Summary
RNA silencing is a sequence‐specific post‐transcriptional gene inactivation mechanism that operates in diverse organisms and that can extend beyond its site of initiation, owing to the movement of the silencing signal, called non‐autonomous gene silencing. Previous studies have shown that several factors manifest the movement of the silencing signal, such as the size (21 or 24 nucleotides) of the secondary small interfering RNA (siRNA) produced, the steady‐state concentration of siRNAs and their cognate messenger RNA (mRNA) or a change in the sink–source status of plant parts affecting phloem translocation. Our study shows that both light intensity and temperature have a significant impact on the systemic movement of the silencing signal in transient agroinfiltration studies in Nicotiana benthamiana. At higher light intensities (≥450 μE/m2/s) and higher temperatures (≥30 °C), gene silencing was localized to leaf tissue that was infiltrated, without any systemic spread. Interestingly, in these light and temperature conditions (≥450 μE/m2/s and ≥30 °C), the N. benthamiana plants showed recovery from the viral symptoms. However, the reduced systemic silencing and reduced viral symptom severity at higher light intensities were caused by a change in the sink–source status of the plant, ultimately affecting the phloem translocation of small RNAs or the viral genome. In contrast, at lower light intensities (<300 μE/m2/s) with a constant temperature of 25 °C, there was strong systemic movement of the silencing signal in the N. benthamiana plants and reduced recovery from virus infections. The accumulation of gene‐specific siRNAs was reduced at higher temperature as a result of a reduction in the accumulation of transcript on transient agroinfiltration of RNA interference (RNAi) constructs, mostly because of poor T‐DNA transfer activity of Agrobacterium, possibly also accompanied by reduced phloem translocation.
Keywords: light intensity, phloem translocation, symptom recovery, systemic silencing, temperature, virus
Introduction
RNA silencing is a sequence‐specific degradation of messenger RNA (mRNA) through the production of small interfering RNA (siRNA) involving an array of enzymes (Ding and Voinnet, 2007; Ruiz‐Ferrer and Voinnet, 2009). The effect of RNA silencing can be perceived in tissues other than the tissue of its origin through systemic spread of the silencing signal (small RNAs), known as non‐cell autonomous silencing (Jose and Hunter, 2007; Voinnet, 2005; Voinnet and Baulcombe, 1997). For many years, the nature of the silencing signal and the factors affecting the systemic spread of the silencing signal remained elusive (Dunoyer et al., 2010a, 2010b; Dunoyer and Voinnet, 2009; Kalantidis et al., 2008; Mlotshwa et al., 2002; Molnar et al., 2010). Earlier studies have shown that the size of siRNA is critical in the determination of the systemic nature of gene silencing (Dunoyer et al., 2005; Voinnet, 2005). Of the two major size classes of siRNAs produced (21 and 24 nucleotides) during gene silencing, the 21‐nucleotide siRNA was considered as a signal for plant cell‐to‐cell movement (Dunoyer et al., 2005). The 21‐nucleotide siRNA is the main component of secondary amplification of gene silencing processed by Dicer Like Protein 4 (DCL4) and RNA‐dependent RNA polymerase 6 (RDR6), which determines the systemic nature of gene silencing (Dunoyer et al., 2005). In addition to the nature and size of the siRNA, the amount of siRNA and its cognate mRNA produced at the primary site of silencing significantly affect the systemic movement of the silencing signal (Kalantidis et al., 2006). The movement of the silencing signal can be classified as short‐distance movement or long‐distance movement (Kalantidis et al., 2008; Voinnet, 2005). Short‐range movement occurs over 10–15 cells, and the 21‐nucleotide siRNA, a product of DCL4, is the most probable nucleic acid component; such reiterated short‐distance signalling events eventually translate into extensive, long‐distance movement (Voinnet, 2005). In addition to DCL4, several other components of gene silencing, such as RDR6, Silencing Defective Protein 3 (SDE3), Silencing Movement Deficient 1 (SMD1), SMD2 and SMD3, and other unidentified cellular factors, are implicated in the systemic movement of the silencing signal (Voinnet, 2005). Using cell‐specific rescue of DCL4 function and cell‐specific inhibition of RNA interference (RNAi) movement, Dunoyer et al. (2010a, b) genetically established that exogenous and endogenous siRNAs act as mobile silencing signals between plant cells, as opposed to their precursor molecules. Long‐distance movement of small RNAs has been analysed by grafting of different Arabidopsis mutants and their deep sequencing, which indicated that the siRNAs produced from all the loci are not mobile (Kalantidis, 2004; Molnar et al., 2010). The mobile siRNAs remain functionally indistinguishable from locally synthesized siRNAs which work through DNA methylation in the recipient cells (Dunoyer et al., 2010a, b; Molnar et al., 2010). All the above reports clearly indicate that plant vasculature systems are the major channels for the transport of gene silencing signals to different plant parts. Studies in green fluorescent protein (GFP)‐transgenic Nicotiana benthamiana have also shown that the change in sink–source status of the plant affects phloem flow and thus manifests the movement of the silencing signal (Tournier et al., 2006).
Light is known to have a direct effect on phloem loading through photosynthesis via the synthesis of sucrose and, similarly, the temperature can also affect sugar transport in the phloem in different ways, involving distinct cell types (intermediary cells, parenchyma transfer cells and sieve elements) (Lemoine et al., 2013). The role of temperature in the manifestation of virus symptoms has been known for several years and, later, this manifestation was shown to be through the direct effect of temperature on post‐transcriptional gene silencing (PTGS) (Chellappan et al., 2005; Harrison, 2008; Hine et al., 1970; Johnson, 1922; Kassanis, 1957; Szittya et al., 2003; Tuttle et al., 2008). However, there have also been contradicting reports that gene silencing is not affected by different temperature conditions (Fu et al., 2006; Sos‐Hegedus et al., 2005). The effect of other environmental factors on gene silencing or virus symptoms has not been investigated thoroughly, and there have been no detailed investigations to unravel the molecular basis of their effect on virus symptoms or virus‐induced gene silencing (VIGS) (Bawden and Roberts, 1947; Colhoun, 1973; Fu et al., 2006; Garrett et al., 2006; Patil and Fauquet, 2011). Recently, a report has been published on the effect of light intensity on the induction and spread of PTGS in the GFP‐transgenic lines of N. benthamiana with spontaneous short‐range silencing (Kalantidis et al., 2006; Kotakis et al., 2010, 2011).
Here, we report the effect of different temperature, light intensity and humidity conditions on the systemic movement of the silencing signal through transient agroinfiltration of RNAi constructs targeting an endogenous gene (Su: a subunit of magnesium chelatase) involved in chlorophyll biosynthesis and a transgene (GFP). The nature of virus symptom phenotypes obtained in different light and temperature conditions is also discussed. Although the systemic movement of the silencing signal and the recovery phenotypes obtained after virus infection are complex, involving several components, here we use different tools and techniques to investigate the basis of these different phenotypes as manifested by different conditions. We show that, at higher temperature, there is reduced systemic silencing as a result of a reduction in the amount of siRNA produced on transient agroinfiltrations because of the low T‐DNA transfer activity, accompanied by reduced phloem translocation. However, at higher light intensities, reduced systemic silencing and reduced viral symptom severity could be an outcome of decreased phloem translocation through the change in the sink–source status of plant leaves.
