Summary
Trichoderma arundinaceum IBT 40837 (Ta37) and Botrytis cinerea produce the sesquiterpenes harzianum A (HA) and botrydial (BOT), respectively, and also the polyketides aspinolides and botcinins (Botcs), respectively. We analysed the role of BOT and Botcs in the Ta37–B. cinerea interaction, including the transcriptomic changes in the genes involved in HA (tri) and ergosterol biosynthesis, as well as changes in the level of HA and squalene‐ergosterol. We found that, when confronted with B. cinerea, the tri biosynthetic genes were up‐regulated in all dual cultures analysed, but at higher levels when Ta37 was confronted with the BOT non‐producer mutant bcbot2Δ. The production of HA was also higher in the interaction area with this mutant. In Ta37–bcbot2Δ confrontation experiments, the expression of the hmgR gene, encoding the 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase, which is the first enzyme of the terpene biosynthetic pathway, was also up‐regulated, resulting in an increase in squalene production compared with the confrontation with B. cinerea B05.10. Botcs had an up‐regulatory effect on the tri biosynthetic genes, with BotcA having a stronger effect than BotcB. The results indicate that the interaction between Ta37 and B. cinerea exerts a stimulatory effect on the expression of the tri biosynthetic genes, which, in the interaction zone, can be attenuated by BOT produced by B. cinerea B05.10. The present work provides evidence for a metabolic dialogue between T. arundinaceum and B. cinerea that is mediated by sesquiterpenes and polyketides, and that affects the outcome of the interaction of these fungi with each other and their environment.
Keywords: Botrytis cinerea, chemical dialogue, fungal interactions, pathway co‐regulation, Trichoderma arundinaceum
Introduction
Botrytis cinerea (teleomorph Botryotinia fuckeliana) is an airborne plant pathogen with a necrotrophic lifestyle which causes ‘grey mould’ on over 200 crops worldwide. The fungus produces two groups of toxins: the sesquiterpene botryanes (Collado et al., 2007) and the polyketides botcinins (Botcs) (Tani et al., 2005). There are multiple botryane analogues, with botrydial (BOT) being the analogue produced in the largest amounts (Colmenares et al., 2002; Pinedo et al., 2008) (Fig. S1a, see Supporting Information), which can induce chlorosis and cell collapse during plant infection (Deighton et al., 2001). Botc production is increased in mutants blocked in BOT production, which show similar virulence levels to those of B. cinerea B05.10 wild‐type strain, indicating a redundant function of both toxins in Botrytis virulence (Pinedo et al., 2008) (Fig. S1a). Botrytis cinerea also produces the polyketide botrylactones, whose biological activities have not been evaluated (Bruns et al., 1995; Massaroli et al., 2013; Moraga et al., 2011). In addition to these toxins, Botrytis produces other virulence factors involved in plant cutin and cell wall degradation, host colonization, host cell death and/or protection against oxidative bursts and defences (Choquer et al., 2007; Nakajima and Akutsu, 2014) which, together with the toxins, make B. cinerea a highly virulent pathogen. Considering the variety of hosts and attack strategies, biocontrol has the potential to be a valid strategy against B. cinerea (Elad and Stewart, 2004).
Fungi of the genus Trichoderma have been reported as efficient antagonists of plant pathogens (Harman et al., 1996; Howell, 2003) and can induce plant systemic defences (De Meyer et al., 1998; Hermosa et al., 2012). Many Trichoderma strains produce secondary metabolites (SMs) and enzymes with diverse industrial interest and biocontrol applications (Hermosa et al., 2014; Lorito et al., 2010). Studies on the Trichoderma–Botrytis interaction have usually focused on the characterization of the effect of Trichoderma on Botrytis growth and/or pathogenicity. Trichoderma arundinaceum has biocontrol activity against B. cinerea and produces harzianum A (HA) (Fig. S1a), an analogue of a family of sesquiterpenes known as trichothecenes (Fig. S1b). HA is involved in the biocontrol activity of Ta37 (T. arundinaceum IBT 40837 strain), as well as in its ability to up‐regulate plant defence‐related genes (Malmierca et al., 2012). Wild‐type, HA‐producing T. arundinaceum reduced the growth of both B. cinerea and Rhizoctonia solani, but HA‐non‐producing mutants of the fungus, which were generated by deletion of the terpene synthase gene (tri5) required for HA biosynthesis, exhibited reduced biocontrol activity against B. cinerea (Malmierca et al., 2013). Wild‐type T. arundinaceum also primed tomato plants by inducing plant defence genes, which were, in turn, induced more rapidly and to higher levels when the plants were subsequently challenged with B. cinerea. In a three‐organism (tomato–B. cinerea–T. arundinaceum) system, HA‐non‐producing mutants of T. arundinaceum were reduced in the ability to control B. cinerea‐induced disease in tomato (Malmierca et al., 2012). Although the culture broth of the HA‐non‐producing mutants failed to prevent B. cinerea‐induced formation of lesions on tomato leaves, the size of the lesions was reduced compared with control broths not inoculated with T. arundinaceum (Malmierca et al., 2013). Together, these findings indicate that HA production contributes to the biocontrol activity of T. arundinaceum. It is not clear, however, whether SMs produced by Botrytis (e.g. BOT and Botcs) are able to regulate or interfere with HA production in T. arundinaceum.
The biosynthesis of HA begins with the cyclization of the isoprenoid intermediate farnesyl diphosphate (FPP) to trichodiene (TD). This reaction is catalysed by the sesquiterpene cyclase enzyme trichodiene synthase (Tri5) (Cardoza et al., 2011). The cytochrome P450 monooxygenase Tri4 then catalyses three consecutive oxygenations of TD to form isotrichodiol, which then undergoes a non‐enzymatic conversion to 12,13‐epoxytrichothec‐9‐ene (EPT = trichothecene). The cytochrome P450 monooxygenase Tri11 oxygenates EPT at C‐4 to produce trichodermol, which is then converted to HA by esterification of octatriendioic acid to the C‐4 oxygen (Fig. S1b). Although it has not yet been demonstrated, it has been proposed that the acetyl transferase, Tri3, catalyses this esterification reaction. Tri3, Tri4 and Tri11 are encoded by genes in the trichothecene biosynthetic gene (tri) cluster, whereas tri5 is located at another locus. In addition to the tri3, tri4 and tri11 genes, the Trichoderma tri cluster includes three additional genes: tri6, tri10 and tri12. These genes have not been functionally characterized in Trichoderma, but have been characterized in the related genus Fusarium. tri12 encodes an efflux pump (Alexander et al., 1999) that facilitates the transport of trichothecenes across the cytoplasmic membranes. tri6 and tri10 genes encode regulatory proteins. Tri6 probably binds to promoter regions of tri genes and positively regulates their expression (Hohn et al., 1999), whereas Tri10 acts upstream of Tri6 and is necessary for full expression of both tri genes and the genes required for the primary metabolic pathway that leads into the trichothecene biosynthetic pathway (Peplow et al., 2003; Tag et al., 2001).
The aim of the present work was to analyse the role of BOT and Botcs in the interaction of B. cinerea and T. arundinaceum. To do this, wild‐type strains of these two fungi, as well as a previously isolated B. cinerea bcbot2Δ mutant, blocked in BOT biosynthesis (Quidde et al., 1999), were used. The production of HA, ergosterol and squalene, and the expression of the genes involved in their biosynthetic pathways, were measured in single cultures of Ta37 strain, as well as in the interaction zones of Ta37 and B. cinerea dual cultures, in the presence or absence of exogenous BOT or Botcs. These analyses allowed us to isolate the effects of BOT or Botcs from the effects of the physical interaction between the two fungi.
Results
Effect of exogenously added BOT on the expression of T. arundinaceum genes involved in HA and ergosterol biosynthesis
To analyse the effect of BOT on the expression of T. arundinaceum genes, strain Ta37 was grown in the absence (control) or presence of 1.5 μg/mL BOT (Fig. 1a), an amount similar to that produced by B05.10 in the interaction zone of Ta37–B05.10 dual cultures (I. G. Collado, personal communication). tri genes exhibited a dramatic reduction in expression when Ta37 was grown in the presence relative to the absence of BOT, with expression ratios ranging from 0.001‐ and 0.069‐fold for tri5 and tri6, respectively (Fig. 1b). The exception to this was the regulatory gene tri10, which did not exhibit a reduction in expression in the presence of BOT. In contrast, the ergosterol biosynthetic genes were not affected by the presence of BOT. The exception to this was hmgR; it was strongly down‐regulated to a level of 0.2‐fold in the presence of BOT (Fig. 1c).
