Summary
The suppressive ability of several strains of cyclic lipopeptide‐producing Bacillus rhizobacteria to grey leaf spot disease caused by Magnaporthe oryzae has been documented previously; however, the underlying mechanism(s) involved in the induced systemic resistance (ISR) activity in perennial ryegrass (Lolium perenne L.) remains unknown. Root‐drench application of solid‐phase extraction (SPE)‐enriched surfactin and live cells of mutant Bacillus amyloliquefaciens strain FZB42‐AK3 (produces surfactin, but not bacillomycin D and fengycin) significantly reduced disease incidence and severity on perennial ryegrass. The application of the treatments revealed a pronounced multilayered ISR defence response activation via timely and enhanced accumulation of hydrogen peroxide (H2O2), elevated cell wall/apoplastic peroxidase activity, and deposition of callose and phenolic/polyphenolic compounds underneath the fungal appressoria in naïve leaves, which was significantly more intense in treated plants than in mock‐treated controls. Moreover, a hypersensitive response (HR)‐type reaction and enhanced expression of LpPrx (Prx, peroxidase), LpOXO4 (OXO, oxalate oxidase), LpPAL (PAL, phenylalanine ammonia lyase), LpLOXa (LOX, lipoxygenase), LpTHb (putative defensin) and LpDEFa (DEFa, putative defensin) in perennial ryegrass were associated with SPE‐enriched surfactin and live AK3 cell treatments, acting as a second layer of defence when pre‐invasive defence responses failed. The results indicate that ISR activity following surfactin perception may sensitize H2O2‐mediated defence responses, thereby providing perennial ryegrass with enhanced protection against M. oryzae.
Keywords: H2O2 accumulation, hypersensitive response, peroxidase activity, real‐time RT‐PCR, solid‐phase extraction (SPE)
Introduction
Perennial ryegrass is an important forage and turf‐type grass because of its superior nutritional and agronomic qualities (Ran et al., 2007; Uddin et al., 2003). Recently, grey leaf spot, caused by Magnaporthe oryzae, has emerged as one of the most perilous diseases of this turfgrass species (Rahman et al., 2014). As a result of heavy reliance on chemical fungicides to control the disease, fungicide‐resistant M. oryzae strains have already emerged (Vincelli and Dixon, 2002), and non‐target effects on beneficial microbes and the possibility of environmental quality deterioration are of serious concern (Pérez‐García et al., 2011). The use of beneficial microbes, together with host resistance, holds great potential as an environmentally friendly approach in the management of plant diseases (Pérez‐García et al., 2011).
Some of the most common responses of induced host resistance in plants following pathogen recognition include cell wall fortification, such as callose deposition in cell wall appositions (CWAs), and localized accumulation of antimicrobial compounds (Ahn et al., 2007; Jones and Dangl, 2006; Pieterse et al., 2009). Such local reactions are often followed by a burst of reactive oxygen species (ROS), e.g. hydrogen peroxide (H2O2), resulting in the onset of programmed cell death (PCD), referred to as a ‘hypersensitive response’ (HR), leading to systemic resistance (Durrant and Dong, 2004; Jones and Dangl, 2006). Regardless of its origin, H2O2 is an important defence signalling molecule and displays direct toxicity towards invading microbial pathogens (Torres, 2010). In addition, pathogen‐induced systemic resistance, known as systemic acquired resistance (SAR), is commonly associated with an enhanced level of endogenous salicylic acid (SA) and pathogenesis‐related 1 (PR‐1), whereas beneficial microorganisms trigger induced systemic resistance (ISR), which is often regulated by jasmonic acid/ethylene (JA/ET)‐dependent signalling pathways with concomitant induction of PDF1.2 (plant defensin 1.2) in Arabidopsis (Kachroo and Kachroo, 2009; Pieterse et al., 2009). Although rhizobacteria‐mediated ISR predominantly involves an SA‐independent JA/ET‐mediated defence response, some rhizobacteria may trigger the SA‐dependent signalling pathway (De Vleesschauwer et al., 2006). A variety of bacterial determinants contributing to ISR‐eliciting activity have been identified, including flagella, lipopolysaccharides, quorum‐sensing molecules, volatile organic compounds (VOCs) and cyclic lipopeptides (CLPs) (Jourdan et al., 2009; Pieterse et al., 2014).
CLPs, namely surfactin, iturin and fengycin family CLPs, are generated by multiple species of Bacillus with an array of biological activity (Raaijmakers et al., 2010). The surfactin family encompasses heptapeptides interlinked with β‐hydroxy fatty acid chains with a length of 12–16 carbon atoms to form a cyclic lactone ring structure, and exhibit strong antiviral and antibacterial, but no marked antifungal, activity (Henry et al., 2011). Surfactin and fengycin, but not iturin, have also been reported to elicit ISR in plants against fungal pathogens, albeit exhibiting differential preference towards different plant cell types (Ongena and Jacques, 2008). The plant root‐colonizing bacterium B. amyloliquefaciens strain FZB42 (Fan et al., 2012) is a naturally occurring isolate capable of stimulating plant growth (Idris et al., 2007). Furthermore, the strain produces all three families of lipopeptides, namely surfactin, bacillomycin D (iturin family member) and fengycin (Koumoutsi et al., 2004).
Studies involving ISR activity in turfgrass species are very scarce. Whether any ISR activity in perennial ryegrass could be elicited following the application of CLPs is yet to be documented. The primary objective of this study was to investigate the role of rhizobacteria‐originating surfactin in suppressing grey leaf spot disease in perennial ryegrass. ISR activity in perennial ryegrass to suppress M. oryzae infection was studied through the application of a mutant B. amyloliquefaciens FZB42‐AK3 strain, which is deficient in the production of bacillomycin D and fengycin (and hence only produces surfactin) (Koumoutsi et al., 2004), and a semi‐purified (solid‐phase extraction, SPE) surfactin preparation.
Results
Suppression of grey leaf spot disease by B. amyloliquefaciens live cells and SPE‐enriched surfactin (SPE‐surfactin) lipopeptide
The purpose of this experiment was to evaluate the potential of root‐drench application of live B. amyloliquefaciens FZB42‐AK3 cells and SPE‐surfactin in suppressing grey leaf spot disease of perennial ryegrass. In order to analyse the disease lesion severity, grey leaf spot lesion types were divided into four groups based on a previous report (Table 1) (Curley et al., 2005). The application of live cells of wild‐type (wt) B. amyloliquefaciens FZB42, ΔbmyAΔfenA B. amyloliquefaciens FZB42‐AK3 strain (bacillomycin D‐ and fengycin‐deficient mutant) and SPE‐surfactin (500 μg/mL) significantly reduced both the disease incidence (DI) and disease severity (DS) of grey leaf spot relative to mock‐treated control plants (P < 0.05) (Fig. 1a). Subsequent bioassays revealed that SPE‐surfactin, when applied at 250 μg/mL (241.3 μm) and 500 μg/mL (488.62 μm), but not at 100 μg/mL (96.52 μm), concentrations significantly suppressed grey leaf spot disease (P < 0.05) (Fig. 1b). However, all four different lesion types (Fig. 1c) observed in treated plants differed significantly from those of mock‐treated control plants (P < 0.05) (Fig. 1d). Lesion type IV (most severe susceptible reaction causing leaf discoloration and blighting) was more prevalent (73.9%) in mock‐treated control plants than in AK3‐ and SPE‐surfactin‐treated plants (30.4% and 20.6%, respectively) (Fig. 1d). In the last two treatments, lesion type III was the most predominant symptom type observed (35.5% and 32.5%, respectively) with type I being the least prevalent (11.8% and 16.9%, respectively) (Fig. 1d). However, there were no significant differences between wt‐FZB42‐ and mutant AK3‐treated plants in the lesion type assay (data not shown).
Table 1.
Grey leaf spot lesion types on perennial ryegrass leaf blades at 5 days post‐inoculation with Magnaporthe oryzae (Curley et al., 2005)
| Type* | Description |
|---|---|
| I | Dark brown to black, non‐sporulating lesions <1.0–3.0 mm in length |
| II | Dark brown to black lesion with central, often sporulating, necrotic area, 1.0–3.0 mm in length |
| III | Circular or small diamond‐shaped lesions with dark brown to black borders with greyish central sporulating areas |
| IV | Completely unbordered, large expanding dark brown lesions with or without sporulating area with chlorotic haloes |
*Lesion types were numbered according to the severity, type IV being the most severe (causing leaf discoloration and blighting) and types I and II being the least severe.
Figure 1.
