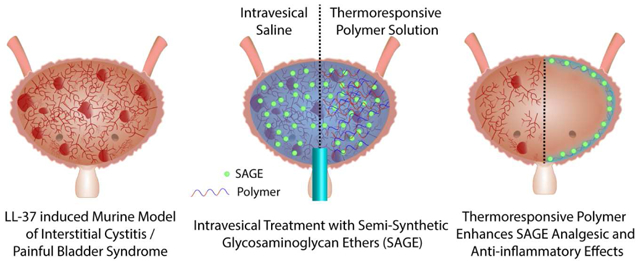
Abstract
Interstitial cystitis (IC), also known as painful bladder syndrome, is a debilitating chronic condition with many patients failing to respond to current treatment options. Rapid clearance, mucosal coating, and tight epithelium create strong natural barriers that reduce the effectiveness of many pharmacological interventions in the bladder. Intravesical drug delivery (IDD) is the administration of therapeutic compounds or devices to the urinary bladder via a urethral catheter. Previous work in improving IDD for IC has focused on the sustained delivery of analgesics within the bladder and other small molecule drugs which do not address underlying inflammation and bladder damage. Therapeutic glycosaminoglycans (GAG) function by restoring the mucosal barrier within the bladder, promoting healing responses, and preventing irritating solutes from reaching the bladder wall. There is an unmet medical need for a therapy that provides both acute relief of symptoms while alleviating underlying physiological sources of inflammation and promoting healing within the urothelium. Semi-synthetic glycosaminoglycan ethers (SAGE) are an emerging class of therapeutic GAG with intrinsic anti-inflammatory and analgesic properties. To reduce SAGE clearance and enhance its accumulation in the bladder, we developed a silk-elastinlike protein polymer (SELP) based system to enhance SAGE IDD. We evaluated in vitro release kinetics, rheological properties, impact on bladder function, pain response, and bladder inflammation and compared their effectiveness to other temperature-responsive polymers including Poloxamer 407 and poly(lactic-co-glycolic acid)-poly(ethylene glycol). SAGE delivered via SELP-enhanced intravesical delivery substantially improved SAGE accumulation in the urothelium, provided a sustained analgesic effect 24 hrs. after administration, and reduced inflammation.
Keywords: Interstitial Cystitis, Painful Bladder Syndrome, Silk-Elastinlike Protein, Temperature-Responsive Polymer, Intravesical Drug Delivery, Glycosaminoglycan
Graphical abstract:
1.0. Introduction:
1.1. Intravesical Therapy
Interstitial Cystitis (IC), also known by descriptive epithet painful bladder syndrome (PBS), is a chronic condition where lower abdominal pain, urinary frequency, sexual dysfunction, and urgency lead to major detrimental impacts on a patient’s quality of life.[1] In spite of afflicting between 3.3 and 7.9 million people in the United States each year, there is a lack of effective treatment options available for these patients. A study of 581 women with IC/PBS over several years found that they underwent 183 different types of therapy in attempts to ameliorate their symptoms.[2] A simple and direct treatment that alleviates symptoms while helping to restore underlying bladder function is needed.
Therapeutic glycosaminoglycans (GAG) are a broadly used treatment for IC/PBS and function by restoring the mucosal barrier within the bladder and preventing irritating solutes from reaching the bladder wall. However, the predominant therapeutic GAG used, pentosan sulfate, lacks analgesic and anti-inflammatory effects.[3] While providing temporary analgesic relief, lidocaine and other analgesic moieties such as botulinum toxin fail to repair underlying damage and offer only superficial bladder anesthesia.[4–7] Even multi-modal treatments where GAGs are combined with analgesics result in only temporary relief lasting up to 12 hours.[8] Intravesical treatment with analgesics provide short-lived and ultimately unsatisfactory relief. There are several ongoing trials for botulinum toxin A delivered in saline, thermoresponsive hydrogels, or liposomes and there are some promising initial results in their potential to prevent bladder urgency and pain, but data reflect that they have limited utility in resolving the underlying pathology.[9–11] There is an unmet medical need for a therapy that provides both acute relief of symptoms while alleviating underlying physiological sources of inflammation and promote healing within the urothelium.
Intravesical delivery of semisynthetic glycosaminoglycan ethers (SAGE) has the potential to overcome the shortcomings of current GAG-based treatment by offering both mucosal restoration and potent analgesic and anti-inflammatory effects.[3] SAGE GM-0111 has previously shown increased anti-inflammatory effects compared to pentosan sulfate and lacks the anticoagulation-associated risks of heparin.[12] However, a prolonged residence time in the bladder for drugs remains a challenge as patients can typically only tolerate intravesical solutions for 60–90 minutes.[4]
Low retention, dilution in urine, and rapid clearance are barriers to effective pharmacological intervention in IC/PBS and result in decreased effect size and frequent treatment failure.[13] Intravesical drug delivery (IDD) via urethral catheter allows for the direct administration of higher concentrations of drugs to the urinary bladder. Intravesical treatment maximizes the concentration of the therapeutic within the bladder while minimizing systemic toxicities. However, the mucous coating of the bladder, tight epithelium, dilution from urine, and rapid clearance during urination create substantial barriers to drugs penetrating the urothelium and achieving sustained therapeutic action.[14] For example, IDD of lidocaine has a residence time of only 20–60 minutes in the bladder prior to clearance. Devices that can prolong delivery have been developed, but must eventually be retrieved and can lead to infection, irritation, obstruction, and other complications.[13] Other formulation approaches for IDD that do not leave behind a device include liposomes, mucoadhesive particles, mucopenetrating particles, and in situ gelling depots such as Poloxamer 407 and poly(lactic-co-glycolic acid) (PLGA)- poly(ethylene glycol) (PEG) copolymers. These systems have been mainly used for small molecule analgesic delivery. While there is extensive research for intravesical delivery of small molecule drugs for the treatment of bladder cancer or urinary tract infections, there are far fewer IDD systems that have been developed for IC/PBS, and none have focused on improving GAG delivery.[4,7,13,15] The delivery of hydrophilic drugs particularly with high molecular weights remains a challenge in the bladder. Successful pharmacological interventions in the bladder is tied to the ability for drugs to reach and then maintain local therapeutic concentrations. Temperature-responsive polymers offer a direct mechanism to enable the transition from an injectable solution into a solid gel to enable localized retention of a therapeutic payload.[16] The physicochemical properties of SAGE can interfere with many standard chemistries used to chemically crosslink polymer systems. Temperature-responsiveness also enables off-the-shelf functionality in the clinic without the need for specialized delivery devices.
Silk-elastinlike protein polymers (SELP) offer substantial potential as drug delivery carriers.[17–19] Exquisite control over polymer structure with tunable gelation and mechanical properties has been achieved based on silk-to-elastin ratio and sequence, incorporation of biodegradable sequences at specific sites, and control over molecular weight.[20–23] Herein, we selected SELPs with appropriate structures to readily be able to transition to form stable gel networks at body temperature and contain positively charged guest residues for interaction with SAGE.
We hypothesized that SAGE GM-0111 is an effective potential therapy for ICP/PBS and its effectiveness can be enhanced via intravesical delivery with SELP. This mixture of protein and GAG mimics the natural components of the mucous membrane within the bladder. To enhance SAGE GM-0111 IDD, we created a silk-elastinlike protein polymer (SELP) intravesical delivery system. We investigated the release kinetics, viscoelastic properties, impact on SAGE accumulation and residence time in the bladder, bladder function, and therapeutic effect in a murine model of IC/PBS. The effectiveness of SELP was tested against two other in situ gelling polymer systems, namely Poloxamer 407 and PLGA-PEG-PLGA that have been explored for improving intravesical therapy in clinical trials.
2.0. Materials and Methods:
2.1. Polymeric Materials
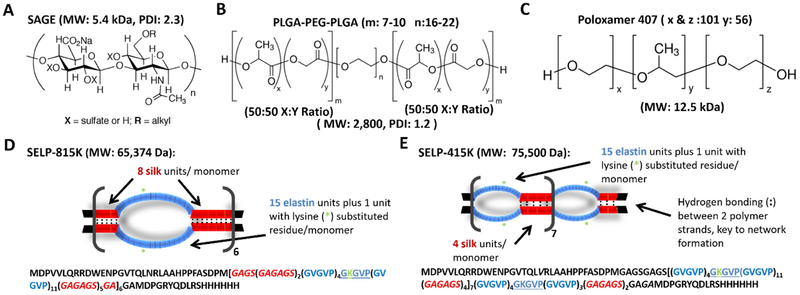
SAGE GM-0111 was purchased from GlycoMira Therapeutics, Inc. (Salt Lake City, UT) (Structure and characteristics shown in Figure 1A). PLGA-PEG-PLGA was purchased from Sigma Aldrich (St. Louis, MO). Poloxamer 407, also known as Polyethylene-Polypropylene Glycol, poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) copolymer (PEO-PPO-PEO), and the tradenames Pluronic® F-127 and Speronic PE/F 127, was purchased from Spectrum Chemical Mfg. Corp. (New Brunswick, NJ). The structures and characteristics of these polymers from manufacturer’s lot characterization are shown in Figure 1B and 1C, respectively. SELP 815K was expressed, characterized, and shear-processed as previously described (structure shown in Figure 1D).[20,22,24,25] SELP 415K was synthesized as previously described (structure shown in Figure 1E).[26] SELP 415K and SELP 815K were selected for consideration as they readily form hydrogels at 37°C and have positively charged lysine residues located in their elastin-like portions of their sequence to facilitate interaction with negatively charged SAGE. SELP was stored at −80° C freezer loaded in luer lock-syringes (Becton Dickinson, Franklin Lakes, NJ) and thawed at room temperature water at the time of use.
Figure 1: Structures and characteristics of polymers used in this study.