Figure 1.
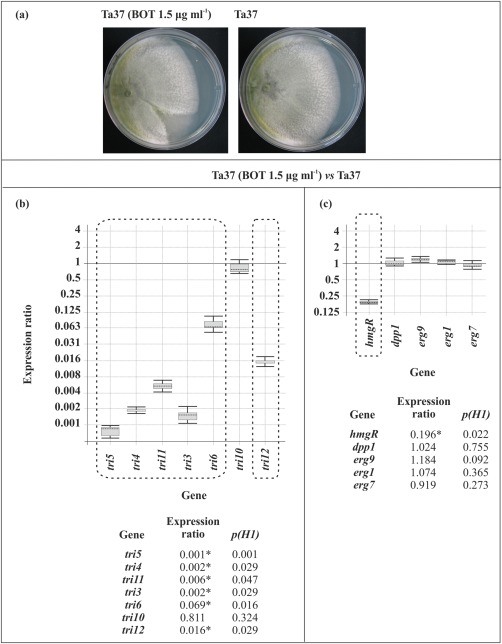
(a) Photographs illustrating the growth of Ta37 in the absence (right panel) and presence (left panel) of 1.5 μg/mL botrydial (BOT). (b, c) Analysis of expression of trichothecene biosynthetic genes (b) and ergosterol biosynthetic genes hmgR, dpp1, erg9, erg1 and erg7 (c) in Ta37 in the presence (1.5 μg/mL) versus absence of BOT. Expression data relative to α‐actin and glyceraldehyde‐3‐phosphate dehydrogenase (gpd) reference (housekeeping) genes are illustrated at the bottom, and statistically significant values (P < 0.05) are indicated with an asterisk and outlined by a broken line in the graphical representation.
HA production and gene expression pattern in Ta37–B05.10 dual cultures
Ta37 produced 44 μg/mL of HA when grown alone on malt extract agar (MEA), but significantly less HA (31.06 μg/mL) when grown in dual culture with B. cinerea strain B05.10. In these dual cultures, the levels of HA detected in the non‐interaction and interaction zones did not differ significantly (Table 1). Although HA production was reduced in dual cultures of Ta37 and B05.10, structural tri genes [=(st)tri] (tri5, tri4, tri11 and tri3) were up‐regulated. In the interaction zone, the expression ratios ranged from 1.7‐ to 6.5‐fold for tri3 and tri5, respectively. In contrast, the expression of the regulatory genes tri6 and tri10 was not affected, whereas expression of the transporter gene tri12 was slightly induced (1.3‐fold) (Fig. 2). In all cases, the comparisons were made versus the expression levels in Ta37 grown alone (i.e. in the absence of B05.10).
Table 1.
Production of harzianum A (HA) by Ta37 growing in dual culture with B05.10 or bcbot2Δ.
| Region | Non‐interaction | Interaction |
|---|---|---|
| Dual culture | (μg HA/mL medium) | (μg HA/mL medium) |
| Ta37–B05.10 | 31.06a ± 5.57 | 36.48a ± 1.87 |
| Ta37–bcbot2Δ | 33.89a ± 3.46 | 54.04b ± 8.91 |
Ta37 growing alone produced 44b ± 4.58 μg/mL of HA.
For each dual culture, values followed by different superscript letters are significantly different.
Kruskal–Wallis test (P < 0.05; n = 3).
Figure 2.
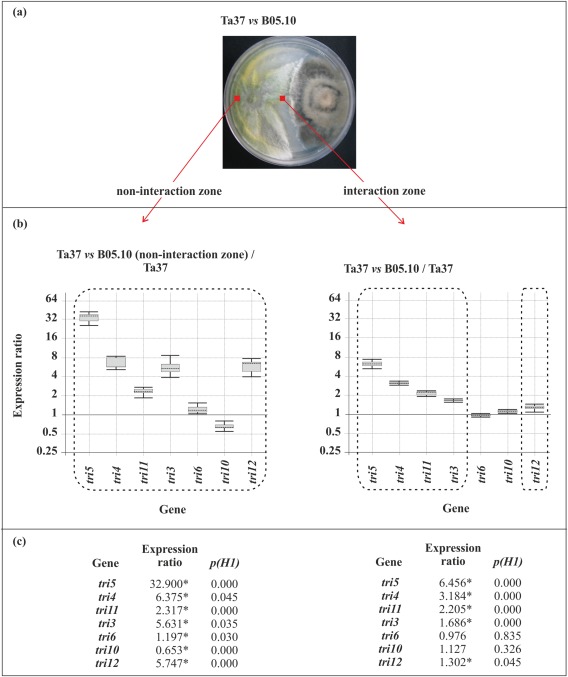
(a) Dual culture of Ta37 and B05.10. (b, c) Analysis of tri gene expression level in Ta37 mycelia isolated from the non‐interaction zone (left panel) and interaction zone (right panel) versus the expression level in Ta37 growing alone. Expression data relative to α‐actin and glyceraldehyde‐3‐phosphate dehydrogenase (gpd) reference (housekeeping) genes are illustrated in (c), and statistically significant values (P < 0.05) are indicated with an asterisk and outlined by a broken line in the graphical representation.
Interestingly, the induction of expression of most (st)tri genes was greater in the non‐interaction zone relative to the interaction zone of these dual cultures. tri11 was the only structural tri gene that was up‐regulated to similar levels in both zones. tri12 was also up‐regulated to a greater degree in the non‐interaction zone (5.7‐fold), whereas the regulatory genes, tri6 and tri10, were slightly up‐regulated (1.2‐fold) and down‐regulated (0.6‐fold), respectively, in the non‐interaction zone (Fig. 2).
These results indicate that the presence of BOT in the interaction zone down‐regulates the expression of the structural and transport tri genes, except for tri11, when compared with expression in the non‐interaction zone, where BOT is not present or is highly diluted (Fig. S2a, see Supporting Information).
tri gene expression during interaction of Trichoderma and a BOT‐non‐producing mutant of B. cinerea
When Ta37 was grown in dual cultures with the previously isolated BOT‐non‐producing mutant bcbot2Δ, there was an increase in HA production (54.04 μg/mL, +74%) in the interaction zone, whereas production in the non‐interaction zone was similar to that in the same region of the Ta37–B05.10 dual cultures (Table 1). The significant increase in the amount of HA detected in the Ta37–bcbot2Δ interaction zone was accompanied by a significant up‐regulation of the (st)tri and tri12 genes. In contrast, the tri regulatory genes were not affected (tri6) or were strongly down‐regulated [tri10 (0.2‐fold)] (Fig. 3).
Figure 3.
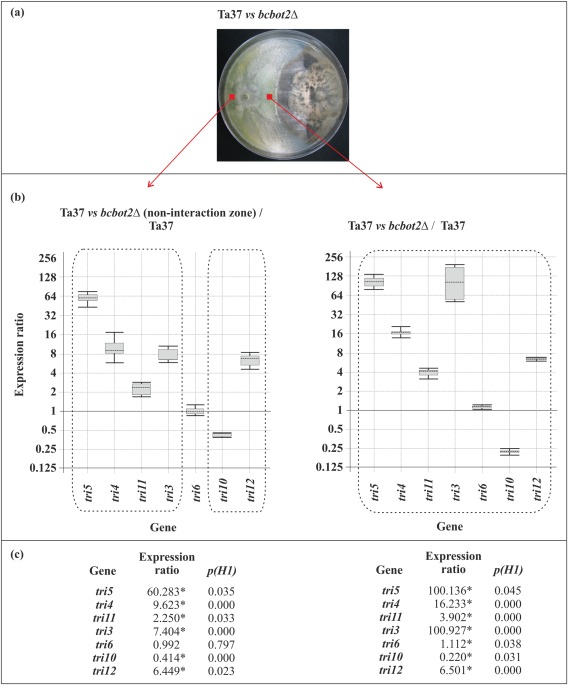
(a) Dual culture of Ta37 and bcbot2Δ. (b, c) Analysis of tri gene expression level in Ta37 mycelia isolated from the non‐interaction zone (left panel) and interaction zone (right panel) versus the expression level in Ta37 growing alone. Expression data relative to α‐actin and glyceraldehyde‐3‐phosphate dehydrogenase (gpd) reference (housekeeping) genes are illustrated in (c), and statistically significant values (P < 0.05) are indicated with an asterisk and outlined by a broken line in the graphical representation.
