Summary
Pepino mosaic virus (PepMV) poses a worldwide threat to the tomato industry. Considerable differences at the genetic level allow for the distinction of four main genotypic clusters; however, the basis of the phenotypic outcome is difficult to elucidate. This work reports the generation of wild‐type PepMV infectious clones of both EU (mild) and CH2 (aggressive) genotypes, from which chimeric infectious clones were created. Phenotypic analysis in three solanaceous hosts, Nicotiana benthamiana, Datura stramonium and Solanum lycopersicum, indicated that a PepMV pathogenicity determinant mapped to the 3′‐terminal region of the genome. Increased aggression was only observed in N. benthamiana, showing that this factor is host specific. The determinant was localized to amino acids 11–26 of the N‐terminal coat protein (CP) region; this is the first report of this region functioning as a virulence factor in PepMV.
Keywords: coat protein, infectious clone, Nicotiana benthamiana, pathogenicity determinant, Pepino mosaic virus, potexvirus, yeast homologous recombination
Pepino mosaic virus is a potexvirus in the family Alphaflexiviridae (Adams et al., 2004; Martelli et al., 2007) and is a major pathogen of tomato. It was first discovered infecting tomato plants in the Netherlands in 1999, and has since achieved a worldwide distribution despite efforts to control its spread (Hanssen and Thomma, 2010).
The genome structure of PepMV is of a typical potexvirus consisting of a single‐stranded (ss) RNA genome possessing both a 5′ methylated guanine cap and 3′ polyadenylated tail. The genome comprises five open reading frames (ORFs), flanked by 5′ and 3′ untranslated regions (UTRs) (Aguilar et al., 2002). ORF 1 encodes the putative RNA‐dependent RNA polymerase (RdRp), containing methyl transferase, helicase and polymerase domains. ORFs 2–4 overlap to form the triple gene block (TGB) element, with roles indicated in viral movement, RNA silencing suppression and symptom development (Hasiów‐Jaroszewska et al., 2011; Morozov and Solovyev, 2003). ORF 5 encodes the coat protein (CP) involved in movement and encapsidation of the virion (Cruz et al., 1998; Morozov and Solovyev, 2003).
PepMV isolates display considerable differences at the genomic level, allowing for the distinction of four main genotypes: EU, CH2, US1 and LP (Hanssen and Thomma, 2010). These are often found in mixed infections from which intergenotype recombinants have been detected, indicating that recombination may be an important factor in shaping PepMV evolutionary dynamics (Gomez et al., 2009; Pagán et al., 2006). Isolates have been found to differ in host range and symptomatology in many solanaceous species, with symptoms ranging from asymptomatic to severe (Salomone and Roggero, 2002). The symptomatic outcome results from a complex interplay between PepMV isolate, host cultivar and climatic conditions, making the impact of the virus difficult to predict (Hanssen et al., 2009; Jordá et al., 2001).
In contrast with the ease with which genotypic analysis may be carried out, no solid basis for phenotypic distinction has been found. Strains from different genotypes may behave in a similar manner and, likewise, a spectrum of isolates ranging from mild to aggressive may be found within the same genotype. Understanding the factors controlling phenotypic outcome is crucial in understanding PepMV epidemiology and in the development of novel control methods. Our current knowledge of PepMV pathogenicity factors is very limited. A single mutation of amino acid 67 of the TGB3 gene was sufficient to convert both EU and CH2 infectious clones (ICs) from mild to aggressive in tomato and Datura inoxia (Hasiów‐Jaroszewska and Borodynko, 2012; Hasiów‐Jaroszewska et al., 2011), but the molecular basis of this effect is unknown. Two mutations in the central part of PepMV CP were involved in yellowing symptoms in tomato, and the ratio of mutated vs. wild‐type (WT) sequences in infected plants determined the severity of yellowing (Hasiów‐Jaroszewska et al., 2013).
The purpose of this study was to investigate potential pathogenicity regions in the PepMV genome through the construction of chimeric viruses combining EU (mild) and CH2 (aggressive) genotypic elements. Phenotypic analysis in three species of solanaceous hosts aimed to highlight regions containing pathogenicity determinants through the symptomatic characteristics displayed by the chimeric viruses. Furthermore, differences in host specificity of such pathogenicity‐containing regions were established.
Viral isolates representing both EU (mild) and CH2 (aggressive) forms of PepMV were obtained courtesy of the Food and Environment Research Agency (Sand Hutton, York, UK) and sequenced to confirm genotypic identity. The viruses were propagated via mechanical inoculation onto Nicotiana benthamiana, and infectious sap was used to mechanically inoculate three species of indicator plant: N. benthamiana, Datura stramonium and Solanum lycopersicum cv. Moneymaker. Comparative viral titres were investigated at 21 days post‐inoculation (dpi) by double antibody sandwich‐enzyme‐linked immunosorbent assay (DAS‐ELISA) (Forde, 1989) on a local and systemic leaf (Fig. 1A), and a phenotypic analysis was conducted (Fig. 1B). The CH2 isolate presented an aggressive phenotype; a high level of rugosity was observed in all indicator species: obvious mosaics on D. stramonium and N. benthamiana, and noticeable vein yellowing on S. lycopersicum. The EU isolate displayed a mild phenotype: mild mosaics on both N. benthamiana and D. stramonium, and an asymptomatic phenotype in S. lycopersicum. Results from DAS‐ELISA showed that the aggressive phenotype of the CH2 virus was not correlated with an increased viral titre (Fig. 1A). The symptoms presented by each isolate provided confirmation of their phenotype.
