Summary
Apple scab, caused by the fungal pathogen Venturia inaequalis, is one of the most severe diseases of apple worldwide. It is the most studied plant–pathogen interaction involving a woody species using modern genetic, genomic, proteomic and bioinformatic approaches in both species. Although ‘Geneva’ apple was recognized long ago as a potential source of resistance to scab, this resistance has not been characterized previously. Differential interactions between various monoconidial isolates of V. inaequalis and six segregating F1 and F2 populations indicate the presence of at least five loci governing the resistance in ‘Geneva’. The 17 chromosomes of apple were screened using genotyping‐by‐sequencing, as well as single marker mapping, to position loci controlling the V. inaequalis resistance on linkage group 4. Next, we fine mapped a 5‐cM region containing five loci conferring both dominant and recessive scab resistance to the distal end of the linkage group. This region corresponds to 2.2 Mbp (from 20.3 to 22.5 Mbp) on the physical map of ‘Golden Delicious’ containing nine candidate nucleotide‐binding site leucine‐rich repeat (NBS‐LRR) resistance genes. This study increases our understanding of the complex genetic basis of apple scab resistance conferred by ‘Geneva’, as well as the gene‐for‐gene (GfG) relationships between the effector genes in the pathogen and resistance genes in the host.
Keywords: apple scab, differential host, fine genetic mapping, gene‐for‐gene relationships, molecular marker, resistance, Venturia inaequalis
Introduction
Scab, caused by the fungus Venturia inaequalis, is one of the most serious diseases of apple (Malus × domestica Borkh.) worldwide. In‐plant resistance to pathogens offers a promising alternative to disease control methods based on the use of pesticides, but often lacks durability as a result of the rapid evolution of pathogen populations (Caffier et al., 2014). Each year, V. inaequalis undergoes a sexual reproduction phase that is the basis for rapid changes in the virulence distributions in populations and raises a continuous threat to the effectiveness of existing scab‐resistant varieties planted for 10–15 years in commercial orchards. Hence, there is an urgent need for strategies to develop varieties with durable resistance to scab. Pyramiding of multiple resistance (R) genes into one cultivar has been an effective route to avoid the selection of new pathogen races and to create more durable resistance in numerous plant–pathogen systems (Huang et al., 1997; Liu et al., 2000; Werner et al., 2005; Witcombe and Hash, 2000). However, this approach is time consuming for classical breeding in tree crops with a long juvenile period, such as apple. Moreover, plants combining two or more R genes are difficult to distinguish phenotypically from plants that have inherited a single resistance. The speed of selection of these plants with pyramided R genes can be greatly increased by the utilization of marker‐assisted selection (MAS) to identify the seedlings that carry multiple resistance alleles.
In the past, at least 17 major scab R genes and 13 quantitative trait loci (QTLs) have been identified and mapped across 11 apple linkage groups (LGs) (Bus et al., 2011; Calenge et al., 2004; Durel et al., 2003, 2004; Gessler et al., 2006; Liebhard et al., 2003; Soufflet‐Freslon et al., 2008). The identity of the genes underlying the resistance loci remains largely unknown as only two major apple scab R genes have been cloned to date: Rvi6 (Vf) encoding for a leucine‐rich repeat receptor‐like family protein (LRR‐RLP) similar to the tomato Cf R gene (Vinatzer et al., 2001), and Rvi15 (Vr2) encoding a nucleotide‐binding site leucine‐rich repeat (NBS‐LRR) family protein (Galli et al., 2010).
The process of resistance mapping and gene discovery has been made more efficient recently, as the draft genome sequence of the domesticated apple cultivar ‘Golden Delicious’ (GD) (Velasco et al., 2010) has given apple researchers access to millions of single nucleotide polymorphism (SNP) markers spanning the whole apple genome. Moreover, the annotation in GD of numerous R gene analogues (RGAs) encoding for NBS‐LRR proteins (Perazzolli et al., 2014) could be the basis of a candidate gene approach to decipher the plant–pathogen interactions between apple and V. inaequalis. These genes show gene‐for‐gene (GfG) relationships with effector genes in the pathogen and, if the corresponding effectors are not essential, are likely to be of a non‐durable nature. In apple, SNP arrays have been developed for the construction of very dense genetic maps (Bianco et al., 2014; Chagné et al., 2012), and genotyping‐by‐sequencing (GBS) based on next‐generation sequencing technologies (Elshire et al., 2011) has been used for association studies and genomics‐assisted breeding (Deng et al., 2014; Gardner et al., 2014).
‘Geneva’ is a scab‐resistant, open‐pollinated selection of Malus pumila (Jefferson, 1970). Resistance symptoms on adult plant material were Class 2 non‐sporulating, irregular chlorotic or necrotic reactions (Shay and Hough, 1952), with the same resistance reactions induced by seven isolates from the USA, the Netherlands and Germany. In contrast, monoconidial isolates from Canada induced symptoms ranging from hypersensitive reactions (HRs) to susceptibility, the latter by an isolate collected from ‘Geneva’ (Julien and Spangelo, 1957), which indicated differential interactions with multiple R genes. Williams and Shay (1957) discerned two distinct resistance classes (Classes 2 and 3) associated with the p‐10 and p‐11 avirulence genes in V. inaequalis identified in the progeny of virulent isolate 651 collected from ‘Geneva’ in Nova Scotia in 1951 (Shay and Williams, 1956). With the 2 → 4 phenotype (Shay and Williams, 1956) assumed to be different from the Classes 2 and 3 resistance reactions, the ‘Geneva’ scab resistance was interpreted as being conditioned by three scab R genes (Bus et al., 2011).
In this study, we use genetic maps constructed by GBS and traditional marker technologies to show that apple scab resistance of ‘Geneva’ is conditioned by at least five resistance loci, suggesting both dominant and recessive genetic control, which all mapped to the lower end of LG4. Primers specific to candidate NBS‐LRR R genes found in the homologous region of the GD genome assembly were designed and used to construct a detailed genetic map around the ‘Geneva’ resistance loci. The consequences of the complexity of the ‘Geneva’ scab resistance for breeding for durable scab resistance and the selection of a differential host (3) (Bus et al., 2011) for V. inaequalis pathotype monitoring ( www.vinquest.ch ) are discussed.
Results
Resistance phenotypes and segregation ratios
Although the F1 families (Table 1) showed very similar segregation ratios of resistant to susceptible (R : S) = 1 : 1 for isolates 1639, EU‐B04 and EU‐NL19, variable segregation ratios were observed for isolates 1770‐3, 1774‐1, NZ188B and EU‐B05, with each time less than one‐half of the seedling population being incompatible (R : S = 1 : 2, 1 : 3 and 1 : 7) (Table 2). The plants resistant to the latter isolates were always also resistant to the former isolates. A notable finding was that isolates 1774‐1 and NZ188B showed segregation ratios in the R : S = 1 : 2 to 1 : 3 range in the cross with ‘Elstar’ (E), but in the 1 : 3 to 1 : 7 range with ‘Braeburn’ (BB).
Table 1.
F1 and F2 families and Venturia inaequalis isolate combinations used in this study to investigate the genetics of scab resistance of ‘Geneva’
| Family | Parents | Generation | Number of progeny | Year of phenotyping | Isolates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | 1639 | 1774‐1 | 1770‐3 | EU‐B04 | EU‐B05 | EU‐D42 | EU‐NL19 | EU‐NL24 | NZ188B | ||||
| M | ‘Elstar’ | ‘Geneva’ | F1 | 125 | 2005 | x | x | x | x | x | ||||
| Q | ‘Geneva’ | ‘Braeburn’ | F1 | 91 | 2005 | x | x | x | x | x | x | |||
| 2011‐050 | ‘Golden Delicious’ | M33 | F2 | 118 | 2012 | x | x | x | x | x | ||||
| 2012‐047 | ‘Elstar’ | Q35 | F2 | 119 | 2013 | x | x | x | x | x | x | |||
| 2012‐037 | Q49 | ‘Gala’ | F2 | 40 | 2013 | (x)* | x | x | (x) | |||||
| 2012‐041 | ‘Gala’ | Q71 | F2 | 119 | 2013 | x | x | x | x | x | ||||
*(x) means inoculum applied, but data not sufficient for genetic analysis.
Table 2.
Segregation data for the various families derived from ‘Geneva’ 21 days after inoculation with conidia from monospore cultures of V enturia inaequalis isolates
| Family | 1639 | EU‐NL19 | EU‐B04 | EU‐D42 | 1770‐3 | 1774‐1 | NZ188B | EU‐B05 | EU‐NL24 | |
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | E × ‘Geneva’ † | |||||||||
| R | 67 | 54 | — | — | 36 | 40 | 41 | — | — | |
| I | 2 | 9 | — | — | 8 | 4 | 9 | — | — | |
| S | 56 | 62 | — | — | 81 | 81 | 75 | — | — | |
| Segregation ratios‡ | 1 : 1*** | 1 : 1*** | — | — | 1 : 2*** | 1 : 2*** | 1 : 2*** | — | — | |
| 1 : 3*** | 1 : 3** | 1 : 3** | — | — | ||||||
| F1 | ‘Geneva’ × BB | |||||||||
| R | 40 | — | 40 | — | — | 13 | 17 | 13 | 0 | |
| I | 4 | — | 4 | — | — | 14 | 5 | 5 | 0 | |
| S | 47 | — | 47 | — | — | 64 | 69 | 73 | 91 | |
| Segregation ratios | 1 : 1*** | — | 1 : 1*** | — | — | 1 : 3*** | 1 : 3*** | 1 : 3** | — | |
| — | — | — | 1 : 7*** | 1 : 7** | 1 : 7*** | — | ||||
| F2 | G × Q71 | |||||||||
| R | 46 | 62 | 63 | — | 14 | 39 | — | — | — | |
| I | 10 | 11 | 5 | — | 8 | 10 | — | — | — | |
| S | 63 | 46 | 51 | — | 97 | 70 | — | — | — | |
| Segregation ratios | 1 : 1*** | 1 : 1*** | 1 : 1*** | — | 1 : 3* | 1 : 3* | — | — | — | |
| — | 1 : 7*** | 1 : 2*** | — | — | — | |||||
| F2 | GD × M33 | |||||||||
| R | 58 | 35 | — | 62 | 46 | 50 | — | — | — | |
| I | 9 | 18 | — | 8 | 5 | 4 | — | — | — | |
| S | 51 | 65 | — | 48 | 67 | 64 | — | — | — | |
| Segregation ratios | 1 : 1*** | 1 : 3** | — | 1 : 1*** | 1 : 1** | 1 : 1*** | — | — | — | |
| 1 : 2*** | — | — | — | — | ||||||
| F2 | E × Q35 | |||||||||
| R | 15 | 71 | 57 | 79 | 15 | 18 | — | — | — | |
| I | 17 | 25 | 7 | 6 | 5 | 11 | — | — | — | |
| S | 90 | 26 | 58 | 37 | 102 | 93 | — | — | — | |
| Segregation ratios | 1 : 3** | 3 : 1*** | 1 : 1*** | 3 : 1** | 1 : 3* | 1 : 3** | — | — | — | |
| 1 : 7*** | 1 : 7*** | 1 : 7*** | — | — | — | |||||
| F2 | Q49 × G | |||||||||
| R | — | — | — | — | 13 | 3 | — | — | — | |
| I | — | — | — | — | 3 | 0 | — | — | — | |
| S | — | — | — | — | 24 | 37 | — | — | — | |
| Segregation ratios | — | — | — | — |
1 : 1**
1 : 2*** 1 : 3*** |
— | — | — |
BB, ‘Braeburn’; E, ‘Elstar’; G, ‘Gala’; GD, ‘Golden Delicious’.
