Summary
The fungal cereal pathogen Fusarium graminearum produces deoxynivalenol (DON) during infection. The mycotoxin DON is associated with Fusarium head blight (FHB), a disease that can cause vast grain losses. Whilst investigating the suitability of Brachypodium distachyon as a model for spreading resistance to F. graminearum, we unexpectedly discovered that DON pretreatment of spikelets could reduce susceptibility to FHB in this model grass. We started to analyse the cell wall changes in spikelets after infection with F. graminearum wild‐type and defined mutants: the DON‐deficient Δtri5 mutant and the DON‐producing lipase disruption mutant Δfgl1, both infecting only directly inoculated florets, and the mitogen‐activated protein (MAP) kinase disruption mutant Δgpmk1, with strongly decreased virulence but intact DON production. At 14 days post‐inoculation, the glucose amounts in the non‐cellulosic cell wall fraction were only increased in spikelets infected with the DON‐producing strains wild‐type, Δfgl1 and Δgpmk1. Hence, we tested for DON‐induced cell wall changes in B. distachyon, which were most prominent at DON concentrations ranging from 1 to 100 ppb. To test the involvement of DON in defence priming, we pretreated spikelets with DON at a concentration of 1 ppm prior to F. graminearum wild‐type infection, which significantly reduced FHB disease symptoms. The analysis of cell wall composition and plant defence‐related gene expression after DON pretreatment and fungal infection suggested that DON‐induced priming of the spikelet tissue contributed to the reduced susceptibility to FHB.
Keywords: Brachypodium distachyon, cell wall, deoxynivalenol, fungal resistance, Fusarium graminearum, mycotoxin, plant defence
Introduction
Based on its phylogeny and morphology, the small annual grass Brachypodium distachyon has emerged as a model system for the investigation of Triticeae (Catalán et al., 1995; Vogel and Bragg, 2009; Vogel et al., 2006). Among this tribe of Poaceae, wheat (Triticum aestivum) has the highest agronomic importance, as it was the third most‐produced cereal after maize (Zea mays) and rice (Oryza sativa) in 2012 according to Food and Agriculture Organization (FAO) statistics (Food and Agriculture Organization of the United Nations, 2014; http://faostat3.fao.org/faostat‐gateway/go/to/download/Q/QC/E). Therefore, yield losses have a direct impact on world food production. Pathogens represent a major cause of yield losses in wheat. Under weather conditions favouring an epidemic outbreak, the fungal crop disease Fusarium head blight (FHB) alone can account for over 40% yield loss on wheat, even in countries with a high degree of mechanization and surveillance systems available, such as the USA (Cowger and Sutton, 2005). The main causative fungal species of FHB is Fusarium graminearum. In recent years, an increasing spread of F. graminearum has further increased its importance in agriculture (Chakraborty and Newton, 2011; Madgwick et al., 2011). New challenges may also emerge from climate change. Changing rainfall patterns have already increased FHB dramatically in the Punjab region of India, e.g. in 2005 (Duveiller et al., 2007). The occurrence of extreme weather situations will decrease the efficiency of prediction of existing agricultural surveillance systems and favour FHB outbreaks. FHB is characterized by a bleaching of the spike. As a result of sterile florets and shrivelled, improperly developed kernels, F. graminearum‐based FHB is associated with severe losses in yield and a reduction in baking and seed quality (McMullen et al., 1997, Pirgozliev, 2003). In addition to yield losses, mycotoxins produced by F. graminearum are a threat to human and animal health. Mycotoxins, such as the potent oestrogenic metabolite zearalenone (Peraica et al., 1999) and the trichothecene deoxynivalenol (DON), can contaminate the remaining grain. DON can depress the immune system and inhibit eukaryotic protein biosynthesis through binding to the 60S ribosomal subunit of eukaryotes (Kimura et al., 1998; Rocha et al., 2005). In plants, DON accumulation induces disease symptoms, including necrosis, chlorosis and wilting (Cutler, 1988). As a result of its importance in agriculture, efforts are being made to enhance the resistance of wheat to F. graminearum and FHB. The application of biotechnological methods to reduce susceptibility to F. graminearum is more complicated than in many other crops. The main reason is the limited genetic access of wheat. The large and complex genome structure, in combination with low transformation efficiency, restricts the identification of putative targets for increased plant resistance and their direct modification. Therefore, most methods follow conventional breeding strategies using wheat cultivars with observed reduced FHB disease symptoms, such as Sumai 3 (del Blanco et al., 2003). However, despite these breeding efforts, most commercially available wheat cultivars used for food production are still susceptible to F. graminearum (Buerstmayr et al., 2009). To overcome restrictions in the analysis of the F. graminearum–wheat pathosystem, B. distachyon was identified as an appropriate plant for genetic and molecular analysis. Peraldi et al. (2011) showed that F. graminearum can infect the spikelet of B. distachyon and DON production is induced during this interaction. In addition, B. distachyon reveals characteristics of a model plant that would support the identification of putative plant defence targets at a functional as well as structural level: (i) a short generation time with only simple growth requirements (Vogel and Bragg, 2009); (ii) high‐throughput genetic studies facilitated by the smallest genome size in the plant family of Poaceae with little repetitive or methylated DNA (Draper et al., 2001); (iii) a publicly available genome sequence (International Brachypodium, 2010); (iv) an easy genetic transformation system (Alves et al., 2009; Christiansen et al., 2005; Vogel and Hill, 2008); and (v) a growing collection of T‐DNA lines (Thole et al., 2010).
Our study aimed to evaluate the suitability of B. distachyon as a model for the study of type II resistance to FHB in wheat caused by the fungal pathogen F. graminearum. On the one hand, we wanted to determine whether similar type II resistance mechanisms were induced in B. distachyon, as in wheat, during interaction with well‐defined F. graminearum mutants. Type II resistance generally describes plant defence mechanisms that do not prevent initial infection, but stop subsequent propagation of the pathogen, e.g. within the cereal spike (Schroeder and Christensen, 1963). The F. graminearum mutants chosen for infection of B. distachyon revealed similar disease phenotypes on susceptible wheat, even though the phenotypes were caused by the disruption of different virulence factors. The Δtri5 mutant is unable to produce the mycotoxin DON because of disruption of the trichodiene synthase gene TRI5 (TRICHODIENE SYNTHASE 5) encoding for the first enzyme in the trichothecene pathway. In wheat, this mutant revealed initial infection of inoculated spikelets, but failed to penetrate the transition zone of the rachilla and rachis, the rachis node, for further colonization of the spike. Cell wall thickenings and appositions were detected at the rachis node during infection with this mutant, which were not formed during infection with the F. graminearum wild‐type (Jansen et al., 2005; Proctor et al., 1995). A similar barrier formation was observed during wheat infection with the Δfgl1 mutant. The disrupted FGL1 (FUSARIUM GRAMINEARUM LIPASE1) gene encodes a secreted lipase, which is required to break type II resistance in wheat (Voigt et al., 2005) as a result of the release of polyunsaturated free fatty acids that can inhibit pathogen‐induced callose deposition in the phloem of infected spikelets (Blümke et al., 2014). We included the Gpmk1 [Gibberella pathogenicity mitogen‐activated protein (MAP) kinase1] disruption mutant Δgpmk1 as an additional F. graminearum mutant (Jenczmionka et al., 2003). The substantially reduced virulence of this mutant is largely based on a strong delay of FGL1 induction (Bluhm et al., 2007; Salomon et al., 2012).
One of our main interests was to examine whether pathogen infection would induce changes in the non‐cellulosic monosaccharide composition of the cell wall in the host model grass B. distachyon. This approach referred to our recent study in the model plant Arabidopsis thaliana, where analysis of the non‐cellulosic monosaccharide composition suggested that cell wall modifications might be involved in the determination of fungal resistance (Ellinger et al., 2013).
Infection with the F. graminearum mutant strains showed that B. distachyon induces similar type II defence mechanisms as in wheat, confirming the suitability of this model grass in F. graminearum interactions. The analysis of the host cell wall after F. graminearum infection revealed that the mycotoxin DON is involved in pathogen‐induced modification. Similar changes in the host cell wall were also induced by DON application at relatively low concentrations without fungal infection. DON pretreatment of B. distachyon spikelets prior to F. graminearum infection supported type II resistance and reduced FHB disease symptoms. The analysis of the cell wall composition and pathogen‐related gene expression after DON application and subsequent F. graminearum infection indicated that DON‐induced priming of the spikelet tissue contributed to the reduction in FHB disease symptoms in pretreated B. distachyon spikelets.