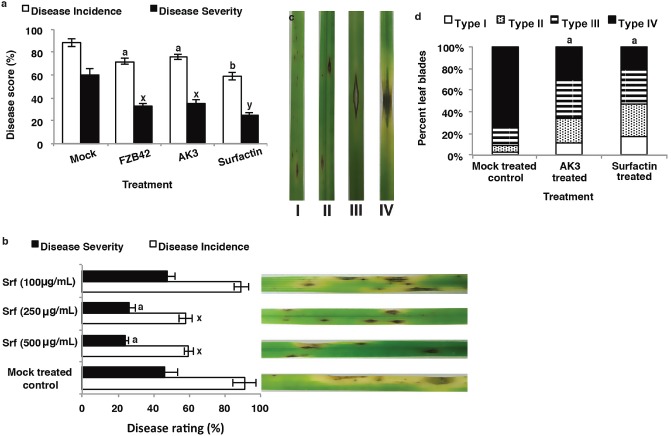
Effects of Bacillus amyloliquefaciens live cells and solid‐phase extraction (SPE)‐enriched surfactin treatment as root‐drench method on grey leaf spot disease incidence (DI) and disease severity (DS) development on perennial ryegrass. (a) DI and DS levels [disease score (%)] in plants treated with live cells of wild‐type B. amyloliquefaciens FZB42 or ΔbmyAΔfenA B. amyloliquefaciens FZB42‐AK3 (AK3) or 500 μg/mL of SPE‐enriched surfactin. (b) DI and DS levels in plants treated with different concentrations (μg/mL) of SPE‐enriched surfactin (Srf). (c) Disease lesion types on the symptomatic leaf blades 5 days post‐inoculation with Magnaporthe oryzae as described in Table 1. (d) Percentage of different lesion types observed on symptomatic leaf blades treated with AK3 live cells and SPE‐enriched surfactin; data from one representative experiment are presented. Mock‐treated control plants were treated with saline (0.85%) water. Different letters indicate significant differences from control and among treatments using Student–Newman–Keul's test (P < 0.05). Each bar represents mean ± standard error for three replicates from one representative experiment. Repetition of experiments led to results very similar to those shown.
We have demonstrated previously that live bacterial cells and enriched lipopeptide extracts from B. amyloliquefaciens FZB42 inhibit M. oryzae mycelial growth and spore germination (Rahman and Uddin, 2010). To rule out the possibility that disease suppression was caused by a direct effect from the possible migration of root‐inoculated bacteria, foliar tissues were analysed for AK3 bacterial cell count. The presence of AK3 colonies was not detected in randomly collected leaf samples from three independent experiments, with a reproducible detection limit of 102 colony‐forming units (CFU)/g tissues, suggesting that bacterial colonization remained confined to the rhizosphere. However, the rhizosphere concentrations of FZB42 and AK3 cells were found to be (6.1 ± 2.1) × 106 and (5.3 ± 3.2) × 106 CFU/g of root fresh weight in 5‐week‐old plants.
Cytological analysis of the perennial ryegrass defence response against M. oryzae
3,3′‐Diaminobenzidine (DAB) staining
To gain an insight into the mechanism(s) associated with AK3‐ and SPE‐surfactin‐mediated ISR, the generation of pathogenesis‐related H2O2 was analysed using DAB staining (Thordal‐Christensen et al., 1997). Consistent differences in DAB reaction types between treated and mock‐treated control plants were observed from 36 h post‐inoculation (hpi) onwards (Fig. S1, see Supporting Information). DAB‐facilitated H2O2 detection was confirmed by 10 mm ascorbate treatment, which abolished the brown colour (Thordal‐Christensen et al., 1997). Starting at 36 hpi, epidermal cells of leaf blades were found to respond to pathogen ingress through the development of various cellular reaction types that were grouped into six categories (Fig. 2a).
Type I: successful fungal invasion with no visible cellular responses in the infected epidermal cell, or neighbouring epidermal or mesophyll cells.
Type II: DAB‐positive partial staining observed as a yellowish‐brown halo surrounding the appressorial penetration point of the epidermal cell, indicating the accumulation of local H2O2 in CWAs.
Type III: pale yellow or brownish staining of anticlinal cell walls of the primary invaded epidermal cell, together with successful fungal invasion of neighbouring cells, without the accumulation of any cytoplasmic vesicles or granules or browning of the whole cell.
Type IV: DAB‐positive intense browning of the infected epidermal cell, as well as neighbouring epidermal and mesophyll cells.
Type V: DAB‐positive whole‐cell staining of the primary infected cell only, associated with the abrupt arrest of intracellular hypha (IH), when present, within the primary penetrated cell.
Type VI: single‐cell infection site associated with the occurrence of DAB‐positive intense brownish granules in the cell cytoplasm.
Figure 2.
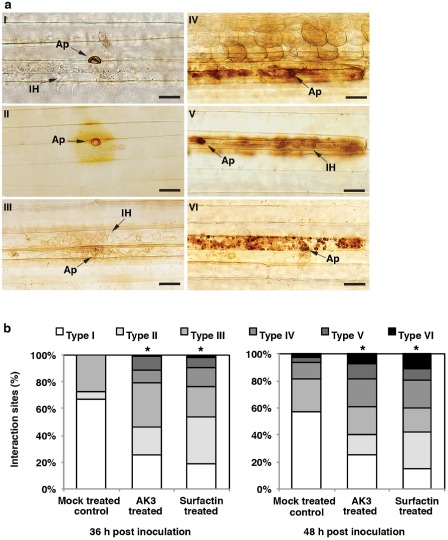
(a) Classification of cytological perennial ryegrass–Magnaporthe oryzae interaction types in leaf epidermal cells following 3,3′‐diaminobenzidine (DAB) staining: I, successful invasion of fungal hyphae with no visible plant cellular responses; II, DAB‐positive H2O2 accumulation in the cell wall appositions (CWAs); III, pale yellow or brownish staining of anticlinal cell walls of the primary infected cell together with intracellular hyphae (IH) in neighbouring cells, but no detectable H2O2 accumulation; IV, DAB‐positive browning of the primary infected and neighbouring epidermal and mesophyll cells; V, DAB‐positive single whole‐cell browning; VI, intense DAB‐positive brownish granules in primary infected cell. Bars, 20 μm. Ap, appressorium. (b) Frequency of different DAB‐associated cellular phenotypes in treated and mock‐treated perennial ryegrass leaves following inoculation with M. oryzae. Data from one experiment are presented. Repetition of the experiments led to very similar results as shown here. Differences in each interaction type observed between treated and mock‐treated control plants were evaluated using Student–Newman–Keul's test. The presence of an asterisk indicates significant differences (P < 0.05) in each interaction type in treated plants relative to mock‐treated plants.
Based on the type of DAB reaction, types I and III were considered to be associated with compatible interaction, whereas types IV–VI represented single‐ or multiple‐cell HR‐type interaction. At 36 hpi, control plants exhibited only the first three types of DAB reaction (I–III), with types I and III comprising the majority (>90%) of all interactions, suggesting the lack of development of any major fungal inhibitory defence responses (Fig. 2b). However, in AK3‐ and SPE‐surfactin‐treated plants, compatible interaction types (I and III) made up less than 60% of all interaction sites, whereas DAB reaction type II appeared to be the most prevalent type in SPE‐surfactin‐treated plants (Fig. 2b). By 48 hpi, type II reaction was no longer visible in mock‐treated control plants with less than one‐fifth of all infection sites associated with HR‐type reactions (Fig. 2a, IV–VI). By contrast, DAB reaction type II was not only visible in both AK3‐ and SPE‐surfactin‐treated plants, but was also the most predominant interaction type in SPE‐surfactin‐treated plants at 48 hpi (Fig. 2b). Furthermore, unlike mock‐treated control plants, in AK3‐ and SPE‐surfactin‐treated plants, more than one‐third of all interaction types belonged to HR‐type reactions (Fig. 2a, IV–VI).
Accumulation of phenolic compounds
Phenolic compounds were found to accumulate at the sites of attempted pathogen entry inside CWAs, as well as in the entire infected cells and in the adjacent neighbouring cells (Fig. 3a,b). Although autofluorescence was detectable irrespective of the treatment, significantly greater numbers of interaction sites in AK3‐ and SPE‐surfactin‐treated plants exhibited augmented deposition of phenolic compounds at 24 and 48 hpi relative to those in mock‐treated control plants (P < 0.05) (Fig. 3c). CWAs containing polyphenolic compounds were also detected using toluidine blue staining (Fig. 4a–c). By 48 and 72 hpi, significantly more CWAs exhibited toluidine blue‐positive lignin/polyphenolic deposition in AK3‐ and SPE‐surfactin‐treated perennial ryegrass relative to those in mock‐treated control plants (P < 0.05) (Fig. 4d). In addition, enhanced and stronger deposition of lignin/polyphenolic compounds was observed in treated plants (Fig. 4a) than in mock‐treated controls (Fig. 4b). Furthermore, mock‐treated control leaf blades more frequently exhibited IH by 36 hpi (Fig. 4c), indicating failure of the lignin/polyphenolic CWAs in these plants to restrict fungal penetration.
Figure 3.
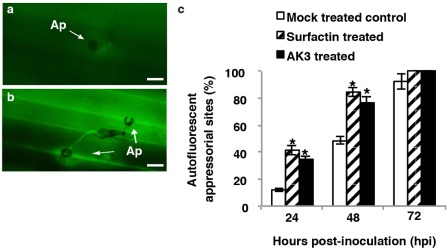
Representative epifluorescence images of mock‐treated control (a) and solid‐phase extraction (SPE)‐enriched surfactin‐treated (b) perennial ryegrass epidermal cells with attempted penetration sites of Magnaporthe oryzae conidia at 24 h post‐inoculation (hpi). Autofluorogenic phenolics are visible in cell wall appositions (CWAs), as well as in whole cells beneath appressoria. Treatment with AK3 live cells generated very similar images to those of the SPE‐enriched surfactin treatment. Bars, 20 μm. Ap, appressorium. (c) Frequency of autofluorogenic phenolic deposition at the attempted penetration sites of M. oryzae conidia on leaf epidermal cells in treated and mock‐treated perennial ryegrass leaves following inoculation with M. oryzae. Values represent the means ± standard deviation. The presence of an asterisk indicates a significant difference from mock‐treated control plants using Student–Newman–Keul's test (P < 0.05).
