Summary
The fungal pathogen Colletotrichum acutatum is the causal agent of strawberry (Fragaria × ananassa) anthracnose. Although the fungus can infect strawberry fruits at both unripe and ripe stages, the symptoms appear only on red ripe fruits. On white unripe fruits, the pathogen becomes quiescent as melanized appressoria after 24 h of interaction. Previous transcriptome analysis has indicated that a mannose‐binding lectin (MBL) gene is the most up‐regulated gene in 24‐h‐infected white strawberries, suggesting a role for this gene in the low susceptibility of unripe stages. A time course analysis of the expression of this MBL gene, named FaMBL1 (Fragaria × ananassa MBL 1a), was undertaken to monitor its expression profile in white and red fruits at early interaction times: FaMBL1 was expressed exclusively in white fruit after 24 h, when the pathogen was quiescent. Agrobacterium‐mediated transient transformation was used to silence and overexpress the FaMBL1 gene in 24‐h‐infected white and red strawberries, respectively. FaMBL1‐silenced unripe fruits showed an increase in susceptibility to C. acutatum. These 24‐h‐infected tissues contained subcuticular hyphae, indicating pathogen penetration and active growth. In contrast, overexpression of FaMBL1 in ripe fruits decreased susceptibility; here, 24‐h‐infected tissues showed a high percentage of ungerminated appressoria, suggesting that the growth of the pathogen had slowed. These data suggest that FaMBL1 plays a crucial role in the resistance of unripe strawberry fruits to C. acutatum.
Keywords: Colletotrichum acutatum, fungal quiescence, mannose binding lectin, strawberry ripening, unripe fruit resistance
Introduction
Fruits undergo dramatic metabolic and structural changes during ripening, which often lead to a totally different susceptibility of unripe and ripe fruit stages to economically important diseases. Generally, unripe fruits are less susceptible to fungal pathogen infections because of the metabolic or mechanical composition of the external cell layers. For this reason, although interacting with unripe fruits, several fungal pathogens become quiescent, arresting their growth until fruits ripen and become susceptible to pathogen colonization. Here, fungi restore their growth, deeply invading the tissues and causing disease symptoms (Prusky, 1996).
Colletotrichum acutatum Simmonds is the primary causal agent of anthracnose disease of strawberry (Fragaria × ananassa). It infects most parts of the plant; however, as for the majority of Colletotrichum species, the highest losses occur on fruits (Bailey and Jeger, 1992). This pathogen attacks fruits at both immature and mature stages; however, the disease symptoms develop only on red ripe fruits, as, on white fruits, C. acutatum becomes quiescent as melanized appressoria after 24 h of interaction (Guidarelli et al., 2011). Only when fruit ripens to the red stage does the pathogen restore its growth, and symptoms become manifest within 3 days.
In an attempt to elucidate the molecular basis of the different susceptibility to C. acutatum, we recently used transcriptome microarray analysis to highlight the differences in gene expression of white unripe and red ripe strawberry fruits after 24 h of interaction with this fungus. Among several genes found to be differentially regulated, a curculin‐like (mannose‐binding) lectin family protein gene was the most up‐regulated gene only in white unripe inoculated fruits (Guidarelli et al., 2011). This gene encodes for a B‐lectin protein of the GNA (Galanthus nivalis agglutinin)‐related mannose‐binding family.
First identified as plant proteins that agglutinate human red blood cells (Van Damme et al., 1998), lectins are ubiquitous proteins in the plant kingdom, capable of recognition and reversible non‐catalytic binding to specific carbohydrates (Peumans and Van Damme, 1995). Although the biological role of plant lectins is still debated, their ability to bind to carbohydrates indicated a role for lectins as plant defence proteins, recognizing and binding glycoconjugates on the surfaces of microorganisms, such as bacteria and fungi (Chrispeels and Raikhel, 1991; De Hoff et al., 2009; Peumans and Van Damme, 1995). Indeed, chitin‐binding lectins from both wheatgerm (Triticum spp.) and stinging nettle (Urtica dioica) have been shown to display inhibitory action against different fungal pathogens on binding to fungal chitin (Broekaert et al., 1989; Mirelman et al., 1975). Similarly, the soybean (Glycine max) lectin β‐glucan‐binding protein recognizes and binds to the β‐glucans, a pathogen‐associated molecular pattern (PAMP) molecule present on the surface of the oomycete Phytophthora sojae (Fliegmann et al., 2004; Mithöfer et al., 2000). In addition, several lectins have been shown to accumulate in plant cells under different biotic and abiotic stresses or displaying antibacterial, antifungal or anti‐insect activity, reinforcing the hypothesis of a role for these proteins in plant defence (Chrispeels and Raikhel, 1991; Peumans and Van Damme, 1995; Van Damme et al., 2004).
Recently, lectins have been classified into 12 families on the basis of their domain structures and phylogenetic analysis (Jiang et al., 2010). Among these, members of the mannose‐binding B‐lectin family, previously known as GNA‐related lectins, or ‘monocot mannose‐binding lectins’ (Van Damme et al., 1995, 2008), have attracted particular attention in plant defence studies, because of their specific ability to interact with oligomannosides and mannose‐type glycans, present on the surface of microbial pathogens (Barre et al., 2002; Van Damme et al., 1998, 2007). Mannose‐binding lectins (MBLs) contain one or two GNA domains, originally characterized from the MBL of snowdrop (Galanthus nivalis) bulbs (Van Damme et al., 1987); each of these contains three similar mannose‐binding sites per subunit (Van Damme et al., 2008).