Results
Temperature has a strong and contrasting effect on symptom recovery compared with light intensity in virus‐infected N. benthamiana
To study the effect of light intensity and temperature on virus symptom initiation and symptom recovery, we used seven distinct species of cassava mosaic geminiviruses (CMGs) [African cassava mosaic virus (ACMV), East African cassava mosaic Cameroon virus (EACMCV), East African cassava mosaic virus (EACMV), East African cassava mosaic Kenya virus (EACMKV), East African cassava mosaic Zanzibar virus (EACMZV) Indian cassava mosaic virus (ICMV) and Sri Lankan cassava mosaic virus (SLCMV)] (Fig. 1A) (Patil et al., 2011). The seven selected geminiviruses show a range of symptom recovery phenotypes at 30 °C and the viruses can be grouped into recovery (ACMV and SLCMV), partial recovery (EACMKV) and non‐recovery (EACMCV, EACMV and ICMV) types. The symptoms of virus infections in N. benthamiana showed contrasting phenotypes at different temperature conditions (21, 25, 27 and 30 °C), with the plants recovering almost completely when infected by recovery‐type viruses at higher temperature. However, the virus symptoms of the recovery phenotypes were not different under four contrasting light intensities (150, 300, 450 and 600 μE/m2/s) in comparison with the symptom phenotypes obtained in different temperature conditions (Fig. 1). The virus infection studies at different light intensities were performed at two different constant temperatures of 25 and 30 °C. At higher light intensities (≥450 μE/m2/s), the recovery of symptoms of CMGs was better than at lower light intensities (≤300 μE/m2/s), and was more prominent at a constant temperature of 30 °C than at a temperature of 25 °C.
Figure 1.
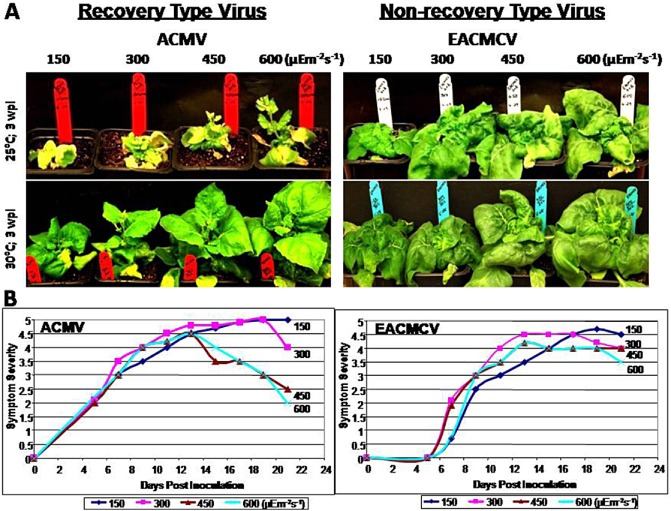
(A) Different symptom phenotypes (3 weeks post‐inoculation; wpi) displayed by Nicotiana benthamiana infected with two distinct species of cassava mosaic geminiviruses (CMGs) (ACMV‐[KE:844:82] and EACMCV‐CM[CI:98]) at different light intensities of 150, 300, 450 and 600 μE/m2/s with two different temperature conditions (25 and 30 °C). (B) Graphical representation of the symptom progression of the CMGs under different light intensities of 150, 300, 450 and 600 μE/m2/s with a constant temperature of 25 °C. ACMV, African cassava mosaic virus; EACMCV, East African cassava mosaic Cameroon virus.
Furthermore, the symptomatic plants from different light intensities were subjected to molecular analysis to quantify the accumulation of viral DNA, viral mRNA (AC2 as probe) and virus‐derived siRNA at 1 and 2 weeks post‐inoculation (wpi) (Fig. 2). There were no significant differences in the accumulation of viral DNA, mRNA and siRNA at different light intensities (150, 300, 450 and 600 μE/m2/s) with a uniform temperature of 25 °C (Fig. 2). The expression level of mi159 was uniform in all different conditions, both with and without viral infections (Fig. 2).
Figure 2.
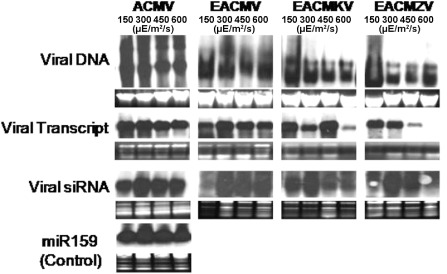
Molecular analysis [viral DNA, viral transcript and viral small interfering RNA (siRNA)] of different symptom phenotypes in Nicotiana benthamiana infected with distinct species of cassava mosaic geminiviruses (ACMV‐[KE:844:82]; EACMV‐KE[KE:Msa:K201:02]; EACMKV[KE:Keh:K229:02]; EACMZV‐[KE:Kib:K275:02]) at different light intensities of 150, 300, 450 and 600 μE/m2/s (with a constant temperature of 25 °C). ACMV, African cassava mosaic virus; EACMKV, East African cassava mosaic Kenya virus; EACMV, East African cassava mosaic virus; EACMZV, East African cassava mosaic Zanzibar virus.
Both very low and high temperatures reduce systemic movement of gene silencing
We wished to determine whether there was a correlation between the extent of gene silencing and the phenomenon of symptom recovery at higher temperatures, as reported by Szittya et al. (2003). Thus, studies were performed with an aim to understand the efficiency of gene silencing at four different temperatures (21, 25, 27 and 30 °C). Transient agroinfiltration studies were performed using the RNAi constructs targeting the endogenous gene ‘Su’ as well as the transgene ‘GFP’ in N. benthamiana plants transgenic for GFP (16C) (Fig. 3). These studies showed that there were significant differences in the systemic spread of gene silencing on transient agroinfiltrations in these temperature conditions, with the maximum systemic spread of the silencing phenotype occurring at 25 °C, whereas systemic silencing was reduced with an increase (>25 °C) or decrease (<25 °C) in temperature. At 30 °C, for both Su and GFP, the silencing phenotype was mostly localized to the site of agroinfiltration (Fig. 3). These contrasting silencing phenotypes were obtained without any pretreatment of the N. benthamiana plants to the above temperature conditions before the agroinfiltrations. As N. benthamiana has a defective RNA‐dependent RNA polymerase (RdRp) (Yang et al., 2004), to rule out any artefacts or misinterpretations, we also confirmed the silencing phenotypes of ‘Su’ in Nicotiana tabacum which has an intact RdRp (Fig. S2B, see Supporting Information).
Figure 3.
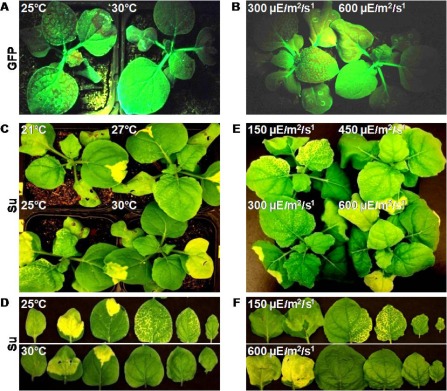
Different levels of systemic silencing obtained for green fluorescent protein (GFP) (A, B) and Su (C–F) genes by RNA interference (RNAi) through transient agroinfiltrations in Nicotiana benthamiana at different temperatures (21, 25, 27 and 30 °C) (A, C, D) and light intensities (150, 300, 450 and 600 μE/m2/s) (B, E, F). Individual leaves of Su‐silenced N. benthamiana from (C) and (E) were removed and placed in ascending order in (D) and (F), respectively. All the plants were photographed at 1 week post‐agroinfiltration of the RNAi construct.
