Summary
Fusarium graminearum, the causal agent of Fusarium head blight, is a common pathogen on small grain cereals worldwide and produces various trichothecenes [deoxynivalenol (DON) is predominant] during infection. A previous study has revealed that DON production is positively correlated with the occurrence of carbendazim (MBC) resistance. Here, we identified and characterized two putative genes encoding hexokinase in F. graminearum (FgHXK1 and FgHXK2), which is a rate‐limiting enzyme in DON biosynthesis. The expression level of hexokinase genes and the production of pyruvate, which is the precursor of DON, were up‐regulated in the MBC‐resistant strain, indicating that hexokinase genes might be involved in increased DON production. Phylogenetic and comparative analyses indicated that FgHXK1 was the predominant hexokinase gene. Gene disruption showed that ΔFgHXK1 severely affected DON production, indicating that FgHXK1 played a role in the regulation of DON biosynthesis. Morphological characterization showed that ΔFgHXK1 led to inhibited vegetative growth and conidiation. Sensitivity tests to MBC and various stresses indicated that both ΔFgHXK1 and ΔFgHXK2 mutants showed no significant difference from parental strains. Pathogencity assays showed that ΔFgHXK1 mutants lost virulence on wheat head and corn stigma; however, they showed no change in sexual reproduction. The FgHXK1‐overexpressing transformants were obtained subsequently. Their pyruvate and DON production was confirmed to be increased, indicating that FgHXK1 positively regulated DON biosynthesis. Although additional defects appeared in overexpression mutants, MBC sensitivity showed no change. All of the results indicated that the transcriptional level of FgHXK1 regulated DON biosynthesis, but showed no direct relationship with MBC resistance.
Keywords: DON, FgHXK1, Fusarium graminearum, hexokinase, MBC resistance
Introduction
The filamentous fungus Fusarium graminearum is a ubiquitous plant pathogen and the causal agent of Fusarium head blight (FHB) (Starkey et al., 2007). Infection by F. graminearum in cereal crops may lead to huge yield losses in severe epidemic years. In addition to economic losses, the pathogen can have a serious impact on human and animal health by contaminating maize, wheat and barley with type B trichothecenes, mainly deoxynivalenol (DON) (Alexander et al., 2009; Goswami and Kistler, 2004). Trichothecenes are potent inhibitors of eukaryotic protein synthesis (Rocha et al., 2005) and constitute a toxin family (Bennett and Klich, 2003).
Since the 1970s, carbendazim (MBC), benomyl and other benzimidazole fungicides have been widely used for the control of FHB during wheat heading and flowering in China (Zhou et al., 1994). The effectiveness of MBC, however, has been threatened by the emergence of resistant pathogen populations in the field (Zhou and Wang, 2001). Point mutations in the β 2‐tub gene at codons 167, 198 and 200 have been detected in MBC‐resistant strains of F. graminearum in the field (Chen et al., 2007). Zhang et al. (2009) first reported that MBC resistance might increase DON production ability of F. graminearum. Global gene expression studies have indicated that a mutation at codon 167 in β2‐tub is involved in the up‐regulation of several developmental processes which are required for pathogenicity and secondary metabolism (J. B. Qiu et al., unpublished data).
Most of the genes involved in DON biosynthesis (TRI genes), which are clustered in a 26‐kb region (Brown et al., 2004), have been identified, allowing studies of the relationship between their transcriptional expression and the level of toxin production. The first step in the DON biosynthetic pathway involves farnesyl pyrophosphate (FPP), which is a common precursor of the sterol pathway and the type B trichothecene pathway (Kimura et al., 2007). FPP is synthesized from geranyl pyrophosphate by the FPP synthetase in the isoprenoid biosynthetic pathway. Acetyl CoA, the main input of the isoprenoid pathway, is converted from pyruvate, which is phosphorylated by hexokinase at the first step of glycolysis (Cody et al., 2000).
In this study, we identified and characterized two putative hexokinase genes in F. graminearum with the goal of clarifying the influence of hexokinase on DON production and MBC sensitivity.
Results
FgHXK1 and FgHXK2 are conserved proteins with hexokinase domains in F. graminearum
FgHXK1 (FGSG_00500) and FgHXK2 (FGSG_03014) were originally identified through sequence homology analysis of the F. graminearum genome using the blastp algorithm with HXK1 and HXK2 of Saccharomyces cerevisiae as query (Fig. S1, see Supporting Information). The genome of F. graminearum was predicted to contain six genes encoding putative hexokinases: FGSG_00500, FGSG_03014, FGSG_08744, FGSG_16878, FGSG_17276 and FGSG_08399 (Fig. S2A, see Supporting Information). All of these genes consisted of two characteristic hexokinase domains: Hexokinase 1 (Pfam accession no. PF00349) and Hexokinase 2 (Pfam accession no. PF03727) (Fig. S2A).
A phylogenetic analysis of the putative hexokinase proteins by neighbour‐joining methods revealed four distinct groups: FGSG_00500 and FGSG_03014 (hexokinase group), FGSG_08744 and FGSG_16878 (glucokinase group), FGSG_08399 (another catalytic hexokinase protein group) and FGSG_17276 (another catalytic hexokinase protein group) (Fig. S2A). According to phylogenetic analysis, FgHXK1 and FgHXK2 in F. graminearum are homologous to those of other fungi (Fig. S2B).
Cluster analysis indicated that FgHXK1 shared 53% and 54% identity with HXK1 and HXK2 of S. cerevisiae, respectively; FgHXK2 shared substantially less identity with HXK1 and HXK2 of S. cerevisiae (43% and 43%, respectively) (Fig. S1). Therefore, there is high homology between the proteins of F. graminearum and S. cerevisiae.
To further confirm FgHXK1 and FgHXK2 transcripts, we sequenced the full‐length genomic DNA and cDNA sequences. FgHXK1 had an open reading frame (ORF) of 2324 bp with five introns, and was predicted to encode a protein of 492 amino acids; FgHXK2 had an ORF of 1520 bp with three introns, and was predicted to encode a protein of 453 amino acids (Figs S1 and S2A).
Pyruvate production analysis
Pyruvate is the precursor of the isoprenoid biosynthetic pathway which is used for DON biosynthesis. According to ultraviolet spectrophotometric detection, the MBC‐resistant strain 167 produced about 1.5‐fold more pyruvate than the MBC‐sensitive strain 2021; furthermore, pyruvate production in ΔFgHXK1 mutants (2hxk1‐14 and 1hxk1‐21) decreased by more than 50% (Table 1).
Table 1.
