SUMMARY
Salicylic acid (SA) biosynthesis, the expression of SA‐related genes and the effect of SA on the Arabidopsis–Plasmodiophora brassicae interaction were examined. Biochemical analyses revealed that, in P. brassicae‐infected Arabidopsis, the majority of SA is synthesized from chorismate. Real‐time monitored expression of a gene for isochorismate synthase was induced on infection. SA can be modified after accumulation, either by methylation, improving its mobility, or by glycosylation, as one possible reaction for inactivation. Quantitative reverse transcription‐polymerase chain reaction (qPCR) confirmed the induction of an SA methyltransferase gene, whereas SA glucosyltransferase expression was not changed after infection. Col‐0 wild‐type (wt) did not provide a visible phenotypic resistance response, whereas the Arabidopsis mutant dnd1, which constitutively activates the immune system, showed reduced gall scores. As dnd1 showed control of the pathogen, exogenous SA was applied to Arabidopsis in order to test whether it could suppress clubroot. In wt, sid2 (SA biosynthesis), NahG (SA‐deficient) and npr1 (SA signalling‐impaired) mutants, SA treatment did not alter the gall score, but positively affected the shoot weight. This suggests that SA alone is not sufficient for Arabidopsis resistance against P. brassicae. Semi‐quantitative PCR revealed that wt, cpr1, dnd1 and sid2 showed elevated PR‐1 expression on P. brassicae and SA + P. brassicae inoculation at 2 and 3 weeks post‐inoculation (wpi), whereas NahG and npr1 showed no expression. This work contributes to the understanding of SA involvement in the Arabidopsis–P. brassicae interaction.
Keywords: Arabidopsis thaliana, defence gene expression, Plasmodiophora brassicae, salicylic acid biosynthesis, salicylic acid mutants
Introduction
Salicylic acid (SA) is a key phytohormone that regulates defence against biotrophic and hemibiotrophic pathogens (Grant and Lamb, 2006) and is often the first chemical signal induced during a resistance response. In higher plants, SA is synthesized via isochorismate (produced by the activity of isochorismate synthase) (Catinot et al., 2008; Mustafa et al., 2009) or via phenylalanine (Phe) (produced by the activity of phenylalanine ammonia lyase, PAL) (Coquoz et al., 1998; Ribnicky et al., 1998). Both isochorismate and Phe are derived from chorismate, the end product of the shikimate pathway (Wildermuth et al., 2001). Stressed Arabidopsis thaliana plants synthesize SA primarily via an isochorismate pathway in the chloroplast, whereas a distinct pathway utilizing Phe as the substrate may contribute to a much lesser extent to SA accumulation (Dempsey et al., 2011).
SA may be further modified by methylation, glycosylation or conjugation to amino acids, making it transportable (Ludwig‐Müller et al., 2015) or inactive (Dean et al., 2003; Zhang et al., 2007), but also determining its sites of accumulation (Rivas‐San Vicente and Plasencia, 2011). Recently, it has been shown that the protist Plasmodiophora brassicae possesses a methyltransferase that is involved in SA methylation and that methylated SA (MeSA) is a mobile form of SA in the Arabidopsis–P. brassicae system (Ludwig‐Müller et al., 2015). In soybean (Glycine max L.), it has been shown that SA is glycosylated in the cytoplasm to SA glycoside (SAG), which is then transported to and stored within the vacuole (Dean and Mills, 2004; Dean et al., 2003). Dean et al. (2005) further revealed that tobacco cells metabolize SA to large amounts of SAG and the glucosylated MeSA 2‐O‐β‐d‐glucose (MeSAG).
SA is a critical component in systemic acquired resistance (SAR), initiated by plants against biotic and abiotic stress (Dempsey et al., 1999; Durrant and Dong, 2004). During SAR, there may be systemic transport of defence metabolites or the de novo production of resistance components activated by translocated signals originating from locally infected tissue (Heil and Ton, 2008). In both cases, compounds that enable SAR need to be translocated from the site of infection to a distal part of the plant. SA is mainly transported in its methylated form, as MeSA (Kumar and Klessig, 2008; Ludwig‐Müller et al., 2015). SAR that is active against P. brassicae has only recently been identified in Brassica oleracea, in which root treatment with SA reduced the rate of disease and induced PR‐1 (pathogenesis‐related gene 1) expression in leaves (Lovelock et al., 2013).
The soil‐borne obligate biotroph P. brassicae is a devastating plant pathogen which affects agricultural crops of the Brassicaceae family, including, broccoli, cabbage and cauliflower, and is the causal agent of clubroot (Dixon, 2009; Donald et al., 2006; Ludwig‐Müller and Schuller, 2008). An internationally recognized classification system for P. brassicae, the European Clubroot Differential (ECD), has been used to identify up to 128 different populations, with indications of a wide range of pathogenicity between individual pathotypes depending also on the genotype of the host plant (Kuginuki et al., 1999). Currently, clubroot has been found on almost every continent and has been isolated from countries such as Australia, Canada, Japan, Germany, UK and USA (Dixon, 2009).
Arabidopsis is especially important in the study of P. brassicae as it belongs to the same family as many of the economically important crops affected by the pathogen (Agarwal et al., 2009; Siemens et al., 2002). Different Arabidopsis ecotypes show various levels of susceptibility to isolates of P. brassicae. Ecotypes such as Col‐0 and Ler are both known to be highly susceptible to most P. brassicae isolates, including isolate ‘e3’ (Siemens et al., 2002) and most Australian isolates (Agarwal et al., 2009). The background for this difference in susceptibility is not clear, nor has the role played by SA been investigated in these interactions.
Most investigations on plant–pathogen interactions utilizing Arabidopsis mutants affected in SA‐related pathways or responses have been undertaken primarily with leaf pathogens. For example, the Arabidopsis defence mutant cpr1 is known to be resistant to the bacterial leaf pathogen Pseudomonas syringae pv. tomato (van Wees et al., 2000). Line cpr1 has elevated PR‐1 expression, which is thought to increase this mutant's resistance to some biotrophic pathogens. Love et al. (2007) found that the Arabidopsis mutants cpr1‐1 and cpr5‐2 were able to control Cauliflower mosaic virus (CaMV) more successfully than could the wild‐type (wt) Col‐0.
The aim of this research was to elucidate the Arabidopsis resistance response against the root pathogen P. brassicae and the role of SA. For this purpose, we investigated: (i) the biosynthetic pathway of SA on infection with P. brassicae; (ii) the quantitative expression of the genes related to the biosynthesis, transport and storage of SA on infection; (iii) the clubroot symptoms in several SA‐related mutants; (iv) the effect of the application of SA on phenotypic disease outcome; and (v) the effect of the application of SA on the expression of the PR‐1 gene in wt and SA mutants. In addition, in lines in which the application of SA did not help to combat clubroot, we looked at the expression of an SA‐independent resistance gene PDF1.2 (plant defensin gene 1.2) in order to obtain an insight into whether this is an alternative pathway through which these mutants react to P. brassicae. The use of labelled SA precursors allowed us to determine the SA biosynthetic pathway, and quantitative reverse transcription‐polymerase chain reaction (qPCR) was employed to obtain insight into the expression levels of the genes involved in SA metabolism as part of a defence response against isolate ‘e3’. Defence‐related mutants may prove to be valuable in the recognition of important components of the SA pathway. For this reason, two key lines with accelerated SA‐dependent responses (cpr1 and dnd1), one SA signalling mutant (npr1) and two SA‐deficient lines (NahG and sid2) were selected to study their susceptibility to an Australian or ‘e3’ isolate of P. brassicae.
Results
Synthesis of labelled chorismic acid (CA)
CA was synthesized from 13C6‐glucose using the Escherichia coli KA12 mutant (Kast et al., 1996). The possible labelling patterns for the resulting product CA are shown in Fig. S1 (see Supporting Information). From 13C6‐glucose, 13C3‐labelled phosphoenolpyruvate (PEP) and 13C4‐erythrose‐4‐phosphate (E‐4‐P) can be made. The resulting CA can be labelled from one (m/z +3) or two (m/z +6) PEP molecules. The incorporation of a label from 13C4‐E‐4‐P results in m/z +4. Alternatively, one labelled PEP and one labelled E‐4‐P molecule give rise to m/z +7 in CA. The last possibility is a labelled CA structure from precursors, which are all labelled (m/z +10). This, according to the mass spectrum (Fig. S2a, see Supporting Information), does not occur, whereas all other masses occur, albeit at different intensities. The identity of 13C‐labelled CA (13C‐CA) was confirmed using mass spectrometry comparing the compound with unlabelled CA (Fig. S2b). The molecular ion was not present, but the daughter ions showed the expected labelling patterns with the highest intensities deriving from +3, +4 and +7, whereas +6 (2 × PEP) was much lower in abundance.
Biosynthesis of SA in Arabidopsis infected by P. brassicae
The biosynthesis of SA could occur from CA directly via isochorismate or Phe as an intermediate (Fig. 1). From the isotope patterns, it is expected that the label in SA, if it is made from 13C‐CA, should be m/z 159, 156, 155 (endogenous m/z 152) or, if synthesized from 13C‐labelled Phe (13C‐Phe) derived from 13C‐CA, should be m/z 158, 156, 155. Exogenous deuterated Phe (PheD5) should result in the molecular ion m/z 157 (Fig. 2A).
