Summary
The tomato [Solanum lycopersicum (Sl)] phosphatidylinositol‐phospholipase C (PI‐PLC) gene family is composed of six members, named SlPLC1 to SlPLC6, differentially regulated on pathogen attack. We have previously shown that the fungal elicitor xylanase induces a raise of SlPLC2 and SlPLC5 transcripts and that SlPLC2, but not SlPLC5, is required for xylanase‐induced expression of defense‐related genes. In this work we studied the role of SlPLC2 in the interaction between tomato and the necrotrophic fungus Botrytis cinerea. Inoculation of tomato leaves with B. cinerea increases SlPLC2 transcript levels. We knocked‐down the expression of SlPLC2 by virus‐induced gene silencing and plant defense responses were analyzed upon B. cinerea inoculation. SlPLC2 silenced plants developed smaller necrotic lesions concomitantly with less proliferation of the fungus. Silencing of SlPLC2 resulted as well in a reduced production of reactive oxygen species. Upon B. cinerea inoculation, transcript levels of the salicylic acid (SA)‐defense pathway marker gene SlPR1a were diminished in SlPLC2 silenced plants compared to non‐silenced infected plants, while transcripts of the jasmonic acid (JA)‐defense gene markers Proteinase Inhibitor I and II (SlPI‐I and SlPI‐II) were increased. This implies that SlPLC2 participates in plant susceptibility to B. cinerea.
Keywords: cell death, defense gene, jasmonic acid, necrotrophic fungus, phospholipid signalling, reactive oxygen species, salicylic acid
Introduction
Phosphatidylinositol‐phospholipase C (PI‐PLC) catalyzes the hydrolysis of the signal molecules phosphatidylinositol 4‐phosphate (PI4P) and phosphatidylinositol (4,5) bisphosphate [PI(4,5)P2] to produce inositol 2‐phosphate (IP2) or inositol 3‐phosphate (IP3) and diacylglycerol (DAG). In plants, IP2 and IP3 can be further phosphorylated to IP6, which acts as a second messenger inducing the release of calcium (Ca+2) from intracellular stores (Meijer and Munnik, 2003). The other PI‐PLC product, DAG, is phosphorylated by DAG kinase (DGK) to produce phosphatidic acid (PA) (Meijer and Munnik, 2003). The activation of PLC is one of the earliest host responses upon treatment of plant cells with pathogen‐associated molecular patterns (PAMPs) (Laxalt and Munnik, 2002). These are conserved compounds of pathogenic microbes that are perceived by immune receptors present in resistant plants. For example, the fungal PAMPs xylanase, chitosan and N‐acetyloligosaccharides, as well as the bacterial flagellin‐derived peptide flg22, induce PA production via PLC/DGK in tomato, alfalfa and rice cells (Bargmann et al., 2006; den Hartog et al., 2003; Laxalt et al., 2007; van der Luit et al., 2000; Raho et al., 2011; Yamaguchi et al., 2003, 2005). Moreover, the race‐specific pathogen effector Avr4, from the fungus Cladosporium fulvum, induces PLC activity in Cf4‐expressing tobacco cells (de Jong et al., 2004). Production of PA via PLC/DGK has also been reported in RPM1/RPS2‐ Arabidopsis plants upon the perception of the specific effectors AvrRpm1 and AvrRpt2 from Pseudomonas syringae (Andersson et al., 2006). It has been well documented that PLC/DGK activation triggers downstream plant defense responses like reactive oxygen species (ROS) production, induction of defense genes and cell death (Testerink and Munnik, 2011). There are seven known functional PLC genes and two pseudogenes in the Arabidopsis thaliana genome (Mueller‐Roeber and Pical, 2002). Multiple PLC genes have been found in several plant species such as rice, potato and tomato (Kopka et al., 1998; Song and Goodman, 2002; Vossen et al., 2010). It has been demonstrated that PLCs are regulated at transcriptional level upon biotic stress. Oryza sativa (Os) PLC1 transcript levels increase upon treatment of rice with P. syringae or the salicylic acid (SA) analogue, benzothiadiazol (Chen et al., 2007; Song and Goodman, 2002). In tomato [Solanum lycopersicum (Sl)], Vossen et al. (2010) characterized a PLC gene family composed of six members named SlPLC1 to SlPLC6. These authors found that the expression levels of SlPLCs are distinctly increased in tomato plants inoculated with C. fulvum (Vossen et al., 2010). By performing virus‐induced gene silencing (VIGS) assays, it was demonstrated that SlPLC4 is specifically involved in the induction of the plant hypersensitive response (HR) upon AVR4 perception (Vossen et al., 2010). Instead, SlPLC6 is a more general component of defense signalling, since it is required for resistance against C. fulvum, Verticillium dahliae and P. syringae (Vossen et al., 2010). Based on this evidence, the authors concluded that there is a differential requirement of PLC isoforms for plant defense. We have recently demonstrated that xylanase induces an increase of SlPLC2 and SlPLC5 transcript levels both in tomato cell suspensions and tomato plants (Gonorazky et al., 2014). We found by VIGS assays that SlPLC2, but not SlPLC5, is required for xylanase‐induced expression of the SA‐defense gene marker Pathogenesis Related1 (SlPR1) and the HR tomato gene marker Hypersensitive Response 203J (SlHSR203J) (Gonorazky et al., 2014). Since xylanase is a PAMP, the aim of this work was to investigate whether SlPLC2 plays a role in plant‐pathogen interactions.
