Summary
The general transcriptional repressor Tup1 proteins play important regulatory roles in the growth and development of fungi. In this report, we characterized MoTup1, a protein homologous to Tup1 of Saccharomyces cerevisiae, from M. oryzae. Disruption of MoTUP1 resulted in severe mycelial growth reduction and a defect in conidiogenesis. We found that MoTup1 is required for the maintenance of cell wall integrity by regulating the expression of the genes involved in cell wall biosynthesis. Pathogenicity assays indicated that the ΔMotup1 mutants lost the ability to invade both rice and barley hosts. Moreover, observation of rice epidermis penetration showed that the hyphal tips of the mutants could still form appressorium‐like structures, but were unable to invade host cells. Taken together, our results demonstrate that M. oryzae MoTup1 is an important regulatory factor in fungal growth, development and pathogenesis on hosts.
Keywords: conidiogenesis, growth, MoTup1, pathogenicity
Introduction
Rice blast, caused by Magnaporthe oryzae, is a destructive disease during rice cultivation that severely threatens the production of rice crops worldwide (Ou, 1980; Talbot, 2003). Control measures, such as the use of disease‐resistant rice cultivars and the application of fungicides, have not completely reduced disease incidence, as newly developed disease‐resistant rice cultivars are prone to lose resistance following the emergence of a new pathogenic trait and/or increased resistance against fungicides (Zeigler et al., 1994). The control of rice blast remains a serious challenge, and there is an urgency to identify new anti‐fungal mechanisms. Thus, the characterization of fungal development and pathogenicity‐related molecular mechanisms is critical for disease management (Wilson and Talbot, 2009).
Like most other fungal pathogens, the conidia of M. oryzae play a central role in the disease cycle by being responsible for disease dissemination. Recently, many genes involved in conidial morphology and development in M. oryzae have been identified. MoHOX4 and MoHOX6, which encode homeobox transcription factors, are important for conidial morphogenesis, and MoHOX2 is essential for asexual reproduction (Kim et al., 2009; Liu et al., 2010). The disruption of MoPDEL and MoPDEH, which encode two phosphodiesterases that regulate intracellular cyclic adenosine monophosphate (cAMP) levels, results in elongated and thinner conidia (Zhang et al., 2011). Core components of the TIG1 complex in M. oryzae, which contains MoTig1, MoSet3, MoSnt1 and MoHos2, are also required for conidiogenesis, as the conidia produced by these gene deletion mutants are abnormal in shape and size (Ding et al., 2010). In addition, recent studies have demonstrated that two soluble N‐ethylmaleimide‐sensitive factor (NSF) attachment protein receptor (SNARE) proteins, MoSec22 and MoVam7, are involved in conidiation. MoSEC22 disruption results in decreased conidiophore development and a lack of conidial production, whereas deletion of MoVAM7 blocks conidial formation (Dou et al., 2011; Song et al., 2010). Moreover, a putative transcriptional activator MoMsn2, which interacts with the mitogen‐activated protein kinase MoOsm1, is required for conidial production and the infection of host plants (Zhang et al., 2014). It is thought that MoMsn2 functions by binding to the promoter region of the gene encoding the zinc‐finger transcription factor MoCos1 to govern conidiophore formation (Zhou et al., 2009).
In eukaryotic cells, the Tup1–Cyc8 complex is an important transcriptional co‐repressor that participates in several important processes through interactions with transcriptional activators or repressors to regulate gene expression, such as those involved in glucose and oxygen sensing, DNA damage and cell fate (Hanlon et al., 2011; Huang et al., 1998; Keleher et al., 1992). Tup1–Cyc8 can shield the activation domain of a repressor–activator DNA‐binding protein, thereby preventing the association with co‐activators (Wong and Struhl, 2011). Tup1 and Cyc8 homologues have been identified in baker's yeast Saccharomyces cerevisiae and the nematode Caenorhabditis elegans. Co‐repressor proteins with similar domain structures and functions have also been found in flies and vertebrates, including the fruit fly Drosophila (Groucho or Gro protein) and mammals (transducin β‐like/transducin β‐like‐related (TBL/TBLR) and transducin‐like enhancer of split (TLE) proteins; Malave and Dent, 2006). These findings suggest that the Tup1–Cyc8 complex is capable of exhibiting conserved and important regulatory functions.
As a transcriptional repressor, Tup1 proteins control morphogenesis, conidiogenesis and pathogenicity in fungi. In Ustilago maydis, deletion of TUP1 decreases spore and plant tumour formation, and UmTup1 is required for full pathogenic development in maize (Elias‐Villalobos et al., 2011). In Candida albicans, hyphal differentiation requires the transcriptional control of hyphal‐specific genes through Tup1, which acts through a third parallel pathway involving the transcriptional regulators Rfg1 and Nrg1 (Braun et al., 2001; Kadosh and Johnson, 2001). Disruption of TUPA, a Tup1 homologue, results in an opposite phenotype that reduces filamentation and promotes yeast phase growth in Penicillium marneffei (Todd et al., 2003). Deletion of TUP1 is negative for glucose derepression, promoting maltose metabolism in S. cerevisiae (Lin et al., 2014). Neurospore crassa Rco1, the homologue of Tup1, is required for hyphal fusion, normal hyphal morphology, and asexual and sexual development (Vandeputte et al., 2012). In Aspergillus niger, TupA is also involved in growth, spore production and nitrogen metabolism, and TupA also controls the expression of a series of genes associated with cell wall biosynthesis and development (Schachtschabel et al., 2013).
In this report, we demonstrate that MoTup1, a Tup1 homologue of M. oryzae, is an important regulatory factor for growth, conidiogenesis, appressorium formation and infection of host plants.
Results
Identification of MoTUP1
Using the S. cerevisiae Tup1 sequence as the trace to blast available genomes (http://www.broadinstitute.org/annotation/genome/magnaporthe_comparative/MultiHome.html) (Dean et al., 2005), we identified the locus MGG_08829 which encodes a Tup1 homologue. MGG_08829 is predicted to encode a 607‐amino‐acid protein that shares 48% identity and 63% similarity with S. cerevisiae Tup1. We named the protein MoTup1. Sequence analysis revealed that MoTup1 contains a Tup_N domain at the N‐terminus and seven repeated WD40 domains towards the C‐terminus (Fig. 1A). The phylogenetic relationship of MoTup1 to other Tup1 proteins revealed that Tup1 proteins in filamentous fungi are separated from those of unicellular yeasts, with M. oryzae MoTup1 being most similar to that of Gaeumannomyces graminis and most distant from that of U. maydis and other yeasts (with identities still close to or above 48%) (Fig. 1B). This finding indicates that Tup1 proteins are highly conserved in fungi.
Figure 1.
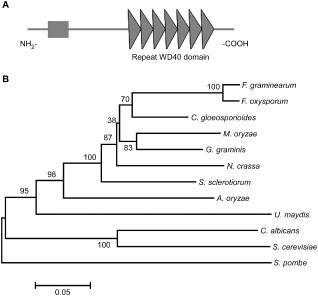
Functional domain identification and phylogenetic tree. A. Prediction of domains in MoTup1 using the SMART website. B. Sequence alignments were performed using the Clustal_W program and the calculated phylogenetic tree was viewed using Mega4.1 Beta program. Neighbor‐joining tree with 500 bootstrap replicates of phylogenetic relationships between Tup1 homologues in fungi. All of the Tup1 proteins were downloaded from the NCBI database and their accession numbers are as following: M. oryzae (Magnaporthe oryzae XP_003717030.1), F. graminearum (Fusarium graminearum XP_382677.1), F. oxysporum (Fusarium oxysporum EGU83576.1), C. gloeosporioides (Colletotrichum gloeosporioides XP_007281086.1), G. graminis (Gaeumannomyces graminis EJT80144.1), N. crassa (Neurospora crassa XP_956212.1), S. sclerotiorum (Sclerotinia sclerotiorum XP_001596040.1), A. oryzae (Aspergillus oryzae XP_001727812.1), U. maydis (Ustilago maydis XP_759427.1), C. albicans (Candida albicans XP_713297.1), S. cerevisiae (Saccharomyces cerevisiae AAA35182.1) and S. pombe (Schizosaccharomyces pombe AAB81475.2).