Figure 1.
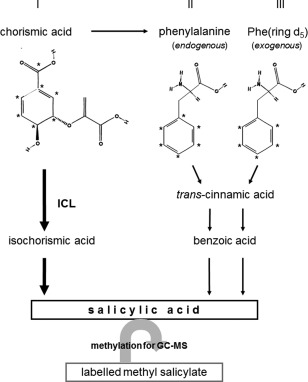
The two possible pathways for the synthesis of salicylic acid (SA) from 13C‐labelled chorismic acid (CA) (indicated by asterisks in the molecule). CA can be converted directly to isochorismic acid (ICA) and then salicylic acid (SA), or via phenylalanine (Phe). The arrows indicate the situation found in clubroot‐infected Arabidopsis roots: bold arrows symbolize the major pathway via ICA and small arrows the minor one via Phe. The roman numbers indicate the three different possibilities. For labelling patterns of CA and the respective mass spectrum, see Figs S1 and S2. GC‐MS, gas chromatography‐mass spectrometry; ICL, isochorismate lyase.
Figure 2.
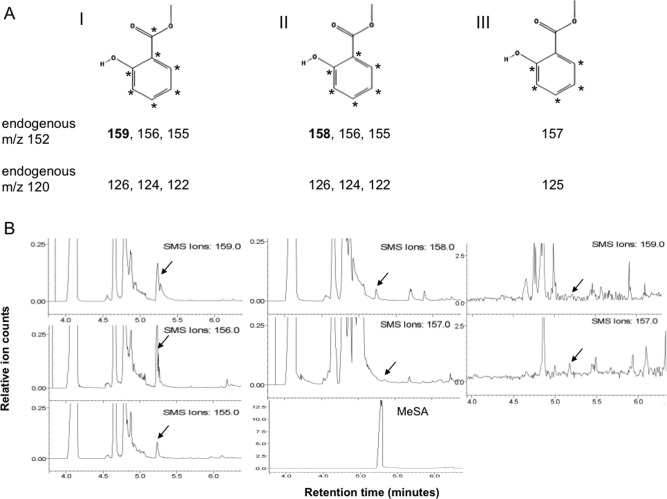
(A) Expected labelling patterns in methylated salicylic acid (MeSA) synthesized from 13C‐labelled chorismic acid (13C‐CA) (I), 13C‐labelled phenylalanine (13C‐Phe) (II) or deuterated phenylalanine (PheD5) (III). The numbers I, II and III refer to the three different pathways in Fig. 1. (B) Extracted ion chromatograms for the expected labelling pattern depicted in (A) for MeSA. The left panels show the molecular ion chromatograms at m/z 159, 156 and 155 (endogenous m/z 152) from roots infected with Plasmodiophora brassicae and treated with 13C‐CA. The middle panels show the ion chromatograms of m/z 158 (derived from 13C‐Phe) and 157 (which could only originate from PheD5) from the same experiment in comparison with the MeSA standard. The right panels show the ion chromatograms at m/z 157 (originating from infected roots after incubation with PheD5) and at m/z 159 (which should only be found when 13C‐CA is used as a precursor).
To investigate the biosynthetic pathway of SA in Arabidopsis clubroots, roots of infected and healthy Arabidopsis were treated 3 and 4 weeks post‐P. brassicae inoculation (wpi) with one or both of the following labelled SA precursors, 13C‐CA and PheD5, followed by extraction of the labelled MeSA (Fig. 2A). Extracted ion chromatograms (for the endogenous molecular ion m/z 152) for the different labels in SA are shown in Fig. 2B. The left and middle panels show the labelling pattern from 13C‐CA in one experiment (infected with P. brassicae), indicating that the ions at m/z 155, 156 and 159 were predominant, whereas m/z 158 (from 13C‐CA via endogenous 13C‐Phe) and 157 (from PheD5) can be neglected. If SA is synthesized via isochorismic acid, the ion at m/z 158 should be lower than that at m/z 159. The right panels show the labelling patterns in the ions at m/z 157 (PheD5) and 159 (which should only occur from 13C‐CA) in an infected root sample. From the values m/z 126, 124, 122 (endogenous m/z 120) and m/z 125 (Fig. 2A), the amounts of SA synthesized from each of the respective branches of the pathways were calculated (Fig. 3A). The values in Fig. 3B were calculated from the molecular ions (Fig. 2B). As shown in Fig. 3A, the highest percentage of labelled SA was found in infected roots incubated in 30 µm 13C‐CA. At the stage at which infection was best developed (3 wpi), a smaller amount of SA was synthesized, even from PheD5 applied at much higher concentration (1 mm), indicating that, when really needed, Arabidopsis will also use the less preferable precursor for SA biosynthesis. When plants were incubated with 13C‐CA at 30 µm and PheD5 at 100 µm simultaneously, the percentage (of total isotopes found) of 13C‐labelled SA was much higher than that of D5‐labelled Phe, at both 3 wpi (70% 13C‐labelled : 22% PheD5‐labelled) and 4 wpi (71% 13C‐labelled : 22% PheD5‐labelled) in infected plants. This pattern was also found in control plants at 3 wpi (58% 13C‐labelled : 6% PheD5‐labelled) and 4 wpi (47% 13C‐labelled : 31% PheD5‐labelled). Moreover, the total percentage of labelled SA was higher in infected than in control plants at both time points.
Figure 3.
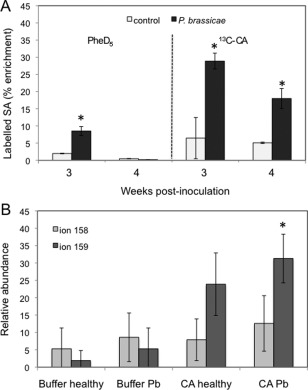
Plant galls/roots were incubated at 3 and 4 weeks post‐inoculation (wpi) in either chorismic acid labelled with 13C (13C‐CA) at 30 µm or deuterated phenylalanine (PheD5) at 1 mm (A). The experiment was repeated three times at 3 wpi and twice at 4 wpi (as the pathogen caused plant death in the third experiment). The reported values represent means ± standard deviation (SD). P. brassicae, Plasmodiophora brassicae‐infected Arabidopsis. Control, healthy Arabidopsis. (B) Additional proof for the synthesis of salicylic acid (SA) by CA and not Phe derived from the relative abundances of the two molecular ions at m/z 159 and m/z 158 of SA in plants at 3 wpi. The ion at m/z 159 is an indication of the synthesis via isochorismic acid and the ion at m/z 158 via phenylalanine after feeding of 13C‐CA. Asterisks indicate significant difference (P ≤ 0.05). Pb, Plasmodiophora brassicae‐infected Arabidopsis.
However, there could still be a limiting step from 13C‐CA to 13C‐Phe. Additional proof for the synthesis of SA from 13C‐CA and not 13C‐Phe comes from the ion intensities of the molecular ions. Only if SA comes directly from 13C‐CA does the molecular ion at m/z 159 occur (Fig. 2). If SA is derived from 13C‐Phe, which is also made from the 13C‐CA precursor, one 13C atom is lost and the molecular ion can only have m/z 158. m/z 159 always occurs at a higher abundance than m/z 158 (Fig. 3B).
Expression of isochorismate synthase, SA methyltransferase and glucosyltransferase genes in Arabidopsis infected by P. brassicae
The effect of P. brassicae infection on the real‐time expression of genes involved in SA metabolism was monitored. The genes tested were isochorismate synthase 1 (ICS1), SA methyltransferase (SAMT1) and SA glucosyltransferase (SAGT) (Fig. 4). The genes for F‐box protein and mitosis protein YLS8 were used as endogenous controls. The data are only presented for normalization on F‐box, because, according to two microarray studies (Agarwal et al., 2011; Siemens et al., 2006) and our results, this reference gene showed a stable expression over the different samples infected with P. brassicae isolates, processed at different time points of the disease and in different laboratories (Table S1, see Supporting Information).
Figure 4.
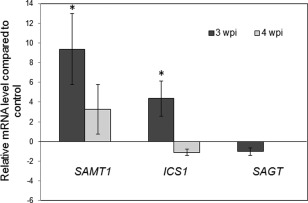
Real‐time polymerase chain reaction (PCR) determination of transcript accumulation of genes for salicylic acid (SA) methyltransferase (SAMT1), isochorismate synthase (ICS1) and SA glucosyltransferase (SAGT) in Arabidopsis roots in response to Plasmodiophora brassicae infection at 3 and 4 weeks post‐inoculation (wpi). Relative amounts were calculated and normalized with respect to the level of F‐box mRNA, and the level of transcripts in the controls was set to 1.0. The comparative CT method for relative quantification was applied. The reported values are the means ± standard deviation (SD) of three independent experiments at 3 wpi or the means ± SD of two independent experiments at 4 wpi, as the pathogen‐caused plant death in the third experiment. Asterisks indicate significant difference (P ≤ 0.05).
qPCR analysis showed an induction of SAMT1 in P. brassicae‐infected roots at 3 wpi of close to 10‐fold relative to control roots of the same age (Fig. 4). At 4 wpi, there was still an induction of SAMT1 transcript in infected roots but, because of the high variation, this was not significantly different from the control. ICS1 was significantly up‐regulated in infected roots at 3 wpi, but only about four‐fold compared with the controls, whereas no up‐regulation was found at 4 wpi. SAGT was not differentially regulated at any time point investigated.