The quantification of transcript and siRNA accumulation in leaf tissues with local and systemic silencing at different temperature conditions was also performed (Fig. 4A). The analysis showed that, in the locally silenced leaf tissue, the amount of siRNA decreased with an increase in temperature from 21 to 30 °C. No signal was obtained for siRNA in any of the systemically silenced leaves, because of the presence of very low levels of siRNA which cannot be detected (data not shown).
Figure 4.
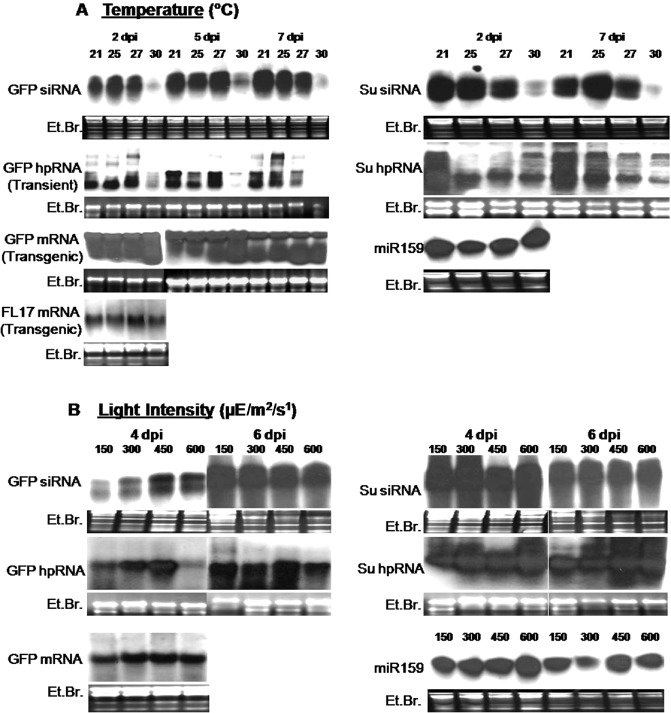
Molecular analysis [hairpin RNA (hpRNA) and small interfering RNA (siRNA)] of different silencing phenotypes obtained for the transgene green fluorescent protein (GFP) and the endogenous gene Su by transient agroinfiltration studies in Nicotiana benthamiana at different temperatures (21, 25, 27 and 30 °C) (A) and light conditions (150, 300, 450 and 600 μE/m2/s) (B). The expression of messenger RNA (mRNA) and siRNA in stable transgenics for GFP (in 16c) and RNA interference Cassava brown streak Uganda virus coat protein (RNAi CBSUV‐CP) (in FL17 line of N. benthamiana) is also shown. Similar expression of mi159 at different light intensities and temperatures is also shown as a control. dpi, days post‐infiltration; Et.Br., ethidium bromide.
Localized silencing at high light intensity is much stronger than that at low light intensity
There were no differences observed in the nature of systemic silencing when the transient silencing studies were performed in different light spectra (wavelengths) with a constant light intensity (300 μE/m2/s) and temperature (25 °C). The two different light spectra were blue (400 nm) and red (700 nm), as the major parts of the photosynthetically active radiation (PAR) obtained using metal halide and high‐pressure sodium lamps, respectively. Thus, we carried out transient silencing studies at different light intensities ranging from 75 to 700 μE/m2/s. The results showed that, with an increase in light intensity, the systemic spread of gene silencing decreased to only localized silencing at a light intensity of more than 600 μE/m2/s (Fig. 3). These contrasting silencing phenotypes were obtained only when N. benthamiana was pretreated for 4–5 days with the above light intensities prior to the agroinfiltration of RNAi constructs. The analyses for transcript and siRNA accumulation showed that, overall, there were no significant differences in their titres at different light intensities (Fig. 4B). Interestingly, the local silencing was initiated a day earlier under high light intensity of ≥600 μE/m2/s compared with low light intensity of 150 μE/m2/s (Fig. S2A), whereas the timescale analysis for the transcript and siRNA did not show a significant difference in their amounts. The siRNAs of both size classes (∼21 and 24 nucleotides) were equally represented in all light intensity and temperature conditions during all days post‐agroinfiltration, thus ruling out the possibility that differences in systemic silencing were a result of the different size classes of siRNAs produced, at least in the case of transient silencing studies, by agroinfiltration of RNAi constructs.
Relative humidity has no effect on systemic spread of the silencing signal
We also checked whether the relative humidity had any influence on the movement of the silencing signal. Silencing experiments were performed at two contrasting relative humidity conditions of 20% and 80% with a constant temperature of 25 °C and a light intensity of 300 μE/m2/s. In both relative humidity conditions, a similar level of systemic spread of the silencing signal was recorded in agroinfiltrated N. benthamiana plants.
Phloem tracer experiments indicate a change in the sink–source status of N. benthamiana leaves under different light intensities
To unravel the possible reasons for increased systemic silencing at lower light intensities, phloem tracer experiments were performed to track the rate of movement of the 32P‐labelled phosphate (Pi) in the form of pyrophosphate and sugar phosphates from source to sink leaves of N. benthamiana (Bieleski, 1969). The phloem tracer experiments showed that, at low light intensities (150 μE/m2/s), with N. benthamiana showing strong systemic silencing, there was significant systemic movement of the radiolabelled Pi from infiltrated leaves (source) to upper young leaves (sink) at both 8 and 16 h post‐infiltration (Fig. 5). However, N. benthamiana at high light intensity (600 μE/m2/s) with local silencing alone did not show any significant systemic movement of radiolabelled Pi, indicating that, as a result of an increase in light intensity accompanied by increased photosynthesis, there was a shift in the sink and source status of N. benthamiana leaves, eventually affecting phloem translocation. Although there was a slight difference in the rates of phloem translocation at different temperatures (25 and 30 °C), it was not as intense as for the two contrasting light intensities (150 and 600 μE/m2/s).
Figure 5.
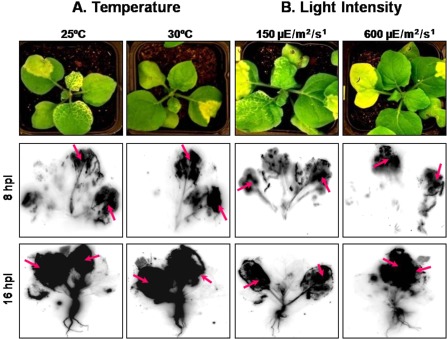
Tracking phloem translocation by infiltration of radioactively labelled inorganic phosphate in Su‐silenced Nicotiana benthamiana maintained at different temperatures (25 and 30 °C) (A) and light intensities (150 and 600 μE/m2/s) (B) at 8 and 16 h post‐infiltration (hpi). The two leaves which have been infiltrated in each plant are marked by arrows.