Botrytis cinerea is a necrotrophic fungus that infects over 200 plant species including several crops such as grape, strawberry and solanaceous plants (Dean et al., 2012). B. cinerea produces ROS, phytotoxins and enzymes, such as xylanase, that are required to induce necrosis of plant tissues (van Baarlen et al., 2007; van Kan, 2006). This pathogen also triggers programmed cell death pathways in the host, activating ROS production and HR, which favors the infection process (van Kan, 2006). It was demonstrated that the growth of B. cinerea is accompanied by H2O2 production and expression of HSR203J in the plant (Govrin and Levine, 2000). Infection of plants with B. cinerea also activates SA‐ and jasmonic acid (JA)‐signalling pathways (Glazebrook, 2005). The expression of PR1a and the transcription factor that regulates its expression, Non‐expressed PR1 (NPR1), is induced upon B. cinerea inoculation (Abuqamar et al., 2009; Diaz et al., 2002; El Oirdi et al., 2011; Flors et al., 2007). Transcript levels of the JA‐gene markers Proteinase Inhibitor I (PI‐I) and Proteinase Inhibitor II (PI‐II) are also augmented by B. cinerea inoculation (Abuqamar et al., 2009; Diaz et al., 2002; El Oirdi et al., 2011).
Previously, we demonstrated that SlPLC2 is required for xylanase‐induced expression of defense genes. The goal of this work was to study whether SlPLC2 regulates defense responses to the necrotrophic pathogen B. cinerea by transient silencing of SlPLC2 in tomato plants.
Results
To investigate whether SlPLC2 plays a role in tomato‐B. cinerea interaction, first we studied the expression of SlPLC2 in tomato leaflets during B. cinerea infection. Figure 1A shows that SlPLC2 transcripts significantly increased in tomato leaflets inoculated with B. cinerea at 48 and 72 h post‐inoculation (hpi). In addition, transcript levels of the other SlPLCs were studied for later analysis of SlPLC2 silencing specificity. At 0 hpi, SlPLC1‐SlPLC6 transcripts were comparable to those reported by Vossen et al. (2010; Fig. 1A). SlPLC1 was the only SlPLC gene whose transcript levels were significantly reduced upon inoculation (Fig. 1A). SlPLC3, SLPLC4 and SlPLC5 were increased in tomato leaflets inoculated with B. cinerea at different time points (Fig. 1A). Transcript levels of SlPLC6 did not significantly change throughout the experiment (Fig. 1A).
Figure 1.
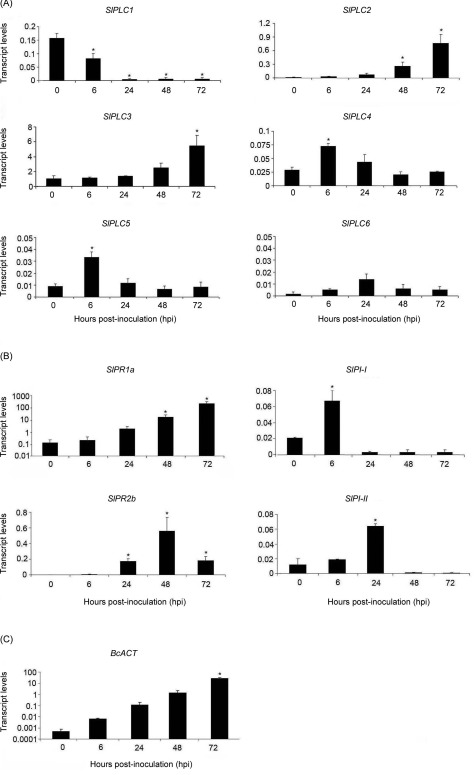
Transcript levels of SlPLC gene family, SA‐ and JA‐ defense gene markers and BcACT during the interaction between tomato and B. cinerea. Detached leaves from 6 weeks old tomato plants were droplet inoculated with B. cinerea isolate B05.10 spore suspension (106 spores.mL−1). Leaflets were harvested at the indicated hours post‐inoculation (hpi). Total RNA was isolated and transcript levels of SlPLC1 – SlPLC6 genes (A), SA‐defense gene markers SlPR1a and SlPR2b and JA‐defense gene markers SlPI‐I and SlPI‐II (B), and BcACT (C), were determined by RT‐qPCR. Transcript levels were normalized to SlACT. Error bars represent standard errors of three independent experiments. Asterisks denote that means are significantly different from 0 hpi samples according to a t‐test (P < 0.05).
Expression of the SA‐defense gene markers, SlPR1a and SlPR2b, and the JA‐defense gene markers, SlPI‐I and SlPI‐II, were used as a read‐out for transcriptional defense‐related gene activation upon B. cinerea infection (Abuqamar et al., 2009; Diaz et al., 2002; El Oirdi et al., 2011; Flors et al., 2007; Uppalapati et al., 2007).Transcript levels of SlPR1a and SlPR2b were significantly increased at 48 hpi, while SlPI‐I and SlPI‐II were induced at 6 and 24 hpi, respectively (Fig. 1B). The transcript levels of the B. cinerea ACTINE (BcACT) gene were quantified by qRT‐PCR at different time points after inoculation, as a quantitative measure for the fungal biomass (Benito et al., 1998). Figure 1C shows that BcACT increased in a time dependent manner from 0 to 72 hpi.
