Abstract
MicroRNAs were discovered more than two decades ago and have profound impact on diverse biological processes. Specific microRNAs have important roles in modulating the innate immune response and their dysregulation has been demonstrated to contribute to inflammatory diseases. MiR-223 in particular, is very highly expressed and tightly regulated in hematopoietic cells. It functions as key modulator for the differentiation and activation of myeloid cells. The central role of miR-223 in myeloid cells, especially neutrophil and macrophage differentiation and activation has been studied extensively. MiR-223 contributes to myeloid differentiation by enhancing granulopoiesis while inhibiting macrophage differentiation. Uncontrolled myeloid activation has detrimental consequences in inflammatory disease. MiR-223 serves as a negative feedback mechanism controlling excessive innate immune responses in the maintenance of myeloid cell homeostasis. This review summarizes several topics covering the function of miR-223 in myeloid differentiation, neutrophil and macrophage functions, as well as in inflammatory diseases including acute respiratory distress syndrome and inflammatory bowel disease. In addition, non-myeloid functions of miR-223 are also discussed in this review. Therapeutic enhancement of miR-223 to dampen inflammatory targets is also highlighted as potential treatment to control excessive innate immune responses during mucosal inflammation.
Introduction:
MicroRNAs (miRNAs) are small, single-stranded RNAs that range from 20–25 nucleotides in length. MiRNAs function through binding of target messenger RNA (mRNA) and thereby alter gene expression via posttranscriptional regulation. MiRNAs are involved in almost all aspects of biological processes involving development, cellular differentiation, immune modulation and pathogenic conditions. MiRNAs alter mRNA function via blockade of translation or RNA degradation and it is predicted that approximately 60% of human genes are controlled by miRNAs [1–7]. The specificity of mRNA targeting relies on a sequence of the miRNA, termed the ‘seed’ sequence, that binds complementary to the 3’ untranslated region (3’UTR) of the mRNA [8]. Thus one miRNA is capable of targeting multiple mRNAs and the function of the targeting mRNAs are usually cell and tissue specific. Being a crucial functional element of gene regulation, miRNAs have significant degree of homology between different species. MiRNAs have been shown to be proficient modulators of immune responses being involved in myeloid cell differentiation and function, in adaptive immune response by modulating B and T cells development, and in the crosstalk between immune and resident cells [9, 10]. Recent reports have identified several miRNAs to be important regulators of immune responses. For instance, expression of miR-146a increases after lipopolysaccharide (LPS) exposure in human THP-1 cells (human monocytic cells) in a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) dependent manner [11]. The same study identified IL-1 receptor associated kinase (IRAK1) and TNF receptor associated factor 6 (TRAF6) as pro-inflammatory targets of miR-146a during toll like receptor (TLR) activation [11]. Murine models of LPS instillation results in massive inflammation led by neutrophil and macrophage activation and also resulted in altered expression of miRNAs, including miR-146a, miR-155, miR-21 and miR-223 [12, 13]. MiR-223, in particular, has been shown to be essential for innate immune responses governing myeloid differentiation and granulocyte functions [14, 15]. Recent studies demonstrated abnormal expression of miR-223 in various pathogenic conditions such as type II diabetes, acute lung injury (ALI), rheumatoid arthritis, and inflammatory bowel disease (IBD) [16–19]. This review discusses the established role of miR-223 in myeloid development of differentiation and highlights the recently acquired insights into the function of miR-223 during innate immunity. We also highlight therapeutic options targeting miR-223 for the treatment of mucosal inflammation such as occurs during IBD or ALI. These studies indicate that overexpression of miR-223 via nano-technology based delivery can be used to treat or prevent uncontrolled inflammation of the lungs or the intestine [2, 18, 19].
MiRNA biosynthesis and function
History of discovery
The first miRNA-mRNA interference was discovered in 1993 in C. elegans with a miRNA named lin-4 [20, 21]. Lin-4 was first identified as a heterochronic gene that functions to control the development of C. elegans. However, there is no conventional start and stop codons in lin-4 gene, which led to the conclusion that lin-4 did not translate into/code for protein [20]. In 1993, Ambros and Ruvkun have independently reported the interaction between lin-4 and the 3’ UTR of its target gene lin-14 [20, 21]. After 7 years of silence in miRNA research, the second miRNA, let-7 was identified in C. elegans in 2000 as essential for the development of C. elegans [22]. Following studies discovered that let-7 is conserved in various species and thus sparked researcher’s interest in miRNA in other organisms including the mammalian system [23]. In the meantime, the discovery of RNA interference (RNAi), a process that utilizes 21–23 nucleotide long double stranded RNA to inhibit gene expression post-transcriptionally, supported a potential mechanism of function for miRNA regulation of target mRNA [24]. Altogether, the discovery of functional miRNAs in C. elegans as well as the advanced understanding of RNAi triggered significant progress in the identification of miRNAs and their mechanism of action in the past decades. With the advancement of RNA sequencing and related technology, exceedingly more miRNAs have been identified. In the most recent miRBase release, there are 28645 entries of hairpin precursor miRNAs that express 35828 mature miRNA products, in 223 species including 2588 human mature microRNAs [25].
Biogenesis and maturation
The biogenesis of miRNAs is unique when compared with other protein-encoding genes and tightly controlled by cellular localization and miRNA specific enzymes (Figure 1). Similar to mRNAs, miRNAs are initially transcribed as long primary miRNAs (pri-miRNAs) in the nucleus [26]. These long pri-miRNAs are poly-adenylated at the 3’ end and capped at the 5’ end. Pri-miRNAs are then cleaved by RNaseII endonuclease III Drosha/ DiGeorge syndrome chromosomal region 8 (DGCR8) complex resulting in a 60–110 nt precursor-miRNA (pre-miRNA) [26, 27]. Pre-miRNAs are subsequently transported from the nucleus to the cytoplasm where they are cleaved by another specific RNase III Dicer-1 into double-stranded miRNA duplexes [28]. Pre-miRNA is further processed into a structure of approximately 20nt mature miRNA. Both Drosha/DGCR8 and Dicer are crucial for miRNA biosynthesis. As a result, mice with conditional or global genetic deletion of Drosha or Dicer all show severe and lethal abnormality in their development [29, 30]. Once a miRNA is fully matured, it will associate with and guide the RNA-induced silencing complex (RISC) to the target mRNA. The binding to mRNA targets with sequences complementary to the sequence of the miRNA will function to regulate the translation and degradation of target transcripts [31].
Figure 1. MiRNA biogenesis and modulation of target gene expression via diverse mechanisms.
