Introduction
Diagnostic errors are a large and underappreciated obstacle to the delivery of high-quality health care in the United States.1 These errors are particularly concerning in cancer care, where the accuracy and timeliness of diagnosis can greatly affect patients’ prospects of survival.
Access to high-quality cancer imaging and pathology services—elements essential for accurate cancer diagnosis and treatment planning—is far from optimal or universal in the United States.1 Drawing on information and ideas discussed at a workshop hosted by the National Cancer Policy Forum,2 this article outlines practical steps to foster improved patient access to cancer imaging expertise.
The Changing Landscape of Oncologic Imaging and the Risk of Diagnostic Errors
For nearly every tumor type, imaging informs care management—from establishing a diagnosis and determining a treatment plan to monitoring response and detecting treatment-related complications and recurrence.3 An accurate cancer diagnosis—one that is both precise and complete—increasingly relies on complex oncologic imaging techniques and the integration of expert imaging interpretations with pathology findings that characterize the disease at both the tissue and molecular levels. As precision oncology care advances, a simple imaging report describing tumor presence, size, and location is no longer adequate for patient care. Similarly, assessing treatment response with traditional criteria on the basis of unidimensional or bidimensional tumor measurements has proven insufficient or inappropriate for evaluating the effects of many new cancer therapies, including targeted agents and immunotherapies. In response, radiologists have developed, and are continuously refining, an array of more sophisticated methods to assess treatment response and toxicity.3,4 For example, the Quantitative Imaging Network has fostered the development and clinical validation of quantitative imaging tools and methods to measure or predict tumor response to therapies in cancer clinical trials.5,6 These methods, combined with new imaging techniques that offer both anatomic and biologic information for patient triage and response assessment, require specialized training and experience for their appropriate use and interpretation. However, training opportunities are often lacking when new methods and technologies are introduced into the clinic.7,8
The increasingly complex process of cancer diagnosis raises the risk for diagnostic errors in three major categories: test selection and execution, image interpretation, and communication, both among physicians and with patients and their families.1 Delays or failures in diagnosing cancer represent approximately 30% of all diagnostic-related malpractice claims. Analyses of data from the CRICO Comparative Benchmarking System, a national database of medical malpractice claims, indicate that errors in clinical judgment, including the misinterpretation of diagnostic testing, contribute to more than two-thirds of these cases.9 In radiology, the top cause of cancer malpractice claims is misinterpretation of imaging results (in nearly 50% of cases).10 These findings point to the critical importance of enhancing physician expertise in cancer diagnosis and follow-up. They also highlight the need for systems-level solutions to improve the diagnostic process and enhance patient safety.
At specialized cancer centers, where care is typically provided by multidisciplinary teams that include treating oncologists, radiologists, and pathologists, cancer mortality rates have been found to be lower than those at community hospitals.11 These differences may be due in part to subspecialty radiologists who participate in multidisciplinary cancer care teams and can provide more clinically relevant oncologic imaging interpretations because they keep abreast of the latest developments in cancer biology and treatment, are well acquainted with the natural history of specific tumor types, and have ready access to the knowledge and expertise of multiple cancer specialists.
