Abstract
Previous studies provide conflicting results regarding the relation between future thinking and executive functioning during early childhood. Further, little is known of the neural mechanisms involved in future thinking during early childhood. We examined the moderating role of frontal electroencephalogram (EEG) activity on the relation between executive functioning and semantic future thinking performance in a sample of 4-year-old children. Our results suggest that frontal EEG moderates the relation between executive functioning and semantic future thinking performance, but only for medium to high levels of frontal EEG power values. These results provide emerging evidence regarding the role of both executive functioning and frontal brain electrical activity on semantic future thinking in 4-year-olds.
Keywords: future thinking, executive functions, early childhood, memory, EEG
Generating scenarios through future oriented thinking is critical for goal directed behaviors (e.g., planning, decision making, etc.). Such thinking may involve specific memory episodes of similar events, knowledge of how similar events typically occur, or a mixture of both (Atance & O’Neill, 2001; Tulving, 2002). For example, when considering what you might have for breakfast tomorrow, you may think of what you ate today (specific episode), think of typical breakfast foods (knowledge), or both. Understanding the development of future oriented thinking, and the associated cognitive and neural contributors, may provide critical insight into the development and structure of goal-directed behaviors. Emerging evidence suggests a possible connection between executive functions (EF; i.e., cognitive control processes; Miyake, Friedman, Emerson, Witzki, Howerter, & Wager, 2000) and future oriented thought in children (Unal & Hohenberger, 2017). Although evidence exists of a behavioral connection between the two constructs during childhood, the underlying neural mechanisms are unknown. Of specific interest is the prefrontal cortex (PFC), a neural region that supports behaviors associated with goal attainment and executive functioning (Devilbiss, Spencer, & Berridge, 2016; Wolfe & Bell, 2004); however, little is known of the role of the PFC in the early development of future thought. Our study had two primary aims: first, to examine contributions of EF and frontal activity, measured through frontal scalp electroencephalogram (EEG) power during task, to early childhood (4 years) future thinking performance; second, to examine whether frontal EEG activity during the future-thinking task moderates the relation between EF and future thinking performance.
Future oriented thinking may be broken down into a dichotomy including episodic and semantic future thought (Atance & O’Neill, 2001). Episodic future thinking incorporates detail rich information (item and context) when forming future scenarios (e.g., imaging oneself at a future birthday party opening presents). Semantic future thinking (SFT) incorporates preexisting knowledge into possible future outcomes, but does not involve self-projection or mental time travel (e.g., knowing that birthday parties typically have balloons, so expecting balloons to be at a party). Children appear to acquire semantic abilities prior to episodic (Nelson, 2001), with children as young as 3 years showing rudimentary semantic memory (Lucariello & Nelson, 1985) and semantic future thought (Prabhakar & Hudson, 2014). To expand, Prabhakar and Hudson (2014) examined 3 and 4 year olds’ SFT abilities using a script involving a birthday party and a tabletop 3-dimensional model of a neighborhood. They found that 4-year-old children were able to maintain larger quantities of information (6 locations) than 3-year-old children (4 locations). They did not, however, look at the possible contributors to SFT performance. Our study was designed to replicate and expand on the findings of Prabhakar and colleagues by examining both cognitive and neural contributors to SFT performance in 4-year-old children.
EFs are higher order cognitive control processes, meaning EF allows for top-down control of cognition (Miyake et al., 2000). For example, EF is involved in problem solving (Zelazo, Carter, Reznick, & Frye, 1997), goal attainment (Novakovic-Agopian et al., 2011), and attentional control (Kaplan & Berman, 2010). EF abilities develop rapidly during early childhood, with significant increases in performance being observed at age 4 (Kerr & Zelazo, 2004; Zelazo et al., 2003). The observed improvements in performance have made early childhood, especially between the ages of 3–5, a popular age range to examine EF development (e.g., Clark, Pritchard, & Woodward, 2010; Cuevas, Deater-Deckard, Kim-Spoon, Watson, Morasch, & Bell, 2014; Kirkham, Cruess, & Diamond, 2003). We focused on age 4 given the rapid development of EF observed at this age. Furthermore, to better understand the relation between EF and future thinking, specifically SFT, we also examined the role of neural processes on future thinking performance.
