Summary
Downy mildew of sunflower is caused by Plasmopara halstedii (Farlow) Berlese & de Toni. Plasmopara halstedii is an obligate biotrophic oomycete pathogen that attacks annual Helianthus species and cultivated sunflower, Helianthus annuus. Depending on the sunflower developmental stage at which infection occurs, the characteristic symptoms range from young seedling death, plant dwarfing, leaf bleaching and sporulation to the production of infertile flowers. Downy mildew attacks can have a great economic impact on sunflower crops, and several Pl resistance genes are present in cultivars to protect them against the disease. Nevertheless, some of these resistances have been overcome by the occurrence of novel isolates of the pathogen showing increased virulence. A better characterization of P. halstedii infection and dissemination mechanisms, and the identification of the molecular basis of the interaction with sunflower, is a prerequisite to efficiently fight this pathogen. This review summarizes what is currently known about P. halstedii, provides new insights into its infection cycle on resistant and susceptible sunflower lines using scanning electron and light microscopy imaging, and sheds light on the pathogenicity factors of P. halstedii obtained from recent molecular data.
Taxonomy
Kingdom Stramenopila; Phylum Oomycota; Class Oomycetes; Order Peronosporales; Family Peronosporaceae; Genus Plasmopara; Species Plasmopara halstedii.
Disease symptoms
Sunflower seedling damping off, dwarfing of the plant, bleaching of leaves, starting from veins, and visible white sporulation, initially on the lower side of cotyledons and leaves. Plasmopara halstedii infection may severely impact sunflower seed yield.
Infection process
In spring, germination of overwintered sexual oospores leads to sunflower root infection. Intercellular hyphae are responsible for systemic plant colonization and the induction of disease symptoms. Under humid and fresh conditions, dissemination structures are produced by the pathogen on all plant organs to release asexual zoosporangia. These zoosporangia play an important role in pathogen dissemination, as they release motile zoospores that are responsible for leaf infections on neighbouring plants.
Disease control
Disease control is obtained by both chemical seed treatment (mefenoxam) and the deployment of dominant major resistance genes, denoted Pl. However, the pathogen has developed fungicide resistance and has overcome some plant resistance genes. Research for more sustainable strategies based on the identification of the molecular basis of the interaction are in progress.
Useful websites
http://www.heliagene.org/HP, http://lipm‐helianthus.toulouse.inra.fr/dokuwiki/doku.php?id=start, https://www.heliagene.org/PlasmoparaSpecies (soon available).
Keywords: effectors, Helianthus annuus, infection modes, life cycle, obligate biotroph oomycete, pathogen virulence, Plasmopara halstedii
Introduction
Downy mildew (caused by Plasmopara halstedii) is one of the major diseases affecting sunflower (Helianthus annuus) production. The pathogen has been reported in most countries in which sunflowers are grown. The global impact on yield has been estimated recently to be 3.5% of commercial seed production in the presence of the control methods currently deployed, but loss of yield can be up to 100% in contaminated fields (Centre Technique Interprofessionnel des Oléagineux Métropolitains, http://www.cetiom.fr). In addition, sunflower cultivation has to be abandoned in fields too heavily contaminated by P. halstedii.
Sunflower, the fourth most widely grown oil crop in the world after oil palm, soybean and rapeseed, but second in the European Union, produces healthy oil rich in unsaturated fatty acids, and is regarded as a plant able to grow under low water input regimes, and with a limited addition of soil fertilizers and fungicides, in contrast with other oil crops. Total sunflower production has increased worldwide by 32% over the past 20 years, reaching 38 million tons in 2012, with an acreage of 23 million hectares (Food and Agriculture Organization).
The pathogen P. halstedii, first identified in North America by Mr Byron D. Halsted, a botanist at the Bussey Institution (Harvard University) (Nishimura, 1922; Young and Morris, 1927), was later reported in Russia and Western Europe around 1960, where it was introduced probably via infected sunflower seeds (Ioos et al., 2007). Since 1992, this pathogen has been submitted to quarantine regulation in the European Union. Thirty‐six pathotypes of P. halstedii, often known as races, have so far been identified worldwide, mostly in zones in which sunflowers are widely grown: in North America, and in Europe, especially France, Spain, Germany, Ukraine and Russia. Plasmopara halstedii pathotypes are defined by an international nomenclature system based on differential virulence profiles on a set of sunflower inbred lines (Gulya et al., 1998, Tourvieille de Labrouhe et al., 2012).
During the last 20 years, new P. halstedii pathotypes showing increased virulence have appeared (Ahmed et al., 2012), and several Pl resistance genes used in sunflower hybrids have become inefficient: for example, the Pl2 gene conferring resistance to the most frequent pathotype has been overcome by different emerging pathotypes (Moinard et al., 2006; Tourvieille de Labrouhe et al., 2000). An understanding of pathotype evolution and sunflower resistance is therefore an agronomic issue.
Nevertheless, despite the economic importance of this crop pathogen, genomic resources for P. halstedii and, more generally, for pathogens of the genus Plasmopara, are limited. The aims of this review are to summarize our knowledge on the sunflower downy mildew pathogen, with a focus on infection and dissemination mechanisms, and to shed light on P. halstedii pathogenicity factors from recent molecular data.
Plasmopara Halstedii Taxonomy and Reproduction
Plasmopara halstedii is a biotrophic oomycete belonging to the Peronosporales, the largest and most devastating group of plant‐pathogenic oomycetes, which also includes the hemibiotrophs of the genus Phytophthora, causing late blight diseases, and an important group of obligate biotrophs causing downy mildews, such as Plasmopara viticola on grapevine and Bremia lactucae on lettuce (Gessler et al., 2011; Michelmore and Wong, 2008; Thines and Kamoun, 2010). Eight hundred species responsible for downy mildews are grouped into 14 genera subdivided into four morphological and ecological subgroups. In subgroup II are included the genera Basidiophora, Benua, Bremia, Paraperonospora, Plasmopara, Plasmoverna and Protobremia, which produce vesicular to pyriform haustoria (Göker et al., 2007). Unfortunately, P. halstedii was not among the eight Plasmopara species included in this study. All downy mildews are obligate biotrophic parasites that require a living plant host to accomplish their life cycle. Most, including P. halstedii, have not been successfully cultured in vitro.
One hundred and forty‐six different Plasmopara species are listed in Index Fungorum. Few are phylogenetically related on the basis of large nuclear subunit ribosomal DNA sequences and other genes (Göker et al., 2007; Riethmüller et al., 2002). Plasmopara species cause downy mildew disease on various plants: P. viticola on grapevine, P. umbelliferarum on Umbelliferae, P. geranii and P. pusilla on geranium and P. obducens on impatiens. Seven species pathogenic on Ranunculaceae, originally referred to as P. pygmaea, but differing by morphological and molecular traits from all the other Plasmopara species, have been placed in the genus Plasmoverna (Constantinescu et al., 2005). The origins of the species P. halstedii remain complex and mostly undetermined (cf. for review, Viranyi and Spring, 2011).
Plasmopara halstedii is a specialized pathogen which attacks several Asteraceae, including annual Helianthus species, e.g. H. argophyllus, H. debilis and H. petiolaris, and wild and cultivated forms of H. annuus, with strong impacts on sunflower yield. Plasmopara halstedii has been reported to infect other Asteraceae (Bidens, Artemisia, Xanthium), which could be potential reservoirs of inoculum.
In oomycetes, two types of sexual reproduction have been described: (i) homothallism, in which sexual reproductive cells (oogonia and antheridia) are produced by the same organism, which could lead to outcrossing or selfing in the absence of self‐incompatibility mechanisms; and (ii) heterothallism, in which sexual reproductive cells are produced by two different organisms, leading only to outcrossing. Plasmopara halstedii is diploid, homothallic and has been shown to reproduce sexually and asexually in laboratory conditions (Spring, 2000; Spring and Zipper, 2006). Homothallic sexual reproduction has been shown to occur after the inoculation of sunflower seedlings with single zoospores (Spring, 2000; Spring et al., 1998; Spring and Zipper, 2000). This is important for epidemiology as a single zoospore in a field can lead to the contamination of soil with oospores, the survival forms of the inoculum. The sexual phase is thus required to produce overwintering oospores, but asexual generations occurring during the sunflower growing season from spring to the end of summer are also important in disease propagation.
Genotypic analysis provided by Ahmed et al. (2012) suggested that reproduction is predominantly by selfing in wild P. halstedii French populations, but this did not prevent the emergence of new virulence (Intelmann and Spring, 2002). Reduced genetic variation and increased linkage disequilibrium have been shown to occur within French P. halstedii populations compared with mostly heterothallic Phytophthora infestans and P. viticola (Delmotte et al., 2006; Delmotte et al., 2008; Grünwald and Flier, 2005). These results confirmed the hypothesis that P. halstedii wild populations are homothallic, and suggested the absence of a self‐incompatibility mechanism, which thus allows selfing and outcrossing mating systems (Ahmed et al., 2012). Spring and Zipper (2006) also provided evidence for genetic recombination through parasexual events.
Plasmopara Halstedii Pathotypes and Sunflower Resistance Genes
Plasmopara halstedii populations have been thoroughly characterized and classified into pathotypes mainly in France (Ahmed et al., 2012) and Germany (Rozynek and Spring, 2000). In France, 17 reference pathotypes have been defined according to their virulence profiles when tested for the infection of differential hosts carrying different Pl resistance genes (Fig. 1; Gulya et al., 1998; Tourvieille de Labrouhe et al., 2012). Fourteen of these pathotypes (defined by triplet name, Fig. 1) have been classified into three distinct clusters, according to 12 single nucleotide polymorphism (SNP) markers designed on P. halstedii expressed sequence tags (ESTs): cluster 1: 100, 300, 304; cluster 2: 307, 700, 703, 707, 730; and cluster 3: 314, 334, 704, 710, 714, 717 (Ahmed et al., 2012; Delmotte et al., 2008). These clusters could have resulted from three independent P. halstedii introductions into France, and intercluster recombinations could have facilitated the emergence of new pathotypes in response to host resistance (Ahmed et al., 2012).
