recombination system for site‐specific excision of chromosomally‐located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene, 212, 77–86. [DOI] [PubMed] [Google Scholar]
- Hopkins, D.L. and Thompson, C.M. (2002) Seed transmission of Acidovorax avenae subsp. citrulli in cucurbits. HorScience, 37, 924–926. [Google Scholar]
- Hsu, F. , Schwarz, S. and Mougous, J.D. (2009) TagR promotes PpkA‐catalysed type VI secretion activation in Pseudomonas aeruginosa . Mol. Microbiol. 72, 1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddleston, J.E. (2011) Bacterial secretion: contact killing by Pseudomonas . Nat. Rev. Microbiol. 9, 632. [DOI] [PubMed] [Google Scholar]
- Jani, A.J. and Cotter, P.A. (2010) Type secretion: not just for pathogenesis anymore. Cell Host Microbe, 8, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K.L. , Minsavage, G.V. and Walcott, R.R. (2009) Effect of type III and type II secretion on Acidovorax avenae subsp. citrulli colonization of watermelon seed and seedling tissue. Phytopathology, 99, S59. [Google Scholar]
- Johnson, K.L. , Minsavage, G.V. , Le, T. , Jones, J.B. and Walcott, R.R. (2011) Efficacy of nonpathogenic Acidovorax citrulli strain as a biocontrol seed treatment for bacterial fruit blotch of cucurbits. Plant Dis. 95, 697–704. [DOI] [PubMed] [Google Scholar]
- Kostakioti, M. , Newman, C.L. , Thanassi, D.G. and Stathopoulos, C. (2005) Mechanisms of protein export across the bacterial outer membrane. J. Bacteriol. 187, 4306–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiman, P.G. , Baslerb, M. , Ramagopal, U.A. , Bonanno, J.B. , Sauder, J.M. , Pukatzki, S. , Burley, S.K. , Almo, S.C. and Mekalanos, J.J. (2009) Type VI secretion apparatus and phage tail‐associated protein complexes share a common evolutionary origin. Proc. Acad. Natl. Sci. USA, 106, 4154–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Coulthurst, S.J. , Pritchard, L. , Hedley, P.E. , Ravensdale, M. , Humphris, S. , Burr, T. , Takle, G. , Brurberg, M.B. , Birch, P.R. , Salmond, G.P. and Toth, I.K. (2008) Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum . PLoS Pathog. 4, e1000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossi, N.S. , Dajani, R. , Freemont, P. and Filloux, A. (2011) Structure–function analysis of HsiF, a gp25‐like component of the type VI secretion system, in Pseudomonas aeruginosa . Microbiology, 157, 3292–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattinen, L. , Nissinen, R. , Riipi, T. , Kalkkinen, N. and Pirhonen, M. (2007) Host‐extract induced changes in the secretome of the plant pathogenic bacterium Pectobacterium atrosepticum . Proteomics, 7, 3527–3537. [DOI] [PubMed] [Google Scholar]
- Merz, J.A. , Enns, C.A. and So, M. (1999) Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol. Microbiol. 32, 1316–1332. [DOI] [PubMed] [Google Scholar]
- Mougous, J.D. , Cuff, M.E. , Raunser, S. , Shen, A. , Zhou, M. , Gifford, C.A. , Goodman, A.L. , Joachimiak, G. , Ordoñez, C.L. , Lory, S. , Walz, T. , Joachimiak, A. and Mekalanos, J. (2006) A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science, 312, 1526–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki, S. , Ma, A.T. , Sturtevant, D. , Krastins, B. , Sarracino, D. , Nelson, W.C. , Heidelberg, J.F. and Mekalanos, J.J. (2006) Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA, 103, 1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki, S. , Ma, A.T. , Revel, A.T. , Sturtevant, D. and Mekalanos, J.J. (2007) Type VI secretion system translocates a phage tail spike‐like protein into target cells where it cross‐links actin. Proc. Natl. Acad. Sci. USA, 104, 15 508–15 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Records, A.R. and Gross, D.C. (2010) Sensor kinases RetS and LadS regulate Pseudomonas syringae type VI secretion and virulence factors. J. Bacteriol. 192, 3584–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T.A. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Sauer, K. , Camper, A.K. , Ehrlich, G.D. , Costerton, J.W. and Davies, D.G. (2002) Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184, 1140–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaad, N.W. , Sowell, G., Jr , Goth, R.W. , Colwell, R.R. and Webb, R.E. (1978) Pseudomonas pseudoalcaligenes subsp. citrulli subsp. nov. Int. J. Syst. Bacteriol. 28, 117–125. [Google Scholar]
- Schaad, N.W. , Postnikova, E. , Sechler, A. , Claflin, L.E. , Vidaver, A.K. , Jones, J.B. , Agarkova, I. , Ignatov, A. , Dickstein, E. and Ramundo, B.A. (2008) Reclassification of subspecies of Acidovorax avenae as A. avenae (Manns 1905) emend, A. cattleyae (Pavarino, 1911) comb. nov., A. citurlli (Schaad et al., 1978) comb. nov., and proposal of A. oryzae sp. nov. Syst. Appl. Microbiol. 31, 434–446. [DOI] [PubMed] [Google Scholar]
