Abstract
PURPOSE
Accurate stratification of patients is an important goal in Hodgkin lymphoma (HL), but the role of pretreatment clinical risk stratification in the context of positron emission tomography (PET) –adapted treatment is unclear. We performed a subsidiary analysis of the RAPID trial to assess the prognostic value of pretreatment risk factors and PET score in determining outcomes.
PATIENTS AND METHODS
Patients with stage IA to IIA HL and no mediastinal bulk underwent PET assessment after three cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine; 143 PET-positive patients (PET score, 3 to 5) received a fourth doxorubicin, bleomycin, vinblastine, and dacarbazine cycle and involved-field radiotherapy, and 419 patients in complete metabolic remission were randomly assigned to receive involved-field radiotherapy (n = 208) or no additional treatment (n = 211). Cox regression was used to investigate the association between PET score and pretreatment risk factors with HL-specific event-free survival (EFS).
RESULTS
High PET score was associated with inferior EFS, before (P < .001) and after adjustment (P = .01) for baseline risk stratification. Only patients with a postchemotherapy PET score of 5 (uptake ≥ three times maximum liver uptake) had an increased risk of progression or HL-related death (hazard ratio, 9.4 v score of 3; 95% CI, 2.8 to 31.3 and hazard ratio, 6.7 v score of 4; 95% CI, 1.4 to 31.7). Patients with a PET score of 5 also had inferior progression-free and overall survival. There was no association between European Organisation for Research and Treatment of Cancer or German Hodgkin Study Group risk group and EFS, before or after adjusting for PET score (all P > .4).
CONCLUSION
In RAPID, a positive PET scan did not carry uniform prognostic weight; only a PET score of 5 was associated with inferior outcomes. This suggests that in future trials involving patients without B symptoms or mediastinal bulk, a score of 5 rather than a positive PET result should be used to guide treatment escalation in early-stage HL.
INTRODUCTION
The goal of Hodgkin lymphoma (HL) treatment is to optimize patient outcomes by maximizing cure while minimizing toxicity. Cure rates for early-stage HL are high, but treatment toxicity reduces long-term survival and confers significant morbidity.1,2 Risk-adapted treatment strategies can potentially address this but are reliant on accurate risk stratification of patients to facilitate individualized treatment approaches. The German Hodgkin Study Group (GHSG) and the European Organisation for Research and Treatment of Cancer (EORTC) have developed clinical prognostic scores for early-stage HL that are frequently used to risk stratify patients for treatment selection,3-5 but it is unclear whether these scores have sufficient specificity to predict outcomes with modern combined-modality treatment.6
Over the past decade, early response to treatment assessed by [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) has emerged as a powerful prognostic indicator in HL.7,8 PET-guided approaches have been evaluated in trials9-13 and successfully implemented in clinical practice,14 as one of the first applications of personalized medicine. The introduction of the 5-point scale for PET reporting helped to standardize image interpretation15 and allowed the threshold used to define a positive PET scan to be adapted according to the research question.16 In trials involving patients with HL, a positive PET scan has been defined as either FDG uptake greater than the normal mediastinum (PET score, 3, 4, or 5) or equal to or greater than the liver uptake (score, 4 or 5), partly dependent on whether the study intervention involves treatment escalation or de-escalation.16 Little is known about the predictive value of individual PET scores, and it remains unclear whether all PET-positive or -negative patients derive equal benefit from PET-adapted approaches.17
The randomized H10 study demonstrated that patients with early-stage HL with a positive PET scan after two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) benefit from treatment intensification.9 Patients with positive PET scans had a progression-free survival (PFS) advantage when switched to escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (escBEACOPP), compared with continuing ABVD, although they experienced greater toxicity. PET positivity in H10 was defined by International Harmonization Project (IHP) criteria (broadly equivalent to PET scores of 3, 4, or 518), and it is unknown whether all patients with a PET score of 3 or higher derive equal benefit from treatment escalation. We performed this subsidiary analysis of the United Kingdom (UK) National Cancer Research Institute (NCRI) RAPID (Randomised Phase III Trial to Determine the Role of FDG-PET Imaging in Clinical Stages IA/IIA Hodgkin’s Disease) study,11 in which all PET-positive patients continued ABVD, to explore whether a subset of PET-positive patients with early-stage HL could be adequately treated with ABVD and radiotherapy. Our aim was to investigate the associations of PET score after three cycles of ABVD, pretreatment risk factors, and clinical prognostic scores with patient outcomes.
PATIENTS AND METHODS
Study Design
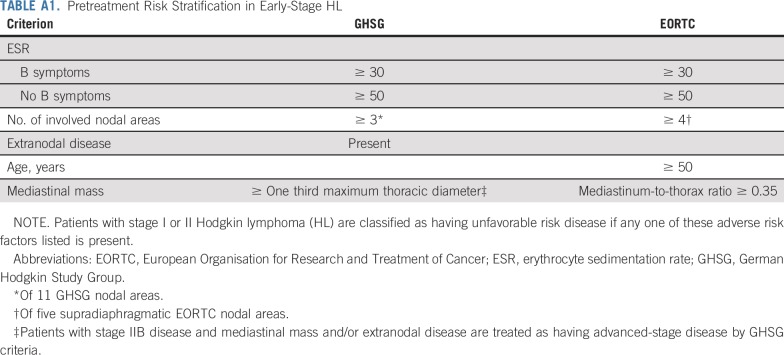
The RAPID trial was one of the first to use a PET-adapted treatment approach in early-stage HL.11 The primary objective of this phase III noninferiority study was to investigate whether PET response could be used to omit radiotherapy in selected patients and reduce late toxicity. The trial design and randomization procedures have been published.11 In brief, patients with newly diagnosed, histologically confirmed stage IA or IIA HL were eligible if age 16 to 75 years and without mediastinal bulk disease. Baseline staging was performed by computed tomography (CT). Clinical risk stratification was retrospectively assessed according to standard criteria (Appendix Table A1, online only).