Removal of sink or source leaves confirms the change in sink–source status of N. benthamiana at different light intensities
The mature leaves, which are photosynthetically more active, function as the source leaves from which the photosynthates are translocated through the phloem to the photosynthetically less active sink leaves, mostly the young unopened leaves. To confirm whether the difference in the systemic movement of gene silencing at different light intensities was mainly caused by a change in the sink and source status of the plant, we artificially altered the sink–source relationship by independently plucking out the sink and source leaves, 2 days post‐agroinfiltration of RNAi‐Su (Fig. S2C). Interestingly, the removal of two old leaves, representing the source, in low light intensities did not produce any systemic silencing, whereas removal of two young leaves from the same set of plants led to enhanced systemic silencing (Fig. S2C). Similar experiments with N. benthamiana maintained at higher light intensities produced systemic silencing on removal of two old leaves and local silencing on removal of two young leaves (Fig. S2C). Thus, this experiment strengthened the above theory that a change in the sink–source status of the plant at different light intensities produced different phenotypes of systemic silencing on transient agroinfiltration with the RNAi constructs.
High temperature reduces transgene expression because of reduced T‐DNA transfer activity of Agrobacterium
To determine whether temperature and light intensity had any effect on the transient expression level of the transgene after agroinfiltration, we agroinfiltrated a GFP‐expressing plasmid in wild‐type (non‐transgenic) N. benthamiana. Both the fluorescent microscopic observations and Northern analysis indicated that GFP expression was significantly reduced at a higher temperature of 30 °C compared with a temperature of 25 °C (Fig. 4A). However, there were no significant differences in GFP expression under different light intensities (Fig. S1, see Supporting Information).
Uniform expression of transcript and siRNA in stable transgenics in different conditions
Two transgenic N. benthamiana plant lines (FL5 and FL17) expressing hairpin RNA targeting the coat protein gene of Cassava brown streak Uganda virus (Patil et al., 2011) were used in this study to analyse their expression levels at different temperatures and light intensities. The Cassava brown streak Uganda virus is now re‐named as Ugandan Cassava brown streak virus (UCBSV) (Mbanzibwa et al., 2011). Three‐week‐old transgenic RNAi lines of N. benthamiana (FL5 and FL17) were maintained for 2 weeks at different temperatures and light intensities, and their leaf samples were analysed. The analysis showed that there were no significant differences in the expression levels of the transgene or corresponding siRNAs, as interpreted from Northern hybridizations (Figs 4A and 5B). Similarly, uniform expression levels were obtained for the GFP transgene in GFP‐transgenic N. benthamiana (line 16c) at different temperatures and light intensities (Figs 4A and 5B).
Discussion
Environmental conditions play a critical role in the determination of symptom severity of virus‐infected plants, and there have been several reports on the manifestation of virus symptoms with varying environmental conditions (Bawden and Roberts, 1947; Fondong et al., 2000; Johnson, 1921, 1922, 1926; Jones, 2009; Patil and Fauquet, 2011; Wingard, 1928). It has been established that the recovery from virus infection is an outcome of PTGS, which controls virus proliferation by destroying the viral RNA or mRNAs transcribed by viral genomes (Ratcliff et al., 1997). PTGS is a natural antiviral defence mechanism present in plants which is part of the host innate immunity and, to counteract this, viruses have evolved gene silencing suppressors (Anandalakshmi et al., 2000; Hamilton et al., 2002; Waterhouse et al., 2001). However, PTGS is manifested by a change in temperature conditions, eventually resulting in the recovery of virus symptoms at higher temperatures because of enhanced PTGS (Chellappan et al., 2005; Fortier and Belote, 2000; Szittya et al., 2003). This recovery phenotype caused by increased PTGS activity at higher temperature has also been reported for geminiviruses with a single‐stranded DNA (ssDNA) genome, with no double‐stranded RNA (dsRNA) phase in their life cycle (Chellappan et al., 2004, 2005). It has been demonstrated that the recovery phenotype obtained on geminivirus infection is caused by complementary action of both PTGS and transcriptional gene silencing (TGS), wherein the methylation of the viral genome acts as an epigenetic defence against geminiviruses (Raja et al., 2008; Rodriguez‐Negrete et al., 2009). Although PTGS has been implicated in the manifestation of viral symptoms at different temperatures, there is no experimental evidence to demonstrate the recovery phenotypes being manifested by light conditions (Fondong et al., 2000). PTGS alone has been considered as a factor responsible for the manifestation of viral symptoms, and other biological phenomena have not been investigated thoroughly for their potential role in the recovery of virus symptoms under different conditions (Chellappan et al., 2005; Szittya et al., 2003).
Like other plant viruses, some of the CMGs also display symptom recovery at different temperature and light conditions (Chellappan et al., 2005; Fondong et al., 2000). The virus recovery phenotypes of cassava plants grown in experimental fields in Africa have shown a correlation with a change in light and temperature conditions (Fondong et al., 2000; Patil and Fauquet, 2011). Therefore, for the first time, we made a systematic effort to characterize the symptom phenotypes of seven different species of geminiviruses under different temperature, light and humidity conditions. Of the three variable environmental factors (temperature, light and humidity) used to study virus infectivity and systemic spread of gene silencing by transient agroinfiltration, temperature had a profound impact, followed by light intensity, whereas humidity had no effect on either factor (Fu et al., 2006). However, in contrast with the strong recovery phenotypes of viral symptoms obtained in different temperature conditions, there were no significant differences in the symptom phenotypes of geminiviruses at different light intensities. Molecular analysis for the accumulation of viral DNA, mRNA and siRNA did not show any significant difference in their accumulation at different light intensities.
The observations with regard to the silencing of an endogenous gene (Su) and a transgene (GFP) at different temperature and light conditions were unexpected. There was good systemic silencing only within a window of temperature (25–27 °C), whereas, at both very low (21 °C) and high (30 °C) temperatures, there was only localized silencing, with no or significantly reduced systemic spread of the silencing signal on transient agroinfiltration. Small RNA analysis showed that, at higher temperature (30 °C), the amount of sequence‐specific siRNA produced was significantly less compared with that at temperatures of 25 and 27 °C, as a result of reduced transient expression at 30 °C. The expression levels for the transcript and siRNA were also reduced slightly at lower temperature (21 °C), when compared with the significant reduction at 30 °C. These observations contradict previous reports on increased gene silencing with an increase in temperature for studies involving both transient and transgenic expression (Szittya et al., 2003). At higher temperature, the level of siRNA produced was much below the threshold levels of siRNA required for the induction of systemic silencing (Kalantidis et al., 2006, 2008). Similar observations were made by Kalantidis et al. (2006), indicating that minor changes in the steady‐state concentration of siRNA and its corresponding mRNA are decisive in whether silencing remains local or shows systemic spread. The transient expression studies with GFP showed that the expression of GFP was significantly reduced at higher temperature (30 °C), because of the reduced T‐DNA transfer activity of Agrobacterium, as interpreted from the low level of GFP fluorescence at 30 °C relative to that at 25 °C (Baron et al., 2001; Fullner and Nester, 1996; Jin et al., 1993). Studies have shown that, at a higher temperature (>32 °C), the Agrobacterium Vir genes are not expressed because of the conformational change in Vir A, and the induction of Vir genes is optimal at 25 °C (Fullner and Nester, 1996; Jin et al., 1993). In addition, it is also possible that temperature may affect phloem transport to some extent, resulting in reduced systemic silencing at higher temperatures (Lemoine et al., 2013).
