Introduction
Seeds, defined, in this Opinion piece, as sexually derived structures of spermatophytes, are involved in the vertical transmission of microorganisms from one plant generation to another and consequently act as a primary source of inoculum for crops. A variety of microorganisms, such as plant growth‐promoting agents and plant or animal pathogens, have been isolated from the seed surfaces or the seed tissues of various plant species. These seed‐associated microorganisms could represent transient colonizers of the seed habitat or, alternatively, be transmitted to the plantlet and influence seedling‐associated microbial assemblages. Therefore, we should differentiate between seed‐borne and seed‐transmitted microorganisms.
Seed transmission of microorganisms can have various detrimental effects on seed physiological quality, including seed discoloration or decrease in germination rate. Moreover, the sanitary quality of seed can also be impacted by the transmission of pathogens through contamination of seeds with mycotoxins or Shiga toxins. From an epidemiological point of view, seed transmission of phytopathogenic microorganisms represents an important means of pathogen dispersion and is therefore significant in the emergence of disease. Even a low level of seed contamination is sufficient to lead to the efficient colonization of plants by bacterial pathogens (Darrasse et al., 2007). Moreover, seed transmission of plant‐pathogenic agents can occur on non‐host plants. For instance, the bacterial pathogen of Brassicas, Xanthomonas campestris pv. campestris (Xcc), is efficiently transmitted from the mother plant to seeds of bean and from bean seeds to seedlings (Darrasse et al., 2010; Darsonval et al., 2008). This non‐host carriage could serve as a potential reservoir of pathogenic agents in new planting areas and may contribute to an increase in the gene pool available for recombination. For all of these reasons, exploring plant–pathogen interactions during the plant reproductive stage is of interest for the control of plant disease.
Vertical transmission guarantees the persistence of a microorganism from parents to offspring. To manage vertically transmitted pathogens, we should either exclude pathogens from the mother plant or treat the seed to eliminate them. Chemical treatments applied to seed‐producing crops or as seed treatments are efficient methods for the control of fungal pathogens. In contrast, these chemical‐based methods are unsatisfactory for bacterial plant pathogens. Therefore, the control of seed‐transmitted bacterial pathogens relies either on alternative seed treatments, such as thermotherapy, or on prophylactic measures performed on crops and seed samples. Nevertheless, none of these strategies guarantees pathogen‐free seeds. Biological control is a promising option, but has been historically hampered by the variation in efficacy of the microbial strains employed, which could partly be explained by the empirical selection of biocontrol agents. The survey of seed‐associated microbial assemblages presented in this Opinion piece should provide novel options for the selection of biocontrol agents.
Processes Contributing to Efficient Seed Transmission of Phytopathogenic Agents
Seeds of different plant species vary greatly in terms of anatomy and morphology (Singh and Mathur, 2004). Despite these variations in structure, mature seeds can be schematically divided into three main compartments: (i) the embryo, which is composed of the embryonic axis and the cotyledon(s); (ii) storage tissues, such as the perisperm and the endosperm; and (iii) the seed coat (also known as the testa). It is noteworthy that non‐endospermic seeds, such as those of Fabaceae, store nutritive resources within the cotyledons and are therefore devoid of storage tissues.
Numerous microorganisms, including bacteria and fungi, have been isolated from the seed coat, whereas contamination of the endosperm and the embryo seed is less frequent (Maude, 1996; Singh and Mathur, 2004). The location of microorganisms within seed tissues is dependent on the stage at which the seed is colonized by these microorganisms. Early colonization of developing seeds can occur through the xylem or non‐vascular tissue of the mother plant (Maude, 1996). These internal seed transmission pathways are restricted to a few pathogenic microorganisms and endophytic microbes. Alternatively, developing seeds can be invaded by microorganisms through the floral pathway via the stigma of the mother plant (Maude, 1996). The internal and floral pathways result in the colonization of all seed tissues from the embryo to the testa. In contrast, late colonization of mature seeds through contact of the seed with microorganisms present on fruits or threshing residues is usually restricted to the seed coat (Singh and Mathur, 2004). As this seed transmission pathway is more permissive than the internal or floral pathway, microbial assemblages associated with the seed surface are probably more diverse than the microbiota of seed internal tissues.