A) The therapeutic semisynthetic glycosaminoglycan (SAGE) GM-0111, B) poly(lactic-co-glycolic) acid (PLGA)-poly(ethylene glycol) (PEG)-PLGA C) Poloxamer 407 D) & E) Graphical depictions of silk-elastinlike protein polymer (SELP) 815K- and SELP-415K structures, respectively. The single letter amino acid code for each polymer is included below the structures. Red represents silk-like units forming a rigid backbone, and blue signifies the flexible elastin-like units that allow for pore formation, : is used to convey the hydrogen bonding between the silk-like units which gives the gels their mechanical strength, and the green asterisk indicates the lysine substituted elastinlike motif. MW: Molecular Weight, PDI: Poly dispersity index.
2.1. In vitro release and polymer formation
The release of SAGE GM-0111 from high and low concentrations of SELP 815K, SELP 415K, PLGA-PEG-PLGA, and Poloxamer 407 was quantified using an Azure A based colorimetric assay method. The high and low concentrations tested were based upon concentrations previously reported in the literature used for the sustained delivery and the dissolution needed to see a substantial difference in rheological properties and gel formation.[27–32] SELP 815K and SELP 415K were evaluated at 12% and 4% (wt/wt). Below 3–4% (wt/wt) these copolymers do not form a cohesive gel matrix, and the possibility of spontaneous gelation during shear processing of the material exists above 12% (wt/wt).[24] Poloxamer 407 and PLGA-PEG-PLGA were dissolved in chilled phosphate buffered saline (referred to as saline through the remaining manuscript) pH 7.4 (ThermoFisher Scientific, Waltham, MA) at 20% and 10% (wt/vol). 20% (wt/vol) Poloxamer 407 is sufficient to form a robust gel, and 10% (wt/vol) is right at the edge of its ability to undergo a temperature responsive transition.[32] 10 mg/ml of lyophilized SAGE GM-0111 was added to each polymer solution and a saline solution as a control. Samples of these solutions were set aside for further characterization. 20 μL of each mixture was placed in a sterile tube and incubated at 37 °C in a humidified incubator for 12 hr. 1 mL of prewarmed Surine™ simulated urine (Sigma Aldrich, St. Louis, MO) was added to each tube and then they were incubated in a SteadyShake™ 757 (Amerex Instruments Inc., Concord, CA) incubator at 37 °C and 120 rpm. 100 μL samples of release media were taken and replaced with clean media after 1 min, 5 min, 15 min, 30 min, 1 hr, 3 hr, 6 hr, 12 hr, and 24 hr. The content of SAGE in the release media was determined using Azure A as previously described.[27]
2.2. Tilt testing
A tilt test was used to observationally assess the gelation of the thermoresponsive polymer under conditions similar to intravesical administration. Solutions of SELP 815K, SELP 415K, PLGA-PEG-PLGA, and Poloxamer 407 at the high and low concentrations loaded with 10 mg/ml of SAGE GM-0111 were cooled to 4 °C. 400 μL of the solution was then placed in glass vial (ThermoFisher Scientific, Waltham, MA) and sealed. The solutions were tilted 90 °C and photographed (images were globally white balanced and cropped to remove extraneous background). They were then placed in a water bath set to 37 °C for 30 sec, 1 min, 2 min, 3 min, 5 min, 10 min, 15 min, 30 min. and 60 min. The samples were then set overnight in a 37 °C water bath prior to further characterization.
2.3. Electron microscopy of SAGE loaded gels
Scanning electron microscopy was used to assess the structure of the gel following formation. Solutions of SELP 815K, SELP 415K, PLGA-PEG-PLGA, and Poloxamer 407 at the high and low concentrations loaded with 10 mg/ml cured overnight in a 37 °C water bath were flash frozen in liquid nitrogen and lyophilized on a FreeZone 12 (Labconco, Kansas City, MO) at ≤ −50 °C and ≤0.005 mBar for 4 days. The lyophilized samples were placed on carbon tape and sputter-coated with 5nm of gold-palladium and imaged on an FEI Quanta 600F (ThermoFisher Scientific, Waltham, MA) with a kV of 10.00.
2.4. Rheological characterization:
The viscosity and rheopectic properties of the gels were assessed using a TA Instruments AR550-Stress Controlled Rheometer (New Castle, DE) with a 20 mm 4° cone-and-plate configuration. Evaporation during the run was prevented using a solvent trap. A viscosity sweep was performed from 18 to 37 °C (5.76 °C/min) at an angular frequency of 6.283 rad/s. A 3 hr oscillatory sweep at 37 °C with 0.1% strain followed as described previously.[23,24] All tests were performed in triplicate using separately prepared samples.
2.5. Animal Care and Ethics Statement
The animal facilities at the University of Utah are American Association for the Accreditation of Laboratory Animal Care (AAALAC) accredited. Before beginning studies, all procedures were reviewed by the Institutional Animal Care and Use Committee of the University of Utah (Protocol #: 1106010), and all experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.[33] Animals were maintained on a standard 12 hr. light/dark cycle and given food and water ad libitum. Mice were randomly allocated to each experimental group and housed together if possible. Experiments were performed during the light portion of the cycle within their care facility or dedicated procedure room. Paperchip® (Shepherd Specialty Papers, Watertown, TN) was used as the primary beading and mice were provided with an Enviropak Nestpak® filled with envirodri® (Fibercore, Cleveland, OH). The health of all mice was assessed daily by either a veterinarian or a trained veterinary technician.
2.6. Qualitative assessment of intravesical accumulation of SAGE:
The impact of thermoresponsive polymer matrices on SAGE accumulation within the bladder urothelium was assessed using fluorescent microscopy. SAGE GM-0111 was labeled with CF™633 Hydrazide (Biotium, Inc., Fremont, CA) as previously described.[12] Labeled SAGE GM-0111 was diluted at a 1:9 ratio to ensure sufficient material for all animals in the study to receive a uniform batch. Animals received 10 mg/ml of fluorescently doped SAGE in Poloxamer 407 20% (wt/vol), PLGA-PEG-PLGA 20% (wt/vol), SELP 415K 12% (wt/wt), SELP 815K 12% (wt/wt), or phosphate buffered saline. Healthy female C57BL/6 mice 11 weeks of age were used for intravesical delivery. Female mice were selected for this study as IC/PBS diagnosis in the clinic has a female-to-male ratio of 10:1.[1] Mice were anesthetized using 1.5–3% isoflurane in oxygen. The bladder was accessed via 1.5 cm long 0.64 mm OD × 0.3 mm ID piece Dow Corning Silastic® (VWR, Radnor, PA) tubing coated in Surgilube® (HR Pharmaceuticals, Inc., York, PA) inserted into the urethra. Urine was then drained through the catheter using gentle suprapubic palpitations. To the empty bladder, 50 μL of test solution was administered followed by immediate withdrawal of the catheter. After 3, 12, and 24 hr. mice (n=3, per group per time point) were sacrificed. The bladders were hemisected at necropsy, fixed in 10% formalin, dehydrated in xylene, embedded in paraffin, sectioned to 0.5 μm thick, and stained with Hoechst 33342. Tissue sections were then imaged on an Olympus Confocal FV1000 microscope at 20x magnification with an excitation of 640±15 nm and emission of 690±15 nm for CF™−633 bound to SAGE GM-0111 and a second channel set to an emission of 350±15 nm and excitation of 460±15 nm for Hoechst 33342.
2.7. Creation of a murine IC/PBS model using LL-37 and treatment with SAGE GM-0111
To evaluate the impact of thermoresponsive polymer formulations on the therapeutic potential of SAGE GM-0111, we treated a mouse model of IC/PBS. The human cathelicidin LL-37, a signaling and anti-microbial peptide found in the urine of IC/PBS patients, was used to induce IC/PBS in 8–10 week old female C57BL/6 mice.[34] This generates a model that mimics the inflammation and pain associated with IC/PBS.[35–37] LL-37 (peptide sequence: LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) was obtained from the DNA/Peptide Synthesis Core at the University of Utah and purified using preparative high-performance liquid chromatography. The peptide was prepared as a 320 μM solution in water and then delivered as a 50 μL treatment via catheter to the bladder as described above. LL-37 was allowed to dwell for 1 hr in order to induce an IC/PBS-like condition. SAGE GM-0111 was delivered via intravesical administration to the bladder at a concentration of 10 mg/ml in 50 μL test solutions including phosphate buffered saline, SELP 815K 12%, SELP 415K 12%, PLGA-PEG-PLGA 20%, and Poloxamer 407 20% (n=6 in each group). The test solution was allowed to dwell for 5 minutes before removing the instillation catheter. The intravesical administration of phosphate buffered saline was used as a sham treatment control in animal studies (n=6). Additionally, a set of mice that were catheterized but did not undergo LL-37 insult referred to as intact mice in this manuscript, were used as reference control to better understand baseline variability in test methods, validate LL-37 induction of an IC/PBS-like disease state, and expected values within a healthy population (n=6).
2.8. Impact on bladder function using void spot assays
The delivery of therapeutic compounds via intravesical delivery of in situ gelling polymers to the bladder can potentially impact bladder function by occupying volume, affecting bladder elasticity, or obstructing clearance through the urethra. Bladder function was assessed using a void spot assay (VSA). Mice were placed into individual wire mesh-bottomed cages over filter paper (ThermoFisher Scientific, Rockford, IL) for 4 hr. in a quiet room during the middle of their day cycle. The filter paper was collected, dried, and then imaged on an Alpha Innotech FluorChem SP Digital Imaging System under ultraviolet light at 365 nm. The area and number of void spots were calculated by analyzing the images using ImageJ. Volume was calculated from the area of the spots compared to a calibration curve. Spots under 6.6 mm2, corresponding to a volume of 0.5 μL, were excluded from consideration. Mice that did not have any voiding events during the test period were excluded from calculation of average voiding volume.