Results
Infection progress of F. graminearum wild‐type and mutants in B. distachyon spikelets
To investigate the potential of B. distachyon as a model host for the study of type II resistance mechanisms to F. graminearum, we point inoculated single florets of spikelets with conidia of F. graminearum wild‐type and the defined, virulence‐deficient mutants Δtri5, Δfgl1 and Δgpmk1, which all constitutively expressed the green fluorescent protein (GFP) to visualize fungal growth in planta. The infection progress was monitored for 2 weeks until ripening started at growth stage 85 referring to the BBCH (Biologische Bundesanstalt, Bundessortenamt and Chemische Industrie) scale (Hong et al., 2011). Statistical analysis of infection progress was achieved by calculation of the disease score at 5, 7, 11 and 14 days post‐inoculation (dpi), where a score of ≥2 indicates a successful fungal colonization of additional florets other than those inoculated directly (Fig. 1).
Figure 1.
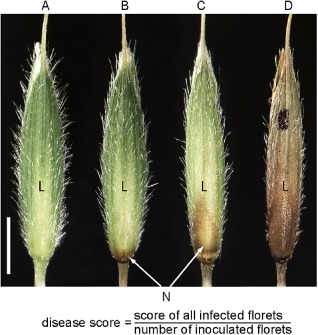
Numerical scoring system for rating the disease severity of Fusarium graminearum‐infected Brachypodium distachyon spikelets. (A) Uninfected floret, disease score: 0.0. (B) Weak infection of a floret, only one small, restricted necrosis (N) visible on the caryopsis or the rachilla, disease score: 0.1. (C) More than one necrotic lesion and/or lesion(s) covering a maximum of 50% of the infected floret, disease score: 0.5. (D) Extended necrosis covering more than half of the floret, highest disease score of 1.0. For each spikelet, single florets were rated at 14 days post‐inoculation with F. graminearum strains and the score was calculated as indicated in the formula. L, lemma. Scale bar, 2 mm.
As expected from the inoculation of wheat spikes, the F. graminearum wild‐type strain wt‐GFP induced the strongest disease symptoms in B. distachyon spikelets. The mycelium colonized the whole spikelet and spread through the rachilla from one floret to another (Fig. 2A). The disease score increased continuously during the monitored 2 weeks of infection, starting with 0.6 at 5 dpi and reaching a final score of 2.3 at 14 dpi (Fig. 2E). In wheat, F. graminearum requires DON production during infection to break type II resistance and to fully colonize the spike (Proctor et al., 1995). The disruption of the trichodiene synthase TRI5 in the F. graminearum mutant Δtri5 prevents DON biosynthesis, which results in reduced virulence of the mutant. In contrast with F. graminearum wild‐type, the Δtri5 mutant is unable to spread through the rachis and to colonize the whole wheat spike (Jansen et al., 2005). In B. distachyon, we observed a similar disease phenotype for the TRI5 disruption mutant Δtri5‐GFP. This mutant failed to cross the rachilla and infection was restricted to the directly inoculated floret (Fig. 2B). A disease score of 1.2 at 14 dpi (Fig. 2E) reflected the reduced virulence compared with the wild‐type. In addition to DON, the secreted lipase FGL1 is required for full virulence of F. graminearum on wheat (Voigt et al., 2005). Similar to Δtri5, the lipase disruption mutant Δfgl1 can only colonize directly inoculated wheat spikelets (Blümke et al., 2014; Voigt et al., 2005). In the initial phase of B. distachyon infection, Δflg1‐GFP exhibited hyphal propagation within the directly inoculated floret, which was similar to that of wt‐GFP infection. However, the infection of the Δflg1‐GFP mutant was restricted to the directly inoculated floret (Fig. 2C). A disease score of 1.2 at 11 and 14 dpi confirmed this observation (Fig. 2E). The disruption of the MAP kinase Gpmk1 in the mutant Δgpmk1‐GFP (Salomon et al., 2012) resulted in a strongly reduced virulence on B. distachyon that was similar to the phenotype reported from wheat (Jenczmionka and Schäfer, 2005; Urban et al., 2003). In B. distachyon, we observed a slight hyphal growth of the Δgpmk1‐GFP mutant only at the point of direct inoculation within the floret (Fig. 2D). The relatively low disease score, with a maximum of 0.2 (Fig. 2E), reflected the inability of this mutant to colonize the host.
Figure 2.
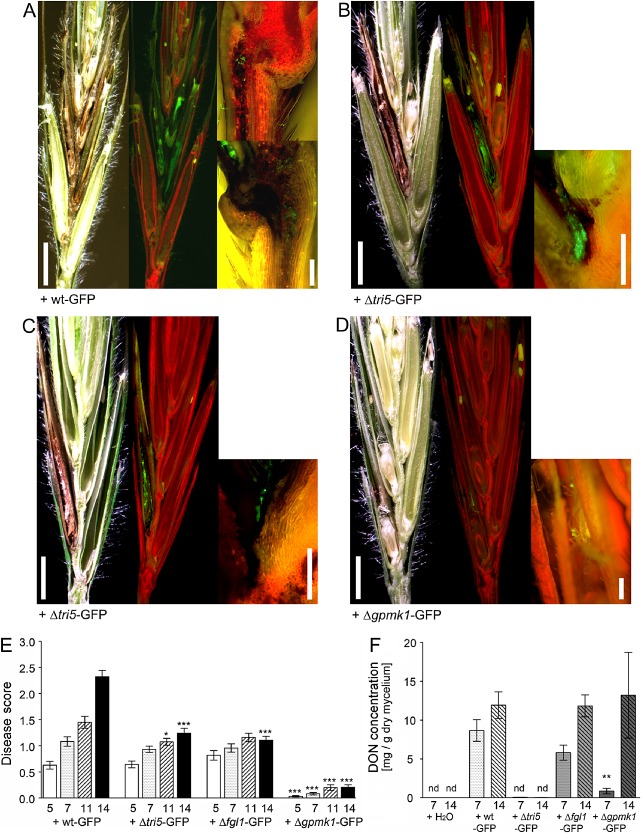
Disease phenotype and deoxynivalenol (DON) accumulation in Brachypodium distachyon spikelets after Fusarium graminearum infection. (A–D) Micrographs of longitudinal sectioned spikelets at 14 days post‐inoculation (dpi) with the green fluorescent protein (GFP)‐tagged F. graminearum strains: (A) wild‐type wt‐GFP; (B) DON‐deficient disruption mutant Δtri5‐GFP; (C) lipase‐deficient disruption mutant Δflg1‐GFP; (D) mitogen‐activated protein (MAP) kinase‐deficient disruption mutant Δgpmk1‐GFP. Left panels: images of spikelet sections with bright field illumination to visualize necrotic tissue; mid‐panels: images of the same sections as in the left panels, but with epifluorescent illumination to visualize GFP‐emitting fungal hyphae; right panels: magnification of the rachilla of inoculated florets with epifluorescent illumination. Scale bars for left and mid‐panels, 2 mm; scale bars for right panels, 0.2 mm. (E) Disease score of infected spikelets at 5, 7, 11 and 14 dpi with GFP‐tagged F. graminearum strains as indicated. *P < 0.05, ***P < 0.005, Dunnett's test. Error bars represent ± SEM and n ≥ 12. (F) DON concentration of infected spikelet tissue at 7 and 14 dpi with GFP‐tagged F. graminearum strains as indicated. Water‐inoculated spikelets served as controls. **P < 0.01, Dunnett's test. Error bars represent ± SEM and n = 3. nd, not detectable.
