Abstract
Objectives:
The purpose of this study was to determine if participant diagnosis, as determined by a health care provider, is associated with dietary supplement (DS) use.
Design/setting:
Surveys from 1255 study participants aged 34–84, part of the Midlife in the US Study (MIDUS 2 Survey) Biomarker Project, were reviewed. Participant data included pharmaceutical use (prescription and overthe- counter medications (OTC)), clinical symptoms and diagnosis, and laboratory results. Associations were calculated between the above participant characteristics and DS use.
Main outcome measures:
Frequency of DS use for physician-reported diagnoses.
Results:
Overall prevalence of DS use was 32.4%. Participants taking DS were more often female (p = .048), white (p < 0.001), and older (mean age 57 years, p < 0.001). Participants taking DS reported taking more OTC (p < .001) and prescription medications (p=.024), and had an increased number of chronic conditions (p = .004). Participants reporting physician-diagnosed diabetes were significantly less likely to be taking DS (p = .0066), while participants with eye disease (p = .001), high cholesterol (p = 0.041), cancer (p = 0.042), and arthritis (p = 0.044) were more likely to be taking DS than those without those conditions. No difference in DS use was found between patients with and without other identified medical conditions. After adjusting for age, race/ethnicity, and gender, only diabetes remained a significant predictor of decreased DS use (OR 0.588, CI 0.388–0.873, p = .01).
Conclusions:
Some physician-reported participant diagnoses were associated, positively or negatively, with DS use.
Introduction
Integrative health (IH), defined by the National Institutes of Health’s (NIH) National Center for Complementary and Integrative Health (NCCIH), involves the incorporation of “…complementary approaches into mainstream healthcare”.1There are a variety of treatment modalities that fall under the umbrella of IH in the United States, including the use of dietary supplements (DS). A DS is a product that is taken by mouth and can contain any of the following ingredients: vitamins, minerals, herbs or botanicals, amino acids, or other substances intended to supplement the diet.2,3 Among IH treatments, DS use is consistently one of the most common4. For instance, as per the 2012 National Health and Nutrition Examination Survey “NHANES”, up to 52% of the US population reported using DS in the past 30 days.5 With respect to economics, the total US expenditure for IH treatments is estimated at $30.2 billion, including $12.8 billion for DS.6
The medical literature details many aspects of DS use. For example, national trends of DS use seem to indicate an increase in DS use, with the second highest rate of growth (7.7%) in over a decade documented in 2016.5,7,8 Of note, even among data that report stable trends of use, there is an annual variability in the popularity of individual DS.4,8 With respect to demographics of DS users, there was an increase in use between 1999–2012 in those 65 and over, but a decrease among those 40–64 years old5. There is some agreement that DS use is more common in some ethnicities (non-Hispanic white) than others,9 and among women more than men.10
While the demographics of DS users remain fairly consistent across medical literature, there remains discrepancy about the conditions DS users have and the reasons why they may choose to use dietary supplements.11 With respect to diagnoses, adults with multiple chronic conditions (including hypertension, CAD, high cholesterol, cancer, diabetes, lung disease, arthritis, and depression) may be more likely to use multivitamins, vitamins, minerals, and non-vitamin or herbal therapies compared to adults without chronic conditions.12 Other studies have found that those with chronic but not life-threatening conditions were more likely to use dietary supplements compared to those with chronic and life-threatening conditions.13 However, an analysis of the 2007–2010 NHANES reported the most common reasons for using supplements were to “improve” or “maintain” overall health and found that organ-specific health reasons were less frequently reported as motivators to use dietary supplements.14 Further research is needed to understand the association of certain conditions with dietary supplement use as it pertains to disease prevention and treatment.