The up‐regulation of tri genes observed in the interaction zone of Ta37–bcbot2Δ was much higher than in the interaction zone of Ta37–B05.10 (see Fig. 2). These differences may be a result of a loss of BOT production in the bcbot2Δ mutant. Thus, to test this hypothesis, tri gene expression was analysed in the non‐interaction zone and compared with the levels of expression in the interaction zone. A relatively strong down‐regulation of tri3 was observed, reaching values of 0.077‐fold (Fig. S2b), which could explain the reduction in the level of HA production in this zone compared with the interaction zone, and also indicates that tri3 may be an important bottleneck in HA biosynthesis. This was also supported by the level of tri3 expression, which was the lowest among all the tri genes, compared with the expression of α‐actin, a constitutively expressed gene used as a reference (Fig. S3, see Supporting Information).
Effect of exogenously added BOT on tri gene expression during interaction of Ta37 with bcbot2Δ
When tri gene expression ratios were determined from the interaction zone of Ta37–bcbot2Δ dual cultures supplemented with 1.5 μg/mL BOT, a concentration similar to that detected in the interaction zone of Ta37–B05.10 dual cultures (I. G. Collado, personal communication), all tri genes, except tri10, were up‐regulated (Fig. 4), with relative expression levels similar to those observed in the interaction zone of the Ta37–bcbot2Δ dual cultures without BOT (see Fig. 3). However, in the non‐interaction zone of these dual cultures, expression of all the (st)tri genes, as well as tri12, reached expression ratios that were significantly lower than those observed in the interaction zone. The regulatory genes were slightly up‐regulated (tri6) or down‐regulated (tri10) (Figs 4 and S2c). Interestingly, the expression ratios of (st)tri genes, observed in the non‐interaction zone, were close to those observed in the interaction zone of Ta37–B05.10 dual cultures, in which BOT was being produced by B05.10 (see Fig. 2).
Figure 4.
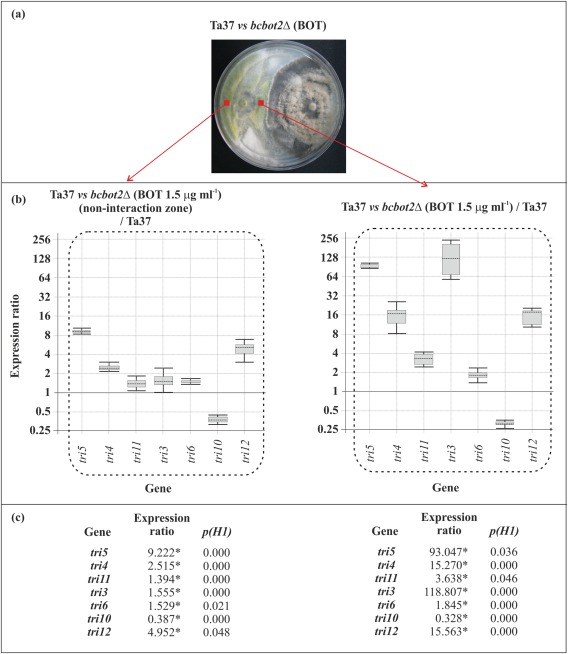
(a) Dual culture of Ta37 and bcbot2Δ in plates supplemented with 1.5 μg/mL botrydial (BOT). (b, c) Analysis of tri gene expression level in Ta37 mycelia isolated from the non‐interaction zone (left panel) and interaction zone (right panel) versus the expression level in Ta37 growing alone without BOT. Expression data relative to α‐actin and glyceraldehyde‐3‐phosphate dehydrogenase (gpd) reference (housekeeping) genes are illustrated in (c), and statistically significant values (P < 0.05) are indicated with an asterisk and outlined by a broken line in the graphical representation.
These results indicate that the up‐regulating effect of (st)tri genes in the interaction zone was not overridden by the addition of exogenous BOT. However, in the non‐interaction zone, the relative down‐regulating effect of BOT on (st)tri gene expression prevailed.
Expression of ergosterol biosynthetic genes and production of ergosterol and squalene during Trichoderma–Botrytis interactions
Confrontation of Ta37 with B05.10 resulted in a significant down‐regulation of hmgR (0.3), and also a slight up‐regulation of all the other ergosterol biosynthetic genes analysed (Fig. 5a), with expression ratios from 1.5‐ to 2.4‐fold for erg1 and erg7, respectively. Interestingly, this was a similar response to that observed for Ta37 grown alone in the presence of 1.5 μg/mL BOT (see Fig. 1), indicating that the expression of these genes, especially hmgR, is regulated by BOT. Furthermore, when the expression ratios of these genes were analysed in Ta37–bcbot2Δ dual cultures, an almost opposite response was observed for hmgR (13.5‐fold) and erg7 (0.5‐fold) (Fig. 5b) to that observed in confrontation with B05.10, whereas the other analysed genes showed similar expression ratios in both analyses.
Figure 5.
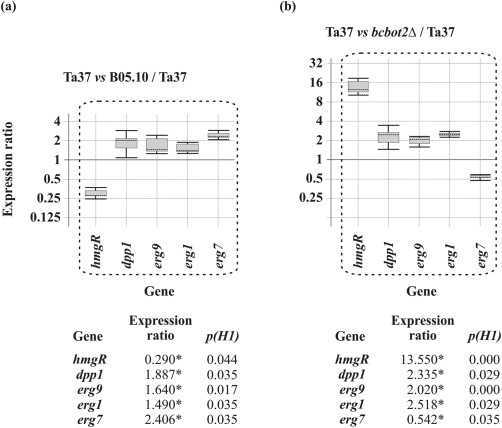
Analysis of terpene/ergosterol biosynthetic gene expression level in Ta37 mycelia isolated from the interaction zone of Ta37–B05.10 dual cultures (a) and the interaction zone of Ta37–bcbot2Δ dual cultures (b), in both cases versus the level of expression of these genes in Ta37 growing alone. Expression data relative to α‐actin and glyceraldehyde‐3‐phosphate dehydrogenase (gpd) reference (housekeeping) genes are illustrated at the bottom, and statistically significant values (P < 0.05) are indicated with an asterisk and outlined by a broken line in the graphical representation.
When the production of ergosterol and squalene was analysed in the interaction zone of these dual cultures, a correlation was observed between the level of expression of hmgR and the amount of squalene produced. Ta37 accumulated more squalene (+88.96%) in the confrontation with bcbot2Δ than in the confrontation with B05.10, where a moderate decrease (−19.80%) was observed, in both cases in comparison with the production data of Ta37 growing alone (Table 2). However, no significant differences were observed in the production of ergosterol, with only a slight increase during the interaction with bcbot2Δ (+9.94%) (Table 2). These results correlated with the almost opposite expression pattern of hmgR and erg7 genes in the dual cultures analysed. The expected reduction in the production of ergosterol by hmgR down‐regulation during the interaction with B05.10 should be compensated by an up‐regulation of erg7. In the case of the interaction with bcbot2Δ, the expected increase in ergosterol production as a result of hmgR up‐regulation was neutralized by the erg7 down‐regulation, which correlates with the increase in the level of squalene accumulation, as well as the increase in HA production in parallel as a result of the up‐regulation of the (st)tri genes (Fig. 5).
Table 2.
Production of squalene and ergosterol by the control of Ta37 growing alone and in confrontation with different Botrytis cinerea strains.
| DW (g) | Squalene (mg/g DW) | Variation (%) | Ergosterol (mg/g DW) | Variation (%) | |
|---|---|---|---|---|---|
| Ta37 | 0.03 | 0.616 ± 0.022a | 68.89 ± 2.87a | ||
| Ta37–B05.10 | 0.029 | 0.494 ± 0.036b | −19.80 | 71.36 ± 1.68a | |
| Ta37–bcbot2Δ | 0.03 | 1.164 ± 0.032c | +88.96 | 75.74 ± 1.43b | +9.94 |
n = 2, analysis of variance (ANOVA). DW, dry weight.
For each time point, values followed by different superscript letters are significantly different (P < 0.05).
Effect of BotcA and BotcB on the expression of trichothecene and ergosterol biosynthetic genes in Ta37 grown alone or in the interaction with B05.10
When BotcA and BotcB were added to cultures of Ta37 growing alone, they up‐regulated the (st)tri and tri12 genes, except tri11. tri6 was not affected and tri10 was down‐regulated with both Botcs. Furthermore, although the effect was very similar with both, BotcA caused greater up‐ or down‐regulation than BotcB (Figs 6 and 7).
Figure 6.