2.9. Evaluation of treatment efficacy via behavioral assessment of lower abdominal pain
Pain is one of the central aspects of IC/PBS and a major contributor to the decrease in patient’s quality of life. SAGE GM-0111 effectiveness as an analgesic intravesical therapy, delivered in solutions of phosphate buffered saline, SELP 815K 12%, SELP 415K 12%, PLGA-PEG-PLGA 20%, or Poloxamer 407 20%, was behaviorally assessed using tactile stimulation. Mice in individual polycarbonate enclosures with a wire mesh base were given at least 10 min prior to testing for acclimation. Von Frey filaments corresponding to 0.04, 0.16, 0.4, 1.0, and 4.0 grams of force (g) were used to generate consistent levels of stimulation to the suprapubic zone over the bladder. Each filament was applied in 10 successive tests for 1 sec with at least 3 sec between each application. Each test was administered to a unique site to prevent wind-up. Positive indicators of pain included a jump, a sharp retraction of the abdomen, or immediate scratching or licking of the stimulated area.[38] The number of positive responses out of the total number of trials at each stimulation level is the response rate. The baseline response rate for each mouse was determined before exposure to LL-37. The effect of treatments was assessed 24 hr. after administration. The degree of analgesic effect was calculated by taking the difference between the post-treatment and baseline response rates at the highest degree of stimulation. The impact on hyperalgesia, or heightened sensitivity to pain, was measured by calculating the area under the curve (AUC) using the trapezoidal rule of the dose-response curve.[39] The AUC was then normalized to the Sham treatment control, where the mice received only saline injections. Allodynia, or the sensitization to normally non-painful stimulation, was assessed by determining the stimulation level at which the animals exceeded a 30% increase in response rate over baseline.
2.10. Examination of bladder inflammation
Inflammation is central to most etiological theories and treatment strategies for IC/PBS. Gross examination of bladder anatomy was conducted at necropsy with attention given to urinary retention, swelling, erythema, and other signs of inflammation. Each bladder was resected and then hemisected to allow for inspection to assess the extent of edema, hemorrhage, urothelial erosion, and ulceration at 10x magnification. Half of the bladder was fixed in formaldehyde and prepared with hematoxylin and eosin for histological evaluation, as described previously.[35] The other half of the tissue was homogenized. As a general marker of bladder inflammation, the myeloperoxidase (MPO) concentration was measured using a Hycult® mouse MPO Kit (Biotech Inc., Plymouth Meeting, PA). The concentration of MPO was normalized to the protein content using a Pierce™ Coomassie Plus (Bradford) assay kit (ThermoFisher Scientific, Rockford, IL).
2.11. Statistical Analysis
Statistics were performed using GraphPad Prism ™ 5.0 (GraphPad Software, CA) for mechanical characterization and release data and R version 3.5.2 (The R Foundation for Statistical Computing, Vienna, Austria). A Grubbs test was used to identify outliers and exclude them from analysis in vitro. Results from in vivo were assumed normal unless they were identified as non-parametric using a Shapiro-Wilks test. A Student’s T-test for paired sets of data and a one-way analysis of variance (ANOVA) with a post-hoc Bonferroni Multiple Comparison Test were used to compare parametrically distributed sets of data with 3 or more groups. For non-parametrically distributed data, a Kruskal-Wallis test followed by a post-hoc Dunn’s test for group comparisons was used to assess statistical significance. No outliers were excluded from in vivo results as they may represent important but infrequent events. A p-value of less than 0.05 was used as the threshold of statistical significance in this manuscript.
3.0. Results:
3.1. Controlled release of SAGE from thermoresponsive polymers
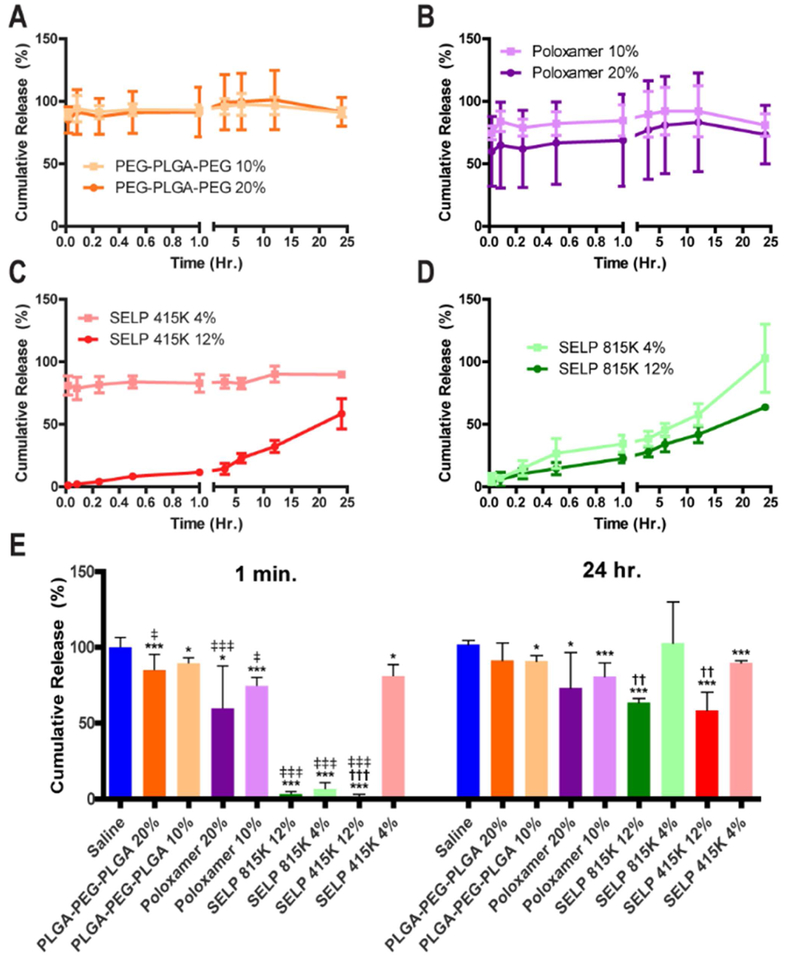
To test if SAGE could be efficiently released from the polymer gels, we measured the release of SAGE over a 24-hour period. A high and low concentration for each polymer was tested based upon past reports in the literature for achieving controlled release of therapeutics for each polymer. The synthetic polymers Poloxamer 407 and PLGA-PEG-PLGA and the low concentration of SELP 415K showed massive burst release with over 80% of the payload being dumped within one min in simulated urine (See Figure 2 A, B, and C). Only SELP 815K and the high concentration of SELP 415K showed controlled release profiles that best fit a zero-order release profile with linear correlation coefficients (R2) of 0.88 and 0.972, respectively within the 24 hr. period of observation (See Figure 2 C, D). Only the SELPs showed statistically significant effects on SAGE release associated with their concentration (See Figure 2 E). All of the high concentrations showed greater than 50% release over 24 hours and were selected for rheological characterization.
Figure 2: Cumulative release of SAGE from different thermoresponsive polymer gels at varying concentrations.
The plots for A) PLGA-PEG-PLGA, B) Poloxamer 407 (Pluronic F127), C) SELP 415K, and D) SELP 815K show the release profile for each structure at a high and low concentration. E) The chart showcases the instantaneous release and cumulative release after 24 hours for each test group demonstrating the distinct advantage of SELPs for the controlled release of SAGE between the synthetic polymers and the recombinant SELP systems. * and *** indicate p-values of less than 0.05 and 0.001 when sample is compared to the PBS control. †† and ††† for comparison of the high and low concentrations polymer indicates p-values of less than 0.01 and 0.001, respectively. ‡, ‡‡, and ‡‡‡ indicate a p-value between the 1 min. and 24 hr. time points of 0.05, 0.01, than 0.001 or less, respectively. Statistical comparisons performed with a 1 way ANOVA followed with a Bonferroni Post Test with a sample size of 6 for each test group. All error bars indicate the standard deviation.
3.2. Mechanical assessment of in situ gelling formulations
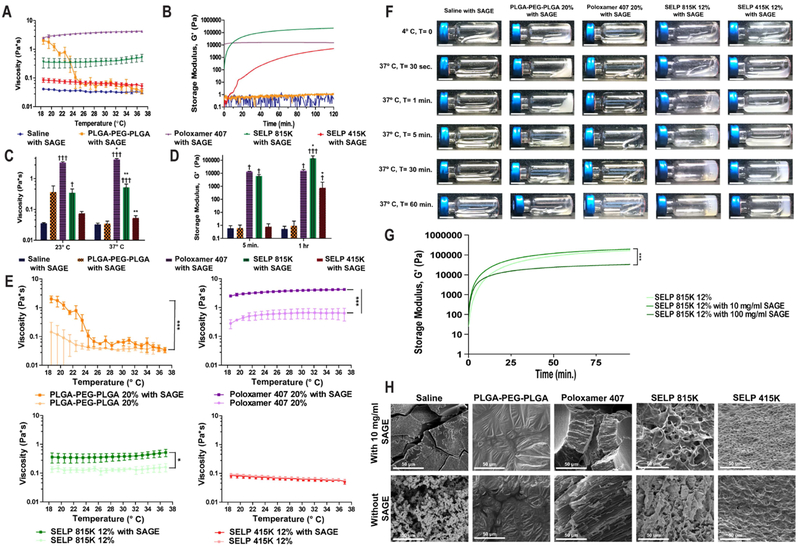
To evaluate the ability of the polymer solutions to be delivered through a catheter into the bladder and then transition into a delivery depot we evaluated rheological properties of the material. Even the most viscous formula tested, Poloxamer 407 20% with SAGE, was able to be easily injected through a 5 Fr (1.7 mm) catheter by hand (see Figure 3A). The SELP 815K and SELP 415K showed increasing modulus with time indicating rheopectic behavior, due to gelation from the intra-polymeric crosslinking of the silk units (See Figure 3B). PLGA-PEG-PLGA had a drastic reduction in viscosity between 23° and 37° C due to phase separation between the polymer and aqueous portions of the media (see Figure 3 A, C, and F). Poloxamer 407 and PLGA-PEG-PLGA showed stable properties over the 2 hr. period indicating that they had reached equilibrium (See Figure 3 D). The addition of 10 mg/ml SAGE to the polymer systems increased their viscosity by a statistically significant amount except for SELP 415K (see Figure 3E). The addition of 100 mg/ml of SAGE to SELP 815K did not prevent matrix formation, but did accelerate gelation and reduce the peak modulus of the gel. The addition of SAGE to SELP 415K and 815K tended to create lower density matrices based upon SEM observations of lyophilized samples. There was no readily apparent effect on PLGA-PEG-PLGA or Poloxamer 407 film structures after the addition of SAGE. SAGE at 10 mg/ml did not interfere with the in situ gelling function of any of the polymers tested in the context of intravesical administration.