Effect of infection by P. brassicae isolates on the fresh weight of Arabidopsis
The Arabidopsis ecotype Col‐0 and ICS1 mutant sid2 were tested for their susceptibility to the isolate ‘e3’, whereas Col‐0 and SA pathway mutants cpr1 and dnd1, as well as the SA‐deficient NahG and SA signalling‐impaired npr1, were tested for their susceptibility to an Australian isolate of P. brassicae. The last two lines were comparable to wt in terms of shoot weight.
Susceptibility was determined by observing the impact of the disease on the shoot weight of the Arabidopsis lines (Table 1; Fig. 5). Arabidopsis ecotype Col‐0 was found to be highly susceptible to both the isolate ‘e3’ and the Australian isolate of P. brassicae at 3 wpi. When inoculated with ‘e3’, an 80% reduction in mean shoot weight in infected plants was recorded, whereas the Australian isolate caused a reduction of 60% mean shoot weight in infected plants relative to controls.
Table 1.
Summary of the effect of salicylic acid (SA) on the development of symptoms associated with infection by Plasmodiophora brassicae (Pb) at 3 weeks post‐inoculation.
| Arabidopsis line | Reduced shoot weight after Pb infection | Positive SA effect on shoot weight after Pb infection | Gall score | SA reduces galls | PDF1.2 expression increased after Pb infection |
|---|---|---|---|---|---|
| Col‐0 | +* | − | 3.5 | − | + |
| cpr‐1 | + | − | 0.8 | − | + |
| dnd‐1 | − | − | 1.2 | + | + |
| NahG | + | + | 3.0 | − | − |
| nprl | + | + | 1.8 | − | − |
| Col‐0† | + | − | 3.4 | − | + |
| sid2 † | + | + | 3.7 | − | + |
*Symbols ‘+'and ‘−’ indicate an effect from the corresponding treatment. Values are the means of two repeats (n = 40) (P < 0.05).
†Inoculated with P. brassicae isolate ‘e3’; all other Arabidopsis inoculated with Australian isolate.
Figure 5.
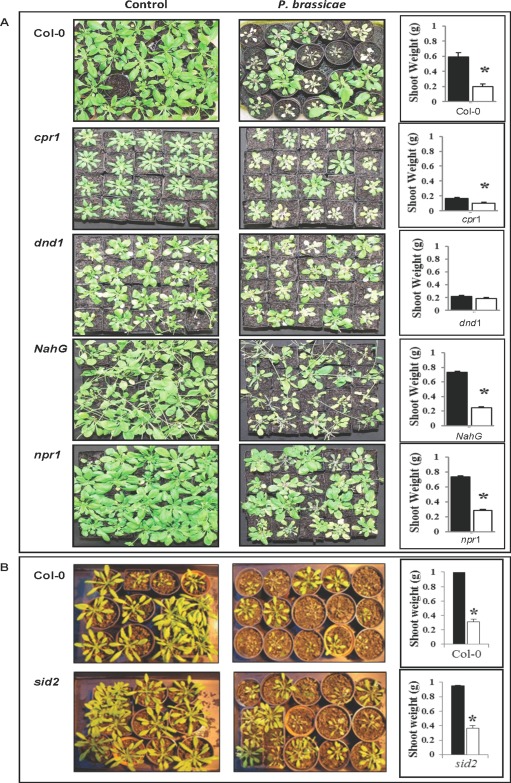
(A) Shoot weight response (bar graphs) of Arabidopsis Col‐0 and mutant lines to the Australian isolate of Plasmodiophora brassicae. (B) Shoot weight response (bar graphs) of Col‐0 and sid2 mutant to ‘e3’. Shoot weight was determined at 3 weeks post‐inoculation (wpi). Asterisks indicate significant difference in shoot weight from the respective control (n = 40) (P ≤ 0.05) (black bars, control; white bars, P. brassicae). The experiment was repeated twice. The photographs show the leaf phenotypes of all plant lines; left panels, control; right panels, P. brassicae‐infected plants.
When inoculated with isolate ‘e3’, sid2 mutants, which are unable to accumulate SA, were found to be highly susceptible with a reduction in shoot weight relative to the control. When inoculated with the Australian isolate of P. brassicae, the Arabidopsis lines cpr1 and dnd1, which are constitutive expressers of the SA pathway, were found to show the least reduction in plant weight. Moreover, dnd1 showed no decrease in shoot weight compared with the control, whereas there was a difference in cpr1; the reduction was about 40%. The Arabidopsis line NahG, which contains the salicylate hydroxylase gene, and npr1, which is unable to up‐regulate PR‐1, were observed to be more susceptible, when shoot weight was taken as a criterion, with a significant reduction in shoot weight associated with disease. A reduction of more than 60% of total shoot weight was observed for these two lines.
Effect of SA on shoot weight of Arabidopsis after P. brassicae inoculation
SA was tested for its direct toxicity to Arabidopsis prior to experiments involving SA, at 1 and 5 mm. No adverse effects, including chlorosis, reduction in plant weight or death, were observed at these concentrations. Root drenching was performed for 15 min and plants were assessed 24, 48 and 72 h later with no observed effect (results not shown).
The Arabidopsis ecotype Col‐0 and the sid2 mutant were assessed for their susceptibility to isolate ‘e3’ in combination with 1 mm SA, whereas Col‐0 and the SA pathway mutants cpr1, dnd1, NahG and npr1 were assessed for their susceptibility to an Australian isolate in combination with 1 mm SA (Fig. 6A). Arabidopsis plants were treated with SA 24 h prior to inoculation of the pathogen and clubroot symptoms were assessed at 2 and 3 wpi. A shoot index was calculated by dividing the fresh weight of infected plants by the weight of control plants (Siemens et al., 2002) to determine the grade of susceptibility or tolerance to the pathogen alone and in combination with SA. A low shoot index is indicative of susceptible plants with a reduction in fresh weight in infected or treated plants relative to controls, whereas a high shoot index indicates tolerant plants.
Figure 6.
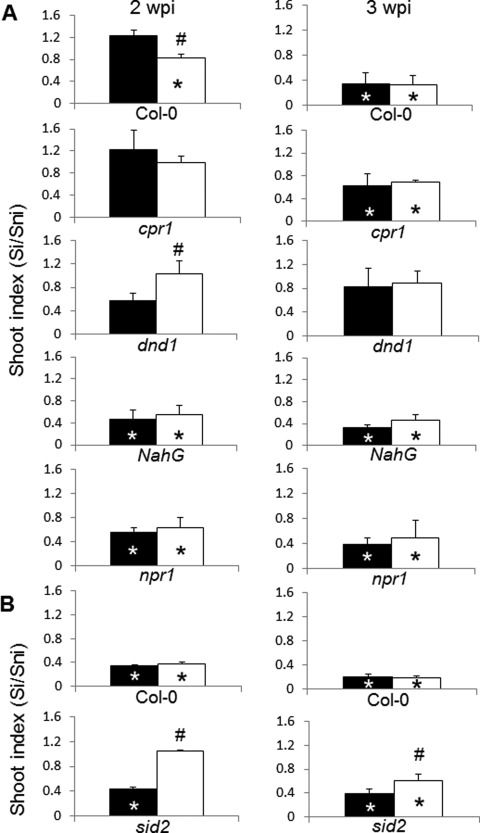
Shoot weight response (bar graphs) of Arabidopsis Col‐0 and mutant lines to the Australian (A) and ‘e3’ (B) isolates of Plasmodiophora brassicae, and in combination with salicylic acid (SA) at 2 and 3 weeks post‐inoculation (wpi). The shoot index is the weight of infected plants divided by the weight of control plants. Asterisks represent significant differences from respective controls (n = 40) and hashtags indicate significant differences between P. brassicae infection and SA + P. brassicae treatment (both at P ≤ 0.05) (black bars, P. brassicae; white bars, SA + P. brassicae). Si, shoot weight of infected plants; Sni, shoot weight of non‐infected plants. For phenotypes of plants, see photographs in Fig. 7.
At 2 wpi, Col‐0 showed a reduction in shoot weight when treated with both ‘e3’ and ‘e3’ with SA (see also Fig. 5). The sid2 mutant showed a reduction in shoot weight following inoculation with ‘e3’, but not with ‘e3’ and SA (Fig. 6B). When inoculated with the Australian isolate, Col‐0 showed a reduction in shoot weight when treated with both SA and P. brassicae, but not with P. brassicae alone. The Arabidopsis line dnd1 showed no significant reduction in shoot weight when treated with the pathogen or in combination with SA. However, the weight of dnd1 shoots after treatment with SA and P. brassicae was significantly higher than that of plants not treated with SA. Lines NahG and npr1 both showed a significant reduction in plant weight when treated with the pathogen or in combination with SA (Fig. 6A).
At 3 wpi, there was a significant reduction in shoot weight in both Col‐0 and sid2 following inoculation with ‘e3’ and with the addition of SA (Fig. 6B). Moreover, treatment with SA led to an increased shoot index of sid2 plants relative to those infected with P. brassicae alone. When inoculated with the Australian isolate, Col‐0 showed a significant reduction in shoot weight in both P. brassicae‐ and SA + P. brassicae‐treated groups. The Arabidopsis line cpr1 showed no significant decrease in shoot weight in either inoculated group. Lines dnd1, NahG and npr1 all showed a significant reduction in shoot weight when inoculated with P. brassicae only and in combination with SA (Fig. 6A).