MoTUP1 expression, mutant generation and complementation
Before exploring the functions of MoTUP1, we evaluated its transcriptional profile. The expression of MoTUP1 was highest during the conidial stage (more than three‐fold; Fig. 2A), and low expression was found during the stage of infection and at 8–72 h post‐inoculation (Fig. 2A). However, the expression of MoTUP1 increased during late infection until 168 h post‐inoculation, when it was comparable with that in the mycelial stage (Fig. 2A). These observations suggest that MoTUP1 may play a major role during conidiogenesis.
Figure 2.
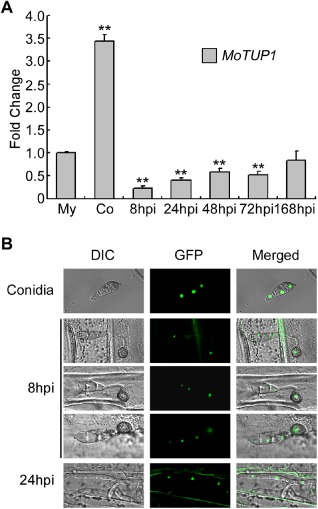
Phase specific expression and subcellular localization of MoTUP1. A. The phase specific expression of MoTUP1 was quantified by quantitative real‐time PCR with synthesis of cDNA from each sample including infectious growth, vegetative growth and conidia. Relative abundance of MoTUP1 transcripts during infectious growth (from ungerminated conidia to in planta fungal cells 168 hpi) was normalized by comparing with vegetative growth in liquid CM (Relative transcript level = 1). Three independent biological experiments with three replicates in each were performed. The double asterisks represent significant difference between other different stages and mycelia stage (p < 0.01). My: Mycelia; Co: Conidia; hpi: hour post inoculation. B. MoTup1 is localized to the nucleus of the conidia in the complemented strain. The subcellular localization of MoTup1 during appressorium and invasive growth was performed by placing conidial suspensions on barley epidermis, and photographs were taken at 8 h and 24 h after incubation, respectively.
MoTUP1 deletion mutants were obtained by replacing the MoTUP1 coding region with the ΔMotup1 mutant allele linked to the hygromycin resistance cassette (HPH) (Fig. S1A, see Supporting Information). Three gene deletion mutants; ΔMotup1#1, ΔMotup1#10 and ΔMotup1#16, were confirmed by Southern blot analysis (Fig. S1B). ΔMotup1#1 and ΔMotup1#16 were selected for further analysis. We first examined the in vivo localization of the MoTUP1‐GFP fusion protein following its introduction into ΔMotup1#16. The resulting transformants exhibited normal growth, conidiation and infection (Figs 3, 4, 5, 6, 7, 8, 9), sufficient for the strain to be considered as a complemented strain. Under an epifluorescence microscope, the green fluorescent protein (GFP) signal was visible in the nuclei of conidia (Fig. 2B). To observe the GFP signal during the germination and infection stages, conidial suspensions were dropped onto barley leaves and bright green fluorescence was observed in the nucleus of appressoria and invasive hyphae (Fig. 2B).
Figure 3.
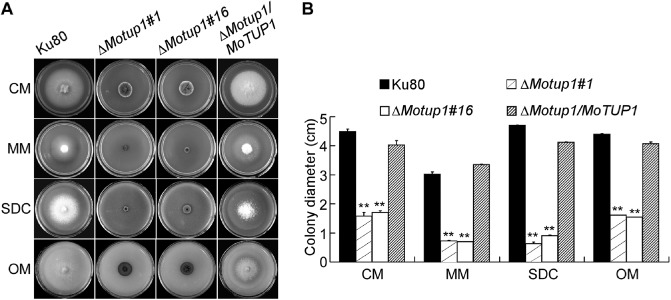
The deletion of MoTUP1 reduced the mycelia growth rate of M. oryzae. A. Growth of Ku80, ΔMotup1 mutants and the complemented strain on CM, MM, SDC and OM media for 7 d. B. Statistical analysis of mycelia growth rate of Ku80, ΔMotup1 mutants and the complemented strain on different media. Three independent biological experiments with three replicates each time were performed and similar results were obtained in each time. Error bars represent the standard deviation, and the double asterisks represent significant difference between Ku80, ΔMotup1 mutants and the complemented strain (p < 0.01).
Figure 4.
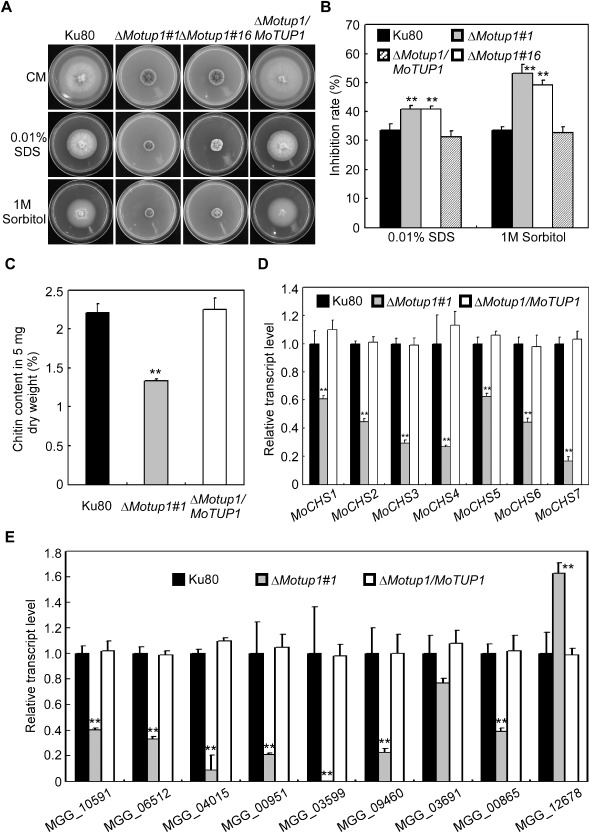
The MoTUP1 deletion mutant had defects in cell wall integrity and osmotic adaption. A. The wild type strain Ku80 and the ΔMotup1 strain were inoculated on CM medium with or without SDS (0.01% w/v) and sorbitol (1 M), and cultured at 28°C for 7 days. B. The growth inhibition rate is estimated relative to the growth rate of each untreated control [Inhibition rate = (the diameter of untreated strain – the diameter of treated strain)/(the diameter of untreated strain × 100%)]. Three repeats were performed and similar results obtained. C. GlcNa determination by the fluorimetric Morgan‐Elson method showed reduced chitin contents in the ΔMotup1 mutant. D. Expression analysis of chitin synthase (CHS 1‐7) genes by qRT‐PCR in ΔMotup1 mutant. E. Expression analysis of cell wall‐related genes by qRT‐PCR in ΔMotup1 mutant. MGG_10591: endo‐beta‐1,3‐glucanase; MGG_06512: glucan 1,3‐beta‐glucosidase 2; MGG_04015: mannan endo‐1,6‐alpha‐mannosidase DCW1; MGG_00951: mannan endo‐1,6‐alpha‐mannosidase DCW1; MGG_03599: acidic endochitinase SE2; MGG_09460: cell wall protein; MGG_03691: putative cell wall protein; MGG_00865: beta‐1,3‐glucan synthases MoFks1; MGG_12678: glucan endo‐1,3‐alpha‐glucosidase MoAgn1. Double asterisks respresent significant differences (p < 0.01).