†I, indeterminate; R, resistant; S, susceptible; ‘I’ seedlings were discarded from the segregation ratio calculations.
‡Significant Mendelian segregation ratios judged at the following levels for P (χ 2) values: ***P > 0.1; **P > 0.01; *P > 0.001.
Most plants that were resistant to all isolates exhibited Class 2 resistance reactions, sometimes with some HR as the result of the high inoculum density, whereas greater proportions of Classes 3A, 3B and 4 were found in plants showing more complex interactions between isolates. Isolate EU‐NL24 was compatible with all ‘Geneva’ × BB progeny (Table 2).
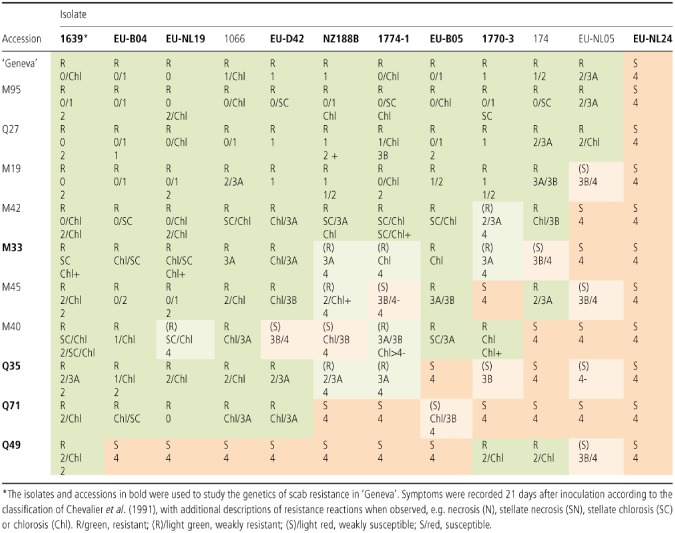
From these F1 families, 10 seedlings were selected to represent the main patterns of differential interactions observed among isolates and subjected, with ‘Geneva’, to a replicated clonal evaluation. Most of the differential interactions were confirmed, with additional isolates providing complementary information (Table 3). Being adult clonal material, the plants showed a higher degree of resistance in that some plants showed no symptoms and/or Class 1 (HR) reactions, which were not observed on the young F1 seedlings. Other plants showed symptoms ranging from Classes 2 to 4, with some isolates inducing varied resistance reactions on the same leaf. Some isolates, such as NZ188B, 1774‐1 and 1770‐3, showed some inconsistencies among the replicates as both resistance and susceptibility symptoms were observed. Eleven of the 12 reference isolates were incompatible with ‘Geneva’, whereas EU‐NL24 was compatible with this accession and all of its F1 derivatives. Isolate 1639 was the only one incompatible with ‘Geneva’ and all of its selected resistant progeny.
Table 3.
Host–pathogen interactions observed between 12 monoconidial isolates of V enturia inaequalis on clonal trees of ‘Geneva’ and selected derivatives
Genotypes M33 from the E × ‘Geneva’ progeny, and Q35, Q71 and Q49 from the ‘Geneva’ × BB progeny, were selected as the resistant parents for the F2 families and were crossed with susceptible parents GD, E or ‘Gala’ (G). (Table 1). Symptoms varied from Class 1 to Class 4 and segregation for resistance was found, even with 1774‐1 and 1770‐3 isolates showing (weak) compatibility with parents M33, Q35 and/or Q71 (Table 2). In these particular cases, less than one‐half of the population was found to be resistant to these isolates (R : S = 1 : 2, 1 : 3 and 1 : 7). Conversely, when these isolates were incompatible with the resistant parent, the resistance often segregated following a ratio of R : S = 1 : 1 in the corresponding F2 progeny (e.g. 1774‐1 in GD × M33 and 1770‐3 in Q49 × G). Resistance to isolates 1639, EU‐NL19, EU‐B04 and EU‐D42 was found in one‐half of the various F2 families (R : S = 1 : 1) or less (R : S = 1 : 2, 1 : 3 and 1 : 7), with only isolates EU‐D42 and EU‐NL19 showing a higher proportion of seedlings resistant, with a tendency towards a R : S = 3 : 1 segregation in the E × Q35 family (Table 2).
Differential interactions
From 3% to 23% of the seedlings in the F1 and F2 progenies were fully incompatible with the set of monoconidial V. inaequalis tested, whereas from 16% to 58% were fully compatible (Table S5, see Supporting Information). The remaining seedlings showed complex differential interactions with V. inaequalis, with some isolates showing very similar differential interactions. For example, in the F1 progenies, isolate 1774‐1 was similar to NZ188B in both F1 families, and to EU‐B05 in the ‘Geneva’ × BB family (except for three plants). Across the various progenies, some seedlings were incompatible with only one isolate among the set of isolates tested (the other isolates were fully compatible with these particular seedlings). This was notably the case for isolates EU‐NL19 (in E × ‘Geneva’, G × Q71, E × Q35, but not in GD × M33), 1639 (in E × ‘Geneva’, ‘Geneva’ × BB, GD × M33, but not in G × Q71 or E × Q35), EU‐B04 (in ‘Geneva’ × BB and G × Q71, but not in E × Q35) and, finally, EU‐D42 (in GD × M33 and E × Q35). Some of the differential interactions and symptoms observed in various progenies are illustrated in Fig. S1 (see Supporting Information).
Based on these observations and considering all the differential interactions among the isolates and the segregation ratios observed, we hypothesized a model for the GfG relationships between the V. inaequalis isolates and the resistance loci (Table 4), the interpretation of which will be developed in the Discussion section.
Table 4.
Model for gene‐for‐gene interactions between the various V enturia inaequalis isolates and the scab resistance loci in ‘Geneva’
| R locus name* | 1639 | EU‐NL19 | EU‐B04 | EU‐D42 | 1770‐3 | 1774‐1 | NZ188B | EU‐B05 |
|---|---|---|---|---|---|---|---|---|
| Rvh3.1 | − | + | + | + | + | + | + | + |
| Rvh3.2 | + | − | − | + | + | + | + | + |
| Rvh3.3 | + | + | − | − | + | + | + | + |
| rvh3.4 | − | − | − | − | + | − | − | − |
| rvh3.5 | − | − | − | − | − | + | + | + |
*Our data suggest dominant genetic control for Rvh3.1, Rvh3.2 and Rvh3.3, and more complex (recessive) genetic control for rvh3.4 and rvh3.5.
GBS and linkage analysis
In total, 23 585 GBS‐derived SNPs polymorphic for the resistant parent Q71 were detected. Of these, 10 631 grouped into 17 LGs with a logarithm of odds (LOD) score of 5, which corresponded to an average of one mapped SNP for every 56 kb (Table S6, see Supporting Information). Loci for resistance to the isolates EU‐NL19, EU‐B04, 1774‐1 and 1639 all mapped to LG4. Figure 1 presents a genetic linkage map of a subset of markers around these loci. No linkage was detected for the locus for resistance to the 1770‐3 isolate.
Figure 1.
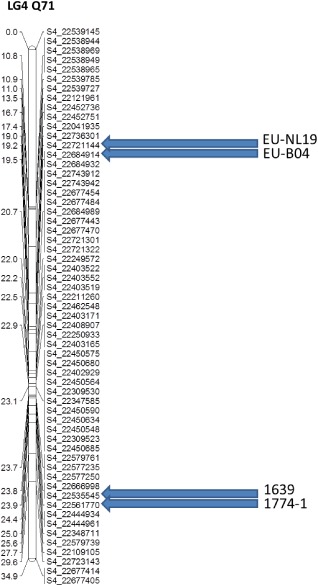
Development of a high‐density linkage map using genotyping‐by‐sequencing (GBS) sequence tags and phenotypic data for reaction to the isolates EU‐NL19, EU‐B04, 1639 and 1774‐1 in ‘Gala’ × Q71 progeny. Marker names indicate the physical location of the single nucleotide polymorphism (SNPs) at the lower end of chromosome 4 of the ‘Golden Delicious’ genome assembly. LG4, linkage group 4.
Fine mapping of the resistance loci on LG4
Genetic maps for apple LG4 were constructed for the resistant parents ‘Geneva’, Q71, M33, Q35 and Q49 using between 9 and 17 SNP markers in each population (Figs 2 and 3). Single sequence repeat (SSR) markers mapped to similar positions compared with previous genetic maps (Liebhard et al., 2002; Silfverberg‐Dilworth et al., 2006). The marker order was consistent between families, indicating the robustness of the genetic maps. No significant segregation distortion was observed for any of the mapped markers on LG4. Before the mapping of the resistance loci commenced, genotype–phenotype incongruent (GPI) plants (Gygax et al., 2004) were identified (ranging from 0 to 16 per family) and excluded from the mapping population. Moreover, in population E × Q35, the R : S = 3 : 1 segregation ratio did not allow us to fine map the R genes interacting with isolates EU‐NL19 and EU‐D42. In agreement with our proposed GfG relationships model, we combined the phenotypes of the isolates EU‐NL19 + EU‐B04 and EU‐D42 + EU‐B04 to map the two resistance loci. In cases of contrasting phenotypes among the pair of isolates, the plants were scored towards susceptibility. The positions of the various R loci identified by the isolate phenotypes used to map them are given in the six parental genetic maps (Figs 2 and 3).