Root gall severity in SA‐treated Arabidopsis following P. brassicae inoculation
In order to determine the effect of SA treatment on disease development, root galling was assessed at 3 wpi (Table 1; Fig. 7), with a maximum attainable score of 5. Arabidopsis ecotype Col‐0 was highly susceptible to the pathogen, with an average root gall score of 3.5 when treated with either isolate ‘e3’ or the Australian isolate of P. brassicae. There was no significant difference in disease severity observed for the isolate ‘e3’ or Australian isolate of P. brassicae following treatment with SA in Col‐0.
Figure 7.
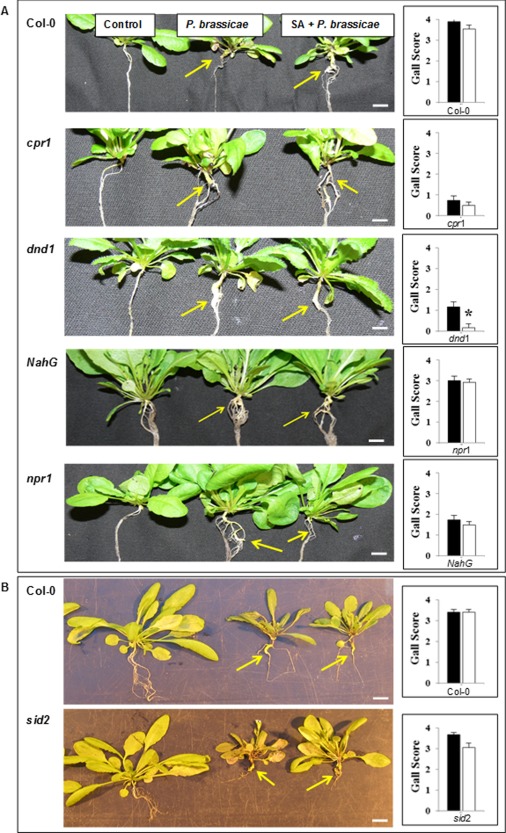
Effect of salicylic acid (SA) on gall scores associated with the Australian isolate of Plasmodiophora brassicae (A) and the ‘e3’ isolate (B). The photographs show the respective Arabidopsis control, P. brassicae‐infected and P. brassicae‐infected + SA‐treated plants. Asterisk indicates significant difference (n = 40) (P ≤ 0.05) between P. brassicae infection and SA + P. brassicae treatment (black bars, P. brassicae; white bars, SA + P. brassicae). Gall scores were determined at 3 weeks post‐inoculation.
Arabidopsis SA constitutive expresser lines, cpr1 and dnd1, both appeared to control the growth of the pathogen as the gall scores were 0.8 and 1.2, respectively. The addition of SA did not significantly reduce galling in cpr1; however, dnd1 showed a significant additional reduction in gall development (0.2) (Fig. 7A).
The Arabidopsis SA‐deficient line NahG was susceptible to the pathogen, with an average score of 3.0 for root galling, which resembled the phenotype of Col‐0. The addition of SA did not significantly reduce disease severity (2.9). Line npr1 was less susceptible to the pathogen than line NahG with an average root gall score of 1.8. The addition of SA did not significantly reduce the severity of disease associated with P. brassicae. The ICS1 mutant sid2, inoculated with ‘e3’, had an average gall score of 3.7; the addition of SA did not reduce the score significantly (Fig. 7B).
Expression of defence genes in Arabidopsis following P. brassicae infection
As SA alone increases PR‐1 expression and P. brassicae infection alone increases the amount of SA in both roots and leaves (Ludwig‐Müller et al., 2015), we were interested to determine PR‐1 gene expression after P. brassicae infection and concomitant SA treatment to investigate whether this treatment could intensify PR‐1 expression. The expression of defence genes PR‐1 and PDF1.2 was measured in leaf material because, in previous work, we found higher SA levels in Arabidopsis leaves than in roots after P. brassicae infection (Ludwig‐Müller et al., 2015) and, in our microarray experiment, PR‐1 was poorly expressed in roots (Siemens et al., 2006). The expression of the defence gene PR‐1 as a marker for SA‐induced defence was assessed at 2 and 3 wpi with either the ‘e3’ or Australian isolate of P. brassicae or SA + P. brassicae (Fig. 8). Col‐0 plants inoculated with ‘e3’ or in combination with SA showed no change in PR‐1 expression at 2 wpi. Line sid2 showed a slightly increased PR‐1 expression after treatment with both SA and ‘e3’. At 3 wpi, PR‐1 was highly expressed in the control and both ‘e3’ and ‘e3’ with SA of Col‐0, but, in sid2, only slightly after both SA and ‘e3’ inoculation.
Figure 8.
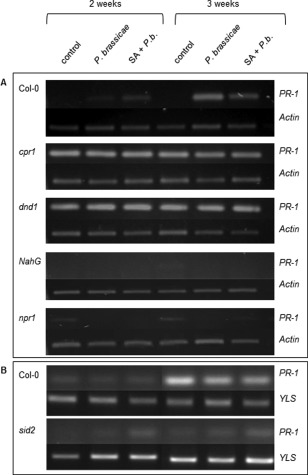
PR‐1 (pathogenesis‐related gene 1) expression in leaf material of Arabidopsis wild‐type and mutant lines following Australian (Col‐0, cpr1, dnd1, NahG, npr1) (A) and ‘e3’ (Col‐0, sid2) (B) Plasmodiophora brassicae infection and infection together with salicylic acid (SA) treatment. Expression levels were determined at 2 and 3 weeks post‐inoculation. Plant actin was used as reference for the Australian isolate, whereas plant YLS was used as reference for ‘e3’.
When inoculated with the Australian isolate, Col‐0 showed an increase in PR‐1 expression at 2 and 3 wpi with P. brassicae and in combination with SA. The SA constitutive expresser lines cpr1 and dnd1 both showed heightened PR‐1 expression in all samples at 2 and 3 wpi. Line NahG, deficient in SA, did not show any PR‐1 expression in any plant material tested. Line npr1, which shows reduced PR gene expression, did not appear to express PR‐1 at 2 wpi in either of the treatment groups; there was, however, a slight increase in combination with SA at 3 wpi.
The data for PR‐1 expression in wt and several SA‐related mutant lines after P. brassicae and SA + P. brassicae treatment suggested that SA is not the only factor controlling disease severity. Therefore, we analysed the expression of a marker gene for the jasmonic acid defence pathway, PDF1.2 (Fig. S3, see Supporting Information). It was shown that, after disruption of SA signalling pathways, defence against several pathogens could be redirected via PDF1.2 (Genger et al., 2008).
PDF1.2 (Fig. S3; Table 1) was observed to be highly expressed at 2 wpi following inoculation with ‘e3’ and in combination with SA in both Col‐0 and sid2 when compared with the controls. PDF1.2 expression was slightly up‐regulated in wt at 2 wpi following inoculation with the Australian P. brassicae isolate, but highly expressed when in combination with SA, whereas this effect could not be observed at 3 wpi. PDF1.2 was not observed to be switched on in cpr1, dnd1 and npr1 plants at 2 wpi; however, at 3 wpi, there was a slight increase when inoculated with the pathogen only in cpr1 and dnd1, but not in npr1. There was a slight increase in PDF1.2 at 2 and 3 wpi with P. brassicae and in combination with SA, respectively, in NahG plants.
Discussion
The aim of this research was to determine the effect of P. brassicae on SA biosynthesis in Arabidopsis and on the expression of Arabidopsis genes related to SA biosynthesis, metabolism and storage. The effect of exogenously applied SA on the Arabidopsis–P. brassicae system was also investigated, as Agarwal et al. (2011) demonstrated that dipping Arabidopsis roots in an SA solution prior to inoculation with P. brassicae reduced clubroot symptoms significantly. The Arabidopsis ecotype Col‐0 and a number of mutant lines enhanced or deficient in the SA pathway were used to determine their susceptibility to P. brassicae with or without the application of SA.
SA is synthesized via isochorismate in P. brassicae‐infected roots
SA can be synthesized from isochorismate and/or Phe (Wildermuth et al., 2001), and it is dependent on the plant–pathogen system as to which of the two pathways is used (Catinot et al., 2008; Ogawa et al., 2006). We performed analyses at 2 (data not shown), 3 and 4 wpi; however, only time points 3 and 4 wpi gave reliable results. This is probably a result of the low level of infection at 2 wpi (infected roots, if at all present, were very small). Our results indicate that, in the Arabidopsis–P. brassicae system, SA is predominantly synthesized from isochorismate. However, at very high infection rates, this system can also produce a smaller amount of SA from Phe, meaning that, when really needed, Arabidopsis will also use the less preferable precursor for SA biosynthesis. A similar result was published by Dempsey et al. (2011), who concluded that stressed Arabidopsis synthesizes SA primarily via an isochorismate pathway, whereas a distinct pathway utilizing Phe as the substrate may also contribute to SA accumulation, although to a much lesser extent.