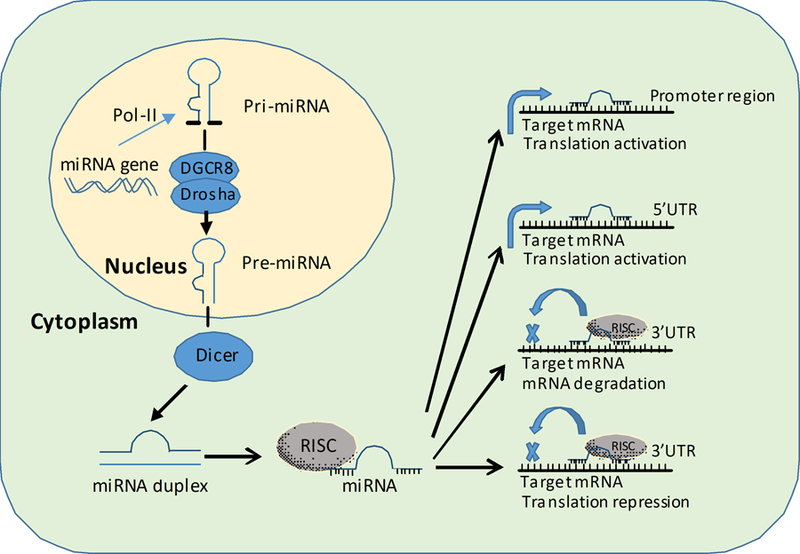
Biogenesis and functions of microRNA. Primary miRNAs (Pri-miRNA) are transcribed in the nucleus by RNA polymerase II (pol II). Pri-miRNAs are cleaved by Drosha complexed with DiGeorge syndrome critical region 8 protein (DGCR 8) to release precursor miRNAs (pre-miRNA). Then pre-miRNAs are transported to the cytosol and further processed by Dicer to reveal a double-stranded miRNA duplex. The mature strand of the miRNA duplex associates with the RNA silencing complex (RISC) to repress target mRNAs via binding to the 3’ untranslated region (3’UTR) region. MiRNAs achieve targeted gene repression via two major mechanisms: inhibition of mRNA translation and degradation of target mRNA. Additionally, recent studies suggest that miRNAs can also bind to 5’UTR region and promoter region of the mRNA target to achieve translation activation. In conclusion, the biogenesis of miRNAs is tightly regulated by specific cellular location and RNase to generate mature RNA. The main function of miRNAs is to modulate protein levels via diverse mechanisms. Adapted from Neudecker et al. MicroRNAs in mucosal inflammation [2]. J Mol Med (Berl).
Function of microRNA via regulation of target genes
The main function of MiRNAs is to repress target genes via inhibition of mRNA translation or degradation of target mRNA (Figure 1). Degradation or destabilization of the target mRNA serves as the dominant mechanisms for the inhibition of protein production while translational inhibition only modestly represses protein production [32]. The most common mRNA site for miRNA binding is the 3’UTR and this mechanism is one of the most accepted for target gene regulation. For instance, the majority of the target prediction databases for miRNAs suggest potential miRNA-mRNA interaction based on the sequence of 3’UTR of mRNAs. However, recent studies suggested that miRNA can also target the 5’UTR site of mRNA to achieve gene repression [33, 34]. Others have also identified binding sites of miRNAs in the coding region of mRNAs, indicating the complexity of mRNA-miRNA interaction [35].
Beside its classic function of gene repression, miRNAs can also serve as transcription and translation activators of target genes (Figure 1). One of the first reports of miRNA mediated induction of target mRNA is from Place and colleagues in 2008, which demonstrated the targeting of miR-373 to the promoter region of E-cadherin and cold-shock domain-containing protein C2 (CSDC2) to induce expression [36]. Another classic example of miRNA activated gene expression is miR-122 mediated activation of hepatitis C (HepC) virus translation, which facilitates the propagation of HepC virus in hepatocytes [37, 38]. In the follow-up study, Jopling and colleagues showed that miR-122 targets the 5’ UTR region of the HepC RNA to facilitate translation activation [39]. Subsequent clinical studies in patients demonstrated that miR-122 can be inhibited in patients to prevent replication of the HepC virus [40]. On the other hand, miR-122 can also target the 3’UTR of CCCTC-binding factor (CTCF) and mediator 1 (MED1) to repress gene expression in hepatoma cells [41]. In conclusion, miRNAs modulate target gene expression via diverse mechanisms.
MiR-223 in innate immunity
MiR-223 is located on the X chromosome governed by an independent promoter not related to other gene products. MiR-223 is preferentially expressed by hematopoietic cells especially the CD34-bone marrow cells but absent in T and B cells [42]. Mice with genetic disruption of miR-223 are viable, fertile and born according to the Mendelian ratio with no gross abnormalities [43]. However, hemizygous miR-223-/y mice showed profound neutrophilia in peripheral blood and 11–12 month old miR-223-/y mice spontaneously develop lung inflammation marked by neutrophil infiltration [43]. There have been no differences between male and female miR-223 deficient mice reported so far. MiR-223-/y mice have been studied in several murine disease models to investigate the impact of miR-223 in innate immunity. In this review we will focus on the function of miR-223 in myeloid differentiation, neutrophil function and macrophage functions. We will also discuss the involvement of miR-223 in non-myeloid cells, showcasing the diverse role of miR-223.
MiR-223 in the regulation of myeloid differentiation
MiR-223 is most highly expressed in the myeloid compartment and, indeed, several studies have demonstrated the important role of miR-223 in myeloid cell differentiation including granulopoiesis and macrophage differentiation. For example, during retinoic acid induced myeloid differentiation, activation of CCAAT-enhancer-binding proteins (C/EBPα) induces the expression of miR-223, which in turn dampens the expression of miR-223 target gene Nuclear factor 1 A-type (NFI-A) [42]. On the other hand, NFI-A inhibits the expression of miR-223 thus suppression of NFI-A by miR-223 comprises a positive feedback loop to maintain the level of miR-223 and leads to granulocyte differentiation [42]. In addition, activation of C/EBPa drives the expression of miR-223 to dampen the expression of E2F1, a master regulator of granulopoiesis and direct target of miR-223 in acute myeloid leukemia (AML) [44]. Subsequently, E2F1 represses miR-223 by binding to the miR-223 promoter in AML blast cells and this negative feedback loop is deregulated in AML resulting in abnormal cell-cycle progression. Due to the interaction between miR-223 with multiple myeloid differentiation transcription factors, such as C/EBPa and NFI-A, one would expect that in the absence of miR-223, granulocyte differentiation would be severely impaired. However, the phenotype of miR-223 deficient mice (miR-223-/y mice) proved otherwise. Johnnidis and colleagues demonstrated that genetic deletion of miR-223 did not impede granulocytes differentiation but caused increased levels of neutrophils due to expanded myeloid progenitor cells [43]. The same study identified myocyte-specific enhancer factor 2C (Mef2c) as a target of miR-223 that promotes expansion of myeloid progenitor cells and disruption of Mef2c in miR-223-/y mice rescued the granulopoietic phenotype [43].
Beside the process of granulopoiesis, the differentiation of monocyte to macrophage is crucial for the activation of innate immune responses and the initiation of adaptive immunity [45]. The expression level of miR-223, alongside with miR-15 and miR-16, decreased significantly during the process of macrophage differentiation induced by inflammatory stimuli such as phorbol 12-myristate 13-acetate (PMA) and LPS [46]. The lowering of those three miRNAs increases the expression of target gene inhibitor of nuclear factor kappa-B kinase subunit alpha (IKKα) to fine-tune the inflammatory responses of macrophages via the alternation of non-canonical NF-κB pathway [46]. As an intriguing example of miR-223’s diverse involvement of granulocyte production and differentiation, a recent study by Vian and colleagues demonstrated upregulation of miR-223 during granulopoiesis and monocytopoiesis in CD34+ HPCs and further described a functional role of miR-223 during myeloid development [47]. Specifically, overexpression of miR-223 in CD34+ HPCs enhances granulopoiesis while concurrently dampening erythroid and macrophage differentiation. On the other hand, inhibition of miR-223 impairs granulopoiesis while enhancing erythroid and macrophage differentiation. In summary, miR-223 is essential and has a diverse functional role in orchestrating myeloid progenitor cell differentiation.