Patients received three cycles of ABVD and then underwent PET and CT assessment. Patients with progressive disease by CT criteria were excluded at this point.19 PET scans were performed within UK NCRI-accredited PET centers using standardized methods for quality control and image acquisition.20 PET images were centrally reviewed by two independent reporters at St Thomas’ Hospital, London, United Kingdom. FDG uptake was prospectively graded using a 5-point scale according to the likelihood of disease response or nonresponse. The central review score determined further management. A similar graded response method was subsequently adopted internationally, widely referred to as the Deauville criteria.15,21 A PET score of 5 was defined as 3 or more times the maximum liver uptake in RAPID, and uptake greater than the mediastinum was considered to represent a positive PET result.
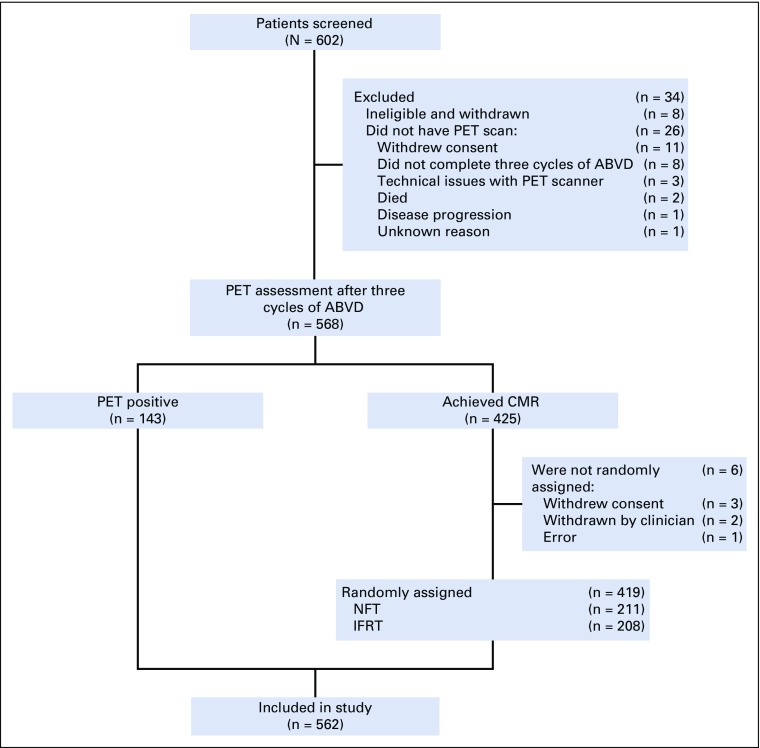
In total, 602 patients were recruited between October 2003 and August 2010, of whom 571 completed three cycles of ABVD and underwent PET evaluation; 145 patients (25.4%) were PET positive (uptake ≥ mediastinum; PET score, 3, 4, or 5) and received a fourth cycle of ABVD and 30 Gy of involved-field radiotherapy (IFRT); 426 patients (74.6%) achieved complete metabolic response (CMR; PET score, 1 or 2) and were randomly assigned using a one-to-one ratio to receive 30-Gy IFRT (n = 209) or no additional treatment (NFT; n = 211). Six patients with CMR withdrew before random assignment. Three additional patients were excluded from this analysis, where review of original diagnostic material at relapse identified a non-HL diagnosis. Outcomes for 562 patients are reported here (PET positive, n = 143; IFRT, n = 208; NFT, n = 211; Fig 1). Patients were monitored for disease progression by regular clinical evaluation and by CT scans at 6, 12, and 24 months post-treatment.
FIG 1.
CONSORT diagram. ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; CMR, complete metabolic response; IFRT, involved-field radiotherapy; NFT, no further treatment; PET, positron emission tomography.
Statistical Considerations
The primary end point of this subsidiary analysis was HL-specific event-free survival (EFS), calculated from the date of registration to relapse or death resulting from HL, censored at the date last seen or date of death resulting from any non-HL cause. PFS was calculated from the date of registration to relapse or death resulting from any cause, censored at the date last seen. Overall survival (OS) was calculated from the date of registration to death resulting from any cause, censored at the date last seen.
EFS, PFS, and OS are described using the Kaplan-Meier method; univariable and multivariable Cox regression analyses were performed to explore the associations with PET score, pretreatment risk factors, and clinical prognostic scores.
RESULTS
