Summary
Plant‐pathogenic fungi produce cellulases. However, little information is available on cellulase as an elicitor in plant–pathogen interactions. Here, an endocellulase (EG1) was isolated from Rhizoctonia solani. It contains a putative protein of 227 amino acids with a signal peptide and a family‐45 glycosyl hydrolase domain. Its aspartic acid (Asp) residue at position 32 was changed to alanine (Ala), resulting in full loss of its catalytic activity. Wild‐type and mutated forms of the endoglucanase were expressed in yeast and purified to homogeneity. The purified wild‐type and mutant forms induced cell death in maize, tobacco and Arabidopsis leaves, and the transcription of three defence marker genes in maize and tobacco and 10 genes related to defence responses in maize. Moreover, they also induced the accumulation of reactive oxygen species (ROS), medium alkalinization, Ca2+ accumulation and ethylene biosynthesis of suspension‐cultured tobacco cells. Similarly, production of the EG1 wild‐type and mutated forms in tobacco induced cell death using the Potato virus X (PVX) expression system. In vivo, expression of EG1 was also related to cell death during infection of maize by R. solani. These results provide direct evidence that the endoglucanase is an elicitor, but its enzymatic activity is not required for its elicitor activity.
Keywords: elicitor, fungal cellulase, Rhizoctonia solani
Introduction
The widespread soil‐borne pathogen Rhizoctonia solani (teleomorph, Thanatephorus cucumeris) is a basidiomycete fungus able to infect many plant species, including many commercially important crops, such as maize, rice, wheat, potato and cotton (Sneh et al., 1996). It infects plants mainly through the formation of infection pegs that penetrate plant tissues (Demirci and Doken, 1998). During the infection process, this fungus secretes cell wall‐degrading enzymes (CWDEs), such as cutinases (Baker and Bateman, 1978), pectinases (Jayasinghe et al., 2004), xylanases (Peltonen, 1995) and cellulases (Jayasinghe et al., 2004).
Cellulose is one of the major cell wall components in most plants. Cellulases catalyse the degradation of the β‐1,4‐glycosidic bonds in cellulose. They have been divided into three types: endoglucanases, exoglucanases and β‐glucosidases. Endoglucanases randomly hydrolyse internal glycosidic linkages, resulting in a rapid decrease in polymer length and a gradual increase in reducing sugar concentration. Exoglucanases hydrolyse cellulose chains by the removal of cellobiose from either the reducing or nonreducing ends, resulting in the rapid release of reducing sugars but little change in polymer length. Endoglucanases and exoglucanases act synergistically on cellulose to produce cellobiose, which is then cleaved by β‐glucosidase to glucose (Beguin and Aubert, 1994).
A number of plant‐pathogenic fungi produce cellulases (Carpita and Gibeaut, 1993). However, little attention has been paid to the role of cellulase in pathogenicity, compared with other CWDEs, such as cutinases, pectinases and xylanases (Annis and Goodwin, 1997; Lagaert et al., 2009). The importance of cellulases to the pathogenicity of plant‐pathogenic fungi is elusive. A few cellulases of plant‐pathogenic fungi have been shown to be involved in pathogenicity (Eshel et al., 2002; Muller et al., 1997; Sexton et al., 2000). It has been proposed that Macrophomina phaseolina utilizes an endocellulase for pathogenicity (Wang and Jones, 1995). A cellobiohydrolase is expressed in the initial infection phase of Claviceps purpurea on Secale cereale (Muller et al., 1997). The phytopathogenic fungus Alternaria alternata produces one endocellulase, which is an important factor in disease development in persimmon fruit (Eshel et al., 2002). Transcription of a cellobiohydrolase from Leptosphaeria maculans is detectable during infection of Brassica napus and Brassica juncea cotyledons and leaves (Sexton et al., 2000). However, disruption of the cellulase gene has almost always failed to show that it is essential for pathogenicity. Disruption of an exoglucanase gene in the maize pathogen Cochliobolus carbonum does not affect pathogenicity on maize (Sposato et al., 1995). Disruption of an endoglucanase gene (cel5A) from Botrytis cinerea results in a strain with identical pathogenicity as the wild‐type (WT) on tomato leaves (Espino et al., 2005). Fungal cellulase systems are very complex in nature. Most studies have revealed that fungi produce multiple forms of cellulase components (Annis and Goodwin, 1997). The multiplicity of individual cellulases with similar activities might be the reason why most gene knockout experiments do not support an essential role of individual cellulases in fungal pathogenicity (Espino et al., 2005).
At present, some conclusive data support the involvement of cellulases in pathogenesis, but much less information is available on cellulase as an elicitor in plant–pathogen interactions. Since cellulase was reported as an elicitor in 1988 (Threlfall and Whitehead, 1988; Whitehead et al., 1988), only a few studies have investigated the elicitor activity of cellulases (Ma, 2008; Mialoundama et al., 2009). In these studies, cellulases from nonpathogenic Trichoderma spp. were shown to induce sesquiterpenoid phytoalexin biosynthesis (Threlfall and Whitehead, 1988; Whitehead et al., 1988) and the accumulation of capsidiol in plants (Ma, 2008; Mialoundama et al., 2009). However, how cellulase functions as an elicitor in plant–pathogen interactions is not understood. In particular, whether the elicitor activity of cellulase is involved in its enzymatic activity is unknown. Here, we report the cloning, site‐directed mutagenesis and heterologous expression of an endoglucanase (EG1) from R. solani. Using the analysis of elicitor responses to study elicitor–plant interaction, together with mutational analysis and the Potato virus X (PVX) expression system, we demonstrate that EG1 is an elicitor, but its elicitor activity is independent of its enzymatic activity.
Results
Cloning and sequence analysis of the endoglucanase gene
Based on the nucleotide sequence of our previously cloned endoglucanase gene (GU372728) from R. solani AG‐1‐IA in 2009, we obtained an open reading frame (ORF) cDNA of the endoglucanase gene (termed Eg1) (Table S1, see Supporting Information). The ORF of the endoglucanase gene encodes a putative protein (termed EG1) with 227 amino acid residues. Using SignalP (http://www.cbs.dtu.dk/service/SignalP), a potential signal peptide was predicted to be amino acids 1–20, indicating that the endoglucanase is a secreted enzyme. The mature endoglucanase consists of 207 amino acids with a calculated molecular weight of 23.66 kDa (http://www.expasy.ch). The blastp search of the putative amino acid sequence of the endoglucanase revealed that it belongs to the family‐45 glycosyl hydrolases (GHs). Rhizoctonia solani EG1 shares 100% identity with an endoglucanase (ADV02787) in the R. solani genome reported by Zheng et al. (2013).
The alignment of R. solani EG1 with three other fungal endoglucanases of family 45, including Melanocarpus albomyces and Humicola insolens endoglucanases, whose domain structures have already been solved (Davies et al., 1996; Hirvonen and Papageorgiou, 2003), indicates that three motifs (TRYWD, PGGGV and WRF) are highly conserved among these endoglucanases (Fig. S1, see Supporting Information). These highly conserved motifs exhibit the characteristic sequence of family‐45 GHs. The mature protein structure of R. solani EG1 comprises a single catalytic domain without a cellulose binding domain, similar to most fungal endoglucanases of family‐45 GHs (Davies et al., 1993, 1996; Hirvonen and Papageorgiou, 2003). The sequence alignment of R. solani EG1 with M. albomyces and H. insolens endoglucanases also showed that the arrangement of the catalytic domain structures was conserved. Among the conserved residues of R. solani EG1, two amino acid residues (D32 and D142) were predicted to be the catalytic sites (Fig. S1).
Expression of WT and mutant forms of EG1 in Pichia pastoris
Using site‐directed mutagenesis, the codon GAC coding for the aspartic acid (Asp, D) residue at position 32 of the WT form of Eg1 was changed to GCC coding for alanine (Ala, A) to produce the mutated form of WT, D32A. The WT and mutant forms of EG1 were expressed in yeast and purified to sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) homogeneity. The molecular weight of purified WT and mutant forms of EG1 was about 28.0 kDa with SDS‐PAGE (Fig. 1A). This value is close to the 23.66 kDa estimated from the deduced amino acid sequence of the endoglucanase. No visible protein band was found by SDS‐PAGE in the corresponding protein preparation (used as a check sample, CK) from culture filtrate of yeast transformed with the empty plasmid pPIC9K. The mutated form of EG1 had lost all enzymatic activity, and CK had no enzymatic activity compared with the WT form (Fig. 1B).
Figure 1.