MiR-223 in the regulation of neutrophil function
The function of miR-223 as a negative regulator of neutrophil activation and chemotaxis has been demonstrated in multiple experimental models of inflammatory diseases. Neutrophils are important for the development and modulation of mucosal inflammation [48]. An earlier study by Johnnidis et al described the regulatory effects of miR-233 on neutrophil function. MiR-223-/y mice have more neutrophils in the bone marrow, as well as peripheral tissue including the blood and the lungs, and those neutrophils are hyperactive with increased inflammatory phenotypes such as respiratory burst and fungistatic activity [43]. Furthermore, miR-223 is expressed stably at high levels in neutrophils and over-expression of miR-223 was shown to diminish NLR family pyrin domain containing 3 (NLRP3) inflammasome activity via direct targeting of NLRP3 and in turn dampens NLRP3-dependent IL-1β production [49]. The NLRP3-miR-223 axis was further studied in acute lung injury (ALI) [50]. Patients with acute respiratory distress syndrome (ARDS) caused by trauma and blood transfusion showed high level of miR-223 in the bronchial alveolar lavage fluid (BALF) when compared with health controls. In ALI induced by mitochondrial damage-associated molecular patterns (DAMPs) (MTDs), miR-223−/+ mice experienced decreased arterial oxygen partial pressure to fractional inspired oxygen ratio (PaO2/FiO2), increased lung wet to dry ratio and elevated infiltrating cells to the airway. Ly-6G+ neutrophils were identified as a key mediator of MTD induced ALI and miR-223 limits the number of Ly6G+ neutrophils and inhibits the NLRP3 inflammasome activity. In the context of neuronal injury and inflammation, miR-233 is located in the neutrophils recruited to the area of spinal cord injury in mice that is associated with high levels of inflammatory cytokines [51]. Additionally, miR-223 was found to control lung recruitment of neutrophils during tuberculosis infection by direct targeting of chemoattractant CXCL2, CCL3 and IL-6 in myeloid cells [52]. Indeed, higher miR-223 levels were identified in the blood and lung tissue of tuberculosis (TB) patients and mice infected with TB. MiR-233 deletion in mice resulted in lethal neutrophil-driven inflammation during TB infection. However, this study didn’t observe any differences in the bacterial killing ability of macrophages and neutrophils. The regulatory role of miR-223 in neutrophil activation and function was further explored in hepatic injury models. MiR-223 acts as a negative regulator to neutrophil activation during acetaminophen (APAP) overdose [53]. APAP overdose elicits the level of miR-223 in neutrophils to limit neutrophil activation and prevent further liver injury. Elevated miR-223 level inhibits the expression of its target gene IKKα and subsequently dampens TLR-NFκB inflammatory pathway during APAP overdose induced liver injury. Furthermore, in alcoholic liver injury, miR-223 was shown to be increased in serum from alcoholic patients and in serum and neutrophils from mice with chronic and binge alcohol consumption [54]. Similarly to APAP overdose model, miR-223-/y mice are more susceptible to alcohol induced liver injury with increased hepatic neutrophil infiltration, ROS production and IL-6 expression, suggesting the regulatory role of miR-223 to control neutrophil activity during hepatic inflammation. In summary, miR-223 is highly expressed in neutrophils and plays essential role to limit the activation and function of neutrophil in inflammatory diseases. Thus miR-223-based therapy would be beneficial to limit neutrophil driven inflammation to avoid excess immune responses.
MiR-223 in the regulation of macrophage function
MiR-223 is involved in both the polarization and activation of macrophages. In adipose tissue inflammation, miR-223 was shown to have regulatory effect on myeloid derived cells, especially macrophages [55]. In short, miR-223 promotes the polarization of macrophage towards an alternative M2 anti-inflammatory phenotype via direct targeting of PBX/Knotted 1 Homeobox 1 (Pknox1), thus reducing high-fat diet induced inflammation. In the absence of miR-223, mice exhibited more severe forms of systemic insulin resistance and adipose tissue inflammation. In addition to its function in macrophage polarization, miR-223 also dampens macrophage inflammatory responses to TLR ligand stimulation [56]. LPS and poly (I:C) activation through TLR4 and TLR3 decreased expression of miR-223 in macrophages that was accompanied by increased protein levels of miR-223 target gene signal transducer and activator of transcription 3 (STAT3). This led to the selective increased expression of IL-6 and IL-1β and formed a positive feedback loop to further amplify the inflammatory responses as the decrease in miR-223 was shown to function via IL-6 classical signaling. Thus, IL-6 may promote macrophage inflammatory signaling by both direct increase in STAT3 expression and by indirect inhibition of miR-223, which targets STAT3 mRNA. Another study by Zhang and colleagues also demonstrated the regulatory function of miR-223 in macrophages during TLR stimulation [57]. Consistent with previous studies, TLR ligand stimulation dramatically reduced miR-223 expression in macrophages. The down regulation of miR-223 led to the increase in the Ras homolog gene family, member B (RhoB) expression, which induced the activation of NF-κB and mitogen-activated protein kinases (MAPK) signaling, promoting TNF-α, IL-6 and IL-1β production upon LPS stimulation. Additionally, miR-223 controls macrophage activation via inhibition of inflammasome activity by targeting NLRP3 [49]. In immortalized murine macrophages, inhibition of miR-223 by sponging resulted in increased level of NLRP3 while overexpression of miR-223 decreased the expression level of NLRP3 and inhibited the IL-1b response to LPS stimulation. Furthermore, miR-223 is shown to negatively regulate activation of NF-κB and decrease the expression of IL-1β, IL-6, TNF-α, and IL-12p40 genes in macrophages [58]. In TB infected patients, increased miR-223 levels in monocyte-derived macrophages correlated with impaired activation and cytokine production when compared to healthy individuals. Mechanistically, miR-223 dampened NF-κB activation in U937 cells (human monocytic cells) by negative regulation of p65 phosphorylation, which in turn resulted in reduced production of inflammatory cytokines. A recent study suggested that miR-223 targets the circadian rhythm molecule brain and muscle ARNT-like 1 (BMAL1), which was shown to alter the matrix metalloproteinase (MMP) production in macrophages in response to mycobacteria tuberculosis infection [59]. TB infection in peritoneal macrophages induced expression of MMPs and miR-223 while inhibited the expression of BMAL1. Overexpression of miR-223 in macrophages reduced BMAL1 expression and 3’UTR assay identified direct binding of miR-223 to the 3’UTR of BMAL1. This novel function of miR-223 participating in the regulation of circadian clock molecules in macrophages further adds to the diverse functions of miR-223. To summarize, miR-223 is crucial for the regulation of macrophage polarization and activation during infection and inflammatory diseases by skewing macrophages toward an anti-inflammatory phenotype. Therapeutic manipulation of miR-223 could dampen aberrant macrophage activation in inflammatory diseases.