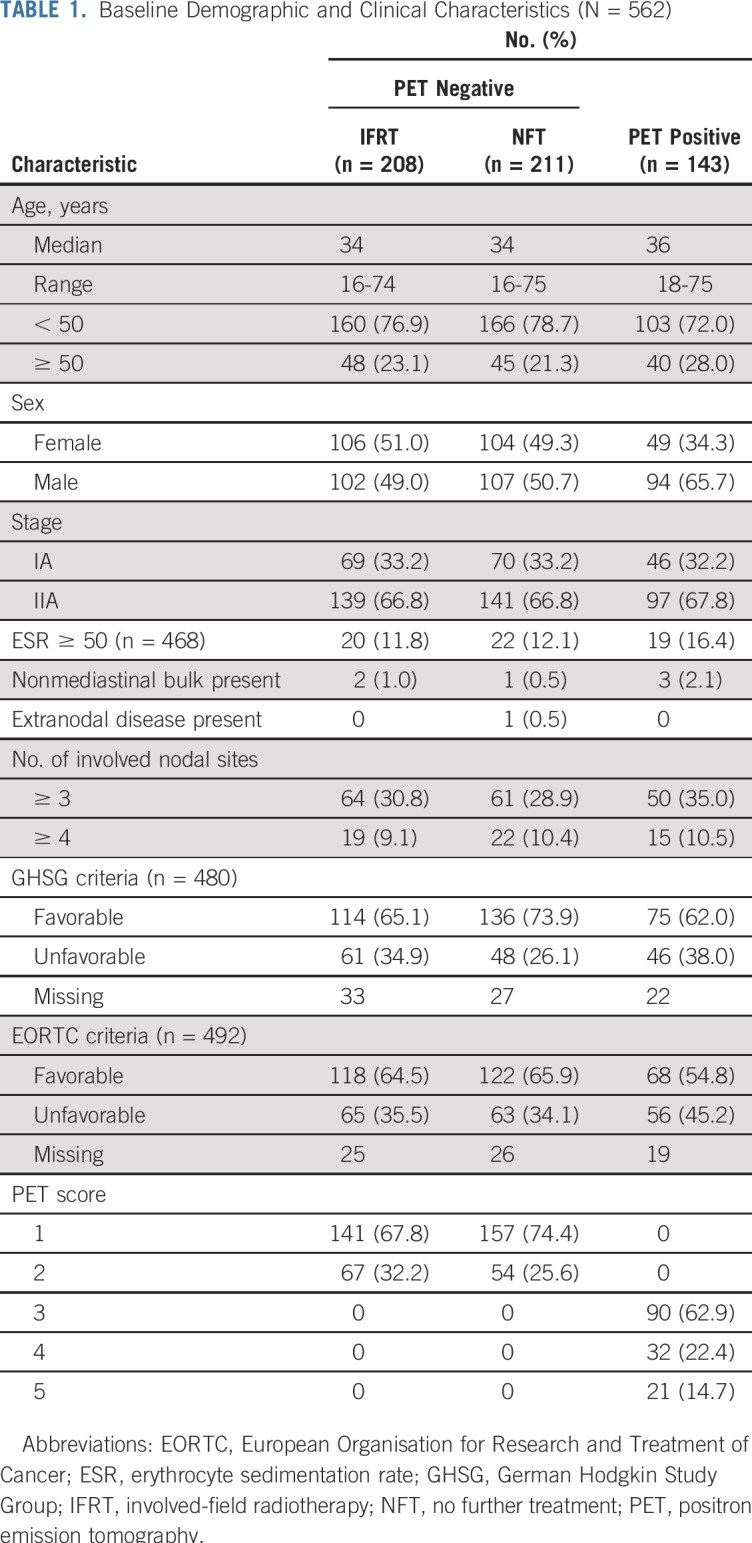
Baseline characteristics are listed in Table 1; data for risk stratification by GHSG criteria were available for 480 patients (85.4%), of whom 155 (32.3%) had unfavorable risk. Data for stratification by EORTC criteria were available for 492 patients (87.5%), of whom 184 (37.4%) were unfavorable risk. Patients were classified as unfavorable risk largely because of the number of involved nodal sites, age (EORTC only), and erythrocyte sedimentation rate; only one patient had extranodal disease, and patients with mediastinal bulk and B symptoms were excluded from RAPID.
TABLE 1.
Baseline Demographic and Clinical Characteristics (N = 562)
After a median follow-up of 61.6 months, 44 patients (7.8%) had an HL-related event, with five deaths resulting from HL and 39 additional disease progressions. Twelve non-HL deaths occurred from pneumonia or pneumonitis related to primary HL treatment (n = 6), other malignancies (n = 4), myocardial fibrosis (n = 1), and intracranial hemorrhage (n = 1). For PET-positive patients, there was no end-of-treatment PET scan; however, no patient received salvage therapy for inadequate response in the absence of confirmed disease progression.
Outcomes by Treatment Arm
EFS was 89.7% (95% CI, 84.6% to 94.8%) at 5 years in the PET-positive group and 93.0% (95% CI, 90.5% to 95.5%) in the PET-negative group (IFRT arm: 96.0%; 95% CI, 93.1% to 98.9%; NFT arm: 90.1%; 95% CI, 85.8% to 94.4%). There was no difference in EFS between patients achieving CMR, who received three cycles of ABVD with or without IFRT, and PET-positive patients treated with four cycles of ABVD and IFRT (hazard ratio [HR], 0.68; 95% CI, 0.36 to 1.29; P = .24). There was an improvement in EFS for patients achieving CMR randomly assigned to receive IFRT compared with PET-positive patients (HR, 0.40; 95% CI, 0.17 to 0.93; P = .03) but not for those randomly assigned to receive NFT compared with PET-positive patients (HR, 0.98; 95% CI, 0.50 to 1.93; P = .95). Similar but nonsignificant results were observed for PFS.
There was better discrimination between PET-positive and -negative patients in terms of both EFS and PFS using the Lugano classification of PET positivity (PET score, 4 or 5; liver threshold) than the mediastinal threshold.21 Patients with a PET score of 4 or 5 had a 5-year EFS of 80.3% (95% CI, 69.3% to 91.3%).
Outcomes According to PET Score After Three Cycles of ABVD
There was strong evidence that higher PET score was associated with increased risk of progression or HL-related death (EFS; P < .001) on univariable analysis, and results remained significant, with similar effect sizes, when adjusted for baseline GHSG (P = .01) or EORTC risk stratification (P = .01). A similar association was identified between PET score and PFS (unadjusted P < .001; adjusted P = .03 and P = .04 for GHSG and EORTC stratification, respectively).
