Summary
Non‐self‐recognition of microorganisms partly relies on the perception of microbe‐associated molecular patterns (MAMPs) and leads to the activation of an innate immune response. Bacillus subtilis produces three main families of cyclic lipopeptides (LPs), namely surfactins, iturins and fengycins. Although LPs are involved in induced systemic resistance (ISR) activation, little is known about defence responses induced by these molecules and their involvement in local resistance to fungi. Here, we showed that purified surfactin, mycosubtilin (iturin family) and plipastatin (fengycin family) are perceived by grapevine plant cells. Although surfactin and mycosubtilin stimulated grapevine innate immune responses, they differentially activated early signalling pathways and defence gene expression. By contrast, plipastatin perception by grapevine cells only resulted in early signalling activation. Gene expression analysis suggested that mycosubtilin activated salicylic acid (SA) and jasmonic acid (JA) signalling pathways, whereas surfactin mainly induced an SA‐regulated response. Although mycosubtilin and plipastatin displayed direct antifungal activity, only surfactin and mycosubtilin treatments resulted in a local long‐lasting enhanced tolerance to the necrotrophic fungus Botrytis cinerea in grapevine leaves. Moreover, challenge with specific strains overproducing surfactin and mycosubtilin led to a slightly enhanced stimulation of the defence response compared with the LP‐non‐producing strain of B. subtilis. Altogether, our results provide the first comprehensive view of the involvement of LPs from B. subtilis in grapevine plant defence and local resistance against the necrotrophic pathogen Bo. cinerea. Moreover, this work is the first to highlight the ability of mycosubtilin to trigger an immune response in plants.
Keywords: Bacillus subtilis, Botrytis cinerea, grapevine, induced resistance, lipopeptides, MAMP
Introduction
Non‐self‐perception in plant innate immunity initially relies on the recognition of microbe‐associated molecular patterns (MAMPs) (Boller and Felix, 2009; Dodds and Rathjen, 2010). MAMP‐triggered immunity (MTI) involves the activation of a wide range of defence responses and provides the plant with a basal level of resistance to pathogenic microorganisms. MTI defence responses are characterized by early signalling events, including ion fluxes, mitogen‐activated protein (MAP) kinase cascade activation and the production of reactive oxygen species (ROS) (Garcia‐Brugger et al., 2006). Key hormone molecules, such as salicylic acid (SA) and jasmonic acid (JA), are involved in the regulation of downstream defence genes (Robert‐Seilaniantz et al., 2011). Interactions between these signal molecules allow the plant to activate and/or modulate an appropriate spectrum of responses, including the strengthening of plant cell physical barriers and the production of antimicrobial compounds that participate in the restriction of the pathogen (Dodds and Rathjen, 2010).
MAMPs are typically essential components of whole classes of pathogens, such as flagellin, EF‐Tu, peptidoglycans, lipopolysaccharides (LPSs) from bacteria or chitin, and elicitins from fungi and oomycetes (Boller and Felix, 2009). Recently, rhamnolipids (RLs), which are bacterial molecules with amphiphilic properties, have been characterized as a new class of MAMPs perceived by plant cells, and activate an MTI in several plant species (Sanchez et al., 2012; Varnier et al., 2009; Vatsa et al., 2010).
Lipopeptides (LPs), another class of amphiphilic molecules, have also emerged as key players in the induction of plant immunity driven by beneficial microorganisms (Falardeau et al., 2013; Ongena and Jacques, 2008; Raaijmakers et al., 2010). LPs are produced by a variety of bacterial genera, such as Streptomyces, Pseudomonas and Bacillus (Raaijmakers et al., 2010). LPs consist of a short peptide chain linked to a lipid tail. They are synthesized by a non‐ribosomal enzyme complex called non‐ribosomal peptide synthetase (NRPS), which confers considerable structural diversity to the molecules and results in the production of linear, branched or cyclic compounds (Strieker et al., 2010). This structural diversity is associated with a large range of functions for the microorganisms. For instance, they are used to modify surface tension, leading to improved motility (Leclere et al., 2006; Raaijmakers et al., 2010), to produce biofilms, which are important for surface attachment and root colonization (Bais et al., 2004), and to chelate metal ions (Raaijmakers et al., 2010). In addition, LPs also show direct antagonist activity toward viruses, mycoplasmas, bacteria, yeast, fungi and oomycetes (Coutte et al., 2010; Fickers et al., 2009; Khong et al., 2012; Ongena and Jacques, 2008; Ongena et al., 2007; Raaijmakers and Mazzola, 2012; Toure et al., 2004; van de Mortel et al., 2009). LPs are able to activate an induced systemic resistance (ISR), which is effective against several diseases in a large range of plant systems (Falardeau et al., 2013; Ongena and Jacques, 2008). For instance, the treatment of tomato roots with massetolide A leads to an increased resistance to the oomycete Phytophthora infestans in leaves (Tran et al., 2007). Fengycin and surfactin are able to trigger an ISR effective against Botrytis cinerea infection in tomato and bean (Ongena et al., 2007). Although LPs are known to be involved in ISR activation, little is known about their perception by plant cells. To date, the only available data have been reported by Jourdan et al. (2009), indicating that surfactin stimulates extracellular medium alkalinization, ROS production and the activity of defence‐related enzymes, such as phenylalanine ammonia‐lyase (PAL) and lipoxygenase (LOX), in tobacco cell suspensions.
Bacillus subtilis is known to produce three main families of cyclic LPs, namely surfactins, iturins and fengycins (Falardeau et al., 2013). In this study, we investigated the perception of these three families of LPs by grapevine plant cells. We also investigated the efficacy of each LP to protect grapevine leaves against the necrotrophic pathogen Bo. cinerea. We showed, for the first time, that grapevine plant cells perceive the three families of LPs, and that these compounds differentially activate the plant innate immunity system and trigger different levels of local resistance to the fungus. Interestingly, mycosubtilin is the most efficient stimulator of the innate immune response in grapevine. We also found that LP overproduction by B. subtilis is not critical in the activation of grapevine innate immunity driven by the bacterium, suggesting that other MAMPs may be involved in the process. Our results provide a comprehensive view of the involvement of LPs in grapevine plant defence and local protection against Bo. cinerea.
Results
LP perception triggers early signalling events with different signatures
Extracellular alkalinization is an essential component of ion fluxes involved in plant defence (van Loon et al., 2008), and has been used as an efficient method to monitor chemosensory perception in cultured plant cells (Felix et al., 1993). We first carried out dose–response experiments on grapevine cell suspension cultures using concentrations of LPs ranging from 10 to 50 μg/mL. All LPs induced an alkalinization of the grapevine cell suspension medium (Fig. 1). However, we observed important differences between the different molecules. Plipastatin only caused a limited alkalinization of the culture medium, whereas cell suspensions incubated with surfactin and mycosubtilin exhibited a clear dose‐dependent response with a strong alkalinization at 50 μg/mL (Fig. 1). Interestingly, mycosubtilin treatment displayed a long‐lasting alkalinization response over several hours that did not return to baseline (data not shown). The subsequent experiments were carried out using a concentration of 50 μg/mL, which displayed a sustained alkalinization response with the different LPs. The kinase inhibitor K‐252a was used to verify whether the proton fluxes were physiologically associated with plant cell signalling activation. The addition of K‐252a strongly inhibited all LP‐induced alkalinizations (Fig. S1, see Supporting Information). Moreover, no refractory state of the proton influx response, indicative of homologous desensitization (Felix et al., 1993; Smith et al., 2014), was observed with either surfactin or plipastatin (Fig. 2). As the alkalinization response was not transient with mycosubtilin, we were unable to test the refractory state with this molecule. Interestingly, only surfactin stimulated a significant and strong oxidative burst (Fig. S2, see Supporting Information). In addition, treatment with the three LPs did not activate calcium influx in grapevine cells (L. Trda and B. Poinssot, personal communication, Université de Bourgogne, UMR 1347 Agroécologie, Pôle Interactions Plantes Micro‐organismes, 17 rue Sully, 21000 Dijon, France).