Figure 1.
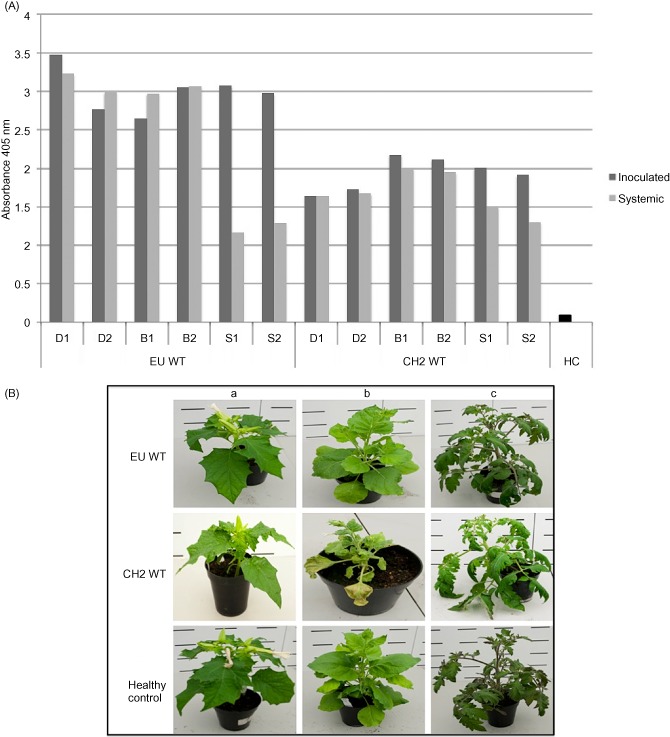
(A) Double antibody sandwich‐enzyme‐linked immunosorbent assay (DAS‐ELISA) absorbance data at 21 days post‐inoculation (dpi) for Datura stramonium (D), Nicotiana benthamiana (B) and Solanum lycopersicum (S) after challenge with the wild‐type (WT) Pepino mosaic virus (PepMV) isolates EU WT and CH2 WT. Local and systemic viral titres are represented by dark grey and light grey bars, respectively. Two plants were inoculated with each viral species in each instance. (B) Phenotypes presented at 21 dpi by the WT PepMV isolates PepMV EU WT and CH2 WT in three species of indicator plant: D. stramonium (a), N. benthamiana (b) and S. lycopersicum (c). Healthy controls are given as a comparison.
WT ICs of both CH2 and EU genotypes, as well as chimeric ICs combining elements of both genotypes, were constructed using a yeast homologous recombination method, involving the inclusion of 30‐bp overlap regions between consecutive fragments to be joined in vivo on introduction into yeast.
The WT EU and CH2 ICs were constructed using first‐strand cDNA, generated from total RNA isolated from PepMV isolates infecting N. benthamiana, as the template in PCR to amplify three overlapping fragments spanning the length of the viral genomes: EU fragments 1, 2 and 3 (Table 1; primer sets 2 + 3, 4 + 5 and 6 + 7, respectively) and CH2 fragments 1, 2 and 3 (Table 1; primer sets 8 + 9, 10 + 11 and 12 + 7, respectively). The unique restriction site KpnI was incorporated into the 3′ terminal primer, 7, to allow for construct linearization after the poly(A) tail. The SP6 promoter sequence was incorporated into the 5′ terminus of the constructs to allow for infectious transcript generation. The three fragments and a linearized pYES2 backbone were transformed into LiAc competent yeast (Gietz and Woods, 2002) to generate full‐length constructs: pYES2_EU and pYES2_CH2. Restriction digestion and sequence analysis confirmed successful construct generation (GenBank accessions EU KJ018164 and CH2 KJ018165).
Table 1.