DON production of F. graminearum wild‐type and mutants in B. distachyon
To analyse the production of DON by the different F. graminearum mutants during infection of B. distachyon spikelets, we determined the relative DON amounts in the floral tissue. At 7 dpi, the infection with wt‐GFP resulted in the highest DON concentration (8.6 mg DON/g dry mycelium). We detected less DON in Δfg1l‐GFP‐infected tissue (5.8 mg/g), and the Δgpmk1‐GFP mutant produced only a small amount of DON in B. distachyon spikelets (0.9 mg/g) (Fig. 2F) at this time point of infection. At 14 dpi, the relative DON amounts of wt‐GFP‐, Δfgl1‐GFP‐ and Δgpmk1‐GFP‐infected tissues were comparable and reached a value of about 12 mg DON/g dry mycelium (Fig. 2F), which was similar to the relative DON amounts reported previously from F. graminearum‐infected wheat spikes (Bormann et al., 2014). As expected from determinations in wheat (Jansen et al., 2005; Maier et al., 2006), we did not detect DON in spikelet tissue infected with the Δtri5‐GFP mutant (Fig. 2F).
Host cell wall changes during F. graminearum infection and DON application
Because the plant cell wall represents the first line of defence against the intruding pathogen and alteration of the cell wall could be involved in pathogen resistance (Ellinger et al., 2013), we determined the non‐cellulosic monosaccharide composition of B. distachyon spikelet tissue after F. graminearum infection using high‐performance anion‐exchange chromatography with pulsed amperometric detection (HPAEC‐PAD). The relative amounts of the main non‐cellulosic cell wall components xylose (c. 70 mol%), glucose (c. 13 mol%), arabinose (c. 12 mol%) and galactose (c. 2 mol%), detected in water‐inoculated control samples at 7 dpi (Fig. 3A), were comparable with the amounts described in previous reports of B. distachyon and other grasses (Christensen et al., 2010; Gomez et al., 2008). Infection of B. distachyon with F. graminearum wt‐GFP, Δfgl1‐GFP and Δgpmk1‐GFP, but not Δtri5‐GFP, altered the spikelet's monosaccharide composition. At 7 dpi, the relative glucose content of wt‐GFP‐ and Δfgl1‐GFP‐infected spikelets was slightly, and of Δgpmk1‐GFP‐infected spikelets significantly, increased, reaching a content of almost 18 mol% (Fig. 3A). Apart from glucose, we did not observe alterations for the other neutral monosaccharides (Fig. 3A). At 14 dpi, we observed a shift in the glucose/xylose ratio in the water‐inoculated control tissue, in which the glucose content increased to almost 30 mol%, which correlated with a decrease in the xylose content to 58 mol%. The relative amounts of arabinose and galactose remained unchanged compared with those at 7 dpi, which also applied to the F. graminearum‐infected samples for these monosaccharides (Fig. 3B). Although we did not detect differences in the cell wall composition between control and Δtri5‐GFP‐infected samples, the infection of spikelets with the F. graminearum strains wt‐GFP, Δfgl1‐GFP and Δgpmk1‐GFP resulted in a significant increase in the glucose amount compared with control tissue (Fig. 3B). Here, the relative glucose amount reached values of almost 40 mol%, which was about 30% higher than in control samples.
Figure 3.
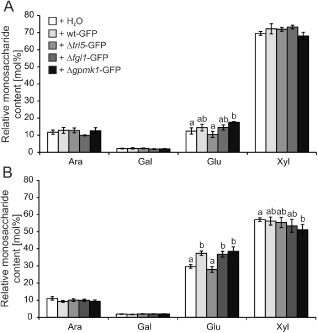
Non‐cellulosic monosaccharide composition of Brachypopdium distachyon spikelets after Fusarium graminearum infection. Cell wall extracts from infected spikelets at 7 days post‐inoculation (dpi) (A) and 14 dpi (B) with the green fluorescent protein (GFP)‐tagged F. graminearum strains wt‐GFP, Δtri5‐GFP, Δflg1‐GFP and Δgpmk1‐GFP were used. Water‐inoculated spikelets served as controls. Non‐cellulosic monosaccharide composition was determined by high‐performance anion‐exchange chromatography with pulsed amperometric detection (HPAEC‐PAD). a,b: P < 0.05 Dunnett's test. Error bars represent ± SEM and n = 3. Ara, l‐arabinose; Gal, d‐galactose; Glu, d‐glucose; Xyl, d‐xylose.
The analysis of the cell wall composition of spikelet tissue revealed that major changes in the glucose content compared with control tissue only occurred after inoculation with those F. graminearum strains that were able to produce the mycotoxin DON during B. distachyon infection (Fig. 2F). Therefore, we tested whether DON alone would be sufficient to induce cell wall changes in the host plant. We applied DON solutions at concentrations ranging from 1 ppb to 500 ppm to unchallenged B. distachyon florets. Seven days after DON application, we observed necrotic tissue, especially at those spikelets that were treated with the highest DON concentration of 500 ppm (Fig. 4A). We occasionally found small necrotic lesions at spikelets treated with DON concentrations of 50 and 100 ppm, whereas lower DON concentrations (1 ppb to 1 ppm) did not induce necrotic lesions. However, the application of relatively low concentrations of DON resulted in an increase in the non‐cellulosic glucose content of the treated spikelet tissue, which was most prominent for concentrations ranging from 1 to 100 ppb, where the relative glucose content was almost twice as high as that in control samples. This increase in glucose content correlated with a slightly decreased xylose content (Fig. 4B). We did not observe an alteration in the glucose content after application of a DON concentration ranging from 50 to 500 ppm (Fig. 4B). The other two neutral monosaccharides arabinose and galactose were not affected in DON‐treated tissue compared with control tissue (Fig. 4B).
Figure 4.
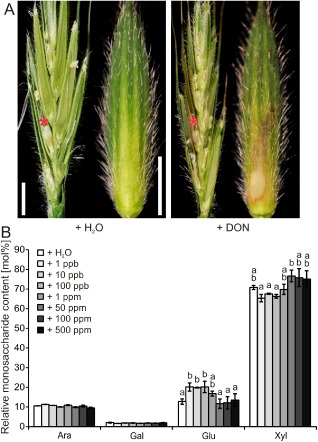
Deoxynivalenol (DON)‐induced cell wall changes in Brachypodium distachyon spikelets. A single floret of a spikelet was point inoculated with DON solutions at concentrations ranging from 1 ppb to 500 ppm. Spikelets inoculated with water and Fusarium graminearum strain wt‐GFP served as controls. (A) Longitudinal spikelet section (left part of each panel) and isolated lemma (right part of each panel) of an inoculated floret 7 days after water and DON application at a concentration of 500 ppm. Red asterisk indicates point‐inoculated floret. Scale bars, 2 mm. (B) Non‐cellulosic monosaccharide composition of cell wall extracts from spikelets treated with DON at the indicated concentrations 7 days after application. a,b: P < 0.05 Dunnett's test. Error bars represent ± SEM and n = 3. Ara, l‐arabinose; Gal, d‐galactose; Glu, d‐glucose; Xyl, d‐xylose.
DON‐induced reduction in susceptibility to F. graminearum in B. distachyon spikelets
Our observations revealed that DON treatment induced cell wall changes in B. distachyon spikelet tissue, which revealed similarities to F. graminearum‐induced cell wall changes during spikelet infection (Figs 3 and 4B). Therefore, we tested whether DON‐induced, putatively defence‐related cell wall changes prior to infection would support resistance of the B. distachyon spikelet to F. graminearum infection. We sprayed untreated B. distachyon spikelets with a DON solution at a concentration of 1 ppm to induce previously observed cell wall changes, which mainly affected the glucose content (Fig. 4B), and with water as control, 7 days prior to anthesis. Subsequently, we inoculated the pretreated spikelets with F. graminearum wt‐GFP at anthesis and determined the disease phenotype at 14 dpi. The water‐pretreated spikelets revealed the same FHB disease phenotype that affected most parts of the spikelet as observed previously with untreated spikelets (Figs 1A and 5A). In contrast with control spikelets, only the directly inoculated floret became necrotic in DON‐pretreated spikelets after infection (Fig. 5A). These observations were statistically confirmed by the decrease in the disease score from 2.7 for water‐pretreated spikelets to 1.6 for DON‐pretreated spikelets at 14 dpi with F. graminearum wt‐GFP (Fig. 5B).
Figure 5.
