Summary
Burkholderia gladioli is a causal agent of bacterial panicle blight and sheath/grain browning in rice in many countries. Many strains produce the yellow pigment toxoflavin, which is highly toxic to plants, fungi, animals and microorganisms. Although there have been several studies on the toxoflavin biosynthesis system of B. glumae, it is still unclear how B. gladioli activates toxoflavin biosynthesis. In this study, we explored the genomic organization of the toxoflavin system of B. gladioli and its biological functions using comparative genomic analysis between toxoflavin‐producing strains (B. glumae BGR1 and B. gladioli BSR3) and a strain not producing toxoflavin (B. gladioli KACC11889). The latter exhibits normal physiological characteristics similar to other B. gladioli strains. Burkholderia gladioli KACC11889 possesses all the genes involved in toxoflavin biosynthesis, but lacks the quorum‐sensing (QS) system that functions as an on/off switch for toxoflavin biosynthesis. These data suggest that B. gladioli has evolved to use the QS signalling cascade of toxoflavin production (TofI/TofR of QS → ToxJ or ToxR → tox operons) similar to that in B. glumae. However, some strains may have evolved to eliminate toxoflavin production through deletion of the QS genes. In addition, we demonstrate that the toxoflavin biosynthetic system enhances the virulence of B. gladioli. These findings provide another line of evidence supporting the differential regulation of the toxoflavin system in Burkholderia strains.
Keywords: Burkholderia gladioli, Burkholderia glumae, genome organization, quorum sensing, toxoflavin, virulence
Introduction
Burkholderia is a genus of gram‐negative, motile and obligately aerobic rod‐shaped proteobacteria (Estrada‐de Los Santos et al., 2013). Burkholderia species exhibit metabolic versatility and inhabit diverse environments, including soils, plants, animals and humans (Coenye and Vandamme, 2003; Elsas et al., 2002; Estrada‐de Los Santos et al., 2001). Although many Burkholderia species are environmentally important and non‐virulent, some species can cause diseases in animals and plants. One of these is B. gladioli, identified by Lucia McCulloch. In 1921, McCulloch initially reported B. gladioli as Pseudomonas marginata and P. gladioli which caused diseases of gladiolus and iris species (McCulloch, 1921, 1924). Burkholderia gladioli can be subdivided into four pathovars: B. gladioli pv. agaricicola, which causes mushroom rot; B. gladioli pv. alliicola, which causes onion bulb rot; B. gladioli pv. gladioli, which causes gladiolus rot; and B. gladioli pv. cocovenenans, which causes severe food poisoning as a result of the ingestion of infected soybean and coconut‐based products (Hildebrand et al., 1973; Jiao et al., 2003; Yabuuchi et al., 1992; Younga et al., 1978). Burkholderia gladioli has been reported to cause bacterial panicle blight, as well as sheath and grain browning, in rice in many countries (Nandakumar et al., 2009; Seo et al., 2011; Ura et al., 2006). These symptoms are very similar to those associated with panicle infection by B. glumae. Although B. gladioli epidemics appear less frequently and this bacterium has relatively weaker virulence than B. glumae, these two bacterial species are often isolated from the same infected rice plants (Fory et al., 2014).
In the stationary phase of bacterial growth, the production of a yellow pigment is a representative phenotype of the B. gladioli pathovars and B. glumae. This unique colour is the result of toxoflavin (1,6‐dimethylpyrimido[5,4‐e]‐1,2,4‐triazine‐5,7[1H,6H]‐dione (molecular mass, 193; Iiyama et al. 1994; Furuya et al. 1997; Jeong et al. 2003). Several plant pathogens can produce a variety of phytotoxins that are toxic to plant cells and affect disease severity (Durbin, 1991; Geng et al., 2014; Renier et al., 2007). Toxoflavin is a bright yellow phytotoxin that is highly toxic to plants, fungi, animals and microorganisms (Choi et al., 2013; Levenberg and Linton, 1966). Recently, interest in the biosynthesis and genetic organization of toxoflavin is growing because the toxicity of bacterial toxoflavin to plants has led to severe losses in rice crops around the world, including in East Asia, South America and the USA (Ham et al., 2011; Jeong et al., 2003; Kim et al., 2004). Toxoflavin causes chlorotic damage to panicles of plants and inhibits the growth of both leaves and roots of rice (Jeong et al., 2003; Suzuki et al., 1998; Yoneyama et al., 1998). Studies have revealed that toxoflavin performs the following functions: active electron transfer between NADH and oxygen, and the generation of hydrogen peroxide, bypassing the cytochrome system (Latuasan and Berends, 1961; Stern, 1934).
The genes involved in toxoflavin production can be classified into two groups in B. glumae: biosynthesis and transport. These genes are polycistronic. The genes involved in biosynthesis include the five genes toxABCDE, and the genes involved in toxoflavin transport include the four genes toxFGHI (Kim et al., 2004). These operons are controlled by a TofI (a LuxI‐family protein)–TofR (a LuxR‐family protein) quorum‐sensing (QS) circuit. QS is a cell‐to‐cell communication mechanism that regulates many physiological systems in B. glumae, including flagellar biosynthesis, toxin production and stress responses (Chun et al., 2009; Kim et al., 2007). TofI of the QS system synthesizes the intercellular signalling molecule N‐octanoyl‐homoserine lactone (C8‐HSL), which is commonly used by gram‐negative bacteria (Kim et al., 2009). C8‐HSL binds to its cognate receptor TofR, and the C8‐HSL–TofR complex induces the expression of the above‐mentioned toxoflavin biosynthetic and transport operons by activating the ToxJ transcriptional regulator. Another regulator, ToxR, also binds toxoflavin as a co‐inducer and enhances the expression of the toxoflavin operons (Kim et al., 2004). Genetic and physical evidence indicates that this regulatory system of toxoflavin production in B. glumae is organized as a serial cascade (TofI/TofR (QS) → ToxJ or ToxR → tox operons; Kim et al., 2004, 2009). Furthermore, Chen et al. (2012) have reported recently that the tofM mutant strain LSUPB286 produces less toxoflavin than the wild‐type strain B. glumae 336gr‐1, which indicates that TofM, acting as a positive regulator, is very important for toxoflavin production.
However, our understanding of the mechanism of toxoflavin production in B. gladioli is at a very rudimentary level. Although it is not difficult to predict the putative functions of genes related to the toxoflavin system through homology searches among DNA sequences, the genomic organization and regulation of the toxoflavin system in B. gladioli remain poorly studied. The complete genome sequence of B. gladioli BSR3 (Seo et al., 2011) provides information on the genomic organization of the QS and toxoflavin systems. The genomic organization of the genes involved in toxoflavin production is the same in B. gladioli BSR3 and B. glumae BGR1, except that, in B. gladioli BSR3, toxJ is located on a different chromosome. The genes encoding the TofI–TofM–TofR QS system in B. gladioli BSR3 show the same genome organization as in B. glumae BGR1.
In this study, we explored the genes related to toxoflavin biosynthesis in B. gladioli. We selected diverse B. gladioli strains isolated from various plants, including B. gladioli BSR3 (rice), B. gladioli KACC11889 (gladiolus), B. gladioli KACC13944 (mushroom) and B. gladioli KCTC12374 (onion). We used the complete genome sequences of B. gladioli BSR3 (Seo et al., 2011) and focused on the genes involved in toxoflavin biosynthesis in B. gladioli KACC11889, because this strain does not produce the yellow pigment in the stationary phase. We determined the genomic organization of the toxoflavin system in various B. gladioli strains and the role of toxoflavin in the enhanced virulence in plant hosts. Furthermore, loss of toxoflavin production as a result of a lack of QS genes in B. gladioli KACC11889 led to variations in toxoflavin regulation among Burkholderia strains.