Figure 1.
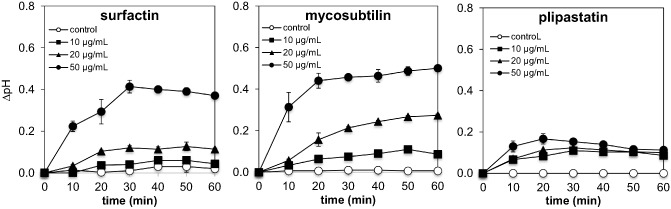
Medium alkalinization of grapevine cell suspensions challenged by lipopeptides (LPs). Cells were treated with surfactin, mycosubtilin or plipastatin at 10, 20 and 50 μg/mL or dimethylsulphoxide (DMSO) 0.1% (control). Data presented are means of triplicate experiments ± standard deviation (SD).
Figure 2.
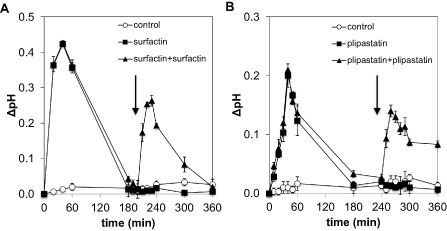
Alkalinization response of grapevine cells exposed to consecutive additions of surfactin and plipastatin. Cells were sequentially treated with surfactin (A) or plipastatin (B) at 50 μg/mL. A second dose of LP was added (arrow) once the alkalinization response had returned to baseline. Data presented are means of triplicate experiments ± standard deviation (SD).
Perception of surfactin and mycosubtilin, but not plipastatin, stimulates defence gene expression
In order to compare grapevine gene expression profiles after LP challenge, we selected several reliable defence gene markers covering a large set of defence classes. We used the chit4c and pin genes (coding for an acidic chitinase and a protease inhibitor, respectively), which are strongly induced in the grapevine plant systems on elicitation (Bordiec et al., 2011). The gluc gene (coding for a basic glucanase) was used as a JA‐regulated gene marker, whereas the Vv17.3 gene was chosen as a marker of the SA‐dependent signalling pathway in grapevine (Bordiec et al., 2011). The expression profile of these genes was monitored 9 and 24 h after inoculation of grapevine cells with the three LPs. Mycosubtilin induced strong expression of all defence markers (Fig. 3). chit4c, pin and Vv17.3 were significantly up‐regulated after challenge with surfactin, but at a lower level compared with mycosubtilin. Surprisingly, plipastatin did not significantly induce defence gene expression in these conditions. Using the Evans blue test, no cell death increase was detected in the cell suspensions treated with surfactin or plipastatin at 50 μg/mL (Fig. S3, see Supporting Information). However, when mycosubtilin was applied, a significant increase in cell death was observed (around 23% of the total cells compared with 11% in the control) at 24 h post‐treatment.
Figure 3.
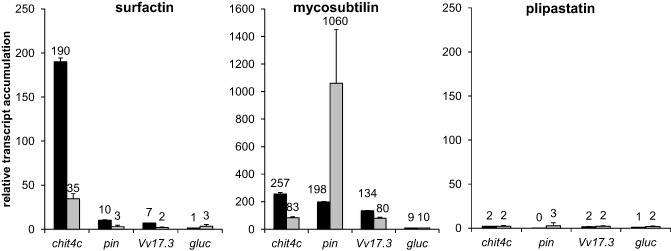
Defence gene expression in response to lipopeptides (LPs). Transcript accumulation of genes encoding a chitinase (chit4c), a protease inhibitor (pin), a salicylic acid (SA)‐regulated marker (Vv17.3) and a glucanase (gluc) was monitored at 9 h (black bars) and 24 h (grey bars) after challenge with surfactin, mycosubtilin and plipastatin. Analyses were performed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). The transcript level was calculated using the standard curve method from duplicate data, with the grapevine EF‐1α gene as the internal control. Results are expressed as relative transcript accumulation (fold increase) over the dimethylsulphoxide (DMSO) 0.1% control. Data presented are means of triplicate experiments ± standard deviation (SD).
As the gene induction profiles triggered by LPs and, especially, the ability of mycosubtilin to trigger an immune response were quite unexpected, we investigated gene expression in Arabidopsis thaliana challenged with LPs. Interestingly, treatment of A. thaliana leaves with purified LPs also led to a differential activation of defence genes and the overall response was comparable with that of grapevine (Fig. S4, see Supporting Information). Indeed, mycosubtilin was the most active in the induction of PR1 expression, followed by surfactin. Plipastatin remained inactive. PR4 expression was only slightly induced by mycosubtilin and surfactin, whereas PDF1.2 expression remained unchanged.
Mycosubtilin and plipastatin, but not surfactin, display direct antifungal activity against Bo. cinerea
Although crude supernatant cultures containing LPs (iturins or fengycins) or B. subtilis strains producing these LPs are known to exhibit antagonistic activities towards a large range of fungi (Coutte et al., 2010; Falardeau et al., 2013; Leclere et al., 2005; Ongena and Jacques, 2008; Ongena et al., 2007; Romero et al., 2007; Toure et al., 2004), direct antifungal activity of the pure compounds against Bo. cinerea has not been described to date. Using an in vitro bioassay, we found that both mycosubtilin and plipastatin inhibited Bo. cinerea spore germination, whereas surfactin remained inactive (Fig. 4).
Figure 4.
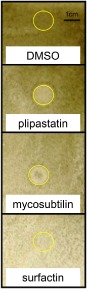
Spore germination assay with lipopeptides (LPs). LPs were spotted onto a TLC (thin layer chromatography) membrane (10 μL for each LP at a concentration of 50 μg/mL; the surface area of the membrane covered by LP solution was delimited by a yellow circle). Conidia (1 × 105) from Botrytis cinerea were applied to the membrane and fungal growth was observed 96 h later. Data present one experiment of three with similar profiles.
Surfactin and mycosubtilin treatments lead to long‐lasting enhanced tolerance to Bo. cinerea in grapevine plants
We then investigated the efficiency of LPs to protect grapevine plants against the necrotrophic fungus Bo. cinerea. Vitro‐plantlets were immersed into solutions of LPs, 48 h prior to infection with Bo. cinerea. A concentration of 10 μg/mL was used to investigate the elicitor properties of surfactin and mycosubtilin and to minimize a direct antifungal effect. However, because plipastatin did not display a strong elicitor activity, we increased the concentration of this molecule to 50 μg/mL. In addition, mycosubtilin was tested in these experiments at an antifungal concentration of 50 μg/mL. Symptoms resulting from fungus colonization were monitored at 2 and 4 days post‐inoculation (Fig. 5). Water control plants infected with Bo. cinerea without previous LP treatment displayed strong disease symptoms, with 41% and 97% of leaves presenting strong necrosis (lesions larger than 50% of the total leaf area) at 2 and 4 days, respectively. Furthermore, 56% of the leaves also presented small necrosis lesions (lesions smaller than 50% of the total leaf area) 2 days after infection. Similar results were observed after DMSO 0.1% treatments. However, 2 days after fungus inoculation of plants pretreated with surfactin (10 μg/mL), mycosubtilin (10 and 50 μg/mL) or plipastatin (50 μg/mL), leaves were significantly protected against Bo. cinerea. Indeed, depending on the LPs, 21%–29% of leaves were symptomless and strong necrosis was only observed in 12%, 10% and 5% of the leaves pretreated with mycosubtilin (10 μg/mL), surfactin (10 μg/mL) and mycosubtilin (50 μg/mL), respectively. Four days after infection, surfactin‐ and mycosubtilin‐driven protection persisted, with more than 35% of the leaves without or with reduced symptoms, whereas plants pretreated with plipastatin completely lost their protection. Interestingly, we did not observe significant differences in the LP‐driven protection by changing the time of elicitation (1–4 days prior to infection), except for mycosubtilin at the highest concentration, which was more efficient when applied 1 day prior to infection (data not shown). The diversity of immune responses, direct effects and local resistance triggered by LPs are summarized in Table 1.
