Summary
The sensing of stress signals and their transduction into appropriate responses are crucial for the adaptation, survival and infection of phytopathogenic fungi and oomycetes. Amongst evolutionarily conserved pathways, mitogen‐activated protein kinase (MAPK) cascades function as key signal transducers that use phosphorylation to convey information. In this study, we identified a gene, designated PsMPK7, one of 14 predicted genes encoding MAPKs in Phytophthora sojae. PsMPK7 was highly transcribed in each tested stage, but was up‐regulated in the zoospore, cyst and cyst germination stages. Silencing of PsMPK7 affected the growth of germinated cysts, oospore production and the pathogenicity of soybean. PsMPK7 transcription was induced by stresses from sorbitol, NaCl and hydrogen peroxide. Transformants in which PsMPK7 expression was silenced (PsMPK7‐silenced) were significantly more sensitive to osmotic and oxidative stress. Aniline blue and diaminobenzidine staining revealed that the silenced lines did not suppress the host reactive oxygen species (ROS) burst, indicating that either the inoculated plants activated stronger defence responses to the transformants and/or the PsMPK7‐silenced transformants failed to overcome plant defences. In addition, extracellular secretion of laccase decreased in the silenced lines. Overall, our results indicate that the PsMPK7 gene encodes a stress‐associated MAPK in P. sojae that is important not only for responses to various stresses, but also for ROS detoxification, cyst germination, sexual oospore production and infection of soybean.
Keywords: cyst germination, MAPK, oospore production, pathogenicity, Phytophthora sojae, stress‐activated protein kinase
Introduction
Phytophthora species are destructive plant pathogens that attack a wide range of ornamentally and agriculturally important plants. At least 116 pathogenic Phytophthora species are currently known, and new species, or new variants of known species, are still being discovered (Kroon et al., 2012). Although Phytophthora species exhibit a filamentous growth morphology that is very similar to that of many fungi, they are not fungi or even related to fungi. The genus Phytophthora belongs to the oomycete group in the Stramenopila kingdom, which constitutes a distinct, major branch of the eukaryotic evolutionary tree and comprises saprophytes and pathogens of plants, animals and insects (Baldauf, 2003; Latijnhouwers and Govers, 2003; Tyler, 2007). One representative plant pathogen is Phytophthora sojae, which is the causal agent of root and stem rot in soybean. Phytophthora sojae has been reported throughout most soybean‐growing regions globally, and causes plant stand reductions and even complete yield losses with damage valued at approximately $1–2 billion each year (Tyler, 2007; Wrather and Koenning, 2006).
In the field, the survival and dispersal of spores are essential for the success of many plant pathogens. Phytophthora sojae produces asexual spores called zoospores and sexual spores called oospores. Zoospores are the most important means of soybean infection, especially in flooded fields. The zoospores swim chemotactically towards compounds released by roots, including the isoflavones daidzen and genistein, and are then triggered to form adhesive cysts, which, in turn, germinate to produce hyphae or secondary zoospores. The hyphae penetrate the plant epidermis directly, but sometimes form appressoria for penetration (Enkerli et al., 1997; Tyler, 2007). The normal behaviour of zoospores has been demonstrated previously to be controlled by a G‐protein α subunit in P. sojae and Phytophthora infestans (Hua et al., 2008; Latijnhouwers et al., 2004). However, the molecular mechanisms of development that occur after zoospore cyst formation, especially germination and the formation of infectious hyphae that penetrate soybean cells, are still largely unknown. As a result of fragility, asexual zoospores are ineffective resting structures, but the thick‐walled and durable sexual oospores, which can survive in inhospitable environments, such as freezing or desiccating conditions, act as resting structures. In the subsequent year, oospores germinate to produce either a hyphal tube to infect the soybean plant or a germ sporangium to release zoospores (Blanco and Judelson, 2005). In Phytophthora, although some developmental stage‐dependent genes have been identified that are active during sexual reproduction (Fabritius et al., 2002; Prakob and Judelson, 2007; Zhao et al., 2011), the signalling pathways governing sexual development are still unknown.
In plants, animals and other eukaryotic organisms, a family of serine/threonine protein kinases, known as mitogen‐activated protein kinases (MAPKs), is involved in the transduction of a variety of extracellular signals and in the regulation of growth and developmental processes. Accumulating evidence has implicated the important roles of MAPK signalling pathways in the control of spore development, infection and sexual reproduction in fungi. For example, the budding yeast Saccharomyces cerevisiae has five MAPK pathways that are involved in sporulation, osmoregulation, cell wall integrity, pheromone response and filamentation (Chen and Thorner, 2007). MAPKs that specifically transmit environmental stress signals are known as stress‐activated protein kinases (SAPKs). The SAPKs have been well studied in fungi and mammalian systems, and are responsible for responses to a wide range of stresses, such as heat shock, hyperosmolarity, ultraviolet (UV) light irradiation and oxidative stress (Degols et al., 1996; Hohmann, 2002; Kato et al., 1996; Kyriakis and Avruch, 1996; Millar et al., 1995; Shiozaki and Russell, 1995). SAPK gene‐silenced transformants of phytopathogenic fungi exhibit impaired osmoregulation and stress responses. For example, Osm1 of Magnaporthe oryzae controls the accumulation of arabitol in response to hyperosmolarity in hyphae (Dixon et al., 1999); MgHog1 of Mycosphaerella graminicola controls pathogenicity, tolerance to osmosensitivity and the transition from yeast‐like growth to filamentous growth (Mehrabi et al., 2006). SRM1 (a Hog1‐type MAPK homologue) of Bipolaris oryzae controls tolerance to hyperosmolarity, hydrogen peroxide (H2O2) and UV exposure (Moriwaki et al., 2006).
Similar to the phytopathogenic fungi, successful infection by oomycetes depends on their ability to overcome defence responses in their hosts, and therefore MAPK signalling pathways are likely to play similar roles in the eukaryotic oomycete plant pathogens. However, because the oomycetes are distantly related and evolutionarily distinct from fungi, major homologues of fungal MAPK genes are difficult to identify in Phytophthora using sequence‐based bioinformatics search methods. In our previous work, we identified 14 MAPK gene candidates in P. sojae (Ye et al., unpublished data) and characterized two of them. PsMPK1 is involved in zoospore reproduction, cell wall integrity and pathogenicity (Li et al., 2014), and PsMPK3 (or PsSAK1) is involved in premature encystment, germination and penetration (Li et al., 2010). In this study, we characterized another P. sojae MAPK gene, designated PsMPK7, which was transcriptionally up‐regulated in the zoospore, cyst and cyst germination stages, and induced by osmotic and oxidative stresses mediated by sorbitol, NaCl and H2O2. In addition, P. sojae transformants in which PsMPK7 expression was silenced were more sensitive to environmental stresses and exhibited abnormal growth during cyst germination and reduced virulence to soybean.