Figure 5.
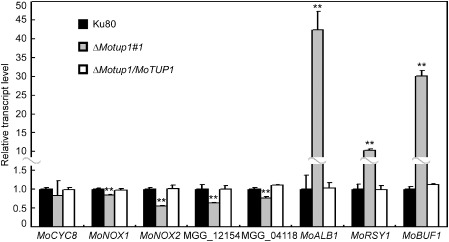
Expression analysis of metabolism ‐related genes by qRT‐PCR in ΔMotup1 mutant. Transcriptional levels of MoCYC8, two NADPH oxidase genes MoNOX1 and MoNOX2, two fatty acid synthase (FAS) genes MGG_12154 and MGG_04118, three genes related to melanin biosynthesis MoALB1, MoRSY1 and MoBUF1 in wild type and ΔMotup1 strains were measured by qRT‐PCR. Double asterisks represent significant differences (p < 0.01).
Figure 6.
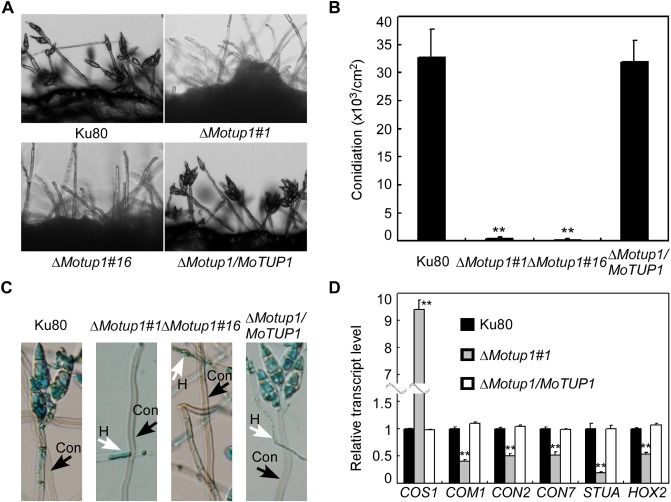
The MoTUP1 deletion mutant has defects in conidiogenesis. A. Development of conidia on conidiophores was affected by the ΔMotup1 mutant. Light microscopic observation was performed on strains grown on SDC medium for 2 days. B. Number of conidia harvested from a 9 cm SDC plate at day 10 after incubation at room temperature. C. Aerial structures stained with lactophenol aniline blue. Conidia and aerial hyphae stained blue, and conidiophores stained gray. D. Expression analysis of conidiation‐related genes by qRT‐PCR in ΔMotup1 mutant. Double asterisks represent significant differences (p < 0.01). H: hyphae; Con: conidiophore.
Figure 7.
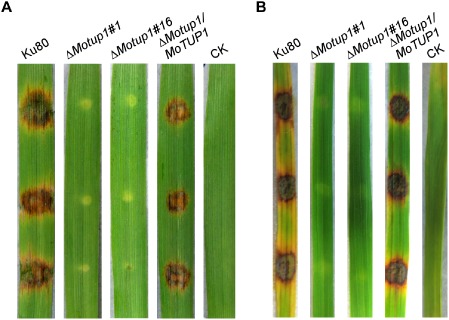
The MoTUP1 deletion mutants were nonpathogenic. A. Disease symptoms on the leaf tissues of barley inoculated by mycelia from WT (Ku80), ΔMotup1 mutants and the complemented strain. Leaves were photographed 5 days after inoculation. B. Disease symptoms on the leaf tissues of rice. The mycelia of WT, ΔMotup1 mutants and the complemented strain were inoculated on the leaf tissue and typical diseased leaves were photographed 7 days after inoculation. CK: suspension without mycelia.
Figure 8.
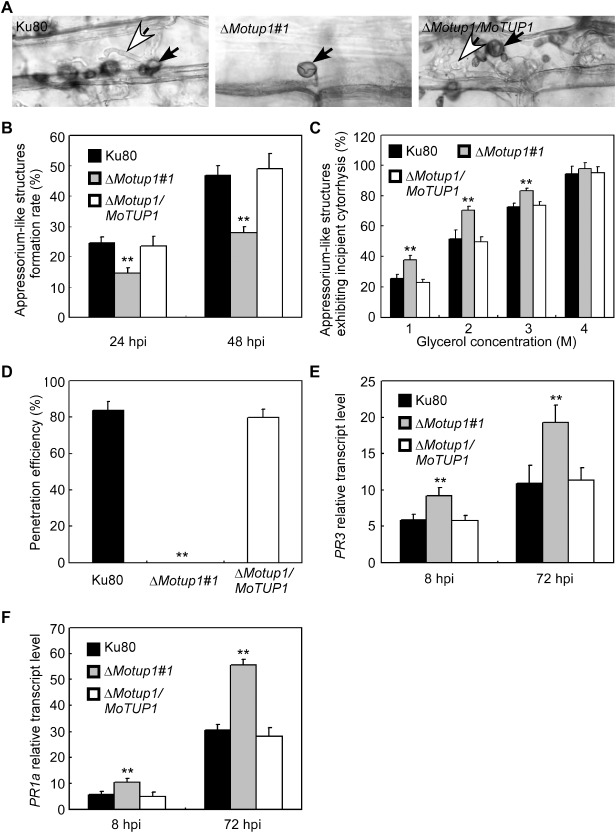
Rice epidermis cell penetration assay. A. The mycelia of Ku80, ΔMotup1 mutant and the complemented strain were inoculated on the rice leaf tissue for 48 h. The rice epidermis cell penetration was observed under light microscope. White arrows point to invasive hyphae; black arrows point to appressorium‐like structures. B. The mycelia of Ku80, ΔMotup1 mutant and the complemented strain were inoculated on the barley leaf epidermis for 24 and 48 h, respectively. The formation rate of appressorium‐like structures was measured under light microscope. C. Quantification of collapsed appressorium‐like structures of Ku80 and the ΔMotup1 mutant strains. For each glycerol concentration, at least 50 appressorium‐like structures were observed and numbers of collapsed appressorium‐like structures were counted. D. Percentage of appressorium‐like structures penetrated and formed invasive hyphae on barley leaves 48 hpi. E and F. The transcription of PR3 and PR1a in the infected host was assayed using qRT‐PCR. RNA samples were collected from rice plants (Oryza sativa cv. CO‐39) 0, 8 and 72 h after inoculation with the ΔMotup1 mutant or the wild‐type strain. The average threshold cycle of triplicate reactions was normalized by the stable‐expressions gene elongation factor 1a (Os03g08020) in O. sativa. Three independent biological experiments with three replicates each time were performed and similar results were obtained in each time. Double asterisks represent significant differences (p < 0.01).
Figure 9.