Figure 5.
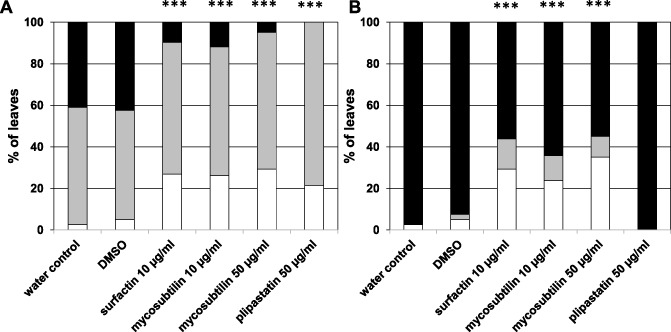
Protection from Botrytis cinerea in grapevine plants challenged by lipopeptides (LPs). Leaves from vitro‐plantlets were sprayed with solutions of LPs at the indicated concentrations or dimethylsulphoxide (DMSO) 0.1% or water. Two days later, leaves were placed on Petri dishes and inoculated with Bo. cinerea conidia (105 conidia/mL). Symptoms were scored 2 days (A) and 4 days (B) after inoculation by defining three lesion surface classes: no lesion (white bars), lesions affecting less than 50% of the leaf surface (grey bars) and lesions affecting more than 50% of the leaf surface (black bars). Results are means of three independent experiments (n = 40). Asterisks indicate statistically significant differences in the percentage of leaves in the different classes compared with the water control (Fisher's test, ***P = 0.001).
Table 1.
Diversity of immune responses, direct antifungal effects and local resistance triggered by lipopeptides (LPs)
| Mycosubtilin | Surfactin | Plipastatin | |
|---|---|---|---|
| ΔpH | +++ | ++ | + |
| Reactive oxygen species | +/− | +++ | +/− |
| Ca2+ | − | − | − |
| Defence gene induction | +++ | ++ | − |
| Local resistance to Botrytis cinerea | +++ | +++ | + |
| Spore germination inhibition | ++ | − | ++ |
Activation of defence responses by B. subtilis in grapevine is not exclusively dependent on LP production
An LP non‐producer and three LP‐overproducing strains of B. subtilis were used to investigate the implication of these molecules in B. subtilis perception. The strain BBG127 does not produce any LPs, whereas BBG131, BBG125 and Bs2504 overproduce solely surfactin, mycosubtilin and plipastatin, respectively. We first monitored the alkalinization response of grapevine cell cultures after inoculation with B. subtilis strains at concentrations of 105 and 107 colony‐forming units (cfu)/mL (Fig. 6). We observed a clear alkalinization response after challenge of cells with the strain BBG127 at the highest concentration, whereas no significant ΔpH was observed with 105 cfu/mL. Perception of this strain stimulated a maximum increase in pH of about 0.7 units within 30 min. Similar ΔpH effects were detected with the strains BBG131 and Bs2504 overproducing surfactin and plipastatin, respectively. BBG125, which produces mycosubtilin, displayed a different profile from that of the other strains. The alkalinization response was significantly lower (ΔpH of about 0.3 units) at the concentration of 107 cfu/mL. The chit4c gene is the classical marker used to monitor defence induction following bacterial challenge in grapevine (Bordiec et al., 2011). We therefore monitored the expression of this defence marker gene after perception of the B. subtilis strains (Fig. 7). Challenge of the grapevine cells with the four strains significantly induced defence marker expression. The strain BBG131, overproducing surfactin, and the strain BBG125, overproducing mycosubtilin, were significantly more active than the non‐producing strain BBG127, especially at the earliest time point. Intriguingly, the strain Bs2504, overproducing plipastatin, stimulated the defence marker gene to a lower level than did the BBG127 control strain. Using Evans blue assay, we did not monitor any cell death at 9 and 24 h after inoculation of cell suspensions with the different strains of B. subtilis (Fig. S3). Surprisingly, when vitro‐plantlets were immersed into LP‐ or non‐LP‐producing bacterial solutions prior to infection with Bo. cinerea, we only observed a slight enhanced protection with the mycosubtilin overproducer strain BBG125 (Fig. S5, see Supporting Information). This result suggests that, although being perceived by grapevine plant cells, B. subtilis is not very effective in triggering local resistance to the fungus in this plant.
Figure 6.
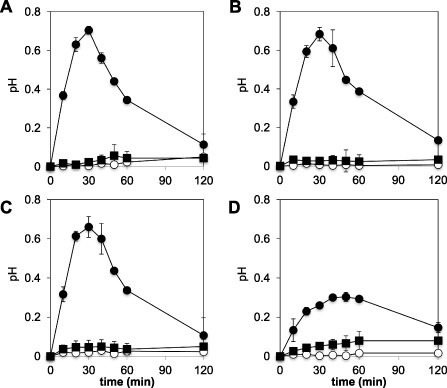
Alkalinization response after perception of Bacillus subtilis not producing or overproducing single lipopeptides (LPs). Cells were treated with LP non‐producer strain BBG127 (A), strain BBG131 overproducing surfactin (B), strain Bs2504 overproducing plipastatin (C) and strain BBG125 overproducing mycosubtilin (D) at 105 colony‐forming units (cfu)/mL (filled squares), 107 cfu/mL (filled circles) or MgCl2 (open circles). Data presented are means of triplicate experiments ± standard deviation (SD).
Figure 7.
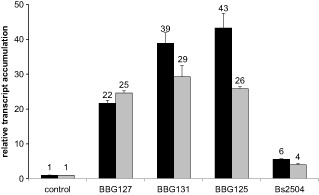
Expression of the chit4c defence marker after perception of Bacillus subtilis strains. Transcript accumulation of the gene marker chit4c was monitored at 9 h (black bars) and 24 h (grey bars) after challenge with the indicated strains or MgCl2 (control). Analyses were performed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). The level of transcripts was calculated using the standard curve method from duplicate data, with the grapevine EF‐1α gene as the internal control. Results are expressed as relative transcript accumulation (fold increase) over the MgCl2 control. Data presented are means of duplicate experiments ± standard deviation (SD).
