Summary
The biocontrol agent Pythium oligandrum and its elicitin‐like proteins oligandrins have been shown to induce disease resistance in a range of plants. In the present study, the ability of two oligandrins, Oli‐D1 and Oli‐D2, to induce an immune response and the possible molecular mechanism regulating the defence responses in Nicotiana benthamiana and tomato were investigated. Infiltration of recombinant Oli‐D1 and Oli‐D2 proteins induced a typical immune response in N. benthamiana including the induction of a hypersensitive response (HR), accumulation of reactive oxygen species and production of autofluorescence. Agrobacterium‐mediated transient expression assays revealed that full‐length Oli‐D1 and Oli‐D2 were required for full HR‐inducing activity in N. benthamiana, and virus‐induced gene silencing‐mediated knockdown of some of the signalling regulatory genes demonstrated that NbSGT1 and NbNPR1 were required for Oli‐D1 and Oli‐D2 to induce HR in N. benthamiana. Subcellular localization analyses indicated that both Oli‐D1 and Oli‐D2 were targeted to the plasma membrane of N. benthamiana. When infiltrated or transiently expressed in leaves, Oli‐D1 and Oli‐D2 induced resistance against Botrytis cinerea in tomato and activated the expression of a set of genes involved in the jasmonic acid/ethylene (JA/ET)‐mediated signalling pathway. Our results demonstrate that Oli‐D1 and Oli‐D2 are effective elicitors capable of inducing immune responses in plants, probably through the JA/ET‐mediated signalling pathway, and that both Oli‐D1 and Oli‐D2 have potential for the development of bioactive formulae for crop disease control in practice.
Keywords: defence response/resistance, hypersensitive response, Nicotiana benthamiana, Oli‐D1/Oli‐D2, oligandrin, tomato
Introduction
The innate immune system in plants contains two types of immune response: pathogen‐associated molecular pattern (PAMP)‐triggered immunity (PTI) and effector‐triggered immunity (ETI) (Boller and He, 2009; Spoel and Dong, 2012). In addition to innate immunity, plants also develop and possess an inducible immunity that is activated on pathogen infection. The forms of inducible immunity are generally referred to as systemic acquired resistance (SAR) and induced systemic resistance (ISR), which are activated by different stimuli and regulated by distinct signalling pathways (Fu and Dong, 2013; Grant and Lamb, 2006; Kachroo and Robin, 2013; Loake and Grant, 2007; van Loon et al., 1998; Shoresh et al., 2010; van Wees et al., 2008). Once activated, the inducible immunity in plants often confers a broad‐spectrum resistance against a wide range of unrelated pathogens (Durrant and Dong, 2004; Grant and Lamb, 2006; van Wees et al., 2008). This great advantage provides a promising strategy for disease control in practice (Walters et al., 2013).
Plant inducible immunity can be triggered by the exogenous application of a number of elicitors (Walters et al., 2013), which are capable of activating inducible immunity and protecting plants from disease damage. Among the elicitors identified so far, the microbe‐derived proteinaceous elicitors show great potential for the development of environmentally friendly biopesticides and for the improvement of crop disease resistance by means of genetic engineering. For example, some of the harpin proteins, produced by Gram‐negative phytopathogenic bacteria (Choi et al., 2013), have been shown to be capable of controlling diseases and insects, as well as improving growth, yield and quality in many crop plants (Chen et al., 2008a, b; Jang et al., 2006; Strobel et al., 1996), and the over‐expression of harpin‐encoding genes from phytopathogenic bacteria in tobacco and rice induces enhanced disease resistance against a number of fungal and bacterial pathogens (Miao et al., 2010; Peng et al., 2004; Shao et al., 2008). Similarly, elicitins produced by a number of pathogenic oomycetes (Grant et al., 1996; Kamoun, 2006; Yu, 1995) have been shown to induce SAR in plants (Bonnet et al., 1996; Cordelier et al., 2003; Kamoun et al., 1993, 1998; Keller et al., 1996), and transgenic plants over‐expressing a Phytophthora elicitin gene display enhanced resistance to Thielaviopsis basicola, Erysiphe cichoracearum and Botrytis cinerea, three fungal pathogens that are unrelated to Phytophthora species (Keller et al., 1999). With increasing demands for the development of environmentally friendly biopesticides, extensive studies have been performed using molecular, biochemical and proteomic approaches to screen and identify novel proteinaceous elicitors from microbes (Kulye et al., 2012; Peng et al., 2011; Qiu et al., 2009; Yang et al., 2009).
Pythium oligandrum, a non‐pathogenic soil‐inhabiting oomycete, has been reported to be an effective biocontrol agent that colonizes the rhizosphere of many crop species and reduces diseases caused by a number of soil‐borne pathogens (Benhamou et al., 2012). It has been reported recently that P. oligandrum can produce two types of proteinaceous elicitor that are distinct from those of the representative elicitins and elicitin‐like proteins from oomycete species (Masunaka et al., 2010; Takenaka et al., 2006). One type of elicitor exists as a cell wall protein (CWP) in mycelial mats of P. oligandrum and has four members, including POD‐1 and POD‐2 (Takenaka et al., 2006) and POS‐1 and Oli‐S1 (Masunaka et al., 2010). POD‐1 and POD‐2 have been shown to induce defence responses in sugarbeet (Takenaka et al., 2003; Takenaka and Tamagake, 2009), wheat (Takenaka et al., 2003), tomato (Hase et al., 2006, 2008; Takenaka et al., 2011) and Arabidopsis (Kawamura et al., 2009a). Another type of elicitor in P. oligandrum, called an oligandrin, is an extracellular protein that can induce resistance in tomato against Phytophthora parasitica, Fusarium oxysporum f. sp. radicis‐lycopersici and B. cinerea (Benhamou et al., 2001; Lou et al., 2011; Mohamed et al., 2007; Picard et al., 2000; Wang et al., 2011). However, the possible mechanism of oligandrins in regulating the immune response remains unclear. In the present study, we examined the ability of two P. oligandrum oligandrins, Oli‐D1 and Oli‐D2 (Masunaka et al., 2010), to induce an immune response and their possible molecular mechanisms in regulating the defence responses in Nicotiana benthamiana and tomato. Our results demonstrate that Oli‐D1 and Oli‐D2 are potential proteinaceous elicitors that can be used to develop bioactive formulae for crop disease control in practice.
Results
Oli‐D1 and Oli‐D2 induce HR in N. benthamiana
Considering that N. benthamiana is a versatile host that can easily be infiltrated by foreign proteins or agrobacteria for the examination of the HR phenotype (Goodin et al., 2008) and that the HR phenotype induced by Oli‐D1 or Oli‐D2 was not seen in tomato in our pilot experiments, N. benthamiana was used for our study. Infiltration with a preparation from bacteria carrying pET‐32a empty vector did not show any HR symptoms during the experimental period (Fig. 1A). Infiltration with Nep1Mo, a necrosis‐ and ethylene‐inducing peptide 1 (Nep1)‐like protein in Magnaporthe oryzae that can produce a typical HR in N. benthamiana (Qutob et al., 2006; Zhang et al., 2010, 2012), rapidly induced a typical HR, and all the infiltrated areas were necrotic at 24 h post‐inoculation (hpi) (Fig. 1A,B). Infiltration with Oli‐D1 and Oli‐D2 at concentrations of 1 and 3 μm also induced a typical HR, but the development of HR was much slower than that induced by Nep1Mo (Fig. 1A,B). The HR was seen only at 48 hpi and approximately 50% of the infiltrated areas were necrotic when 1 μm of Oli‐D1 or Oli‐D2 was applied. In leaves infiltrated with 3 μm of Oli‐D1 or Oli‐D2, the HR developed as small necrotic lesions at 24 hpi and eventually spread to nearly all the infiltrated areas at 72 hpi (Fig. 1B), indicating that Oli‐D1 and Oli‐D2 at higher concentrations induced a relatively more rapid and stronger HR. We further examined whether the expression of HSR203J and harpin‐induced 1 (HIN1), two HR‐specific marker genes whose expression is induced during pathogen‐triggered HR (Gopalan et al., 1996; Pontier et al., 1994, 1999; Takahashi et al., 2004a), and is independent of the salicylic acid (SA)‐ or jasmonic acid (JA)/ethylene (ET)‐mediated signalling pathways (Takahashi et al., 2004b; Varet et al., 2002; Zheng et al., 2004), could be induced by Oli‐D1 or Oli‐D2. As shown in Fig. 1C, expression of HSR203J and HIN1 was significantly up‐regulated by Oli‐D1 and Oli‐D2 as early as 6 hpi. Taken together, these data indicate that Oli‐D1 and Oli‐D2 induce typical HR in N. benthamiana.
Figure 1.
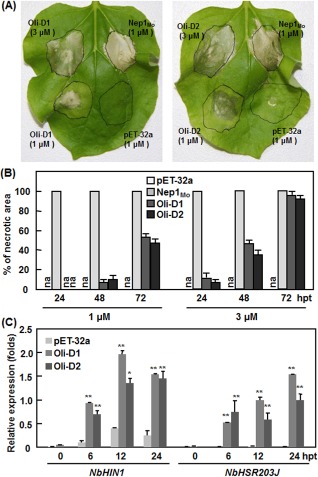
Recombinant Oli‐D1 and Oli‐D2 induce a hypersensitive response (HR) in the leaves of Nicotiana benthamiana plants. (A) Representative HR in leaves of N. benthamiana plants induced by recombinant Oli‐D1 and Oli‐D2 at 1 and 3 μm. Nep1Mo and a preparation from bacteria carrying pET‐32a empty vector were used as positive and negative controls, respectively. Photographs were taken at 48 h after infiltration. (B) Development of HR. Percentages of necrosis in the infiltration areas were estimated at different time points as indicated. (C) Expression of HR marker genes induced by Oli‐D1 and Oli‐D2. Infiltrated leaves were collected at the indicated time points and used to analyse the expression of NbHIN1 and NbHSR203J by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). Data presented are the mean ± SD from three independent experiments. Significant difference at *P < 0.05 and **P < 0.01. hpt, hours post‐treatment.