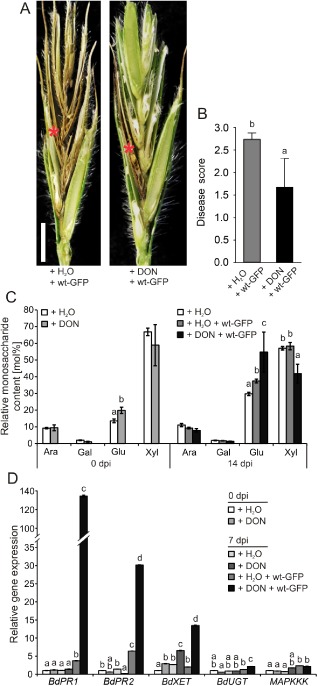
Deoxynivalenol (DON)‐induced resistance to Fusarium graminearum colonization of Brachypodium distachyon spikelets. (A) Longitudinal section of spikelets infected with the wild‐type F. graminearum strain wt‐GFP to highlight disease phenotype at 14 days post‐inoculation (dpi). Spikelets were pretreated by spraying a DON solution (concentration, 1 ppm) and water as control 7 days before F. graminearum inoculation. Red asterisk indicates point‐inoculated floret. Scale bar, 2 mm. (B) Disease score of water‐ and DON‐pretreated spikelets at 14 dpi with F. graminearum wt‐GFP. a,b: P < 0.05 Dunnett's test. Error bars represent ± SEM and n ≥ 12. (C) Non‐cellulosic monosaccharide composition of cell wall extracts from spikelets 7 days after spraying (0 dpi, time point of F. graminearum inoculation) of DON or water as control [as described in (A)] and at 14 dpi of the pretreated spikelets with F. graminearum wt‐GFP. a,b,c: P < 0.05 Dunnett's test. Error bars represent ± SEM and n = 3. Ara, l‐arabinose; Gal, d‐galactose; Glu, d‐glucose; Xyl, d‐xylose. (D) Expression analysis of pathogen‐ and DON‐inducible genes in DON‐ and water‐pretreated B. distachyon spikelets at the time point of F. graminearum wt‐GFP inoculation (0 dpi) and at 7 dpi. Water‐sprayed, unchallenged spikelets served as control. a,b,c,d: P < 0.05 Dunnett's test. Error bars represent ± SEM and n = 3. A repeat experiment gave similar results. BdPR1.1/BdPR2, pathogenesis‐related genes; BdXET, xyloglucan xyloglucosyl transferase; BdUGT, UDP‐glycosyltransferase; BdMAPKKK, mitogen‐activated protein (MAP) kinase kinase kinase.
To analyse the putative basis of the observed resistance in DON‐pretreated spikelets, we compared the non‐cellulosic cell wall composition and expression of plant defence‐related genes before and after F. graminearum infection in control and pretreated tissue. Similar to our previous results with direct DON treatment at relatively low concentrations (Fig. 4B), DON spraying of the spikelet also induced cell wall changes. The relative glucose content in DON‐pretreated spikelets was significantly higher than in water‐sprayed control spikelets at the time point of F. graminearum inoculation (time point of anthesis, 7 days after spraying), reaching a value of 20 mol%, compared with 14 mol% in control tissue (Fig. 5C). The impact of DON pretreatment on cell wall alteration was strongly enhanced after F. graminearum infection. At 14 dpi, the relative glucose content increased from about 30 mol% in untreated spikelet tissue to 38 mol% in infected spikelets which were only pretreated with water, whereas the glucose amount reached about 55 mol% in DON‐pretreated and subsequently inoculated spikelets (Fig. 5C). Only in this pretreated and infected tissue was the relative xylose content reduced compared with control tissue. With a relative amount of about 45 mol%, the xylose content was lower than the glucose content in this tissue (Fig. 5C) .
Similar to the observed cell wall changes after DON pretreatment, we observed both a direct effect on gene expression prior to infection and strong induction of gene expression after infection. The expression analysis comprised genes that showed highest homology to F. graminearum‐ and DON‐responsive genes in barley (Hordeum vulgare) and wheat, i.e. the pathogenesis‐related genes BdPR1.1 and BdPR2, the putative UDP‐glucosyltransferase gene BdUGT, the MAP kinase kinase kinase gene BdMAPKKK, and BdXET, encoding a putative xyloglucosyl transferase with a possible involvement in the observed alterations of the cell wall composition after DON application and F. graminearum infection (Fig. 5C).
We detected a transcriptional up‐regulation after DON pretreatment and prior infection only for BdXET, where the expression level was 3.5 times higher than in control tissue. Also, at 7 dpi, the expression of BdXET was higher in DON‐pretreated tissue than in control tissue (Fig. 5D). Although we did not observe a pathogen‐induced increase in BdXET expression in floret tissue that was only pretreated with water, a strong induction of BdXET expression occurred in DON‐pretreated and F. graminearum‐infected tissue, where the expression level was about five times higher than in infected, water‐pretreated tissue (Fig. 5D). We also measured a strong induction of gene expression after infection for BdPR1.1 and BdPR2. However, DON pretreatment did not affect gene expression prior to infection, but strongly promoted transcriptional up‐regulation after infection (Fig. 5D). At 7 dpi, BdPR1.1 expression was about four times higher in infected floral tissue pretreated with water only than in unchallenged tissue, but 135 times higher in infected and DON‐pretreated tissue (Fig. 5D). We obtained a similar result for BdPR2, where expression was about seven times higher in infected and water only‐pretreated tissue, but 30 times higher in infected and DON‐pretreated tissue, than in control tissue (Fig. 5D). For BdUGT, we observed a transcriptional up‐regulation only after combined DON pretreatment and F. graminearum infection (Fig. 5D). In contrast, transcriptional up‐regulation of BdMAPKKK seemed to be independent of DON pretreatment because the gene expression level was twice as high in infected tissue, with and without DON pretreatment, than in control tissue (Fig. 5D).
Discussion
In our study, we confirmed previous results on the general suitability of B. distachyon as a host plant for the agronomically important plant‐pathogenic fungus F. graminearum. Similar to the disease phenotype described by Peraldi et al. (2011) for B. distachyon after infection with the F. graminearum isolate UK1, we observed a strong colonization of the B. distachyon spikelet by the GFP‐tagged F. graminearum isolate 8/1 (Fig. 2A) used in our experiments. In both studies, the disease phenotype at 14 dpi with F. graminearum resembled FHB disease symptoms reported from wheat head infection with this fungal pathogen (Blümke et al., 2014; Walter et al., 2010). In addition to the macroscopically observed similarity of the FHB disease phenotype in wheat and B. distachyon, the induction and production of the mycotoxin DON by F. graminearum in B. distachyon spikelets (Fig. 2F) was comparable with the DON concentration determined in infected wheat heads (Bormann et al., 2014; Nguyen et al., 2011; Peraldi et al., 2011). In addition to F. graminearum wild‐type, we were able to show that defined, virulence‐deficient F. graminearum mutant strains induced a similar disease phenotype during B. distachyon infection as previously reported from infected wheat heads. The infection of the DON‐deficient disruption mutant Δtri5‐GFP, as well as the infection of the lipase disruption mutant Δfgl1‐GFP, was restricted to the directly inoculated floret (Fig. 2B,C,E), which confirmed the importance of these F. graminearum virulence factors during B. distachyon infection, as previously reported from wheat infection (Blümke et al., 2014; Jansen et al., 2005; Proctor et al., 1995; Voigt et al., 2005). The strongly reduced disease phenotype of the MAP kinase disruption mutant Δgpmk1 reported from wheat (Jenczmionka et al., 2003) was also confirmed on B. distachyon spikelets (Fig. 2D,E). This suggests the presence of comparable type II defence mechanisms (Schroeder and Christensen, 1963) in these Triticeae species, and supports the applicability of the model pathosystem B. distachyon—F. graminearum for the study and modification of essential plant defence responses to improve fungal resistance.