Discussion
The substantial structural diversity of LPs produced by Bacillus (but also Pseudomonas and other bacterial genera) suggests that these metabolites may have different modes of action related to their capacity to enhance plant protection (Raaijmakers et al., 2010). Iturins and fengycins, but not surfactins, are known to possess direct antifungal activities (Ongena and Jacques, 2008; Perez‐Garcia et al., 2011). To date, only surfactins and fengycins have been described as ISR inducers (Falardeau et al., 2013; Ongena et al., 2007). Interestingly, whereas ISR to pathogens by LPs has been investigated in numerous studies, local defence responses after LP perception have been poorly documented (Falardeau et al., 2013). Here, we demonstrate, for the first time, elicitor properties for mycosubtilin, a member of the iturin family. Mycosubtilin is able to activate ion fluxes and to stimulate defence gene expression in grapevine. Mycosubtilin‐triggered innate immune responses in this plant system are even stronger, in terms of intensity, than are those of surfactin and the fengycin member, plipastatin. The defence response signature is reminiscent of that observed with RLs, another amphiphilic MAMP produced by Pseudomonas and Burkholderia species (Varnier et al., 2009), especially as cell death could be observed above a concentration threshold. Plipastatin is clearly perceived by grapevine plant cells, as illustrated by a proton influx, but, surprisingly, this early signalling event is not accompanied by the induction of our defence gene markers. Fengycin family members have been shown to be involved as MAMPs in tomato and potato, but not in bean (Ongena et al., 2005b, 2007), suggesting a clear plant‐dependent response for these molecules. Similarly, plant specificities in the recognition of the typical MAMP flagellin from a beneficial rhizobacterium have recently been described (Trda et al., 2014). Surfactin displays an intermediate MAMP profile in grapevine, with a strong alkalinization response, but a weaker induction of defence genes, than mycosubtilin. Significant ROS production is only observed in surfactin‐treated cells. In tobacco, the addition of surfactin, but not fengycin or iturin, induces defence‐related early events, such as extracellular medium alkalinization and ROS production (Henry et al., 2011; Jourdan et al., 2009). Surfactin is also able to stimulate the defence enzymes PAL and LOX, and to modify the pattern of phenolics produced by tobacco‐elicited cells (Jourdan et al., 2009). Like surfactin, synthetic ultrashort cationic LPs have also been described as potent inducers of medium alkalinization of tobacco suspension‐cultured cells and are able to stimulate the expression of defence‐related genes in cucumber and Arabidopsis seedlings (Brotman et al., 2009).
Interestingly, the defence gene signature observed in grapevine after treatment with the three LPs was not restricted to this plant. Challenge of A. thaliana with purified LPs also led to a differential activation of defence genes with a similar pattern. As in grapevine, mycosubtilin was the most active in the induction of defence gene expression, followed by surfactin. Plipastatin remained inactive. The strong activation of PR1 by mycosubtilin and, to a lesser extent, by surfactin suggests that these two LPs activate the SA‐dependent signalling pathway in A. thaliana. In grapevine, we observed a clear up‐regulation of the JA‐regulated gene marker gluc and the Vv17.3 gene marker of the SA‐dependent signalling pathway after challenge with mycosubtilin. Therefore, it is plausible that SA‐ and JA‐dependent signalling pathways are involved in the activation of defence responses in grapevine plant cells perceiving this LP. Results obtained with surfactin are more difficult to interpret given the low level of gene expression, especially with these two markers. Nevertheless, a significant induction of Vv17.3 (seven‐fold over the control) was constantly monitored at 9 h with surfactin, suggesting that the SA signalling pathway may also be activated by this MAMP in grapevine. In agreement with our results, a recent study has demonstrated that surfactin is a major determinant for the stimulation of an immune response in melon plants and that this response is SA and JA dependent (Garcia‐Gutierrez et al., 2013).
Interestingly, homologous desensitization experiments confirmed that surfactin did not display a refractory state in grapevine cells similar to tobacco cells (Henry et al., 2011). We also found that plipastatin did not exhibit a refractory state profile, suggesting that, like surfactin, recognition of the molecule could be independent of a specific receptor and may involve direct perception at the plasma membrane level by a lipid‐driven process (Henry et al., 2011). Mycosubtilin has been shown to interact with cholesterol, ergosterol and phospholipid membrane models (Nasir and Besson, 2011, 2012; Nasir et al., 2010), but it is not known whether this LP could interfere with plant plasma membranes, especially by forming pores and inducing important modifications of the membrane permeability, as demonstrated for fungal membranes (Nasir and Besson, 2011). Physiological relevance of LP‐driven elicitation was also confirmed by the use of K‐252a, a potent inhibitor of protein kinases in animals and plants (Ruegg and Burgess, 1989). K‐252a is known to cause significant changes in phosphorylated protein patterns within minutes after elicitation and to inhibit or greatly decrease alkalinization in tomato cells (Felix et al., 1991, 1993). The same effect on pH reduction was observed in our grapevine cell system after surfactin, mycosubtilin and plipastatin treatment, indicating that the alkalinization triggered by all these molecules is related to changes in the phosphorylation of proteins and is an active MAMP response.
Bacillus subtilis perception by Arabidopsis cells, although suppressing some defence responses at the root level (probably allowing colonization) (Lakshmanan et al., 2012), has also been shown to trigger transcriptomic changes in this plant organ with the up‐regulation of several defence‐related candidate genes (Lakshmanan et al., 2013). In addition, B. subtilis was able to induce systemic expression of defence genes (Rudrappa et al., 2010) or MTI‐triggered responses, such as stomatal closure, in Arabidopsis leaves (Kumar et al., 2012). In tobacco and tomato, this bacterium is able to stimulate systemic defence reactions (Ongena et al., 2005a). Bacillus subtilis supernatants have also been shown to stimulate an alkalinization response and ROS production in tobacco cell suspensions (Cawoy et al., 2014; Jourdan et al., 2009). Here, we show that the model non‐LP‐producing strain BBG127 of B. subtilis is able to trigger an innate immune response in grapevine cells, involving early signalling perception and defence gene induction. These results are consistent with a recent study on grapevine using a natural B. subtilis strain originating from vineyards that induced ROS production and trans‐resveratrol synthesis following bacterial perception (Verhagen et al., 2011). Here, we further demonstrate that the B. subtilis‐triggered immune response does not require LPs as the non‐LP‐producing strain fully retains its ability to induce defence responses. Our results suggest that other MAMPs are probably involved in the process. In agreement with this hypothesis, the ability of B. subtilis to induce stomatal closure is lost in fls2 Arabidopsis mutants, suggesting that flagellin is necessary for B. subtilis‐triggered MTI in this plant (Kumar et al., 2012). Moreover, volatile compounds and, more particularly, 2,3‐butanediol and acetoin, were also identified as determinants for elicitation by Bacillus spp. (Farag et al., 2013; Rudrappa et al., 2010; Ryu et al., 2004). A saturating threshold level of elicitation by several MAMPs may explain the similar profile of alkalinization observed in our conditions after challenge with the Bacillus strains producing or not producing LPs. However, our results on defence genes also show that the overproduction of surfactin or mycosubtilin by B. subtilis strains can enhance the level of defence markers, especially at early time points. This indicates a potential role of these LPs in the potentiation of defence responses in grapevine following bacterial perception. Intriguingly, the plipastatin‐overproducing strain exhibited a lower defence‐inducing profile. Whether plipastatin may retain some effector properties suppressing plant defence responses remains to be investigated. Interestingly, a cell culture filtrate of B. subtilis strain B17 was found to suppress flg22‐induced MAMP‐activated root defence responses (Lakshmanan et al., 2012). Nevertheless, the effector(s) involved in the process have not been characterized to date.