Figure 3: Polymer solutions maintain injectable profiles and gelation after the incorporation of SAGE.
A) Viscosity profile of SAGE containing polymer solutions over a temperature range simulating a typical intravesical administration. B) Rheology trace of polymers over a 2-hour period demonstrates the distinct difference between SELP 815K, SELP 415K, Poloxamer 407, and PLGA-PEG-PLGA gelation profiles. C) Comparison of polymer solution viscosity at room and physiological temperatures. * indicates statistically significant differences between the viscosity at 23° and 37° C. † indicates statistical differences between the saline and polymer solutions. D) Gelation over time was observed in the SELP samples but not either of the synthetic polymers or saline control. * indicates statistically significant differences between the modulus at 5 min. and 1 hr. † indicates statistical differences between the saline and polymer solutions. E) Viscosity traces showing that 10 mg/ml SAGE increases the viscosity of the polymer solutions, but the degree of impact varies by temperature and polymer type. F) Images of polymer solutions in HPLC vials over time visually demonstrating gelation kinetics for each of the solutions. The scale bar represents 1 cm. G) A rheology trace showing the impact of adding 10 mg/ml and 100 mg/ml SAGE to SELP 815K 12% solutions on gelation. H) SEM images of lyophilized polymers after 24 hr. at 37° C, demonstrating SELP network formation. Statistical comparisons performed with a 1-way ANOVA followed with a Bonferroni Post Test. All points indicate the mean of 3 independent samples and error bars indicating the standard deviation.
The structure of the materials was evaluated via SEM of lyophilized samples of the gels. SELP samples formed distinct crosslinked matrices while PLGA-PEG-PLGA and Poloxamer 407 formed a film and highly viscous fluid respectively that depended on hydrophobic-hydrophilic interactions to stabilize their structures (see Figure 3G and 3H). SELP structure persisted through lyophilization due to crosslinking between the beta sheets formed between the silk-like motifs. SELP 415K produced a denser matrix than SELP 815K, but had slower gelation and lower peak modulus owing to the higher silk-like:elastin-like motif ratio. These materials gave us a broad base of properties to evaluate for enhancing SAGE accumulation in the bladder.
3.3. Polymer enhanced accumulation in healthy mice
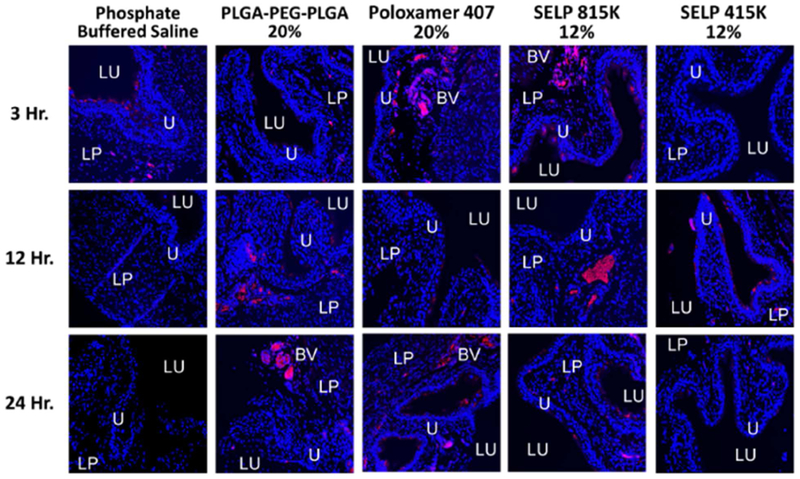
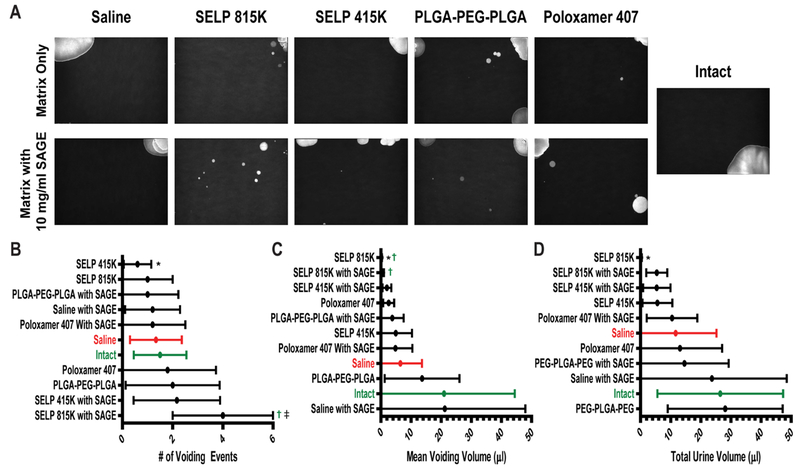
We assessed the impact on SAGE accumulation in the bladder and ease of intravesical administration for each of the thermoresponsive polymers. All solutions were easily administered by hand through a small silicone 0.3 mm diameter catheter into the bladder. This represents a greater challenge for administration than clinically used catheters which have diameters ranging from 2.67 to 6 mm in diameter (8–18 Fr). After the 3-hr. time point, no SAGE-633 was observed in the animals that received SAGE in a traditional intravesical administration using saline (See Figure 4). The PLGA-PEG-PLGA and SELP 415K showed limited accumulation of SAGE-633 at the 3-hr. time point, but did show increased retention in the bladder wall at the 12 and 24-hr time points. Poloxamer and SELP 815K showed enhancement of accumulation at all three-time points (See Figure 4). SAGE-633 was observed within the mucus coating, penetrating the urothelium, and associating with the endovasculature. These results indicate that the addition of thermoresponsive polymers to intravesical delivery enhances the accumulation of SAGE compared to saline and polymers.
Figure 4: Thermoresponsive polymers enhance SAGE accumulation within the urothelium.
Representative fluorescent micrographs of bladder tissue samples. SAGE accumulation was enhanced when delivered via a thermoresponsive polymer matrix to when delivered in phosphate buffered saline at time points greater than 3 hours. The SAGE GM-0111 labeled with CF™633 is shown in red and the tissue stained with Hoechst 3342 is shown in blue. Key anatomical features are labeled as follows: bladder lumen (LU), urothelium (U), lamina propria (LP), and blood vessel (BV).
3.4. Effect of thermoresponsive polymers on urinary function after intravesical administration
We used VSA to evaluate the influence of SAGE with the intravesical polymer administration on bladder function in mice exposed to LL-37 induced IC/PBS. Healthy intact mice tended to have 1–2 large voiding events in the corners of their cages during the 4-hr observational period (See Figure 5A). This is typical behavior for female C57BL/6 mice.[40] The induction of IC/PBS did not significantly alter standard urinary behavior, but intravesical delivery with polymers did impact bladder function.
Figure 5: Assessment of bladder function in IC/PBS mouse model after treatment with SAGE loaded thermoresponsive polymers.
A) Representative images of void spot assay (VSA) sheets under ultra violet illumination. B) The number of voiding events with greater than 0.5 μL calculated volume, C) mean voiding volume, and D) total volume of urination in a 4 hr. period was determined by analyzing the images. *, †, and ‡ indicate a statistical difference with a p value of less than 0.05 to the saline control group, intact mice, and the polymer without SAGE, respectively. Samples were statistically analyzed via a 1-way ANOVA with Bonferroni Post-Test. Each experimental group contained 6 animals.
Intravesical administration of matrix forming gels negatively impacted bladder function, with a stiffer faster gelling formulation having the most deleterious outcomes. SELP 415K and 815K reduced both the mean and total urination volume (See Figure 5C and 5D). The faster gelation and stiffer modulus of SELP 815K lead to the formation of a free-floating gel mass within the bladder leading to reduced capacity and potential bladder outlet obstruction. These effects were manifested by frequent small urination events in the center of the cage, indicating an increased feeling of urgency (See Figure 5). SELP 415K due to its slower gelling and more elastic character formed a stronger association with the urothelium and demonstrated reduced impact on urination compared to SELP 815K.
Film forming and soluble polymers had minimal impact on bladder function 24 hours after intravesical delivery. Both PLGA-PEG-PLGA and Poloxamer 407 did not significantly alter the frequency or volume of urination (See Figure 5 B, C, and D). The addition of SAGE to Poloxamer 407 and PLGA-PEG-PLGA did not lead to any apparent change in urinary behavior after 24 hours. This may be due to rapid clearance from the bladder during subsequent urination. No residual Poloxamer 407 or PLGA-PEG-PLGA polymer was observed in the bladder after administration in either healthy or IC/PBS mice and were likely cleared with urination.
3.5. Amelioration of LL-37 induced pain in murine IC/PBS model by SAGE
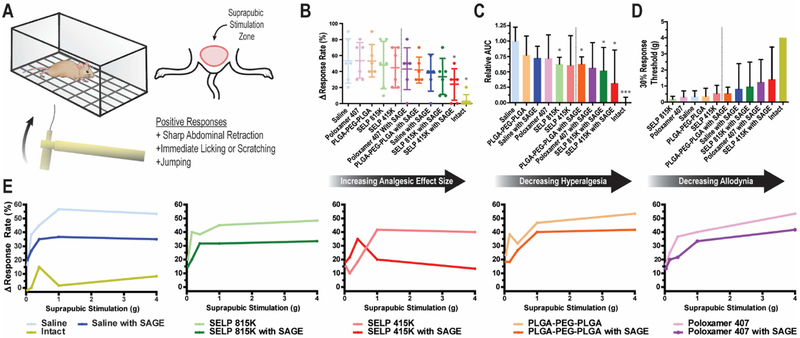
SAGE ameliorated the pain associated with an LL-37 induced model of IC/PBS, and this effect was enhanced by thermoresponsive polymers. Pain response was assessed using mechanical stimulation to the suprapubic area of the mice using Von Frey filaments after LL-37 insult (See Figure 6A). LL-37 exposure significantly increased the relative maximal pain response rate by 250% and induced a highly significant allodynic and hyperalgesic response to suprapubic stimulation (See Figure 6 B, C, and D). Treatment with SAGE via a traditional saline injection provided a reduction in pain response. All of the solutions that contained SAGE demonstrated superior analgesic effects to those that did not contain SAGE (See Figure 6E) as seen by the repression of elevation pain response from baseline in the dose-response curves. Poloxamer 407, PLGA-PEG-PLGA, saline, SELP 815K SELP 415K with SAGE reduced the pain response by 19%, 22%, 28%, 38%, and 56%, respectively, compared to the sham treatment control (See Figure 6B).