Understanding the relative importance of seed transmission pathways in relation to specific diseases is a prerequisite for the development of efficient control methods. Indeed, the location of microorganisms in seed can affect the transmission efficiency to developing seedlings. On the one hand, the location of any microorganism within the embryo guarantees its transfer to the seedling, therefore representing successful seed transmission. On the other, microorganisms present on the surface of the embryo or in other tissues of the seed (e.g. endosperm or perisperm, testa) have yet to colonize the seedlings. Indeed, the microorganism needs to survive multiple anthropogenic processes, such as harvest, cleaning, treatment or storage of seeds, and also intense microbial competition near the site of exudation once the seed germinates. Therefore, successful colonization of a seedling can be dependent on a minimum bacterial population size of 1 × 102 colony‐forming units (CFU) per seed (Darrasse et al., 2007). Provided that this minimal inoculum density is reached, it seems that the transmission of phytopathogenic microorganisms from seed to seedlings is relatively permissive in both compatible and incompatible interactions. For example, the transmission rates of Clavibacter michiganensis pv. michiganensis, Pseudomonas syringae pv. tomato and Xanthomonas vesicatoria on tomato seedlings is around 50% for each phytopathogenic microorganism (J.‐F. Guimbaud et al., unpublished results). This transmission rate can reach, on average, 80% for Xanthomonas fuscans ssp. fuscans (Xff) strains on bean seedlings (J.‐F. Guimbaud et al., unpublished results) and 100% for Acidovorax citrulli on watermelon plantlets (Dutta et al., 2012). Moreover, the multiplication rates of Xcc and Escherichia coli on bean seedlings are not significantly different from the multiplication rate of Xff, suggesting that, whatever the type of interaction, bacteria behave similarly at emergence (Darrasse et al., 2010). This similar colonization pattern could be explained by the absence of the induction of plant defences during emergence (Darrasse et al., 2010). In contrast, during development, seeds respond to pathogen presence through the activation of plant defences and subsequent repression of seed maturation pathways (Terrasson et al., 2015). Based on these results, it is tempting to conclude that the transmission of microorganisms from seed to seedling is relatively permissive in comparison with the transfer from mother plant to seeds. Therefore, the development of control methods aimed at decreasing the efficiency of plant to seed transmission of plant pathogens could represent an interesting strategy for the production of seeds with optimal sanitary quality. Alternatively, the selection of biocontrol agents possessing key molecular determinants required for successful persistence during germination and emergence is also of interest for the exclusion of phytopathogenic agents on plantlets.
Potential Influences of Seed‐Associated Microbial Assemblages on Seed Transmission of Plant Pathogens
Although the host immune system is undoubtedly an important environmental filter that prevents the establishment of plant pathogens, host‐associated microbial assemblages may also restrain this invasion. The resistance of a microbial community to invasion is usually correlated with its level of diversity as a result of enhanced competition for resources within species‐rich communities (Mallon et al., 2015). Hence, assessments of the microbial diversity associated with seeds and the processes involved in the assembly of seed‐associated microbial communities are important preliminary steps for the design of new biocontrol‐based methods.
There is an abundant literature relating to the isolation of microorganisms from seeds of various plant species through classical microbiological techniques. Conversely, few studies have performed an in‐depth investigation of the composition of seed‐associated microbial assemblages with culture‐independent approaches. Recent profiling of seed‐associated microbial assemblages performed on various plant species has revealed that the seed microbiota contains, on average, less bacterial and fungal taxa than the microbial community associated with the rhizosphere and a comparable level of diversity to the phyllosphere (Barret et al., 2015; Klaedtke et al., 2015; Links et al., 2014). Interestingly, most of the seed‐associated bacterial and fungal operational taxonomic units (OTUs) are linked to species whose members can be easily recovered from culture‐dependent approaches. Although some microbial taxa, such as Pantoea or Alternaria, are frequently identified in seeds of various plant species, an important variation in the composition of microbial assemblages exists between seed samples. For example, the number of microbial entities (expressed as OTUs) observed on seeds ranged from 10 to 500 OTUs depending on the molecular marker and analytical workflow employed (Barret et al., 2015; Links et al., 2014). According to the samples analysed, the composition of seed‐associated microbial assemblages is not driven by the plant genotypes (Barret et al., 2015). However, the structure of fungal assemblages could be explained, in part, by the geographical location of the production region. In order to confirm or refute this observation, the relative influence of the environment and the host genotypes on the structure of the seed microbiota was assessed on five bean cultivars cropped in two distinct production regions (Klaedtke et al., 2015). The structure of seed‐associated fungal assemblages is mostly determined by the production region. This finding may have implications for the restriction of seed transmission of phytopathogenic fungi through the selection of a specific production area, where the presence of these plant pathogens has not been detected.