The role of MBLs in plant defence has been confirmed for a number of plant species: the MBL CaMBL1 was isolated from Capsicum annuum leaves infected with Xanthomonas campestris pv. vesicatoria (Xcv). Its GNA domain is capable of specific binding to high‐mannose N‐glycans, present on the Xcv surface. CaMBL1 localizes to plasma membranes and is necessary for the activation of defence‐related genes, for salicylic acid accumulation and for cell death induction in infected leaves. The overexpression of the CaMBL1 gene in Arabidopsis confers enhanced resistance to bacterial and fungal pathogens (Hwang and Hwang, 2011). Furthermore, a number of MBLs have been shown to display direct antifungal activity. For example, the monomeric mannose/glucose‐binding lectin, isolated from the seed of red cluster pepper, Capsicum frutescens, is capable of inhibiting conidia germination and hyphal growth of the two fungi Aspergillus flavus and Fusarium moniliforme (Ngai and Ng, 2007); the mannose/glucose‐binding lectin Dgui, extracted from the leguminous plant Dioclea guianensis, inhibits the conidia germination of C. gloeosporioides, suggesting that this protein recognizes a specific target on the surface of this fungus (Araujo‐Filho et al., 2010); similarly, the orchid mannose‐binding proteins, gastrodianins, are active against the fungal pathogen Valsa ambiens (Wang et al., 2001), and also against Botrytis cinerea, Gibberella zeae, Ganoderma lucidum, Rhizoctonia solani and Phytophthora nicotianae (Cox et al., 2006; Xu et al., 1998); finally, the mycelial growth of Alternaria alternata is inhibited by an MBL from the orchid Dendrobium findleyanum (Sudmoon et al., 2008).
Despite the large number of reports on the defence role of plant MBLs, their mode of action and molecular mechanisms are still poorly understood. In addition, there is no evidence of the possible role of these proteins in determining the low susceptibility of unripe fruits to microbial pathogens (Guidarelli et al., 2011).
The previously reported data on the up‐regulation of a gene encoding for an MBL only in white strawberries interacting with quiescent C. acutatum suggests that the encoded lectin could possibly have a role in the resistance of unripe strawberries to anthracnose.
Here, to investigate this hypothesis, we first performed a time course analysis of the expression of this lectin gene in both white and red fruits, to score possible differences in the regulation of its transcription, before 24 h of interaction with the fungus. Subsequently, Agrobacterium tumefaciens‐mediated transient transformation was used to silence the expression of the lectin gene in white 24‐h‐infected fruits and, in parallel, to overexpress it in red ripe fruits, similarly infected. The observation of anthracnose symptoms of transfected white and red strawberries inoculated with C. acutatum, and histological analysis of the infected tissues, indicated that this gene strongly influences the susceptibility of ripening strawberry fruits, possibly playing an important role in the different response of these fruits to the pathogen.
Results
FaMBL1 gene encodes for a B‐lectin protein
In our previous study, we found that the mannose‐binding lectin (MBL) gene (TA10594_57918) was the most up‐regulated gene after 24 h of C. acutatum infection only in white unripe strawberry fruits, where the pathogen becomes quiescent (Guidarelli et al., 2011). No difference in the expression of this gene was found in inoculated red fruits. The full‐length cDNA of this gene, named FaMBL1 (GenBank accession number: KF962716), was isolated from a 24‐h‐infected white fruit cDNA library (Fragaria × ananassa MBL): it is 1329 bp long and encodes for a protein of 442 amino acids.
The FaMBL1 cDNA sequence was analysed using the blastx program (http://blast.ncbi.nlm.nih.gov); the encoded protein contains an N‐terminus signal peptide (SP) necessary for protein secretion into the extracellular environment, a B‐lectin domain containing the three‐fold internal repeat QXDXNXVXY consensus sequence motif, involved in α‐d‐mannose recognition, and a C‐terminal PAN‐Apple domain, widespread in plant lectins (Van Damme et al., 2008), mediating protein–protein and protein–carbohydrate interactions (Fig. 1). These features allow us to ascribe FaMBL1 to the plant B‐lectin family (Jiang et al., 2010).
Figure 1.
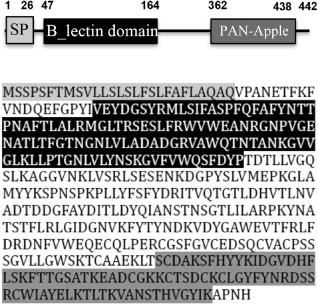
FaMBL1 primary structure. Amino acid sequence of FaMBL1 and blastx domain prediction analysis. The signal peptide, B‐lectin domain and PAN‐Apple domain are highlighted.
FaMBL1 shares 54% sequence identity with the Arabidopsis thaliana curculin‐like (mannose‐binding) lectin family protein (accession number NP_178007), 57% with the carrot (Daucus carota) cell attachment protein (BAD24818), 63% with the sugar beet (Beta vulgaris) SIEP1L protein (CAA61158), 67% with a Theobroma cacao d‐mannose‐binding lectin protein (EOY13258) and 100% with the woodland strawberry (Fragaria vesca) epidermis‐specific secreted glycoprotein (XP_004294119). However, the recently characterized pepper (Capsicum annuum) CaMBL1 (ADG04234) shares similarity with FaMBL1, but is a shorter protein, lacking 144 amino acids at its C‐terminus (Fig. S1, see Supporting Information).