Primers and annealing conditions used in this investigation
| Primer number | Primer sequence (5′–3′) | Annealing temperature (°C) |
|---|---|---|
| 1 | GACATGAAGCATTCATACCAAATGGG | 58 |
| 2 | GAGCGGCCGCCAGTGTGATGGATATCTGCAAATTACTAGTATTTAGGTGACACTATAGAAAACAAAATAAATAAATAAATATA | 61.5 |
| 3 | ATGGTGGAACAAATAGGCTTCAATGTAACC | 61.5 |
| 4 | TGATTGGGTGAACAAATTGTGTAAGCTACC | 61.5 |
| 5 | TAACCCCTCAATGTGTTGCTTTTGGAGGGC | 61.5 |
| 6 | AAACATTCATACCAAATGGGTGATGAGCTG | 61.5 |
| 7 | CTCACTATAGGGAATATTAAGCTTGGTACCAATTGGTACCACGCGTTTTTTTTTTTTTTTTTTTTTTTTTT | 61.5 |
| 8 | GAGCGGCCGCCAGTGTGATGGATATCTGCAAATTACTAGTATTTAGGTGACACTATAGAAAACAAAACATAACACATAATATC | 60 |
| 9 | GTAATGCCTGGGATTTGCCAGAACCTCCGC | 60 |
| 10 | TATGCCATCCCAATAAGCCACAGGAGGGC | 60 |
| 11 | GTCACCCATTTGGTATGAATGCTTCATGTC | 60 |
| 12 | GGGGATTACGCTGAATTTTGCGGTTGGAC | 60 |
| 13 | GGTAAAGTTTGACCCCTTTTGAATTGGGGAGTTACACAACGAAAACCTCCCGAGAAAAGTGGTTC | 58 |
| 14 | TGATGAGCTGCACAATTACTTAACACCAGATGAAGCTGAACAACACTTCCTTGCTGTTC | 58 |
| 15 | AGAGTCCCACATTACACTTCCTTC | 58 |
| 16 | TGGGTTAGAAGCTGTAGGTTGGTTTTCCATGATTGTTTATTGAAGTTGAT | 58 |
| 17 | ATGGAAAACCAACCTACAGCTTCTAACCCATCAAGTGCACCACCCACAGCC | 58 |
| 18 | ATGGAAAACCAACCTACAGCTTC | 58 |
| 19 | GGCTGGGCTCTGGGCACCAGC | 58 |
| 20 | CTGCTGCTCAAGCTGGTGCCCAGAGCCCAGCCGACTTCTCAAATCCCAATACAG | 58 |
The WT ICs were used as a framework with which to construct chimeric ICs containing both EU and CH2 elements (Fig. 2A). The EU+ 3′ CH2 chimera was designed to contain the 5′ UTR to RdRp of EU origin, and the remainder of CH2 origin. A 2.2‐kb CH2 fragment, encompassing the UTR upstream from the TGB (bp 4407) to the end of the poly(A) tail, was amplified from a pYES2_CH2 template (Table 1; primer set 1 + 13). This was entered into a yeast recombination with a BstBI‐digested pYES2_EU and linearized pYES2 backbone to produce the construct EU+ 3′ CH2, contained within a pYES2 vector: pYES2_EU+ 3′ CH2 (GenBank accession KJ026530). The EU+ CP CH2 chimera was designed to contain EU‐derived sequence from the 5′ UTR to the end of TGB3, and the remainder CH2 derived. A 770‐bp fragment, from bp 5595 to the end of the poly(A) tail, was amplified from a pYES2_CH2 template (Table 1; primer set 1 + 14) incorporating homology to an SspI‐digested pYES2_EU and linearized pYES2 backbone. The sections were entered into a yeast homologous recombination to produce the construct EU+ CP CH2, contained within a pYES2 vector: pYES2_EU+ CP CH2 (GenBank accession KJ018166).
Figure 2.
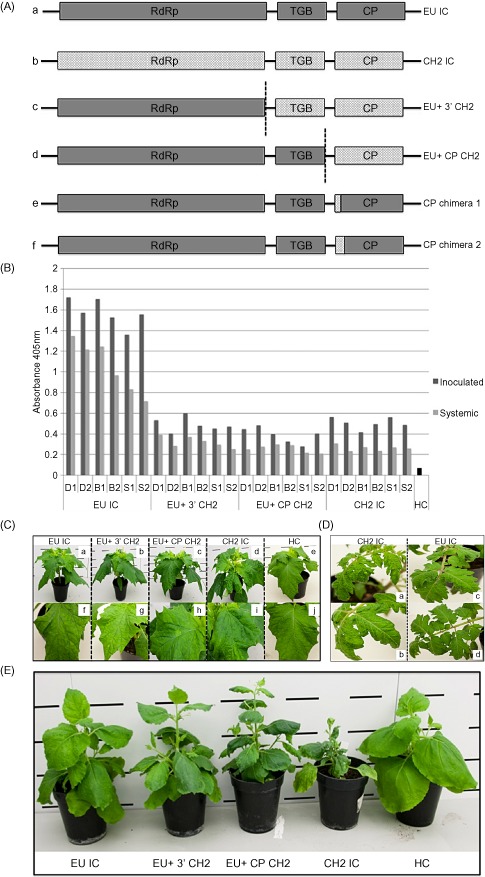
(A) Schematic diagrams of the infectious clones (ICs) generated in this study. Wild‐type (WT) ICs: EU IC (A) and CH2 IC (B). Chimeric ICs: EU+ 3′ CH2 (C), consisting of EU‐derived sequence from the 5′ terminus to the end of the RNA‐dependent RNA polymerase (RdRp) and the remainder CH2 derived to the end of the poly(A) tail; EU+ CP CH2 (D), consisting of EU sequence from the 5′ terminus to the end of TGB3 and the rest CH2 derived to the end of the poly(A) tail; CP chimera 1 and CP chimera 2 (E, F), first 10 and 26 coat protein (CP) amino acids of CH2 origin, respectively, in a mild EU background. (B) Double antibody sandwich‐enzyme‐linked immunosorbent assay (DAS‐ELISA) absorbance data at 21 days post‐inoculation (dpi), displaying local and systemic viral titres (given in dark grey and light grey, respectively) in three species of indicator plant, Datura stramonium (D), Nicotiana benthamiana (B) and Solanum lycopersicum (S), inoculated with EU IC, EU+ 3′ CH2, EU+ CP CH2 and CH2 