With respect to DS documentation, previous studies have prompted study participants choose the type of DS from pre-made lists without the opportunity for the participant to record their particular DS. Furthermore, specific combination products, brand names, or DS dose relevant to actual use may be lacking.15,16 There are some concerns that these methodologies for DS documentation may have limited research generalizability, and some experts are recommending a more complete description of DS use to provide standardization among DS studies.17
The MIDUS longitudinal study is a national survey intended to investigate the consequences of behavioral and psychosocial factors on health. The Biomarker Project of MIDUS 2 contains data from 1255 participants with clinical and biological assessments added to the medical history questionnaire of a subsample of respondents. The advantages of the MIDUS Biomarker Project include exploration of both self-reported diagnoses and those that originated with the participants’ health care provider. The database includes clinical assessments and laboratory data lending an additional layer of credibility to the information collected. Furthermore, with comprehensive DS use data collected from observation and recording the exact DS by research staff during site visits, there is a level of detail about the specific DS products being used that extends beyond prior studies. Overall, the goal of this study was to utilize the depth of information present in the Midlife in the United States (MIDUS) 2 Biomarker Project database to look for trends in DS use with a focus on participant diagnoses.
Methods
MIDUS 2 Project 4 Description
The Biomarker Project of MIDUS 2 contains data from 1,255 respondents from 2004–2009. These respondents include two distinct subsamples, all of whom completed the Project 1 Survey: (1) longitudinal survey sample (n = 1,054) and (2) Milwaukee sample (n = 201). The purpose of the Biomarker Project was to add comprehensive biological assessments on a subsample of MIDUS respondents, thus facilitating analyses that integrate behavioral and psychosocial factors with biology. A complete description of the MIDUS project and methodology is available in prior publications.10,18 To augment the self-reported data collected in the MIDUS survey project, participants completed a medical history, self-administered questionnaire, and self-reported sleep assessments (see below).
Dietary Supplement Collection and Charting
Respondents were instructed to bring all their medications (prescription and OTC) and DS, in the original bottles, to the inpatient study site for review by study personnel. See Table 1, below, for relevant information regarding the three categories documented. Of note, “alternative medicines”, or “Alt Med”, included non-vitamin, non-mineral DS; vitamins and minerals were included under the OTC category in MIDUS.
Table 1:
MIDUS “alternative medicines”, OTC medicine, pharmaceuticals, and their definitions and examples.
| Label, code, and description | Common examples (% of use by study participants) |
|---|---|
| Alternative medications (B4XAM) | |
| Include herbs, herbal blends (not including herbal teas), homeopathic remedies, and other alternative remedies. These may be purchased over the counter or they may be “prescribed” by a health care practitioner trained in a nonwestern tradition. | Fish Oil (10.68%) Miscellaneous (10.20%) Glucosamine/chondroitin (5.98%) Flaxseed oil (3.35%) Garlic (2.55%) CoQ10 (2.15%) |
| Over-the-counter (OTC) medications and products (B4XOM) | |
| Include vitamins, minerals, non-prescription pain, antacids, anti-diarrheas, fiber, lubricating eye or nose preparations etc. that the subject uses regularly and can be purchased “Over the Counter” (OTC) without a prescription. | Misc. vitamins (29.7%) Aspirin (28.7%) Multivitamins (26.5%) Vitamin C (14.7%) Calcium with Vitamin D (12.5%) Calcium (12.0%) |
| Prescription medications (B4XPM) | |
| Prescription pharmaceuticals | Lipitor (9.72%) Levothroid (8.61%) Simvastatin (7.97%) Hydrochlorothiazide (6.45%) Lisinopril (6.22%) Metformin (6.22%) |
During the inpatient study visit the research staff recorded medication information including medication name, dosage, frequency and route of administration, and how long the participant has been taking a given medication. In addition, each study participant was asked about their reasons for using a medication on a questionnaire “Why are you taking it?” and responses to this question were recorded verbatim. Standardized protocols as outlined in “Documentation for MIDUS and MIDJA Medication Data”19 were then applied to code text data describing reasons why participants think they are taking a given medication. Many participants were able to name specific conditions or diseases, but others gave more general responses such as “general health” or “bone health”. Each response was coded into two mutually exclusive sets of categorical codes based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) or a MIDUS code. The MIDUS code utilizes key words and common phrases in participant responses to form a standardized code label for each response. A separate MIDUS Biomarker Project Medical History questionnaire20 was also used that asked each participant about their extensive medical history including symptoms and conditions. If a participant had a certain symptom or condition, they were asked if it was or was not diagnosed by a physician.