Strains used in this study, their pyruvic acid concentration and carbendazim (MBC) sensitivity
| Strain | Genotype | Source | Concentration of pyruvic acid (μg/g)b | MBC sensitivity | |
|---|---|---|---|---|---|
| EC50 (μg/mL) | R 2 c | ||||
| 2021 | Wild‐type | This laboratorya | 1533.56 ± 190 a | 0.56 ± 0.05 a | 0.9943 |
| 167 | Point mutant at codon 167 of β2‐tub from 2021 | This laboratorya | 2324.81 ± 159 c | 8.25 ± 1.2 b | 0.9748 |
| 2hxk1‐14 | ΔFgHXK1 mutant of 2021 | This study | 724.52 ± 95 b | 0.54 ± 0.06 a | 0.9917 |
| 1hxk1‐21 | ΔFgHXK1 mutant of 167 | This study | 952.53 ± 182 b | 9.08 ± 0.9 b | 0.9876 |
| 2hxk2‐19 | ΔFgHXK2 mutant of 2021 | This study | 1773.71 ± 186 a | 0.49 ± 0.04 a | 0.9947 |
| 1hxk2‐33 | ΔFgHXK2 mutant of 167 | This study | 2697.14 ± 257 c | 8.15 ± 1.7 b | 0.9803 |
| 2hxk1‐C4 | Genetic complement strain of 2hxk1‐14 | This study | 1436.14 ± 162 a | 0.59 ± 0.07 a | 0.9924 |
| 1hxk1‐C5 | Genetic complement strain of 1hxk1‐21 | This study | 2433.23 ± 212 c | 7.94 ± 0.7 b | 0.9747 |
| 2hxk2‐C1 | Genetic complement strain of 2hxk2‐19 | This study | 1359.22 ± 117 a | 0.51 ± 0.09 a | 0.9942 |
| 1hxk2‐C6 | Genetic complement strain of 1hxk2‐33 | This study | 2249.58 ± 198 c | 9.02 ± 1.6 b | 0.9872 |
| 2Ohxk1‐11 | FgHXK1 overexpression mutant of 2021 | This study | 8112.57 ± 719 d | 0.54 ± 0.05 a | 0.9923 |
| 2Ohxk1‐12 | FgHXK1 overexpression mutant of 2021 | This study | 6952.06 ± 689 d | 0.58 ± 0.07 a | 0.9961 |
In this study, 2021 and 167 were used as parental strains. 2021 was the wild‐type and sensitive to MBC; 167 was resistant to MBC and was derived from 2021 through two steps of homologous double‐crossover gene manipulation: deletion of the β2‐tub gene of 2021, followed by a homologous replacement with a point mutation β2‐tub fragment at codon 167 (Phe → Tyr) (Qiu et al., 2011). The only difference in genetic background between strain 2021 and 167 was the amino acid sequence in codon 167 of β2‐tub.
Values are the mean of three replicates (± standard error of the mean). Means followed by the same letter in each column are not significantly different between strains by the least‐significant difference (LSD) test at P = 0.05.
R 2 is the correlation coefficient.
To further confirm the finding that FgHXK1 could affect pyruvate production, we assayed the expression of hexokinase encoding genes, which were necessary for pyruvate biosynthesis. Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis showed that the expression levels of FgHXK1 and FgHXK2 in 167 were increased by 2.3‐fold and 2.6‐fold, respectively, in comparison with those in 2021 (Fig. 1). These results indicated that hexokinase activity affected pyruvate biosynthesis, and the MBC‐resistant strain displayed significantly greater pyruvate production.
Figure 1.
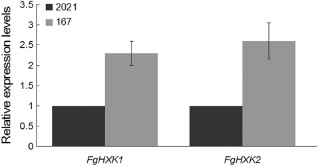
Expression levels of FgHXK genes. The relative quantities of the FgHXK1 and FgHXK2 transcripts were compared between the carbendazim (MBC)‐sensitive strain 2021 and the MBC‐resistant strain 167. Means and standard deviations were calculated with data from three replicates.
Disruption of FgHXK1 and FgHXK2 in F. graminearum
Transformants that grew on potato dextrose agar (PDA) plates amended with 150 μg/mL hygromycin were tentatively considered to be the target transformants. The primer pair Fghxk1‐F/Fghxk1‐R was used to specifically amplify the partial FgHXK1 (400 bp) in parental and complement strains. The primer pairs Fghxk1‐FF/Hph‐R and Hph‐F/Fghxk1‐RR were used to amplify the two homologous arms with a partial fragment of the connecting area (1757 and 2068 bp). Probe 1 detected a 2549‐bp fragment in parental and complement strains, and probe 2 detected a single band of the 6979‐bp fragment in the two corresponding mutants (2hxk1‐14 and 1hxk1‐21) (Fig. 2C). The primer pair Fghxk2‐F/Fghxk2‐R was used to specifically amplify the partial FgHXK2 (520 bp) in parental and complement strains. The primer pairs Fghxk2‐FF/Hph‐R and Hph‐F/Fghxk2‐RR were used to amplify the two homologous arms with a partial fragment of the connecting area (1727 and 2463 bp). Probe 3 detected a 3582‐bp fragment in parental and complement strains, and probe 2 detected a single band of the 5912‐bp fragment in the two corresponding mutants (2hxk2‐19 and 1hxk2‐33) (Fig. 2D). PCR and Southern blotting confirmed that these mutants were null mutants resulting from a single homologous recombination event at the FgHXK1 and FgHXK2 loci of 2021 and 167.
Figure 2.
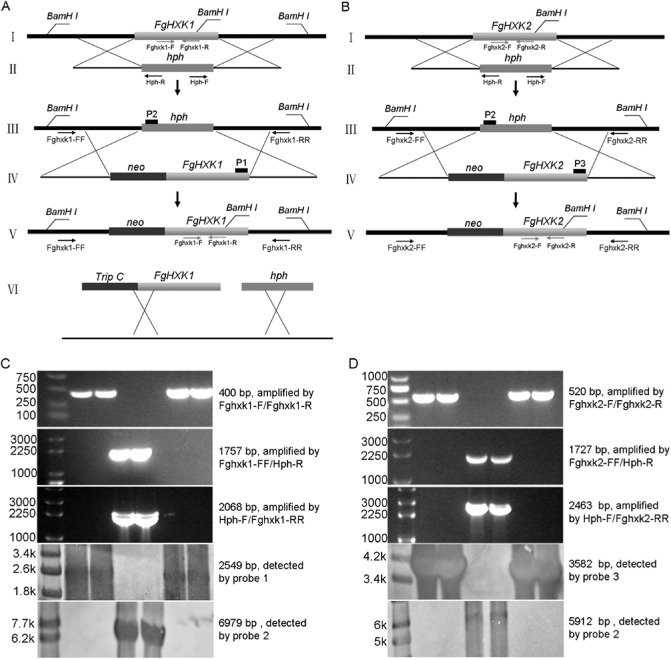
Generation and identification of hexokinase gene deletion and FgHXK1 overexpression mutants. (A, B) Homologous gene replacement and complementation strategy for FgHXK1 and FgHXK2, and FgHXK1 overexpression of 2021. (C) Confirmation of FgHXK1 deletion mutants by polymerase chain reaction (PCR) and Southern blot analysis. Probe 1 detected a 2549‐bp fragment in parental strains and complement strains, and probe 2 detected a single band of a 6979‐bp fragment in the two corresponding mutants. (D) Confirmation of FgHXK2 deletion mutants. Probe 3 detected a 3582‐bp fragment in parental strains and complement strains, and probe 2 detected a single band of a 5912‐bp fragment in the two corresponding mutants.
Involvement of FgHXK1 and FgHXK2 in the regulation of DON biosynthesis
The production of DON by F. graminearum is a prerequisite for the colonization of wheat (Maier et al., 2006; Proctor et al., 1995). Thus, the alteration in DON production of ΔFgHXK1 and ΔFgHXK2 mutants was examined in this study. After culture in GYEP (50 g/L glucose, 1 g/L yeast extract and 1 g/L peptone) for 7 days, the amount of DON produced by ΔFgHXK1 mutants was much lower than that by parental strains and ΔFgHXK2 mutants (Fig. 3A).
Figure 3.
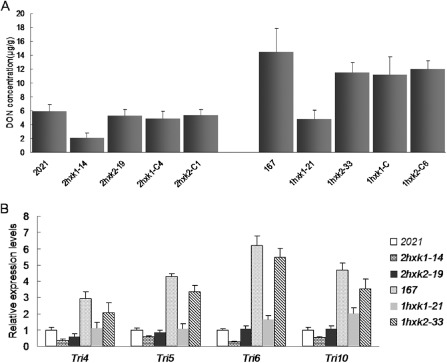
Deoxynivalenol (DON) production in shake flasks and expression of TRI genes. Line bars in each column denote the standard errors of three repeated experiments. (A) The amount of DON (μg/g of fungal DNA) produced by ΔFgHXK1 mutants after 7 days in GYEP (50 g/L glucose, 1 g/L yeast extract and 1 g/L peptone) was remarkably lower than that produced by parental strains and ΔFgHXK2 mutants. (B) The relative expression levels of TRI genes in ΔFgHXK1 mutants decreased significantly when compared with those in parental strains and ΔFgHXK2 mutants.