We found that the three LPs were able to enhance grapevine leaf tolerance against the necrotrophic pathogen Bo. cinerea. However, plipastatin's protection was transient. This could be related to the fact that this LP exhibits antibiosis, but does not have significant elicitation properties. In contrast, surfactin does not exhibit any antifungal properties and we can therefore assume that the resistance associated with this compound is related to the activation of the innate immune response in grapevine. Mycosubtilin clearly displayed both anti‐botrycidal and elicitation activities, and it is plausible that both are involved in Bo. cinerea restriction. Similar properties have been described for RLs (Varnier et al., 2009), chitosan (Aziz et al., 2006) and phosphites (Massoud et al., 2012). However, the importance of the innate immune response in the RL‐ and phosphate‐induced resistances has been clearly revealed using signalling‐ and defence‐defective Arabidopsis mutants (Massoud et al., 2012; Sanchez et al., 2012). Bacillus subtilis is known to be a very efficient ISR‐inducing strain in many plants, such as cucumber, bean, tomato and tobacco (Ongena and Jacques, 2008; Perez‐Garcia et al., 2011), mainly through the production of surfactins and, to a lesser extent, of plipastatin (Ongena et al., 2007). However, biocontrol activity of this bacterium on grapevine does not seem to be as effective. Only the mycosubtilin‐overproducing strain of B. subtilis was able to slightly enhance grapevine protection against Bo. cinerea. These results are in agreement with a previous study showing that a natural B. subtilis originating from the vineyard was much less efficient than Pantoea agglomerans, Acinetobacter lwoffii and Pseudomonas fluorescens in triggering an ISR in grapevine (Verhagen et al., 2011). Interestingly, the plipastatin‐overproducing strain used by Ongena et al. (2007) and in the present work also exhibited contrasting ISR properties towards Bo. cinerea infection in bean and tomato (Ongena et al., 2007). In our experiments, leaves from vitro‐plantlets were immersed directly in bacterial solutions and, a few days later, were inoculated with Bo. cinerea conidia. In these conditions, we investigated local resistance induced by the bacterial strains and not a typical ISR. These two experimental procedures could account for the differences observed in the protection results. As iturins are involved as key factors in the direct antagonism of B. subtilis towards fungi, such as Podosphaera fusca (Romero et al., 2007), and because purified mycosubtilin is directly active against Bo. cinerea, it is likely that the antifungal properties of this compound are involved in the biocontrol of Bo. cinerea by B. subtilis in our plant system. It remains to be determined whether the eliciting properties of the compound are also important for B. subtilis‐driven grapevine enhanced protection. Unfortunately, defence gene markers (‘resistance marker’) closely linked to pathogen resistance are not available in grapevine (Delaunois et al., 2014), making a direct correlation difficult to establish.
Altogether, our results demonstrate a high diversity in the defence responses triggered by B. subtilis LPs. Moreover, they highlight an interesting potential for these compounds in the protection of grapevine from Bo. cinerea. LPs act as MAMPs in grapevine, and the combination of elicitor activity with antifungal properties (in the case of plipastatin and mycosubtilin) may participate in a high level of plant protection (Fig. 8). Natural B. subtilis strains usually produce the three classes of LPs. How the combination of these LPs acts in grapevine protection is a key question that remains to be investigated. It is likely that the different LPs are necessary in their natural concentrations and combinations in relation to each other to be most effective in triggering innate immunity and as protective agents. However, natural Bacillus strains are known to produce variable concentrations of each LP, depending on the time of interaction with the plant or during the establishment of biofilms (Cawoy et al., 2014; Debois et al., 2014). It is therefore challenging to determine which LP is involved at a given time and for which effect. Further research is needed to clarify this.
Figure 8.
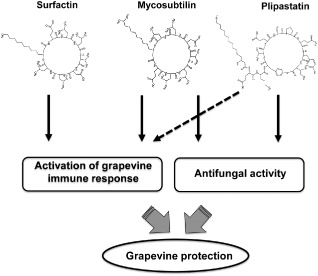
Proposed model showing the mechanisms by which the lipopeptides (LPs) produced by Bacillus subtilis enhance grapevine protection.
Experimental Procedures
Grapevine cell suspension culture and vitro‐plantlets
Cell suspensions of 41B (Vitis vinifera L. cv. Chasselas × V. berlandieri) were cultured in Murashige–Skoog (MS) medium (pH 5.8) containing vitamins (×1.5), sucrose (30 g/L), 2,4‐dichlorophenoxyacetic acid (2,4‐D, 0.2 mg/L) and 6‐benzylaminopurine (BAP, 0.5 mg/L), and were propagated in the dark at 25 °C with shaking at 120 rpm. They were subcultured every 7 days for maintenance in the exponential phase. For the experiments, cells subcultured for 7 days were filtered, weighed and resuspended in derived MS medium for 3–4 days at a concentration of 8 g/100 mL for a total volume of 10 mL. Grapevine vitro‐plantlets (V. vinifera L. cv. Chardonnay 7535) were grown in modified MS medium at 26 °C with a photoperiod of 16 h of light according to Bézier et al. (2002).
Microorganisms and LP treatments
Botrytis cinerea strain 630 cultures were initiated by transferring pieces of solid tomato/agar medium [tomato juice 25% (v/v), agar 3.75% (w/v)] containing mycelium to fresh solid tomato/agar medium and incubated at 21 °C. Conidia were collected from 2‐week‐old plates with 8 mL of growth culture medium (MgSO4, 3 mm; KH2PO4, 12.8 mm; d‐glucose, 22 mm; peptone, 4 g/L; citric acid, 10 mm; final pH 3.1). Bacillus subtilis strains BBG127 and BBG131 used in this study have been described previously by Coutte et al. (2010). Strain BBG125 was characterized by Bechet et al. (2013). Strain Bs2504 was characterized by Ongena et al. (2007). The strain BBG127 is unable to produce any LPs and was used as a negative control in our experiments. The strains BBG131, BBG125 and Bs2504 overproduce surfactin, mycosubtilin and plipastatin, respectively. LP production under controlled conditions in shaken flask cultures of these strains led to a production of 1540 ± 38.2 mg/L of surfactin by BBG131 (Coutte et al., 2010), 104 ± 9.57 mg/L of mycosubtilin by BBG125 (Bechet et al., 2013) and 452 mg/L of plipastatin by Bs2504 (Ongena et al., 2007). Strains of B. subtilis were grown at 37 °C with shaking at 130 rpm in Luria–Bertani (LB) medium. Before any inoculation treatment, overnight bacterial cultures were collected by centrifugation (4500 g, 10 min) and washed with sterile MgCl2 (10 mm). Final concentrations of bacteria in grapevine cell suspensions were expressed in cfu/mL. The three main LPs used in this study, surfactin, mycosubtilin (iturin family) and plipastatin (fengycin family), were produced and purified from the different strains of B. subtilis using an integrated bioprocess recently described by Coutte et al. (2013). LPs were resuspended in 0.1% dimethylsulphoxide (DMSO; Sigma‐Aldrich, Saint‐Quentin Fallavier, France) and were added directly to the grapevine cell suspension medium at the indicated concentrations.
Bioassays in cell cultures
Medium alkalinization measurements were performed according to Felix et al. (1993) using a standard pH‐meter (Basic, Denver Instrument, Goettingen, Germany). In order to test the physiological relevance of the proton fluxes, medium alkalinization was also measured after treatment of the cell suspension with 1 μm of the protein kinase inhibitor K‐252a (Sigma‐Aldrich; diluted in DMSO 0.05%) according to Felix et al. (1993). The inhibitor was added to plant cell suspensions 5 min prior to LPs (50 μg/mL). For refractory state experiments, 50 μg/mL of LPs were added to the cells and a second addition of LPs was performed when the pH of the cells returned to baseline. Cell death and ROS quantifications were performed according to Bordiec et al. (2011).
RNA extraction and real‐time PCR analysis
Cell suspension cultures treated with LPs and/or bacterial strains were filtered and frozen in liquid nitrogen. RNA extraction and qRT‐PCR were performed as described previously by Le Henanff et al. (2013). The sequences of the defence gene primers used for qRT‐PCR have been described previously by Bordiec et al. (2011).