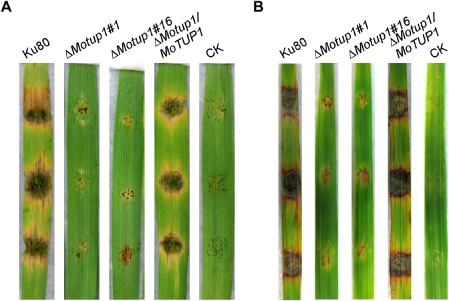
Pathogenicity test of the MoTUP1 deletion mutants on the abrade barley and rice leaves. A. The mycelia of the wild type strain Ku80, ΔMotup1 mutants and the complemented strain were inoculated on the abrade barley leaves and then cultured under the moist conditions for 5 days for the disease lesion to appear. The abrade leaves with nothing on was used as control. Leaves were photographed 5 days after inoculation. B. Disease symptoms on the abrade rice leaf. The mycelia of WT, ΔMotup1 mutants and the complemented strain were inoculated on the abrade rice leaf and typical diseased leaves were photographed 7 days after inoculation. CK: suspension without mycelia.
MoTUP1 deletion affects vegetative growth
We first evaluated the growth of ΔMotup1 mutants on complete medium (CM), minimal medium (MM), straw decoction and corn agar (SDC) and oatmeal medium (OM) plates. The growth rates of the ΔMotup1 mutants were significantly reduced compared with the wild‐type (Ku80) and complemented strains (Fig. 3A), and the colony diameters of the ΔMotup1 mutants were also significantly lower than that of Ku80 (Fig. 3B). In addition, the colonies of ΔMotup1 mutants appeared corrugated towards the edge and exhibited more pigmentation (Fig. S2A, see Supporting Information). We further incubated Ku80, the ΔMotup1 mutants and the complemented strain in liquid CM for 48 h, and found that the mutant strains formed compact mycelial masses, in contrast with the sparse mycelial masses formed by the reference strains (Fig. S2B). Overall, these results indicate that MoTup1 is essential for correct hyphal branching and vegetative growth.
MoTup1 is involved in cell wall integrity and osmotic adaptation
The fungal cell wall plays an important role in maintaining hyphal morphology and in adaptation to the environmental conditions. As the ΔMotup1 mutants showed apparent defects in hyphal growth, the cell wall integrity and osmotic adaptation of the ΔMotup1 mutants were examined. Mycelial growth was measured on CM containing sodium dodecylsulfate (SDS, cell wall stressor) (0.01%, w/v) or sorbitol (1 m) (osmotic stressor). The ΔMotup1 mutants were more sensitive to these agents (Fig. 4A), with the inhibition rates higher than those of the wild‐type Ku80 by 8% and 20%, respectively (Fig. 4B). This indicates that MoTup1 plays a role in cell wall integrity and osmotic adaptation.
As chitin is one of the main cell wall components, and the synthesis of chitin depends on the activity of the chitin synthases (Roncero, 2002), we quantified the accumulation of chitin and found that the chitin content was reduced by 60% in the ΔMotup1 mutant relative to that in the wild‐type strain (Fig. 4C). We then examined the expression of seven genes that are involved in chitin synthesis of M. oryzae (Kong et al., 2012) in Ku80 and the ΔMotup1 strains. The expression of these genes was significantly lower in the ΔMotup1 mutant than in the Ku80 strain (Fig. 4D). As Tup1 could regulate the expression of a series of cell wall biosynthesis‐associated genes in A. niger (Schachtschabel et al., 2013), we examined the expression of these genes and found that they were down‐regulated in the ΔMotup1 mutant. The expression of the β‐1,3‐glucan synthase MoFKS1 (MGG_00865) (Fujikawa et al., 2009) was also repressed in the ΔMotup1 mutant. Interestingly, the expression of MGG_12678, an α‐glucosidase MoAgn1, was increased (Fig. 4E). These results suggest that MoTup1 is involved in cell wall integrity through the regulation of chitin and cell wall synthesis genes.
MoTUP1 also regulates the expression of metabolic genes
In addition to the genes found above, MoTup1 also regulates the expression of genes related to metabolism through the Tup1–Cyc8 complex (Keleher et al., 1992; Schachtschabel et al., 2013). We therefore examined the expression of the genes known to be involved in metabolism. First, we examined MoCYC8, as MoCyc8 is predicted to be the functional partner of MoTup1 through the Tup1–Cyc8 complex. We found that MoTUP1 disruption did not affect the expression of MoCYC8 (Fig. 5). As genes encoding NADPH oxidase and melanin biosynthesis have been reported to play very important roles in the growth, development and pathogenicity of M. oryzae (Chumley and Valent, 1990; Egan et al., 2007), and genes encoding fatty acid synthase (FAS) are involved in appressorium development (Soanes et al., 2012), we analysed the transcript abundance of two NADPH oxidase genes, MoNOX1 and MoNOX2 (Egan et al., 2007), two FAS genes, MGG_12154 and MGG_04118 (Soanes et al., 2012), and three genes related to melanin biosynthesis (Chumley and Valent, 1990). We found that MoNOX1 and MoNOX2, and MGG_12154 and MGG_04118, were significantly down‐regulated in the ΔMotup1 mutant (Fig. 5). In contrast, the expression of the three melanin biosynthesis genes, MoALB1, MoRSY1 and MoBUF1, was increased by over 40‐, 10‐, and 30‐fold, respectively, in the ΔMotup1 mutant (Fig. 5). These results suggest that MoTup1 is involved in the regulation of the expression of genes related to metabolism.
MoTUP1 disruption blocks conidial formation
Conidia play an important role during the infection stage as propagules. Thus, we examined the effect of MoTUP1 disruption on conidial production. Conidial formation was drastically reduced in the ΔMotup1 mutants, and the conidium number was less than 1% compared with that of the wild‐type Ku80 and complemented strains (Fig. 6A, B). However, examination by light microscopy of the colony stained with lactophenol aniline blue revealed that the ΔMotup1 mutant developed aerial hyphae and conidiophores in SDC (Fig. 6C). This indicates that the deletion of MoTUP1 significantly reduces conidial formation.
To elucidate the mechanism by which MoTup1 is required for conidial formation, we examined the expression of six conidiation‐related genes by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). The expression level of MoCOS1, a C2H2 zinc‐finger transcription factor encoding gene essential for conidiophore development (Zhou et al., 2009), was significantly increased in the ΔMotup1 mutant (Fig. 6D). In contrast, the transcript levels of MoCOM1, MoCON2, MoCON7, MoSTUA and MoHOX2 were distinctively lower in the ΔMotup1 mutant than in the Ku80 strain (Fig. 6D) (Kim et al., 2009; Nishimura et al., 2009; Shi and Leung, 1995; Yang et al., 2010). This indicates that MoTup1 is involved in the regulation of the expression of conidiation‐related genes.
MoTup1 is required for appressorium‐like structure‐mediated penetration
Normally, M. oryzae invades rice cells using appressoria, which develop from conidia (Howard et al., 1991). However, M. oryzae also forms appressorium‐like structures at the hyphal tips on plant surfaces to facilitate breaching of the plant cuticle and the induction of disease (Kim et al., 2009; Kong et al., 2013). As the ΔMotup1 mutants produced few conidia in the present study, mycelia of the ΔMotup1 mutants were used instead to inoculate the detached barley and rice leaves to assess pathogenicity. No disease symptoms were observed on the surfaces of the barley or rice leaves after 5 and 7 days, respectively, in contrast with leaves infected with wild‐type Ku80 and the complemented strains, which exhibited typical rice blast lesions (Fig. 7A, B). These observations indicate that MoTup1 is essential for M. oryzae pathogenicity.