To confirm the above results, we checked the expression level of a transgene, ‘Cassava brown streak Uganda virus full‐length coat protein’ (CBSUV FL‐CP), and the accumulation of the corresponding siRNAs in the stable RNAi‐transgenic N. benthamiana plant lines (Patil et al., 2011). No marked differences in the expression levels of CBSUV FL‐CP at different temperature conditions were observed, thus emphasizing the fact that the reduced expression of the transgene in transient studies is caused by the reduced T‐DNA transfer activity of Agrobacterium at high temperatures, also known as plasmid curing (Baron et al., 2001). Studies by Sos‐Hegedus et al. (2005) have shown that RNA silencing is active at even lower temperatures (15 °C) in potato antisense lines. Tuttle et al. (2008) observed that even though the accumulation of Cotton leaf crumple virus (Geminiviridae) was reduced at higher temperature compared to the lower temperatures, there was no corresponding increase in the accumulation of siRNA at higher temperature, in contrast there was a reduction in the siRNA levels at higher temperature compared to the lower temperatures. Similarly, enhanced VIGS was observed at lower temperatures, when the VIGS vector derived from Tobacco rattle virus was infiltrated in tomato plants (Fu et al., 2006). Thus, these observations do not agree with the previous hypothesis of virus symptom recovery as a result of increased gene silencing with an increase in temperature. Recently, Ghoshal and Sanfacon (2014) have shown that temperature‐dependent symptom recovery in N. benthamiana plants infected with a nepovirus, Tomato ring spot virus, is associated with reduced translation of viral RNA2, and the silencing of Argonaute1‐like (Ago1) genes prevent RNA2 translation repression and symptom recovery. However, we hypothesize that there is enhanced virus replication at higher temperature, leading to the production of more transcripts, possibly containing an increased amount of aberrant transcripts to trigger gene silencing, thus leading to greater accumulation of siRNA and, eventually, symptom recovery.
Viruses move from the site of infection to the systemic tissues via the symplastic continuity created by plasmodesmata interconnecting the cells and the phloem long‐distance translocation system (Lemoine et al., 2013). As phloem translocation is reduced at high temperature and light intensities, it may also be possible that there is reduced systemic movement of viruses, eventually leading to increased localized accumulation of viral genomes and their products (Scholthof, 2005). Thus, the different virus symptom phenotypes obtained in different hosts or environmental conditions represent an outcome of several different physiological processes which may affect gene silencing indirectly. It has also been shown that the movement of many small RNAs is controlled in response to nutrient conditions, and these reports propose that small RNAs in phloem might signal the changes in environmental conditions (Buhtz et al., 2010).
The studies at different light intensities indicated that there was significant reduction in the systemic spread of the silencing signal with an increase in light intensity. These differences were mostly caused by the change in sink and source status of the leaves of N. benthamiana, as indicated by the phloem translocation experiments, and the phloem flow has a strong influence on the systemic spread of the silencing signal (Kalantidis et al., 2008). Nicotiana benthamiana required at least 4–5 days of pretreatment with the above light intensities to facilitate a shift in the source–sink status of the leaves. The experiment involving the removal of source and sink leaves to change the source and sink status of the leaves of N. benthamiana plants further strengthened this hypothesis. Timescale studies to quantify the siRNA specific to Su or GFP sequences indicated that there was no significant difference in their quantities at different light intensities at different time periods. There have been no reports on the change in T‐DNA transfer activity of Agrobacterium at different light intensities, although it has been demonstrated that, with an increase in photoperiod, there is an increase in T‐DNA transfer activity (Zambre et al., 2003). Transient GFP expression studies in N. benthamiana by agroinfiltration of GFP‐expressing vector at different light intensities did not show a drastic difference in their expression level. Analysis of stable RNAi‐based‐transgenic N. benthamiana plants expressing hairpin CBSUV FL‐CP under different light intensities did not show a significant difference in their expression level of the transgene (hairpin RNA; Patil et al., 2011) or the siRNA produced. Further, experiments with combinations of different temperatures (25 and 30 °C) and light intensities indicated that temperature had a stronger effect than light intensity on the systemic movement of the silencing signal in transient agroinfiltration studies, and also on the recovery of virus symptoms.
To conclude, these experiments have attempted to explain the natural phenomenon exhibited by plant viruses and gene silencing under the influence of different environmental conditions. However, there are several potential applications of these important findings in the field of functional genomics and virus control using gene silencing as a method of choice. These studies will help significantly in carrying out successful functional genomics studies using the VIGS approach in ideal temperature and light conditions in which the best silencing phenotypes can be obtained. Recently, most virus‐resistant transgenic plants are based on the RNAi strategy, and it is important that these transgenes express efficiently to effectively ward off the viruses. Such RNAi‐based transgenic plants, such as vegetable or flower crops, which are grown in greenhouse conditions, can be subjected to optimum light and temperature conditions for the effective expression of small RNAs. This information can also be useful in the management of RNAi‐based transgenic root stocks of horticultural plants like grapes (Vigne et al., 2004), where in the movement of siRNA from the transgenic root stock to the scion can be regulated by varying environmental conditions. Transient virus protection can also be performed in favourable light and temperature conditions by spraying synthetic small RNAs on the plant foliage.
Experimental Procedures
Plant growth conditions
To study the effect of the quality of light, two growth chambers (PGW36, Conviron, Pembina, ND, USA) providing two different spectra of light with the majority of the radiation falling in the blue (400 nm) or red (700 nm) regions, artificially created using metal halides for the blue light spectrum and sodium lamps for the red light spectrum, were used. The light intensity was maintained at 300 μE/m2/s in both spectra and a constant temperature of 25 °C was used. Both the light quality and intensity were measured using a spectroradiometer (models PAR‐NIR and UV‐PAR, Apogee Instruments Inc., Logan, UT, USA). To study the effect of temperature, light intensity and humidity, four controlled growth chambers (PGR14, Conviron) set at different conditions (temperature, light intensity and humidity) were used. The sources of light used were a mixture of fluorescent tubes and incandescent bulbs, which were adjusted to varying light intensities to study their effect on virus symptoms and movement of the silencing signal. The experiments were performed with a combination of two different temperatures, 25 and 30 °C, and four different light intensities (150, 300, 450 and 600 μE/m2/s). The four growth chambers were also set at four different temperatures of 21, 25, 27 and 30 °C, with a constant light intensity of 300 μE/m2/s and a uniform relative humidity of 50%, to study the effect of temperature. The experiments were also performed at two contrasting relative humidities of 20% and 80% with a constant temperature of 25 °C and a light intensity of 300 μE/m2/s.
RNAi vectors to silence GFP and Su (magnesium chelatase)
The binary vectors carrying the RNAi cassettes to silence GFP and Su (sulfur gene), encoding for a subunit of magnesium chelatase, an enzyme involved in chlorophyll biosynthesis, were used for transient gene silencing studies in N. benthamiana. For GFP silencing studies, the GFP‐transgenic N. benthamiana (16c) from Dr David Baulcombe (University of Cambridge, Cambridge, UK) was used (Ruiz et al., 1998). The GFP‐transgenic N. benthamiana (16c) plants were screened for silencing of GFP under a 100‐W hand‐held long‐wave ultraviolet lamp (Black Ray model B 100AP, UV Products, Upland, CA, USA) and photographed using a Nikon digital camera (Nikon D80 digital SLR camera, Nikon Inc. Melville, NY, USA). The images were processed for clarity using Adobe Photoshop CS2.