With regard to plant defence, we focused on cell wall changes of B. distachyon spikelets that were infected with F. graminearum wild‐type and the virulence‐deficient mutants Δtri5‐GFP, Δfgl1‐GFP and Δgpmk1‐GFP. Because the plant cell wall represents a main barrier against intruding pathogens that can be actively remodelled after infection (Sanchez‐Rodriguez et al., 2009; Underwood, 2012), we anticipated a correlation between the strength of the infection and the alteration of the cell wall composition. However, we found a correlation between F. graminearum strains with intact DON production and plant cell wall changes, but not between the strength of infection and cell wall changes (Figs 2F and 3B). Therefore, we wanted to determine whether the mycotoxin DON alone would be sufficient to induce the cell wall changes observed after infection with DON‐producing F. graminearum strains. In B. distachyon spikelets point inoculated with DON solutions at relatively low concentrations ranging from 1 to 100 ppb, we determined an increase in glucose content in the non‐cellulosic monosaccharide fraction 7 days after DON application (Fig. 4B). The higher applied DON concentrations of 50–500 ppm did not induce cell wall changes, but led to necroses of the inoculated florets (Fig. 4A), which were not found after application of low DON concentrations. The observation that DON induces disease symptoms, including necrosis and chlorosis (Cutler, 1988; Peraldi et al., 2011), has been associated with the protein synthesis‐inhibiting properties of DON through binding to the 60S ribosomal subunit of eukaryotes and induction of ribosomal RNA cleavage, which can result in hypersensitive responses and cell death (He et al., 2012; Kimura et al., 1998; Rocha et al., 2005; Zhou et al., 2005).
Because the polysaccharide composition of the plant cell wall has been proposed to play a role in host–pathogen interactions (Vorwerk et al., 2004), which was also indicated by our recent study in A. thaliana (Ellinger et al., 2013), we assumed that the increase in glucose content of the non‐cellulosic monosaccharide fraction of the cell wall could be an indication of a pathogen‐ and DON‐induced plant defence reaction. Therefore, we tested whether pretreatment of floral tissue with DON would increase resistance to subsequent F. graminearum infection. Interestingly, the DON pretreatment of B. distachyon spikelets resulted in an increased resistance to F. graminearum wild‐type (Fig. 4A,B). Because the disease phenotype resembled the phenotype observed after infection with the virulence‐deficient mutants Δtri5‐GFP and Δfgl1‐GFP (Fig. 2B,C), we concluded that application of DON at a relatively low concentration might activate defence mechanisms that induce type II resistance in the host floral tissue. Moreover, the analysis of the cell wall composition and the expression of genes reported previously to be associated with a pathogen‐ and DON‐dependent stress response suggest that a relatively low‐dose DON pretreatment could have a dual effect on triggering plant defence responses. On the one hand, we identified cell wall changes that were induced by low‐dose DON application without subsequent pathogen infection, namely the increased non‐cellulosic glucose content. Because the expression of the putative xyloglucan xyloglucosyl transferase‐encoding gene, BdXET, correlated with the observed changes in the plant cell wall (Fig. 5C,D), it is likely that these DON‐induced cell wall alterations are the result of DON‐induced transcriptional changes in genes encoding cell wall‐modifying enzymes. The extent to which the observed cell wall change, namely the DON‐induced increase in glucose in the non‐cellulosic fraction, is a direct defence response of the plant cannot be answered conclusively. Because reference data for pathogen‐ and mycotoxin‐induced cell wall modifications and their consequence on host–pathogen interaction for grasses are not available, our data could be considered as a starting point for additional cell wall analyses. Interestingly, the observed increased resistance of tobacco (Nicotiana tabacum) to the fungal pathogen Botrytis cinerea as a result of overexpression of polygalacturonase‐inhibiting protein from grapevine (Vitis vinifera) was associated with a reorganization of the cellulose–xyloglucan network in advance of infection (Nguema‐Ona et al., 2013). Further cell wall studies in B. distachyon might reveal whether the DON‐ and pathogen‐induced increase in the glucose content is also a consequence of the cellulose–xyloglucan network modification.
However, we observed a hyperactivation of pathogenesis‐related gene expression in DON‐pretreated spikelet tissue after F. graminearum infection. Unlike BdXET, the expression of the two analysed pathogenesis‐related genes BdPR1.1 and BdPR2 in DON‐pretreated tissue was no different from that of control tissue without infection. As expected from pathogenesis‐related genes, the expression of BdPR1.1 and BdPR2 was induced after F. graminearum infection of untreated B. distachyon spikelets; however, gene expression was 30 times higher for BdPR1.1 and four times higher for BdPR2 in DON‐pretreated tissue after infection (Fig. 5D). Hence, resistance to FHB could be based on the hyperactivation of pathogenesis‐related genes, which would support plant defence reactions and type II resistance.
The observed hyperactivation of plant defence responses after DON pretreatment shows clear similarities to defence priming of plants, a process of enhanced activation of defence responses following the recognition of pathogen‐derived molecular patterns, effectors or compound treatment (Conrath, 2011). In primed plants, the enhanced activation of plant defence is often linked to local and systemic resistance, leading to higher stress and pathogen tolerance (Conrath et al., 2002, 2006; Jung et al., 2009). In this concept of plant defence priming, DON, if applied at a relatively low concentration, would function as a cellular signal amplifier (Conrath, 2011), resulting in enhanced defence. In contrast with relatively low concentrations of DON application and the associated priming effect in B. distachyon, Peraldi et al. (2011) showed that high concentrations of DON not only induced lesions and necroses, but also strongly supported the F. graminearum infection of B. distachyon.
A dose‐dependent effect of the mycotoxin DON on immunity has also been shown in animal systems, where DON targets monocytes, macrophages and lymphocytes of the immune system. Mice fed with sublethal doses of 0.5 and 1.0 mg DON/kg body weight/day in their basal diet showed a significant reduction in the serum levels of alpha‐1‐ and alpha‐2‐globulins and a reduced time‐to‐death interval when challenged with the infectious bacterium Listeria monocytogenes, whereas a DON dose of 0.25 mg/kg did not influence these parameters (Tryphonas et al., 1986). One reason for the DON‐induced symptoms in mice is a modulation of the genes associated with immunity, inflammation and chemotaxis (Kinser et al., 2004), which was recently confirmed in chickens fed with basal diet contaminated with DON (Ghareeb et al., 2013). Further in vitro analysis of the dose‐dependent effect of DON on immunity revealed that an immune stimulation followed a transcriptional up‐regulation of cytokines, chemokines and inflammatory genes at low DON concentrations, whereas high DON concentrations induced a ribotoxic stress response which promoted leucocyte apoptosis. The intracellular DON signalling involved different kinases, including MAP kinases and RNA‐activated protein kinases, that were activated after the binding of DON to ribosomes (He et al., 2012; Pestka, 2008). Interestingly, dormant MAP kinases accumulated during priming in A. thaliana (Beckers et al., 2009) and supported the assumption that kinases play a key role in the molecular mechanisms and signalling pathways of defence priming.
Therefore, plant kinases and especially MAP kinases would constitute a promising target in gene expression and protein studies after DON application to elucidate the DON signaling pathway in plants leading to plant defence priming because MAP kinases would represent a link between the known mode of action of DON in eukaryotes, which is the binding to ribosomes, and the molecular basis of priming in plants.
Experimental Procedures
Plant material and growth conditions
Brachypodium distachyon (L.) Beauv. inbred line Bd21 (Vogel et al., 2006) was cultivated in two parts of soil (Einheitserdewerk, Uetersen, Germany, ED 73 + 10% sand) and one part of sand at 22 °C and with a 20‐h photoperiod in a growth chamber. Approximately 6 weeks after sowing, the plants reached anthesis and were inoculated. After inoculation, the plants were transferred to a growth cabinet and grown for 14 days at 22 °C, with a photoperiod of 16 h and at 50%–60% humidity.
Fungal strains and culture conditions
All fungal mutants used in this study originated from the transformation of the F. graminearum isolate Fg 8/1 (Miedaner et al., 2000). The trichodiene synthase disruption mutant Δtri5‐GFP with constitutive GFP expression was derived from the study of Jansen et al. (2005), the Gpmk1 MAP kinase disruption mutant Δgpmk1‐GFP with constitutive GFP expression was derived from the study of Salomon et al. (2012), and the GFP‐expressing wild‐type strain wt‐GFP as well as the GFP‐expressing lipase‐deficient disruption mutant Δfgl1‐GFP were derived from our recent study (Blümke et al., 2014). Media, induction of conidiation and culture conditions were applied according to Jenczmionka et al. (2003). Fusarium graminearum conidia were stored in aqueous suspensions at −70 °C.