As noted, the role of the PFC in the early development of future thinking has received little attention. The adult literature examining the neural underpinnings of future oriented thought suggests that the PFC is a critical component of a neural “core network” consisting of temporal, parietal and frontal regions (Schacter, Benoit, & Szpunar, 2017). Further supporting the role of the PFC in future thinking, Peters and Buchel (2010) reported that dorsolateral prefrontal activity was associated with future oriented thinking during a decision-making task. Little is known, however, of the neural mechanisms involved in childhood future thought. What is known is that the dorsolateral PFC is involved in EF (Smith & Jonides, 1999), which has been behaviorally associated with childhood future oriented thinking (Unal & Hohenberger, 2016). We examined frontal scalp activity within the child alpha (6–9 HZ) frequency band, which is associated with EF in children (e.g., Broomell & Bell, 2017; Swingler, Willoughby, & Calkins, 2011). High alpha activity in childhood is associated with greater EF abilities (e.g., Swingler et al., 2011; Wolfe & Bell, 2004, 2007). Thus, high levels of child alpha during a SFT task may reflect the degree of cognitive control used during the SFT task.
The PFC undergoes significant development during early childhood (Diamond, 2002; Paus et al., 2001). The neurological development of the PFC that occurs between the ages of 3 and 5 corresponds with changes in behavioral measures of cognition and memory during the preschool period (e.g., Diamond, 2002). For example, at age 3 many children are unable to comprehend the rules of simple laboratory cognitive tasks, but by age 5 most children are proficient at these same tasks (Carlson, 2005). This developmental pattern makes preschool an important time to investigate prefrontal activity during cognitive tasks, as variability in brain function can inform task performance in both adults (e.g., Baldassarre, Lewis, Committeri, Snyder, Romani, & Corbetta, 2012) and children (Blankenship & Bell, 2015; Wolfe & Bell, 2014). Evidence of the development that occurs during preschool is seen in the specific increases in medial prefrontal EEG power from baseline to working memory tasks that occurs in 4 year olds, while a less localized cortical power increase with task performance is seen during a working memory task in infancy (Bell & Wolfe, 2007). Given the association of EF and PFC activity to future oriented thinking, we examined the contributions of both EF and the PFC (via frontal EEG activity) to SFT.
The relation between preschool SFT and EF has not been examined, but the function of EF is theoretically similar for both episodic and semantic future oriented memory (Schacter, Addis, & Buckner, 2008). Specifically, planning for the future requires remembering past information (semantic or episodic) and then organizing and using that information in anticipation of a future event. This type of prospective thinking, regardless of the type of past information used (semantic or episodic), would theoretically require working memory (organization of information), inhibitory control (suppression of immediate outcomes), and cognitive flexibility (anticipation of outcomes; Kliegel, Martin, McDaniel, & Einstein, 2002). In terms of episodic future thinking, there are inconsistencies in the literature regarding the role of EF during early childhood, with some researchers suggesting that EF is crucial for future thinking abilities (Unal & Hohenberger, 2017), and others suggesting these constructs are not related during early childhood (Hanson et al., 2014). One potential reason for the inconsistencies is that EF may be related to future thinking performance under specific conditions (e.g., level of frontal EEG activity during future thinking tasks).
A substantial literature exists examining individual differences in cognition in relation to frontal EEG activity and EF performance (e.g., Alvarez & Emory, 2006; Sauseng, Klimesch, Schabus, & Doppelmayr, 2005; Swingler et al., 2011; Wolfe & Bell, 2014, 2007, 2004). A possible reason for the inconsistencies in links between EF and future thinking found in the developmental literature may be the lack of information regarding individual differences during SFT development. In fact, our understanding of the relation between EF and theory of mind, a construct theorized to be involved in future oriented thought (Russell, Alexis, & Clayton, 2010), has greatly improved through methods incorporating individual differences (e.g., controlling for age, gender, verbal ability etc.; Carlson & Moses, 2001). One potential way to address the individual differences question in research on SFT is through examining the interactive function of PFC activity, measured using frontal EEG power, on the relation between EF and SFT. Such EEG methodology has successfully been used to examine the moderating role of frontal EEG asymmetry in the emotion regulation literature (e.g., Lopez-Duran, Nusslock, & Kovacs, 2012). Given the relation of both frontal activation and EF to adults’ future thinking performance (de Vito et al., 2012; Schacter et al., 2008), and the literature suggesting informative interactions between cognitive and electrophysiological measures, it is possible that frontal EEG activity and EF interact to impact SFT performance. We propose that that if children have lower frontal EEG activity during future thinking then the relation between SFT and EF may not be detected, which is possibly what occurred in Hanson and colleagues’ study (2014). Given the relation between frontal activation and EF to future thinking in adults, we hypothesized that frontal EEG activity during our SFT task would moderate the relation between EF and SFT in a sample of 4-year-old children.