Figure 2.
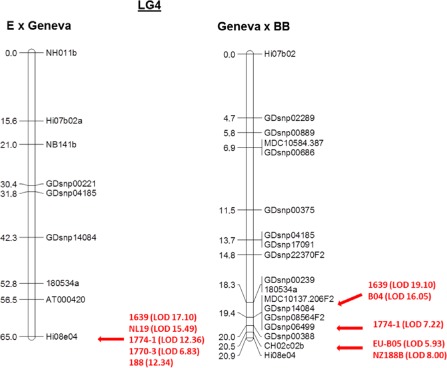
Genetic linkage analysis for the resistant parents ‘Geneva’ among single nucleotide polymorphism (SNP) and single sequence repeat (SSR) molecular markers on linkage group 4 (LG4) of F1 families ‘Elstar’ × ‘Geneva’ and ‘Geneva’ × ‘Braeburn’, and resistance towards various monoconidial Venturia inaequalis isolates. BB, ‘Braeburn’; E, ‘Elstar’; LOD, logarithm of odds.
Figure 3.
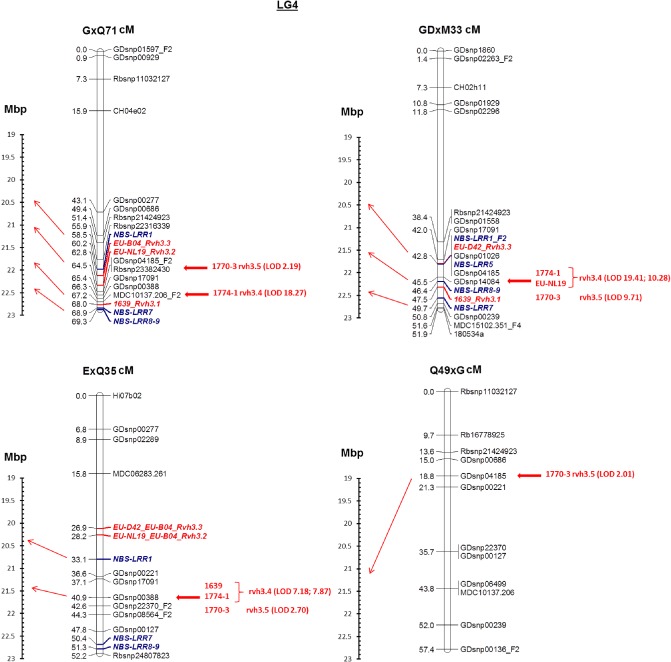
Genetic maps of linkage group 4 (in cM) of F1 resistant parents Q71, M33, Q35 crossed with ‘Gala’ (G), ‘Golden Delicious’ (GD) and ‘Elstar’ (E), respectively, and resistance towards various monoconidial Venturia inaequalis isolates. On the left of each map, arrows indicate the position on the reference ‘Golden Delicious’ physical map (in Mbp) of the closest molecular markers flanking each resistance locus. LOD, logarithm of odds.
In the F1 progenies, resistance to all the isolates mapped to the lower end of LG4 (Fig. 2). In the F2 families (Fig. 3), resistance to the various isolates was resolved to three distinct genomic regions of the denser maps: (1) resistance to isolate 1639 was linked to MDC10137.206 and GDsnp00239 in Q71 and M33, respectively, which corresponds to a position located around 22 Mbp on LG4 of the reference GD assembly; (2) resistance to isolates 1774‐1 and 1770‐3 was linked to Rbsnp23382430, MDC10137.206, GDsnp14084, GDsnp00388 and GDsnp04185 in Q71, M33, Q35 and Q49, respectively, which corresponds to 21.5 Mbp of GD LG4; (3) resistance to isolates EU‐NL19, EU‐B04 and EU‐D42 was linked to GDsnp04185, GDsnp01026 and GDsnp00221 in Q71, M33 and Q35, respectively, which corresponds to 21 Mbp in the GD LG4 assembly. The resistance loci for isolates 1639 and EU‐NL19 co‐located with 1774‐1 and 1770‐3 when mapped in the E × Q35 and GD × M33 families, respectively. All resistance loci were in coupling phase.
Co‐segregation between fully informative markers that were heterozygous in both parents (‘ef × eg’ segregation type in JoinMap format) and resistance to isolates that segregated 1 : 3 was examined (Table S7, see Supporting Information). In family G × Q71 and E × Q35, most of the resistant seedlings for isolates 1774‐1, 1639 and 1770‐3 co‐segregated with only one class of allelotypic data, among the four possible classes ‘ef’, ‘eg’, ‘ee’ and ‘fg’, whereas susceptible plants mostly co‐segregated with the remaining three classes. None of the ‘ef × eg’ markers deviated significantly from the expected Mendelian segregation.
Co‐localization of disease resistance loci with analogues of disease R genes
Based on the GD LG4 assembly (Velasco et al., 2010), nine predicted genes encoding proteins belonging to the NBS‐LRR disease R gene family (NBS‐LRR 1–9, Table S4, see Supporting Information) were identified within a 2.2‐Mbp genomic region (from 20.3 to 22.5 Mbp) between the markers flanking the resistance loci in the various families (Fig. 4). As two NBS‐LRRs had the same DNA sequence (NBS‐LRR 8 and 9), a single primer set was designed for these two analogues. Five candidate genes (NBS‐LRR 1, 5, 7, 8 and 9) mapped to LG4 of ‘Geneva’ derivatives and were linked to the resistance loci (Fig. 3), enabling a reduction of the intervals between markers and the three resistance loci. These loci mapped to the homologous region of GD at 20.5, 21.5 and 22.5 Mbp, respectively. NBS‐LRR 2, 3, 4 and 6 did not map to LG4. No analogues of R genes showing homology to the Cladiosporium fulvum R gene family of tomato (and Rvi6 scab R gene) were identified in this region.
Figure 4.
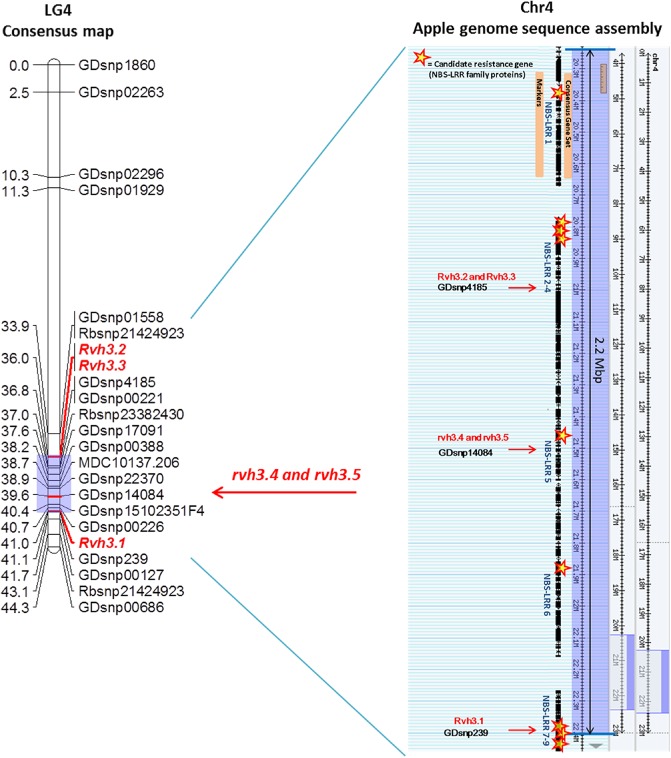
Consensus map of linkage group 4 (LG4) of ‘Geneva’ derived from the populations ‘Geneva’ × BB, GD × M33, G × Q71 and E × Q35, aligned with the homologous region of the reference apple genome (Velasco et al., 2010), showing the relative positions of the mapped disease resistance loci (Rvh3.1–3 and rvh3.4–5) to candidate disease resistance genes [nucleotide‐binding site leucine‐rich repeat (NBS‐LRR) family protein] on the genome assembly. BB, ‘Braeburn’; E, ‘Elstar’; G, ‘Gala’; GD, ‘Golden Delicious’.
Discussion
The hypothesis from previous research (Shay and Williams, 1956; Williams and Shay, 1957) was that the apple scab resistance of ‘Geneva’ was conditioned by a spontaneous pyramided complex involving three major R genes (Bus et al., 2011). We now propose that at least five genes underlie the ‘Geneva’ resistance. Our conclusions were drawn from the differential interactions of nine monoconidial V. inaequalis isolates on six F1 and F2 progenies and were based on GfG theory, which postulates that, for each R gene in a plant, there is a corresponding avirulence gene in the pathogen (Flor, 1971). Some resistant seedlings were incompatible with only one isolate (1639, EU‐NL19 or EU‐D42/EU‐B04) among the set of isolates tested, suggesting the existence of at least three independent resistance loci, the first incompatible with isolate 1639, the second incompatible with EU‐NL19, and the third incompatible with both EU‐B04 and EU‐D42. As such specific GfG relationships with isolates 1639 and EU‐NL19 were not observed in the F2 families GD × M33 and E × Q35, we suggest that the first two loci probably did not segregate in the resistant M33 and Q35 families. In contrast, no differential interactions of this type were observed for the other isolates tested (1770‐3, 1774‐1, NZ188B and EU‐B05). However, our data indicated that these isolates were able to overcome all of these first three resistance loci, suggesting the presence of two additional resistance loci. The number of resistance loci segregating in Q49 × G remains hypothetical as inoculation failed with isolates 1639 and EU‐B04 in this population. Nevertheless, we did observe segregation for resistance with isolate 1770‐3, whereas isolate 1639 was shown to be incompatible with clonal Q49 (Tables 2 and 3). This suggested the presence of at least one resistance locus in this progeny, which is in agreement with earlier findings of one of the Avr genes of 1639 showing a GfG relationship with a gene in Q49 (Broggini et al., 2011).
