Summary
Taxonomy
Iris yellow spot virus (IYSV) is in the genus Tospovirus, family Bunyaviridae, with a single‐stranded, tri‐segmented RNA genome with an ambisense genome organization. Members of the other genera in the family infect predominantly vertebrates and insects.
Geographical distribution
IYSV is present in most Allium‐growing regions of the world.
Physical properties
Virions are pleomorphic particles of 80–120 nm in size. The particle consists of RNA, protein, glycoprotein and lipids.
Genome
IYSV shares the genomic features of other tospoviruses: a segmented RNA genome of three RNAs, referred to as large (L), medium (M) and small (S). The L RNA codes for the RNA‐dependent RNA polymerase (RdRp) in negative sense. The M RNA uses an ambisense coding strategy and codes for the precursor for the GN/GC glycoprotein in the viral complementary (vc) sense and a non‐structural protein (NSm) in the viral (v) sense. The S RNA also uses an ambisense coding strategy with the coat protein (N) in vc sense and a non‐structural protein (NSs) in the v sense.
Transmission
The virus is transmitted by Thrips tabaci Lindeman (Order: Thysanoptera; Family: Thripidae; onion thrips) and with less efficiency by Frankliniella fusca Hinds (tobacco thrips).
Host
IYSV has a relatively broad host range, including cultivated and wild onions, garlic, chives, leeks and several ornamentals. Some weeds are naturally infected by IYSV and may serve as alternative hosts for the virus.
Symptoms
IYSV symptoms in Allium spp. are yellow‐ to straw‐coloured, diamond‐shaped lesions on leaves and flowering scapes. Diamond‐shaped lesions are particularly pronounced on scapes. As the disease progresses, the lesions coalesce, leading to lodging of the scapes. In seed crops, this could lead to a reduction in yield and quality. Early to mid‐season infection in bulb crops results in reduced vigour and bulb size.
Control
Resistant varieties are not available, but a limited number of accessions with field tolerance have been identified. Integrated disease management tactics, including sanitation, crop rotation, thrips management, maintenance of optimal plant vigour, soil fertility, irrigation and physical separation of bulb and seed crops, can mitigate the effect of the disease.
Virus code: 00.011.0.85.009
Useful link: http://www.alliumnet.com/
Keywords: Bunyaviridae, thrips, tospoviruses, viruses
Introduction
Iris yellow spot virus (IYSV) is an economically important virus and a threat to onion seed and bulb crops in many parts of the world (Gent et al., 2006; Mandal et al., 2012; Pappu et al., 2009). Iris yellow spot (IYS) disease is most damaging to high‐value onion crops, where it can reduce bulb size and seed yields (Gent et al., 2004) and may cause crop losses of up to 100% (Pozzer et al., 1999). IYSV is a member of the genus Tospovirus, family Bunyaviridae. Members of the genus Tospovirus are the only plant‐infecting viruses in a family of viruses that infect vertebrates or insects. IYSV is transmitted by onion thrips (Thrips tabaci L.) in a persistent and propagative manner (Kritzman et al., 2001; Nagata et al., 1999). Recently, tobacco thrips (Frankliniella fusca Hind) have been shown to transmit IYSV, albeit at a lower efficiency relative to T. tabaci (Srinivasan et al., 2012).
The disease was first reported on onion from Brazil in 1981 (de Avila et al., 1981), and was characterized by chlorotic and necrotic eye spots with a green island at the centre, known as diamond eyes. In the late 1980s, similar symptoms were observed in the onion‐growing regions of the Treasure Valley (southwestern Idaho and southeastern Oregon) in the USA (Hall et al., 1993; Israel (Gera et al., 1998), and on Iris hollandica in the Netherlands (Cortês et al., 1998). In the USA, although IYSV was found in the late 1980s, it initially caused concern as a potential threat to onion production in the northwestern USA; however, it remained ‘dormant’ without causing much economic damage for a decade. Interestingly, since 2000, several states in the USA, beginning with Arizona, California and Utah, have reported its occurrence (Abad et al., 2003; Bag et al., 2009a; Evans et al., 2009a, b; Moyer et al., 2003), and IYSV began to re‐emerge as the cause of disease epidemics in the Pacific Northwestern states of Idaho, Oregon and Washington State beginning in 2003. Since then, the virus has been reported from several more states with reports of economic losses in both onion seed and bulb crops (Gent et al., 2006; Pappu et al., 2009). IYSV has now been reported from other major onion‐growing countries (Pappu et al., 2009; Mandal et al., 2012; Turina et al., 2012) (Fig. 1).
Figure 1.
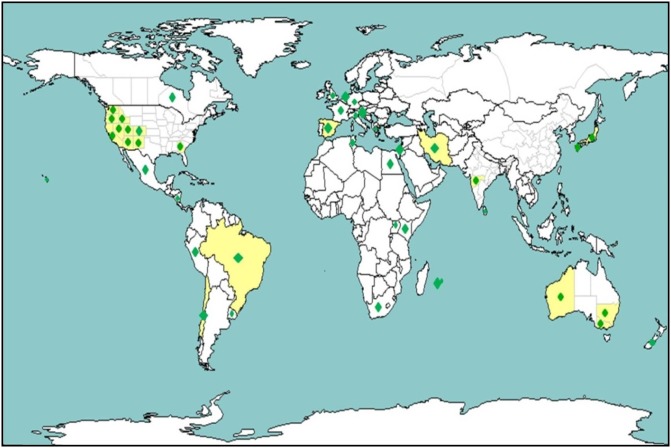
World map showing the countries in which Iris yellow spot virus (IYSV) has been reported. Yellow and green regions denote the presence of IYSV within a country.
The ever‐increasing globalization and trade of plants, sets and bulbs, and the small size of its primary vector, T. tabaci, (Fig. 2A), have probably contributed to the spread of IYSV to other onion‐growing regions. With improvement in diagnostics, the virus has been reported from an increasing number of countries in the last decade. The continued spread and increasing economic impact of the virus on an economically important crop, such as onion, and the threat to other Allium crops, have provided impetus to gain a better understanding of its biology, epidemiology and molecular biology, and to develop management options. For several onion growers in the USA and abroad, thrips and IYSV have become major concerns and are considered important research priorities (http://www.alliumnet.com/), resulting in several multi‐state, multi‐institutional and multi‐agency collaborations funded by onion grower associations, and regional and federal funding agencies.
Figure 2.

(A) Adult Thrips tabaci Lindeman (onion thrips). (B) Electron micrograph of Iris yellow spot virus (IYSV) from a thin section of infected Nicotiana benthamiana (photograph credit: Dr Abed Gera, Volcani Center, Bet Dagan, Israel). (C) Symptoms of IYSV in onion. Early symptoms display characteristically yellow‐ to straw‐coloured chlorotic or necrotic lesions, elongated, lenticular or spindle shaped, from the mid to lower portion of the infected plant. As the disease develops and plants grow, the lesions elongate and coalesce together, completely covering the leaves and scapes. Sometimes, green tissue in the centre of the lesions is present in the shape of a diamond: when present, this is considered as a specific diagnostic symptom for IYSV. (D) Reproduction of symptoms characteristic of IYSV infection in mechanically inoculated onion plants under controlled conditions.
Physical Properties
Hall et al. (1993), using transmission electron microscopy, showed the presence of virions that shared similar morphological features to known tospoviruses. Virions consist of 80–120‐nm pleomorphic particles (Fig. 2B) (Kritzman et al., 2001). Cortês et al. (1998) purified the nucleocapsid protein from infected Nicotiana benthamiana Domin, and subsequently purified the viral RNA and showed RNA segments corresponding to three sizes: small (2.9 kb), medium (4.8 kb) and large (8.9 kb).
Symptomatology and Host Range
IYSV infection on onion plants produces a wide range of disease symptoms. Early symptoms display yellow‐ to straw‐coloured chlorotic or necrotic lesions, elongated, lenticular or spindle shaped, from the mid to lower portion of the infected plant. As the disease develops and plants grow, the lesions elongate and coalesce, covering the leaves and scapes. Sometimes, green tissue in the centre of the lesions is present in the shape of a diamond which could be used as a diagnostic feature (Fig. 2C). Symptoms observed in the field have been reproduced in healthy onions by mechanical inoculation under controlled glasshouse conditions (Fig. 2D; S. Bag and H. R. Pappu, unpublished data). However, the rate of transmission remains relatively low (30%). Hence, the virus is maintained on experimental hosts, such as Nicotiana benthamiana or Datura stramonium. Infection and subsequent development of foliar symptoms result in a decline in photosynthetic activity and, as a result, reduced bulb size (Gent et al., 2006). Later in the season, the coalesced lesions cause the scape to lodge. In other Allium hosts, similar symptoms were observed, whereas, in weeds, mild chlorosis, necrosis, streaking or purpling was also observed (Evans et al., 2009a, b). The virus tends to be localized in and within close proximity to the lesions on leaves and scapes, and the highest virus titre is usually found in the inner leaves at the centre where thrips reside and feed (Kritzman et al., 2001). In a recent study, Boateng and Schwartz (2013) determined the virus distribution in various parts of the infected onion plants obtained from the field. IYSV could not be detected in roots, basal plates, bulb scales or dead leaves, and virus accumulation, based on the optical density, was significantly higher in younger leaves than in intermediate and older leaves under field conditions (Fig. S1, see Supporting Information). Within an individual symptomatic leaf, virus accumulation was greater in the basal section relative to the middle or top sections (Boateng and Schwartz, 2013).