Results
PsMPK7 gene encodes a MAPK
We identified a MAPK gene from P. sojae, designated PsMPK7 (http://www.jgi.doe.gov; JGI v1.1, protein ID: 129213). The corrected PsMPK7 gene model is 4185 bp in length, intronless and encodes a protein of 1394 amino acids [scaffold_7:138979–143163(‐); Fig. S1, see Supporting Information]. PsMPK7 contains a typical serine/threonine protein kinase catalytic (STKc) MAPK domain (E = 5.74e‐172; amino acids 1008–1334) and a WW domain (E = 4.58e‐03; amino acids 1360–1390) which were predicted using the CD‐Search program at the National Center for Biotechnology Information (NCBI) website (Marchler‐Bauer et al., 2011). The PsMPK7 full‐length protein is highly conserved with its orthologues in Phytophthora ramorum (87%), Phytophthora parasitica (88%), Phytophthora capsici (88%) and Hyaloperonospora arabidopsidis (81%). PsMPK7 and its orthologues have a predicted dual phosphorylation lip sequence TEY (amino acids 1168–1170; Fig. S2, see Supporting Information).
PsMPK7 transcripts were abundant in various developmental and infection stages, and were up‐regulated twofold in the zoospore and cyst stages and more than fivefold during cyst germination, when compared with mycelia (Fig. 1A). A consistent transcription pattern was found from the published Digital Gene Expression profiling data (Ye et al., 2011), although the strong up‐regulation during the germinating cyst stage was not detected (Fig. S3, see Supporting Information). This difference may be a result of differences in technologies or samples. However, the stage‐specific up‐regulation suggested that PsMPK7 may play important roles in the physiological processes of zoospores and cysts, and during cyst germination.
Figure 1.
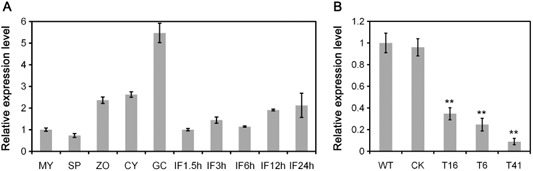
PsMPK7 gene expression. (A) PsMPK7 expression was profiled using quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) throughout the Phytophthora sojae life cycle, including vegetative mycelia (MY), sporulating hyphae (SP), zoospores (ZO), cysts (CY), cyst germination (GC) and during the early stages of infection, including 1.5, 3, 6, 12 and 24 h post‐inoculation (hpi) of the susceptible soybean cv. Williams. (B) Relative gene expression levels of the control strain (CK) and PsMPK7‐silenced transformants (T16, T6 and T41) using the wild‐type (WT) as a reference (value, 1.0). The qRT‐PCR assays were performed using cDNAs synthesized from mycelial RNAs. Expression of the P. sojae actinA gene was used as a constitutively expressed endogenous gene control, and the level of PsMPK7 expression was determined relative to that of actinA. Three biological replicates, each containing three technical replicates for each sample, were performed. Statistical significance was analysed using Student's t‐test between the WT/CK and WT/transformant comparisons (**P < 0.01).
Association of PsMPK7 with P. sojae pathogenicity
To determine whether PsMPK7 was required for P. sojae pathogenicity, stable PsMPK7‐silenced strains of P. sojae (P6497) were generated using a polyethylene glycol (PEG) transformation‐mediated gene silencing method (see Experimental procedures). We obtained three PsMPK7‐silenced lines, T16, T6 and T41, in which PsMPK7 expression was reduced by 65%, 75% and 91%, respectively, compared with the wild‐type (WT) strain, whereas PsMPK7 expression was not affected in the control strain [CK; green fluorescent protein (GFP)‐expressing transformant; see Experimental procedures] (Fig. 1B).
To examine the roles of PsMPK7 in P. sojae pathogenicity in its native host (soybean), inoculation assays were performed by applying zoospore suspensions of WT, CK and the three silenced transformants (T16, T6 and T41) onto etiolated seedlings of the soybean cultivar Hefeng 47. At 2 days post‐inoculation (dpi), the hypocotyls of the etiolated seedlings inoculated with WT and CK zoospores showed typical disease symptoms and water‐soaked lesions (Fig. 2). In contrast, the three silenced transformants produced almost no lesions or very small lesions that did not expand beyond the inoculation site (Fig. 2). To determine whether the pathogenicity defect was associated with penetration, the hypocotyls of etiolated seedlings were wounded with a pipette tip prior to inoculation with the zoospore suspensions. At 2 dpi under the same conditions, disease symptoms did not expand on the hypocotyls inoculated with zoospores of the silenced transformants (Fig. 2). However, the hypocotyls of the etiolated seedlings inoculated with WT and CK zoospores showed typical disease symptoms. These results indicated that PsMPK7 was associated with P. sojae pathogenicity in soybean and that the loss of pathogenicity was not caused directly by an impaired ability to penetrate.
Figure 2.
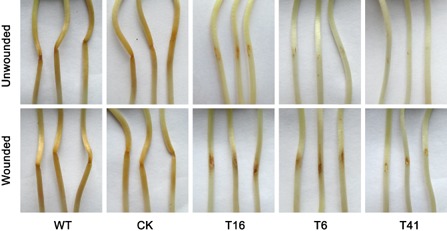
Pathogenicity assay performed on etiolated soybean seedlings. Unwounded and wounded etiolated soybean seedlings (Hefeng 47) were inoculated with zoospores [approximately 100 in 10 μL for wild‐type (WT) and control strain (CK) zoospores, and 150 for transformant zoospores). Images were taken after 48 h, and the experiments were repeated at least three times.