Figure 4.
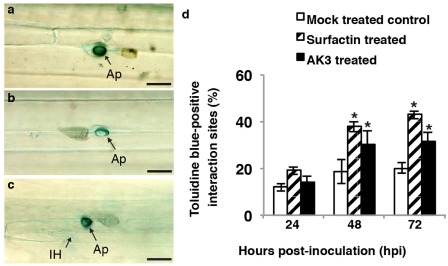
Representative images of toluidine blue staining for lignin/polyphenolics (appear turquoise blue) of solid‐phase extraction (SPE)‐enriched surfactin‐treated (a) and mock‐treated control (b) perennial ryegrass epidermal cells with attempted penetration sites of Magnaporthe oryzae conidia at 24 h post‐inoculation (hpi). (c) Penetrated lignin/polyphenolic‐deposited cell wall appositions (CWAs) in which M. oryzae had established intracellular hyphae (IH) at 36 hpi were frequently observed in control but not treated plants. Treatment with AK3 live cells generated very similar images to those of SPE‐enriched surfactin treatment. Bars, 20 μm. Ap, appressorium. (d) Frequency of lignin/polyphenolic deposition at the attempted penetration sites of M. oryzae conidia on leaf epidermal cells in treated and mock‐treated perennial ryegrass leaves following inoculation with M. oryzae. Values represent the means ± standard deviation. The presence of an asterisk indicates significant difference from mock‐treated control plants using Student–Newman–Keul's test (P < 0.05).
Callose deposition and IH propagation
When present in CWAs, callose deposition can either prevent the penetration of appressorial penetration pegs (Fig. 5a,b) or form a haustorial collar neck (Fig. 5c,d) when breached (Zeyen et al., 2002). Callose deposition was observed regardless of the treatment type by 24 hpi (Table 2). However, both AK3 and SPE‐surfactin treatment resulted in significantly higher proportions of callose deposition at 48 and 72 hpi relative to that in mock‐treated control plants (P < 0.05). Furthermore, significantly higher proportions of all deposited callose were penetrated by fungal penetration pegs in mock‐treated control plants (6.5% of all attempted penetration sites were non‐penetrated) relative to that in AK3‐ and SPE‐surfactin‐treated plants (15% and 31% remained non‐penetrated, respectively) at 48 hpi (P < 0.05) (Table 2).
Figure 5.
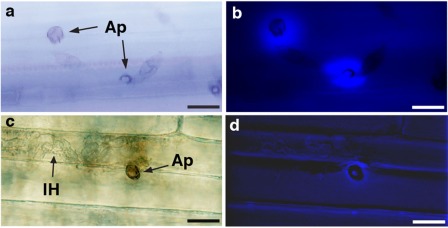
Representative images of aniline blue‐stained callose deposition inside cell wall appositions (CWAs) during attempted penetration by Magnaporthe oryzae conidia on perennial ryegrass epidermal cells. Non‐penetrated papillae showing localized deposition of callose beneath the attempted penetration sites as seen by epifluorescent microscopy with white light (a) and UV filter (b). Penetrated callose‐deposited papilla on epidermal cell, as viewed by epifluorescent microscopy with white light (c) and UV filter (d), in which M. oryzae has established intracellular hyphae (IH). Treatment with AK3 live cells generated very similar images to those of solid‐phase extraction (SPE)‐enriched surfactin treatment. Bars, 20 μm. Ap, appressorium.
Table 2.
Callose deposition on perennial ryegrass leaf blades at M agnaporthe oryzae infection sites during different time points
| Time (hpi) | Interaction sites with deposited callose* (%) | ||
|---|---|---|---|
| Mock‐treated control | SPE‐surfactin treated | AK3 treated | |
| 24 | 16.8 a† ± 2.6 | 22.7 a ± 2.9 | 21.0 a ± 3.1 |
| 48 | 19.3 a ± 1.7 | 42.4 b ± 3.9 | 30 c ± 2.7 |
| 72 | 21.5 a ± 3.0 | 45.5 b ± 5.3 | 31.6 c ± 2.5 |
| Non‐penetrated‡ | |||
| 48 | 33.7 a ± 4.9 | 72.7 b ± 2.6 | 50.4 c ± 7.8 |
*Data represent the mean ± standard deviation of 75–125 interaction sites at each time point with three replicates. AK3, live cells of B. amyloliquefaciens FZB42‐AK3; SPE‐surfactin, solid‐phase extraction‐enriched surfactin at 250 μg/mL concentration.
†Values followed by different letters within the row are significantly different according to Student–Newman–Keul's multiple range test (P < 0.05).
‡Percentage of callose‐deposited sites that were not penetrated by a M. oryzae penetration peg at the designated time point.
A detailed examination of the temporal changes in IH proliferation was visualized using KOH–aniline blue staining in M. oryzae‐inoculated plants (Fig. 6a–h). In mock‐treated control plants, a rapid growth of IH was observed by 36 hpi and, within 48 hpi, resulted in secondary cell (secondary epidermal cell) infection (Fig. 6a,b). However, in AK3‐ and SPE‐surfactin‐treated plants, M. oryzae IH was contained within the primary infected cell (almost 80% of infection sites when IH was present), even at 48 hpi, and invasion into secondary cells was not observed until 60 hpi (Fig. 6f,g). Conversely, in mock‐treated control plants, by 60 hpi, multiple surrounding epidermal cells from the initial focal point were invaded by IH (Fig. 6c) and, by 72 hpi, extensive proliferation of IH and massive fungal biomass encompassing multiple layers of neighbouring cells were observed (almost 80% of all infection sites) (Fig. 6d). In contrast, in AK3‐ and SPE‐surfactin‐treated plants, by 72 hpi, M. oryzae IH were contained within the secondary infected cells in approximately 30% and 45% of all infected sites examined, respectively (Fig. 6h).
Figure 6.
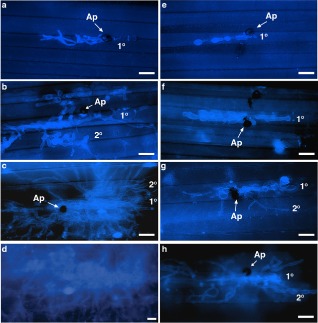
Representative images of KOH–aniline blue staining of fungal hyphae inside epidermal cells of perennial ryegrass leaves infected with Magnaporthe oryzae observed under an epifluorescent microscope. (a–d) Mock‐treated control. (e–h) Solid‐phase extraction (SPE)‐enriched surfactin‐treated perennial ryegrass. (a, e) 36 h post‐inoculation (hpi); (b, f) 48 hpi; (c, g) 60 hpi; (d, h) 72 hpi. Treatment with AK3 live cells generated very similar images to those of SPE‐enriched surfactin treatment. Bars, 20 μm. Ap, appressorium; IH, intracellular hyphae; 1°, primary infected epidermal cell; 2°, secondary infected epidermal cell.
Defence‐related gene expression analysis
To investigate the resistance mechanisms at a molecular level, gene expression of selected defence‐related genes, namely LpPrx (Prx, peroxidase), LpOXO4 (OXO, oxalate oxidase), LpPAL (PAL, phenylalanine ammonia lyase), LpLOXa (LOX, lipoxygenase), LpTHb (putative defensin) and LpDEFa (DEFa, putative α‐defensin), was analysed using real‐time quantitative reverse transcription‐polymerase chain reaction (RT‐PCR). These PR genes were selected on the basis of previous reports on transcriptome analysis of H2O2‐ and pathogenic fungus‐mediated induction of defence‐related genes in plant pathosystems (Eaton et al., 2010; Huckelhoven, 2007; Jourdan et al., 2009).
An induction of LpPrx expression was observed in AK3‐ and SPE‐surfactin‐treated plants by 24 hpi, which was more than 2.0‐fold and 3.6‐fold higher, respectively, than in mock‐treated control plants (P < 0.05) (Fig. 7a). Similarly, in AK3‐ and SPE‐surfactin‐treated plants, an increased (4.2‐fold and 10.4‐fold, respectively) expression level of OXO4, another candidate for the production of defence‐related extracellular H2O2 in cereal plants (Molla et al., 2013; Zhou et al., 1998), was observed by 24 hpi (Fig. 7b). In both instances, only SPE‐surfactin‐treated plants exhibited significantly higher expression (P < 0.05) than mock‐treated control plants at all times. In addition, a much stronger and significant induction of both PAL (Fig. 7c) and LOXa (Fig. 7d) genes was associated with AK3‐ (7.9‐fold and 6.9‐fold, respectively) and SPE‐surfactin‐ (13.5‐fold and 13.2‐fold, respectively) treated plants by 24 hpi, relative to that in mock‐treated control plants (P < 0.05). However, by 72 hpi, the relative expression levels of both genes were comparable in all plants with no significant difference. Plant defensins (PR‐12 proteins) exhibit membrane‐permeabilizing ability (Edreva, 2005; Huckelhoven, 2007) and are deposited together with callose following pathogen inoculation (Jensen et al., 2008). The analysis of two putative defensin genes, LpTHb and LpDEFa, indicated an elevated expression level in both AK3‐ (1.7‐fold and 3.7‐fold, respectively) and SPE‐surfactin‐ (8.1‐fold and 3.8‐fold, respectively) treated plants relative to control plants within 24 hpi (P < 0.05) (Fig. 7e,f). Although the expression level of LpTHb peaked at 48 hpi, LpDEFa exhibited a peak expression level at 72 hpi in both AK3‐ and SPE‐surfactin‐treated plants. However, the relative expression of LpTHb in AK3‐treated plants was found to be no different from that of mock‐treated control plants at 72 hpi. In addition, in mock‐treated control plants, the relative expression levels of both LpTHb and LpDEFa were higher at 72 hpi relative to earlier time points. Collectively, these gene expression analyses indicated that AK3 and SPE‐surfactin treatment resulted in an attacker‐induced augmented expression of defence‐related genes relative to that in mock‐treated control plants.