Results
The toxoflavin biosynthesis of Burkholderia species
On the basis of previous studies, we isolated the chemicals secreted by Burkholderia strains using thin‐layer chromatography (TLC) (Devescovi et al., 2007; Kim et al., 2004). The results of TLC analysis are shown in Fig. 1. Toxoflavin production was visible to the naked eye in most B. gladioli strains and in B. glumae BGR1. Under UV light at 365 nm, their yellow colouring was even more vivid. Burkholderia glumae BGR1 exhibited the strongest yellow colouring, whereas B. gladioli BSR3 produced much less toxoflavin. The strains KCTC12374 and KACC13944 produced similar amounts of toxoflavin to that produced by B. gladioli BSR3. By contrast, strain KACC11889, which was isolated from gladiolus, did not produce any toxoflavin according to both simple visual inspection and UV light examination. Based on the genome sequence of B. gladioli BSR3, this result may indicate that the toxoflavin system was inactive in this strain, possibly at the level of biosynthesis, transport, transcriptional regulation or QS.
Figure 1.
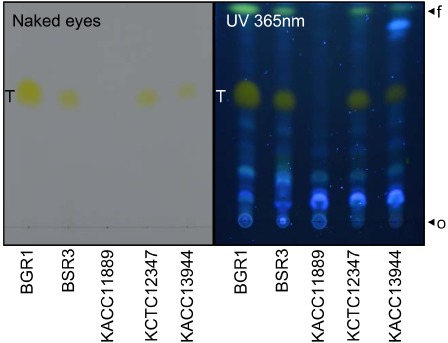
Toxoflavin production by Burkholderia. Five wild‐type strains of Burkholderia were tested: B. glumae BGR1, B. gladioli BSR3, B. gladioli KACC11889, B. gladioli KCTC12347 and B. gladioli KACC13944. The supernatant of the bacterial culture was extracted with chloroform and the mixture was centrifuged; the resulting infranatant was dried in a centrifugal vaporizer. The solutes were separated in 80% methanol on a thin‐layer chromatography (TLC) silica gel plate. The toxoflavin produced was examined visually in daylight and under UV light at 365 nm (T, toxoflavin). The origin (o) and solvent front (f) on the TLC plate are shown.
Comparative analysis of toxoflavin‐related systems
In order to determine whether the toxoflavin system was inactivated in strain KACC11889, we performed a comparative analysis of all gene clusters of the biosynthetic, transport and QS components. First, we determined the nucleotide sequence of the 11 genes in the tox operons that are directly responsible for toxoflavin production. These include toxABCDE for toxoflavin biosynthesis, toxFGHI for toxoflavin transport and toxJR for transcriptional regulation (Kim et al., 2004). Using ClustalW2 software, we compared all sequence data with genes of B. glumae BGR1 to assess their homology. The resulting homology percentages are listed in Table 1. All toxoflavin biosynthetic genes (toxABCDE) of B. gladioli strains showed very high homology (over 90%) with B. glumae BGR1. Similarly, genes of the transporter (toxFGHI) showed 89.94%–96.07% homology to the gene in B. glumae BGR1, in most cases. Although toxJ among transcriptional regulators (toxJR) showed a relatively low level of homology, the percentage itself was >80% in all strains. Compared with the genes in strains BSR3, KCTC12374 and KACC13944, the genes in KACC11889 were not notably different (Table 1). In addition, we generated three phylogenetic trees of systems related to toxoflavin production in Phylogeny.fr software (http://www.phylogeny.fr/). The data from the phylogenetic trees are shown in Figs S1–S3 (see Supporting Information). All phylogenetic trees indicated that 11 genes of tox operons of the strain KACC11889 are very similar to the corresponding genes of the other strains, and there were no significant differences among the homologous sequences.
Table 1.
Homology of the tox operon between Burkholderia glumae BGR1 and B . gladioli strains
| Gene | Homology (%) | |||
|---|---|---|---|---|
| BSR3 | KACC11889 | KCTC12374 | KACC13944 | |
| toxA | 95.93 | 95.66 | 94.71 | 95.66 |
| toxB | 95.78 | 96.18 | 95.65 | 96.05 |
| toxC | 95.47 | 93.26 | 94.68 | 93.44 |
| toxD | 95.11 | 94.80 | 95.51 | 95.41 |
| toxE | 93.77 | 93.94 | 93.68 | 94.11 |
| toxF | 95.09 | 94.44 | 94.10 | 95.14 |
| toxG | 93.83 | 93.74 | 93.93 | 92.99 |
| toxH | 96.07 | 92.99 | 95.77 | 95.48 |
| toxI | 91.06 | 90.53 | 90.73 | 89.94 |
| toxJ | 83.33 | 83.27 | 83.60 | 83.60 |
| toxR | 95.25 | 95.47 | 95.47 | 95.81 |
Subsequently, we characterized the tofIMR genes of the QS system, which regulates the operons of toxoflavin biosynthesis (Chen et al., 2012; Kim et al., 2004). The presence of the tofIMR genes was confirmed by PCR and electrophoresis. The PCR products are shown in Fig. 2. According to the specific primers used for each gene, the predicted PCR products are 850 bp for the tofI (AHL synthase) region, 832 bp for the tofM (quorum‐sensing regulatory protein) region and 1267 bp for the tofR (quorum‐sensing LuxR family sensor regulator) region. Our results indicate that the strain KACC11889 has no genes similar to tofIMR. The outer regions of tofM were not amplified because the agarose gel did not show an ∼800‐bp PCR band for strain KACC11889 (Fig. 2b). In Fig. 2a,c, PCR bands of sizes that differed from those of the original products were obtained, and sequencing analysis showed that the PCR bands were amplified from unrelated genes. However, the other strains, such as BSR3, KCTC12374, and KACC13944, yielded PCR products of the expected size in all lanes of tofIMR electrophoresis. Therefore, we assume that the QS genes required for toxoflavin biosynthesis are inactive in strain KACC11889.
Figure 2.
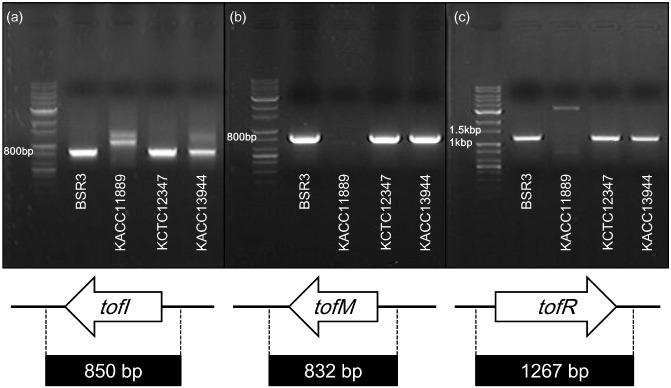
Molecular genetic confirmation of the identity of tof genes in Burkholderia gladioli strains. The tofIMR genes were confirmed using polymerase chain reaction (PCR) in four B. gladioli strains (BSR3, KACC11889, KCTC12347 and KACC13944). The amplicons were analysed using agarose gel electrophoresis. The positions of the DNA molecular weight markers are indicated to the right of the gels. Black arrows show the position and transcriptional direction of tofIMR, and thick bars below the arrows indicate the expected sizes of the PCR products as follows: 850 bp, tofI; 832 bp, tofM; 1267 bp, tofR. (a) The tofI gene. (b) The tofM gene. (c) The tofR gene.