Figure 6: Behavioral assessment of suprapubic pain in murine IC/PBS model after SAGE administration.
A) Graphical depiction of Von Frey filament testing of bladder pain via suprapubic stimulation. B) The change in response rate for mice from baseline at the 4 g stimulation level. Each dot represents the individual mouse (n=6). Dashed line shows the demarcation between groups that did or did not receive SAGE. Intact mice were not subjected to LL-37 insult. C) Area Under the Curve (AUC) of the response curve after the subtraction of baseline response rates for each animal normalized to the saline, sham treatment, group (n=6). D) Plot showing treatment effects on allodynic response based on the level of stimulation needed to increase positive response rate from baseline by 30%. None of the 6 mice from the intact group exceeded the 30% response threshold, so the maximum stimulation of 4 g is shown for reference. All plots are of the mean with error bars representing the standard deviation when included. The dashed line shows the clear demarcation between groups that did or did not receive SAGE. E) Traces of the increased positive response rate from baseline for mice (n=6) 24 hours after LL-37-induced IC/PBS model and treatment with polymer solutions with or without 10 mg/ml of SAGE. * and *** indicate a p-value of less than 0.05 or 0.001 when compared to the saline control group, respectively, as determined by a 1-way ANOVA with Bonferroni Post Test. Each experimental group contained 6 animals.
Polymers that formed gel networks and mediated SAGE release showed improved analgesic effects over non-network forming systems. Poloxamer 407 and PLGA-PEG-PLGA solutions reduced the size of the analgesic efficacy compared to either SELP 815K and SELP 415K which showed a greater degree of analgesic effect (See Figure 6B). Only SELP 415K showed significant amelioration of hyperalgesia, based upon the reduction in the AUC of the stimuli response curves (See Figure 6C). SELP 815K was second only to SELP 415K, and likely has its effectiveness reduced due to untoward effects on urination. In particular, this is seen in the reduced response threshold for SELP 815K, which is negatively impacted by the retention of urine in the bladder. The ability of polymers to increase retention of therapeutic payload in the bladder leads to improved analgesic outcomes.
3.6. Evaluation of anti-inflammatory effects
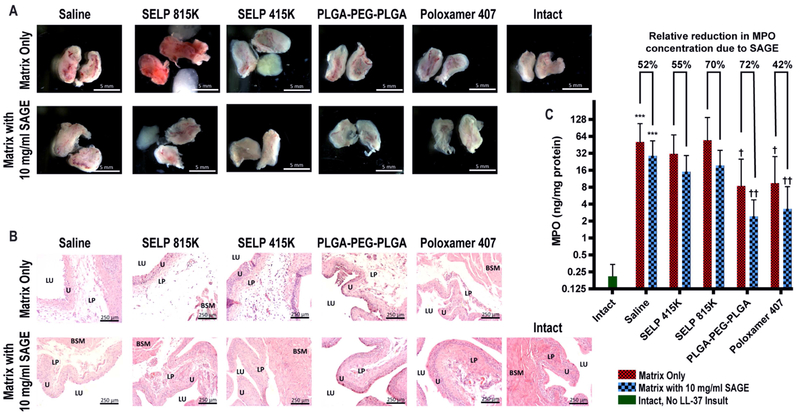
Administering SAGE to the bladder visibly reduced inflammation and ulceration in the urothelium. Gross anatomical inspection showed clear signs of swelling, hyperemia erythema, and ulceration 24 hr. after the administration of LL-37 (See Figure 7A). The addition of SAGE at 10 mg/ml drastically reduced the visible signs of inflammation and in many cases were visually indistinguishable from intact bladders which were never exposed to LL-37. Histology substantiated this observation of inflammation as indicated by significant edema within the urothelium and cellular infiltrates (See Figure 7B). This indicates that SAGE substantially improved local inflammation. Quantitative assessment of MPO, a general marker for the degree of bladder inflammation, demonstrated a barrier function in the polymer systems in preventing LL-37 induced inflammation. MPO rose 245-fold between the saline control and intact mice, which were never exposed to LL-37 (See Figure 7C). PLGA-PEG-PLGA, Poloxamer 407, and SELP 415K without SAGE decreased MPO concentration by 70%, 67%, and 35% compared to the sham treatment, respectively. The addition of SAGE further enhanced reductions in MPO concentration by an additional 42–72% depending on the mode of delivery. The intravesical administration of SAGE had a clear beneficial effect on reducing bladder inflammation induced from LL-37 insult.
Figure 7: Examination of inflammation within the mouse bladder.
A) Gross anatomy of hemisected bladders 24 hours after treatment showed visually apparent reduction in ulceration, swelling, erythema, and hyperemia within the mucosa of the bladder with the administration of SAGE. Bladders administered SELP 815K or 415K contained intact gels that were in intimate contact with the urothelium prior to dissection. Scale bar represents 5 mm. B) Histological inspection showed that the addition of SAGE substantially reduced edema and signs of vessel dilation. SELP 815K without SAGE showed especially pronounced edema. Key anatomical features are labeled as follows: bladder lumen (LU), urothelium (U), lamina propria (LP), bladder smooth muscle (BSM). Scale bar represents 250 μm. C) Myeloperoxidase (MPO) for each bladder was quantified as a general marker of the degree of inflammation. LL-37 exposure induced a highly inflamed state and drastically increased MPO production. Pretreatment with PLGA-PEG-PLGA and Poloxamer 407 had a prophylactic protective effect. *** indicates a p-value of less than 0.001 when the sample is compared to the intact mice. † and †† indicate statistical difference between the sham treatment group, saline without SAGE, with p-values of less than 0.05 and 0.01, respectively. A Kruskal-Wallis test followed by a post-hoc Dunn’s test for group comparisons were used to assess the statistical significance (n=6 per group) of the MPO data.
Intravesical delivery of thermoresponsive polymer to the bladder exhibited a moderate amelioration of LL-37 induced inflammation, except SELP 815K which visually exacerbated inflammatory response. PLGA-PEG-PLGA and Poloxamer 407 reduced the signs of erythema and swelling and other signs of inflammation and no signs of the material 24 hours after administration (See Figure 7A). Substantial urinary retention and distention of the bladder was noted in all of the animals that received SELP 815K with or without SAGE. Swelling due to urinary retention lead to increased inflammation in animals that received SELP 815K. This effect was not observed with the other polymers. Depots of 815K and 415K were observed in the bladders at the time of resection indicating a greater degree of retention. Distension leading to increased inflammation for SELP 815K was also apparent in histology by the greater degree of edema and in increased MPO expression (See Figure 7B and 7C). The masses of SELP 415K were far softer and more pliable than 815K at the time of resection and tended to conform tightly to the surface of the bladder like a coating. Poloxamer 407 and PLGA-PEG-PLGA were no longer visibly present in the bladder after 24 hrs. However, Poloxamer 407 and PLGA-PEG-PLGA did significantly reduce MPO expression due to LL-37 insult even without the incorporation of SAGE.
4.0. Discussion:
IC/PBS is a chronic condition with few treatments that are effective on most patients. The intrinsic symptomology of urinary urgency, sexual dysfunction, and pain result in a substantial reduction to patient quality of life. Oral pentosan sulfate is the only currently US FDA approved drug for IC/PBS and has shown limited efficacy in placebo-controlled trials. Poor oral absorption and degradation is a challenge for therapeutic glycosaminoglycans, such as pentosan sulfate, with less than 6% of the ingested dose reaching the bladder, and then mostly as desulphated and depolymerized metabolites. [41–43] Given the proposed therapeutic mechanism of restoring the mucosal barrier, low molecular weight fragments have limited restorative potential. Intravesical hyaluronic acid and heparin have been used extensively to treat patients with IC/PBS in the US and Europe.[5] Patients treated with intravesical GAGs typically receive no immediate amelioration of pain, and it can take 3–6 months to show any clinical signs of improvement and frequently result in only a modest amelioration of symptoms.[41,44,45] While intravesical delivery of analgesics such as lidocaine offer immediate relief, the effects are short-lived and fail to result in adequate resolution of patients’ symptoms in the long term. Here we show preliminary data demonstrating that SAGE GM-0111 when combined with a SELP polymer for enhanced delivery is able to both ameliorate bladder pain and reduce the underlying inflammatory source of IC/PBS.
SAGE as a therapeutic for IC/PBS has multiple potential modes of action that could be enhanced by polymer mediated intravesical drug delivery. SAGE has numerous purported mechanisms of action whose mode is tied to accumulation within target tissues. The most prevalent explanation of intravesical GAG therapeutic action in IC/PBS is fortification of the protective urothelial barrier that reduces ingress of molecules with danger-associated molecular patterns (DAMP).[46] However, this explanation can only partially explain the observed efficacy of SAGE in IC/PBS models. SAGE has improved efficacy compared to pentosan sulfate, hyaluronan, heparin, or chondroitin sulfate in spite of similar or greater anionic charge density and molecular weight.[3] Indicating, that this mechanism only partially contributes to the observed therapeutic effects of SAGE GM-0111. SAGE GM-0111 additionally blocks receptors for advanced glycosylation end products (RAGE), Toll-like receptor (TLR) 2 and TLR4 from activating downstream inflammatory pathways through competitive inhibition.[46,47] This can then inhibit Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) mediated cellular responses, decrease the extracellular release of ATP, reduce apoptosis in urothelial cells and leukocyte release of elastase, and lessen immune cell infiltration into the bladder leading to overall reduction in pain response, mast cell infiltration and degranulation, deterioration of bladder wall, and suprapubic pain responses.[36,37,46–48] These pharmacological aspects of SAGE GM-0111 action are likely the source of the observed SAGE-associated amelioration of IC/PBS symptomology seen in the model in this study.