Figure 7.
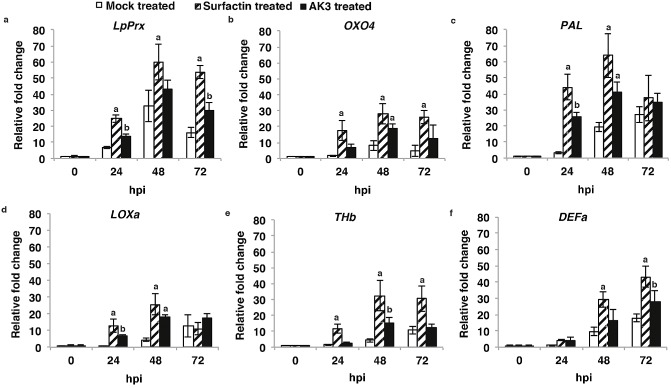
Expression pattern of perennial ryegrass genes [(a) Prx, (b) OXO4, (c) PAL, (d) LOXa, (e) THb, (f) DEFa] following inoculation with Magnaporthe oryzae. Filled white bars, mock‐treated control; striped bars, solid‐phase extraction (SPE)‐enriched surfactin treated; filled black bars, AK3 live cell treated. Each bar represents the average relative expression (± SD) of PR genes normalized to the housekeeping gene eEF1A(s) (stably expressed eukaryotic elongation factor 1α) for three replicates. Fold changes were calculated by calibrating all data to the 0‐h time point of a healthy control plant leaf tissue sample using the comparative threshold method (2–ΔΔCt) (Livak and Schmittgen, 2001). Different letters indicate significant differences from mock‐treated control using Student–Newman–Keul's test (P < 0.05).
Discussion
Root treatment with B. amyloliquefaciens live cells and SPE‐surfactin suppresses grey leaf spot on perennial ryegrass
Our aim in this study was to investigate the possible role of surfactin in eliciting ISR in the perennial ryegrass–M. oryzae pathosystem by taking advantage of the ΔbmyAΔfenA mutant B. amyloliquefaciens FZB42‐AK3 strain (Koumoutsi et al., 2004). Bacillus amyloliquefaciens FZB42 is a well‐known rhizobacterium with in vitro antifungal activity against multiple plant‐pathogenic fungi, such as Fusarium oxysporum and Gaeumannomyces graminis (Grosch et al., 2001; Koumoutsi et al., 2004). Furthermore, the fungal disease suppression ability of this strain has been observed in multiple plant pathosystems, including tomato, cucumber, cotton and, most recently, lettuce (Chowdhury et al., 2013). However, to our knowledge, this is the first report showing that live cells of B. amyloliquefaciens and SPE‐enriched surfactin are able to suppress grey leaf spot disease in perennial ryegrass.
Comparable suppression of grey leaf spot disease through root application of live cells of wt B. amyloliquefaciens FZB42 and its mutant AK3 (ΔbmyA::Emr, ΔfenA::Cmr) indicates that bacillomycin D and fengycin are dispensable for ISR activity in perennial ryegrass (Fig. 1a). Furthermore, 250 μg/mL (241.3 μm) SPE‐surfactin was sufficient for ISR‐mediated suppression of M. oryzae (Fig. 1b), with no inhibitory effect on fungal spore germination and appressorium formation (Fig. S2, see Supporting Information). Another CLP, massetolide A (100 μg/mL), from Pseudomonas fluorescence SS101, was found to suppress late blight severity on tomato leaves (Tran et al., 2007), suggesting that different plant pathosystems could vary considerably in resistance activity depending on the application rate of specific bacterial determinants. Fourier transform‐mass spectrometric fragmentation pattern analyses of different surfactin homologues from AK3 and standard surfactin (Sigma‐Aldrich, St. Louis, MO, USA) were an exact match, suggesting that the amino acid sequences of the heptapeptide ring of surfactin isomers were identical (ELLDVLL) (Fig. S3, see Supporting Information). AK3 strain‐generated surfactin exhibited a relative proportion of C12 : C13 : C14 : C15 = 2:7:42:49 (%) (Fig. S4, see Supporting Information), which is in agreement with the requirement of C13 or longer acyl chain‐bearing surfactin homologues for the generation of any ISR activity (Henry et al., 2011).
The FZB42 strain has been observed to efficiently colonize root surfaces of Arabidopsis, corn (Fan et al., 2012), rice (Wang et al., 2013), lettuce (Chowdhury et al., 2013) and wheat (Talboys et al., 2014). Recovery of live FZB42 and AK3 cells from the perennial ryegrass rhizosphere indicated that they also efficiently colonize the root zone of this grass species. Furthermore, symptomatic leaf blades of mock‐treated control plants exhibited predominantly lesion type IV, whereas treated plant infected leaves shared almost equal proportions of lesion types II, III and IV (Fig. 1d). The appearance of different types of lesions suggests that systemic resistance induced by AK3 cells and SPE‐surfactin resembles the resistance phenotype of quantitative trait loci‐governed partial resistance (Curley et al., 2005; De Vleesschauwer et al., 2006).
H2O2‐mediated defence responses in perennial ryegrass following AK3 live cell and SPE‐surfactin treatment against M. oryzae
Similar to surfactin‐induced H2O2 generation in tobacco cell culture, and in tomato and tobacco root tissues (Henry et al., 2011; Jourdan et al., 2009), SPE‐surfactin application resulted in enhanced H2O2 accumulation in perennial ryegrass root tissues (Fig. S5, see Supporting Information). Based on the DAB‐positive cellular phenotypes observed, treatment with AK3 cells and SPE‐surfactin resulted in an enhanced attacker‐induced ROS (H2O2) production that was characterized by a timely oxidative burst and often encompassed one or more underlying layers of mesophyll cells at the attempted penetration sites by the fungal appressoria (Fig. 2a,b). Among all the DAB‐positive interaction types, type II reaction was present in the treated plants at both 36 and 48 hpi (Fig. 2b), but only weakly present at 36 hpi in mock‐treated control plants (Figs 2b and S1b). Comparable strong deposition of DAB‐positive H2O2 inside CWAs in barley (Hordium vulgare) leaves thwarted Blumeria graminis f. sp. hordei (Bgh) penetration attempts (Thordal‐Christensen et al., 1997). Similarly, in the WRKY45‐overexpressing rice–M. oryzae interaction, H2O2 accumulated in the cell wall, between the cell wall and plasma membrane, immediately underneath the fungal appressorium and around the penetration peg, thereby blocking fungal invasion (Shimono et al., 2012). This phenotypic similarity prompted us to investigate the peroxidase activity in the different cellular fractions of infected leaves. Indeed, a rapid enhancement of peroxidase activity in the cell wall and intercellular fluid (ICF) fractions of the treated plants was observed (Fig. S6, see Supporting Information). However, DAB type III reaction closely resembled a DAB‐negative reaction type observed by De Vleesschauwer et al. (2008) in the pseudobactin (from Pseudomonas fluorescens WCS374r rhizobacterium)‐treated rice–M. oryzae interaction. In addition, IH were observed to proliferate and spread to secondary cells which, together with the lack of cytoplasmic granulation or browning of the whole cell (Fig. 2a, III), indicated that type III reaction was a sensitive/compatible‐type interaction. However, the presence of DAB type IV–VI reactions revealed that a proportion of appressoria triggered additional layers of defence response, including the HR‐type reaction, following successful penetration (Fig. 2a, IV–VI). A comparative analysis of DAB reaction types at two different time periods suggested that application of AK3 live cells and SPE‐surfactin generated higher frequencies of enhanced and pathogen‐blocking H2O2‐positive CWAs (type II), as well as whole‐cell HR‐type incompatible interactions (types IV–VI), relative to that in mock‐treated control plants (Fig. 2b).