As mentioned previously, it was demonstrated that SlPLC2 is involved in xylanase‐induced defense gene expression (Gonorazky et al., 2014). To investigate whether SlPLC2 plays a role in tomato‐B. cinerea interaction, SlPLC2 expression was knocked down by VIGS employing a tobacco‐rattle virus (TRV) construct (Gonorazky et al., 2014). As a negative control we used a TRV with the β‐glucuronidase (GUS) gene (TRV:GUS), which has no homologues in plants. Under normal growth conditions, the TRV:SlPLC2 plants displayed no apparent morphological alterations, as reported earlier (Gonorazky et al., 2014). Silencing specificity of SlPLC2 was studied by measuring transcript levels of the six SlPLCs in TRV:GUS and TRV:SlPLC2 leaflets inoculated with B. cinerea. For this, we analyzed only the time points at which the expression of each SlPLC changed significantly upon B. cinerea inoculation of wild type plants (Fig. 1A). For SlPLC6, which expression did not change upon infection, we chose 24 hpi as an intermediate point. Figure 2 shows that there was a 40% reduction of SlPLC2 transcript levels in TRV:SlPLC2 leaflets upon 72 hpi. This result demonstrates that SlPLC2 was knocked down. Differences in SlPLC2 transcript levels could not be detected between TRV:GUS and TRV:SlPLC2 plants at 0 hpi, since the SlPLC2 transcript levels are very low (0,01 in relation to SlACT). Transcript levels of the other five SlPLC genes were not reduced in TRV:SlPLC2 leaflets (Fig. 2). It was observed as well that transcripts of SlPLC1 were significantly higher in TRV:SlPLC2 than in TRV:GUS leaflets (Fig. 2). This indicates that transient silencing of SlPLC2 was specific. Analysis of the expression of all six SlPLC genes in TRV:GUS and TRV:SlPLC2 plants at additional time points was included as Supporting Information (Fig. S1).
Figure 2.
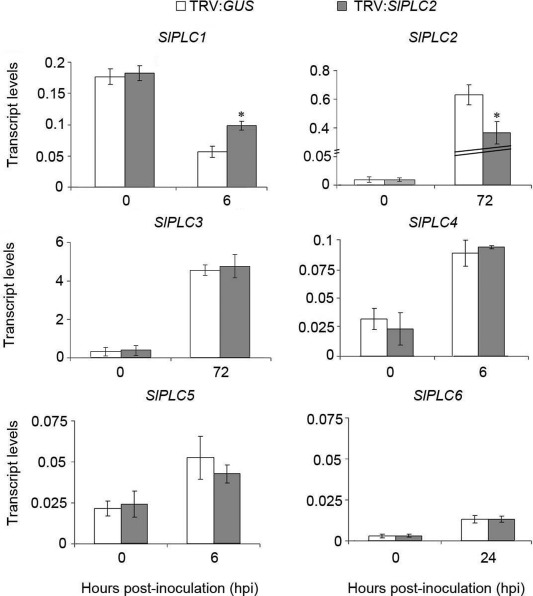
Specificity of virus‐induced gene silencing of SlPLC2 on tomato. Fourteen days old tomato seedlings were agroinfiltrated with the tobacco rattle virus (TRV) silencing constructs TRV:GUS (control) or TRV:SlPLC2. After 4 weeks, detached leaves were droplet inoculated with B. cinerea isolate B05.10 spore suspension (106 spores.mL−1). Leaflets were harvested at the indicated hours post‐inoculation (hpi). Total RNA was isolated and transcript levels of the six SlPLC genes were determined by RT‐qPCR. Transcript levels were normalized to SlACT. Error bars represent standard errors of three independent experiments. Asterisks denote that means are significantly different from inoculated TRV:GUS samples according to a t‐test (P < 0.05).
Once established that SlPLC2 was silenced, we analyzed the role of SlPLC2 in the tomato ‐ B. cinerea interaction. Figure 3A shows that necrotic lesions produced in tomato leaflets by B. cinerea inoculation were smaller in TRV:SlPLC2 plants than in TRV:GUS plants. To quantitatively confirm this phenotype, the lesion expansion rate produced by B. cinerea between 48 and 72 hpi was measured. As shown in Fig. 3B, the average lesion expansion rate was 21% lower in TRV:SlPLC2 than in TRV:GUS plants. To determine whether B. cinerea growth was affected in TRV:SlPLC2 infected plants, the transcript levels of BcACT were quantified in TRV:GUS and TRV:SlPLC2 leaflets inoculated with B. cinerea at 72 hpi. These experiments showed that BcACT transcripts were 70% lower in TRV:SlPLC2 than in TRV:GUS leaflets (Fig. 3C). This indicates that B. cinerea proliferation was significantly lower in TRV:SlPLC2 plants. Altogether, it can be concluded that SlPLC2 silenced plants were less susceptible to B. cinerea infection.
Figure 3.
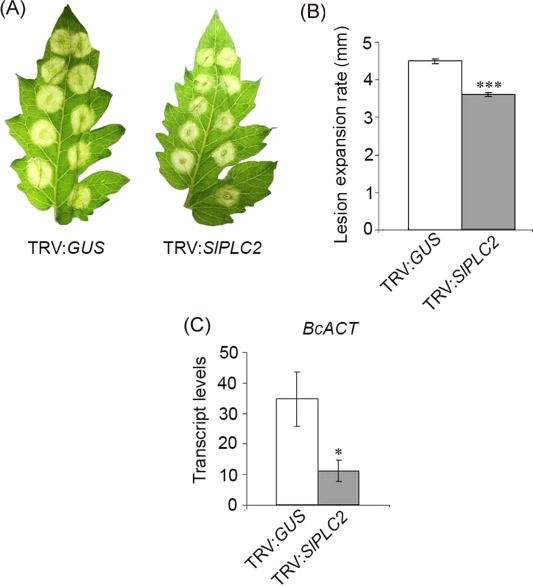
Susceptibility of SlPLC2 silenced tomato plants to B. cinerea infection. Fourteen days old tomato seedlings were agroinfiltrated with the constructs TRV:GUS (control) or TRV:SlPLC2. After 4 weeks, detached leaves were droplet inoculated with B. cinerea isolate B05.10 spore suspension (106 spores.mL−1). (A) Pictures were taken from representative leaflets at 72 h post‐inoculation (hpi). (B) Lesion diameter of 300‐400 inoculation sites was measured at 48 and 72 hpi and the average lesion expansion rate was calculated. Error bars represent standard errors of three independent experiments. Asterisks denote that means are significantly different from inoculated TRV:GUS samples according to a t‐test (P < 0.0001). (C) Leaflets were harvested at 72 hpi. Total RNA was isolated and transcript levels of BcACT were determined by RT‐qPCR. Transcript levels were normalized to SlACT. Error bars represent standard errors of three independent experiments. Asterisks denote that means are significantly different from inoculated TRV:GUS samples according to a t‐test (P < 0.05).