In various experimental hosts (Bag and Pappu, 2009; Bulajic et al., 2009; Gent et al., 2006; Srinivasan et al., 2011), the virus causes chlorotic ring spots, chlorotic local lesions and necrotic spots, which coalesce and lead to the early senescence of infected leaves. The symptomatology depends on the virus isolate (Bulajic et al., 2009) and environmental conditions. The virus causes local infections in most experimental hosts, with only a few exceptions, such as N. benthamiana, where it produces necrosis in veins, stem and buds, leading to lodging of infected plants and early senescence (Bag and Pappu, 2009) (Fig. S2, see Supporting Information); in another study, it remained symptomless, but could be detected serologically (Bulajic et al., 2009). In Eustoma russellianum Salisb., the virus causes necrosis in systemic leaf tissues (Srinivasan et al., 2011). No local lesions or other symptoms developed in a number of other plant species tested (Bulajic et al., 2009), including D. stramonium (Bulajic et al., 2009). However, IYSV was shown to produce local lesions (Bag et al., 2012a; Bag and Pappu, 2009), further suggesting that symptom development is dependent on isolates and environmental conditions. Isolates were characterized that differ in symptom development and disease severity in experimental hosts, suggesting the existence of biologically distinct strains (Bag et al., 2012b).
Apart from the cultivated onion, IYSV has also been reported from other Allium spp., such as A. galanthum (Cramer et al., 2011), A. porrum (Gent et al., 2007; Schwartz et al., 2007), A. pskemense (Pappu et al., 2006b), A. roylei (Cramer et al., 2011), A. sativum (Bag et al., 2009b), A. schoenoprasum and A. tuberosum (Cramer et al., 2011) and A. vavilovii (Cramer et al., 2011; Pappu et al., 2006b). In addition to Allium spp. and some ornamentals, several weeds have been identified that are naturally infected with IYSV. Recent reports of natural infections of weeds include Ameranthus retroflexus, Chenopodium album, Kochia scoparia, Lactuca serriola and Tribulus terrestris (Sampangi et al., 2007), Atriplex micrantha and Setaria viridis (Evans et al., 2009a, b), and Cichorium intybus, Arctium minus, Rumex cripus and Taraxacum officinale (Hsu et al., 2011; Smith et al., 2011). A recent search of IYSV in weeds within and next to onion fields with a high IYSV disease incidence in northern Italy failed to reveal any possible reservoir hosts (M. Turina, unpublished data). A current listing of the host range of IYSV is available at http://www.alliumnet.org.
Genome Organization
As a typical tospovirus, the IYSV genome consists of three single‐stranded RNAs—large (L), medium (M) and small (S) (Fig. 3A). The L RNA is 8880 nucleotides long and contains a single open reading frame (ORF) of 8621 nucleotides in the viral complementary (vc) strand (Bag et al., 2010). The ORF potentially codes for a protein of 2873 amino acids with a predicted molecular weight of 331.17 kDa, sharing many of the features of the viral RNA‐dependent RNA polymerase (RdRp) coded by L RNAs of known tospoviruses. The 5′ and 3′ termini of IYSV L RNA (vc) contain two untranslated regions of 33 and 226 nucleotides, respectively, and both termini have eight nucleotides conserved at the 5′ terminal end (5′AGAGCAAT3′) and reverse complementary to the 3′ end (5′TCTCGTTA3′), another common feature of tospovirus genomic RNAs (Bag et al., 2010; Nichol et al., 2005).
Figure 3.
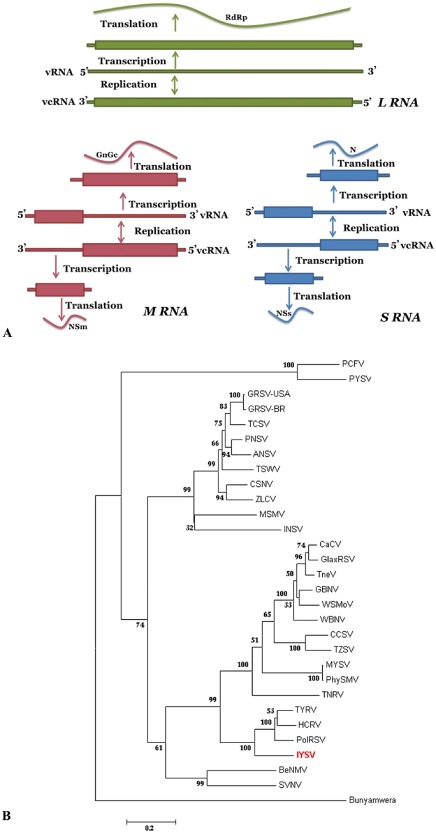
(A) Schematic representation of the genome organization and replication strategy of tospoviruses, showing the three RNAs: large (L), medium (M) and small (S). The rectangular boxes indicate the proteins coded. (B) Phylogeny based on amino acid sequences of nucleocapsid protein of known tospoviruses. The tospoviruses fall into two major groups based on their prevalence and distribution: Eurasia consisting of Europe, Asia and Oceania; the Americas consisting of North and South America. The representative gene sequence was obtained from the National Center for Biotechnology Information (NCBI) GenBank. Virus acronyms used in TREE are: Groundnut ringspot virus (GRSV); Groundnut bud necrosis virus (GBNV); Impatiens necrotic spot virus (INSV); Iris yellow spot virus (IYSV); Peanut yellow spot virus (PYSV); Polygonum ringspot virus (PolRSV); Tomato chlorotic spot virus (TCSV); Tomato spotted wilt virus (TSWV); Watermelon bud necrosis virus (WBNV); Zucchini lethal chlorosis virus (ZLCV); Alstroemeria necrotic streak virus (ANSV); Bean necrotic mosaic virus (BeNMV); Calla lily chlorotic spot virus (CCSV); Capsicum chlorosis virus (CaCV); Chrysanthemum stem necrosis virus (CSNV); Groundnut ringspot virus‐USA (GRSV‐USA); Gloxinia tospovirus (GlaxRSV); Hippeastrum chlorotic ringspot virus (HCRV); Melon severe mosaic virus (MSMV); Melon yellowspot virus (MYSV); Pepper necrotic spot virus (PNSV); Peanut chlorotic fanspot virus (PCFV); Physalis silver mottle virus (PhySMV); Soybean vein necrosis virus (SVNV); Tomato necrosis virus (TNeV); Tomato necrotic ringspot virus (TNRV); Tomato yellow fruit ring virus (TYFRV); Tomato zonate spot virus (TZSV); Watermelon silver mottle virus (WSMoV).
The M RNA is 4821 nucleotides long with two ORFs in ambisense arrangement expressed through two subgenomic RNAs, possibly capped by a ‘cap snatch’ mechanism (Duijsings et al., 2001; van Knippenberg et al., 2005). The smaller ORF of 935 nucleotides is located at the 5′ end of the viral (v) sense strand, potentially encoding a 311‐amino‐acid protein with a predicted molecular mass of 34.7 kDa, coding for a non‐structural protein (NSm) homologue to the NSm of Tomato spotted wilt virus (TSWV), which has been shown to be a movement protein. The second ORF, the homologue to TSWV glycoprotein precursor (GN/GC), is in the vc sense, and is 3410 nucleotides in length, potentially coding for a 1136‐amino‐acid protein of 128.8 kDa. The two ORFs are separated by a 380‐nucleotide intergenic region (Bag et al., 2009c; Cortês et al., 2002). The GN protein in TSWV has been shown to serve as a ligand for the virus that mediates the uptake by thrips receptors (Sin et al., 2005; Whitfield et al., 2005).
The S RNA segment of IYSV is 3105 nucleotides long (Cortês et al., 1998) and codes for two non‐overlapping ORFs in an ambisense arrangement. The ORF in v sense is 1329 nucleotides long, coding for a 50.1‐kDa non‐structural protein (NSs) shown to be a silencing suppressor for TSWV (Takeda et al., 2002), and potentially plays a similar role in other tospoviruses. The second ORF in vc sense is 819 nucleotides long, and potentially codes for the nucleocapsid protein of 30.5 kDa (Cortês et al., 1998) encapsulating each tospovirus genomic RNA.
The three IYSV genomic RNAs share several genome‐wide characteristics with those of other tospoviruses. The first eight nucleotides of the 5′ and 3′ terminal sequences are identical for all three RNA segments. Furthermore, the three RNA segments have complementary ends forming stable panhandle termini (Fig. S3A, see Supporting Information). Both coding ORFs of S and M RNA are separated by intergenic regions rich in A and U residues with potential for internal pairing, and are probably involved in transcription termination of the subgenomic RNAs (Fig. S3B) (Adkins, 2000; Nichol et al., 2005). The tetraloop motif is speculated to determine the binding of translation factors or other proteins (Clabbers et al., 2014).
Based on the N gene amino acid sequence of known tospovirus species, IYSV forms a clade with recently described tospoviruses from Eurasia, such as Tomato yellow ring virus (TYRV), Hippeastrum chlorotic ringspot virus (HCRV) and Polygonum ringspot virus (PolRSV), with 69.7%–72.9% and 51%–71.3% identity at amino acid and nucleotide levels, respectively (Fig. 3B). Similarly, based on various genes coded by M and L RNAs, IYSV forms a clade within the Eurasian group, distinct from the American clade (Bag et al., 2009c, 2010), suggesting its European origin. Among the known IYSV isolates, the N protein amino acid sequence identity ranges between 89% and 90%. Phylogenetic analysis of the complete N protein sequences of 99 IYSV isolates available in GenBank showed that IYSV isolates formed two distinct clades (Fig. 4A). Clade I mainly consists of the Asian isolates (India, Japan, Sri Lanka), three isolates from the Oceania (Australia and New Zealand) and three isolates from the USA. Another clade consisted of the rest of the isolates from the Americas and Eurasia. Most of the isolates formed close clusters based on the collection sites, suggesting limited evolution within a given geographical region. Based on this study and an earlier study by Turina et al. (2012), the little variability observed in some clades and the higher variability seen in isolates from the Mediterranean area support the hypothesis that the origin of IYSV could be from this region and, to some extent, the clustering of isolates could be based on geographical origin.
Figure 4.