Statistical Analysis
Data were extracted from the MIDUS database to determine the associations between participant characteristics and DS use. Participants taking all DS were identified by the variable B4XAM (number of alternative medications). Participant characteristics considered were doctor diagnosed diseases (heart disease, high blood pressure, anemia, high cholesterol, diabetes, asthma, cancer, arthritis, depression, thyroid disease, glaucoma, and other eye disease), health characteristics (BMI, number of chronic conditions, smoking status), prescription and OTC medication usage, and demographic information (age, gender, white or non-white race).
In an unadjusted analysis, patients were stratified by whether they were taking at least one alternative medication or none. Chi-square tests were used to determine associations between categorical variables and alternative medication use. The Mann-Whitney-Wilcoxon test was used to compare continuous numerical variables and alternative medication use. Despite the high number of unadjusted tests (27 tests), no p-value correction for Type I error rates were applied. When overall significance was found for a variable with more than two categories, post-hoc analysis was conducted using tests of proportions and correcting the p-value using the Bonferroni method.
The probability of taking an alternative medication with and without one of the doctor diagnosed diseases was reported along with 95% Agresti-Coull confidence intervals.
Adjusted models were fit to determine the association between taking alternative medications and each of the eleven doctor diagnosed conditions while adjusting for age, race (white or non-white), and gender. Estimated odds ratios with 95% confidence intervals and p-values from these models are reported. Significance was assessed with a type I error rate of 0.05.
Statistical analysis was performed with R 3.4.3 using the binom package, 1.1–1 by Sundar Dorai-Raj (https://CRAN.R-project.org/package=binom) to calculate confidence intervals.
Results
Records from 1,255 study participants were extracted. The overall prevalence of participants who take DS was 32.4% (95% Agresti-Coull CI 29.9–35.1), equivalent to an odds of 0.48 (CI 0.426–0.540).
Demographics and diagnostic differences between users and nonusers of DS are listed in Table 2. Participants taking DS tended to be older (p < 0.001), female (p = 0.041), and white (p < 0.001). Participants taking DS reported taking 2.20 supplements on average, and took more OTC (p < 0.001) and prescription medications (p = 0.024) than participants with no DS. DS use was associated with the number of chronic conditions (p = 0.004), but not with body mass index (p = 0.078). Furthermore, an association was found between DS use and tobacco use (p < 0.001); participants taking DS were less often current smokers (6.14% to 19.2%, p < 0.001), but there was no difference observed for former smokers (37.1% to 30.4%, p = 0.055) or never smokers (56.8% to 50.4%, p = 0.100).
Table 2:
Demographics and health characteristics of DS users and non-users and users of dietary supplements.
| Measure | No DS | DS | p-value |
|---|---|---|---|
| Total | 848 | 407 | |
| Demographics (count (%)) | |||
| Race, white | 626 (3.1) | 352 (1.8) | < 0.001 |
| Smoker | < 0.001 | ||
| Never | 427 (2.1) | 231 (1.2) | |
| Current | 163 (0.8) | 25 (0.1) | < 0.001 |
| Former | 258 (1.3) | 151 (0.8) | < 0.001 |
| Gender, female | 465 (2.3) | 248 (1.2) | 0.041 |
| Demographics (mean (sd)) | |||
| Age | 53.34 (11.74) | 56.98 (11.27) | 0.004 |
| BMI | 29.98 (6.78) | 29.32 (6.28) | 0.078 |
| Chronic conditions | 0.92 (1.29) | 1.13 (1.39) | 0.004 |
| Medications (mean (sd)) | |||
| DS | 2.20 (2.18) | ||
| OTC | 1.68 (1.71) | 3.22 (2.18) | < 0.001 |
| Prescriptions | 2.69 (2.91) | 2.98 (2.94) | 0.024 |
With respect to laboratory values (see Table 3), DS users had higher HDL (median 55 to 52, p = 0.017) and lower glucose values (median 96 to 97, p = 0.018). No difference was found in HbA1c (p = 0.212), total cholesterol (p = 0.124),triglycerides (p = 0.184), LDL (p = 0.403), CRP (p = 0.057), and insulin (p = 0.051).