Oli‐D1 and Oli‐D2 activate HR biochemical responses in N. benthamiana
To examine whether Oli‐D1 and Oli‐D2 activated HR biochemical responses, we analysed the accumulation of reactive oxygen species (ROS), generated by plasma membrane‐localized NADPH oxidase complex (Mur et al., 2008), and the appearance of autofluorescence, probably resulting from the accumulation of phenolic compounds in and around the cells that had died (Dietrich et al., 1994), in N. benthamiana leaves after infiltration with Oli‐D1 or Oli‐D2. In all leaves assayed, significant brown 3,3′‐diaminobenzidine (DAB)‐stained precipitates were easily and clearly observed in the areas infiltrated with 1 μm Oli‐D1 or Oli‐D2 at 4 hpi, but only very weak brown DAB‐stained precipitates was seen in the same leaves infiltrated with a preparation from bacteria carrying pET‐32a empty vector (Fig. 2A). Similarly, Oli‐D1 and Oli‐D2 also induced the appearance of autofluorescence symptoms in N. benthamiana leaves (Fig. 2B). At 24 hpi, significant autofluorescence was seen in Oli‐D1‐ and Oli‐D2‐infiltrated areas in N. benthamiana leaves and was comparable with that induced by Nep1Mo (Fig. 2B). No autofluorescence was observed in negative control leaves (Fig. 2B). These results indicate that Oli‐D1 and Oli‐D2 can activate HR biochemical responses, such as the accumulation of H2O2 and the appearance of autofluorescence.
Figure 2.
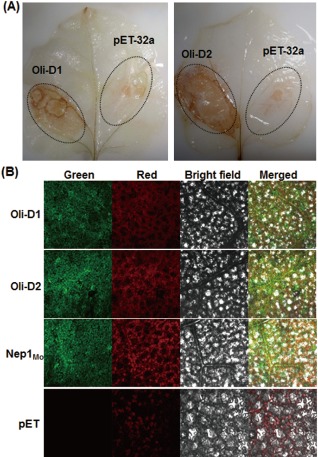
Recombinant Oli‐D1 and Oli‐D2 activate the defence response in Nicotiana benthamiana plants. (A) Accumulation of H2O2 in Oli‐D1‐ and Oli‐D2‐infiltrated areas. Recombinant Oli‐D1 and Oli‐D2 at 1 μm were infiltrated into N. benthamiana leaves and in situ detection of H2O2 using 3,3′‐diaminobenzidine (DAB) staining was performed on leaves at 4 h post‐inoculation (hpi). (B) Autofluorescence in N. benthamiana leaves induced by Oli‐D1 and Oli‐D2. Recombinant Oli‐D1 and Oli‐D2 at 1 μm were infiltrated into N. benthamiana leaves and autofluorescence was visualized at 48 hpi. Photographs were taken in dark field for green and red fluorescence, in bright field for the morphology of the cells and in combination.
Full‐length Oli‐D1 and Oli‐D2 are required for HR‐inducing activity
The Oli‐D1 and Oli‐D2 proteins are 120 amino acids (aa) in size and contain a signal peptide (SP) (1–18 aa) and an elicitin superfamily domain (20–116 aa) (Masunaka et al., 2010). To examine whether there is a region/motif responsible for the HR‐inducing activity in the Oli‐D1 and Oli‐D2 proteins, five deletion mutants were generated (Fig. 3A) and analysed for their ability to induce HR in N. benthamiana. When transiently expressed in leaves of N. benthamiana plants, full‐length Oli‐D1 and Oli‐D2 induced typical HR (Fig. 3B), similar to the HR induced by the purified recombinant Oli‐D1 and Oli‐D2 proteins (Fig. 1A), and the green fluorescent protein (GFP) negative control did not induce HR, indicating that the transient expression system worked properly in N. benthamiana. However, no HR was observed when the deletion mutants for Oli‐D1 or Oli‐D2 were transiently expressed (Fig. 3B). To rule out the possibility that an error may have been introduced during sequence amplification and vector construction, we examined through Western blotting the detection of the FLAG tags that had been fused to the C‐termini of the proteins of the deletion mutants in N. benthamiana leaves. As shown in Fig. 3C, all the deletion mutant proteins and the full‐length Oli‐D1 and Oli‐D2 were detected at 24 hpi in N. benthamiana leaves infiltrated with the corresponding constructs, indicating that all the deletion mutants were expressed correctly in N. benthamiana leaves. Collectively, these results demonstrate that full‐length Oli‐D1 and Oli‐D2 are required for HR‐inducing activity and the deletion of any part of Oli‐D1 or Oli‐D2 abolishes their HR‐inducing activity.
Figure 3.
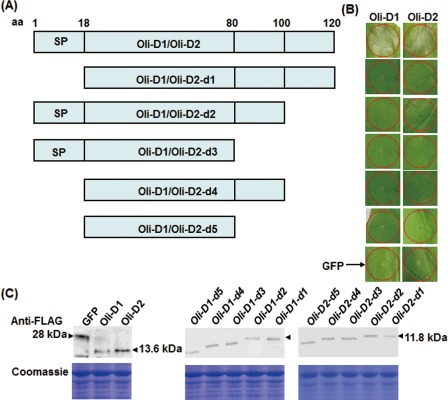
Full‐length Oli‐D1 and Oli‐D2 are required for hypersensitive response (HR)‐inducing activity in Nicotiana benthamiana plants. (A) Diagram of the deletion mutants for Oli‐D1 and Oli‐D2 proteins. Amino acid (aa) positions in Oli‐D1 and Oli‐D2 are indicated above the diagram. SP, signal peptide. (B) HR induced by Oli‐D1 and Oli‐D2 and their deletion mutants when transiently expressed in N. benthamiana leaves. Photographs were taken 3 days after infiltration. GFP, green fluorescent protein. (C) Western blotting of Oli‐D1 and Oli‐D2 and their deletion mutant proteins in N. benthamiana leaves. Leaf samples were collected at 36 h post‐inoculation and soluble proteins were extracted for Western blotting analysis. Top row: detection of target proteins with anti‐FLAG antibody; bottom row, Coomassie blue staining showing equal loading of the samples.
NbSGT1 and NbNPR1 are required for Oli‐D1‐ and Oli‐D2‐induced HR
To gain an insight into the molecular mechanism involved in Oli‐D1 and Oli‐D2 function, we examined the requirements of some important regulatory components in the defence signalling pathways for Oli‐D1‐ and Oli‐D2‐induced HR using a standard virus‐induced gene silencing (VIGS) procedure (Liu et al., 2002). In our experiments, the silencing effectiveness for a single target gene was >70%, as revealed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) using gene‐specific primers (Fig. 4A). Infiltration with pGR106‐GFP (as a negative control) did not cause HR in all the plants; however, infiltration with pGR106‐Nep1Mo (as a positive control) induced HR in NbLRK1‐silenced and pTRV2‐GUS‐infiltrated plants, but did not cause HR in NbSGT1‐, NbNRP1‐, NbHSP90.2‐, NbCOI1‐ and NbTGA2.2‐silenced plants (Fig. 4B). Typical HR was observed in NbLRK1‐, NbHSP90.2‐, NbCOI1‐ and NbTGA2.2‐silenced plants after infiltration with pGR106‐Oli‐D1 or pDR106‐Oli‐D2, as in pTRV2‐GUS‐infiltrated plants (Fig. 4B). However, infiltration with pGR106‐Oli‐D1 or pGR106‐Oli‐D2 did not induce HR in NbSGT1‐ and NbNPR1‐silenced plants (Fig. 4B). These results indicate that NbSGT1 and NbNPR1 are required for Oli‐D1‐ and Oli‐D2‐induced HR in N. benthamiana.
Figure 4.
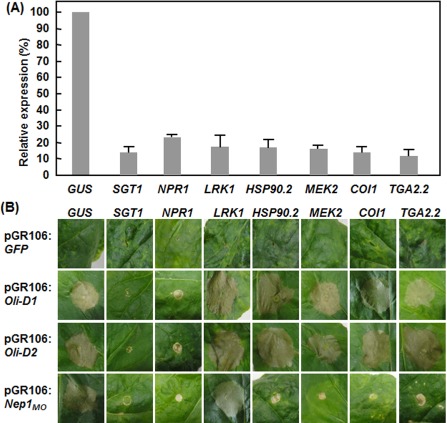
NbSGT1 and NbNPR1 are required for the Oli‐D1‐ and Oli‐D2‐induced hypersensitive response (HR) in Nicotiana benthamiana. Leaves of N. benthamiana plants at the five‐ to six‐leaf stage were infiltrated with a mixed suspension of agrobacteria carrying pTRV1 and pTRV2‐GUS, pTRV2‐SGT1, pTRV2‐NPR1, pTRV2‐LRK1, pTRV2‐HSP90.2, pTRV2‐COI1, pTRV2‐MEK2 or pTRV2‐TGA2.2, and the upper leaves were infiltrated with agrobacteria carrying pGR106‐D1, pGR106‐D2, pGR106‐Nep1MO (as a positive control) or pGR106‐GFP (as a negative control) 3 weeks after virus‐induced gene silencing (VIGS) infiltration. (A) Silencing effectiveness of the target genes in corresponding VIGS‐silenced plants. Leaf samples were collected 2 weeks after VIGS infiltration and the transcript levels of the target genes were analysed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). Relative expression was expressed as the percentages of the transcript levels of the target gene‐silenced plants vs. that in pTRV‐GUS‐infiltrated plants. (B) Development of HR induced by transiently expressed Oli‐D1 or Oli‐D2 in the target gene‐silenced plants. GUS, β‐glucuronidase; SGT1, suppressor of G2 allele of skp1; NPR1, nonexpresser of PR genes 1; LRK1, lectin‐like receptor kinases 1; HSP90.2, heat shock protein 90.2; MEK2, mitogen‐activated protein kinase kinase 2; COI1, CORONATINE INSENSITIVE 1; TGA2.2, TGACG sequence‐specific binding protein 2.2.