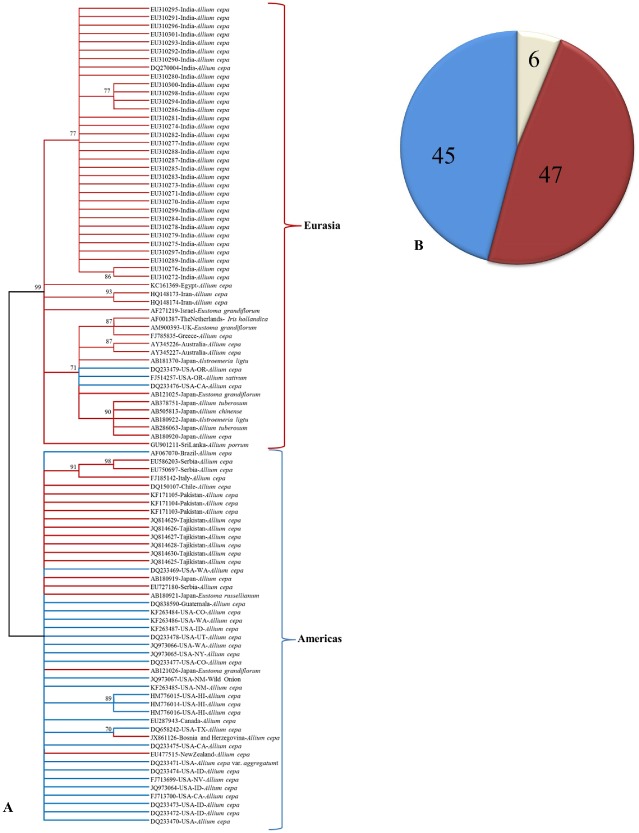
(A) Phylogeny based on amino acid sequences of the nucleocapsid protein of Iris yellow spot virus (IYSV) available in GenBank. The evolutionary history was inferred using the unweighted pair group method with arithmetic average (UPGMA). The optimal tree with the sum of branch length = 0.76736606 is shown. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. The analysis involved 99 amino acid sequences. All positions containing gaps and missing data were eliminated. There were a total of 270 positions in the final dataset. Evolutionary analyses were conducted in MEGA6. (B) Genotyping of IYSV accessions based on in silico restriction fragment length polymorphism (RFLP) simulation of nucleocapsid (N) gene (percentage of accessions under various genotypes).
IYSV isolates could be grouped into Netherlands (NL) and Brazil (BR) genotypes based on restriction fragment length polymorphism (RFLP; Zen et al., 2005). A global analysis of 99 complete N gene sequences available in GenBank showed an almost even distribution of known IYSV isolates into NL and BR genotypes (Fig. 4B). Temporal and spatial analysis of the N protein sequences showed that the observed genetic diversity among IYSV populations could be attributed to the combined action of genetic recombination, purifying selection and genetic drift (Iftikhar et al., 2014).
Localization and interaction studies of viral proteins provide important information about virus replication in their host plants. Tripathi et al. (2013) studied the in vivo localization and interaction patterns of IYSV proteins in infected N. benthamiana, and identified the interacting partners. IYSV genes were cloned into binary pSITE‐BiFC vectors and agroinfiltrated into marker N. benthamiana plants expressing cyan or red fluorescent protein (CFP/RFP) in their nuclei. Bimolecular fluorescence complementation (BiFC) analysis was used to test the interactions between IYSV proteins (Fig. 5A). IYSV proteins localized in the cell periphery of cells, and homotypic and heterotypic interactions between IYSV nucleocapsid (N) and movement (NSm) proteins were observed. These interactions were further confirmed by pull‐down assays. Interacting regions of IYSV N and NSm were identified by the yeast two‐hybrid assay and β‐galactosidase assay. The N protein self‐association was found to be mediated through the N‐ and C‐terminal regions making head to tail combinations. Self‐interaction of IYSV NSm was shown to occur through multiple interacting regions. The N‐ and C‐terminal regions of IYSV N interacted with an N‐terminal region of IYSV NSm in a yeast two‐hybrid assay (Tripathi, 2014). These studies provided new insights into the localization and interactions of IYSV proteins.
Figure 5.
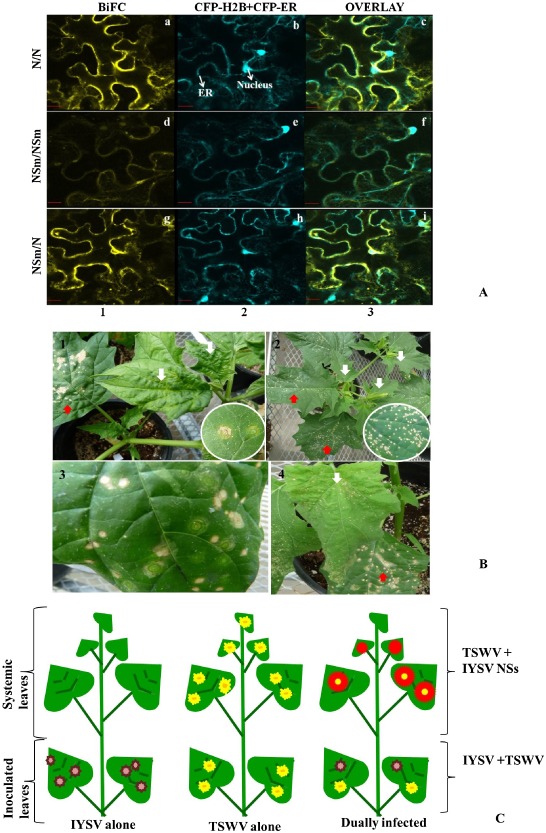
(A) Confocal micrographs showing the Iris yellow spot virus (IYSV) protein interactions examined. Interaction assays were performed in leaf epidermal cells of transgenic Nicotiana benthamiana plants expressing cyan fluorescent protein fused to the nuclear marker histone 2B (CFP‐H2B) and the endoplasmic reticulum marker (CFP‐ER). Shown are the interaction assay [bimolecular fluorescence complementation (BiFC), column 1], localization of CFP‐H2B and CFP‐ER (nucleus and ER, column 2) and a merge of all panels (overlay, column 3). Proteins listed first in the pair of interactors were expressed as C‐terminal fusions to the amino‐terminal half of yellow fluorescent protein (YFP). Those listed second were expressed as C‐terminal fusions to the carboxy‐terminal half of YFP. All protein fusions to each half of YFP were tested in all pairwise combinations, a subset of detectable interactions of which is shown here: (a–c) N/N; (d–f) NSm/NSm; (g–i) NSm/N. Micrographs shown are representative of at least 50 cells examined. Scale bar, 20 μm. (B) Differential response of Datura stramonium to mechanical inoculation with IYSV and Tomato spotted wilt virus (TSWV). Top left: D. stramonium is a permissive host for TSWV, wherein infection results in concentric ring spots in inoculated leaves, and leaf curling, mottling and necrosis in systemic leaves, shown by white arrows. Top right: D. stramonium is a restrictive host for IYSV. IYSV infection produces small necrotic spots. Bottom left: distinctive concentric rings and necrotic spots develop in inoculated leaves of Datura infected with both viruses. Bottom right: more severe symptoms develop in inoculated and systemic leaves of dually infected Datura. Red arrows show symptoms in inoculated leaves and white arrows show symptoms in systemic infection. Insets show close‐up of TSWV and IYSV symptoms in inoculated leaves. (C) Schematic representation of differential response of D. stramonium to IYSV and TSWV.
Mixed infections of commercial crops with two or more distinct tospoviruses have been reported from different cucurbits and Allium crops (Kunkalikar et al., 2011; Mullis et al., 2004). These mixed infections could be the contributing factor to the observed genetic diversity of segmented viruses, such as tospoviruses. Using Datura, Bag et al. (2012a) found potential genetic complementation between IYSV and another distinct tospovirus, TSWV, in plants that were mechanically inoculated with both viruses. In Datura, TSWV causes systemic infection (Fig. 5B), whereas IYSV is restricted to inoculated leaves (Fig. 5B). In contrast, when Datura plants were mechanically inoculated with a mixture of TSWV and IYSV, symptoms characteristic for each of the viruses (concentric ring spots for TSWV and necrotic spots for IYSV) were produced in inoculated leaves (Fig. 5B). Interestingly, more severe symptoms were observed in younger, uninoculated leaves relative to those produced by TSWV infection alone (Fig. 5B). On further molecular analysis of different symptomatic leaves, it was found that TSWV facilitates the selective movement of the IYSV NSs gene to younger, uninoculated leaves (Fig. 5B), causing more severe systemic infection by overcoming plant defence more effectively. Further investigation revealed that, in systemically infected leaves of dually infected plants, there is a reduced level of TSWV N gene‐specific small interfering RNAs (siRNAs). There was no TSWV NSs‐specific siRNAs in inoculated and systemically infected leaves of dually infected plants, suggesting effective and efficient overcoming of the plant silencing mechanism under the influence of both NSs genes relative to the presence of individual NSs genes (Bag et al., 2012a).
Diagnosis
Tospoviruses generally produce necrotic and chlorotic symptoms in their host plants in both localized and systemic infections. In the case of IYSV, the virus tends to remain localized and produces characteristic symptoms as discussed above. The diamond‐shaped lesions are more obvious on scapes than on leaves, and are unique to IYSV infection in onion and could be used as a diagnostic aid.
For immunodiagnosis, IYSV‐specific polyclonal antibodies have been developed against the N protein and enzyme‐linked immunosorbent assay (ELISA) reagents and immunostrips are commercially available for rapid and accurate diagnosis (Pappu et al., 2006a). Although the N gene (and the protein) is conserved among known IYSV isolates, some differences in the efficiency among the various commercial kits that utilize different polyclonal antisera have been reported (Tomassoli et al., 2009). For the detection of IYSV in individual thrips, a polyclonal antiserum was produced against the recombinant NSs protein that was expressed in Escherichia coli. The antiserum has been used to determine the seasonal dynamics of thrips transmitters in field‐collected thrips populations (Bag et al., 2014).