Our data suggest both dominant control for isolates fitting a 1 : 1 segregation ratio (1639, EU‐NL19, EU‐B04 and EU‐D42), and more complex genetic control for resistance to isolates 1774‐1, 1770‐3, NZ188B and EU‐B05. With these isolates, the following observations were made: (i) incompatibility with less than one‐half of the progenies and segregation ratios approximating R : S = 1 : 3 in some families; (ii) the single genotype obtained for resistant plants with fully informative markers (segregating 1 : 1 : 1 : 1); and (iii) the observation that the resistance could ‘skip’ one generation (segregation for resistance was found in progenies resulting from the cross of two susceptible parents). At first glance, these results could be interpreted as a genetic recessive control of the ‘Geneva’ resistance against the subset of V. inaequalis isolates, with resistance to isolates conferred in homozygous plants. Two susceptible heterozygous parents for the recessive R gene (Sr × Sr) would lead to the R : S = 1 : 3 segregation ratio observed in the progeny (e.g. interaction of 1774‐1 and 1770‐3 with G × Q71). In contrast, a resistant homozygous (rr) parent would give an R : S = 1 : 1 segregation ratio when crossed with a susceptible heterozygous (Sr) parent (e.g. interaction of 1774‐1 and 1770‐3 with E × Q35). Finally, two susceptible homozygous parents (Ss) would result in the whole progeny being susceptible (e.g. interaction of 1774‐1 with Q49 × G). Such recessive genetic control has been found in many plant–pathogen relationships, particularly in the resistance of plants to viruses, with about 100 recessive R genes identified (Diaz‐Pendon et al., 2004), but also to bacteria (Deslandes et al., 2002; Iyer and McCouch, 2004; Li et al., 2001) and fungi (Büschges et al., 1997; Calvo et al., 2008; Hua et al., 2009; Peng et al., 2014; Perchepied et al., 2005; ). In apple, recessive genetic control of resistance was proposed for bitter rot (Glomerella cingulata) (Thompson and Taylor, 1971), blotch (Phyllosticta solitaria) (Mowry, 1964) and Alternaria blotch (Alternaria alternata) (Moriya et al., 2011), and could also form the basis of segregation observed for the partial resistance to race (6) isolates of V. inaequalis in the cross ‘Prima’ × ‘Fiesta’ (Durel et al., 2003).
Various biological mechanisms have been proposed to explain a recessive resistance. Either a mutation or loss of a susceptibility allele that facilitates infection of the plant by the pathogen (e.g. the negative regulator of the defence response mlo gene in barley) and hence causes an abnormal defence response in the host (Büschges et al., 1997; Devoto et al., 1999; van Schie and Takken, 2014), or a dosage‐dependent effect of each allele of the recessive R gene that might accumulate until a sufficient resistance is reached to be effective (e.g. xa5 gene in rice) (Iyer and McCouch, 2004; Vanderplank, 1984). Nevertheless, a homozygous recessive nature of R genes in ‘Geneva’ would be remarkable from a genetics point of view, given the fact that the segregation of resistance to isolates 1774‐1, 1770‐3, NZ188B and EU‐B05 has been found in almost all families under investigation. It suggests that the same resistance allele is present in all four susceptible genotypes used to develop the F1 and F2 families and has conferred the homozygous resistant status in part of the subsequent progenies. In addition, this theory cannot explain the segregation ratios observed with some isolates in F1: crossing the homozygous resistant parent ‘Geneva’ (rr) with a heterozygous susceptible cultivar E of BB (Sr) should have led to an R : S = 1 : 1 segregation ratio instead of the 1 : 2, 1 : 3 or 1 : 7 observed.
In the Arabidopsis plant model, the recessive resistance locus RRS1 (Resistant to Ralstonia solanacearum 1) has received increasing attention from researchers. Although genetically defined as recessive in segregating populations (R : S = 1 : 3), this R gene encodes a protein whose structure combines the Toll‐interleukin‐like receptor (TIR)‐NBS‐LRR domains found in several R proteins with an additional WRKY DNA‐binding motif characteristic of some plant transcriptional factors, and behaves as a dominant gene in transgenic susceptible plants (Deslandes et al., 2002, 2003). More recently, it has been suggested that RRS1 alone would not be sufficient to confer resistance, but its protein has been found to physically associate with an R protein from another gene, RPS4 (Resistant to Pseudomonas syringae 4), to cooperatively trigger immunity against a range of pathogens (Narusaka et al., 2009; Sohn et al., 2014; Williams et al., 2014). The R gene pairs RRS1 and RPS4 would function as a heterocomplex receptor with each partner having a different role: RPS4 acting as an inducer of disease resistance signalling and RRS1 acting as an effector‐binding receptor and repressor of RPS4 TIR signalling activity. The action of complementary R gene pairs, in which both proteins are required to confer resistance against a pathogen isolate, has been found to function in the recognition of bacterial, viral, oomycete and fungal pathogens in both monocotyledonous and dicotyledonous plants, suggesting that this is a common and widespread mechanism in plant immunity (Cesari et al., 2014; Griebel et al., 2014). Moreover, as there is increasing structural evidence for both coiled coil (CC) and TIR domains of NBS‐LRR proteins being able to form dimers (Maekawa et al., 2011; Williams et al., 2014), and also because of the lack of polymorphism within this family protein that might hide such dimerizations, R gene action in pairs may be even more general than already demonstrated. It might also form the basis of the complex resistance of ‘Geneva’ against V. inaequalis.
Interestingly, all of the R protein pairs identified in the literature are encoded by two tightly linked resistance loci, most of which are transcribed in opposite directions with a relatively short intergenic region. This conserved genomic organization could be important for the co‐regulation of these R genes or to prevent recombination events leading to the separation or inappropriate pairing of R genes, which could cause a loss of resistance or spontaneous necrosis (Cesari et al., 2014). However, it cannot explain the R : S = 1 : 3 segregation observed in some Arabidopsis progenies deriving from the Nd‐0 and Ws‐2 resistant RRS1/RPS4 parents and the Col‐0 susceptible parent. One possible interpretation of this recessive nature of RRS1 is that the Col‐0 susceptible allele encoding a protein would interfere in trans with the pathogen effector responsiveness and thus act as a ‘poison subunit’ of the RRS1/RPS4 R gene complex (Sohn et al., 2014). Such a model, as well as potential recombination events between the R gene pair loci if not tightly linked, could be at the origin of the distorted segregation ratio R : S = 1 : 2, 1 : 3 or 1 : 7 observed with some V. inaequalis isolates across the various F1 and F2 ‘Geneva’ families. Further genetic studies, including the creation of new segregating populations involving backcrosses or the cloning of the resistance loci, could help confirm the status of the genetic control of the complex resistance of ‘Geneva’ against V. inaequalis. Moreover, we are currently proceeding with the genome resequencing of the ‘Geneva’ resistance loci to investigate the potential presence of pairs of NBS‐LRR genes.
Based on these observations and considering all the differential interactions among the isolates and the segregation ratios observed, we hypothesized a model of the GfG relationships between the V. inaequalis isolates and the resistance loci (Table 4). In this model, we propose a dominant genetic control for the first three loci revealed by the isolates 1639, EU‐NL19 and EU‐B04/EU‐D42, and more complex (recessive) genetic control for the last two loci revealed by the isolates 1774‐1, 1770‐3, NZ188B and EU‐B05. We have accordingly assigned temporary names to the resistance alleles: Rvh3.1, Rvh3.2 and Rvh3.3 for the dominant loci, and rvh3.4 and rvh3.5 for the other two alleles to indicate that their control does not fit the classic dominant model (Table 4). Nevertheless, some interactions remain unexplained. For example, the proposed model does not explain why, in GD × M33, EU‐NL19 was compatible with some seedlings for which incompatibility was shown with 1774‐1 and 1770‐3. Such interactions were not observed in the other families and were not included in our GfG relationships model. Such unexplained interactions could be caused by mis‐phenotyping of some genotypes, either as ‘escapes’ during the inoculation procedures or of plants being erroneously scored as susceptible whilst carrying resistance factors. This is possible as some resistant symptoms allowed considerable sporulation when exposed to a longer period of humidity, as shown in previous studies (symptoms 2 → 4; Shay and Williams, 1956). This could also explain some inconsistencies in the phenotypes of the replicated clonal trees of the selected F1 progeny. Finally, we cannot exclude the possibility of additional R genes governing the resistance of ‘Geneva’. In this regard, we noticed the presence of resistant seedlings carrying the marker alleles for susceptibility for all markers tested. These plants were classified as GPI plants (Gygax et al., 2004), and their genetic data were discarded from the JoinMap analysis; however, they could suggest the involvement of a sixth R gene independently inherited from another LG. Finally, epistatic interactions among the R genes were not considered in our GfG relationships model. Epistasis leading to increased resistance has already been shown in pyramided dominant resistances against apple scab (Bus et al., 2002), as well as in recessive resistance of rice against Xanthomonas oryzae pv. oryzae (Xoo) races, for example, between the recessive R genes xa5 and xa13, or among these two recessive genes and a dominant one (Li et al., 2001).
To further elucidate the genetic organization of ‘Geneva’ resistance loci related to specific strains of V. inaequalis, we performed fine mapping of resistance to nine isolates in six segregating F1 and F2 populations derived from ‘Geneva’. In order to construct a particularly dense genetic map, we employed GBS to obtain 23 585 SNPs polymorphic in the resistant parent Q71. The resulting map revealed the location of resistances to isolates EU‐NL19, EU‐B04, 1639 and 1774‐1 at the distal end of LG4 (Fig. 1). Fine mapping of the individual resistance loci was then carried out in six families derived from ‘Geneva’. Our genetic linkage analysis identified three close but distinct chromosomal regions on LG4 where resistance loci were detected. These regions are located on the homologous GD sequence assembly at about: (1) 22.5 Mbp for isolate 1639; (2) 21.5 Mbp for 1774‐1 and 1770‐3, and (3) 20.5 Mbp for EU‐NL19, EU‐B04 and EU‐D42 (Figs 2 and 3). These three chromosomal regions would encompass the five resistance loci governing the resistance of ‘Geneva’ against V. inaequalis that were indicated by our initial analysis of phenotypic segregation by family and isolate (Table 4). Region (1) would carry the dominant Rvhi3.1 locus, region (2) the rhvi3.4 and rhvi3.5 loci harbouring a more complex (recessive) genetic control, and region (3) the dominant Rvhi3.2 and Rvhi3.3 loci. We noted that resistance to isolates 1639 and EU‐NL19 mapped at the same position as 1774‐1 in families E × Q35 and GD × M33, respectively, suggesting that Rvhi3.1 might be missing in Q35 and the dominant Rvhi3.2 in M33.