Oli‐D1 and Oli‐D2 are targeted to the plasma membrane
Subcellular localization assays in N. benthamiana revealed that, when transiently expressed, the GFP fluorescence from pGFP‐Oli‐D1‐ or pGFP‐Oli‐D2‐infiltrated and pGFP‐Oli‐D1‐d1‐ or pGFP‐Oli‐D2‐d2‐infiltrated leaves was observed mainly on the plasma membrane of the cells, whereas GFP fluorescence in pFGC‐Egfp empty vector‐infiltrated leaves was observed throughout the entire cells, including the plasma membrane and nucleus, without specific compartmental localization (Fig. 5). These data indicate that not only the full‐length, but also the SP‐deleted mutants of Oli‐D1 and Oli‐D2 proteins, are targeted to the plasma membrane of the cells.
Figure 5.
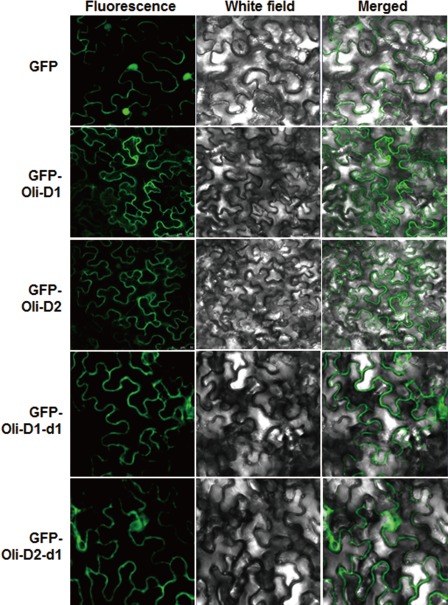
Oli‐D1 and Oli‐D2 are targeted to the plasma membrane. Agrobacteria carrying pGFP‐Oli‐D1, pGFP‐Oli‐D1‐d1, pGFP‐Oli‐D2, pGFP‐Oli‐D2‐d1 or pFGC‐Egfp empty vector were infiltrated into Nicotiana benthamiana leaves for transient expression, and leaf samples were collected at 16 h post‐inoculation for observation under a confocal laser scanning microscope. Photographs were taken in dark field for green fluorescence (left), in white field for the morphology of the cells (middle) and in combination (right). GFP, green fluorescent protein.
Oli‐D1 and Oli‐D2 induce disease resistance against B. cinerea in tomato
Considering that Oli‐D1 and Oli‐D2 trigger immune responses in N. benthamiana, we examined whether they had the ability to induce disease resistance in other plants, such as tomato. As shown in Fig. 6, B. cinerea‐induced lesions developed and expanded much more slowly in leaves treated with either recombinant or transiently expressed Oli‐D1 and Oli‐D2 than in corresponding control plants (Fig. 6A,D). At 3 days post‐inoculation (dpi), the lesion sizes in leaves treated with either recombinant or transiently expressed Oli‐D1 and Oli‐D2 were markedly reduced by ∼30% when compared with those in corresponding control plants (Fig. 6B,E). Consistent with the disease phenotypes observed in Oli‐D1‐ and Oli‐D2‐treated plants, in planta growth of B. cinerea, as measured by the transcript level of the BcActinA gene (Benito et al., 1998), in either recombinant or transiently expressed Oli‐D1‐ and Oli‐D2‐treated leaves, was significantly reduced, showing a reduction of 55–65% when compared with that in corresponding control plants (Fig. 6C,F). These results suggest that either recombinant or transiently expressed Oli‐D1 and Oli‐D2 can induce resistance against B. cinerea in tomato, resulting in decreased disease severity and in planta growth of B. cinerea.
Figure 6.
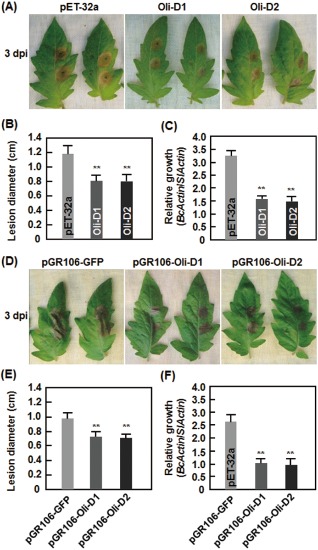
Oli‐D1 and Oli‐D2 induce resistance in tomato against Botrytis cinerea. (A–C) Resistance induced by recombinant Oli‐D1 and Oli‐D2 proteins. (D–F) Resistance induced by transiently expressed Oli‐D1 and Oli‐D2. (A, D) Representative phenotypes of disease caused by B. cinerea in Oli‐D1‐ and Oli‐D2‐induced plants. (B, E) Size of lesions caused by B. cinerea in detached leaves from Oli‐D1‐ and Oli‐D2‐induced plants at 3 days post‐inoculation (dpi). (C, F) In planta growth of B. cinerea in detached leaves from Oli‐D1‐ and Oli‐D2‐induced plants. One half of the leaf was infiltrated with 1 μm Oli‐D1, Oli‐D2 or a preparation from bacteria carrying pET‐32 empty vector (A–C) or with agrobacteria carrying pGR106‐Oli‐D1, pGR106‐Oli‐D2 or pGR106‐GFP (D–F) and the opposite half of the same leaf was inoculated by dropping 5 μL of spore suspension of B. cinerea at 24 h after infiltration. Photographs, lesion sizes and in planta fungal growth were taken or analysed at 3 dpi. Data presented in (B), (C), (E) and (F) are the means ± SD from three independent experiments. Significant difference at **P < 0.01.
Oli‐D1 and Oli‐D2 activate the defence response in tomato plants
As Oli‐D1 and Oli‐D2 induced resistance against B. cinerea in tomato, we examined whether they could activate the defence response. Similar to the observations in N. benthamiana, Nep1Mo induced HR in tomato leaves, but a preparation from bacteria carrying the pET‐32a empty vector did not (Fig. 7A). In contrast with Nep1Mo‐induced HR, however, a weak HR phenotype was observed at 72 hpi in leaves infiltrated with recombinant Oli‐D1 and Oli‐D2, and HR was evident in leaves infiltrated with a relatively high concentration of Oli‐D1 and Oli‐D2 (Fig. 7A). The expression levels of SlLapA1, SlPin2, SlLOX‐E and SlERF2, which are considered to be involved in the JA/ET‐mediated signalling pathway (Kawamura et al., 2009b; Kawazu et al., 2012), were significantly increased at 12 and 24 hpi in Oli‐D1‐ and Oli‐D2‐treated plants, giving increases of three‐ to seven‐fold (Fig. 7B) when compared with the expression levels at 0 h after treatment. The expression levels of SlPR1a and SlPR‐P2, two SA‐dependent defence genes (Kawazu et al., 2012), were not induced in Oli‐D1‐ and Oli‐D2‐treated tomato plants (Fig. 7C); however, the expression of SlPR1b, another SA‐dependent defence gene (Kawazu et al., 2012), was increased by two‐ to three‐fold when compared with the level at 0 h after treatment (Fig. 7C). These results indicate that Oli‐D1 and Oli‐D2 can activate the expression of a set of genes associated with the JA‐mediated signalling pathway in tomato.
Figure 7.
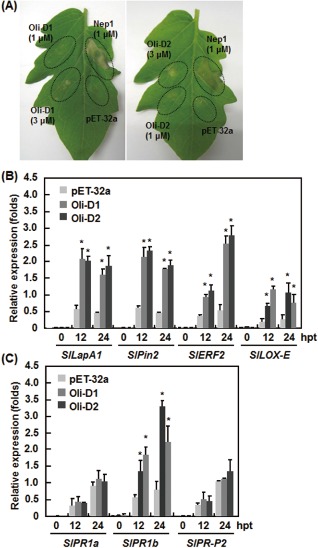
The hypersensitive response (HR) and expression of defence genes induced by Oli‐D1 and Oli‐D2 in tomato plants. (A) Representative HR in tomato leaves induced by recombinant Oli‐D1 and Oli‐D2 at 1 and 3 μm. Nep1Mo and a preparation from bacteria carrying pET‐32a empty vector were used as positive and negative controls, respectively. Photographs were taken at 48 h after infiltration. (B, C) Expression of jasmonic acid/ethylene (JA/ET)‐responsive (B) and salicylic acid (SA)‐responsive (C) genes in Oli‐D1‐ and Oli‐D2‐infiltrated tomato leaves. Leaf samples were collected at different time points as indicated after infiltration with 1 μm Oli‐D1 or Oli‐D2, and expression of defence genes was analysed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). Data presented in (B) and (C) are the means ± SD from three independent experiments. *Significant difference at P < 0.05. hpt, hours post‐treatment. LapA1, leucine aminopeptidase A1; Pin2, proteinase inhibitor 2; ERF2, ETHYLENE RESPONSIVE ELEMENT BINDING FACTOR 2; LOX‐E, lipoxygenase‐E; PR1a, pathogenesis‐related protein 1a; PR1b, pathogenesis‐related protein 1a; PR‐P2, pathogenesis‐related protein P2.