Molecular diagnostics based on ‘traditional’ PCR and real‐time PCR for IYSV detection and diagnosis have been developed and the N gene was used as a target for amplification for this purpose (Chingandu et al., 2012; Pappu et al., 2006a). In most cases, the resulting amplicons have been sequenced, and more than 100 complete N gene sequences are now available in GenBank. This dataset on IYSV N gene sequences has facilitated some detailed genetic diversity studies on a global scale (Iftikhar et al., 2014).
Transmission and Epidemiology
Of the 14 species of thrips reported to transmit tospoviruses (reviewed by Riley et al., 2011; Turina et al., 2012), onion thrips are the confirmed vector for IYSV (Cortês et al., 1998; Hsu et al., 2010; Kritzman et al., 2001; Nagata et al., 1999) (Fig. 2A). Recently, Srinivasan et al. (2012) have reported that Frankliniella fusca (Hinds) (tobacco thrips) can also transmit IYSV, albeit with a lower efficiency, under experimental conditions. Infestation by T. tabaci was correlated with a higher incidence of IYSV in onion (Kritzman et al., 2001). Similar to TSWV, IYSV is considered to be acquired at the larval stage and transmission occurs by second‐instar larvae and adult thrips in a propagative and persistent manner (Inoue et al., 2010; Ullman et al., 1992a, b, c, 1993; Wetering et al., 1996; Wijkamp et al., 1993). In addition to being a primary vector for IYSV, onion thrips are a serious pest of onion and their leaf‐feeding damage can cause up to 60% yield loss (Waiganjo et al., 2008). There is no evidence that IYSV is transmitted through either onion bulbs or seeds (Boateng and Schwartz, 2013; Bulajic et al., 2009; Kritzman et al., 2001).
Originating in the Mediterranean region, T. tabaci is now a cosmopolitan pest and is present in almost every Allium‐growing region in the world (Jones, 2005). The insect reproduces by thelytokous (Kendall and Capinera, 1990; Lewis, 1973), parthenogenesis (Moritz, 1997) and arrhenotoky (Diaz‐Montano et al., 2011; Kendall and Capinera, 1990). Onion thrips are polyphagous, infest plants belonging to ∼25 species, but show preference for Alliaceae over other plant species. In Allium spp., they have been shown to have a mixed population of both sexes, in contrast with only females in other crops (Bosco and Tavella, 2010). This preferential habitat towards Allium spp. could be correlated with the increased number of Allium spp. known to be susceptible to IYSV infection. Recent reports have highlighted the genetic basis of the differential ability of specific T. tabaci populations to transmit TSWV (Inoue et al., 2010; Jacobson and Kennedy, 2013; Jacobson et al., 2013; Westmore et al., 2013; Wijkamp et al., 1995), and TYRV (Golnaraghi et al., 2007), which might influence T. tabaci–IYSV transmission and virus–host specificity. Adaptation to specific T. tabaci populations through co‐evolution could explain the recent outbreaks of the disease in various onion‐growing areas in the USA and elsewhere. Because of its rapid growth cycle and abundance in both open field and glasshouse environments, T. tabaci usually overwinters in weeds and other vegetable and fruit crops under field conditions.
Several studies have investigated the role of transplants, crop volunteers and weeds present in and around onion fields. IYSV infection has been detected in different weeds collected from onion fields from different onion‐growing regions (Evans et al., 2009a, b; Hsu et al., 2011; Nischwitz et al., 2007; Sampangi et al., 2007; Smith et al., 2011), suggesting that weeds could serve as alternative hosts and potential sources of inoculum for virus transmission and infection of new Allium plantings. Infected transplants carrying viruliferous thrips may also be a probable cause of long‐distance disease spread, especially in island states and nations, such as Hawaii (Sether et al., 2010) and Mauritius (Lobin et al., 2010). Sequence comparisons of the N gene suggested that IYSV in Georgia, USA might have originated in Peru from either infected onions or onions harbouring viruliferous thrips (Nischwitz et al., 2006). Similarly, it has also been shown that IYSV could have been introduced into Colorado (Mahaffey and Cranshaw, 2010) and New York (Hsu et al., 2011) by either infected transplants or plants carrying viruliferous thrips imported from states from which IYSV had already been reported.
Hsu et al. (2011) and Smith et al. (2011) have suggested that volunteer onions, bulbs, imported bare root transplants and weeds could be major sources of IYSV introduction into commercial onion fields of New York. The seasonal dynamics of T. tabaci in onion fields in Oregon suggested that the largest number of viruliferous thrips were present in the middle of July near overwintering onion fields, compared with fewer viruliferous thrips in onion fields that were located further from overwintering onion fields, suggesting that overwintering onion fields are probable sources of primary infection in this study (Bag et al., 2014). As suggested by Mo et al. (2009), thrips typically invade onion fields early in the season, with subsequent buildup of the population during the season. A different pest and disease dynamic was described by Hsu et al. (2010), who found higher thrips numbers later in the season, migrating from adjacent fields that had been harvested.
Disease Management
Although the virus has been known to infect Allium spp., especially onion, for over 25 years, an organized effort to develop management tactics has started only recently after the virus‐induced disease outbreaks have become increasingly damaging and widespread in the western USA. Scientifically validated management tactics have just begun to emerge for IYSV. Research on the development of integrated disease management has been considered a priority area by several state‐based onion grower associations, state and federal funding agencies in the USA, and support from these various sources has resulted in a nationwide organized effort of an interdisciplinary team of scientists, including virologists, plant pathologists, horticulturalists, entomologists, geneticists and breeders. As a result, various production practices that could influence disease outbreaks have been identified. Based on these observations, a risk index to estimate and predict the likelihood of disease outbreaks has been developed and is being validated (Schwartz et al., 2010).
Similar to any other insect‐transmitted virus, an integrated disease management approach needs to be developed and adapted to reduce the impact of the disease. The central part of such an integrated strategy is to grow virus‐resistant or virus‐tolerant cultivars and to develop sound thrips management practices. For disease resistance, possible approaches include the development of resistant cultivars to either the virus or its thrips vector, or both. The evaluation of cultivars and breeding material for virus resistance must be carried out under field conditions relying on natural disease pressure, as there is no reliable and efficient method for the inoculation of onion by mechanical inoculation. Attempts to develop a screening method based on mechanical inoculation have so far yielded limited results—the disease could be reproduced under glasshouse conditions using manual inoculation (Fig. 2D), but the inoculation efficiency was no more than 30%, which is not acceptable for screening for virus resistance (S. Bag and H. R. Pappu, unpublished data); another study reported a transmission rate of 60% by mechanical inoculation (Kumar and Dhawan, 2013).
Field resistance could be manifested as reduced disease severity and/or delayed symptom development with the goal that bulb yield and grade are not negatively affected. The evaluation of onion germplasm and existing cultivars has been ongoing in several states in the USA. No resistant variety has been identified to date, but germplasm and cultivars with tolerance to the virus and to thrips feeding have been identified. Shock et al. (2008) and Diaz‐Montano et al. (2010) evaluated a number of onion cultivars for resistance to T. tabaci, and found that two cultivars, Colorado 6 and NMSU 03‐52‐1, had the smallest numbers of thrips per plant, but neither cultivar was resistant to IYSV. During a further evaluation of 17 additional cultivars, none was found to be resistant to thrips and/or IYSV (Diaz‐Montano et al., 2010). In a study conducted by Multani et al. (2009), 18 winter‐sown onion entries were screened for reduced IYSV symptom expression, with NMSU 03‐52‐1, NMSU 04‐41, NMSU 04‐44‐1 and ‘NuMex Jose Fernandez’ exhibiting the fewest IYSV symptoms. Moghadam et al. (2011) evaluated 13 winter‐sown onion entries for IYSV symptom expression, and NMSU 05‐33‐1 showed a delayed symptom expression, suggesting that differences exist among entries for IYSV symptoms.
Singh (2013) evaluated the breeding materials for reduced IYSV‐induced symptom expression after one cycle of selection. He found a limited number of instances in which selection was able to reduce virus damage. The effect of onion foliage colour on thrips feeding behaviour has been well documented: yellow–green‐coloured foliage is less attractive than blue–green‐coloured foliage to thrips (Diaz‐Montano et al., 2012; Jones et al., 1934; Molenaar, 1984). Although foliage colour depends on nutritional level, these foliage types can be distinguished by different amounts of epicuticular waxes on leaf surfaces. Blue–green foliage carries large amounts of epicuticular waxes relative to lighter green foliage types, such as glossy (essentially no waxes) and semi‐glossy (intermediate to waxy and glossy) foliage types (Damon and Havey, 2013; Molenaar, 1984). This trait is being exploited to develop onion cultivars showing less damage by thrips and IYSV. However, early research suggests that this trait may not be the sole determinant for thrips feeding, as plants with blue–green foliage have been observed with a reduced number of thrips per plant, and plants with light green foliage have been observed with a high number of thrips per plant (Singh, 2013).
Results from numerous groups have indicated that thrips populations are often lower on onions with less epicuticular wax when compared with waxier types (Diaz‐Montano et al., 2012; Jones et al., 1934; Molenaar, 1984). This may be advantageous to growers by reducing the frequencies of insecticide sprays over the growing season. However, there is no evidence that reduced waxiness of foliage conditions resistance to thrips or IYSV. Smaller amounts of epicuticular waxes are recessive to waxier types (Molenaar, 1984). Two chromosome regions have been identified controlling the amounts of epicuticular waxes on onion foliage (Damon and Havey, 2013). Molecular markers are being identified to facilitate the backcrossing of less waxy phenotypes into diverse onion populations.