B. cinerea actively triggers an oxidative burst during plant cuticle penetration and primary lesion formation to favor its growth (van Kan, 2006). It was examined whether there was a difference between TRV:GUS and TRV:SlPLC2 plants in H2O2 accumulation during the tomato – B. cinerea interaction. H2O2 production was detected by DAB staining. As shown in Fig. 4A, TRV:SlPLC2 leaflets inoculated with B. cinerea displayed less DAB stained area than TRV:GUS leaflets. DAB precipitation was 40% lower in TRV:SlPLC2 than in TRV:GUS leaflets (Fig. 4B). This result indicates that H2O2 production was reduced in SlPLC2 silenced tomato plants inoculated with B. cinerea.
Figure 4.
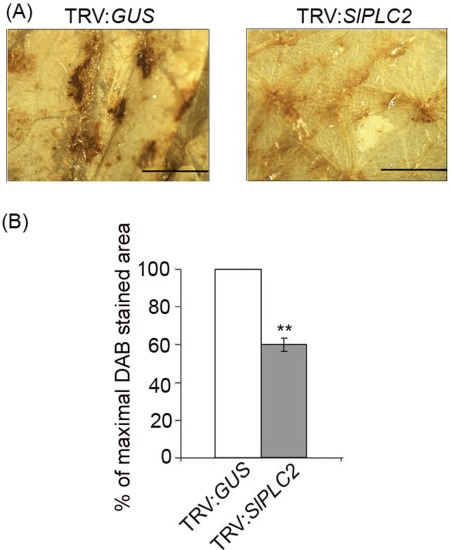
Production of H2O2 on SlPLC2 silenced tomato plants inoculated with B. cinerea. Fourteen days old tomato seedlings were agroinfiltrated with the constructs TRV:GUS (control) or TRV:SlPLC2. After 4 weeks, detached leaves were spray inoculated with B. cinerea isolate B05.10 spore suspension (106 spores.mL−1). Leaflets were harvested at 24 hpi and H2O2 production was detected by 3,3‐diaminobenzidine (DAB) staining immediately after harvesting. (A) DAB stained tissue was macroscopically observed. Pictures were taken from representative leaflets (scale bars=1.5 mm). (B) Quantification of DAB stained area was performed using ImageJ 1.3 software and expressed as a percentage of inoculated TRV:GUS samples which was set to 100%. Error bars represent standard errors of three independent experiments. Asterisks denote that means are significantly different according to a t‐test (P < 0.001).
The expression of SA‐ and JA‐defense gene markers was quantified in TRV:GUS and TRV:SlPLC2 leaflets inoculated with B. cinerea. Transcript levels of SlPR1a were 30% lower in TRV:SlPLC2 than in TRV:GUS, while no significant differences were detected in SlPR2b transcripts (Fig. 5). In contrast, transcript levels of SlPI‐I and SlPI‐II were over 3‐fold higher in TRV:SlPLC2 than in TRV:GUS leaflets (Fig. 5).
Figure 5.
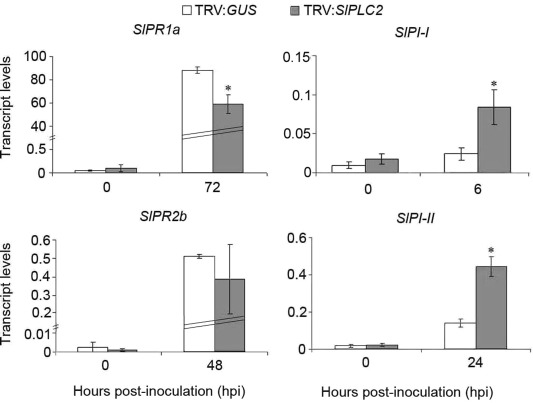
Transcript levels of SA‐ and JA‐defense gene markers on SlPLC2 silenced tomato plants inoculated with B. cinerea. Fourteen days old tomato seedlings were agroinfiltrated with the constructs TRV:GUS (control) or TRV:SlPLC2. After 4 weeks, detached leaves were droplet inoculated with B. cinerea isolate B05.10 spore suspension (106 spores.mL−1). Leaflets were harvested at the indicated hours post‐inoculation (hpi). Total RNA was isolated and transcript levels of the SA‐defense gene markers SlPR1a and SlPR2b, and the JA‐defense gene markers SlPI‐I and SlPI‐II were determined by RT‐qPCR. Transcript levels were normalized to SlACT. Error bars represent standard errors of three independent experiments. Asterisks denote that means are significantly different from inoculated TRV:GUS samples according to a t‐test (P < 0.05).
Discussion
In this report we show that transient silencing of SlPLC2 resulted in a reduction of the lesion expansion rate, together with a diminished B. cinerea growth, less H2O2 production and differential expression pattern of defense genes upon B. cinerea inoculation. This implies that SlPLC2 participates in plant susceptibility to B. cinerea.