Discussion
In the present study, we have shown that Oli‐D1 and Oli‐D2 proteins, two elicitin‐like elicitors produced by P. oligandrum, can trigger typical immune responses in N. benthamiana and induce resistance in tomato against B. cinerea. We also found that full‐length Oli‐D1 and Oli‐D2 are required for HR‐inducing activity, which is dependent on the functions of NbSGT1 and NbNPR1. The data presented in this study not only expand our understanding of the molecular mechanism for the action of oligandrins in the regulation of the plant immune response, but also provide experimental evidence for the development of potential bioactive formulae with Oli‐D1 and Oli‐D1 for crop disease control.
It is well known that some elicitins from Pythium and Phytophthora spp. can induce HR in Nicotiana species (Ponchet et al., 1999); for example, 100 nm INF1, an elicitin from Phytophthora infestans, can induce HR‐like cell death in tobacco (Ponchet et al., 1999). By contrast, it has been reported previously that both CWPs (POD‐1 and POD‐2) and oligandrin elicitors from P. oligandrum are unable to induce HR in tobacco and tomato leaves (Kawamura et al., 2009b; Picard et al., 2000; Takahashi et al., 2006; Takenaka et al., 2006). Unlike these observations, however, we found, in this study, that recombinant Oli‐D1 and Oli‐D2 can induce visible HR in both N. benthamiana and tomato leaves, as verified by the appearance of rapid cell death, expression of HR marker genes (Fig. 1) and activation of the defence response, such as the accumulation of ROS and the production of autofluorescence (Fig. 2). Several reasons may explain the differences in induction of HR by oligandrins and CWP elicitors from P. oligandrum. First, the induction of HR requires appropriate concentrations of CWP and oligandrins (Oli‐D1 and Oli‐D2). For example, it was found that CWP at 300 nmol/mL and 1 μm did not cause visible HR‐like necrosis in leaves of Arabidopsis and tobacco plants, respectively (Kawamura et al., 2009a; Takenaka et al., 2006). Indeed, our results imply that the HR‐inducing activity of Oli‐D1 and Oli‐D2 is dosage dependent, as Oli‐D1 and Oli‐D2 at relatively higher concentrations triggered a more rapid and stronger HR (Fig. 1). This feature of dosage‐dependent HR‐inducing activity for Oli‐D1 and Oli‐D2 is similar to the function of quercinin, an elicitin from Phytophthora quercina, which induces a dose‐dependent cell death and oxidative burst in tobacco cell suspension cultures (Koehl et al., 2003, 2007). Second, Oli‐D1 and Oli‐D2, comprising 120 aa with an elicitin domain (Masunaka et al., 2010), are structurally similar to class I elicitins, whereas CWPs, comprising 145–151 aa containing an elicitin domain and an additional C‐terminus with glycosylation sites, are structurally similar to class III elicitins (Kamoun et al., 1997; Masunaka et al., 2010). Our analyses of the different deletion mutants demonstrated that full‐length Oli‐D1 and Oli‐D2 proteins are required for HR‐inducing activity in N. benthamiana (Fig. 3). This is reasonable as Oli‐D1 and Oli‐D2 are small proteins (<10 kDa) and deletion of any part could destroy their high‐order structures. Third, Oli‐D1 and Oli‐D2 are phylogenetically distinct from CWPs, showing approximately 52% identity at the amino acid level (Masunaka et al., 2010), and thus may have different activity in HR induction. Based on the speed and degree of HR development in N. benthamiana and tomato leaves, Oli‐D1 and Oli‐D2 seem to be weak inducers of HR when compared with Nep1Mo (Figs 1 and 7) (Zhang et al., 2010, 2012). However, this should be an advantage for the development of bioactive formulae for disease control because significant HR or necrosis induced by the application of strong HR inducers may not be acceptable for practical use.
Both Oli‐D1 and Oli‐D2 belong to the oligandrin type of elicitors in P. oligandrum and contain typical SPs at N‐termini (Masunaka et al., 2010), suggesting that Oli‐D1 and Oli‐D2 might be extracellularly secreted proteins. This is supported by the fact that oligandrin was first identified and purified from a culture filtrate of P. oligandrum (Picard et al., 2000). In our subcellular localization experiments, we found that Oli‐D1 and Oli‐D2 proteins were targeted to the plasma membrane when transiently expressed in N. benthamiana leaves (Fig. 4), implying that receptors for Oli‐D1 and Oli‐D2 may be localized on the plasma membrane of plant cells. This is consistent with common knowledge from the identification of plasma membrane surface‐localized pattern recognition receptors for some well‐known PAMPs, such as FLS2 for flg22 and EPR for Tu‐EF (Boller and Felix, 2009; Greeff et al., 2012). It has also been shown that some oomycete‐secreted elicitins, such as cryptogein and INF1, have binding sites on the plasma membranes (Kanzaki et al., 2008; Wendehenne et al., 1995). Thus, it is likely that similar but distinct plasma membrane‐localized receptors might be involved in the perception of the diverse range of elicitins and elicitin‐like elicitors produced by oomycetes (Kanzaki et al., 2008). However, silencing of NbLRK1, encoding for a lectin‐like receptor kinase that interacts with INF1, did not affect the development of HR induced by Oli‐D1 and Oli‐D2 (Fig. 4B), indicating that NbLRK1 is not a shared receptor for both INF1 and oligandrins. Considering that plant genomes encode a large number of receptor‐like kinases (Shiu et al., 2004), further identification of the Oli‐D1 and Oli‐D2 receptors or receptor complex and its associated components will broaden our understanding of the molecular action mechanism of these elicitin‐like elicitors, especially the clarification of the earliest events and downstream signalling pathway involved in Oli‐D1‐ and Oli‐D2‐induced HR.
Pythium oligandrum, as a biocontrol agent, can protect a variety of plants from disease damage through induced resistance (Benhamou et al., 2012). For instance, direct application of P. oligandrum can trigger not only local resistance, but also systemic induced resistance, against fungal, oomycete and bacterial pathogens in tomato and grapevine plants (Le Floch et al., 2003; Masunaka et al., 2009; Mohamed et al., 2007). The P. oligandrum‐triggered induced resistance might result from the action of the elicitors, because CWP and oligandrin elicitors induced resistance in sugarbeet, wheat, tomato and Arabidopsis against a range of pathogens (Hase et al., 2006, 2008; Kawamura et al., 2009a; Lou et al., 2011; Mohamed et al., 2007; Picard et al., 2000; Takenaka et al., 2003, 2011; Wang et al., 2011). In the present study, we found that either the recombinant or transiently expressed Oli‐D1 and Oli‐D2 induced resistance to B. cinerea by reducing disease severity and decreasing in planta fungal growth (Fig. 7). The latter phenomenon is somewhat similar to the observation that the growth of Ph. parasitica was restricted to the outermost tissues and its viability was decreased in oligandrin‐treated tomato plants (Picard et al., 2000). It is worth noting that, in our study, the Oli‐D1‐ and Oli‐D2‐induced resistance to B. cinerea was observed in the untreated half of the tomato leaves, which were treated on one half with recombinant or transiently expressed Oli‐D1 or Oli‐D2 (Fig. 7). This is consistent with previous results indicating that foliar application or soil drenching with oligandrin induced resistance against Ph. parasitica and B. cinerea (Lou et al., 2011; Picard et al., 2000). These observations indicate that oligandrins, such as Oli‐D1 and Oli‐D2, trigger the generation of signal(s) in the treated tissues and the signal(s) are then transmitted to the untreated parts, where they activate effective multiple defence responses, including the accumulation of ROS and autofluorescence, increased activity of defence‐related enzymes and up‐regulated expression of defence and signalling regulatory genes (Figs 2 and 7) (Lou et al., 2011). However, there is still a possibility that Oli‐D1 and Oli‐D2 may have a direct effect on B. cinerea within the plants, as oligandrin was found to be translocated within tomato plants through the vascular system (Picard et al., 2000).