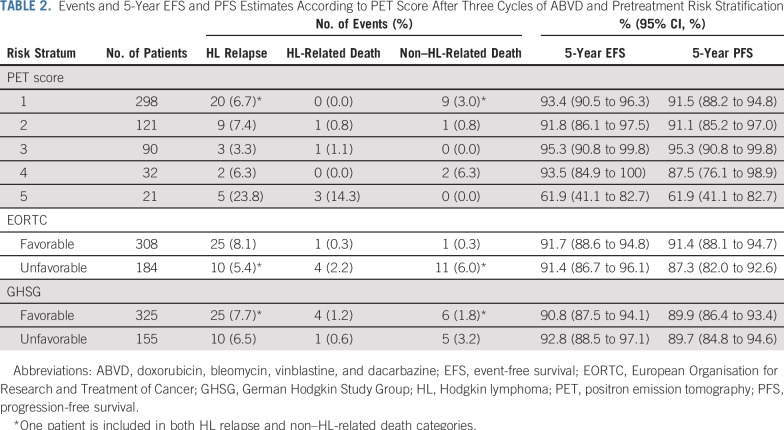
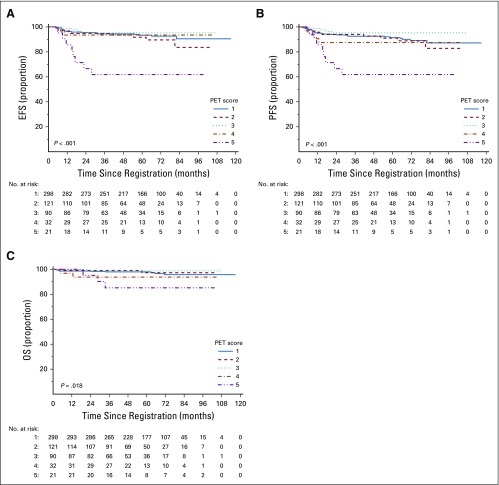
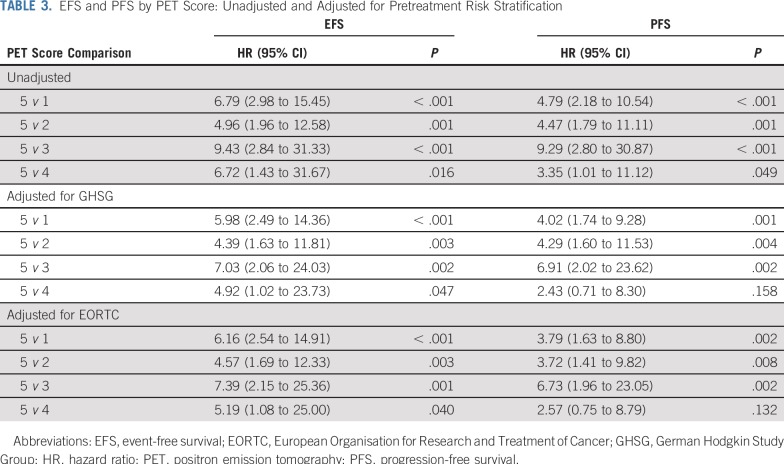
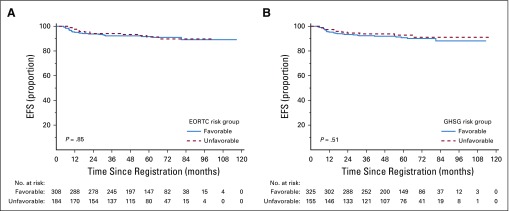
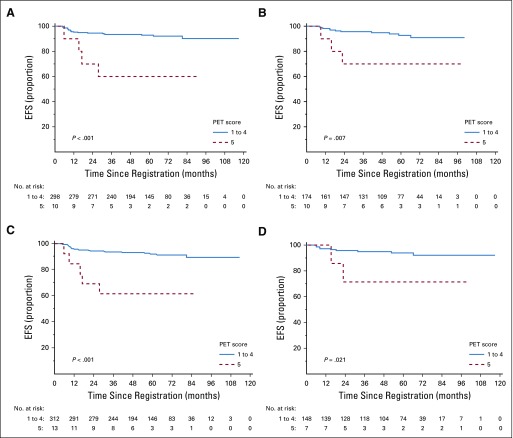
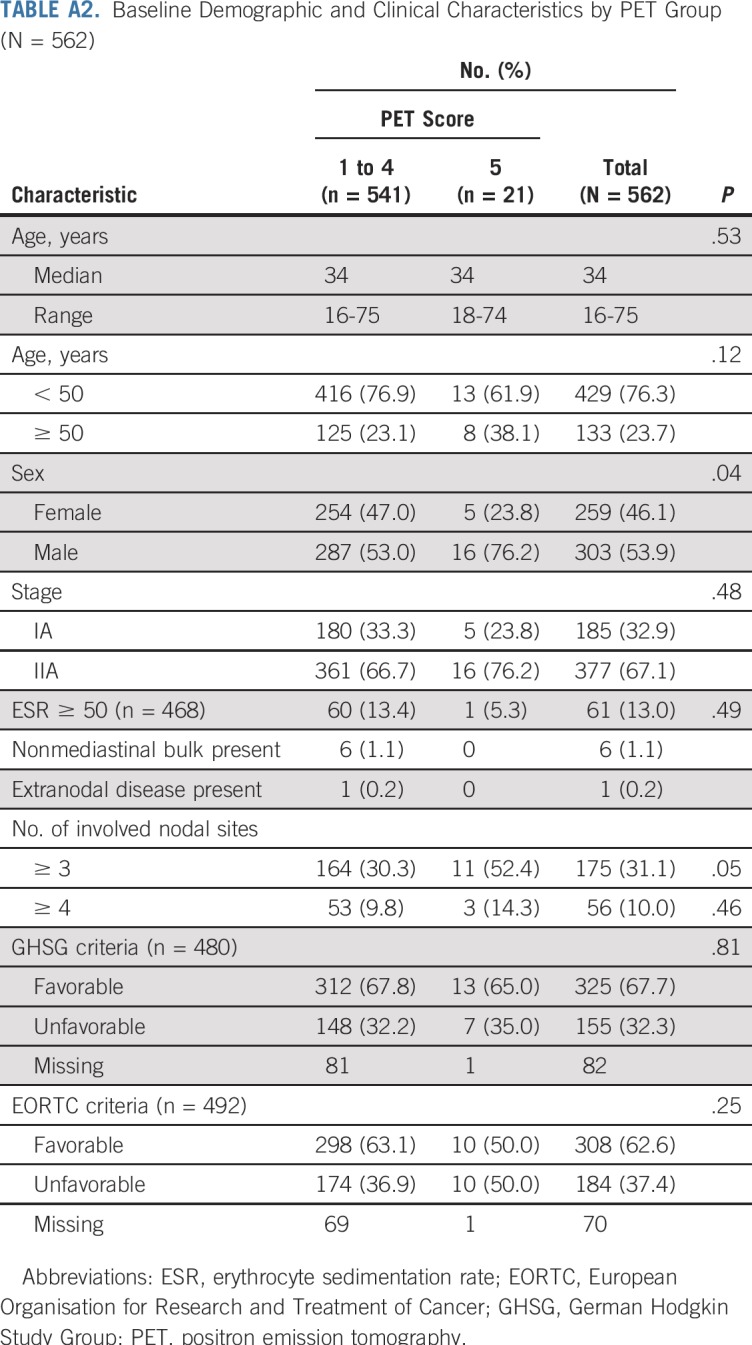
EFS and PFS by individual PET score are listed in Table 2 and Figures 2A and 2B. Patients with a score of 5 had a significantly higher risk of progression or HL-related death than those with all other PET scores (Table 3; P < .001 for both EFS and PFS). Furthermore, a score of 5 identified poor-prognosis patients among the favorable EORTC or GHSG groups, and similarly, a lower PET score identified good-prognosis patients in the unfavorable group (Appendix Figs A1A to A1D, online only). A similar association was observed for OS (P = .002; Fig 2C). The 5-year OS rate was 85.2% (95% CI, 69.7% to 100%) in patients with a score of 5, compared with 97.8% (95% CI, 96.4% to 99.2%) in patients with a score of 1 to 4. Compared with those with a score of 1 to 4, patients with a score of 5 were more likely to be male (76.2% v 53.0%; P = .04) and have 3 or more involved nodal sites (52.4% v 30.3%; P = .05), but there was a similar proportion of patients with unfavorable risk disease (Appendix Table A2, online only). Excluding patients with a PET score of 5, there was no evidence of pairwise differences between any other PET scores for EFS or PFS.