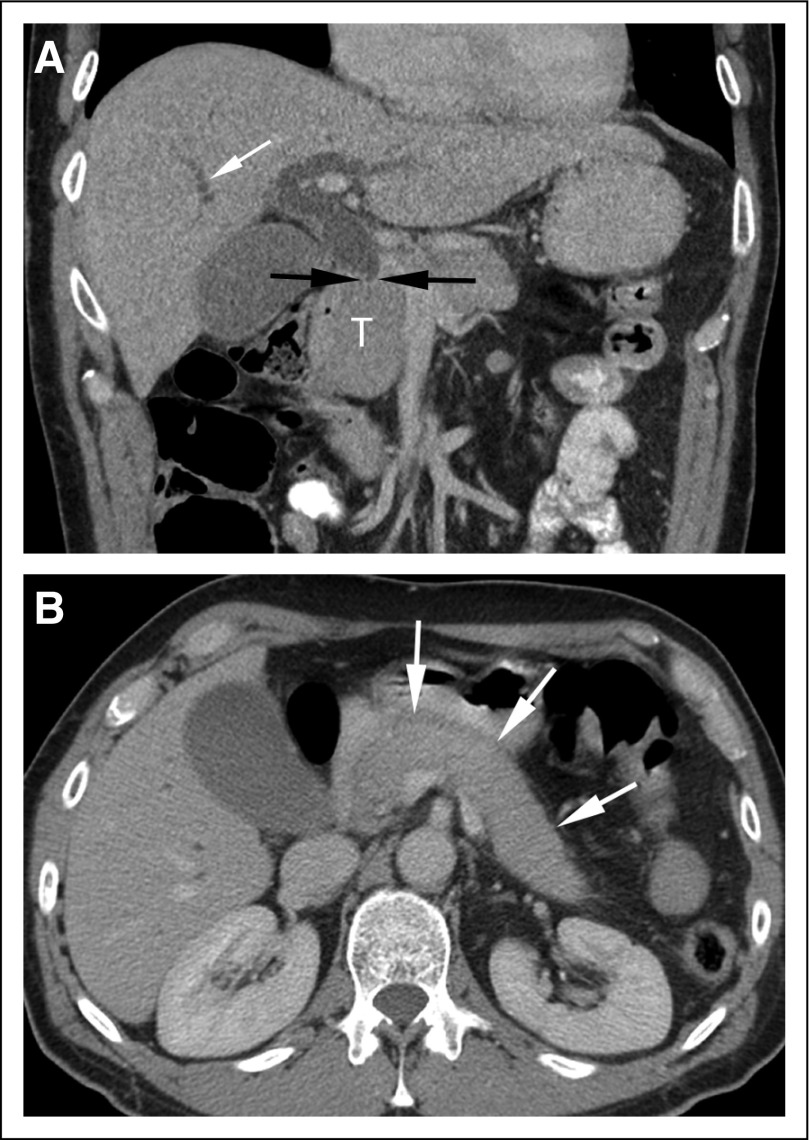
As reviewed by Schlemmer et al,3 studies have repeatedly shown substantial rates of disagreement—ranging from 13% to 56%—between initial cancer imaging reports and second-opinion readings by radiologists specializing in cancer imaging, with second-opinion reports indicating the need for changes in patient management in as many as 13% to 53.5% of cases.7-19 Second-opinion review can prevent unnecessary biopsies while improving cancer detection, as was shown by a recent study that included 147 patients who self-referred for second-opinion readings of breast images.20 On secondary review, 24 (25%) of 96 lesions originally reported as suspicious were downgraded to benign or probably benign, preventing biopsy in 21 patients, all of whom remained disease free on follow-up imaging. Furthermore, out of 87 biopsies performed, 28 (32%) were recommended only after second-opinion review, and eight of 28 (28%) yielded cancer. Thus, out of a total of 23 cancers ultimately identified, more than a third (35%) were not detected in initial image interpretations. Second-opinion readings can also reduce unnecessary hospital admissions and surgeries, increase accuracy in staging and post-treatment follow-up, and enhance the clinical relevance of imaging reports.15,21-23 An example of the importance of second readings by oncology imaging experts is shown in Figure 1.
FIG 1.
Coronal (A) and axial (B) contrast-enhanced computed tomography (CT) images. (A) The coronal image demonstrates abrupt cutoff of the common bile duct (black arrows) at the level of the enlarged pancreatic head (T), with intrahepatic biliary duct dilatation (white arrow). The study was initially interpreted as demonstrating a 3.9-cm cancer in the pancreatic head. The patient was scheduled for a Whipple procedure but decided to seek a second opinion before surgery. At the request of the consulting surgeon, the same set of CT images was reviewed by a specialized radiologist who participates in a hepato-pancreato-biliary tumor board. Although the head of the pancreas is enlarged, the axial CT image (B) also shows diffuse pancreatic enlargement (arrows), with no visible pancreatic duct. The imaging findings were thus considered suspicious for autoimmune pancreatitis, and measurement of circulating IgG4 levels was recommended. Subsequent testing revealed elevated IgG4 levels. Response to steroid therapy confirmed the diagnosis of autoimmune pancreatitis. Images courtesy of Richard Do, MD, PhD, Memorial Sloan Kettering Cancer Center.