- Schuch, R. and Maurelli, A.T. (2001) MxiM and MxiJ, base elements of the Mxi‐Spa type III secretion system of Shigella, interact with and stabilize the MxiD secretin in the cell envelope. J. Bacteriol. 183, 6991–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchik, V.E. and Condemine, G. (1998) Functional characterization of the Erwinia chrysanthemi OutS protein, an element of a type II secretion system. Microbiology, 144, 3219–3228. [DOI] [PubMed] [Google Scholar]
- Shrivastava, S. and Mande, S.S. (2008) Identification and functional characterization of gene components of type VI secretion system in bacterial genomes. PLoS ONE, 3, e2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, R. , Priefer, U. and Pühler, A. (1983) ) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology, 1, 784–790. [Google Scholar]
- Smits, T.H.M. , Rezzonico, F. , Kamber, T. , Blom, J. , Goesmann, A. , Frey, J.E. and Duffy, B. (2010) Complete genome sequence of the fire blight pathogen Erwinia amylovora CFBP 1430 and comparison to other Erwinia spp. Mol. Plant–Microbe Interact. 23, 384–393. [DOI] [PubMed] [Google Scholar]
- Southey‐Pillig, C. , Davies, D. and Sauer, K. (2005) Characterization of temporal protein production in Pseudomonas aeruginosa biofilms. J. Bacteriol. 187, 8114–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez, G. , Sierra, J.C. , Sha, J. , Wang, S. , Erova, T.E. , Fadl, A.A. , Foltz, S.M. , Horneman, A.J. and Chopra, A.K. (2008) Molecular characterization of a functional type VI secretion system from a clinical isolate of Aeromonas hydrophila . Microb. Pathog. 44, 344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng, T.T. , Tyler, B.M. and Setubal, J.C. (2009) Protein secretion systems in bacterial–host associations, and their description in the Gene Ontology. BMC Microbiol. 9, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott, R.R. , Castro, A.C. , Fessehaie, A. and Ling, K. (2006) Progress towards commercial‐scale detection of A. avenae subsp. citrulli in cucurbit seed using immunomagnetic separation and the polymerase chain reaction. Seed Sci Technol. 34, 101–116. [Google Scholar]
- Wang, Y.Y. (2008) Characterization of type six secretion systems in Pseudomonas syringae pv. tomato DC3000. MSc Thesis, National University of Taiwan.
- Weber, B. , Hasic, M. , Chen, C. , Wai, S.N. and Milton, D.L. (2009) Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum . Environ. Microbiol. 11, 3018–3028. [DOI] [PubMed] [Google Scholar]
- Willems, A. , Goor, M. , Thielemans, S. , Gillis, M. , Kersters, K. and De Ley, J. (1992) Transfer of several phytopathogenic Pseudomonas species to Acidovorax as Acidovorax avenae subsp. avenae subsp. nov., comb. nov., Acidovorax avenae subsp. citrulli, Acidovorax avenae subsp. cattleyae, and Acidovorax konjaci . Int. J. Syst. Bacteriol. 42, 107–119. [DOI] [PubMed] [Google Scholar]
- Wu, C.F. , Lin, J.S. , Shaw, G.C. and Lai, E.M. (2012) Acid‐induced type VI secretion system is regulated by ExoR‐ChvG/ChvI signaling cascade in Agrobacterium tumefaciens . PLoS Pathog. 8, e1002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H.Y. , Chung, P.C. , Shih, H.W. , Wen, S.R. and Lai, E.M. (2008) Secretome analysis uncovers an Hcp‐family protein secreted via a type VI secretion system in Agrobacterium tumefaciens . J. Bacteriol. 190, 2841–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, Z.C. , Liu, P. , Saenkham, P. , Kerr, K. and Nester, E.W. (2008) Transcriptome profiling and functional analysis of Agrobacterium tumefaciens reveals a general conserved response to acidic conditions (pH 5.5) and a complex acid‐mediated signaling involved in Agrobacterium–plant interactions. J. Bacteriol. 190, 494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. and Leung, K.Y. (2007) Dissection of a type VI secretion system in Edwardsiella tarda . Mol. Microbiol. 66, 1192–1206. [DOI] [PubMed] [Google Scholar]
- Zheng, J. , Ho, B. and Mekalanos, J.J. (2011) Genetic analysis of anti‐amoebae and antibacterial activities of the type VI secretion system in Vibrio cholerae . PLoS ONE, 6, e23876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, L.F. , Li, Y.R. and Chen, G.Y. (2011) A non‐marker mutagenesis strategy to generate poly‐hrp gene mutants in the rice pathogen Xanthomonas oryzae pv. oryzicola . Agric. Sci. China, 10, 1139–1150. [Google Scholar]
- Zoued, A. , Durand, E. , Bebeacua, C. , Brunet, Y.R. , Douzi, B. , Cambillau, C. , Cascales, E. and Journet, L. (2013) TssK is a trimeric cytoplasmic protein interacting with components of both phage‐like and membrane anchoring complexes of the Type VI secretion system. J. Biol. Chem. 288, 27 031–27 041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Oligonucleotide primers used in this study.
Table S2 Valine–glycine repeat G (VgrG) proteins in the Acidovorax citrulli AAC00‐1 genome and homologues in Vibrio cholerae, Edwardsiella tarda and Rhizobium leguminosarum.