The lack of pathogenicity of ΔMotup1 mutants on rice and barley leaves may be a result of either the mutants not forming appressorium‐like structures or the appressorium‐like structures being unable to penetrate the host epidermal cell. To investigate this, mycelia of the ΔMotup1 mutant, Ku80 and the complemented strains were inoculated onto detached rice leaves. After 48 h, leaves were processed and observed by microscopy. The ΔMotup1 mutant formed normal appressorium‐like structures, but the formation rate was significantly lower than that in Ku80 (Fig. 8A, B), and the ΔMotup1 mutant could not produce invasive hyphae; as a consequence, it could not penetrate cells, in contrast with Ku80 and the complemented strains (Fig. 8D). We also examined the turgor pressure of appressorium‐like structures, and found that appressorium‐like structures of the ΔMotup1 mutant showed an increased collapse rate in 1, 2 and 3 m glycerol solution (Fig. 8C), indicating that MoTup1 is required for normal turgor of appressorium‐like structures. Finally, we examined the expression of the plant pathogenesis‐related defence genes PR3 and PR1a (van Loon et al., 2006), and found higher transcription levels in rice infected with the ΔMotup1 mutant compared with the wild‐type Ku80 (Fig. 8E, F).
The above results indicate that the deletion of MoTUP1 results in failed penetration of the plant cell. We also examined whether MoTup1 is involved in invasive hyphal growth after artificial penetration by inoculation of mycelium on wounded barley and rice leaves. The ΔMotup1 mutants produced very small lesions on detached barley (5 days) and rice (7 days) leaves compared with Ku80, which caused visible diffuse lesions (Fig. 9A, B). These results suggest that the reduced pathogenicity in wound tissue by the ΔMotup1 mutant is probably a result of activation of the plant defence response.
Discussion
Previous studies have demonstrated that the fungal Tup1–Cyc8 complex is an important transcriptional co‐repressor that plays a role during the growth of vegetative cells. In C. albicans, the homozygous tup1/tup1 mutant strain is constitutively filamentous and the mutant cells grow as pseudohyphae rather than as true hyphae on most media (Braun and Johnson, 1997). It is thought that Tup1 is recruited by Nrg1 to repress several filament‐specific genes, such as ECE1 and HWP1 (Braun et al., 2001). In P. marneffei, the tupA deletion mutant shows growth reduction and severe morphological defects when grown at 25 °C (Todd et al., 2003). Deletion of TUP1 also affects growth at both 25 and 30 °C in Cryptococcus neoformans (Lee et al., 2005). Our results show that ΔMotup1 mutants exhibit a reduced growth rate in natural or synthetic media and form compact mycelial masses, which indicates that the ΔMotup1 mutant fails to assimilate and utilize nutrients for normal metabolism. Collectively, our study suggests that Tup1 plays a conservative role in fungal growth and development.
The ΔMotup1 mutants show defects in cell wall integrity and are more sensitive to cell wall stress inducers than is the wild‐type strain. Previous studies have suggested that chitin synthase genes play important roles in various developmental and plant infection processes of M. oryzae (Kong et al., 2012), and that MoTup1 may affect chitin synthesis. Indeed, the expression of seven chitin synthase genes was markedly decreased in the ΔMotup1 mutant. Moreover, the expression of genes encoding cell wall protein and glycoprotein was also repressed in the ΔMotup1 mutant.
In this study, the ΔMotup1 mutants hardly produced any conidia, suggesting that MoTup1 is primarily involved in conidial formation. The expression analysis indicated that MoTUP1 was up‐regulated during the conidial formation stage, and five conidiation‐related genes were markedly decreased in the ΔMotup1 mutant; this supports the proposition that MoTup1 plays an important role in conidiogenesis. This is also consistent with previous studies indicating that Tup1 plays an important role in asexual and sexual development (Cr. neoformans and N. crassa; Lee et al., 2005; Vandeputte et al., 2012) and mating, infective filament formation and conidial formation (U. maydis; Elias‐Villalobos et al., 2011). Conversely, the ΔtupA mutant prematurely initiates asexual development and promotes conidial production in P. marneffei (Todd et al., 2003). Nevertheless, these results indicate that MoTup1 shares conserved functions with most, but not all, fungal Tup1 proteins in the regulation of asexual and sexual processes.
Disruption of TUP1 is known to reduce the virulence of C. albicans and Cr. neoformans (Lee et al., 2009; Liu, 2002). In U. maydis, there was also a significant decrease in the number of Δtup1‐infected plants that developed tumours compared with the wild‐type strain. In addition, U. maydis Δtup1 lost its ability to complete the sexual cycle in infected host plants (Elias‐Villalobos et al., 2011). Magnaporthe oryzae adopts the formation of appressoria at the hyphal tips as a strategy for host infection (Kim et al., 2009; Kong et al., 2013). Although ΔMotup1 mutants form appressorium‐like structures at the hyphal tips, the formation rate and turgor pressure of the appressorium‐like structures are lower than in the wild‐type Ku80. This may account for the attenuation in pathogenicity. However, it is possible that certain plant defence responses may also be activated by the ΔMotup1 mutant, leading to failure in host penetration and loss of the ability to form typical lesions in leaves.
To summarize, we have demonstrated that MoTup1 plays several important roles during the development and pathogenicity of M. oryzae. Our findings have increased our understanding of the regulatory mechanism of conidiation and hyphal growth during plant infection, and have provided new insights into the conserved nature of Tup1 proteins in the growth, development and virulence of various fungi.
Experimental Procedures
Strains and culture conditions
The M. oryzae Ku80 strain was used as the wild‐type strain in this study. All strains were cultured on CM agar plates for 3–15 days at 28 °C. Mycelia were harvested from liquid CM and used for genomic DNA and RNA extractions. Protoplasts were prepared and transformed as described by Sweigard et al. (1992). Transformants were selected on TB3 medium (3 g of yeast extract, 3 g of casamino acids, 200 g of sucrose, 7.5 g of agar in 1 L of distilled water) with 300 μg/mL hygromycin B (Roche, San Francisco, CA, USA) or 200 μg/mL zeocin (Invitrogen, Carlsbad, CA, USA). For conidiation, strain blocks were maintained on SDC at 28 °C for 7 days in the dark, followed by 3 days of continuous illumination under fluorescent light (Zhang et al., 2009).
MoTUP1 gene disruption
To construct the MoTUP1 gene replacement construct, a 1‐kb upstream flanking sequence fragment and a 1‐kb downstream flanking sequence fragment were amplified from M. oryzae genomic DNA by PCR using the primer pairs FL10581 (F)/FL10582 (R) and FL10583 (F)/FL10584 (R), respectively (Table S1, see Supporting Information). The upstream flanking sequence was digested by XhoI and SalI, and cloned into a pCX62 vector to generate plasmid pCX62‐MoTUP1‐U. The plasmid pCX62‐MoTUP1‐U and the downstream flanking sequence were digested by SpeI and XbaI, respectively, and linked to generate the final disruption construct pCX62‐MoTUP1‐U‐D.
Vegetative growth, conidiation and stress adaption
Vegetative growth of ΔMotup1 and Ku80 was measured on CM (50 mL 20× nitrate salts, 1 mL trace elements, 10 g glucose, 2 g peptone, 1 g yeast extract, 1 g casamino acids, 1 mL vitamin solution, 15 g agar in 1 L distilled water), MM (6 g NaNO3, 0.52 g KCl, 0.52 g MgSO4, 1.52 g KH2PO4, 10 g glucose, 0.5% biotin and 15 g agar in 1 L distilled water), OM (30 g oatmeal and 10 g agar in 1 L distilled water) and SDC (100 g straw, 40 g corn powder, 15 g agar in 1 L distilled water) for 7 days. Equal plugs of mycelia, from 5‐day‐old CM plates, were transferred into liquid CM. The mycelia were cultured with shaking (150 rpm) at 28 °C for 2 days (Chen et al., 2014).