Time course of FaMBL1 gene expression in strawberry fruits infected with C. acutatum
In our previous study, we showed that, depending on the fruit ripening stage, at 24 h post‐inoculation (hpi), C. acutatum infecting strawberries changes its colonization strategy: on white unripe fruits, it arrests its growth and becomes quiescent as melanized appressoria, whereas, in red ripe fruits, it is actively growing as subcuticular intercellular hyphae. In order to establish whether the up‐regulation of the FaMBL1 gene, unique to white strawberries at 24 hpi, was a specific response of unripe fruits interacting with a quiescent pathogen, or whether it also occurred in red ripe strawberries at earlier times of infection, a time course analysis of FaMBL1 expression was performed. In particular, the FaMBL1 transcript level was quantified by quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) in white and red fruits at earlier stages of the interaction, namely at 8 h (when conidia are still ungerminated on the fruit surface), at 16 h (when germinative tubules are produced from most conidia), at 20 h (when appressoria become apparent) and at 24 h (when C. acutatum proceeds with its infection only in red ripe fruits with subcuticular hyphae, whereas, in white fruits, it becomes quiescent as melanized appressoria) (Guidarelli et al., 2011).
By comparing inoculated fruits with mock‐inoculated ones, the expression of the FaMLB1a gene varied significantly exclusively in white strawberry fruits at 24 hpi: here, in accordance with our previous microarray data, the FaMLB1a transcript level increased by about five‐fold with respect to mock‐inoculated fruits. The expression level of FaMBL1 did not vary on infection in white fruits at earlier time points (0, 8, 16 and 20 hpi) (Fig. 2). In red inoculated fruits, the expression level of FaMLB1a did not undergo any variation with respect to mock‐inoculated fruits at any of the examined time points. This indicates that the increase in the expression level of this gene is indeed specific to white unripe strawberry fruits interacting with quiescent melanized appressoria of C. acutatum, and could suggest that FaMBL1 has a specific role for the resistance of white unripe strawberries to this pathogen.
Figure 2.
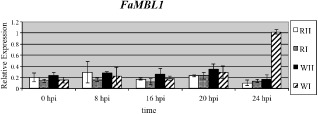
Time course of gene expression analysis of FaMBL1 in white and red strawberry fruits on Colletotrichum acutatum inoculation. The expression level of FaMBL1 was quantified in white and red fruits inoculated with C. acutatum (WI and RI, respectively) or mock inoculated (WH and RH), and scored at 0, 8, 16, 20 and 24 h post‐inoculation (hpi). Data were normalized to the transcript level of the housekeeping elongation factor 1α gene. Values are the means of three biological replicates.
FaMBL1 silencing and T‐DNA integration are effectively accomplished in white and red strawberry fruits 3 days after injection with A. tumefaciens
To investigate the possible role of FaMBL1 in the difference in susceptibility to C. acutatum of unripe and ripe strawberry fruits, we used Agrobacterium transient transformation to silence the increase in expression of this gene typical of white fruits after 24 hpi with C. acutatum and, in parallel, to induce its overexpression in red infected fruits.
To set up the best timing of Agrobacterium‐mediated gene silencing, white strawberries were transfected for 1, 2 or 3 days with A. tumefaciens carrying FaMBL1 silencing vectors, harvested and inoculated with C. acutatum. The FaMBL1 expression level was scored by qRT‐PCR at 24 hpi. The silencing vector (pk7:FaMBL1) consisted of an intron‐containing hairpin (ihp) construct generated by inserting a partial fragment of the FaMBL1 gene in the sense and antisense orientation interrupted by an intron, which determined the formation of self‐complementary hairpin RNA (ihp‐RNA), leading to an RNAi‐induced degradation of the specific gene target mRNAs. As a negative control, to check that agroinfiltration did not interfere with FaMBL1 gene expression independent of silencing, white strawberries were transformed with Agrobacterium carrying the empty vector (pk7:00) for 1, 2 or 3 days, harvested and assayed for FaMBL1 gene expression after 24 h of C. acutatum infection. The increase in gene expression, normally induced by the 24‐h C. acutatum inoculation in wild‐type strawberries, was drastically decreased in strawberry transformed with pk7:FaMBL1 even after 1 day of agroinfiltration. Silencing was still effective after 3 days (Fig. 3A). In the white control strawberries, transfected with empty vector and infected with C. acutatum, FaMBL1 gene expression increased, indicating that 1, 2 or 3 days of agroinfiltration did not alter significantly the FaMBL1 transcriptional response.
Figure 3.
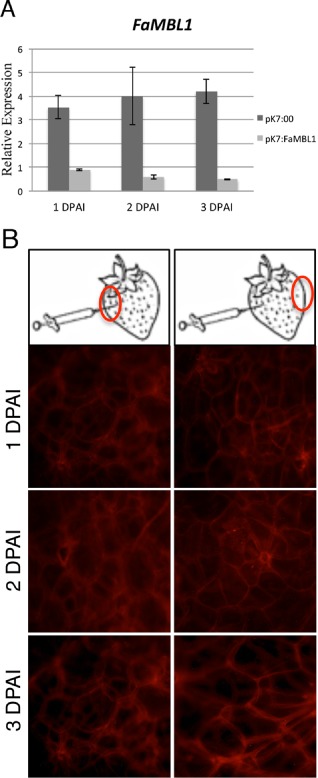
Timing of T‐DNA integration in strawberry fruits. (A) Quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis of FaMBL1 expression in silenced white strawberry fruits infiltrated with Agrobacterium carrying the construct pk7:FaMBL1 (pk7:FaMBL1) or the empty vector control (pk7:00). FaMBL1 expression was scored from 1 to 3 days post‐agroinjection (DPAI). Data were normalized to the expression level of the housekeeping elongation factor 1α gene. Values are the means of three biological replicates. (B) Fluorescence microscope analysis of strawberry tissues at 1–3 DPAI carrying the plasmid pK7GWIWG2 II‐RedRoot. Transfected strawberries were observed both proximal (left) and distal (right) to the Agrobacterium infiltration point.
