Abstract
Osteoporosis is an aging-related disease of reduced bone mass that is particularly prevalent in post-menopausal women, but also affects the aged male population and is associated with increased fracture risk. Osteoporosis is the result of an imbalance whereby bone formation by osteoblasts no longer keeps pace with resorption of bone by osteoclasts. Osteocytes are the most abundant cells in bone and, although previously thought to be quiescent, they are now known to be active, multifunctional cells that play a key role in the maintenance of bone mass by regulating both osteoblast and osteoclast activity. They are also thought to regulate bone mass through their role as mechanoresponsive cells in bone that coordinate adaptive responses to mechanical loading. Osteocytes form an extensive interconnected network throughout the mineralized bone matrix and receive their nutrients as well as hormones and signaling factors through the lacunocanalicular system. Several studies have shown that the extent and connectivity of the lacunocanalicular system and osteocyte networks degenerates in aged humans as well as in animal models of aging. It is also known that the bone anabolic response to loading is decreased with aging. This review summarizes recent research on the degenerative changes that occur in osteocytes and their lacunocanalicular system as a result of aging and discusses the implications for skeletal health and homeostasis as well as potential mechanisms that may underlie these degenerative changes. Since osteocytes are such key regulators of skeletal homeostasis, maintaining the health of the osteocyte network would seem critical for maintenance of bone health. Therefore, a more complete understanding of the structure and function of the osteocyte network, its lacunocanalicular system, and the degenerative changes that occur with aging should lead to advances in our understanding of age related bone loss and potentially lead to improved therapies.
Keywords: Aging, osteocytes, osteoporosis, dendrite degeneration, bone fragility
Introduction:
Osteoporosis is an aging related disease of reduced bone mass that is particularly prevalent in post-menopausal women, but also affects the aged male population (reviewed in [1, 2]). In the United States, approximately 1.5 million fractures per year result from osteoporosis [3]. Worldwide, osteoporotic fractures result in the loss of an estimated 5.8 million Disability Adjusted Life Years due to the increased morbity and mortality associated with fracture, particularly of the hip [4]. With the expanding aged population resulting in a projected 6.26 million hip fractures per year by 2050 [5], it is clear that a comprehensive understanding of the factors contributing to bone fragility is of utmost importance.
Osteoporosis is the result of dysregulated bone remodeling, where the laying down of new bone by osteoblasts no longer keeps pace with resorption of bone by osteoclasts. Osteocytes are the most abundant cells in bone and, although these cells were previously thought to be quiescent, they are now known to be active, multifunctional cells that play a key role in maintenance of bone mass through regulation of both osteoblast and osteoclast activity (for reviews see [6–9]). The osteocyte is therefore poised to be a key therapeutic target for the treatment of osteoporosis. Osteocytes express sclerostin, a natural inhibitor of Wnt/β-catenin signaling, which is a major pathway that promotes bone formation [10, 11]. Clinical trials using anti-sclerostin antibodies have shown considerable promise in treating osteoporosis and increasing bone mass [12–15]. However, this initial enthusiasm has been tempered by recent reports of potential cardiovascular adverse events [16, 17], therefore further research is needed before these drugs can be FDA approved for clinical use. Another means by which osteocytes control bone mass is by regulating bone resorption. They express M-CSF and recent studies suggest that they provide a major source of RANKL, both of which promote osteoclast formation [18–20]. Additionally, osteocytes are thought to regulate bone mass through their role as the major cell type responsible for mechanotransduction and coordination of adaptive responses to mechanical loading [21–28]. Studies in mice and rats have shown that the bone anabolic response to loading is decreased with age [29, 30] and in vitro studies suggest impaired mechanresponsiveness in bone cells from osteoporotic patients[31]. Using low magnitude loading by whole body vibration, Kiel et al. showed no significant effect on femoral bone mineral density in elderly men and women [32], in contrast to earlier studies that showed beneficial effects in younger women[33]. This age-related impairment in the anabolic response to mechanical loading may further compromise skeletal homeostasis. Overall, a new view of the osteocyte is emerging as a central orchestrator within the skeleton that can integrate mechanical, hormonal and growth factor inputs to regulate bone mass.
Since osteocytes are such key regulators of osteoblast and osteoclast activity, maintaining the health of the osteocyte network is critical for maintenance of bone health. Therefore, a more complete understanding of the structure and function of the osteocyte network, its lacunocanalicular system, and the degenerative changes that occur with aging should lead to improvements in our understanding of age related bone loss and potentially lead to improved therapies. This article will briefly review imaging approaches used to study osteocytes and their lacunocanalicular system and will then discuss what is currently known concerning the degenerative changes that occur in this system with aging, the underlying mechanisms for these changes, as well as the implications for skeletal health and homeostasis.
Osteocytes and the Lacunocanalicular System
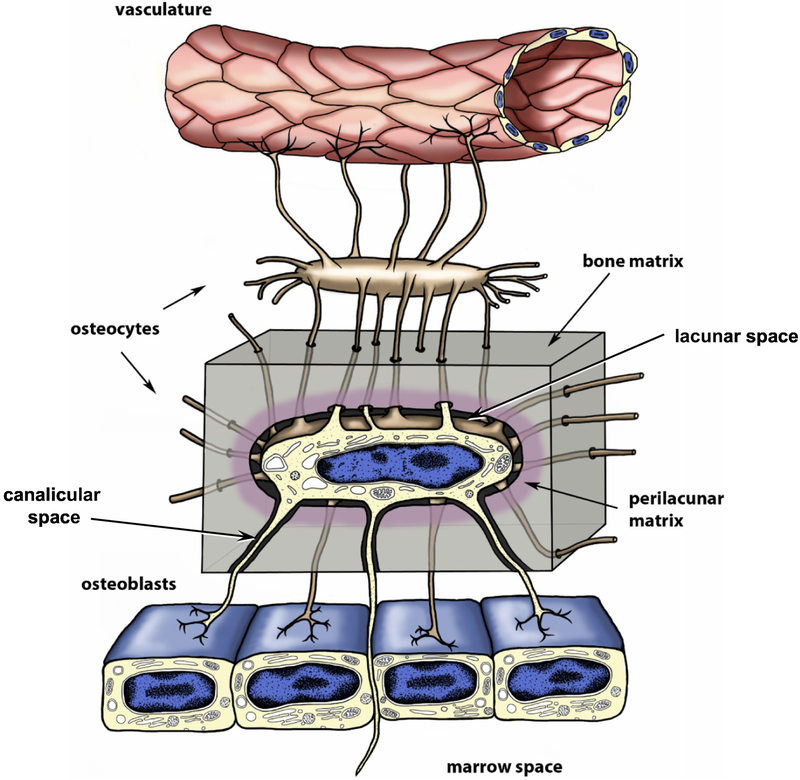
Osteocytes are terminally differentiated osteoblasts and comprise over 90% of all bone cells. They have a highly dendritic morphology and are located in a unique environment, embedded within the mineralized bone matrix, where they can be viable for decades (reviewed in [6–9]). The osteocyte cell bodies are housed within a network of lacunae that are interconnected by numerous canaliculi, through which the osteocyte dendritic processes run (see figure 1). Together, the osteocyte lacunae and canaliculi comprise the lacunocanalicular system. This system allows for the flow of canalicular fluid in the lacunocanalicular space that carries nutrients and signaling factors to and from the osteocytes via the circulation. It also allows signaling between osteocytes and is connected to the marrow space. The intimate connection of the lacunocanalicular system with the vasculature has been elegantly demonstrated by dye injection studies, which have shown permeation of the canalicular space with dye only minutes after injection into the circulation [34]. These types of studies have shown that dye molecules <40kDa can rapidly reach the lacunocanalicular space from the bloodstream but that molecules >70kDa take much longer and molecules as large as 440kDa do not enter [35, 36]. As discussed later in this review, the flow of canalicular fluid around osteocytes during mechanical loading of bone may provide an important stimulus to the osteocyte and/or its dendrites to mediate mechanotransduction (reviewed in [35, 37–40].
Figure 1: Schematic Representation of Osteocytes and Lacunocanalicular System.
Schematic representation of an embedded osteocyte located within its lacuna, illustrating its dendritic processes passing through the bone matrix (grey shading) within narrow tunnels termed canaliculi. The osteocyte’s dendritic processes interconnect with other osteocytes and surface osteoblasts. Note that some osteocyte processes may extend beyond the osteoblast layer to potentially interact with cells in the marrow and that osteocyte dendrites are also in intimate contact with the vasculature. Modified and reproduced from Dallas et al 2013 [7] with permission.
Approaches for Visualizing the Osteocyte Network and Lacunocanalicular System
Because they are embedded within the mineralized bone matrix, osteocytes are not readily accessible and our understanding of their role in bone remodeling and age-related bone loss is incomplete. Imaging of osteocytes in bone in situ is challenging due to the need to develop techniques for sectioning and imaging of undecalcified specimens or techniques for decalcifying samples to facilitate use of conventional sectioning and imaging methods. Advanced imaging techniques are starting to provide new insight into the structure and function of osteocytes and combining these with in vivo and transgenic mouse models to integrate structural information with quantitative biochemical data has the potential to provide a more in depth understanding of osteocyte function and the effects of aging. Here, we provide a brief review of some of the available methods for imaging osteocytes and their lacunocanalicular system, but the reader is referred to other recent articles for a more in depth discussion of osteocyte imaging methods [40–42].