SELP improves the intravesical delivery of GAG therapeutics. The primary proposed mode of action for current clinically used GAG therapies is the restoration of mucosal barrier function within the bladder. SELP polymers were able to retain GM-0111 over at least a 24 hr. period (see Figure 1), where PLGA-PEG-PLGA and Poloxamer 407 released nearly their entire cargo within 1 min at both the 20% and 10% concentrations tested. In spite of exhibiting very different viscoelastic properties, these concentrations had minimal impact on SAGE release. Poloxamer 407 at 18.75% and 11.6% have a peak modulus of 9kPa to 24kPa.[32] This indicates that structure of the gel is unable to substantially impede the diffusional release of the SAGE. Floating Poloxamer 407 hydrogels achieved sustained release of rhodamine B for 10 hr. after installation within the rabbit bladder.[49] In spite of having a rapid release profile, Poloxamers form the basis for a number of clinically investigated thermoresponsive gels for intravesical delivery including in situ gelling Theracoat, RTGel™, Mitogel™, Vesigel™, BOTUGEL™, and TC-3 Gel formulations.[10,50–52] These Poloxamer gels are being explored in various clinical trials for the delivery of mitomycin and botulinum toxin.[10,50,53] PLGA-PEG-PLGA underwent a macroscopic phase separation during heating. This resulted in very low observed viscosities, lack of a defined modulus, and an essentially instantaneous release of its SAGE payload into the aqueous phase. Even the modest increase in intravesical dwell time of approximately 6 hr. leads to improved clinical outcomes. The ability of SELP 815K and 415K to extend release out to 24 hr. is a significant step forward for non-device based IDD. SELP’s extended release profile is attributable to its formation of a distributed hydrogel network limiting diffusion and the presence of positively charged lysines within the matrix that interact with the negatively charged sulfate groups in SAGE GM-0111.
The use of a murine model for IC/PBS to assess therapeutic effects comes with inherent limitations. Voiding behavior is dependent upon mouse strain and other factors. Factors such as cage shape, time of day, age, cage flooring, and animal sex can have significant impacts on void volume, frequency, and spatial patterning.[40,54] To facilitate interpretation of data with this study, we used both healthy mice and sham treatment controls for reference. The LL-37 dose administered in this study did not increase voiding frequency, but did reduce voiding volume which is part of the canonical symptomology for IC/PBS. The lack of increased frequency observed in this study is likely caused due to post-procedural reduced water intake.[55] Past work has shown that LL-37 does also induce bladder frequency if the model is allowed to further mature past the acute phase and allow time for water intake to normalize. The purpose of the urodynamics studies in this report was to predominantly evaluate the impact of the polymers on bladder function.
SELP-SAGE solutions are easily administered via a catheter to the bladder and gel in place. By virtue of its polymeric nature, incorporation of SAGE to other polymer solutions increased the viscosity by increasing the frequency of polymer-polymer interactions and drag created by water-polymer interactions. While increased viscosity was observed for PLGA-PEG-PLGA, Poloxamer 407, SELP 815K, and SLEP 415K in no case did this make the solution too viscous for intravesical delivery. Substantial phase separation for PLGA-PEG-PLGA stemming from the precipitation of polymer into a micellar coating resulted in a large apparent drop in viscosity and a low storage modulus. This phase separation also likely led to the near instantaneous release of SAGE during the release study. SELP 415K and SELP 815K were both retained within the bladder 24 hr after treatment. SELP 415K, in particular, was observed as a tight elastic film bladder urothelium, whereas SELP 815K was more of a floating mass with only moderate interaction with the bladder wall. This was likely due to the differences in gelation kinetics, where the SELP 415K had a greater time as a liquid allowing it to more deeply penetrate the mucus and set within the native mucus to form an interpenetrating network. While SELP mucus interactions have been noted previously, further work is needed to investigate such interactions.[27] This, in turn, led to distinct differences in how SELP 815K and SELP 415K impacted bladder function and treatment outcomes, in spite of only minor differences in polymer architecture. We attribute the exacerbated urinary symptomology from 815K to the biomechanical impact and stimulation of a rigid mass within the bladder, coupled with partial obstruction of the urethra. The slower gelation, softer gels, potential for clearance, along with the lower relative volumes occupied by the gel likely lead to a decreased impact on bladder function for SELP 415K, PEG-PLGA-PEG, and Poloxamer 407.
Intravesical delivery of Poloxamer 407 and PLGA-PEG-PLGA reduced the impact of LL-37 insult on bladder inflammation. This type of protective effect is also seen clinically where intravesical liposomes with or without drugs function as a protective lipid barrier over the urothelium that prevents inflammatory solutes from inducing bladder inflammation and pain in areas where the protective mucus coating has eroded.[56–59] The Poloxamer 407 or PLGA-PEG-PLGA may have produced a similar protective coating or acted as a detergent that increased the elimination of LL-37 from the bladder. This effect was not observed in either SELP 815K or SELP 415K indicating that by themselves they did not create a barrier to diffusion. In all cases however the addition of SAGE GM-0111 to the polymer solution resulted in a visible decrease in inflammation.
SELPs impacted bladder function to a greater extent than Poloxamer 407 or PLGA-PEG-PLGA. The incorporation of 50 μL of hydrogel into the bladder of mice occupied approximately a third of the typical 150 μL volume of the adult mouse urinary bladder. This resulted in reduced filling prior to the induction of the voiding urge. Additionally, SELP 815K with its rapid gelation formed a mass that potentially obstructed the bladder outlet and urethra in some of the mice. The distinct differences between SELP 815K and 415K indicate that more soft elastic gels with slower gelation may potentially yield superior results as intravesical delivery vehicles. These types of obstructive events have been previously observed where pumps or polymer swelling drives the release of analgesics such as lidocaine into the bladder.[4,60] The narrow diameter of the mouse urethra compounds any potential blockage issues, and may not be relevant in larger animal models.
The quantification of MPO expression as a general marker of inflammation and visually apparent level of inflammation did not correspond with the observed pain responses. This observational precedent both clinically and within animal models of IC/PBS. Pain in IC/PBS patients can be independent of inflammation or the extent of visually observed pathological changes in bladder tissue.[35,61] In the LL-37-induced IC/PBS model used this has also been noted. While increased pain response remains at peak levels 7 days after LL-37 insult, MPO levels and visual inflammation return to near baseline by day 3.[35] In a chemically-induced, mustard oil, mouse model of visceral pain, morphine analgesic efficacy was assessed using abdominal stimulation with von Frey filaments, as in this study.[62] Morphine, 1.9 ± 1 mg/kg, in this model of visceral pain was required to illicit a 50% reduction in pain response.[62] SELP 415K with 10 mg/ml of SAGE GM-0111 reduced the mean pain response rate to suprapubic stimulation rate by 56% compared to the sham treatment control 24 hr. after administration. It is important to consider both inflammation and analgesic effects when evaluating treatments for IC/PBS.
The clinical relevance of small animal modeling for urodynamics is also challenging as the stiffness, voiding frequency, forces generated, and volumes differ from what is encountered clinically or in large animal models.[63] These features alter the potential dimensions and stiffness of delivery modalities that can be deployed without impairing bladder function. The viscoelastic properties of materials and relative volumes of polymer gels used in this study had an impact on mouse bladder function, but these effects may not carry into the clinic or larger animal models. Additionally, there are substantial physiological differences between the urinary bladder in rodents and larger animal models such as the locations of parasympathetic cell ganglia that can affect the potential clinical translation of these findings.[64] The work in mice in this paper lays the ground work for potential future work in large animal and alternative models of IC/PBS for evaluating the impact of SAGE treatment on bladder pathophysiology.
SELP 415K resulted in the greatest degree of analgesic improvement in spite of limited accumulation. Several factors may have contributed to this including the slow release rate of SAGE GM-0111 resulting in low accumulation at the 3 hr. time point. During preparation for histology, residual 815K and 415K was removed as the drug remaining in the gel wasn’t relevant to the therapeutic effect and would create an imaging artifact that would overshadow the SAGE that accumulated in tissues. The slow release, improved retention of SAGE, and lack of bladder obstruction were likely the main contributors to why SELP 415K exhibited the greatest degree of therapeutic analgesia at the 24 hr. time point.
5.0. Conclusion:
Improving intravesical delivery is key to increasing the effectiveness of pharmaceutical interventions for bladder diseases including IC/PBS. SAGE represents an effective potential treatment for IC/PBS and demonstrated both analgesic and anti-inflammatory effects within a mouse model. SELPs were able to provide a superior intravesical delivery platform for sustained release of SAGE GM-0111 compared to Poloxamer 407 or PLGA-PEG-PLGA. SELP 415K and 815K were both able to generate a sustained release profile and remain within the bladder and provide a sustained therapeutic effect for at least 24 hr. Intravesical delivery of SAGE GM-0111 was improved by thermoresponsive in situ gelling polymers with SELP 415K exhibiting the greatest enhancement of analgesic efficacy for the treatment of IC/PBS.
Highlights:
Temperature-responsive polymers improved intravesical drug delivery
Glycosaminoglycans exhibited analgesic and anti-inflammatory properties
Glycosaminoglycans altered polymer solution viscosity and gelation kinetics
Temperature-responsive polymer enhanced therapeutic potential of Glycosaminoglycans
Soft and erodible polymer gels have less impact on bladder function
Acknowledgements:
We are grateful to the University of Utah and Surface Characterization Lab for assistance with SEM imaging supported by the Utah Science Technology and Research Initiative (USTAR), Office of the Vice President for Research at the University of Utah, the College of Engineering at the University of Utah, and Materials Research Science and Engineering Center. Imaging was performed at the Fluorescence Microscopy Core Facility, a part of the Health Sciences Cores at the University of Utah. Microscopy equipment was obtained using a NCRR Shared Equipment Grant # 1S10RR024761–01.
Funding:
This work was supported by the National Institutes of Health (R01 CA227225), University of Utah Huntsman Cancer Institute Experimental Therapeutics Seed Grant, and a fellowship from the National Science Foundation Graduate Research Fellowship program [1256065 (MJ)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest:
HG is co-founder of TheraTarget, Inc., a polymeric drug delivery company based in Salt Lake City, Utah.