PsMPK7 is required for reactive oxygen species (ROS) detoxification
Plant defence responses play important roles during plant—microbe interactions. The recognition of microbe‐associated molecules by plant immune systems causes reinforcement of the cell wall (e.g. callose deposition), the accumulation of pathogenesis‐related (PR) proteins and rapid generation of ROS in the infected cell (Abramovitch et al., 2003; Bradley et al., 1992; Levine et al., 1994; Tanaka et al., 2006; Torres and Dangl, 2005). Callose deposition was observed at the infection sites of soybean epidermal cells at 16 h post‐inoculation (hpi) in the transformants. It was normal and similar to that of WT (Fig. 3A). ROS accumulation at the infection site, also known as the oxidative burst, is regarded as one of the earliest events during incompatible interactions between plants and pathogens (Apostolet et al., 1989; Yoshioka et al., 2008). Thus, we examined host‐derived ROS levels by staining with 3,3′‐diaminobenzidine (DAB) at 10 hpi. After DAB staining, a limited reddish‐brown precipitate was observed near the infection site of WT P. sojae. However, increased amounts of reddish‐brown precipitate were observed in soybean cells around the infection site of PsMPK7‐silenced transformants (Fig. 3B), indicating that the PsMPK7‐silenced transformants failed to scavenge ROS that accumulated at the infection site.
Figure 3.
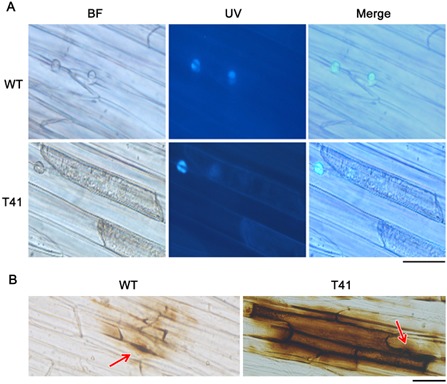
PsMPK7‐silenced transformants did not suppress host callose deposition or the reactive oxygen species (ROS) burst. (A) Etiolated soybean seedlings were infected with zoospores and stained with aniline blue after 16 h. The epidermal cells were peeled for microscopic observation. Callose in the host tissues beneath the zoospore penetration sites was seen as fluorescent solid circular shapes (see the corresponding light microscopic fields). Bar represents 50 μm. (B) 3,3′‐Diaminobenzidine staining of soybean seedlings infected by PsMPK7‐silenced transformants and the wild‐type (WT) after 10 h. Epidermal cells were peeled for microscopic observation. The penetration sites are indicated by red arrows. Bar represents 50 μm.
PsMPK7 plays a role in cyst germination
Because increased PsMPK7 transcript levels were detected during the zoospore, cyst and cyst germination stages, and these stages are the primary steps for host infection by Phytophthora pathogens, we examined the role of PsMPK7 in cyst germination. Zoospore suspensions of the three transformants, WT and CK were centrifuged to induce encystment, and then incubated in 1% V8 liquid at 25 °C for 48 h. Although we found no differences in the formation or morphology of sporangia, or in the development, release or encystment of zoospores, abnormal germinated cysts were found in the three transformants. As shown in Fig. 4A, the abnormal germinated cysts revealed apical swelling, indicating that polarized growth might be affected. The rates of abnormal cysts ranged from approximately 15% to 23%, which were significantly (P < 0.01) higher than the values of 3% and 4% for WT and CK, respectively (Fig. 4B). Although the germination of cysts was impaired by PsMPK7 silencing, the colony growth rates of PsMPK7‐silenced transformants were not affected and remained similar to those of WT and CK (Fig. 5A).
Figure 4.
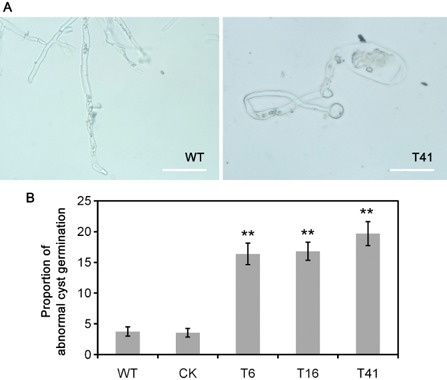
Abnormal cyst germination of PsMPK7‐silenced transformants. (A) Zoospores of the wild‐type (WT0 and the PsMPK7‐silenced transformant T41 were encysted by vortexing for 90 s, and the cysts were incubated in clarified 1% V8 liquid medium at 25 °C and stained in 0.3 mm nitroblue tetrazolium chloride (NBT) aqueous solution for 20 min after 48 h. Three independent experiments were performed and yielded similar results. Representative images are presented. Bars represent 50 μm. (B) The proportions of abnormally germinated cysts (cysts that did not grow) were counted at 48 h under a microscope in three independent experiments. CK, control strain.
Figure 5.
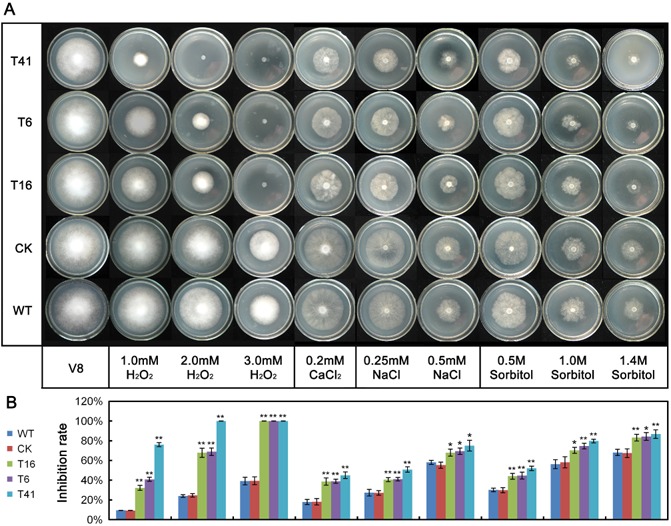
Assay of mycelial growth under different stress conditions. (A) Assay of mycelial growth of wild‐type (WT), control strain (CK) and PsMPK7‐silenced transformants on V8 agar medium only or supplemented with 1, 2 or 3 mm H2O2, 0.2 m CaCl2, 0.25 or 0.5 m NaCl and 0.5, 1.0 or 1.4 m sorbitol. (B) Colony diameters were measured in each independent biological experiment after 5.5 days of growth. Rates of growth inhibition were calculated for each treatment relative to growth on V8 agar medium only. Three independent biological experiments were performed with three replicates each. Error bars represent the standard deviation and asterisks denote significant differences between the PsMPK7‐silenced transformants and the WT (**P < 0.01; *P < 0.05).
PsMPK7 and oxidative, osmotic and salt stress tolerance
To determine whether PsMAPK7 was a stress‐associated MAPK, its expression was investigated under oxidative, osmotic and salt stress conditions using H2O2 (1.0, 2.0 and 3.0 mm), sorbitol (1.0 m) and NaCl (0.25 m) treatments, respectively. PsMAPK7 expression increased significantly (P < 0.01) by approximately twofold in response to sorbitol and NaCl. PsMAPK7 expression also increased in response to the various concentrations of H2O2 and, although not significant, the increases were positively correlated with the increasing H2O2 concentrations (Fig. S4, see Supporting Information).