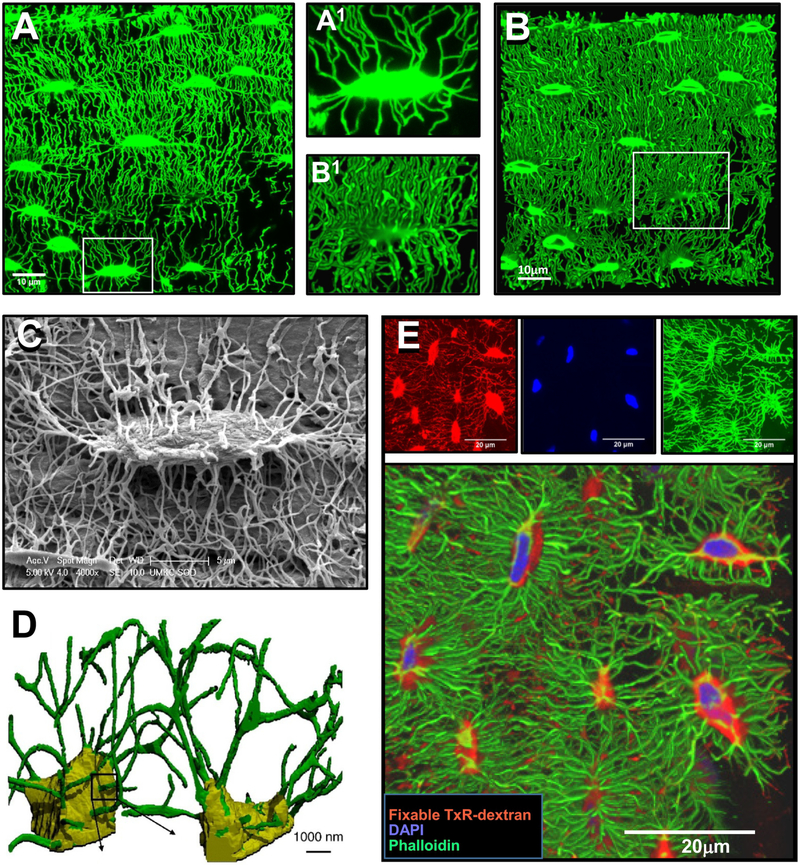
The majority of studies that have examined changes in osteocyte networks with aging have used methods that are imaging the lacunocanalicular network rather than directly imaging the osteocytes themselves. Changes in the lacunocanalicular network have then been used to infer age-related changes in osteocytes and in their dendrite connectivity. The lacunocanalicular system has been imaged using light microscopy based methods such as basic fuchsin, procion red or FITC staining of undecalcified bone sections, which can be combined with widefield epifluorescence microscopy or confocal microscopy [43–45]. Standard light microscopy/histomorphometric methods of quantitation have the limitation that they are inherently two-dimensional. In contrast, confocal microscopy has the advantage that optical sectioning can be used to acquire images at multiple focal planes through the specimen thickness, which can then be stacked together to generate 3D rendered images of the lacunocanalicular networks [46–48] (reviewed in[41]) (see figure 2A, B). A disadvantage is that the focal plane depth is limited to 100–150 μm, unless combined with clearing methods such as clarity imaging or clearing with TDE (2,2’-thiodiethanol) mounting buffer [47, 49, 50] together with use of long working distance lenses. Also, there may be image artifacts, such as attenuation of signal with increasing focal plane depth or aberrations caused by refractive index mismatch. Additionally, while confocal imaging can distinguish canaliculi and can be reliably used for quantifying canalicular number, it is less accurate for volumetric measurements of canaliculi due to inadequate resolution for these small diameter structures. Another widely used method for imaging the lacunocanalicular network is acid-etching of resin embedded bone (which provides a relief cast of the lacunocanalicular system). This is combined with scanning electron microscopy to provide high resolution images of the lacunocanalicular system [51–53] (see figure 2C).
Figure 2: Methods for Imaging Osteocytes and the Lacunocanalicular System.
A) Confocal image of FITC stained, undecalcified mouse femur, showing the lacunocanalicular system (maximal Z-projection, 40 planes with 0.126 μm Z plane separation, 100x oil objective, 1.7x digital zoom). The image in A1 shows an enlarged image of the boxed area in A), bar = 10μm. B) 3D rendered view of the lacunocanalicular system shown in A) (250 planes, 0.126 μm Z plane separation), bar = 10μm. The image in B1 shows an enlarged image of the boxed area in B). C) Acid-etch resin embedded SEM image of an osteocyte lacuna and its canaliculi, bar = 5μm. D) 3D representation of osteocyte lacunocanalicular networks imaged using serial FIB/SEM with the lacunae indicated in yellow and the canaliculi indicated in green, bar = 1μm. E) Multiplexed confocal imaging of osteocytes and their lacunocanalicular system in mouse femur using combined staining with phalloidin, DAPI and fixable Dextran. Top row shows maximal Z-projected confocal images of fixable Texas Red-Dextran [red] to show the lacunocanalicular network, DAPI [blue], to show the nuclei and alexa fluor 488-phalloidin [green], to show the actin cytoskeleton of the osteocytes (50 planes with 0.13μm Z plane separation, 100x oil lens, 1x digital zoom). Lower panel shows a 3D rendered view of the same field (242 planes 0.13μm Z plane separation). Note that the labeling of the lacunae with fixable dextran (red) extends out beyond the limits of the osteocyte cell body and that the dendrites can be seen to traverse across the lacunar space, bar = 20μm. Panel D) is reprinted from Schneider et al. 2011 [42] and panel E) is modified and reprinted from Kamel-ElSayed et al. 2015 [47] with permission.
The application of high resolution micro-computed tomography (CT) methods and synchrotron radiation-based CT to bone imaging has now made it possible to image osteocyte lacunae in bone samples non-invasively to generate 3D images and quantify lacunar volumes and morphology [54–57]. These methods do not usually resolve the canaliculi, however, Pacureanu et al. developed an enhanced protocol for synchrotron based CT imaging that improved the spatial resolution so that the larger canaliculi could also be imaged in human femoral samples [58]. An advantage of the CT-based methods is that they allow analysis of larger sample sizes of osteocyte lacunae (thousands as opposed to hundreds) compared to microscopy based methods.
The above methods are all techniques for imaging the lacunocanalicular system rather than the osteocytes themselves. For imaging osteocytes directly, standard histological or immunostaining methods can be used. Additionally, Bodian stain, which is known as a stain for neuronal cells, can be used to image osteocyte networks [59]. Standard transmission electron microscopy (TEM) and scanning electron microscopy (SEM) can be used for examining osteocyte ultrastructure and techniques such as serial block face sectioning and serial focused ion beam scanning electron microscopy (FIB/SEM) can be used to add 3D capabilities to EM-based imaging of osteocytes and their lacunae/canaliculi [42, 60] (reviewed in [41]) (see figure 2D). However, these methods can only sample a relatively small number of osteocytes, typically no more than 10 to 20. A widely used approach for confocal imaging of osteocytes developed by Kamioka and colleagues uses phalloidin staining of the actin cytoskeleton [61, 62]. Our group has adapted these approaches to develop multiplexed confocal imaging methods for imaging osteocytes combined with using fixable tracer dyes that permeate the lacunocanalicular system [47]. These approaches have allowed us to simultaneously image several aspects of the osteocyte, such as its cell membrane, nucleus and cytoskeleton, together with the lacunocanalicular fluid space in three dimensions (see figure 2E). This has enabled us to examine the effects of aging on the osteocytes themselves as well as their lacunocanalicular networks in aged male and female mice and obtain volumetric data on both the osteocytes and their lacunae in the same specimens [48]. All of the above approaches have been used in various combinations for studies from several laboratories examining the effects of aging on the osteocyte and lacunocanalicular networks, as discussed in the following sections.
Changes in Osteocytes and the Lacunocanalicular System with Aging
The changes to bone mass and geometry that occur with aging have been well documented in human patient studies and are paralleled by similar changes in aged animal models. In aging men and women, bone mineral density, cortical bone thickness and trabecular bone volume decrease with increasing age and there is a dramatic increase in cortical bone porosity, which is more pronounced in post-menopausal women and leads to increased fragility [3, 63, 64]. In animal models, analogous reductions in trabecular bone volume, cortical bone thickness and bone strength occur in association with aging, with females affected more severely than males [65–67]. Mouse aging models also show expanded cortical diameter and increased cortical porosity, analogous to the porosities seen in humans, which appears to be more prominent in female mice than males [48, 68]. Accompanying these changes in bone volume and bone geometry are changes in the osteocyte lacunocanalicular system. For the purposes of this review, we have subdivided these into age-related changes to the lacunae, to the osteocytes themselves and to canaliculi/dendrite connectivity, although clearly these are all interrelated.
Age Related Lacunar Changes –
Changes in osteocyte lacunar density with aging have been studied using a variety of the imaging methodologies described above. In a 2010 study by Busse et al., lacunar density of femurs from human organ donors with ages ranging from in the teens to the nineties was measured by histomorphometry. Their study showed that the osteocyte lacunar density decreased linearly in both males and females for periosteal and endosteal regions of the bone, with the effect more pronounced in the endosteal compartment [69]. These findings have been confirmed by other studies in women and men, which have reported significant reductions of between 15 and 30% in osteocyte lacunar density in aged compared to young bone [70–73]. A recent study by Ashique et al. [46] using FITC staining in conjunction with confocal imaging in femoral samples from young (20–23 yr) and aged (70–86 yr) females similarly reported an approximately 20% decline in lacunar number per bone area with aging. In contrast, a later synchrotron-based micro CT study from the same group using human femurs from adults of ages 20–86 reported no significant relationship between age and lacunar density [74], although these authors did find a downward trend in lacunar density with aging. They proposed that the discrepancy between this study and prior studies may be because the synchrotron-based method allows the analysis of a much higher number of lacunae (thousands as opposed to hundreds) in the patient samples and therefore may provide a more accurate sampling. Additionally, the variation between individuals was high. Interestingly, in addition to measuring lacunar density, the synchrotron-based technique can provide volumetric information, and the same study found that there was a significant reduction in lacunar volumes in the femurs of aged women, such that the lacunae were approximately 30% smaller and were less flattened (i.e. more spherical) compared to those in the young specimens.
Rodent models seem to recapitulate the decrease in lacunar density with advanced aging that is seen in humans. Heveran et al. compared male C57BL/6 mice between the ages of 6 and 24 months using confocal-based imaging combined with automated 3D volumetric analysis [75].Their data confirmed decreased lacunar density and surface area in aged mice and an increase in spacing between lacunae. Their study also showed similar volumetric changes, including reduced lacunar size and a change to a less flattened and more spherical lacunar morphology. In contrast, Hemmatian et al., using micro computed tomography in the fibulae of 5 and 23 month old female mice, showed no significant difference in lacunar density overall, and actually found a statistically significant increase with age in the middle region of the fibula, but not the posterior or anterior regions [57]. This suggests that the variation of lacunar density with age may be dependent on the specific bone and on the location within the bone, and likely varies between bones within the same animal. The study by Hemmatian et al. also confirmed age-related volumetric changes in the lacunae, including reduced lacunar size and a more spherical morphology.