To further confirm these results, we analysed the expression levels of four DON biosynthesis genes by qRT‐PCR. The expression levels of TRI genes in ΔFgHXK1 mutants decreased significantly relative to those in parental strains and ΔFgHXK2 mutants (Fig. 3B). All of the defects were restored by genetic complementation of the ΔFgHXK1 mutant with the wild‐type FgHXK1 gene (Fig. 3). These results were consistent with the profiles of DON production in liquid culture, which indicated that FgHXK1 played an important role in the regulation of DON biosynthesis in F. graminearum.
Deletion of FgHXK1 reduces growth rate and dry hyphal weight
Pleiotropic growth defects were observed for the hxk1 mutant in Botrytis cinerea (Rui and Hahn, 2007). Deletion of FgHXK1 also dramatically affected the development of aerial hyphae in F. graminearum. The radial growth of all ΔFgHXK1 mutants was significantly retarded (by approximately 50%) and there was reduced aerial hyphal growth, whereas the growth of the ΔFgHXK2 mutant was similar to that of the parental strains (Fig. 4A). After 3 days of culture in liquid PDA medium, fungal biomass showed a significant reduction in ΔFgHXK1 mutants compared with ΔFgHXK2 mutants and parental strains (Fig. 4B), indicating that FgHXK1 was important for the vegetative growth of F. graminearum. All of the defects were restored by genetic complementation of the ΔFgHXK1 mutant with the wild‐type FgHXK1 gene (Fig. 4).
Figure 4.
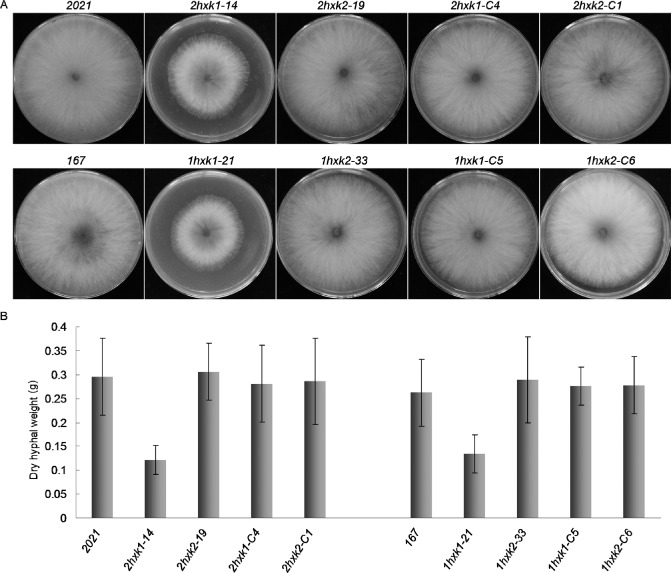
Impact of FgHXK1 and FgHXK2 deletion on colony morphology and dry hyphal weight. (A) Colony growth rate was assayed in potato dextrose agar (PDA) according to colony diameter after inoculation at 25 °C for 3 days. (B) Fungal biomass production was measured by weighing mycelia after freeze–drying at −40 °C for 48 h.
FgHXK1 is involved in conidiation and germination
Hexokinase has been shown to regulate asexual development in several fungal species (Calvo, 2008). Therefore, we investigated conidiation and conidial germination of ΔFgHXK1 and ΔFgHXK2 mutants. The results showed that the sporulation of ΔFgHXK1 mutants was strongly reduced (Fig. 5A). For instance, only (1.6 ± 0.25) × 106 and (1.1 ± 0.21) × 106 conidia were produced by 2hxk1‐14 and 1hxk1‐21, respectively, after 7 days of incubation, in comparison with (3.4 ± 0.88) × 106 and (2.9 ± 0.65) × 106 conidia of the parental strains (2021 and 167, respectively). Furthermore, ΔFgHXK1 mutants formed smaller conidia (2hxk1‐14, 3.2 ± 0.7 mm; 1hxk1‐21, 2.88 ± 0.6 mm) than those of the parental strains (2021, 5.99 ± 1.2 mm; 167, 5.53 ± 1 mm) and ΔFgHXK2 mutants (2hxk2‐19, 4.41 ± 1.3 mm; 1hxk2‐33, 4.19 ± 1.6 mm) (Fig. 5B).
Figure 5.
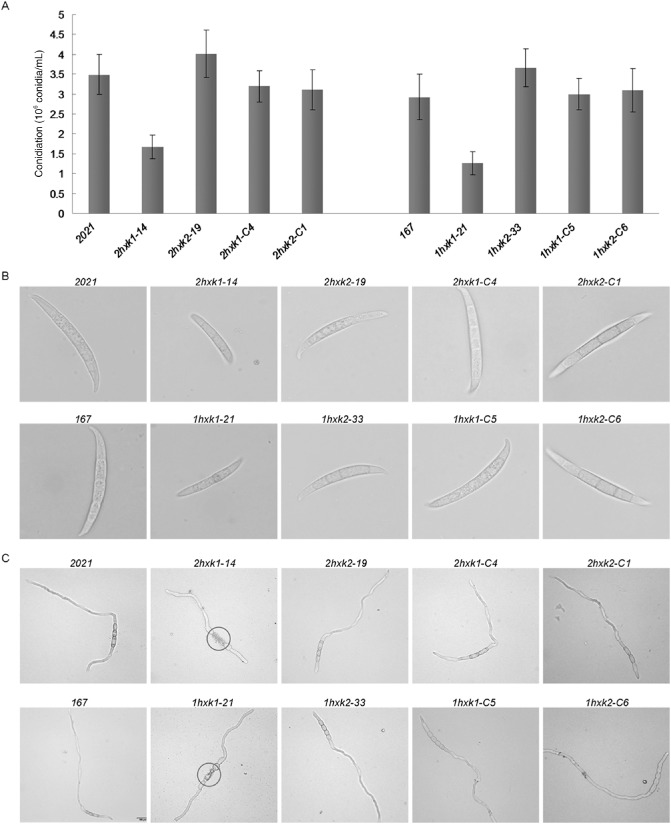
Comparison of conidiation and conidial germination among different strains. (A) All the strains were cultured in mung bean broth (MBB) on a shaker for 7 days (175 rpm, 25 °C). Line bars in each column denote standard errors of three experiments. (B) ΔFgHXK1 mutants formed smaller conidia than those of the parental strains and ΔFgHXK2 mutants. Bar, 10 μm. (C) Germ tubes were photographed after 6 h of culture in YEPD (10 g/L peptone, 3 g/L yeast extract and 20 g/L glucose). The black circle indicates that the cell wall of the ΔFgHXK1 mutant appears to have collapsed in the middle. Bar, 100 μm.
When incubated in YEPD (10 g/L peptone, 3 g/L yeast extract and 20 g/L glucose), conidial germination of the ΔFgHXK1 and ΔFgHXK2 mutants was similar to that of the parental strains. Interestingly, the germinated conidial cell wall of ΔFgHXK1 mutants appeared to show collapse in the middle (Fig. 5C). This phenotype might contribute to the defects in the growth parameters tested. These results indicated that FgHXK1 was involved in the regulation of conidiation and conidial germination. All the defects were repaired by genetic complementation of the ΔFgHXK1 mutant with the wild‐type FgHXK1 gene (Fig. 5).
Sensitivity of ΔFgHXK1 and ΔFgHXK2 mutants to MBC, osmotic stress and carbon utilization
FgHXK1 and FgHXK2 of F. graminearum showed no direct association with MBC sensitivity. The 50% effective concentrations (EC50) of all the deletion mutants showed no significant difference from those of the parental strains (Table 1).