Recovery of toxoflavin biosynthesis in B. gladioli KACC11889
To confirm that toxoflavin biosynthesis could be restored by introducing the QS genes, we generated KACC11889 complemented strains in which various QS genes were introduced (tofI, tofM, tofR or tofIMR from B. glumae BGR1 or B. gladioli BSR3) following the method described in Experimental procedures. All genes of B. glumae BGR1 and B. gladioli BSR3 were amplified by PCR and inserted into pBBR1MCS‐2 via common restriction sites of the PCR product and the vector (Table S1, see Supporting Information). Genetic validation of the complementation strains involved PCR and DNA sequencing using the PBBR1MCS2_MCS primer set. Eight complementation strains were obtained (Table 2). The complementation strains contained the following introduced genes: 11889ATOFI contained tofI from B. gladioli BSR3; 11889ATOFM contained tofM from B. gladioli BSR3; 11889ATOFR contained tofR from B. gladioli BSR3; 11889UTOFI contained tofI from B. glumae BGR1; 11889UTOFM contained tofM from B. glumae BGR1; and 11889UTOFR contained tofR from B. glumae BGR1. Furthermore, two additional complementation strains, 11889ATOFIMR and 11889UTOFIMR, were created by introduction of the entire tofIMR genetic region from B. gladioli BSR3 and B. glumae BGR1, respectively.
Table 2.
Bacterial strains and plasmids used in this study
| Name | Description | Source |
|---|---|---|
| Bacterial strains | ||
| BGR1 | Burkholderia glumae isolate from rice, wild‐type | Jeong et al. (2003) |
| BGS2 | BGR1 tofI::Ω | Kim et al. (2004) |
| BSR3 | B. gladioli isolate from rice, wild‐type | Seo et al. (2011) |
| COK94 | BSR3 tofI::lacZ | Laboratory collection |
| KACC11889 | B. gladioli isolate from gladiolus, wild‐type | KACC |
| KCTC12374 | B. gladioli isolate from onion, wild‐type | KCTC |
| KACC13944 | B. gladioli isolate from mushroom, wild‐type | KACC |
| 11889EMPTY | KACC11889 transformed with empty vector pBBR1MCS‐2 | This study |
| 11889ATOFI | KACC11889 transformed with pATOFI | This study |
| 11889ATOFM | KACC11889 transformed with pATOFM | This study |
| 11889ATOFR | KACC11889 transformed with pATOFR | This study |
| 11889ATOFIMR | KACC11889 transformed with pATOFIMR | This study |
| 11889UTOFI | KACC11889 transformed with pUTOFI | This study |
| 11889UTOFM | KACC11889 transformed with pUTOFM | This study |
| 11889UTOFR | KACC11889 transformed with pUTOFR | This study |
| 11889UTOFIMR | KACC 11889 transformed with pUTOFIMR | This study |
| DH5α λpir | Escherichia coli used for cloning and propagation of plasmids | Laboratory collection |
| S17‐1 λpir | E. coli used for analysis of conjugal transfer functions | Kalogeraki and Winans (1997) |
| Plasmids | ||
| pBBR1MCS‐2 | Broad‐host‐range vector containing mobilization gene and kanamycin resistance gene | Kovach et al. (1995) |
| pATOFI | pBBR1MCS‐2 containing bgla_2g11050 (tofI) gene of BSR3 | This study |
| pATOFM | pBBR1MCS‐2 containing bgla_2g11060 (tofM) gene of BSR3 | This study |
| pATOFR | pBBR1MCS‐2 containing bgla_2g11070 (tofR) gene of BSR3 | This study |
| pATOFIMR | pBBR1MCS‐2 containing bgla_2g11050‐70 (tofIMR) genes of BSR3 | This study |
| pUTOFI | pBBR1MCS‐2 containing bglu_2g14490 (tofI) gene of BGR1 | This study |
| pUTOFM | pBBR1MCS‐2 containing bglu_2g14480 (tofM) gene of BGR1 | This study |
| pUTOFR | pBBR1MCS‐2 containing bglu_2g14470 (tofR) gene of BGR1 | This study |
| pUTOFIMR | pBBR1MCS‐2 containing bglu_2g14470‐90 (tofIMR) genes of BGR1 | This study |
KACC, Korean Agricultural Culture Collection (http://www.genebank.go.kr); KCTC, Korea Collection Type Cultures (http://kctc.kribb.re.kr).
The ability of the complementation strains to produce toxoflavin was evaluated using the above‐mentioned TLC assay including wild‐type strains (BSR3 and KACC11889). All results were inspected visually in daylight and under UV light at 365 nm. Images of the TLC silica gel plates are shown in Fig. 3a,b. When the TLC silica gel plate with samples of wild‐type and complementation strains was developed, the parent strain KACC11889 and the six complementation strains (11889ATOFI, 11889ATOFM, 11889ATOFR, 11889UTOFI, 11889UTOFM and 11889UTOFR) containing the introduced tofI, tofM and tofR genes from B. gladioli and B. glumae, respectively, did not show any yellow pigment production. However, 11889ATOFIMR and 11889UTOFIMR, which received the entire tofIMR from B. glumae BGR1 and B. gladioli BSR3, respectively, exhibited the production of toxoflavin at a level similar to that of B. gladioli BSR3. In addition, these patterns were evaluated on a plate containing a rich medium [Luria–Bertani (LB); Fig. 3c]. 11889EMPTY, which received the empty vector, was used as a negative control. Burkholderia gladioli BSR3 and B. glumae BGR1 showed abundant production of toxoflavin. Similar to KACC11889, the strain 11889EMPTY did not produce any yellow colouring around the bacterial patch. In contrast, strains 11889ATOFIMR and 11889UTOFIMR fully recuperated from the loss of the ability to produce toxoflavin. All of these results indicate that inactivation of the toxoflavin system involves the loss of the entire tofIMR, and complementation with tofIMR can bring about recovery of toxoflavin biosynthesis at a level similar to that in B. glumae BGR1 and B. gladioli BSR3.
Figure 3.
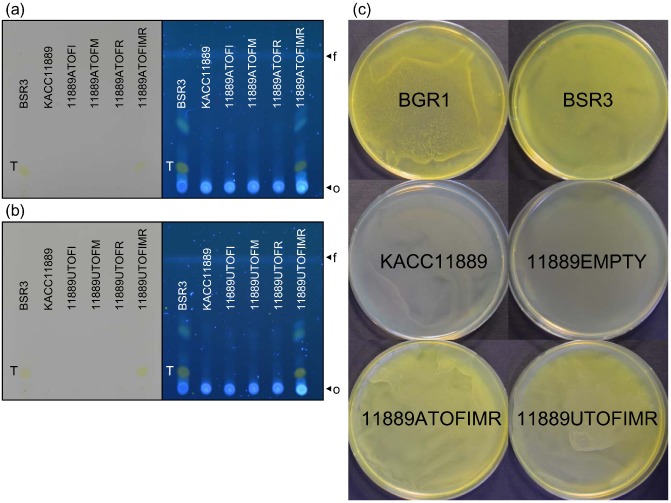
Restoration of toxoflavin production by genetic complementation of strains of Burkholderia gladioli KACC11889. (a) Thin‐layer chromatography (TLC) analysis in complementation strains receiving the tof genes of B. gladioli BSR3. Four complementation strains were tested: 11889ATOFI, 11889ATOFM, 11889ATOFR and 11889ATOFIMR. Burkholderia gladioli BGR3 and KACC11889 are indicated to the left of the plates as a control. The toxoflavin produced was evaluated visually in daylight and under UV light at 365 nm (T, toxoflavin). The origin (o) and solvent front (f) on the TLC plate are shown. (b) TLC analysis of the complementation strains that received the tof genes of B. glumae BGR1. Four complementation strains were tested: 11889UTOFI, 11889UTOFM, 11889UTOFR and 11889UTOFIMR. (c) The pigmentation phenotype of the complementation strains 11889ATOFIMR and 11889UTOFIMR. Burkholderia gladioli BSR3 and B. glumae BGR1 were used as positive controls. The strain 11889EMPTY, which was created by introduction of the empty vector pBBR1MCS‐2, served as a negative control. Freshly grown bacterial culture was spread on a Luria–Bertani (LB) agar plate and photographed after 48 h of incubation at 30 °C post‐inoculation.