H2O2‐dependent, peroxidase‐mediated cross‐linking of cell wall‐localized phenolic compounds plays an important role in strengthening plant cell walls and limiting pathogen invasion (Almagro et al., 2009). Robust accumulation of fluorescent phenolic compounds inside CWAs, and in multiple cells surrounding the attempted penetration sites (Fig. 3b,c), suggested a rapid onset of cell wall fortification in AK3‐ and SPE‐surfactin‐treated plants. Similar deposition of phenolic compounds in the barley–Bgh (Huckelhoven, 2007) and rice–M. oryzae (Faivre‐Rampant et al., 2008) interactions was directly proportional to fungal penetration failure. In addition, callose (β‐1,3‐glucan) deposition is recognized to reinforce weakened or compromised sections of the plant cell wall (Daudi et al., 2012). Augmented deposition of callose was observed in treated plants, together with significantly more non‐penetrated callose spots, relative to mock‐treated controls (Fig. 5 and Table 2). Rhizobacteria‐mediated activation of ISR, involving cell wall reinforcement and accumulation of H2O2 and defence‐related phenolics, following pathogen challenge, has been described (Ahn et al., 2007; De Vleesschauwer et al., 2008). In Arabidopsis, Prx33 and Prx34 knockdown lines (AtPrx33–34 kd), which are deficient in generating cell wall/apoplastic H2O2 following microbe‐associated molecular pattern (MAMP) (Flg22) treatment, exhibited a significantly reduced level of callose deposition (Daudi et al., 2012). These AtPrx33–34 kd lines also exhibited larger leaves than wt, indicating their inverse relationship with leaf expansion (Daudi et al., 2012). Noticeably, drought‐induced ionically bound cell wall peroxidases showed a similar relationship to leaf expansion in annual ryegrass (Lolium temulentum L.) (Bacon et al., 1997). In this study, we also observed pathogen‐mediated induction of comparable ionically bound cell wall peroxidase activity in AK3‐ and SPE‐surfactin‐treated perennial ryegrass (Fig. S6). Depending on the physiological conditions, peroxidases may act as either H2O2 scavengers or generators (Almagro et al., 2009; O'Brien et al., 2012). Hence, our results suggest that peroxidase activity was up‐regulated in AK3‐ and SPE‐surfactin‐treated perennial ryegrass following M. oryzae inoculation, which resulted in the enhanced generation of H2O2, thereby activating a cascade of events leading to the augmented deposition of callose and phenolic cross‐linking of lignin precursors. However, progression of IH in leaf cells of treated plants was quite limited over the 72‐hpi time period relative to that in mock‐treated control plants (Fig. 6a–h). Similar restricted intracellular proliferation of M. oryzae was observed in resistant (ABR5) Brachypodium distachyon leaf epidermal cells (Parker et al., 2009).
Enhanced expression of defence‐related genes is associated with the protection of perennial ryegrass against M. oryzae
ISR‐facilitated induction of defence‐related PR genes following pathogen challenge has been identified in several plant pathosystems (Ahn et al., 2007; Niu et al., 2011), including Harpophora oryzae‐primed defence genes in the rice–M. oryzae interaction (Su et al., 2013) and B. amyloliquefaciens CM‐2‐induced PR genes in the tomato–Ralstonia solanacearum interaction (Tan et al., 2013). Similarly, comparative analyses of the relative expression levels of all defence‐related genes at 0 hpi and later time points suggested a possible ‘priming’ (Pieterse et al., 2014) effect of AK3 live cell and SPE‐surfactin treatments (Fig. 7a–f).
LpPrx (Eaton et al., 2010), a homologue of barley Prx7 class III peroxidase, which exhibits targeted localization in the CWAs following inoculation with Bgh (An et al., 2006; Kristensen et al., 1999), displayed a rapid accumulation in AK3‐ and SPE‐surfactin‐treated plants (Fig. 7a). Notably, the relative expression pattern of LpPrx complemented the temporal distribution of cell wall and ICF peroxidase activity. Another well‐known candidate for pathogen‐induced H2O2 generation in plant cells is mesophyll‐localized germin‐like OXO (Almagro et al., 2009). OXO4, a perennial ryegrass wound‐, H2O2‐ and methyljasmonate (MeJA)‐inducible OXO (Le Deunff et al., 2004), exhibited a stronger magnitude of expression in treated plants relative to mock‐treated control plants (Fig. 7b), suggesting that OXO4 generated delayed accumulation of H2O2 in leaf mesophyll tissue cell wall, resulting in HR‐type PCD (Fig. 2a, IV).
Although the timing of the onset of both PAL and LOXa up‐regulation was similar in treated and mock‐treated control plants following M. oryzae inoculation, the magnitude of expression, by 24 hpi, was much higher in the former than in the latter (Fig. 7c,d). Rapid up‐regulation of these transcripts in treated plants suggested the accumulation of defence‐related phenolic metabolites, generated through the activation of phenylpropanoid and lipoxygenase pathways. Enriched activity of both PAL and LOX, and only LOX, was observed in tobacco cell culture and tomato plants, respectively, following surfactin application (Jourdan et al., 2009; Ongena et al., 2007).
We have demonstrated previously that the SAR activator benzothiadiazole (BTH) suppresses grey leaf spot in perennial ryegrass with enhanced expression of PR‐1, PR‐3.1 and PR‐5 (Rahman et al., 2014). However, no significant differential expression of any of these PR genes was detected in the present study. However, enhanced expression of two putative defensin genes, LpTHb and LpDEFa, which were found to be associated with partial resistance against multiple fungal diseases in perennial ryegrass (Dracatos et al., 2008), was observed in our study. In Arabidopsis NAC transcription factor ATAF1 knocked‐down lines, suppression of defensin genes PDF1.1 and PDF1.2 was associated with an increased penetration rate by Bgh, although deposition of callose at the attempted penetration sites remained unaffected (Jensen et al., 2008). Induced expression of OXO4, LOXa and putative defensins (LpTHb and LpDEFa) suggested that AK3 and SPE‐surfactin treatment might have potentiated the JA‐mediated defence response(s) in perennial ryegrass to impede M. oryzae infection. On the contrary, we have found previously that exogenous JA application increases the susceptibility of perennial ryegrass to M. oryzae infection (Rahman et al., 2014). However, the final nature of the plant defence responses to be manifested perhaps depends on the synergistic effect of SA‐ and JA‐mediated pathways, as co‐application of 0.1 mm JA and 0.1 mm SA in the apoplast resulted in higher accumulation of H2O2 and HR‐like PCD compared with lone application of each hormone in Arabidopsis (Mur et al., 2006). Based on multiple gene expression analyses, we speculate that physiological concentrations of both SA and JA activated (if any) by M. oryzae attack in treated perennial ryegrass were sufficient to exhibit a coordinated defence response tailored towards the restriction of fungal growth in infected plant cells. However, our experiments to determine the endogenous SA concentration showed inconclusive results to identify any differential expression of free SA between treated and mock‐treated control plants (data not shown). Further studies involving microarray‐ and metabolomics‐based analyses might help to comprehensively identify the signalling pathways associated with AK3‐ and SPE‐surfactin‐mediated ISR in perennial ryegrass.
Conclusions
Pronounced induction of multilayered defence responses, including the rapid accumulation of H2O2, phenolic/polyphenolic compounds and callose at the sites of attempted pathogen entry, together with the timely oxidative burst driving single or multicellular HR‐type PCD, following M. oryzae challenge in treated plants, supports the ISR effect of AK3 cells and SPE‐surfactin treatment in perennial ryegrass. Whether or not AK3‐induced ISR is strictly dependent on in situ surfactin biosynthesis could not be verified as surfactin‐deficient mutants of B. amyloliquefaciens FZB42 strains were observed by Raaijmakers et al. (2010) to exhibit an extremely poor growth rate and lacked motility. Evidently, the role of surfactin is indispensable for ISR activation, root colonization, biofilm formation and as a signalling molecule during extracellular matrix formation by B. subtilis (Pérez‐García et al., 2011). It is therefore conceivable that the release of surfactin by B. amyloliquefaciens FZB42‐AK3 into the rhizosphere of perennial ryegrass played a vital role in the ISR‐mediated suppression of M. oryzae infection. Surfactin molecules predominantly bind to the plant cell membrane, and such perception of surfactin molecules by plant cells has been primarily attributed to its ISR activity (Henry et al., 2011). Presumably, such perception could be responsible for the sensitization of the resistance activity of plants, resulting in a surveillance state that is extremely sensitive to M. oryzae penetration attempts, thereby triggering a rigorous activation of H2O2‐mediated defence responses. However, M. oryzae‐induced modification of host cell metabolic profiles (increased anti‐oxidant molecules) (Parker et al., 2009), as well as the requirement of peroxidase and laccase activity by pathogenic M. oryzae to scavenge/neutralize host cell‐accumulated H2O2 activity (Chi et al., 2009), amply supports the importance of H2O2‐induced defence responses in suppressing M. oryzae proliferation. The most recent demonstration of the M. oryzae redox potential to surmount the H2O2‐mediated oxidation potential in non‐induced susceptible plants (Samalova et al., 2014) supports our results indicating that prompt and robust induction of H2O2‐mediated multifaceted defence responses in AK3‐ and SPE‐surfactin‐treated perennial ryegrass plays a major role in grey leaf spot disease suppression.
Experimental Procedures
Plant materials and microbial culture conditions
All experiments were conducted in controlled‐environment chambers with perennial ryegrass (Lolium perenne L.) cv. Legacy‐II, a highly susceptible cultivar to infection by M. oryzae, grown in plastic pots (diameter, 10.2 cm) with a seeding rate of 20 g/m2 in peat Farfard mix no. 2 : sand (2:1, v/v), as described previously (Rahman et al., 2014). Rhizobacterium B. amyloliquefaciens FZB42 and its mutant strain AK3 (ΔbmyA::Emr, ΔfenA::Cmr) were collected from the Bacillus Genetic Stock Center (BGSC, Columbus, OH, USA) (Dean and Zeigler, 1994) and were maintained as recommended by BGSC. The pathogenic isolate of M. oryzae O5T was routinely grown on potato dextrose agar (PDA) at 25 °C. The preparation of M. oryzae conidia was identical to that described previously (Rahman et al., 2014).