TABLE 2.
Events and 5-Year EFS and PFS Estimates According to PET Score After Three Cycles of ABVD and Pretreatment Risk Stratification
FIG 2.
Kaplan-Meier curves of (A) event-free survival (EFS), (B) progression-free survival (PFS), and (C) overall survival (OS) by positron emission tomography (PET) score.
TABLE 3.
EFS and PFS by PET Score: Unadjusted and Adjusted for Pretreatment Risk Stratification
Outcomes According to Pretreatment Risk Stratification
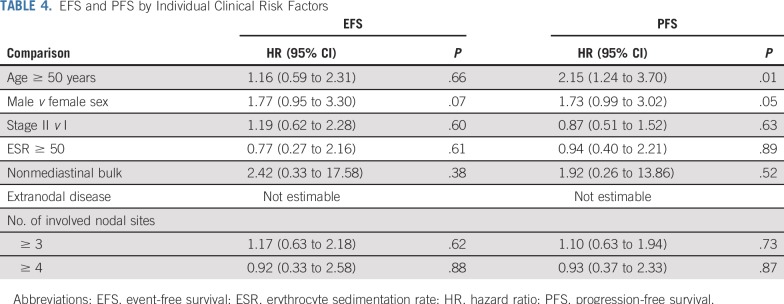
There was no evidence of an association between baseline GHSG (HR, 1.26; 95% CI, 0.63 to 2.53; P = .51) or EORTC (HR, 1.06; 95% CI, 0.56 to 2.04; P = .85) risk group and EFS on univariable analysis (Table 3) or after adjusting for PET score (GHSG: HR, 1.31; 95% CI, 0.65 to 2.62; P = .45; EORTC: HR, 1.19; 95% CI, 0.61 to 2.29; P = .61). Survival curves according to risk stratification are shown in Figures 3A and 3B. There were also no strong associations between any individual pretreatment clinical risk factors and EFS, although age was associated with PFS (Table 4), with 11 of 12 non-HL deaths occurring in patients age 50 years or older. There was no evidence of an association between baseline risk stratification and PFS, even though age 50 years or older is an unfavorable risk factor according to EORTC risk stratification.
FIG 3.
Kaplan-Meier curves of event-free survival (EFS) by (A) European Organisation for Research and Treatment of Cancer (EORTC) and (B) German Hodgkin Study Group (GHSG) risk group.
TABLE 4.
EFS and PFS by Individual Clinical Risk Factors
DISCUSSION
The RAPID trial was a large prospective phase III randomized study and the first to our knowledge to use a graded 5-point scale for response adaptation in lymphoma, which has become the modern standard for response assessment.16,21 Contemporaneous studies used the now outdated IHP18 criteria, with binary positive versus negative outcomes. This subsidiary analysis from RAPID assessed the prognostic relevance of early PET, using graded response and pretreatment risk factors in early-stage HL. Our results demonstrate that PET score after three cycles of ABVD has greater prognostic value than pretreatment risk stratification. These findings support the continuing use of early PET response assessment as part of risk-adapted treatment strategies for early-stage HL.
Using a binary definition of PET positivity (score, 3 to 5) did not sufficiently discriminate outcomes in RAPID. PET-positive patients had a 5-year PFS of 88.4% (95% CI, 83.1% to 93.7%), compared with 91.4% (95% CI, 88.5% to 94.3%) in those achieving CMR, although the two groups had divergent treatment strategies. Some authors have interpreted the results of RAPID and similar studies to mean that PET assessment has limited prognostic value in early-stage HL.22,23 However, the value of a positive PET scan is dependent on the threshold used. Our results demonstrate that individual PET scores are strongly associated with outcomes and reinforce the role of PET in individualized treatment planning in early-stage HL. Using the more widely accepted definition of PET positivity in the Lugano classification (score, 4 or 5)21 provided better discrimination, although only a score of 5 was clearly associated with adverse outcomes in RAPID.
In RAPID, patients with a PET score of 3 had excellent outcomes after ABVD and IFRT without chemotherapy intensification. With a 5-year EFS of 95.3% (95% CI, 90.8% to 99.8%) with ABVD and IFRT alone, our results do not support treatment escalation in this cohort. Whether these patients can be treated with ABVD alone remains unclear; in the PET-adapted Cancer and Leukemia Group B (CALGB) 50604 study, patients with a Deauville score of 3 had inferior outcomes to those with a score of 1 or 2 after receiving four cycles of ABVD alone, although patient numbers were small.12
Patients with a score of 4 also had good outcomes with ABVD and IFRT, with a 5-year EFS of 93.5% (95% CI, 84.9% to 100%), similar to patients with a score of 1 to 3. Although PFS was slightly lower in patients with a score of 4 (87.5%; 95% CI, 76.1% to 98.9%), this included two treatment-related non-HL deaths (bronchopneumonia and pneumonitis), where treatment escalation would not have been beneficial or feasible. None of the deaths in this group were attributable to HL.