In contrast with the results obtained for seed‐associated fungal assemblages, variance in bacterial assemblage structure cannot be explained by the production region or the host genotype (Klaedtke et al., 2015). Therefore, we might expect that neutral processes, such as assembly history, may determine the structure of seed‐associated bacterial assemblages. This could mean that the first bacterial taxa that colonize the seed probably exclude other equivalent taxa sharing the same functional potential from colonizing this habitat. Therefore, the inoculation of a plant at the flowering stage with microorganisms having a high overlap in resource use is likely to restrict the subsequent colonization of phytopathogenic microorganisms and could be used as a promising biological treatment strategy.
As stated earlier, an understanding of the nature, succession and activities of seed‐borne microorganisms is relevant for the determination of their successful transmission to the next plant generation. Seed‐associated microorganisms must display great physiological adaptation capacity to the changing conditions encountered during seed developmental stages, as well as during seed germination. Functions such as adhesion, resistance to hydric and osmotic stresses, quorum sensing and secretion of microbial effectors have been demonstrated to be key determinants for the transmission of fungal pathogens, such as Alternaria brassicicola (Pochon et al., 2013), or bacterial pathogens, such as Xanthomonas spp. (Darsonval et al., 2008, 2009) or Acidovorax (Johnson and Walcott, 2013; Tian et al., 2015), to seeds. In addition, a number of broad functional categories, such as chemotaxis, attachment, carbohydrate use and iron acquisition, are necessary for the successful colonization of germinating seeds by endophytes (Truyens et al., 2015).
The dynamics of different seed‐associated microbial assemblages have been investigated recently on germinating seeds and seedlings of numerous plant genotypes (Barret et al., 2015). Based on sequences of different bacterial and fungal markers, we found that fungal and bacterial diversity was markedly decreased during emergence (Fig. 1). This reduction in microbial diversity within seedlings reflected an increase in the relative abundance of some bacterial (e.g. Pantoea and Pseudomonas) and fungal (e.g. Cladosporium and Alternaria) taxa possessing fast‐growing abilities and diverse diet breadths. The enrichment of these microbial taxa on seedlings is ultimately associated with the extinction of transient seed colonizers that were initially detected as low population sizes on seeds and germinating seeds (Barret et al., 2015). Therefore, for any microorganism, efficient seed transmission is clearly dependent on a minimum inoculum threshold (Darrasse et al., 2007), together with a strong physiological adaptation capacity to the changing conditions encountered during germination and emergence.
Figure 1.
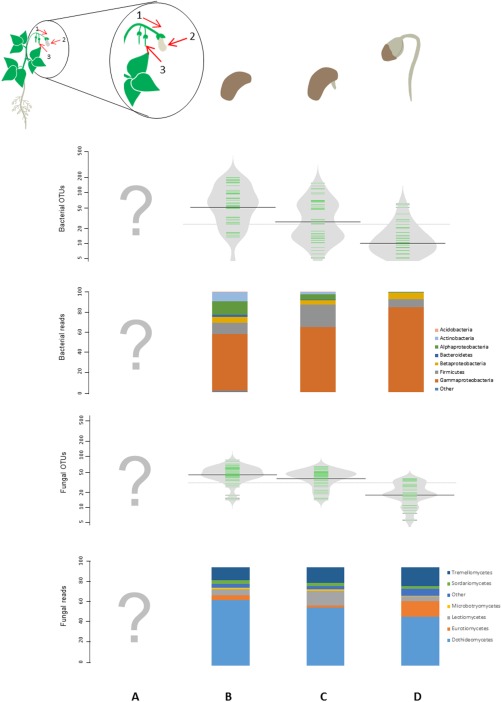
Seed transmission of microbial assemblages at different plant development stages. Community profiling of microbial assemblages associated with the developing seed (A), seed (B), germinating seed (C) and seedling (D). The microbial richness [presented here as bacterial and fungal operational taxonomic units (OTUs)] and relative abundance of microbial taxa (presented here as bacterial and fungal phyla) have been assessed for stages (B), (C) and (D) (Barret et al., 2015). However, the composition of microbial assemblages associated with developing seeds (A) is currently unknown. Notably, the relative influence of the systemic (1), floral (2) and external (3) pathways in seed transmission of the plant microbiota remains to be determined.