Virulence of the strains with restored toxoflavin production
In order to determine whether recovery of toxoflavin production affected virulence in rice plants, we performed a seed germination assay with wild‐type strains (BGR1, BSR3 and KACC11889) and the complemented toxoflavin‐producing strains (11889ATOFIMR and 11889UTOFIMR). Seven days after pre‐germination of the seeds, all results were analysed (Fig. 4). In this assay, an LB broth sample served as a control. Strains KACC11889 and 11889EMPTY induced mild disease symptoms, including a slight growth reduction with yellowing (Fig. 4). In contrast, B. glumae BGR1 and B. gladioli BSR3 caused a severe growth reduction with discoloured stems. Strains 11889ATOIMR and 11889UTOFIMR were virulent, and the damage caused was similar to that caused by B. glumae BGR1 and B. gladioli BSR3 (Fig. 4).
Figure 4.

Virulence of the complemented toxoflavin‐producing strains in rice according to changes in seed germination. (a) Damage to rice seeds. Pre‐germinated seeds of rice were inoculated with the bacterial culture [optical density at 600 nm (OD 600) of 0.5], as described in Experimental procedures. The LB liquid medium was used as a negative control. The photographs were taken 7 days after inoculation. (b) The length of stems. Average length of the inoculated seeds was measured/calculated 7 days after inoculation. Each bar shows the standard error from five replicates.
Furthermore, the enhanced virulence of the complemented toxoflavin‐producing strains (11889ATOFIMR and 11889UTOFIMR) was in agreement with the results of the onion tissue maceration assay (Fig. 5). After inoculation, the area of macerated tissue was monitored for 72 h. The strains KACC11889 and 11889EMPTY yielded 0–1 cm of macerated tissue, whereas the complemented toxoflavin‐producing strains 11889ATOFIMR and 11889UTOFIMR yielded macerated areas >2 cm and severe water‐soaking symptoms. Thus, the complemented toxoflavin‐producing strains were able to cause severe damage to host plants as a result of the introduction of the QS genes from B. glumae BGR1 or B. gladioli BSR3.
Figure 5.
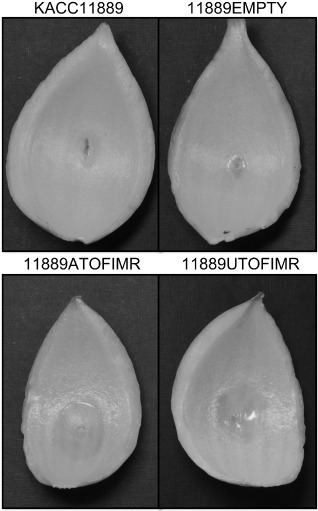
Virulence of the complemented toxoflavin‐producing strains in onion, judged by tissue maceration. Onion tissue samples were inoculated with the wild‐type strain (KACC11889), negative control (11889EMPTY) and the complemented toxoflavin‐producing strains (11889ATOFIMR and 11889UTOFIMR). All samples were incubated at 30 °C for 72 h. The photographs were taken 3 days after inoculation.
Swarming motility of Burkholderia
Swarming motility is a typical phenotypic trait of QS‐related downstream systems. Burkholderia glumae BGR1 is able to move about using flagella to reach the site of infection in host plants (Kim et al., 2007). We tested the swarming motility of wild‐type strains (BGR1, BSR3 and KACC11889), QS‐deficient mutants (BGS2, B. glumae tofI mutant; COK94, B. gladioli tofI mutant) and the complemented toxoflavin‐producing strains (11889ATOFIMR and 11889UTOFIMR). All results are shown in Fig. 6. The wild‐type strain BGR1 showed swarming motility on 0.5% agar plates, whereas the mutant BGS2 (tofI::Ω mutant strain) did not show any movement (Kim et al., 2007). Likewise, B. gladioli BSR3 exhibited swarming motility, whereas the mutant COK94 (tofI::lacZ mutant strain) did not (Kim et al., 2014). The strain KACC11889 exhibited normal swarming motility on 0.5% agar plates. The complemented toxoflavin‐producing strains exhibited swarming motility similar to that of the wild‐type strain KACC11889.
Figure 6.
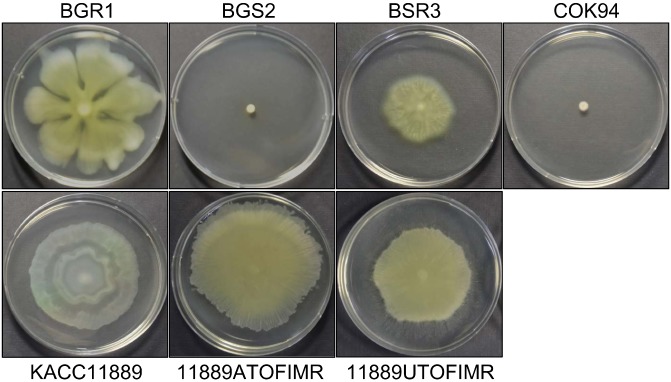
Swarming motility of Burkholderia. A swarming motility assay was performed to confirm bacterial motility in three wild‐type strains (B. glumae BGR1, B. gladioli BSR3 and KACC11889), two quorum‐sensing (QS)‐deficient strains (B. glumae BGS2 and B. gladioli COK94) and two complemented toxoflavin‐producing strains (11889ATOFIMR and 11889UTOFIMR, derived from KACC11889). The cells were seeded onto 0.5% Luria–Bertani (LB) agar plates and photographed after 24 h of incubation at 30 °C.
Discussion
Plant‐pathogenic bacteria produce diverse phytotoxins, including albicidin, coronatine, fervenulin, reumycin and toxoflavin (Geng et al., 2014; Levenberg and Linton, 1966; Renier et al., 2007). Albicidin produced by Xanthomonas albilineans causes the chlorotic symptoms of leaf scald disease in sugarcane by blocking chloroplast development (Renier et al., 2007). Numerous strains of Pseudomonas syringae produce the phytotoxin coronatine, which is a multifunctional virulence factor that induces leaf chlorosis, hypertrophic growth on potato and inhibition of root growth in rice (Geng et al., 2014; Mittal and Davis, 1995). Among the above‐mentioned toxins, toxoflavin is potent in plants, fungi, animals and microorganisms, and acts as an azapteridine antibiotic (Fenwick et al., 2011; Levenberg and Linton, 1966; Sato et al., 1989). As a prosthetic group, toxoflavin acts as an effective electron carrier and helps to produce superoxide and hydrogen peroxide in the presence of oxygen and light (Latuasan and Berends, 1961; Stern, 1934). In plants, this toxic property of toxoflavin is essential for the inhibition of elongation of sprouts and roots and for the induction of grain rot in rice (Suzuki et al., 1998; Yoneyama et al., 1998). To date, there have been several studies of the toxoflavin biosynthesis system, especially in B. glumae. The toxoflavin biosynthesis system contains two polycistronic operons: a biosynthetic operon (toxABCDE) and a transport operon (toxFGHI) (Kim et al., 2004). It has also been reported that the toxoflavin biosynthesis system is controlled by the QS system (Kim et al., 2009), depending on the homoserine lactone (HSL) synthesized by TofI and the cognate receptor TofR, by activating ToxJ and ToxR as transcriptional regulators of toxoflavin biosynthesis. It is still unclear how B. gladioli activates the toxoflavin biosynthesis system. Therefore, we focused on whether toxoflavin‐related operons are present in and show homology among the genomes of B. gladioli strains, and whether the B. gladioli strains utilize the general cascade (TofI/TofR of QS → ToxJ or ToxR → tox operons) of toxoflavin biosynthesis of B. glumae.