Table 3:
Summary statistics and associations of physician-diagnosed conditions between users and non-users of dietary supplements.
| Measure | No DS | DS | Unadj. p-value | Adj. p-value |
|---|---|---|---|---|
| Total | 848 | 407 | ||
| Physician diagnosed conditions (count (%)) | ||||
| Heart disease | 93 (0.5) | 52 (0.3) | 0.348 | 0.992 |
| High blood pressure | 305 (1.5) | 154 (0.8) | 0.519 | 0.987 |
| Liver disease | 19 (0.1) | 9 (0.0) | 0.974 | 0.884 |
| Thyroid disease | 97 (0.5) | 58 (0.3) | 0.156 | 0.876 |
| Cancer | 104 (0.5) | 67 (0.3) | 0.042 | 0.663 |
| Depression | 161 (0.8) | 84 (0.4) | 0.489 | 0.469 |
| Glaucoma | 35 (0.2) | 13 (0.1) | 0.420 | 0.254 |
| Arthritis | 270 (1.3) | 153 (0.8) | 0.044 | 0.241 |
| High cholesterol | 338 (1.7) | 187 (0.9) | 0.041 | 0.224 |
| Anemia | 117 (0.6) | 72 (0.4) | 0.071 | 0.179 |
| Eye disease | 179 (0.9) | 122 (0.6) | < 0.001 | 0.089 |
| Asthma | 97 (0.5) | 57 (0.3) | 0.195 | 0.053 |
| Diabetes | 121 (0.6) | 36 (0.2) | 0.007 | 0.010 |
| Lab values (mean(sd)) | ||||
| Cholesterol | 185.50 (39.68) | 188.88 (41.13) | 0.124 | |
| CRP | 3.18 (5.04) | 2.70 (4.16) | 0.057 | |
| Glucose | 104.06 (32.46) | 98.12 (16.45) | 0.018 | |
| HA1C | 6.15 (1.30) | 5.98 (0.78) | 0.212 | |
| HDL | 54.60 (17.78) | 56.97 (18.28) | 0.017 | |
| Insulin | 13.88 (12.32) | 12.67 (14.58) | 0.051 | |
| LDL | 104.82 (34.79) | 107.00 (36.62) | 0.403 | |
| Triglycerides | 135.03 (148.52) | 127.25 (86.94) | 0.184 | |
Dietary supplement use and diagnosis
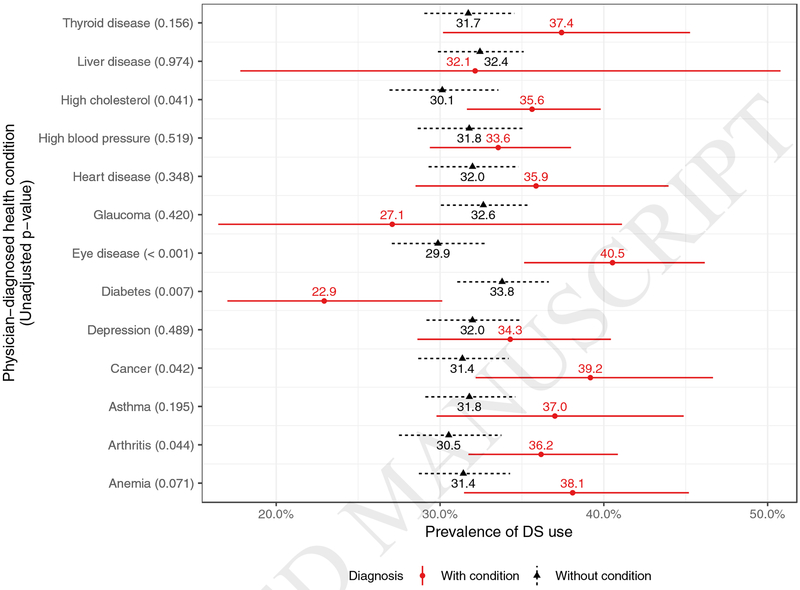
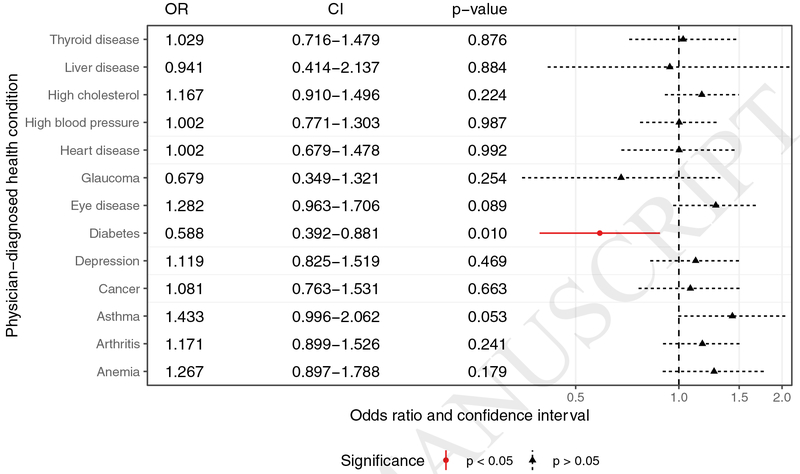
With respect to diagnoses that were reportedly diagnosed by physicians, study participants with diabetes were less likely to be taking DS (22.9% to 33.8%, p = 0.007) (Table 3, Figures 1–2). Alternatively, patients were more likely to be taking DS if they had eye disease (40.5% to 29.9%, p < 0.001), high cholesterol (35.6% to 30.1%, p = 0.041), cancer (39.2% to 31.4%, p = 0.042), and arthritis (36.2% to 30.5%, p =0.047), compared to study participants without those conditions. No difference in DS use was found between study participants with or without heart disease (p = 0.348), high blood pressure (p = 0.519), anemia (p = 0.071), asthma (p = 0.195), glaucoma (p= 0.420), liver disease (p = 0.974), depression (p = 0.489), or thyroid disease (p = 0.156). After adjusting for age, race, and gender, only diabetes remained a significant predictor of alternative medication use (Figure 2). Patients with diabetes had a 0.588 times lower odds of taking alternative medications than patients without diabetes of the same age, race, and gender (CI 0.388–0.873, p = 0.01).
Figure 1:
Rates of DS use for participants with and without each of the eleven physician-diagnosed diseases with illustrated 95% confidence intervals and Chi-square p-values.
Figure 2:
Adjusted odds ratios of taking DS between participants with and without each physician-diagnosed disease.
Discussion
The current analysis both supports and adds to the past literature on DS use. In the MIDUS 2 dataset, higher DS use was seen in study participants who were white, female, of an older mean age, nonsmokers, and who also used OTC and prescription medications. This is not dissimilar from an oft-referenced national study, the 2012 NHANES, which found DS users to be healthy individuals who were older, non-Hispanic white, reported lower alcohol use, and were nonsmokers.14 Other studies have corroborated the correlation between DS use and the use of OTC and prescription medications.10,21
With respect to diagnoses, significantly increased DS use was found among participants with eye disease (including glaucoma), cancer, elevated lipids, and arthritis, though these trends were found to be non-significant with the adjusted statistical analysis. Although study participants with physician-diagnosed elevated lipid levels showed borderline increased DS use (unadjusted), there was no difference in mean total cholesterol or triglycerides lab values between groups, though there was higher HDL (median 55 to 52) and lower glucose (median 96 to 97) amongst DS users. Though these latter values may be statistically significant, arguably they are not clinically significant. Objective measurements of disease may be an imperfect guide to accurate diagnosis; normal laboratory values could indicate controlled pathology, or, alternatively, a misdiagnosis. Further analysis of the dataset could help to differentiate the laboratory variables most likely to indicate disease or diagnoses, and most strongly associated with DS use..