Recent studies have demonstrated that the JA/ET‐mediated signalling pathway plays an important role in the signalling network involved in P. oligandrum CWP‐mediated induced resistance (Hase et al., 2006, 2008; Kawamura et al., 2009a). Our VIGS‐based analyses indicate that the HR‐inducing activity in N. benthamiana plants requires the functions of NbSGT1 and NbNPR1, but not NbCOI1, NbMEK2, NbTGA2.2 and NbHSP90.2 (Fig. 4). This is in agreement with the action of CWP elicitors in Arabidopsis, in which the CWP‐induced defence response is dependent on AtSGT1, AtRAR1 and AtNPR1 (Kawamura et al., 2009a). Although SGT1 and NPR1 are known to be critical regulators in R protein‐triggered disease resistance (Shirasu, 2009) and SA signalling‐mediated SAR (Dong, 2004; Durrant and Dong, 2004), the requirements for SGT1 in nonhost resistance and the JA response (Meldau et al., 2011; Peart et al., 2002), and for NPR1 in JA/ET signalling‐mediated ISR (Pieterse and van Loon, 2004), have also been documented. Surprisingly, the silencing of NbCOI1 did not affect the development of HR induced by Oli‐D1 and Oli‐D2 (Fig. 4), differing from the observation that AtCOI1 and AtJAR1 are required for the CWP‐induced defence response in Arabidopsis (Kawamura et al., 2009a). Consistent with the fact that INF1‐induced cell death is independent of TGA2.2 and MEK2 (Kanneganti et al., 2006), our results also revealed that both NbTGA2.2 and NbMEK2 are not involved in Oli‐D1‐ and Oli‐D2‐induced HR (Fig. 4). However, treatment with recombinant Oli‐D1 and Oli‐D2 activated the expression of some selected JA/ET signalling‐responsive genes, such as SlLapA1, SlPin2, SlLOX‐E and SlERF2 (Kawamura et al., 2009b; Kawazu et al., 2012), but did not affect the expression of two SA signalling‐responsive genes, SlPR1a and SlPR‐P2 (Kawazu et al., 2012) (Fig. 7), indicating that the JA/ET signalling pathway is involved in the Oli‐D1‐ and Oli‐D2‐induced defence response in tomato. This is similar to previous observations indicating that CWP or INF1 up‐regulated the expression of JA/ET‐responsive genes, but not SA‐responsive genes (Hase et al., 2008; Kawamura et al., 2009b; Takahashi et al., 2006), and that CWP‐induced resistance is dependent on the JA/ET signalling pathway, but not the SA signalling pathway (Hase et al., 2008; Kawamura et al., 2009b). Collectively, our data indicate that the JA/ET signalling pathway and the functions of SGT1 and NPR1 are involved in the regulation of Oli‐D1‐ and Oli‐D2‐induced HR and disease resistance. Further genetic studies are required to identify key regulatory components and to elucidate the detailed signalling pathway regulating the Oli‐D1‐ and Oli‐D2‐induced defence response.
Experimental Procedures
Plants and growth conditions
Plants of N. benthamiana and tomato (Solanum lycopersicum cv. Suhong 2003) were grown in compost soil mix (perlite : vermiculite : plant ash, 1:6:2) in a growth room under fluorescent light (200 μE/m2/s) at 22–24 °C with 60% relative humidity in a 14 h light/10 h dark regimen. Four‐ to five‐week‐old plants were used for all experiments.
Cloning of the Oli‐D1 and Oli‐D2 genes
The open reading frames (ORFs) of the Oli‐D1 and Oli‐D2 genes, encoding for the ologandrins of P. oligandrum, were synthesized by Invitrogen, Shanghai, China according to the sequences of GenBank accessions AB474242 and AB474243, and cloned into pMD19, yielding pMD19‐Oli‐D1 and pMD19‐Oli‐D2. The sequences of Oli‐D1 and Oli‐D2 in plasmids pMD19‐Oli‐D1 and pMD19‐Oli‐D2 were confirmed by sequencing and used as templates for further study. Nep1Mo (MGG_08454) was cloned from M. oryzae and used as a positive control (Zhang et al., 2010).
Preparation of recombinant Oli‐D1 and Oli‐D2 proteins
The ORF sequences of Oli‐D1 and Oli‐D2 were amplified from plasmids pMD19‐Oli‐D1 and pMD19‐Oli‐D2 using pairs of specific primers (Table S1, see Supporting Information) and cloned into pET‐32a, yielding plasmids pET32a‐Oli‐D1 and pET32a‐Oli‐D2, which were then transformed into Escherichia coli rosetta strain. The E. coli strains carrying pET32a‐Oli‐D1 and pET32a‐Oli‐D2 were grown at 28 °C with shaking (220 rpm) in Luria–Bertani (LB) liquid medium to an optical density at 600 nm (OD600) = 0.6 and were induced for 4 h by the addition of isopropyl β‐d‐1‐thiogalactopyranoside to a final concentration of 0.1 nm. Bacteria were collected by centrifugation and the recombinant proteins were purified using Ni‐NTA‐Agarose (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Protein concentration was determined with a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA). Sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and Western blotting analysis with a 6 × histidine (6 × His) tag antibody revealed that the purified Oli‐D1 and Oli‐D2 proteins with 6 × His tags were homogeneous with a single band and migrated with the same molecular weights as calculated from protein sequences (data not shown). A similar procedure was used to purify the recombinant Nep1Mo protein. All the purified Oli‐D1, Oli‐D2 and Nep1Mo proteins were stored at −70 °C and used for further experiments.
Construction of vectors
Five deletion mutants of Oli‐D1 and Oli‐D2 proteins were designed. The d1 mutant deleted the SP, but kept the entire elicitin domain. The d2 and d3 mutants kept the SP, but deleted 20 and 40 aa from the C‐terminus, respectively, which disrupted the integrity of the elicitin domain. The d4 and d5 mutants deleted both the SP and 20 or 40 aa from the C‐terminus, respectively. For transient expression assays, the Oli‐D1 and Oli‐D2 ORFs without stop codons and five deletion mutants (d1–d5) for Oli‐D1 and Oli‐D2 were amplified using specific primers (Table S1) from plasmids pMD19‐Oli‐D1 and pMD19‐Oli‐D2 and cloned into pFLAG‐CTS to add FLAG tags at the C‐termini. The FLAG‐tagged target fragments were then amplified and inserted into vector pGR106, yielding pGR106‐Oli‐D1, pGR106‐Oli‐D1‐d1–d5, pGR106‐Oli‐D2 and pGR106‐Oli‐D2‐d1–d5. For subcellular localization analysis, the ORFs and d1 deletion mutants of Oli‐D1 and Oli‐D2 were amplified from plasmids pMD19‐Oli‐D1 and pMD19‐Oli‐D2 and cloned into vector pFGC‐Egfp, yielding pGFP‐Oli‐D1, pGFP‐Oli‐D2, pGFP‐Oli‐D1‐d1 and pGFP‐Oli‐D2‐d1. For VIGS assay, cDNA fragments for NbSGT1.1 (AF516180), NbHSP90.2 (AY368905), NbMEK2 (AB360636), NbLRK1 (AB247455), NbCOI1 (NtCOI1, AB433899), NbTGA2.2 (NtTGA2.2, AF031487) and NbNPR1 (NtNPR1, DQ837218) were amplified from cDNAs of N. benthamiana using primers designed on the basis of corresponding N. benthamiana or tobacco sequences. After confirmation of the sequences of interest of the genes by cloning and sequencing, the cDNA fragments were amplified and cloned into the pTRV2 vector (Liu et al., 2002), resulting in pTRV2‐NbSGT1.1, pTRV2‐NbHSP90.2, pTRV2‐NbMEK2, pTRV2‐NbLRK1, pTRV2‐NbCOI1, pTRV2‐NbTGA2.2 and pTRV2‐NbNPR1. All of these constructed vectors were introduced into Agrobacterium tumefaciens strain GV3101 by electroporation using a GENE PULSER II Electroporation System (Bio‐Rad Laboratories, Hercules, CA, USA). Agrobacteria carrying different constructs were grown in YEP medium (50 μg/mL rifampicin, 50 μg/mL kanamycin and 25 μg/mL gentamicin) for 24 h with continuous shaking at 28 °C. Cells were centrifuged and resuspended in infiltration buffer [10 mm MgCl2, 10 mm 2‐(N‐morpholino)ethanesulphonic acid (MES), 200 μm acetosyringone, pH 5.7].
HR‐inducing activity assays
The purified Oli‐D1 and Oli‐D2 at concentrations of 1 and 3 μm were infiltrated into leaves of 4‐week‐old N. benthamiana plants using 1‐mL needleless plastic syringes. A preparation from empty vector pET‐32a and the recombinant Nep1Mo protein at a concentration of 1 μm were used as negative and positive controls, respectively. Leaves were harvested at the indicated time points to monitor the development of HR and to analyse the expression of marker genes.
VIGS in N. benthamiana
A standard VIGS procedure was performed according to the method described previously (Liu et al., 2002). The recombinant plasmids carrying the inserts of the genes of interest or pTRV2‐GUS (as a control) were transformed into A. tumefaciens strain GV3101. Bacterial suspensions were infiltrated into leaves of 3‐week‐old N. benthamiana plants using 1‐mL plastic needleless syringes. Plants infiltrated with VIGS constructs were allowed to grow in a glasshouse at 22 °C for another 2 weeks, and leaf samples were collected for the analysis of silencing efficiencies for each gene of interest by qRT‐PCR. Only when the silencing efficiency in an independent experiment was >70% were the VIGS‐infiltrated plants used for further experiments.
Transient expression assays in N. benthamiana
Fully expanded leaves of 4‐week‐old or VIGS‐infiltrated N. benthamiana plants were infiltrated using 1‐mL plastic needleless syringes with suspensions of agrobacteria carrying different constructs (pGR106‐Oli‐D1, pGR106‐Oli‐D1‐d1–d5, pGR106‐Oli‐D2, pGR106‐Oli‐D2‐d1–d5) or pGR106‐GFP (as a control), and leaf samples were collected at 48 h after infiltration for the visualization of HR and for Western blotting analyses.