Figure 1.
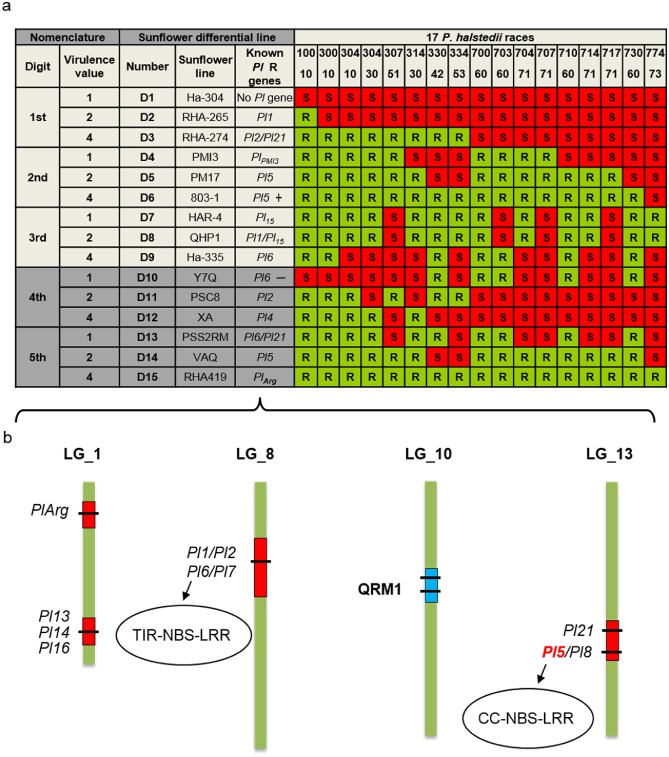
Nomenclature of Plasmopara halstedii pathotypes and genetic map of sunflower resistance genes. (a) Plasmopara halstedii pathotypes were defined according to an international nomenclature based on the virulence profile of a given isolate on 15 sunflower differential lines (D1–D15) selected according to their resistance patterns (Gulya et al., 1998; Tourvieille de Labrouhe et al., 2012). Susceptibility (S) and resistance (R) were defined by the presence or absence of disease symptoms and sporulation on leaves 2–3 weeks after inoculation of sunflower root seedlings, grown in soil (Mouzeyar et al., 1993). A triplet coding system was initially set up on nine sunflower lines (D1–D9, Gulya et al., 1998), but the occurrence of new P. halstedii pathotypes led to the addition of six other sunflower lines (in dark grey) and to a five‐digit coding system (D10–D15, Tourvieille de Labrouhe et al., 2012). The phenotyping results on each triplet of sunflower differential lines give the pathotype digit values. If the first differential line of a set of three is susceptible, a value of ‘1’ is assigned to the pathotype. If the second line is S, a value of ‘2’, and, for the third line, a value of ‘4’. When the line is resistant, a value of ‘0’ is assigned to the pathotype. The virulence code is additive within each set. For example, virulence code 710 is explained by ‘7’ (S for D1–D3, 1 + 2 + 4 = 7), ‘1’ (S for D4) and ‘0’ (R for D7–D9). Dominant genes giving resistance to P. halstedii are called Pl genes. The known functional R genes are indicated in the sunflower differential lines. ‘+’ or ‘–’ after the Pl gene indicates the strength of resistance alleles. (b) Eleven sunflower Pl genes have been mapped on three of 17 sunflower linkage groups (LGs) (Bachlava et al., 2011; Bouzidi et al., 2002; Franchel et al., 2013; Liu et al., 2012; Mulpuri et al., 2009; Radwan et al., 2003; Vincourt et al., 2012; Wieckhorst et al., 2010). Pl1/Pl2/Pl6 and Pl7 are localized in a Toll/interleukin‐1 receptor‐nucleotide‐binding site‐leucine‐rich repeat (TIR‐NBS‐LRR)‐rich region. Pl5/Pl8 and Pl21 are localized in a coiled‐coil (CC)‐NBS‐LRR‐rich region. QRM1, the major quantitative trait locus (QTL), conferring a quantitative resistance to pathotype 710 of P. halstedii, was mapped on LG10 (Vear et al., 2008b; Vincourt et al., 2012).
Two pathotypes showing new virulence profiles (304 30 and 774 73) have been recorded more recently and can be differentiated by the addition of six further sunflower lines (Fig. 1a, dark grey rows), extending the pathotype nomenclature to a five‐digit code (Tourvieille de Labrouhe et al., 2012) (Fig. 1). The addition of these six differential hosts was also a prospective approach aimed to improve the distinction of emerging pathotypes. Assuming a strong correlation between the two codes for a given pathotype, the triplet code is used in the rest of this article.
To avoid downy mildew attacks, modern sunflower cultivars carry one or more dominant resistance genes, denoted Pl. Pl genes originated mostly from wild H. annuus, but also from other Helianthus species (H. argophyllus and H. tuberosus) (Vear et al., 2008a). More than 20 Pl genes (Pl1, Pl2, Pl5–Pl8, Pl13–Pl16, Pl21, Pl Arg; Fig. 1), conferring resistance to one or more pathotypes of P. halstedii, have been described, and 10 have been mapped in sunflower (Fig. 1b). Pl genes are grouped into at least four complex genomic regions on three linkage groups (LGs). Toll/interleukin‐1 receptor‐nucleotide‐binding site‐leucine‐rich repeat (TIR‐NBS‐LRR) and coiled‐coil (CC)‐NBS‐LRR resistance gene analogues (RGAs) have been localized genetically to these regions (Fig. 1; Bouzidi et al., 2002; Gentzbittel et al., 1998; Radwan et al., 2003, 2004, 2008), but no Pl gene has yet been cloned. Quantitative resistance to pathotypes 710 and 703 of P. halstedii has been described in the sunflower line XRQ (Tourvieille de Labrouhe et al., 2008) and is conferred by three quantitative trait loci (QTLs) (Vear et al., 2008b; Vincourt et al., 2012). These QTLs have been mapped on LG10, LG8 and LG7 (Vincourt et al., 2012). The major QTL, QRM1 (Fig. 1), explaining 65% of the variability, was mapped on LG10 in a genetic region of 0.8 cM (Vincourt et al., 2012). A map‐based cloning approach based on recombinant lines segregating only for QRM1 is underway in our group (As‐Sadi, 2011; S. Muños, LIPM, Castanet‐Tolosan, France, personal communication).
Mildew Symptoms Caused by P . Halstedii on Sunflower
Contrary to the related P. viticola, which attacks only green parts and fruits of grapevine (Musetti et al., 2005), field infections of P. halstedii in sunflower usually result from root attacks by zoospores released at the beginning of spring from zoosporangia derived from oospores (primary infections, Fig. 3). Root infection leads to either seedling damping off, or to severe symptoms, in particular plant dwarfing (Fig. 2a), leaf discoloration (Fig. 2b) and sporulation (Fig. 2f,g), ultimately resulting in important yield losses caused by the production of infertile flowers (Sackston, 1981). Dwarfing of sunflower plants is currently not explained, although it might be the result of hormonal manipulation or nutrient uptake by P. halstedii.
Figure 3.
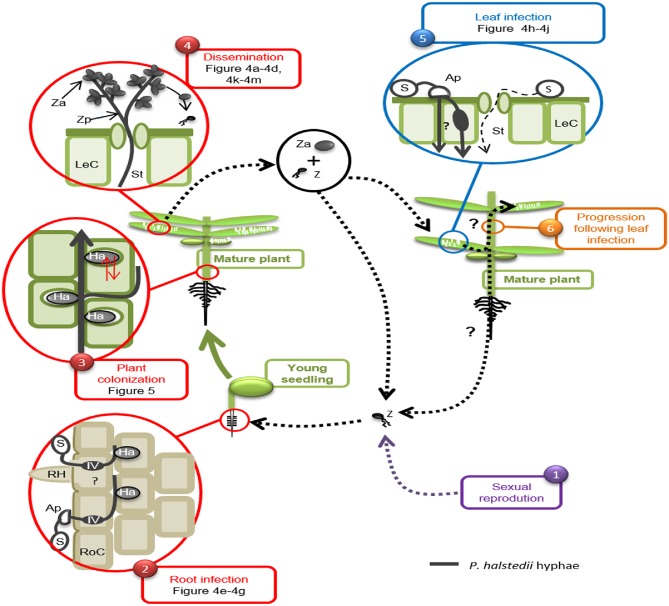
Life cycle of Plasmopara halstedii. (1) In spring, biflagellate zoospores (Z) originating from zoosporangia produced by overwintering sexual oospores are released in soil free water. Spore (S) encystment occurs in contact with a sunflower root. (2) Zoospores germinate in a few hours and enter the root by two methods: (i) by direct penetration into a root cell (RoC) with or without the formation of an appressorium (Ap), a bulge in which the pathogen increases osmotic pressure and drills root epidermal cells; and (ii) through injuries frequently present at the base of root hairs (RH). The formation of infection vesicles (IV) then occurs in plant epidermal cells infected with appressoria (Wehtje et al., 1979). (3) Pathogen hyphae (grey thick line) progress further through the intercellular spaces between cortical root cells to reach and colonize shoot tissues. At this stage of infection, P. halstedii establishes a large number of infection/nutrition structures, named haustoria (Ha). (4) Under high humidity conditions and moderate temperature (late spring and summer), P. halstedii will emerge first on the abaxial surface of leaves and cotyledons via stomata (St), close to a leaf cell (LeC). The branched structures that are produced are dissemination structures, named zoosporangiophores (Zp), carrying zoosporangia (Za). Zoosporangia can be disseminated and contaminate the leaves of other plants. They release up to 20 zoospores (Z). (5) Spores grouped around leaf trichomes or near veins encyst and germinate in a few hours to penetrate leaf tissues at intercellular junctions by forming appressoria (thick gray line). Rare events of penetration via stomata are also observed (thin dotted line). (6) Pathogen progression in plant tissue following leaf infection remains poorly characterized.