In a previous study, NaCl and KCl were used for ionic osmotic stress tests, and caffeine and Congo red caused damage to fungal cell walls by binding to chitin and cellulose (Jiang et al., 2011). Therefore, we tested the sensitivity of ΔFgHXK1 and ΔFgHXK2 mutants to various stresses. As shown in Fig. 6A, 2hxk1‐14 and 1hxk1‐21 exhibited increased sensitivity to Congo red and sodium dodecylsulfate (SDS), but not to another cell wall toxicant, caffeine. These results indicated that FgHXK1 might be involved in the regulation of cell wall integrity. ΔFgHXK1 and ΔFgHXK2 mutants showed similar sensitivity to the parental strains to ionic osmotic stress (Fig. 6A), which indicated that FgHXK1 and FgHXK2 in F. graminearum did not participate in the regulation of ionic osmotic stress.
Figure 6.
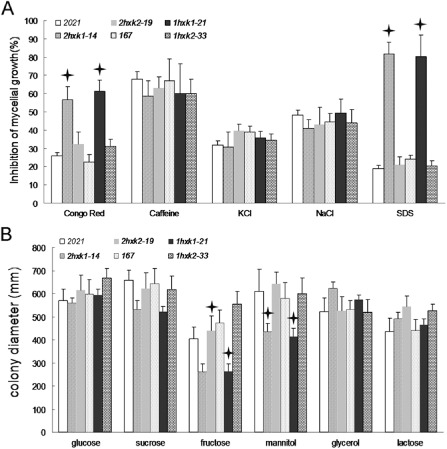
Sensitivity of all strains to various stress and carbon utilization tests. Bars with stars are significantly different compared with parental strains at the P < 0.05 level according to Duncan's multiple comparison. (A) Sensitivity of all strains to ionic osmotic and cell wall‐damaging agents. (B) Carbon utilization tests under different conditions were performed on minimal medium (MM) supplemented with different carbon sources.
Hexokinase plays a central role in the initiation of sugar metabolism of living organisms and has also been implicated in carbon catabolite repression in yeasts and plants (Rui and Hahn, 2007). Therefore, we determined the carbon source consumption ability of the mutants. As shown in Fig. 6B, the growth of ΔFgHXK1 mutants on minimal medium (MM) with mannitol or fructose as carbon source was reduced, but not completely inhibited; the growth rate of ΔFgHXK2 mutants on MM amended with different sugars was not significantly different from that on PDA. These results indicated that FgHXK1 played an important role in mannitol and fructose utilization.
Sexual reproduction and pathogenicity analysis
Forcibly discharged into air from perithecia, ascospores are the primary inoculum of the disease (Parry et al., 1995; Trail et al., 2002). ΔFgHXK1 and ΔFgHXK2 mutants produced equal amounts of perithecia (Fig. 7A), indicating that these two genes were not required for sexual reproduction.
Figure 7.
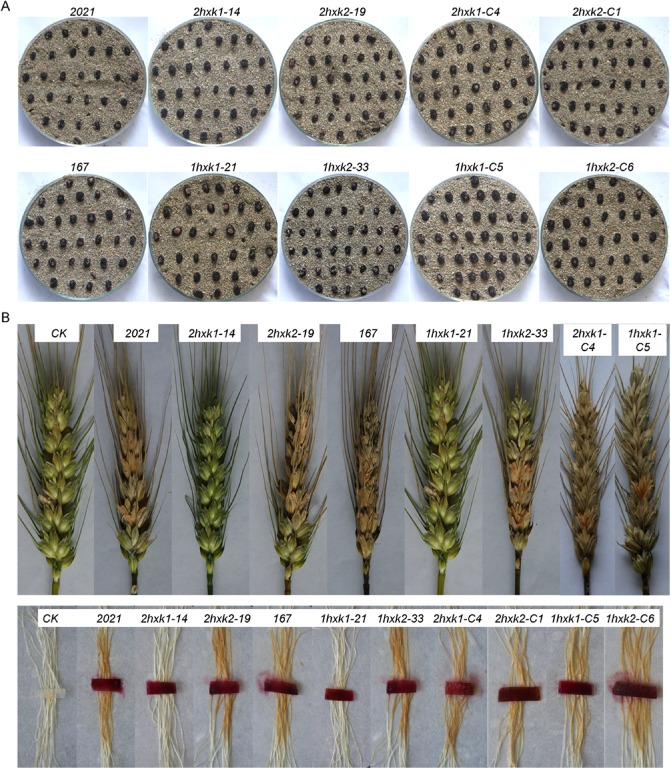
Effects of ΔFgHXK1 and ΔFgHXK2 on perithecium production and virulence. (A) Perithecium production ability was evaluated according to the area covered with perithecia. (B) The parental strains and ΔFgHXK2 mutants were able to colonize inoculated spikelets and corn stigmas; however, the ΔFgHXK1 mutants lost pathogenicity absolutely.
To check the pathogenic performance of the mutants, we performed infection tests on wheat spikelets and corn stigma. The infection abilities of the mutants were uniform on the two hosts. Both the parental strains and ΔFgHXK2 mutants were able to colonize inoculated spikelets (more than 80% of the spikelets were colonized) and corn stigma, resulting in scab symptoms (Fig. 7B). Under the same conditions, however, the ΔFgHXK1 mutants showed a complete loss of pathogenicity (no spikelet or corn stigma was colonized) (Fig. 7B). All the defects were restored by genetic complementation of the ΔFgHXK1 mutant with the wild‐type FgHXK1 gene (Fig. 7). These results demonstrated that FgHXK1 was essential for full virulence in F. graminearum.
Effects of FgHXK1 overexpression on DON production
The positive FgHXK1 overexpression clones, which grew on PDA containing 150 μg/mL hygromycin, were selected for qRT‐PCR analysis. Gene expression indicated that several strains showed significantly increased FgHXK1 expression relative to the parental strain 2021. Two transformants (2Ohxk1‐11 and 2Ohxk1‐12) were randomly chosen for the following research (Fig. 8A).
Figure 8.
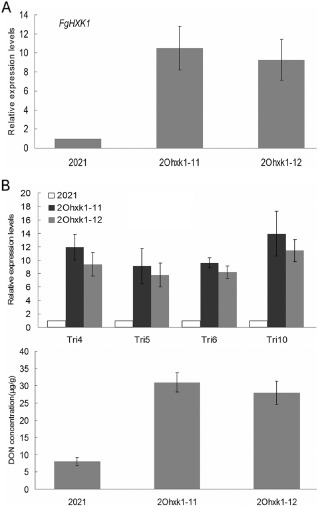
Confirmation of FgHXK1 overexpression mutants and their deoxynivalenol (DON) biosynthesis test. (A) Confirmation of FgHXK1 overexpression mutants by detection of the FgHXK1 expression level. (B) DON biosynthesis test of FgHXK1 overexpression mutants by detection of the amount of DON (μg/g of fungal DNA) and the relative expression levels of TRI genes. Line bars in each column denote standard errors of three repeated experiments.
The TRI gene expression of 2Ohxk1‐11 and 2Ohxk1‐12 was up‐regulated, and DON production and pyruvate concentration were significantly higher than in the parental strain 2021 (Table 1, Fig. 8B). Biological phenotype measurement showed that the growth rate and conidiation of 2Ohxk1‐11 and 2Ohxk1‐12 displayed some defects, but the MBC sensitivity showed no change (Table 1, Fig. S3, see Supporting Information). These results indicated that the enhanced expression level of FgHXK1 could induce higher DON production, but had no influence on MBC sensitivity.
Discussion
Fusarium graminearum is one of the most destructive species of Fusarium, responsible for FHB in the field, producing mycotoxins in mouldy corn and wheat that are toxic to animals and humans (Cleveland et al., 2003; Placinta et al., 1999; Windels, 2000). Among these mycotoxins, DON is frequently encountered in cereal crops. Fungicides have been used extensively to fight against FHB, but the cocktail of fungicides currently spread on cereals is not fully operative in controlling the accumulation of DON. Moreover, in some cases, the application of ineffective doses of some fungicides or the development of resistance to MBC could trigger certain cellular mechanisms in the fungus leading to the enhancement of DON production (Audenaert et al., 2010; Zhang et al., 2009). Recently, MBC resistance resulting from a mutation at codon 167 of β2‐tub has been shown to be the mutation predominantly observed in the field (Zhang et al., 2013). Accordingly, there is a need to investigate the effect of the potential induction factor of MBC resistance on DON biosynthesis.