Plant inoculation
All plants were inoculated in the late afternoon. For infection studies with F. graminearum, the third or fourth floret of a B. distachyon spikelet was point inoculated with 1 μL of water containing 40 conidia, or with sterile water only as negative control, at growth stages 61–65 (mid‐anthesis) following the BBCH scale (Hong et al., 2011). The conidial suspension was placed between lemma and palea. The floret was gently closed and the plant was covered with a plastic bag for 2 days to increase humidity and to promote fungal infection. Samples for cell wall analysis were taken at 7 and 14 dpi. Repeat inoculation experiments were performed with the same −70 °C conidial stock suspensions.
Point inoculation of florets with 1 μL of DON solutions at different concentrations (1, 10 and 100 ppb, 1, 50, 100 and 500 ppm) and water only as control followed the above description for inoculation with conidia, except that plants were not covered with plastic bags after inoculation. Samples for cell wall analysis were taken at 7 dpi.
Spray inoculation of B. distachyon spikelets with a DON solution (concentration, 1 ppm) was performed 7 days before mid‐anthesis (growth stage 55–53; Hong et al., 2011). Spraying with water served as a control. Samples for cell wall analysis were taken 7 days after spraying at the time point of F. graminearum inoculation (0 dpi) and at 14 dpi.
A single spikelet of at least three individual plants grown in separate pots was inoculated with fungal strains or treated with DON as described above. Inoculation experiments were repeated three times in a weekly interval.
Disease scoring
At 14 dpi with F. graminearum, when ripening started and no further infection progress was detectable, the infection was monitored using a numerical scoring system (Fig. 1). The infection was rated as 0.1 for slight infection of a floret when only small brownish dots were visible on the caryopsis or the rachilla. A floret was rated as 0.5 when the infection resulted in at least two necrotic spots and a maximum of 50% necrotic tissue. In the case of over 50% necrotic tissue, the floret was rated as 1.0. For each spikelet, the single florets were examined and the score was calculated.
Microscopy
To visualize the spread and propagation of the F. graminearum strains within the infected floret and spikelet, a Nikon AZ100 fluorescence microscope was used in bright field microscopy and fluorescence microscopy with the filter B‐2A (Nikon, Tokyo, Japan), with excitation at 450–490 nm (epifluorescence illuminator C‐HGFI, Nikon), to visualize GFP fluorescence emitted from tagged fungal strains.
DON quantification
To determine the concentration of the mycotoxin DON, inoculated B. distachyon florets were harvested at 7 and 14 dpi with F. graminearum. At least three spikelets from individually inoculated plants of four biologically independent experiments were pooled (pool 1, spikelets from experiment 1; pool 2, spikelets from experiment 2; pool 3, spikelets from experiments 3 and 4; see description in section on ‘Plant inoculation’), ground under liquid nitrogen and freeze–dried.
DON was extracted from 50 mg of dried sample by the Ridascreen DON enzymatic immunoassay following the manufacturer's instructions (R‐Biopharm, Darmstadt, Germany). The determined amount of DON was normalized to the amount of fungal mycelium in the respective plant tissue. Quantification of fungal mycelium was performed in real‐time polymerase chain reaction (PCR) analysis using DNA from dried fungal mycelium as a standard, as previously described by Voigt et al. (2007).
Cell wall analysis
The determination of the non‐cellulosic monosaccharide composition of the cell wall from B. distachyon spikelet tissue followed the description in Ellinger et al. (2013). Extracted monosaccharides were quantified by HPAEC‐PAD on an ICS‐5000 system equipped with an electrochemical detector and a CarboPac PA 20 column (Dionex, Sunnyvale, CA, USA). Fucose, arabinose, rhamnose, galactose, mannose, xylose, glucose, glucuronic acid and galacturonic acid (all from Sigma‐Aldrich, Steinheim, Germany) were used as standards. The generation of three pools of spikelets for cell wall analysis followed the description in the section on ‘DON quantification’.
Quantitative real‐time PCR
To study gene expression in B. distachyon before and after F. graminearum infection, samples were collected from spikelets that were sprayed with DON or water only as control at the time point of F. graminearum inoculation (0 dpi) and at 7 dpi. Samples without fungal inoculation served as controls. For RNA isolation, complete spikelets were used. The generation of three pools of spikelets for quantitative real‐time PCR followed the description in the section on ‘DON quantification’. Subsequent procedures for RNA isolation and cDNA generation were performed according to Voigt et al. (2006). Quantitative real‐time PCR was performed on a LightCyler 480 (Roche Diagnostics, Mannheim, Germany) using the LightCycler 480 SYBR Green I Master mix (Roche Diagnostics). In quantitative real‐time PCRs, cDNA samples were normalized to the expression of the constitutive Actin gene [Gramene database (Monaco et al., 2014); Bradi4g41850; primer: fwd, 5′‐GCTGGGCGTGACCTAACTGAC; rev, 5′‐ATGAAAGATGGCTGGAAAAGGACT].
The genes used for expression analysis were identified on the basis of their sequence homology to known F. graminearum‐ and DON‐induced genes in wheat and barley. The pathogenesis‐related genes BdPR1.1 (Bradi1g57540; fwd, 5′‐AAGAACGCCGTGGACATGTG; rev, 5′‐ACCCGGAGGATCATAACTAC) and BdPR2 (Bradi2g60490; fwd, 5′‐AGCCATCCAGCTCAACTAC; rev, 5′‐CCTTGCCAACATGGTCAATC) showed highest homology to respective wheat genes with a transcriptional induction after DON treatment (Desmond et al., 2008); the putative UDP‐glycosyltransferase encoding gene BdUGT (Bradi2g05050; fwd, 5′‐CGCGGCTTCCGTGGTGTA; rev, 5′‐GTTGCCGTCGCCCACGTC) and the putative MAP kinase kinase kinase gene BdMAPKKK (Bradi2g17840; fwd, 5′‐CCATGCCGACCTTGATAGAG; rev, 5′‐CCTGAAACTTTGGGCGAGAG) showed highest homology to respective F. graminearum‐ and DON‐induced barley genes (Boddu et al., 2007); and BdXET (Bradi1g33827; fwd, 5′‐AGCACAGGAACAGGGAGAC; rev, 5′‐GTCCAGCTCCTGGTACATC) showed highest homology to the cell wall‐modifying xyloglucan xyloglucosyl transferase gene XET6 from barley (Hrmova et al., 2009).
Statistical analysis
Statistical analysis was performed using SPSS Statistics (release 20.0.0, IBM, Armonk, NY, USA). Parametric data from the disease score, DON content and monosaccharide composition were analysed using one‐way analysis of variance (ANOVA), followed by a Dunnett post‐hoc test to identify significant samples. If variance was not homogeneous, data were compared via Welch (t‐test) tests. P < 0.05 was considered to be significant. All statistical values represent the mean of the respective dataset and error bars show the standard error of the mean (± SEM).
Acknowledgements
We would like to thank John Vogel for providing the seeds of the B. distachyon inbred line Bd21, and Wilhelm Schäfer for providing the F. graminearum strain Δtri5‐GFP.