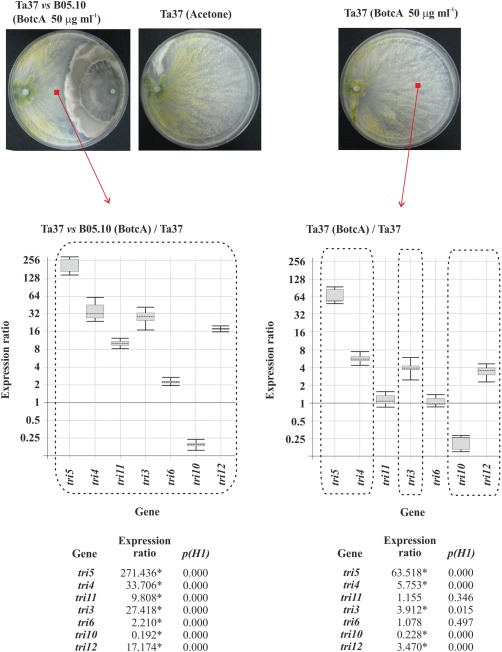
Top: photographs illustrating the growth of Ta37 in confrontation with B05.10 in medium supplemented with 50 μg/mL botcinin A (BotcA) (left), alone in the presence of acetone (solvent) (centre) and alone in the presence of 50 μg/mL BotcA (right). Bottom: analysis of tri gene expression levels in Ta37 mycelium isolated from the interaction zone of Ta37–B05.10 dual cultures supplemented with 50 μg/mL BotcA (left) and Ta37 mycelium grown in isolated cultures supplemented with 50 μg/mL BotcA (right), in both cases versus the level of expression in Ta37 grown alone with acetone. Expression data relative to α‐actin and glyceraldehyde‐3‐phosphate dehydrogenase (gpd) reference (housekeeping) genes are illustrated at the bottom, and statistically significant values (P < 0.05) are indicated with an asterisk and outlined by a broken line in the graphical representation.
Figure 7.
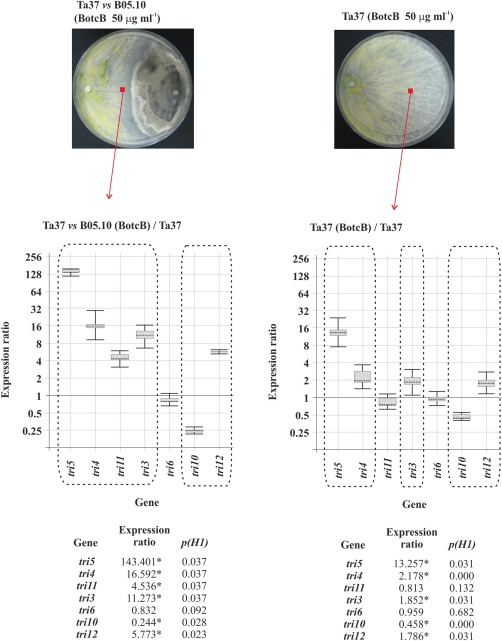
Top: photographs illustrating the growth of Ta37 in confrontation with B05.10 in medium supplemented with 50 μg/mL botcinin B (BotcB) (left) and alone in the presence of 50 μg/mL BotcB (right). Bottom: analysis of tri gene expression levels in Ta37 mycelium isolated from the interaction zone of Ta37–B05.10 dual cultures supplemented with 50 μg/mL BotcB (left) and Ta37 mycelium grown in isolated cultures supplemented with 50 μg/mL BotcB (right), in both cases versus the level of expression in Ta37 grown alone with acetone. Expression data relative to α‐actin and glyceraldehyde‐3‐phosphate dehydrogenase (gpd) reference (housekeeping) genes are illustrated at the bottom, and statistically significant values (P < 0.05) are indicated with an asterisk and outlined by a broken line in the graphical representation.
The addition of BotcA or BotcB to Ta37–B05.10 dual cultures also resulted in an up‐regulation of all the structural and transport tri genes; tri6 was up‐regulated by BotcA, but was not affected by BotcB, and tri10 was down‐regulated with both Botcs. However, as was the case in the experiments with Ta37 growing alone, the level of up‐ or down‐regulation was significantly stronger with BotcA (Figs 6 and 7).
The expression of other terpene biosynthetic genes was only analysed in dual cultures supplemented with Botcs. An up‐regulation of hmgR and erg7 was observed with both Botcs, with slightly higher up‐regulation with BotcA. The other three genes analysed were only slightly up‐regulated (dpp1) or down‐regulated (erg9, erg1) by BotcA and were not affected by BotcB (Fig. S4). These data correlate with the levels of squalene and ergosterol produced in the confrontation regions of these supplemented dual cultures. Thus, the production of these compounds was increased in both cases compared with their levels in the confrontation without Botcs (Table 3). Interestingly, the production of squalene was much higher in the presence of BotcB, which could be caused by the relative up‐ and down‐regulation of erg1 and erg7 genes, respectively (Fig. S4, see Supporting Information), compared with the levels detected in the presence of BotcA, and emphasizes the important role of these genes as bottlenecks in the ergosterol biosynthetic pathway.
Table 3.
Production of squalene and ergosterol by the control of Ta37 growing alone and in confrontation with B05.10, in the presence of BotcA or BotcB.
| DW (g) | Squalene (mg/g DW) | Variation (%) | Ergosterol (mg/g DW) | Variation (%) | |
|---|---|---|---|---|---|
| Ta37–B05.10 (acetone) | 0.017 | 2.067 ± 0.019a | 50.69 ± 3.18a | ||
| Ta37–B05.10 (BotcA) | 0.012 | 2.247 ± 0.043b | +8.70 | 73.69 ± 3.25b | +45.37 |
| Ta37–B05.10 (BotcB) | 0.014 | 3.173 ± 0.028c | +53.50 | 66.87 ± 2.18c | +31.91 |
n = 2, analysis of variance (ANOVA). DW, dry weight.
For each time point, values followed by different superscript letters are significantly different (P < 0.05).
Discussion
Co‐cultures of fungi are an effective tool to simulate the physiological conditions that occur during the interactions of fungi in natural or agricultural systems (Brakhage, 2013). Such cultures have facilitated the detection of volatile compounds which are not produced when individual fungi are cultured alone (Hynes et al., 2007) and metabolite profiles that change depending on the interacting fungi (Peiris et al., 2008; Rodríguez‐Estrada et al., 2011). In order to elucidate the chemical communications that may affect the outcome of the interaction of the biocontrol agent T. arundinaceum and the plant pathogen B. cinerea, we examined the two zones of growth (interaction and non‐interaction) that form in dual cultures of these fungi.
The results observed in the interaction zone between Ta37 and B05.10 contrasted with the strong down‐regulation of tri genes observed when Ta37 was grown alone in the presence of BOT. Exogenously added BOT elicited the strong down‐regulation of tri genes, except tri10, when Ta37 was grown alone. These results indicate the importance of the Ta37–B. cinerea interaction in tri gene regulation, but also the role of BOT in this interaction. For example, in the confrontation of Ta37 with bcbot2Δ, all the tri biosynthetic genes were up‐regulated compared with the confrontation of Ta37 with B05.10, because of the lack of BOT in the interaction zone and an up‐regulatory effect by the confrontation between both fungi.
In addition, important differences were observed when Ta37–bcbot2Δ dual cultures supplemented with exogenous BOT were compared with Ta37 confronted with the wild‐type B05.10 strain, which may be caused by the way in which Ta37 interacts with BOT in these two assays. In the Ta37–B05.10 dual cultures, BOT was produced by Botrytis and contacts with Ta37 in the confrontation area, with a gradient of BOT towards the distal areas of the Ta37 culture. In these cultures, the regulatory effect was mainly detected in the interaction zone, where most tri genes were up‐regulated at a lower level than observed in the non‐interaction zone (see Fig. 8 for a synopsis). However, when BOT was exogenously added, Ta37 grew in direct contact with it throughout the incubation period, even before contacting with the Botrytis mycelium, and the effect of BOT could be overridden by the up‐regulatory effect in the interaction zone. Most of the tri genes were relatively down‐regulated in the non‐interaction zone, where the effects of the elicitor signals transmitted from the interaction zone were weaker (Figs 4 and S5, see Supporting Information).
Figure 8.