Data availability:
The raw/processed data sets needed to reproduce the findings reported in this manuscript are available from the corresponding authors upon reasonable request.
References:
- [1].Hanno PM, Erickson D, Moldwin R, Faraday MM, American Urological Association, Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment, J. Urol 193 (2015) 1545–1553. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- [2].Elbadawi A, Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis, Urology. 49 (1997) 14–40. doi: 10.1016/S0022-5347(01)63337-8. [DOI] [PubMed] [Google Scholar]
- [3].Lee WY, Savage JR, Zhang J, Jia W, Oottamasathien S, Prestwich GD, Prevention of Anti-microbial Peptide LL-37-Induced Apoptosis and ATP Release in the Urinary Bladder by a Modified Glycosaminoglycan, PLoS One. 8 (2013) e77854. doi: 10.1371/journal.pone.0077854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nickel JC, Jain P, Shore N, Anderson J, Giesing D, Lee H, Kim G, Daniel K, White S, Larrivee-Elkins C, Lekstrom-Himes J, Cima M, Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: safety and efficacy of a new drug delivery device., Sci. Transl. Med 4 (2012) 143ra100. doi: 10.1126/scitranslmed.3003804. [DOI] [PubMed] [Google Scholar]
- [5].Parsons CL, Housley T, Schmidt JD, Lebow D, Treatment of interstitial cystitis with intravesical heparin, Br. J. Urol 73 (1994) 504–507. doi: 10.1111/j.1464-410X.1994.tb07634.x. [DOI] [PubMed] [Google Scholar]
- [6].Welk BK, Teichman JMH, Dyspareunia Response in Patients with Interstitial Cystitis Treated with Intravesical Lidocaine, Bicarbonate, and Heparin, Urology. 71 (2008) 67–70. doi: 10.1016/j.urology.2007.09.067. [DOI] [PubMed] [Google Scholar]
- [7].Nickel JC, Moldwin R, Lee S, Davis EL, Henry RA, Wyllie MG, Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome., BJU Int. 103 (2009) 910–8. doi: 10.1111/j.1464-410X.2008.08162.x. [DOI] [PubMed] [Google Scholar]
- [8].Parsons CL, Zupkas P, Proctor J, Koziol J, Franklin A, Giesing D, Davis E, Lakin CM, Kahn BS, Garner WJ, Alkalinized lidocaine and heparin provide immediate relief of pain and urgency in patients with interstitial cystitis., J. Sex. Med 9 (2012) 207–12. doi: 10.1111/j.1743-6109.2011.02542.x. [DOI] [PubMed] [Google Scholar]
- [9].Lamarre NS, Bjorling DE, Treatment of painful bladder syndrome/interstitial cystitis with botulinum toxin A: Why isn’t it effective in all patients?, Transl. Androl. Urol 4 (2015) 543–554. doi: 10.3978/j.issn.2223-4683.2015.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Krhut J, Navratilova M, Sykora R, Jurakova M, Gärtner M, Mika D, Pavliska L, Zvara P, Intravesical instillation of onabotulinum toxin A embedded in inert hydrogel in the treatment of idiopathic overactive bladder: A double-blind randomized pilot study, Scand. J. Urol 50 (2016) 200–205. doi: 10.3109/21681805.2015.1121406. [DOI] [PubMed] [Google Scholar]
- [11].Chuang Y-C, Kuo H-C, A Prospective, Multicenter, Double-Blind, Randomized Trial of Bladder Instillation of Liposome Formulation OnabotulinumtoxinA for Interstitial Cystitis/Bladder Pain Syndrome., J. Urol 198 (2017) 376–382. doi: 10.1016/j.juro.2017.02.021. [DOI] [PubMed] [Google Scholar]
- [12].Pulsipher A, Qin X, Thomas AJ, Prestwich GD, Oottamasathien S, Alt JA, Prevention of sinonasal inflammation by a synthetic glycosaminoglycan, Int. Forum Allergy Rhinol 7 (2017) 177–184. doi: 10.1002/alr.21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kaufman J, Tyagi V, Anthony M, Chancellor MB, Tyagi P, State of the art in intravesical therapy for lower urinary tract symptoms., Rev. Urol 12 (2010) e181–9. [PMC free article] [PubMed] [Google Scholar]
- [14].GuhaSarkar S, Banerjee R, Intravesical drug delivery: Challenges, current status, opportunities and novel strategies, J. Control. Release 148 (2010) 147–159. doi: 10.1016/j.jconrel.2010.08.031. [DOI] [PubMed] [Google Scholar]
- [15].Zacchè MM, Srikrishna S, Cardozo L, Novel targeted bladder drug-delivery systems: a review., Res. Reports Urol 7 (2015) 169–78. doi: 10.2147/RRU.S56168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Karimi M, Sahandi Zangabad P, Ghasemi A, Amiri M, Bahrami M, Malekzad H, Ghahramanzadeh Asl H, Mahdieh Z, Bozorgomid M, Ghasemi A, Rahmani Taji Boyuk MR, Hamblin MR, Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances., ACS Appl. Mater. Interfaces 8 (2016) 21107–33. doi: 10.1021/acsami.6b00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Price R, Poursaid A, Ghandehari H, Controlled release from recombinant polymers, J. Control. Release 190 (2014) 304–313. doi: 10.1016/j.jconrel.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ciofani G, Genchi GG, Mattoli V, Mazzolai B, Bandiera A, The potential of recombinant human elastin-like polypeptides for drug delivery, Expert Opin. Drug Deliv 11 (2014) 1507–1512. doi: 10.1517/17425247.2014.926885. [DOI] [PubMed] [Google Scholar]
- [19].Megeed Z, Cappello J, Ghandehari H, Genetically engineered silk-elastinlike protein polymers for controlled drug delivery, Adv. Drug Deliv. Rev 54 (2002) 1075–1091. doi: 10.1016/S0169-409X(02)00063-7. [DOI] [PubMed] [Google Scholar]
- [20].Dandu R, Von Cresce A, Briber R, Dowell P, Cappello J, Ghandehari H, Silk- elastinlike protein polymer hydrogels: Influence of monomer sequence on physicochemical properties, Polymer. 50 (2009) 366–374. doi: 10.1016/j.polymer.2008.11.047. [DOI] [Google Scholar]
- [21].Price R, Poursaid A, Cappello J, Ghandehari H, In vivo evaluation of matrix metalloproteinase responsive silk–elastinlike protein polymers for cancer gene therapy, J. Control. Release 213 (2015) 96–102. doi: 10.1016/j.jconrel.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Poursaid A, Jensen MM, Nourbakhsh I, Weisenberger M, Hellgeth J, Sampath S, Cappello J, Ghandehari H, Silk-elastinlike protein polymer liquid chemoembolic for localized release of doxorubicin and sorafenib, Mol. Pharm 13 (2016) 2736–2748. doi: 10.1021/acs.molpharmaceut.6b00325. [DOI] [PubMed] [Google Scholar]
- [23].Poursaid A, Price R, Tiede A, Olson E, Huo E, McGill L, Ghandehari H, Cappello J, In situ gelling silk-elastinlike protein polymer for transarterial chemoembolization, Biomaterials. 57 (2015) 142–152. doi: 10.1016/j.biomaterials.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Price R, Poursaid A, Cappello J, Ghandehari H, Effect of shear on physicochemical properties of matrix metalloproteinase responsive silk-elastinlike hydrogels, J. Control. Release 195 (2014) 92–98. doi: 10.1016/j.jconrel.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gustafson JA, Price RA, Frandsen J, Henak CR, Cappello J, Ghandehari H, Synthesis and characterization of a matrix-metalloproteinase responsive silk–elastinlike protein polymer, Biomacromolecules. 14 (2013) 618–625. doi: 10.1021/bm3013692. [DOI] [PubMed] [Google Scholar]
- [26].Haider M, Leung V, Ferrari F, Crissman J, Powell J, Cappello J, Ghandehari H, Molecular engineering of silk-elastinlike polymers for matrix-mediated gene delivery: biosynthesis and characterization, Mol. Pharm 2 (2005) 139–150. doi: 10.1021/mp049906s. [DOI] [PubMed] [Google Scholar]
- [27].Jensen MM, Jia W, Isaacson KJ, Schults A, Cappello J, Prestwich GD, Oottamasathien S, Ghandehari H, Silk-elastinlike protein polymers enhance the efficacy of a therapeutic glycosaminoglycan for prophylactic treatment of radiation-induced proctitis, J. Control. Release 263 (2017) 46–56. doi: 10.1016/j.jconrel.2017.02.025. [DOI] [PubMed] [Google Scholar]
- [28].Price R, Gustafson J, Greish K, Cappello J, McGill L, Ghandehari H, Comparison of silk-elastinlike protein polymer hydrogel and poloxamer in matrix-mediated gene delivery, Int. J. Pharm 427 (2012) 97–104. doi: 10.1016/j.ijpharm.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dandu R, Ghandehari H, Cappello J, Characterization of structurally related adenovirus-laden silk-elastinlike hydrogels, J. Bioact. Compat. Polym 23 (2008) 5–19. doi: 10.1177/0883911507085278. [DOI] [Google Scholar]
- [30].Gao Y, Sun Y, Ren F, Gao S, PLGA-PEG-PLGA hydrogel for ocular drug delivery of dexamethasone acetate., Drug Dev. Ind. Pharm 36 (2010) 1131–8. doi: 10.3109/03639041003680826. [DOI] [PubMed] [Google Scholar]
- [31].Yu L, Chang GT, Zhang H, Ding JD, Injectable block copolymer hydrogels for sustained release of a PEGylated drug., Int. J. Pharm 348 (2008) 95–106. doi: 10.1016/j.ijpharm.2007.07.026. [DOI] [PubMed] [Google Scholar]
- [32].Fakhari A, Corcoran M, Schwarz A, Thermogelling properties of purified poloxamer 407., Heliyon. 3 (2017) e00390. doi: 10.1016/j.heliyon.2017.e00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Council National Council, Guide for the Care and Use of Laboratory Animals, 8th edition, 8th Editio, National Academies Press, Washington, DC, 2011. doi: 10.17226/12910. [DOI] [Google Scholar]