MiR-223 in adaptive immunity
Although mounting evidence suggests that the expression of miR-223 is most critical to regulation of myeloid-lineage cells, it has also been implicated to have functional roles in lymphoid cells. By utilizing next-generation sequencing, Fehniger and colleagues identified miR-223 in natural killer cells (NK cells) as one of the top genes differentially expressed upon cytokine stimulation [60]. The expression levels of miR-223 significantly decreased following IL-15 stimulation in NK cells, which was confirmed by multiple methods of deep sequencing. Granzyme B (GzmB) was further identified as a direct target of miR-223 in NK cells as overexpression of miR-223 inhibits luciferase activity in cells transfected with granzyme B 3’UTR but not mutated granzyme B 3’UTR. It is suggested that miR-223 controls granzyme B expression in NK cells under resting state, however upon NK cell activation the level of miR-223 decreases and in turn releases the repression of granzyme B to switches on NK cells. Besides the function of miR-223 in NK cells, another study also identified a potential detrimental role of miR-223 in IL-17 mediated inflammation during murine inflammatory bowel diseases [61]. IL-10−/−mice spontaneously develop inflammatory bowel disease and miR-223 was shown to be upregulated in inflamed colon tissue from these mice. In colonic intraepithelial lymphocytes, inhibition of miR-223 increased the expression of Roquin, a predicted target of miR-223. Roquin inhibits IL-17 production in colonic intraepithelial lymphocytes (cIELs), suggesting that miR-223 indirectly increases IL-17 production. Another interesting example of functions in lymphoid cells is the involvement of miR-223 in CD4+ T cells during HIV infection [62]. MiR-223, alongside of miR-28, miR-125b, miR-150 ,and miR-382, was enriched in resting primary CD4+ T cells selected from HIV-infected patients with highly active antiretroviral therapy (HAART) treatment to suppress viral replication. Those resting CD4+ T cells are crucial for HIV latency, a major obstacle for the complete clearance of virus. Those miRNAs were capable to target 3’ end of the HIV genome, subsequently inhibiting the HIV activity in CD4+ T cells. Moreover, miR-223 was also detected in human pulmonary artery smooth muscle cells (HPASMC), normal human bronchial epithelial cells (NHBEC), and human pulmonary artery endothelial cells (HPAEC), in the later especially at higher levels after stimulation by IL-1β and TNFα [63]. The authors identified histone deacetylase 2 (HDAC2) as a direct target of miR-223 in HPAEC, and demonstrated the potential disease promoting function of miR-223 during chronic obstructive pulmonary diseases. These findings together showcase the diverse function of miR-223 in non-myeloid cells.
Functional role of miR-223 in nonhaematologic cells
Besides the function of miR-223 in haematologic cells, increasing evidence shows the potential role of miR-223 in nonhaematologic malignancies [14, 64]. For example, miR-223 is one of the most upregulated miRNAs in recurrent ovarian cancer when compared with primary ovarian cancer [65]. MiR-223 is also shown to be upregulated in gastric cancer and promotes invasion and metastasis driven by Twist, a transcription factor highly expressed in metastatic gastric cancer [66]. Overexpression of miR-223 in gastric cancer cells promotes the invasion while miR-223 antisense oligos inhibited invasion in vitro and in vivo. Further the identification of erythrocyte membrane protein band 4.1 like 3 (EPB41L3) as a tumor suppressive target of miR-223, mechanistically explained the function miR-223 during gastric cancer cell metastasis and invasion. Additionally, the function of miR-223 was explored in prostate cancer [67]. Indeed, upregulation of miR-223 was observed in prostate cancer tissue and cell lines. Overexpression of miR-223 in prostate cancer cell lines promoted proliferation, inhibited apoptosis and enhanced invasion activity. Septin 6 (SEPT6) was further identified as a direct target of miR-223 in prostate cancer cells and overexpression of SEPT6 dampened cell proliferation and invasion. In addition to its involvement in promoting cancer cell invasion and proliferation, miR-223 also shares tumor suppressive functions. In hepatocellular carcinoma (HCC), miR-223 was repressed in cell lines and HCC tumors, when compared with normal livers, and paired adjacent non-tumoral livers [68]. Ectopic expression of miR-223 in Hep3B, HKCI-10, and HKCI-C3 cells steadily suppressed cell viability by direct targeting of tumor promotor Stathmin 1 (STMN1). To summarize, miR-223 not only controls myeloid cell differentiation and activation but also is involved in multiple cells and tissues such as NK cells, T cells and various cancers. Modulating miR-223 therapeutically could potentially alter the function of non-myeloid immune cells to improve disease outcomes. Targeting miR-223 in cancer also has potential to prevent cancer metastasis.
Functional roles of myeloid miR-223 during inflammatory diseases
Shuttling of miR-223 during acute respiratory distress syndrome
Besides the cell intrinsic function of miR-223 in myeloid cells to modulate innate immunity, miR-223 is also contained in microvesicles to perform intercellular communication by regulation of its target genes. During inflammatory diseases as well as cancer, intercellular communication between resident cells and infiltrating immune cells is essential for the regulation and proper activation of immune responses [69]. Recent studies haves implicated the transfer of exosome-derived miRNAs to target cells as a novel mechanism to modulate biological functions. Several studies have identified the important role of miR-223 during intercellular communication. For example, IL-4 activated macrophages can shuttle fluorescent labelled exogenous miRNAs to breast cancer cells in the absence of direct cell-cell contact [70]. MiR-223 was detected in exosomes released by IL-4 activated macrophages and the shuttling of miR-223 from macrophage to breast cancer cells increases cell invasiveness via the Mef2c-b-catenin pathway. The specificity of miR-223 in exosome released from macrophage was demonstrated by miR-223 antisense oligo treatment, which abolished the shuttling promoted invasiveness in breast cancer cells. Additional evidence points out the liver protective function of miR-223 containing exosomes in murine liver injury [71]. Indeed, exosomes containing miR-223 were detected in bone marrow-derived mesenchymal stem cells (BMSCs) and overexpression of miR-223 in BMSCs exosomes conferred protection in murine experimental autoimmune hepatitis. BMSC derived miR-223 containing exosomes also targeted NLRP3 in murine models and in hepatocytes to dampen inflammation in a similar fashion to the regulation network between miR-223 and the NLRP3 inflammasome mentioned in previous sections. In conclusion, the above studies indicate a potential role of miR-223 shuttling to mediate biological functions in cells other than myeloid cells, which opens a new door to extend the function of miR-223 above beyond innate immunity.
Shuttling of miR-223 has also been investigated beyond the scope of cancer and liver inflammation. A recent study found a novel role of miR-223 shuttling to orchestrate the pulmonary immune responses in acute respiratory distress syndrome (ARDS) (Figure 2) [18]. ARDS is a syndrome characterized by acute respiratory failure in the setting of non-cardiogenic pulmonary edema [72]. Among its hallmarks is the initiation of a very robust inflammatory response, a disruption of the alveolar-capillary barrier, pulmonary edema and attenuated gas exchange. ARDS contributes significantly to morbidity and mortality of critical ill patients with a mortality rate as high as 60% [73], and long-term disability and decreased quality of life are dramatic [74]. In the search of potential therapeutic target for ARDS, Neudecker et al. discovered a novel signaling pathway involving the intercellular communication of miR-223 between alveolar epithelial cells and lung-infiltrating polymorphonuclear neutrophils (PMNs) to dampen acute lung injury. Initial studies in alveolar epithelial cells and N-formylmethionine-leucyl-phenylalanine (fMLP)-activated PMNs identified a unique interaction between these two cell types with robust increase in epithelial miR-223 levels following co-culture with activated PMNs. Subsequent studies in ventilation induced lung injury (VILI) also supported the transfer of miR-223 from bone marrow (BM) derived cells to epithelial cells, due to the fact that transfer of miR-223-/y BM to irradiated WT mice abolished miR-223 elevation in ATII cells following acute lung injury. To determine the function of miR-223 during acute lung injury, mice deficient in miR-223 (miR-223-/y mice) were subjected to VILI. After VILI, the mice presented exacerbated clinical and histologic signs of acute lung injury, characterized by increased albumin leakage to the airway and more pulmonary inflammation. The authors characterized the cytokine response in the lung following VILI exposure and discovered elevated IL-6 and CXCL1 levels in the BAL. A similar phenotype was also observed in murine pulmonary bacterial infection models, suggesting a central role of miR-223 during acute lung injury. As discussed in this review, miR-223 targets many genes involved in innate immunity. In the scope of murine ARDS, poly(ADP-ribose) polymerase 1 (PARP-1) was identified as a direct epithelial target of miR-223 and silencing of PARP-1 in vivo dampened VILI induced pulmonary inflammation in miR-223-/y mice. Finally, pre-treatment with intratracheal nanoparticle based delivery of miR-223 dampened VILI and pulmonary inflammation. In conclusion, shuttling of miR-223 between innate immune cells and resident cells forms a new paradigm for the regulation of innate immunity during inflammatory disease. Inhalation of miR-223 mimetics to directly target alveolar epithelial cells could potentially serve as treatment for ARDS to dampen inflammation.