Workforce and Care Delivery Challenges
As demand for radiology expertise increases, radiologists with subspecialty expertise are in short supply in many locations. Recent data from the Centers for Medicare & Medicaid Services showed that only 21.8% of 3,143 counties in the United States had at least one subspecialty radiologist; most subspecialty radiologists are located in urban areas. Even if subspecialists could be redistributed geographically, the total supply is insufficient to achieve widespread geographic coverage.24 The data from the Centers for Medicare & Medicaid Services do not specifically capture oncologic imaging as a subspecialty, because it is not a formally recognized subspecialty in the United States or most other countries. Subspecialization in radiology is generally by organ or system, which does not ensure intensive training or expertise in the pertinent areas of cancer imaging.
Expansion of dedicated oncologic imaging fellowship programs could help alleviate a shortage of subspecialized expertise in cancer imaging, which is usually gained through fellowship training and/or by participation on multidisciplinary cancer care teams. Out of the more than 240 radiology fellowships currently offered in the United States, only a handful (approximately five) are dedicated to oncologic imaging.3 Furthermore, modern cancer imaging principles are not treated in depth in medical school or residency training, and few continuing medical education courses are dedicated to cancer imaging.
As a result, most radiology practice models are not organized to deliver consistent, high-quality oncologic imaging services. Optimal practice models could include either referral to tertiary subspecialty multidisciplinary tumor board–style groups or blended generalist/specialist staffing in large community practices with a formalized conduit to subspecialty second opinions. Telemedicine or telementoring, with continuous supervision, feedback, and coaching for all team members, could enable broad implementation of these models.2
The Increase of Clinical Decision Support and Standardized Reporting
Radiology in general, and oncologic imaging in particular, stands to benefit greatly from advances in augmented intelligence—that is, the application of machine learning and artificial intelligence (AI) to automate routine tasks and enhance users’ performance of nonroutine tasks.25 The use of AI to provide focused clinical decision support at the point of care has the potential to reduce unwarranted variations in cancer care and decrease diagnostic errors. AI could streamline clinician workflow by automatically extracting pertinent clinical information from the electronic health record, ordering the appropriate imaging examination on the basis of appropriate use criteria, and selecting the correct imaging protocol for initial and follow-up examinations on the basis of the clinical question.2 AI algorithms could also enhance the work of radiologists by facilitating more accurate image analysis as well as improved early detection and disease quantification.26 For example, software programs for automated tumor volume measurement and tumor segmentation that enable consistency in longitudinal assessments are already available, although not yet widely implemented.2,27
In recent years, there has been a major shift from narrative, free-style reports to structured image reporting.28 Although it is still in its infancy, synoptic reporting, involving use of discrete entry options and standardized lexicons with embedded learning modules, is expected to facilitate complete capture of necessary information in a standardized and structured format. Structured and synoptic reporting is conceptually similar to the use of checklists in other areas of medicine, such as intensive care and surgery, to improve patient safety and decrease medical errors.
Ensuring the Quality and Accessibility of Oncologic Imaging Expertise
To expand patient access to oncologic imaging expertise, leaders in radiology and the broader health care community will need to work together to develop and implement strategies to enhance expertise in oncologic imaging, including improved education and training, expert consultations, telemedicine and telementoring, clinical decision support, collaboration, and data sharing (Box 1). With the emergence of precision oncology and the frequent introduction of new treatment strategies, the essential role for precise oncologic imaging to guide treatment decisions is growing. The potential for harm when patients lack access to high-quality oncologic imaging can no longer be ignored. Policymakers and leaders in imaging and oncology should take action to raise awareness and address this critical gap in cancer care.