Inducers of systemic acquired resistance (SAR), such as Actigard (1,2,3‐benzothiadiazole‐7‐thiocarboxylic acid‐S‐methyl‐ester; Syngenta, Wilmington, DE, USA), have been used on onions under field conditions and the final disease incidence was determined. Although Actigard reduced the final disease incidence in some years, the effect was not consistent from year to year, indicating that other factors, such as environment, could influence the resistance response (Gent et al., 2006). Efforts to understand the mechanism behind the SAR induction against IYSV are being carried out under controlled conditions and by using experimental hosts, Datura and N. benthamiana. With Datura, a local lesion host for IYSV, three descriptors were used—lesion number, lesion size and relative virus levels in Actigard‐treated plants. As determined by ELISA and PCR, Actigard‐treated plants showed a significant level of reduction in virus levels relative to buffer‐inoculated control plants. In addition, Actigard also reduced the IYSV lesion number and size in treated Datura plants (Fig. S4, see Supporting Information) (Tripathi and Pappu, 2011).
In the absence of any significant host plant resistance to IYSV, management options are currently centred around cultural or production practices, such as sanitation (removal of crop volunteers, better weed management), crop rotation, straw mulching, irrigation scheduling, avoidance of the green bridge by physical separation of bulb and seed crops, fertilizer management, especially for nitrogen (Buckland et al., 2013; Larentzaki et al., 2008; Malik et al., 2009), and sound thrips control practices through the judicious use of reduced‐risk insecticides (Nault et al., 2012). The use of straw mulch resulted in the reduction of thrips numbers by 30% (Schwartz et al., 2009). In a recent study in Utah, USA, it was found that reduced soil nitrogen in the absence of biostimulants can reduce onion thrips densities and final disease incidence without a significant loss in yield. It also created a favourable environment for soil microbial activity, and reduced the risk of leaching soil nitrate (Buckland et al., 2013). It was further suggested that adult onion thrips prefer onion leaf tissues with higher nitrogen contents caused by maximum use of nitrogen fertilizers (Buckland et al., 2013; Malik et al., 2009). It has been widely noted and accepted that plants that are under stress (such as moisture stress) show more disease relative to those that are not. The field tolerance (expressed as reduced symptoms) to IYSV observed in some cultivars and breeding lines could be caused by an increased tolerance to plant stress. This is a worthwhile topic for future research. Using multiple approaches, such as crop rotations, together with soil and fertilizer managements, to control thrips infestation during the season, especially in the early stages of the crop, could reduce the damage caused by both thrips feeding and IYSV infection.
The use of RNA interference (RNAi) by the expression of IYSV genomic sequences to generate transgenic plants with resistance to IYSV is an approach that could complement ongoing efforts to develop virus‐resistant varieties using traditional plant breeding. However, efficient transformation and regeneration of onion continue to be a challenge. Transgenic onion expressing an RNAi construct was developed to reduce lachrymatory synthase activity, significantly reducing the level of tear‐inducing lachrymatory factor (Eady et al., 2008), and this strategy could be further exploited to develop IYSV‐resistant cultivars.
Summary
The initial emergence of IYSV and the lull, which was followed by resurgence and the subsequent rapid spread of IYSV in the USA, could be considered a textbook example of basic epidemiological principles at work: virus inoculum levels, levels and distribution of vector species, climatic effects, production practices, regional and international trade of onion sets and transplants may all have played an important role in virus spread and subsequent outbreaks. Although the virus was first reported in the 1980s, it remained of little concern because of its negligible impact in major onion‐growing regions of the world until 2000. Virus epidemics and their rapid spread to new areas have been witnessed since then. However, the trigger of this re‐emergence resulting in outbreaks is not known. New strains, new vector biotypes, increased trade of onion planting material, global warming and the expansion of the virus host range, including weeds, might have contributed to an increase in the levels of virus inoculum and its subsequent spread by thrips. Considering the continuing new reports of IYSV from many parts of the onion‐growing areas of the world, and the importance of Allium spp. for many regional and national economies, research should continue in an attempt to understand the epidemiology and to develop control options.
In a relatively short period of time of 10 years since IYSV outbreaks became economically damaging, considerable progress has been made in our understanding of the virus and the vector, and the multitude of their interactions, in diverse managed ecosystems. A package of practical disease management recommendations and a risk index have been developed that can be used as decision‐making tools for growers in some parts of the USA. This risk index must be adjusted and fine‐tuned to meet the different climatic conditions and production practices in different parts of the onion‐growing regions. This index was made possible because of the sustained financial support of research on this disease by onion growers, industry, state and federal funding agencies in the USA and elsewhere. This is a fine example of a public–private partnership to address a potential food security issue in a coordinated, collaborative and interdisciplinary manner.
Challenges remain however. A lack of a reliable assay based on mechanical inoculation for the infection of onion is a bottleneck, and biological studies have to rely on experimental hosts, such as N. benthamiana. Considering the critical role of thrips in virus spread, the options for the management of vector thrips are still fairly limited, and chemicals utilizing different chemistries are few and research should continue in this area. In addition, the use of molecular approaches to target the developmental genes of thrips through RNAi is another fertile ground for research that could yield effective ways to intervene and interrupt the IYSV–thrips association, and thus contribute to the limitation of the role of thrips in virus outbreaks.
Overall, the significant progress made on IYSV bodes well for the future of research on this important disease of onion and its potential to reduce the impact of the disease. This is contingent upon continued adherence to the proven model that made the progress possible: a strong private–public partnership, and a sustained, long‐term funding for area‐wide, collaborative, interdisciplinary and mission‐oriented research.
Supporting information
Fig. S1 Distribution of Iris yellow spot virus (IYSV) in plants. (A) Onion plant with bulb. (B) Virus accumulation was found to be significantly greater in younger leaves (8–11) than in intermediate and older leaves. (C) IYSV was not detected in roots, basal plates, bulb scales or dead leaves.
Fig. S2 Comparison of biologically distinct isolates of Iris yellow spot virus (IYSV) that differed in disease development in Nicotiana benthamiana under controlled glasshouse conditions. Initial symptom differentiation was superimposed for each isolate. IYSV‐Idaho (IYSV‐ID) and IYSV‐Washington (IYSV‐WA) were found to be more severe than IYSV‐California (IYSV‐CA). IYSV‐ID and IYSV‐WA isolates had a more severe phenotype at 22 days post‐inoculation (dpi) compared with the mild strain, IYSV‐CA, as shown in the top panel. By 50 dpi, IYSV‐ID and IYSV‐WA isolates were lethal to the plants, whereas the effect on the plant infected with IYSV‐CA was less severe, as shown in the bottom panel.
Fig. S3 Secondary RNA structure of Iris yellow spot virus (IYSV) genomic RNAs based on minimum free energy (MFE) fold algorithm prediction using the RNA Fold Web Server (http://rna.tbi.univie.ac.at/cgi‐bin/RNAfold.cgi). (A) Secondary structure of small RNA. Inset shows the panhandle structural forms in all three RNAs as a result of the presence of the complementary structure. (B) Structure of the intergenic region (IGR) of IYSV M RNA forming a stem‐loop structure. The apical Y‐shaped loop formed (dG = −63.70) is shown as an enlarged image.
Fig. S4 Acibenzolar‐S‐methyl (ASM)‐induced resistance response against Iris yellow spot virus (IYSV) in Datura stramonium and Nicotiana benthamiana plants. (A, B) IYSV symptoms in D. stramonium plants. Datura stramonium leaves showing lesions induced by IYSV in buffer‐ and ASM‐treated plants at 14 days post‐inoculation (dpi). Different sets of plants were treated with buffer or ASM (0.1 mm). Seven days later, treated plants were inoculated with IYSV. The diameters of IYSV lesions were measured at 14 days post‐IYSV inoculations. (C, D) Reverse transcription‐polymerase chain reaction (RT‐PCR) of IYSV nucleocapsid (N) gene. Relative levels of expression of nucleocapsid (N) gene of IYSV in ASM‐ and buffer‐treated D. stramonium (C) and N. benthamiana (D) plants. Different sets of plants were treated with buffer or ASM (0.1 mm). Seven days later, treated plants were inoculated with IYSV. RT‐PCR was conducted on samples collected at 14 days post‐IYSV inoculations. I, IYSV‐inoculated plants; B + I, buffer‐treated plants inoculated with IYSV; A + I, ASM‐treated plants inoculated with IYSV; H, healthy plants; A, ASM‐non‐inoculated plants. (E) RT‐PCR of pathogenesis‐related (PR‐1a) genes. Detection of PR‐1a genes in buffer‐ and ASM‐treated N. benthamiana plants. Different sets of plants were treated with buffer or ASM (0.1 mm). A set of three plants per treatment was used for analysis. Leaf samples were harvested after 2 days of treatment, and expression of the PR1a gene was detected by RT‐PCR using PR‐1a gene‐specific primers.
Acknowledgements
This research was funded in part by the United States Department of Agriculture ‐ National Institute of Food and Agriculture (USDA‐NIFA) Specialty Crop Research Initiative Grant numbers 2008‐51180‐04875, 2008‐4804 and 2010‐01193. PPNS no. 0575, Department of Plant Pathology, College of Agricultural, Human and Natural Resource Sciences, Agricultural Research Center, Project # WNPO 0545, Washington State University, Pullman, WA 99164‐6430, USA.