Infectious clones of CMGs
The agroinfectious clones of ICMV (ICMV‐IN[IN:Mah:88]), SLCMV (SLCMV‐LK[LK:Col:98]), ACMV (ACMV‐[KE:844:82]), EACMV (EACMV‐KE[KE:Msa:K201:02]), EACMCV (EACMCV‐CM[CI:98]), EACMKV (EACMKV[KE:Keh:K229:02]) and EACMZV‐[KE:Kib:K275:02] were used to study the variation in symptom phenotypes under contrasting temperatures and light intensities (Patil and Fauquet, 2009, 2010).
Agroinfiltration of N. benthamiana, viral symptom score and data analysis
Agroinfiltrations of the infectious virus clones and RNAi constructs were performed as discussed in Patil and Fauquet (2010). The inoculated plants were maintained in controlled growth chambers set at different light intensities and temperatures and with an alternating light and dark period of 16 h and 8 h, respectively. All the experiments were repeated at least four times in N. benthamiana, and symptom progression was recorded every alternate day for a period of 1 month, beginning from the day of appearance of the first symptoms. Symptoms were scored on a scale of 0–5 (0, absence of symptoms; 5, necrosis/death of the plant) (Patil and Fauquet, 2010). The symptomatic plants were photographed using a Nikon digital camera (Nikon D80 digital SLR camera, Nikon Inc. Melville, NY, USA) and the images were processed for clarity using Adobe Photoshop CS2.
Analysis of siRNA, mRNA and viral DNA/RNA accumulation
Total RNA was isolated from agroinfiltrated leaves using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions, and fractionated using an RNeasy Plant mini kit (Qiagen, Valencia, CA, USA) and the RNA cleanup protocol, as described by Akbergenov et al. (2006) with minor modifications. Further, small RNAs were subjected to electrophoresis and hybridizations were performed as described in Patil et al. (2011). The segments of GFP and Su used to prepare the RNAi constructs were cloned in the vector pSPT19, and the probes were synthesized by in vitro transcription with T7 RNA polymerase using a digoxigenin (DIG) RNA labelling kit (Roche Applied Science, Indianapolis, IN, USA). The labelled RNA was hydrolysed for 10–15 min in 50 mm sodium bicarbonate/carbonate at 95 °C. Oligonucleotides with the microRNA159 (miRNA159) sequence end‐labelled with dUTP at the 3′ end, employing the DIG oligonucleotide 3′‐end labelling kit (Roche Applied Science), were used to study the expression level of miRNA159 in different conditions.
Similarly, 20 μg of total RNA were fractionated in agarose gel prepared in 3(N‐morpholino)propanesulfonic acid (MOPS) buffer (Ambion Inc., Austin, TX, USA) and transferred to a Hybond N+ membrane by capillary action using 20 × saline‐sodium citrate (SSC) (Sambrook and Russell, 2001). Northern hybridizations were performed using polymerase chain reaction (PCR)‐amplified DNA products employing the DIG High Prime DNA labelling and detection kit (Roche Applied Science). All the other processes are similar to the descriptions given in Patil et al. (2011). Detection of geminiviral DNA was performed by Southern hybridizations, as described in Patil and Fauquet (2010).
Phloem labelling experiment using radiolabelled inorganic phosphate
The radioisotope γ32P‐ATP (New England Nuclear Research Products, Boston, MA, USA) was treated with alkaline phosphatase (1 μCi, with 2 units of alkaline phosphatase for 1 h at 37 °C in 50 μL of 1 × buffer from New England Biolabs Inc., Beverly, MA, USA) to separate the labelled inorganic phosphate (32Pi) from the rest of the ATP from γ32P, as described in Tournier et al. (2006). This CIP‐treated γ32P was diluted with 7% sucrose solution to an activity of 10 μCi/μL. Nicotiana benthamiana plants (4 weeks old), pre‐silenced for the magnesium chelatase gene using the RNAi‐Su construct, were infiltrated with γ32P in 7% sucrose solution with 1 mL of solution per plant. In each plant, two medium‐sized leaves were infiltrated with a 1‐mL needleless syringe and the plants were incubated for 4 and 16 h. After incubation at both 4 and 16 h post‐infiltration, the N. benthamiana plants were taken out from the pots and gently wrapped with saran wrap without any damage to the plants, keeping them intact. The plants were then exposed to the phosphor‐imager screen for 1 h or overnight depending on the signal intensity, and scanned in a Typhoon PhosphorImager (Typhoon 9400, GE Healthcare, Piscataway, NJ, USA).
Supporting information
Fig. S1 Fluorescence microscopy for expression of the reporter gene, green fluorescent protein (GFP), in the leaf mesophyll tissue of Nicotiana benthamiana, after the agroinfiltration of the GFP‐expressing construct, in two different temperature conditions (25 and 30 °C) under a constant light intensity of 300 μE/m2/s.
Fig. S2 Early and strong local (L) silencing in high light intensity (600 μE/m2/s) in contrast with weak local silencing, but strong systemic (S) silencing, in low light intensity (150 μE/m2/s) (A). Silencing of Su in Nicotiana tabacum at different light intensities (150 and 600 μE/m2/s) (B). Removal of sink or source leaves changes the movement of the silencing signal in different light intensities (150, 300, 450 and 600 μE/m2/s) in agroinfiltrated Nicotiana benthamiana with the RNA interference (RNAi) construct for Su (C). [Correction added on 30 January 2015, after first online publication: In the original publication, the top left hand panel of this figure contained an error. The authors inadvertently used an image of a treated plant instead of a control plant. The resupplied figure correctly shows a control plant in the top left hand panel. The authors apologise for the error and any confusion caused.]
Acknowledgements
We acknowledge funding from the Monsanto Fund and the United States Agency for International Development (USAID). We thank Dr Chris Taylor for providing the RNAi constructs targeting GFP and Su genes and Dr David Baulcombe for the seeds of the GFP‐transgenic N. benthamiana (16c). We acknowledge the Donald Danforth Plant Science Center (DDPSC) glasshouse staff for excellent care of the plants.
The authors have no conflicts of interest to declare.