SDS‐PAGE and Western blotting
Leaf samples were ground in 200 μL of extraction buffer [4 m urea, 100 mm dithiothreitol (DTT)], followed by the addition of 100 μL of loading buffer. After 5 min of boiling and subsequent 10 min of centrifugation at 10 000 × g, 20 μL of the supernatant were separated on a 15% SDS‐PAGE gel and transferred onto nitrocellulose by wet electroblotting. The detection of FLAG tags was performed using a mouse monoclonal antibody (1:1000 dilution) (Huaan Company, Hangzhou, China) and a peroxidase‐conjugated antimouse antibody (1:8000 dilution) (Huaan Company) according to the manufacturer's instructions. Proteins in the SDS‐PAGE gel were detected by an ECL Plus detection system (Huaan Company).
Subcellular localization
The subcellular localization of Oli‐D1 and Oli‐D2, as well as the mutants (Oli‐D1‐d1 and Oli‐D2‐d1) with deletion of the SP, was examined. Agrobacteria carrying pGFP‐Oli‐D1, pGFP‐Oli‐D1‐d1, pGFP‐Oli‐D2 and pGFP‐Oli‐D2‐d1, or pFGC‐Egfp empty vector, were infiltrated into N. benthamiana leaves using 1‐mL needleless syringes. Leaves were collected at 24 h after agroinfiltration and GFP was detected with a confocal laser scanning microscope (Zeiss LSM 510 META; argon laser excitation wavelength, 488 nm) (Zeiss, Jena GmbH, Jena, Germany).
Disease assays
Inoculation of B. cinerea was performed using a spore suspension at a spore density of 4 × 105 spores/mL according to a previously reported procedure (AbuQamar et al., 2008). One half of the leaves of 4‐week‐old tomato plants were infiltrated with recombinant Oli‐D1 or Oli‐D2 proteins, or with agrobacteria carrying pGR106‐GFP (as a negative control), pGR106‐Oli‐D1 or pGR106‐Oli‐D2. Two days later, the agrobacteria‐infiltrated leaves were detached and inoculated by dropping 5 μL of a spore suspension on the opposite half of the same leaves. The inoculated leaves were kept in a high‐humidity chamber and disease severity was recorded by measurement of the lesion size. The inoculated leaves were collected at 0 and 3 dpi, and the fungal growth in planta was measured by qRT‐PCR, analysing the transcript of the B. cinerea ActinA gene as an indicator of fungal growth (Benito et al., 1998) using a pair of primers (BcActin‐F and BcActin‐R; Table S1) and with a tomato Actin gene as an internal control. Relative growth was expressed as the fold change of BcActinA transcripts/tomato SlActin transcripts.
qRT‐PCR analysis of gene expression
Total RNA was extracted from leaf samples using TRIZOL reagent (Invitrogen) and the first‐strand cDNA was synthesized from 1 μg of total RNA with a SuperScript III Kit (Invitrogen) according to the manufacturer's instructions. qRT‐PCR was performed with SYBR Premix Ex Taq (Takara, Dalian, China) on a CFX96 Real‐time System (Bio‐Rad Laboratories). Tomato and N. benthamiana Actin genes were used as internal controls for normalization of the data obtained. Relative expression was calculated using the 2–ΔΔCT method. The primers used are listed in Table S1.
Detection of H2O2 and autofluorescence
Detection of H2O2 in planta was performed using the DAB staining method as described previously (Thordal‐Christensen et al., 1997). Nicotiana benthamiana leaves infiltrated with 1 μm of recombinant Oli‐D1 or Oli‐D2 were collected 4 h after infiltration, dipped into DAB solution (1 mg/mL, pH 3.8) and incubated for 10 h in the dark at room temperature. After incubation with DAB, the leaves were removed, placed into acetic acid–glycerol–ethanol (1:1:1, v/v/v) and boiled for 5 min, followed by several changes of the solution. Brownish‐red precipitates formed in the presence of H2O2 were visualized using a digital camera. Examination of autofluorescence was performed according to Dietrich et al. (1994) with slight modifications. Detached leaves were fixed in an autofluorescence‐fixing solution (10% formaldehyde, 5% acetic acid and 45% ethanol). Samples were cleared in alcoholic lactophenol, rinsed with distilled water and then equilibrated in 50% glycerol before mounting on slides. Autofluorescence was visualized under an EGFP filter set (excitation, 488 nm; emission, 500/600 nm; dichroic, 495 nm) with a confocal laser scanning microscope (Zeiss LSM 510 META).
Statistical analysis
All experiments were repeated independently three times and at least six plants were included in each treatment in an independent experiment. Data obtained from three biological replications were subjected to statistical analysis by Student's t‐test using DPS software (http://www.chinadps.net/) and the results are presented as mean ± standard deviation. Probability values of P < 0.05 were considered as significant between different treatments.
Supporting information
Table S1 Primers used in this study.
Acknowledgements
This work was supported by the National High‐Tech R & D Program (No. 2012AA101504), the National Key Technology R & D Program of China (2011BAD12B04), the National Basic Research Program of China (2009CB119005) and the Research Fund for the Doctoral Program of Higher Education of China (20120101110070).
References
- AbuQamar, S. , Chai, M.F. , Luo, H. , Song, F. and Mengiste, T. (2008) Tomato protein kinase 1b mediates signaling of plant responses to necrotrophic fungi and insect herbivory. Plant Cell, 20, 1964–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou, N. , Belanger, R. , Rey, P. and Tirilly, Y. (2001) Oligandrin, the elicitin‐like protein produced by the mycoparasite Pythium oligandrum, induces systemic resistance to Fusarium crown root rot in tomato plants. Plant Physiol. Biochem. 39, 681–696. [Google Scholar]
- Benhamou, N. , le Floch, G. , Vallance, J. , Gerbore, J. , Grizard, D. and Rey, P. (2012) Pythium oligandrum: an example of opportunistic success. Microbiology, 158, 2679–2694. [DOI] [PubMed] [Google Scholar]
- Benito, E.P. , ten Have, A. , van't Klooster, J.W. and van Kan, J.A.L. (1998) Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea . Eur. J. Plant Pathol. 104, 207–220. [Google Scholar]
- Boller, T. and Felix, G. (2009) A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Boller, T. and He, S.Y. (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science, 324, 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet, P. , Bourdon, E. , Ponchet, M. , Blein, J.P. and Ricci, P. (1996) Acquired resistance triggered by elicitins in tobacco and other plants. Eur. J. Plant Pathol. 102, 181–192. [Google Scholar]
- Chen, L. , Zhang, S.J. , Zhang, S.S. , Qu, S. , Ren, X. , Long, J. , Yin, Q. , Qian, J. , Sun, F. , Zhang, C. , Wang, L. , Wu, X. , Wu, T. , Zhang, Z. , Cheng, Z. , Hayes, M. , Beer, S.V. and Dong, H. (2008a) A fragment of the Xanthomonas oryzae pv. oryzicola harpin HpaG Xooc reduces disease and increases yield of rice in extensive grower plantings. Phytopathology, 98, 792–802. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Qian, J. , Qu, S. , Long, J. , Yin, Q. , Zhang, C. , Wu, X. , Sun, F. , Wu, T. , Hayes, M. , Beer, S.V. and Dong, H. (2008b) Identification of specific fragments of HpaG Xooc, a harpin from Xanthomonas oryzae pv. oryzicola, that induce disease resistance and enhance growth in plants. Phytopathology, 98, 781–791. [DOI] [PubMed] [Google Scholar]
- Choi, M.S. , Kim, W. , Lee, C. and Oh, C.S. (2013) Harpins, multifunctional proteins secreted by gram‐negative plant‐pathogenic bacteria. Mol. Plant–Microbe Interact. 26, 1115–1122. [DOI] [PubMed] [Google Scholar]
- Cordelier, S. , de Ruffray, P. , Fritig, B. and Kauffmann, S. (2003) Biological and molecular comparison between localized and systemic acquired resistance induced in tobacco by a Phytophthora megasperma glycoprotein elicitin. Plant Mol. Biol. 51, 109–118. [DOI] [PubMed] [Google Scholar]
- Dietrich, R.A. , Delaney, T.P. , Uknes, S.J. , Ward, E.R. , Ryals, J.A. and Dangl, J.L. (1994) Arabidopsis mutants simulating disease resistance response. Cell, 77, 565–577. [DOI] [PubMed] [Google Scholar]
- Dong, X. (2004) NPR1, all things considered. Curr. Opin. Plant Biol. 7, 547–552. [DOI] [PubMed] [Google Scholar]
- Durrant, W.E. and Dong, X. (2004) Systemic acquired resistance. Annu. Rev. Phytopathol. 42, 185–209. [DOI] [PubMed] [Google Scholar]
- Fu, Z.Q. and Dong, X. (2013) Systemic acquired resistance: turning local infection into global defense. Annu. Rev. Plant Biol. 64, 839–863. [DOI] [PubMed] [Google Scholar]