Figure 2. Shuttling of miR-223 in acute respiratory distress syndrome.
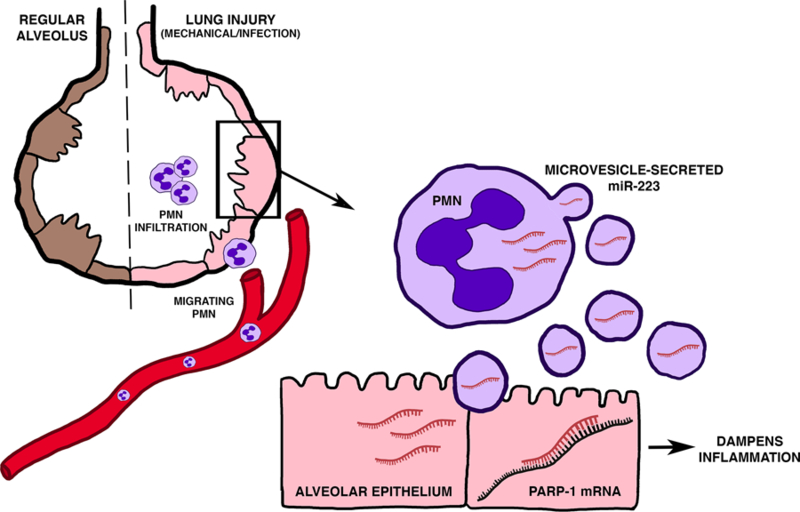
MiR-223 protects tissue inflammation and injury in acute respiratory distress syndrome (ARDS). During mechanical ventilation or infection induced lung injury, neutrophils are recruited from the vasculature to the sites of pulmonary inflammation and tissue injury. Although highly expressed by myeloid cells, miR-223 is secreted in microvesicles from neutrophils upon pulmonary injury and then shuttled to alveolar type II epithelial cells (ATII) to confer lung protection. Once shuttled into ATII cells, miR-223 inhibits expression of poly(ADP-ribose) polymerase 1 (PARP-1) by directly targeting the 3’UTR region to dampen epithelial inflammation and tissue injury in acute lung injury. Nanoparticle-based delivery of miR-223 into the airway confers lung protection in murine models of acute lung injury. Future studies should focus on miR-223 as a promising therapeutic target for the treatment and prevention of ARDS. Adapted from Neudecker et al. MicroRNAs in mucosal inflammation [2]. J Mol Med (Berl).
MiR-223 in inflammatory bowel diseases
IBD, including Crohn’s disease and ulcerative colitis, are characterized by disrupted epithelial barrier function, increased bacterial translocation and dysregulated immunological environment in the gut [75–84]. Adaptive immunity, T cell helper 17 (Th17) responses especially, plays a crucial role in the propagation of inflammation during IBD [85, 86]. On the other hand, dendritic cell (DC) and macrophages are also crucial in the maintenance of intestinal immune homeostasis [87]. Given the fact that miR-223 modulates the differentiation of monocytes, several studies have indicated the involvement of miR-223 in the regulation of innate immunity in IBD [19, 88]. Although miR-223 deficiency in mice resulted in reduction of CX3CR1hi macrophages at steady state, both CX3CR1hi macrophage and CD103+ DCs showed a pro-inflammatory phenotypes. Consistent with previous studies, miR-223 also controls differentiation of monocyte-derived DCs. In mouse models of colitis, miR-223 deficiency resulted in more severe colitis via direct targeting of C/EBPβ [88]. Another study by Neudecker et. al also demonstrated the protective role of miR-223 in a murine model of colitis (Figure 3) [19]. The expression level of miR-223 was shown to increase in intestinal biopsies as well as inflamed colon tissue of mice treated with dextran sodium sulfate (DSS). To determine the function of miR-223 in intestinal inflammation, miR-223 deficient mice were exposed to DSS in the drinking water to chemically induce colitis. Exacerbated intestinal inflammation was observed in miR-223 deficient mice characterized by increased weight loss and histological signs. Due to the function of miR-223 in myeloid cells, the authors used bone marrow chimeric mice to study the tissue contribution of miR-223 during murine colitis. Indeed, irradiated wild type mice receiving miR-223 deficient bone marrow recapitulated the phenotype of miR-223 knockout mice, suggesting the function of miR-223 in intestinal inflammation stems from the myeloid compartment. Further studies using antibody depletion of neutrophil or monocytes pointed out the important role of monocytes in miR-223 mediated dampening of immune responses. Mechanistically, miR-223 directly targeted NLRP3 in monocytes to inhibit inflammasome activity and IL-1β production during murine colitis. Indeed, mice with targeted deletion of miR-223 binding site in the 3’UTR region of NLRP3 pheno-copied miR-223-/y mice in DSS-induced colitis. Finally, systemic overexpression of miR-223 attenuated intestinal inflammation and clinical and histological signs of DSS-induced colitis, suggesting miR-223 as a therapeutic agent for inflammatory bowel diseases. In conclusion, miR-223 is protective during experimental colitis by dampening myeloid cell mediated innate immune responses. Future studies should therefore focus on therapeutic enhancement of miR-223 in IBD patients to control intestinal inflammation.
Figure 3. MiR-223 in inflammatory bowel disease.
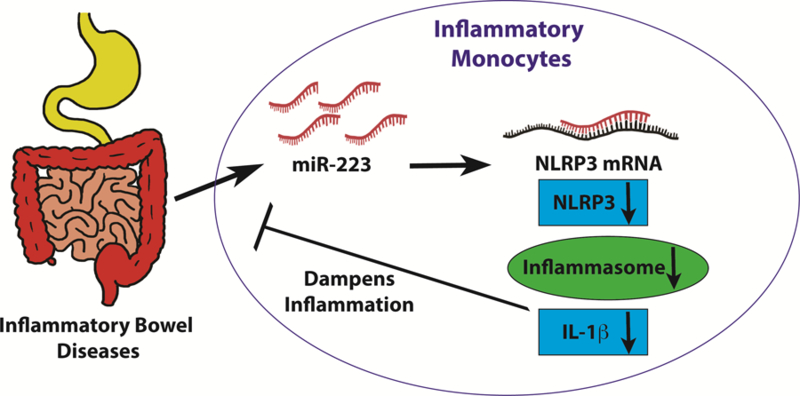
MiR-223 dampens intestinal inflammation in inflammatory bowel diseases. MiR-223 is induced in inflammatory monocytes during murine intestinal injury resulted from DSS exposure. Elevated miR-223 levels in inflammatory monocytes inhibit NLR family pyrin domain containing 3 (NLRP3), a direct miR-223 target gene. The inhibition of NLRP3 reduces inflammasome activity, and subsequently turns down IL-1b production. Reduced IL-1b levels result in amelioration of intestinal inflammation and improvement of clinical and histological signs of murine colitis. Therapeutic overexpression of miR-223 by systemic nanoparticle delivery decreeses disease severity in murine experimental colitis. Enhancing miR-223 therapeutically might serve as a promising treatment of inflammatory bowel disease to dampen intestinal inflammation [19].