Method
Participants
Forty-one children were recruited for participation in this study using existing participant databases and flyers distributed in areas populated by young families, including parks, recreation centers, and daycare facilities. One child did not have EF data due to parental interference, resulting in a final sample of 40 children. Children were between 48 and 59 months at the time of appointment and no history of medical or psychological disorders. The average age of children included in the study was 4.46 years (SD = 0.30), the average age for mothers was 32.83 years (SD = 4.55), and for fathers 32.37 years (SD = 5.05). The parents of the sample were well-educated, as 75.6% of mothers and 73.2% of fathers had completed a college degree or higher (6 sets of parents did not report education). In terms of ethnicity, 7.3% identified as Hispanic, 78% identified as non-Hispanic, and 14.6% did not report ethnicity. When asked about race, 4.9% of children were reported by their parents to be Asian, 2.4% Black or African American, 9.8% Other/Multiracial, and 82.9% Caucasian.
Procedure
Upon arrival to the lab, parents consented and children assented to the protocol. After consent and assent, the EEG cap was placed and children completed the EF and SFT tasks. Children completed a series of additional cognitive tasks that were not part of the current study. As compensation for participation, parents received a $10 gift card and children received a small gift.
EEG Acquisition, Processing and Analysis
EEG data were collected during all of the tasks; the focus here is on the EEG collected during the SFT task. Recordings were made from 26 left, right, and midline scalp sites [frontal pole (Fp1, Fp2), frontal (F3, F4, Fz, F7, F8), central (C3, C4), central frontal (FC1, FC2, FC5, FC6), temporal (T7, T8), parietal (P3, P4, Pz, P7, P8), central parietal (CP1, CP2, CP5, CP6), occipital (O1, O2)]. All electrodes were referenced to Cz during the recordings. We recorded EEG using a stretch cap (Electro-Cap, Inc.; Eaton, OH; E1-series cap) with electrodes in the 10/20 system pattern. We placed a small amount of abrasive gel into each recording site and gently rubbed the scalp. We then added conductive gel to the recording sites. Electrode impedances were measured and accepted if they were below 20 KΩ. The electrical activity from each lead was amplified using separate James Long Company Bioamps (James Long Company; Caroga Lake, NY). The EEG activity for each scalp electrode was displayed on the monitor of the acquisition computer. The signal was digitized on-line at 512 samples per second for each channel in order to eliminate the effects of aliasing. This calibration signal was digitized for 30 seconds and stored for subsequent analysis. The acquisition software used was Snapshot-Snapstream (HEM Data Corp., Southfield, MI) and the raw data were stored for later analyses.
EEG data were examined and analyzed using EEG Analysis software developed by the James Long Company. Average reference EEG data were then artifact scored for eye movements using a peak-to-peak criterion of 100μV or greater. Gross motor movements over 200μV peak to peak were also scored. These artifact scored epochs were eliminated from all analyses. The data were then analyzed with a discrete Fourier transform (DFT) using a Hanning window of 1 second width and 50% overlap. Based on research regarding EF in childhood, we examined frontal (F3 and F4) regions within the child alpha (6–9 hz) power band for our analyses (Marshall, Bar-Haim, & Fox, 2002). We focused on the alpha power given its association with EF in adults and preschool children (e.g., Klimesch, Sauseng, & Hanslmayr, 2007; Wolfe & Bell, 2004), and F3/F4 electrode sites because of their spatial proximity to the dorsolateral PFC (Herwig, Satrapi, & Schonfeldt-Lecuona, 2003) and their use in previous studies of PFC functioning (e.g., Blankenship et al., 2015; Gomot, Giard, Adrien, Barthelemy, & Bruneau, 2002); however, we acknowledge that specific spatial conclusions cannot be made when using EEG methodology. In early childhood, higher EEG alpha power values are associated with cognitive engagement through increases in EF performance (e.g., Wolfe & Bell, 2004, 2007). EEG power during the learning phase of the SFT task (described below) was the measures of frontal EEG activity used in our analyses. We focused on EEG during the learning portion (composite across SFT conditions) of our task because it required less movement, resulting in fewer artifacts, than the test portion but still allowed for brain-related activity during the explicit formation and usage of memories associated with the SFT task (e.g., house location etc.). Because we had no hypotheses regarding hemisphere, we averaged across left and right hemisphere EEG power values for our analyses.