For the quantification of conidial production, 10‐day‐old conidia were collected with 5 mL of distilled water, filtered through three layers of lens paper and counted with a haemocytometer under a microscope (Guo et al., 2010).
Strain blocks (5 mm × 5 mm) were placed onto freshly prepared CM agar plates with SDS (0.01%, w/v) and sorbitol (1 M), and cultured in the dark at 28 °C for 7 days. The experiment was repeated three times with three replicates each time.
Staining with lactophenol aniline blue and measurement of the chitin content
To stain conidiophores, a tablet of SDC inoculated with the fungal strain of interest was placed on a sterile glass slide on a plate, kept moist and grown for 2 days at 28 °C under continuous fluorescent light. The agar tablet was then removed. A few drops of 1:3 dilution of lactophenol aniline blue stain were mounted and a coverslip was immediately applied to the slide. The slide was examined under a light microscope immediately after staining.
Chitin (N‐acetylglucosamine, GlcNAc) content was determined as described by Bulik et al. (2003). Mycelia of Ku80 and the ΔMotup1 mutant were harvested from liquid CM cultured for 2 days and freeze–dried; the subsequent detailed experimental procedure was performed according to the previous description in Song et al. (2010).
Nucleic acid manipulation and Southern blotting
The standard Southern blot protocol was utilized (Sambrook and Russell, 2001). The target gene probe and HPH probe were amplified with primer pairs FL10585/FL10586 (for MoTUP1) (Table S1) and FL1111/FL1112 (for HPH), respectively. Probe labelling, hybridization and detection were performed with the DIG High Prime DNA Labelling and Detection Starter Kit (Roche Applied Science, Penzberg, Germany). Total RNA was isolated from frozen fungal mycelia using the RNA extraction kit (Invitrogen). To measure the relative abundance of gene transcripts, RNAs were extracted from mycelia grown in liquid CM for 2 days at 28 °C in an orbital shaker (150 rpm) (Wang et al., 2013). To measure the relative abundance of MoTUP1 transcripts during diverse fungal developmental stages, the total RNA samples were extracted from mycelia grown in liquid CM, conidia and plants inoculated with the conidia of Ku80 (1 × 108 spores/mL) for 8, 24, 48, 72 and 168 h by the method described above. The crude RNA was treated with DNase I (TaKaRa, Dalian, China) before being reverse transcribed. qRT‐PCR was performed on an ABI 7500 real‐time PCR system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. A 20‐μL reaction volume contained 2 μL of reverse transcription product, 10 μL of SYBR premix Ex Taq (2×), 0.4 μL of ROX reference dye (50×, SYBR PrimeScript RT‐PCR kit; TaKaRa) and 0.4 μL of each primer (10 μL). The stable expression ACTIN gene (MGG_03982), amplified by primers FL4362 and FL4363 (Table S1), was used as the internal control. Normalization and comparison of the mean Ct value were performed as described by Livak and Schmittgen (2001). All statistical analyses were performed according to Duncan's test. The experiment was repeated three times with three replicates each time.
Plant infection assays, appressorium‐like structure formation, turgor analysis and examination of the level of PR gene transcription
For pathogenicity assessment, mycelia cultured in liquid CM for 2 days were harvested, washed twice with distilled water and fragmented into a length of 30–50 μm using a homogenizer. The mycelial suspension was adjusted to 5 × 104 pieces/mL and inoculated onto intact or abraded leaves of the susceptible rice variety Oryza sativa cv. CO39 or 7‐day‐old barley seedlings (Dou et al., 2011). The mycelia of Ku80 and the ΔMotup1 mutant were inoculated onto rice leaf tissue for 48 h to observe appressorium‐like structure formation by the hyphal tips. The same method was used to measure the formation rate of appressorium‐like structures on the epidermis of barley leaves. After the formation of an appressorium‐like structure on the barley leaf epidermis, droplets of glycerol at concentrations ranging from 1.0 to 4.0 m were applied. The numbers of appressorium‐like structures that collapsed after 10 min were recorded. To measure the relative abundance of rice PR gene transcripts, mycelial suspensions of Ku80 and the ΔMotup1 mutant were adjusted to 5 × 104 pieces/mL and inoculated onto rice leaf tissue for 8 and 72 h, respectively, and plant total RNA samples were extracted according to the method described above.
Generation of the MoTUP1‐GFP fusion construct and complemented strains, and cellular localization of MoTup1‐GFP
For the generation of the MoTUP1‐GFP fusion construct, a 3.6‐kb fragment of the MoTUP1 genes containing the native promoter regions was amplified and co‐transformed with XhoI‐digested pKB04 into S. cerevisiae strain XK1‐25. pYC5 plasmid was rescued from tryptophan‐positive (Trp+) yeast transformants and inserts were verified by sequencing analysis to confirm the in‐frame MoTUP1‐GFP fusion constructs. Complemented zeocin‐resistant transformants were isolated after transformation of the ΔMotup1#16 mutant with pYC5.
To investigate the cellular localization of MoTup1 during infection‐related development, conidial suspensions (5 × 104 conidia/mL) of the ΔMotup1/MoTUP1‐GFP transformant were inoculated on 5‐cm‐long leaf segments from 8‐day‐old barley plants. After 8 and 24 h of incubation under humid conditions at 28 °C, the leaf segments were observed under a fluorescence microscope.
Bioinformatics analysis
The full MoTUP1 sequence was obtained from the M. oryzae database (http://www.broadinstitute.org/annotation/genome/magnaporthe_comparative/MultiHome.html). Protein domain and motif prediction was performed with smart software. Amino acid sequence alignments were performed using the clustal_w program, and the calculated phylogenetic tree was viewed using the Mega4.1 Beta program.
Supporting information
Fig. S1 Construction strategies for MoTUP1 knock‐out vector and validation of mutants. A. MoTUP1 targeted gene replacement strategy. A. 2.16 kb fragment of the MoTUP1 coding region was replaced with a 1.4 kb fragment containing the hygromycin B resistance cassette (HPH) to create the MoTUP1 deletion mutant. The DNA fragment at the inner space of MoTUP1 deletion region was used as the probe to validate the MoTUP1 deletion transformants by PCR amplification. B. Southern hybridization was used to analyze the deletion of MoTUP1 gene and the single copy integration of the HPH gene in the mutants. The genomic DNA of Ku80 and ΔMotup1 mutant was digested with EcoRV and then hybridized with a DNA fragment at the inner space of MoTUP1 deletion region and a 1.4 kb HPH fragment.
Fig. S2 The deletion of MoTUP1 has an effect on the phenotypes of mycelia growth. A. Phenotypes of the wild type strain Ku80, the ΔMotup1 mutant and the complemented strain grown on CM media at 28°C in darkness for 7 days. B. Phenotype of mycelia growth in liquid CM media. The mutants, Ku80, and the complemented strain were inoculated in liquid CM media and cultured for 48 h at 28°C in darkness, and then photographed.
Table S1 Primers used in this study.
Acknowledgements
This research was supported by the National Science Foundation for Distinguished Young Scholars of China (Grant No.31325022 to ZZ), Natural Science Foundation of China (Grant No: 31271998, ZZ) and the specially appointed professorship (Jiangsu, China).