- Goodin, M.M. , Zaitlin, D. , Naidu, R.A. and Lommel, S.A. (2008) Nicotiana benthamiana: its history and future as a model for plant–pathogen interactions. Mol. Plant–Microbe Interact. 21, 1015–1026. [DOI] [PubMed] [Google Scholar]
- Gopalan, S. , Wei, W. and He, S.Y. (1996) hrp gene‐dependent induction of hin1: a plant gene activated rapidly by both harpins and the avrPto gene‐mediated signal. Plant J. 10, 591–600. [DOI] [PubMed] [Google Scholar]
- Grant, B.R. , Ebert, D. and Gayler, K.R. (1996) Elicitins: proteins in search of a role. Aust. Plant Pathol. 25, 148–157. [Google Scholar]
- Grant, M. and Lamb, C. (2006) Systemic immunity. Curr. Opin. Plant Biol. 9, 414–420. [DOI] [PubMed] [Google Scholar]
- Greeff, C. , Roux, M. , Mundy, J. and Petersen, M. (2012) Receptor‐like kinase complexes in plant innate immunity. Front. Plant Sci. 3, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase, S. , Shimizu, A. , Nakaho, K. , Takenaka, S. and Takahashi, H. (2006) Induction of transient ethylene and reduction in severity of tomato bacterial wilt by Pythium oligandrum. Plant Pathol. 55, 537–543. [Google Scholar]
- Hase, S. , Takahashi, S. and Takenaka, S. (2008) Involvement of jasmonic acid signalling in bacterial wilt disease resistance induced by biocontrol agent Pythium oligandrum in tomato. Plant Pathol. 57, 870–876. [Google Scholar]
- Jang, Y.‐S. , Sohn, S.‐I. and Wang, M.‐H. (2006) The hrpN gene of Erwinia amylovora stimulates tobacco growth and enhances resistance to Botrytis cinerea . Planta, 223, 449–456. [DOI] [PubMed] [Google Scholar]
- Kachroo, A. and Robin, G.P. (2013) Systemic signaling during plant defense. Curr. Opin. Plant Biol. 16, 527–533. [DOI] [PubMed] [Google Scholar]
- Kamoun, S. (2006) A catalogue of the effector secretome of plant pathogenic oomycetes. Annu. Rev. Phytopathol. 44, 41–60. [DOI] [PubMed] [Google Scholar]
- Kamoun, S. , Young, M. , Glascock, C.B. and Tyler, B.M. (1993) Extracellular protein elicitors from Phytophthora: host‐specificity and induction of resistance to bacterial and fungal phytopathogens. Mol. Plant–Microbe Interact. 6, 15–25. [Google Scholar]
- Kamoun, S. , Lindqvist, H. and Govers, F. (1997) A novel class of elicitin‐like genes from Phytophthora infestans . Mol. Plant–Microbe Interact. 10, 1028–1030. [DOI] [PubMed] [Google Scholar]
- Kamoun, S. , Van West, P. , Vleeshouwers, V.G.A. , de Groot, K.E. and Govers, F. (1998) Resistance of Nicotiana benthamiana to Phytophthora infestans is mediated by the recognition of the elicitor protein INF1. Plant Cell, 10, 1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti, T.D. , Huitema, E. , Cakir, C. and Kamoun, S. (2006) Synergistic interactions of the plant cell death pathways induced by Phytophthora infestans Nepl‐like protein PiNPP1.1 and INF1 elicitin. Mol. Plant–Microbe Interact. 19, 854–863. [DOI] [PubMed] [Google Scholar]
- Kanzaki, H. , Saitoh, H. , Takahashi, Y. , Berberich, T. , Ito, A. , Kamoun, S. and Terauchi, R. (2008) NbLRK1, a lectin‐like receptor kinase protein of Nicotiana benthamiana, interacts with Phytophthora infestans INF1 elicitin and mediates INF1‐induced cell death. Planta, 228, 977–987. [DOI] [PubMed] [Google Scholar]
- Kawamura, Y. , Takenaka, S. , Hase, S. , Kubota, M. , Ichinose, Y. , Kanayama, Y. , Nakaho, K. , Klessing, D. and Takahashi, H. (2009a) Enhanced defense responses in Arabidopsis induced by the cell wall protein fractions from Pythium oligandrum require SGT1, RAR1, NPR1, and JAR1 . Plant Cell Physiol. 50, 924–934. [DOI] [PubMed] [Google Scholar]
- Kawamura, Y. , Hase, S. , Takenaka, S. , Kanayama, Y. , Yoshioka, H. , Kamoun, S. and Takahashi, H. (2009b) INF1 elicitin activates jasmonic acid‐ and ethylene‐mediated signaling pathways and induces resistance to bacterial wilt disease in tomato. J. Phytopathol. 157, 287–297. [Google Scholar]
- Kawazu, K. , Mochizuki, A. , Sato, Y. , Sugeno, W. , Murata, M. , Seo, S. and Mitsuhara, I. (2012) Different expression profiles of jasmonic acid and salicylic acid inducible genes in the tomato plant against herbivores with various feeding modes. Arthropod–Plant Interact. 6, 221–230. [Google Scholar]
- Keller, H. , Blein, J.P. , Bonnet, P. and Ricci, P. (1996) Physiological and molecular characteristics of elicitin‐induced systemic acquired resistance in tobacco. Plant Physiol. 110, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, H. , Pamboukdjian, N. , Ponchet, M. , Poupet, A. , Delon, R. , Verrier, J.L. , Roby, D. and Ricci, P. (1999) Pathogen‐induced elicitin production in transgenic tobacco generates a hypersensitive response and nonspecific disease resistance. Plant Cell, 11, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl, J. , Oβwald, W. , Kohn, H. , Elstner, E.F. and Heiser, I. (2003) Different responses of two tobacco cultivars and their cell suspension cultures to quercinin, a novel elicitin from Phytophthora quercina . Plant Physiol. Biochem. 41, 261–269. [Google Scholar]
- Koehl, J. , Djulic, A. , Kirner, V. , Nguyen, T.T. and Heiser, I. (2007) Ethylene is required for elicitin‐induced oxidative burst but not for cell death induction in tobacco cell suspension cultures. J. Plant Physiol. 164, 1555–1563. [DOI] [PubMed] [Google Scholar]
- Kulye, M. , Liu, H. , Zhang, Y. , Zeng, H. , Yang, X. and Qiu, D. (2012) Hrip1, a novel protein elicitor from necrotrophic fungus, Alternaria tenuissima, elicits cell death, expression of defence‐related genes and systemic acquired resistance in tobacco. Plant Cell Environ. 35, 2104–2120. [DOI] [PubMed] [Google Scholar]
- Le Floch, G. , Rey, P. , Deniel, F. , Benhamou, N. , Picard, K. and Tirilly, Y. (2003) Enhancement of development and induction of resistance in tomato plants by the antagonist, Pythium oligandrum . Agronomie, 23, 455–460. [Google Scholar]
- Liu, Y.L. , Schiff, M. and Dinesh‐Kumar, S.P. (2002) Virus‐induced gene silencing in tomato. Plant J. 31, 777–786. [DOI] [PubMed] [Google Scholar]
- Loake, G. and Grant, M. (2007) Salicylic acid in plant defence—the players and protagonists. Curr. Opin. Plant Biol. 10, 466–472. [DOI] [PubMed] [Google Scholar]
- van Loon, L.C. , Bakker, P.A. and Pieterse, C.M. (1998) Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36, 453–483. [DOI] [PubMed] [Google Scholar]
- Lou, B.G. , Wang, A.Y. , Lin, C. , Xu, T. and Zheng, X.D. (2011) Enhancement of defense responses by oligandrin against Botrytis cinerea in tomatoes. Afr. J. Biotechnol. 10, 11 442–11 449. [Google Scholar]
- Masunaka, A. , Nakaho, K. , Sakai, M. , Takahashi, H. and Takenaka, S. (2009) Visualization of Ralstonia solanacearum cells during biocontrol of bacterial wilt disease in tomato with Pythium oligandrum . J. Gen. Plant Pathol. 75, 281–287. [Google Scholar]
- Masunaka, A. , Sekiguchi, H. , Takahashi, H. and Takenaka, S. (2010) Distribution and expression of elicitin‐like protein genes of the biocontrol agent Pythium oligandrum . J. Phytopathol. 158, 417–426. [Google Scholar]
- Meldau, S. , Baldwin, I.T. and Wu, J. (2011) SGT1 regulates wounding‐ and herbivory‐induced jasmonic acid accumulation and Nicotiana attenuata's resistance to the specialist lepidopteran herbivore Manduca sexta . New Phytol. 189, 1143–1156. [DOI] [PubMed] [Google Scholar]
- Miao, W. , Wang, X. , Li, M. , Song, C. , Wang, Y. , Hu, D. and Wang, J. (2010) Genetic transformation of cotton with a harpin‐encoding gene hpaXoo confers an enhanced defense response against different pathogens through a priming mechanism. BMC Plant Biol. 10, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, N. , Lherminier, J. , Farmer, M.J. , Fromentin, J. , Béno, N. , Houot, V. , Milat, M.L. and Blein, J.P. (2007) Defense responses in grapevine leaves against Botrytis cinerea induced by application of a Pythium oligandrum strain or its elicitin, oligandrin, to roots. Phytopathology, 97, 611–620. [DOI] [PubMed] [Google Scholar]
- Mur, L.A. , Kenton, P. , Lloyd, A.J. , Ougham, H. and Prats, E. (2008) The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 59, 501–520. [DOI] [PubMed] [Google Scholar]
- Peart, J.R. , Lu, R. , Sadanandom, A. , Malcuit, I. , Moffett, P. , Brice, D.C. , Schauser, L. , Jaggard, D.A. , Xiao, S. , Coleman, M.J. , Dow, M. , Jones, J.D. , Shirasu, K. and Baulcombe, D.C. (2002) Ubiquitin ligase‐associated protein SGT1 is required for host and nonhost disease resistance in plants. Proc. Natl. Acad. Sci. USA, 99, 10 865–10 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, D.H. , Qiu, D.W. , Ruan, L.F. , Zhou, C.F. and Sun, M. (2011) Protein elicitor PemG1 from Magnaporthe grisea induces systemic acquired resistance (SAR) in plants. Mol. Plant–Microbe Interact. 24, 1239–1246. [DOI] [PubMed] [Google Scholar]