Protection assays
Conidia of Bo. cinerea were collected from 2‐week‐old plates with 8 mL of growth culture medium (MgSO4, 3 mm; KH2PO4, 12.8 mm; d‐glucose, 22 mm; peptone, 4 g/L; citric acid, 10 mm; final pH 3.1), filtered to remove mycelia and counted. The suspension was adjusted to 5 × 105 conidia/mL of culture medium and placed under 150 rpm agitation at 21 °C for 6 h. Six‐week‐old vitro‐plantlets were completely immersed in solutions of LPs at the indicated concentration or in water or DMSO 0.1% (controls) for 30 s, and were placed in growth chambers; 1, 2 or 4 days later, leaves were excised from grapevine vitro‐plantlets and placed on wet (Whatman Velizy‐Villacoublay, France) 3MM paper in plastic Petri dishes. A drop of 5 μL of the 1 × 105 conidia/mL solution was inoculated on the upper face of the leaves. Symptoms were scored 2 or 4 days after inoculation. Infected leaves were assigned to three different classes based on lesion surfaces: no lesion, lesions affecting less than 50% of the leaf surface and lesions affecting more than 50% of the leaf surface. Fisher's test was performed to show significant differences between treatments and water control.
Protection from Bo. cinerea in grapevine plants challenged by B. subtilis strains was investigated by immersing leaves from vitro‐plantlets in bacterial solutions (107 cfu/mL) cultured as described above. Two days later, leaves were placed on Petri dishes and inoculated with Bo. cinerea conidia (105 conidia/mL). Symptoms were observed 2 days after inoculation and quantified as described above.
Antibiosis assay
Antibiosis assay on Bo. cinerea germination was conducted on TLC (thin layer chromatography) membranes. LPs were spotted onto a CCM membrane (10 μL for each LP at a concentration of 50 μg/mL) which was allowed to dry for 10 min. Freshly harvested conidia (105 conidia/mL) were mixed with Peptone/Tween agar medium (45 °C), poured on the CCM membrane and then cooled down to 20 °C for 20 min. Fungal growth was observed 4 days later.
Supporting information
Fig. S1 Effect of K‐252a on the extracellular alkalinization induced by lipopeptides (LPs). K‐252a (1 μm) was added 5 min before treatment with LPs (50 μg/mL). Data presented are means of triplicate experiments ± standard deviation (SD).
Fig. S2 Differential activation of reactive oxygen species (ROS) production after perception of lipopeptides (LPs). Cell suspensions were treated with LPs at 50 μg/mL. ROS production after treatment with surfactin (filled circles), mycosubtilin (filled squares), plipastatin (filled diamonds) and dimethylsulphoxide (DMSO) 0.1% (open circles). Data presented are means of duplicate experiments ± standard deviation (SD).
Fig. S3 Cell death assays using Evans blue staining. Control cells were challenged with MgCl2 or dimethylsulphoxide (DMSO) 0.1% as indicated. Surfactin, mycosubtilin and plipastatin were used at 50 μg/mL. Bacterial strains BBG127, BBG131, BBG125 and Bs2504 were used at 107 colony‐forming units (cfu)/mL. Cell death was monitored 24 h post‐treatment. In these experiments, 100% of dead cells corresponds to an optical density at 600 nm (OD600) of 1.6. Data presented are means of triplicate experiments ± standard deviation (SD). ‘b’ indicates statistically significant differences compared with the control according to Tukey's multiple comparison test (P < 0.05).
Fig. S4 Arabidopsis defence gene expression in response to lipopeptides (LPs). Leaves from 5‐week‐old Arabidopsis thaliana col‐0 plants were sprayed with LPs (0.5 mg/mL) or dimethylsulphoxide (DMSO) 0.1% (control) according to Sanchez et al. (2012). Transcript accumulation of PR1, PDF1.2 and PR4 genes was monitored 24 h post‐treatment. Analyses were performed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) according to Sanchez et al. (2012). Results are expressed as relative transcript accumulation (fold increase) over the control. Data presented are means of duplicate experiments ± standard deviation (SD).
Fig. S5 Protection from Botrytis cinerea in grapevine plants challenged by Bacillus subtilis strains. Leaves from vitro‐plantlets were immersed in bacterial solutions at 107 colony‐forming units (cfu)/mL. Two days later, leaves were placed on Petri dishes and inoculated with Bo. cinerea conidia (105 conidia/mL). Symptoms were scored 2 days after inoculation by defining three lesion surface classes: no lesion (white bars), lesions affecting less than 50% of the leaf surface (grey bars) and lesions affecting more than 50% of the leaf surface (black bars). Results are means of two independent experiments (n = 20). Asterisks indicate statistically significant differences in the percentage of leaves in the different classes compared with the MgCl2 control (Fisher's test, **P = 0.005).
Acknowledgements
This work was supported by the European Funds of INTERREG IV PhytoBio Project (Giovanni Farace and Olivier Fernandez) and the Region Champagne‐Ardenne. We acknowledge Corinne Boistel, Amandine Silvain and Bertrand Fertin from the Viollette Institute (Université Lille 1, Lille, France) for their precious help in lipopeptide production and purification. We also acknowledge Barbara Courteaux (Université de Reims Champagne‐Ardenne, Reims, France) for her excellent technical assistance in plant molecular experiments.
References
- Aziz, A. , Trotel‐Aziz, P. , Dhuicq, L. , Jeandet, P. , Couderchet, M. and Vernet, G. (2006) Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology, 96, 1188–1194. [DOI] [PubMed] [Google Scholar]
- Bais, H.P. , Fall, R. and Vivanco, J.M. (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 134, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechet, M. , Castera‐Guy, J. , Guez, J.S. , Chihib, N.E. , Coucheney, F. , Coutte, F. , Fickers, P. , Leclere, V. , Wathelet, B. and Jacques, P. (2013) Production of a novel mixture of mycosubtilins by mutants of Bacillus subtilis . Bioresour. Technol. 145, 264–270. [DOI] [PubMed] [Google Scholar]
- Bézier, A. , Lambert, B. and Baillieul, F. (2002) Study of defense‐related gene expression in grapevine leaves and berries infected with Botrytis cinerea . Eur. J. Plant Pathol. 108, 111–120. [Google Scholar]
- Boller, T. and Felix, G. (2009) A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Bordiec, S. , Paquis, S. , Lacroix, H. , Dhondt, S. , Ait Barka, E. , Kauffmann, S. , Jeandet, P. , Mazeyrat‐Gourbeyre, F. , Clément, C. , Baillieul, F. and Dorey, S. (2011) Comparative analysis of defence responses induced by the endophytic plant growth‐promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non‐host bacterium Pseudomonas syringae pv. pisi in grapevine cell suspensions. J. Exp. Bot. 62, 595–603. [DOI] [PubMed] [Google Scholar]
- Brotman, Y. , Makovitzki, A. , Shai, Y. , Chet, I. and Viterbo, A. (2009) Synthetic ultrashort cationic lipopeptides induce systemic plant defense responses against bacterial and fungal pathogens. Appl. Environ. Microbiol. 75, 5373–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawoy, H. , Mariutto, M. , Henry, G. , Fisher, C. , Vasilyeva, N. , Thonart, P. , Dommes, J. and Ongena, M. (2014) Plant defense stimulation by natural isolates of bacillus depends on efficient surfactin production. Mol. Plant–Microbe Interact. 27, 87–100. [DOI] [PubMed] [Google Scholar]