Figure 2.

Disease symptoms caused by Plasmopara halstedii on sunflower in field (a, b) and hydroponic (c–g) conditions. Plant dwarfing (a, arrow) and leaf discoloration (b) of sunflower plants. (c–g) Two‐day‐old sunflower seedlings were root inoculated by P. halstedii, pathotype 710 (Mouzeyar et al., 1993) and grown in oxygenated liquid growth medium under hydroponic conditions, shown in (c). A saturated atmosphere, obtained by placing for 2 days plastic covers over the trays, 12 days after inoculation, induced sporulation on cotyledons of the susceptible line TS (c, f, g), but not on the resistant near‐isogenic line TSRM (c), as for inoculation tests performed in soil (Mouzeyar et al., 1993). Dwarfing symptoms are visible on infected (inf.) TS plants, but not on TSRM (c), compared with uninfected (ctrl) TS and TSRM plants (d, e). Infection of TSRM did not affect shoot growth compared with non infected TSRM plants (e). (d, e) Note the brownish coloration on infected roots of both lines. (f, g) Typical ‘downy’ appearance on abaxial and adaxial cotyledon surfaces caused by sporulation on TS. (g) Leaf discoloration on 14‐day‐old plants. (d, e) Scale bars: 5 cm.
During sunflower growth, under humid and cool conditions, zoosporangia produced mainly on the surfaces of leaves and cotyledons are responsible for the contamination of the shoots of neighbouring plants, where they release zoospores (secondary infections, Fig. 3). Symptoms induced by secondary infections are often less severe than those induced by primary infections (Gulya et al., 1998). In contrast, in grapevine, yield losses by P. viticola are mostly attributed to leaf infections (Schruft and Kassemeyer, 2000).
Two types of resistance to downy mildew conferred by Pl genes have been described in sunflower: type I resistance is characterized by the absence of symptoms on shoots and the absence of the pathogen above the base of hypocotyls; type II resistance is characterized by weak sporulation symptoms restricted to cotyledons, and the absence of symptoms in the upper part of the plant with the pathogen never reaching the true leaves (Mouzeyar et al., 1994). Resistant sunflower genotypes present either type I or type II resistance depending on their Pl resistance genes (for example, the Pl5 allele from the inbred line XRQ confers type II resistance, whereas Pl6 confers type I resistance). These resistances have been used to protect crops against P. halstedii pathotypes, but, except for PlArg (Fig. 1a), they have all been overcome during the last 15 years. For example, the Pl2 and Pl6 genes have been overcome by eight pathotypes (Delmotte et al., 2008; Moinard et al., 2006).
To study P. halstedii resistance in laboratory conditions, we infected sunflower seedlings and grew them in hydroponic cultures (Fig. 2c), such that they showed the same symptoms as plants in the field. In our experiments, we compared a pair of downy mildew‐susceptible and downy mildew‐resistant near‐isogenic sunflower lines (NILs), called TS and TSRM, respectively. The inbred line TS has no identified Pl gene and is susceptible to all P. halstedii pathotypes. TSRM was obtained by backcrossing of the Pl5 gene from the inbred line XRQ into TS (six back‐crosses and two selfings to obtain homozygous Pl5 resistance in a TS genetic background). TSRM presents type II resistance to P. halstedii pathotypes 100, 304, 703 and 710, as shown for Pl5 in other genetic backgrounds.
Two weeks after infection of TS and TSRM by pathotype 710, infected TS plants showed dwarfing (Fig. 2d), leaf discoloration (Fig. 2g) and sporulation on cotyledons and leaves (Fig. 2f,g). In contrast, infected TSRM plants grew as well as non‐infected plants (Fig. 2e), showed no leaf discoloration and only rare sporulation on cotyledons, but never on leaves, as described previously for type II resistance (Mouzeyar et al., 1993, 1994). In addition, hydroponic culture revealed root phenotypes (Fig. 2d,e). In infected plants of both TS and TSRM, roots turned brown and showed reduced growth, especially in the case of TS, compared with uninfected roots (Fig. 2d,e). It is not clear whether the brown root phenotype could be attributed to a general plant defence reaction to P. halstedii infection or to disease symptoms of roots. In TSRM plants, the root phenotype was less marked compared with TS plants, and probably not sufficiently strong to impact TSRM shoot development (Fig. 2d,e).
Life Cycle of P. Halstedii
Two modes of spore germination preceding plant infection have been described for obligate biotroph oomycetes: (i) indirect germination occurring by the release of motile zoospores from mature sporangia, their encystment on the plant surface and spore germination; (ii) direct germination: production of a germ tube by the sporangium itself. Although direct germination is the only method of germination for the genera Hyaloperonospora and Peronospora, which never produce mobile zoospores, the genus Plasmopara mainly uses the indirect germination of zoospores (Grenville‐Briggs and Van West, 2005).
Root infection
Although P. halstedii can be dispersed by wind, water and via contaminated seeds (see section on Pathogen sporulation and dissemination), it is mostly a soil‐borne pathogen (Ioos et al., 2007). In the field, root infections of young sunflower plants are responsible for the most severe symptoms and have a strong impact on yield. Infections have been shown to have a maximum efficiency during the first 5 days after seed germination, but can still occur until sunflower plants reach the four‐leaf stage (2–3 weeks; Zimmer, 1971, 1975). Plasmopara halstedii zoospores released either from oospore sporangia (in the spring) or from asexual sporangia (from spring to summer) (Fig. 4a) can infect young sunflower plants (Cohen and Sackston, 1973; Nishimura, 1922; Spring and Zipper, 2000).
Figure 4.
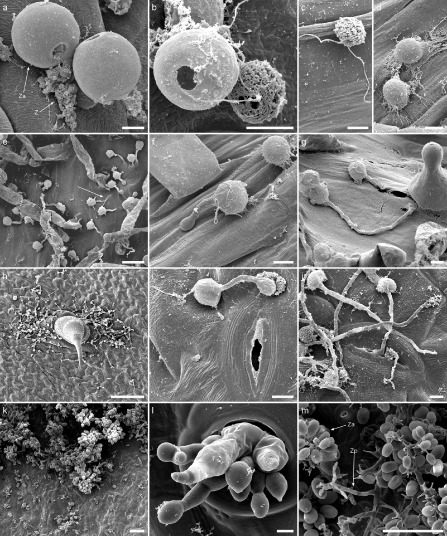
Scanning electron microscopy (SEM) images illustrating key steps of the Plasmopara halstedii life cycle. (a–d) Release and encystment of P. halstedii zoospores from zoosporangia. (a) Zoospores (Z) released from zoosporangia (Za) opercula. (b) A swollen zoospore on the right of a zoosporangium shows a lumpy surface and uses two flagella to move towards plant tissues (c). (d) Encysted spores lose their flagella and show a smooth surface. Scale bars: (a–d) 5 μm. Germination of spores into sunflower roots (e–g) and leaves (h–j). (e) Germinating spores in the root hair emerging zone. White arrow indicates penetration of a germ tube directly through the epidermis without appressorium formation. (f) Spore with a germ tube forming an appressorium. (g) Spore penetrating at the base of a root hair without a visible appressorium. (h) Spores germinating on leaves, grouped around a trichome. (i) Spore germination with appressorium formation near a stomata. (j) Rare entry of P. halstedii in leaves through stomata. Scale bars: 20 μm (e); 5 μm (f, g, i, j); 100 μm (h). (k–m) Dissemination structures of P. halstedii. (k) Sporulation of P. halstedii on the lower face of a cotyledon. (l) Enlargement of a zone from (k) showing zoosporangiophores exiting the plant through stomata. (m) Zoosporangia (Za) carried by a zoosporangiophore (Zp). Scale bars: 100 μm (k, m), 5 μm (l).
To decipher the different strategies deployed by the pathogen to colonize sunflower roots, scanning electronic microscopy (SEM) was used to follow root infection on TS and TSRM plants inoculated by pathotype 710 and grown in hydroponic conditions. Plasmopara halstedii zoosporangia release up to 20 mobile zoospores each (Fig. 4a,b). These mobile zoospores of P. halstedii possess two flagella (Fig. 4b,c) used to swim in soil free water and to reach epidermal root cells. In our infection conditions, all germinated zoospores were randomly dispersed on epidermal cells without apparent chemoattraction or electrotaxis, as reported for other root oomycete pathogens, such as Phytophthora sojae and Phytophthora palmivora (Morris et al., 1998; Tyler et al., 1996), and for P. halstedii by Delanoë (1972), who described zoospore attraction to sunflower root surfaces by unidentified chemical compounds secreted by the plant.