References
- Abad, J.A. , Speck, J. , Mohan, S.K. and Moyer, J.W. (2003) Diversity of the Iris yellow spot virus N gene in the USA. Phytopathology, 93, S1.18944040 [Google Scholar]
- Adkins, S. (2000) Tomato spotted wilt virus—positive steps towards negative success. Mol. Plant Pathol. 1, 151–157. [DOI] [PubMed] [Google Scholar]
- de Avila, A.C. , Gama, M.I.C. , Kitajima, E.W. and Pereira, W. (1981) Um virus do grupo vira‐cabeca do tomateiro isolado de cebola (Allium cepa L.). Fitopatol. Bras. 6, 525. [Google Scholar]
- Bag, S. and Pappu, H.R. (2009) Symptomatology of Iris yellow spot virus in selected indicator hosts. Plant Health Prog. doi: 10.1094/PHP-2009-0824-01-BR. [DOI] [Google Scholar]
- Bag, S. , Singh, J. , Davis, R.M. , Chounet, W. and Pappu, H.R. (2009a) Iris yellow spot virus in Nevada and northern California. Plant Dis. 93, 674. [DOI] [PubMed] [Google Scholar]
- Bag, S. , Rogers, P. , Watson, R. and Pappu, H.R. (2009b) First report of natural infection of garlic with Iris yellow spot virus in the United States. Plant Dis. 93, 839. [DOI] [PubMed] [Google Scholar]
- Bag, S. , Druffel, K.L. , Salewsky, T. and Pappu, H.R. (2009c) Nucleotide sequence and genome organization of the medium RNA of Iris yellow spot virus (genus Tospovirus, family Bunyaviridae) from the Unites States. Arch. Virol. 154, 715–718. [DOI] [PubMed] [Google Scholar]
- Bag, S. , Druffel, K.L. and Pappu, H.R. (2010) Structure and genome organization of the large RNA of Iris yellow spot virus (genus Tospovirus, family Bunyaviridae). Arch. Virol. 155, 275–279. [DOI] [PubMed] [Google Scholar]
- Bag, S. , Mitter, N. , Eid, S. and Pappu, H.R. (2012a) Complementation between two tospoviruses facilitates the systemic movement of a plant virus silencing suppressor in an otherwise restrictive host. PLoS ONE, 7, e44803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag, S. , Schwartz, H.F. and Pappu, H.R. (2012b) Identification and virus characterization of biologically distinct Iris yellow spot virus (genus Tospovirus, family Bunyaviridae), a serious pathogen of onion. Eur. J. Plant Pathol. 134, 97–104. [Google Scholar]
- Bag, S. , Rondon, S.I. , Druffel, K.L. , Riley, D.G. and Pappu, H.R. (2014) Seasonal dynamics of thrips (Thrips tabaci) transmitters of Iris yellow spot virus, a serious viral pathogen of onion bulb and seed crops. J. Econ. Entomol. 107, 75–82. [DOI] [PubMed] [Google Scholar]
- Boateng, C.O. and Schwartz, H.F. (2013) Temporal and localized distribution of Iris yellow spot virus within tissues of infected onion plants. Southwest. Entomol. 38, 183–199. [Google Scholar]
- Bosco, L. and Tavella, L. (2010) Population dynamics and integrated pest management of Thrips tabaci on leek under field conditions in northwest Italy. Entomol. Exp. Appl. 135, 276–287. [Google Scholar]
- Buckland, K. , Reeve, J.R. , Alston, D. , Nischwitz, C. and Drost, D. (2013) Effects of nitrogen fertility and crop rotation on onion growth and yield, thrips densities, Iris yellow spot virus and soil properties. Agr. Ecosyst. Environ. 177, 63–74. [Google Scholar]
- Bulajic, A. , Djekic, I. , Jovic, J. , Krnjajic, S. , Vucurovic, A. and Krstic, B. (2009) Incidence and distribution of Iris yellow spot virus on onion in Serbia. Plant Dis. 93, 976. [DOI] [PubMed] [Google Scholar]
- Chingandu, N. , Druffel, K.L. , Schroeder, K. , Okubara, P. and Pappu, H.R. (2012) Quantitative molecular assays for investigating virus–host and virus–virus interactions using negative‐stranded RNA viruses as a model system. Phytopathology, 102 (Suppl. 6), S6.8. [Google Scholar]
- Clabbers, M.T.B. , Olsthoorn, R.C.L. and Gultyaev, A.P. (2014) Tospovirus ambisense genomic RNA segments use almost complete repertoire of stable tetraloops in the intergenic region. Bioinformatics, 30, 1800–1804. [DOI] [PubMed] [Google Scholar]
- Cortês, I. , Livieratos, I.C. , Derks, A. , Peters, D. and Kormelink, R. (1998) Molecular and serological characterization of Iris yellow spot virus, a new and distinct tospovirus species. Phytopathology, 88, 1276–1282. [DOI] [PubMed] [Google Scholar]
- Cortês, I. , Aires, A. , Pereira, A.M. , Goldbach, R. , Peters, D. and Kormelink, R. (2002) Genetic organization of Iris yellow spot virus M RNA: indications for functional homology between the G(C) glycoproteins of tospoviruses and animal‐infecting bunyaviruses. Arch. Virol. 147, 2313–2325. [DOI] [PubMed] [Google Scholar]
- Cramer, C.S. , Bag, S. , Schwartz, H.F. and Pappu, H.R. (2011) Susceptibility of onion relative (Allium spp.) to Iris yellow spot virus . Plant Dis. 95, 1319. [DOI] [PubMed] [Google Scholar]
- Damon, S. and Havey, M.J. (2013) Reduced epicuticular waxes are associated with insect tolerance. Abstracts from the 2013 Conference of the American Society of Agronomy. Available at: https://scisoc.confex.com/crops/2013am/webprogram/Paper81114.html [accessed on August 20, 2014].
- Diaz‐Montano, J. , Fuchs, M. , Nault, B.A. and Shelton, A.M. (2010) Evaluation of onion cultivars for resistance to onion thrips (Thysanoptera: Thripidae) and Iris yellow spot virus . J. Econ. Entomol. 103, 925–937. [DOI] [PubMed] [Google Scholar]
- Diaz‐Montano, J. , Fuchs, M. , Nault, B.A. , Fail, J. and Shelton, A.M. (2011) Onion thrips (Thysanoptera: Thripidae): a global pest of increasing concern in onion. J. Econ. Entomol. 104, 1–13. [DOI] [PubMed] [Google Scholar]
- Diaz‐Montano, J. , Fail, J. , Deutschlander, M. , Nault, B.A. and Shelton, A.M. (2012) Characterization of resistance, evaluation of the attractiveness of plant odors, and effect of leaf color on different onion cultivars to onion thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 105, 632–641. [DOI] [PubMed] [Google Scholar]
- Duijsings, D. , Kormelink, R. and Goldbach, R. (2001) In vivo analysis of the TSWV cap‐snatching mechanism: single base complementarity and primer length requirements. EMBO J. 20, 2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady, C.C. , Kamoi, T. , Kato, M. , Porter, N.G. , Davis, S. , Shaw, M. , Kamoi, A. and Imai, S. (2008) Silencing onion lachrymatory factor synthase causes a significant change in the sulfur secondary metabolite profile. Plant Physiol. 147, 2096–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, C.K. , Bag, S. , Frank, E. , Reeve, J. , Ransom, C. , Drost, D. and Pappu, H.R. (2009a) Green Foxtail (Setaria viridis), a naturally infected grass host of Iris yellow spot virus in Utah. Plant Dis. 93, 670. [DOI] [PubMed] [Google Scholar]
- Evans, C.K. , Bag, S. , Frank, E. , Reeve, J. , Ransom, C. , Drost, D. and Pappu, H.R. (2009b) Natural infection of Iris yellow spot virus in two scale saltbush (Atriplex micrantha) growing in Utah. Plant Dis. 93, 430. [DOI] [PubMed] [Google Scholar]
- Gent, D.H. , Schwartz, H.F. and Khosla, R. (2004) Distribution and incidence of Iris yellow spot virus and its relation to onion plant population and yield. Plant Dis. 88, 446–452. [DOI] [PubMed] [Google Scholar]
- Gent, D.H. , du Toit, L. , Fichtner, S.F. , Krishna Mohan, S. , Pappu, H.R. and Schwartz, H.F. (2006) Iris yellow spot virus: an emerging threat to onion bulb and seed production. Plant Dis. 90, 1468–1479. [DOI] [PubMed] [Google Scholar]
- Gent, D.H. , Martin, R.R. and Ocamb, C.M. (2007) First report of Iris yellow spot virus on onion and leek in western Oregon. Plant Dis. 91, 468. [DOI] [PubMed] [Google Scholar]
- Gera, A. , Cohen, J. , Salomon, R. and Raccah, B. (1998) Iris yellow spot tospovirus detected in onion (Allium cepa) in Israel. Plant Dis. 82, 127. [DOI] [PubMed] [Google Scholar]
- Golnaraghi, A.R. , Pourrahim, R. , Farzadfar, S. , Ohshima, K. , Shahraeen, N. and Ahoonmanesh, A. (2007) Incidence and distribution of Tomato yellow fruit ring virus on soybean in Iran. Plant Pathol J. 6, 14–21. [Google Scholar]
- Hall, J.M. , Mohan, K. , Knott, E.A. and Moyer, J.W. (1993) Tospoviruses associated with scape blight of onion (Allium cepa) seed crops in Idaho. Plant Dis. 77, 952. [Google Scholar]
- Hsu, C.L. , Hoepting, C.A. , Fuchs, M. , Sheltom, A.M. and Nault, B.A. (2010) Temporal dynamics of Iris yellow spot virus and its vector, Thrips tabaci (Thysanoptera: Thripidae), in seeded and transplanted onion fields. Environ. Entomol. 39, 266–277. [DOI] [PubMed] [Google Scholar]
- Hsu, C.L. , Hoepting, C.A. , Fuchs, M. , Smith, E.A. and Nault, B.A. (2011) Sources of Iris yellow spot virus in New York. Plant Dis. 95, 735–743. [DOI] [PubMed] [Google Scholar]
- Iftikhar, R. , Ramesh, S.V. , Bag, S. , Ashfaq, M. and Pappu, H.R. (2014) Global analysis of population structure, spatial and temporal dynamics of genetic diversity, and evolutionary lineages of Iris yellow spot virus (Tospovirus: Bunyaviridae). Gene, 547, 111–118. [DOI] [PubMed] [Google Scholar]