The PsMPK7‐silenced lines, T16, T6 and T41, and the WT and CK lines were cultured on V8 agar medium supplemented with different concentrations of H2O2, sorbitol, NaCl or CaCl2 (Fig. 5A). All of the PsMPK7‐silenced transformants exhibited significantly (P < 0.01) higher inhibition of growth rates than WT and CK at 1.0 mm H2O2, and did not grow at 2.0 or 3.0 mm H2O2, whereas the WT and CK lines survived (Fig. 5A, B). In addition, under the stresses of NaCl, CaCl2 and sorbitol, the PsMPK7 transformants showed significantly (P < 0.01 or P < 0.05) higher rates of growth inhibition than WT or CK (Fig. 5A, B). These results indicated that the function of PsMPK7 was related to osmoregulation and tolerance to various stresses.
Requirement of PsMPK7 for oospore production
To analyse the involvement of PsMPK7 in sexual reproduction, the oospore development of the silenced transformants was examined by microscopy. As shown in Fig. 6A, oospore production was reduced markedly after growth for 30 days on lima bean agar (LBA) medium in the PsMPK7‐silenced transformants. The average numbers of oospores in each 1‐cm2 zone adjacent to the inoculation site declined from more than 670 in WT and 660 in CK to no more than 32 in the PsMPK7‐silenced transformants (T16, 32; T6, 23; T41, 17; P < 0.01; Fig. 6A, B). In addition, many more oospores produced by the transformants were abnormal (60%) than were those produced by WT and CK (both 5%; P < 0.01; Fig. 6A, C).
Figure 6.
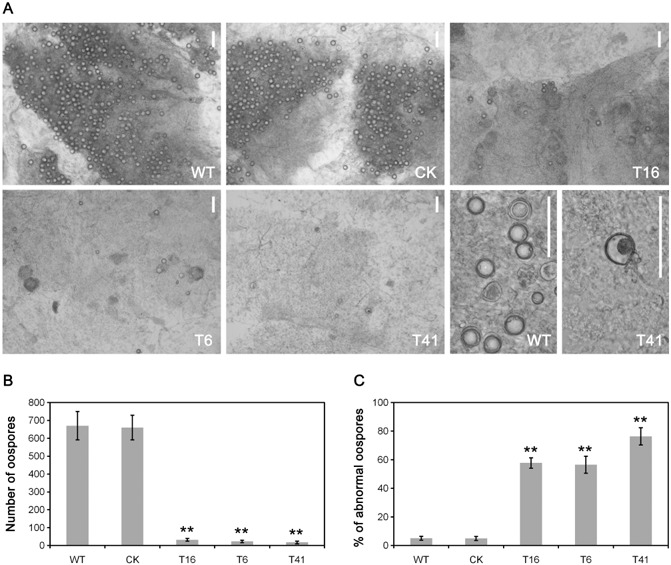
Oospores of PsMPK7‐silenced transformants. (A) Microscopic observation of oospore production for wild‐type (WT), control strain (CK) and PsMPK7‐silenced transformant strains (T6, T16 and T41). All strains were grown for 30 days on lima bean agar (LBA) medium to induce oospore formation. Bars represent 100 μm. (B) Mean values of oospores per square centimetre around the inoculation site. Data represent three independent experiments in triplicate. **P < 0.01. (C) Abnormal oospores were counted under the microscope. The ratios of abnormal oospores to the total number of oospores were calculated. The data represent three independent experiments in triplicate. **P < 0.01.
Silencing of PsMPK7 expression decreases extracellular laccase activity
We examined the secretion of extracellular enzymes to learn more about the effects of PsMPK7 silencing. The activity of the laccase enzyme was measured because laccase is a type of secreted extracellular protein that is widely distributed in plants and fungi. The secreted laccase activity of WT, CK and the transformants grown on solid lima bean medium was measured using 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid) (ABTS). Significantly decreased laccase activity was observed for the three transformants when compared with WT and CK (Fig. S5A, see Supporting Information). Transcription levels of six predicted signal peptide‐containing laccase genes (IDs: 137983, 137984, 137978, 137979, 137990 and 129781) were then measured in WT and T41 at the mycelial stage. The transcripts of all six genes were significantly reduced in T41 when compared with WT (Fig. S5B). Hence, the silencing of PsMPK7 expression affected the transcription of laccase genes and led to the decrease in extracellular laccase activity. Whether PsMPK7 silencing broke the secretion pathway is still unknown.
Discussion
In this study, we identified and functionally characterized a putative P. sojae MAPK gene, PsMPK7. The PsMPK7 gene was transcribed constitutively throughout the P. sojae life cycle, with the highest expression levels occurring during the zoospore, cyst and cyst germination stages. Silencing of PsMPK7 expression in P. sojae affected cyst germination and oospore production, and failed to scavenge ROS that accumulated at the infection site. PsMPK7‐silenced transformants lost pathogenicity to soybean, but this was not a direct result of impaired penetration ability. Gene expression profiling and colony growth rate assays indicated that PsMPK7 is a stress‐associated MAPK that contributes to the tolerance to various stresses.
The ability of fungi to sense and respond to environmental stress is critical for their survival. In the fission yeast Schizosaccharomyces pombe, the MAPK Sty1, in combination with the transcription factor Yap1, regulates genes in response to oxidative stress (D'Autreaux and Toledano, 2007), and the Sty1 regulatory pathway is conserved amongst other fungi, such as Colletotrichum lagenarium, Botrytis cinerea and Fusarium graminearum (Kojima et al., 2004; Segmuller et al., 2007; Zheng et al., 2012). However, oomycetes are distantly related and evolutionarily distinct from fungi, which makes the identification of any Yap1 or Sty1 homologues in P. sojae using sequence‐based bioinformatics search methods difficult. In addition, all of the 14 predicted MAPKs in P. sojae lack the dual phosphorylation lip sequence TGY, which is a hallmark of SAPKs in fungal and mammalian systems (Hamel et al., 2012). In this study, PsMPK7‐silenced lines that exhibited increased sensitivity to oxidative, osmotic and salt stresses were very similar to the phenotype of the Sty1 mutant in S. pombe (Toone and Jones, 1998), demonstrating that the sensing and response to varied environmental stresses are critical for the survival and pathogenicity of oomycete pathogens, and that one or more MAPK pathways may also function in these processes.