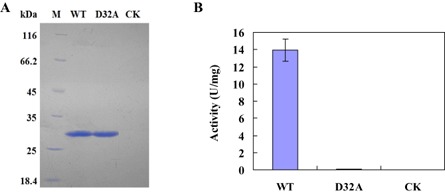
(A) Sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) of the purified enzymes of EG1 from Rhizoctonia solani. WT, the wild‐type form of EG1; D32A, the mutated form (D32A) of EG1; M, molecular weight marker; CK, check sample. (B) Cellulase activity (U) of the wild‐type (WT) form of EG1, the mutant form (D32A) of EG1 and the check sample (CK). One unit (U) of cellulase activity was defined as the amount of cellulase that catalysed the liberation of reducing sugar equivalent to 1.0 μg glucose/min under assay conditions. Three independent biological replicates were used for each protein.
Elicitor responses induced by WT and mutant forms of EG1
It is common for elicitors to induce cell death in plants. Purified WT and mutant forms of EG1 at concentrations of 0–100 nm were injected into maize leaves to test their capacity to induce cell death. WT and mutant forms of EG1 induced cell death in maize, tobacco and Arabidopsis leaves (Fig. 2A,C,D). Obvious symptoms in maize leaves appeared at 3 days after inoculation of EG1 at a concentration of 40 nm. Injection of CK did not result in any visible symptoms in maize leaves. In addition, a cellulase (C61F) and a manganese superoxide dismutase (SOD) of Chaetomium thermophilum and a cellulase (T61F) of Thermoascus aurantiacus were purified using nickel affinity chromatography from a culture filtrate of yeast transformed with the plasmids pPIC9K/C61F, pPIC9K/T61F and pPIC9K/SOD (Fig. S2A–C, see Supporting Information). The three unrelated protein samples, C61F, T61F and SOD, were injected into maize leaves and did not induce cell death (Fig. 2B).
Figure 2.
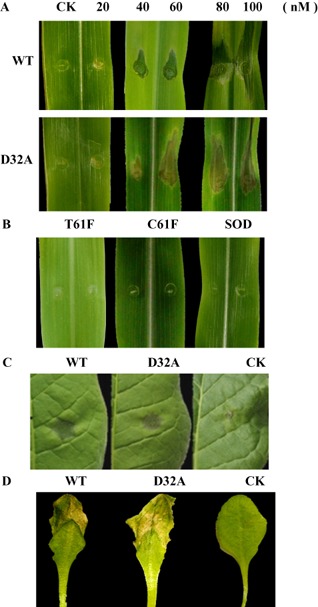
Induction of death in plant leaves by EG1 from Rhizoctonia solani and unrelated enzymes from other fungi. (A) Maize leaves 3 days after inoculation with the wild‐type (WT) form of EG1, the mutant (D32A) form of EG1 and the check sample (CK) at concentrations of 0–100 nm for WT and D32A. (B) Maize leaves 3 days after inoculation with three unrelated proteins at a concentration of 40 nm: a cellulase (C61F, KC441879) of Chaetomium thermophilum, a manganese superoxide dismutase (SOD, EF569978) of Chaetomium thermophilum and a cellulase (T61F, KF170230) of Thermoascus aurantiacus. (C) Tobacco leaves 3 days after inoculation with the wild‐type (WT) form of EG1, the mutant (D32A) form of EG1 and the check sample (CK) at a concentration of 40 nm. (D) Arabidopsis leaves 2 days after inoculation with the wild‐type (WT) form of EG1, the mutant (D32A) form of EG1 and the check sample (CK) at a concentration of 40 nm. Three independent biological replicates were used for each plant leaf.
As it was shown that EG1 could induce cell death of maize and tobacco leaves, we investigated the expression patterns of defence marker genes in these plants after EG1 treatment by reverse transcription‐polymerase chain reaction (RT‐PCR). These marker genes included the pathogenesis‐related 1a (PR1a) gene, peroxidase (POD) gene and phenylalanine ammonia‐lyase (PAL) gene. WT and mutant forms of EG1 clearly induced greater expression of PAL, POD and PR1a in maize and tobacco leaves than did CK (Fig. 3A). In addition to the above three defence marker genes, the expression of 10 selected genes related to defence responses in the maize genome, such as chitinase, β‐1,3‐glucanase, pathogenesis‐related protein, alternative oxidase, thaumatin and glutathione transferase (Table S1), was analysed by real‐time quantitative RT‐PCR (RT‐qPCR). The analysis showed that these 10 genes exhibited similar up‐regulated expression patterns in maize leaves injected with WT and mutant forms of EG1 (Fig. 3B).
Figure 3.

Induction of defence response genes by EG1 from Rhizoctonia solani. (A) Detection of transcripts of three defence response genes [phenylalanine ammonia‐lyase (PAL), peroxidase (POD) and pathogen‐related 1a (PR1a)] and elongation factor 1 (EF1‐a) using reverse transcription‐polymerase chain reaction (RT‐PCR) in maize and tobacco leaves 3 days after inoculation with the wild‐type (WT) form of EG1, mutant (D32A) form of EG1 and check sample (CK) at a concentration of 40 nm. Three independent biological replicates were used for each plant leaf. (B) Patterns of gene expression of 10 selected genes (1–10) in WT and D32A compared with CK in maize leaves 3 days after inoculation with the wild‐type (WT) form of EG1, mutant (D32A) form of EG1 and check sample (CK) at a concentration of 40 nm. The fold changes of these genes were calculated as the expression ratios (Relative Fold Expression) of WT and D32A compared with CK. Real‐time quantitative RT‐PCR (RT‐qPCR) data from three biological replicates for each maize leaf: WT, blue columns; D32A, red columns. 1, Thaumatin (GRMZM2G006853); 2, thaumatin (GRMZM2G042631); 3, histamine receptor activity (GRMZM2G041493); 4, 1,3‐β‐glucanase (GRMZM2G065585); 5, pathogenesis‐related protein (GRMZM2G112524); 6, alternative oxidase (GRMZM2G125669); 7, zinc finger homeodomain protein (GRMZM2G353076); 8, chitinase (GRMZM2G453805); 9, pathogenesis‐related protein (GRMZM2G465226); 10, glutathione transferase (GRMZM2G475059).
Reactive oxygen species (ROS) are considered to be cytotoxic byproducts of cellular metabolism, and the accumulation of ROS in cells may promote cell death. To investigate the mechanism of cell death induced by EG1, we analysed the production of ROS in suspension‐cultured tobacco cells induced by EG1 using 2′,7′‐dichlorodihydrofluorescein diacetate (H2DCFDA), which is particularly sensitive to ROS, forming the highly fluorescent 2′,7′‐dichlorofluorescein compound. The WT and mutant forms of EG1 strongly induced cellular ROS accumulation relative to CK (Fig. 4A).
Figure 4.

Production of reactive oxygen species (ROS) and cytosolic Ca2+ accumulation induced by EG1 from Rhizoctonia solani. (A) Production of ROS induced by EG1. Suspension‐cultured tobacco cells were treated with the wild‐type (WT) form of EG1, the mutant (D32A) form of EG1 and the check sample (CK) for 30 min at 25 °C at a concentration of 40 nm. Afterwards, suspension‐cultured tobacco cells were incubated with 2′,7′‐dichlorodihydrofluorescein diacetate (H 2 DCFDA) and ROS production was visualized under a fluorescence microscope with fluorescence (top) and phase contrast (bottom). Scale bar, 50 μm. (B) Induction of cytosolic Ca2+ accumulation in suspension‐cultured tobacco cells by EG1. Cytosolic Ca2+ accumulation of suspension‐cultured tobacco cells was investigated for the wild‐type (WT) form of EG1, the mutant (D32A) form of EG1 and the check sample (CK) at a concentration of 40 nm. Afterwards suspension‐cultured tobacco cells were incubated with Fura 2‐AM and cytosolic Ca2+ accumulation was visualized under a fluorescence microscope with fluorescence (top) and phase contrast (bottom). Scale bar, 100 μm. Three independent biological replicates were used for suspension‐cultured tobacco cells.
Ca2+ plays a pivotal role in the physiology and biochemistry of organisms and cells. It plays an important role in signal transduction pathways, where it acts as a second messenger. To investigate the role of EG1 in the induction of cytosolic Ca2+ ion accumulation, we analysed the concentration of cytosolic Ca2+ ions in suspension‐cultured tobacco cells treated with EG1. Treatment of suspension‐cultured tobacco cells with WT and mutant forms of EG1 produced a characteristic increase in cytosolic Ca2+ ions. In contrast, no comparable increase was detected on treatment with CK (Fig. 4B).