Conclusion and perspectives
To summarize, several exciting research studies suggest that miR-223 serves as a crucial regulator in innate immunity, ranging from myeloid differentiation to neutrophil and macrophage functions. From a mechanistic perspective, various immune related targets of miR-223 have been identified in multiple inflammatory diseases conditions. For example, miR-223 directly targets multiple myeloid differentiation transcription factors to regulate myeloid differentiation. However, studies in gene-targeted mice suggests a profound role of miR-223 in immune modulation of granulocyte and macrophage activation. In multiple diseases settings, including ARDS or IBD, recent evidences point to an essential role of miR-223 in the control of innate immunity to confer protection against excess inflammation and tissue damage. MiR-223 can function in a cell intrinsic manner to modulate the immune response to inflammatory stimuli in myeloid cells. Moreover, it can also function in crosstalk between immune cells and resident cells via miRNA shuttling. Shuttling of miR-223 provides new insights of immune regulation and future research directions to study the role of miR-223 to regulate non-myeloid cells functions. In this process, the generation of miR-223 conditional knockout mice will remain indispensable for future studies in order to investigate the tissue and cell specific functions of miR-223. Moving from bench to bedside, miR-223 has been explored in several clinical trials as immunological biomarkers for diseases pathophysiology and interventions (Table 1). Based on recent translational studies, we are anticipating that there will also be clinical trials targeting miR-223 function during inflammatory disease. In conclusion, already utilized as biomarker in several inflammatory diseases, the tissue specific role and therapeutic potential of miR-223 will likely be further investigated as we search for safe and effective treatment for inflammatory diseases.
Table 1.
Summary of clinical trials utilizing miR-223 as biomarker
| Biomarkers | Study population | Purpose of Study | ClinicalTrial.gov Number (Status) |
|---|---|---|---|
| MicroRNAs (miR-9, miR-16, miR-125, miR-132, miR-146a, miR-150, miR-155, miR-181 and miR-223) | Rheumatoid arthritis (RA) patients treated with tocilizumab | To investigate the effects of tocilizumab on the expression of biomarkers in peripheral blood leukocytes and in serum samples from RA patients | NCT03149796 (Completed) |
| MiR-223, miR-15a and miR-16 | Sepsis patients within 24 hours admitted to the ICU | To study the regulatory mechanism of those miRNAs in lymphocyte in sepsis | NCT02756559 (Recruiting) |
| MiR-223, miR-133b and miR-1229 | Patients with recurrent implantation failure (RIF) | To study the immunomodulatory effects of rapamycin on pregnancy rate of patient with RIF | NCT03161340 (Recruiting) |
| MicroRNAs (miR-208a, miR-499, miR-1, miR-133, miR-126, miR-29, miR-233, miR-222 and miR-4454) | Patients with previous diagnosis of acromegaly with stable parameters of acromegaly disease in the last 3 months | To evaluate heart remodeling and performance changes and metabolic/immunological/molecular parameters after 5-months of Tadalafil 20 mg in acromegaly cardiomyopathy | NCT02611336 (Recruiting) |
| MicroRNAs (miR-208a, miR-499, miR-1, miR-133, miR-126, miR-29, miR-233, miR-222 and miR-4454) | Patients with previous diagnosis of Cushing Syndrome (CS) with concomitant cardiac hypertrophy and/or diastolic dysfunction | To evaluate heart remodeling and performance changes and metabolic/immunological/molecular parameters after 5-months of Tadalafil 20 mg in CS cardiomyopathy | NCT02611258 (Recruiting) |
Acknowledgments
Funding: R01 DK097075, POI-HL114457, R01-HL109233, R01-DK109574, R01-HL119837 and R01-HL133900 to HKE
Abbreviation
- 3’ UTR
3’ untranslated region
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- BALF
bronchial alveolar lavage fluid
- BM
bone marrow
- DAMP
damage-associated molecular patterns
- DC
dendritic cell
- DGCR8
DiGeorge syndrome chromosomal region 8
- DSS
dextran sodium sulfate
- IBD
inflammatory bowel disease
- LPS
lipopolysaccharide
- MiRNA
microRNA
- Mrna
messenger RNA
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK cells
natural killer cells
- PaO2/FiO2
arterial oxygen partial pressure to fractional inspired oxygen ratio
- PMNs
polymorphonuclear neutrophils
- Pre-miRNA
Precursor miRNA
- RISC
RNA-induced silencing complex
- RNAi
RNA interference
- TLR
Toll like receptor
- VILI
ventilation induced lung injury
Footnotes
Conflicts of interest: None
References:
- 1.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R (2006) Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20, 515–24. [DOI] [PubMed] [Google Scholar]
- 2.Neudecker V, Yuan X, Bowser JL, Eltzschig HK (2017) MicroRNAs in mucosal inflammation. J Mol Med (Berl) 95, 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari D, Bianchi N, Eltzschig HK, Gambari R (2016) MicroRNAs Modulate the Purinergic Signaling Network. Trends Mol Med [DOI] [PubMed]
- 4.Neudecker V, Brodsky KS, Kreth S, Ginde AA, Eltzschig HK (2016) Emerging Roles for MicroRNAs in Perioperative Medicine. Anesthesiology 124, 489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eltzschig HK and Eckle T (2011) Ischemia and reperfusion--from mechanism to translation. Nat Med 17, 1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan X, Lee JW, Bowser JL, Neudecker V, Sridhar S, Eltzschig HK (2017) Targeting Hypoxia Signaling for Perioperative Organ Injury. Anesth Analg [DOI] [PMC free article] [PubMed]
- 7.Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19, 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai EC (2002) Micro RNAs are complementary to 3’ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet 30, 363–4. [DOI] [PubMed] [Google Scholar]
- 9.O’Connell RM, Rao DS, Baltimore D (2012) microRNA regulation of inflammatory responses. Annu Rev Immunol 30, 295–312. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay MA (2008) microRNAs and the immune response. Trends Immunol 29, 343–51. [DOI] [PubMed] [Google Scholar]
- 11.Taganov KD, Boldin MP, Chang KJ, Baltimore D (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103, 12481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee S, Meng J, Das S, Krishnan A, Haworth J, Charboneau R, Zeng Y, Ramakrishnan S, Roy S (2013) Morphine induced exacerbation of sepsis is mediated by tempering endotoxin tolerance through modulation of miR-146a. Sci Rep 3, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moschos SA, Williams AE, Perry MM, Birrell MA, Belvisi MG, Lindsay MA (2007) Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genomics 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haneklaus M, Gerlic M, O’Neill LA, Masters SL (2013) miR-223: infection, inflammation and cancer. J Intern Med 274, 215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aziz F (2016) The emerging role of miR-223 as novel potential diagnostic and therapeutic target for inflammatory disorders. Cell Immunol 303, 1–6. [DOI] [PubMed] [Google Scholar]
- 16.Fulci V, Scappucci G, Sebastiani GD, Giannitti C, Franceschini D, Meloni F, Colombo T, Citarella F, Barnaba V, Minisola G, Galeazzi M, Macino G (2010) miR-223 is overexpressed in T-lymphocytes of patients affected by rheumatoid arthritis. Hum Immunol 71, 206–11. [DOI] [PubMed] [Google Scholar]