References
- Braun, B.R. and Johnson, A.D. (1997) Control of filament formation in Candida albicans by the transcriptional repressor TUP1 . Science, 277, 105–109. [DOI] [PubMed] [Google Scholar]
- Braun, B.R. , Kadosh, D. and Johnson, A.D. (2001) NRG1, a repressor of filamentous growth in C. albicans, is down‐regulated during filament induction. EMBO J. 20, 4753–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik, D.A. , Olczak, M. , Lucero, H.A. , Osmond, B.C. , Robbins, P.W. and Specht, C.A. (2003) Chitin synthesis in Saccharomyces cerevisiae in response to supplementation of growth medium with glucosamine and cell wall stress. Eukaryot. Cell, 2, 886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Zhai, S. , Zhang, H.F. , Zuo, R.F. , Wang J., Guo, M. , Zheng, X.B. , Wang, P. and Zhang, Z.G. (2014) Shared and distinct functions of two Gti1/Pac2 family proteins in growth, morphogenesis and pathogenicity of Magnaporthe oryzae . Environ. Microbiol. 16, 788–801. [DOI] [PubMed] [Google Scholar]
- Chumley, F.G. and Valent, B. (1990) Genetic analysis of melanin‐deficient, nonpathogenic mutants of Magnaporthe grisea . Mol. Plant‐Microbe Interact. 3, 135–143. [Google Scholar]
- Dean, R.A. , Talbot, N.J. , Ebbole, D.J. , Farman, M.L. , Mitchell, T.K. , Orbach, M.J. , Thon, M. , Kulkarni, R. , Xu, J.R. , Pan, H. , Read, N.D. , Lee, Y.H. , Carbone, I. , Brown, D. , Oh, Y.Y. , Donofrio, N. , Jeong, J.S. , Soanes, D.M. , Djonovic, S. , Kolomiets, E. , Rehmeyer, C. , Li, W. , Harding, M. , Kim, S. , Lebrun, M.H. , Bohnert, H. , Coughlan, S. , Butler, J. , Calvo, S. , Ma, L.J. , Nicol, R. , Purcell, S. , Nusbaum, C. , Galagan, J.E. and Birren, B.W. (2005) The genome sequence of the rice blast fungus Magnaporthe grisea . Nature, 434, 980–986. [DOI] [PubMed] [Google Scholar]
- Ding, S.L. , Liu, W. , Iliuk, A. , Ribot, C. , Vallet, J. , Tao, A. , Wang, Y. , Lebrun, M.H. and Xu, J.R. (2010) The tig1 histone deacetylase complex regulates infectious growth in the rice blast fungus Magnaporthe oryzae . Plant Cell, 22, 2495–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou, X.Y. , Wang, Q. , Qi, Z.Q. , Song, W.W. , Wang, W. , Guo, M. , Zhang, H.F. , Zhang, Z.G. , Wang, P. and Zheng, X.B. (2011) MoVam7, a conserved SNARE involved in vacuole assembly, is required for growth, endocytosis, ROS accumulation, and pathogenesis of Magnaporthe oryzae . PLoS One, 6, e16439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan, M.J. , Wang, Z.Y. , Jones, M.A. , Smirnoff, N. and Talbot, N.J. (2007) Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. USA, 104, 11 772–11 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias‐Villalobos, A. , Fernandez‐Alvarez, A. and Ibeas, J.I. (2011) The general transcriptional repressor Tup1 is required for dimorphism and virulence in a fungal plant pathogen. PLoS Pathog. 7, e1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa, T. , Kuga, Y. , Yano, S. , Yoshimi, A. , Tachiki, T. , Abe, K. and Nishimura, M. (2009) Dynamics of cell wall components of Magnaporthe grisea during infectious structure development. Mol. Microbiol. 73, 553–570. [DOI] [PubMed] [Google Scholar]
- Guo, M. , Guo, W. , Chen, Y. , Dong, S. , Zhang, X. , Zhang, H.F. , Song, W.W. , Wang, W. , Wang, Q. , Lv, R.L. , Zhang, Z.G. , Wang, Y.C. and Zheng, X.B. (2010) The basic leucine zipper transcription factor Moatf1 mediates oxidative stress responses and is necessary for full virulence of the rice blast fungus Magnaporthe oryzae . Mol. Plant–Microbe Interact. 23, 1053–1068. [DOI] [PubMed] [Google Scholar]
- Hanlon, S.E. , Rizzo, J.M. , Tatomer, D.C. , Lieb, J.D. and Buck, M.J. (2011) The stress response factors Yap6, Cin5, Phd1, and Skn7 direct targeting of the conserved co‐repressor Tup1‐Ssn6 in S. cerevisiae . PLoS One, 6, e19060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, R.J. , Ferrari, M.A. , Roach, D.H. and Money, N.P. (1991) Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc. Natl. Acad. Sci. USA, 88, 11 281–11 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M.X. , Zhou, Z. and Elledge, S.J. (1998) The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell, 94, 595–605. [DOI] [PubMed] [Google Scholar]
- Kadosh, D. and Johnson, A.D. (2001) Rfg1, a protein related to the Saccharomyces cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in Candida albicans . Mol. Cell. Biol. 21, 2496–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleher, C.A. , Redd, M.J. , Schultz, J. , Carlson, M. and Johnson, A.D. (1992) Ssn6‐Tup1 is a general repressor of transcription in yeast. Cell, 68, 709–719. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Park, S.Y. , Kim, K.S. , Rho, H.S. , Chi, M.H. , Choi, J. , Park, J. , Kong, S. , Park, J. , Goh, J. and Lee, Y.H. (2009) Homeobox transcription factors are required for conidiation and appressorium development in the rice blast fungus Magnaporthe oryzae . Plos Genet. 5, e1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L.A. , Yang, J. , Li, G.T. , Qi, L.L. , Zhang, Y.J. , Wang, C.F. , Zhao, W.S. , Xu, J.R. and Peng, Y.L. (2012) Different chitin synthase genes are required for various developmental and plant infection processes in the rice blast fungus Magnaporthe oryzae . PLoS Pathog. 8, e1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L.A. , Li, G.T. , Liu, Y. , Liu, M.G. , Zhang, S.J. , Yang, J. , Zhou, X.Y. , Peng, Y.L. and Xu, J.R. (2013) Differences between appressoria formed by germ tubes and appressorium‐like structures developed by hyphal tips in Magnaporthe oryzae . Fungal Genet. Biol. 56, 33–41. [DOI] [PubMed] [Google Scholar]
- Lee, H. , Chang, Y.C. and Kwon‐Chung, K.J. (2005) TUP1 disruption reveals biological differences between MATa and MATalpha strains of Cryptococcus neoformans . Mol. Microbiol. 55, 1222–1232. [DOI] [PubMed] [Google Scholar]
- Lee, H. , Chang, Y.C. , Varma, A. and Kwon‐Chung, K.J. (2009) Regulatory diversity of TUP1 in Cryptococcus neoformans . Eukaryot. Cell, 8, 1901–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X. , Zhang, C.Y. , Bai, X.W. , Song, H.Y. and Xiao, D.G. (2014) Effects of MIG1, TUP1 and SSN6 deletion on maltose metabolism and leavening ability of baker's yeast in lean dough. Microb. Cell Fact. 13, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H.P. (2002) Co‐regulation of pathogenesis with dimorphism and phenotypic switching in Candida albicans, a commensal and a pathogen. Int. J. Med. Microbiol. 292, 299–311. [DOI] [PubMed] [Google Scholar]