Executive Function Task
The Dimensional Change Card Sort task (DCCS; Zelazo, 2006) is used as a measure of general EF ability during early childhood (Zelazo et al., 2014). During the task, children were instructed to sort cards based on two dimensions (i.e., color, shape). First, children sorted six cards based on one dimension (preswitch). They were then instructed to switch and to sort the remaining six cards by another dimension (postswitch). Dimensions were counterbalanced throughout conditions and across children. Our variable of interest was the proportion of correct on post-switch responses.
Semantic Future Thinking Task
The SFT task was the one reported by Prabhakar and colleagues (2014), with the inclusion of an additional condition. Children were introduced to two separate models, one at the beginning of the appointment, and the other at the end of the appointment (counterbalanced). One model was of a neighborhood (Prabhakar et al., 2014) and the other a playground. Buildings (neighborhood) and play equipment (park) were labeled based on their color and name (e.g., “The blue store is the toy store” or “The gray slide is the big kids’ slide”). All building and play equipment locations were distinct from one another in terms of color. Houses, stores, sandboxes, and slides were distinct shapes. There were two primary phases of the study, learning and test.
During the learning phase, the experimenter indicated what each of the shapes on the respective model represented, and the children were prompted to identify each location. For example, “ Can you show me where the big kids’ slide is?” and “This is Mary’s sandbox.” This was repeated until children were able to correctly identify all locations. Then the test phase began. Using the park condition as an example, children were given a target sandbox (final goal) and a target slide (subgoal). There were distractor sandboxes and slides in the model (i.e., four sandboxes and two slides). Children began by placing a doll at a starting location and were then told a story resulting in the two goal locations (subgoal and final goal). “Mary left her favorite Elsa doll at the big kids’ slide. You want to give it back to her. Where should you go first?” The subgoal was moving the doll to the correct first location (i.e., big kid’s slide). If the children correctly identified the subgoal then the experimenter prompted the children to continue. “Where should you go next?” The final goal was the correct sandbox associated with the character (i.e., Mary’s sandbox). Children were given two attempts at each location. If children were correct on their first attempt they received 2 points, and if they were correct on their second attempt they received 1 point (total of 4 points possible for each model). All children completed both versions of the task (neighborhood, playground; counterbalanced). There was ~30 minutes between the presentation of each SFT task to prevent carryover effects. Our variable of interest was the total number of points received across tasks (total of 8 points possible).
Results
SFT performance was not correlated with frontal EEG power values during the learning portion of the SFT task or with EF performance. Further, EF performance was not correlated with frontal EEG during the SFT task. For correlation values and descriptive statistics refer to Table 1. Hierarchical regression equation was used to examine a possible moderation effect of frontal EEG activity on the relation between EF and SFT. Both frontal EEG power values and EF values were centered and an interaction term was generated. Frontal EEG power and EF performance were entered into the first step of the equation, and the interaction term was entered into the second step.
Table 1.
Correlations and Descriptive Statistics
| Variables | 1 | 2 | 3 |
|---|---|---|---|
| 1. SFT | -- | ||
| 2. EF | .19 | -- | |
| 3. Frontal Activity | −.01 | .24 | -- |
| Mean | 5.78 | 0.89 | 3.16 |
| SD | 1.41 | 0.30 | 0.38 |
| Min | 2.00 | 0.00 | 2.31 |
| Max | 8.00 | 1.00 | 4.10 |
Note: SFT is Semantic Future Thinking, EF is Executive Functioning. Scores represent values prior to centering variables.
Interaction Analyses
Frontal EEG activity during the learning phase of SFT task and EF performance in Step 1 separately accounted for a nonsignificant 6% of the variance in SFT performance. The variables in Step 2 accounted for an additional 14% of the variance in SFT performance, with frontal EEG, EF, and the interaction between frontal EEG and EF contributing unique variance (see Table 2). The interaction term was significant; we therefore probed the interaction.
Table 2.