- Inoue, T. , Murai, T. and Natsuaki, T. (2010) An effective system for detecting Iris yellow spot virus transmission by Thrips tabaci . Plant Pathol. 59, 422–428. [Google Scholar]
- Jacobson, A.L. and Kennedy, G.G. (2013) Specific insect–virus interactions are responsible for variation in competency of different Thrips tabaci isolines to transmit different Tomato spotted wilt virus isolates. PLoS ONE, 8, e54567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, A.L. , Booth, W. , Vargo, E.L. and Kennedy, G.G. (2013) Thrips tabaci population genetic structure and polyploidy in relation to competency as a vector of Tomato spotted wilt virus . PLoS ONE, 8, e54484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D.R. (2005) Plant viruses transmitted by thrips. Eur. J. Plant Pathol. 113, 119–157. [Google Scholar]
- Jones, H.A. , Bailey, S.F. and Emsweller, S.L. (1934) Thrips resistance in onion. Hilgardia, 8, 215–232. [Google Scholar]
- Kendall, D.M. and Capinera, J.L. (1990) Geographic and temporal variation in the sex ratio of onion thrips. Southwest. Entomol. 15, 80–88. [Google Scholar]
- van Knippenberg, I. , Lamine, M. , Goldbach, R. and Kormelink, R. (2005) Tomato spotted wilt virus transcriptase in vitro displays a preference for cap donors with multiple base complementarity to the viral template. Virology, 335, 122–130. [DOI] [PubMed] [Google Scholar]
- Kritzman, A. , Lampal, M. , Raccah, B. and Gera, A. (2001) Distribution and transmission of Iris yellow spot virus . Plant Dis. 85, 838–842. [DOI] [PubMed] [Google Scholar]
- Kumar, P. and Dhawan, P. (2013) Biological and serological diagnosis of Iris yellow spot virus in onion from northern India. Ann. Agri‐Bio Res. 18, 48–54. [Google Scholar]
- Kunkalikar, S.R. , Poojari, S. , Arun, B.M. , Rajagopalan, P.A. , Chen, T.C. , Yeh, S.D. , Naidu, R.A. , Zehr, U.B. and Ravi, K.S. (2011) Importance and genetic diversity of vegetable‐infecting tospoviruses in India. Phytopathology, 101, 367–376. [DOI] [PubMed] [Google Scholar]
- Larentzaki, E. , Plate, J. , Nault, B.A. and Shelton, A.M. (2008) Impact of straw mulch on populations of onion thrips (Thysanoptera: Thripidae) in onion. J. Econ. Entomol. 101, 1317–1324. [DOI] [PubMed] [Google Scholar]
- Lewis, T. (1973) Thrips: Their Biology, Ecology and Economic Importance. London: Academic Press. [Google Scholar]
- Lobin, K. , Saison, A. , Hostachy, B. , Benimadhu, S.P. and Pappu, H.R. (2010) First report of Iris yellow spot virus in onion in Mauritius. Plant Dis. 94, 645. [DOI] [PubMed] [Google Scholar]
- Mahaffey, L.A. and Cranshaw, W.S. (2010) Thrips species associated with onion in Colorado. Southwest. Entomol. 35, 45–50. [Google Scholar]
- Malik, M.F. , Nawaz, M. , Ellington, J. , Sanderson, R. and El‐Heneidy, A.H. (2009) Effect of different nitrogen regimes on onion thrips, Thrips tabaci Lindemann, on onions, Allium cepa L. Southwest. Entomol. 34, 219–225. [Google Scholar]
- Mandal, B. , Jain, R.K. , Krishnareddy, M. , Krishna Kumar, N.K. , Ravi, K.S. and Pappu, H.R. (2012) Emerging problems of Tospoviruses (Bunyaviridae) and their management in the Indian subcontinent. Plant Dis. 96, 468–479. [DOI] [PubMed] [Google Scholar]
- Mo, J. , Stevens, M. , Liu, D.L. and Herron, G. (2009) Investigating the effect of invasion characteristics on onion thrips (Thysanoptera: Thripidae) populations in onions with a temperature‐driven process model. Environ. Entomol. 38, 1575–1584. [DOI] [PubMed] [Google Scholar]
- Moghadam, M.M. , Cramer, C.S. , Steiner, R.L. and Creamer, R. (2011) Evaluating winter‐sown onion entries for Iris yellow spot virus susceptibility. HortScience, 46, 1224–1229. [Google Scholar]
- Molenaar, N. (1984) Thrips (Thrips tabaci L.) resistance and epicuticular wax characteristics of nonglossy and glossy onions (Allium cepa L.). PhD Thesis, University of Wisconsin‐Madison, WI, ADB1108UW.
- Moritz, G. (1997) Structure, growth and development In: Thrips as Crop Pests (Lewis T., ed.), pp. 15–63. New York: CAB International. [Google Scholar]
- Moyer, J.W. , Abad, J.A. , Ullman, D. and Mohan, K. (2003) INSV and IYSV: the other tospoviruses in the United States. Phytopathology, 93, S–115. [Google Scholar]
- Mullis, S.W. , Langston, J.D.B. , Gitaitis, R.D. , Sherwood, J.L. and Csinos, A.C. (2004) First report of Vidalia onion (Allium cepa) naturally infected with Tomato spotted wilt virus and Iris yellow spot virus (family Bunyaviridae, genus Tospovirus) in Georgia. Plant Dis. 88, 1285. [DOI] [PubMed] [Google Scholar]
- Multani, P.S. , Cramer, C.S. , Steiner, R.L. and Creamer, R. (2009) Screening winter‐sown onion entries for Iris yellow spot virus tolerance. HortScience, 44, 627–632. [Google Scholar]
- Nagata, T. , Almeida, A.C.L. , Resende, R. de O. and de Avila, A.C. (1999) The identification of the vector species of Iris yellow spot tospovirus occurring on onion in Brazil. Plant Dis. 83, 399. [DOI] [PubMed] [Google Scholar]
- Nault, B.A. , Hsu, C.L. and Hopeting, C.A. (2012) Consequences of co‐applying insecticides and fungicides for managing Thrips tabaci (Thysanoptera: Thripidae) on onion. Pest Manag. Sci. 69, 841–849. [DOI] [PubMed] [Google Scholar]
- Nichol, S.T. , Beaty, B.J. , Elliott, R.M. , Goldbach, R. , Plyusnin, A. , Schmaljohn, C.S. and Tesh, R.B. (2005) Bunyaviridae In: Virus Taxonomy, VIIIth Report of the ICTV (Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U. and Ball L.A., eds), pp. 695–716. San Diego, CA: Elsevier/Academic Press. [Google Scholar]
- Nischwitz, C. , Mullis, S.W. , Csinos, A.S. , Langston, D.B. , Sparks, A.N. , Torrance, R.L. , Rafael Mallaupoma, Z.C. , Inguil Rojas, E.H. and Gitaitis, R.D. (2006) Phylogenetic analysis of the N gene links Georgia strains of Iris yellow spot virus to strains from Peru. Phytopathology, 96, S84. [Google Scholar]
- Nischwitz, C. , Gitaitis, R.D. , Mullis, S.W. , Csinos, A.S. , Langston, D.B. and Sparks, A.N. (2007) First report of Iris yellow spot virus in spiny sowthistle (Sonchus asper) in the United States. Plant Dis. 91, 1518. [DOI] [PubMed] [Google Scholar]
- Pappu, H.R. , du Toit, L.J. , Schwartz, H.F. and Mohan, S.K. (2006a) Sequence diversity of the nucleoprotein gene of Iris yellow spot virus (genus Tospovirus, family Bunyaviridae) isolates from the western region of the United States. Arch. Virol. 151, 1015–1023. [DOI] [PubMed] [Google Scholar]
- Pappu, H.R. , Hellier, B.C. and Dugan, F.M. (2006b) Wild Allium spp. as natural hosts of Iris yellow spot virus . Plant Dis. 90, 378. [DOI] [PubMed] [Google Scholar]
- Pappu, H.R. , Jones, R.A. and Jain, R.K. (2009) Global status of tospovirus epidemics in diverse cropping systems: successes achieved and challenges ahead. Virus Res. 141, 219–236. [DOI] [PubMed] [Google Scholar]
- Pozzer, L. , Bezerra, I.C. , Kormelink, R. , Prins, M. , Peters, D. , Resende, R. , De, O. and de Ávila, A.C. (1999) Characterization of a tospovirus isolate of Iris yellow spot virus associated with a disease in onion fields in Brazil. Plant Dis. 83, 345–350. [DOI] [PubMed] [Google Scholar]
- Riley, D.G. , Joseph, S.V. , Srinivasan, R. and Diffie, S. (2011) Thrips vectors of tospoviruses. J. Int. Pest. Manag. 2, 1–10. [Google Scholar]
- Sampangi, R.K. , Mohan, S.K. and Pappu, H.R. (2007) Identification of new alternative weed hosts for Iris yellow spot virus in the Pacific Northwest. Plant Dis. 91, 1683. [DOI] [PubMed] [Google Scholar]
- Schwartz, H.F. , Otto, K. and Pappu, H.R. (2007) First report of Iris yellow spot virus in commercial leek (Allium porrum) in the United States. Plant Dis. 91, 113. [DOI] [PubMed] [Google Scholar]
- Schwartz, H.F. , Gent, D.H. , Fichtner, S.M. , Hammon, R. , Cranshaw, W.S. , Mahaffey, L. , Otto, K. and McMillan, M. (2009) Straw mulch and reduced‐risk pesticide impact on thrips and Iris yellow spot virus on Western grown onion. Southwest. Entomol. 34, 13–29. [Google Scholar]
- Schwartz, H.F. , Gent, D.H. , Fichtner, S.M. , Mahaffey, L.A. , Camper, M.A. and Cranshaw, W.S. (2010) Spatial and temporal distribution of Iris yellow spot virus and thrips in Colorado onion fields. Plant Health Prog. doi: 10.1094/PHP-2010-0820-01-RS. [DOI] [Google Scholar]
- Sether, D.M. , Borth, W.B. , Shimabuku, R.S. , Pappu, H.R. , Melzer, M.J. and Hu, J.S. (2010) First report of Iris yellow spot virus in onion in Hawaii. Plant Dis. 94, 1508. [DOI] [PubMed] [Google Scholar]
- Shock, C.C. , Feibert, E. , Jensen, L. , Mohan, S.K. and Saunders, L.D. (2008) Onion variety response to Iris yellow spot virus . Horttechnology, 18, 539–544. [Google Scholar]
- Sin, S.H. , McNulty, B.C. , Kennedy, G.G. and Moyer, J.W. (2005) Viral genetic determinants for thrips transmission of Tomato spotted wilt virus . Proc. Natl. Acad. Sci. USA, 02, 5168–5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N. (2013) Selection progress for reduced Iris yellow spot symptom expression in onion. MS Thesis, New Mexico State University, Las Cruces, NM.