Similarly, the T‐DNA integration and overexpression timing in strawberry fruit on agroinfiltration was scored by transfecting fruits with the plasmid pK7GWIWG2 II‐RedRoot, allowing the overexpression of the DsRED fluorescent protein. RedRoot fluorescence was monitored after 1, 2 and 3 days of agroinfiltration. Fluorescence became apparent even after 1 day of agroinfection and remained clearly visible up to 3 days after infiltration (Fig. 3B), indicating that the overexpression of the transfected gene is maintained very efficiently during this time. Furthermore, tissues both close and far from the injection point became fluorescent, indicating that the transfection occurred in the whole fruit (Fig. 3B).
Taking into account these data, 3 days was chosen as the agroinfiltration time for the subsequent experiments.
Silencing of FaMBL1 in white unripe strawberries affects fruit resistance to C. acutatum
The induction of FaMBL1 gene expression at 24 hpi of C. acutatum was evaluated by qRT‐PCR in pk7:FaMBL1 [3 days post‐agroinfiltration (DPAI) with the FaMBL1 silencing vector], pk7:00 (3 DPAI with empty silencing vector, as control) and in wild‐type white strawberries. After 24 h of infection, FaMBL1 expression was strongly induced by C. acutatum in both wild‐type and control infiltrated strawberries (pk7:00), where its transcript level increased by about 10‐ and eight‐fold, respectively. In contrast, in the pk7:FaMBL1 transfected strawberries, the induction of FaMBL1 gene expression was almost abolished (Fig. 4A).
Figure 4.
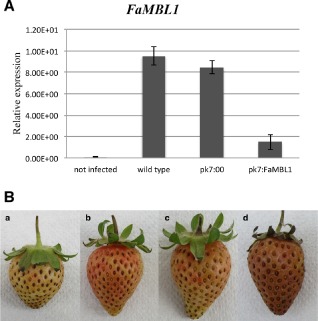
Infection of Colletotrichum acutatum in FaMBL1‐silenced white strawberry fruits. (A) Quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis of FaMBL1 expression in white wild‐type or transfected strawberries 24 h after inoculation with C. acutatum. The transcript levels of mock‐inoculated wild‐type fruits (not infected) and 24‐h C. acutatum‐inoculated fruits (wild‐type) were compared with those of mock‐silenced fruits (pK7:00) and FaMBL1‐silenced fruits (pK7:FaMBL1), both 24 h after inoculation with C. acutatum. Data were normalized to the expression level of the housekeeping elongation factor 1α gene. Values are the means of three biological replicates. (B) Disease symptom analysis in mock‐infected white fruits (not infected) (a), wild‐type white fruits (b), mock‐silenced white fruits (pk7:00) (c) and FaMBL1‐silenced white fruits (pK7:FaMBL1) (d), all (b–d) infected for 5 days with C. acutatum.
With respect to disease susceptibility, 5 days after C. acutatum inoculation, the wild‐type and pk7:00 control agroinfiltrated whitestrawberries did not show anthracnose symptoms. This is consistent with our previously published results, showing that C. acutatum infecting white unripe strawberries becomes quiescent (Guidarelli et al., 2011), and indicates that agroinfiltration does not alter the resistance of unripe fruits to this pathogen. However, at this time (5 days post‐infection), the white pk7:FaMBL1‐silenced fruits showed typical anthracnose symptoms with brown necrosis in the whole fruit (Fig. 4B), suggesting that FaMBL1 gene silencing strongly affects the resistance of white fruits to C. acutatum.
The histological analysis of the 24‐hpi‐infected tissues highlighted the presence of melanized appressoria in wild‐type and pk7:00 control strawberries, whereas penetration events with hyphae internal to the epidermal layers were distinguishable in pk7:FaMBL1‐silenced fruits (Fig. 5). This suggests that, in these fruits, C. acutatum does not become quiescent, but proceeds in its colonization process, whereas, in wild‐type and pk7:00 control strawberries, the pathogen becomes quiescent.
Figure 5.
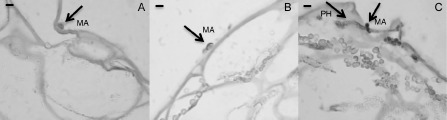
Histological analysis of 24‐h Colletotrichum acutatum‐infected FaMBL1‐silenced white strawberry fruits. Optical microscopy of wild‐type white fruits (A), mock‐silenced white fruits (pk7:00) (B) and silenced white fruits (pk7:FaMBL1) (C), all inoculated for 24 h with C. acutatum. Tissue slices were stained with haematoxylin and eosin. Melanized appressoria (MA) and intercellular hyphae (PH) are indicated. Bar, 10 μm.
Overexpression of FaMBL1 in red ripe strawberries decreases the fruit susceptibility to C. acutatum
The overexpression of FaMBL1 was induced in red ripe strawberries by infiltration for 3 days with Agrobacterium carrying the plasmid 35S:FaMBL1. Overexpression in these strawberries was evaluated by qRT‐PCR at 24 hpi of C. acutatum; here, the expression of the FaMBL1 gene increased more than 400‐fold with respect to the control red fruits (non‐infected, infected wild‐type or infected 35S:00) (Fig. 6A), indicating that overexpression was very efficient and that agroinfiltration itself did not alter FaMBL1 expression.
Figure 6.