Our in‐depth analysis of the ‘Geneva’ scab resistance enables us to clarify the preliminary findings suggesting two linked genes, temporarily named Vh3.1 and Vh3.2, which were mapped to LG4 in the E × ‘Geneva’ (using the isolates EU‐NL19 and 1770‐3, respectively) and ‘Geneva’ × BB (using the isolates 1639 and EU‐B05, respectively) progenies (Bus et al., 2011). Considering our GfG relationships model (Table 4) and the isolate phenotypes used to map the resistance loci, it is most likely that Vh3.1 corresponds to Rvh3.1 and/or Rvh3.2 and Vh3.2 to rvh3.4 and/or rvh3.5 loci in our study. However, in the study by Broggini et al. (2011), Vh3.2 corresponds to Rvh3.1 as the other V. inaequalis parent, EU‐B04, is compatible with Q49 (Table 3); hence, avirulence gene AvrVh3.2 in V. inaequalis isolate 1639 (Broggini et al., 2011) should now be named AvrRvh3.1. Alternatively, the differential interaction in Q49 also involves the rvh3.5 locus as suggested by the segregation of resistance to the 1770‐3 isolate in the Q49 × G family, but the location of the corresponding avirulence loci in V. inaequalis could not be mapped employing the EU‐B04 × 1639 progeny (Broggini et al., 2011) as both isolates were incompatible with hosts carrying this gene (Table 4).
One of the criteria for assigning a single Rvik name to a locus is that it has a sufficiently wide spectrum against V. inaequalis isolates to be of value for breeding (Bus et al., 2011). Only rvh3.4 appears to meet this criterion from the small sample of V. inaequalis isolates in our study, and hence is the best candidate for Rvi3, the name assigned in the international nomenclature system to the main scab R gene in the ‘Geneva’ host (Bus et al., 2011). However, extensive evaluations need to be carried out to confirm the resistance spectrum of this gene in a host only carrying this gene, which, at the same time, would become the differential host used for pathotype monitoring (Patocchi et al., 2009; www.vinquest.ch ). With ‘Geneva’ having been reported as considerably infected under field infections in multiple years (Beckerman et al., 2009; Bengtsson et al., 2000), races may readily develop in orchard populations of V. inaequalis, despite the presence of a spontaneous pyramided complex of at least five R genes. This seems in contradiction with previous theories suggesting durable resistance in cultivars pyramided with various disease R genes with complementary modes of action (Joshi and Nayak, 2010; Mundt, 1990). Further investigation of the GfG relationships at the molecular level should be performed to understand why V. inaequalis was able to overcome all five genes. Nevertheless, based on the considerable sporulation on ‘Geneva’ by, for example, EU‐NL24, a race (1,3,6,7) isolate (Caffier et al., 2015), we can hypothesize that the R genes do not impose a high penalty to the pathogen for adaptation (Leach et al., 2001). Nevertheless, (part of) the ‘Geneva’ R gene complex has demonstrated field resistance in New Zealand (V. Bus et al., unpublished data), and hence will still be useful for gene pyramiding with other scab genes by breeders applying MAS enabled by our fine mapping of five ‘Geneva’ resistance loci.
We have demonstrated that five NBS‐LRR candidate genes are tightly linked to the dominant R genes Rvh3.1, Rvh3.2 and Rvh3.3, and these were anchored on our ‘Geneva’ genetic maps. This co‐localization indicates the potential involvement of NBS‐LRR family proteins in the resistance of ‘Geneva’ to V. inaequalis. In contrast, no HcrVf paralogues were identified on LG4 of GD. In future studies, complemented by effector gene research in V. inaequalis (Bowen et al., 2009, 2010), it would be of interest to investigate the possible complementary mode of action of pairs of NBS‐LRR genes among the ‘Geneva’ resistance loci, or the co‐localization of our recessive resistance loci with genes that do not conform to typical R gene structural classes, as cloning studies indicate that recessive plant R genes usually encode proteins with no previously known role in disease resistance (Antony et al., 2010; Büschges et al., 1997; Deslandes et al., 2002; Devoto et al., 1999; Diaz‐Pendon et al., 2004; Iyer and McCouch, 2004; Iyer‐Pascuzzi and McCouch, 2007).
Experimental Procedures
Plant material and V. inaequalis isolates
The characterization of host–pathogen interactions was performed at Plant Research International in Wageningen, the Netherlands on F1 and F2 progenies derived from ‘Geneva’ using nine reference monoconidial isolates of V. inaequalis (Caffier et al., 2015), including isolate 1774‐1, which was distributed as a race (3) isolate (Bus, 2006), but recently characterized as race (1,7) (Caffier et al., 2015) (Tables 1 and S1, see Supporting Information).
Seeds of the F1 families ‘Geneva’ × BB and E × ‘Geneva’ were stratified at 0–1 °C for 8 weeks, and then germinated in the glasshouse. At the four‐ to five‐leaf stage, they were transplanted and raised under a 20 °C day/14 °C night temperature and 16 h light/8 h dark regime using daylight‐incandescent lights when required. Inoculations started at the 10‐leaf stage.
For the identification of the four resistant parents required to develop F2 populations, ‘Geneva’ and selected F1 progeny grafted onto ‘M9’ rootstock (clonal trees) were inoculated with 12 reference isolates, with each host–pathogen interaction evaluated in triplicate. These were crossed with susceptible parents GD, E and G, and the F2 seedlings were raised as described for the F1 progenies (Table 1).
Inoculation procedure and phenotyping
Inoculum was prepared from dried scab‐infected leaves stored at −20 °C, as described by Bus et al. (2005). Venturia inaequalis inoculations were performed using 130 μL of inoculum at a concentration of 1 × 105 conidia/mL using the droplet inoculation technique (Bus et al., 2005), followed by symptom development in the glasshouse with the roof ventilation set at 20 °C and 80% relative humidity.
Symptoms were recorded 21 days after inoculation. On clonal trees and on F1 progenies, phenotypic data were scored according to the classification of Chevalier et al. (1991), with additional descriptions of resistance reactions when observed, e.g. necrotic (N), stellate necrotic (SN) or stellate chlorotic (SC). On F2 progenies, sporulation severity was also scored using a quantitative scale, ranging from ‘1’ (slight) to ‘5’ (heavy). For genetic mapping, plants were classified as being resistant (R) in the presence of resistance reactions with low/moderate sporulation (levels 1–3) and as susceptible (S) in the absence of resistance reactions with moderate to high sporulation (level 4–5) or in the presence of chlorosis with very high sporulation (level 5). Finally, plants with an indeterminate phenotype were scored as ‘I’. Uninoculated leaves from each individual of the progenies and their parents were harvested and immediately frozen in dry ice and lyophilized for storage at −80 °C until DNA extraction.
GBS
GBS was used to screen 58 of the 119 individuals from the F2 segregating population G × Q71. Genomic DNA (gDNA) was extracted from leaf material of both parents and each individual by milling with ball bearings for 1 min at 3.55 m/s in a cetyltrimethylammonium bromide (CTAB)‐based buffer (Table S2, see Supporting Information). The homogenate was incubated at 65 °C for 30 min, cooled and a chloroform extraction was performed. gDNA was precipitated with the addition of a two‐thirds volume of isopropanol and centrifuged. The gDNA pellet was washed twice with 70% ethanol and resuspended in TE buffer [10 : 0.1, 10 mm Tris‐Cl, 0.1 mm ethylenediaminetetraacetic acid (EDTA), pH 8]. gDNA concentrations were determined by fluorometry (Qubit, Life Technologies, Carlsbad, CA, USA) and quality was checked by running 100–150 ng of each gDNA sample on a 1% w/v agarose gel.
Fragment libraries were prepared from 1 μg of gDNA using a modified GBS protocol (Elshire et al., 2011). gDNA was digested with ApeKI type II restriction endonuclease (New England Biolabs, Ipswich, MA, USA). Fragments were barcoded and common linker DNA oligonucleotides were annealed and ligated to the gDNA ends using T4 DNA ligase (Promega, Madison, WI, USA). The libraries were amplified with high‐fidelity Taq DNA polymerase (Accuprime, Life Technologies) as follows: an initial denaturation step at 94 °C for 2 min, followed by 25 amplification cycles of [94 °C for 30 s, 65 °C for 30 s, 68 °C for 30 s], and then a final extension step at 68 °C for 5 min. An aliquot from each library amplification was run on a 3% agarose gel to check for product. Amplified libraries were pooled and purified on a spin column (Qiagen, Venlo, Netherlands) prior to sequencing. Sequencing was performed by Macrogen Inc. (Seoul, South Korea) on the HiSeq2000 Illumina® platform.
GBS data were analysed using the TASSEL‐GBS pipeline (Glaubitz et al., 2014). The sequencing reads from the barcoded 58 genotypes and both parents were deconvoluted and aligned to the GD genome assembly v1.0 (http://www.rosaceae.org/). GBS‐derived SNPs were called using TASSEL for the efficient processing of raw GBS sequence data into SNP genotypes, with SNP nomenclature indicating the position of the SNP on the apple LG and position in base pair (bp) in the assembly. SNPs heterozygous for the Q71 resistant parent and homozygous for the susceptible G parent were used for linkage map analysis.
SSR and SNP genotyping
The F1 and F2 families were genotyped using SSRs (Liebhard et al., 2002; Silfverberg‐Dilworth et al., 2006; Yamamoto et al., 2002) and SNPs. DNA was extracted from the lyophilized leaves by Slipstream Automation Ltd. (Palmerston North, New Zealand) (http://www.slipstream‐automation.co.nz) on a subset of the F1 families (94 and 91 individuals for the E × ‘Geneva’ and ‘Geneva’ × BB populations, respectively) and on all the F2 progenies. Both SNP and SSR markers were initially screened over DNA extracted from both parents and six individuals of each progeny to identify polymorphic markers, which were then screened over the entire populations. The primer sequences used to generate these are listed in Table S3 (see Supporting Information).
SNP markers were genotyped using the high‐resolution melting (HRM) technique (Liew et al., 2004) as described by Chagné et al. (2008). SNPs were scored using the LightCycler 480 Gene Scanning Software (Roche, Basel, Switzerland).
PCR amplifications for SSR markers were performed in a final volume of 12.5 μL containing 5 ng of genomic DNA, 1 × buffer, 1.5 mm MgCl2, 200 μm of each deoxynucleoside diphosphate (dNTP), 13 nm forward primer with the 5′‐end labelled with the fluorescent FAM, HEX or NED, 200 nm reverse primer and 0.5 U of Platinum Taq DNA Polymerase (Life Technologies). All SSR amplifications were performed under touchdown PCR conditions as described by Bus et al. (2005). The amplified fragments were run on an ABI 3500 Genetic Analyzer (Applied Biosystems by Life Technologies) with an internal 50–500‐bp size standard, and fragment sizing was performed with GeneMarker software v. 2.2.0 (SoftGenetics LLC, State College, PA, USA).