Magnaporthe oryzae inoculation and disease rating
Four‐week‐old perennial ryegrass plants were inoculated with (3–4) × 104 spores/mL conidial suspension by atomizing the leaves until run‐off, as described previously (Rahman et al., 2014). Pots of plants were arranged in a randomized complete block design with three replications for each treatment. DI and DS were assessed 5 days after inoculation with M. oryzae, as described previously (Rahman et al., 2014). In brief, DI was assessed by quantifying the percentage of symptomatic leaves in each treatment. DS was assessed using an index of 0–10 (0, no necrosis; 10, >90% necrotic leaf area on individual blades). The rating scale used for different lesion types on the symptomatic leaf blades was measured according to the severity of the lesions described in Table 1 (Curley et al., 2005). Between 100 and 150 leaf blades from each pot of plants were analysed. DI and DS experiments were repeated twice and disease lesion type (DLT) experiments were repeated once. All lesion type data generated from control and treated plants in each experiment were used for significance analysis independently. Data from each experiment were subjected to analysis of variance (ANOVA) using MINITAB (version 16.1.0; Minitab Inc., State College, PA, USA). Multiple comparisons of means for DI and DS, and for each lesion type (for DLTs), were made using Student–Newman–Keul's (SNK) test (P < 0.05).
Bacterial inocula preparation and surfactin extraction
Bacillus amyloliquefaciens strains were prepared as described previously (Ongena et al., 2005). In brief, bacterial cells were grown at 30 °C for 72 h and 180 rpm in ‘Opt medium’ (Jourdan et al., 2009). Cultures were then centrifuged at 15 000 g for 15 min, washed twice with sterile saline water (0.85% NaCl) and re‐suspended in sterile saline water to make 5 × 107 colony‐forming units (CFU)/mL. An enriched surfactin lipopeptide extract was prepared using the SPE method described previously (Ongena et al., 2005) from a culture supernatant of the AK3 strain grown in ‘Opt medium’ (Jourdan et al., 2009).
ISR experiments
ISR experiments were performed using both live AK3 cells and SPE‐surfactin, as described previously (De Vleesschauwer et al., 2008). Briefly, ‘Legacy‐II’ perennial ryegrass (L. perenne L.) seeds were first surface sterilized with 1% sodium hypochlorite and air dried. Seeds were then soaked in AK3 live cell suspension in sterile saline (5 × 107 CFU/mL) for 30 min. In addition, the bacterial inoculum was thoroughly mixed with autoclaved potting mix (pot diameter, 10.2 cm) to a final density of 5 × 107 CFU/g and again after 3 weeks as a root drench. Mock‐treated control plants were treated with equal volumes of 0.85% sterile saline water. A 10‐mL solution of SPE‐surfactin (250 μg/mL) was applied once as a soil drench 2 days prior to pathogen challenge, unless otherwise stated.
Evaluation of bacterial colonization of plant roots
Plant root colonization and possible migration of root‐inoculated bacterial cells to distal leaf tissues were determined according to Kinsella et al. (2009) with minor modifications (Ongena et al., 2005). Briefly, after 5 weeks of growth, 1‐g samples of leaf and shoot tissue or root fresh weight (FW) were randomly collected from three independent pots of plants per experiment. Root samples were lightly washed with sterile Millipore water to remove clinging potting medium. Both tissue samples were then homogenized in 20 mL of sterile Millipore (Darmstadt, Germany) water with 0.05% Tween 20 and incubated for 1 h at 140 rpm. Following incubation, aliquots of 100 μL of serial dilutions were plated on ‘Turner and Backman selective medium’ (TBSM) (Kinsella et al., 2009) and incubated for 3 days at 24 °C. Furthermore, cyclohexamide (45 μg/mL) was added to inhibit fungi and polymixin B (22.5 μg/mL) to inhibit Gram‐negative bacteria. When required, chloramphenicol (5 μg/mL) and erythromycin (1 μg/mL) were added for AK3 selection. FZB42 colonies on the TBSM plates had a characteristic morphology, which was easily distinguishable, and formed the large majority of the total CFUs.
Visualization of defence responses
The production of H2O2 and reactive oxygen intermediates (ROIs) was detected using the ‘DAB‐uptake method’ (Thordal‐Christensen et al., 1997) with modifications (Daudi et al., 2012). Pieces of leaves (3 cm) were infiltrated with 1 mg/mL DAB containing Tween 20 (0.05% v/v) and 10 mm sodium phosphate buffer (pH 7.0) for 5 h. Leaves were then fixed in ethanol : glycerol : acetic acid (3:1:1, v/v) until clear, mounted in 60% glycerol (v/v) and visualized by bright‐field (BF) microscopy. Phenolic compounds were analysed as autofluorescence in cleared leaf segments and visualized under blue light epifluorescence (Nikon Eclipse E600, Melville, NY, USA; GFP filter set 480/30 nm; DM 400 dichroic beam splitter 505 nm and emission filter 535/40 nm) or by their blue/turquoise appearance when stained in a solution of 0.05% toluidine blue in 50 mm citrate buffer, pH 3.5, visualized by BF microscopy (Mellersh et al., 2002). For the visualization of callose deposition at the infection sites, an identical technique with 0.05% aniline blue stain was employed as described previously (Rahman et al., 2014). IH were visualized using a modified KOH–aniline blue technique (Hood and Shew, 1996). In brief, cleared leaves were boiled for 10 min in 1 m KOH, followed by staining with 0.05% aniline blue, and visualized using BF microscopy with a UV filter (Olympus BX‐61, Melville, NY, USA; excitation filter 350/50 nm, replicated DM 400 dichroic beam splitter and emission filter 460/50 nm).
In each experiment, 75–125 single‐cell interaction sites on leaf segments (length, 3 cm), randomly collected from the middle part of inoculated leaves from all experimental pots (replicates) and pooled together, were inspected and scored independently. All experiments were repeated twice. All BF and UV‐filtered images were acquired digitally [Hamamatsu cooled digital camera: ORCA ER (Model C4742‐80) (Bridgewater, NJ, USA) and Olympus DP71 (Center Valley, PA, USA)] and further processed with CellSens Dimension software.
Analysis of gene expression using quantitative real‐time PCR
The collection of leaf samples, RNA extraction, cDNA synthesis and quantitative RT‐PCR were identical to those described previously (Rahman et al., 2014). Specific primers for the perennial ryegrass genes LpOXO, LpPrx, LpPALa, LpLOXa, LpTHb, LpDEFa and LpeEF1A(s) (stably expressed eukaryotic elongation factor 1α) were used for each cDNA sample (Table 3). For each gene, the sample value at the 0‐h time point of healthy control plants was defined as the calibrator sample and the results were expressed as the fold change of mRNA over this sample using the comparative threshold method (2–ΔΔCt) (Livak and Schmittgen, 2001). Primers for each gene were chosen on the basis of the amplification efficiency between a target and the reference gene [eEF1A(s)], which was determined as described previously (Livak and Schmittgen, 2001). The specification of each pair of primers was also confirmed by randomly sequencing PCR products and further consolidated by melting curve analysis using real‐time PCR. All experiments were performed three times and each real‐time PCR sample was run in triplicate.
Table 3.
DNA primers used to assay the gene expression by quantitative real‐time PCR
| Gene | Primer | Primer sequence (5′–3′) | Product size (base pair) | Reference |
|---|---|---|---|---|
| Prx | Forward | CCGGAACACACCAGAATGAA | 111 | Eaton et al. (2010) |
| Reverse | TATGACGTACGGGTCGATGA | |||
| OXO4 | Forward | CGACTCTGGGAACAAGCTATAC | 139 | Le Deunff et al. (2004) |
| Reverse | CGACTCTGGGAACAAGCTATAC | |||
| PAL | Forward | GCTTGAAGGGTGCTGAGATT | 131 | Sawbridge et al. (2003) |
| Reverse | AGAGATGAGACCGAGAGAGTTG | |||
| LOX | Forward | TCGCTGAATCGCTTGAACA | 136 | Eaton et al. (2010) |
| Reverse | TCCAGACCATCATCGGGATA | |||
| THb | Forward | GTGAGCTCGATCGGTTAGTG | 74 | Dracatos et al. (2008) |
| Reverse | AGAAAGTGCCATCTCTGCTTG | |||
| DEFa | Forward | CTGTGTTGCTAATCTTGTGCG | 84 | Dracatos et al. (2008) |
| Reverse | CAGCACTTAATTTCAGGGTCTAC | |||
| eEF1A(s) | Forward | CCGTTTTGTCGAGTTTGGT | 113 | Lee et al. (2010) |
| Reverse | AGCAACTGTAACCGAACATAGC |
Supporting information
Fig. S1 Representative images of perennial ryegrass leaf blades treated with 3,3′‐diaminobenzidine (DAB), 24 and 36 h following inoculation with Magnaporthe oryzae. Ap, appressorium.
Fig. S2 Effect of solid‐phase extraction (SPE)‐enriched cyclic lipopeptide(s) from wild‐type (wt) Bacillus amyloliquefaciens FZB42 and AK3 strains on germination of ZsGreen‐tagged Magnaporthe oryzae. Ap, appressorium.
Fig. S3 Reverse phase high‐performance liquid chromatography (RP‐HPLC) and mass spectrometry (MS) analysis of surfactin isomers in solid‐phase extraction‐enriched surfactin (SPE‐surfactin) (a1, a2 and a3) and standard surfactin (b1, b2 and b3) (Sigma‐Aldrich).