A property of signalling enzymes in general is that treatments that activate them often rapidly enhance expression of their genes. The response could be a positive feedback mechanism to prime the cell for further stimulation (Hirt, 1999; Yamamoto, 1998). Accordingly, distinct evidence indicates that PI‐PLCs are also modulated at a transcriptional level in response to biotic stress (Chen et al., 2007; Gonorazky et al., 2014; Vossen et al., 2010). Particularly in tomato, xylanase treatment increases SlPLC2 and SlPLC5 transcript levels (Gonorazky et al., 2014). From these two SlPLC genes, SlPLC2 showed the highest induction (Gonorazky et al., 2014). Here we demonstrate that SlPLC2 expression was induced in tomato plants inoculated with B. cinerea, together with SlPLC3, SlPLC4 and SlPLC5. Induction of SlPLC2 occurred simultaneously with an enhanced expansion of B. cinerea lesions between 48 and 72 hpi. Vossen et al. (2010) showed that expression of all six SlPLCs is differentially induced during interaction between tomato and C. fulvum. SlPLC2 maximum expression coincided with the time point at which C. fulvum biomass starts to increase significantly (Vossen et al., 2010). Altogether, these results point out that SlPLC2 is induced upon perception of distinct fungal pathogens.
In order to study the involvement of SlPLC2 in the tomato – B. cinerea interaction, SlPLC2 was transiently silenced in tomato plants by VIGS. As a necrotrophic pathogen, B. cinerea requires induction of plant cell death to grow and infect the host (van Kan, 2006). It has been demonstrated that accelerated cell death (acd) mutants are more susceptible to B. cinerea (van Baarlen et al., 2007). Inversely, mutation of type 2 metacaspases, which have been associated with induction of plant cell death, resulted in plants significantly less susceptible to this fungus (van Baarlen et al., 2007). Virus‐induced gene silencing of SlPLC2 resulted in a significant reduction of the lesion expansion rate in tomato leaflets inoculated with B. cinerea. In addition, there was a drastically reduced growth of B. cinerea in SlPLC2 silenced plants. Therefore, it can be concluded that SlPLC2 is required for plant susceptibility to B. cinerea. We have previously reported that xylanase‐induced cell death requires PLC activation (Laxalt et al., 2007). Interestingly, it has been demonstrated that xylanase is required by B. cinerea to be fully virulent on tomato plants (Brito et al., 2006). It remains to be elucidated whether xylanase and/or other molecules produced by B. cinerea induce SlPLC2 activity.
Induction of the plant oxidative burst is required by B. cinerea to infect the host (van Kan, 2006). This is partially dependent on NADPH oxidase activity, since inhibition of this enzyme significantly diminishes ROS production and reduces fungal colonization (Govrin and Levine, 2000). SlPLC2 silenced tomato plants showed less H2O2 production than non‐silenced plants inoculated with B. cinerea. This is consistent with the less susceptible phenotype of SlPLC2 silenced plants to this fungus. Host cell death requires the active participation of both, the pathogen and the host (van Kan, 2006). Therefore, the reduced H2O2 production, required to induce cell death, leads to smaller B. cinerea lesions. At the same time, less B. cinerea proliferation induces less H2O2 production. It has been demonstrated that activation of PLC is required for ROS production induced by xylanase, chitosan, N‐acetychitooligosaccharide and the race specific elicitor Avr4 (de Jong et al., 2004; Laxalt et al., 2007; Raho et al., 2011; Yamaguchi et al., 2003). In addition, PLC activation correlates in time with early oxidative burst upon pathogen recognition (Andersson et al., 2006). It has been reported that PA and Ca2+ positively regulate NADPH oxidase activity with the consequent increase in generation, which is a precursor of H2O2 (Zhang et al., 2009). Therefore, it could be speculated that the second messengers derived from SlPLC2 activation positively regulate the NADPH oxidase activity in tomato‐B. cinerea interaction.
It has been postulated that, in general terms, SA‐regulated defense responses favor plant infection by necrotrophs like B. cinerea, while JA‐regulated defense responses are involved in restricting the disease produced by this kind of pathogens. The inverse model is proposed for (hemi)biotrophs (Glazebrook, 2005; Pieterse et al., 2009). The final balance between SA‐ and JA‐signalling pathways would determine the establishment of the infection. Therefore, a reduction of plant susceptibility to necrotrophic pathogens results in an increase of susceptibility to (hemi)biotrophs, and vice versa (Glazebrook, 2005; Pieterse et al., 2009). Partial silencing of SlPLC2 in tomato plants infected with B. cinerea resulted in lower transcript levels of SlPR1a and higher transcripts of SlPI‐I and SlPI‐II. This indicates that silencing of SlPLC2 increases the basal resistance of tomato to B. cinerea, and slower disease development results in a lower expression of SlPR1a and higher expression of SlPI‐I and SlPI‐II. These results are in accordance to El Oirdi et al. (2011), who demonstrated that transient silencing of the transcription factor that induces SlPR1a gene expression, SlNPR1, diminishes susceptibility of tomato plants to B. cinerea. Tomato SlNPR1 silenced plants presented higher transcript levels of SlPI‐I and SlPI‐II, indicating that SlNPR1 negatively regulates SlPI‐I and SlPI‐II (El Oirdi et al., 2011). Inversely, silencing of SlPI‐I and SlPI‐II by VIGS increased susceptibility to B. cinerea (El Oirdi et al., 2011). It has been previously reported that NPR1 modulates both SA‐ and JA‐signalling pathways (Pieterse et al., 2009). A follow up of our work will be to determine the connection between NPR1 and SlPLC2.