Another typical early event after the incubation of suspension‐cultured tobacco cells with EG1 is alkalinization of the tobacco cell culture medium; therefore, this was also analysed. The pH of a tobacco cell suspension treated with WT and mutant forms of EG1 increased significantly relative to that treated with CK (Fig. 5A).
Figure 5.
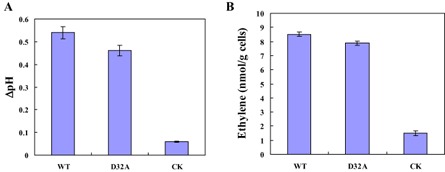
Alkalinization response and induction of ethylene biosynthesis in suspension‐cultured tobacco cells by EG1. (A) Alkalinization response in suspension‐cultured tobacco cells to EG1. Extracellular pH was measured with a pH electrode after tobacco cells had been treated with the wild‐type (WT) form of EG1, the mutant (D32A) form of EG1 and the check sample (CK) at 25 °C for 15 min at a concentration of 40 nm. ΔpH was the difference between the final pH reading of the cells after addition of the different proteins and the pH reading of the cells before addition of the different proteins. (B) Induction of ethylene biosynthesis in suspension‐cultured tobacco cells by EG1. Ethylene accumulation in the free‐air phase of the cultures was measured after treatment with the wild‐type (WT) form of EG1, the mutant (D32A) form of EG1 and the check sample (CK) for 4 h at 25 °C at a concentration of 40 nm. Three independent biological replicates were used for suspension‐cultured tobacco cells.
Ethylene production is also known to be a response to pathogen‐derived elicitors in plants. As for other representative elicitors, EG1 was an elicitor of ethylene biosynthesis. WT and mutant forms of EG1 induced ethylene biosynthesis of suspension‐cultured tobacco cells, whereas no comparable induction of ethylene biosynthesis was observed with CK (Fig. 5B).
Elicitor responses with EG1 produced in planta using the PVX‐based expression system
The PVX‐based vector has been comprehensively applied in expression systems to produce heterologous proteins more rapidly and stably. In particular, the PVX‐based expression system does not require purification of the proteins under study, being very convenient for the functional identification of WT and mutated proteins. In this study, the PVX‐based expression system was used to further demonstrate the elicitor nature of EG1 and the independence of its elicitor activity. cDNAs of the WT (WT) and mutant form (D32A) of EG1, containing the signal peptide of EG1, were cloned into a PVX‐based expression vector (pGR106) and transformed into Agrobacterium tumefaciens. Colonies were individually injected onto leaves of tobacco plants. Tobacco leaves injected with pGR106/WT and pGR106/D32A exhibited a necrotic lesion after 3 days, whereas plants injected with pGR106 did not display a necrotic lesion at this time (Fig. 6A). Protein gel blot analysis with antibodies against EG1 showed that leaves injected with pGR106/WT and pGR106/D32A contained substantial amounts of EG1 after 3 days (Fig. 6B). Cellulase activity assay showed that death was not related to cellulase activity in infected leaves at 3 days after inoculation (Fig. 6C). These observations also confirm that the death‐inducing activity of EG1 is independent of its enzymatic activity.
Figure 6.
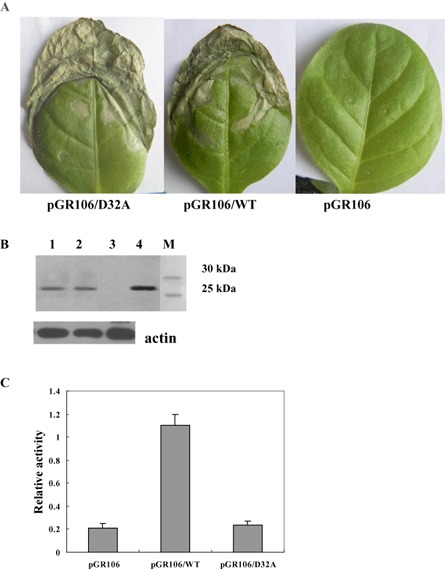
Elicitor activity of EG1 produced in planta using the Potato virus X (PVX)‐based expression system. (A) Symptoms of tobacco leaves injected with Agrobacterium tumefaciens strains carrying the pGR106, pGR106/WT or pGR106/D32A vector. Photographs were taken at 5 days after inoculation. (B) Protein blot analysis of EG1 production in plants. Tobacco leaf samples were collected at 5 days after inoculation, and total protein was extracted and subjected to protein gel blot analysis using an anti‐EG1 polyclonal antiserum. Lane 1, tobacco leaves injected with the A. tumefaciens strain carrying the pGR106/D32A vector; lane 2, tobacco leaves injected with the A. tumefaciens strain carrying the pGR106/WT vector; lane 3, tobacco leaves injected with the A. tumefaciens strain carrying the pGR106 vector; lane 4, EG1; lane M, molecular weight marker. Anti‐plant actin mouse monoclonal antibody (Abbkine) was used as a loading control to detect actin expression. (C) Cellulase activity assay of tobacco leaves. Leaf samples were collected at 5 days after inoculation, and total protein was extracted and subjected to cellulase activity assay. Relative activity was the difference between the activity (A 540) of tobacco leaves inoculated with A. tumefaciens strains carrying pGR106, pGR106/WT or pGR106/D32A and the activity (A 540) of tobacco leaves not inoculated with A. tumefaciens. Three independent biological replicates were used for each tobacco leaf.
Dose–response studies and half‐maximal effective concentration (EC 50) determination of EG1
To quantitatively analyse the EG1 activity causing leaf death, a simple bioassay was developed using dose–response curves of lesion size of leaf death to the concentration of WT and mutant (D32A) forms of EG1 in plant leaves. WT and mutant forms of EG1 induced cell death of maize, tobacco and Arabidopsis plant leaves at a concentration of 40–100 nm, but with no visible macroscopic symptoms on maize and tobacco plant leaves at a concentration of 20 nm (Fig. 7). According to the percentage ratio of a certain lesion size at 0, 20, 40, 60, 80 or 100 nM of WT or D32A to the maximum lesion size of leaf death in maize, tobacco and Arabidopsis plant leaves, EC50 of EG1 (WT and D32A) was determined using the Data Processing System version 7.5 (DPS 7.5). The results showed that the EC50 values of WT were 47.98 nm in maize, 47.99 nm in tobacco and 34.02 nm in Arabidopsis, and the EC50 values of D32A were 48.80 nm in maize, 48.51 nm in tobacco and 42.48 nm in Arabidopsis. Significant differences were observed for EC50 of WT or D32A on different plants. Arabidopsis plants were more sensitive than maize and tobacco to EG1.
Figure 7.

Dose–response curves of lesion size of leaf death in maize at 3 days (A), tobacco at 3 days (B) and Arabidopsis at 2 days (C). Leaves were injected with the wild‐type form (WT) of EG1 and the mutant form (D32A) of EG1. The lesion size of leaf death was estimated using the lesion length (mm) for maize leaves, the lesion diameter (mm) for tobacco leaves and the lesion length (mm) for Arabidopsis leaves. Three independent biological replicates were used for each plant leaf.
Cell death caused by EG1 is a hypersensitive response (HR)‐type cell death
The terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end labelling (TUNEL) stain has been used extensively for the in situ detection of DNA fragmentation sites associated with HR‐type cell death. Green fluorescence in this study marks the site at which terminal deoxynucleotidyl transferase (TdT)‐mediated incorporation of fluorescein‐labelled dUTP occurs during fragmentation of the DNA. Suspension‐cultured tobacco cells incubated with CK showed no symptoms of DNA fragmentation, whereas incubation of suspension‐cultured tobacco cells with EG1 induced widespread and more extensive DNA fragmentation (Fig. 8). We conclude that EG1 can induce DNA fragmentation in suspension‐cultured tobacco cells, suggesting that cell death caused by EG1 is an HR‐type cell death.
Figure 8.