The fact that, in P. brassicae‐infected Arabidopsis, SA is synthesized from chorismate, that it is transported to distant sites in a methylated form and is accumulated in a greater amount than in control plants suggest that the expression of genes encoding proteins involved in these processes could also be affected by P. brassicae infection. Therefore, we investigated the real‐time expression patterns of the corresponding genes in Arabidopsis roots at 3 and 4 wpi, because, at (data not shown) the level of infection was very low or not present at all. The expression of the ICS1 gene associated with the biosynthesis of SA was increased at 3 wpi on P. brassicae infection of Arabidopsis. This is consistent with our result indicating that the biosynthetic precursor relating to chorismate is a predominant source of SA biosynthesis in this system, similar to the observations in other plant–pathogen systems (Wildermuth et al., 2001). The other enzyme(s) involved in the initial conversion of Phe is PAL. However, the expression of PAL genes was not investigated as: (i) the contribution of the PAL pathway, addressed at the biochemical level, was significantly lower than that of the ICS pathway; (ii) PAL is an enzyme responsible for many other secondary metabolite pathways that could also be involved in the Arabidopsis–P. brassicae interaction, and so changes in PAL regulation could be a result of a plethora of pathways; (iii) the four isoforms of PAL were regulated in a different manner in Arabidopsis clubroots according to microarray data (Päsold et al., 2010); and (iv) it was found that PAL activity is not a part of the defence mechanism against P. brassicae in the related Brassica species Chinese cabbage (Ludwig‐Müller et al., 1995).
Another gene whose real‐time expression was monitored is the SAMT1 gene associated with SA methylation. The significant increase in SAMT1 expression at 3 wpi follows the findings in previous studies which suggest that the methylation of SA is needed for its long‐distance transport through Tobacco mosaic virus‐infected tobacco (Park et al., 2007), and that MeSA is a mobile form of SA during clubroot disease of Arabidopsis (Ludwig‐Müller et al., 2015). However, a mutant in the methyltransferase, AtBSMT, did not show altered disease symptoms (Ludwig‐Müller et al., 2015).
As the interaction between Arabidopsis and P. brassicae suggests an elevated level of the defence hormone SA in tissues, the expression of the SAGT gene encoding SA glucosyltransferase was monitored. SAGT is believed to be indirectly linked to the storage of SA in plant cells; however, the expression of SAGT in this research was not significantly altered in infected relative to control roots. In addition to glycosylation, the conjugation of SA to amino acids has been reported. The protein AtGH3.5 was found to conjugate both auxin and SA to several amino acids (Zhang et al., 2007). The expression of AtGH3.5 was slightly up‐regulated on P. brassicae infection during a period of 10 to 28 days after inoculation (Jahn et al., 2013).
Some SA‐related mutants show altered responses to the clubroot pathogen
Arabidopsis SA‐related mutant lines showing a constitutively activated immune system (cpr1 and dnd1) and SA‐deficient lines (NahG, sid2 and npr1) impaired in SA signalling are essential tools in understanding the critical components of the SA pathway in the defence response; therefore, we compared the interactions of the wt and these lines with the Australian and ‘e3’ isolates of P. brassicae. Mutants with a constitutively activated immune system (cpr1), constant expression of PR genes and elevated SA levels, and dnd1, which does not produce a hypersensitive response (HR), but also has elevated SA levels and constant PR expression, may possess the ability to control the disease associated with P. brassicae. Our results revealed that all of the tested lines, except cpr1 and dnd1, were highly susceptible to the Australian isolate; however, SA treatment was only able to further enhance disease resistance after P. brassicae inoculation in the dnd1, but not cpr1, mutant. This suggests that their resistance might not be a result of PR‐1 expression alone, but also caused by other immune reactions that cannot be triggered only by SA. Both mutants have pleiotropic phenotypes, as the mutations do not affect SA biosynthesis or metabolism directly. The mutated gene in dnd1 is a cyclic nucleotide‐gated ion channel protein (Genger et al., 2008), whereas the cpr1 mutation affects an F‐box protein that is thought to negatively regulate a resistance protein (Gou et al., 2012). Additional functions might be responsible for the different responses of these two mutant lines in relation to P. brassicae infection.
So far, the dnd1 mutant has been used primarily with foliar pathogens, such as Pseudomonas syringae. Yu et al. (1998) compared the disease severity in Col‐0 and dnd1 following P. syringae inoculation and discovered that the lack of an HR in dnd1 did not affect the overall defence response to the pathogen, whereas Col‐0 was severely infected. They probed for PR‐1 using an RNA blot and determined that the expression level of PR‐1 in dnd1 was almost 10 times greater than that in Col‐0 in both infected and control tissue. This is in agreement with our findings that PR‐1 is constantly expressed in Arabidopsis infected by the Australian isolate of P. brassicae. However, our results showed that dnd1 was susceptible to P. brassicae, and this suggests that constant PR‐1 expression is not crucial for Arabidopsis resistance against P. brassicae. Thus, SA‐mediated resistance of dnd1 shows different effectiveness against different pathogens.
It has been shown that the Arabidopsis npr1 line, deficient in SA signalling, is susceptible to the ‘e3’ isolate of P. brassicae (Siemens et al., 2002). We found that the mutants npr1, NahG and sid2 were highly susceptible to infection when shoot weight was taken as a criterion, which indicates that SA does not play a role in the interaction. We further recorded that, although the line npr1 showed a significantly reduced shoot weight after P. brassicae infection, relative to Col‐0 and NahG, the gall score was low (1.8). This suggests that P. brassicae‐induced production of the signals needed for growth in npr1 is significantly lower than that in Col‐0 and NahG root cells. The SA‐deficient line NahG (gall score 3.0) and the SA biosynthesis‐deficient line sid2 (gall score 3.7) showed a susceptible interaction, indicating that the SA levels that are synthesized on infection do not elicit sufficient defence responses to restrict the pathogen.
We found that the biosynthesis of SA in Arabidopsis is increased on P. brassicae infection (Figs 3A and 4). Agarwal et al. (2011) found that dipping Arabidopsis roots in SA prior to inoculation increased disease resistance, and Lovelock et al. (2013) revealed that exogenously applied SA increased the intracellular SA levels in P. brassicae‐infected Brassica oleracea which, in turn, resulted in more resistant plants. Therefore, we investigated whether exogenously applied SA would affect clubroot symptoms in Arabidopsis wt and SA mutants cpr1, dnd1, NahG, npr1 and sid2.
Exogenous SA did not protect wt against the pathogen, contrary to the results of Agarwal et al. (2011), but it can be assumed that the different application methods (treatment of the soil vs. dipping of the roots) or different isolates of P. brassicae could be responsible for these discrepancies. From the tested lines, the most resistant was cpr1; however, application of exogenous SA to the infected mutant did not affect the gall size or shoot weight. In the dnd1 mutant, SA reduced gall size, but did not affect shoot weight. It could be that, in the dnd1 mutant, SA transport from the roots, where it was applied, to the shoots was limited or, simply, the applied SA was fully utilized in the roots in order to reduce symptom development. This suggests that SA alone is not sufficient, and other SA‐independent effects triggered in these lines contribute to resistance.
The application of SA prior to P. brassicae inoculation did not reduce significantly the gall score in sid2 plants, but showed a positive effect on shoot weight and PR‐1 gene expression at both 2 and 3 wpi, whereas, in NahG, the application of SA did not show an effect on gall score or PR‐1 gene expression, and only a positive effect on shoot weight was observed. This discrepancy can be explained by the different SA reduction mechanisms in these two lines: sid2 is defective in the biosynthetic pathway, whereas, in NahG, a bacterial SA hydroxylase is constitutively expressed, resulting in the continuous degradation of SA. In the former case, SA can therefore reconstitute the phenotype, whereas, in the latter, the hydroxylase is also active towards the applied SA.
As, in some of the lines, the application of SA did not help to reduce galls or to maintain shoot weight, we further investigated the expression of an SA‐independent resistance gene PDF1.2 after P. brassicae infection in order to obtain information on whether this could be a resistance pathway in which future research should be directed. However, for this gene, no pattern of induction in P. brassicae or concomitant SA treatment was found in wt or mutants, even though it was shown for cpr mutants that an NPR (non‐expressor of pathogenesis‐related protein)‐independent, but SA‐mediated resistance response involved players in the jasmonate (JA)/ethylene pathways (Clarke et al., 2000). In addition, the broad spectrum resistance of dnd1 was shown to involve multiple resistance pathways (Genger et al., 2008), and therefore other possible signalling components should be investigated for possible interactions in the Arabidopsis–P. brassicae system. Recently, Gravot et al. (2012) and Lemarié et al. (2015) have found that JA signalling is induced in the Arabidopsis ecotype Col‐0 on P. brassicae infection. However, Lemarié et al. (2015) concluded that the SA pathway appears to be more efficient than the JA pathway against clubroot infection in a partially resistant interaction with the ecotype Bur‐0. It is known that ‘these two pathways do not function independently, but rather influence each other through a complex network of regulatory interactions’ (Kunkel and Brooks, 2002). Proietti et al. (2013) found a negative crosstalk on the combined action of SA and JA in Arabidopsis, and revealed that, generally, SA exerts a negative crosstalk compared with the JA pathway. Their results support the idea that the combination of SA and JA induces reprogramming of the plant transcriptome (Proietti et al., 2013).