Patients with a PET score of 5 after three cycles of ABVD had particularly poor outcomes, with five progressions and three HL-related deaths in only 21 patients. It is clear that treatment with ABVD and IFRT alone is inadequate for these patients, and alternative strategies should be explored. These might include escalation of chemotherapy intensity, as demonstrated by the H10 study,9 or introduction of novel agents.
One of the main limitations of this study is that relatively few patients had a PET score of 4, with a small number of events. Although our findings require additional confirmation, they are supported by emerging data in HL and other lymphoma subtypes that demonstrate patients with a PET score of 5 have significantly worse outcomes than those with a PET score of 4.10,24-27 Baseline PET scans were not performed; therefore, we cannot determine whether patients with a score of 5 had appearances suggestive of progressive metabolic disease, which may have a worse prognosis. However, there was no evidence of progression by CT criteria for patients in this analysis, and early progression is rare in early-stage HL.28 In other studies10,27 and international guidance,16 a score of 5 refers to uptake markedly above liver, without distinguishing whether findings also suggest disease progression, such as increasing metabolic activity and/or new lesions. There was no formal monitoring of the discrepancy rate among central PET reviewers, but several studies have demonstrated that concordance between PET readers using the 5-point scale is high (76% to 84%).8,29,30
It is unclear whether the results of the H10 study can be generalized to the RAPID population, given significant differences in inclusion criteria, particularly with respect to B symptoms and mediastinal bulk. PET scans were also performed earlier in H10, after two cycles of ABVD. However, it is notable that, although H10 was randomized, PET scans were reported only as positive or negative by IHP criteria.18 PET score was not used to stratify patients, and it is unknown whether patients with a score of 5 were balanced between treatment arms. We propose using a score of 5 as a basis for treatment escalation and/or as a stratification factor in future PET-adapted trials.
The PET scoring system used here and in other UK NCRI trials29 evolved directly into the 5-point Deauville scale.15 A PET score of 5 is defined as three times the maximum liver uptake in UK NCRI-led trials. The Lymphoma Study Association and Fondazione Italiana Linfomi use the lower threshold of twice the maximum liver uptake. Improvements in imaging technology, especially new reconstruction algorithms, mean that today, PET is more sensitive, and there may be a shift toward more scans being scored as 3 or 4.31 Treatment efficacy may also affect the predictive ability of PET.32 Quantitative PET(qPET), which is a ratio between residual FDG and mean liver uptake, replaces an ordinal with a continuous scale and may help to refine the threshold between adequate and inadequate response for treatment optimization and allow individualized risk estimates in the future.33,34
In RAPID, neither GHSG nor EORTC risk score was associated with outcomes. Unlike most early-stage HL studies, treatment in RAPID was not adapted according to baseline risk, and this is one of the first studies to explore the prognostic relevance of clinical risk stratification in the context of PET-adapted treatment. Given the much stronger association with PET score, our findings suggest that pretreatment risk stratification may have diminished relevance with PET-adapted treatment, particularly for patients without mediastinal bulk or B symptoms. It is unclear whether our findings are applicable to the wider early-stage HL population, particularly patients with mediastinal bulk, who were excluded from RAPID and in whom the association with adverse outcomes may be stronger.6 However, all risk factors are weighted equally within EORTC and GHSG groupings, which are designed to apply to all patients with early-stage HL, including the RAPID population; therefore, our findings highlight weaknesses in current risk stratification models.
Our results are similar to those of retrospective studies in advanced-stage HL, where the International Prognostic Score failed to retain independent prognostic significance over interim PET assessment.7,35 Indeed, in a subsidiary analysis of H10, only PET assessment, but not baseline risk stratification, was prognostic on multivariable analysis, although treatment was adapted according to EORTC stratification.28 A subsidiary analysis of the GHSG early-stage HL trials in the pre-PET era showed a small absolute difference in PFS between favorable and unfavorable risk groups for patients treated with ABVD and IFRT (9.4% for GHSG and 6.7% for EORTC risk stratification).6 These findings emphasize the need to re-evaluate the use of clinical prognostic grouping in early-stage HL in the era of PET-adapted therapy. Incorporation of biological or baseline PET parameters may be required to improve pretreatment risk stratification.28,36,37
In conclusion, this subsidiary analysis of the RAPID trial demonstrates that PET response assessment after chemotherapy has a much stronger association with outcomes than clinical risk stratification in early-stage HL. We have shown that a positive PET scan does not carry uniform prognostic weight, with only a PET score of 5 associated with inferior outcomes in RAPID; patients with nonbulky early-stage HL and a PET score of 3 or 4 after three cycles of ABVD were treated effectively with a fourth cycle of ABVD and IFRT. In future trials, we propose reserving treatment escalation with its attendant toxicity in this patient group for those with a PET score of 5, who have significantly worse outcomes than those patients with PET scores of 1 to 4. These results support the continued development and use of PET-adapted strategies in early-stage HL.
ACKNOWLEDGMENT
We thank Professor Andrew Lister for his critical review of the manuscript and the investigators, positron emission tomography centers, and patients from all parts of the United Kingdom for their support.
Appendix
FIG A1.
Kaplan-Meier curves of event-free survival (EFS) by positron emission tomography score (PET) of 1 to 4 versus 5 for (A) EORTC favorable risk, (B) EORTC unfavorable risk, (C) GHSG favorable risk, and (D) GHSG unfavorable risk.
TABLE A1.
Pretreatment Risk Stratification in Early-Stage HL
TABLE A2.
Baseline Demographic and Clinical Characteristics by PET Group (N = 562)
Footnotes
Presented at the 58th Annual Meeting of the British Society of Haematology, Liverpool, United Kingdom, April 16-18, 2018, and in part at the 13th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 17-20, 2015.
Supported by the Leukaemia and Lymphoma Research Fund grant reference 0220 (now Bloodwise), Lymphoma Research Trust, Teenage Cancer Trust, and UK Department of Health and Social Care and by the National Institute for Health Research Grant No. RP-2-16-07-001 (S.F.B.). The King’s College London and University College London Comprehensive Cancer Imaging Centre is funded by Cancer Research UK and the Engineering and Physical Sciences Research Council in association with the Medical Research Council and UK Department of Health.