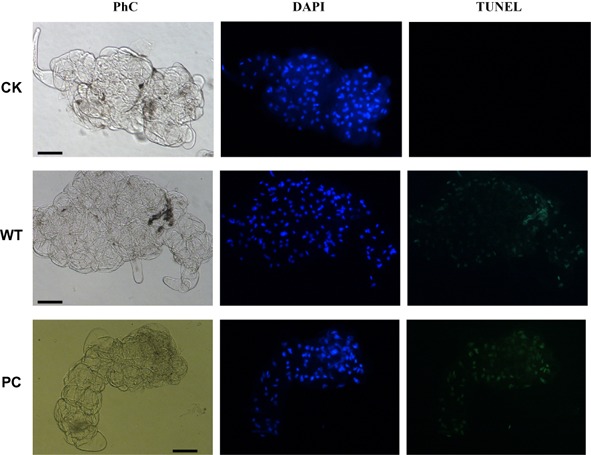
EG1‐induced DNA fragmentation in suspension‐cultured tobacco cells at 12 h. The cells were counterstained in situ with 4′,6‐diamidino‐2‐phenylindole (DAPI) (blue colour represents nuclei) followed by terminal deoxynucleotidyl transferase‐mediated dUTP nick‐end labelling (TUNEL) reagents (green colour represents DNA fragmentation). Corresponding phase contrast images (PhC) of suspension‐cultured tobacco cells are also shown. Bars, 50 μm. WT, suspension‐cultured tobacco cells treated with the wild‐type of EG1 (40 nm). CK, suspension‐cultured tobacco cells treated with the check sample. PC, positive control. Three independent biological replicates were used for suspension‐cultured tobacco cells.
Expression of EG1 during the infection of maize by R. solani
To determine whether R. solani EG1 is involved in cell death during infection, a plant inoculation test was performed. We injected maize leaves with R. solani. As a result, necrotic lesions were clearly formed on inoculation with R. solani (Fig. 9A), and transcription of the EG1 gene was detected during infection of maize by R. solani using RT‐PCR. Transcription of Eg1 was detected at 2 and 3 days post‐inoculation, and a faint band was also seen at 4–7 days post‐inoculation (Fig. 9B). Protein gel blot analysis with antibodies against EG1 showed that leaves injected with R. solani contained substantial amounts of EG1 after 3–7 days (Fig. 9C). These data indicate that EG1 may be involved in cell death.
Figure 9.
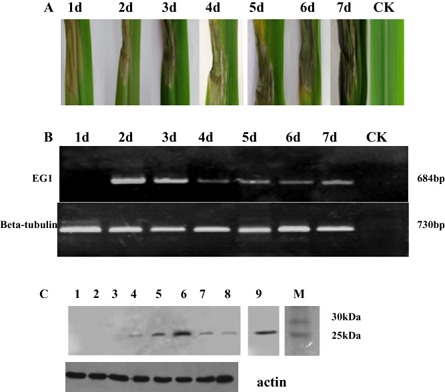
Maize leaves injected by Rhizoctonia solani. The fungus was injected onto potato dextrose agar (PDA) plates. One disc (diameter, 3 mm) of PDA from the margin of an actively growing colony of the fungus on PDA was placed on maize leaves at 100% relative humidity for 12 h. (A) The symptoms of maize leaves injected with R. solani. (B) Expression of EG1 of R. solani during inoculation of maize. (C) Protein blot analysis of EG1 in maize. Maize leaf samples were collected at 1–7 days (lanes 2–8) after inoculation, and total protein was extracted and subjected to protein gel blot analysis using an anti‐EG1 polyclonal antiserum. Anti‐plant actin mouse monoclonal antibody (Abbkine) was used as a loading control to detect actin expression. M, molecular weight marker; lane 1, check sample (CK); lane 9, EG1.
Discussion
A model has emerged in which pathogen‐associated molecular patterns (PAMPs) are molecules associated with groups of pathogens that are recognized by pattern recognition receptors (PRRs) in hosts (Jones and Dangl, 2006). In plants, PAMPs, as elicitors, activate innate immune responses, protecting plants from infection (Ausubel, 2005; Chinchilla et al., 2007; Jones and Dangl, 2006; Nurnberger et al., 2004; Postel and Kemmerling, 2009). So far, many proteinaceous elicitors of various structures have been isolated from a variety of phytopathogenic and nonpathogenic microorganisms (Ahuja et al., 2012; Ebel and Cosio, 1994; Postel and Kemmerling, 2009; Ricci et al., 1993; Zhao et al., 2005), such as flagellin (Felix et al., 1999), elongation factor (Kunze et al., 2004), transglutaminase (Brunner et al., 2002; Nurnberger et al., 1994), xylanase (Enkerli et al., 1999), cellulose binding elicitor lectin (Gaulin et al., 2006), harpin (Wei et al., 1992) and elicitin (Takemoto et al., 2005). However, there is little detailed information available about cellulase as an elicitor to date. Our results show that cellulase EG1 of the fungus R. solani is able to induce cell death, the transcription of defence genes, the production of ROS, medium alkalinization, Ca2+ accumulation and ethylene biosynthesis, displaying features of a representative proteinaceous elicitor (Ahuja et al., 2012; Angelova et al., 2006; Bailey et al., 1990; Enkerli et al., 1999; Postel and Kemmerling, 2009; Ron et al., 2000; Zhao et al., 2005).
As with the fungal proteins ethylene‐inducing xylanase (EIX) from Trichoderma reesei (Enkerli et al., 1999) and endopolygalacturonase 1 from Botrytis cinerea (Poinssot et al., 2003), EG1 has two biological activities, enzymatic activity and elicitor activity, which are independent of each other. The independence of EG1 was clearly demonstrated using the analysis of elicitor responses for the study of elicitor–plant interactions, together with mutational analysis and the PVX expression system, in our study. We mutated the catalytic site (D32) necessary for the cellulase activity of EG1. Although the mutation drastically reduced the enzymatic activity, it did not affect the elicitor activity of EG1. This is unsurprising, because it is a common phenomenon that a protein has different structural domains which function independently. The active site for enzymatic activity of hydrolytic enzymes is usually found in a three‐dimensional groove or pocket on the enzyme; the active site for elicitor activity has been shown to be located on surface‐exposed residues of the enzyme (Rotblat et al., 2002; this research).
How plant cells recognize EG1 is not understood. We hypothesize that the elicitor activity of EG1 may be based on the specific recognition between the EG1 elicitor and a possible potential protein receptor in plants. It has been demonstrated that plants have evolved to recognize PAMPs through the presence of PRRs (Chinchilla et al., 2007; Gomez‐Gomez and Boller, 2000; Kunze et al., 2004; Nurnberger et al., 1994; Zhang et al., 2013; Zipfel et al., 2006). Several PRRs in plants have been identified (Postel and Kemmerling, 2009), such as FLAGELLIN‐SENSING 2 (FLS2) (Gomez‐Gomez and Boller, 2000), elongation factor Tu receptor (EFR) (Zipfel et al., 2006), LeEIX1/2 (Ron and Avni, 2004) and RESPONSIVENESS TO BOTRYTIS POLYGALACTURONASES1 (RBPG1) (Zhang et al., 2013). FLS2 was the first identified and best studied PRR, and is a representative leucine‐rich repeat receptor‐like kinase (LRR‐RLK). In Arabidopsis thaliana, FLS2 contributes to resistance against bacterial pathogens with a related LRR‐RLK, BAK1, which positively regulates FLS2 function (Chinchilla et al., 2007). EFR is a close homologue of FLS2 and belongs to the same subclade XII of LRR‐RLKs (Zipfel et al., 2006). LeEIX1/2 are LRR receptor‐like proteins (LRR‐RLPs), similar to the tomato Ve resistance genes (Bar et al., 2010; Ron and Avni, 2004; Sharfman et al., 2011). RBPG1 is an Arabidopsis LRR‐RLP that recognizes fungal endopolygalacturonases (Zhang et al., 2013). We believe that these currently known PAMP receptors will provide some clues to the identification of the possible potential protein receptor to EG1.
Experimental Procedures
Plant growth conditions
Maize (Zea mays) plants (Nongda 108 from Shandong, China, the most widely cultured hybrid in China) and tobacco plants (Nicotiana tabacum cv. NC89 from China Tobacco Seed Co. Ltd., Beijing, China) were grown under standard glasshouse conditions at 25 °C with a cycle of 12 h of light and 12 h of dark. Arabidopsis plants were grown at 20–21 °C with an 8‐h photoperiod.