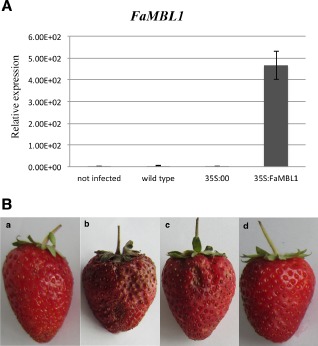
Infection of Colletotrichum acutatum in FaMBL1‐overexpressing red strawberry fruits. (A) Quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) analysis of FaMBL1 expression in red wild‐type or transfected strawberries 24 h after inoculation with C. acutatum. The transcript levels of wild‐type mock‐inoculated fruits (not infected) and 24‐h C. acutatum‐inoculated fruits (wild‐type) were compared with those of mock‐overexpressing fruits (35S:00) and FaMBL1‐overexpressing fruits (35S:FaMBL1), both at 24 hpi with C. acutatum. Data were normalized to the expression level of the housekeeping elongation factor 1α gene. Values are the means of three biological replicates. (B) Disease symptom analysis in red wild‐type mock‐infected fruits (a), red wild‐type fruits (wild‐type) (b), mock‐overexpressing red fruits (35S:00) (c) and FaMBL1‐overexpressing red fruits (35S:FaMBL1) (d), all (b–d) infected for 5 days with C. acutatum.
Five days after C. acutatum inoculation, wild‐type red fruits and 35S:00 agroinfiltrated control fruits showed the typical anthracnose symptoms with dark and sunken lesions on the fruit surface (Fig. 6B). Symptom development was drastically slowed in 35S:FaMBL1 red fruits, supporting the hypothesis that this gene plays a role in the susceptibility of strawberries to C. acutatum.
Sections of 24‐hpi‐infected red fruits were analysed microscopically; penetrated hyphae underneath the fruit surface were detected in 35S:00 control fruits and in wild‐type ripe strawberry fruits (Fig. 7); in contrast, in 35S:FaMBL1 fruits, a higher percentage of melanized appressoria of C. acutatum without penetration events was clearly visible in the tissue, suggesting that here the pathogen slowed its growth.
Figure 7.
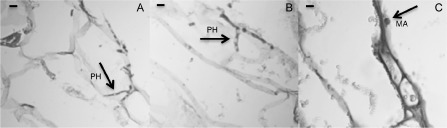
Histological analysis of 24‐h Colletotrichum acutatum‐infected FaMBL1‐overexpressing red strawberry fruits. Optical microscopy of wild‐type red fruits (A), mock‐overexpressing red fruits (35S:00) (B) and overexpressing red fruits (35S:FaMBL1) (C), all inoculated for 24 h with C. acutatum. Tissue slices were stained with haematoxylin and eosin. Melanized appressoria (MA) and intercellular hyphae (PH) are indicated. Bar, 10 μm.
Discussion
The majority of post‐harvest losses in fruit production are caused by fungal pathogen infections occurring at the pre‐harvest immature stage. Here fungi become quiescent until the fruits ripen, provoking symptoms only in mature fruits, when the commercial value is at its highest. For this reason, knowledge of the molecular basis of the low susceptibility of unripe fruits is of great importance, in particular for the development of new control strategies.
In this study, we isolated and functionally characterized the strawberry FaMBL1 gene, previously found to be up‐regulated in unripe strawberry fruits interacting with quiescent melanized appressoria of C. acutatum (Guidarelli et al., 2011).
MBLs are B‐lectin proteins believed to play a role in the innate immune response through their ability to bind to carbohydrates on the surface of microbial pathogens (Barre et al., 2001; Peumans and Van Damme, 1995; Vijayan and Chandra, 1999;). The molecular basis of their mode of action is still uncharacterized; furthermore, their structure is very variable in terms of peptide length and domain composition, as indicated by Van Damme et al. (2008).
Following the description reported by Van Damme et al. (2008), FaMBL1 encodes a protein similar to the GNA‐related lectins identified in the Brassicaceae family; it includes an N‐terminus signal peptide necessary for protein secretion into the extracellular environment, followed by three mannose‐binding domains, with the QxDxNxVxYx consensus motif for α‐d‐mannose recognition and a C‐terminal PAN‐Apple domain (Fig. 1). FaMBL1 lacks the S‐locus glycoprotein domain (SLP), playing a role in Brassica self‐incompatibility, which is anyway poorly conserved among GNA‐related lectins. For several MBL proteins, the ability to bind in vitro to mannose residues has been established: many bind with a weak affinity to mannose, but strongly interact with oligomannosides and high‐mannose N‐glycans (Van Damme et al., 2008).
For most plant lectins, the ability to recognize and bind to glycoconjugates on the surface of microorganisms through GNA‐related domains is strictly related to their defence role (Peumans and Van Damme, 1995). On establishment of contacts with a host surface, pathogens release extracellular matrix (ECM) material containing glycoproteins with mannose residues, which are recognized by and bind to the mannose‐binding domains of MBLs (Barre et al., 2001; Sugui et al., 1998). In particular, for most Colletotrichum species, it was found that the production and secretion of ECM material are associated with the developing appressorium process and are highest when melanized appressoria formation is accomplished (Bailey et al., 1992). Using lectin cytochemistry, the specific sugar composition and abundance on the surface of Colletotrichum spp. appressoria were monitored during appressoria formation: α‐d‐mannose residues, in the form of mannans or mannoproteins, and galactose, were detected as the most abundant sugars in ECM material at the stage of melanized appressoria (O'Connel et al., 1992; Pain et al., 1996).