- Liu, W. , Xie, S. , Zhao, X. , Chen, X. , Zheng, W. , Lu, G. , Xu, J.R. and Wang, Z. (2010) A homeobox gene is essential for conidiogenesis of the rice blast fungus Magnaporthe oryzae . Mol. Plant–Microbe Interact. 23, 366–375. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2−ΔΔCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- van Loon, L.C. , Rep, M. and Pieterse, C.M. (2006) Significance of inducible defense‐related proteins in infected plants. Annu. Rev. Phytopathol. 44, 135–162. [DOI] [PubMed] [Google Scholar]
- Malave, T.M. and Dent, S.Y. (2006) Transcriptional repression by Tup1‐Ssn6. Biochem. Cell Biol. 84, 437–443. [DOI] [PubMed] [Google Scholar]
- Nishimura, M. , Fukada, J. , Moriwaki, A. , Fujikawa, T. , Ohashi, M. , Hibi, T. and Hayashi, N. (2009) Mstu1, an APSES transcription factor, is required for appressorium‐mediated infection in Magnaporthe grisea . Biosci. Biotechnol. Biochem. 73, 1779–1786. [DOI] [PubMed] [Google Scholar]
- Ou, S. (1980) Pathogen variability and host resistance in rice blast disease. Annu. Rev. Phytopathol. 18, 167–187. [Google Scholar]
- Roncero, C. (2002) The genetic complexity of chitin synthesis in fungi. Curr. Genet. 41, 367–378. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. and Russell, D. (2001) Molecular Cloning – A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Schachtschabel, D. , Arentshorst, M. , Nitsche, B.M. , Morris, S. , Nielsen, K.F. , van den Hondel, C.A. , Klis, F.M. and Ram, A.F. (2013) The transcriptional repressor TupA in Aspergillus niger is involved in controlling gene expression related to cell wall biosynthesis, development, and nitrogen source availability. PLoS One, 8, e78102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Z.X. and Leung, H. (1995) Genetic analysis of sporulation in Magnaporthe grisea by chemical and insertional mutagenesis. Mol. Plant–Microbe Interact. 8, 949–959. [Google Scholar]
- Soanes, D.M. , Chakrabarti, A. , Paszkiewicz, K.H. , Dawe, A.L. and Talbot, N.J. (2012) Genome‐wide transcriptional profiling of appressorium development by the rice blast fungus Magnaporthe oryzae . PLoS Pathog. 8, e1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.W. , Dou, X.Y. , Qi, Z.Q. , Wang, Q. , Zhang, X. , Zhang, H.F. , Guo, M. , Dong, S.M. , Zhang, Z.G. , Wang, P. and Zheng, X.B. (2010) R‐SNARE homolog MoSec22 is required for conidiogenesis, cell wall integrity, and pathogenesis of Magnaporthe oryzae . PLoS One, 5, e13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweigard, J.A. , Chumley, F.G. and Valent, B. (1992) Disruption of a Magnaporthe grisea cutinase gene. Mol. Gen. Genet. 232, 183–190. [PubMed] [Google Scholar]
- Talbot, N.J. (2003) On the trail of a cereal killer: exploring the biology of Magnaporthe grisea . Annu. Rev. Microbiol. 57, 177–202. [DOI] [PubMed] [Google Scholar]
- Todd, R.B. , Greenhalgh, J.R. , Hynes, M.J. and Andrianopoulos, A. (2003) TupA, the Penicillium marneffei Tup1p homologue, represses both yeast and spore development. Mol. Microbiol. 48, 85–94. [DOI] [PubMed] [Google Scholar]
- Vandeputte, P. , Pradervand, S. , Ischer, F. , Coste, A.T. , Ferrari, S. , Harshman, K.A. and Sanglard, D. (2012) Identification and functional characterization of Rca1, a transcription factor involved in both antifungal susceptibility and host response in Candida albicans . Eukaryot. Cell, 11, 916–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.M. , Du, Y. , Zhang, H.F. , Zhou, C. , Qi, Z.Q. , Zheng, X.B. , Wang, P. and Zhang, Z.G. (2013) The actin‐regulating kinase homologue MoArk1 plays a pleiotropic function in Magnaporthe oryzae . Mol. Plant Pathol. 14, 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, R.A. and Talbot, N.J. (2009) Under pressure: investigating the biology of plant infection by Magnaporthe oryzae . Nature Rev. Micriobiol. 7, 185–195. [DOI] [PubMed] [Google Scholar]
- Wong, K.H. and Struhl, K. (2011) The Cyc8‐Tup1 complex inhibits transcription primarily by masking the activation domain of the recruiting protein. Genes Dev. 25, 2525–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Zhao, X. , Sun, J. , Kang, Z. , Ding, S. , Xu, J.R. and Peng, Y.L. (2010) A novel protein Com1 is required for normal conidium morphology and full virulence in Magnaporthe oryzae . Mol. Plant–Microbe Interact. 23, 112–123. [DOI] [PubMed] [Google Scholar]
- Zeigler, R.S. , Leong, S.A. and Teng, P. (1994) Rice Blast Disease. Wallingford, Oxon (United Kingdom): International Rice Research Institute. [Google Scholar]
- Zhang, H.F. , Liu, K.Y. , Zhang, X. , Tang, W. , Wang, J.S. , Guo, M. , Zhao, Q. , Zheng, X.B. , Wang, P. and Zhang, Z.G. (2011) Two phosphodiesterase genes, PDEL and PDEH, regulate development and pathogenicity by modulating intracellular cyclic AMP levels in Magnaporthe oryzae . PLoS One, 6, e17241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H.F. , Zhao, Q. , Guo, X.X. , Guo, M. , Qi, Z.Q. , Tang, W. , Dong, Y.H. , Ye, W.W. , Zheng, X.B. , Wang, P. and Zhang, Z.G. (2014) Pleiotropic function of the putative zinc‐finger protein MoMsn2 in Magnaporthe oryzae . Mol. Plant–Microbe Interact. 27, 446–460. [DOI] [PubMed] [Google Scholar]
- Zhang, H.F. , Zhao, Q. , Liu, K.Y. , Zhang, Z.G. , Wang, Y.C. and Zheng, X.B. (2009) MgCRZ1, a transcription factor of Magnaporthe grisea, controls growth, development and is involved in full virulence. FEMS Microbiol. Lett. 293, 160–169. [DOI] [PubMed] [Google Scholar]
- Zhou, Z.Z. , Li, G.H. , Lin, C.H. and He, C.Z. (2009) Conidiophore Stalk‐less1 encodes a putative zinc‐finger protein involved in the early stage of conidiation and mycelial infection in Magnaporthe oryzae . Mol. Plant–Microbe Interact. 22, 402–410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Construction strategies for MoTUP1 knock‐out vector and validation of mutants. A. MoTUP1 targeted gene replacement strategy. A. 2.16 kb fragment of the MoTUP1 coding region was replaced with a 1.4 kb fragment containing the hygromycin B resistance cassette (HPH) to create the MoTUP1 deletion mutant. The DNA fragment at the inner space of MoTUP1 deletion region was used as the probe to validate the MoTUP1 deletion transformants by PCR amplification. B. Southern hybridization was used to analyze the deletion of MoTUP1 gene and the single copy integration of the HPH gene in the mutants. The genomic DNA of Ku80 and ΔMotup1 mutant was digested with EcoRV and then hybridized with a DNA fragment at the inner space of MoTUP1 deletion region and a 1.4 kb HPH fragment.
Fig. S2 The deletion of MoTUP1 has an effect on the phenotypes of mycelia growth. A. Phenotypes of the wild type strain Ku80, the ΔMotup1 mutant and the complemented strain grown on CM media at 28°C in darkness for 7 days. B. Phenotype of mycelia growth in liquid CM media. The mutants, Ku80, and the complemented strain were inoculated in liquid CM media and cultured for 48 h at 28°C in darkness, and then photographed.
Table S1 Primers used in this study.