The trial was run by the Cancer Research UK and University College London Cancer Trials Centre. The views expressed are those of the authors and not necessarily those of the National Health Service, National Institute for Health Research, or UK Department of Health and Social Care.
Listen to the podcast by Dr Cheson at ascopubs.org/jco/podcasts
Clinical trial information: NCT00943423.
AUTHOR CONTRIBUTIONS
Conception and design: Sally F. Barrington, Nicholas Counsell, Barry Hancock, Ruth Pettengell, Peter Johnson, Peter Hoskin, Michael J. O’Doherty, Tim Illidge, John Radford
Administrative support: Bilyana Popova
Collection and assembly of data: Sally F. Barrington, Elizabeth H. Phillips, Nicholas Counsell, Ruth Pettengell, Peter Johnson, William Townsend, Bilyana Popova, Andrew McMillan, Peter Hoskin, Michael J. O’Doherty
Data analysis and interpretation: Sally F. Barrington, Elizabeth H. Phillips, Nicholas Counsell, Ruth Pettengell, Peter Johnson, William Townsend, Dominic Culligan, Laura Clifton-Hadley, Andrew McMillan, Peter Hoskin, Michael J. O’Doherty, Tim Illidge, John Radford
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Positron Emission Tomography Score Has Greater Prognostic Significance Than Pretreatment Risk Stratification in Early-Stage Hodgkin Lymphoma in the UK RAPID Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Sally F. Barrington
Speakers’ Bureau: F. Hofmann-La Roche
Research Funding: Bristol-Myers Squibb (Inst), Celgene (Inst), F. Hofmann-La Roche (Inst), Amgen (Inst)
Elizabeth H. Phillips
Research Funding: F. Hoffman-La Roche (Inst)
Ruth Pettengell
Honoraria: CTI Life Sciences, Immune Design, Pfizer, Roche/Genentech, Servier, Takeda Pharmaceuticals, TEVA Pharmaceuticals Industries
Travel, Accommodations, Expenses: Takeda Pharmaceuticals, Pfizer, Servier
Peter Johnson
Honoraria: Takeda Pharmaceuticals, Bristol-Myers Squibb, Novartis, Celgene, Kite Pharma, Genmab, Incyte, MorphoSys
Consulting or Advisory Role: Janssen, Epizyme, Boehringer Ingelheim
Research Funding: Epizyme (Inst), Janssen (Inst)
Patents, Royalties, Other Intellectual Property: Combined use of Fc gamma RIIb (CD32b)– and CD20-specific antibodies, WO patent, PCT/GB2011/051572; EU11760819.0
William Townsend
Honoraria: Roche
Consulting or Advisory Role: Roche
Travel, Accommodations, Expenses: Roche
Dominic Culligan
Honoraria: AbbVie, Pfizer, Takeda Pharmaceuticals, Daiichi Sankyo, Merck Sharp & Dohme, Jazz Pharmaceuticals
Consulting or Advisory Role: AbbVie, Daiichi Sankyo, Pfizer, Takeda Pharmaceuticals, Merck Sharp & Dohme, Jazz Pharmaceuticals
Speakers’ Bureau: Takeda Pharmaceuticals, Celgene
Travel, Accommodations, Expenses: AbbVie, Celgene/Jazz Pharmaceuticals, Daiichi Sankyo
Laura Clifton-Hadley
Research Funding: Pfizer (Inst), Bristol-Myers Squibb (Inst), Takeda Pharmaceuticals (Inst), Janssen (Inst)
Andrew McMillan
Honoraria: Roche/Genentech, Celgene, Bristol-Myers Squibb, MSD Oncology, Novartis
Consulting or Advisory Role: Celgene (I)
Speakers’ Bureau: Roche/Genentech
Research Funding: Pfizer, Roche/Genentech (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Celgene, Takeda Pharmaceuticals
Peter Hoskin
Research Funding: Varian Medical Systems (Inst), Astellas Pharma (Inst), Bayer HealthCare Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Elekta
Tim Illidge
Consulting or Advisory Role: Takeda Pharmaceuticals, Nordic Nanovector
Speakers’ Bureau: Takeda Pharmaceuticals, Bristol-Myers Squibb, Roche
Research Funding: AstraZeneca/MedImmune, MSD Oncology
Travel, Accommodations, Expenses: Roche
John Radford
Stock and Other Ownership Interests: GlaxoSmithKline (I), AstraZeneca (I)
Honoraria: Takeda Pharmaceuticals
Consulting or Advisory Role: Takeda Pharmaceuticals, Seattle Genetics, Novartis
Speakers’ Bureau: Takeda Pharmaceuticals, Seattle Genetics, Novartis
Research Funding: Takeda Pharmaceuticals
Travel, Accommodations, Expenses: Takeda Pharmaceuticals
Travel, Accommodations, Expenses: ADC Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366:399–408. doi: 10.1056/NEJMoa1111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373:2499–2511. doi: 10.1056/NEJMoa1505949. [DOI] [PubMed] [Google Scholar]
- 3. Raemaekers JMM, Andre MPE, Federico M, et al: Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 32:1188-1194, 2014. [DOI] [PubMed]
- 4.Behringer K, Goergen H, Hitz F, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): An open-label, randomised, non-inferiority trial. Lancet. 2015;385:1418–1427. doi: 10.1016/S0140-6736(14)61469-0. [DOI] [PubMed] [Google Scholar]
- 5.von Tresckow B, Plütschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: Final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol. 2012;30:907–913. doi: 10.1200/JCO.2011.38.5807. [DOI] [PubMed] [Google Scholar]
- 6.Klimm B, Goergen H, Fuchs M, et al. Impact of risk factors on outcomes in early-stage Hodgkin’s lymphoma: An analysis of international staging definitions. Ann Oncol. 2013;24:3070–3076. doi: 10.1093/annonc/mdt413. [DOI] [PubMed] [Google Scholar]
- 7.Gallamini A, Barrington SF, Biggi A, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica. 2014;99:1107–1113. doi: 10.3324/haematol.2013.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biggi A, Gallamini A, Chauvie S, et al. International validation study for interim PET in ABVD-treated, advanced-stage hodgkin lymphoma: Interpretation criteria and concordance rate among reviewers. J Nucl Med. 2013;54:683–690. doi: 10.2967/jnumed.112.110890. [DOI] [PubMed] [Google Scholar]