- 17.Zhu H and Leung SW (2015) Identification of microRNA biomarkers in type 2 diabetes: a meta-analysis of controlled profiling studies. Diabetologia 58, 900–11. [DOI] [PubMed] [Google Scholar]
- 18.Neudecker V, Brodsky KS, Clambey ET, Schmidt EP, Packard TA, Davenport B, Standiford TJ, Weng T, Fletcher AA, Barthel L, Masterson JC, Furuta GT, Cai C, Blackburn MR, Ginde AA, Graner MW, Janssen WJ, Zemans RL, Evans CM, Burnham EL, Homann D, Moss M, Kreth S, Zacharowski K, Henson PM, Eltzschig HK (2017) Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neudecker V, Haneklaus M, Jensen O, Khailova L, Masterson JC, Tye H, Biette K, Jedlicka P, Brodsky KS, Gerich ME, Mack M, Robertson AAB, Cooper MA, Furuta GT, Dinarello CA, O’Neill LA, Eltzschig HK, Masters SL, McNamee EN (2017) Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med 214, 1737–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–54. [DOI] [PubMed] [Google Scholar]
- 21.Wightman B, Ha I, Ruvkun G (1993) Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–62. [DOI] [PubMed] [Google Scholar]
- 22.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403, 901–6. [DOI] [PubMed] [Google Scholar]
- 23.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Muller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G (2000) Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408, 86–9. [DOI] [PubMed] [Google Scholar]
- 24.Zamore PD, Tuschl T, Sharp PA, Bartel DP (2000) RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101, 25–33. [DOI] [PubMed] [Google Scholar]
- 25.Kozomara A and Griffiths-Jones S (2014) miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 42, D68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, Sohn SY, Cho Y, Zhang BT, Kim VN (2006) Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 125, 887–901. [DOI] [PubMed] [Google Scholar]
- 27.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R (2004) The Microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–40. [DOI] [PubMed] [Google Scholar]
- 28.Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW (2004) Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117, 69–81. [DOI] [PubMed] [Google Scholar]
- 29.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K (2005) Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev 19, 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JS and Lai EC (2011) Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell 43, 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–97. [DOI] [PubMed] [Google Scholar]
- 32.Guo H, Ingolia NT, Weissman JS, Bartel DP (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee I, Ajay SS, Yook JI, Kim HS, Hong SH, Kim NH, Dhanasekaran SM, Chinnaiyan AM, Athey BD (2009) New class of microRNA targets containing simultaneous 5’-UTR and 3’-UTR interaction sites. Genome Res 19, 1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grey F, Tirabassi R, Meyers H, Wu G, McWeeney S, Hook L, Nelson JA (2010) A viral microRNA down-regulates multiple cell cycle genes through mRNA 5’UTRs. PLoS Pathog 6, e1000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brummer A and Hausser J (2014) MicroRNA binding sites in the coding region of mRNAs: extending the repertoire of post-transcriptional gene regulation. Bioessays 36, 617–26. [DOI] [PubMed] [Google Scholar]
- 36.Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R (2008) MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A 105, 1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P (2005) Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 309, 1577–81. [DOI] [PubMed] [Google Scholar]
- 38.Jopling CL (2008) Regulation of hepatitis C virus by microRNA-122. Biochem Soc Trans 36, 1220–3. [DOI] [PubMed] [Google Scholar]
- 39.Roberts AP, Lewis AP, Jopling CL (2011) miR-122 activates hepatitis C virus translation by a specialized mechanism requiring particular RNA components. Nucleic Acids Res 39, 7716–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR (2013) Treatment of HCV Infection by Targeting MicroRNA. N Engl J Med [DOI] [PubMed]
- 41.Chowdhary V, Teng KY, Thakral S, Zhang B, Lin CH, Wani N, Bruschweiler-Li L, Zhang X, James L, Yang D, Junge N, Bruschweiler R, Lee WM, Ghoshal K (2017) miRNA-122 Protects Mice and Human Hepatocytes from Acetaminophen Toxicity by Regulating Cytochrome P450 Family 1 Subfamily A Member 2 and Family 2 Subfamily E Member 1 Expression. Am J Pathol 187, 2758–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, Bozzoni I (2005) A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell 123, 819–31. [DOI] [PubMed] [Google Scholar]
- 43.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD (2008) Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451, 1125–9. [DOI] [PubMed] [Google Scholar]
- 44.Pulikkan JA, Dengler V, Peramangalam PS, Peer Zada AA, Muller-Tidow C, Bohlander SK, Tenen DG, Behre G (2010) Cell-cycle regulator E2F1 and microRNA-223 comprise an autoregulatory negative feedback loop in acute myeloid leukemia. Blood 115, 1768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakubzick CV, Randolph GJ, Henson PM (2017) Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 17, 349–362. [DOI] [PubMed] [Google Scholar]
- 46.Li T, Morgan MJ, Choksi S, Zhang Y, Kim YS, Liu ZG (2010) MicroRNAs modulate the noncanonical transcription factor NF-kappaB pathway by regulating expression of the kinase IKKalpha during macrophage differentiation. Nat Immunol 11, 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vian L, Di Carlo M, Pelosi E, Fazi F, Santoro S, Cerio AM, Boe A, Rotilio V, Billi M, Racanicchi S, Testa U, Grignani F, Nervi C (2014) Transcriptional fine-tuning of microRNA-223 levels directs lineage choice of human hematopoietic progenitors. Cell Death Differ 21, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall CHT, Campbell EL, Colgan SP (2017) Neutrophils as Components of Mucosal Homeostasis. Cell Mol Gastroenterol Hepatol 4, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V (2012) NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol 189, 4175–81. [DOI] [PubMed] [Google Scholar]
- 50.Feng Z, Qi S, Zhang Y, Qi Z, Yan L, Zhou J, He F, Li Q, Yang Y, Chen Q, Xiao S, Li Q, Chen Y, Zhang Y (2017) Ly6G+ neutrophil-derived miR-223 inhibits the NLRP3 inflammasome in mitochondrial DAMP-induced acute lung injury. Cell Death Dis 8, e3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Izumi B, Nakasa T, Tanaka N, Nakanishi K, Kamei N, Yamamoto R, Nakamae T, Ohta R, Fujioka Y, Yamasaki K, Ochi M (2011) MicroRNA-223 expression in neutrophils in the early phase of secondary damage after spinal cord injury. Neurosci Lett 492, 114–8. [DOI] [PubMed] [Google Scholar]
- 52.Dorhoi A, Iannaccone M, Farinacci M, Fae KC, Schreiber J, Moura-Alves P, Nouailles G, Mollenkopf HJ, Oberbeck-Muller D, Jorg S, Heinemann E, Hahnke K, Lowe D, Del Nonno F, Goletti D, Capparelli R, Kaufmann SH (2013) MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Invest 123, 4836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Y, Feng D, Li M, Gao Y, Ramirez T, Cao H, Kim SJ, Yang Y, Cai Y, Ju C, Wang H, Li J, Gao B (2017) Hepatic mitochondrial DNA/Toll-like receptor 9/MicroRNA-223 forms a negative feedback loop to limit neutrophil overactivation and acetaminophen hepatotoxicity in mice. Hepatology 66, 220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao Y, Ross RA, Cao H, Cai Y, Xu M, Feng D, Zhang P, Liangpunsakul S, Gao B (2017) MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47(phox)-oxidative stress pathway in neutrophils. Gut 66, 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S, Wu C, Zhou B (2012) A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation 125, 2892–903. [DOI] [PubMed] [Google Scholar]