- Peng, J.L. , Bao, Z.L. , Ren, H.Y. , Wang, J.S. and Dong, H.S. (2004) Expression of harpin (Xoo) in transgenic tobacco induces pathogen defense in the absence of hypersensitive cell death. Phytopathology, 94, 1048–1055. [DOI] [PubMed] [Google Scholar]
- Picard, K. , Ponchet, M. , Blein, J.P. , Rey, P. , Tirilly, Y. and Benhamou, N. (2000) Oligandrin. A proteinaceous molecule produced by the mycoparasite Pythium oligandrum induces resistance to Phytophthora parasitica infection in tomato plants. Plant Physiol. 124, 379–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse, C.M.J. and Van Loon, L.C. (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr. Opin. Plant Biol. 7, 456–464. [DOI] [PubMed] [Google Scholar]
- Ponchet, M. , Panabières, F. , Milat, M.‐L. , Mikes, V. , Montillet, J.L. , Suty, L. , Triantaphylides, C. , Tirilly, Y. and Blein, J.P. (1999) Are elicitins cryptograms in plant–oomycete communications? Cell. Mol. Life Sci. 56, 1020–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontier, D. , Godiard, L. , Marco, Y. and Roby, D. (1994) hsr203J, a tobacco gene whose activation is rapid, highly localized and specific for incompatible plant/pathogen interactions. Plant J. 5, 507–521. [DOI] [PubMed] [Google Scholar]
- Pontier, D. , Gan, S. , Amasino, R.M. , Roby, D. and Lam, E. (1999) Markers for hypersensitive response and senescence show distinct patterns of expression. Plant Mol. Biol. 39, 1243–1255. [DOI] [PubMed] [Google Scholar]
- Qiu, D. , Mao, J. , Yang, X. and Zeng, H. (2009) Expression of an elicitor‐encoding gene from Magnaporthe grisea enhances resistance against blast disease in transgenic rice. Plant Cell Rep. 28, 925–933. [DOI] [PubMed] [Google Scholar]
- Qutob, D. , Kemmerling, B. , Brunner, F. , Kufner, I. , Engelhardt, S. , Gust, A.A. , Luberacki, B. , Seitz, H.U. , Stahl, D. , Rauhut, T. , Glawischnig, E. , Schween, G. , Lacombe, B. , Watanabe, N. , Lam, E. , Schlichting, R. , Scheel, D. , Nau, K. , Dodt, G. , Hubert, D. , Gijzen, M. and Nürnberger, T. (2006) Phytotoxicity and innate immune responses induced by Nep1‐like proteins. Plant Cell, 18, 3721–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, M. , Wang, J. , Dean, R.A. , Lin, Y. , Gao, X. and Hu, S. (2008) Expression of a harpin‐encoding gene in rice confers durable nonspecific resistance to Magnaporthe grisea . Plant Biotechnol. J. 6, 73–81. [DOI] [PubMed] [Google Scholar]
- Shirasu, K. (2009) The HSP90‐SGT1 chaperone complex for NLR immune sensors. Annu. Rev. Plant Biol. 60, 139–164. [DOI] [PubMed] [Google Scholar]
- Shiu, S.‐H. , Karlowski, W.M. , Pan, R. , Tzeng, Y.‐H. , Mayer, K.F.X. and Li, W.‐H. (2004) Comparative analysis of the receptor‐like kinase family in Arabidopsis and rice. Plant Cell, 16, 1220–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoresh, M. , Harman, G.E. and Mastouri, F. (2010) Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 48, 21–43. [DOI] [PubMed] [Google Scholar]
- Spoel, S.H. and Dong, X. (2012) How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12, 89–100. [DOI] [PubMed] [Google Scholar]
- Strobel, N.E. , Ji, C. , Gopalan, S. , Kuc, J.A. and He, S.Y. (1996) Induction of systemic acquired resistance in cucumber by Pseudomonas syringae pv. syringae 61 HrpZPss protein. Plant J. 9, 431–439. [Google Scholar]
- Takahashi, H. , Ishihara, T. , Hase, S. , Chiba, A. , Nakaho, K. , Arie, T. , Teraoka, T. , Iwata, M. , Tugane, T. , Shibata, D. and Takenaka, S. (2006) Beta‐cyanoalanine synthase as a molecular marker for induced resistance by fungal glycoprotein elicitor and commercial plant activators. Phytopathology, 96, 908–916. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y. , Berberich, T. , Yamashita, K. , Uehara, Y. , Miyazaki, A. and Kusano, T. (2004a) Identification of tobacco HIN1 and two closely related genes as spermine‐responsive genes and their differential expression during the Tobacco mosaic virus‐induced hypersensitive response and during leaf‐ and flower‐senescence. Plant Mol. Biol. 54, 613–622. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y. , Uehara, Y. , Berberich, T. , Ito, A. , Saitoh, H. , Miyazaki, A. , Terauchi, R. and Kusano, T. (2004b) A subset of hypersensitive response marker genes, including HSR203J, is the downstream target of a spermine signal transduction pathway in tobacco. Plant J. 40, 586–595. [DOI] [PubMed] [Google Scholar]
- Takenaka, S. and Tamagake, H. (2009) Foliar spray of a cell wall protein fraction from the biocontrol agent Pythium oligandrum induces defence‐related genes and increases resistance against Cercospora leaf spot in sugar beet. J. Gen. Plant Pathol. 75, 340–348. [Google Scholar]
- Takenaka, S. , Nishio, Z. and Nakamura, Y. (2003) Induction of defense reactions in sugar beet and wheat by treatment with cell wall protein fractions from the mycoparasite Pythium oligandrum . Phytopathology, 93, 1228–1232. [DOI] [PubMed] [Google Scholar]
- Takenaka, S. , Nakamura, Y. , Kono, T. , Sekiguchi, H. , Masunaka, A. and Takahashi, H. (2006) Novel elicitin‐like proteins isolated from cell wall of the biocontrol agent Pythium oligandrum induce defense‐related genes in sugar beet. Mol. Plant Pathol. 7, 325–339. [DOI] [PubMed] [Google Scholar]
- Takenaka, S. , Yamaguchi, K. , Masunaka, A. , Hase, S. , Inoue, T. and Takahashi, H. (2011) Implications of oligomeric forms of POD‐1 and POD‐2 proteins isolated from cell walls of the biocontrol agent Pythium oligandrum in relation to their ability to induce defense reactions in tomato. J. Plant Physiol. 168, 1972–1979. [DOI] [PubMed] [Google Scholar]
- Thordal‐Christensen, H. , Zhang, Z. , Wei, Y. and Collinge, D.B. (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J. 11, 1187–1194. [Google Scholar]
- Varet, A. , Parker, J. , Tornero, P. , Nass, N. , Nürnberger, T. , Dangl, J.L. , Scheel, D. and Lee, J. (2002) NHL25 and NHL3, two NDR1/HIN1‐1ike genes in Arabidopsis thaliana with potential role(s) in plant defense. Mol. Plant–Microbe Interact. 15, 608–616. [DOI] [PubMed] [Google Scholar]
- Walters, D.R. , Ratsep, J. and Havis, N.D. (2013) Controlling crop diseases using induced resistance: challenges for the future. J. Exp. Bot. 64, 1263–1280. [DOI] [PubMed] [Google Scholar]
- Wang, A. , Lou, B. , Xu, T. and Lin, C. (2011) Defense responses in tomato fruit induced by oligandrin against Botrytis cinerea. Afri. J. Biotechnol. 10, 4596–4601. [Google Scholar]
- van Wees, S.C. , Van der Ent, S. and Pieterse, C.M. (2008) Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 11, 443–448. [DOI] [PubMed] [Google Scholar]
- Wendehenne, D. , Binet, M.N. , Blein, J.P. , Ricci, P. and Pugin, A. (1995) Evidence for specific, high affinity binding sites for a proteinaceous elicitor in tobacco plasma membrane. FEBS Lett. 374, 203–207. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Zhang, H. , Li, G. , Li, W. , Wang, X. and Song, F. (2009) Ectopic expression of MgSM1, a Cerato‐platanin family protein from Magnaporthe grisea, confers broad‐spectrum disease resistance in Arabidopsis. Plant Biotechnol. J. 7, 763–777. [DOI] [PubMed] [Google Scholar]
- Yu, L.M. (1995) Elicitins from Phytophthora and basic resistance in tobacco. Proc. Natl. Acad. Sci. USA, 92, 4088–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Dong, S. , Wang, M. , Wang, W. , Song, W. , Dou, X. , Zheng, X. and Zhang, Z. (2010) The role of vacuolar processing enzyme (VPE) from Nicotiana benthamiana in the elicitor‐triggered hypersensitive response and stomatal closure. J. Exp. Bot. 61, 3799–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Li, D. , Wang, M. , Liu, J. , Teng, W. , Cheng, B. , Huang, Q. , Wang, M. , Song, W. , Dong, S. , Zheng, X. and Zhang, Z. (2012) The Nicotiana benthamiana mitogen‐activated protein kinase cascade and WRKY transcription factor participate in Nep1(Mo)‐triggered plant responses. Mol. Plant–Microbe Interact. 25, 1639–1653. [DOI] [PubMed] [Google Scholar]
- Zheng, M.S. , Takahashi, H. , Miyazaki, A. , Hamamoto, H. , Shah, J. , Yamaguchi, I. and Kusano, T. (2004) Up‐regulation of Arabidopsis thaliana NHL10 in the hypersensitive response to Cucumber mosaic virus infection and in senescing leaves is controlled by signalling pathways that differ in salicylate involvement. Planta, 218, 740–750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Primers used in this study.