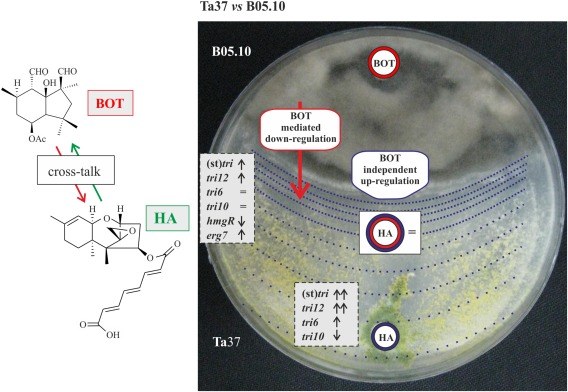
Synopsis of the most remarkable phenomena observed in the Ta37–B05.10 dual cultures. Arrows pointing up/down indicate gene up‐/down‐regulation. Arrows, boxes or circles in red indicate the responses that can be attributed to botrydial (BOT)‐mediated regulation. Lines, arrows or circles in blue represent the up‐regulatory effect caused by the signals generated in the interaction zone. The blue dotted lines originating in the interaction zone, projected over the Ta37 colony, represent the weakening of this confrontation effect in the zones located far from the confrontation line. ‘BOT‐mediated down‐regulation’ (red) and ‘BOT‐independent up‐regulation’ (blue) boxes illustrate the down‐regulatory effect of BOT produced by B05.10, which is compensated by the up‐regulatory effect of the Ta37 structural tri genes [(st)tri], caused by the elicitors released in the interaction zone. It should be noted that, in the non‐interaction zone, the BOT effect does not exist or is much weaker, and there is an up‐regulation of (st)tri genes by the distal effect of elicitors released in the interaction zone.
The results presented in this work lead us to deduce that the signals generated in the interaction zones are detected by the entire Ta37 culture, and that the entire culture responds to these signals, although the response reaches lower levels in the most distant non‐interaction zones (see Fig. 8). Thus, when the expression of genes encoding several key hydrolytic enzymes was analysed in the interaction zone versus the level of expression in the non‐interaction zone, a remarkable up‐regulation was observed for several of the genes analysed: sprT (27.6‐fold), glyH (6.6‐fold) and gh92 (9.4‐fold). Surprisingly, the expression of chit37 was not detected, whereas chit42 and papA expression was not significantly affected (Fig. S6, see Supporting Information). These enzymes hydrolyse proteins and glycoproteins, generating oligomers that could act as signalling molecules, which could be, at least in part, responsible for the up‐regulation of tri genes. The expression of chit42 was not affected by the interaction with B. cinerea, which correlates with the fact that the chitin content in B. cinerea cell walls is unusually low compared with that in other filamentous fungi, with neutral sugars and proteins as the two main components (Cantu et al., 2009). In addition, sprT and glyH have been identified as two of the most highly up‐regulated genes in the interaction between T. harzianum and Sclerotinia sclerotiorum (Steindorff et al., 2014). In the same study, the expression of ptr, a gene encoding an oligopeptide transporter (Vizcaíno et al., 2006), was strongly down‐regulated, which would indicate that, in the interaction zone, secondary metabolism prevails over mycelial growth and that the oligomers generated in the interaction zone play a main role as elicitors rather than in fungal growth. In addition, recent studies based on transcriptomic analysis have indicated that proteolysis is a major biological process involved in the mycoparasitism of Trichoderma overgrowing its prey (Atanasova et al., 2013; Steindorff et al., 2014). This conclusion is supported by the fact that, in Ta37–bcbot2Δ dual cultures, tri genes followed a similar expression pattern in both zones, with the exception of tri11 and tri3, which were comparatively up‐regulated in the interaction region, as a response to the confrontation effect in that region (Figs 3 and S7, see Supporting Information). However, BOT produced by B05.10 preferentially affected those areas of the culture that were in direct contact with this sesquiterpene, and this would explain the relative down‐regulation of tri genes in the interaction zone of Ta37–B05.10 dual cultures, compared with the non‐interaction zone. In addition, the presence of BOT down‐regulates Ta37 hmgR expression in the confrontation with this strain, which could be a mechanism used to reduce the flux of precursors towards the terpene pathway and, in this way, prevent a possible increase in HA production as a result of the interaction with this pathogen. This implies that there is a sesquiterpenic cross‐talk between these two strains, by which the sesquiterpene produced by one fungus regulates the biosynthesis of the sesquiterpene produced by the other partner.
However, in this cross‐regulation, not all the tri genes are equally affected. tri3 was up‐regulated to a very high level in the interaction zone of Ta37–bcbot2Δ dual cultures with or without BOT, compared with its expression in the non‐interaction zone or with the expression in Ta37 confrontation with B05.10. Interestingly, a similar level of expression was reached by all the genes in both zones in Ta37–bcbot2Δ dual cultures, except that tri3 was strongly down‐regulated in the non‐interaction zone, indicating that, in spite of the elicitor effect produced as a result of the confrontation between both fungi, some other factor/s may be produced by bcbot2Δ that could contribute to this specific up‐regulation of tri3 expression in the interaction zone.
In previous studies, the analysis of the bcbot2Δ metabolome led to the conclusion that BcBOT2 disruption abolishes BOT biosynthesis, but also results in the overproduction of botcinolides BotcA and BotcB, which are polyketide compounds also produced by the wild‐type strain, but at a much lower level (Moraga et al., 2011; Pinedo et al., 2008). Thus, the effect of addition of Botcs to Ta37 cultures grown alone or in confrontation with B05.10 was analysed to determine their effect in tri gene regulation. The effect of exogenous Botcs on the expression of tri genes in cultures of Ta37 grown alone was almost the opposite of that observed when the experiment was performed with exogenous BOT. Botcs have a redundant role with BOT in their phytotoxicity (Pinedo et al., 2008), although, based on the results presented here, they seem to have a very different function in the interaction with other environmental fungi and, more specifically, with other sesquiterpene‐producing fungi. Furthermore, the synthesis of polyketide compounds as a result of the disruption of the synthesis of sesquiterpenoid compounds has also been described in T. arundinaceum, where a block in the production of the trichothecene (sesquiterpene) HA resulted in the production at high levels of several aspinolides, polyketide compounds that are not produced or produced at low levels in the wild‐type strain (Malmierca et al., 2015). Interestingly, similar cross‐pathway interactions have been described for other fungal gene clusters. Thus, O'Keeffe et al. (2014) have demonstrated that an intact gliotoxin self‐protection mechanism, mediated by GliT, is essential to regulate the biosynthesis of apparently unrelated metabolites in Aspergillus fumigatus, such as pseurotin A, fumagillin and fumitremorgins. In another example, Bergmann et al. (2010) have shown that overexpression of the silent inp putative secondary metabolism cross‐pathway regulator gene (scpR) in A. nidulans results in the expression of both the inp and asperfuranone silent gene clusters.
The results indicate that the presence of Botcs in the interaction zone overrides the relative down‐regulation effect caused by BOT. In addition, the pattern of expression and the expression ratio levels observed are closer to those observed in the confrontation area of Ta37–bcbot2Δ, when BOT is added to the medium. Especially noteworthy is the case of tri3, which showed an up‐regulation that was significantly higher in the interaction zone between Ta37 and bcbot2Δ than in the cultures with exogenous BOT, or in the B05.10 confrontation with exogenous Botcs, indicating that there is an effect of the exogenous addition of BOT or BOT produced by B05.10 in the interaction zone. Thus, even when Botcs induce the expression of (st)tri genes, it seems that tri3 expression is specifically and more drastically affected by BOT. Taking together all the results concerning tri3 regulation, an important role can be attributed to this gene as a bottleneck in HA biosynthesis in confrontation with B. cinerea. Indeed, the variations observed in the level of HA production (Table 1) correlate with tri3 expression levels in the analysed zones.
In addition to the SMs produced by both fungal partners, oligomers released from the fungal prey cell wall can elicit a response in the producer fungus, mediated by a transduction signal mechanism, which, finally, results in the production of SMs and in an increase in the production of hydrolytic enzymes (Druzhinina et al., 2011). These responses, which are localized in the interaction zone, can be co‐responsible for the up‐regulatory effect of tri gene expression. The effect observed in the non‐interaction zones against B05.10 or bcbot2Δ without exogenous BOT or BotcA can be understood as a response to the signals that originate in the interaction zone. Thus, the eliciting activity of the oligomers released from the pathogen's cells would have effects on the entire Ta37 culture (even far away from the interaction zone) and (st)tri genes would be up‐regulated. However, the relative down‐regulation of these genes in the non‐interaction zones of Ta37 versus bcbot2Δ in the presence of BOT did not support this hypothesis. This effect can be explained by the direct effect of BOT added to the medium, which cannot be overridden by the elicitor activity generated in the interaction zone. It should be understood that, in the non‐interaction zone, the elicitor activity would be weaker than in the interaction zone, and could be easily overridden by exogenous BOT. Another important point to consider is the time course of the confrontation. When BOT is exogenously added to cultures, it affects Ta37 before the generation of the eliciting activity that results from the physical confrontation between the fungi. This sequence of events could be responsible for the relative down‐regulation of tri genes in the non‐interaction zone of Ta37–bcbot2Δ dual cultures in the presence of BOT.