Candidate gene mining and design of primers
To delimit the physical interval in which the scab resistance loci are located, the sequences of the molecular markers flanking the resistance loci were searched in silico in the GD genome assembly v1.0 (Velasco et al., 2010) using blastn. This interval was mined for RGAs of the NBS‐LRR family and the Cladiosporium fulvum R gene family, using the apple GenomeBrowser. Primers were designed using Primer3 (Rozen and Skaletsky, 2000) to amplify amplicons of 50 to 120 bp in size located in the promoter region of each candidate R gene. NBS‐LRR candidate R genes identified in the homologous region of GD and their specific primer sequences used in this study are listed in Tables S3 and S4. PCR was conducted on both parents and the individuals of the F2 progenies G × Q71, GD × M33 and E × Q35, and SNPs were detected using the HRM technique as described above.
Genetic map construction
Genetic maps were constructed for the resistant parents for each family using JoinMap 3.0 and 4.1 with the double pseudo‐testcross mapping strategy (Grattapaglia and Sederoff, 1994) and a LOD score of 6 for grouping. The phenotypic data obtained with the various monoconidial isolates of V. inaequalis were integrated into the JoinMap file, with the phenotypes scored as I and plants showing genotype–phenotype incongruence (GPI plants; Gygax et al., 2004) recorded as missing data. The final locus order was determined following extensive proofreading and by minimizing the number of double crossovers flanking single loci. Chi‐squared tests were used to identify any segregation distortions of parental alleles and segregation ratios of resistance phenotypes. For the GBS mapping analysis, the SNP data were divided into 17 files comprising SNPs informative for the resistant Q71 parent (ab × aa backcross‐type segregation). Each file contained SNPs located on the GD primary assembly for a particular LG. A LOD score of 5 was used for grouping and genetic maps were drawn using MapChart 2.2. A consensus map using the phenotypic and genotypic data of the F2 populations derived from M33, Q35 and Q71 was constructed using JoinMap 3.0.
Supporting information
Fig. S1 Illustrations of various symptoms and differential interaction patterns (incompatible in green background, compatible in red background) between monoconidial isolates of Venturia inaequalis and ‘Geneva’ and ‘Braeburn’ cultivars, as well as some individuals of various segregating populations (BB, ‘Braeburn’; E, ‘Elstar’; GD, ‘Golden Delicious’).
Table S1 Origin and race status of monoconidial Venturia inaequalis isolates used in this study.
Table S2 Composition of the cetyltrimethylammonium bromide (CTAB)‐based buffer for extraction of genomic DNA from apple leaf samples.
Table S3 List of primers for single sequence repeat (SSR) and single nucleotide polymorphism (SNP) molecular markers used in this study (Liebhard et al., 2002; Silfverberg‐Dilworth et al., 2006; Yamamoto et al., 2002).
Table S4 Resistance gene analogues [nucleotide‐binding site leucine‐rich repeat (NBS‐LRR) family proteins] identified within the 2.2‐Mbp region between the markers flanking the resistance loci, based on the ‘Golden Delicious’ genome assembly (Velasco et al., 2010).
Table S5 Differential host–pathogen interactions observed between monoconidial isolates of Venturia inaequalis, and seedlings of the F1 and F2 progenies derived from ‘Geneva’ and crossed with ‘Elstar’ (E), ‘Braeburn’ (BB), ‘Gala’ (G) and ‘Golden Delicious’ (GD). Based on the gene‐for‐gene relationship governing the interaction between the R gene in the host and the avirulence gene in the pathogen, we hypothesized from these differential interactions the number and organization of the resistance genes in ‘Geneva’.
Table S6 Repartition of genotyping‐by‐sequencing (GBS)‐derived single nucleotide polymorphisms (SNPs) on the 17 linkage groups of apple using the ApeKI restriction enzyme.
Table S7 Segregation of allelotypes with the phenotype of reaction to Venturia inaequalis isolates exhibiting 1 : 3, 1 : 2 or 1 : 7 segregation (resistant : susceptible, R : S) for co‐dominant molecular marker data.
Acknowledgements
We would like to thank the Wallonie‐Bruxelles International (WBI) grant for the financial support provided for the 1‐year scientific stay of Héloïse Bastiaanse at Plant & Food Research (PFR), Havelock North and Palmerston North. We thank the Unifarm glasshouse staff at Wageningen University for the excellent plant care and climate control services, and Yolanda Noordijk for assisting in the leaf sampling and processing. We are also grateful to Dr Erik Rikkerink from PFR, Auckland, for his valuable comments on the draft manuscript. The research was supported by the New Zealand Foundation for Science, Research and Technology contracts C02X0406 and C06X0810, and PFR Pipfruit Core programme 1433.
References
- Available at www.vinquest.ch [accessed 21 January 2015].
- Antony, G. , Zhou, J. , Huang, S. , Li, T. , Liu, B. , White, F. and Yang, B. (2010) Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os‐11N3. Plant Cell, 22, 3864–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerman, J. , Chatfield, J. and Draper, E. (2009) A 33‐year evaluation of resistance and pathogenicity in the apple scab–crabapples pathosystem. HortScience, 44, 599–608. [Google Scholar]
- Bengtsson, M. , Lindhard, H. and Grauslund, J. (2000) Occurence of races of Venturia inaequalis in apple scab race screening orchard in Denmark. IOBC Wprs Bull. 23, 225–229. [Google Scholar]
- Bianco, L. , Cestaro, A. , Sargent, D.J. , Banchi, E. , Derdak, S. , Di Guardo, M. , Salvi, S. , Jansen, J. , Viola, R. , Gut, I. , Laurens, F. , Chagné, D. , Velasco, R. , van de Weg, E. and Troggio, M. (2014) Development and validation of a 20K single nucleotide polymorphism (SNP) whole genome genotyping array for apple (Malus × domestica Borkh). PLoS ONE, 9, e110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen, J.K. , Mesarich, C.H. , Rees‐George, J. , Cui, W. , Fitzgerald, A. , Win, J. , Plummer, K.M. and Templeton, M.D. (2009) Candidate effector gene identification in the ascomycete fungal phytopathogen Venturia inaequalis by expressed sequence tag analysis. Mol. Plant Pathol. 10, 431–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen, J.K. , Mesarich, C.H. , Bus, V.G.M. , Beresford, R.M. , Plummer, K.M. and Templeton, M.D. (2010) Venturia inaequalis: the causal agent of apple scab. Mol. Plant Pathol. 12, 105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broggini, G.A. , Bus, V.G.M. , Parravicini, G. , Kumar, S. , Groenwold, R. and Gessler, C. (2011) Genetic mapping of 14 avirulence genes in an EU‐B04 × 1639 progeny of Venturia inaequalis . Fungal Genet. Biol. 48, 166–176. [DOI] [PubMed] [Google Scholar]
- Bus, V. , White, A. , Gardiner, S. , Weskett, R. , Ranatunga, C. , Samy, A. , Cook, M. and Rikkerink, E. (2002) An update on apple scab resistance breeding in New Zealand. Acta Hortic. 595, 43–47. [Google Scholar]
- Bus, V.G.M. (2006) Differential host–pathogen interactions of Venturia inaequalis and Malus . PhD Thesis, University of Auckland, Auckland. [DOI] [PubMed]
- Bus, V.G.M. , Laurens, F.N.D. , van de Weg, W.E. , Rusholme, R.L. , Rikkerink, E.H.A. , Gardiner, S.E. , Bassett, H.C.M. , Kodde, L.P. and Plummer, K.M. (2005) The Vh8 locus of a new gene‐for‐gene interaction between Venturia inaequalis and the wild apple Malus sieversii is closely linked to the Vh2 locus in Malus pumila R12740‐7A. New Phytol. 166, 1035–1049. [DOI] [PubMed] [Google Scholar]
- Bus, V.G.M. , Rikkerink, E.H.A. , Caffier, V. , Durel, C.E. and Plummer, K.M. (2011) Revision of the nomenclature of the differential host–pathogen interactions of Venturia inaequalis and Malus . Annu. Rev. Phytopathol. 49, 391–413. [DOI] [PubMed] [Google Scholar]
- Büschges, R. , Hollricher, K. , Panstruga, R. , Simons, G. , Wolter, M. , Frijters, A. , van Daelen, R. , van der Lee, T. , Diergaarde, P. , Groenendijk, J. , Töpsch, S. , Vos, P. , Salamini, F. and Schulze‐Lefert, P. (1997) The Barley Mlo gene: a novel control element of plant pathogen resistance. Cell, 88, 695–705. [DOI] [PubMed] [Google Scholar]
- Caffier, V. , Lasserre‐Zuber, P. , Giraud, M. , Lascostes, M. , Stievenard, R. , Lemarquand, A. , van de Weg, E. , Expert, P. , Denancé, C. , Didelot, F. , Le Cam, B. and Durel, C. (2014) Erosion of quantitative host resistance in the apple × Venturia inaequalis pathosystem. Infect. Genet. Evol. 27, 481–489. [DOI] [PubMed] [Google Scholar]
- Caffier, V. , Patocchi, A. , Expert, P. , Bellanger, M.N. , Durel, C.E. , Hilber‐Bodmer, M. , Broggini, G.A.L. , Groenwold, R. and Bus, V.G.M. (2015) Virulence characterisation of Venturia inaequalis reference isolates on the differential set of Malus hosts. Plant Dis. 99, 370–375. doi: 10.1094/PDIS-07-14-0708-RE. [DOI] [PubMed] [Google Scholar]
- Calenge, F. , Faure, A. , Goerre, M. , Gebhardt, C. , Van de Weg, W.E. , Parisi, L. and Durel, C.E. (2004) Quantitative Trait Loci (QTL) analysis reveals both broad‐spectrum and isolate‐specific QTL for scab resistance in an apple progeny challenged with eight isolates of Venturia inaequalis . Phytopathology, 94, 370–379. [DOI] [PubMed] [Google Scholar]