In this study, we tested B. gladioli BSR3 isolated from rice; the genome sequence of this strain is well known (Seo et al., 2011). We also evaluated three representative strains of the main pathovars: B. gladioli KACC11889, a strain isolated from gladiolus; B. gladioli KACC13944, a strain isolated from mushrooms; and B. gladioli KCTC12374, a strain isolated from onions. Using TLC analysis, we showed that all strains, except KACC11889, produced the yellow pigment toxoflavin, which was visible to the naked eye and under UV light (Fig. 1). However, KACC11889 showed normal growth during all experiments. These data provided a very useful starting point to conduct comparative analysis between a functional and defective toxoflavin system for the identification of genes that activate toxoflavin biosynthesis in B. gladioli. First, we predicted that the defective toxoflavin system of strain KACC11889 was the result of the loss of either the biosynthetic or the transport operon, because it has been reported that mutations in these operons result in the loss of the ability to produce toxoflavin (Suzuki et al., 2004). Nevertheless, the biosynthetic operon toxABCDE, the transport operon toxFGHI and the transcriptional regulators toxJR of strain KACC11889 showed very high sequence homologies to those of B. gladioli BSR3 (Table 1 and Figs S1–S3). However, the genetic defect in strain KACC11889 is located in the QS system (tofIMR) of chromosome 2 of B. gladioli BSR3 (Fig. 2). The QS system is a cell‐to‐cell communication mechanism and regulates a diverse range of bacterial behavioural patterns, including the formation of biofilms, flagellar biosynthesis, stress responses and the production of virulence factors (Chun et al., 2009; Miller and Bassler, 2001). Kim et al. (2004) reported that a QS‐deficient mutant (BGS2) produces neither the HSL signalling molecule nor toxoflavin. According to these data, we believe that B. gladioli has evolved to utilize the general cascade (TofI/TofR of QS → ToxJ or ToxR → tox operons) of B. glumae, as well as to selectively accommodate the original QS system, serving as an on/off switch of toxoflavin biosynthesis. Furthermore, a complemented strain of B. gladioli KACC11889, in which tofI/R from B. gladioli BSR3 was introduced, did not produce any toxoflavin that was detectable in the liquid medium or on a TLC plate (Fig. S5, see Supporting Information). This result supports the function of TofM as a positive regulator of toxoflavin biosynthesis in B. glumae 336gr‐1 (Chen et al., 2012).
It should be noted that B. gladioli BSR3 contains another QS system (bgla_1p1740‐60), which is located on plasmid 1 (Choudhary et al., 2013). Previously, we have reported the total gene expression profile of B. gladioli BSR3 in the exponential phase and stationary phase using RNA sequencing (Kim et al., 2014). We confirmed that the QS system (bgla_1p1740‐60) on plasmid 1 yields stronger responses to population density than the other QS genes (bgla_2g11050‐70) on chromosome 2 (Fig. S4, see Supporting Information). Furthermore, the tox genes of B. gladioli are divided between two chromosomes (the toxJ gene is in chromosome 2, whereas the others are in chromosome 1), in contrast with the neighbouring genes in chromosome 2 of B. glumae (Lim et al., 2009; Seo et al., 2011). These observations indicate that B. gladioli may utilize a specialized or dichotomous regulatory mechanism through two QS systems. Moreover, the present experimental results add weight to our ideas relating to the regulation of the two QS genes. The introduction of the tof genes from B. glumae BGR1 or from B. gladioli BSR3 restores toxoflavin biosynthesis in strain KACC11889 (Fig. 3). This restoration, which is mediated by the tof genes of B. glumae, indicates that B. gladioli also uses QS‐mediated toxoflavin biosynthesis in a similar manner to that in B. glumae. In addition, there is systemic flexibility, which may change according to environmental factors, via the loss of the on/off switch of the QS system rather than inactivation of the biosynthesis system itself. In other words, B. gladioli has evolved the ability to utilize a regulatory system different from the strong regulation produced by the original QS mechanism. The swarming motility of strain KACC11889 is a good illustrative example. Kim et al. (2007) reported that the mutated tofI strain BGS2 shows significantly reduced swimming motility and no swarming motility. In contrast (Fig. 6), we observed active swarming motility in strain KACC11889, just as in B. glumae BGR1 and B. gladioli BSR3, on 0.5% agar plates. Thus, this phenomenon could be explained by the presence of a different regulatory system in strain KACC11889. In addition, the QS‐deficient B. glumae mutant strains (deletion mutants of tofI or tofR) produced toxoflavin on LB agar medium (Chen et al., 2012). This indicates that B. glumae and B. gladioli have diverse regulatory systems for toxoflavin production through QS.
What are the other possible functions of the toxoflavin biosynthesis system in plant‐pathogenic Burkholderia strains? As an azapteridine antibiotic, toxoflavin is toxic to other microorganisms and also causes stress to the producer itself. For example, it has been reported that the growth of Candida albicans is inhibited by farnesol (Shirtliff et al., 2009). Farnesol is a well‐known virulence factor and QS signalling molecule in C. albicans (Cells et al., 2008; Hornby et al., 2001). Hence, strain KACC11889 might have evolved to use virulence factors other than toxoflavin. Indeed, Suzuki et al. (2004) found that a toxoflavin‐deficient mutant strain of B. glumae is still able to cause damage to rice seedlings and rice panicles. These data indicate that toxoflavin is a very important virulence factor, but that Burkholderia exhibits a complex pathogenesis mechanism because of multiple virulence factors. In contrast, toxoflavin biosynthesis confers enhanced virulence in rice to B. gladioli KACC11889. We also found that the complemented toxoflavin‐producing strains (11889ATOFIMR and 11889UTOFIMR, derived from KACC11889) inflict a greater severity of damage than the parental strain on rice and onion (Figs 4 and 5).
In conclusion, we have characterized the genomic organization of the toxoflavin system and the different modes of regulation for toxoflavin biosynthesis in B. gladioli. In addition, we have demonstrated that the capacity for toxoflavin biosynthesis confers enhanced virulence to this phytopathogen. Our findings provide another line of evidence supporting the differential regulation of the toxoflavin system in Burkholderia strains. Further studies should lead to a better understanding of the global regulatory system, which involves the double QS systems of B. gladioli.
Experimental Procedures
Bacterial strains, plasmids, oligonucleotide primers and culture conditions
The bacterial strains and plasmids used in this study are shown in Table 2. The PCR primers used in this study are shown in Table S1. A single colony grown on an LB agar plate was incubated in liquid medium in a shaker‐incubator at 200 rpm. The culture temperature for all strains was 30 °C.
TLC analysis
Each strain was cultured in a 2‐mL microcentrifuge tube containing 1.5 mL of LB broth at 30 °C for 1 day. Then, 1 mL of the liquid culture was pelleted by centrifugation at 12 000 g for 1 min; 500 μL of the supernatant was transferred into a new tube containing 500 μL of chloroform. After one round of vortexing, the mixture was again pelleted by centrifugation at 12 000 g for 10 min. Next, 500 μL of the infranatant was transferred to a new tube and dried in a centrifugal vaporizer (Tokyo Rikakikai, Japan). The solutes were then dissolved in 10 μL of 80% methanol, and 1 μL of the solution was spotted onto a TLC silica gel plate (Merck Millipore, Darmstadt, Germany). This step was repeated 10 times. The TLC silica gel plate was placed in a TLC chamber (the solvent was chloroform–methanol, 95: 5) to start chromatography. 3UV™ Multi‐Wavelength lamps (UVP, Upland, CA, USA) were used to examine the band pattern on the TLC silica gel plate.