Furthermore, there are several elements that are clearly involved in this interaction. These include BOT exogenously added or produced by B05.10, Botcs added to the medium or produced by bcbot2Δ, HA produced by Ta37, other SMs produced by B05.10 (botcinic acid, Moraga et al., 2011; botrylactones, Massaroli et al., 2013) and Ta37 (aspinolides, Malmierca et al., 2015), and the hydrolytic enzymes and signalling molecules generated in the interaction zone that can act as signals even far away from the confrontation region. The cross‐pathway interactions established between these SMs, together with the hydrolytic enzyme activity, observed in the present work, and with the ability of HA to inhibit the germination of B. cinerea spores (Malmierca et al., 2013), contribute to explain why T. arundinaceum is able to overcome and suppress the virulence mechanisms exhibited by the pathogen.
In the present work, we tried to isolate the effect of BOT, as the main virulence factor produced by B. cinerea, in the regulation of tri genes during the confrontation interaction with Ta37. We found that the production of BOT relatively down‐regulates the expression of tri genes and HA production, and also drives the channelling of precursors towards the ergosterol biosynthetic pathway. Botcs, with redundant roles with BOT on B. cinerea pathogenicity, nevertheless have an opposite effect on tri and ergosterol gene regulation. Together with these SMs, hydrolytic enzymes produced by Ta37 in the confrontation region are also involved in these responses, being responsible for the production of elicitors that up‐regulate tri gene expression, in both the interaction and non‐interaction zones, but in the latter at a lower level.
Experimental Procedures
Strains and culture media
Botrytis cinerea B05.10 (=B05.10) is derived from a vineyard field isolate (Quidde et al., 1998). Botrytis cinerea B05.10 cnd15 strain is a BOT‐non‐producer mutant (now referred to as bcbot2Δ) in which BcBOT2 is deleted (Pinedo et al., 2008). This gene encodes the presilphiperfolan‐8β‐ol synthase which catalyses the multistep cyclization of FPP to presilphiperfolan‐8β‐ol, the precursor of BOT. The bcbot2Δ mutant overproduces Botcs, but these compounds are produced in very small amounts by B05.10 (Pinedo et al., 2008).
Trichoderma arundinaceum IBT 40837 (=Ta37) (IBT Culture Collection of Fungi at the Department of Biotechnology, Technical University of Denmark) was kindly provided by Ulf Thrane.
Botrytis cinerea and T. arundinaceum strains were maintained on MEA (2% glucose, 2% malt extract, 1% peptone, 2% agar, pH 5.6) and PPG (2% mashed potatoes, 2% glucose, 2% agar) respectively.
For the isolation of toxins, B. cinerea strains were initially grown on malt agar medium at 25 °C; these cultures were used to inoculate Roux bottles or Erlenmeyer flasks containing 150 mL of modified Czapek–Dox medium. Cultures were grown at room temperature (Pinedo et al., 2008).
Confrontation assays
Confrontation assays between T. arundinaceum and B. cinerea strains were carried out using 7‐mm‐diameter agar plugs cut from the growing edge of 5‐day‐old colonies of T. arundinaceum strains grown on PPG at 28°C, and 7‐day‐old colonies of B. cinerea strains grown on MEA at 21°C, with a photoperiod of 16 h light/8 h dark. Each confrontation was initiated by placing a B. cinerea agar plug 2 cm from the edge of a 15‐cm‐diameter Petri dish containing 50 mL of MEA medium covered with a sterile cellophane sheet. Botrytis cinerea was allowed to grow at 21°C under the same conditions. After 4 days, the T. arundinaceum agar plug was placed at 2 cm from the edge on the opposite side of the same Petri dish. Dual cultures were incubated at 28°C for 7 days (Fig. S8a, see Supporting Information). Mycelia were collected for RNA extraction, in triplicate, from 1‐cm bands from two locations: (i) the interaction zone between both microorganisms; and (ii) the non‐interaction zone of the T. arundinaceum colony (Fig. S8b).
Single cultures, incubated under the same conditions as the dual cultures, were used for comparative studies.
When BOT or BotcA and B were used to supplement confrontation or single cultures, they were mixed with the melted medium before distribution onto the Petri dishes. Thus, the strains grow in contact with these metabolites from the beginning of the incubation period.
Extraction and chemical analysis of HA, BOT and Botcs
For HA quantification in solid medium, samples were collected from the different T. arundinaceum–B. cinerea cultures, grown as described above. After the cellophane and mycelia had been removed from the surface of the medium, eight 1‐cm‐diameter MEA plugs were cut from both the interaction and non‐interaction zones of the T. arundinaceum colony (Fig. S8c), or from single cultures of Ta37, and separately placed in 10‐mL tubes. Four millilitres of ethyl acetate were added, vortexed for 1 min and recovered. Then, another 4 mL of ethyl acetate were added, shaken for 3 h and recovered. The two extracts were pooled and then evaporated to dryness in a rotary evaporator at room temperature. The residue was resuspended in 150 µL of acetonitrile and stored at −20°C. The HA quantities were determined by high‐performance liquid chromatography (HPLC), as described previously (Cardoza et al., 2011).
BOT and BotcA and B were isolated and characterized from strains B05.10 and bcbot2Δ, respectively, as described previously (Moraga et al., 2011; Pinedo et al., 2008). Extensive spectroscopic analyses by 1H‐nuclear magnetic resonance (1H‐NMR) and 13C‐NMR were used to identify the various toxins in each fraction.
RNA extraction and cDNA synthesis
Mycelia collected from the different zones indicated above (Fig. S8b), or from T. arundinaceum grown in single cultures, were used for RNA extraction using Trizol reagent (Life Technologies, Carlsbad, CA, USA), following the manufacturer's instructions, treated with RNase‐free DNase and purified through Zymo‐Spin™ IC Columns (Zymo Research Corporation, Irvine, CA, USA).
cDNA was synthesized using 1 µg of total RNA and a reverse transcription system based on the use of an Oligo(dT)15 primer (Promega, Madison, WI, USA). cDNAs were quantified using a Nanodrop 2000 (Thermo Scientific, Wilmington, DE, USA).
Quantitative polymerase chain reaction (qPCR) experiments
In order to perform comparative studies, previously described oligonucleotides of the Ta37 tri, ergosterol biosynthetic and housekeeping genes were used (Cardoza et al., 2011). PCR primers for Ta37 genes encoding hydrolytic enzymes [chitinase 37 (chit37), chitinase 42 (chit42), serine endopeptidase (sprT), glycoside hydrolase (glyH), α‐mannosidase (gh92), aspartic protease (papA)] and oligopeptide transporter (ptr) were designed based on the genomic sequence data of the fungus. To obtain the data, approximately 0.5 g of mycelial growth of Ta37 was removed from the surface of a V‐8 juice agar medium (Tuite, 1969). Genomic DNA was purified from the grown mycelia using the Fungal/Bacterial DNA MiniPrep kit (Zymo Research Corporation), and genome sequencing was performed using the Ion Proton System (Life Technologies), as specified by the manufacturers. The resulting sequence reads were trimmed and assembled using the program CLC Genomics Workbench. Sequences of the T. harzianum orthologues of these genes were then used to query the resulting assembled genome sequence and thereby recover the sequences of the Ta37 orthologue genes. Oligonucleotide pairs designed from the genomic sequences of the chit37, chit42, sprT, glyH, gh92, papA and ptr genes, and the amplification efficiencies, are included in Table S1 (see Supporting Information). According to GeNorm software (Vandesompele et al., 2002) results, the α‐actin and glyceraldehyde‐3‐phosphate dehydrogenase (gpd) genes were used as Ta37 endogenous controls. The qPCRs and comparative analyses were carried out as described previously (Cardoza et al., 2011; Pfaffl et al., 2002). Each measurement was made in triplicate using cDNA pooled from three biological replications.
Statistical analysis
Kruskal–Wallis tests were performed with IBM SSPS Statistics 19 Software (www.ask.com/IBM+Spss+Statistics+19).