The Sty1 homology pathway varies significantly among different fungal pathogens for plant infection. It is essential for pathogenicity in F. graminearum (Zheng et al., 2012), M. graminicola (Mehrabi et al., 2006), B. cinerea (Segmuller et al., 2007), Cryphonectria parasitica (Park et al., 2004), Alternaria alternata (Lin and Chung, 2010), Cochliobolus heterostrophus (Igbaria et al., 2008) and Alternaria brassicicola (Joubert et al., 2011), whereas it is dispensable in Magnaporthe oryzae, Colletotrichum lagenarium (Kojima et al., 2004) and Bipolaris oryzae (Moriwaki et al., 2006). We found that the virulence of the PsMPK7 mutants was lost regardless of unwounding or wounding, indicating that the wound does not help the mutant to penetrate soybean tissue, and PsMPK7 plays a critical role in pathogenesis.
During plant interactions with fungi or oomycetes, plants use many different defence mechanisms to protect themselves from the pathogens. The host plant cellular environment may act as one of the first major challenges to pathogens (Kim et al., 2009). Fungi or oomycetes require adaptive mechanisms to survive. We showed that the PsMPK7‐silenced transformants were more sensitive to H2O2, salt and hyperosmotic stress, and DAB staining indicated that higher levels of H2O2 accumulated in soybean cells around the PsMPK7‐silenced transformant infection sites, suggesting reasons why the PsMPK7‐silenced transformants lost pathogenicity. Thus, we speculate that the loss of pathogenicity of the transformants may be caused by a lost or severely diminished ability to degrade H2O2 and/or reductions in other stress tolerance capabilities during infection. ROS production may function as a second messenger to induce various plant defence responses, and is essential for pathogen‐associated molecular pattern (PAMP)‐triggered immunity (PTI) in plants (Gan et al., 2009; Nurnberger et al., 2004; Torres and Dangl, 2005; Zhang et al., 2009). Callose is another plant defensive compound known to be involved in cell wall appositions, and is deposited during both compatible and incompatible interactions (Huckelhoven, 2007). However, PsMPK7 transformants induced normal callose deposition. The silencing of PsMPK7 expression affected the transcription of laccase genes and resulted in decreasing extracellular laccase activity. However, whether PsMPK7 silencing broke the secretion pathway is still unknown. If so, the secretion, or even production, of effector proteins to suppress plant defence responses might also be affected. This is another possibility to explain the lost pathogenicity of PsMPK7‐silenced transformants.
This is the first study to report that MAPK gene‐silenced Phytophthora transformants display abnormal (possibly polar) growth during cyst germination. A nitroblue tetrazolium chloride (NBT) staining experiment demonstrated that ROS accumulation was high in the transformants (Fig. 3A), which may explain why the zoospores could not mature. In contrast, during sexual development, oospores produced by the transformants were critically reduced, similar to observations in the mutants that lack Sty1 in F. graminearum (Zheng et al., 2012) and Aspergillus nidulans (Kawasaki et al., 2002). In addition, a large proportion of the transformant‐produced oospores were abnormal. Whether the loss of pathogenicity was a direct or indirect effect of PsMPK7 silencing remains unclear. Nevertheless, we have demonstrated that PsMPK7 is a stress‐associated protein kinase in P. sojae that plays a role in various important physiological processes.
Experimental Procedures
Phytophthora sojae strains and culture conditions
The P. sojae genome sequencing strain P6497 (Race 2) was provided by Professor Brett Tyler (Oregon State University, Corvallis, OR, USA). The WT strain and silenced transformants were grown routinely on 10% V8 medium at 25 °C in darkness (Erwin and Ribiero, 1996). To test mycelial growth, the strains were subcultured twice, and a 5‐mm‐diameter agar disc was removed from the edge of an actively growing culture and transferred to the centre of the plate. Oospores were obtained after growth for 30 days on LBA medium at 25 °C in darkness. For use in an extracellular laccase assay, strains were cultured on LBA medium containing 0.8 mm ABTS, which is commonly used with H2O2 as a substrate for laccase. To test the expression of PsMPK7 under different stress conditions, strain blocks (5 mm × 5 mm) were placed into freshly prepared 10% V8 liquid plates for 3 days; H2O2, sorbitol or NaCl was added to the liquid and mycelia were collected after 10–30 min for RNA extraction. To test for stress sensitivity, strain blocks (5 mm × 5 mm) were placed onto freshly prepared 10% V8 agar plates supplemented with H2O2 (1–3 mm), NaCl (0.25 and 0.5 m), CaCl2 (0.2 m) or sorbitol (0.5, 1.0 and 1.4 m), and cultured in darkness at 25 °C for 5.5 days. To examine the germination of zoospores, 500‐μL zoospore suspensions were incubated in 1% V8 liquid medium in 1.5‐mL tubes and vortexed for 90 s to induce encystment. The tubes were incubated at 25 °C in darkness for 48 h. At least 100 cysts were examined for each treatment and all treatments were performed in triplicate.
Plasmid construction and generation of P. sojae transformants
A partial sequence of the PsMPK7 open reading frame (ORF) was amplified from cDNA by polymerase chain reaction (PCR) using PrimeStar polymerase (TaKaRa Bio, Otsu, Japan) and the primers PsMPK7‐F (5′‐GCCAAGAATGGAGCTGAGTC‐3′) and PsMPK7‐R (5′‐GCAAGTCCCAGGTCACAAAT‐3′). The amplified fragment was digested with EcoRI and XbaI, and ligated in the antisense orientation into the pTOR vector to produce the plasmid pTOR‐PsMPK7. The insert sequence was confirmed by sequencing. Phytophthora sojae was transformed with pTOR‐PsMPK7 and pTOR‐GFP (control, CK) using a PEG‐mediated protoplast transformation strategy (McLeod et al., 2004).
Putative PsMPK7‐silenced transformants were screened by quantitative reverse transcription‐PCR (qRT‐PCR) using the primers PsMPK7‐RT‐F (5′‐CATGACTGTGGACCTGATGG‐3′) and PsMPK7‐RT‐R (5′‐GCAAGTCCCAGGTCACAAAT‐3′). The primers actinA‐F (5′‐ACTGCACCTTCCAGACCATC‐3′) and actinA‐R (5′‐CCACCACCTTGATCTTCATG‐3′) were used to detect actinA (Gene ID: 108986) expression, which served as a constitutively expressed endogenous control gene. Total RNA was isolated using an RNA extraction kit (OMEGA R 6834‐1; Omega Bio‐Tek, Norcross, GA, USA) following the manufacturer's protocol. First‐strand cDNA was synthesized from 1–5 μg of total RNA by oligo(dT) priming using an M‐MLV reverse transcriptase kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer's protocol. For gene expression analysis, SYBR green qRT‐PCR assays were performed using an ABI 7500 Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. A 20‐μL reaction volume contained 2 μL of reverse transcription product, 10 μL of SYBR premix Ex Taq, 0.4 μL of ROX reference dye (SYBR Prime Script RT‐PCR kit; TaKaRa Bio) and 0.4 μL of each primer (10 mm). Target gene expression was determined relative to actinA expression using the ΔΔCt method. qRT‐PCR experiments were repeated in triplicate with independent RNA isolations.