DNA sequencing and data analysis
Primer sets for PCR and sequencing are listed in Table S1. The reaction mixture for PCR was as follows: 5 μL, 10 × Pfu buffer; 1 μL, deoxynucleoside triphosphates (dNTPs) (10 mm stock); 2 μL, Primer 1 (10 pmol/μL); 2 μL, Primer 2 (10 pmol/μL); 0.5 μL, Pfu DNA polymerase (SolGent, Seoul, South Korea); 10 μL, 5X Band Doctor™; x μL (∼10 ng), template; y μL (up to the total volume of 100 μL), distilled water. PCR was performed under the following conditions: one cycle of 10 min at 95 °C, followed by 35 cycles of 1 min at 95 °C, 1 min at 53 °C and 2 min at 72 °C. After an electrophoresis step, the amplified fragment was extracted from the gel using the AccuPrep® PCR Purification Kit (Bioneer, Daejeon, South Korea). DNA sequencing was performed by Macrogen Inc., using the 3730xl DNA analyser (Applied Biosystems®, Waltham, Massachusetts, USA). The DNA sequence data were analysed using ClustalW2 software (http://www.ebi.ac.uk/Tools/msa/clustalw2) and Phylogeny.fr (http://www.phylogeny.fr/).
Creation of transformant strains
To create each transformant clone, general and standard techniques were used for DNA manipulations, cloning, restriction digestion and agarose gel electrophoresis (Sambrook et al., 1989). Genes of B. gladioli BSR3 and B. glumae BGR1 were amplified by means of Pfu DNA polymerase. To insert a gene into the pBBR1MCS‐2 vector, restriction sites were added to both PCR primers. Table S1 shows the restriction sites of all primers. The amplified fragment and pBBR1MCS‐2 were digested using appropriate restriction enzymes to obtain common restriction sites and were ligated using DNA ligase. Escherichia coli DH5α λpir competent cells were transformed with the resulting vector, and the transformed cells were cultured in a kanamycin‐containing (50 μg/mL) medium. To confirm correct ligation, we extracted plasmid DNA from the cells and performed restriction digestion and electrophoresis. In the conjugation procedure, the properly assembled plasmid DNA was again used for the transformation of E. coli S17‐1 λpir, and the resulting bacterial cells were mated with the strain KACC11889. Transformant cells that were grown on a plate with two antibiotics (tetracycline, 20 μg/mL; kanamycin, 50 μg/mL) were collected and the genetic composition was confirmed by PCR with two primers designed to bind outside the multiple cloning site of pBBR1MCS‐2 (Table S1).
Seed germination assay
Each strain was incubated in 2 mL of LB liquid medium at 30 °C for 12–14 h with shaking, and then subcultured at 30 °C until an optical density at 600 nm (OD600) of 0.5 was reached in 5 mL of LB liquid medium. Seeds of rice (Oryza sativa L. cv. Dongjin) were pre‐germinated at 30 °C in a bacterial suspension. Two days later, the germinating seeds were transferred to a sterile plant culture dish (SPL Life Sciences, Pocheon, South Korea) containing 20 mL of distilled water, and were grown for 7 days under the following conditions: 30 °C, high relative humidity (close to 100%), 16 h of light provided by 400‐V lamps, during the day; 28 °C, high relative humidity (close to 100%), 8 h of darkness, at night. The damage to and length of rice seeds were assessed 7 days after inoculation. The experiments were repeated five times.
Onion tissue maceration assay
All papery skins and external layers of onions were removed. An onion was quartered with a sterilized knife. Onion scales were placed in sterilized plant culture dishes (SPL Life Sciences) containing 5 mL of distilled water, and each onion scale was wounded on the inner surface by a sterile 1–10‐μL pipette tip. Then, 10 μL of bacterial culture adjusted to OD600 = 0.5 was placed onto the wound of the onion scale. All samples were incubated at 30 °C for 72 h. Tissue maceration was assessed 3 days after inoculation.
Bacterial swarming motility assay
This assay was performed using LB broth in agar (0.5%, w/v). Each strain was incubated at 30 °C for 12–14 h in 2 mL of LB liquid medium. Then, 1 mL of cultured cells was pelleted by centrifugation at 900 g for 2 min. The harvested cells were washed with 1 mL of fresh LB liquid medium, and the centrifugation step was repeated. The washed pellet was resuspended in 100 μL of distilled water, and 1 μL of the suspension was spotted onto the assay plate. The assay plate was incubated at 30 °C for 24 h.
Supporting information
Fig. S1 The phylogenetic tree of toxoflavin biosynthesis genes in Burkholderia. Five wild‐type strains of Burkholderia were analysed: B. glumae BGR1, B. gladioli BSR3, B. gladioli KACC11889, B. gladioli KCTC12347 and B. gladioli KACC13944. The phylogenetic tree of toxABCDE was constructed using Phylogeny.fr software. The bootstrap consensus was inferred from 1000 replicates showing values of >30.
Fig. S2 The phylogenetic tree of toxoflavin transport genes in Burkholderia. Five wild‐type strains of Burkholderia were analysed: B. glumae BGR1, B. gladioli BSR3, B. gladioli KACC11889, B. gladioli KCTC12347 and B. gladioli KACC13944. The phylogenetic tree of toxFGHI was constructed using Phylogeny.fr software. The bootstrap consensus was inferred from 1000 replicates showing values of >30.
Fig. S3 The phylogenetic tree of toxoflavin transcriptional regulator genes in Burkholderia. Five wild‐type strains of Burkholderia were analysed: B. glumae BGR1, B. gladioli BSR3, B. gladioli KACC11889, B. gladioli KCTC12347 and B. gladioli KACC13944. The phylogenetic tree of toxJR was constructed using Phylogeny.fr software. The bootstrap consensus was inferred from 1000 replicates showing values of >30.
Fig. S4 Expression of quorum sensing (QS) genes in Burkholderia gladioli BSR3. Expression levels of two QS systems (bgla_2g11050‐70 and bgla_1p1740‐60) were measured using RNA sequencing in B. gladioli BSR3. The results were calculated as RPKM (reads per kilobase of exon per million mapped sequence reads). Blue and red bars indicate the exponential phase and stationary phase, respectively.
Fig. S5 Toxoflavin production and reverse transcription‐polymerase chain reaction (RT‐PCR) in a tofI/R‐complemented strain (11889ATOFIR) of Burkholderia gladioli KACC11889. (a) Thin‐layer chromatography (TLC) analysis of toxoflavin production in 11889ATOFIR, in which the tofI/R genes of B. gladioli BSR3 were introduced. Burkholderia gladioli BGR3 and KACC11889 are shown on the right side of the plates as controls. Toxoflavin production was evaluated visually in both daylight and under UV light at 365 nm (T, toxoflavin). The origin (o) and solvent front (f) on the TLC plate are indicated. (b) RT‐PCR analysis of the tofIMR genes. The expression of the tofIMR genes was confirmed using RT‐PCR of total RNA from four B. gladioli strains (BSR3, KACC11889, 11889ATOFIMR and 11889ATOFIR). The 16s ribosomal RNA was used as a positive control. The amplified products were 225 bp for tofI (lane 1: primers 5′‐GTACCTGCTGCACGAGGTG‐3′ and 5′‐CGCTCGATACTGCAGAAGGT‐3′), 201 bp for tofM (lane 2: primers 5′‐TTTCGCACACCTGACCGTT‐3′ and 5′‐GTGAGGGCCTGGTTGACC‐3′), 208 bp for tofR (lane 3: primers 5′‐CTGGATGGCCCATTACCAGG‐3′ and 5′‐GATGGTCAGCAGTCCGAACA‐3′) and 282 bp for the control (lane 4: primers 5′‐CCAGCAGCCGCGGTAATACG‐3′ and 5′‐TACCAGGGTATCTAATCC‐3′).
Table S1 PCR primers used in this study.
Acknowledgements
This research was supported by grants from the Rural Development Administration (No. PJ009774).