- Calvo, E.S. , Kiihl, R.A. , Garcia, A. , Harada, A. and Hiromoto, D.M. (2008) Two major recessive soybean genes conferring soybean rust resistance. Crop Sci. 48, 1350–1354. [Google Scholar]
- Cesari, S. , Bernoux, M. , Moncuquet, P. , Kroj, T. and Dodds, P.N. (2014) A novel conserved mechanism for plant NLR protein pairs: the ‘integrated decoy’ hypothesis. Front. Plant Sci. 5, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagné, D. , Gasic, K. , Crowhurst, R.N. , Han, Y. , Bassett, H.C. , Bowatte, D.R. , Lawrence, T.J. , Rikkerink, E.H.A. , Gardiner, S.E. and Korban, S.S. (2008) Development of a set of SNP markers present in expressed genes of the apple. Genomics, 92, 353–358. [DOI] [PubMed] [Google Scholar]
- Chagné, D. , Crowhurst, R.N. , Troggio, M. , Davey, M.W. , Gilmore, B. , Lawley, C. , Vanderzande, S. , Hellens, R.P. , Kumar, S. , Cestaro, A. , Velasco, R. , Main, D. , Rees, J.D. , Iezzoni, A. , Mockler, T. , Wilhelm, L. , Van de Weg, E. , Gardiner, S.E. , Bassil, N. and Peace, C. (2012) Genome‐wide SNP detection, validation, and development of an 8K SNP array for apple. PLoS ONE, 12, e31745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier, M. , Lespinasse, Y. and Renaudin, S. (1991) A microscopic study of the different classes of symptoms coded by the Vf gene in apple for resistance to scab (Venturia inaequalis). Plant Pathol. 40, 249–256. [Google Scholar]
- Deng, C.H. , Hilario, I. , Datson, P. , Gardiner, S.E. , Barron, L. , Kirk, C. , Kumar, S. , Tahir, J. , De Silva, N. and Chagné, D. (2014) Genotyping by sequencing in fruit tree species In: Plant and Animal Genome XXII Conference, 11–15 January 2014, San Diego, CA, USA. Available at https://pag.confex.com/pag/xxii/webprogram/Paper11998.html [Google Scholar]
- Deslandes, L. , Olivier, J. , Theulières, F. , Hirsch, J. , Feng, D.X. , Bittner‐Eddy, P. , Beynon, J. and Marco, Y. (2002) Resistance to Ralstonia solanacearum in Arabidopsis thaliana is conferred by the recessive RRS1‐R gene, a member of a novel family of resistance genes. Proc. Natl. Acad. Sci. USA, 99, 2404–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes, L. , Olivier, J. , Peeters, N. , Feng, D.X. , Khounlotham, M. , Boucher, C. , Somssich, I. , Genin, S. and Marco, Y. (2003) Physical interaction between RRS1‐R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc. Natl. Acad. Sci. USA, 100, 8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto, A. , Piffanelli, P. , Nilsson, I. , Wallin, E. , Panstruga, R. , von Heijne, G. and Schulze‐Lefert, P. (1999) Topology, subcellular localization, and sequence diversity of the Mlo family in plants. J. Biol. Chem. 274, 34 993–35 004. [DOI] [PubMed] [Google Scholar]
- Diaz‐Pendon, J.A. , Truniger, V. , Nieto, C. , Garcia‐Mas, J. , Bendahmane, A. and Aranda, M.A. (2004) Advances in understanding recessive resistance to plant viruses. Mol. Plant Pathol. 5, 223–233. [DOI] [PubMed] [Google Scholar]
- Durel, C.E. , Parisi, L. , Laurens, F. , Van de Weg, W.E. , Liebhard, R. and Jourjon, M.F. (2003) Genetic dissection of partial resistance to race 6 of Venturia inaequalis in apple. Genome, 46, 224–234. [DOI] [PubMed] [Google Scholar]
- Durel, C.E. , Calenge, F. , Parisi, L. , van de Weg, W.E. , Kodde, L.P. , Liebhard, R. , Gessler, C. , Thiermann, M. , Dunemann, F. , Gennari, F. and Tartarini, S. (2004) An overview of the position and robustness of scab resistance QTLs and major genes by aligning genetic maps of five apple progenies. Acta Hortic. 663, 135–140. [Google Scholar]
- Elshire, R.J. , Glaubitz, J.C. , Sun, Q. , Poland, J.A. , Kawamoto, K. , Buckler, E.S. and Mitchell, S.E. (2011) A robust, simple genotyping‐by‐sequencing (GBS) approach for high diversity species. PLoS ONE, 6, e19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor, H.H. (1971) Current status of the gene‐for‐gene concept. Annu. Rev. Phytopathol. 9, 275–296. [Google Scholar]
- Galli, P. , Patocchi, A. , Broggini, G.A. and Gessler, C. (2010) The Rvi15 (Vr2) apple scab resistance locus contains three TIR‐NBS‐LRR genes. Mol. Plant–Microbe Interact. 23, 608–617. [DOI] [PubMed] [Google Scholar]
- Gardner, K.M. , Brown, P. , Cooke, T.F. , Cann, S. , Costa, F. , Bustamante, C. , Velasco, R. , Troggio, M. and Myles, S. (2014) Fast and cost‐effective genetic mapping in apple using next‐generation sequencing. G3: Genes Genom. Genet. 4, 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler, C. , Patocchi, A. , Sansavini, S. , Tartarini, S. and Gianfranceschi, L. (2006) Venturia inaequalis resistance in apple. Crit. Rev. Plant Sci. 25, 473–503. [Google Scholar]
- Glaubitz, J.C. , Casstevens, T.M. , Lu, F. , Harriman, J. , Elshire, R.J. , Sun, Q. and Buckler, E.S. (2014) TASSEL‐GBS: a high capacity genotyping by sequencing analysis pipeline. PLoS ONE, 9, e90346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattapaglia, D. and Sederoff, R. (1994) Genetic linkage maps of Eucalyptus grandis and Eucalyptus urophylla using a pseudo‐testcross: mapping strategy and RAPD markers. Genetics, 137, 1121–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel, T. , Maekawa, T. and Parker, J.E. (2014) NOD‐like receptor cooperativity in effector‐triggered immunity. Trends Immunol. 35, 562–570. [DOI] [PubMed] [Google Scholar]
- Gygax, M. , Gianfranceschi, L. , Liebhard, R. , Kellerhals, M. , Gessler, C. and Patocchi, A. (2004) Molecular markers linked to the apple scab resistance gene Vbj derived from Malus baccata jackii . Theor. Appl. Genet. 109, 1702–1709. [DOI] [PubMed] [Google Scholar]
- Hua, W. , Liu, Z. , Zhu, J. , Xie, C. , Yang, T. , Zhou, Y. , Duan, X. , Sun, Q. and Liu, Z. (2009) Identification and genetic mapping of pm42, a new recessive wheat powdery mildew resistance gene derived from wild emmer (Triticum turgidum var. dicoccoides). Theor. Appl. Genet. 119, 223–230. [DOI] [PubMed] [Google Scholar]
- Huang, N. , Angeles, E.R. , Domingo, J. , Magpantay, G. , Singh, S. , Zhang, G. , Kumaravadivel, N. , Bennett, J. and Khush, G.S. (1997) Pyramiding of bacterial blight resistance genes in rice: marker‐assisted selection using RFLP and PCR. Theor. Appl. Genet. 95, 313–320. [Google Scholar]
- Iyer, A.S. and McCouch, S.R. (2004) The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol. Plant–Microbe Interact. 17, 1348–1354. [DOI] [PubMed] [Google Scholar]
- Iyer‐Pascuzzi, A.S. and McCouch, S.R. (2007) Recessive resistance genes and the Oryza sativa–Xanthomonas oryzae pv. oryzae pathosystem. Mol. Plant–Microbe Interact. 20, 731–739. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.M. (1970) History, Progeny, and Location of Crabapples of Documented Authentic Origin. Washington, DC: US Department of Agriculture. [Google Scholar]
- Joshi, R.K. and Nayak, S. (2010) Gene pyramiding—a broad spectrum technique for developing durable stress resistance in crops. Biotechnol. Mol. Biol. Rev. 5, 51–60. [Google Scholar]
- Julien, J.B. and Spangelo, L.P.S. (1957) Physiological races of Venturia inaequalis . Can. J. Plant Sci. 37, 102–107. [Google Scholar]
- Leach, J.E. , Vera Cruz, C.M. , Bai, J. and Leung, H. (2001) Pathogen fitness penalty as a predictor of durability of disease resistance genes. Annu. Rev. Phytopathol. 39, 187–224. [DOI] [PubMed] [Google Scholar]
- Li, Z.K. , Sanchez, A. , Angeles, E. , Singh, S. , Domingo, J. , Huang, N. and Khush, G.S. (2001) Are the dominant and recessive plant disease resistance genes similar?: a case study of rice R genes and Xanthomonas oryzae pv. oryzae races. Genetics, 159, 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhard, R. , Gianfranceschi, L. , Koller, B. , Ryder, C.D. , Tarchini, R. , Van de Weg, E. and Gessler, C. (2002) Development and characterization of 140 new microsatellites in apple (Malus × domestica Borkh.). Mol. Breed. 10, 217–241. [Google Scholar]
- Liebhard, R. , Koller, B. , Patocchi, A. , Kellerhals, M. , Pfammatter, W. , Jermini, M. and Gessler, C. (2003) Mapping quantitative field resistance against apple scab in a ‘Fiesta’ × ‘Discovery’ progeny. Phytopathology, 93, 493–501. [DOI] [PubMed] [Google Scholar]
- Liew, M. , Pryor, R. , Palais, R. , Meadows, C. , Erali, M. , Lyon, E. and Wittwer, C. (2004) Genotyping of single‐nucleotide polymorphisms by high‐resolution melting of small amplicons. Clin. Chem. 50, 1156–1164. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Liu, D. , Tao, W. , Lio, W. , Wang, S. , Chen, P. , Cheng, S. and Gao, D. (2000) Molecular marker‐facilitated pyramiding of different genes for powdery mildew resistance in wheat. Plant Breed. 119, 21–24. [Google Scholar]
- Maekawa, T. , Cheng, W. , Spiridon, L.N. , Töller, A. , Lukasik, E. , Saijo, Y. , Liu, P. , Shen, Q.H. , Micluta, M.A. , Somssich, I.E. , Takken, F.L.W. , Petrescu, A.J. , Chai, J. and Schulze‐Lefert, P. (2011) Coiled‐coil domain‐dependent homodimerization of intracellular barley immune receptors defines a minimal functional module for triggering cell death. Cell Host Microbe, 9, 187–199. [DOI] [PubMed] [Google Scholar]