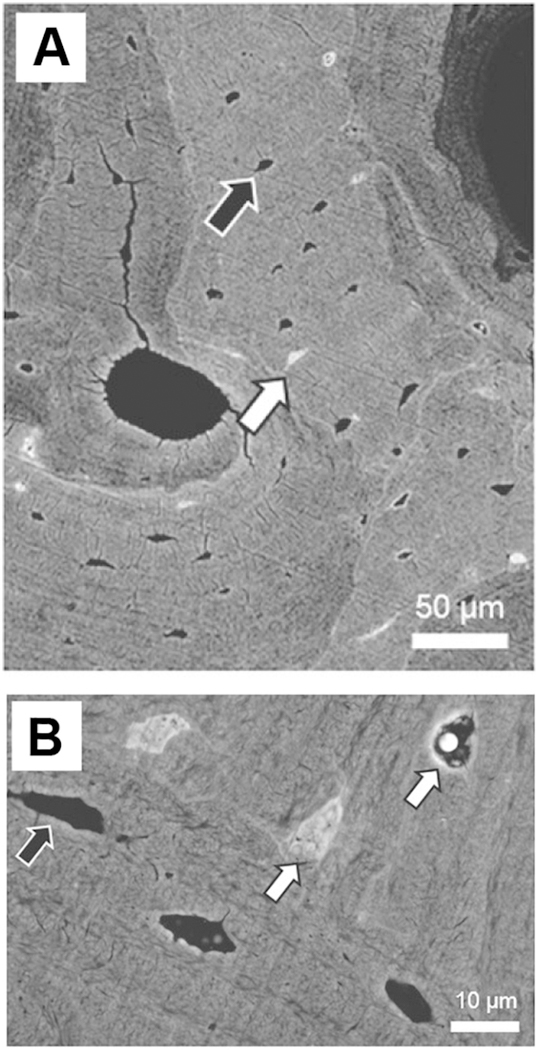
Micropetrosis –
An interesting feature that occurs in association with decreased numbers of lacunae in aged human bone is “micropetrosis”, originally identified by Frost in 1960 from basic fuchsin stained ground sections of human bone [76]. This is a process by which a lacuna that has become empty due to the death of an osteocyte fills in with mineral [69, 76–78] (see figure 3). These micropetrotic lacunae are more readily identified from electron microscopy images as they are hypermineralized, making them more electron dense, and therefore distinct from surrounding bone. Busse et al. [69] showed that decreased lacunar density was accompanied by an increase in the number of hypermineralized (micropetrotic) lacunae in aging bone in men and women, which they attributed to a failure of normal bone remodeling with age. In a 2012 study, Carpentier et al. reported that hypermineralized lacunae were increased in osteoporotic samples, supporting the idea that micropetrosis might be an important component of dysfunctional bone remodeling in osteoporosis [77]. A later study by Milovanovic et al. [78] showed that hypermineralized lacunae increased with age in human femurs. They further showed that mineralization of the osteocyte lacunae and their canaliculi occurs via a mineralization process that is distinct from normal bone formation and involves accumulation and fusion of nanospherites in the absence of a collagen matrix. The filling in of lacunae with mineral may result in impaired canalicular fluid flow and the hypermineralization could potentially increase the brittleness of the bone. It has also been suggested that impaired canalicular fluid flow may impede detection of microdamage, which is important in initiating remodeling responses to repair damaged bone [69]. Micropetrosis appears to be a phenomenon specific to human aged bone and does not seem to occur in rodent models [69, 76]. One potential explanation is that this process may take years to complete and therefore is not seen in rodent systems due to their shorter lifespan, however, this remains to be determined.
Figure 3: Micropetrosis of Osteocyte Lacunae in Aged Mice.
A) Backscattered SEM image of aged human osteoporotic bone showing normal osteocyte lacunae (black arrow) and mineralized micropetrotic lacunae (white arrow), bar = 50μm. B) Higher power backscattered SEM image showing normal (black arrow) and mineralized (white arrow) lacunae, bar = 10μm. The micropetrotic lacunae are hypermineralized compared to the surrounding matrix and therefore appear lighter than the surrounding matrix. Panels A) and B) are reproduced from figures 3 and 1, respectively, from Milovanovic et al, 2017 [78] with permission.
Age Related Changes to Osteocytes –
Measurements of changes in lacunar properties have been informative and useful as a surrogate for examining the osteocytes directly. However, these lacunar measurements may not always accurately reflect the changes to the cells themselves, as lacunae can be unoccupied and the osteocyte may not occupy the full volume of its lacuna. In a microscopy study of iliac crest biopsies from 94 women ages 20–73 years old, Qiu et al. showed decreased osteocyte density and lacunar density with age [43], with the density of osteocytes being dependent on the distance from the bone surface. In their study, the lacunar number was consistently higher than osteocyte number, reflecting the presence of empty lacunae and the percentage of empty lacunae was also found to increase with age.
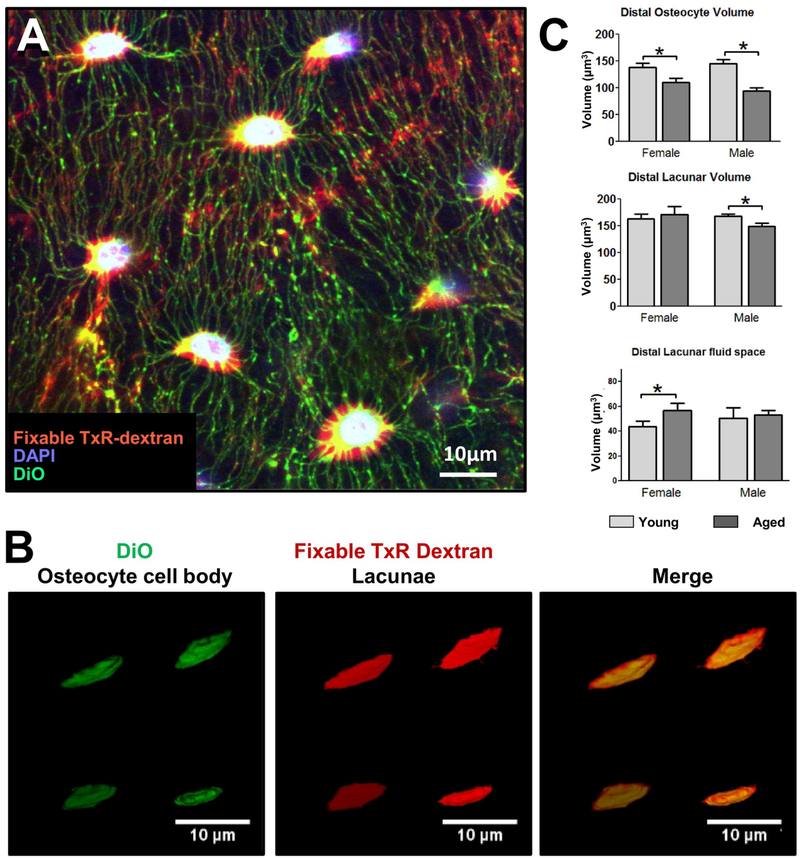
Our own work in aged male and female C57BL/6 mice showed a 20–30% decline in osteocyte density in the femurs of 22 month old mice compared to 5 month old animals [48]. Histomorphometric data further showed that even in relatively young mice at 5 months of age there is a small percentage of lacunae (<2%) that are not occupied by an osteocyte. This number increases significantly to about 3% in the females at 22 months but did not significantly increase in males. Piemontese et al. reported similar increases in empty lacunae in both endosteal and periosteal bone in mice between 7 and 21 months [68]. Another study in mice reported percentages of empty lacunae as high as ~22–23% in young and ~32–34% in aged mice [79] but, based our experience, this may be due to shrinkage of osteocytes during processing for paraffin embedding leading to overestimation and we believe the lower values obtained from plastic sectioning to be more reliable. In our study, multiplexed confocal imaging was also used, combined with fixable tracer dyes that allowed us to simultaneously obtain 3D images of both the osteocytes and their lacunae in young and aged mice [48], which was previously only possible by TEM. Volumetric measurements on the osteocyte cell bodies and their corresponding lacunae revealed gender differences (see figure 4). Both males and females showed an approximately 15–20% reduction in cell body volume, but only males showed a corresponding reduction in lacunar volume. These data imply that the female osteocytes “shrink” inside their lacunae by reducing their cell body but not lacunar volume, thereby increasing their lacunocanalicular fluid volume. In contrast, the male osteocytes showed reductions in both their cell body and lacunar size without significantly altering the lacunar fluid volume. These sex dependent differences in lacunar fluid volumes could result in differences in mechanosensitivity, as the lacunar and canalicular fluid flow shear stresses may be different in males and females. Such effects could be missed in analyses that only consider the lacunar volumes.
Figure 4: Multiplexed Imaging and Volumetric Quantitation of Osteocyte Cell body and Lacunae in Young and Aged Mice.
A) Multiplexed confocal imaging of the osteocyte cell body and lacunae in mouse femur using combined staining with fixable Texas Red-Dextran [red] to show the lacunocanalicular network, DAPI [blue], to show the nuclei and DiO [green], to show the cell membrane (single Z plane, 40x oil objective, 4x digital zoom), bar = 10μm. B) Examples of 3D renderings of osteocyte cell bodies labeled with DiO and lacunae labeled with fixable Tx-Red-Dextran for volumetric calculations, with merged image shown on the right, bar = 10μm. C) quantitation of osteocyte cell volume in the distal region of the femur in young and aged mice with quantitation of the corresponding lacunar volume and lacunar fluid space volume (Data are mean ± SEM, * = p< 0.05, ANOVA/Tukey’s; n= 5). Panels B) and C) are reproduced from Tiede-Lewis et al, 2017 [48].
Overall, while there is still some degree of controversy amongst studies from different laboratories, the preponderance of data in human and animal studies seem to support an age-related decline in lacunar density and osteocyte density of approximately 20–30%. It is also noteworthy that Rolvien et al. [80] showed an age related decrease in lacunar density as well as evidence of micropetrosis even in the auditory ossicles, making it seem likely that decreased lacunar density is a ubiquitous hallmark of bone aging in humans. The majority of studies also support that there is a reduction in osteocyte cell volume that may be combined with reduced lacunar size and a change with aging to a less flattened, more spherical morphology.