Strains, plasmids and culture media
Rhizoctonia solani AG‐1‐IA was isolated from a sample of maize sheath blight in Shandong, China and preserved by our laboratory. The isolate was identified through a combined study of rDNA‐ITS sequences and cultural characteristics. Anastomosis grouping was determined by a reported technique (Parmeter et al., 1969). Escherichia coli DH5α and JM109 were used for cloning and nucleotide sequencing. Pichia pastoris GS115 and plasmid vector pPIC9K were obtained from Invitrogen (USA). The vector pMD18‐T was purchased from Takara (Japan). Pichia pastoris GS115 was used as a host for the expression of cellulase cDNA. Recombinant plasmids were constructed from pMD18‐T for subcloning and sequencing, and from pPIC9K for the expression of cellulase cDNA. Escherichia coli was grown at 37 °C in Luria–Bertani Medium (LB). The Minimal Dextrose Medium (MD), Minimal Methanol Medium (MM), Yeast Extract Peptone Dextrose Medium (YPD), Buffered Minimal Glycerol‐complex Medium (BMGY) and Buffered Minimal Methanol‐complex Medium (BMMY), were used for the culture of Pichia pastoris GS115 according to the Pichia Expression System Kit (Invitrogen).
Fungal growth conditions
For the induction of cellulase of R. solani, the actively growing fungal mycelium was transferred from a potato dextrose agar (PDA) plate to medium containing the following: carboxymethylcellulose (CMC), 10.0 g; asparagines, 0.5 g; K2HPO4.3H2O, 1.0 g; KNO3, 2.0 g; KCl, 0.5 g; FeSO4, 0.01 g; MgSO4.7H2O, 0.5 g; vitamin B1 (VB1), 0.01 mg; dissolved in 1 L of distilled water (Marcus et al., 1986). The fungus was cultured in a rotary shaker (200 rpm) at 28 °C. After 2 days of growth at 28 °C, the mycelia were collected for total RNA isolation.
Culture of suspension‐cultured tobacco cells
Nicotiana tabacum NC89 suspension‐cultured cells were maintained as described previously (Liu et al., 2010). The cell density was adjusted with medium to achieve an average fresh weight of 100 mg/mL.
Enzyme assays and protein determination
Enzyme activity towards CMC was measured according to the method of Stewart and Leatherwood (1976). The reaction mixture was composed of 0.8 mL of 0.5% CMC (sodium salt) in 50 mm acetate buffer, pH 5.0, and 0.2 mL of enzyme solution. After incubation at 50 °C for 30 min, the reaction was stopped by the addition of 0.5 mL of 3,5‐dinitrosalicylic acid reagent (Miller, 1959), and A 540 was measured using a Shimadzu UV‐160A spectrophotometer (Kyoto, Japan). One unit (U) of enzyme activity was defined as the amount of enzyme that catalysed the liberation of reducing sugar equivalent to 1.0 μg glucose/min under assay conditions. Protein was determined by the method of Lowry et al. (1951), with crystalline bovine serum albumin as the standard. The protein content of column eluents was monitored by measurement of the absorbance at 280 nm.
cDNA cloning
Total RNA was isolated from the mycelia of R. solani using the Trizol® Reagent (Invitrogen) according to the manufacturer's instructions. Reverse transcription was carried out according to the RNA PCR Kit 3.0 instructions (Takara). Previously, we have cloned an endoglucanase gene (GU372728) from R. solani. To obtain the ORF cDNA of the endoglucanase, a pair of specific oligonucleotide primers (Table S1) for PCR was synthesized based on the nucleotide sequence of the endoglucanase gene. PCR products were amplified on the ORF cDNA (termed Eg1) according to the manufacturer's instructions, and then sequenced.
Site‐directed mutagenesis
For the loss of cellulase activity of EG1, site‐directed mutagenesis of Eg1 was performed to modify the Asp residue at position 32, predicted to be critical for the enzymatic activity of EG1 according to the QuickChange™ Site‐Directed Mutagenesis Kit (Stratagene,USA). The mutant was designed in which Asp‐32 was replaced by Ala. The mutation was confirmed by sequencing of the gene. The WT and mutated form of Eg1 were designated as WT and D32A, respectively.
Construction of the expression system
The coding regions of WT and D32A were amplified by PCR using a pair of specific sense and antisense primers (Table S1). To facilitate the cloning of the amplified fragment, the sense and antisense primers contained an EcoRI and a NotI restriction site, respectively. In addition, the sense primer also contained an N‐terminal 6 × histidine tag sequence for expressed product purification. The amplified product was digested with EcoRI and NotI, and ligated to pPIC9K (also digested with EcoRI and NotI), producing the P. pastoris secretion expression plasmids pPIC9K/WT and pPIC9K/D32A. The DNA manipulations were carried out using standard procedures (Sambrook et al., 1989). These recombinant plasmids were subsequently confirmed by PCR analysis, restriction analysis and DNA sequencing.
Transformation and analysis of the genomic DNA of P. pastoris GS115
Pichia pastoris GS115 was transformed with 10 μg of the SacI‐linearized recombinant plasmid or the empty plasmid pPIC9K by electroporation with an Eppendorf Electroporator 2510 (Eppendorf, Germany). Screening was performed by streaking single colonies on MD and MM plates, and the multi‐copy integrants were selected on YPD plates containing various concentrations of G418 (Invitrogen). Verification of the gene integrated into the P. pastoris genome and determination of the phenotype were carried out according to the method of the Pichia Expression System Kit (Invitrogen).
Endoglucanase induction in P. pastoris and purification of recombinant enzymes
Endoglucanase induction in P. pastoris was carried out according to the method of the Pichia Expression System Kit (Invitrogen). To obtain purified protein of the recombinant endoglucanase, the transformed yeast with the highest activity was cultured at 30 °C for 6 days in BMMY according to the Pichia Expression System Kit (Invitrogen). The culture filtrate was centrifuged at 10 000 g for 10 min at 4 °C, and the resultant supernatant was dialysed overnight against three changes of 50 mm phosphate‐buffered saline (PBS) (pH 7.4, buffer A). The histidine‐tagged EG1 was purified using nickel affinity chromatography (HisTrap™ FF crude, GE Healthcare, USA). The volume of the fractions was 2 mL. Fractions with endoglucanase were pooled, dialysed overnight against three changes of water and concentrated for the determination of purity by SDS‐PAGE and elicitor activity. The corresponding protein preparation from the culture filtrate of control yeast transformed with the empty plasmid pPIC9K was prepared by the same method. The corresponding protein preparation was used as CK.
To demonstrate the purity of EG1 purified using nickel affinity chromatography from yeast, we carried out mass spectrometry (MS) and high‐pressure liquid chromatography (HPLC). The analysis indicated that the WT and mutant (D32A) forms of EG1 showed a protein peak, and had a high purity above 95% (Fig. S3, see Supporting Information). The purified WT form of EG1, the purified mutated form (D32A) of EG1 and the corresponding protein preparation (CK) of the culture filtrate of yeast transformed with pPIC9K were used for further determination of elicitor activity.
SDS‐PAGE
The purity and molecular weight of the enzyme were confirmed by SDS‐PAGE according to Laemmli (1970).
Assays for elicitor activity
To test for the induction of death, purified WT and mutated forms of EG1, and CK, were injected into maize leaves, tobacco seedling leaves and Arabidopsis plant leaves with injector. The injected maize and tobacco seedlings were kept in a glasshouse after incubation at 25 °C with a cycle of 12 h of light and 12 h of dark. The injected Arabidopsis plants were kept in a biochemical incubator at 25 °C with an 8‐h photoperiod.
To examine the induction of transcription of the three defence marker genes PAL, POD and PR1a in maize and tobacco after incubation, leaves of maize and tobacco seedlings, 3 days after inoculation with purified WT and mutated forms of EG1, and CK, were harvested in liquid nitrogen and freeze–dried. RT‐PCR was used to detect the transcription of PAL, POD and PR1a genes in total RNA from injected leaves.
The extracellular alkalinization response in suspension‐cultured tobacco cells was analysed as described previously (Felix et al., 1993). Purified WT and mutated forms of EG1, and CK, were added to 1 mL of suspension‐cultured tobacco cells. After the suspension‐cultured tobacco cells had been incubated at 25 °C on a rotary shaker at 200 rpm, the extracellular pH in tobacco cells treated for 15 min with purified WT and mutated forms of EG1, and CK, was measured with a pH electrode.
The induction of ethylene biosynthesis was assayed as described previously (Felix et al., 1991). Purified WT and mutated forms of EG1, and CK, were added to 1 mL of suspension‐cultured tobacco cells. After suspension‐cultured tobacco cells had been incubated for 4 h at 25 °C on a rotary shaker at 200 rpm, ethylene accumulation was measured by a GC‐2010ATF (Shimadzu).