Hierarchical Regression Analyses of the Interaction between Frontal Activity and Executive Functioning on Semantic Future Thinking Performance
| R | R2 | R2Δ | FΔ | F | β | t | sr2 | |
|---|---|---|---|---|---|---|---|---|
| Step 1. | .25 | .06 | 1.21 | |||||
| Frontal Activity | −.16 | −.97 | .02 | |||||
| Executive Functioning | .24 | 1.44 | .05 | |||||
| Step 2: | .45 | .20 | .14 | 6.52* | 3.10* | |||
| Frontal Activity | −.37 | −2.12* | .10 | |||||
| Executive Functioning | .83 | 2.98** | .20 | |||||
| Frontal Activity X Executive Functioning | .68 | 2.55* | .14 |
Note:
p ≤ .01;
p ≤ .05.
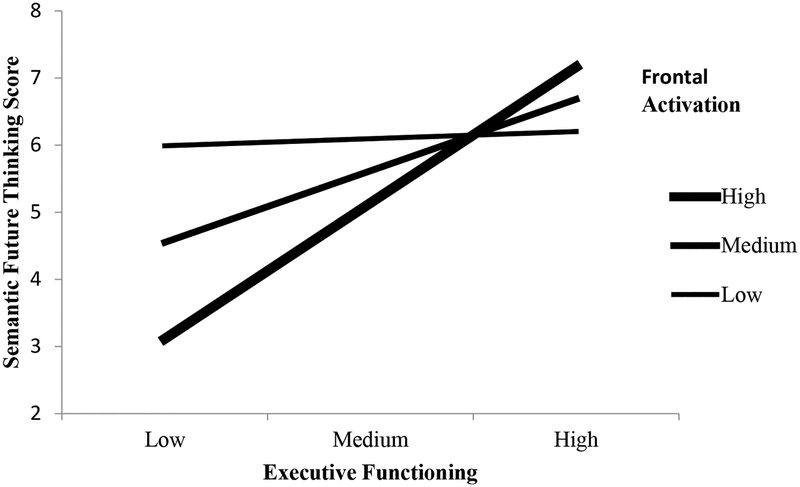
We tested the interaction at three values (mean, +1SD, -1SD) of the moderating variable (frontal EEG activity). Our low value was 1 SD below the mean EEG alpha power value, our medium value was the mean, and our high value was 1 SD above the mean (Aiken & West, 1991). For EF and SFT values for low, medium, and high EEG power groups see Table 3. The simple slopes for the high, t (6) = 2.88, p = .006, and medium, t (13) = 2.98, p =.005, but not low t (17) = 0.50, p = .619, were significant. These results suggest that frontal EEG activity moderates the relation between EF and SFT, but only when the frontal EEG power values are high or medium, but not when they are low (see Figure 1).
Table 3.
Descriptive Statistics of Semantic Future Thinking and Executive Functioning Performance at low, medium, and high levels of EEG activity
| Variables | Low | Medium | High |
|---|---|---|---|
| SFT | 6.38 | 5.58 | 5.86 |
| (0.92) | (1.58) | (1.07) | |
| EF | 0.75 | 0.90 | 1.00 |
| (0.46) | (0.28) | (0.00) | |
Note: SFT is Semantic Future Thinking, EF is Executive Functioning. Values represent the Means and Standard Deviations (in parentheses below Mean) of SFT and EF for low, medium and high EEG activity groups.
Figure 1.
Interaction between frontal activation and executive functioning on semantic future thinking. High and medium levels of frontal activation significantly moderated the relation between executive functioning and semantic future thinking.
Discussion
The primary goal of our study was to examine the moderating role of frontal EEG activity on the relation between EF and SFT task in 4-year-old children. We hypothesized that frontal brain electrical activity would modulate the relation between EF and SFT. Our hypothesis was supported, with medium to high frontal EEG power values resulting in a stronger positive relation between EF and SFT performance. Our study provides emerging evidence of the relation between frontal EEG activity and future thinking performance in children. Further, our results suggest that EF is related to SFT, but that this relation is conditional on the frontal EEG values during the learning portion of our SFT task.