Pyruvate production controlled by hexokinase supplies the main substrate for the biosynthesis of many secondary metabolites, including trichothecene, DON, fumonisins, penicillin and aflatoxin. The hexokinase encoding gene HXK1 has been shown to regulate carbon catabolism, sporulation, fumonisin B1 production and pathogenesis in F. verticillioides (Kim et al., 2011). Our qRT‐PCR assay showed that two hexokinase genes were expressed at a two‐fold higher level in F. graminearum MBC‐resistant strain than in the wild‐type (Fig. 1). A similar tendency was observed in the pyruvate concentration test (Table 1), which indicated that the mutation at codon 167 of β2‐tub promoted pyruvate production and might be involved in DON biosynthesis.
Fungi and plants contain several hexokinases known to be involved in signalling networks for the control of growth and development in response to the changing environment (Fleck and Brock, 2010; Moore et al., 2003). Similar to the situation in Aspergillus spp. and B. cinerea, F. graminearum probably contains two functional hexokinases. We obtained these two hexokinase genes (FgHXK1 and FgHXK2) containing Hexokinase 1 and Hexokinase 2 domains through the blastp program with the HXK1 and HXK2 genes of S. cerevisiae as query (Figs S1 and S2).
According to recent research, the lack of Hxk1 attenuates the virulence of B. cinerea on leaves, and the strain shows a pleiotropic growth defect, including retarded vegetative growth and strongly reduced conidial formation (Rui and Hahn, 2007). The F. verticillioides HXK1 mutant is substantially less virulent than the wild‐type on maize kernels, and has a pronounced effect on trehalose and fumonisin B1 biosynthesis (Kim et al., 2011). In this study, we generated ΔFgHXK1 and ΔFgHXK2 mutants, and found that these two genes displayed different biochemical properties during growth and development. The ΔFgHXK1 mutants (2hxk1‐14 and 1hxk1‐21) were viable, but exhibited various defects, including growth, pathogenicity, conidial and perithecia production. In contrast, deletion of FgHXK2 had no effect on fungal development (Figs 4, 5 and 7). Taken together, it can be concluded that FgHXK2 is dispensable for growth in F. graminearum, whereas FgHXK1 is absolutely required for normal growth, sporulation and infection in F. graminearum.
A previous study has shown that the F. verticillioides Δhxk1 mutant displays increased sensitivity to NaCl stress, but the growth rates of the mutant and wild‐type on Congo red are similar (Kim et al., 2011). In the current study, however, we found that the ΔFgHXK1 mutants displayed identical growth on exposure to ionic osmotic stress, but decreased tolerance to SDS and Congo red in F. graminearum (Fig. 6A). This might be related to cell wall integrity in conidial germination (Fig. 5C), which indicated that the role of F. graminearum hexokinase in stress defence had unique characteristics.
Ma and Botstein (1986) constructed null mutations of both hexokinase genes and found that hxk1 and hxk2 single null mutants could ferment fructose, but hxk1hxk2 double mutants could not; the hxk2 single mutant, as well as the double mutant, failed to show catabolite repression, whereas the hxk1 null mutation had little or no effect. A deletion mutant of HXK1 in F. verticillioides was unable to grow when supplied with fructose as the sole carbon source, and growth was repaired when glucose, sucrose or maltotriose was provided (Kim et al., 2011). However, in our study, growth of ΔFgHXK1 mutants on MM with mannitol or fructose as carbon source was reduced, but not completely inhibited (Fig. 6B). The lack of agreement with former research indicated that carbon utilization by hexokinase was species specific. In contrast, ΔFgHXK2 mutants showed great adaptation to all kinds of sugar supplied as carbon source (Fig. 6B).
Most importantly, ΔFgHXK1 mutants produced a dramatically low level of DON (Fig. 3), which has been identified as an important virulence factor and plays a significant role in the spread of F. graminearum within spikes (Desjardins et al., 1996; Proctor et al., 1995). The low level of DON production in ΔFgHXK1 mutants was also consistent with the observation of scab symptoms in spikelets and corn stigma (Fig. 7B). These results demonstrated that FgHXK1 was involved in DON biosynthesis in F. graminearum. In contrast, ΔFgHXK2 mutants showed no difference in TRI gene expression and DON production relative to parental strains.
As strain 167, characterized by an up‐regulation of the FgHXK1 level, displayed high DON production, and disruption of FgHXK1 resulted in a reverse phenotype, we constructed a FgHXK1 overexpression fragment to verify its effect on DON production and MBC sensitivity. The target transformants (2Ohxk1‐11 and 2Ohxk1‐12) presented defects in biological characteristics, such as growth rate and conidiation (Fig. S3), but less so than in the ΔFgHXK1 mutants. Remarkably, 2Ohxk1‐11 and 2Ohxk1‐12 showed greatly improved DON production (Fig. 8), indicating that the activity of hexokinase controlled DON biosynthesis. As a predominant isozyme involved in glucose metabolism and ATP generation, overexpression of hexokinase (Hexokinase 1 and Hexokinase 2) often occurs in humans and mammals (Ye et al., 2005). To our knowledge, this was the first time that hexokinase had been overexpressed in plant‐pathogenic fungi to explore its potential function in the promotion of secondary metabolism. However, the sensitivity of 2Ohxk1‐11 and 2Ohxk1‐12 to MBC showed no difference from that of parental strain 2021 (Table 1), indicating that the hexokinase activity change of the pathogen contributed little to MBC resistance. The F. graminearum MBC‐resistant gene, β2‐tub, is a component of microtubules, which are essential in cell division and intracellular transport; however, hexokinases are multifunctional enzymes, with roles in catabolism (e.g. required for the first step of glycolysis) and signalling (e.g. glucose sensors) (Harrington and Bush, 2003; Santangelo, 2006). The weak connection in function between β2‐tub and hexokinases might explain why there were no differences in MBC resistance between the wild‐type and transgenic strains (including deletion and overexpression mutants). It seemed more likely that MBC resistance perturbed cellular homeostasis in some other way, and increased expression of FgHXK1 was simply an indirect byproduct. According to these results, the transcriptional level of FgHXK1 regulated DON production through an effect on the substrate (pyruvate) yield; MBC resistance also induced DON production, but showed no direct relationship with the expression level of FgHXK1.
In summary, we have investigated the relationship between mutation of β2‐tub at codon 167 and hexokinase activity; one of the encoding genes, FgHXK1, played a considerable role in DON production and fungal development.
Experimental Procedures
Strains and culture conditions
We used the F. graminearum MBC‐sensitive strain 2021 [formerly called ZF21 (Zhang et al., 2009) and subsequently unified as ‘2021'] and MBC‐resistant strain 167 as our parental strains. Strain 167 was obtained from strain 2021 through point mutation at codon 167 (Phe → Tyr) of the β 2‐tub gene by two steps of homologous double‐crossover gene manipulation (Qiu et al., 2011). The only difference in genetic background between 2021 and 167 was the amino acid sequence in codon 167 of β2‐tub. All the strains used in this study and their genotypes are listed in Table 1.
PDA (200 g/L potato, 20 g/L glucose and 20 g/L agar) was used for colony morphology examination and sensitivity test to MBC in vitro. Mung bean broth (MBB, 30 g/L mung bean) was prepared for conidiation assays. YEPD was used for conidial germination. GYEP was employed for DON production. MM was used for carbon utilization tests.
DNA and RNA extraction
Genomic DNA was isolated from mycelia using the optimized cetyltrimethylammonium bromide (CTAB) procedure (Möller et al., 1992). Total RNA was extracted from mycelia using the TaKaRa RNAiso Reagent (TaKaRa, Dalian, China), and for reverse transcription using a PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa). The integrity of the DNA and RNA was validated by agarose gel electrophoresis and absorbance determination.