Intracellular generation of ROS was analysed with the H2DCFDA fluorescence probe using a ROS Assay Kit according to the manufacturer's protocol (Haimen, Jiangsu, China). Briefly, purified WT and mutated forms of EG1, and CK, were added to 1 mL of suspension‐cultured tobacco cells. After the suspension‐cultured tobacco cells had been incubated for 15 min at 25 °C on a rotary shaker at 200 rpm, they were collected at 20‐min intervals through at least 80 min and resuspended in 100 μm H2DCFDA. Intracellular H2DCFDA was de‐esterified to dichlorodihydrofluorescein, which is oxidized by ROS to produce the fluorescent compound dichlorofluorescein. After 30 min of incubation at 37 °C, the fluorescence was visualized under a fluorescence microscope (Eclipse 90I, Nikon, Japan) with an excitation filter of 450–490 nm and a barrier filter of 510 nm, and the fluorescence intensity was measured by a fluorescence spectrophotometer (F‐4600, Hitachi, Japan) at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Cytosolic Ca2+ was measured with a Ca2+‐sensitive dye (Fura 2‐AM) according to the manufacturer's protocol (Dojindo, Kyushu, Japan). Purified WT and mutated forms of EG1, and CK, were added to 1 mL of suspension‐cultured tobacco cells in N‐2‐hydroxyethylpiperazine‐N’‐2‐ethanesulphonic acid (HEPES)‐buffered saline. After the suspension‐cultured tobacco cells had been incubated for 15 min at 25 °C on a rotary shaker at 200 rpm, they were loaded with 5 μm Fura 2‐AM for 60 min at 37 °C. The suspension‐cultured tobacco cells were then washed twice with HEPES‐buffered saline and resuspended in the same buffer for fluorescent Ca2+ ion detection. The fluorescence was visualized under a fluorescence microscope (Eclipse 90I, Nikon) with an excitation filter of 340–380 nm and a barrier filter of 510 nm.
RT‐PCR and RT‐qPCR
RT‐PCR and RT‐qPCR were performed using a TaKaRa One Step RNA PCR Kit (AMV) and SYBR Premix Ex Taq™ Kit (Takara), respectively. The sequences of the primers used are given in Table S1.
Construction of recombinant A. tumefaciens PVX vectors and PVX expression assay
The constructs pGR106/WT and pGR106/D32A were based on the coding sequences of WT and D32A, containing the signal peptide sequence of EG1. The sequences were amplified by PCR using their cDNA as template. The PCR fragments of WT and D32A were ligated into pGR106 to form pGR106/WT and pGR106/D32A, respectively. The binary expression constructs were introduced into A. tumefaciens strain GV3101 by electroporation and transformed bacteria were selected. The selected agrobacteria containing the constructs pGR106/WT and pGR106/D32A were grown for 2 days at 28 °C on LB agar plates. The colonies were injected onto leaflets of tobacco plants. The injected plants were grown under standard glasshouse conditions at 25 °C with a cycle of 12 h of light and 12 h of dark. Control plants were injected with bacteria containing the empty vector pGR106.
Polyclonal antibodies of EG1
Purified EG1 was used as an antigen for the preparation of polyclonal antibodies. A 6‐month‐old rabbit was immunized with purified EG1. The initial immunization was carried out with 1 mg of EG1 suspended in water and mixed 1:1.5 with complete Freund's adjuvant. One month later, the next immunization was carried out with 0.5 mg of EG1 suspended in water and mixed 1:1 with incomplete Freund's adjuvant. Two weeks later, the rabbit was bled and polyclonal serum was prepared.
Protein extraction and analysis
Tobacco leaves (100 mg) infected by A. tumefaciens were frozen in liquid nitrogen and ground in a mortar with a pestle. Extraction was achieved in 50 mm Tris (hydroxymethyl) aminomethane hydrochloride (Tris‐HCL) buffer, pH 7.0. The suspension was centrifuged at 10 000 g for 10 min at 4 °C. The resulting supernatant was used for protein gel blot analysis and cellulase activity analysis. Protein gel blot analysis was performed as described by Towbin et al. (1979). Anti‐plant actin mouse monoclonal antibody (Abbkine, California, USA) was used as a loading control to detect actin expression. Cellulase activity analysis was performed as described by Stewart and Leatherwood (1976).
TUNEL staining
TUNEL was performed using an In Situ Cell Death Detection kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions. Briefly, suspension‐cultured tobacco cells treated with EG1 (WT) for 12 h were fixed in 4% formaldehyde for 1 h at 25 °C, followed by three washes in PBS, permeabilization with 0.1% Triton X‐100 and 0.1% sodium citrate, and incubation at 37 °C for 60 min with TdT and fluorescein‐conjugated nucleotides in the dark. To visualize nuclei in suspension cells, samples were stained with 4′,6‐diamidino‐2‐phenylindole (DAPI; Sigma, USA) at 1 μg/mL in PBS buffer for 10 min. DAPI‐ and TUNEL‐positive staining was observed with a fluorescence microscope (Eclipse 90I, Nikon) with an excitation filter of 488 nm and an emission of 515–530 nm. DAPI was excited at 405 nm, and emission was filtered at 430–460 nm.
Transcriptional analysis of the R. solani endoglucanase gene (Eg1)
To examine the transcript of the R. solani endoglucanase gene (Eg1) during infection, leaves of maize seedlings were injected with R. solani AG‐1‐IA. Leaves were harvested in liquid nitrogen at 1, 2, 3, 4, 5, 6 or 7 days after inoculation and freeze–dried. RT‐PCR was used to detect the transcription of the Eg1 gene in total RNA from infected leaves. The β‐tubulin gene of R. solani was used as an internal control for RT‐PCR. Protein gel blot analysis was performed as described by Towbin et al. (1979). Anti‐plant actin mouse monoclonal antibody (Abbkine) was used as a loading control to detect actin expression.
Supporting information
Fig. S1 Alignment of the deduced amino acid sequences of Rhizoctonia solani endoglucanase (GU372728), Humicola insolens (A21793), Schizophyllum commune (EFI93868) and Melanocarpus albomyces (AJ515703). ▼, catalytic sites (D).
Fig. S2 Sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) analysis of the purified enzymes. (A) The unrelated cellulase (C61F, KC441879) of Chaetomium thermophilum. M, molecular weight marker. (B) The unrelated cellulase (T61F, KF170230) of Thermoascus aurantiacus. M, molecular weight marker. (C) The unrelated manganese superoxide dismutase (SOD, EF569978) of Chaetomium thermophilum. M, molecular weight marker. The three unrelated proteins were purified using nickel affinity chromatography from culture filtrates of yeast transformed with the plasmids pPIC9K/C61F, pPIC9K/T61F and pPIC9K/SOD. All methods of expression and purification of C61F, T61F and SOD were carried out according to those of the wild‐type (WT) and D32A. Three independent biological replicates were used for each protein.
Fig. S3 Mass spectrometry (MS) and high‐pressure liquid chromatography (HPLC) of purified EG1. (A) MS of purified EG1 (WT and D32A). Peak 1, EG1 with two charges; peak 2, EG1 with one charge; peak 3, two dimeric EG1 with one charge. (B) HPLC of purified EG1 (WT and D32A). Three independent biological replicates were used for each protein.
Table S1 List of primers used in the study for polymerase chain reaction (PCR). Ten selected genes of maize injected with EG1: thaumatin (GRMZM2G006853), histamine receptor (GRMZM2G041493), β‐1,3‐glucanase (GRMZM2G065585), pathogenesis‐related protein (GRMZM2G112524), alternative oxidase (GRMZM2G125669), zinc finger homeodomain protein (GRMZM2G353076), thaumatin (GRMZM2G042631), chitinase (GRMZM2G453805), pathogenesis‐related protein (GRMZM2G465226) and glutathione transferase (GRMZM2G475059).
Acknowledgements
We thank Professor Zhaohuei Chu for kindly providing pGR106. We are grateful to Professor Silke Robatzek, Professor Zhaohuei Chu, Professor Xiangdong Li, Professor Xinhua Ding and Dr Anna Li for technical assistance and helpful discussions. This work was supported by the Chinese National Nature Science Foundation (grant no. 31071723), Chinese National Programs for High Technology Research and Development (grant no. 2012AA10180402), Chinese Project of Transgenic Organisms (grant no. 2013X08001‐002) and the National Department Public Benefit Research Foundation (grant no. nyhyzx07‐049).