Age Related Changes to Canaliculi and Dendrites –
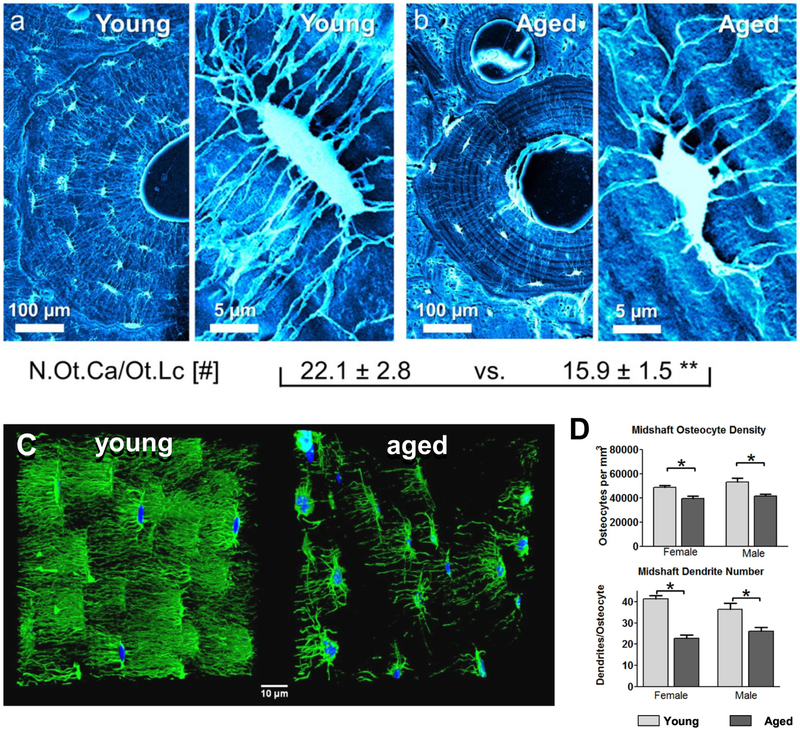
In addition to the age-related decline in the number of lacunae and osteocytes per bone volume, several studies have shown a dramatic loss of osteocyte dendricity with aging that may further compromise the connectivity of osteocyte networks (see figure 5). Using acid-etching/SEM of resin embedded bone Milovanovic et al. showed an approximately 30% decrease in canalicular number per osteocyte in the femurs of aged (70–95 yr) compared to young (20–40 yr) women [51] (Figure 5A, B). The canaliculi also had fewer branches and frequently did not cross cement lines, in contrast to samples from younger individuals. A similar reduction in osteocyte dendrite number was found using histomorphometry combined with a silver staining based method in young (3 month) compared to aged (24 month) mouse femurs [81]. Recent studies from our laboratory in aging male and female C57BL/6 mice using multiplexed confocal imaging examined both the osteocyte dendrites and their corresponding canaliculi in the same specimens [48]. A 27% reduction in the number of dendrites per osteocyte was observed in 22 month old male femurs compared to 5 month old animals and a 45% reduction was observed in females (figure 5C, D). The study further showed that in males, the percent reduction in canalicular number was comparable to the reduction in dendrites, but females showed only a 26% reduction in canalicular number, which was much less severe than the reduction in dendrites[48]. This suggests that measuring changes in canalicular number in females may considerably underestimate the actual loss of dendrite connectivity between osteocytes. Moreover, in terms of absolute numbers of canaliculi and dendrites per osteocyte, we showed that the canaliculi outnumbered primary dendrites by ~1.2–1.4 fold in young mice and by ~1.5–1.7 fold in aged mice, indicating that many canaliculi are not occupied by a dendrite and that canalicular occupancy decreases with age. Timecourse studies in the female mice revealed a linear decline in dendrite number per osteocyte between 5, 12, 18 and 22 months of age and showed a negative linear correlation between age and dendrite number. Interestingly, osteocyte dendrite number correlated positively with cortical thickness and negatively with cortical bone perimeter, indicating that maintenance of osteocyte connectivity may be important in preventing bone loss on the endosteal surface and limiting bone expansion on the periosteal surface. In addition to reduced osteocyte density and decreased dendrite number, we observed that the aged bones had more interfaces (cement lines from bone remodeling), across which the dendrites did not appear to extend. Similar to the porosities seen in aged humans, we observed increased cortical porosity, particularly in females, due to intracortical remodeling [48]. This further reduced osteocyte connectivity by creating “islands” of osteocytes that were disconnected from other osteocytes.
Figure 5: Degeneration of the Osteocyte and Lacunocanalicular Network with Aging –
Scanning electron micrographs of resin embedded acid-etched osteonal bone from A) young and B) aged human bone samples. The right panel in each image pair shows a magnified view of a single osteocyte lacuna. Note that the young osteon is larger, with more osteocyte lacunae that are interconnected extensively via numerous canaliculi. The magnified images show that more canaliculi radiate from the osteocyte lacuna in the young case than the aged case. The numbers below show quantitation of the number of osteocyte canaliculi per lacuna in young (n=9) vs. aged (n=11) bone samples (** p< 0.001). C) Confocal 3D rendered images of osteocyte networks stained with alexa-488 phalloidin [green] and DAPI [blue] in young (5 month old) compared to aged (22 month old) female C57BL/6 mice. Note the dramatic loss of osteocyte connectivity. D) Quantitation of osteocyte number and dendrite number from phalloidin/DAPI stained sections in the femurs of young and aged male and female C57BL/6 mice (Data are mean ± SEM, * = p< 0.05, ANOVA/Tukey’s) (males n=6, females n=5). Panels A) and B) are reproduced from Milovanovic et al. 2103 [51] with permission, panel C) is a still frame from a movie published in Tiede-Lewis et al. 2017 [48] and panel D) is reproduced from Tiede-Lewis et al. 2017 [48].
Overall, there appears to be good agreement between the above studies that aging is associated with a decline in canalicular number of around 30% in males and females in humans and in rodent models. The mouse studies suggest that this is accompanied by a decline in dendrite number that may even be more severe than the loss of canaliculi, particularly in females.
Implications of the Age Related Changes in the Lacunocanalicular System and Decline in Osteocyte Connectivity
Following on from the above discussions, if aging is associated with an approximately 20–30% decrease in lacunar or osteocyte density and this is combined with a ~30% decline in canalicular or dendrite number per osteocyte, one can infer that the overall loss in connectivity of the entire osteocyte network may be as high as ~50%, which could have profound implications for skeletal health. Since osteocyte dendrites, as opposed to the cell body, are thought to be critical for mechanotransduction [82–84], reduced dendrite connectivity may be partly responsible for the attenuated bone anabolic response to mechanical loading with aging [29, 30, 32]. Theoretically, this could be due to both a reduced number of dendrites available to sense and integrate information from strain inputs, as well as impaired fluid flow through canalicular networks that are poorly connected to each other, with groups of osteocytes isolated from each other. Fewer dendritic connections between osteocytes could also affect the osteocyte signal propagation that occurs in response to loading [85], further attenuating load-related bone anabolic responses.
Changes in the lacunae may also affect mechanotransduction. Nicolella et al. showed that changes in lacunar morphology altered the local strains experienced by osteocytes [86] and micropetrotic lacunae may provide a barrier to lacunar fluid flow between osteocytes. Furthermore, changes in lacunar fluid volumes due to shrinkage of the osteocyte lacunae and/or cell bodies could lead to alterations in fluid flow shear stress that could impact mechanotransduction. Interestingly, in a mouse model, osteocytes have been shown to alter their lacunar size, shape and even alignment in response to hindlimb unloading [87], raising the intriguing possibility that reduced physical activity and associated reduced mechanical loading in the aged population could provide a stimulus for lacunar remodeling. Although the implications of the age related changes in osteocyte lacunar morphology are still not fully understood, it is noteworthy that other disease models also exhibit differences in lacunar volume and morphology, such as diabetes mellitus [88] and osteogenesis imperfecta [89], further suggesting that lacunar morphology as well as number may play an important role in bone health. An increase in lacunar size has also been shown in lactating mice, due to osteocyte mediated perilacunar resorption [90]. This has been shown to result in a 13% reduction in the elastic modulus of the bone tissue, which returned to normal after lactation, suggesting that osteocyte mediated perilacunar remodeling can change the mechanical properties of the bone [91].
Ma and colleagues have reported that osteocyte density is correlated with the biomechanical quality of bone [92] and studies in human bone also suggest that a dense osteocyte network is correlated with better bone material quality [93]. Studies by Vashishth et al. have linked reduced osteocyte viability and density with accumulation of microdamage and microcracks [73], suggesting that a healthy osteocyte network may be important in initiating remodeling responses and/or resisting damage in order to maintain healthy and mechanically robust bone. However, it remains to be clarified whether the osteocyte cell death occurs in response to microdamage or whether the microdamage occurs after loss of osteocyte viability, or both. Either way, the accumulation of microdamage and/or lack of repair in areas of aged bone with reduced osteocyte viability may compromise the mechanical integrity of the tissue, resulting in increased bone fragility.
Reduced numbers of viable osteocytes may also have effects on bone remodeling, as osteocytes regulate osteoblast activity through production of sclerostin, a natural inhibitor of Wnt/β-catenin signaling, and regulate osteoclast activity through production of RANKL. Piemontese et al. have shown that osteocyte cell death is associated with increased expression of RANKL and they proposed that this may be a trigger for intracortical remodeling [68]. In support of this, Cabahug-Zuckerman et al. showed that osteocyte apoptosis in response to hindlimb unloading in mice is required for induction of RANKL expression in osteocytes and stimulation of bone resorption[28]. Evidence is therefore accumulating that loss of osteocyte viability may disrupt the bone remodeling balance in favor of bone resorption.