Contact with the root surface induced P. halstedii zoospore encystment involving ultrastructural changes, including the loss of flagella and the appearance of a smooth surface (Fig. 4d). Other changes, not checked in our experiments, including the synthesis of a cell wall and deposition on the spore surface of an amorphous electron‐dense material, were described by Gray et al. (1985) in P. halstedii as in other oomycetes. Twenty‐four hours after infection, the majority of spores were encysted and then germinated in the root hair emerging zone (Fig. 4e). Plasmopara halstedii germ tubes can enter root tissues by three different ways: mostly by the formation of an appressorium structure (Fig. 4f), but also through injuries, especially at the bases of root hairs (Fig. 4g), or by direct penetration without an appressorium through the wall of epidermal cells (Fig. 4e). These three penetration modes have been described previously for pathotypes other than 710 (Nishimura, 1922; Wehtje et al., 1979; Wehtje and Zimmer, 1978). Furthermore, it has been shown that the penetration of P. halstedii pathotype 300 via an appressorium leads to the formation of an infection vesicle in the root epidermal cell, suggesting a successful infection (Wehtje et al., 1979). Subsequently, the pathogen may grow through one or two cortical cells before it becomes intercellular (Wehtje et al., 1979).
In our conditions, no differences were observed in the establishment of infection structures between interactions that were incompatible or compatible as a result of the presence/absence of the Pl5 resistance gene. In contrast, Wehtje and Zimmer (1978) reported that resistance conditioned by the Pl2 gene (type I resistance) could inhibit the penetration or pre‐penetration processes during or soon after zoospore encystment.
Plant colonization and defence reactions
Following root penetration, P. halstedii grows intercellularly through plant tissues by coenocytic hyphae (Fig. 5a–c), producing infection/nutrition invaginations, named haustoria, in root, hypocotyl and cotyledon cells (Fig. 5a–c). During the compatible interaction between TS and pathotype 710, P. halstedii hyphae reach the top of the plant and infection becomes systemic (Fig. 2g), which is not observed for the resistant line TSRM.
Figure 5.
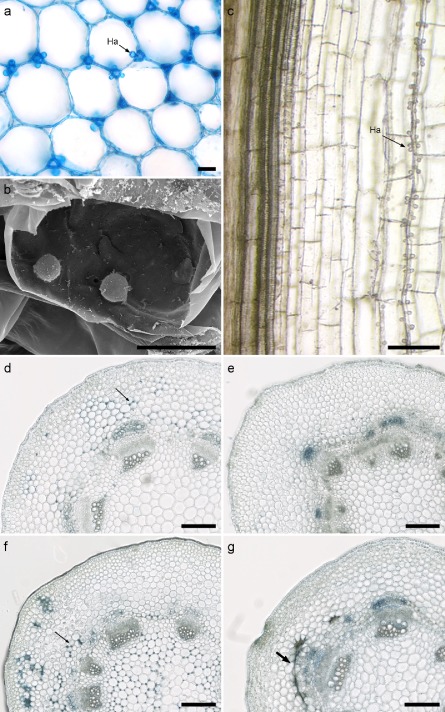
Plasmopara halstedii hyphae and haustoria in hypocotyl and cotyledon tissues of susceptible and resistant sunflower lines. (a) Transverse sections of hypocotyls stained with ‘cotton blue’ in a lactophenol blue solution. Intercellular hyphae form haustoria (Ha) in adjacent cortical cells (black arrow). (b) Several haustoria were frequently observed in single hypocotyl and cotyledon cells [scanning electron microscopy (SEM) observations]. (c) Longitudinal section of a hypocotyl showing hyphal growth parallel to vascular bundles as a single track from the bottom to the top, and haustoria (Ha) in adjacent cells. (d–g) Time course of P. halstedii (pathotype 710) in a hypocotyl of susceptible (d, f) and resistant (e, g) near‐isogenic lines of sunflower. Transverse section at 6 (d, e) and 10 (f, g) days post‐inoculation stained with lactophenol blue. In the susceptible line TS, P. halstedii grew in both cortical and medullar cells of the hypocotyl (see arrows in d and f), whereas, in the resistant line TSRM, no (e) or very few (g) hyphae were visible and spider web hypersensitive response (HR)‐like necroses (thick arrow, g) developed within the cortical parenchyma. Scale bars: 20 μm (a, b); 200 μm (c–g).
However, in roots of both susceptible and resistant sunflower lines, a few hyphae and haustoria were observed, suggesting that, in type II resistance, the growth of the pathogen is not completely blocked at the root level (data not shown). However, from 6 days post‐inoculation (dpi) in hypocotyl sections, large numbers of hyphae could be observed between cortical cells of TS plants (Fig. 5d, arrow), whereas no hyphae were detected in its NIL counterpart TSRM (Fig. 5e). This trend was confirmed at 10 dpi. In TS, all hypocotyl tissues, except vascular bundles, were fully infected by P. halstedii (Fig. 5f), whereas, in TSRM, far fewer hyphae were observed and none were seen in the central cylinder (Fig. 5g). Moreover, defence reactions, visualized by groups of brown collapsed cells formed in contact with the pathogen, were only observed in TSRM cortex at 10 dpi (Fig. 5g, thick arrow). These reactions resembled the hypersensitive response (HR)‐like reactions of hypocotyls and cotyledons described for type II (Pl1, Pl5 and Pl8) and type I (Pl2) resistances (Mouzeyar et al., 1993; Radwan et al., 2005, 2011). Early HR‐like reactions (before 6 dpi), resulting from contact with the pathogen, could explain the difference in P. halstedii colonization levels between TS and TSRM plants.
HR‐like reactions described in type I and type II resistances were accompanied by callose deposition, phenolic compound accumulation, and cell divisions around necrotic cells in several studies (Mouzeyar et al., 1993; Radwan et al., 2005, 2011). HR‐like reactions observed in the hypocotyl might increase sunflower resistance by limiting pathogen growth. The induction of sunflower defence genes during P. halstedii interactions is, however, poorly documented (Radwan et al., 2005, 2011; As‐Sadi, 2011).
The presence of HR‐like reactions does not completely stop pathogen progression in hypocotyls and cotyledons of TSRM (as shown by weak sporulation on cotyledons). However, the absence of P. halstedii in TSRM leaves suggests that the region of the cotyledon node may serve as a mechanical or physiological barrier to oomycete spread, as hypothesized by Delanoë (1972) and Allard (1978).
Defence gene activation, leading to the production of antimicrobial compounds, HR‐like reactions and other unknown mechanisms, is probably sufficient in resistant plants to impair mycelium growth in leaves and to stop systemic spread that would result in the formation of dissemination structures.
Pathogen sporulation and dissemination
At about 14 dpi in the growth chamber at a cool temperature (18–20 °C), after 2 days under saturating humidity conditions, sporulation occurred; a dense white down, visible to the naked eye, was produced on the cotyledon and/or leaf surfaces (Figs 2f,g and 3). This down was composed of zoosporangiophores (or conidiophores) (Fig. 4k–m). In the field, P. halstedii sporulation is only observed under favourable environmental conditions and notably high humidity.
Plasmopara halstedii hyphae were seen to exit the plant mainly through the stomata of leaves and cotyledons (Fig. 4l). At these sites, the hyphae developed branched zoosporangiophores, carrying the dissemination organs, zoosporangia, at the ends of the branches [Figs 4m and S1 (see Supporting Information)]. Zoosporangiophores were also formed on hypocotyl tissues and on roots (Fig. S1), but we cannot exclude the possibility that their formation on roots was facilitated by the hydroponic conditions used. It should be noted that P. halstedii zoosporangiophores and zoosporangia that formed on sunflower cotyledons, hypocotyls or roots showed different shapes (Fig. S1), as already described by Zahka and Viranyi (1991). Nishimura (1922) observed zoosporangia already formed by the pathogen in the substomatal cavity before the leaf emergence of zoosporangiophores. In addition, Bouterige et al. (2003) studied zoosporangia maturation in vitro leading to the formation and liberation of zoospores by the addition of 1% sucrose. At the end of the maturation, an apical papilla was formed at the top of zoosporangia through which the zoospores would be released (Figs 4a,b and S1).
The same types of dissemination structure have been observed for other phytopathogenic oomycetes, such as P. viticola on grapevine (Gessler et al., 2011). The shape of zoosporangiophores has been used to classify oomycetes, but this method has been overemphasized and is controversial (Thines, 2014; Voglmayr et al., 2004).
Shoot infection
Following sporulation, the dissemination of zoosporangia can lead to the secondary spread of infections (Fig. 3). This step in the life cycle of P. halstedii is responsible for late infections of neighbouring plants and contributes to pathogen dissemination (Spring, 2009).
In order to mimic shoot contaminations by zoosporangia in the field, a drop infection method, by the deposition of a suspension of zoosporangia on sunflower TS and TSRM leaves, was used. Grouped around leaf trichomes (Fig. 4h) and main veins (data not shown), zoospore encystment involved similar ultrastructural changes as in root infections (Bouterige et al., 2003; Nishimura, 1922). Encysted zoospores germinated rapidly [starting from 4 h post‐inoculation (hpi)] and subsequently penetrated plant tissues. Generally, the penetration of leaf tissues was enabled by appressoria formation close to intercellular junctions (Fig. 4i). Although Nishimura (1922) reported that P. halstedii enters leaves by stomatal openings, we rarely observed this on TS and TSRM leaves inoculated by pathotype 710 (Fig. 4j). These results contrast with P. viticola grapevine leaf infections, in which zoospores germinate on stomata (Dièsez‐Navajas et al., 2007; Kiefer et al., 2002) or differentiate a germ tube towards stomatal openings (Burruano, 2000). In contrast with root infections by P. halstedii, direct entry in leaf tissues was not observed, probably because of the cuticle present on the sunflower leaf surface. Following appressorium formation, it is still unclear how P. halstedii invades leaf tissues: does it establish an infection vesicle in the epidermal cell as in roots, and how does it develop in leaf parenchyma?