BOX 1: Potential Actions to Improve Patient Access to High-Quality Oncologic Imaging
Improve Education and Training in Oncologic Imaging
Update core and continuing radiology curricula, training, and evaluation to include a greater emphasis on modern oncologic imaging competencies
Expand fellowship training programs in oncologic imaging
Facilitate and recognize oncology subspecialization in radiology
Use peer-learning programs to promote quality improvement among radiologists
Prepare radiologists to incorporate machine learning algorithms into clinical practice
Emphasize communication and intra- and interdisciplinary collaboration
Adopt automated tracking tools for assessing tumor characteristics longitudinally
Expand Access to Expertise in Oncologic Imaging
Form second-opinion networks and cancer imaging consortia
Develop tools and mechanisms for imaging referrals at cancer centers
Build community capacity in oncologic imaging through telementoring
Create oncologic imaging expertise within radiology departments
Increase Integration and Collaboration Among Specialties in Cancer Care
Engage tumor boards to help integrate specialties for diagnosis and care management
Provide incentives for interdisciplinary collaboration
Improve and Adopt Use of Clinical Decision Support
Collaborate with patients and physicians to design decision support tools
Effectively embed decision support tools into clinical workflow
Incorporate patient-reported outcome measures within these systems
Create machine-readable clinical practice guidelines
Support Innovation in Oncologic Imaging
Develop machine learning methods to process complex, multimodality, time-based data
Use artificial intelligence and interconnectivity to create a more dynamic, proficient, precise, and efficient health care workforce
Ensure appropriate validation and workforce training before disseminating new technologies into clinical practice
Improve Data Curation, Integration, and Sharing
Develop systematic approaches for data curation, anonymization, and aggregation
Standardize data elements and information nomenclature
Use structured or synoptic reporting to ensure data completeness and quality
Adhere to the FAIR principles (findable, accessible, interoperable, and reusable)
Include data from diverse populations
Adapted with permission from the NASEM, 2018, by the National Academy of Sciences, Courtesy of the National Academies Press, Washington, DC.2
ACKNOWLEDGMENT
We thank the speakers and participants for their contributions to the workshop.
The activities of the National Cancer Policy Forum are supported by its sponsoring members, which currently include the Centers for Disease Control and Prevention, the National Institutes of Health/National Cancer Institute, the American Association for Cancer Research, the American Cancer Society, the American College of Radiology, the American Society of Clinical Oncology, the Association of American Cancer Institutes, the Association of Community Cancer Centers, Bristol-Myers Squibb, the Cancer Support Community, the CEO Roundtable on Cancer, Flatiron Health, Helsinn Therapeutics (US), the LIVESTRONG Foundation, Merck, the National Comprehensive Cancer Network, Novartis Oncology, the Oncology Nursing Society, and Pfizer.
Footnotes
Supported by Contract No. HHSN263201200074I (Task Order No. HHSN26300120) and Contract No. 200-2011-38807 (Task Order No. 0051) between the National Academy of Sciences and the National Cancer Institute/National Institutes of Health and the Centers for Disease Control and Prevention, respectively.
R.L.S. and H.H. are co-senior authors.
The responsibility for the content of this article rests with the authors and does not necessarily represent the views of the National Academies of Sciences, Engineering, and Medicine, its committees, its sponsors, or its convening activities.
Preprint version available on bioRxiv.
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Improving Cancer Diagnosis and Care: Patient Access to Oncologic Imaging Expertise
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Sharyl J. Nass
Research Funding: Bristol-Myers Squibb (Inst), Flatiron Health (Inst), Helsinn Healthcare (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst)
Christopher R. Cogle
Consulting or Advisory Role: Celgene
Patents, Royalties, Other Intellectual Property: Patent on a method for preventing graft-versus-host disease using an oncolytic virus.
Other Relationship: CancerPOP
James A. Brink
Patents, Royalties, Other Intellectual Property: Patent on a method of an apparatus for predicting computed tomography contrast enhancement with feedback.