Mechanisms Responsible for Age Related Changes in the Lacunocanalicular Network and in Osteocyte Connectivity
The above discussions have provided an overview of the changes in the osteocyte lacunocanalicular system that occur with aging and their implications for bone homeostasis, however, the mechanisms underlying these age-related changes are still not fully determined. Timecourse studies from our laboratory have suggested that the decline in osteocyte dendrite number in aged female mouse femurs precedes the decrease in osteocyte number [48]. Therefore, loss of dendrite connectivity could be a trigger for subsequent loss of cell viability. This is analogous to neurodegeneration, whereby dendrite and/or axonal pruning is thought to precede loss of cell viability [94, 95]. Decreased osteocyte connectivity through a combination of cell death and loss of dendrites could then lead to a further downward spiral in osteocyte cell viability, as essential nutrients and survival factors are obtained by osteocytes though their canalicular fluid and signaling factors are transferred through cell-cell contact via the dendrites. As more and more osteocytes are lost and their connectivity is further decreased, this may lead to increased stress on the remaining osteocytes and further loss of viability.
Osteocyte apoptosis can be induced by many different stimuli, including microdamage [96, 97], disuse [98], excess glucocorticoids [99–101], estrogen deficiency [102–105] and oxidative stress [81, 104], any of which could potentially contribute to the age-related decline in osteocyte number. Oxidative stress, which occurs due to overproduction of reactive oxygen species (ROS) and resultant induction of mitochondrial DNA damage, is known as an important mechanism of aging in many cell systems [106–108] and accumulating evidence supports a role for oxidative stress in bone aging [81, 104]. Almeida et al. showed an age related increase in the levels of ROS in the bone marrow of C57BL/6 mice between 4 and 31 months of age [104]. This was accompanied by a decrease in glutathione reductase, an enzyme that protects against oxidative stress. They also found increased phosphorylation of p53 and p66shc, suggesting activation of signaling pathways associated with ROS-induced apoptosis. These changes were associated with increased apoptosis of osteoblasts and osteocytes, suggesting that oxidative stress could be an important trigger for osteocyte degeneration with aging. Interestingly, loss of estrogens in females or androgens in males via gonadectomy mimicked these changes in oxidative stress pathways [104]. This indicates that oxidative stress may mediate the effects of sex steroid deficiency on osteocyte viability and suggests that estrogen deficiency in postmenopausal women may further exacerbate the increase in oxidative stress that occurs with normal aging.
Kobayashi et al. showed a similar increase in superoxide, a reactive oxygen species, in mice at 24 months of age compared to 2 months. They also performed an osteocyte targeted deletion of mitochondrial superoxide dismutase (Sod2) and showed that these mice had elevated levels of superoxide together with a low bone mass phenotype resembling accelerated aging [81]. Loss of Sod2 also resulted in degeneration of the lacunocanalicular network, decreased osteocyte numbers and reduced osteocyte connectivity. The bone loss was due to increased bone resorption and decreased bone formation and was associated with increased osteocyte expression of RANKL and sclerostin. Overall, the data make a compelling case for an important role for oxidative stress in age related bone loss and potentially in degeneration of the osteocyte network.
Autophagy is another pathway that may play a role in skeletal aging. This is a “self-devouring” (autophagocytotic) pathway that is activated as an adaptive response to stress to maintain cell viability and function by degrading and recycling damaged organelles and macromolecules (reviewed in [109]). This pathway serves to promote the health and viability of long lived cells and it therefore makes sense that the pathway would be relevant in osteocytes. A recent study by Onal et al. has shown that mice lacking Atg7 in osteocytes (a key protein in the autophagy pathway) showed a bone phenotype as early as 6 months that mimics the changes that occur with aging [110]. This included decreased trabecular bone volume and cortical thickness as well as increased cortical porosity. Suppression of autophagy by deletion of Atg7 in the osteoblast lineage also reduced osteocyte dendricity [111], suggesting a potential role for autophagy in maintenance of osteocyte viability and connectivity.
A recent paper by Farr et al. [112] has highlighted the possibility that cellular senescence may play a role in age-related bone loss. This is a process activated in response to excessive extracellular or intracellular stress, whereby the cell remains metabolically active but is locked into cell cycle arrest and stops dividing, preventing the propagation of DNA damage to future cell generations (reviewed in [113]). Senescent cells undergo characteristic phenotypic changes, including upregulation of senescence marker genes, acquisition of a senescence associated secretory phenotype (SASP) as well as telomere shortening. This is thought to be beneficial in healthy individuals as a protective mechanism against malignant transformation. However, senescent cells accumulate in many tissues with aging and the factors they secrete as part of the senescence associated secretory phenotype promote inflammation, tissue degradation and possibly cancer. The work of Farr and colleagues showed that expression of senescence associated markers was increased in bone and marrow cell populations from aged male and female mice, including osteoblasts and more prominently in osteocytes [112]. Osteocytes also showed unravelling of their pericentromeric satellite DNA, another hallmark of senescence. A related study by Piemontese et al. showed that the loss of cortical bone and increased bone porosity in aging mice was associated with higher levels of DNA damage and elevated expression of senescence associated markers in osteocytes [68]. The targeting of senescent cells and factors associated with the SASP has become a promising approach for preventing various age-related diseases and Farr and colleagues went on to show that inhibiting senescent cells in mice using genetic approaches or targeting senescence using “senolytic” drugs, protected the mice from age-related bone loss [114].
The oxidative stress, autophagy and cellular senescence pathways may not be mutually exclusive and are likely interconnected. For example, oxidative stress can cause DNA damage, which could in turn activate cellular senescence pathways and autophagy pathways could be simultaneously activated in an attempt to maintain cell survival. Future studies are needed to further understand these complex pathways, but the exciting discoveries that have been made so far suggest that one or all of these pathways could be targeted as treatments for maintaining osteocyte viability and as a potential treatment for osteoporosis.
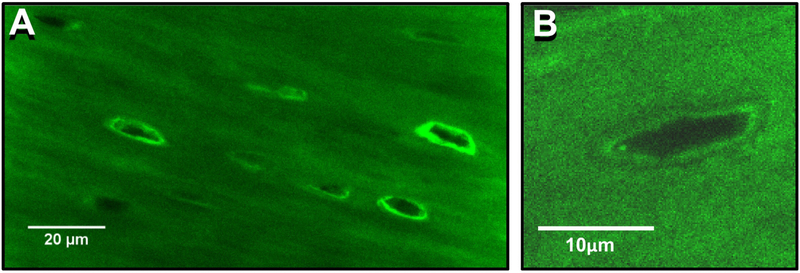
The mechanisms responsible for the age-related changes in lacunar morphology are not yet defined, but several studies have shown that osteocytes are capable of remodeling their perilacunar matrix [90, 115]. These studies have shown that during lactation, osteocytes express genes normally associated with osteoclastic resorption, such as tartrate resistant acid phosphatase, cathepsin K, Vacuolar-type ATPase dependent proton pumps and MMP-13 [90, 115]. Furthermore, Jahn et al. recently showed that osteocytes can acidify their microenvironment [116]. Interestingly, in the study by Qing et al., lacunar size was increased in lactating female mice, but was restored post-lactation, suggesting that not only can osteocytes remove mineral from their lacunae, but they can lay down new bone as well [90]. Using transgenic mice expressing a GFP-tagged collagen construct, we have found that many osteocytes are surrounded by bright rings of perilacunar GFP-collagen fluorescence, which appears to represent cycles of addition and removal of collagen by osteocytes in their perilacunar matrix [47] (see figure 6). Thus, osteocytes appear to be capable of expressing all the requisite genes to dissolve mineral and remodel their perilacunar matrix, as well as to lay down new bone matrix, suggesting that this may be the mechanism by which lacunar morphology is changed with aging.
Figure 6: Evidence of Osteocyte Perilacunar Matrix Formation.
A) Confocal image from femoral cortical bone from a transgenic mouse expressing a GFP-tagged collagen construct. Note that there are numerous osteocyte lacunae surrounded by bright rings of GFP-collagen fluorescence, suggesting that the osteocytes may be able to add collagen to their perilacunar matrix, bar = 20μm (single Z-plane, 100x oil objective), bar = 20μm. B) Enlarged image of a single osteocyte lacuna from a GFP-collagen mouse showing interfaces around the lacuna that suggest GFP-collagen addition and removal, bar = 10μm.
Are There Parallels between Loss of Osteocyte Connectivity and Neurodegeneration?
Osteocytes are not the only cells that lose their dendrites as a function of aging. In neuronal cells, dendrite pruning is a hallmark of aging (for review see[117]). Loss of dendricity has been linked with impaired nervous system function in disorders such as Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis (ALS) and ischemic brain injury [94, 118]. Interestingly, osteocytes show many similarities with neuronal cells. They are both terminally differentiated cells that are highly dendritic and form complex interconnected networks. A recent theoretical article by Buenzli et al. has highlighted the similarities between osteocyte and neuronal networks by mathematically estimating their orders of magnitude in the human body [119]. Their calculations suggest that the lacunocanalicular system of the human skeleton has a total volume of ~35.8 cm3 with an estimated surface area of ~215 m2. They calculate that the lacunocanalicular system houses an estimated 42 billion osteocytes, which is in the same order of magnitude as the ~86 billion neurons in the brain [120]. The cumulative length of osteocyte dendritic processes in bone was estimated at 175,000km, which falls in a similar range as the total length of nerve fibers, 150,000–180,000km, in a typical human brain [121]. They further estimate a total of 23 trillion osteocyte dendritic connections in the skeleton as compared to approximately 150 trillion neural cortex synapses [121]. These theoretical calculations show that osteocyte networks are comparable to neural systems in scale and connectivity, but osteocytes show many other similarities with neuronal cells, including staining with Bodian stain, a histological stain for nerve fibers that is specific for neurofilament proteins [59]. Additionally, osteocytes exhibit calcium oscillations and calcium spikes [27, 122] and they express a number of neuronal related genes, including neuropeptide Y, cholinergic receptors, the glutamate aspartate transporter (GLAST), and the receptor associated protein of the synapse Rapsn [123–125]. Because of the similarities between osteocyte and neuronal networks, it is intriguing to speculate that there may be common molecular mechanisms underlying osteocyte degeneration and neurodegeneration. Neurotrophic factors are known to act to maintain dendrites by interacting with receptors and preventing the pruning back of axons and dendrites. When these neurotrophic factors decrease, as with aging, dendrites and/or axons die back and after a critical loss occurs, neuronal apoptosis tends to follow (reviewed in [95, 126]). The cell death and elimination of axons and dendrites is a normal developmentally regulated process that is important for the proper wiring of the nervous system and axonal degeneration also occurs in response to injury. It has been proposed that neurodegeneration in aging and in various “dying back” neurodegenerative conditions could represent a misregulation of this normal process. Unpublished studies in our laboratory using a neurotrophin and receptor targeted qPCR array have shown that an osteocyte-like cell line, OmGFP66, expresses several genes associated with neurogenesis and neurite formation, including, nerve growth factor (Ngf) and its receptor (Ngfr), brain derived neurotrophic factor (Bdnf), glial cell line derived neurotrophic factor (Gdnf), ciliary neurotrophic factor (Cntf), fibroblast growth factor 9 (Fgf9), Artemin (Artn) and Neurotrophin 5 (Ntf5), among others, and it will be interesting to determine whether expression of these changes with aging and/or whether these neurogenesis related genes play a role in osteocyte dendrite formation and maintenance. It will also be interesting to determine whether damage to osteocyte dendrites via bone microdamage may lead to injury related responses similar to those that occur in damaged neurons.