More generally, following leaf infection, little is known about further P. halstedii colonization of the entire plant and how it impacts sunflower yield. However, leaf infections leading to systemic infection in upper plant parts and, conversely, basipetal growth of P. halstedii hyphae in the shoot, have been reported (Meliala et al., 2000; Spring, 2009).
Molecular Resources and Pathogenicity Effectors
Plant‐pathogenic oomycetes, either obligate biotrophs or hemibiotrophs, rely for their developmental cycle on pathogenicity factors, called effectors, that modify the metabolism of host plants to their benefit and thus enable pathogenicity (Bozkurt et al., 2012). Repertoires of hundreds of effector proteins that can be localized in either the apoplasm or cytoplasm of plant cells have been described to be encoded in oomycete genomes (Stassen and Van den Ackerveken, 2011). Within the class of cytoplasmic effectors, the RXLR and Crinkler (CRN) families have been shown to be secreted by the pathogen from haustoria and translocated into host cells (Schornack et al., 2010; Whisson et al., 2007). In addition to a signal peptide, RXLR proteins exhibit a characteristic RXLR amino acid motif that is sometimes associated with a (D)EER motif, whereas CRN proteins show a characteristic LXLFLAK motif (Rehmany et al., 2005; Torto et al., 2003). The different virulence profiles shown by pathotypes of P. halstedii (Fig. 1) could be explained by an intricate repertoire of effectors, and this has motivated our effort to develop P. halstedii genomics.
Despite the economic importance of downy mildew diseases caused by Plasmopara species, genomic resources were very limited until recently, even for P. viticola, the agent of grapevine downy mildew (As‐Sadi et al., 2011; Bouzidi et al., 2007; Mestre et al., 2012). The obligate biotrophic requirements of these organisms, and our consequent inability to grow them in vitro, could explain the lack of genomic resources. The genome size of P. halstedii was estimated at 100 Mbp (Q. Gascuel, O. Catrice, L. Godiard LIPM, Castanet‐Tolosan, France, unpublished data), close to the genome sizes of other Peronosporales, such as Hyaloperonospora arabidopsidis (100 Mbp) and Phytophthora sojae (95 Mbp) (Baxter et al., 2010; Tyler et al., 2006). In contrast with these model oomycetes that can be transformed, genetic tools must be improved for P. halstedii. For example, transient expression of green fluorescent protein (GFP) was shown to be possible in P. halstedii using electroporation of sporangia, but GFP expression was lost during subsequent infections (Hammer et al., 2007).
The first characterization of P. halstedii ESTs used a subtractive hybridization method applied to infected sunflower seedlings, and led to the identification of 230 non‐redundant ESTs, 145 of which were assigned by polymerase chain reaction (PCR) tests on genomic DNA to be from P. halstedii (Bouzidi et al., 2007). Among the predicted proteins, three shared significant homology to a Phytophthora infestans elicitor and to protease inhibitors related to pathogenicity, and five corresponded to unknown proteins having a signal peptide suggesting that they could be secreted proteins, but no RXLR or CRN effector was identified (Bouzidi et al., 2007).
Extensive P. halstedii EST sequencing and search for RXLR and CRN effectors have been initiated by our group (As‐Sadi et al., 2011). 454® pyrosequencing of cDNA was performed using two sunflower genotypes, XRQ and PSC8, infected by pathotype 710. These genotypes are resistant and susceptible, respectively (As‐Sadi et al., 2011). About 45 000 non‐redundant clusters, that may be from either sunflower or pathogen origin, were assembled and made available through a web‐based portal http://www.heliagene.org/cgi/HP.cgi. This resource was screened on dedicated plant or oomycete libraries using in silico blast filtering, and 577 clusters predicted to have an oomycete origin were identified, including 389 new P. halstedii genes (As‐Sadi et al., 2011). Most of the genes encode predicted proteins with unknown functions (only 32 had an Interpro motif), but the highest blast scores were obtained with proteins of hemibiotroph Phytophthora species (73% of the genes) and, to a lesser extent (17%), with the obligate biotroph Hyaloperonospora arabidopsidis, suggesting that P. halstedii does not share closer sequence homology with a pathogen causing downy mildew than with other Peronosporales oomycetes causing late blight disease. Homology searches based on known sequences of oomycete cytoplasmic effectors led to the characterization of 20 potential effectors having the conserved motifs found in RXLR and CRN effectors, showing, for the first time, that P. halstedii probably has the same kind of cytoplasmic effectors as found in other oomycetes (As‐Sadi et al., 2011).
To increase the number of P. halstedii expressed sequences, we performed a deeper transcriptome 454® sequencing based on the following materials: hypocotyls from infected susceptible sunflower plants of the inbred GB line and in vitro‐germinated spores corresponding to a pre‐infectious stage (the last stage at which it is possible to obtain P. halstedii material free of plant tissue). Four pathotypes of P. halstedii (100, 304‐10, 703 and 710), representatives of the three groups of pathotypes described by Delmotte et al. (2008), were used; 80 354 non‐redundant EST clusters were assembled and compared with draft genomic assemblies from P. halstedii in order to select only the oomycete clusters (S. Carrère, J. Gouzy, L. Godiard, LIPM, Castanet‐Tolosan, France, unpublished data). The selected 24 597 P. halstedii clusters (mean size of 621 nucleotides) and 17 417 predicted peptides (mean size of 163 amino acids) will soon be made available in a new database, named PlasmoparaSp. This database was screened to identify the repertoire of expressed RXLR and CRN effectors from P. halstedii. Forty‐nine expressed putative RXLRs and 54 CRNs were identified (L. Godiard, LIPM, Castanet‐Tolosan, France, unpublished data); these numbers are consistent with data from other oomycetes (Cabral et al., 2011; Haas et al., 2009). All the selected RXLRs have a predicted signal peptide, suggesting that they could be secreted from the pathogen. In contrast, 80% of P. halstedii CRNs were not predicted to have a canonical signal peptide, as reported for CRNs from other oomycete species (Stam et al., 2013), suggesting that the highly conserved N‐terminal sequences detected in P. halstedii CRN effectors could not be recognized by the prediction programs used. Nevertheless, the role of signal peptides in effector secretion must be shown experimentally. The putative translocation motifs of P. halstedii RXLR and CRN effectors were very similar to the published consensus sequences. Forty per cent of the RXLRs showed the exact RXLR consensus followed, or not, by the EER motif, and 29% had only the (D)EER motif. Plasmopara halstedii CRN effectors presented the characteristic modular structure composed of an N‐terminal conserved region including the LXLFLAK and HVLVVVP motifs, followed by a variable C‐terminal domain. Variant motifs of LXLFLAK (28%) were observed, including LXLSLAK (35%) and LXLYLAK (20%), as observed for other oomycetes (Gaulin et al., 2008). Our analyses revealed the presence in P. halstedii CRN effectors of nine different CRN C‐terminal domains (DBE, DBF, DFB, DN5, DN17, DO, DXZ, newD2 and SN6) already described in P. infestans (Haas et al., 2009). Comparing the putative effectors of P. halstedii with those of other oomycetes identified both conserved and P. halstedii‐specific effectors, and revealed that the effector repertoire of P. halstedii is overall closely related to that of Phytophthora species (P. Mestre, INRA, Colmar, France and L. Godiard, LIPM, Castanet‐Tolosan, France, unpublished results).
Future Prospects
Plasmopara halstedii, an obligate biotroph oomycete causing downy mildew on the important crop plant sunflower, offers puzzling traits, such as diversified infection modes on plant roots and shoots, and a still enigmatic reproduction cycle. The adaptable characteristics of P. halstedii might favour the rapid evolution of its virulence, illustrated by the occurrence of novel pathotypes overcoming the resistance used in sunflower cultivars. In addition, evolutionary questions raised about its increasing virulence make plant genetic control of this pathogen a major stake and render this pathogen particularly interesting to study. Moreover, a global population genetic study should provide information on the origin of this invasive pathogen from North America and elucidate its worldwide dispersion and co‐evolution with sunflower.
Pathogen effectors are probably major players in the interaction between P. halstedii and sunflower, as illustrated in other oomycete–plant interactions. An essential issue will be to define P. halstedii effectors that may have an impact on its capacity to infect the plant, on host specificity and on the pathogen developmental cycle in planta. The establishment of in vitro growth conditions and the development of genetic tools for P. halstedii would be good assets to solve these challenges.
To circumvent these difficulties, we developed genomic data on several P. halstedii pathotypes, and aimed to describe the complete set of effectors. A comprehensive study of effector polymorphism will be performed in P. halstedii pathotypes in order to find key determinants of pathogen virulence and host specialization. The identification of effectors with large variability between P. halstedii isolates and under positive selection should constitute a starting point to understand pathogen evolution. In contrast, the identification of the effectors most conserved between P. halstedii pathotype isolates, corresponding to essential pathogen genes, could be used to screen sunflower genetic resources to find more sustainable resistance genes.
In parallel, functional studies should be used to specify the roles of these proteins in sunflower cells and how they condition pathogen virulence. For this purpose, agroinfection methods have been set up in sunflower plants, allowing the transient expression of P. halstedii effectors (Q. Gascuel, unpublished data). Ultimately, these studies should unveil molecular and physiological mechanisms, and hence lead to a better understanding of sunflower–P. halstedii interactions.