IC. Healthy (HC) material is included for comparison. (C) Characteristic phenotypes presented by EU IC (a, f), EU+ 3′ CH2 (b, g), EU+ CP CH2 (c, h) and CH2 IC (d, i) in D. stramonium at 21 dpi. Healthy control (HC) plant is given as a comparison (e, j). (D) Characteristic phenotypes presented by EU IC and CH2 IC in S. lycopersicum at 21 dpi. The CH2 isolate displayed a high level of rugosity (a) and vein yellowing (b); EU IC displays an asymptomatic phenotype (c, d). (E) Phenotypes presented by EU IC, EU+ 3′ CH2, EU+ CP CH2 and CH2 IC in N. benthamiana at 21 dpi. A healthy control plant (HC) is given as a comparison.
Infectious transcripts were synthesized in vitro from KpnI linearized plasmids (Foster and Turner, 1998; Turner et al., 1994, 1999) using an SP6 Riboprobe® System (Promega, Madison, USA), in conjunction with a Ribo m7G Cap Analog (Promega). Each reaction was mechanically inoculated onto two N. benthamiana plants at the three‐leaf developmental stage, delivering approximately 1 μg to each. Plants were investigated for viral presence at 21 dpi by DAS‐ELISA on a systemic leaf. All RNA transcripts proved infectious, as indicated by positive ELISA values and sequence analysis (data not shown).
Infectious sap representing each construct (EU IC, EU+ 3′ CH2, EU+ CP CH2 and CH2 IC) from the primary N. benthamiana infections was used to mechanically inoculate three species of indicator plant: D. stramonium, N. benthamiana and S. lycopersicum. Both inoculated and systemic leaves were investigated for viral presence at 21 dpi by DAS‐ELISA (Fig. 2B) and a phenotypic analysis was conducted. Two independent experimental repeats were performed and the results were reproducible. Both WT ICs gave symptoms that were typical of the WT viruses from which they were created, showing them to behave in a biologically relevant manner. The EU IC produced mild mosaics on both D. stramonium and N. benthamiana, and an asymptomatic phenotype on S. lycopersicum. The CH2 IC displayed a high level of rugosity in all indicator species: obvious mosaics and stunting on D. stramonium and N. benthamiana, and noticeable vein yellowing on S. lycopersicum.
EU+ 3′ CH2 and EU+ CP CH2 displayed mild phenotypes typical of the EU IC in D. stramonium and S. lycopersicum, presenting mild mosaics in D. stramonium and an asymptomatic outcome in S. lycopersicum (Fig. 2C, D). However, these chimeric clones displayed increased stunting, mosaics, rugosity and leaf deformation in N. benthamiana (Fig. 2E). DAS‐ELISA data demonstrated that the chimeric viruses reach titres equivalent to that of a WT CH2 IC infection (Fig. 2B).
Increased aggression of the chimeric ICs EU+ 3′ CH2 and EU+ CP CH2 in N. benthamiana suggested that a pathogenicity factor was present in the 3′ terminal CP region, common to both chimeric ICs. Sequence alignment was conducted for both EU IC and CH2 IC 3′ terminal regions in order to investigate differences that may be contributory to the differential phenotypes displayed. A marked level of variation (60%) was observed in the N‐terminal region of the CP gene (Fig. 3A), whereas the remaining CP region and 3′ UTR displayed high levels of nucleotide identity (97% and 75%, respectively). Two chimeric ICs were constructed, consisting of CH2‐derived sections of the low homology N‐terminal region in a mild EU background: CP chimera 1, first 10 amino acids CH2 derived; CP chimera 2, first 26 amino acids CH2 derived (GenBank accessions KJ018167 and KJ018168, respectively).
Figure 3.