The time course analysis of FaMBL1 gene expression revealed that this gene is up‐regulated exclusively in white unripe strawberry fruits after 24 h of infection with C. acutatum, at the same time as the formation of quiescent melanized appressoria. No change in the expression of this gene was detected in white fruits at earlier time points. Furthermore, FaMBL1 gene expression was not altered in red fruits interacting with the pathogen, either during the early infection stages, such as conidia germination and appressoria formation, or after 24 h of infection, when penetration had occurred and subcuticular hyphae were visible (Guidarelli et al., 2011) (Fig. 2). Such a specific up‐regulation of the MBL gene in unripe fruits at 24 hpi with C. acutatum could suggest that, similar to other GNA‐related lectins, FaMBL1 is capable of the recognition of and binding to carbohydrates present on the surface of quiescent melanized appressoria. To test this hypothesis, the binding ability of the FaMBL1 GNA domain to mannans and mannoproteins of C. acutatum appressoria should be tested. Similarly, it would be interesting to characterize the downstream effects of this interaction on both fungal pathogen growth and strawberry fruit susceptibility.
We have shown that silencing of FaMBL1 expression in 24‐h C. acutatum‐inoculated white fruits leads to enhanced disease susceptibility and the appearance of early disease symptoms at unripe stages (Fig. 4). The histological analysis of infected tissues showed that this phenotype is associated with pathogen penetration events, characterized by the presence of subcuticular hyphae, a clear sign of active pathogen colonization (Fig. 5), normally absent in white unripe fruits. However, 24‐h C. acutatum‐inoculated red ripe fruits overexpressing the FaMBL1 gene were shown to have delayed anthracnose symptoms, which normally appear 3 days after infection. A high percentage of melanized appressoria was observed in these fruits (Figs 6 and 7).
The altered susceptibility of transfected strawberry fruits supports the hypothesis that FaMBL1 plays a crucial role in the differential susceptibility of fruits to C. acutatum. Consistently, silencing of the homologous CaMBL1 gene in pepper leaves infected with Xcv enhanced disease susceptibility, decreased the accumulation of reactive oxygen species (ROS) and reduced the expression of pathogenesis‐related (PR) genes. By contrast, its overexpression in Arabidopsis conferred resistance to Alternaria brassicicola (Hwang and Hwang, 2011). The GNA‐related domain of CaMBL1 is capable of binding to mannose and N‐acetylgalactosamine residues, and is responsible for cell death induction; it is believed that its binding activity is related to its defence role (Hwang and Hwang, 2011). Similarly, on recognition and binding to specific mannose residues on C. acutatum appressoria, FaMBL1 could induce downstream signals leading to the expression and activation of defence signals in strawberry fruits. However, FaMBL1 could also act directly against the fungus, inhibiting its growth on binding to fungal oligomannoside residues.
Furthermore, the expression of the FaMBL1 gene only in white infected strawberry fruits indicates that the ripening process dramatically changes the regulation of transcription of this specific lectin gene.
Interestingly, our past microarray study revealed that, together with the FaMBL1 gene, several genes encoding hormone biosynthesis enzymes were expressed exclusively in 24‐h‐infected white fruits, and not in red ones: among these were, for example, aminocyclopropane carboxylate oxidase (ACO), and allene oxide cyclase (AOC) genes, involved in synthesis of ethylene (ET) and jasmonic acid (JA), respectively, and the abscisic acid (ABA)‐responsive element (ARE) and phosphatase 2C (PP2C) genes, involved in the ABA biosynthesis (Guidarelli et al., 2011). These data suggest that ET, JA and ABA could play roles in regulating FaMBL1 transcription during ripening. In support of this, AtLEC, a lectin gene in Arabidopsis, was shown to be induced by fungal cell wall oligosaccharides, as well as JA and ET hormones (Lyou et al., 2009), and jasmonate‐inducible lectins were identified from Nicotiana spp. (Lannoo et al., 2006). In addition, a recent transcriptome analysis of ripening tomato interacting with Botrytis highlighted a role for ET, JA and ABA hormones in the resistance of unripe fruits to fungal pathogens (Blanco‐Ulate et al., 2013).
Our results indicate that an MBL protein plays an important role in the resistance of unripe stages of fruits to fungal pathogens. Future investigations should be addressed to clarify the molecular basis of the function of the FaMBL1 protein in unripe fruit resistance, to elucidate the upstream events regulating its transcription and to reveal the downstream events leading to the inhibition of fungal growth.
Experimental Procedures
Fungal and plant material
Colletotrichum acutatum was isolated from strawberry fruits showing severe anthracnose symptoms and identified by morphological analysis and sequencing of ribosomal DNA internal transcribed spacer (ITS) regions. The isolate was grown on potato dextrose agar (Sigma, St. Louis, MO, USA) at 20 °C for 10 days. For fruit infections, stock solutions (≥100×) of conidial suspensions (≥108 conidia/mL) were prepared by washing the colonies with 5 mL of sterile distilled water containing 0.05% (v/v) Tween‐80 and quantifying the conidia concentration with a haemocytometer.
Fragaria × ananassa cv. ‘Alba’ plants cultivated in pots in a glasshouse were used for all experiments. Standard growing conditions were maintained at 20 °C with a 16‐h photoperiod.