- Smith, E.A. , Ditommaso, A. , Fuchs, M. , Shelton, A.M. and Nault, B.A. (2011) Weed hosts for onion thrips (Thysanoptera: Thripidae) and their potential role in the epidemiology of Iris yellow spot virus in an onion ecosystem. Environ. Entomol. 40, 194–203. [Google Scholar]
- Srinivasan, R. , Diffie, S. , Sundaraj, S. , Mullis, S.W. , Riley, D.G. , Gitaitis, R. and Pappu, H.R. (2011) Evaluation of Lisianthus (Eustoma russellianum) as an indicator host for Iris yellow spot virus . Plant Dis. 95, 1520–1527. [DOI] [PubMed] [Google Scholar]
- Srinivasan, R. , Sundaraj, S. , Pappu, H.R. , Diffie, S. , Riley, D. and Gitaitis, R. (2012) Transmission of Iris yellow spot virus by Frankliniella fusca and Thrips tabaci (Thysanoptera: Thripidae). J. Econ. Entomol. 105, 40–47. [DOI] [PubMed] [Google Scholar]
- Takeda, A. , Sugiyama, K. , Nagano, H. , Mori, M. , Kaido, M. , Mise, K. , Tsuda, S. and Okuno, T. (2002) Identification of a novel RNA silencing suppressor, NSs protein of Tomato spotted wilt virus. FEBS Lett. 532, 75–79. [DOI] [PubMed] [Google Scholar]
- Tomassoli, L. , Tiberini, A. , Masenga, V. , Vicchi, V. and Turina, M. (2009) Characterization of Iris yellow spot virus isolates from onion crops in northern Italy. J. Plant Pathol. 91, 733–739. [Google Scholar]
- Tripathi, D. (2014) In vivo localization and interaction studies of tospovirus (genus Tospovirus, family Bunyaviridae) proteins. PhD Thesis, Washington State University.
- Tripathi, D. and Pappu, H.R. (2011) Use of Datura stramonium and Nicotiana benthamiana to study Acibenzolar‐S‐methyl‐induced SAR against Iris yellow spot virus (genus Tospovirus). Phytopathology, 101 (Suppl. 1), 78. [Google Scholar]
- Tripathi, D. , Goodin, M. , Dietzen, R.G. and Pappu, H.R. (2013) Interaction between tospovirus proteins in mixed infections using bimolecular fluorescence complementation (BiFC). Phytopathology, 103 (Suppl. 2), 148. [Google Scholar]
- Turina, M. , Tavella, L. and Ciuffo, M. (2012) Tospoviruses in the Mediterranean Area. Adv. Virus Res. 84, 403–437. [DOI] [PubMed] [Google Scholar]
- Ullman, D.E. , Cho, J.J. , Mau, R.F.L. , Westcot, D.M. and Custer, D.M. (1992a) A midgut barrier to Tomato spotted wilt virus acquisition by adult western flower thrips. Phytopathology, 82, 1333–1342. [Google Scholar]
- Ullman, D.E. , Cho, J.J. , Mau, R.F.L. , Hunter, W.B. , Westcot, D.M. and Custer, D.M. (1992b) Thrips–Tomato spotted wilt virus interactions: morphological, behavioral and cellular components influencing thrips transmission In: Advances in Disease Vector Research, Vol. 9 (Harris K.F., ed.), pp. 195–240. New York: Springer‐Verlag. [Google Scholar]
- Ullman, D.E. , Westcot, D.M. , Cantone, F.A. , Sherwood, J.L. and German, T.L. (1992c) Immunocytochemical evidence for Tomato spotted wilt virus (TSWV) replication in cells of the western flower thrips, Frankliniella occidentalis (Pergande). Phytopathology, 82, 1087. [Google Scholar]
- Ullman, D.E. , German, T.L. , Sherwood, J.L. , Westcot, W. and Cantone, F.A. (1993) Tospovirus replication in insect vector cells: immuno‐cytochemical evidence that the nonstructural protein encoded by the S RNA of Tomato spotted wilt tospovirus is present in the thrips vector cells. Phytopathology, 83, 456–463. [Google Scholar]
- Waiganjo, M.M. , Mueke, J.M. and Gitonga, L.M. (2008) Susceptible onion growth stages for selective and economic protection from onion thrips infestation. Acta Hortic. 767, 193–200. [Google Scholar]
- Westmore, G.C. , Poke, F.S. , Allen, G.R. and Wilson, C.R. (2013) Genetic and host‐associated differentiation within Thrips tabaci Lindeman (Thysanoptera: Thripidae) and its links to Tomato spotted wilt virus–vector competence. Heredity, 111, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetering, F. , Goldbach, R. and Peter, D. (1996) Tomato spotted wilt tospovirus ingestion by first instar larvae of Frankliniella occidentalis is a prerequisite for transmission. Phytopathology, 86, 900–905. [Google Scholar]
- Whitfield, A.E. , Ullman, D.E. and German, T.L. (2005) Tospovirus–thrips interaction. Annu. Rev. Phytopathol. 43, 459–489. [DOI] [PubMed] [Google Scholar]
- Wijkamp, I. , van Lent, J. , Kormelink, R. , Goldbach, R. and Peters, D. (1993) Multiplication of Tomato spotted wilt virus in its insect vector, Frankliniella occidentalis . J. Gen. Virol. 74, 341–349. [DOI] [PubMed] [Google Scholar]
- Wijkamp, I. , Almarza, N. , Goldbach, R. and Peters, D. (1995) Distinct levels of specificity in thrips transmission of tospoviruses. Phytopathology, 85, 1069–1074. [Google Scholar]
- Zen, S. , Okuda, M. , Ebihara, K. , Uematsu, S. , Hanada, K. , Iwanami, T. and Nakashima, S. (2005) Genetic characterization of Iris yellow spot virus on onion (Allium cepa) and pathogenicity of two IYSV strains on onion and leaf onion (A. schoenoprasum). Jpn. J. Phytopathol. 71, 123–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Distribution of Iris yellow spot virus (IYSV) in plants. (A) Onion plant with bulb. (B) Virus accumulation was found to be significantly greater in younger leaves (8–11) than in intermediate and older leaves. (C) IYSV was not detected in roots, basal plates, bulb scales or dead leaves.
Fig. S2 Comparison of biologically distinct isolates of Iris yellow spot virus (IYSV) that differed in disease development in Nicotiana benthamiana under controlled glasshouse conditions. Initial symptom differentiation was superimposed for each isolate. IYSV‐Idaho (IYSV‐ID) and IYSV‐Washington (IYSV‐WA) were found to be more severe than IYSV‐California (IYSV‐CA). IYSV‐ID and IYSV‐WA isolates had a more severe phenotype at 22 days post‐inoculation (dpi) compared with the mild strain, IYSV‐CA, as shown in the top panel. By 50 dpi, IYSV‐ID and IYSV‐WA isolates were lethal to the plants, whereas the effect on the plant infected with IYSV‐CA was less severe, as shown in the bottom panel.
Fig. S3 Secondary RNA structure of Iris yellow spot virus (IYSV) genomic RNAs based on minimum free energy (MFE) fold algorithm prediction using the RNA Fold Web Server (http://rna.tbi.univie.ac.at/cgi‐bin/RNAfold.cgi). (A) Secondary structure of small RNA. Inset shows the panhandle structural forms in all three RNAs as a result of the presence of the complementary structure. (B) Structure of the intergenic region (IGR) of IYSV M RNA forming a stem‐loop structure. The apical Y‐shaped loop formed (dG = −63.70) is shown as an enlarged image.
Fig. S4 Acibenzolar‐S‐methyl (ASM)‐induced resistance response against Iris yellow spot virus (IYSV) in Datura stramonium and Nicotiana benthamiana plants. (A, B) IYSV symptoms in D. stramonium plants. Datura stramonium leaves showing lesions induced by IYSV in buffer‐ and ASM‐treated plants at 14 days post‐inoculation (dpi). Different sets of plants were treated with buffer or ASM (0.1 mm). Seven days later, treated plants were inoculated with IYSV. The diameters of IYSV lesions were measured at 14 days post‐IYSV inoculations. (C, D) Reverse transcription‐polymerase chain reaction (RT‐PCR) of IYSV nucleocapsid (N) gene. Relative levels of expression of nucleocapsid (N) gene of IYSV in ASM‐ and buffer‐treated D. stramonium (C) and N. benthamiana (D) plants. Different sets of plants were treated with buffer or ASM (0.1 mm). Seven days later, treated plants were inoculated with IYSV. RT‐PCR was conducted on samples collected at 14 days post‐IYSV inoculations. I, IYSV‐inoculated plants; B + I, buffer‐treated plants inoculated with IYSV; A + I, ASM‐treated plants inoculated with IYSV; H, healthy plants; A, ASM‐non‐inoculated plants. (E) RT‐PCR of pathogenesis‐related (PR‐1a) genes. Detection of PR‐1a genes in buffer‐ and ASM‐treated N. benthamiana plants. Different sets of plants were treated with buffer or ASM (0.1 mm). A set of three plants per treatment was used for analysis. Leaf samples were harvested after 2 days of treatment, and expression of the PR1a gene was detected by RT‐PCR using PR‐1a gene‐specific primers.