Conclusions—Future Directions
Seed transmission of microorganisms is the primary source of inoculum for plants and, accordingly, may play a crucial role in plant growth and plant health. To date, the structure of seed‐associated microbial assemblages and the regulators of assemblage structure have been overlooked. Preliminary studies of microbial assemblages associated with seeds of various plant species have unveiled a relatively low microbial diversity in comparison with other plant habitats, such as the phyllosphere and the rhizosphere, with the gradual enrichment of general seed colonizers during germination and emergence (Fig. 1). These seed‐associated microbial assemblages seem to be structured by a combination of deterministic and stochastic processes. How are microbial entities assembled throughout the seed developmental stages (from fertilization to maturation drying; Fig. 1)? This is currently unknown and deserves to be tested experimentally through the profiling of microbial assemblages on different host plants. In theory, successional changes occurring over the seed developmental stages within the microbial community should ultimately result in a stable‐state community. This theoretical framework is particularly relevant for mature seeds that have low moisture content and are almost metabolically inactive, which suggests that associated microorganisms are probably dormant and thus no longer interact with each other.
The stability of a microbial community could be impacted by a number of events, called disturbances, which could be chemical (e.g. fungicide), physical (e.g. temperature changes) or biological (e.g. invader) in nature. The response of the seed‐associated microbial community to biological disturbance caused by the seed transmission of phytopathogenic agents is of great interest when proposing biocontrol‐based strategies. Future experiments should assess the influence of distinct microbial invaders differing in their seed transmission pathways (systemic, floral and external) on the composition of seed‐associated microbial assemblages. These studies could highlight a number of positive and negative associations between the invaders and the resident member(s) of the seed microbiota, which might be related to mutualistic or competitive interactions between these entities. As most of the members of the seed microbiota can be cultivated on synthetic media, the isolation of strains representative of these resident members potentially interacting with the pathogenic agent should be relatively straightforward. The invasion success of the plant pathogen could be tested first in a laboratory microcosm containing these microbial strains, and subsequently assessed during co‐inoculation experiments performed in planta.
According to multiple metagenomics studies, it seems that the assembly of the host‐associated microbial community is based on its functional potential rather than on its species composition. Indeed, species having similarities in ecological function, such as resource consumption, probably compete for nutrients and space, and therefore rarely coexist in the same ecological niche. Therefore, it also seems important to assess the functional potential of the seed microbiome at various seed and seedling developmental stages. Metagenomics‐ and metatranscriptomics‐based approaches are undoubtedly of interest for the assessment of the genetic content and gene expression patterns of these microbial assemblages. Abundance comparisons of the proteins encoded by seed‐associated assemblages with those encoded in the germinating seed and seedling microbiota would reveal insights into the traits involved in the establishment of microbes in young plants. These traits might again be useful indicators for the selection of microbial inoculants possessing biocontrol activities.
Conflict of Interest
The authors report no conflicts of interest.