Pathogenicity test
For plant inoculation, P6497 and PsMPK7‐silenced transformants were incubated in 10% V8 broth in darkness for 2 days. Zoospores were then induced by washing with sterilized distilled water. The soybean cultivar Hefeng 47, which is compatible with P. sojae strain P6497, was planted in plastic pots containing vermiculite and grown in darkness for 4 days. Because almost 20% of zoospores could not mature normally, both wounded and unwounded etiolated soybean seedlings were inoculated with 100 zoospores of WT and CK and 150 zoospores of the transformants. The etiolated seedlings were then maintained in 80% humidity and darkness at 25 °C. Pathogenicity symptoms were evaluated after 2 days.
Staining
The ROS levels were stained with the oxidant‐sensitive probe NBT (Sigma, Seelze, Germany), and viewed by an Olympus IX71 bright‐field microscope (Olympus, Tokyo, Japan). The NBT staining was performed in three independent biological experiments, with three replicates in each test. Etiolated soybean seedlings infected with zoospores were stained with aniline blue after 16 h to measure callose deposition.
Supporting information
Fig. S1 Correction of PsMPK7 gene model. (A) The automatic model (Ps129213) was suggested to be nine introns and almost 7 kb (red blocks). According to the distribution of transcript tags (Ye et al., 2011) and the alignments against homologues in other close species, the new gene model was suggested to be 4613 bp in length and intronless. Three primer pairs were designed to verify this model, i.e. F1‐R, F2‐R and F3‐R. (B) Polymerase chain reaction (PCR) products could be obtained from Phytophthora sojae cDNA using F2‐R and F3‐R, but not F1‐R. The F2‐R product was sequenced and perfectly matched the prediction. The primers are listed.
Fig. S2 Protein sequence alignment of PsMPK7 and its orthologues. The protein sequences of PsMPK7 orthologues were identified from available genome assemblies of Phytophthora ramorum, Phytophthora infestans, Phytophthora capsici, Hyaloperonospora arabidopsidis and Pythium ultimum. The alignment was generated using BioEdit software (Hall, 1999). The STKc_MAPK domain, WW domain and dual phosphorylation lip sequence were predicted using the CD‐Search program at the National Center for Biotechnology Information (NCBI) website (Marchler‐Bauer et al., 2011)
Fig. S3 PsMPK7 gene expression. PsMPK7 expression was profiled throughout the Phytophthora sojae life cycle, including vegetative mycelia (MY), sporulating hyphae (SP), zoospores (ZO), cysts (CY), cyst germination (GC) and during the early stages of infection including 1.5, 3, 6, 12 and 24 h post‐inoculation (hpi) of the susceptible soybean cv. Williams. Data were obtained from Digital Gene Expression profiling (Ye et al., 2011).
Fig. S4 PsMPK7 expression under hydrogen peroxide (H2O2), sorbitol and NaCl stress conditions. Mycelia were cultured in liquid 10% V8 medium only or supplemented with 1, 2 or 3 mm H2O2 for 10 min or with 1.0 m sorbitol or 0.25 m NaCl for 30 min. Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) assays were performed using cDNAs synthesized from mycelial RNAs, and the Phytophthora sojae actinA gene served as a constitutively expressed endogenous control gene. The level of PsMPK7 expression was determined relative to that of actinA, and the relative gene expression levels shown were determined relative to expression on V8 medium alone (value, 1.0).
Fig. S5 Analysis of extracellular laccase activity. (A) Laccase activity was detected in lima bean agar (LBA) medium supplemented with 0.8 mm 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid) (ABTS) after 6 days of incubation. (B) Transcription levels of six predicted signal peptide‐containing laccase genes (IDs: 137983, 137984, 137978, 137979, 137990 and 129781) were measured in wild‐type (WT) and T41 at the mycelial stage by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR).
Acknowledgements
This work was supported by grants to YW from the China National Funds for Distinguished Young Scientists (31225022), China Agriculture Research System (CARS‐004‐PS14) and Special Fund for Agro‐scientific Research in the Public Interest (201303018). We thank the reviewer for providing good suggestions with regard to gene model correction.