References
- Alves, S.C. , Worland, B. , Thole, V. , Snape, J.W. , Bevan, M.W. and Vain, P. (2009) A protocol for Agrobacterium‐mediated transformation of Brachypodium distachyon community standard line Bd21. Nat. Protoc. 4, 638–649. [DOI] [PubMed] [Google Scholar]
- Beckers, G.J. , Jaskiewicz, M. , Liu, Y. , Underwood, W.R. , He, S.Y. , Zhang, S. and Conrath, U. (2009) Mitogen‐activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana . Plant Cell, 21, 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Blanco, I.A. , Frohberg, R.C. , Stack, R.W. , Berzonsky, W.A. and Kianian, S.F. (2003) Detection of QTL linked to Fusarium head blight resistance in Sumai 3‐derived North Dakota bread wheat lines. Theor. Appl. Genet. 106, 1027–1031. [DOI] [PubMed] [Google Scholar]
- Bluhm, B.H. , Zhao, X. , Flaherty, J.E. , Xu, J.R. and Dunkle, L.D. (2007) RAS2 regulates growth and pathogenesis in Fusarium graminearum . Mol. Plant–Microbe Interact. 20, 627–636. [DOI] [PubMed] [Google Scholar]
- Blümke, A. , Falter, C. , Herrfurth, C. , Sode, B. , Bode, R. , Schäfer, W. , Feussner, I. and Voigt, C.A. (2014) Secreted fungal effector lipase releases free fatty acids to inhibit innate immunity‐related callose formation during wheat head infection. Plant Physiol. 165, 346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddu, J. , Cho, S. and Muehlbauer, G.J. (2007) Transcriptome analysis of trichothecene‐induced gene expression in barley. Mol. Plant–Microbe Interact. 20, 1364–1375. [DOI] [PubMed] [Google Scholar]
- Bormann, J. , Boenisch, M.J. , Bruckner, E. , Firat, D. and Schäfer, W. (2014) The adenylyl cyclase plays a regulatory role in the morphogenetic switch from vegetative to pathogenic lifestyle of Fusarium graminearum on wheat. PLoS One, 9, e91135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerstmayr, H. , Ban, T. and Anderson, J.A. (2009) QTL mapping and marker‐assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breed. 128, 1–26. [Google Scholar]
- Catalán, P. , Shi, Y. , Armstrong, L. , Draper, J. and Stace, C.A. (1995) Molecular phylogeny of the grass genus Brachypodium P. Beauv. based on RFLP and RAPD analysis. Bot. J. Linn. Soc. 117, 263–280. [Google Scholar]
- Chakraborty, S. and Newton, A.C. (2011) Climate change, plant diseases and food security: an overview. Plant Pathol. 60, 2–14. [Google Scholar]
- Christensen, U. , Alonso‐Simon, A. , Scheller, H.V. , Willats, W.G.T. and Harholt, J. (2010) Characterization of the primary cell walls of seedlings of Brachypodium distachyon—a potential model plant for temperate grasses. Phytochemistry, 71, 62–69. [DOI] [PubMed] [Google Scholar]
- Christiansen, P. , Andersen, C.H. , Didion, T. , Folling, M. and Nielsen, K.K. (2005) A rapid and efficient transformation protocol for the grass Brachypodium distachyon . Plant Cell Rep. 23, 751–758. [DOI] [PubMed] [Google Scholar]
- Conrath, U. (2011) Molecular aspects of defence priming. Trends Plant Sci. 16, 524–531. [DOI] [PubMed] [Google Scholar]
- Conrath, U. , Pieterse, C.M. and Mauch‐Mani, B. (2002) Priming in plant–pathogen interactions. Trends Plant Sci. 7, 210–216. [DOI] [PubMed] [Google Scholar]
- Conrath, U. , Beckers, G.J. , Flors, V. , Garcia‐Agustin, P. , Jakab, G. , Mauch, F. , Newman, M.A. , Pieterse, C.M. , Poinssot, B. , Pozo, M.J. , Pugin, A. , Schaffrath, U. , Ton, J. , Wendehenne, D. , Zimmerli, L. and Mauch‐Mani, B. (2006) Priming: getting ready for battle. Mol. Plant–Microbe Interact. 19, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Cowger, C. and Sutton, A.L. (2005) The southeastern U.S. Fusarium head blight epidemic of 2003. Plant Health Progress , doi: 10.1094/PHP-2005-1026-01-RS. [DOI]
- Cutler, H.G. (1988) Trichothecenes and their role in the expression of plant disease In: Biotechnology for Crop Protection (Russell R.B., ed.), pp. 50–72. Washington DC: American Chemical Society. [Google Scholar]
- Desmond, O.J. , Manners, J.M. , Stephens, A.E. , Maclean, D.J. , Schenk, P.M. , Gardiner, D.M. , Munn, A.L. and Kazan, K. (2008) The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol. Plant Pathol. 9, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper, J. , Mur, L.A. , Jenkins, G. , Ghosh‐Biswas, G.C. , Bablak, P. , Hasterok, R. and Routledge, A.P. (2001) Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol. 127, 1539–1555. [PMC free article] [PubMed] [Google Scholar]
- Duveiller, E. , Singh, R.P. and Nicol, J.M. (2007) The challenges of maintaining wheat productivity: pests, diseases, and potential epidemics. Euphytica, 157, 417–430. [Google Scholar]
- Ellinger, D. , Naumann, M. , Falter, C. , Zwikowics, C. , Jamrow, T. , Manisseri, C. , Somerville, S.C. and Voigt, C.A. (2013) Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiol. 161, 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareeb, K. , Awad, W.A. , Soodoi, C. , Sasgary, S. , Strasser, A. and Bohm, J. (2013) Effects of feed contaminant deoxynivalenol on plasma cytokines and mRNA expression of immune genes in the intestine of broiler chickens. PLoS One, 8, e71492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, L.D. , Bristow, J.K. , Statham, E.R. and McQueen‐Mason, S.J. (2008) Analysis of saccharification in Brachypodium distachyon stems under mild conditions of hydrolysis. Biotechnol. Biofuels, 1, 15, doi: 10.1186/1754-6834-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, K. , Zhou, H.R. and Pestka, J.J. (2012) Targets and intracellular signaling mechanisms for deoxynivalenol‐induced ribosomal RNA cleavage. Toxicol. Sci. 127, 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, S.Y. , Park, J.H. , Cho, S.H. , Yang, M.S. and Park, C.M. (2011) Phenological growth stages of Brachypodium distachyon: codification and description. Weed Res. 51, 612–620. [Google Scholar]
- Hrmova, M. , Farkas, V. , Harvey, A.J. , Lahnstein, J. , Wischmann, B. , Kaewthai, N. , Ezcurra, I. , Teeri, T.T. and Fincher, G.B. (2009) Substrate specificity and catalytic mechanism of a xyloglucan xyloglucosyl transferase HvXET6 from barley (Hordeum vulgare L). FEBS J. 276, 437–456. [DOI] [PubMed] [Google Scholar]
- International Brachypodium, I. (2010) Genome sequencing and analysis of the model grass Brachypodium distachyon . Nature, 463, 763–768. [DOI] [PubMed] [Google Scholar]
- Jansen, C. , von Wettstein, D. , Schafer, W. , Kogel, K.H. , Felk, A. and Maier, F.J. (2005) Infection patterns in barley and wheat spikes inoculated with wild‐type and trichodiene synthase gene disrupted Fusarium graminearum . Proc. Natl. Acad. Sci. USA, 102, 16 892–16 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenczmionka, N.J. and Schäfer, W. (2005) The Gpmk1 MAP kinase of Fusarium graminearum regulates the induction of specific secreted enzymes. Curr. Genet. 47, 29–36. [DOI] [PubMed] [Google Scholar]
- Jenczmionka, N.J. , Maier, F.J. , Lösch, A.P. and Schäfer, W. (2003) Mating, conidiation and pathogenicity of Fusarium graminearum, the main causal agent of the head‐blight disease of wheat, are regulated by the MAP kinase gpmk1. Curr. Genet. 43, 87–95. [DOI] [PubMed] [Google Scholar]
- Jung, H.W. , Tschaplinski, T.J. , Wang, L. , Glazebrook, J. and Greenberg, J.T. (2009) Priming in systemic plant immunity. Science, 324, 89–91. [DOI] [PubMed] [Google Scholar]
- Kimura, M. , Kaneko, I. , Komiyama, M. , Takatsuki, A. , Koshino, H. , Yoneyama, K. and Yamaguchi, I. (1998) Trichothecene 3‐O‐acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. J. Biol. Chem. 273, 1654–1661. [DOI] [PubMed] [Google Scholar]