The case of physician-diagnosed cancer and borderline, unadjusted, correlation with DS use was less than would have been expected based on the literature. Per the 2012 NHIS, cancer survivors were more likely to report using vitamins and minerals (75% vs. 61%, p<.001), as well as non-vitamin/mineral natural products (24% vs. 19%, p < .001).22 The Breast Cancer Quality of Care (BQUAL) Study identified breast cancer patients as the highest users of complementary and alternative medicine among all types of cancer. They found the prevalence of CAM use to be 87% among patients with dietary supplements used in 70% of patients.23 The current iteration of the MIDUS dataset did not differentiate those participants currently undergoing cancer treatment versus previous cancer diagnosis and treatment. It is not unfathomable, given themes in the medical literature regarding DS safety in oncological cases, that patients undergoing cancer treatment may be dissuaded from DS use by their oncology providers due to potential interactions with conventional treatments.
The only truly significant finding when adjusting for gender, age, and race/ethnicity was a decreased DS use among study participants with physician-diagnosed diabetes. There was, however, no clinically significant difference in mean A1C between DS users and nonusers, potentially indicating that people in this study with diabetes had adequate glycemic control. The lack of an association with DS use, though, is perplexing given that research findings have shown that people with diabetes may use medicinal plants either in association or instead of conventional physician-prescribed treatments.24,25 The Medical Expenditure Panel Surveys showed those with diabetes were 1.6 times more likely to use CAM than those without diabetes. The 2012 NHIS reported that in the past year more than 2 million older adults with diabetes (25%) used IH treatments. Of those older adults utilizing some sort of IH treatment, 62.8% utilized herbal therapies.26 Additionally, several recent randomized controlled trials have shown certain “natural health products” to lower A1C by at least 0.5% such as aloe (Aloe vera), Hintonia latiflora, and various traditional Chinese medicine herbs.27 The result of decreased use of DS in our study could be due to limitations in diversity of our predominantly white study population. Perhaps health care providers are acting on published concerns about supplement-pharmaceutical interactions28 and counseling their patients with diabetes to discontinue their DS use.
Interestingly, this analysis did not document an increase in DS use among patients with coronary artery disease and hypertension, in contrast to other recent analyses.21,29 Since 2000, reports on adverse effects of dietary supplements and interactions have drastically increased28 and much of this research is targeted towards clinicians to be aware of these potential adverse events and talk to their patients about them. It is possible that physicians are telling their patients now more than ever to stop taking their dietary supplements especially with life-threatening chronic diseases. Such a situation would agree with one analysis of prescription medication users with coronary artery disease and a history of MI who were found to have the lowest rates of nonvitamin dietary supplements.13 People in that analysis with chronic but not life-threatening conditions like menopause, chronic GI conditions, and headaches tended to have higher DS use while those with chronic and life-threatening conditions (CV disease, stroke, and diabetes), had lowest rates of DS use.
Limitations of this analysis include the fact that the majority of the study participants are “healthy”, and of a narrow age, race, and ethnicity, perhaps limiting the generalizability of the results to the US population. In addition, of this study cohort, a relatively low number were taking DS, restricting the analysis of sub-groups to even smaller numbers, contributing to study biases and statistical deficiencies. Finally, the physician-diagnosed conditions reported in the MIDUS dataset had little overlap with the participants’ verbatim responses for their reasons for dietary supplement use. Further analysis should be performed to determine the association between the participants’ physician-diagnosed conditions and their reasons for DS use.