- Coutte, F. , Leclere, V. , Bechet, M. , Guez, J.S. , Lecouturier, D. , Chollet‐Imbert, M. , Dhulster, P. and Jacques, P. (2010) Effect of pps disruption and constitutive expression of srfA on surfactin productivity, spreading and antagonistic properties of Bacillus subtilis 168 derivatives. J. Appl. Microbiol. 109, 480–491. [DOI] [PubMed] [Google Scholar]
- Coutte, F. , Lecouturier, D. , Leclère, V. , Béchet, M. , Jacques, P. and Dhulster, P. (2013) New integrated bioprocess for the continuous production, extraction and purification of lipopeptides produced by Bacillus subtilis in membrane bioreactor. Process Biochem. 48, 25–32. [Google Scholar]
- Debois, D. , Jourdan, E. , Smargiasso, N. , Thonart, P. , De Pauw, E. and Ongena, M. (2014) Spatiotemporal monitoring of the antibiome secreted by Bacillus biofilms on plant roots using MALDI mass spectrometry imaging. Anal. Chem. 86, 4431–4438. [DOI] [PubMed] [Google Scholar]
- Delaunois, B. , Farace, G. , Jeandet, P. , Clement, C. , Baillieul, F. , Dorey, S. and Cordelier, S. (2014) Elicitors as alternative strategy to pesticides in grapevine? Current knowledge on their mode of action from controlled conditions to vineyard. Environ. Sci. Pollut. Res. Int. 21, 4837–4846. [DOI] [PubMed] [Google Scholar]
- Dodds, P.N. and Rathjen, J.P. (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Falardeau, J. , Wise, C. , Novitsky, L. and Avis, T.J. (2013) Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J. Chem. Ecol. 39, 869–878. [DOI] [PubMed] [Google Scholar]
- Farag, M.A. , Zhang, H. and Ryu, C.M. (2013) Dynamic chemical communication between plants and bacteria through airborne signals: induced resistance by bacterial volatiles. J. Chem. Ecol. 39, 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, G. , Grosskopf, D.G. , Regenass, M. and Boller, T. (1991) Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc. Natl. Acad. Sci. USA, 88, 8831–8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, G. , Regenass, M. and Boller, T. (1993) Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 4, 307–316. [Google Scholar]
- Fickers, P. , Guez, J.S. , Damblon, C. , Leclere, V. , Bechet, M. , Jacques, P. and Joris, B. (2009) High‐level biosynthesis of the anteiso‐C(17) isoform of the antibiotic mycosubtilin in Bacillus subtilis and characterization of its candidacidal activity. Appl. Environ. Microbiol. 75, 4636–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Brugger, A. , Lamotte, O. , Vandelle, E. , Bourque, S. , Lecourieux, D. , Poinssot, B. , Wendehenne, D. and Pugin, A. (2006) Early signaling events induced by elicitors of plant defenses. Mol. Plant–Microbe Interact. 19, 711–724. [DOI] [PubMed] [Google Scholar]
- Garcia‐Gutierrez, L. , Zeriouh, H. , Romero, D. , Cubero, J. , de Vicente, A. and Perez‐Garcia, A. (2013) The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate‐ and salicylic acid‐dependent defence responses. Microb. Biotechnol. 6, 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, G. , Deleu, M. , Jourdan, E. , Thonart, P. and Ongena, M. (2011) The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune‐related defence responses. Cell. Microbiol. 13, 1824–1837. [DOI] [PubMed] [Google Scholar]
- Jourdan, E. , Henry, G. , Duby, F. , Dommes, J. , Barthelemy, J.P. , Thonart, P. and Ongena, M. (2009) Insights into the defense‐related events occurring in plant cells following perception of surfactin‐type lipopeptide from Bacillus subtilis . Mol. Plant–Microbe Interact. 22, 456–468. [DOI] [PubMed] [Google Scholar]
- Khong, N.G. , Randoux, B. , Tayeh, C. , Coutte, F. , Bourdon, N. , Tisserant, B. , Laruelle, F. , Jacques, P. and Reignault, P. (2012) Induction of resistance in wheat against powdery mildew by bacterial cyclic lipopeptides. Commun. Agric. Appl. Biol. Sci. 77, 39–51. [PubMed] [Google Scholar]
- Kumar, A.S. , Lakshmanan, V. , Caplan, J.L. , Powell, D. , Czymmek, K.J. , Levia, D.F. and Bais, H.P. (2012) Rhizobacteria Bacillus subtilis restricts foliar pathogen entry through stomata. Plant J. 72, 694–706. [DOI] [PubMed] [Google Scholar]
- Lakshmanan, V. , Kitto, S.L. , Caplan, J.L. , Hsueh, Y.H. , Kearns, D.B. , Wu, Y.S. and Bais, H.P. (2012) Microbe‐associated molecular patterns‐triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol. 160, 1642–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan, V. , Castaneda, R. , Rudrappa, T. and Bais, H.P. (2013) Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta, 238, 657–668. [DOI] [PubMed] [Google Scholar]
- Le Henanff, G. , Profizi, C. , Courteaux, B. , Rabenoelina, F. , Gerard, C. , Clement, C. , Baillieul, F. , Cordelier, S. and Dhondt‐Cordelier, S. (2013) Grapevine NAC1 transcription factor as a convergent node in developmental processes, abiotic stresses, and necrotrophic/biotrophic pathogen tolerance. J. Exp. Bot. 64, 4877–4893. [DOI] [PubMed] [Google Scholar]
- Leclere, V. , Bechet, M. , Adam, A. , Guez, J.S. , Wathelet, B. , Ongena, M. , Thonart, P. , Gancel, F. , Chollet‐Imbert, M. and Jacques, P. (2005) Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism's antagonistic and biocontrol activities. Appl. Environ. Microbiol. 71, 4577–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclere, V. , Marti, R. , Bechet, M. , Fickers, P. and Jacques, P. (2006) The lipopeptides mycosubtilin and surfactin enhance spreading of Bacillus subtilis strains by their surface‐active properties. Arch. Microbiol. 186, 475–483. [DOI] [PubMed] [Google Scholar]
- van Loon, L.C. , Bakker, P.A. , van der Heijdt, W.H. , Wendehenne, D. and Pugin, A. (2008) Early responses of tobacco suspension cells to rhizobacterial elicitors of induced systemic resistance. Mol. Plant–Microbe Interact. 21, 1609–1621. [DOI] [PubMed] [Google Scholar]
- Massoud, K. , Barchietto, T. , Le Rudulier, T. , Pallandre, L. , Didierlaurent, L. , Garmier, M. , Ambard‐Bretteville, F. , Seng, J.M. and Saindrenan, P. (2012) Dissecting phosphite‐induced priming in Arabidopsis infected with Hyaloperonospora arabidopsidis . Plant Physiol. 159, 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Mortel, J.E. , Tran, H. , Govers, F. and Raaijmakers, J.M. (2009) Cellular responses of the late blight pathogen Phytophthora infestans to cyclic lipopeptide surfactants and their dependence on G proteins. Appl. Environ. Microbiol. 75, 4950–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir, M.N. and Besson, F. (2011) Specific interactions of mycosubtilin with cholesterol‐containing artificial membranes. Langmuir, 27, 10 785–10 792. [DOI] [PubMed] [Google Scholar]
- Nasir, M.N. and Besson, F. (2012) Interactions of the antifungal mycosubtilin with ergosterol‐containing interfacial monolayers. Biochim. Biophys. Acta, 1818, 1302–1308. [DOI] [PubMed] [Google Scholar]
- Nasir, M.N. , Thawani, A. , Kouzayha, A. and Besson, F. (2010) Interactions of the natural antimicrobial mycosubtilin with phospholipid membrane models. Colloids Surf. B Biointerfaces, 78, 17–23. [DOI] [PubMed] [Google Scholar]
- Ongena, M. and Jacques, P. (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16, 115–125. [DOI] [PubMed] [Google Scholar]
- Ongena, M. , Duby, F. , Jourdan, E. , Beaudry, T. , Jadin, V. , Dommes, J. and Thonart, P. (2005a) Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl. Microbiol. Biotechnol. 67, 692–698. [DOI] [PubMed] [Google Scholar]