Supporting information
Fig. S1 Various Plasmopara halstedii zoosporangiophores formed on sunflower tissues. Zoosporangiophore (Zp) carrying zoosporangia (Za) on cotyledon (a), hypocotyl tissue (b), emerging probably through a lenticel. (c, d) Zoosporangiophores formed on roots, near root hairs. Scale bars: 100 μm (a, b); 50 μm (c, d).
Acknowledgements
The authors thank Patrick Vincourt, Clare Gough (LIPM, Castanet‐Tolosan, France) and Nicolas Frei dit Frey (Université Toulouse III/CNRS, LRSV, Castanet‐Tolosan, France) for critical reading of the manuscript, and Pere Mestre (INRA, Colmar, France) for allowing the citation of unpublished data. We acknowledge Denis Tourvieille de Labrouhe (INRA, Clermont‐Ferrand, France) for his expertise on sunflower downy mildew and for providing P. halstedii pathotypes, Nicolas Blanchet (LIPM, Castanet‐Tolosan, France) for preparing plant material and Isabelle Fourquaux (Université Toulouse III, France) for preparing scanning electron microscopy samples. We are indebted to the TRI Platform, Toulouse, France for allowing access to imaging facilities. This work was supported by the French Laboratory of Excellence project ‘TULIP’ (ANR‐10‐LABX‐41; ANR‐11‐IDEX‐0002‐02).
References
- Ahmed, S. , Tourvieille de Labrouhe, D. and Delmotte, F. (2012) Emerging virulence arising from hybridisation facilitated by multiple introductions of the sunflower downy mildew pathogen Plasmopara halstedii . Fungal Genet. Biol. 49, 847–855. [DOI] [PubMed] [Google Scholar]
- Allard, C. (1978) Invasion et colonisation systémique de la plantule de tournesol (Helianthus annuus L.) par le Plasmopara halstedii (Farl.) Berl. et de Toni. Ann. Phytopathol. 10, 197–217. [Google Scholar]
- As‐Sadi, F. (2011) Genetic and genomic based approaches towards a better sustainability of sunflower Helianthus annuus resistance to downy mildew Plasmopara halstedii . PhD, Université Toulouse III Paul Sabatier.
- As‐Sadi, F. , Carrere, S. , Gascuel, Q. , Hourlier, T. , Rengel, D. , Le Paslier, M.‐C. , Bordat, A. , Boniface, M.C. , Brunel, D. , Gouzy, J. , Godiard, L. and Vincourt, P. (2011) Transcriptomic analysis of the interaction between Helianthus annuus and its obligate parasite Plasmopara halstedii shows single nucleotide polymorphisms in CRN sequences. BMC Genomics, 12, 498–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachlava, E. , Radwan, O.E. , Abratti, G. , Tang, S. , Gao, W. , Heesacker, A.F. , Bazzalo, M.E. , Zambelli, A. , Leon, A.J. and Knapp, S.J. (2011) Downy mildew Pl8 and Pl14 and rust RAdv resistance genes reside in close proximity to tandemly duplicated clusters of non‐TIR‐like NBS‐LRR‐encoding genes on sunflower chromosomes 1 and 13. Theor. Appl. Genet. 122, 1211–1221. [DOI] [PubMed] [Google Scholar]
- Baxter, L. , Tripathy, S. , Ishaque, N. , Boot, N. , Cabral, A. , Kemen, E. , Thines, M. , Ah‐Fong, A. , Anderson, R. , Badejoko, W. , Bittner‐Eddy, P. , Boore, J.L. , Chibucos, M.C. , Coates, M. , Dehal, P. , Delehaunty, K. , Dong, S. , Downton, P. , Dumas, B. , Fabro, G. , Fronick, C. , Fuerstenberg, S.I. , Fulton, L. , Gaulin, E. , Govers, F. , Hughes, L. , Humphray, S. , Jiang, R.H.Y. , Judelson, H. , Kamoun, S. , Kyung, K. , Meijer, H. , Minx, P. , Morris, P. , Nelson, J. , Phuntumart, V. , Qutob, D. , Rehmany, A. , Rougon‐Cardoso, A. , Ryden, P. , Torto‐Alalibo, T. , Studholme, D. , Wang, Y. , Win, J. , Wood, J. , Clifton, S.W. , Rogers, J. , Van den Ackerveken, G. , Jones, J.D.G. , McDowell, J.M. , Beynon, J. and Tyler, B.M. (2010) Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science, 330, 1549–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouterige, S. , Tronchin, G. , Lesourd, M. , Marot‐Leblond, A. , Molinero, V. , Bouchara, J.P. and Robert, R. (2003) Ultrastructural and immunochemical changes during the in vitro development of Plasmopara halstedii . Phytopathology, 93, 1023–1030. [DOI] [PubMed] [Google Scholar]
- Bouzidi, M.F. , Badaoui, S. , Cambon, F. , Vear, F. , De Labrouhe, D.T. , Nicolas, P. and Mouzeyar, S. (2002) Molecular analysis of a major locus for resistance to downy mildew in sunflower with specific PCR‐based markers. Theor. Appl. Genet. 104, 592–600. [DOI] [PubMed] [Google Scholar]
- Bouzidi, M.F. , Parlange, F. , Nicolas, P. and Mouzeyar, S. (2007) Expressed Sequence Tags from the oomycete Plasmopara halstedii, an obligate parasite of the sunflower. BMC Microbiol. 7, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt, T.O. , Schornack, S. , Banfield, M.J. and Kamoun, S. (2012) Oomycetes, effectors, and all that jazz. Curr. Opin. Plant Biol. 15, 483–492. [DOI] [PubMed] [Google Scholar]
- Burruano, S. (2000) The life‐cycle of Plasmopara viticola, cause of downy mildew of vine. Mycologist, 14, 179–182. [Google Scholar]
- Cabral, A. , Stassen, J.H. , Seidl, M.F. , Bautor, J. , Parker, J.E. and Van den Ackerveken, G. (2011) Identification of Hyaloperonospora arabidopsidis transcript sequences expressed during infection reveals isolate‐specific effectors. PLoS One, 6, e19328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, Y. and Sackston, W.E. (1973) Factors affecting infection of sunflower by Plasmopara halstedii . Can. J. Bot. 51, 15–22. [Google Scholar]
- Constantinescu, O. , Voglmayr, H. , Fatehi, J. and Thines, M. (2005) Plasmoverna gen. nov., and the taxonomy and nomenclature of Plasmopara (Chromista, Peronosporales). Taxon, 54, 813–821. [Google Scholar]
- Delanoë, D. (1972) Biologie et épidémiologie du mildiou du tournesol (Plasmopara helianthi Novot.). Inf. Tech. CETIOM, 29, 1–47. [Google Scholar]
- Delmotte, F. , Chen, W.J. , Richard‐Cervera, S. , Greif, C. , Papura, D. , Giresse, X. , Mondor‐Genson, G. and Corio‐Costet, M.F. (2006) Microsatellite DNA markers for Plasmopara viticola, the causal agent of downy mildew of grapes. Mol. Ecol. Notes. 6, 379–381. [Google Scholar]
- Delmotte, F. , Giresse, X. , Richard‐Cervera, S. , M'Baya, J. , Vear, F. , Tourvieille, J. , Walser, P. and Tourvieille de Labrouhe, D. (2008) Single nucleotide polymorphisms reveal multiple introductions into France of Plasmopara halstedii, the plant pathogen causing sunflower downy mildew. Infect. Genet. Evol. 8, 534–540. [DOI] [PubMed] [Google Scholar]
- Dièsez‐Navajas, A.M. , Greif, C. , Poutaraud, A. and Merdinoglu, D. (2007) Two simplified fluorescent staining techniques to observe infection structures of the oomycete Plasmopara viticola in grapevine leaf tissues. Micron, 38, 680–683. [DOI] [PubMed] [Google Scholar]
- Franchel, J. , Bouzidi, M.F. , Bronner, G. , Vear, F. , Nicolas, P. and Mouzeyar, S. (2013) Positional cloning of a candidate gene for resistance to the sunflower downy mildew, Plasmopara halstedii race 300. Theor. Appl. Genet. 126, 359–367. [DOI] [PubMed] [Google Scholar]
- Gaulin, E. , Madoui, M.A. , Bottin, A. , Jacquet, C. , Mathe, C. , Couloux, A. , Wincker, P. and Dumas, B. (2008) Transcriptome of Aphanomyces euteiches: new oomycete putative pathogenicity factors and metabolic pathways. PLoS One, 3, e1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzbittel, L. , Mouzeyar, S. , Badaoui, S. , Mestries, E. , Vear, F. , De Labrouhe, D.T. and Nicolas, P. (1998) Cloning of molecular markers for disease resistance in sunflower, Helianthus annuus L. Theor. Appl. Genet. 96, 519–525. [DOI] [PubMed] [Google Scholar]
- Gessler, C. , Pertot, I. and Perazzolli, M. (2011) Plasmopara viticola: a review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 50, 3–44. [Google Scholar]
- Göker, M. , Voglmayr, H. , Riethmüller, A. and Oberwinkler, F. (2007) How do obligate parasites evolve? A multi‐gene phylogenetic analysis of downy mildews. Fungal Genet. Biol. 44, 105–122. [DOI] [PubMed] [Google Scholar]
- Gray, A.B. , Sackston, W.E. and Thauvette, L. (1985) The development of infection structures of Plasmopara halstedii in suspensions of sunflower cells. Can. J. Bot. 63, 1817–1819. [Google Scholar]
- Grenville‐Briggs, L.J. and Van West, P. (2005) The biotrophic stages of oomycete–plant interactions. Adv. Appl. Microbiol. 57, 217–243. [DOI] [PubMed] [Google Scholar]
- Grünwald, N.J. and Flier, W.G. (2005) The biology of Phytophthora infestans at its center of origin. Annu. Rev. Phytopathol. 43, 171–190. [DOI] [PubMed] [Google Scholar]
- Gulya, T.J. , Tourvieille de Labrouhe, D. , Masirevic, S. , Penaud, A. , Rashid, K. and Viranyi, F. (1998) Proposal for the standardized nomenclature and identification of races of Plasmopara halstedii (sunflower downy mildew). In: Sunflower Downy Mildew Symposium, Proceedings of Sunflower Downy Mildew Symposium, International Sunflower Association Symposium III, Fargo, ND, USA , pp. 130–136.