Some of the same mechanisms discussed above in relation to osteocyte apoptosis have been shown to play a role in neuronal disorders. For example, mitochondrial health plays a key role in neuronal health and oxidative stress is thought to play a role in the development of Parkinson’s disease and Alzheimer’s disease [107, 127]. Furthermore, mutations in superoxide dismutase 1 are responsible for some forms of ALS [128]. The resulting mitochondrial dysfunction may induce neural degeneration by causing a cellular energy crisis. Dysregulated autophagy has been linked to synaptic pruning defects [129] and neurite and axonal degeneration [130]. Additionally, Rapamycin, which has been shown to induce neuronal autophagy [131] and protect against neuronal death in Parkinson’s disease models [132], has been shown to protect against bone loss in aged rats by increasing autophagy and decreasing apoptosis in osteocytes [133]. Zonisamide, a drug used in treating epilepsy and Parkinson’s disease, was shown to promote neurite elongation in primary motor neurons and increase axon size in sciatic nerve autograft in mice and was protective against oxidative stress [134]. Similar approaches could be investigated as a potential means of preserving osteocyte connectivity and improving bone health and future cross disciplinary research between bone biologists and neurobiologists could therefore prove to be a productive avenue of research.
Summary and Perspective
It is clear that aging is associated with loss of bone mass, intracortical remodeling and increased bone fragility and accumulating evidence suggests that the age related degeneration of the osteocyte network and its lacunocanalicular system may play a role in this deterioration of bone quality. If, as we suspect, loss of dendricity provides a trigger for loss of osteocyte viability, this raises the question as to whether therapeutic interventions directed towards maintaining dendritic connections may improve osteocyte viability and skeletal health. Also, is the loss of osteocyte dendrites an irreversible process or can dendrites can be regenerated? Since not all canaliculi appear to be occupied, it is tempting to speculate that new dendrites could be extended into unoccupied canaliculi. Some studies also hint at the possibility for dendrite regeneration as osteocytes are capable of remodeling their perilacunar matrix [90, 116]. Furthermore, Holmbeck et al. have reported that osteocyte dendrite formation is an active, invasive process involving collagen degradation by the osteocytes and their data suggest that more dendrites are formed as bone matures [59]. Future research is clearly needed to identify novel approaches for maintaining a healthy osteocyte network. Exciting areas of research include drugs targeting pathways such as oxidative stress and autophagy as well as senolytic drugs that target cellular senescence. It will be important to determine which of these pathways triggers the loss of dendricity that appears to precede osteocyte cell death.
Another important question is whether exercise intervention could have beneficial effects on osteocyte networks, since exercise is known to reduce the risk of mortality from all causes [135] and has positive effects on many systems in the body, including the neural, metabolic, and musculoskeletal systems. An exciting recent study by Kitase et al. has shown that β-aminoisobutyric acid (L-BAIBA), a factor secreted by exercising muscles, has protective effects to maintain osteocyte viability under conditions of oxidative stress by preventing mitochondrial fragmentation and depolarization [136]. Their work also showed that BAIBA protected mice against bone loss induced by hindlimb suspension. This identifies a naturally produced muscle-derived factor that has positive effects on the skeleton, and supports the idea that there is a beneficial molecular crosstalk between muscle and bone. These observations imply that exercise may have beneficial effects in maintaining a healthy osteocyte network. Support from this comes from our own recent studies using voluntary wheel running in 12 month old female C57BL/6 mice [137]. Our data showed that 6 months of voluntary wheel running (harvest age 18 months) prevented the loss of osteocyte dendrite connectivity that occurred in non exercised mice. However, this effect was not maintained in the older 10 month exercised group (harvest age 22 months), which may be due to a tailing off in the amount of daily voluntary exercise in the older animals. Optimization of the wheel running protocols, initiation at an earlier age and/or the inclusion of more resistance may be useful approaches to enhance the effects of wheel running.
Clearly, it is an exciting time in age related research and our understanding of age related changes in the skeleton is advancing rapidly. Combining innovative methods for imaging of osteocytes and the lacunocanalicular system with mouse genetics and studies in aged humans and animal models should lead to an advanced understanding of how the health of osteocyte networks regulates bone health and quality. This may lead to new innovations in treatments for age related bone loss.
Acknowledgements
The work was supported by NIH grants P01AG039355, R21AR054449, R21AR062346 and R01AR051517. We acknowledge use of the UMKC Confocal Microscopy Core supported by NIH grants S10RR027668 and S10OD021665, the UMKC Office of Research Services and UMKC Center of Excellence in Dental and Musculoskeletal Tissues. We thank Anita Xie (UMKC Dept. Oral and Craniofacial Biology) for providing the acid-etched electron microscopy image in figure 2C and Loretta Laughrey and Ganesh Thiagarajan (UMKC Dept. Computing and Engineering) for collaboration in generating the multiplexed confocal image in figure 4A.
Footnotes
DECLARATIONS OF INTEREST: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harvey ND E and Cooper C (2013). The Epidemiology of Osteoporotic Fractures. In: Rosen CJ, ed. Primer on the Metabolic Diseases and Disorders of Bone: John Wiley & Sons, Inc.), pp. 348–56. [Google Scholar]
- 2.Tarantino U, Iolascon G, Cianferotti L, Masi L, Marcucci G, Giusti F, Marini F, Parri S, Feola M, Rao C, Piccirilli E, Zanetti EB, Cittadini N, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017; 18: 3–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black DM, Rosen CJ. Clinical Practice. Postmenopausal Osteoporosis. N Engl J Med. 2016; 374: 254–62. [DOI] [PubMed] [Google Scholar]
- 4.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006; 17: 1726–33. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992; 2: 285–9. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Senda T, Kubo KY. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med Mol Morphol. 2015; 48: 61–8. [DOI] [PubMed] [Google Scholar]
- 7.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013; 34: 658–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jilka RL, O’Brien CA. The Role of Osteocytes in Age-Related Bone Loss. Curr Osteoporos Rep. 2016; 14: 16–25. [DOI] [PubMed] [Google Scholar]
- 9.Prideaux M, Findlay DM, Atkins GJ. Osteocytes: The master cells in bone remodelling. Curr Opin Pharmacol. 2016; 28: 24–30. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 2005; 280: 19883–7. [DOI] [PubMed] [Google Scholar]
- 11.Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S, Krumlauf R. Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res 2006; 21: 1738–49. [DOI] [PubMed] [Google Scholar]
- 12.Padhi D, Allison M, Kivitz AJ, Gutierrez MJ, Stouch B, Wang C, Jang G. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 2014; 54: 168–78. [DOI] [PubMed] [Google Scholar]
- 13.Recker RR, Benson CT, Matsumoto T, Bolognese MA, Robins DA, Alam J, Chiang AY, Hu L, Krege JH, Sowa H, Mitlak BH, Myers SL. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res 2015; 30: 216–24. [DOI] [PubMed] [Google Scholar]
- 14.McColm J, Hu L, Womack T, Tang CC, Chiang AY. Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res. 2014; 29: 935–43. [DOI] [PubMed] [Google Scholar]
- 15.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014; 370: 412–20. [DOI] [PubMed] [Google Scholar]
- 16.Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med 2017; 377: 1417–27. [DOI] [PubMed] [Google Scholar]
- 17.Lewiecki EM, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD, Miyauchi A, Maddox J, Chen L, Horlait S. A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men With Osteoporosis. J Clin Endocrinol Metab 2018; 103: 3183–93. [DOI] [PubMed] [Google Scholar]
- 18.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med 2011; 17: 1235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res 2002; 17: 2068–79. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 2011; 17: 1231–4. [DOI] [PubMed] [Google Scholar]
- 21.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007; 5: 464–75. [DOI] [PubMed] [Google Scholar]
- 22.Burra S, Nicolella DP, Jiang JX. Dark horse in osteocyte biology: Glycocalyx around the dendrites is critical for osteocyte mechanosensing. Commun Integr Biol 2011; 4: 48–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012; 50: 209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javaheri B, Stern AR, Lara N, Dallas M, Zhao H, Liu Y, Bonewald LF, Johnson ML. Deletion of a single beta-catenin allele in osteocytes abolishes the bone anabolic response to loading. J Bone Miner Res. 2014; 29: 705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J. Mechanical loading prevents the stimulating effect of IL-1beta on osteocyte-modulated osteoclastogenesis. Biochem Biophys Res Commun. 2012; 420: 11–6. [DOI] [PubMed] [Google Scholar]
- 26.Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of beta-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: Implications for the study of mechanosensation in bone. Bone. 2010; 47: 872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jing D, Baik AD, Lu XL, Zhou B, Lai X, Wang L, Luo E, Guo XE. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB J. 2014; 28: 1582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cabahug-Zuckerman P, Frikha-Benayed D, Majeska RJ, Tuthill A, Yakar S, Judex S, Schaffler MB. Osteocyte Apoptosis Caused by Hindlimb Unloading is Required to Trigger Osteocyte RANKL Production and Subsequent Resorption of Cortical and Trabecular Bone in Mice Femurs. J Bone Miner Res. 2016; 31: 1356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holguin N, Brodt MD, Silva MJ. Activation of Wnt Signaling by Mechanical Loading Is Impaired in the Bone of Old Mice. J Bone Miner Res. 2016; 31: 2215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res. 1995; 10: 1544–9. [DOI] [PubMed] [Google Scholar]