Both EF and frontal brain activity are related to future thinking in adults (Baird, Smallwood, & Schooler, 2011; Schacter et al., 2017); however, research focused on early development of future thinking is mixed, with some researchers finding a relation between EF and future thinking performance in children (Unal & Hohenberger, 2016) and others not (Hanson et al., 2014). Our study is the first to report evidence of a moderation effect of frontal EEG activity on the relation between EF and SFT. This interaction may explain why some developmental studies have been unable to find a connection between EF and future thinking performance. In fact, the first step of our regression (without the interaction term) if examined alone would have suggested that neither frontal EEG nor EF contributes to SFT performance. The previous studies, however, specifically examined episodic future thinking and not SFT. Therefore, additional research is needed before concrete conclusions may be drawn connecting EF to episodic future thinking in early childhood.
Our study examined frontal EEG activity (i.e., low, medium, high levels of brain electrical activity) within the child alpha rhythm (6–9 Hz). Our results suggest that low level alpha power does not interact with EF to impact SFT performance, but that medium and high levels of alpha power do. The interaction between medium and high frontal EEG power and EF performance may reflect children’s recruitment of cognitive resources or effort during the SFT task, given that increases in alpha power are associated with performance benefits during childhood (Watson & Bell, 2013; Wolfe & Bell, 2004, 2007, 2014).
Children with low EEG power values during the learning phase of the SFT task performed well on the SFT task regardless of their EF abilities. This was an unexpected finding. Although it is typical for higher alpha power to be related to EF performance and thus cognitive control during early childhood (e.g., Wolfe & Bell, 2014), lower alpha power is associated with these higher order processes in adults (Sauseng, Klimesch, Doppelmayr, Pecherstorfer, Freunberger, & Hanslmayr, 2005). This age-related differences in how alpha power relates to cognitive performance may be related to alpha being at the 6–9 Hz level for children (Saby & Marshall, 2012) and at the 8–13 Hz level for adults. This finding may suggest that children with low alpha power values were engaging in more mature encoding strategies during the SFT task, and thus may not have relied as heavily on EF to aid in SFT performance. An alternative explanation is that our results are a product of high performance during the EF task (M=.89). Future studies should examine this relation using a more diverse sample, thus increasing performance variability.
There were some limitations to our work. First, we focused on semantic based future oriented processes given children’s success with such tasks early in development (Prabhakar & Hudson, 2014). Future studies should use our moderation model to examine episodic future orienting thinking in children. Given that episodic and semantic memory are dissociable processes (Vargha-Khadem, Gadian, Watkins, Connelly, Van Paesschen, & Mishkin, 1997), it is possible that different interaction patterns will be found when examining episodic future thinking. Second, we only used one measure of SFT. We did administer two versions of our SFT task, whereas other studies have used multiple future thinking measures when assessing SFT during early childhood (e.g., Hanson et al., 2014). Future studies should replicate our results using multiple measures of SFT. Third similar to SFT, we only used one measure of EF. Although the DCCS task has been argued to measure general EF ability (Zelazo et al., 2014), others argue that the DCCS primarily measures cognitive flexibility (Kloo, Perner, Aichorn, & Schmidhuber, 2010). In order to mitigate concerns regarding task specificity, future studies should replicate our results using multiple measures of EF. Fourth, we measured EEG activity during the learning portion of our SFT task. Thus, the EEG activity reflects the formation of information that was later used during the task, but it does not reflect activity during the actual test portion of SFT task. We propose that the learning portion still occurred within the context of the SFT task, and thus provides information regarding activity during future thinking. Future studies should examine EEG for both learning and test portions of a SFT task. Finally, our sample lacked variability in terms of race and parent education level, with the majority of children being Caucasian with highly educated parents. Future studies should examine children from more diverse backgrounds to increase the generalizability of our results.
Our study provides emerging evidence for the role of EF and frontal EEG activity in SFT performance during early childhood. Specifically, frontal EEG power values during the learning portion of the SFT task moderated the relation between EF and SFT performance in a sample of 4-year-old children. These results inform literature examining the mechanism associated with future oriented thinking in early childhood.
Acknowledgments
This research was supported by grant HD049878 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to Martha Ann Bell. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health. We are grateful to the families for their participation in our research. We thank Ran Liu for her assistance with task coding.