- Haas, B.J. , Kamoun, S. , Zody, M.C. , Jiang, R.H.Y. , Handsaker, R.E. , Cano, L.M. , Grabherr, M. , Kodira, C.D. , Raffaele, S. , Torto‐Alalibo, T. , Bozkurt, T.O. , Ah‐Fong, A.M.V. , Alvarado, L. , Anderson, V.L. , Armstrong, M.R. , Avrova, A. , Baxter, L. , Beynon, J. , Boevink, P.C. , Bollmann, S.R. , Bos, J.I.B. , Bulone, V. , Cai, G. , Cakir, C. , Carrington, J.C. , Chawner, M. , Conti, L. , Costanzo, S. , Ewan, R. , Fahlgren, N. , Fischbach, M.A. , Fugelstad, J. , Gilroy, E.M. , Gnerre, S. , Green, P.J. , Grenville‐Briggs, L.J. , Griffith, J. , Grünwald, N.J. , Horn, K. , Horner, N.R. , Hu, C. , Huitema, E. , Jeong, D. , Jones, A.M.E. , Jones, J.D.G. , Jones, R.W. , Karlsson, E.K. , Kunjeti, S.G. , Lamour, K. , Liu, Z. , Ma, L. , Maclean, D. , Chibucos, M.C. , McDonald, H. , McWalters, J. , Meijer, H.J.G. , Morgan, W. , Morris, P.F. , Munro, C.A. , O'Neill, K. , Ospina‐Giraldo, M. , Pinzón, A. , Pritchard, L. , Ramsahoye, B. , Ren, Q. , Restrepo, S. , Roy, S. , Sadanandom, A. , Savidor, A. , Schornack, S. , Schwartz, D.C. , Schumann, U.D. , Schwessinger, B. , Seyer, L. , Sharpe, T. , Silvar, C. , Song, J. , Studholme, D.J. , Sykes, S. , Thines, M. , van de Vondervoort, P.J.I. , Phuntumart, V. , Wawra, S. , Weide, R. , Win, J. , Young, C. , Zhou, S. , Fry, W. , Meyers, B.C. , van West, P. , Ristaino, J. , Govers, F. , Birch, P.R.J. , Whisson, S.C. , Judelson, H.S. and Nusbaum, C. (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans . Nature, 461, 393–398. [DOI] [PubMed] [Google Scholar]
- Hammer, T.R. , Thines, M. and Spring, O. (2007) Transient expression of gfp in the obligate biotrophic oomycete Plasmopara halstedii using electroporation and a mechanoperforation method. Plant Pathol. 56, 177–182. [Google Scholar]
- Intelmann, F. and Spring, O. (2002) Analysis of total DNA by minisatellite and simple‐sequence repeat primers for the use of population studies in Plasmopara halstedii . Can. J. Microbiol. 48, 555–559. [DOI] [PubMed] [Google Scholar]
- Ioos, R. , Laugustin, L. , Rose, S. , Tourvieille, J. and de Labrouhe, D.T. (2007) Development of a PCR test to detect the downy mildew causal agent Plasmopara halstedii in sunflower seeds. Plant Pathol. 56, 209–218. [Google Scholar]
- Kiefer, B. , Riemann, M. , Buche, C. , Kassemeyer, H.H. and Nick, P. (2002) The host guides morphogenesis and stomatal targeting in the grapevine pathogen Plasmopara viticola . Planta, 215, 387–393. [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Gulya, T.J. , Seiler, G.J. , Vick, B.A. and Jan, C.C. (2012) Molecular mapping of the Pl(16) downy mildew resistance gene from HA‐R4 to facilitate marker‐assisted selection in sunflower. Theor. Appl. Genet. 125, 121–131. [DOI] [PubMed] [Google Scholar]
- Meliala, C. , Vear, F. and Tourvieille de Labrouhe, D. (2000) Relation between date of infection of sunflower downy mildew (Plasmopara halstedii) and symptoms development. Helia, 23, 35–44. [Google Scholar]
- Mestre, P. , Piron, M.C. and Merdinoglu, D. (2012) Identification of effector genes from the phytopathogenic oomycete Plasmopara viticola through the analysis of gene expression in germinated zoospores. Fungal Biol. 116, 825–835. [DOI] [PubMed] [Google Scholar]
- Michelmore, R.W. and Wong, J. (2008) Classical and molecular genetics of Bremia lactucae, cause of lettuce downy mildew. Eur. J. Plant Pathol. 122, 19–30. [Google Scholar]
- Moinard, J. , Mestries, E. , Penaud, A. , Pinochet, X. , Tourvieille de Labrouhe, D. , Vear, F. , Tardin, M.‐C. , Pauchet, I. and Eychenne, N. (2006) An overview of sunflower downy mildew. Phytoma—La Défense des Végétaux, 589, 34–38. [Google Scholar]
- Morris, P.F. , Bone, E. and Tyler, B.M. (1998) Chemotropic and contact responses of Phytophthora sojae hyphae to soybean isoflavonoids and artificial substrates. Plant Physiol. 117, 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouzeyar, S. , Tourvieille de Labrouhe, D. and Vear, F. (1993) Histopathological studies of resistance of sunflower (Helianthus annuus L.) to downy mildew (Plasmopara halstedii). J. Phytopathol. 139, 289–297. [Google Scholar]
- Mouzeyar, S. , Delabrouhe, D.T. and Vear, F. (1994) Effect of host–race combination on resistance of sunflower, Helianthus annuus L., to downy mildew Plasmopara halstedii . J. Phytopathol. 141, 249–258. [Google Scholar]
- Mulpuri, S. , Liu, Z. , Feng, J. , Gulya, T.J. and Jan, C.C. (2009) Inheritance and molecular mapping of a downy mildew resistance gene, Pl (13) in cultivated sunflower (Helianthus annuus L.). Theor. Appl. Genet. 119, 795–803. [DOI] [PubMed] [Google Scholar]
- Musetti, R. , Stringher, L. , Borselli, S. , Vecchione, A. , Zulini, L. and Pertot, I. (2005) Ultrastructural analysis of Vitis vinifera leaf tissues showing atypical symptoms of Plasmopara viticola . Micron, 36, 73–80. [DOI] [PubMed] [Google Scholar]
- Nishimura, M. (1922) Studies in Plasmopara halstedii . J. Coll. Agric. Hokkaido Imp. Univ. XI (Pt 3), 185–210. [Google Scholar]
- Radwan, O. , Bouzidi, M.F. , Vear, F. , Philippon, J. , de Labrouhe, D.T. , Nicolas, P. and Mouzeyar, S. (2003) Identification of non‐TIR‐NBS‐LRR markers linked to the Pl5/Pl8 locus for resistance to downy mildew in sunflower. Theor. Appl. Genet. 106, 1438–1446. [DOI] [PubMed] [Google Scholar]
- Radwan, O. , Bouzidi, M.F. , Nicolas, P. and Mouzeyar, S. (2004) Development of PCR markers for the Pl5/Pl8 locus for resistance to Plasmopara halstedii in sunflower, Helianthus annuus L. from complete CC‐NBS‐LRR sequences. Theor. Appl. Genet. 109, 176–185. [DOI] [PubMed] [Google Scholar]
- Radwan, O. , Mouzeyar, S. , Venisse, J.S. , Nicolas, P. and Bouzidi, M.F. (2005) Resistance of sunflower to the biotrophic oomycete Plasmopara halstedii is associated with a delayed hypersensitive response within the hypocotyls. J. Exp. Bot. 56, 2683–2693. [DOI] [PubMed] [Google Scholar]
- Radwan, O. , Gandhi, S. , Heesacker, A. , Whitaker, B. , Taylor, C. , Plocik, A. , Kesseli, R. , Kozik, A. , Michelmore, R.W. and Knapp, S.J. (2008) Genetic diversity and genomic distribution of homologs encoding NBS‐LRR disease resistance proteins in sunflower. Mol. Gen. Genom. 280, 111–125. [DOI] [PubMed] [Google Scholar]
- Radwan, O. , Bouzidi, M.F. and Mouzeyar, S. (2011) Molecular characterization of two types of resistance in sunflower to Plasmopara halstedii, the causal agent of downy mildew. Phytopathology, 101, 970–979. [DOI] [PubMed] [Google Scholar]
- Rehmany, A.P. , Gordon, A. , Rose, L.E. , Allen, R.L. , Armstrong, M.R. , Whisson, S.C. , Kamoun, S. , Tyler, B.M. , Birch, P.R.J. and Beynon, J.L. (2005) Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines. Plant Cell, 17, 1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmüller, A. , Voglmayr, H. , Goker, M. , Weiss, M. and Oberwinkler, F. (2002) Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia, 94, 834–849. [DOI] [PubMed] [Google Scholar]
- Rozynek, B. and Spring, O. (2000) Pathotypes of sunflower downy mildew in southern parts of Germany. Helia, 23, 27–34. [Google Scholar]
- Sackston, W.E. (1981) Downy mildew of sunflower In: The Downy Mildews (Spencer D.M., ed.), pp. 545–575. London: Academic Press. [Google Scholar]
- Schornack, S. , van Damme, M. , Bozkurt, T.O. , Cano, L.M. , Smoker, M. , Thines, M. , Gaulin, E. , Kamoun, S. and Huitema, E. (2010) Ancient class of translocated oomycete effectors targets the host nucleus. Proc. Natl. Acad. Sci. USA, 107, 17 421–17 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schruft, G. and Kassemeyer, H.H. (2000) Krankheiten und Schadlinge der Weinrebe. Gelsenkirchen: Verlag Th. Mann. [Google Scholar]