Fig. S4 Relative proportions of different surfactin homologues in standard surfactin (Sigma‐Aldrich) (a) and solid‐phase extraction‐enriched surfactin (SPE‐surfactin) (b).
Fig. S5 Hydrogen peroxide (H2O2) content in perennial ryegrass roots following application of 250 μg/mL of solid‐phase extraction‐enriched surfactin (SPE‐surfactin).
Fig. S6 Time course of peroxidase enzyme activity in different cellular fractions of perennial ryegrass leaf cells following infection with Magnaporthe oryzae: (a) cell wall (CW); (b) intercellular fluid (ICF); (c) cytoplasm.
Acknowledgements
We thank the Huck Institute of Life Sciences and Genomic Core Facility, The Pennsylvania State University, University Park, PA, USA for the use of facilities in this study.
References
- Ahn, I.P. , Lee, S.W. and Suh, S.C. (2007) Rhizobacteria‐induced priming in Arabidopsis is dependent on ethylene, jasmonic acid, and NPR1. Mol. Plant–Microbe Interact. 20, 759–768. [DOI] [PubMed] [Google Scholar]
- Almagro, L. , Ros, L.V.G. , Belchi‐Navarro, S. , Bru, R. , Barcelo, A.R. and Pedreno, M.A. (2009) Class III peroxidases in plant defence reactions. J. Exp. Bot. 60, 377–390. [DOI] [PubMed] [Google Scholar]
- An, Q.L. , Huckelhoven, R. , Kogel, K.H. and Van Bel, A.J.E. (2006) Multivesicular bodies participate in a cell wall‐associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell. Microbiol. 8, 1009–1019. [DOI] [PubMed] [Google Scholar]
- Bacon, M.A. , Thompson, D.S. and Davies, W.J. (1997) Can cell wall peroxidase activity explain the leaf growth response of Lolium temulentum L. during drought? J. Exp. Bot. 48, 2075–2085. [Google Scholar]
- Chi, M.H. , Park, S.Y. , Kim, S. and Lee, Y.H. (2009) A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host. PLoS Pathog. 5, e1000401. doi: 10.1371/journal.ppat.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury, S.P. , Dietel, K. , Randler, M. , Schmid, M. , Junge, H. , Borriss, R. , Hartman, A. and Grosch, R. (2013) Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE, 8, e68818. doi: 10.1371/journal.pone.0068818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley, J. , Sim, S.C. , Warnke, S. , Leong, S. , Barker, R. and Jung, G. (2005) QTL mapping of resistance to gray leaf spot in ryegrass. Theor. Appl. Genet. 111, 1107–1117. [DOI] [PubMed] [Google Scholar]
- Daudi, A. , Cheng, Z.Y. , O'Brien, J.A. , Mammarella, N. , Khan, S. , Ausubel, F.M. and Bolwell, G.P. (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern‐triggered immunity. Plant Cell, 24, 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vleesschauwer, D. , Cornellis, P. and Höfte, M. (2006) Redox‐active pyocyanin secreted by Pseudomonas aeruginosa 7NSK2 triggers systemic resistance to Magnaporthe grisea but enhances Rhizoctonia solani susceptibility in rice. Mol. Plant–Microbe Interact. 19, 1406–1419. [DOI] [PubMed] [Google Scholar]
- De Vleesschauwer, D. , Djavaheri, M. , Bakker, P.A.H.M. and Höfte, M. (2008) Pseudomonas fluorescens WCS374r‐induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin‐mediated priming for a salicylic acid‐repressible multifaceted defense response. Plant Physiol. 148, 1996–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, D. and Zeigler, D. (1994) Bacillus Genetic Stock Center Strains & Data. 6th ed. Columbus, OH, USA: Ohio State University Press. [Google Scholar]
- Dracatos, P. , Cogan, N. , Dobrowolski, M. , Sawbridge, T. , Spangenberg, G. , Smith, K. and Forster, J. (2008) Discovery and genetic mapping of single nucleotide polymorphisms in candidate genes for pathogen defence response in perennial ryegrass (Lolium perenne L.). Theor. Appl. Genet. 117, 203–219. [DOI] [PubMed] [Google Scholar]
- Durrant, W.E. and Dong, X. (2004) Systemic acquired resistance. Annu. Rev. Phytopathol. 42, 185–209. [DOI] [PubMed] [Google Scholar]
- Eaton, C.J. , Cox, M.P. , Ambrose, B. , Becker, M. , Hesse, U. , Schardl, C.L. and Scott, B. (2010) Disruption of signaling in a fungal–grass symbiosis leads to pathogenesis. Plant Physiol. 153, 1780–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edreva, A. (2005) Pathogenesis‐related proteins: research progress in the last 15 years. Gen. Appl. Plant Physiol. 31, 105–124. [Google Scholar]
- Faivre‐Rampant, O. , Thomas, J. , Allegre, M. , Morel, J.B. , Tharreau, D. , Notteghem, J.L. , Lebrun, M.H. , Schaffrath, U. and Piffanelli, P. (2008) Characterization of the model system rice–Magnaporthe for the study of nonhost resistance in cereals. New Phytol. 180, 899–910. [DOI] [PubMed] [Google Scholar]
- Fan, B. , Borriss, R. , Bleiss, W. and Wu, X.Q. (2012) Gram‐positive rhizobacterium Bacillus amyloliquefaciens FZB42 colonizes three types of plants in different patterns. J. Microbiol. 50, 38–44. [DOI] [PubMed] [Google Scholar]
- Grosch, R. , Kofoet, A. and Junge, H. (2001) Biological control of root pathogens in soilless culture using bacteria. Acta Hortic. 548, 393–400. [Google Scholar]
- Henry, G. , Deleu, M. , Jourdan, E. , Thonart, P. and Ongena, M. (2011) The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune‐related defence responses. Cell. Microbiol. 13, 1824–1837. [DOI] [PubMed] [Google Scholar]
- Hood, M.E. and Shew, H.D. (1996) Applications of KOH–aniline blue fluorescence in the study of plant–fungal interactions. Phytopathology, 86, 704–708. [Google Scholar]
- Huckelhoven, R. (2007) Cell wall‐associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 45, 101–127. [DOI] [PubMed] [Google Scholar]
- Idris, E.E. , Iglesias, D.J. , Talon, M. and Borriss, R. (2007) Tryptophan‐dependent production of indole‐3‐acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant–Microbe Interact. 20, 619–626. [DOI] [PubMed] [Google Scholar]
- Jensen, M.K. , Hagedorn, P.H. , de Torres‐Zabala, M. , Grant, M.R. , Rung, J.H. , Collinge, D.B. and Lyngkjaer, M.F. (2008) Transcriptional regulation by an NAC (NAM‐ATAF1,2‐CUC2) transcription factor attenuates ABA signalling for efficient basal defence towards Blumeria graminis f. sp hordei in Arabidopsis . Plant J. 56, 867–880. [DOI] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Jourdan, E. , Henry, G. , Duby, F. , Dommes, J. , Barthélemy, J. , Thonart, P. and Ongena, M. (2009) Insights into the defense‐related events occurring in plant cells following perception of surfactin‐type lipopeptide from Bacillus subtilis . Mol. Plant–Microbe Interact. 22, 456–468. [DOI] [PubMed] [Google Scholar]
- Kachroo, A. and Kachroo, P. (2009) Fatty acid‐derived signals in plant defense. Annu. Rev. Phytopathol. 47, 153–176. [DOI] [PubMed] [Google Scholar]
- Kinsella, K. , Schulthess, C.P. , Morris, T.F. and Stuart, J.D. (2009) Rapid quantification of Bacillus subtilis antibiotics in the rhizosphere. Soil Biol. Biochem. 41, 374–379. [Google Scholar]
- Koumoutsi, A. , Chen, X.‐H. , Henne, A. , Liesegang, H. , Hitzeroth, G. , Franke, P. , Vater, J. and Borriss, R. (2004) Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 186, 1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen, B.K. , Bloch, H. and Rasmussen, S.K. (1999) Barley coleoptile peroxidases. Purification, molecular cloning, and induction by pathogens. Plant Physiol. 120, 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Deunff, E. , Davoine, C. , Le Dantec, C. , Billard, J.P. and Huault, C. (2004) Oxidative burst and expression of germin/oxo genes during wounding of ryegrass leaf blades: comparison with senescence of leaf sheaths. Plant J. 38, 421–431. [DOI] [PubMed] [Google Scholar]
- Lee, J.M. , Roche, J.R. , Donaghy, D.J. , Thrush, A. and Sathish, P. (2010) Validation of reference genes for quantitative RT‐PCR studies of gene expression in perennial ryegrass (Lolium perenne L.). BMC Mol. Biol. 11, doi: 10.1186/1471-2199-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2–ΔΔCt method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Mellersh, D.G. , Foulds, I.V. , Higgins, V.J. and Heath, M.C. (2002) H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. Plant J. 29, 257–268. [DOI] [PubMed] [Google Scholar]