Activation of PI‐PLCs modulate levels of their substrates, PI4P and PI(4,5)P2, and generate IP2, IP3 and DAG (Meijer and Munnik, 2003). PI4P and PI(4,5)P2 act as molecular signals that can bind to proteins, thus modifying their localization and/or their activity (Munnik and Nielsen, 2011). Arabidopsis mutants expressing a mammalian type I inositol polyphosphate 5‐phosphatase, characterized by low levels of IP3, had a reduced cytosolic Ca+2 increase in response to flagellin, delayed induction of defense gene expression such as PR1 and compromised plant defense to P. syringae (Ma et al., 2012; Hung et al., 2014). Ca+2 activates diverse proteins involved in plant defense such as phospholipase D (PLD), which produces PA from structural phospholipids, NADPH oxidase and Ca+2‐dependent protein kinases (Kadota et al., 2015; Meijer and Munnik, 2003; Romeis and Herde, 2014). In plants, DAG produced by PI‐PLCs is subsequently phosphorylated to the signal molecule PA, which is involved in the induction of distinct defense responses such as ROS production, expression of defense genes and cell death (Testerink and Munnik, 2011). The biochemical mechanisms by which SlPLC2 regulates downstream signalling during tomato ‐ B. cinerea interaction remain to be elucidated.
In summary, we demonstrated that SlPLC2 contributes to plant susceptibility to B. cinerea. Future work will be carried out to determine whether other SlPLCs are involved in the tomato‐B. cinerea interaction. Also, a future challenge will be to study the role of SlPLC2 in the interaction between tomato and (hemi)biotrophs pathogens.
Experimental Procedures
Plant and fungal material
MM‐Cf0 tomato plants were grown in soil under a 16 h light/8 h dark regime, at 21°C and 70% relative humidity. B. cinerea strain B05.10 was maintained and conidia was isolated as described (Benito et al., 1998).
Virus‐induced gene silencing (VIGS) assays and inoculation of tomato leaves
SlPLC2 gene of 10‐day‐old tomato seedlings was silenced employing a tobacco rattle virus (TRV) as previously described (Gonorazky et al., 2014). As a negative control a TRV conteining part of the sequence of the β‐glucuronidase (GUS) gene was used (Gonorazky et al., 2014). Compound leaves of 6‐week‐old TRV:GUS and TRV:SlPLC2 plants were detached for B. cinerea inoculation. Harvest and pre‐incubation of conidia and tomato leaf handling were performed as described (Benito et al., 1998). Leaflets were droplet inoculated (for lesion diameter measurements and RNA isolation) or spray inoculated (for detection of H2O2 production) with B. cinerea spore suspension (106 spores.mL−1). For droplet inoculations, 8‐10 of 4 µL droplets were applied to each leaflet of detached compound leaves, except the apical. Incubations of droplet or sprayed inoculated leaves were performed in humid chambers at 20°C in the dark (Benito et al., 1998). Lesion diameters of 300‐400 inoculation sites were measured with a caliper at 48 and 72 h post‐inoculation (hpi). The average lesion expansion rate was calculated by subtracting to each 72 hpi lesion diameter the corresponding measure made at 48 hpi.
Detection of H2O2 production
Sprayed inoculated leaflets were harvested at 24 hpi and incubated with 20 mg.mL−1 3,3‐diaminobenzidine (DAB) in 50 mM sodium acetate at room temperature in the dark for 5 h, immediately after harvesting. DAB locally polymerizes as soon as it comes into contact with H2O2 in the presence of peroxidase, and it is visualized as a brown precipitate (Thordal‐Christensen et al., 1997). Leaflets were bleached with 100% ethanol. Stained areas were quantified using the program ImageJ after generating an extension for the plugin Phenotype Quant (Abd‐El‐Haliem, 2012). The new extension (called ‘DAB Quant’) was generated by training the program to recognize and measure the surfaces of DAB stained areas in scanned images of DAB stained leaves. [Correction added on 22 September 2016, after first online publication: the wording has been amended to include more details on the Phenotype Quant plugin.]
cDNA synthesis and quantitative PCR analysis
Total RNA was extracted using Trizol as described by the manufacturer (Invitrogen, NY, USA). Complementary DNA (cDNA) was synthesized using MMLV reverse transcriptase (RT) from Promega (Madison, USA) and an oligo‐dT primer on 1 µg of total RNA as a template. The cDNA was diluted to a final volume of 200 µL and 2.5 µL was used for quantitative PCR (qPCR). The Fast Universal SYBR Green Master mix from Roche (Mannheim, Germany) was employed, using a Step‐one Real‐time PCR machine from Applied Biosystems (California, USA). The standard amplification program was used. The nucleotide sequences of the specific primers for qPCR analysis of SlPLC1 to SlPLC6, SlPR1a, SlPR2b, SlPI‐II, SlACT and BcACT were previously reported (ten Have et al., 2010; Lopez‐Raez et al., 2010; Uppalapati et al., 2007; Vossen et al., 2010). The primers used for SlPI‐I were 5’‐GACTCTAACTTGATGTGCGAAGG‐3’ (forward primer) and 5’‐TCAAAAAGACGAACTCGATCAC‐3’ (reverse primer). Stepone Software v2.1 (Applied Biosystems) was used to analyze the transcript amounts of all genes.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Silencing specificity of S1PLC2 on tomato.
Acknowledgements
This work was financially supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Universidad Nacional de Mar del Plata (UNMdP) and the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). G. Gonorazky and A.M. Laxalt are members of the research career of CONICET. M.C. Guzzo is member of the professional career of the Instituto Nacional de Tecnología Agropecuaria (INTA). [Correction added on 22 September 2016, after first online publication: The acknowledgement to Matthieu H.A.J. Joosten has been deleted as he is now listed as one of the authors.]
[Correction added on 22 September 2016, after first online publication: Two authors, Ahmed M. Abd‐El‐Haliem and Matthieu H.A.J. Joosten, were not listed in the original version. This was an error and they have now been added as authors.]