- 31.Sterck JG, Klein-Nulend J, Lips P, Burger EH. Response of normal and osteoporotic human bone cells to mechanical stress in vitro. Am J Physiol. 1998; 274: E1113–20. [DOI] [PubMed] [Google Scholar]
- 32.Kiel DP, Hannan MT, Barton BA, Bouxsein ML, Sisson E, Lang T, Allaire B, Dewkett D, Carroll D, Magaziner J, Shane E, Leary ET, Zimmerman S, et al. Low-Magnitude Mechanical Stimulation to Improve Bone Density in Persons of Advanced Age: A Randomized, Placebo-Controlled Trial. J Bone Miner Res. 2015; 30: 1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006; 21: 1464–74. [DOI] [PubMed] [Google Scholar]
- 34.Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998; 22: 107–17. [DOI] [PubMed] [Google Scholar]
- 35.Fritton SP, Weinbaum S. Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annu Rev Fluid Mech. 2009; 41: 347–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Ciani C, Doty SB, Fritton SP. Delineating bone’s interstitial fluid pathway in vivo. Bone. 2004; 34: 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonewald LF. Mechanosensation and Transduction in Osteocytes. Bonekey Osteovision. 2006; 3: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008; 42: 606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinbaum S, Duan y, Thia MM, You L. An Integrative Review of Mechanotransduction in Endothelial, Epithelial (Renal) and Dendritic Cells (Osteocytes). Cellular and Molecular Bioengineering. 2011; 4: 510–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemmatian H, Bakker AD, Klein-Nulend J, van Lenthe GH. Aging, Osteocytes, and Mechanotransduction. Curr Osteoporos Rep. 2017; 15: 401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webster DJ, Schneider P, Dallas SL, Muller R. Studying osteocytes within their environment. Bone. 2013; 54: 285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider P, Meier M, Wepf R, Muller R. Serial FIB/SEM imaging for quantitative 3D assessment of the osteocyte lacuno-canalicular network. Bone. 2011; 49: 304–11. [DOI] [PubMed] [Google Scholar]
- 43.Qiu S, Rao DS, Palnitkar S, Parfitt AM. Relationships between osteocyte density and bone formation rate in human cancellous bone. Bone. 2002; 31: 709–11. [DOI] [PubMed] [Google Scholar]
- 44.Ciani C, Doty SB, Fritton SP. An effective histological staining process to visualize bone interstitial fluid space using confocal microscopy. Bone. 2009; 44: 1015–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006; 38: 1310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashique AM, Hart LS, Thomas CDL, Clement JG, Pivonka P, Carter Y, Mousseau DD, Cooper DML. Lacunar-canalicular network in femoral cortical bone is reduced in aged women and is predominantly due to a loss of canalicular porosity. Bone Rep. 2017; 7: 916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamel-ElSayed SA, Tiede-Lewis LM, Lu Y, Veno PA, Dallas SL. Novel approaches for two and three dimensional multiplexed imaging of osteocytes. Bone. 2015; 76: 129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiede-Lewis LM, Xie Y, Hulbert MA, Campos R, Dallas MR, Dusevich V, Bonewald LF, Dallas SL. Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging (Albany NY). 2017; 9: 2190–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coutu DL, Kokkaliaris KD, Kunz L, Schroeder T. Multicolor quantitative confocal imaging cytometry. Nat Methods. 2018; 15: 39–46. [DOI] [PubMed] [Google Scholar]
- 50.Greenbaum A, Chan KY, Dobreva T, Brown D, Balani DH, Boyce R, Kronenberg HM, McBride HJ, Gradinaru V. Bone CLARITY: Clearing, imaging, and computational analysis of osteoprogenitors within intact bone marrow. Sci Transl Med. 2017; 9. [DOI] [PubMed] [Google Scholar]
- 51.Milovanovic P, Zimmermann EA, Hahn M, Djonic D, Puschel K, Djuric M, Amling M, Busse B. Osteocytic canalicular networks: morphological implications for altered mechanosensitivity. ACS Nano. 2013; 7: 7542–51. [DOI] [PubMed] [Google Scholar]
- 52.Okada S, Yoshida S, Ashrafi SH, Schraufnagel DE. The canalicular structure of compact bone in the rat at different ages. Microsc Microanal. 2002; 8: 104–15. [DOI] [PubMed] [Google Scholar]
- 53.Kubek DJ, Gattone VH, Allen MR. Methodological assessment of acid-etching for visualizing the osteocyte lacunar-canalicular networks using scanning electron microscopy. Microscopy Research and Technique. 2010; 73: 182–6. [DOI] [PubMed] [Google Scholar]
- 54.Peyrin F, Salome M, Cloetens P, Laval-Jeantet AM, Ritman E, Rüegsegger P. Micro-CT examinations of trabecular bone samples at different resolutions: 14, 7 and 2 micron level. Technology and Health Care. 1998; 6: 391–401. [PubMed] [Google Scholar]
- 55.Schneider P, Stauber M, Voide R, Stampanoni M, Donahue LR, Müller R. Ultrastructural properties in cortical bone vary greatly in two inbred strains of mice as assessed by synchrotron light based micro- and nano-CT. J Bone Miner Res. 2007; 22: 1557–70. [DOI] [PubMed] [Google Scholar]
- 56.van Hove RP, Nolte PA, Vatsa A, Semeins CM, Salmon PL, Smit TH, Klein-Nulend J. Osteocyte morphology in human tibiae of different bone pathologies with different bone mineral density - Is there a role for mechanosensing? Bone. 2009; 45: 321–9. [DOI] [PubMed] [Google Scholar]
- 57.Hemmatian H, Laurent MR, Bakker AD, Vanderschueren D, Klein-Nulend J, van Lenthe GH. Age-related changes in female mouse cortical bone microporosity. Bone. 2018; 113: 1–8. [DOI] [PubMed] [Google Scholar]
- 58.Pacureanu A, Langer M, Boller E, Tafforeau P, Peyrin F. Nanoscale imaging of the bone cell network with synchrotron X-ray tomography: optimization of acquisition setup. Medical Physics. 2012; 39: 2229–38. [DOI] [PubMed] [Google Scholar]
- 59.Holmbeck K, Bianco P, Pidoux I, Inoue S, Billinghurst RC, Wu W, Chrysovergis K, Yamada S, Birkedal-Hansen H, Poole AR. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 2005; 118: 147–56. [DOI] [PubMed] [Google Scholar]
- 60.Kamioka H, Murshid SA, Ishihara Y, Kajimura N, Hasegawa T, Ando R, Sugawara Y, Yamashiro T, Takaoka A, Takano-Yamamoto T. A method for observing silver-stained osteocytes in situ in 3-micrometer sections using ultra-high voltage electron microscopy tomography. Microscopy and Microanalysis. 2009; 15: 377–83. [DOI] [PubMed] [Google Scholar]
- 61.Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001; 28: 145–9. [DOI] [PubMed] [Google Scholar]
- 62.Sugawara Y, Kamioka H, Honjo T, Tezuka K, Takano-Yamamoto T. Three-dimensional reconstruction of chick calvarial osteocytes and their cell processes using confocal microscopy. Bone. 2005; 36: 877–83. [DOI] [PubMed] [Google Scholar]
- 63.Seeman E Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci. 2013; 68: 1218–25. [DOI] [PubMed] [Google Scholar]
- 64.Bilezikian JP, Kurland ES, Rosen CJ. Male Skeletal Health and Osteoporosis. Trends Endocrinol Metab. 1999; 10: 244–50. [DOI] [PubMed] [Google Scholar]
- 65.Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res. 2002; 17: 1044–50. [DOI] [PubMed] [Google Scholar]
- 66.Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell. 2010; 9: 851–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007; 22: 1197207. [DOI] [PubMed] [Google Scholar]
- 68.Piemontese M, Almeida M, Robling AG, Kim HN, Xiong J, Thostenson JD, Weinstein RS, Manolagas SC, O’Brien CA, Jilka RL. Old age causes de novo intracortical bone remodeling and porosity in mice. JCI Insight. 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Busse B, Djonic D, Milovanovic P, Hahn M, Puschel K, Ritchie RO, Djuric M, Amling M. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010; 9: 1065–75. [DOI] [PubMed] [Google Scholar]
- 70.Mori S, Harruff R, Ambrosius W, Burr DB. Trabecular bone volume and microdamage accumulation in the femoral heads of women with and without femoral neck fractures. Bone. 1997; 21: 521–6. [DOI] [PubMed] [Google Scholar]
- 71.Mullender MG, van der Meer DD, Huiskes R, Lips P. Osteocyte density changes in aging and osteoporosis. Bone. 1996; 18: 109–13. [DOI] [PubMed] [Google Scholar]
- 72.Qiu S, Rao DS, Palnitkar S, Parfitt AM. Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone. 2002; 31: 313–8. [DOI] [PubMed] [Google Scholar]
- 73.Vashishth D, Verborgt O, Divine G, Schaffler MB, Fyhrie DP. Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone. 2000; 26: 375–80. [DOI] [PubMed] [Google Scholar]
- 74.Carter Y, Thomas CDL, Clement JG, Cooper DML. Femoral osteocyte lacunar density, volume and morphology in women across the lifespan. J Struct Biol. 2013; 183: 519–26. [DOI] [PubMed] [Google Scholar]
- 75.Heveran CM, Rauff A, King KB, Carpenter RD, Ferguson VL. A new open-source tool for measuring 3D osteocyte lacunar geometries from confocal laser scanning microscopy reveals age-related changes to lacunar size and shape in cortical mouse bone. Bone. 2018; 110: 115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frost HM. Micropetrosis. J Bone Joint Surg Am. 1960; 42-A: 144–50. [PubMed] [Google Scholar]
- 77.Carpentier VT, Wong J, Yeap Y, Gan C, Sutton-Smith P, Badiei A, Fazzalari NL, Kuliwaba JS. Increased proportion of hypermineralized osteocyte lacunae in osteoporotic and osteoarthritic human trabecular bone: implications for bone remodeling. Bone. 2012; 50: 688–94. [DOI] [PubMed] [Google Scholar]
- 78.Milovanovic P, Zimmermann EA, Vom Scheidt A, Hoffmann B, Sarau G, Yorgan T, Schweizer M, Amling M, Christiansen S, Busse B. The Formation of Calcified Nanospherites during Micropetrosis Represents a Unique Mineralization Mechanism in Aged Human Bone. Small. 2017; 13. [DOI] [PubMed] [Google Scholar]