Sequence analysis of hexokinase genes in F. graminearum
FgHXK1 (FGSG_00500) and FgHXK2 (FGSG_01347) were originally identified through homology searches of the F. graminearum genome sequence at the Munich Information Centre of Protein Sequences (MIPS) and the Fusarium comparative database from the Broad Institute (http://www.broadinstitute.org) using blast with HXK1 and HXK2 from S. cerevisiae (Desjardins et al., 1996; Lobo and Maitra, 1977; Rodriguez et al., 2001) as query. The full‐length genomic DNA and cDNA of FgHXK1 and FgHXK2 were amplified and sequenced to verify the existence and size of the introns. Phylogenetic analysis was performed by the neighbour‐joining method using Mega 4.0.2 software (Kumar et al., 2008) according to amino acid sequences.
Pyruvate production and hexokinase gene expression analysis
Pyruvate was determined using a Pyruvate Assay Kit (Jiancheng, Nanjing, China) according to the ultraviolet spectrophotometry procedure. Conidia of all strains were harvested and cultured in liquid YEPD medium for 3 days. Fresh mycelia were collected and ground with a mortar and pestle using quartz sand and liquid nitrogen. The powder was suspended in extraction buffer and subsequently used for pyruvate detection according to the manufacturer's instructions.
Expression levels of FgHXK1 and FgHXK2 related to pyruvate biosynthesis were determined by qRT‐PCR assays, which were conducted in an ABI PRISM 7500 DNA Engine (Applied Biosystems, Foster City, CA, USA) using SYBR Premix Ex Taq™ (Tli RNaseH Plus) (TaKaRa). PCR amplification of the glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) gene was performed as a reference. Gene expression levels were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001). There were four replicates for each sample and the experiment was repeated three times with independent RNA isolations.
Generation of FgHXK1 and FgHXK2 deletion mutants
To explore the biological role of FgHXK1 and FgHXK2 in F. graminearum, we generated gene disruption mutants of the parental strains via homologous recombination by replacing them with the hygromycin resistance gene (hph) (Fig. 2A,B). Among the hygromycin‐resistant transformants, several ΔFgHXK1 and ΔFgHXK2 mutants were identified by PCR analyses with the coding region primer pairs Fghxk1‐F/Fghxk1‐R and Fghxk2‐F/Fghxk2‐R [Table S1 (see Supporting Information), Fig. 2A,B] to confirm that the genes had been knocked out. Primer pairs Fghxk1‐FF/HyR and HyF/Fghxk1‐RR for ΔFgHXK1, and Fghxk2‐FF/HyR and HyF/Fghxk2‐RR for ΔFgHXK2 (Table S1, Fig. 2A,B), were used to amplify the two homologous arms, respectively, in order to confirm that the target genes had been homologously replaced by the hph gene.
For further verification through Southern blotting analysis, ΔFgHXK1 mutants were digested with BamHI and hybridized with probe 1 (554 bp), which corresponded to part of the FgHXK1 coding region, and with probe 2 (485 bp), which corresponded to part of the hph coding region, whereas ΔFgHXK2 mutants were digested with BamHI and hybridized with probe 3 (405 bp), which corresponded to part of the coding region, and with probe 2 (Fig. 2A,B). Southern blotting was performed using the Dig High Primer DNA Labelling and Detection Starter Kit I (Roche Diagnostics, Mannhein, Germany), according to the manufacturer's instructions.
The ΔFgHXK1 and ΔFgHXK2 mutants were complemented with full‐length FgHXK1 and FgHXK2 genes to confirm that the phenotype changes in disruption mutants were caused by the disruption of the genes. The complement fragments were constructed by fusing the neocassette and full‐length FgHXK genes (Fig. 2A,B). Transformation was conducted as described above, except that geneticin was used as a selection agent.
Generation of FgHXK1 overexpression mutants
In addition, an FgHXK1 overexpression fragment and hph gene were co‐transformed into the parental strain 2021 to confirm the relationship between high hexokinase expression and high DON production. Transformation was conducted as described above. The fragment was constructed by fusing the TrpC promoter and full‐length FgHXK1 gene (Fig. 2A). Transformation was verified by qRT‐PCR and hygromycin was used as a selection agent.
Analysis of DON production and expression levels of TRI genes
The amount of DON produced in liquid GYEP for 7 days, which included the sum of DON and 3ADON (3‐Acetyldeoxynivalenol), or of DON and 15ADON (15‐Acetyldeoxynivalenol), was measured by gas chromatography as described previously (Zhang et al., 2009). DON production ability in shake culture was expressed as the amount of DON produced per dry weight of mycelia (μg/g of dry mycelia). The experiment was repeated three times independently.
To determine the expression levels of TRI genes (Tri4, Tri5, Tri6, Tri10), conidia of each strain were inoculated into GYEP and cultured for 3 days at 25 °C in the dark. Total RNA was extracted, and the expression levels of TRI genes were determined by qRT‐PCR assays with the primers listed in Table S1. Four replicates were used for each strain, and the experiment was repeated three times independently.
Assays for mycelial growth
Colony growth rate was assayed with PDA according to colony diameter after inoculation at 25 °C for 3 days. To determine the change in dry hyphal weight, 10 mycelial plugs of each strain were cut and transferred to a conical flask containing 150 mL of PDA, and the flask was placed on a shaker for 3 days (175 rpm, 25 °C). Fungal biomass production was measured by weighing mycelia after freeze–drying at −40 °C for 48 h. Three replicates were used for each strain, and the experiment was repeated three times.
Asexual development and conidial morphology assays
After the strains had been cultured on PDA at 25 °C for 3 days, 10 mycelial plugs of each strain were cut and transferred to a conical flask containing 150 mL of MBB, and the flask was placed on a shaker for 7 days (175 rpm, 25 °C). The number of spores produced per flask was determined with a haemocytometer. Three replicates were used for each strain, and the experiment was repeated three times.
Conidial morphology was observed subsequently. To assay conidial germination and germ tube growth, freshly harvested conidia were cultured in YEPD for 6 h. The germ tubes were then examined with an Olympus IX71 microscope equipped with an Olympus DP72 lens (Olympus, Tokyo, Japan).
MBC sensitivity, osmotic stress and carbon utilization test
MBC was dissolved in 0.1 m hydrochloric acid (HCl) at 10 mg/mL as a stock solution. A 5‐mm mycelial plug of each strain was added to the centre of a Petri dish and grown in the dark at 25 °C. The radial growth from the edge of the plug to the edge of the colony of each strain was measured once larger than 60 mm. For each plate, the average radial growth, measured in two perpendicular directions, was used for the calculation of EC50. There were three replicate plates for each treatment and each experiment was repeated three times independently.
Osmotic stress tests under different conditions were performed on PDA supplemented with the following products: 1.2 m NaCl, 1.2 m KCl, 5 mm caffeine, 0.05 mg/mL SDS and 0.3 mg/mL Congo red. Each plate was inoculated with a plug as before. After incubation at 25 °C for 3 days, the colony diameter was measured and the percentage of mycelial radial growth inhibition (RGI) was calculated using the formula RGI = [(C − N)/(C − 5)] × 100, where C is the colony diameter of the control and N is the colony diameter of a treatment. Three replicates were used for each strain, and the experiment was repeated three times.
Carbon utilization tests under different conditions were performed on MM supplemented with the following reagents as carbon source: glucose, sucrose, fructose, mannitol, glycerol and lactose at concentrations of 2%. The strain inoculation and RGI measurements were carried out as described above.