- Moriya, S. , Terakami, S. , Iwanami, H. , Haji, T. , Okada, K. , Yamamoto, T. and Abe, K. (2011) Genetic mapping and marker‐assisted selection of the gene conferring susceptibility to Alternaria blotch caused by Alternaria alternata apple pathotype in apple. Acta Hortic. 976, 555–560. [Google Scholar]
- Mowry, J.B. (1964) Inheritance of susceptibility to Gymnosporangium juniperi‐virginianae . Phytopathology, 54, 1363–1366. [Google Scholar]
- Mundt, C.C. (1990) Probability of mutation to multiple virulence and durability of resistance gene pyramids. Phytopathology, 80, 221–223. [Google Scholar]
- Narusaka, M. , Shirasu, K. , Noutoshi, Y. , Kubo, Y. , Shiraishi, T. , Iwabuchi, M. and Narusaka, Y. (2009) RRS1 and RPS4 provide a dual resistance‐gene system against fungal and bacterial pathogens. Plant J. 60, 218–226. [DOI] [PubMed] [Google Scholar]
- Patocchi, A. , Frei, A. , Frey, J.E. and Kellerhals, M. (2009) Towards improvement of marker assisted selection of apple scab resistant cultivars: Venturia inaequalis virulence surveys and standardization of molecular marker alleles associated with resistance genes. Mol. Breed. 24, 337–347. [Google Scholar]
- Peng, F. , Song, N. , Shen, H. , Wu, H. , Dong, H. , Zhang, J. , Li, Y. , Peng, H. , Ni, Z. , Liu, Z. , Yang, T. , Li, B. , Xie, C. and Sun, Q. (2014) Molecular mapping of a recessive powdery mildew resistance gene in spelt wheat cultivar Hubel. Mol. Breed. 34, 491–500. [Google Scholar]
- Perazzolli, M. , Malacarne, G. , Baldo, A. , Righetti, L. , Bailey, A. , Fontana, P. , Velasco, R. and Malnoy, M. (2014) Characterization of resistance gene analogues (RGAs) in apple (Malus × domestica Borkh.) and their evolutionary history of the Rosaceae family. PLoS ONE, 9, e83844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchepied, L. , Dogimont, C. and Pitrat, M. (2005) Strain‐specific and recessive QTLs involved in the control of partial resistance to Fusarium oxysporum f. sp. melonis race 1.2 in a recombinant inbred line population of melon. Theor. Appl. Genet. 111, 65–74. [DOI] [PubMed] [Google Scholar]
- Rozen, S. and Skaletsky, H.J. (2000) Primer3 on the www for general users and for biologist programmers In: Bioinformatics Methods and Protocols: Methods in Molecular Biology (Krawetz S. and Misener S., eds), pp. 365–386. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- van Schie, C.C. and Takken, F.L. (2014) Susceptibility genes 101: how to be a good host. Annu. Rev. Phytopathol. 52, 551–581. [DOI] [PubMed] [Google Scholar]
- Shay, J.R. and Hough, L.F. (1952) Evaluation of apple scab resistance in selection of Malus . Am. J. Bot. 39, 288–297. [Google Scholar]
- Shay, J.R. and Williams, E.B. (1956) Identification of three physiologic races of Venturia inaequalis . Phytopathology, 46, 190–193. [Google Scholar]
- Silfverberg‐Dilworth, E. , Matasci, C.L. , Van de Weg, W.E. , Van Kaauwen, M.P.W. , Walser, M. , Kodde, L.P. , Soglio, V. , Gianfranceschi, L. , Durel, C.E. , Costa, F. , Yamamoto, T. , Koller, B. , Gessler, C. and Patocchi, A. (2006) Microsatellite markers spanning the apple (Malus × domestica Borkh.) genome. Tree Genet. Genomes, 2, 202–224. [Google Scholar]
- Sohn, K.H. , Segonzac, C. , Rallapalli, G. , Sarris, P.F. , Woo, J.Y. , Williams, S.J. , Newman, T.E. , Paek, K.H. , Kobe, B. and Jones, J.D.G. (2014) The nuclear immune receptor RPS4 is required for RRS1SLH1‐dependent constitutive defense activation in Arabidopsis thaliana . Plos Genet. 10, e1004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufflet‐Freslon, V. , Gianfranceschi, L. , Patocchi, A. and Durel, C.‐E. (2008) Inheritance studies of apple scab resistance and identification of Rvi14, a new major gene that acts together with other broad‐spectrum QTL. Genome, 51, 657–667. [DOI] [PubMed] [Google Scholar]
- Thompson, J.M. and Taylor, J. (1971) Genetic susceptibility to Glomerella leaf blotch in apple. J. Hered. 62, 303–306. [Google Scholar]
- Vanderplank, J.E. (1984) Disease Resistance in Plants. San Diego: Academic Press, Inc. [Google Scholar]
- Velasco, R. , Zharkikh, A. , Affourtit, J. , Dhingra, A. , Cestaro, A. , Kalyanaraman, A. , Fontana, P. , Bhatnagar, S.K. , Troggio, M. , Pruss, D. , Salvi, S. , Pindo, M. , Baldi, P. , Castelletti, S. , Cavaiuolo, M. , Coppola, G. , Costa, F. , Cova, V. , Dal Ri, A. , Goremykin, V. , Komjanc, M. , Longhi, S. , Magnago, P. , Malacarne, G. , Malnoy, M. , Micheletti, D. , Moretto, M. , Perazzolli, M. , Si‐Ammour, A. , Vezzulli, S. , Zini, E. , Eldredge, G. , Fitzgerald, L.M. , Gutin, N. , Lanchbury, J. , Macalma, T. , Mitchell, J.T. , Reid, J. , Wardell, B. , Kodira, C. , Chen, Z. , Desany, B. , Niazi, F. , Palmer, M. , Koepke, T. , Jiwan, D. , Schaeffer, S. , Krishnan, V. , Wu, C. , Chu, V.T. , King, S.T. , Vick, J. , Tao, Q. , Mraz, A. , Stormo, A. , Stormo, K. , Bogden, R. , Ederle, D. , Stella, A. , Vecchietti, A. , Kater, M.M. , Masiero, S. , Lasserre, P. , Lespinasse, Y. , Allan, A.C. , Bus, V. , Chagné, D. , Crowhurst, R.N. , Gleave, A.P. , Lavezzo, E. , Fawcett, J.A. , Proost, S. , Rouzé, P. , Sterck, L. , Toppo, S. , Lazzari, B. , Hellens, R.P. , Durel, C.E. , Gutin, A. , Bumgarner, R.E. , Gardiner, S.E. , Skolnick, M. , Egholm, M. , Van de Peer, Y. , Salamini, F. and Viola, R. (2010) The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 42, 833–839. [DOI] [PubMed] [Google Scholar]
- Vinatzer, B.A. , Patocchi, A. , Gianfranceschi, L. , Tartarini, S. , Zhang, H.B. , Gessler, C. and Sansavini, S. (2001) Apple contains receptor‐like genes homologous to the Cladiosporium fulvum resistance gene family of tomato with a cluster of genes cosegregating with Vf apple scab resistance. Mol. Plant–Microbe Interact. 14, 508–515. [DOI] [PubMed] [Google Scholar]
- Werner, K. , Friedt, W. and Ordon, F. (2005) Strategies for pyramiding resistance genes against the barley yellow mosaic virus complex (BaMMV, BaYMV, BaYMV‐2). Mol. Breed. 16, 45–55. [Google Scholar]
- Williams, E.B. and Shay, J.R. (1957) The relationship of genes for pathogenicity and certain other characters in Venturia inaequalis (Cke.) Wint. Genetics, 42, 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, S.J. , Sohn, K.H. , Wan, L. , Bernoux, M. , Sarris, P.F. , Segonzac, C. , Ve, T. , Ma, Y. , Saucet, S.B. , Ericsson, D.E. , Casey, L.W. , Lonhienne, T. , Winzor, D.J. , Zhang, X. , Coerdt, A. , Parker, J.E. , Dodds, P.N. , Kobe, B. and Jones, J.D.G. (2014) Structural basis for assembly and function of a heterodimeric plant immune receptor. Science, 344, 299–303. [DOI] [PubMed] [Google Scholar]
- Witcombe, J.R. and Hash, C.T. (2000) Resistance gene deployment strategies in cereal hybrids using marker‐assisted selection: gene pyramiding, three‐way hybrids, and synthetic parent populations. Euphytica, 112, 175–186. [Google Scholar]
- Yamamoto, T. , Kimura, T. , Shoda, M. , Imai, T. , Saito, T. , Sawamura, Y. , Kotobuki, K. , Hayashi, T. and Matsuta, N. (2002) Genetic linkage maps constructed by using an interspecific cross between Japanese and European pears. Theor. Appl. Genet. 106, 9–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Illustrations of various symptoms and differential interaction patterns (incompatible in green background, compatible in red background) between monoconidial isolates of Venturia inaequalis and ‘Geneva’ and ‘Braeburn’ cultivars, as well as some individuals of various segregating populations (BB, ‘Braeburn’; E, ‘Elstar’; GD, ‘Golden Delicious’).
Table S1 Origin and race status of monoconidial Venturia inaequalis isolates used in this study.
Table S2 Composition of the cetyltrimethylammonium bromide (CTAB)‐based buffer for extraction of genomic DNA from apple leaf samples.
Table S3 List of primers for single sequence repeat (SSR) and single nucleotide polymorphism (SNP) molecular markers used in this study (Liebhard et al., 2002; Silfverberg‐Dilworth et al., 2006; Yamamoto et al., 2002).
Table S4 Resistance gene analogues [nucleotide‐binding site leucine‐rich repeat (NBS‐LRR) family proteins] identified within the 2.2‐Mbp region between the markers flanking the resistance loci, based on the ‘Golden Delicious’ genome assembly (Velasco et al., 2010).
Table S5 Differential host–pathogen interactions observed between monoconidial isolates of Venturia inaequalis, and seedlings of the F1 and F2 progenies derived from ‘Geneva’ and crossed with ‘Elstar’ (E), ‘Braeburn’ (BB), ‘Gala’ (G) and ‘Golden Delicious’ (GD). Based on the gene‐for‐gene relationship governing the interaction between the R gene in the host and the avirulence gene in the pathogen, we hypothesized from these differential interactions the number and organization of the resistance genes in ‘Geneva’.
Table S6 Repartition of genotyping‐by‐sequencing (GBS)‐derived single nucleotide polymorphisms (SNPs) on the 17 linkage groups of apple using the ApeKI restriction enzyme.
Table S7 Segregation of allelotypes with the phenotype of reaction to Venturia inaequalis isolates exhibiting 1 : 3, 1 : 2 or 1 : 7 segregation (resistant : susceptible, R : S) for co‐dominant molecular marker data.