References
- Abd‐El‐Haliem, A. (2012) An unbiased method for the quantitation of disease phenotypes using a custom‐built macro plugin for the program ImageJ. Methods Mol. Biol. 835, 635–644. [DOI] [PubMed] [Google Scholar]
- Abuqamar, S. , Luo, H. , Laluk, K. , Mickelbart, M.V. and Mengiste, T. (2009) Crosstalk between biotic and abiotic stress responses in tomato is mediated by the AIM1 transcription factor. Plant J. 58, 347–360. [DOI] [PubMed] [Google Scholar]
- Andersson, M.X. , Kourtchenko, O. , Dangl, J.L. , Mackey, D. and Ellerstrom, M. (2006) Phospholipase‐dependent signalling during the AvrRpm1‐ and AvrRpt2‐induced disease resistance responses in Arabidopsis thaliana. Plant J. 47, 947–959. [DOI] [PubMed] [Google Scholar]
- van Baarlen, P. , Woltering, E.J. , Staats, M. and van Kan, J.A.L. (2007) Histochemical and genetic analysis of host and non‐host interactions of Arabidopsis with three Botrytis species: an important role for cell death control. Mol. Plant Pathol. 8, 41–54. [DOI] [PubMed] [Google Scholar]
- Bargmann, B.O. , Laxalt, A.M. , Riet, B.T. , Schouten, E. , van Leeuwen, W. , Dekker, H.L. , de Koster, C.G. , Haring, M.A. and Munnik, T. (2006) LePLDbeta1 activation and relocalization in suspension‐cultured tomato cells treated with xylanase. Plant J. 45, 358–368. [DOI] [PubMed] [Google Scholar]
- Benito, E.P. , ten Have, A. , van 't Klooster, J.W. and van Kan, J.A.L. (1998) Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea. Eur. J. Plant Pathol. 104, 207–220. [Google Scholar]
- Brito, N. , Espino, J.J. and Gonzalez, C. (2006) The endo‐beta‐1,4‐xylanase xyn11A is required for virulence in Botrytis cinerea. Mol. Plant Microbe. Interact. 19, 25–32. [DOI] [PubMed] [Google Scholar]
- Chen, J. , Zhang, W. , Song, F. and Zheng, Z. (2007) Phospholipase C/diacylglycerol kinase‐mediated signalling is required for benzothiadiazole‐induced oxidative burst and hypersensitive cell death in rice suspension‐cultured cells. Protoplasma, 230, 13–21. [DOI] [PubMed] [Google Scholar]
- Dean, R. , Van Kan, J.A. , Pretorius, Z.A. , Hammond‐Kosack, K.E. , Di Pietro, A. , Spanu, P.D. , Rudd, J.J. , Dickman, M. , Kahmann, R. , Ellis, J. and Foster, G.D. (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, J. , ten Have, A. and van Kan, J.A. (2002) The role of ethylene and wound signaling in resistance of tomato to Botrytis cinerea. Plant Physiol. 129, 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong, C.F. , Laxalt, A.M. , Bargmann, B.O. , de Wit, P.J. , Joosten, M.H. and Munnik, T. (2004) Phosphatidic acid accumulation is an early response in the Cf‐4/Avr4 interaction. Plant J. 39, 1–12. [DOI] [PubMed] [Google Scholar]
- den Hartog, M. , Verhoef, N. and Munnik, T. (2003) Nod factor and elicitors activate different phospholipid signaling pathways in suspension‐cultured alfalfa cells. Plant Physiol. 132, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oirdi, M. , El Rahman, T.A. , Rigano, L. , El Hadrami, A. , Rodriguez, M.C. , Daayf, F. , Vojnov, A. and Bouarab, K. (2011) Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. Plant Cell, 23, 2405–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flors, V. , Leyva Mde, L. , Vicedo, B. , Finiti, I. , Real, M.D. , Garcia‐Agustin, P. , Bennett, A.B. and Gonzalez‐Bosch, C. (2007) Absence of the endo‐beta‐1,4‐glucanases Cel1 and Cel2 reduces susceptibility to Botrytis cinerea in tomato. Plant J. 52, 1027–1040. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Gonorazky, G. , Ramirez, L. , Abd‐El‐Haliem, A. , Vossen, J.H. , Lamattina, L. , ten Have, A. , Joosten, M.H. and Laxalt, A.M. (2014) The tomato phosphatidylinositol‐phospholipase C2 (SlPLC2) is required for defense gene induction by the fungal elicitor xylanase. J. Plant Physiol. 171, 959–965. [DOI] [PubMed] [Google Scholar]
- Govrin, E.M. and Levine, A. (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 10, 751–757. [DOI] [PubMed] [Google Scholar]
- Hirt, H. (1999) Transcriptional upregulation of signaling pathways: more complex than anticipated? Trends Plant Sci. 4, 7–8. [DOI] [PubMed] [Google Scholar]
- Hung, C.Y. , Aspesi, P., Jr. , Hunter, M.R. , Lomax, A.W. and Perera, I.Y. (2014) Phosphoinositide‐signaling is one component of a robust plant defense response Front Plant Sci. 5, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota, Y. , Shirasu, K. and Zipfel, C. (2015) Regulation of the NADPH Oxidase RBOHD During Plant Immunity. Plant Cell Physiol. 56, 1472–1480. [DOI] [PubMed] [Google Scholar]
- Kopka, J. , Pical, C. , Gray, J.E. and Muller‐Rober, B. (1998) Molecular and enzymatic characterization of three phosphoinositide‐specific phospholipase C isoforms from potato. Plant Physiol. 116, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxalt, A.M. and Munnik, T. (2002) Phospholipid signalling in plant defence. Curr. Opin. Plant Biol. 5, 332–338. [DOI] [PubMed] [Google Scholar]