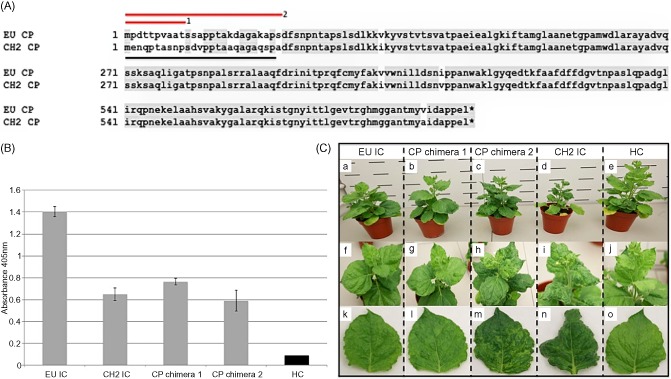
(A) Amino acid alignment of the coat protein (CP) regions of EU IC and CH2 IC. A marked level of variation is present in the N‐terminal region of the CP, indicated with a black line. Amino acids of CH2 origin comprising CP chimera 1 and CP chimera 2 are indicated with red lines 1 and 2, respectively. (B) Double antibody sandwich‐enzyme‐linked immunosorbent assay (DAS‐ELISA) absorbance data at 21 days post‐inoculation (dpi), showing systemic viral titres in Nicotiana benthamiana inoculated with EU IC, CH2 IC, CP chimera 1 and CP chimera 2. Healthy (HC) material is included for comparison. (C) Characteristic phenotypes presented by EU IC (a, f, k), CP chimera 1 (b, g, l), CP chimera 2 (c, h, m) and CH2 IC (d, i, n) in N. benthamiana at 21 dpi. Healthy control (HC) plant is given as a comparison (e, j, o). The single leaves depicted (k–o) are the third most developed leaves from the apex of the plant.
CP chimera 1 (amino acids 1–10) was constructed through amplification of 900‐ and 800‐bp fragments (Table 1; primer sets 15 + 16 and 17 + 7, respectively) from a pYES2_EU template, entered into a yeast recombination with digested pYES2_EU and HpaI‐SacI‐digested pYES2. CP chimera 2 (amino acids 1–26) was constructed through amplification of three overlapping sections: 900‐bp section 1 and 780‐bp section 3 from a pYES2_EU template, and 80‐bp section 2 from a pYES2_CH2 template (Table 1: primer sets 15 + 16, 18 + 19 and 20 + 7, respectively). The three sections were entered into yeast recombination with digested pYES2_EU and HpaI‐SacI‐digested pYES2. In vitro transcripts representing CP chimeras 1 and 2 were synthesized from KpnI linearized templates, and infections were established in N. benthamiana as described previously. DAS‐ELISA and sequencing analysis at 21 dpi confirmed viral presence. Infectious sap representing each construct [EU IC, CP chimera 1 (amino acids 1–10), CP chimera 2 (amino acids 1–26) and CH2 IC] from the primary N. benthamiana infections was used to mechanically inoculate three N. benthamiana plants at the three‐leaf developmental stage. Systemic leaves were investigated for viral presence at 21 dpi by DAS‐ELISA (Fig. 3B) and a phenotypic analysis was conducted (Fig. 3C). Both WT ICs presented phenotypes typical of the WT viruses from which they were created. CP chimera 1 (amino acids 1–10) gave a mild mosaic phenotype, characteristic of a mild EU IC infection; however, the phenotype presented by CP chimera 2 (amino acids 1–26) was significantly more aggressive than that of the mild EU IC from which it was created; obvious leaf mosaics and rugosity were observed over the entire plant surface. DAS‐ELISA data showed that CP chimera ICs reached titres equivalent to that of a CH2 IC infection (Fig. 3B). Progeny viruses were sequenced from the plants, providing confirmation of sequence integrity. No secondary mutations were found in the N‐terminal CP region of either CP chimera, and both constructs appeared to be stable in planta.
Current knowledge of PepMV pathogenicity factors is very limited. A single mutation in the TGB3 protein (Hasiów‐Jaroszewska and Borodynko, 2012) and the proportion of a centrally located CP mutation present in the viral quasispecies (Hasiów‐Jaroszewska et al., 2013) are the only known pathogenicity determinants to date. Understanding the biological basis for phenotypic severity is crucial in the development of novel control methods to reduce the impact of this devastating pathogen, as well as to ensure efficient and safe functioning of expression vectors based on PepMV (Sempere et al., 2011).
This work clearly displays a novel role for the N‐terminal CP extension in increased symptom severity of PepMV in the N. benthamiana host. This effect was not observed in S. lycopersicum or D. stramonium displaying the host specificity with which these pathogenicity determinants may function. Indeed, host specificity in the functioning of the TGB3 necrosis determinant has also been observed (Hasiów‐Jaroszewska and Borodynko, 2012), in line with the results of this investigation. The host specificity component displays the interaction of the PepMV viral CP with one or more host factors to be crucial in determining the outcome of infection; however, the nature of this interaction remains to be elucidated. At present we cannot say whether the significant increase in aggression observed is caused solely by differences in the region of amino acids 11–26, or within the broader context of amino acids 1–26. In the future, this could be assessed by exchanging this smaller internal region. It would also be interesting to study the diversity of natural isolates of PepMV in this N‐terminal region and its putative relationship with symptom severity.