References
- Cells, C. , Scheper, M.A. , Shirtliff, M.E. , Meiller, T.F. , Peters, B.M. and Jabra‐rizk, M.A. (2008) Farnesol, a fungal quorum‐sensing molecule triggers apoptosis in human oral squamous carcinoma cells. Neoplasia, 10, 954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. , Barphagha, I.K. , Karki, H.S. and Ham, J.H. (2012) Dissection of quorum‐sensing genes in Burkholderia glumae reveals non‐canonical regulation and the new regulatory gene tofM for toxoflavin production. Plos ONE, 7, e52150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, O. , Lee, Y. , Han, I. , Kim, H. , Goo, E. , Kim, J. and Hwang, I. (2013) A simple and sensitive biosensor strain for detecting toxoflavin using β‐galactosidase activity. Biosens. Bioelectron. 50, 256–261. [DOI] [PubMed] [Google Scholar]
- Choudhary, K.S. , Hudaiberdiev, S. , Gelencsér, Z. , Gonçalves Coutinho, B. , Venturi, V. and Pongor, S. (2013) The organization of the quorum sensing luxI/R family genes in Burkholderia . Int. J. Mol. Sci. 14, 13 727–13 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun, H. , Choi, O. , Goo, E. , Kim, N. , Kim, H. , Kang, Y. , Kim, J. , Moon, J.S. and Hwang, I. (2009) The quorum sensing‐dependent gene katG of Burkholderia glumae is important for protection from visible light. J. Bacteriol. 191, 4152–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenye, T. and Vandamme, P. (2003) Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 5, 719–729. [DOI] [PubMed] [Google Scholar]
- Devescovi, G. , Bigirimana, J. , Degrassi, G. , Cabrio, L. , LiPuma, J.J. , Kim, J. , Hwang, I. and Venturi, V. (2007) Involvement of a quorum‐sensing‐regulated lipase secreted by a clinical isolate of Burkholderia glumae in severe disease symptoms in rice. Appl. Environ. Microbiol. 73, 4950–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin, R.D. (1991) Bacterial phytotoxins: mechanisms of action. Experientia, 47, 776–783. [Google Scholar]
- van Elsas, J.D. , Garbeva, P. and Salles, J. (2002) Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil‐borne plant pathogens. Biodegradation, 13, 29–40. [DOI] [PubMed] [Google Scholar]
- Estrada‐de Los Santos, P. , Bustillos‐cristales, R. and Caballero‐mellado, J. (2001) Burkholderia, a genus rich in plant‐associated nitrogen fixers with wide environmental and geographic distribution. Appl. Environ. Microbiol. 67, 2790–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada‐de Los Santos, P. , Vinuesa, P. , Martínez‐Aguilar, L. , Hirsch, A.M. and Caballero‐Mellado, J. (2013) Phylogenetic analysis of Burkholderia species by multilocus sequence analysis. Curr. Microbiol. 67, 51–60. [DOI] [PubMed] [Google Scholar]
- Fenwick, M.K. , Philmus, B. , Begley, T.P. and Ealick, S.E. (2011) Toxoflavin lyase requires a novel 1‐His‐2‐carboxylate facial triad. Biochemistry, 50, 1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fory, P.A. , Triplett, L. , Ballen, C. , Abello, J.F. , Duitama, J. , Aricapa, M.G. , Prado, G.A. , Correa, F. , Hamilton, J. , Leach, J.E. , Tohme, J. and Mosquera, G. (2014) Comparative analysis of two emerging rice seed bacterial pathogens. Phytopathology, 104, 436–444. [DOI] [PubMed] [Google Scholar]
- Furuya, N. , Iiyama, K. , Shiozaki, N. and Matsuyama, N. (1997) Phytotoxin produced by Burkholderia gladioli . J. Fac. Agric. 42, 33–37. [Google Scholar]
- Geng, X. , Jin, L. , Shimada, M. , Kim, M.G. and Mackey, D. (2014) The phytotoxin coronatine is a multifunctional component of the virulence armament of Pseudomonas syringae . Planta, 240, 1149–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham, J.H. , Melanson, R.A. and Rush, M.C. (2011) Burkholderia glumae: next major pathogen of rice? Mol. Plant Pathol. 12, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand, D.C. , Palleroni, N.J. and Doudoroff, M. (1973) Synonymy of Pseudomonas gladioli Severini 1913 and Pseudomonas marginata (McCulloch 1921) Stapp 1928. Int. J. Syst. Bacteriol. 23, 433–437. [Google Scholar]
- Hornby, J.M. , Jensen, E.C. , Lisec, A.D. , Tasto, J.J. , Jahnke, B. , Shoemaker, R. , Dussault, P. and Nickerson, K.W. (2001) Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67, 2982–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iiyama, K. , Furuya, N. , Hara, K. and Nakashima, N. (1994) Phytotoxin produced by Pseudomonas glumae Kurita et Tabei, a causal bacterium of the grain and seedling rot of rice. J. Fac. Agric. 38, 175–181. [Google Scholar]
- Jeong, Y. , Kim, J. , Kim, S. , Kang, Y. , Nagamatsu, T. and Hwang, I. (2003) Toxoflavin produced by Burkholderia glumae causing rice grain rot is responsible for inducing bacterial wilt in many field crops. Plant Dis. 87, 890–895. [DOI] [PubMed] [Google Scholar]
- Jiao, Z. , Kawamura, Y. , Mishima, N. , Yang, R. , Li, N. , Liu, X. and Ezaki, T. (2003) Need to differentiate lethal toxin‐producing strains of Burkholderia gladioli, which cause severe food poisoning: description of B. gladioli pathovar cocovenenans and an emended description of B. gladioli . Microbiol. Immunol. 47, 915–925. [DOI] [PubMed] [Google Scholar]
- Kalogeraki, V.S. and Winans, S.C. (1997) Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene, 188, 69–75. [DOI] [PubMed] [Google Scholar]
- Kim, J. , Kim, J.G. , Kang, Y. , Jang, J.Y. , Jog, G.J. , Lim, J.Y. , Kim, S. , Suga, H. , Nagamatsu, T. and Hwang, I. (2004) Quorum sensing and the LysR‐type transcriptional activator ToxR regulate toxoflavin biosynthesis and transport in Burkholderia glumae . Mol. Microbiol. 54, 921–934. [DOI] [PubMed] [Google Scholar]
- Kim, J. , Kang, Y. , Choi, O. , Jeong, Y. , Jeong, J.E. , Lim, J.Y. , Kim, M. , Moon, J.S. , Suga, H. and Hwang, I. (2007) Regulation of polar flagellum genes is mediated by quorum sensing and FlhDC in Burkholderia glumae . Mol. Microbiol. 64, 165–179. [DOI] [PubMed] [Google Scholar]
- Kim, J. , Oh, J. , Choi, O. , Kang, Y. , Kim, H. , Goo, E. , Ma, J. , Nagamatsu, T. , Moon, J.S. and Hwang, I. (2009) Biochemical evidence for ToxR and ToxJ binding to the tox operons of Burkholderia glumae and mutational analysis of ToxR. J. Bacteriol. 191, 4870–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Park, J. , Choi, O. , Kim, J. and Seo, Y.S. (2014) Investigation of quorum sensing‐dependent gene expression in Burkholderia gladioli BSR3 through RNA‐seq analyses. J. Microbiol. Biotechnol. 24, 1609–1621. [DOI] [PubMed] [Google Scholar]
- Kovach, M.E. , Elzer, P.H. , Hill, D.S. , Robertson, G.T. , Farris, M.A. , Roop, R.M. and Peterson, K.M. (1995) Four new derivatives of the broad‐host‐range cloning vector pBBR1MCS, carrying different antibiotic‐resistance cassettes. Gene, 166, 175–176. [DOI] [PubMed] [Google Scholar]
- Latuasan, H.E. and Berends, W. (1961) On the origin of the toxicity of toxoflavin. Biochim. Biophys. Acta, 30, 502–508. [DOI] [PubMed] [Google Scholar]