Conclusions
The infection of Arabidopsis with P. brassicae induced the expression of ICS1 and SAMT1 genes at 3 and 4 wpi. ICS1 expression confirms that SA is synthesized from isochorismate, whereas SAMT1 expression, together with previous data (Ludwig‐Müller et al., 2015), suggests that MeSA is a mobile form of SA in this plant–pathogen system. The plant accumulates SA as a defence signal to combat the pathogen, but, as shown for the addition of SA to roots, SA alone is insufficient for the induction of a resistance response. This might be a result of: (i) the way in which SA is applied (see also Agarwal et al., 2011; Lovelock et al., 2013); (ii) the P. brassicae isolate; or (iii) the existence of a methyltransferase from P. brassicae which is capable of methylating SA (Ludwig‐Müller et al., 2015). It was speculated in the work of Ludwig‐Müller et al. (2015) that this is one way in which the pathogen is able to decrease SA locally to reduce the defence response.
Some data from our mutant analyses support a role for SA in the homeostasis between host root and pathogen.
The Arabidopsis mutant cpr1, revealing a constitutive resistance response, provided evidence that PR gene‐mediated resistance could protect plants against the Australian isolate of P. brassicae; however, exogenous application of SA in cpr1 did not reduce severity.
Line dnd1 showed an interesting pattern after SA application: galls were reduced and the shoot recovered.
The addition of SA to SA‐deficient mutants failed to significantly reduce disease severity; this was expected as NahG degrades exogenous SA and the npr1 mutant is deficient in SA signalling.
The sid2 shoots, after addition of SA, positively reacted against P. brassicae infection in terms of fresh weight because, here, the defects in SA biosynthesis could be restored.
For future studies, it might be useful to include Arabidopsis overexpressing ICS1 in order to determine whether a constitutive SA supply can alter clubroot symptoms more effectively than treatment with SA. However, it should be noted that a constitutive SA supply to the plant might also alter the phenotype in a non‐desirable manner.
As the constitutive activation of the immune system yields small plants, the goal of future research will be to find a way to elicit the resistance response on infection. SA treatment alone does not seem to be sufficient. One solution could be by biocontrol organisms, which could induce the plant's immune system, as shown for the interaction of Brassica napus with P. brassicae, where JA/ethylene pathways could be induced (Lahlali et al., 2014).
Experimental Procedures
Growth and maintenance of A. thaliana and Brassica rapa
To investigate the biosynthesis of SA and its effect on the plant–pathogen interaction, A. thaliana ecotype Col‐0 and mutant lines cpr, dnd1, NahG, sid2 and npr1 were used. Brassica rapa ssp. pekinensis was used as a host for P. brassicae spore cultivation. Plants were either grown on filtered and autoclaved soil (Stender B400, Schermbeck, Germany) mixed with sand in a 4 : 1 ratio or on a commercial propagation mix (Debco, Pty Ltd. Tyabb, Victoria, Australia). Arabidopsis seeds were sterilized and sown on wet filter paper in a covered Petri dish for 3 days at 4 °C. Plants were grown under standard glasshouse conditions at 21–23 ºC.
Synthesis and identification of 13C‐CA
Synthesis of 13C‐CA was undertaken using 13C6‐labelled glucose (Campro Scientific, Berlin, Germany). For the production of 13C‐CA, the E. coli KA12 mutant constructed by Kast et al. (1996) was used. Isolation of 13C‐CA was performed according to Gibson (1970). The identity of 13C‐CA was confirmed by comparison of its mass spectrum with that of an unlabelled standard using direct injection on a Finnigan MAT95 mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with electron collision ionization at 70 eV and 250 °C, positively charged ion mode and a resolution of 1000.
Plasmodiophora brassicae and infection of plants
The single spore isolate ‘e3’ was used in qPCR, and experiments with sid2 and experiments on SA biosynthesis, whereas the Australian isolate was used in experiments assessing the plant–pathogen interaction with all other mutants.
The single spore isolate ‘e3’ was prepared from clubroot galls of B. rapa in Dresden, Germany. The Australian P. brassicae isolate was obtained from inoculated cabbage from Lindenow, Vic., Australia (Department of Primary Industries; ECD code 16/2/31). Inoculi were prepared according to Siemens et al. (2002) or Agarwal et al. (2011).
Arabidopsis plants were 7 or 14 days old when inoculated with P. brassicae spores. Each plant was inoculated by injecting the soil around the plant with 1 mL of isolate ‘e3’ (7 × 106 spores/mL) or 125 µL of the Australian isolate (106 spores/mL). Gall scoring was as follows: 0, no galling; 1, small gall on lateral root, with no adverse effect to plant health; 2, more than one small gall on lateral root, with no adverse effect on plant health; 3, galling on tap root, small galls on laterals, plant appears to be stressed; 4, large galling on taproot and lateral roots, plant health is severely compromised (Klewer et al., 2001). The shoot index was calculated by dividing the fresh weight of infected plants by the weight of control plants (Siemens et al., 2002).
Collection and storage of plant material and analysis of shoot weight
Arabidopsis plants were collected at 2, 3 and 4 wpi. On collection, plants were either incubated with SA precursors (13C‐CA and/or PheD5) for the purpose of biosynthesis experiments or were frozen under liquid nitrogen and stored at −20 °C. Appropriate tissue was dried by lyophilization for 48 h in a CHRIST ALPHA lyophilizer 1‐2 (Christ, Osterode am Harz, Germany) at −60 °C and 0.01 mbar; tissue was chopped on a TissueLyser (Qiagen, Retsch, Hombrechtikon, Switzerland) and stored at −20 °C until use for an experiment. Shoot weight (leaf rosette and inflorescence) was assessed at 2 and 3 wpi.
Treatment of Arabidopsis with SA precursors (PheD5 and/or 13C‐CA)
Arabidopsis plants were immersed in 100 mm 2‐(N‐morpholino)ethanesulfonic acid (MES) buffer (pH 6.5) to which was added: (i) PheD5 at a final concentration of 1 mm; (ii) 13C‐CA at a final concentration of 30 μm or (iii) PheD5 at a final concentration of 1 mm and 13C‐CA at a final concentration of 30 μm. Plants were incubated for 24 h, and then the roots/galls were cut off, thoroughly washed with water, frozen under liquid nitrogen and stored at −20 °C.
Extraction of SA from Arabidopsis roots and gas chromatography‐mass spectrometry (GC‐MS) analysis
SA was extracted according to Chen et al. (1988), with modifications. Briefly Arabidopsis roots were separately homogenized in a mortar with a (65% isopropanol) : (35% 200 mm imidazole, pH 7.0) buffer. The homogenate was centrifuged and the supernatant was dried under nitrogen flux until an aqueous phase was reached. The pH value of the aqueous phase was set to 3.0 and the same volume (as that of the aqueous phase) of ethyl acetate was added; this was followed by vortexing and centrifugation. The upper organic phase containing SA was removed and retained. The extraction step was repeated three times and the organic phases were pooled and dried under a nitrogen flux. SA was derivatized before analysis by GC‐MS according to Cohen (1984): 50 μL of ethyl acetate and 950 μL of diazomethane were added to SA, incubated for 15 min at room temperature, the mixture was dried under nitrogen flux and the MeSA obtained was redissolved in 50 μL of ethyl acetate.
A Phenomenex (Aschaffenburg, Germany) ZB‐5 column (length 30 m, inner diameter 0.25 mm, film thickness 0.25 μm) was used to separate the compounds. Aliquots of 1 μL were injected into the GC‐MS system. The temperature program was 60 °C for 1 min, going to 220 °C at a rate of 25 °C/min, and then to 280 °C at 20 °C/min, and held for 5 min at 280 °C. MeSA eluted at 5.5 min. The diagnostic ion used for the quantification of endogenous MeSA was m/z 120, whereas those used for the quantification of exogenous MeSA were m/z 125 for MeSA that originates from PheD5, and m/z 122, m/z 124 and m/z 126 for MeSA that originates from 13C‐CA.
Isolation of RNA, cDNA synthesis and gene expression in Arabidopsis
Infected and control root tissues at 3 and 4 wpi were used for the isolation of RNA and the production of cDNA. Total RNA for qPCR was isolated using commercial product TRI Reagent Solution (Ambion, Austin, TX, USA) according to the manufacturer's instructions. This RNA was further processed with TURBO DNase (Ambion) according to the manufacturer's instructions in order to be cleaned from DNA residues. The concentration of RNA was determined spectrophotometrically using a NanoDrop 2000c (Thermo Fisher Scientific) device. Prepared RNA was further used for cDNA synthesis.
Synthesis of cDNA was performed using MuLV reverse transcriptase according to the manufacturer's instructions (Applied Biosystems, Carlsbad, CA, USA). As a template, 10 μg of total RNA processed with DNase was used; cDNA was stored at −80 °C. All primers, oligo d(T)16, random hexamers and those from the already prepared reaction mixtures, for the determination of expression of a certain gene were from Applied Biosystems.
RNA isolation from Arabidopsis shoots inoculated with P. brassicae for RT‐PCR was performed at 2 and 3 wpi following the protocol of Lovelock et al. (2013).