The analysis of systemic viral titres in infected N. benthamiana, by DAS‐ELISA, found that the highly aggressive symptoms of the CH2 IC and chimeric viruses were not accompanied by an increase in viral titre. Other studies have also found no correlation between PepMV viral titre and virulence, in support of the findings of this investigation (Gomez et al., 2009; Hanssen et al., 2008; Hasiów‐Jaroszewska and Borodynko, 2012; Pagán et al., 2006). It should be noted that a calibrated ELISA was not performed. However, the use of polyclonal antibodies means that it is highly unlikely that the antibody specificity, in response to different ICs, was affected.
Evidence for a role of the N‐terminal CP extension in symptomatology has also been demonstrated in the potexvirus Bamboo mosaic virus (BaMV) (Lan et al., 2010). Site‐directed mutagenesis and deletion analysis displayed a unique 35‐amino‐acid glycine‐rich motif (GRM) in the N‐terminus of the BaMV CP to function as a symptom determinant. This GRM is unique to BaMV among potexvirus groups; however, interestingly, its location coincides with that of the PepMV pathogenicity determinant found in this investigation.
Examples of the CP playing a direct role in symptom determination have been found in species of other viral families, such as Alfalfa mosaic virus (AMV) (Neeleman et al., 1991), Cucumber mosaic virus (CMV) (Shintaku et al., 1992) and Tobacco mosaic virus (TMV) (Banerjee et al., 1995; Knorr and Dawson, 1988; Shintaku et al., 1992). Mutation of specific CP residues resulted in increased phenotypic aggression, thought to originate from conformational changes induced in the CP structure. However, the surface location of the potexvirus N‐terminal CP extension indicates a non‐structural role and it is unlikely that the differential symptomatology observed in this work is based on a mechanism involving a conformational change in the CP (Baratova et al., 1992a, 1992b; Rioux et al., 2012).
The N‐terminal CP extension has also been implicated in potyviral pathogenicity. Differential pathogenicity of Potato virus Y (PVY) isolates has been mapped to the CP gene, especially the 5′ proximal segment (Bukovinszki et al., 2007; Hu et al., 2011). It has also been implicated in resistance gene evasion and symptomatic development (Decroocq et al., 2009; Ullah and Grumet, 2002), as well as functioning as a determinant of systemic infectivity for a number of potyviruses (Andersen and Johansen, 1998; Desbiez et al., 2014; Tatineni et al., 2011). Interestingly, potyviral pathogenicity determinants mapping to this region may also display host specificity in their functioning (Pérez et al., 2013), and play a key role in host adaptation (Carbonell et al., 2013; Salvador et al., 2008).
In conclusion, the use of chimeric ICs has helped to identify a host‐specific symptom determinant mapping to the N‐terminal CP region of the PepMV genome. The elucidation of the role of the PepMV N‐terminal CP extension in host factor interaction will be integral in understanding the molecular basis for its role in host‐specific symptomatic development. The construction of PepMV ICs provides a powerful tool with which to investigate the molecular basis for phenotypic aggression of PepMV isolates, the elucidation of which will be integral in the fight against this destructive pathogen.
Acknowledgements
This work was supported by funding from the Lady Emily Smyth Agricultural Research Station (LESARS). We would like to thank the Food and Environment Research Agency (FERA) for the kind use of their facilities, provision of viral isolates and the invaluable work of the glasshouse team in growing the plants used in this investigation. This work was carried out under Department for Environment, Food and Rural Affairs (DEFRA) licence 50973/198463/1.
References
- Adams, M.J. , Antoniw, J.F. , Bar‐Joseph, M. , Brunt, A.A. , Candresse, T. , Foster, G.D. , Martelli, G.P. , Milne, R.G. and Fauquet, C.M. (2004) The new plant virus family Flexiviridae and assessment of molecular criteria for species demarcation. Arch. Virol. 149, 1045–1060. [DOI] [PubMed] [Google Scholar]
- Aguilar, J.M. , Hernández‐Gallardo, M.D. , Cenis, J.L. , Lacasa, A. and Aranda, M.A. (2002) Complete sequence of the Pepino mosaic virus RNA genome. Arch. Virol. 147, 2009–2015. [DOI] [PubMed] [Google Scholar]
- Andersen, K. and Johansen, I.E. (1998) A single conserved amino acid in the coat protein gene of pea seed‐borne mosaic potyvirus modulates the ability of the virus to move systemically in Chenopodium quinoa . Virology, 241, 304–311. [DOI] [PubMed] [Google Scholar]