The MIDUS dataset does provide advantages in the information about DS use and related factors. For example, there is an extension of self-reported diagnoses to diagnoses as mentioned by health care providers, potentially improving the accuracy of documented diagnoses. Furthermore, study participants had objective data (laboratory findings), lending another layer of commentary on health status relevant to diagnoses and subsequent associations with DS use. In addition, ingested DS were also verified by study personnel; most prior work was based merely on survey data and self-report. These factors, arguably, contribute to improved accuracy of information and clinical applicability.
Conclusions
Participants reporting physician-diagnosed diabetes were significantly less likely to be taking DS, while participants with eye disease, high cholesterol, cancer, and arthritis were more likely to be taking DS than those without those conditions. No difference in DS use was found between patients with and without other identified medical conditions. After adjusting for age, ethnicity, and gender, only diabetes remained a significant predictor of decreased DS use.
Highlights.
We do not have a Graphical Highlights option for our paper.
Acknowledgements
Funding for this project came from several sources, including the University of Wisconsin Department of Family Medicine and Community Health Summer Student Research and Clinical Assistantship (SSCRA) program (JF), and National Institute on Aging grants #U19AG051426 and #P01AG020166 (DK, GL).
Funding sources:
National Institute on Aging grants #U19AG051426 and #P01AG020166 University of Wisconsin Department of Family Medicine and Community Health Summer Student Research and Clinical Assistantship (SSCRA) program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Contributor Information
Julie Friedman, University of Wisconsin School of Medicine and Public Health
Jen Birstler, University of Wisconsin-Madison Department of Biostatistics and Informatics
Gayle Love, University of Wisconsin-Madison Institute on Aging
David Kiefer, University of Wisconsin Department of Family Medicine and Community Health.
References
- 1.National Center for Complementary and Integrative Health. Complementary, Alternative, or Integrative Health: What’s In a Name? [Internet]. National Center for Complementary and Integrative Health. Complementary, Alternative, or Integrative Health: What’s In a Name? 2017. [cited 2018 Jul 23];Available from: http://nccih.nih.gov/health/integrative-health#integrative.
- 2.U.S. Food and DrugAdministration. Dietary Supplements: What You Need To Know [Internet]. U.S. Food and Drug Administration. Dietary Supplements: What You Need To Know. 2017. [cited 2018 Jul 23];Available from: https://www.fda.gov/Food/DietarySupplements/UsingDietarySupplements/ucm109760.htm
- 3.National Center forComplementary and Integrative Health. Dietary and Herbal Supplements [Internet]. National Center forComplementary and Integrative Health. Dietary and Herbal Supplements. 2018. [cited 2018 Jul 23];Available from: https://nccih.nih.gov/health/supplements
- 4.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report 2015;(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA 2016;316(14):1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahin RL, Barnes PM, Stussman BJ. Expenditures on complementary health approaches: united states, 2012. Natl Health Stat Report 2016;(95):1–11. [PubMed] [Google Scholar]
- 7.Wu C-H, Wang C-C, Tsai M-T, Huang W-T, Kennedy J. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 national health interview surveys. Evid Based Complement Alternat Med 2014;2014:872320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith T; Kawa K; Eckl V; Morton C; Stredney R American Botanical Council: US Sales of Herbal Supplements Increase by 7.7% in 2016. Herbal Gram: The Journal of the American Botanical Council 2017;(115):56–65. [Google Scholar]