PCR primers and conditions
The real‐time expression of the following genes was determined: the gene for SA methyltransferase (AT4G39460 labelled as SAMT1), for isochorismate synthase 1 associated with the biosynthesis of SA (AT1G74710 labelled as ICS1) and for SA glucosyltransferase (AT2G43820 labelled as SAGT). The genes for F‐box protein (AT5G15710) and mitosis protein YLS8 (AT5G08290) were used as endogenous controls. Each sample for each gene was performed in triplicate. The comparative CT method for relative quantification was applied. The measurements were performed on a 7300 Real‐Time PCR machine (Applied Biosystems). Two microlitres of cDNA were mixed with 1 μL of reaction mixture prepared for the determination of the expression of a certain gene and 10 μL of a corresponding buffer (TaqMan Gene Expression Master Mix and Assays, Applied Bioystems), and made up to 20 μL with Diethylpyrocarbonate‐water pre‐cleaned from nucleases. The reaction was carried out as follows: 10 min at 95 °C for template denaturation, 40 cycles each of 15 s at 95 °C for denaturation and 1 min at 60 °C for primer ligation and elongation.
The following gene expression assays were used for SA metabolism analysis: At02174493_g1 for AT4G39460, At02286260_g1 for AT1G74710, At02327040_gH for AT2G43820, At02200102_s1 for AT5G15710, and At02183416_g1 for AT5G08290.
The following primers were used for gene expression analysis of the plant–pathogen interaction: PR‐1, 5′‐AAGAGGCAACTGCAGACTCA‐3′ and 5′‐TCTCGCTAACCCACATGTTC‐3′; PDF 1.2, 5′‐TTGCTGCTTTCGACGCA‐3′ and 5′‐TGTCCCACTTGGCTTCTCG‐3′; actin (reference gene), 5′‐ACGTGGACATCAGGAAGGAC‐3′ and 5′‐GAACCACCGATCCAGACACT‐5′; YLS (Yellow leaf specific 8, reference gene), 5′‐AAGAGCGTCTCGTCGTCATT‐3′ and 5′‐CGGCTTCCAAATTTCATTTC‐3′.
PCR for plant–pathogen interaction was performed using the following cycling conditions: 95 °C for 3 min, followed by 28 cycles for PDF1.2 and PR‐1, and 35 cycles for actin of 95 °C for 30 s, 52 °C for 30 s and 72 °C for 30 s. PCR products were visualized with ethidium bromide‐stained 2% agarose gels under UV after running at 80 V for 40 min.
Statistical analysis
Statistical analysis was performed using SPSS (PASW 18.0) (IBM, Armonk, NY, USA) and a statistics package (Microsoft Excel 2010). In all experiments, one‐way analyses of variance (ANOVAs), specifically Tukey's test and Fisher's least‐significant difference (LSD) test, with P = 0.05, were used to analyse significant differences between each treatment group.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Expected labelling pattern for chorismic acid [molecular weight (MW), 226] synthesized by Escherichia coli KA12 mutant from 13C6‐glucose‐containing medium. Only those labelling positions which are present in the molecule as determined by mass spectrometry are shown (indicated by asterisks) (see Fig. S2). A 13C‐label in all 10 possible C‐positions did not occur in chorismic acid. PEP, phosphoenolpyruvate; E‐4‐P, erythrose‐4‐phosphate.
Fig. S2 Mass spectra of 13C‐labelled (a) and unlabelled (b) chorismic acid. The numbers show the expected isotope masses for the different labelling possibilities (see Fig. S1).
Fig. S3 PDF1.2 expression in Arabidopsis wild‐type and mutant lines following Australian (Col‐0, cpr1, dnd1, NahG, npr1) (A) and ‘e3’ (Col‐0, sid2) (B) Plasmodiophora brassicae infection and infection together with salicylic acid (SA) treatment. Expression levels were determined at 2 and 3 weeks post‐inoculation. Note that this experiment is from the same reverse transcription‐polymerase chain reaction (RT‐PCR) as PR‐1 expression, and so the reference gene expression can be found in Fig. 7.
Table S1 Expression of the F‐box gene AT5G15710 in Plasmodiophora brassicae‐infected vs. control roots of Arabidopsis thaliana determined by two different microarray experiments. The European Clubroot Differential (ECD) code is given for the Australian field isolate. The German isolate is ‘e3’.
Acknowledgements
This research was partially funded by the Department of Primary Industries (DPI) Victoria and Horticulture Australia Limited (HAL) using the vegetable levy and matched funds from the Australian Government. This work was also partially supported by a German–Croatian Mobility Grant from the Deutsche Akademische Austauschdienst (DAAD) to JL‐M and GR. DAL was funded by a Deakin University‐DPI Scholarship. We thank Christine Lüth (Biochemistry, TU Dresden) for the preparation of chorismic acid. We would also like to acknowledge Dr Rosalia Deeken, University of Wuerzburg, Germany, for donating the sid2 seeds.
Contributor Information
David A. Lovelock, Email: david.lovelock@nt.gov.au
Ivana Šola, Email: ivana.sola@biol.pmf.hr.
References
- Agarwal, A. , Kaul, V. , Faggian, R. and Cahill, D.M. (2009) Development and use of a model system to monitor clubroot disease progression with an Australian field population of Plasmodiophora brassicae . Australas. Plant Pathol. 38, 120–127. [Google Scholar]
- Agarwal, A. , Kaul, V. , Faggian, R. , Rookes, J.E. , Ludwig‐Müller, J. and Cahill, D.M. (2011) Analysis of global host gene expression during the primary phase of the Arabidopsis thaliana–Plasmodiophora brassicae interaction. Funct. Plant Biol. 38, 462–478. [DOI] [PubMed] [Google Scholar]
- Attaran, E. , Zeier, T.E. , Griebel, T. and Zeier, J. (2009) Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell, 21, 954–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catinot, J. , Buchala, A. , Abou‐Mansour, E. and Metraux, J.P. (2008) Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana . FEBS Lett. 582, 473–478. [DOI] [PubMed] [Google Scholar]
- Chen, K.H. , Miller, A.N. , Patterson, G.W. and Cohen, J.D. (1988) A rapid and simple procedure for purification of indole‐3‐acetic acid prior to GC‐SIM‐MS analysis. Plant Physiol. 86, 822–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, J.D. , Volko, S.M. , Ledford, H. , Ausubel, F.M. and Dong, X. (2000) Roles of salicylic acid, jasmonic acid, and ethylene in cpr‐induced resistance in Arabidopsis. Plant Cell, 12, 2175–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J.D. (1984) Convenient apparatus for the generation of small amounts of diazomethane. J. Chromatogr. A, 303, 193–196. [Google Scholar]
- Coquoz, J.L. , Buchala, A. and Métraux, J.P. (1998) The biosynthesis of salicylic acid in potato plants. Plant Physiol. 117, 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, J.V. and Mills, J.D. (2004) Uptake of salicylic acid 2‐O‐b‐D‐glucose into soybean tonoplast vesicles by an ATP‐binding cassette transporter‐type mechanism. Physiol. Plant. 120, 603–612. [DOI] [PubMed] [Google Scholar]
- Dean, J.V. , Shah, R.P. and Mohammed, L.A. (2003) Formation and vacuolar localization of salicylic acid glucose conjugates in soybean cell suspension cultures. Physiol. Plant. 118, 328–336. [DOI] [PubMed] [Google Scholar]
- Dean, J.V. , Mohammed, L.A. and Fitzpatrick, T. (2005) The formation, vacuolar localization, and tonoplast transport of salicylic acid glucose conjugates in tobacco cell suspension cultures. Planta, 221, 287–296. [DOI] [PubMed] [Google Scholar]
- Dempsey, D.A. , Shah, J. and Klessig, D.F. (1999) Salicylic acid and disease resistance in plants. Crit. Rev. Plant Sci. 18, 547–575. [Google Scholar]
- Dempsey, D.A. , Vlot, A.C. , Wildermuth, M.C. and Klessig, D.F. (2011) Salicylic acid biosynthesis and metabolism In: The Arabidopsis Book (Torii K., ed.). The American Society of Plant Biologists, Rockville, USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, G.R. (2009) Plasmodiophora brassicae in its environment. J. Plant Growth Regul. 28, 212–228. [Google Scholar]
- Donald, E.C. , Cross, S.J. , Lawrence, J.M. and Porter, I.J. (2006) Pathotypes of Plasmodiophora brassicae, the cause of clubroot, in Australia. Ann. Appl. Biol. 148, 239–244. [Google Scholar]
- Durrant, W.E. and Dong, X. (2004) Systemic acquired resistance. Annu. Rev. Phytopathol. 42, 185–209. [DOI] [PubMed] [Google Scholar]
- Genger, R.K. , Jurkowski, G.I. , McDowell, J.M. , Lu, H. , Jung, H.W. , Greenberg, J.T. and Bent, A.F. (2008) Signaling pathways that regulate the enhanced disease resistance of Arabidopsis “defense, no death” mutants. Mol. Plant–Microbe Interact. 21, 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, F. (1970) Preparation of chorismic acid. Methods Enzymol. 17, 362–364. [Google Scholar]
- Gou, M. , Shi, Z. , Zhu, Y. , Bao, Z. , Wang, G. and Hua, J. (2012) The F‐box protein CPR1/CPR30 negatively regulates R protein SNC1 accumulation. Plant J. 69, 411–420. [DOI] [PubMed] [Google Scholar]