- Molla, K.A. , Karmakar, S. , Chanda, P.K. , Ghosh, S. , Sarkar, S.N. , Datta, S.K. and Datta, K. (2013) Rice oxalate oxidase gene driven by green tissue‐specific promoter increases tolerance to sheath blight pathogen (Rhizoctonia solani) in transgenic rice. Mol. Plant Pathol. 14, 910–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur, L.A. , Kenton, P. , Atzorn, R. , Miersch, O. and Wasternack, C. (2006) The outcomes of concentration‐specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 140, 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, D.D. , Liu, H.X. , Jiang, C.H. , Wang, Y.P. , Wang, Q.Y. , Jin, H.L. and Guo, J.H. (2011) The plant growth‐promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate‐ and jasmonate/ethylene‐dependent signaling pathways. Mol. Plant–Microbe Interact. 24, 533–542. [DOI] [PubMed] [Google Scholar]
- O'Brien, J.A. , Daudi, A. , Butt, V.S. and Bolwell, G.P. (2012) Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta, 236, 765–779. [DOI] [PubMed] [Google Scholar]
- Ongena, M. and Jacques, P. (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16, 115–125. [DOI] [PubMed] [Google Scholar]
- Ongena, M. , Jacques, P. , Toure, Y. , Destain, J. , Jabrane, A. and Thonart, P. (2005) Involvement of fengycin‐type lipopeptides in the multifaceted biocontrol potential of Bacillus subtilis . Appl. Microbiol. Biotechnol. 69, 29–38. [DOI] [PubMed] [Google Scholar]
- Ongena, M. , Jourdan, E. , Adam, A. , Paquot, M. , Brans, A. , Joris, B. , Arpigny, J.L. and Thonart, P. (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 9, 1084–1090. [DOI] [PubMed] [Google Scholar]
- Parker, D. , Beckmann, M. , Zubair, H. , Enot, D.P. , Caracuel‐Rios, Z. , Overy, D.P. , Snowdon, S. , Talbot, N.J. and Draper, J. (2009) Metabolomic analysis reveals a common pattern of metabolic re‐programming during invasion of three host plant species by Magnaporthe grisea . Plant J. 59, 723–737. [DOI] [PubMed] [Google Scholar]
- Pérez‐García, A. , Romero, D. and de Vicente, A. (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 22, 187–193. [DOI] [PubMed] [Google Scholar]
- Pieterse, C.M. , Zamioudis, C. , Berendsen, R.L. , Weller, D.M. , Van Wees, S.C. and Bakker, P.A. (2014) Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375. [DOI] [PubMed] [Google Scholar]
- Pieterse, C.M.J. , Leon‐Reyes, A. , Van der Ent, S. and Van Wees, S.C.M. (2009) Networking by small‐molecule hormones in plant immunity. Nat. Chem. Biol. 5, 308–316. [DOI] [PubMed] [Google Scholar]
- Raaijmakers, J.M. , de Bruijn, I. , Nybroe, O. and Ongena, M. (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. Fems Microbiol. Rev. 34, 1037–1062. [DOI] [PubMed] [Google Scholar]
- Rahman, A. and Uddin, W. (2010) Inhibition of Magnaporthe oryzae using cells and cell‐free extracts of several strains of Bacillus . Phytopathology, 100, S106. [Google Scholar]
- Rahman, A. , Kuldau, G.A. and Uddin, W. (2014) Induction of salicylic acid‐mediated defense response in perennial ryegrass against infection by Magnaporthe oryzae . Phytopathology, 104, 614–623. [DOI] [PubMed] [Google Scholar]
- Ran, Y. , Ramage, C. , Felitti, S. , Emmerling, M. , Chalmers, J. , Cummings, N. , Petrovska, N. , Mouradov, A. and Spangenberg, G. (2007) Ryegrasses In: Biotechnology in Agriculture and Forestry: Transgenic Crops VI (Pua E.‐C. and Davey M., eds), pp. 373–395. Verlag Berlin Heidelberg: Springer. [Google Scholar]
- Samalova, M. , Meyer, A.J. , Gurr, S.J. and Fricker, M.D. (2014) Robust anti‐oxidant defences in the rice blast fungus Magnaporthe oryzae confer tolerance to the host oxidative burst. New Phytol. 201, 556–573. [DOI] [PubMed] [Google Scholar]
- Sawbridge, T. , Ong, E.K. , Binnion, C. , Emmerling, M. , McInnes, R. , Meath, K. , Nguyen, N. , Nunan, K. , O'Neill, M. , O'Toole, F. , Rhodes, C. , Simmonds, J. , Tian, P. , Wearne, K. , Webster, T. , Winkworth, A. and Spangenberg, G. (2003) Generation and analysis of expressed sequence tags in perennial ryegrass (Lolium perenne L.). Plant Sci. 165, 1089–1100. [Google Scholar]
- Shimono, M. , Koga, H. , Akagi, A. , Hayashi, N. , Goto, S. , Sawada, M. , Kurihara, T. , Matsushita, A. , Sugano, S. and Jiang, C.J. (2012) Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol. Plant Pathol. 13, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, Z.Z. , Mao, L.J. , Li, N. , Feng, X.X. , Yuan, Z.L. , Wang, L.W. , Lin, F.C. and Zhang, C.L. (2013) Evidence for biotrophic lifestyle and biocontrol potential of dark septate endophyte Harpophora oryzae to rice blast disease. PLoS ONE, 8, e61332. doi: 10.1371/journal.pone.0061332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboys, P.J. , Owen, D.W. , Healey, J.R. , Withers, P.J. and Jones, D.L. (2014) Auxin secretion by Bacillus amyloliquefaciens FZB42 both stimulates root exudation and limits phosphorus uptake in Triticum aestivium . BMC Plant Biol. 14, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, S.Y. , Dong, Y. , Liao, H.P. , Huang, J.F. , Song, S. , Xu, Y.C. and Shen, Q. (2013) Antagonistic bacterium Bacillus amyloliquefaciens induces resistance and controls the bacterial wilt of tomato. Pest Manag. Sci. 69, 1245–1252. [DOI] [PubMed] [Google Scholar]
- Thordal‐Christensen, H. , Zhang, Z.G. , Wei, Y.D. and Collinge, D.B. (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J. 11, 1187–1194. [Google Scholar]
- Torres, M.A. (2010) ROS in biotic interactions. Physiol. Plant. 138, 414–429. [DOI] [PubMed] [Google Scholar]
- Tran, H. , Ficke, A. , Asiimwe, T. , Höfte, M. and Raaijmakers, J.M. (2007) Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens . New Phytol. 175, 731–742. [DOI] [PubMed] [Google Scholar]
- Uddin, W. , Viji, G. and Vincelli, P. (2003) Gray leaf spot (blast) of perennial ryegrass turf: an emerging problem for the turfgrass industry. Plant Dis. 87, 880–889. [DOI] [PubMed] [Google Scholar]
- Vincelli, P. and Dixon, E. (2002) Resistance to Q(o)I (strobilurin‐like) fungicides in isolates of Pyricularia grisea from perennial ryegrass. Plant Dis. 86, 235–240. [DOI] [PubMed] [Google Scholar]
- Wang, W. , Chen, L.N. , Wu, H. , Zang, H. , Gao, S. , Yang, Y. , Xie, S. and Gao, X. (2013) Comparative proteomic analysis of rice seedlings in response to inoculation with Bacillus cereus . Lett. Appl. Microbiol. 56, 208–215. [DOI] [PubMed] [Google Scholar]
- Zeyen, R. , Carver, T.L. , Lyngkjaer, M.F. , Bélanger, R. , Bushnell, W. and Dik, A. (2002) Epidermal cell papillae In: The Powdery Mildews: A Comprehensive Treatise (Bélanger R.R., Bushnell W.R., Dik A.J. and Carver T.L.W., eds), pp. 107–125. St. Paul, MN: American Phytopathological Society Press. [Google Scholar]
- Zhou, F.S. , Zhang, Z.G. , Gregersen, P.L. , Mikkelsen, J.D. , de Neergaard, E. , Collinge, D.B. and Thordal‐Christensen, H. (1998) Molecular characterization of the oxalate oxidase involved in the response of barley to the powdery mildew fungus. Plant Physiol. 117, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Representative images of perennial ryegrass leaf blades treated with 3,3′‐diaminobenzidine (DAB), 24 and 36 h following inoculation with Magnaporthe oryzae. Ap, appressorium.
Fig. S2 Effect of solid‐phase extraction (SPE)‐enriched cyclic lipopeptide(s) from wild‐type (wt) Bacillus amyloliquefaciens FZB42 and AK3 strains on germination of ZsGreen‐tagged Magnaporthe oryzae. Ap, appressorium.
Fig. S3 Reverse phase high‐performance liquid chromatography (RP‐HPLC) and mass spectrometry (MS) analysis of surfactin isomers in solid‐phase extraction‐enriched surfactin (SPE‐surfactin) (a1, a2 and a3) and standard surfactin (b1, b2 and b3) (Sigma‐Aldrich).
Fig. S4 Relative proportions of different surfactin homologues in standard surfactin (Sigma‐Aldrich) (a) and solid‐phase extraction‐enriched surfactin (SPE‐surfactin) (b).
Fig. S5 Hydrogen peroxide (H2O2) content in perennial ryegrass roots following application of 250 μg/mL of solid‐phase extraction‐enriched surfactin (SPE‐surfactin).
Fig. S6 Time course of peroxidase enzyme activity in different cellular fractions of perennial ryegrass leaf cells following infection with Magnaporthe oryzae: (a) cell wall (CW); (b) intercellular fluid (ICF); (c) cytoplasm.