- Banerjee, N. , Wang, J.Y. and Zaitlin, M. (1995) A single nucleotide change in the coat protein gene of Tobacco mosaic virus is involved in the induction of severe chlorosis. Virology, 207, 234–239. [DOI] [PubMed] [Google Scholar]
- Baratova, L.A. , Grebenshchikov, N.I. , Shishkov, A.V. , Kashirin, I.A. , Radavsky, J.L. , Jarvekulg, L. and Saarma, M. (1992a) The topography of the surface of Potato virus X: tritium planigraphy and immunological analysis. J. Gen. Virol. 73, 229–235. [DOI] [PubMed] [Google Scholar]
- Baratova, L.A. , Grebenshchikov, N.I. , Dobrov, E.N. , Gedrovich, A.V. , Kashirin, I.A. , Shishkov, A.V. , Efimov, A.V. , Järvekülg, L. , Radavsky, Y.L. and Saarma, M. (1992b) The organization of Potato virus X coat proteins in virus particles studied by tritium planigraphy and model building. Virology, 188, 175–180. [DOI] [PubMed] [Google Scholar]
- Bukovinszki, A. , Götz, R. , Johansen, E. , Maiss, E. and Balázs, E. (2007) The role of the coat protein region in symptom formation on Physalis floridana varies between PVY strains. Virus Res. 127, 122–125. [DOI] [PubMed] [Google Scholar]
- Carbonell, A. , Maliogka, V.I. , Pérez, J.D.J. , Salvador, B. , León, D.S. , García, J.A. and Simón‐Mateo, C. (2013) Diverse amino acid changes at specific positions in the N‐terminal region of the coat protein allow Plum pox virus to adapt to new hosts. Mol. Plant–Microbe Interact. 26, 1211–1224. [DOI] [PubMed] [Google Scholar]
- Cruz, S. , Roberts, A. , Prior, D. , Chapman, S. and Oparka, K. (1998) Cell‐to‐cell and phloem‐mediated transport of Potato virus X. The role of virions. Plant Cell, 10, 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroocq, V. , Salvador, B. , Sicard, O. , Glasa, M. , Cosson, P. , Svanella‐Dumas, L. , Revers, F. , García, J. and Candresse, T. (2009) The determinant of potyvirus ability to overcome the RTM resistance of Arabidopsis thaliana maps to the N‐terminal region of the coat protein. Mol. Plant–Microbe Interact. 22, 1302–1311. [DOI] [PubMed] [Google Scholar]
- Desbiez, C. , Chandeysson, C. and Lecoq, H. (2014) A short motif in the N‐terminal part of the coat protein is a host‐specific determinant of systemic infectivity for two potyviruses. Mol. Plant Pathol. 15, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde, S.M.D. (1989) Strain differentiation of Barley yellow dwarf virus isolates using specific monoclonal antibodies in immunosorbent electron microscopy. J. Virol. Methods, 23, 313–319. [DOI] [PubMed] [Google Scholar]
- Foster, G.D. and Turner, R. (1998) Plant Virology Protocols: From Virus Isolation to Transgenic Resistance Methods in Molecular Biology. Foster G.D. and Taylor S.C., eds. Totowa, NJ: Humana Press Inc, pp. 293–299. [Google Scholar]
- Gietz, R.D. and Woods, R.A. (2002) Transformation of yeast by lithium acetate/single‐stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350, 87–96. [DOI] [PubMed] [Google Scholar]
- Gomez, P. , Sempere, R.N. , Elena, S.F. and Aranda, M.A. (2009) Mixed infections of Pepino mosaic virus strains modulate the evolutionary dynamics of this emergent virus. J. Virol. 83, 12 378–12 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen, I.M. and Thomma, B.P.H. (2010) Pathogen profile Pepino mosaic virus: a successful pathogen that rapidly evolved from emerging to endemic in tomato crops. Mol. Plant Pathol. 11, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen, I.M. , Paeleman, A. , Wittemans, L. , Goen, K. , Lievens, B. , Bragard, C. , Vanachter, A.C.R.C. and Thomma, B.P.H.J. (2008) Genetic characterization of Pepino mosaic virus isolates from Belgian greenhouse tomatoes reveals genetic recombination. Eur. J. Plant Pathol. 121, 131–146. [Google Scholar]
- Hanssen, I.M. , Paeleman, A. , Vandewoestijne, E. , van Bergen, L. , Bragard, C. , Lievens, B. , Vanachter, A.C.R.C. and Thomma, B.P.H.J. (2009) Pepino mosaic virus isolates and differential symptomatology in tomato. Plant Pathol. 58, 450–460. [Google Scholar]
- Hasiów‐Jaroszewska, B. and Borodynko, N. (2012) Characterization of the necrosis determinant of the European genotype of Pepino mosaic virus by site‐specific mutagenesis of an infectious cDNA clone. Arch. Virol. 157, 337–341. [DOI] [PubMed] [Google Scholar]
- Hasiów‐Jaroszewska, B. , Borodynko, N. , Jackowiak, P. , Figlerowicz, M. and Pospieszny, H. (2011) Single mutation converts mild pathotype of the Pepino mosaic virus into necrotic one. Virus Res. 159, 57–61. [DOI] [PubMed] [Google Scholar]