References
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage. [Google Scholar]
- Alvarez JA, & Emory E (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychology review, 16, 17–42. [DOI] [PubMed] [Google Scholar]
- Atance CM, & O’Neill DK (2001). Episodic future thinking. Trends in cognitive sciences, 5, 533–539. [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, & Schooler JW (2011). Back to the future: autobiographical planning and the functionality of mind-wandering. Consciousness and cognition, 20, 1604–1611. [DOI] [PubMed] [Google Scholar]
- Baldassarre A, Lewis CM, Committeri G, Snyder AZ, Romani GL, & Corbetta M (2012). Individual variability in functional connectivity predicts performance of a perceptual task. Proceedings of the National Academy of Sciences, 109, 3516–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, & Wolfe CD (2007). Changes in brain functioning from infancy to early childhood: Evidence from EEG power and coherence during working memory tasks. Developmental neuropsychology, 31(1), 21–38. [DOI] [PubMed] [Google Scholar]
- Blankenship TL, & Bell MA (2015). Frontotemporal coherence and executive functions contribute to episodic memory during middle childhood. Developmental neuropsychology, 40, 430–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomell AP, & Bell MA (2017). Inclusion of a Mixed Condition Makes the Day/Night Task More Analogous to the Adult Stroop. Developmental Neuropsychology, 42, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM (2005). Developmentally sensitive measures of executive function in preschool children. Developmental neuropsychology, 28, 595–616. [DOI] [PubMed] [Google Scholar]
- Carlson SM, & Moses LJ (2001). Individual differences in inhibitory control and children’s theory of mind. Child development, 72, 1032–1053. [DOI] [PubMed] [Google Scholar]
- Clark CA, Pritchard VE, & Woodward LJ (2010). Preschool executive functioning abilities predict early mathematics achievement. Developmental psychology, 46, 1176. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Deater‐Deckard K, Kim‐Spoon J, Watson AJ, Morasch KC, & Bell MA (2014). What’s mom got to do with it? Contributions of maternal executive function and caregiving to the development of executive function across early childhood. Developmental Science, 17, 224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2002). Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. Principles of frontal lobe function, 466–503. [Google Scholar]
- Devilbiss DM, Spencer RC, & Berridge CW (2016). Stress degrades prefrontal cortex neuronal coding of goal-directed behavior. Cerebral Cortex, 27, 2970–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vito S, Gamboz N, Brandimonte MA, Barone P, Amboni M, & Della Sala S (2012). Future thinking in Parkinson’s disease: An executive function?. Neuropsychologia, 50(7), 1494–1501 [DOI] [PubMed] [Google Scholar]
- Gomot M, Giard MH, Adrien JL, Barthelemy C, & Bruneau N (2002). Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology, 39, 577–584. [DOI] [PubMed] [Google Scholar]
- Hanson LK, Atance CM, & Paluck SW (2014). Is thinking about the future related to theory of mind and executive function? Not in preschoolers. Journal of experimental child psychology, 128, 120–137. [DOI] [PubMed] [Google Scholar]
- Herwig U, Satrapi P, & Schönfeldt-Lecuona C (2003). Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain topography, 16, 95–99. [DOI] [PubMed] [Google Scholar]
- Kaplan S, & Berman MG (2010). Directed attention as a common resource for executive functioning and self-regulation. Perspectives on Psychological Science, 5, 43–57. [DOI] [PubMed] [Google Scholar]
- Kerr A, & Zelazo PD (2004). Development of “hot” executive function: The children’s gambling task. Brain and cognition, 55, 148–157. [DOI] [PubMed] [Google Scholar]
- Kirkham NZ, Cruess L, & Diamond A (2003). Helping children apply their knowledge to their behavior on a dimension‐switching task. Developmental Science, 6, 449–467. [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, & Einstein GO (2002). Complex prospective memory and executive control of working memory: A process model. Psychological Test and Assessment Modeling, 44, 303. [Google Scholar]
- Klimesch W, Sauseng P, & Hanslmayr S (2007). EEG alpha oscillations: the inhibition–timing hypothesis. Brain research reviews, 53, 63–88. [DOI] [PubMed] [Google Scholar]
- Kloo D, Perner J, Aichhorn M, & Schmidhuber N (2010). Perspective taking and cognitive flexibility in the Dimensional Change Card Sorting (DCCS) task. Cognitive Development, 25, 208–217. [Google Scholar]
- Lopez‐Duran NL, Nusslock R, George C, & Kovacs M (2012). Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology, 49, 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucariello J, & Nelson K (1985). Slot-filler categories as memory organizers for young children. Developmental psychology, 21, 272. [Google Scholar]