References
- Akbergenov, R. , Si‐Ammour, A. , Blevins, T. , Amin, I. , Kutter, C. , Vanderschuren, H. , Zhang, P. , Gruissem, W. , Meins, F. Jr , Hohn, T. and Pooggin, M.M. (2006) Molecular characterization of geminivirus‐derived small RNAs in different plant species. Nucleic Acids Res. 34, 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandalakshmi, R. , Marathe, R. , Ge, X. , Herr, J.M. Jr , Mau, C. , Mallory, A. , Pruss, G. , Bowman, L. and Vance, V.B. (2000) A calmodulin‐related protein that suppresses posttranscriptional gene silencing in plants. Science, 290, 142–144. [DOI] [PubMed] [Google Scholar]
- Baron, C. , Domke, N. , Beinhofer, M. and Hapfelmeier, S. (2001) Elevated temperature differentially affects virulence, VirB protein accumulation, and T‐pilus formation in different Agrobacterium tumefaciens and Agrobacterium vitis strains. J. Bacteriol. 183, 6852–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawden, F.C. and Roberts, F.M. (1947) The influence of light intensity on the susceptibility of plants to certain viruses. Ann. Appl. Biol. 34, 286–296. [DOI] [PubMed] [Google Scholar]
- Bieleski, R.L. (1969) Phosphorus compounds in translocating phloem. Plant Physiol. 44, 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhtz, A. , Pieritz, J. , Springer, F. and Kehr, J. (2010) Phloem small RNAs, nutrient stress responses, and systemic mobility. BMC Plant Biol. 10, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan, P. , Vanitharani, R. and Fauquet, C.M. (2004) Short interfering RNA accumulation correlates with host recovery in DNA virus‐infected hosts and gene silencing targets specific viral sequences. J. Virol. 78, 7465–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan, P. , Vanitharani, R. , Ogbe, F. and Fauquet, C.M. (2005) Effect of temperature on geminivirus‐induced RNA silencing in plants. Plant Physiol. 138, 1828–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colhoun, J. (1973) Effects of environmental factors on plant disease. Annu. Rev. Phytopathol. 11, 343–364. [Google Scholar]
- Ding, S.W. and Voinnet, O. (2007) Antiviral immunity directed by small RNAs. Cell, 130, 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunoyer, P. and Voinnet, O. (2009) Movement of RNA silencing between plant cells: is the question now behind us? Trends Plant Sci. 14, 643–644. [DOI] [PubMed] [Google Scholar]
- Dunoyer, P. , Himber, C. and Voinnet, O. (2005) DICER‐LIKE 4 is required for RNA interference and produces the 21‐nucleotide small interfering RNA component of the plant cell‐to‐cell silencing signal. Nat. Genet. 37, 1356–1360. [DOI] [PubMed] [Google Scholar]
- Dunoyer, P. , Brosnan, C.A. , Schott, G. , Wang, Y. , Jay, F. , Alioua, A. , Himber, C. and Voinnet, O. (2010a) An endogenous, systemic RNAi pathway in plants. EMBO J. 29, 1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dunoyer, P. , Schott, G. , Himber, C. , Meyer, D. , Takeda, A. , Carrington, J.C. and Voinnet, O. (2010b) Small RNA duplexes function as mobile silencing signals between plant cells. Science, 328, 912–916. [DOI] [PubMed] [Google Scholar]
- Fondong, V.N. , Thresh, J.M. and Fauquet, C. (2000) Field experiments in Cameroon on cassava mosaic virus disease and the reversion phenomenon in susceptible and resistant cassava cultivars. Int. J. Pest Manag. 46, 211–217. [Google Scholar]
- Fortier, E. and Belote, J.M. (2000) Temperature‐dependent gene silencing by an expressed inverted repeat in Drosophila. Genesis, 26, 240–244. [DOI] [PubMed] [Google Scholar]
- Fu, D.Q. , Zhu, B.Z. , Zhu, H.L. , Zhang, H.X. , Xie, Y.H. , Jiang, W.B. , Zhao, X.D. and Luo, K.B. (2006) Enhancement of virus‐induced gene silencing in tomato by low temperature and low humidity. Mol. Cells, 21, 153–160. [PubMed] [Google Scholar]
- Fullner, K.J. and Nester, E.W. (1996) Temperature affects the T‐DNA transfer machinery of Agrobacterium tumefaciens . J. Bacteriol. 178, 1498–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, K.A. , Dendy, S.P. , Frank, E.E. , Rouse, M.N. and Travers, S.E. (2006) Climate change effects on plant disease: genomes to ecosystems. Annu. Rev. Phytopathol. 44, 489–509. [DOI] [PubMed] [Google Scholar]
- Ghoshal, B. and Sanfacon, H. (2014) Temperature‐dependent symptom recovery in Nicotiana benthamiana plants infected with tomato ringspot virus is associated with reduced translation of viral RNA2 and requires ARGONAUTE 1. Virology, 456–457, 188–197. [DOI] [PubMed] [Google Scholar]
- Hamilton, A. , Voinnet, O. , Chappell, L. and Baulcombe, D. (2002) Two classes of short interfering RNA in RNA silencing. EMBO J. 21, 4671–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, B.D. (2008) Studies on the effect of temperature on virus multiplication in inoculated leaves. Ann. Appl. Biol. 44, 215–226. [Google Scholar]
- Hine, R.B. , Osborne, W.E. and Dennis, R.E. (1970) Elevation and temperature effects on severity of maize dwarf mosaic virus in sorghum in Arizona. Plant Dis. Rep. 54, 1064–1068. [Google Scholar]
- Jin, S. , Song, Y.N. , Deng, W.Y. , Gordon, M.P. and Nester, E.W. (1993) The regulatory VirA protein of Agrobacterium tumefaciens does not function at elevated temperatures. J. Bacteriol. 175, 6830–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, J. (1921) The relation of air temperature to certain plant diseases. Phytopathology, 11, 446–458. [Google Scholar]
- Johnson, J. (1922) The relation of air temperature to the mosaic disease of potatoes and other plants. Phytopathology, 12, 438–440. [Google Scholar]
- Johnson, J. (1926) The attenuation of plant viruses and the inactivating influence of oxygen. Science, 64, 210. [DOI] [PubMed] [Google Scholar]
- Jones, R.A. (2009) Plant virus emergence and evolution: origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res. 141, 113–130. [DOI] [PubMed] [Google Scholar]
- Jose, A.M. and Hunter, C.P. (2007) Transport of sequence‐specific RNA interference information between cells. Annu. Rev. Genet. 41, 305–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantidis, K. (2004) Grafting the way to the systemic silencing signal in plants. Plos Biol. 2, E224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantidis, K. , Tsagris, M. and Tabler, M. (2006) Spontaneous short‐range silencing of a GFP transgene in Nicotiana benthamiana is possibly mediated by small quantities of siRNA that do not trigger systemic silencing. Plant J. 45, 1006–1016. [DOI] [PubMed] [Google Scholar]
- Kalantidis, K. , Schumacher, H.T. , Alexiadis, T. and Helm, J.M. (2008) RNA silencing movement in plants. Biol. Cell, 100, 13–26. [DOI] [PubMed] [Google Scholar]
- Kassanis, B. (1957) Effects of changing temperature on plant virus diseases. Adv. Virus Res. 4, 221–241. [DOI] [PubMed] [Google Scholar]