- Hasiów‐Jaroszewska, B. , Paeleman, A. , Ortega‐Parra, N. , Borodynko, N. , Minicka, J. , Czerwoniec, A. , Thomma, B.P. and Hanssen, I.M. (2013) Ratio of mutated versus wild‐type coat protein sequences in Pepino mosaic virus determines the nature and severity of yellowing symptoms on tomato plants. Mol. Plant Pathol. 14, 923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. , Nie, X. , He, C. and Xiong, X. (2011) Differential pathogenicity of two different recombinant PVY(NTN) isolates in Physalis floridana is likely determined by the coat protein gene. Virol. J. 8, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordá, C. , Pérez, A.L. , Martínez Culebras, P.V. and Lacasa, A. (2001) First report of Pepino mosaic virus on natural hosts. Plant Dis. 85, 1292–1292. [DOI] [PubMed] [Google Scholar]
- Knorr, D.A. and Dawson, W.O. (1988) A point mutation in the Tobacco mosaic virus capsid protein gene induces hypersensitivity in Nicotiana sylvestris . Proc. Natl. Acad. Sci. USA, 85, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, P. , Yeh, W.‐B. , Tsai, C.‐W. and Lin, N.‐S. (2010) A unique glycine‐rich motif at the N‐terminal region of Bamboo mosaic virus coat protein is required for symptom expression. Mol. Plant–Microbe Interact. 23, 903–914. [DOI] [PubMed] [Google Scholar]
- Martelli, G.P. , Adams, M.J. , Kreuze, J.F. and Dolja, V.V. (2007) Family Flexiviridae: a case study in virion and genome plasticity. Annu. Rev. Phytopathol. 45, 73–100. [DOI] [PubMed] [Google Scholar]
- Morozov, S.Y.U. and Solovyev, A.G. (2003) Triple gene block: modular design of a multifunctional machine for plant virus movement. J. Gen. Virol. 84, 1351–1366. [DOI] [PubMed] [Google Scholar]
- Neeleman, L. , Van Der Kuyl, A.C. and Bol, J.F. (1991) Role of Alfalfa mosaic virus coat protein gene in symptom formation. Virology, 181, 687–693. [DOI] [PubMed] [Google Scholar]
- Pagán, I. , Córdoba‐Sellés, M.C. , Martínez‐Priego, L. , Fraile, A. , Malpica, J.M. , Jordá, C. and García‐Arenal, F. (2006) Genetic structure of the population of Pepino mosaic virus infecting tomato crops in Spain. Phytopathology, 96, 274–279. [DOI] [PubMed] [Google Scholar]
- Pérez, J.D.J. , Udeshi, N.D. , Shabanowitz, J. , Ciordia, S. , Juárez, S. , Scott, C.L. , Olszewski, N.E. , Hunt, D.F. and García, J.A. (2013) O‐GlcNAc modification of the coat protein of the potyvirus Plum pox virus enhances viral infection. Virology, 442, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux, G. , Majeau, N. and Leclerc, D. (2012) Mapping the surface‐exposed regions of Papaya mosaic virus nanoparticles. FEBS J. 279, 2004–2011. [DOI] [PubMed] [Google Scholar]
- Salomone, A. and Roggero, P. (2002) Host range, seed transmission and detection by ELISA and lateral flow of an Italian isolate of Pepino mosaic virus. J. Plant Pathol. 84, 65–68. [Google Scholar]
- Salvador, B. , Delgadillo, M.O. , Sáenz, P. , García, J. and Simón‐Mateo, C. (2008) Identification of Plum pox virus pathogenicity determinants in herbaceous and woody hosts. Mol. Plant–Microbe Interact. 21, 20–29. [DOI] [PubMed] [Google Scholar]
- Sempere, R.N. , Gomez, P. , Truniger, V. and Aranda, M.A. (2011) Development of expression vectors based on Pepino mosaic virus. Plant Methods, 7, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintaku, M.H. , Lee, Z. and Palukaitis, P. (1992) A single amino acid substitution in the coat protein of Cucumber mosaic virus induces chlorosis in tobacco. Plant Cell, 4, 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatineni, S. , Van Winkle, D.H. and French, R. (2011) The N‐terminal region of Wheat streak mosaic virus coat protein is a host and strain‐specific long‐distance transport factor. J. Virol. 85, 1718–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, R. , Bate, N. , Twell, D. and Foster, G.D. (1994) Analysis of a translational enhancer upstream from the coat protein open reading frame of Potato virus S. Arch. Virol. 134, 321–333. [DOI] [PubMed] [Google Scholar]
- Turner, R.L. , Glynn, M. , Taylor, S.C. , Cheung, M.‐K. , Spurr, C. , Twell, D. and Foster, G.D. (1999) Analysis of a translational enhancer present within the 5′‐terminal sequence of the genomic RNA of Potato virus S. Arch. Virol. 144, 1451–1461. [DOI] [PubMed] [Google Scholar]
- Ullah, Z. and Grumet, R. (2002) Localization of Zucchini yellow mosaic virus to the veinal regions and role of viral coat protein in veinal chlorosis conditioned by the zym potyvirus resistance locus in cucumber. Physiol. Mol. Plant Pathol. 60, 79–89. [Google Scholar]