- Spring, O. (2000) Homothallic sexual reproduction in Plasmopara halstedii, the downy mildew of sunflower. Helia, 23, 19–26. [Google Scholar]
- Spring, O. (2009) Transition of secondary to systemic infection of sunflower with Plasmopara halstedii—an underestimated factor in the epidemiology of the pathogen. Fungal Ecol. 2, 75–80. [Google Scholar]
- Spring, O. and Zipper, R. (2000) Isolation of oospores of sunflower downy mildew, Plasmopara halstedii, and microscopical studies on oospore germination. J. Phytopathol. 148, 227–231. [Google Scholar]
- Spring, O. and Zipper, R. (2006) Evidence for asexual genetic recombination in sunflower downy mildew, Plasmopara halstedii . Mycol. Res. 110, 657–663. [DOI] [PubMed] [Google Scholar]
- Spring, O. , Rozynek, B. and Zipper, R. (1998) Single spore infections with sunflower downy mildew. J. Phytopathol. 146, 577–579. [Google Scholar]
- Stam, R. , Jupe, J. , Howden, A.J.M. , Morris, J.A. , Boevink, P.C. , Hedley, P.E. and Huitema, E. (2013) Identification and Characterisation CRN Effectors in Phytophthora capsici Shows Modularity and Functional Diversity. PLoS One. 8, e59517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen, J.H.M. and van den Ackerveken, G. (2011) How do oomycete effectors interfere with plant life? Curr. Opin. Plant Biol. 14, 407–414. [DOI] [PubMed] [Google Scholar]
- Thines, M. (2014) Phylogeny and evolution of plant pathogenic oomycetes—a global overview. Eur. J. Plant Pathol. 138, 431–447. [Google Scholar]
- Thines, M. and Kamoun, S. (2010) Oomycete–plant coevolution: recent advances and future prospects. Curr. Opin. Plant Biol. 13, 427–433. [DOI] [PubMed] [Google Scholar]
- Torto, T.A. , Li, S.A. , Styer, A. , Huitema, E. , Testa, A. , Gow, N.A.R. , van West, P. and Kamoun, S. (2003) EST mining and functional expression assays identify extracellular effector proteins from the plant pathogen Phytophthora . Genome Res. 13, 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourvieille de Labrouhe, D. , Pilorgé, E. , Nicolas, P. and Vear, F. (2000) Le Mildiou du Tournesol. Points Techniques. Paris: INRA and CETIOM. [Google Scholar]
- Tourvieille de Labrouhe, D. , Serre, F. , Walser, P. , Roche, S. and Vear, F. (2008) Quantitative resistance to downy mildew (Plasmopara halstedii) in sunflower (Helianthus annuus). Euphytica, 164, 433–444. [Google Scholar]
- Tourvieille de Labrouhe, D. , Walser, P. , Joliovot, D. , Roche, S. , Serre, F. , Delmotte, F. , Bordat, A. , Godiard, L. , Vincourt, P. and Vear, F. (2012) Proposal for improvement of sunflower downy mildew race nomenclature In: Proceedings of the 18th International Sunflower Conference, Mar del Plata, Argentina, March 2012, pp. 322–327. Paris: International Sunflower Association; ed. [Google Scholar]
- Tyler, B.M. , Wu, M.H. , Wang, J.M. , Cheung, W. and Morris, P.F. (1996) Chemotactic preferences and strain variation in the response of Phytophthora sojae zoospores to host isoflavones. Appl. Environ. Microbiol. 62, 2811–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, B.M. , Tripathy, S. , Zhang, X. , Dehal, P. , Jiang, R.H.Y. , Aerts, A. , Arredondo, F.D. , Baxter, L. , Bensasson, D. , Beynon, J.L. , Chapman, J. , Damasceno, C.M.B. , Dorrance, A.E. , Dou, D. , Dickerman, A.W. , Dubchak, I.L. , Garbelotto, M. , Gijzen, M. , Gordon, S.G. , Govers, F. , Grünwald, N.J. , Huang, W. , Ivors, K.L. , Jones, R.W. , Kamoun, S. , Krampis, K. , Lamour, K.H. , Lee, M. , McDonald, W.H. , Medina, M. , Meijer, H.J.G. , Nordberg, E.K. , Maclean, D.J. , Ospina‐Giraldo, M.D. , Morris, P.F. , Phuntumart, V. , Putnam, N.H. , Rash, S. , Rose, J.K.C. , Sakihama, Y. , Salamov, A.A. , Savidor, A. , Scheuring, C.F. , Smith, B.M. , Sobral, B.W.S. , Terry, A. , Torto‐Alalibo, T.A. , Win, J. , Xu, Z. , Zhang, H. , Grigoriev, I.V. , Rokhsar, D.S. and Boore, J.L. (2006) Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science, 313, 1261–1266. [DOI] [PubMed] [Google Scholar]
- Vear, F. , Serieys, H. , Petit, A. , Serre, F. , Boudon, J.P. , Roche, S. , Walser, P. and Tourvieille de Labrouhe, D. (2008a) Origins of major genes for downy mildew resistance in sunflower In: Proceeedings of the 17th International Sunflower Conference, Cordoba, Spain, pp. 125–130. Paris: International Sunflower Association; ed. Junta de Andalucia. [Google Scholar]
- Vear, F. , Serre, F. , Jouan‐Dufournel, I. , Bert, P.F. , Roche, S. , Walser, P. , Tourvieille de Labrouhe, D. and Vincourt, P. (2008b) Inheritance of quantitative resistance to downy mildew (Plasmopara halstedii) in sunflower (Helianthus annuus L.). Euphytica, 164, 561–570. [Google Scholar]
- Vincourt, P. , As‐Sadi, F. , Bordat, A. , Langlade, N.B. , Gouzy, J. , Pouilly, N. , Lippi, Y. , Serre, F. , Godiard, L. , Tourvieille de Labrouhe, D. and Vear, F. (2012) Consensus mapping of major resistance genes and independent QTL for quantitative resistance to sunflower downy mildew. Theor. Appl. Genet. 125, 909–920. [DOI] [PubMed] [Google Scholar]
- Viranyi, F. and Spring, O. (2011) Advances in sunflower downy mildew research. Eur. J. Plant. Pathol. 129, 207–220. [Google Scholar]
- Voglmayr, H. , Rietmuller, A. , Goker, M. , Weiss, M. and Oberwinkler, F. (2004) Phylogenetic relationships of Plasmopara, Bremia and other genera of downy mildew pathogens with pyriform haustoria based on Bayesian analysis of partial LSU rDNA sequence data. Mycol. Res. 108, 1011–1024. [DOI] [PubMed] [Google Scholar]
- Wehtje, G. and Zimmer, D.E. (1978) Downy mildew of sunflower—biology of systemic infection and the nature of resistance. Phytopathology, 68, 1568–1571. [Google Scholar]
- Wehtje, G. , Littlefield, L.J. and Zimmer, D.E. (1979) Ultrastructure of compatible and incompatible reactions of sunflower to Plasmopara halstedii . Can. J. Bot. 57, 315–323. [Google Scholar]
- Whisson, S.C. , Boevink, P.C. , Moleleki, L. , Avrova, A.O. , Morales, J.G. , Gilroy, E.M. , Armstrong, M.R. , Grouffaud, S. , van West, P. , Chapman, S. , Hein, I. , Toth, I.K. , Pritchard, L. and Birch, P.R.J. (2007) A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature, 450, 115–119. [DOI] [PubMed] [Google Scholar]
- Wieckhorst, S. , Bachlava, E. , Dussle, C.M. , Tang, S. , Gao, W. , Saski, C. , Knapp, S.J. , Schoen, C.C. , Hahn, V. and Bauer, E. (2010) Fine mapping of the sunflower resistance locus PlARG introduced from the wild species Helianthus argophyllus . Theor. Appl. Genet. 121, 1633–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, P.A. and Morris, H.E. (1927) Plasmopara downy mildew of cultivated sunflowers. Am. J. Bot. 14, 551–558. [Google Scholar]
- Zahka, G.A. and Viranyi, F. (1991) Axenic culture of the downy mildew fungus Plasmopara halstedii in Agrobacterium rhizogenes‐induced roots of sunflower (Helianthus annuus). Can. J. Bot. 69, 2709–2715. [Google Scholar]
- Zimmer, D.E. (1971) A serious outbreak of downy mildew in the principal sunflower production area of the United States. Plant Dis. Rep. 55, 11–12. [Google Scholar]
- Zimmer, D.E. (1975) Some biotic and climatic factors influencing sporadic occurrence of sunflower downy mildew. Phytopathology, 65, 751–754. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Various Plasmopara halstedii zoosporangiophores formed on sunflower tissues. Zoosporangiophore (Zp) carrying zoosporangia (Za) on cotyledon (a), hypocotyl tissue (b), emerging probably through a lenticel. (c, d) Zoosporangiophores formed on roots, near root hairs. Scale bars: 100 μm (a, b); 50 μm (c, d).