- 9.André MPE, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: Final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35:1786–1794. doi: 10.1200/JCO.2016.68.6394. [DOI] [PubMed] [Google Scholar]
- 10.Johnson P, Federico M, Kirkwood A, et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374:2419–2429. doi: 10.1056/NEJMoa1510093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
- 12. Straus DJ, Jung SH, Pitcher B, et al: CALGB 50604: Risk-adapted treatment of nonbulky early-stage Hodgkin lymphoma based on interim PET. Blood 132:1013-1021, 2018. [DOI] [PMC free article] [PubMed]
- 13. Fuchs M, Goergen H, Kobe C, et al: PET-guided treatment of early-stage favorable Hodgkin lymphoma: Final results of the international, randomized phase 3 trial HD16 by the German Hodgkin Study Group. Blood 132, 2018 pp 925. [Google Scholar]
- 14.Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin lymphoma version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:608–638. doi: 10.6004/jnccn.2017.0064. [DOI] [PubMed] [Google Scholar]
- 15.Meignan M, Gallamini A, Meignan M, et al. Report on the First International Workshop on interim-PET scan in lymphoma. Leuk Lymphoma. 2009;50:1257–1260. doi: 10.1080/10428190903040048. [DOI] [PubMed] [Google Scholar]
- 16.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maraldo MV. Continued conundrum of PET-CT and Hodgkin’s lymphoma. Lancet. 2018;390:2744–2745. doi: 10.1016/S0140-6736(17)32343-7. [DOI] [PubMed] [Google Scholar]
- 18.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 20.Barrington SF, Mackewn JE, Schleyer P, et al. Establishment of a UK-wide network to facilitate the acquisition of quality assured FDG-PET data for clinical trials in lymphoma. Ann Oncol. 2011;22:739–745. doi: 10.1093/annonc/mdq428. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams HJA, Kwee TC. The predictive value of interim FDG-PET in early-stage Hodgkin lymphoma is not well established. Ann Oncol. 2018;29:510–512. doi: 10.1093/annonc/mdx644. [DOI] [PubMed] [Google Scholar]
- 23.Coyle M, Kostakoglu L, Evens AM. The evolving role of response-adapted PET imaging in Hodgkin lymphoma. Ther Adv Hematol. 2016;7:108–125. doi: 10.1177/2040620715625615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ceriani L, Martelli M, Gospodarowicz MK, et al: Positron emission tomography/computed tomography assessment after immunochemotherapy and irradiation using the Lugano classification criteria in the IELSG-26 study of primary mediastinal B-cell lymphoma. Int J Radiat Oncol Biol Phys 97:42-49, 2017. [DOI] [PubMed]
- 25.Hertzberg M, Gandhi MK, Trotman J, et al. Early treatment intensification with R-ICE and 90Y-ibritumomab tiuxetan (Zevalin)-BEAM stem cell transplantation in patients with high-risk diffuse large B-cell lymphoma patients and positive interim PET after 4 cycles of R-CHOP-14. Haematologica. 2017;102:356–363. doi: 10.3324/haematol.2016.154039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mikhaeel GN, Brady J, McMillan A, et al: Blinded evaluation of the prognostic value of FDG-PET after 2 cycles of R-CHOP in DLBCL UK-NCRI study. Hematol Oncol 33, 2015 (abstr 258a) [Google Scholar]
- 27.Melani C, Advani R, Roschewski M, et al. End-of-treatment and serial PET imaging in primary mediastinal B-cell lymphoma following dose-adjusted EPOCH-R: A paradigm shift in clinical decision making. Haematologica. 2018;103:1337–1344. doi: 10.3324/haematol.2018.192492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cottereau AS, Versari A, Loft A, et al. Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood. 2018;131:1456–1463. doi: 10.1182/blood-2017-07-795476. [DOI] [PubMed] [Google Scholar]
- 29.Barrington SF, Kirkwood AA, Franceschetto A, et al. PET-CT for staging and early response: Results from the Response-Adapted Therapy in Advanced Hodgkin Lymphoma study. Blood. 2016;127:1531–1538. doi: 10.1182/blood-2015-11-679407. [DOI] [PubMed] [Google Scholar]
- 30.Barrington SF, Qian W, Somer EJ, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2010;37:1824–1833. doi: 10.1007/s00259-010-1490-5. [DOI] [PubMed] [Google Scholar]
- 31.Boellaard R, Kobe C, Zijlstra JM, et al. Does PET reconstruction method affect Deauville scoring in lymphoma patients? J Nucl Med. 2018;59:1167–1169. doi: 10.2967/jnumed.118.211607. [DOI] [PubMed] [Google Scholar]
- 32.Johnson P, Longley J. Should response-adapted therapy now be the standard of care for advanced Hodgkin’s lymphoma? Curr Treat Options Oncol. 2017;18:15. doi: 10.1007/s11864-017-0460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasenclever D, Kurch L, Mauz-Körholz C, et al. qPET: A quantitative extension of the Deauville scale to assess response in interim FDG-PET scans in lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:1301–1308. doi: 10.1007/s00259-014-2715-9. [DOI] [PubMed] [Google Scholar]
- 34.Barrington SF, Kluge R. FDG PET for therapy monitoring in Hodgkin and non-Hodgkin lymphomas. Eur J Nucl Med Mol Imaging. 2017;44(s) uppl 1:97–110. doi: 10.1007/s00259-017-3690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–3752. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 36.Pike LC, Kirkwood AA, Patrick P, et al. Can baseline PET-CT features predict outcomes in advanced Hodgkin lymphoma? A prospective evaluation of UK patients in the RATHL trial (CRUK/07/033) Hematol Oncol 3537–38.2017. 28591427 [Google Scholar]
- 37.Mottok A, Steidl C. Biology of classical Hodgkin lymphoma: Implications for prognosis and novel therapies. Blood. 2018;131:1654–1665. doi: 10.1182/blood-2017-09-772632. [DOI] [PubMed] [Google Scholar]