- Marshall PJ, Bar-Haim Y, & Fox NA (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113, 1199–1208. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Nelson K (2001). Language and the self: From the” Experiencing I” to the” Continuing Me”. Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Novakovic-Agopian T, Chen AJW, Rome S, Abrams G, Castelli H, Rossi A, … & D’esposito M (2011). Rehabilitation of executive functioning with training in attention regulation applied to individually defined goals: a pilot study bridging theory, assessment, and treatment. The Journal of head trauma rehabilitation, 26, 325–338. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, & Zijdenbos A (2001). Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain research bulletin, 54, 255–266. [DOI] [PubMed] [Google Scholar]
- Peters J, & Büchel C (2010). Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron, 66(1), 138–148. [DOI] [PubMed] [Google Scholar]
- Prabhakar J, & Hudson JA (2014). The development of future thinking: Young children’s ability to construct event sequences to achieve future goals. Journal of experimental child psychology, 127, 95–109. [DOI] [PubMed] [Google Scholar]
- Russell J, Alexis D, & Clayton N (2010). Episodic future thinking in 3-to 5-year-old children: The ability to think of what will be needed from a different point of view. Cognition, 114(1), 56–71. [DOI] [PubMed] [Google Scholar]
- Saby JN, & Marshall PJ (2012). The utility of EEG band power analysis in the study of infancy and early childhood. Developmental Neuropsychology, 37, 253–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, & Buckner RL (2008). Episodic simulation of future events. Annals of the New York Academy of Sciences, 1124(1), 39–60. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, & Doppelmayr M (2005). Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. International Journal of Psychophysiology, 57, 97–103. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Doppelmayr M, Pecherstorfer T, Freunberger R, & Hanslmayr S (2005). EEG alpha synchronization and functional coupling during top‐down processing in a working memory task. Human brain mapping, 26, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, & Buckner RL (2008). Episodic simulation of future events. Annals of the New York Academy of Sciences, 1124, 39–60. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Benoit RG, & Szpunar KK (2017). Episodic future thinking: mechanisms and functions. Current Opinion in Behavioral Sciences, 17, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, & Jonides J (1999). Storage and executive processes in the frontal lobes. Science, 283, 1657–1661. [DOI] [PubMed] [Google Scholar]
- Swingler MM, Willoughby MT, & Calkins SD (2011). EEG power and coherence during preschoolers’ performance of an executive function battery. Developmental Psychobiology, 53, 771–784. [DOI] [PubMed] [Google Scholar]
- Tulving E (2002). Episodic memory: from mind to brain. Annual review of psychology, 53, 1–25. [DOI] [PubMed] [Google Scholar]
- Ünal G, & Hohenberger A (2017). The cognitive bases of the development of past and future episodic cognition in preschoolers. Journal of Experimental Child Psychology, 162, 242–258. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, & Mishkin M (1997). Differential effects of early hippocampal pathology on episodic and semantic memory. Science, 277, 376–380. [DOI] [PubMed] [Google Scholar]
- Watson AJ, & Bell MA (2013). Individual differences in inhibitory control skills at three years of age. Developmental neuropsychology, 38, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe CD, & Bell MA (2004). Working memory and inhibitory control in early childhood: Contributions from physiology, temperament, and language. Developmental psychobiology, 44, 68–83. [DOI] [PubMed] [Google Scholar]
- Wolfe CD, & Bell MA (2007). Sources of variability in working memory in early childhood: A consideration of age, temperament, language, and brain electrical activity. Cognitive Development, 22, 431–455. [Google Scholar]
- Wolfe CD, & Bell MA (2014). Brain electrical activity of shy and non-shy preschool-aged children during executive function tasks. Infant and Child Development, 23, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD (2006). The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nature protocols, 1, 297. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Anderson JE, Richler J, Wallner-Allen K, Beaumont JL, Conway KP, … & Weintraub S (2014). NIH Toolbox Cognition Battery (CB): Validation of executive function measures in adults. Journal of the International Neuropsychological Society, 20, 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Carter A, Reznick JS, & Frye D (1997). Early development of executive function: A problem-solving framework. Review of general psychology, 1, 198. [Google Scholar]
- Zelazo PD, Müller U, Frye D, Marcovitch S, Argitis G, Boseovski J, … & Carlson SM (2003). The development of executive function in early childhood. Monographs of the society for research in child development, i–151. [DOI] [PubMed] [Google Scholar]