Time course of gene expression analysis
For the time course of FaMBL1 gene expression analysis, white unripe (about 20 days after anthesis, daa) and red ripe (about 30 daa) strawberry fruits were harvested and inoculated with C. acutatum by dipping for 1 min in a solution containing 106 conidia/mL suspension (diluted in water from the prepared stock) or with distilled water (mock control), and stored at 20 °C and 70% relative humidity for 8, 16, 20 and 24 h. After that time, the fruit surface was excised with a clean scalpel and immediately frozen in liquid nitrogen and transferred to −80 °C until use. Total RNA was prepared as described by Lopez‐Gomez and Gomez‐Lim (1992) with minor modifications. For qRT‐PCR experiments, first‐strand cDNA was synthesized from 1 μg of total RNA in a volume of 20 μL with oligo‐d(T)17 and Superscript III (Invitrogen Life Technology, Carlsbad, CA, USA), following the manufacturer's instructions. The cDNA concentration in the RT mix was quantified using a ND‐1000 UV spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA), and 1 μg of cDNA was used for qRT‐PCR experiments, employing an MX3000 thermal cycler (Stratagene, La Jolla, CA, USA) and Platinum Sybr‐Green Kit (Invitrogen Life Technology), according to the manufacturer's instructions.
The elongation factor 1α gene, having constitutive expression, was used to normalize raw data and to calculate relative transcript levels. Experiments were performed in three independent biological replicates of cDNA, each run in three technical replicates. Means were subjected to analysis of variance (ANOVA).
Plasmid construction, Agrobacterium transformation and plant transfection
The pK7GWIWG2(II), pK7GWIWG2 II‐RedRoot and pK7WG2 vectors, described in Karimi et al. (2002), were obtained from the VIB Department of Plant Systems Biology, Ghent University, Belgium (http://gateway.psb.ugent.be).
For FaMBL1 silencing, the partial sequence of the FaMBL1 gene was amplified from the white inoculated strawberry cDNA pool using the FaMBL1‐silencing forward primer (5′‐CACCATGCAAGTCCCGGCAAACGAAAC‐3′) and FaMBL1‐silencing reverse primer (5′‐TTTGCCATGCAACGCGGCCGTCGGCGT‐3′). The PCR product was directionally cloned into entry vector pENTR/D‐TOPO (Invitrogen Life Technology). The FaMBL1 gene fragment was subsequently cloned into GATEWAY ready pK7GWIWG2(II) RNAi vector in sense and antisense orientation spaced by an intron, using the LR reaction of GATEWAY recombination‐based cloning (Invitrogen Life Technology). To confirm the correct orientation, the resulting RNAi construct was checked using PCR [two reactions: one with the CaMV35S promoter forward primer (5′‐ATTACAATTTACTATTCTAGTCG‐3′) and FaMBL1‐silencing reverse primer; a second with the CaMV35S terminator forward primer (5′‐TTTTGCGGACTCTAGCATGGCCG‐3′) and the FaMBL1‐silencing reverse primer] and digested using XbaI and HindIII restriction enzymes. Sequencing of PCR products was performed to ensure the specificity of the gene target.
In order to overexpress FaMBL1 in red fruits, the full‐length sequence of FaMBL1 was isolated from the cDNA library of white unripe strawberry fruits infected with C. acutatum and directionally cloned into entry vector pENTR/D‐TOPO using the primers FaMBL1Over forward (5′‐CACCATGTCTTCTCCTTCATTCACAAT‐3′) and FaMBL1Over reverse (5′‐TATACTAGTGATTAGGTGCCTTGATGT‐3′). The FaMBL1 gene was, in turn, cloned into pK7WG2 using GATEWAY cloning technology, as described above. The plasmid constructs were checked by PCR [CaMV35S promoter forward primer and attB2 reverse primer (5′‐ACCATTTGTACAAGAAA‐3′)], by digestion (XbaI and HindIII restriction enzymes) and by DNA sequencing.
The resulting plasmids (pK7:FaMBL1 for silencing, 35S:FaMBL1 for overexpression) were introduced into A. tumefaciens strain EHA105 using the freeze–thaw shock method (Holsters et al., 1978).
The A. tumefaciens strains EHA105, containing pK7:FaMBL1, and 35S:FaMBL1 were grown at 28 °C in Luria–Bertani (LB) medium with appropriate antibiotics. When the culture reached an optical density at 600 nm (OD600) of about 0.8, Agrobacterium cells were harvested and resuspended in a modified MacConkey agar (MMA) medium [Murashige and Skoog salts, 10 mm 2‐(N‐morpholino)ethanesulphonic acid (MES), pH 5.6, 20 g/L sucrose and 200 μm acetosyringone], according to Spoalore et al. (2001). After 1 h of incubation at 22 °C, the Agrobacterium suspension was injected into fruits still attached to the plant.
The silencing time was scored by agroinfiltrating white fruits with the plasmid pK7:FaMBL1. After 1, 2 or 3 days, fruits (10 fruits for each transfection time) were harvested and inoculated with C. acutatum conidia for 24 h, as described previously. Tissues from the surface of the whole fruit were collected and RNA was isolated, as described previously. qRT‐PCR was used to evaluate the FaMBL1 transcript level, as described above. Similarly, for the determination of the integration timing of T‐DNA on the strawberry genome, nine white fruits were agroinfiltrated with a plasmid pK7GWIWG2 II‐RedRoot containing a red fluorescent protein gene. Strawberry fruits were harvested after 1, 2 or 3 days (three fruits per condition), and tissues were analysed using a Nikon Eclipse TE2000‐E fluorescence microscope (Nikon, Melville, NY, USA).
The A. tumefaciens strains EHA105, containing pK7:FaMBL1, and 35S:FaMBL1 were grown at 28 °C in LB medium with appropriate antibiotics. When the culture reached an OD600 of about 0.8, Agrobacterium cells were harvested and resuspended in MMA medium. After 1 h of incubation at 22 °C, the Agrobacterium suspension was evenly injected into fruits still attached to the plant using a sterile 5‐mL syringe.