- Kinser, S. , Jia, Q. , Li, M. , Laughter, A. , Cornwell, P. , Corton, J.C. and Pestka, J. (2004) Gene expression profiling in spleens of deoxynivalenol‐exposed mice: immediate early genes as primary targets. J. Toxicol. Environ. Health A, 67, 1423–1441. [DOI] [PubMed] [Google Scholar]
- Madgwick, J.W. , West, J.S. , White, R.P. , Semenov, M.A. , Townsend, J.A. , Turner, J.A. and Fitt, B.D.L. (2011) Impacts of climate change on wheat anthesis and fusarium ear blight in the UK. Eur. J. Plant Pathol. 130, 117–131. [Google Scholar]
- Maier, F.J. , Miedaner, T. , Hadeler, B. , Felk, A. , Salomon, S. , Lemmens, M. , Kassner, H. and Schäfer, W. (2006) Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Mol. Plant Pathol. 7, 449–461. [DOI] [PubMed] [Google Scholar]
- McMullen, M. , Jones, R. and Gallenberg, D. (1997) Scab of wheat and barley: a re‐emerging disease of devastating impact. Plant Dis. 81, 1340–1348. [DOI] [PubMed] [Google Scholar]
- Miedaner, T. , Reinbrecht, C. and Schilling, A.G. (2000) Association among aggressiveness, fungal colonization, and mycotoxin production of 26 isolates of Fusarium graminearum in winter rye head blight. J. Plant Dis. Protect. 107, 124–134. [Google Scholar]
- Monaco, M.K. , Stein, J. , Naithani, S. , Wei, S. , Dharmawardhana, P. , Kumari, S. , Amarasinghe, V. , Youens‐Clark, K. , Thomason, J. , Preece, J. , Pasternak, S. , Olson, A. , Jiao, Y. , Lu, Z. , Bolser, D. , Kerhornou, A. , Staines, D. , Walts, B. , Wu, G. , D'Eustachio, P. , Haw, R. , Croft, D. , Kersey, P.J. , Stein, L. , Jaiswal, P. and Ware, D. (2014) Gramene 2013: comparative plant genomics resources. Nucleic Acids Res. 42, D1193–D1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguema‐Ona, E. , Moore, J.P. , Fagerstrom, A.D. , Fangel, J.U. , Willats, W.G. , Hugo, A. and Vivier, M.A. (2013) Overexpression of the grapevine PGIP1 in tobacco results in compositional changes in the leaf arabinoxyloglucan network in the absence of fungal infection. BMC Plant Biol. 13, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, L.N. , Bormann, J. , Le, G.T. , Stärkel, C. , Olsson, S. , Nosanchuk, J.D. , Giese, H. and Schäfer, W. (2011) Autophagy‐related lipase FgATG15 of Fusarium graminearum is important for lipid turnover and plant infection. Fungal Genet. Biol. 48, 217–224. [DOI] [PubMed] [Google Scholar]
- Peraica, M. , Radic, B. , Lucic, A. and Pavlovic, M. (1999) Toxic effects of mycotoxins in humans. Bull. World Health Org. 77, 754–766. [PMC free article] [PubMed] [Google Scholar]
- Peraldi, A. , Beccari, G. , Steed, A. and Nicholson, P. (2011) Brachypodium distachyon: a new pathosystem to study Fusarium head blight and other Fusarium diseases of wheat. BMC Plant Biol. 11, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka, J.J. (2008) Mechanisms of deoxynivalenol‐induced gene expression and apoptosis. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 25, 1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirgozliev, S.R. , Edwards, S.G. , Hare, M.C. and Jenkinson, P. (2003) Strategies for the control of Fusarium head blight in cereals. Eur. J. Plant Pathol. 109, 731–742. [Google Scholar]
- Proctor, R.H. , Hohn, T.M. and McCormick, S.P. (1995) Reduced virulence of Gibberella zeae caused by disruption of a trichthecine toxin biosynthetic gene. Mol. Plant–Microbe Interact. 8, 593–601. [DOI] [PubMed] [Google Scholar]
- Rocha, O. , Ansari, K. and Doohan, F.M. (2005) Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 22, 369–378. [DOI] [PubMed] [Google Scholar]
- Salomon, S. , Gácser, A. , Frerichmann, S. , Kröger, C. , Schäfer, W. and Voigt, C. (2012) The secreted lipase FGL1 is sufficient to restore the initial infection step to the apathogenic Fusarium graminearum MAP kinase disruption mutant Δgpmk1 . Eur. J. Plant Pathol. 134, 23–37. [Google Scholar]
- Sanchez‐Rodriguez, C. , Estevez, J.M. , Llorente, F. , Hernandez‐Blanco, C. , Jorda, L. , Pagan, I. , Berrocal, M. , Marco, Y. , Somerville, S. and Molina, A. (2009) The ERECTA receptor‐like kinase regulates cell wall‐mediated resistance to pathogens in Arabidopsis thaliana . Mol. Plant–Microbe Interact. 22, 953–963. [DOI] [PubMed] [Google Scholar]
- Schroeder, H.W. and Christensen, J.J. (1963) Factors affecting resistance of wheat to scab caused by Gibberella zeae . Phytopathology, 53, 831–838. [Google Scholar]
- Thole, V. , Worland, B. , Wright, J. , Bevan, M.W. and Vain, P. (2010) Distribution and characterization of more than 1000 T‐DNA tags in the genome of Brachypodium distachyon community standard line Bd21. Plant Biotechnol. J. 8, 734–747. [DOI] [PubMed] [Google Scholar]
- Tryphonas, H. , Iverson, F. , So, Y. , Nera, E.A. , McGuire, P.F. , O'Grady, L. , Clayson, D.B. and Scott, P.M. (1986) Effects of deoxynivalenol (vomitoxin) on the humoral and cellular immunity of mice. Toxicol. Lett. 30, 137–150. [DOI] [PubMed] [Google Scholar]
- Underwood, W. (2012) The plant cell wall: a dynamic barrier against pathogen invasion. Front. Plant Sci. 3, 85, doi: 10.3389/fpls.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban, M. , Mott, E. , Farley, T. and Hammond‐Kosack, K. (2003) The Fusarium graminearum MAP1 gene is essential for pathogenicity and development of perithecia. Mol. Plant Pathol. 4, 347–359. [DOI] [PubMed] [Google Scholar]
- Vogel, J. and Bragg, J. (2009) Brachypodium distachyon, a new model for the Triticeae In: Genetics and Genomics of the Triticeae (Feuillet C. and Muehlbauer G.J., eds.), pp. 427–449. New York: Springer. [Google Scholar]
- Vogel, J. and Hill, T. (2008) High‐efficiency Agrobacterium‐mediated transformation of Brachypodium distachyon inbred line Bd21‐3. Plant Cell Rep. 27, 471–478. [DOI] [PubMed] [Google Scholar]
- Vogel, J.P. , Gu, Y.Q. , Twigg, P. , Lazo, G.R. , Laudencia‐Chingcuanco, D. , Hayden, D.M. , Donze, T.J. , Vivian, L.A. , Stamova, B. and Coleman‐Derr, D. (2006) EST sequencing and phylogenetic analysis of the model grass Brachypodium distachyon . Theor. Appl. Genet. 113, 186–195. [DOI] [PubMed] [Google Scholar]
- Voigt, C.A. , Schäfer, W. and Salomon, S. (2005) A secreted lipase of Fusarium graminearum is a virulence factor required for infection of cereals. Plant J. 42, 364–375. [DOI] [PubMed] [Google Scholar]
- Voigt, C.A. , Schäfer, W. and Salomon, S. (2006) A comprehensive view on organ‐specific callose synthesis in wheat (Triticum aestivum L.): glucan synthase‐like gene expression, callose synthase activity, callose quantification and deposition. Plant Physiol. Biochem. 44, 242–247. [DOI] [PubMed] [Google Scholar]
- Voigt, C.A. , von Scheidt, B. , Gácser, A. , Kassner, H. , Lieberei, R. , Schäfer, W. and Salomon, S. (2007) Enhanced mycotoxin production of a virulence‐reduced Fusarium graminearum mutant correlates to toxin‐related gene expression. Eur. J. Plant Pathol. 117, 1–12. [Google Scholar]
- Vorwerk, S. , Somerville, S. and Somerville, C. (2004) The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 9, 203–209. [DOI] [PubMed] [Google Scholar]
- Walter, S. , Nicholson, P. and Doohan, F.M. (2010) Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 185, 54–66. [DOI] [PubMed] [Google Scholar]
- Zhou, H.R. , Islam, Z. and Pestka, J.J. (2005) Induction of competing apoptotic and survival signaling pathways in the macrophage by the ribotoxic trichothecene deoxynivalenol. Toxicol. Sci. 87, 113–122. [DOI] [PubMed] [Google Scholar]