- Kotakis, C. , Vrettos, N. , Kotsis, D. , Tsagris, M. , Kotzabasis, K. and Kalantidis, K. (2010) Light intensity affects RNA silencing of a transgene in Nicotiana benthamiana plants. BMC Plant Biol. 10, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotakis, C. , Vrettos, N. , Daskalaki, M.G. , Kotzabasis, K. and Kalantidis, K. (2011) DCL3 and DCL4 are likely involved in the light intensity–RNA silencing cross talk in Nicotiana benthamiana . Plant Signal. Behav. 6, 1180–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine, R. , La Camera, S. , Atanassova, R. , Dedaldechamp, F. , Allario, T. , Pourtau, N. , Bonnemain, J.L. , Laloi, M. , Coutos‐Thevenot, P. , Maurousset, L. , Faucher, M. , Girousse, C. , Lemonnier, P. , Parrilla, J. and Durand, M. (2013) Source‐to‐sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 4, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbanzibwa, D.R. , Tian, Y.P. , Tugume, A.K. , Patil, B.L. , Yadav, J.S. , Bagewadi, B. , Abarshi, M.M. , Alicai, T. , Changadeya, W. , Mkumbira, J. , Muli, M.B. , Mukasa, S.B. , Tairo, F. , Baguma, Y. , Kyamanywa, S. , Kullaya, A. , Maruthi, M.N. , Fauquet, C.M. and Valkonen, J.P. (2011) Evolution of cassava brown streak disease‐associated viruses. J. Gen. Virol. 92, 974–987. [DOI] [PubMed] [Google Scholar]
- Mlotshwa, S. , Voinnet, O. , Mette, M.F. , Matzke, M. , Vaucheret, H. , Ding, S.W. , Pruss, G. and Vance, V.B. (2002) RNA silencing and the mobile silencing signal. Plant Cell, 14 (Suppl), S289–S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar, A. , Melnyk, C.W. , Bassett, A. , Hardcastle, T.J. , Dunn, R. and Baulcombe, D.C. (2010) Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science, 328, 872–875. [DOI] [PubMed] [Google Scholar]
- Patil, B.L. and Fauquet, C.M. (2009) Cassava mosaic geminiviruses: actual knowledge and perspectives. Mol. Plant Pathol. 10, 685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, B.L. and Fauquet, C.M. (2010) Differential interaction between cassava mosaic geminiviruses and geminivirus satellites. J. Gen. Virol. 91, 1871–1882. [DOI] [PubMed] [Google Scholar]
- Patil, B.L. and Fauquet, C.M. (2011) Ecology of plant viruses, with special reference to geminiviruses In: Studies in Viral Ecology, Vol. I, Microbial and botanical host systems (Hurst C.J., ed.), pp. 273–306. New York and Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Patil, B.L. , Ogwok, E. , Wagaba, H. , Mohammed, I.U. , Yadav, J.S. , Bagewadi, B. , Taylor, N.J. , Alicai, T. , Kreuze, J.F. , Maruthi, M.N. , Alicai, T. and Fauquet, C.M. (2011) RNAi mediated resistance to diverse isolates belonging to two virus species involved in Cassava brown streak disease. Mol. Plant Pathol. 12, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja, P. , Sanville, B.C. , Buchmann, R.C. and Bisaro, D.M. (2008) Viral genome methylation as an epigenetic defense against geminiviruses. J. Virol. 82, 8997–9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff, F. , Harrison, B.D. and Baulcombe, D.C. (1997) A similarity between viral defense and gene silencing in plants. Science, 276, 1558–1560. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Negrete, E.A. , Carrillo‐Tripp, J. and Rivera‐Bustamante, R.F. (2009) RNA silencing against geminivirus: complementary action of posttranscriptional gene silencing and transcriptional gene silencing in host recovery. J. Virol. 83, 1332–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz, M.T. , Voinnet, O. and Baulcombe, D.C. (1998) Initiation and maintenance of virus‐induced gene silencing. Plant Cell, 10, 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Ferrer, V. and Voinnet, O. (2009) Roles of plant small RNAs in biotic stress responses. Annu. Rev. Plant Biol. 60, 485–510. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. and Russell, D.W. (2001) Molecular Cloning: A Laboratory Manual, 3rd edn New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Scholthof, H.B. (2005) Plant virus transport: motions of functional equivalence. Trends Plant Sci. 10, 376–382. [DOI] [PubMed] [Google Scholar]
- Sos‐Hegedus, A. , Lovas, A. , Kondrak, M. , Kovacs, G. and Banfalvi, Z. (2005) Active RNA silencing at low temperature indicates distinct pathways for antisense‐mediated gene‐silencing in potato. Plant Mol. Biol. 59, 595–602. [DOI] [PubMed] [Google Scholar]
- Szittya, G. , Silhavy, D. , Molnar, A. , Havelda, Z. , Lovas, A. , Lakatos, L. , Banfalvi, Z. and Burgyan, J. (2003) Low temperature inhibits RNA silencing‐mediated defence by the control of siRNA generation. EMBO J. 22, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier, B. , Tabler, M. and Kalantidis, K. (2006) Phloem flow strongly influences the systemic spread of silencing in GFP Nicotiana benthamiana plants. Plant J. 47, 383–394. [DOI] [PubMed] [Google Scholar]
- Tuttle, J.R. , Idris, A.M. , Brown, J.K. , Haigler, C.H. and Robertson, D. (2008) Geminivirus‐mediated gene silencing from Cotton leaf crumple virus is enhanced by low temperature in cotton. Plant Physiol. 148, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne, E. , Komar, V. and Fuchs, M. (2004) Field safety assessment of recombination in transgenic grapevines expressing the coat protein gene of Grapevine fanleaf virus. Transgenic Res. 13, 165–179. [DOI] [PubMed] [Google Scholar]
- Voinnet, O. (2005) Induction and suppression of RNA silencing: insights from viral infections. Nat. Rev. Genet. 6, 206–220. [DOI] [PubMed] [Google Scholar]
- Voinnet, O. and Baulcombe, D.C. (1997) Systemic signalling in gene silencing. Nature, 389, 553. [DOI] [PubMed] [Google Scholar]
- Waterhouse, P.M. , Wang, M.B. and Lough, T. (2001) Gene silencing as an adaptive defence against viruses. Nature, 411, 834–842. [DOI] [PubMed] [Google Scholar]
- Wingard, S.A. (1928) Hosts and symptoms of ring spot, a virus disease of plants. J. Agric. Res. 37, 127–153. [Google Scholar]
- Yang, S.J. , Carter, S.A. , Cole, A.B. , Cheng, N.H. and Nelson, R.S. (2004) A natural variant of a host RNA‐dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana . Proc. Natl. Acad. Sci. USA, 101, 6297–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambre, M. , Terryn, N. , De Clercq, J. , De Buck, S. , Dillen, W. , Van Montagu, M. , Van Der Straeten, D. and Angenon, G. (2003) Light strongly promotes gene transfer from Agrobacterium tumefaciens to plant cells. Planta, 216, 580–586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Fluorescence microscopy for expression of the reporter gene, green fluorescent protein (GFP), in the leaf mesophyll tissue of Nicotiana benthamiana, after the agroinfiltration of the GFP‐expressing construct, in two different temperature conditions (25 and 30 °C) under a constant light intensity of 300 μE/m2/s.
Fig. S2 Early and strong local (L) silencing in high light intensity (600 μE/m2/s) in contrast with weak local silencing, but strong systemic (S) silencing, in low light intensity (150 μE/m2/s) (A). Silencing of Su in Nicotiana tabacum at different light intensities (150 and 600 μE/m2/s) (B). Removal of sink or source leaves changes the movement of the silencing signal in different light intensities (150, 300, 450 and 600 μE/m2/s) in agroinfiltrated Nicotiana benthamiana with the RNA interference (RNAi) construct for Su (C). [Correction added on 30 January 2015, after first online publication: In the original publication, the top left hand panel of this figure contained an error. The authors inadvertently used an image of a treated plant instead of a control plant. The resupplied figure correctly shows a control plant in the top left hand panel. The authors apologise for the error and any confusion caused.]