- Grant, M. and Lamb, C. (2006) Systemic immunity. Curr. Opin. Plant Biol. 9, 414–420. [DOI] [PubMed] [Google Scholar]
- Gravot, A. , Deleu, C. , Wagner, G. , Lariagon, C. , Lugan, R. , Todd, C. , Wendehenne, D. , Delourme, R. , Bouchereau, A. and Manzanares‐Dauleux, M.J. (2012) Arginase induction represses gall development during clubroot infection in Arabidopsis. Plant Cell Physiol. 53, 901–911. [DOI] [PubMed] [Google Scholar]
- Heil, M. and Ton, J. (2008) Long‐distance signalling in plant defence. Trends Plant Sci. 13, 264–272. [DOI] [PubMed] [Google Scholar]
- Jahn, L. , Mucha, S. , Bergmann, S. , Horn, C. , Siemens, J. , Staswick, P. , Steffens, B. and Ludwig‐Müller, J. (2013) The clubroot pathogen (Plasmodiophora brassicae) influences auxin signaling to regulate auxin homeostasis. Plants, 2, 726–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast, P. , Asif‐Ullah, M. and Hilvert, D. (1996) Is chorismate mutase a prototypic entropy trap? – Activation parameters for the Bacillus subtilis enzyme. Tetrahedron Lett. 37, 2691–2694. [Google Scholar]
- Klewer, A. , Luerßen, H. , Graf, H. and Siemens J. (2001) RFLP markers to characterise Plasmodiophora brassicae single‐spore‐isolates with different virulence patterns. J. Phytopathol. 149, 1–7. [Google Scholar]
- Kuginuki, Y. , Yoshikawa, H. and Hirai, M. (1999) Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot‐cultivars of Chinese cabbage (Brassica rapa L. ssp. Pekinensis). Eur. J. Plant Pathol. 105, 327–332. [Google Scholar]
- Kumar, D. and Klessig, D.F. (2008) The search for the salicylic acid receptor led to discovery of the SAR signal receptor. Plant Signal. Behav. 3, 691–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel, B.N. and Brooks, D.M. (2002) Cross talk between signalling pathways in pathogen defense. Curr. Opin. Plant Biol. 5, 325–331. [DOI] [PubMed] [Google Scholar]
- Lahlali, R. , McGregor, L. , Song, T. , Gossen, B.D. , Narisawa, K. and Peng, G. (2014) Heteroconium chaetospira induces resistance to clubroot via upregulation of host genes involved in jasmonic acid, ethylene, and auxin biosynthesis. PLoS One, 9(4), e94144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarié, S. , Robert‐Seilaniantz, A. , Lariagon, C. , Lemoine, J. , Marnet, N. , Jubault, M. , Manzanares‐Dauleux M.J. and Gravot, A. (2015) Both the jasmonic acid and the salicylic acid pathways contribute to resistance to the biotrophic clubroot agent Plasmodiophora brassicae in Arabidopsis. Plant Cell Physiol. 56, 2158–2168. [DOI] [PubMed] [Google Scholar]
- Love, A.J. , Laval, V. , Geri, C. , Laird, J. , Tomos, A.D. , Hooks, M.A. and Milner, J.J. (2007) Components of Arabidopsis defense‐ and ethylene‐signalling pathways regulate susceptibility to Cauliflower mosaic virus by restricting long‐distance movement. Mol. Plant–Microbe Interact. 20, 659–670. [DOI] [PubMed] [Google Scholar]
- Lovelock, D. , Donald, C. , Conlan, X. and Cahill, D. (2013) Salicylic acid suppression of clubroot in broccoli (Brassica oleracea var. italica) caused by the obligate biotroph Plasmodiophora brassicae . Australas. Plant Pathol. 42, 141–153. [Google Scholar]
- Ludwig‐Müller, J. and Schuller, A. (2008) What can we learn from clubroots: alterations in host roots and hormone homeostasis caused by Plasmodiophora brassicae . Eur. J. Plant Pathol. 121, 291–302. [Google Scholar]
- Ludwig‐Müller, J. , Kasperczyk, N. , Schubert, B. and Hilgenberg, W. (1995) Identification of salicylic acid in Chinese cabbage and its possible role in the development of the clubroot disease. Curr. Top. Phytochem. (Life Sci. Adv.) 14, 39–45. [Google Scholar]
- Ludwig‐Müller, J. , Jülke, S. , Geiß, K. , Richter, F. , Šola, I. , Rusak, G. , Mithöfer, A. , Keenan, S. and Bulman, S. (2015) A novel methyltransferase from the intracellular pathogen Plasmodiophora brassicae participates in methylation of salicylic acid. Mol. Plant Pathol. 16, 349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa, N.R. , Kim, H.K. , Choi, Y.H. , Erkelens, C. , Lefeber, A.W.M. , Spijksma, G. , van der Heijden, R. and Verpoorte, R. (2009) Biosynthesis of salicylic acid in fungus elicited Catharanthus roseus cells. Phytochemistry, 70, 532–539. [DOI] [PubMed] [Google Scholar]
- Ogawa, D. , Nakajima, N. , Seo, S. , Mitsuhara, I. , Kamada, H. and Ohashi, Y. (2006) The phenylalanine pathway is the main route of salicylic acid biosynthesis in Tobacco mosaic virus‐infected tobacco leaves. Plant Biotechnol. 23, 395–398. [Google Scholar]
- Park, S.W. , Kaimoyo, E. , Kumar, D. , Mosher, S. and Klessig, D.F. (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science, 318, 113–116. [DOI] [PubMed] [Google Scholar]
- Päsold, S. , Siegel, I. , Seidel, C. , and Ludwig‐Müller, J. (2010) Flavonoid accumulation in Arabidopsis thaliana root galls caused by the obligate biotrophic pathogen Plasmodiophora brassicae . Mol. Plant Pathol., 11, 545–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti, S. , Bertini, L. , Timperio, A.M. , Zolla, L. , Caporale, C. and Caruso, C. (2013) Crosstalk between salicylic acid and jasmonate in Arabidopsis investigated by an integrated proteomic and transcriptomic approach. Mol. BioSyst. 9, 1169–1187. [DOI] [PubMed] [Google Scholar]
- Ribnicky, D.M. , Shulaev, V. and Raskin, I. (1998) Intermediates of salicylic acid biosynthesis in tobacco. Plant Physiol. 118, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas‐San Vicente, M. and Plasencia, J. (2011) Salicylic acid beyond defence: its role in plant growth and development. J. Exp. Bot. 62, 3321–3338. [DOI] [PubMed] [Google Scholar]
- Siemens, J. , Nagel, M. , Ludwig‐Müller, J. and Sacristán, M.D. (2002) The interaction of Plasmodiophora brassicae and Arabidopsis thaliana: parameters for disease quantification and screening of mutant lines. J. Phytopathol. 150, 592–605. [Google Scholar]
- Siemens, J. , Keller, I. , Sarx, J. , Kunz, S. , Schuller, A. , Nagel, W. , Schmülling, T. , Parniske, M. and Ludwig‐Müller, J. (2006) Transcriptome analysis of Arabidopsis clubroots indicate a key role for cytokinins in disease development. Mol. Plant–Microbe Interact. 19, 480–494. [DOI] [PubMed] [Google Scholar]
- van Wees, S.C.M. , de Swart, E.A.M. , van Pelt, J.A. , van Loon, L.C. and Pieterse, C.M. (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate‐ and jasmonate‐dependent defense pathways in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA, 97, 8711–8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth, M.C. , Dewdney, J. , Wu, G. and Ausubel, F.M. (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature, 414, 562–565. [DOI] [PubMed] [Google Scholar]
- Yu, I.C. , Parker, J. and Bent, A.F. (1998) Gene‐for‐gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc. Natl. Acad. Sci. USA, 95, 7819–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Li, Q. , Li, Z. , Staswick, P.E. , Wang, M. , Zhu, Y. and He, Z. (2007) Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis–Pseudomonas syringae interaction. Plant Physiol. 145, 450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Expected labelling pattern for chorismic acid [molecular weight (MW), 226] synthesized by Escherichia coli KA12 mutant from 13C6‐glucose‐containing medium. Only those labelling positions which are present in the molecule as determined by mass spectrometry are shown (indicated by asterisks) (see Fig. S2). A 13C‐label in all 10 possible C‐positions did not occur in chorismic acid. PEP, phosphoenolpyruvate; E‐4‐P, erythrose‐4‐phosphate.
Fig. S2 Mass spectra of 13C‐labelled (a) and unlabelled (b) chorismic acid. The numbers show the expected isotope masses for the different labelling possibilities (see Fig. S1).
Fig. S3 PDF1.2 expression in Arabidopsis wild‐type and mutant lines following Australian (Col‐0, cpr1, dnd1, NahG, npr1) (A) and ‘e3’ (Col‐0, sid2) (B) Plasmodiophora brassicae infection and infection together with salicylic acid (SA) treatment. Expression levels were determined at 2 and 3 weeks post‐inoculation. Note that this experiment is from the same reverse transcription‐polymerase chain reaction (RT‐PCR) as PR‐1 expression, and so the reference gene expression can be found in Fig. 7.
Table S1 Expression of the F‐box gene AT5G15710 in Plasmodiophora brassicae‐infected vs. control roots of Arabidopsis thaliana determined by two different microarray experiments. The European Clubroot Differential (ECD) code is given for the Australian field isolate. The German isolate is ‘e3’.