- Levenberg, B. and Linton, S.N. (1966) On the biosynthesis of toxoflavin, an azapteridine produced by Pseudomonas cocovenenans . J. Biol. Chem. 241, 846–852. [PubMed] [Google Scholar]
- Lim, J. , Lee, T.H. , Nahm, B.H. , Choi, Y.D. , Kim, M. and Hwang, I. (2009) Complete genome sequence of Burkholderia glumae BGR1. J. Bacteriol. 191, 3758–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch, L. (1921) A bacterial disease of gladiolus. Science, 54, 115–116. [DOI] [PubMed] [Google Scholar]
- McCulloch, L. (1924) A bacterial blight of gladioli. J. Agric. Res. 27, 225–230. [Google Scholar]
- Miller, M.B. and Bassler, B.L. (2001) Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199. [DOI] [PubMed] [Google Scholar]
- Mittal, S. and Davis, K.R. (1995) Role of the phytotoxin coronatine in the infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato . Am. Phytopathol. Soc. 8, 165–171. [DOI] [PubMed] [Google Scholar]
- Nandakumar, R. , Shahjahan, A.K.M. , Yuan, X.L. , Dickstein, E.R. , Groth, D.E. , Clark, C.A. , Cartwright, R.D. and Rush, M.C. (2009) Burkholderia glumae and B. gladioli cause bacterial panicle blight in rice in the southern United States. Plant Dis. 93, 896–905. [DOI] [PubMed] [Google Scholar]
- Renier, A. , Vivien, E. , Cociancich, S. , Letourmy, P. , Perrier, X. , Rott, P.C. and Royer, M. (2007) Substrate specificity‐conferring regions of the nonribosomal peptide synthetase adenylation domains involved in albicidin pathotoxin biosynthesis are highly conserved within the species Xanthomonas albilineans . Appl. Environ. Microbiol. 73, 5523–5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J. , Maniatis, T. , Fritsch, E.F. and Laboratory, C.S.H. (1989) Molecular Cloning: A Laboratory Manual (Sambrook J., Fritsch E.F. and Maniatis T., eds). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Sato, Z. , Koiso, Y. , Iwasaki, S. , Matsuda, I. and Shirata, A. (1989) Toxins produced by Pseudomonas glumae . Ann. Phytopathol. Soc. Jpn. 55, 353–356. [Google Scholar]
- Seo, Y.S. , Lim, J. , Choi, B.S. , Kim, H. , Goo, E. , Lee, B. , Lim, J.S. , Choi, I.Y. , Moon, J.S. , Kim, J. and Hwang, I. (2011) Complete genome sequence of Burkholderia gladioli BSR3. J. Bacteriol. 193, 3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtliff, M.E. , Krom, B.P. , Meijering, R.A.M. , Peters, B.M. , Zhu, J. , Scheper, M.A. , Harris, M.L. and Jabra‐Rizk, M.A. (2009) Farnesol‐induced apoptosis in Candida albicans . Antimicrob. Agents Chemother. 53, 2392–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, K.G. (1934) Oxidation–reduction potentials of toxoflavin. Biochem. J. 28, 949–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, F. , Zhu, Y. , Sawada, H.A. and Matsuda, I. (1998) Identification of proteins involved in toxin production by Pseudomonas glumae . Ann. Phytopathol. Soc. Jpn. 64, 75–79. [Google Scholar]
- Suzuki, F. , Sawada, H. , Azegami, K. and Tsuchiya, K. (2004) Molecular characterization of the tox operon involved in toxoflavin biosynthesis of Burkholderia glumae . J. Gen. Plant Pathol. 70, 97–107. [Google Scholar]
- Ura, H. , Furuya, N. , Iiyama, K. , Hidaka, M. , Tsuchiya, K. and Matsuyama, N. (2006) Burkholderia gladioli associated with symptoms of bacterial grain rot and leaf‐sheath browning of rice plants. J. Gen. Plant Pathol. 72, 98–103. [Google Scholar]
- Yabuuchi, E. , Kosako, Y. , Oyaizu, H. , Yano, I. , Hotta, H. , Hashimoto, Y. , Ezaki, T. and Arakawa, M. (1992) Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group ii to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiol. Immunol. 36, 1251–1275. [DOI] [PubMed] [Google Scholar]
- Yoneyama, K. , Kono, Y. , Yamaguchi, I. , Horikoshi, M. and Hirooka, T. (1998) Toxoflavin is an essential factor for virulence of Burkholderia glumae causing rice seedling rot disease. Ann. Phytopathol. Soc. Jpn. 64, 91–96. [Google Scholar]
- Younga, J.M. , Dyea, D.W. , Bradburyb, J.F. , Panagopoulosc, C.G. and Robbsd, C.F. (1978) A proposed nomenclature and classification for plant pathogenic bacteria. N. Z. J. Agric. Res. 21, 153–177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 The phylogenetic tree of toxoflavin biosynthesis genes in Burkholderia. Five wild‐type strains of Burkholderia were analysed: B. glumae BGR1, B. gladioli BSR3, B. gladioli KACC11889, B. gladioli KCTC12347 and B. gladioli KACC13944. The phylogenetic tree of toxABCDE was constructed using Phylogeny.fr software. The bootstrap consensus was inferred from 1000 replicates showing values of >30.
Fig. S2 The phylogenetic tree of toxoflavin transport genes in Burkholderia. Five wild‐type strains of Burkholderia were analysed: B. glumae BGR1, B. gladioli BSR3, B. gladioli KACC11889, B. gladioli KCTC12347 and B. gladioli KACC13944. The phylogenetic tree of toxFGHI was constructed using Phylogeny.fr software. The bootstrap consensus was inferred from 1000 replicates showing values of >30.
Fig. S3 The phylogenetic tree of toxoflavin transcriptional regulator genes in Burkholderia. Five wild‐type strains of Burkholderia were analysed: B. glumae BGR1, B. gladioli BSR3, B. gladioli KACC11889, B. gladioli KCTC12347 and B. gladioli KACC13944. The phylogenetic tree of toxJR was constructed using Phylogeny.fr software. The bootstrap consensus was inferred from 1000 replicates showing values of >30.
Fig. S4 Expression of quorum sensing (QS) genes in Burkholderia gladioli BSR3. Expression levels of two QS systems (bgla_2g11050‐70 and bgla_1p1740‐60) were measured using RNA sequencing in B. gladioli BSR3. The results were calculated as RPKM (reads per kilobase of exon per million mapped sequence reads). Blue and red bars indicate the exponential phase and stationary phase, respectively.
Fig. S5 Toxoflavin production and reverse transcription‐polymerase chain reaction (RT‐PCR) in a tofI/R‐complemented strain (11889ATOFIR) of Burkholderia gladioli KACC11889. (a) Thin‐layer chromatography (TLC) analysis of toxoflavin production in 11889ATOFIR, in which the tofI/R genes of B. gladioli BSR3 were introduced. Burkholderia gladioli BGR3 and KACC11889 are shown on the right side of the plates as controls. Toxoflavin production was evaluated visually in both daylight and under UV light at 365 nm (T, toxoflavin). The origin (o) and solvent front (f) on the TLC plate are indicated. (b) RT‐PCR analysis of the tofIMR genes. The expression of the tofIMR genes was confirmed using RT‐PCR of total RNA from four B. gladioli strains (BSR3, KACC11889, 11889ATOFIMR and 11889ATOFIR). The 16s ribosomal RNA was used as a positive control. The amplified products were 225 bp for tofI (lane 1: primers 5′‐GTACCTGCTGCACGAGGTG‐3′ and 5′‐CGCTCGATACTGCAGAAGGT‐3′), 201 bp for tofM (lane 2: primers 5′‐TTTCGCACACCTGACCGTT‐3′ and 5′‐GTGAGGGCCTGGTTGACC‐3′), 208 bp for tofR (lane 3: primers 5′‐CTGGATGGCCCATTACCAGG‐3′ and 5′‐GATGGTCAGCAGTCCGAACA‐3′) and 282 bp for the control (lane 4: primers 5′‐CCAGCAGCCGCGGTAATACG‐3′ and 5′‐TACCAGGGTATCTAATCC‐3′).
Table S1 PCR primers used in this study.