References
- Abramovitch, R.B. , Kim, Y.J. , Chen, S.R. , Dickman, M.B. and Martin, G.B. (2003) Pseudomonas type III effector AvrPtoB induces plant disease susceptibility by inhibition of host programmed cell death. EMBO J. 22, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol, I , Heinstein, P.F. and Low, P.S. (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: Role in defense and signal transduction. Plant Physiol. 90, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf, S.L. (2003) The deep roots of eukaryotes. Science, 300, 1703–1706. [DOI] [PubMed] [Google Scholar]
- Blanco, F.A. and Judelson, H.S. (2005) A bZIP transcription factor from Phytophthora interacts with a protein kinase and is required for zoospore motility and plant infection. Mol. Microbiol. 56, 638–648. [DOI] [PubMed] [Google Scholar]
- Bradley, D.J. , Kjellbom, P. and Lamb, C.J. (1992) Elicitor‐induced and wound‐induced oxidative cross‐linking of a proline‐rich plant‐cell wall protein – a novel, rapid defense response. Cell, 70, 21–30. [DOI] [PubMed] [Google Scholar]
- Chen, R.E. and Thorner, J. (2007) Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae . BBA‐Mol. Cell. Res. 1773, 1311–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Autreaux, B. and Toledano, M.B. (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824. [DOI] [PubMed] [Google Scholar]
- Degols, G. , Shiozaki, K. and Russell, P. (1996) Activation and regulation of the Spc1 stress‐activated protein kinase in Schizosaccharomyces pombe . Mol. Cell. Biol. 16, 2870–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, K.P. , Xu, J.R. , Smirnoff, N. and Talbot, N.J. (1999) Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium‐mediated plant infection by Magnaporthe grisea . Plant Cell, 11, 2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkerli, K. , Hahn, M.G. and Mims, C.W. (1997) Ultrastructure of compatible and incompatible interactions of soybean roots infected with the plant pathogenic oomycete Phytophthora sojae . Can. J. Bot. 75, 1493–1508. [Google Scholar]
- Erwin, D.C. and Ribiero, O.K. (1996) Phytophthora Diseases Worldwide. St. Paul, MN: APS Press. [Google Scholar]
- Fabritius, A.L. , Cvitanich, C. and Judelson, H.S. (2002) Stage‐specific gene expression during sexual development in Phytophthora infestans . Mol. Microbiol. 45, 1057–1066. [DOI] [PubMed] [Google Scholar]
- Gan, Y.Z. , Zhang, L.S. , Zhang, Z.G. , Dong, S.M. , Li, J. , Wang, Y.C. et al (2009) The LCB(2) subunit of the sphingolip biosynthesis enzyme serine palmitoyltransferase can function as an attenuator of the hypersensitive response and Bax‐induced cell death. New Phytol. 181, 127–146. [DOI] [PubMed] [Google Scholar]
- Hall, T.A. (1999) BioEdit: a user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98. [Google Scholar]
- Hamel, L.P. , Nicole, M.C. , Duplessis, S. and Ellis, B.E. (2012) Mitogen‐activated protein kinase signaling in plant‐interacting fungi: distinct messages from conserved messengers. Plant Cell, 24, 1327–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann, S. (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, C.L. , Wang, Y.L. , Zheng, X.B. , Dou, D.L. , Zhang, Z.G. , Govers, F. et al (2008) A Phytophthora sojae G‐protein alpha subunit is involved in chemotaxis to soybean isoflavones. Eukaryot. Cell, 7, 2133–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckelhoven, R. (2007) Cell wall‐associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 45, 101–127. [DOI] [PubMed] [Google Scholar]
- Igbaria, A. , Lev, S. , Rose, M.S. , Lee, B.N. , Hadar, R. , Degani, O. et al (2008) Distinct and combined roles of the MAP kinases of Cochliobolus heterostrophus in virulence and stress responses. Mol. Plant–Microbe Interact. 21, 769–780. [DOI] [PubMed] [Google Scholar]
- Joubert, A. , Bataille‐Simoneau, N. , Campion, C. , Guillemette, T. , Hudhomme, P. , Iacomi‐Vasilescu, B. et al (2011) Cell wall integrity and high osmolarity glycerol pathways are required for adaptation of Alternaria brassicicola to cell wall stress caused by brassicaceous indolic phytoalexins. Cell. Microbiol. 13, 62–80. [DOI] [PubMed] [Google Scholar]
- Kato, T. , Okazaki, K. , Murakami, H. , Stettler, S. , Fantes, P.A. and Okayama, H. (1996) Stress signal, mediated by a HOG1‐like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 378, 207–212. [DOI] [PubMed] [Google Scholar]
- Kawasaki, L. , Sanchez, O. , Shiozaki, K. and Aguirre, J. (2002) SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans . Mol. Microbiol. 45, 1153–1163. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Park, S.Y. , Kim, K.S. , Rho, H.S. , Chi, M.H. , Choi, J. et al (2009) Homeobox transcription factors are required for conidiation and appressorium development in the rice blast fungus Magnaporthe oryzae . PLoS Genet. 5, e1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, K. , Takano, Y. , Yoshimi, A. , Tanaka, C. , Kikuchi, T. and Okuno, T. (2004) Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol. 53, 1785–1796. [DOI] [PubMed] [Google Scholar]
- Kroon, L.P. , Brouwer, H. , de Cock, A.W. and Govers, F. (2012) The genus Phytophthora anno 2012. Phytopathology, 102, 348–364. [DOI] [PubMed] [Google Scholar]
- Kyriakis, J.M. and Avruch, J. (1996) Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 271, 24 313–24 316. [DOI] [PubMed] [Google Scholar]
- Latijnhouwers, M. and Govers, F. (2003) A Phytophthora infestans G‐protein beta subunit is involved in sporangium formation. Eukaryot. Cell, 2, 971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latijnhouwers, M. , Ligterink, W. , Vleeshouwers, V.G.A.A. , van West, P. and Govers, F. (2004) A G alpha subunit controls zoospore motility and virulence in the potato late blight pathogen Phytophthora infestans . Mol. Microbiol. 51, 925–936. [DOI] [PubMed] [Google Scholar]
- Levine, A. , Tenhaken, R. , Dixon, R. and Lamb, C. (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell, 79, 583–593. [DOI] [PubMed] [Google Scholar]
- Li, A. , Zhang, M. , Wang, Y. , Li, D. , Liu, X. , Tao, K. et al (2014) PsMPK1, an SLT2‐type mitogen‐activated protein kinase, is required for hyphal growth, zoosporogenesis, cell wall integrity, and pathogenicity in Phytophthora sojae . Fungal Genet. Biol. 65, 14–24. [DOI] [PubMed] [Google Scholar]
- Li, A.N. , Wang, Y.L. , Tao, K. , Dong, S.M. , Huang, Q.A. , Dai, T.T. et al (2010) PsSAK1, a stress‐activated map kinase of Phytophthora sojae, is required for zoospore viability and infection of soybean. Mol. Plant–Microbe Interact. 23, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Lin, C.H. and Chung, K.R. (2010) Specialized and shared functions of the histidine kinase‐ and HOG1 MAP kinase‐mediated signaling pathways in Alternaria alternata, a filamentous fungal pathogen of citrus. Fungal Genet. Biol. 47, 818–827. [DOI] [PubMed] [Google Scholar]