References
- Ahuja, I. , Kissen, R. and Bones, A.M. (2012) Phytoalexins in defense against pathogens. Trends Plant Sci. 17, 73–90. [DOI] [PubMed] [Google Scholar]
- Angelova, Z. , Georgiev, S. and Roos, W. (2006) Elicitation of plants. Biotechnol. Biotechnol. Equip. 20, 72–83. [Google Scholar]
- Annis, S.L. and Goodwin, P.H. (1997) Recent advances in the molecular genetics of plant cell wall‐degrading enzymes produced by plant pathogenic fungi. Eur. J. Plant Pathol. 103, 1–14. [Google Scholar]
- Ausubel, F.M. (2005) Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 6, 973–979. [DOI] [PubMed] [Google Scholar]
- Bailey, B.A. , Dean, J.F.D. and Anderson, J.D. (1990) An ethylene biosynthesis‐inducing endoxylanase elicits electrolyte leakage and necrosis in Nicotiana tabacum cv. xanthi leaves. Plant Physiol. 94, 1849–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, C.J. and Bateman, D.F. (1978) Cutin degradation by plant pathogenic fungi. Phytopathology, 68, 1577–1584. [Google Scholar]
- Bar, M. , Sharfman, M. , Ron, M. and Avni, A. (2010) BAK1 is required for the attenuation of ethylene‐inducing xylanase (Eix)‐induced defense responses by the decoy receptor LeEix1. Plant J. 63, 791–800. [DOI] [PubMed] [Google Scholar]
- Beguin, P. and Aubert, J.P. (1994) The biological degradation of cellulose. FEMS Microbiol. Rev. 13, 25–58. [DOI] [PubMed] [Google Scholar]
- Brunner, F. , Rosahl, S. , Lee, J. , Rudd, J.J. , Geiler, S. , Kauppinen, S. , Rasmussen, G. , Scheel, D. and Nurnberger, T. (2002) Pep‐13, a plant defense‐inducing pathogen‐associated pattern from Phytophthora transglutaminases. EMBO J. 21, 6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita, N.C. and Gibeaut, D.M. (1993) Structural models of primary cell walls in flowering plants, consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3, 1–30. [DOI] [PubMed] [Google Scholar]
- Chinchilla, D. , Zipfel, C. , Robatzek, S. , Kemmerling, B. , Nurnberger, T. , Jones, J.D.G. , Felix, G. and Boller, T. (2007) A flagellin‐induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature, 448, 497–500. [DOI] [PubMed] [Google Scholar]
- Davies, G.J. , Dodson, G.G. , Moore, M.H. , Tolley, S.P. , Dauter, Z. , Wilson, K.S. , Rasmussen, G. and Schuelein, M. (1996) Structure determination and refinement of the Humicola insolens endoglucanase V at 1.5‐Å resolution. Acta Crystallogr. D, 52, 7–17. [DOI] [PubMed] [Google Scholar]
- Davies, G.T. , Dodson, G.G. , Hubbard, R.E. , Tolley, S.P. , Dauter, Z. , Wilson, K.S. , Hjort, C. , Mikkelsen, J.M. , Rasmussen, G. and Schulein, M. (1993) Structure and function of endoglucanase V. Nature, 365, 362–364. [DOI] [PubMed] [Google Scholar]
- Demirci, E. and Doken, M.T. (1998) Host penetration and infection by the anastomosis groups of Rhizoctonia solani Kuhn isolated from potatoes. Trans. J. Agric. Forest. 22, 609–613. [Google Scholar]
- Ebel, J. and Cosio, E.G. (1994) Elicitors of plant defense responses. Int. Rev. Cytol. 148, 1–36. [Google Scholar]
- Enkerli, J. , Felix, G. and Boller, T. (1999) The enzymatic activity of fungal xylanase is not necessary for its elicitor activity. Plant Physiol. 121, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel, D. , Miyara, I. , Ailing, T. , Dinoor, A. and Prusky, D. (2002) pH regulates endoglucanase expression and virulence of Alternaria alternata in persimmon fruit. Mol. Plant–Microbe Interact. 15, 774–779. [DOI] [PubMed] [Google Scholar]
- Espino, J.J. , Brito, N. , Noda, J. and Gonzalez, C. (2005) Botrytis cinerea endo‐β‐1,4‐glucanase Cel5A is expressed during infection but is not required for pathogenesis. Physiol. Mol. Plant Pathol. 66, 213–221. [Google Scholar]
- Felix, G. , Grosskopf, D.G. , Regenass, M. , Basse, C.W. and Boller, T. (1991) Elicitor‐induced ethylene biosynthesis in tomato cells, characterization and use as a bioassay for elicitor action. Plant Physiol. 97, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, G. , Regenass, M. and Boller, T. (1993) Specific perception of subnanomolar concentrations of chitin fragments by tomato cells, induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 4, 307–316. [Google Scholar]
- Felix, G. , Duran, J.D. , Volko, S. and Boller, T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18, 265–276. [DOI] [PubMed] [Google Scholar]
- Gaulin, E. , Drame, N. , Lafitte, C. , Torto‐Alalibo, T. , Martinez, Y. , Ameline‐Torregrosa, C. , Khatib, M. , Mazarguil, H. , Villalba‐Mateos, F. , Kamoun, S. , Mazars, C. , Dumas, B. , Bottin, A. , Esquerre‐Tugaye, M.T. and Rickauer, M. (2006) Cellulose binding domains of a Phytophthora cell wall protein are novel pathogen‐associated molecular patterns. Plant Cell, 18, 1766–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Gomez, L. and Boller, T. (2000) FLS2, an LRR receptor‐like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis . Mol. Cell, 5, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Hirvonen, M. and Papageorgiou, A.C. (2003) Crystal structure of a family 45 endoglucanase from Melanocarpus albomyces, mechanistic implications based on the free and cellobiose‐bound forms. J. Mol. Biol. 329, 403–410. [DOI] [PubMed] [Google Scholar]
- Jayasinghe, C.K. , Wijayaratne, S.C.P. and Fernando, T.H.P.S. (2004) Characterization of cell wall degrading enzymes of Thanatephorus cucumeris . Mycopathologia, 157, 73–79. [DOI] [PubMed] [Google Scholar]
- Jones, D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kunze, G. , Zipfel, C. , Robatzek, S. , Niehaus, K. , Boller, T. and Felix, G. (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell, 16, 3496–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970) Clearage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lagaert, S. , Belien, T. and Volckaert, G. (2009) Plant cell walls, protecting the barrier from degradation by microbial enzymes. Semin. Cell Dev. Biol. 20, 1064–1073. [DOI] [PubMed] [Google Scholar]
- Liu, F. , Wei, F.F. , Wan, L.L. , Liu, H. , Zhu, X.P. and Liang, Y.C. (2010) Riboflavin activates defense responses in tobacco and induces resistance against Phytophthora parasitica and Ralstonia solanacearum . Physiol. Mol. Plant Pathol. 74, 330–336. [Google Scholar]
- Lowry, O.H. , Rosebrough, N.J. , Farr, A.V. and Randall, R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- Ma, C. (2008) Cellulase elicitor induced accumulation of capsidiol in Capsicum annuum L. suspension cultures. Biotechnol. Lett. 30, 961–965. [DOI] [PubMed] [Google Scholar]
- Marcus, L. , Barash, I. , Sneh, B. , Koltin, Y. and Finkler, A. (1986) Purification and characterization of pectolytic enzymes produced by virulent and hypovirulent isolates of Rhizoctonia solani Kuhn. Physiol. Mol. Plant Pathol. 29, 325–326. [Google Scholar]
- Mialoundama, A.S. , Heintz, D. , Debayle, D. , Rahier, A. , Camara, B. and Bouvier, F. (2009) Abscisic acid negatively regulates elicitor‐induced synthesis of capsidiol in wild tobacco. Plant Physiol. 150, 1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G.L. (1959) Use of dinitrosalicylic reagent for determination of reducing sugar. Anal. Chem. 31, 426–428. [Google Scholar]
- Muller, U. , Tenberge, K.B. , Oeser, B. and Tudzynski, P. (1997) Cel1, probably encoding a cellobiohydrolase lacking the substrate binding domain, is expressed in the initial infection phase of Claviceps purpurea on Secale cereale . Mol. Plant–Microbe Interact. 10, 268–279. [DOI] [PubMed] [Google Scholar]
- Nurnberger, T. , Nennstiel, D. , Jabs, T. , Sacks, W.R. , Hahlbrock, K. and Scheel, D. (1994) High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell, 78, 449–460. [DOI] [PubMed] [Google Scholar]
- Nurnberger, T. , Brunner, F. , Kemmerling, B. and Piater, L. (2004) Innate immunity in plants and animals, striking similarities and obvious differences. Immunol. Rev. 198, 249–266. [DOI] [PubMed] [Google Scholar]