Other Relationship: American College of Radiology
Curtis P. Langlotz
Stock and Other Ownership Interests: Whiterabbit.ai, Galileo CDS, Nines.ai, Bunker Hill
Consulting or Advisory Role: Whiterabbit.ai, Galileo CDS, Nines.ai, Bunker Hill
Research Funding: Philips Healthcare (Inst), GE Healthcare (Inst), Siemens Healthineers (Inst), Google (Inst)
Travel, Accommodations, Expenses: Sectra
Other Relationship: Radiological Society of North America
Erin P. Balogh
Research Funding: Bristol-Myers Squibb (Inst), Flatiron Health (Inst), Helsinn Therapeutics (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst)
Other Relationship: American Society of Clinical Oncology (I)
Richard L. Schilsky
Research Funding: AstraZeneca (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Genentech (Inst), Eli Lilly (Inst), Merck (Inst), Pfizer (Inst)
Hedvig Hricak
Leadership: Ion Beam Applications
No other potential conflicts of interest were reported.
REFERENCES
- 1.Institute of Medicine . Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 2.National Academies of Sciences, Engineering, and Medicine . Proceedings of a Workshop. Washington, DC: The National Academies Press; 2018. Improving Cancer Diagnosis and Care. Patient Access to Oncologic Imaging and Pathology Expertise and Technologies. [PubMed] [Google Scholar]
- 3.Schlemmer H-P, Bittencourt LK, D’Anastasi M, et al. Global challenges for cancer imaging. J Glob Oncol. 2018;4:1–10. doi: 10.1200/JGO.17.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxnard GR, Morris MJ, Hodi FS, et al. When progressive disease does not mean treatment failure: Reconsidering the criteria for progression. J Natl Cancer Inst. 2012;104:1534–1541. doi: 10.1093/jnci/djs353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mountz JM, Yankeelov TE, Rubin DL, et al. Letter to cancer center directors: Progress in quantitative imaging as a means to predict and/or measure tumor response in cancer therapy trials. J Clin Oncol. 2014;32:2115–2116. doi: 10.1200/JCO.2014.55.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yankeelov TE, Mankoff DA, Schwartz LH, et al. Quantitative imaging in cancer clinical trials. Clin Cancer Res. 2016;22:284–290. doi: 10.1158/1078-0432.CCR-14-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffey K, D’Alessio D, Keating DM, et al. Second-opinion review of breast imaging at a cancer center: Is it worthwhile? AJR Am J Roentgenol. 2017;208:1386–1391. doi: 10.2214/AJR.16.16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulaner GA, Mannelli L, Dunphy M. Value of second-opinion review of outside institution PET-CT examinations. Nucl Med Commun. 2017;38:306–311. doi: 10.1097/MNM.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman J, editor. 2014 Annual Benchmarking Report: Malpractice Risks in the Diagnostic Process. CRICO Strategies. 2014 https://psnet.ahrq.gov/resources/resource/28612/2014-Annual-Benchmarking-Report-Malpractice-Risks-in-the-Diagnostic-Process
- 10.Siegal D, Stratchko LM, DeRoo C. The role of radiology in diagnostic error: A medical malpractice claims review. Diagnosis (Berl) 2017;4:125–131. doi: 10.1515/dx-2017-0025. [DOI] [PubMed] [Google Scholar]
- 11.Pfister DG, Rubin DM, Elkin EB, et al. Risk adjusting survival outcomes in hospitals that treat patients with cancer without information on cancer stage. JAMA Oncol. 2015;1:1303–1310. doi: 10.1001/jamaoncol.2015.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen NLKB, Koo BC, Gallagher FA, et al. Comparison of initial and tertiary centre second opinion reads of multiparametric magnetic resonance imaging of the prostate prior to repeat biopsy. Eur Radiol. 2017;27:2259–2266. doi: 10.1007/s00330-016-4635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo S, Kim SY, Cho JY, et al. Assessment of deep myometrial invasion of endometrial cancer on MRI: Added value of second-opinion interpretations by radiologists subspecialized in gynaecologic oncology. Eur Radiol. 2017;27:1877–1882. doi: 10.1007/s00330-016-4582-1. [DOI] [PubMed] [Google Scholar]