- Laxalt, A.M. , Raho, N. , ten Have, A. and Lamattina, L. (2007) Nitric oxide is critical for inducing phosphatidic acid accumulation in xylanase‐elicited tomato cells. J. Biol. Chem. 282, 21160–21168. [DOI] [PubMed] [Google Scholar]
- Lopez‐Raez, J.A. , Verhage, A. , Fernandez, I. , Garcia, J.M. , Azcon‐Aguilar, C. , Flors, V. and Pozo, M.J. (2010) Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J. Exp. Bot. 61, 2589–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Walker, R.K. , Zhao, Y. and Berkowitz, G.A. (2012) Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proc. Natl. Acad. Sci. U S A 109, 19852–19857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer, H.J. and Munnik, T. (2003) Phospholipid‐based signaling in plants. Annu. Rev. Plant Biol. 54, 265–306. [DOI] [PubMed] [Google Scholar]
- Mueller‐Roeber, B. and Pical, C. (2002) Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide‐specific phospholipase C. Plant Physiol. 130, 22–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik, T. and Nielsen, E. (2011) Green light for polyphosphoinositide signals in plants. Curr. Opin. Plant Biol. 14, 489–497. [DOI] [PubMed] [Google Scholar]
- Pieterse, C.M.J. , Leon‐Reyes, A. , Van der Ent, S. and Van Wees, S.C.M. (2009) Networking by small‐molecule hormones in plant immunity. Nat. Chem. Biol. 5, 308–316. [DOI] [PubMed] [Google Scholar]
- Raho, N. , Ramirez, L. , Lanteri, M.L. , Gonorazky, G. , Lamattina, L. , Ten Have, A. and Laxalt, A.M. (2011) Phosphatidic acid production in chitosan‐elicited tomato cells, via both phospholipase D and phospholipase C/diacylglycerol kinase, requires nitric oxide. J. Plant Physiol. 168, 534–539. [DOI] [PubMed] [Google Scholar]
- Romeis, T. and Herde, M. (2014) From local to global: CDPKs in systemic defense signaling upon microbial and herbivore attack. Curr. Opin. Plant Biol. 20, 1–10. [DOI] [PubMed] [Google Scholar]
- Song, F. and Goodman, R.M. (2002) Molecular cloning and characterization of a rice phosphoinositide‐specific phospholipase C, OsPI‐PLC1, that is activated in systemic acquired resistance. Physiol. Mol. Plant Pathol. 61, 31–40. [Google Scholar]
- Testerink, C. and Munnik, T. (2011) Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. J. Exp. Bot. 62, 2349–2361. [DOI] [PubMed] [Google Scholar]
- ten Have, A. , Espino, J.J. , Dekkers, E. , Van Sluyter, S.C. , Brito, N. , Kay, J. , Gonzalez, C. and van Kan, J.A. (2010) The Botrytis cinerea aspartic proteinase family. Fungal Genet. Biol. 47, 53–65. [DOI] [PubMed] [Google Scholar]
- Thordal‐Christensen, H. , Zhang, S. , Wie, Y. and Collinge, D. (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 11, 1187–1194. [Google Scholar]
- Uppalapati, S.R. , Ishiga, Y. , Wangdi, T. , Kunkel, B.N. , Anand, A. , Mysore, K.S. and Bender, C.L. (2007) The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe. Interact. 20, 955–965. [DOI] [PubMed] [Google Scholar]
- van der Luit, A.H. , Piatti, T. , van Doorn, A. , Musgrave, A. , Felix, G. , Boller, T. and Munnik, T. (2000) Elicitation of suspension‐cultured tomato cells triggers the formation of phosphatidic acid and diacylglycerol pyrophosphate. Plant Physiol. 123, 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kan, J.A. (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 11, 247–253. [DOI] [PubMed] [Google Scholar]
- Vossen, J.H. , Abd‐El‐Haliem, A. , Fradin, E.F. , van den Berg, G.C. , Ekengren, S.K. , Meijer, H.J. , Seifi, A. , Bai, Y. , ten Have, A. , Munnik, T. , Thomma, B.P. and Joosten, M.H. (2010) Identification of tomato phosphatidylinositol‐specific phospholipase‐C (PI‐PLC) family members and the role of PLC4 and PLC6 in HR and disease resistance. Plant J. 62, 224–239. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, T. , Minami, E. and Shibuya, N. (2003) Activation of phospholipases by N‐acetylchitooligosaccharide elicitor in suspension‐cultured rice cells mediates reactive oxygen generation. Physiol. Plant, 118, 361–370. [Google Scholar]
- Yamaguchi, T. , Minami, E. , Ueki, J. and Shibuya, N. (2005) Elicitor‐induced activation of phospholipases plays an important role for the induction of defense responses in suspension‐cultured rice cells. Plant Cell Physiol. 46, 579–587. [DOI] [PubMed] [Google Scholar]
- Yamamoto, Y.Y. , Matsui, M. and Deng, X.‐W. (1998) Positive feedback in plant signaling pathways. Trends Plant Sci. 3, 374–375. [Google Scholar]
- Zhang, Y. , Zhu, H. , Zhang, Q. , Li, M. , Yan, M. , Wang, R. , Wang, L. , Welti, R. , Zhang, W. and Wang, X. (2009) Phospholipase Dα1 and Phosphatidic Acid Regulate NADPH Oxidase Activity and Production of Reactive Oxygen Species in ABA‐Mediated Stomatal Closure in Arabidopsis. Plant Cell, 21, 2357–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Silencing specificity of S1PLC2 on tomato.