Sequence accession numbers
Sequences of genes encoding Ta37 hydrolytic enzymes have been deposited in the GenBank database. Accession numbers are as follows: LN890267, chit37; LN890268, chi42; LN890269, sprT; LN890270, papA; LN890272, gh92.
Conflict of Interest
The authors declare no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Chemical structures of secondary metabolites (SMs) produced by Trichoderma arundinaceum IBT 40837 (harzianum A) and Botrytis cinerea (botrydial and botcinins A and B) (a) and a schematic representation of the Trichoderma terpene pathway (b).
Fig. S2 Relative expression levels of tri genes in the non‐interaction zone compared with the expression levels in the interaction zone of Ta37 cultures confronted with B05.10 (a), bcbot2Δ (b) or bcbot2Δ + 1.5 μg/mL botrydial (BOT) (c).
Fig. S3 Analysis of tri gene expression levels in Ta37 growing alone compared with the level of expression of the α‐actin gene.
Fig. S4 Analysis of terpene/ergosterol biosynthetic gene expression levels in Ta37 mycelia isolated from the interaction zone of Ta37–B05.10 dual cultures supplemented with 50 μg/mL botcinin A (BotcA) or 50 μg/mL botcinin B (BotcB) versus the level of expression of these genes in Ta37 growing alone.
Fig. S5 Synopsis of the most remarkable phenomena observed in the Ta37–bcbot2Δ dual cultures supplemented with 1.5 μg/mL botrydial (BOT).
Fig. S6 Analysis of the relative expression levels of chit42, sprT, glyH, gh92, papA and ptr in Ta37 mycelia isolated from the interaction zone versus the expression level in Ta37 mycelia isolated from the non‐interaction zone with B05.10.
Fig. S7 Synopsis of the most remarkable phenomena observed in the Ta37–bcbot2Δ dual cultures.
Fig. S8 Photographs illustrating a Trichoderma arundinaceum–Botrytis cinerea dual culture, the different zones sampled for quantitative polymerase chain reaction (qPCR) analysis and the agar plugs that were collected to extract and quantify harzianum A (HA).
Table S1 Sequences of the oligonucleotides used to analyse the expression of Ta37 genes encoding hydrolytic enzymes.
Acknowledgements
Funding was obtained from the Junta de Castilla y León (SA260A11‐2, LE125A12‐2 and LE228U14) and Spanish Government grants MICINN‐AGL2009‐13431‐C02‐02, MINECO‐AGL2012‐40041‐C02‐01, AGL2012‐40041‐C02‐02 and AGL2012‐39798‐C02‐01. MGM and II‐B were granted fellowships from the Spanish Ministry of Science and Innovation (AP2007‐02835, BES‐2013‐063411). We thank Dr Muriel Viaud (INRA, France) for providing an isolate of the Botrytis cinerea B05.10 cnd15 mutant.
We thank José Álvarez (University of León, Spain) and Marcie Moore and Nathane Orwing (National Center for Agricultural Utilization Research, Peoria, IL, USA) for their excellent technical assistance.
References
- Alexander, N.J. , McCormick, S.P. and Hohn, T.M. (1999) TRI12, a trichothecene efflux pump from Fusarium sporotrichioides: gene isolation and expression in yeast. Mol. Gen. Genet. 26, 977–984. [DOI] [PubMed] [Google Scholar]
- Atanasova, L. , Le Crom, S. , Gurber, S. , Coulpier, D. , Seidl‐Seiboth, V. , Kubicek, C.P. and Druzhinina, E.S. (2013) Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genomics, 14, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann, S. , Funk, A.N. , Scherlach, K. , Schroeckh, V. , Shelest, E. , Horn, U. , Hertweck, C. and Brakhage, A.A. (2010). Activation of a silent fungal polyketide biosynthesis pathway through regulatory cross talk with a cryptic nonribosomal peptide synthetase gene cluster. Appl . Envrion. Microbiol. 76, 8143–8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakhage A.A. (2013) Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 11, 21–32. [DOI] [PubMed] [Google Scholar]
- Bruns, W. , Horns, S. and Redlich, H. (1995) Botrylactone synthesis and structure revision: a convergent approach from D‐glucose. Synthesis, 3, 335–342. [Google Scholar]
- Cantu, D. , Carl Greve, L. , Labavitch, J.M. and Powell, A.L.T. (2009) Characterization of the cell wall of the ubiquitous plant pathogen Botrytis cinerea . Mycol. Res. 113, 1396–1403. [DOI] [PubMed] [Google Scholar]
- Cardoza, R.E. , Malmierca, M.G. , Hermosa, M.R. , Alexander, N.J. , McCormick, S.P. , Proctor, R.H. , Tijerino, A.M. , Rumbero, A. , Monte, E. and Gutiérrez, S. (2011) Identification of loci and functional characterization of trichothecene biosynthetic genes in the filamentous fungus Trichoderma . Appl. Environ. Microbiol. 77, 4867–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquer, M. , Fournier, E. , Kunz, C. , Levis, C. , Pradier, J.M. , Simon, A. and Viaud, M. (2007). Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 277, 1–10. [DOI] [PubMed] [Google Scholar]
- Collado, I.G. , Sánchez, A.J.M. and Hanson, J.R. (2007) Fungal terpene metabolites: biosynthetic relationships and the control of the phytopathogenic fungus Botrytis cinerea . Nat. Prod. Rep. 24, 674–686. [DOI] [PubMed] [Google Scholar]
- Colmenares, A.J. , Aleu, J. , Duran‐Patron, R. , Collado, I.G. and Hernandez‐Galan, R. (2002) The putative role of botrydial and related metabolites in the infection mechanism of Botrytis cinerea . J. Chem. Ecol. 28, 997–1005. [DOI] [PubMed] [Google Scholar]
- Deighton, N. , Muckenschnabel, I. , Colmenares, A.J. , Collado, I.G. and Williamson, B. (2001) Botrydial is produced in plant tissues infected by Botrytis cinerea . Phytochemistry, 57, 689–692. [DOI] [PubMed] [Google Scholar]
- De Meyer, G. , Bigirimana, J. , Elad, Y. and Höfte, M. (1998) Induced systemic resistance in Trichoderma harzianum T39 biocontrol of Botrytis cinerea . Eur. J. Plant Pathol. 104, 279–286. [Google Scholar]
- Druzhinina, I.S. , Seidl‐Seiboth, V. , Herrera‐Estrella, A. , Horwitz, B.A. , Kenerley, C.M. , Monte, E. , Mukherjee, P.K. , Zeilinger, S. , Grigoriev, I.V. and Kubicek, C.P. (2011) Trichoderma: the genomics of opportunistic success. Nat. Rev. Microbiol. 9, 749–759. [DOI] [PubMed] [Google Scholar]
- Elad, Y. and Stewart, A. (2004) Microbial control of Botrytis spp In: Botrytis: Biology, Pathology and Control (Elad Y., Williamson B., Tudzynski P. and Delen N., eds), pp. 223–241. Dordrecht: Kluwer Academic. [Google Scholar]
- Harman, G.E. , Latorre, B. , Agosin, E. , San Martin, R. , Riegel, D.G. , Nielsen, P.A. , Tronsmo, A. and Pearson, R.C. (1996) Biological and integrated control of Botrytis bunch rot of grape using Trichoderma spp. Biol. Control, 7, 259–266. [Google Scholar]
- Hermosa, H , Cardoza, R.E. , Rubio, M.B. , Gutiérrez, S. and Monte, E. (2014) Secondary metabolism and antimicrobial metabolites of Trichoderma In: Biotechnology and Biology of Trichoderma (Gupta V.K., Schmoll M., Herrera‐Estrella A., Upadhyay R.S., Druzhinina I. and Tuohy M., eds), Chapter 10, pp. 125–137. Amsterdam, Elsevier. [Google Scholar]
- Hermosa, R. , Viterbo, A. , Chet, I. and Monte, E. (2012) Plant‐beneficial effects of Trichoderma and of its genes. Microbiology, 158, 17–25. [DOI] [PubMed] [Google Scholar]
- Hohn, T.M. , Krishna, R. and Proctor, R.H. (1999) Characterization of a transcriptional activator controlling trichothecene toxin bosynthesis. Fungal Genet. Biol. 26, 224–235. [DOI] [PubMed] [Google Scholar]
- Howell, C.R. ( 2003) Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis. 87, 4–10. [DOI] [PubMed] [Google Scholar]
- Hynes, J. , Müller, C.T. , Jones, T.H. and Boddy, L. (2007) Changes in volatile production during the course of fungal mycelia interactions between Hypholoma fasciculare and Resinicium bicolor . J. Chem. Ecol. 33, 43–57. [DOI] [PubMed] [Google Scholar]