References
- Barret, M. , Briand, M. , Bonneau, S. , Préveaux, A. , Valière, S. , Bouchez, O. , Hunault, G. , Simoneau, P. and Jacquesa, M.A. (2015) Emergence shapes the structure of the seed microbiota. Appl. Environ. Microbiol. 81, 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrasse, A. , Bureau, C. , Samson, R. , Morris, C.E. and Jacques, M.A. (2007). Contamination of bean seeds by Xanthomonas axonopodis pv. phaseoli associated with low bacterial densities in the phyllosphere under field and greenhouse conditions. Eur. J. Plant Pathol. 119, 203–215. [Google Scholar]
- Darrasse, A. , Darsonval, A. , Boureau, T. , Brisset, M.‐N. , Durand, K. and Jacques, M.‐A. (2010) Transmission of plant‐pathogenic bacteria by nonhost seeds without induction of an associated defense reaction at emergence. Appl. Environ. Microbiol. 76, 6787–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsonval, A. , Darrasse, A. , Meyer, D. , Demarty, M. , Durand, K. , Bureau, C. , Manceau, C. and Jacques, M.A. (2008) The Type III secretion system of Xanthomonas fuscans subsp. fuscans is involved in the phyllosphere colonization process and in transmission to seeds of susceptible beans. Appl. Environ. Microbiol. 74, 2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsonval, A. , Darrasse, A. , Durand, K. , Bureau, C. , Cesbron, S. and Jacques, M.A. (2009) Adhesion and fitness in the bean phyllosphere and transmission to seed of Xanthomonas fuscans subsp. fuscans . Mol. Plant–Microbe Interact. 22, 747–757. [DOI] [PubMed] [Google Scholar]
- Dutta, B. , Avci, U. , Hahn, M.G. and Walcott, R.R. (2012) Location of Acidovorax citrulli in infested watermelon seeds is influenced by the pathway of bacterial invasion. Phytopathology, 102, 461–468. [DOI] [PubMed] [Google Scholar]
- Johnson, K.L. and Walcott, R.R. (2013). Quorum sensing contributes to seed‐to‐seedling transmission of Acidovorax citrulli on watermelon. J. Phytopathol. 161, 562–573. [Google Scholar]
- Klaedtke, S. , Jacques, M.‐A. , Raggi, L. , Préveaux, A. , Bonneau, S. , Negri, V. , Chable, V. and Barret, M. (2015) Terroir is a key driver of seed‐associated microbial assemblages. Environ. Microbiol. doi: 10.1111/1462-2920.12977. [DOI] [PubMed] [Google Scholar]
- Links, M.G. , Demeke, T. , Grafenhan, T. , Hill, J.E. , Hemmingsen, S.M. and Dumonceaux, T.J. (2014) Simultaneous profiling of seed‐associated bacteria and fungi reveals antagonistic interactions between microorganisms within a shared epiphytic microbiome on Triticum and Brassica seeds. New Phytol. 202, 542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallon, C.A. , Poly, F. , Le Roux, X. , Marring, I. , van Elsas, J.D. and Salles, J.F. (2015). Resource pulses can alleviate the biodiversity–invasion relationship in soil microbial communities. Ecology, 96, 915–926. [DOI] [PubMed] [Google Scholar]
- Maude, R.B. (1996) Seedborne Diseases and Their Control: Principles and Practice. Wallingford, Oxfordshire: CAB International. [Google Scholar]
- Pochon, S. , Simoneau, P. , Pigne, S. , Balidas, S. , Bataille‐Simoneau, N. , Campion, C. , Jaspard, E. , Calmes, B. , Hamon, B. , Berruyer, R. , Juchaux, M. and Guillemette, T. (2013) Dehydrin‐like proteins in the necrotrophic fungus Alternaria brassicicola have a role in plant pathogenesis and stress response. PLoS One, 8, e75143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, D. and Mathur, S.B. (2004) Histopathology of Seed‐Borne Infections. Boca Raton, FL: CRC Press. [Google Scholar]
- Terrasson, E. , Darrasse, A. , Righetti, K. , Buitink, J. , Lalanne, D. , Ly Vu, B. , Pelletier, S. , Bolingue, W. , Jacques, M.A. and Leprince, O. (2015) Identification of a molecular dialogue between developing seeds of Medicago truncatula and seedborne xanthomonads. J. Exp. Bot. 66, 3737–3752. doi: 10.1093/jxb/erv167. [DOI] [PubMed] [Google Scholar]
- Tian, Y. , Zhao, Y. , Wu, X. , Liu, F. , Hu, B. and Walcott, R.R. (2015). The type VI protein secretion system contributes to biofilm formation and seed‐to‐seedling transmission of Acidovorax citrulli on melon. Mol. Plant Pathol. 16, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyens, S. , Weyens, N. , Cuypers, A. and Vangronsveld, J. (2015) Bacterial seed endophytes: genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 7, 40–50. [Google Scholar]