- 79.Meakin LB, Galea GL, Sugiyama T, Lanyon LE, Price JS. Age-related impairment of bones’ adaptive response to loading in mice is associated with sex-related deficiencies in osteoblasts but no change in osteocytes. J Bone Miner Res. 2014; 29: 1859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rolvien T, Schmidt FN, Milovanovic P, Jahn K, Riedel C, Butscheidt S, Puschel K, Jeschke A, Amling M, Busse B. Early bone tissue aging in human auditory ossicles is accompanied by excessive hypermineralization, osteocyte death and micropetrosis. Sci Rep. 2018; 8: 1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobayashi K, Nojiri H, Saita Y, Morikawa D, Ozawa Y, Watanabe K, Koike M, Asou Y, Shirasawa T, Yokote K, Kaneko K, Shimizu T. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Sci Rep. 2015; 5: 9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole K, Jiang JX. Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc Natl Acad Sci U S A 2010; 107: 13648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci U S A 2004; 101: 16689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thi MM, Suadicani SO, Schaffler MB, Weinbaum S, Spray DC. Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require alphaVbeta3 integrin. Proc Natl Acad Sci U S A 2013; 110: 21012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lara-Castillo N, Kim-Weroha NA, Kamel MA, Javaheri B, Ellies DL, Krumlauf RE, Thiagarajan G, Johnson ML. In vivo mechanical loading rapidly activates beta-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone. 2015; 76: 5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006; 39: 1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sugawara Y, Kamioka H, Ishihara Y, Fujisawa N, Kawanabe N, Yamashiro T. The early mouse 3D osteocyte network in the presence and absence of mechanical loading. Bone. 2013; 52: 189–96. [DOI] [PubMed] [Google Scholar]
- 88.Lai X, Price C, Modla S, Thompson WR, Caplan J, Kirn-Safran CB, Wang L. The dependences of osteocyte network on bone compartment, age, and disease. Bone Res. 2015; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carriero A, Doube M, Vogt M, Busse B, Zustin J, Levchuk A, Schneider P, Muller R, Shefelbine SJ. Altered lacunar and vascular porosity in osteogenesis imperfecta mouse bone as revealed by synchrotron tomography contributes to bone fragility. Bone. 2014; 61: 116–24. [DOI] [PubMed] [Google Scholar]
- 90.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, Wysolmerski J, Bonewald LF. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012; 27: 1018–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaya S, Basta-Pljakic J, Seref-Ferlengez Z, Majeska RJ, Cardoso L, Bromage TG, Zhang Q, Flach CR, Mendelsohn R, Yakar S, Fritton SP, Schaffler MB. Lactation-Induced Changes in the Volume of Osteocyte Lacunar-Canalicular Space Alter Mechanical Properties in Cortical Bone Tissue. J Bone Miner Res. 2017; 32: 688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma YL, Dai RC, Sheng ZF, Jin Y, Zhang YH, Fang LN, Fan HJ, Liao EY. Quantitative associations between osteocyte density and biomechanics, microcrack and microstructure in OVX rats vertebral trabeculae. J Biomech. 2008; 41: 1324–32. [DOI] [PubMed] [Google Scholar]
- 93.Kerschnitzki M, Kollmannsberger P, Burghammer M, Duda GN, Weinkamer R, Wagermaier W, Fratzl P. Architecture of the osteocyte network correlates with bone material quality. J Bone Miner Res. 2013; 28: 1837–45. [DOI] [PubMed] [Google Scholar]
- 94.Koleske AJ. Molecular mechanisms of dendrite stability. Nat Rev Neurosci. 2013; 14: 536–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yaron A, Schuldiner O. Common and Divergent Mechanisms in Developmental Neuronal Remodeling and Dying Back Neurodegeneration. Curr Biol. 2016; 26: R628–R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012; 50: 1115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000; 15: 60–7. [DOI] [PubMed] [Google Scholar]
- 98.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006; 21: 605–15. [DOI] [PubMed] [Google Scholar]
- 99.Kogianni G, Mann V, Ebetino F, Nuttall M, Nijweide P, Simpson H, Noble B. Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci. 2004; 75: 2879–95. [DOI] [PubMed] [Google Scholar]
- 100.O’Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004; 145: 1835–41. [DOI] [PubMed] [Google Scholar]
- 101.Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O’Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010; 9: 147–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lirani-Galvao AP, Chavassieux P, Portero-Muzy N, Bergamaschi CT, Silva OL, Carvalho AB, Lazaretti-Castro M, Delmas PD. Low-intensity electrical stimulation counteracts the effects of ovariectomy on bone tissue of rats: effects on bone microarchitecture, viability of osteocytes, and nitric oxide expression. Calcif Tissue Int. 2009; 84: 502–9. [DOI] [PubMed] [Google Scholar]
- 103.Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS. The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res. 1998; 13: 1243–50. [DOI] [PubMed] [Google Scholar]
- 104.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007; 282: 27285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002; 57: 385–409. [DOI] [PubMed] [Google Scholar]
- 106.Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013; 8: 2003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hwang O Role of oxidative stress in Parkinson’s disease. Exp Neurobiol. 2013; 22: 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lenaz G Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta. 1998; 1366: 53–67. [DOI] [PubMed] [Google Scholar]
- 109.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013; 15: 713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Onal M, Piemontese M, Xiong J, Wang Y, Han L, Ye S, Komatsu M, Selig M, Weinstein RS, Zhao H, Jilka RL, Almeida M, Manolagas SC, et al. Suppression of autophagy in osteocytes mimics skeletal aging. J Biol Chem. 2013; 288: 17432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Piemontese M, Onal M, Xiong J, Han L, Thostenson JD, Almeida M, O’Brien CA. Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage. Sci Rep. 2016; 6: 24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL, Bonewald LF, Pignolo RJ, Monroe DG, et al. Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res. 2016; 31: 1920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010; 5: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 2017; 23: 1072–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang SY, Herber RP, Ho SP, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012; 27: 1936–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jahn K, Kelkar S, Zhao H, Xie Y, Tiede-Lewis LM, Dusevich V, Dallas SL, Bonewald LF. Osteocytes Acidify Their Microenvironment in Response to PTHrP In Vitro and in Lactating Mice In Vivo. J Bone Miner Res. 2017; 32: 1761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007; 6: 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McNeill TH, Brown SA, Rafols JA, Shoulson I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res. 1988; 455: 148–52. [DOI] [PubMed] [Google Scholar]
- 119.Buenzli PR, Sims NA. Quantifying the osteocyte network in the human skeleton. Bone. 2015; 75: 144–50. [DOI] [PubMed] [Google Scholar]
- 120.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009; 513: 532–41. [DOI] [PubMed] [Google Scholar]
- 121.Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJ, Nyengaard JR, Regeur L. Aging and the human neocortex. Exp Gerontol. 2003; 38: 95–9. [DOI] [PubMed] [Google Scholar]
- 122.Lu XL, Huo B, Chiang V, Guo XE. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. J Bone Miner Res. 2012; 27: 563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Igwe JC, Jiang X, Paic F, Ma L, Adams DJ, Baldock PA, Pilbeam CC, Kalajzic I. Neuropeptide Y is expressed by osteocytes and can inhibit osteoblastic activity. J Cell Biochem. 2009; 108: 621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, Kuo L, Shin DG, Rowe DW, Harris SE, Kalajzic I. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009; 45: 682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mason DJ, Suva LJ, Genever PG, Patton AJ, Steuckle S, Hillam RA, Skerry TM. Mechanically regulated expression of a neural glutamate transporter in bone: a role for excitatory amino acids as osteotropic agents? Bone. 1997; 20: 199–205. [DOI] [PubMed] [Google Scholar]
- 126.Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002; 296: 868–71. [DOI] [PubMed] [Google Scholar]
- 127.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39: 44–84. [DOI] [PubMed] [Google Scholar]
- 128.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993; 362: 59–62. [DOI] [PubMed] [Google Scholar]
- 129.Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, Yue Z, Arancio O, Peterson BS, et al. Loss of mTORdependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014; 83: 1131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang Y, Coleman M, Zhang L, Zheng X, Yue Z. Autophagy in axonal and dendritic degeneration. Trends Neurosci. 2013; 36: 418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rubinsztein DC, Nixon RA. Rapamycin induces autophagic flux in neurons. Proc Natl Acad Sci U S A. 2010; 107: E181; author reply E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci. 2010; 30: 1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Luo D, Ren H, Li T, Lian K, Lin D. Rapamycin reduces severity of senile osteoporosis by activating osteocyte autophagy. Osteoporos Int. 2016; 27: 1093–101. [DOI] [PubMed] [Google Scholar]
- 134.Yagi H, Ohkawara B, Nakashima H, Ito K, Tsushima M, Ishii H, Noto K, Ohta K, Masuda A, Imagama S, Ishiguro N, Ohno K. Zonisamide Enhances Neurite Elongation of Primary Motor Neurons and Facilitates Peripheral Nerve Regeneration In Vitro and in a Mouse Model. PLoS One. 2015; 10: e0142786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blair SN, Kohl HW 3rd, Barlow CE, Paffenbarger RS Jr., Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995; 273: 1093–8. [PubMed] [Google Scholar]
- 136.Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jahn K, Yi J, Zhou J, Brotto M, Bonewald LF. beta-aminoisobutyric Acid, l-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018; 22: 1531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tiede-Lewis LM, Kitase Y, Tom K, Dallas MR, Zhao H, Xie Y, Wacker M, Brotto M, Bonewald L,F, Dallas SL. Voluntary Wheel Running Exercise Maintains Osteocyte Connectivity and Muscle-Secreted Osteocyte Protective Factors in Aged C57BL/6 Mice. 2018 Annual Meeting of the American Society for Bone and Mineral Research: Journal of Bone and Mineral Research. 2018; 33 (S1): 383 [Google Scholar]