- 56.Chen Q, Wang H, Liu Y, Song Y, Lai L, Han Q, Cao X, Wang Q (2012) Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1beta production in macrophages by targeting STAT3. PLoS One 7, e42971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang N, Fu L, Bu Y, Yao Y, Wang Y (2017) Downregulated expression of miR-223 promotes Toll-like receptor-activated inflammatory responses in macrophages by targeting RhoB. Mol Immunol 91, 42–48. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Wang R, Jiang J, Yang B, Cao Z, Cheng X (2015) miR-223 is upregulated in monocytes from patients with tuberculosis and regulates function of monocyte-derived macrophages. Mol Immunol 67, 475–81. [DOI] [PubMed] [Google Scholar]
- 59.Lou J, Wang Y, Zhang Z, Qiu W (2017) Activation of MMPs in Macrophages by Mycobacterium tuberculosis via the miR-223-BMAL1 Signaling Pathway. J Cell Biochem 118, 4804–4812. [DOI] [PubMed] [Google Scholar]
- 60.Fehniger TA, Wylie T, Germino E, Leong JW, Magrini VJ, Koul S, Keppel CR, Schneider SE, Koboldt DC, Sullivan RP, Heinz ME, Crosby SD, Nagarajan R, Ramsingh G, Link DC, Ley TJ, Mardis ER (2010) Next-generation sequencing identifies the natural killer cell microRNA transcriptome. Genome Res 20, 1590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaefer JS, Montufar-Solis D, Vigneswaran N, Klein JR (2011) Selective upregulation of microRNA expression in peripheral blood leukocytes in IL-10−/−mice precedes expression in the colon. J Immunol 187, 5834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang J, Wang F, Argyris E, Chen K, Liang Z, Tian H, Huang W, Squires K, Verlinghieri G, Zhang H (2007) Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med 13, 1241–7. [DOI] [PubMed] [Google Scholar]
- 63.Leuenberger C, Schuoler C, Bye H, Mignan C, Rechsteiner T, Hillinger S, Opitz I, Marsland B, Faiz A, Hiemstra PS, Timens W, Camici GG, Kohler M, Huber LC, Brock M (2016) MicroRNA-223 controls the expression of histone deacetylase 2: a novel axis in COPD. J Mol Med (Berl) 94, 725–34. [DOI] [PubMed] [Google Scholar]
- 64.Gao Y, Lin L, Li T, Yang J, Wei Y (2017) The role of miRNA-223 in cancer: Function, diagnosis and therapy. Gene 616, 1–7. [DOI] [PubMed] [Google Scholar]
- 65.Laios A, O’Toole S, Flavin R, Martin C, Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, D’Arcy T, McGuinness E, Sheils O, Sheppard B, J, O. L. (2008) Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M, Sun L, Ji G, Shi Y, Han Z, Han S, Nie Y, Chen X, Zhao Q, Ding J, Wu K, Daiming F (2011) miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res 9, 824–33. [DOI] [PubMed] [Google Scholar]
- 67.Wei Y, Yang J, Yi L, Wang Y, Dong Z, Liu Z, Ou-yang S, Wu H, Zhong Z, Yin Z, Zhou K, Gao Y, Yan B, Wang Z (2014) MiR-223–3p targeting SEPT6 promotes the biological behavior of prostate cancer. Sci Rep 4, 7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong QW, Lung RW, Law PT, Lai PB, Chan KY, To KF, Wong N (2008) MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology 135, 257–69. [DOI] [PubMed] [Google Scholar]
- 69.Pitt JM, Kroemer G, Zitvogel L (2016) Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest 126, 1139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang J-D, Song E (2011) Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Molecular cancer 10, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L, Lu FB, Chen DZ, Wu JL, Hu ED, Xu LM, Zheng MH, Li H, Huang Y, Jin XY, Gong YW, Lin Z, Wang XD, Chen YP (2018) BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol 93, 38–46. [DOI] [PubMed] [Google Scholar]
- 72.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307, 2526–33. [DOI] [PubMed] [Google Scholar]
- 73.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and Outcomes of Acute Lung Injury. N Engl J Med 353, 1685–1693. [DOI] [PubMed] [Google Scholar]
- 74.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM (2011) Functional disability 5 years after acute respiratory distress syndrome. The New England journal of medicine 364, 1293–304. [DOI] [PubMed] [Google Scholar]
- 75.Bowser JL, Phan LH, Eltzschig HK (2018) The Hypoxia-Adenosine Link during Intestinal Inflammation. J Immunol 200, 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bowser JL, Lee JW, Yuan X, Eltzschig HK (2017) The Hypoxia-Adenosine Link during Inflammation. J Appl Physiol (1985), jap 00101 2017. [DOI] [PMC free article] [PubMed]
- 77.Bowser JL, Lee JW, Yuan X, Eltzschig HK (2017) The hypoxia-adenosine link during inflammation. J Appl Physiol (1985) 123, 1303–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aherne CM, Saeedi B, Collins CB, Masterson JC, McNamee EN, Perrenoud L, Rapp CR, Curtis VF, Bayless A, Fletcher A, Glover LE, Evans CM, Jedlicka P, Furuta GT, de Zoeten EF, Colgan SP, Eltzschig HK (2015) Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal Immunol 8, 1324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aherne CM, Collins CB, Eltzschig HK (2013) Netrin-1 guides inflammatory cell migration to control mucosal immune responses during intestinal inflammation. Tissue Barriers 1, e24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colgan SP and Eltzschig HK (2012) Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol 74, 153–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK (2009) Cutting Edge: A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol 182, 3965–8. [DOI] [PubMed] [Google Scholar]
- 82.Eltzschig HK, Sitkovsky MV, Robson SC (2012) Purinergic signaling during inflammation. N Engl J Med 367, 2322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eltzschig HK and Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364, 656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neudecker V, Colgan SP, Eltzschig HK (2017) Novel therapeutic concepts for inflammatory bowel disease-from bench to bedside. J Mol Med (Berl) 95, 899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doherty GA, Bai A, Hanidziar D, Longhi MS, Lawlor GO, Putheti P, Csizmadia E, Nowak M, Cheifetz AS, Moss AC, Robson SC (2012) CD73 is a phenotypic marker of effector memory Th17 cells in inflammatory bowel disease. Eur J Immunol 42, 3062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bai A, Moss A, Kokkotou E, Usheva A, Sun X, Cheifetz A, Zheng Y, Longhi MS, Gao W, Wu Y, Robson SC (2014) CD39 and CD161 modulate Th17 responses in Crohn’s disease. J Immunol 193, 3366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coombes JL and Powrie F (2008) Dendritic cells in intestinal immune regulation. Nat Rev Immunol 8, 435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou H, Xiao J, Wu N, Liu C, Xu J, Liu F, Wu L (2015) MicroRNA-223 Regulates the Differentiation and Function of Intestinal Dendritic Cells and Macrophages by Targeting C/EBPbeta. Cell Rep 13, 1149–1160. [DOI] [PubMed] [Google Scholar]