- 9.Gardiner P, Whelan J, White LF, Filippelli AC, Bharmal N, Kaptchuk TJ. A systematic review of the prevalence of herb usage among racial/ethnic minorities in the United States. J Immigr Minor Health 2013;15(4):817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiefer DS, Chase JC, Love GD, Barrett BP. The overlap of dietary supplement and pharmaceutical use in the MIDUS national study. Evid Based Complement Alternat Med 2014;2014:823853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardiner P, Graham R, Legedza ATR, Ahn AC, Eisenberg DM, Phillips RS. Factors associated with herbal therapy use by adults in the United States. Altern Ther Health Med 2007;13(2):22–9. [PubMed] [Google Scholar]
- 12.Falci L, Shi Z, Greenlee H. Multiple chronic conditions and use of complementary and alternative medicine among US adults: results from the 2012 national health interview survey. Prev Chronic Dis 2016;13:E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardiner P, Graham RE, Legedza ATR, Eisenberg DM, Phillips RS. Factors associated with dietary supplement use among prescription medication users. Arch Intern Med 2006;166(18):1968–74. [DOI] [PubMed] [Google Scholar]
- 14.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med 2013;173(5):355–61. [DOI] [PubMed] [Google Scholar]
- 15.CDC National Health and Nutrition Examination Survey. National Health and Nutrition Examination Survey: 2013–2014 Data Documentation, Codebook, and Frequencies [Internet]. 2016. [cited 2019 Feb 14];Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/DS1IDS_H.htm
- 16.2012 NHIS Sample Adult Alternative Health Questionnaire2012 NHIS Questionnaire - Adult CAM [Internet]. CDC 2012 NHIS; 2013. [cited 2019 Feb 14]. Available from: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Survey_Questionnaires/NHIS/2012/English/qalthealt.pdf
- 17.Gagnier JJ, Boon H, Rochon P, et al. Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration. J Clin Epidemiol 2006;59(11):1134–49. [DOI] [PubMed] [Google Scholar]
- 18.Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS national study of health andwell-being. J Aging Health 2010;22(3):307–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryff CD, Kawakami N, Kitayama S, Karasawa M, Markus H, Coe C. MIDUS and MIDJA Medication Data Documentation. University of Wisconsin Institute on Aging; 2016. [Google Scholar]
- 20.Ryff CD, Seeman T, Weinstein M. Midlife in the United States (MIDUS 2): Biomarker Project, 2004–2009 Medical History Questionnaire.
- 21.Rashrash M, Schommer JC, Brown LM. Prevalence and predictors of herbal medicine use among adults in the united states. J Patient Exp 2017;4(3):108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John GM, Hershman DL, Falci L, Shi Z, Tsai W-Y, Greenlee H. Complementary and alternative medicine use among US cancer survivors. J Cancer Surviv 2016;10(5):850–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenlee H, Neugut AI, Falci L, et al. Association between complementary and alternative medicine use and breast cancer chemotherapy initiation: the breast cancer quality of care (BQUAL) study. JAMA Oncol 2016;2(9):1170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemley M, Spies LA. Traditional beliefs and practices among Mexican American immigrants with type II diabetes: A case study. J Am Assoc Nurse Pract 2015;27(4):185–9. [DOI] [PubMed] [Google Scholar]
- 25.Giovannini P, Howes M-JR, Edwards SE. Medicinal plants used in the traditional management of diabetes and its sequelae in Central America: A review. J Ethnopharmacol 2016;184:58–71. [DOI] [PubMed] [Google Scholar]
- 26.Rhee TG, Westberg SM, Harris IM. Use of complementary and alternative medicine in older adults with diabetes. Diabetes Care 2018;41(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diabetes Canada Clinical Practice Guidelines Expert Committee, Grossman LD, Roscoe R, Shack AR. Complementary and alternative medicine for diabetes. Can J Diabetes 2018;42 Suppl 1:S154–61. [DOI] [PubMed] [Google Scholar]
- 28.Choi JG, Eom SM, Kim J, et al. A Comprehensive Review of Recent Studies on Herb-Drug Interaction: A Focus on Pharmacodynamic Interaction. J Altern Complement Med 2016;22(4):262–79. [DOI] [PubMed] [Google Scholar]
- 29.Grant SJ, Bin YS, Kiat H, Chang DH-T. The use of complementary and alternative medicine by people with cardiovascular disease: a systematic review. BMC Public Health 2012;12:299. [DOI] [PMC free article] [PubMed] [Google Scholar]