- Ongena, M. , Jacques, P. , Toure, Y. , Destain, J. , Jabrane, A. and Thonart, P. (2005b) Involvement of fengycin‐type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis . Appl. Microbiol. Biotechnol. 69, 29–38. [DOI] [PubMed] [Google Scholar]
- Ongena, M. , Jourdan, E. , Adam, A. , Paquot, M. , Brans, A. , Joris, B. , Arpigny, J.L. and Thonart, P. (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 9, 1084–1090. [DOI] [PubMed] [Google Scholar]
- Perez‐Garcia, A. , Romero, D. and de Vicente, A. (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of bacilli in agriculture. Curr. Opin. Biotechnol. 22, 187–193. [DOI] [PubMed] [Google Scholar]
- Raaijmakers, J.M. and Mazzola, M. (2012) Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 50, 403–424. [DOI] [PubMed] [Google Scholar]
- Raaijmakers, J.M. , De Bruijn, I. , Nybroe, O. and Ongena, M. (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol. Rev. 34, 1037–1062. [DOI] [PubMed] [Google Scholar]
- Robert‐Seilaniantz, A. , Grant, M. and Jones, J.D. (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate–salicylate antagonism. Annu. Rev. Phytopathol. 49, 317–343. [DOI] [PubMed] [Google Scholar]
- Romero, D. , de Vicente, A. , Rakotoaly, R.H. , Dufour, S.E. , Veening, J.W. , Arrebola, E. , Cazorla, F.M. , Kuipers, O.P. , Paquot, M. and Perez‐Garcia, A. (2007) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca . Mol. Plant–Microbe Interact. 20, 430–440. [DOI] [PubMed] [Google Scholar]
- Rudrappa, T. , Biedrzycki, M.L. , Kunjeti, S.G. , Donofrio, N.M. , Czymmek, K.J. , Pare, P.W. and Bais, H.P. (2010) The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana . Commun. Integr. Biol. 3, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegg, U.T. and Burgess, G.M. (1989) Staurosporine, K‐252 and UCN‐01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol. Sci. 10, 218–220. [DOI] [PubMed] [Google Scholar]
- Ryu, C.M. , Farag, M.A. , Hu, C.H. , Reddy, M.S. , Kloepper, J.W. and Pare, P.W. (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 134, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez, L. , Courteaux, B. , Hubert, J. , Kauffmann, S. , Renault, J.H. , Clement, C. , Baillieul, F. and Dorey, S. (2012) Rhamnolipids elicit defense responses and induce disease resistance against biotrophic, hemibiotrophic, and necrotrophic pathogens that require different signaling pathways in Arabidopsis and highlight a central role for salicylic acid. Plant Physiol. 160, 1630–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J.M. , Salamango, D.J. , Leslie, M.E. , Collins, C.A. and Heese, A. (2014) Sensitivity to flg22 is modulated by ligand‐induced degradation and de novo synthesis of the endogenous flagellin‐receptor FLAGELLIN‐SENSING2. Plant Physiol. 164, 440–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieker, M. , Tanovic, A. and Marahiel, M.A. (2010) Nonribosomal peptide synthetases: structures and dynamics. Curr. Opin. Struct. Biol. 20, 234–240. [DOI] [PubMed] [Google Scholar]
- Toure, Y. , Ongena, M. , Jacques, P. , Guiro, A. and Thonart, P. (2004) Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J. Appl. Microbiol. 96, 1151–1160. [DOI] [PubMed] [Google Scholar]
- Tran, H. , Ficke, A. , Asiimwe, T. , Hofte, M. and Raaijmakers, J.M. (2007) Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens . New Phytol. 175, 731–742. [DOI] [PubMed] [Google Scholar]
- Trda, L. , Fernandez, O. , Boutrot, F. , Heloir, M.C. , Kelloniemi, J. , Daire, X. , Adrian, M. , Clement, C. , Zipfel, C. , Dorey, S. and Poinssot, B. (2014) The grapevine flagellin receptor VvFLS2 differentially recognizes flagellin‐derived epitopes from the endophytic growth‐promoting bacterium Burkholderia phytofirmans and plant pathogenic bacteria. New Phytol. 201, 1371–1384. [DOI] [PubMed] [Google Scholar]
- Varnier, A.L. , Sanchez, L. , Vatsa, P. , Boudesocque, L. , Garcia‐Brugger, A. , Rabenoelina, F. , Sorokin, A. , Renault, J.H. , Kauffmann, S. , Pugin, A. , Clément, C. , Baillieul, F. and Dorey, S. (2009) Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 32, 178–193. [DOI] [PubMed] [Google Scholar]
- Vatsa, P. , Sanchez, L. , Clément, C. , Baillieul, F. and Dorey, S. (2010) Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int. J. Mol. Sci. 11, 5095–5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen, B. , Trotel‐Aziz, P. , Jeandet, P. , Baillieul, F. and Aziz, A. (2011) Improved resistance against Botrytis cinerea by grapevine‐associated bacteria that induce a prime oxidative burst and phytoalexin production. Phytopathology, 101, 768–777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Effect of K‐252a on the extracellular alkalinization induced by lipopeptides (LPs). K‐252a (1 μm) was added 5 min before treatment with LPs (50 μg/mL). Data presented are means of triplicate experiments ± standard deviation (SD).
Fig. S2 Differential activation of reactive oxygen species (ROS) production after perception of lipopeptides (LPs). Cell suspensions were treated with LPs at 50 μg/mL. ROS production after treatment with surfactin (filled circles), mycosubtilin (filled squares), plipastatin (filled diamonds) and dimethylsulphoxide (DMSO) 0.1% (open circles). Data presented are means of duplicate experiments ± standard deviation (SD).
Fig. S3 Cell death assays using Evans blue staining. Control cells were challenged with MgCl2 or dimethylsulphoxide (DMSO) 0.1% as indicated. Surfactin, mycosubtilin and plipastatin were used at 50 μg/mL. Bacterial strains BBG127, BBG131, BBG125 and Bs2504 were used at 107 colony‐forming units (cfu)/mL. Cell death was monitored 24 h post‐treatment. In these experiments, 100% of dead cells corresponds to an optical density at 600 nm (OD600) of 1.6. Data presented are means of triplicate experiments ± standard deviation (SD). ‘b’ indicates statistically significant differences compared with the control according to Tukey's multiple comparison test (P < 0.05).
Fig. S4 Arabidopsis defence gene expression in response to lipopeptides (LPs). Leaves from 5‐week‐old Arabidopsis thaliana col‐0 plants were sprayed with LPs (0.5 mg/mL) or dimethylsulphoxide (DMSO) 0.1% (control) according to Sanchez et al. (2012). Transcript accumulation of PR1, PDF1.2 and PR4 genes was monitored 24 h post‐treatment. Analyses were performed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) according to Sanchez et al. (2012). Results are expressed as relative transcript accumulation (fold increase) over the control. Data presented are means of duplicate experiments ± standard deviation (SD).
Fig. S5 Protection from Botrytis cinerea in grapevine plants challenged by Bacillus subtilis strains. Leaves from vitro‐plantlets were immersed in bacterial solutions at 107 colony‐forming units (cfu)/mL. Two days later, leaves were placed on Petri dishes and inoculated with Bo. cinerea conidia (105 conidia/mL). Symptoms were scored 2 days after inoculation by defining three lesion surface classes: no lesion (white bars), lesions affecting less than 50% of the leaf surface (grey bars) and lesions affecting more than 50% of the leaf surface (black bars). Results are means of two independent experiments (n = 20). Asterisks indicate statistically significant differences in the percentage of leaves in the different classes compared with the MgCl2 control (Fisher's test, **P = 0.005).