- Marchler‐Bauer, A. , Lu, S. , Anderson, J.B. , Chitsaz, F. , Derbyshire, M.K. , DeWeese‐Scott, C. et al (2011) CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39, D225–D229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod, A. , Smart, C.D. and Fry, W.E. (2004) Core promoter structure in the oomycete Phytophthora infestans . Eukaryot. Cell, 3, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabi, R. , Zwiers, L.H. , de Waard, M.A. and Kema, G.H.J. (2006) MgHog1 regulates dimorphism and pathogenicity in the fungal wheat pathogen Mycosphaerella graminicola . Mol. Plant–Microbe Interact. 19, 1262–1269. [DOI] [PubMed] [Google Scholar]
- Millar, J.B.A. , Buck, V. and Wilkinson, M.G. (1995) Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing map kinase controlling cell‐size at division in fission yeast. Genes Dev. 9, 2117–2130. [DOI] [PubMed] [Google Scholar]
- Moriwaki, A. , Kubo, E. , Arase, S. and Kihara, J. (2006) Disruption of SRM1, a mitogen‐activated protein kinase gene, affects sensitivity to osmotic and ultraviolet stressors in the phytopathogenic fungus Bipolaris oryzae . FEMS Microbiol. Lett. 257, 253–261. [DOI] [PubMed] [Google Scholar]
- Nurnberger, T. , Brunner, F. , Kemmerling, B. and Piater, L. (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunol. Rev. 198, 249–266. [DOI] [PubMed] [Google Scholar]
- Park, S.M. , Choi, E.S. , Kim, M.J. , Cha, B.J. , Yang, M.S. and Kim, D.H. (2004) Characterization of HOG1 homologue, CpMK1, from Cryphonectria parasitica and evidence for hypovirus‐mediated perturbation of its phosphorylation in response to hypertonic stress. Mol. Microbiol. 51, 1267–1277. [DOI] [PubMed] [Google Scholar]
- Prakob, W. and Judelson, H.S. (2007) Gene expression during oosporogenesis in heterothallic and homothallic Phytophthora . Fungal Genet. Biol. 44, 726–739. [DOI] [PubMed] [Google Scholar]
- Segmuller, N. , Ellendorf, U. , Tudzynski, B. and Tudzynski, P. (2007) BcSAK1, a stress‐activated mitogen‐activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea . Eukaryot. Cell, 6, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki, K. and Russell, P. (1995) Cell‐cycle control linked to extracellular environment by map kinase pathway in fission yeast. Nature, 378, 739–743. [DOI] [PubMed] [Google Scholar]
- Tanaka, A. , Christensen, M.J. , Takemoto, D. , Park, P. and Scott, B. (2006) Reactive oxygen species play a role in regulating a fungus–perennial ryegrass mutualistic interaction. Plant Cell, 18, 1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toone, W.M. and Jones, N. (1998) Stress‐activated signalling pathways in yeast. Genes Cells, 3, 485–498. [DOI] [PubMed] [Google Scholar]
- Torres, M.A. and Dangl, J.L. (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 8, 397–403. [DOI] [PubMed] [Google Scholar]
- Tyler, B.M. (2007) Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol. Plant Pathol. 8, 1–8. [DOI] [PubMed] [Google Scholar]
- Wrather, J.A. and Koenning, S.R. (2006) Estimates of disease effects on soybean yields in the United States 2003 to 2005. J. Nematol. 38, 173–180. [PMC free article] [PubMed] [Google Scholar]
- Ye, W.W. , Wang, X.L. , Tao, K. , Lu, Y.P. , Dai, T.T. , Dong, S.M. et al (2011) Digital gene expression profiling of the Phytophthora sojae transcriptome. Mol. Plant–Microbe Interact. 24, 1530–1539. [DOI] [PubMed] [Google Scholar]
- Yoshioka, H. , Bouteau, F. and Kawano, T. (2008) Discovery of oxidative burst in the field of plant immunity: Looking back at the early pioneering works and towards the future development. Plant Signal Behav. 3, 153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H.F. , Zhao, Q. , Liu, K.Y. , Zhang, Z.G. , Wang, Y.C. and Zheng, X.B. (2009) MgCRZ1, a transcription factor of Magnaporthe grisea, controls growth, development and is involved in full virulence. FEMS Microbiol. Lett. 293, 160–169. [DOI] [PubMed] [Google Scholar]
- Zhao, W. , Dong, S.M. , Ye, W.W. , Hua, C.L. , Meijer, H.J.G. , Dou, X.Y. et al (2011) Genome‐wide identification of Phytophthora sojae SNARE genes and functional characterization of the conserved SNARE PsYKT6. Fungal Genet. Biol. 48, 241–251. [DOI] [PubMed] [Google Scholar]
- Zheng, D.W. , Zhang, S.J. , Zhou, X.Y. , Wang, C.F. , Xiang, P. , Zheng, Q. et al (2012) The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum . PLoS ONE, 7, e49495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Correction of PsMPK7 gene model. (A) The automatic model (Ps129213) was suggested to be nine introns and almost 7 kb (red blocks). According to the distribution of transcript tags (Ye et al., 2011) and the alignments against homologues in other close species, the new gene model was suggested to be 4613 bp in length and intronless. Three primer pairs were designed to verify this model, i.e. F1‐R, F2‐R and F3‐R. (B) Polymerase chain reaction (PCR) products could be obtained from Phytophthora sojae cDNA using F2‐R and F3‐R, but not F1‐R. The F2‐R product was sequenced and perfectly matched the prediction. The primers are listed.
Fig. S2 Protein sequence alignment of PsMPK7 and its orthologues. The protein sequences of PsMPK7 orthologues were identified from available genome assemblies of Phytophthora ramorum, Phytophthora infestans, Phytophthora capsici, Hyaloperonospora arabidopsidis and Pythium ultimum. The alignment was generated using BioEdit software (Hall, 1999). The STKc_MAPK domain, WW domain and dual phosphorylation lip sequence were predicted using the CD‐Search program at the National Center for Biotechnology Information (NCBI) website (Marchler‐Bauer et al., 2011)
Fig. S3 PsMPK7 gene expression. PsMPK7 expression was profiled throughout the Phytophthora sojae life cycle, including vegetative mycelia (MY), sporulating hyphae (SP), zoospores (ZO), cysts (CY), cyst germination (GC) and during the early stages of infection including 1.5, 3, 6, 12 and 24 h post‐inoculation (hpi) of the susceptible soybean cv. Williams. Data were obtained from Digital Gene Expression profiling (Ye et al., 2011).
Fig. S4 PsMPK7 expression under hydrogen peroxide (H2O2), sorbitol and NaCl stress conditions. Mycelia were cultured in liquid 10% V8 medium only or supplemented with 1, 2 or 3 mm H2O2 for 10 min or with 1.0 m sorbitol or 0.25 m NaCl for 30 min. Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) assays were performed using cDNAs synthesized from mycelial RNAs, and the Phytophthora sojae actinA gene served as a constitutively expressed endogenous control gene. The level of PsMPK7 expression was determined relative to that of actinA, and the relative gene expression levels shown were determined relative to expression on V8 medium alone (value, 1.0).
Fig. S5 Analysis of extracellular laccase activity. (A) Laccase activity was detected in lima bean agar (LBA) medium supplemented with 0.8 mm 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid) (ABTS) after 6 days of incubation. (B) Transcription levels of six predicted signal peptide‐containing laccase genes (IDs: 137983, 137984, 137978, 137979, 137990 and 129781) were measured in wild‐type (WT) and T41 at the mycelial stage by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR).