- Parmeter, J.R. , Sherwood, R.T. and Platt, W.D. (1969) Anastomosis grouping among isolates of Thanatephorus cucumeris . Phytopathology, 59, 1270–1278. [Google Scholar]
- Peltonen, S. (1995) Comparison of xylanase production by fungal pathogens of barley with special reference to Bipolaris sorokiniana . Mycol. Res. 99, 717–723. [Google Scholar]
- Poinssot, B. , Vandelle, E. , Bentejac, M. , Adrian, M. , Levis, C. , Brygoo, Y. , Garin, J. , Sicilia, F. , Coutos‐Thevenot, P. and Pugin, A. (2003) The endopolygalacturonase 1 from Botrytis cinerea activates grapevine defense reactions unrelated to its enzymatic activity. Mol. Plant–Microbe Interact. 16, 553–564. [DOI] [PubMed] [Google Scholar]
- Postel, S. and Kemmerling, B. (2009) Plant systems for recognition of pathogen‐associated molecular patterns. Semin. Cell Dev. Biol. 20, 1025–1031. [DOI] [PubMed] [Google Scholar]
- Ricci, P. , Panabieres, F. , Bonnet, P. , Maia, N. , Ponchet, M. , Devergne, J.C. , Marais, A. , Cardin, L. , Milat, M.L. and Blein, J.P. (1993) Proteinaceous elicitors of plant defense responses In: Mechanisms of Plant Defense Responses (Legrand M. and Fritig B., eds), pp. 121–135. Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- Ron, M. and Avni, A. (2004) The receptor for the fungal elicitor ethylene inducing xylanase is a member of a resistance‐like gene family in tomato. Plant Cell, 16, 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron, M. , Kantety, R. , Martin, G.B. , Avidan, N. , Eshed, Y. , Zamir, D. and Avni, A. (2000) High‐resolution linkage analysis and physical characterization of the EIX‐responding locus in tomato. Theor. Appl. Genet. 100, 184–189. [Google Scholar]
- Rotblat, B. , Enshell‐Seijffers, D. , Gershoni, J.M. , Schuster, S. and Avni, A. (2002) Identification of an essential component of the elicitation active site of the EIX protein elicitor. Plant J. 32, 1049–1055. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. , Frotsch, E.F. and Maniatis, T. (1989) Molecular Cloning, a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Sexton, A.C. , Paulsen, M. , Woestemeyer, J. and Howlett, B.J. (2000) Cloning, characterization and chromosomal location of three genes encoding host‐cell‐wall‐degrading enzymes in Leptosphaeria maculans, a fungal pathogen of Brassica spp. Gene, 248, 89–97. [DOI] [PubMed] [Google Scholar]
- Sharfman, M. , Bar, M. , Ehrlich, M. , Schuster, S. , Melech‐Bonfil, S. , Ezer, R. , Sessa, G. and Avni, A. (2011) Endosomal signaling of the tomato leucine‐rich repeat receptor‐like protein LeEix2. Plant J. 68, 413–423. [DOI] [PubMed] [Google Scholar]
- Sneh, B. , Jabaji‐Hare, S. , Neate, S. and Dijst, G. (1996) Rhizoctonia Species, Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control. Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- Sposato, J.P. , Ahn, J.H. and Walton, J.D. (1995) Characterization and disruption of a gene in the maize pathogen Cochliobolus carbonum encoding a cellulase lacking a cellulose binding domain and hinge region. Mol. Plant–Microbe Interact. 8, 602–609. [DOI] [PubMed] [Google Scholar]
- Stewart, B.J. and Leatherwood, J.M. (1976) Derepressed synthesis of cellulase by Cellulomonas . J. Bacteriol. 128, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto, D. , Hardham, A.R. and Jones, D.A. (2005) Differences in cell death induction by Phytophthora elicitins are determined by signal components downstream of MAP kinase in different species of Nicotiana and cultivars of Brassica rapa and Raphanus sativus . Plant Physiol. 138, 1491–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall, D.R. and Whitehead, I.M. (1988) Coordinated inhibition of squalene synthetase and induction of enzymes of sesquiterpenoid phytoalexin biosynthesis in cultures of Nicotiana tabacum . Phytochemistry, 27, 2567–2580. [Google Scholar]
- Towbin, H. , Staehelin, T. and Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets, procedure and some applications. Proc. Natl. Acad. Sci. USA, 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. and Jones, R.W. (1995) A unique endoglucanase‐encoding gene cloned from the phytopathogenic fungus Macrophomina phaseolina . Appl. Environ. Microbiol. 61, 2004–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Z.M. , Laby, R.J. , Zumoff, C.H. , Bauer, D.W. , He, S.Y. , Collmer, S. and Beer, S.V. (1992) Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora . Science, 257, 85–88. [DOI] [PubMed] [Google Scholar]
- Whitehead, I.M. , Ewing, D.F. and Threlfall, D.R. (1988) Sesquiterpenoids related to the phytoalexin debneyol from elicited cell suspension cultures of Nicotiana tabacum . Phytochemistry, 27, 1365–1370. [Google Scholar]
- Zhang, L. , Kars, I. , Essenstam, B. , Liebrand, T.W. , Wagemakers, L. , Elberse, J. , Tagkalaki, P. , Tjoitang, D. , van den Ackerveken, G. and van Kan, J.A. (2013) Fungal endopolygalacturonases are recognized as microbe‐associated molecular patterns by the Arabidopsis receptor‐like protein RESPONSIVENESS TO BOTRYTIS POLYGALACTURONASES1. Plant Physiol. 164, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Davis, L.C. and Verpoorte, R. (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 23, 283–333. [DOI] [PubMed] [Google Scholar]
- Zheng, A. , Lin, R. , Zhang, D. , Qin, P. , Xu, L. , Ai, P. , Ding, L. , Wang, Y. , Chen, Y. , Liu, Y. , Sun, Z. , Feng, H. , Liang, X. , Fu, R. , Tang, C. , Li, Q. , Zhang, J. , Xie, Z. , Deng, Q. , Li, S. , Wang, S. , Zhu, J. , Wang, L. , Liu, H. and Li, P. (2013) The evolution and pathogenic mechanisms of the rice sheath blight pathogen. Nat. Commun. 4, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel, C. , Kunze, G. , Chinchilla, D. , Caniard, A. , Jones, J.D. , Boller, T. and Felix, G. (2006) Perception of the bacterial PAMP EF‐Tu by the receptor EFR restricts Agrobacterium‐mediated transformation. Cell, 125, 749–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Alignment of the deduced amino acid sequences of Rhizoctonia solani endoglucanase (GU372728), Humicola insolens (A21793), Schizophyllum commune (EFI93868) and Melanocarpus albomyces (AJ515703). ▼, catalytic sites (D).
Fig. S2 Sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) analysis of the purified enzymes. (A) The unrelated cellulase (C61F, KC441879) of Chaetomium thermophilum. M, molecular weight marker. (B) The unrelated cellulase (T61F, KF170230) of Thermoascus aurantiacus. M, molecular weight marker. (C) The unrelated manganese superoxide dismutase (SOD, EF569978) of Chaetomium thermophilum. M, molecular weight marker. The three unrelated proteins were purified using nickel affinity chromatography from culture filtrates of yeast transformed with the plasmids pPIC9K/C61F, pPIC9K/T61F and pPIC9K/SOD. All methods of expression and purification of C61F, T61F and SOD were carried out according to those of the wild‐type (WT) and D32A. Three independent biological replicates were used for each protein.
Fig. S3 Mass spectrometry (MS) and high‐pressure liquid chromatography (HPLC) of purified EG1. (A) MS of purified EG1 (WT and D32A). Peak 1, EG1 with two charges; peak 2, EG1 with one charge; peak 3, two dimeric EG1 with one charge. (B) HPLC of purified EG1 (WT and D32A). Three independent biological replicates were used for each protein.
Table S1 List of primers used in the study for polymerase chain reaction (PCR). Ten selected genes of maize injected with EG1: thaumatin (GRMZM2G006853), histamine receptor (GRMZM2G041493), β‐1,3‐glucanase (GRMZM2G065585), pathogenesis‐related protein (GRMZM2G112524), alternative oxidase (GRMZM2G125669), zinc finger homeodomain protein (GRMZM2G353076), thaumatin (GRMZM2G042631), chitinase (GRMZM2G453805), pathogenesis‐related protein (GRMZM2G465226) and glutathione transferase (GRMZM2G475059).