- 14.Hatzoglou V, Omuro AM, Haque S, et al. Second-opinion interpretations of neuroimaging studies by oncologic neuroradiologists can help reduce errors in cancer care. Cancer. 2016;122:2708–2714. doi: 10.1002/cncr.30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakhman Y, D’Anastasi M, Miccò M, et al. Second-opinion interpretations of gynecologic oncologic MRI examinations by sub-specialized radiologists influence patient care. Eur Radiol. 2016;26:2089–2098. doi: 10.1007/s00330-015-4040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spivey TL, Carlson KA, Janssen I, et al. Breast imaging second opinions impact surgical management. Ann Surg Oncol. 2015;22:2359–2364. doi: 10.1245/s10434-014-4205-5. [DOI] [PubMed] [Google Scholar]
- 17.Wibmer A, Vargas HA, Donahue TF, et al. Diagnosis of extracapsular extension of prostate cancer on prostate MRI: Impact of second-opinion readings by subspecialized genitourinary oncologic radiologists. AJR Am J Roentgenol. 2015;205:W73-78. doi: 10.2214/AJR.14.13600. [DOI] [PubMed] [Google Scholar]
- 18.Lysack JT, Hoy M, Hudon ME, et al. Impact of neuroradiologist second opinion on staging and management of head and neck cancer. J Otolaryngol Head Neck Surg. 2013;42:39. doi: 10.1186/1916-0216-42-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenzen J, Finck-Wedel AK, Lisboa B, et al. Second opinion assessment in diagnostic mammography at a breast cancer centre. Geburtshilfe Frauenheilkd. 2012;72:734–739. doi: 10.1055/s-0032-1315107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coffey K, Mango V, Keating DM, et al. The impact of patient-initiated subspecialty review on patient care. J Am Coll Radiol. 2018;15:1109–1115. doi: 10.1016/j.jacr.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Corrias G, Huicochea Castellanos S, Merkow R, et al. Does second reader opinion affect patient management in pancreatic ductal adenocarcinoma? Acad Radiol. 2018;25:825–832. doi: 10.1016/j.acra.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dudley RA, Hricak H, Scheidler J, et al. Shared patient analysis: A method to assess the clinical benefits of patient referrals. Med Care. 2001;39:1182–1187. doi: 10.1097/00005650-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Sawan P, Rebeiz K, Schoder H, et al. Specialized second-opinion radiology review of PET/CT examinations for patients with diffuse large B-cell lymphoma impacts patient care and management. Medicine (Baltimore) 2017;96:e9411. doi: 10.1097/MD.0000000000009411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenkrantz AB, Wang W, Hughes DR, et al. A county-level analysis of the US radiologist workforce: Physician supply and subspecialty characteristics. J Am Coll Radiol. 2018;15:601–606. doi: 10.1016/j.jacr.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Rouse WB, Spohrer JC. Automating versus augmenting intelligence. Journal of Enterprise Transformation. 2018. 10.1080/19488289.2018.1424059.
- 26.Jha S, Topol EJ. Adapting to artificial intelligence: Radiologists and pathologists as information specialists. JAMA. 2016;316:2353–2354. doi: 10.1001/jama.2016.17438. [DOI] [PubMed] [Google Scholar]
- 27.Trebeschi S, van Griethuysen JJM, Lambregts DMJ, et al. Deep learning for fully-automated localization and segmentation of rectal cancer on multiparametric. MR. Sci Rep. 2017;7:5301. doi: 10.1038/s41598-017-05728-9. [Erratum: Sci Rep 8:2589, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn CE, Jr, Langlotz CP, Burnside ES, et al. Toward best practices in radiology reporting. Radiology. 2009;252:852–856. doi: 10.1148/radiol.2523081992. [DOI] [PubMed] [Google Scholar]