Perithecium production analysis
Moist, autoclaved wheat seeds (Huaimai NO.4) in sterile 50‐mL flasks were inoculated with 10 mycelial plugs of each strain and incubated at 25 °C for 5–7 days; the seeds were then placed in Petri dishes covered with sterile wet sand, and incubated in a humid room (relative humidity, 60%) at 25 °C and in a 12 h : 12 h light : dark photoperiod for 15 days. Perithecium production ability was evaluated according to the area covered with perithecia. Three replicates were used for each strain, and the experiment was repeated twice.
Pathogenicity assay
Freshly harvested conidia were resuspended in sterilized water to a concentration of 1 × 106 conidia/mL. The two outer florets of the centre spikelet were injected with 10 μL of conidial suspension. Control heads were injected with distilled water. For each strain, more than 60 replicate heads were injected. After inoculation, wheat heads were capped with a plastic bag for 48 h to maintain moisture. Disease severity was calculated as the percentage of blighted spikelets in each head 2 weeks after inoculation.
Moreover, 30 strips of 10‐cm corn stigmas were placed on a filter paper, and inoculated with a brick‐shaped mycelial plug on each corn stigma cluster. We surveyed the pathogenic performance after 3 days of cultivation in a humid room (relative humidity, 60%) at 25 °C and in a 12 h : 12 h light : dark photoperiod.
Supporting information
Fig. S1 Alignment of the protein sequences of FgHXK1 (A) and FgHXK2 (B) in Fusarium graminearum with HXK1 and HXK2 of Saccharomyces cerevisiae by ClustalX. Identical amino acids are highlighted in grey.
Fig. S2 Phylogenetic trees generated by the neighbour‐joining method with Mega 4.0.2 software. (A) A phylogenetic analysis of the putative hexokinase proteins in Fusarium graminearum by neighbour‐joining methods revealed four distinct groups: FGSG_00500 and FGSG_03014 (hexokinase group), FGSG_08744 and FGSG_16878 (glucokinase group), FGSG_08399 (another catalytic hexokinase protein group) and FGSG_17276 (another catalytic hexokinase protein group). There are four hexokinase isozymes which vary in subcellular location and kinetics in different substrates and conditions, and physiological function, resulting from the alternative splicing of the same genes. All of these genes consist of two characteristic hexokinase domains: Hexokinase 1 (Pfam accession no. PF00349) and Hexokinase 2 (Pfam accession no. PF03727). (B) Phylogenetic tree generated on the basis of the deduced amino acid sequences of hexokinase genes from F. graminearum (HXK1, XP_380676.1; HXK2, XP_383190.1) and from the following fungal species: F. verticillioides (HXK1, ABY89285.1; HXK2, EWG51741.1), F. oxysporum (HXK1, EXK48243.1; HXK2, EWZ83160.1), F. fujikuroi (HXK1, CCT62433.1; HXK2, CCT75899.1), Trichoderma reesei (HXK, XP_006961576.1), Verticillium dahliae (HXK, EGY22649.1), Botryotinia fuckeliana (HXK, EMR90813.1), Colletotrichum gloeosporioides (HXK, XP_007275677.1), Magnaporthe oryzae (HXK, ADD84641.1), Aspergillus fumigatus (HXK, XP_749720.1), Neurospora crassa (HXK, XP_965673.2) and Saccharomyces cerevisiae (ScHXK1, YFR053C; ScHXK2, YGL253W).
Fig. S3 Impacts of overexpression of FgHXK1 on growth rate and conidial production. (A) The radial growth of 2Ohxk1‐11 and 2Ohxk1‐12 was retarded by approximately 16%–19%. The colony morphology of the mutant was similar to that of parental strain 2021. (B) (2.3 ± 0.26) × 106 and (2.5 ± 0.17) × 106 conidia were produced by 2Ohxk1‐11 and 2Ohxk1‐12, in comparison with (3.3 ± 0.1) × 106 conidia of the parental strain 2021. Conidial morphology showed no difference from that of 2021. Line bars in each column denote standard errors of three experiments. Bar, 10 μm.
Table S1 Primers used in this study.
Acknowledgements
This work was supported by the National Key Basic Research Programme (973 Programme, Grant No: 2012CB114000); National Science and Technology Support Programme (Grant No: 2012BAD19B01) and the Special Fund for Agro‐scientific Research in the Public Interest (Grant No: 201303023).
The authors have no conflicts of interest to declare.
References
- Alexander, N.J. , Proctor, R.H. and McCormick, S.P. (2009) Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium . Toxin Rev. 28, 198–215. [Google Scholar]
- Audenaert, K. , Callewaert, E. , Höfte, M. , De Saeger, S. and Haesaert, G. (2010) Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum . BMC Microbiol. 10, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, J.W. and Klich, M. (2003) Mycotoxins. Clin. Microbiol. Rev. 16, 497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D.W. , Dyer, R.B. , McCormick, S.P. , Kendra, D.F. and Plattner, R.D. (2004) Functional demarcation of the Fusarium core trichothecene gene cluster. Fungal Genet. Biol. 41, 454–462. [DOI] [PubMed] [Google Scholar]
- Calvo, A.M. (2008) The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 45, 1053–1061. [DOI] [PubMed] [Google Scholar]
- Chen, C.J. , Wang, J.X. , Luo, Q.Q. , Yuan, S.K. and Zhou, M.G. (2007) Characterization and fitness of carbendazim‐resistant strains of Fusarium graminearum (wheat scab). Pest Manag. Sci. 63, 1201–1207. [DOI] [PubMed] [Google Scholar]
- Cleveland, T.E. , Dowd, P.F. , Desjardins, A.E. , Bhatnagar, D. and Cotty, P.J. (2003) United States Department of Agriculture‐Agricultural Research Service research on pre‐harvest prevention of mycotoxins and mycotoxigenic fungi in US crops. Pest Manag. Sci. 59, 629–642. [DOI] [PubMed] [Google Scholar]
- Cody, G.D. , Boctor, N.Z. , Filley, T.R. , Hazen, R.M. , Scott, J.H. , Sharma, A. and Yoder, J.H.S. (2000) Primordial carbonylated iron–sulfur compounds and the synthesis of pyruvate. Science, 289, 1337–1340. [DOI] [PubMed] [Google Scholar]
- Desjardins, A.E. , Proctor, R.H. , Bai, G. , McCormick, S.P. , Shaner, G. , Buechley, G. , Hohn T.M. (1996) Reduced virulence of trichothecene‐nonproducing mutants of Gibberella zeae in wheat field tests. Mol. Plant–Microbe Interact. 9, 775–781. [Google Scholar]
- Fleck, C.B. and Brock, M. (2010) Aspergillus fumigatus catalytic glucokinase and hexokinase: expression analysis and importance for germination, growth, and conidiation. Eukaryot. Cell, 9, 1120–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami, R.S. and Kistler, H.C. (2004) Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 5, 515–525. [DOI] [PubMed] [Google Scholar]
- Harrington, G.N. and Bush, D.R. (2003) The bifunctional role of hexokinase in metabolism and glucose signaling. Plant Cell, 15, 2493–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Yun, Y. , Yang, Q. , Shim, W.B. , Wang, Z. and Ma, Z. (2011) A type 2C protein phosphatase FgPtc3 is involved in cell wall integrity, lipid metabolism, and virulence in Fusarium graminearum . Plos ONE, 6, e25311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. , Smith, J.E. , Ridenour, J.B. , Woloshuk, C.P. and Bluhm, B.H. (2011) HXK1 regulates carbon catabolism, sporulation, fumonisin B1 production and pathogenesis in Fusarium verticillioides . Microbiology, 157, 2658–2669. [DOI] [PubMed] [Google Scholar]