- Lorito, M. , Woo, S.L. , Harman, G.E. and Monte, E. (2010) Translational research on Trichoderma: from 'omics to the field. Annu. Rev. Phytopathol. 48, 395–417. [DOI] [PubMed] [Google Scholar]
- Malmierca, M.G. , Cardoza, R.E. , Alexander, N.J. , McCormick, S.P. , Hermosa, R. , Monte, E. and Gutiérrez, S. (2012) Involvement of Trichoderma trichothecenes in the biocontrol activity and induction of plant defense‐related genes. Appl . Environ. Microbiol. 78, 4856–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca, M.G. , Cardoza, R.E. , Alexander, N.J. , McCormick, S.P. , Collado, I.G. , Hermosa, R. , Monte, E. and Gutiérrez, S. (2013) Relevance of trichothecenes in the fungal physiology: disruption of tri5 gene in Trichoderma arundinaceum . Fungal Genet. Biol. 53, 22–33. [DOI] [PubMed] [Google Scholar]
- Malmierca, M.G. , Barua, J. , McCormick, S.P. , Izquierdo‐Bueno, I. , Cardoza, R.E. , Alexander, N.J. , Hermosa, R. , Collado, I.G. , Monte, E. and Gutiérrez, S. (2015) Novel aspinolide production by Trichoderma arundinaceum with a potential role in Botrytis cinerea antagonistic activity and plant defence priming. Environ. Microbiol. 17, 1103–1118 [DOI] [PubMed] [Google Scholar]
- Massaroli, M. , Moraga, J. , Bastos‐Borges, K. , Ramírez‐Fernández, J. , Viaud, M. , Gollado, I.G. , Durán‐Patrón, R. and Hernández‐Galán, R. (2013) A shared biosynthetic pathway for botcinins and botrylactones revealed through gene deletions. ChemBioChem, 14, 132–136. [DOI] [PubMed] [Google Scholar]
- Moraga, J. , Pinedo, C. , Durán‐Patrón, R , Collado, I.G. and Hernández‐Galán, R. (2011) Botrylactone: new interest in an old molecule—review of its absolute configuration and related compounds. Tetrahedron, 67, 417–420. [Google Scholar]
- Nakajima, M. and Akutsu, K. (2014) Virulence factors of Botrytis cinerea . J. Gen. Plant Pathol. 80, 15–23. [Google Scholar]
- O'Keeffe, G. , Hammel, S. , Owens, R.A. , Keane, T.M. , Fizpatrick, S.A. , Jones, G.W. and Doyle, S. (2014). RNA‐seq reveals the pan‐transcriptomic impact of attenuating the gliotoxin self‐protection mechanism in Aspergillus fumigatus . BMC Genomics, 15, 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris, D. , Dunn, W.B. , Brown, M. , Kell, D.B. , Roy, I. and Hedger, J.N. (2008) Metabolite profiles of interacting mycelia fronts differ for pairings of the wood decay basidiomycete fungus, Stereum hirsutum with its competitors Coprinus micaceus and Coprinus disseminatus . Metabolomics, 4, 52–62. [Google Scholar]
- Peplow, A.W. , Tag, A.G. , Garifullina, G.F. and Beremand, M.N. (2003) Identification of new genes positively regulated by Tri10 and a regulatory network for trichothecene mycotoxin production. Appl. Envrion. Microbiol. 69, 2731–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M.W. , Horgan, G.W. and Dempfle, L. (2002) Relative expression software tool (REST) for group‐wise comparison and statistical analysis of relative expression results in real‐time PCR. Nucleic Acids Res. 30, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinedo, C. , Wang, C.M. , Pradier, J.M. , Dalmais, B. , Choquer, M. , Le Pêcheur, P. , Morgant, G. , Collado, I.G. , Cane, D.E. and Viaud, M. (2008) Sesquiterpene synthase from the botrydial biosynthetic gene cluster of the phytopathogen Botrytis cinerea . ACS Chem. Biol. 3, 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quidde, T. , Osbourn, A.E. and Tudzynski, P. (1998) Detoxification of α‐tomatine by Botrytis cinerea . Physiol. Mol. Plant Pathol. 52, 151–165. [Google Scholar]
- Quidde, T. , Büttner, P. and Tudzynski, P. (1999) Evidence for three different specific saponin‐deoxifying activities in Botrytis cinerea and cloning and functional analysis of a gene coding for a putative avenacinase. Eur. J. Plant Pathol. 105, 273–283. [Google Scholar]
- Rodríguez‐Estrada, A.E. , Hegeman, A. , Kistler, H.C. and May, G. (2011) In vitro interactions between Fusarium verticilloides and Ustilago maydis through real‐time PCR and metabolic profiling. Fungal Genet. Biol. 48, 874–885. [DOI] [PubMed] [Google Scholar]
- Steindorff, A.S. , Ramada, M. H. S. , Coelho, A. S. G. , Miller, R. N. G. , Pappas, G. J. Jr. , Uhloa, C. J. and Noronha, E.F. (2014) Identification of mycoparasitism‐related genes against the phytopathogen Sclerotinia sclerotiorum through transcriptome and expression profile analysis in Trichoderma harzianum . BMC Genomics, 15, 204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tag, A.G. , Garifullina, G.F. , Peplow, A.W. , Ake, C. , Phillips, T.D. , Hohn, T.M. and Beremand, M.N. (2001) A novel regulatory gene, Tri10, controls trichothecene toxin production and gene expression. Appl. Environ. Microbiol. 67, 5294–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani, H. , Koshino, H. , Sakuno, E. and Nakajima, H. (2005) Botcinins A, B, C, and D, metabolites produced by Botrytis cinerea, and their antifungal activity against Magnaporthe grisea, a pathogen of rice blast disease. J. Nat. Prod. 68, 1768–1772. [DOI] [PubMed] [Google Scholar]
- Tuite, J.F. (1969) Plant Pathological Methods: Fungi and Bacteria. Minneapolis, MN: Burgess Publishing Company, University of Minnesota. [Google Scholar]
- Vandesompele, J. , De Preter, K. , Pattyn, F. , Poppe, B. , Van Roy, N. , De Paepe, A. and Speleman, F. (2002) Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, R34.1–R34.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaíno, J.A. , Cardoza, R.E. , Hauser, M. , Hermosa, R. , Rey, M. , Llobell, A. , Becker, J.M. , Gutiérrez, S. and Monte, E. (2006) ThPTR2, a di/tri‐peptide transporter gene from Trichoderma harzianum . Fungal Genet. Biol. 43, 234–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Chemical structures of secondary metabolites (SMs) produced by Trichoderma arundinaceum IBT 40837 (harzianum A) and Botrytis cinerea (botrydial and botcinins A and B) (a) and a schematic representation of the Trichoderma terpene pathway (b).
Fig. S2 Relative expression levels of tri genes in the non‐interaction zone compared with the expression levels in the interaction zone of Ta37 cultures confronted with B05.10 (a), bcbot2Δ (b) or bcbot2Δ + 1.5 μg/mL botrydial (BOT) (c).
Fig. S3 Analysis of tri gene expression levels in Ta37 growing alone compared with the level of expression of the α‐actin gene.
Fig. S4 Analysis of terpene/ergosterol biosynthetic gene expression levels in Ta37 mycelia isolated from the interaction zone of Ta37–B05.10 dual cultures supplemented with 50 μg/mL botcinin A (BotcA) or 50 μg/mL botcinin B (BotcB) versus the level of expression of these genes in Ta37 growing alone.
Fig. S5 Synopsis of the most remarkable phenomena observed in the Ta37–bcbot2Δ dual cultures supplemented with 1.5 μg/mL botrydial (BOT).
Fig. S6 Analysis of the relative expression levels of chit42, sprT, glyH, gh92, papA and ptr in Ta37 mycelia isolated from the interaction zone versus the expression level in Ta37 mycelia isolated from the non‐interaction zone with B05.10.
Fig. S7 Synopsis of the most remarkable phenomena observed in the Ta37–bcbot2Δ dual cultures.
Fig. S8 Photographs illustrating a Trichoderma arundinaceum–Botrytis cinerea dual culture, the different zones sampled for quantitative polymerase chain reaction (qPCR) analysis and the agar plugs that were collected to extract and quantify harzianum A (HA).
Table S1 Sequences of the oligonucleotides used to analyse the expression of Ta37 genes encoding hydrolytic enzymes.