- [34].Chromek M, Slamová Z, Bergman P, Kovács L, Podracká L, Ehrén I, Hökfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A, The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection, 12 (2006). doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- [35].Jia W, Schults AJ, Jensen MM, Ye X, Alt JA, Prestwich GD, Oottamasathien S, Bladder pain in an LL-37 interstitial cystitis and painful bladder syndrome model, Am. J. Clin. Exp. Urol 5 (2017) 10–17. http://www.ncbi.nlm.nih.gov/pubmed/29034266 (accessed June 7, 2019). [PMC free article] [PubMed] [Google Scholar]
- [36].Oottamasathien S, Jia W, Roundy LM, Zhang J, Wang L, Ye X, Hill AC, Savage J, Lee WY, Hannon AM, Milner S, Prestwich GD, Physiological relevance of LL-37 induced bladder inflammation and mast cells, J. Urol 190 (2013) 1596–1602. doi: 10.1016/j.juro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Oottamasathien S, Jia W, McCoard L, Slack S, Zhang J, Skardal A, Job K, Kennedy TP, Dull RO, Prestwich GD, A murine model of inflammatory bladder disease: cathelicidin peptide induced bladder inflammation and treatment with sulfated polysaccharides, J. Urol 186 (2011) 1684–1692. doi: 10.1016/j.juro.2011.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rudick CN, Schaeffer AJ, Klumpp DJ, Pharmacologic attenuation of pelvic pain in a murine model of interstitial cystitis, BMC Urol. 9 (2009) 1. doi:101186/1471-2490-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cappelleri JC, Bushmakin AG, Zlateva G, Sadosky A, Pain Responder Analysis: Use of Area Under the Curve to Enhance Interpretation of Clinical Trial Results, Pain Pract. 9 (2009) 348–353. doi: 10.1111/j.1533-2500.2009.00293.x. [DOI] [PubMed] [Google Scholar]
- [40].Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Hill WG, Zeidel ML, Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait, Am. J. Physiol. Physiol 306 (2014) F1296–F1307. doi: 10.1152/ajprenal.00074.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Erickson DR, Sheykhnazari M, Bhavanandan VP, Molecular Size Affects Urine Excretion of Pentosan Polysulfate, 175 (2006) 1143–1147. doi: 10.1016/S0022-5347(05)00319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Faaij RA, Srivastava N, van Griensven JMT, Schoemaker RC, Kluft C, Burggraaf J, Cohen AF, The oral bioavailability of pentosan polysulphate sodium in healthy volunteers, Eur. J. Clin. Pharmacol 54 (1999) 929–935. doi: 10.1007/s002280050577. [DOI] [PubMed] [Google Scholar]
- [43].Janssen Pharmaceuticals Inc., ELMIRON ®−100 MG [prescribing information], Titusville, New Jersey, 2018. [Google Scholar]
- [44].Davis EL, El Khoudary SR, Talbott EO, Davis J, Regan LJ, Safety and Efficacy of the Use of Intravesical and Oral Pentosan Polysulfate Sodium for Interstitial Cystitis: A Randomized Double-Blind Clinical Trial, J. Urol 179 (2008) 177–185. doi: 10.1016/j.juro.2007.08.170. [DOI] [PubMed] [Google Scholar]
- [45].Cox A, Golda N, Nadeau G, Curtis Nickel J, Carr L, Corcos J, Teichman J, CUA guideline: Diagnosis and treatment of interstitial cystitis/bladder pain syndrome., Can. Urol. Assoc. J 10 (2016) E136–E155. doi: 10.5489/cuaj.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang J, Xu X, Rao NV, Argyle B, McCoard L, Rusho WJ, Kennedy TP, Prestwich GD, Krueger G, Novel sulfated polysaccharides disrupt cathelicidins, inhibit RAGE and reduce cutaneous inflammation in a mouse model of rosacea., PLoS One. 6 (2011) e16658–e16658. doi: 10.1371/journal.pone.0016658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Savage JR, Pulsipher A, Rao NV, Kennedy TP, Prestwich GD, Ryan ME, Lee WY, A Modified Glycosaminoglycan GM -0111, Inhibits Molecular Signaling Involved in Periodontitis, PLoS One. 11 (2016) e0157310. doi: 10.1371/journal.pone.0157310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jensen M. Martin , Jia W, Schults AJ, Ye X, Prestwich GD, Oottamasathien S, IL-33 mast cell axis is central in LL-37 induced bladder inflammation and pain in a murine interstitial cystitis model, Cytokine. 110 (2018) 420–427. doi: 10.1016/J.CYTO.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lin T, Zhao X, Zhang Y, Lian H, Zhuang J, Zhang Q, Chen W, Wang W, Liu G, Guo S, Wu J, Hu Y, Guo H, Lin T, Zhao X, Zhang Y, Lian H, Zhuang J, Zhang Q, Chen W, Wang W, Liu G, Guo S, Wu J, Hu Y, Guo H, Floating Hydrogel with Self-Generating Micro-Bubbles for Intravesical Instillation, Mater. 9 (2016) E1005. doi: 10.3390/ma9121005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Donin NM, Strauss-Ayali D, Agmon-Gerstein Y, Malchi N, Lenis AT, Holden S, Pantuck AJ, Belldegrun AS, Chamie K, Serial retrograde instillations of sustained release formulation of mitomycin C to the upper urinary tract of the Yorkshire swine using a thermosensitive polymer: Safety and feasibility, Urol. Oncol. Semin. Orig. Investig 35 (2017) 272–278. doi: 10.1016/j.urolonc.2016.11.019. [DOI] [PubMed] [Google Scholar]
- [51].Holzer A, Daniel D, Mullerad M, De La Zerda U, Jaime Shpolanskyn N Dollberg Malchi, Y., Tal D, Yavin Y, Konorty M, US20130046275A1 Material and method for treating internal cavities, (2013). [Google Scholar]
- [52].Leoni L, Maj R, Pattarino F, Vecchio C, US 2011/0319442 A1 Pharmaceutical compositions comprising imidazoquinolin(amines) and dervatives thereof suitable for local administration, 2011. [Google Scholar]
- [53].Lin JS, Kleinmann N, Wirth GJ, Matin SF, Mayer G, Nativ O, Witjes A, Shvero A, Chamie K, Pantuck AJ, Smith A, Schoenberg M, Malchi N, Hakim G, Agmon-Gerstein Y, Jeshurun-Gutshtat M, Klein I, Kopelen H, Lerner SP, Thermo reversible hydrogel based delivery of mitomycin C for treatment of upper tract urothelial carcinoma (UTUC)., J. Clin. Oncol 35 (2017) e16089–e16089. doi: 10.1200/JCO.2017.35.15_suppl.e16089. [DOI] [Google Scholar]
- [54].Chen H, Zhang L, Hill WG, Yu W, Evaluating the voiding spot assay in mice: a simple method with complex environmental interactions, Am. J. Physiol Physiol. 313 (2017) F1274–F1280. doi: 10.1152/ajprenal.00318.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jacobsen KR, Kalliokoski O, Teilmann AC, Hau J, Abelson KS, Postsurgical Food and Water Consumption, Fecal Corticosterone Metabolites, and Behavior Assessment as Noninvasive Measures of Pain in Vasectomized BALB/c Mice, J. Am. Assoc. Lab. Anim. Sci 51 (2012) 69–75. https://www.ingentaconnect.com/content/aalas/jaalas/2012/00000051/00000001/art00011# (accessed June 7, 2019). [PMC free article] [PubMed] [Google Scholar]
- [56].Janicki JJJ, Gruber MMAM, Chancellor MMB, Intravesical liposome drug delivery and IC/BPS, Transl. Androl. Urol 4 (2015) 572–578. doi: 10.3978/j.issn.2223-4683.2015.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chuang YC, Lee WC, Lee WC, Chiang PH, Intravesical Liposome Versus Oral Pentosan Polysulfate for Interstitial Cystitis/Painful Bladder Syndrome, J. Urol 182 (2009) 1393–1400. doi: 10.1016/j.juro.2009.06.024. [DOI] [PubMed] [Google Scholar]
- [58].Tyagi P, Kashyap M, Majima T, Kawamorita N, Yoshizawa T, Yoshimura N, Intravesical liposome therapy for interstitial cystitis, Int. J. Urol 24 (2017) 262–271. doi: 10.1111/iju.13317. [DOI] [PubMed] [Google Scholar]
- [59].Janicki JJ, Chancellor MB, Kaufman J, Gruber MA, Chancellor DD, Potential Effect of Liposomes and Liposome-Encapsulated Botulinum Toxin and Tacrolimus in the Treatment of Bladder Dysfunction, Toxins (Basel). 8 (2016) 81. doi: 10.3390/toxins8030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chancellor MB, Future trends in the treatment of urinary incontinence., Rev. Urol. 3 Suppl 1 (2001) S27–34. [PMC free article] [PubMed] [Google Scholar]
- [61].Killinger KA, Boura JA, Peters KM, Pain in interstitial cystitis/bladder pain syndrome: do characteristics differ in ulcerative and non-ulcerative subtypes?, Int. Urogynecol. J 24 (2013) 1295–1301. doi: 10.1007/s00192-012-2003-9. [DOI] [PubMed] [Google Scholar]
- [62].Laird JMAA, Martinez-Caro L, Garcia-Nicas E, Cervero F, A new model of visceral pain and referred hyperalgesia in the mouse, Pain. 92 (2001) 335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- [63].Birder L, Andersson K-E, Animal Modelling of Interstitial Cystitis/Bladder Pain Syndrome, Int. Neurourol. J 22 (2018) S3. doi: 10.5213/INJ.1835062.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tyagi P, Smith PP, Kuchel GA, de Groat WC, Birder LA, Chermansky CJ, Adam RM, Tse V, Chancellor MB, Yoshimura N, Pathophysiology and animal modeling of underactive bladder, Int. Urol. Nephrol 46 (2014) 11–21. doi: 10.1007/s11255-014-0808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]