- Kimura, M. , Tokai, T. , Takahashi‐Ando, N. , Ohsato, S. and Fujimura, M. (2007) Molecular and genetic studies of Fusarium trichothecene biosynthesis: pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 71, 2105–2123. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Nei, M. , Dudley, J. and Tamura, K. (2008) MEGA: a biologist‐centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lobo, Z. and Maitra, P.K. (1977) Physiological role of glucose‐phosphorylating enzymes in Saccharomyces cerevisiae . Arch. Biochem. Biophys. 182, 639–645. [DOI] [PubMed] [Google Scholar]
- Ma, H. and Botstein, D. (1986) Effects of null mutations in the hexokinase genes of Saccharomyces cerevisiae on catabolite repression. Mol. Cell. Biol. 6, 4046–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, F.J. , Miedaner, T. , Hadeler, B. , Felk, A. , Salomon, S. , Lemmens, M. , Kassner, H. and Schäfer, W. (2006) Involvement of trichothecenes in fusarioses of wheat, barley and maize evaluated by gene disruption of the trichodiene synthase (Tri5) gene in three field isolates of different chemotype and virulence. Mol. Plant Pathol. 7, 449–461. [DOI] [PubMed] [Google Scholar]
- Möller, E. , Bahnweg, G. , Sandermann, H. and Geiger, H. (1992) A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 20, 6115–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, B. , Zhou, L. , Rolland, F. , Hall, Q. , Cheng, W.H. , Liu, Y.X. , Hwang, I. , Jones, T. and Sheen, J. (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science, 300, 332–336. [DOI] [PubMed] [Google Scholar]
- Parry, D. , Jenkinson, P. and McLeod, L. (1995) Fusarium ear blight (scab) in small grain cereals—a review. Plant Pathol. 44, 207–238. [Google Scholar]
- Placinta, C. , D'mello, J. and Macdonald, A. (1999) A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 78, 21–37. [Google Scholar]
- Proctor, R.H. , Hohn, T.M. , McCormick, S.P. and Desjardins, A.E. (1995) Tri6 encodes an unusual zinc finger protein involved in regulation of trichothecene biosynthesis in Fusarium sporotrichioides . Appl. Environ. Microbiol. 61, 1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, J.B. , Xu, J.Q. , Yu, J.J. , Bi, C.W. , Chen, C.J. and Zhou, M.G. (2011) Localisation of the benzimidazole fungicide binding site of Gibberella zeae β 2‐tubulin studied by site‐directed mutagenesis. Pest Manag. Sci. 67, 191–198. [DOI] [PubMed] [Google Scholar]
- Rocha, O. , Ansari, K. and Doohan, F. (2005) Effects of trichothecene mycotoxins on eukaryotic cells: a review. Food Addit. Contam. 22, 369–378. [DOI] [PubMed] [Google Scholar]
- Rodriguez, A. , De La Cera, T. , Herrero, P. and Moreno, F. (2001) The hexokinase 2 protein regulates the expression of the GLK1, HXK1 and HXK2 genes of Saccharomyces cerevisiae . Biochem. J. 355, 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui, O. and Hahn, M. (2007) The Botrytis cinerea hexokinase, Hxk1, but not the glucokinase, Glk1, is required for normal growth and sugar metabolism, and for pathogenicity on fruits. Microbiology, 153, 2791–2802. [DOI] [PubMed] [Google Scholar]
- Santangelo, G.M. (2006) Glucose signaling in Saccharomyces cerevisiae . Microbiol. Mol. Biol. Rev. 70, 253–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey, D.E. , Ward, T.J. , Aoki, T. , Gale, L.R. , Kistler, H.C. , Geiser, D.M. , Suga, H. , Tóth, B. , Varga, J. and O'Donnell, K. (2007) Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genet. Biol. 44, 1191–1204. [DOI] [PubMed] [Google Scholar]
- Trail, F. , Xu, H. , Loranger, R. and Gadoury, D. (2002) Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia, 94, 181–189. [PubMed] [Google Scholar]
- Windels, C.E. (2000) Economic and social impacts of Fusarium head blight: changing farms and rural communities in the Northern Great Plains. Phytopathology, 90, 17–21. [DOI] [PubMed] [Google Scholar]
- Ye, G. , Donthi, R.V. , Metreveli, N.S. and Epstein, P.N. (2005) Overexpression of hexokinase protects hypoxic and diabetic cardiomyocytes by increasing ATP generation. Cardiovasc. Toxicol. 5, 293–300. [DOI] [PubMed] [Google Scholar]
- Zhang, L.G. , Jia, X.J. , Chen, C.J. and Zhou, M.G. (2013) Characterization of carbendazim sensitivity and trichothecene chemotypes of Fusarium graminearum in Jiangsu Province of China. Physiol. Mol. Plant Pathol. 84, 53–60. [Google Scholar]
- Zhang, Y.J. , Yu, J.J. , Zhang, Y.N. , Zhang, X. , Cheng, C.J. , Wang, J.X. , Hollomon, D.W. , Fan, P.S. and Zhou, M.G. (2009) Effect of carbendazim resistance on trichothecene production and aggressiveness of Fusarium graminearum . Mol. Plant–Microbe Interact. 22, 1143–1150. [DOI] [PubMed] [Google Scholar]
- Zhou, M.G. and Wang, J.X. (2001) Study on sensitivity base‐line of Fusarium graminearum to carbendazim and biological characters of MBC‐resistant strains. Acta Phytopathol. Sin. 31, 365–370. [Google Scholar]
- Zhou, M.G. , Ye, Z.Y. and Liu, J.F. (1994) Progress of fungicide resistance. J. Nanjing Agric. Univ. 17, 33–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Alignment of the protein sequences of FgHXK1 (A) and FgHXK2 (B) in Fusarium graminearum with HXK1 and HXK2 of Saccharomyces cerevisiae by ClustalX. Identical amino acids are highlighted in grey.
Fig. S2 Phylogenetic trees generated by the neighbour‐joining method with Mega 4.0.2 software. (A) A phylogenetic analysis of the putative hexokinase proteins in Fusarium graminearum by neighbour‐joining methods revealed four distinct groups: FGSG_00500 and FGSG_03014 (hexokinase group), FGSG_08744 and FGSG_16878 (glucokinase group), FGSG_08399 (another catalytic hexokinase protein group) and FGSG_17276 (another catalytic hexokinase protein group). There are four hexokinase isozymes which vary in subcellular location and kinetics in different substrates and conditions, and physiological function, resulting from the alternative splicing of the same genes. All of these genes consist of two characteristic hexokinase domains: Hexokinase 1 (Pfam accession no. PF00349) and Hexokinase 2 (Pfam accession no. PF03727). (B) Phylogenetic tree generated on the basis of the deduced amino acid sequences of hexokinase genes from F. graminearum (HXK1, XP_380676.1; HXK2, XP_383190.1) and from the following fungal species: F. verticillioides (HXK1, ABY89285.1; HXK2, EWG51741.1), F. oxysporum (HXK1, EXK48243.1; HXK2, EWZ83160.1), F. fujikuroi (HXK1, CCT62433.1; HXK2, CCT75899.1), Trichoderma reesei (HXK, XP_006961576.1), Verticillium dahliae (HXK, EGY22649.1), Botryotinia fuckeliana (HXK, EMR90813.1), Colletotrichum gloeosporioides (HXK, XP_007275677.1), Magnaporthe oryzae (HXK, ADD84641.1), Aspergillus fumigatus (HXK, XP_749720.1), Neurospora crassa (HXK, XP_965673.2) and Saccharomyces cerevisiae (ScHXK1, YFR053C; ScHXK2, YGL253W).
Fig. S3 Impacts of overexpression of FgHXK1 on growth rate and conidial production. (A) The radial growth of 2Ohxk1‐11 and 2Ohxk1‐12 was retarded by approximately 16%–19%. The colony morphology of the mutant was similar to that of parental strain 2021. (B) (2.3 ± 0.26) × 106 and (2.5 ± 0.17) × 106 conidia were produced by 2Ohxk1‐11 and 2Ohxk1‐12, in comparison with (3.3 ± 0.1) × 106 conidia of the parental strain 2021. Conidial morphology showed no difference from that of 2021. Line bars in each column denote standard errors of three experiments. Bar, 10 μm.
Table S1 Primers used in this study.