FaMBL1 silencing and overexpression
For silencing, 50 white strawberry fruits at about 17 daa (3 days before the ‘white’ stage) were agroinfiltrated with pK7:FaMBL1 for 3 days. They were harvested and inoculated with C. acutatum, as described above. Twenty of these fruits were collected 24 h after inoculation: 15 fruits were used for FaMBL1 gene expression analysis to confirm gene silencing. For this, RNA was isolated and qRT‐PCR was performed, as described above. Five fruits were used for histological analysis of the fungal infection, as described by Guidarelli et al. (2011). The other 30 fruits were phenotypically observed after 5 days of inoculation for anthracnose symptom evaluation.
Similarly, for overexpression, 50 red fruits at about 27 daa (3 days before the ‘red’ stage) were agroinfiltrated with plasmid 35S:FaMBL1 and treated as for silencing, except that, for better phenotype evaluation, 30 fruits were observed 3 days instead of 5 days after C. acutatum inoculation.
As controls for silencing and overexpression, the same number (50) of white and red fruits were agroinfiltrated with pK7:00 (the empty silencing vector) and 35S:00 (the empty overexpression vector) plasmids, or left in the wild‐type condition. Phenotype observation, gene expression and histological analysis were performed as described above.
Supporting information
Fig. S1 Multiple sequence alignment of FaMBL1 and mannose‐binding lectins (MBLs) from other species. FaMBL1 protein sequence (KF962716) was aligned with woodland strawberry (Fragaria vesca) epidermis‐specific secreted glycoprotein (XP_004294119), cacao (Theobroma cacao) MBL (EOY13258), sugar beet (Beta vulgaris) SIEP1L protein (CAA61158), pepper (Capsicum annuum) CaMBL1 (ADG04234), carrot (Daucus carota) cell attachment protein (BAD24818) and Arabidopsis curculin‐like (mannose‐binding) lectin protein (NP_178007). The mannose‐binding domain is boxed.
References
- Araujo‐Filho, J.H. , Vasconcelos, I.M. , Martins‐Miranda, A.S. , Gondim, D.M. and Oliveira, J.T. (2010) A ConA‐like lectin from Dioclea guianensis Benth. has antifungal activity against Colletotrichum gloeosporioides, unlike its homologues, ConM and ConA. J. Agric. Food Chem. 58, 4090–4096. [DOI] [PubMed] [Google Scholar]
- Bailey, J.A. and Jeger, M.J. (1992) Colletotrichum: Biology, Pathology and Control. Wallingford, Oxfordshire: CAB International. [Google Scholar]
- Bailey, J.A. , O'Connel, R.J. , Pring, R.J. and Nash, C. (1992) Infection strategies of Colletotrichum species In: Colletotrichum: Biology, Pathology and Control (Bailey J.A. and Jeger M.J., eds), pp. 88–120. Wallingford, Oxfordshire: CAB International. [Google Scholar]
- Barre, A. , Bourne, Y. , Van Damme, E.J.M. , Peumans, W.J. and Rougé, P. (2001) Mannose‐binding plant lectins: different structural scaffolds for a common sugar‐recognition process. Biochimie, 83, 645–651. [DOI] [PubMed] [Google Scholar]
- Barre, A. , Herve, C. , Lescure, B. and Rougé, P. (2002) Lectin receptor kinases in plants. Crit. Rev. Plant Sci. 21, 379–399. [Google Scholar]
- Blanco‐Ulate, B. , Vincenti, E. , Powell, A.L.T. and Cantu, D. (2013) Tomato transcriptome and mutant analyses suggest a role for plant stress hormones in the interaction between fruit and Botrytis cinerea . Front. Plant Sci. 4, 142. doi: 10.3389/fpls.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert, W.F. , van Parijs, J. , Leyns, F. , Joos, H. and Peumans, W.J. (1989) A chitin‐ binding lectin from stinging nettle rhizomes with antifungal properties. Science, 245, 1100–1102. [DOI] [PubMed] [Google Scholar]
- Chrispeels, M.J. and Raikhel, N.V. (1991) Lectins, lectin genes and their role in plant defense. Plant Cell, 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, K.D. , Layne, D.R. , Scorza, R. and Schnabel, G. (2006) Gastrodia anti‐fungal protein from the orchid Gastrodia elata confers disease resistance to root pathogens in transgenic tobacco. Planta, 224, 1373–1383. [DOI] [PubMed] [Google Scholar]
- De Hoff, P.L. , Brill, L.M. and Hirsch, A.M. (2009) Plant lectins: the ties that bind in root symbiosis and plant defense. Mol. Genet. Genomics, 282, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegmann, J. , Mithofer, A. , Wanner, G. and Ebel, J. (2004) An ancient enzyme domain hidden in the putative β‐glucan elicitor receptor of soybean may play an active part in the perception of pathogen‐associated molecular patterns during broad host resistance. J. Biol. Chem. 279, 1132–1140. [DOI] [PubMed] [Google Scholar]
- Guidarelli, M. , Carbone, F. , Mourgues, F. , Perrotta, G. , Rosati, C. , Bertolini, P. and Baraldi, E. (2011) Colletotrichum acutatum interactions with unripe and ripe strawberry fruits and differential responses at histological and transcriptional levels. Plant Pathol. 60, 685–697. [Google Scholar]
- Holsters, M. , de Waele, D. , Depicker, A. , Messens, E. , Van Montagu, M. and Schell, J. (1978) Transfection and transformation of Agrobacterium tumefaciens . Mol. Gen. Genet. 163, 181–187. [DOI] [PubMed] [Google Scholar]
- Hwang, B.K. and Hwang, I.S. (2011) The pepper mannose‐binding lectin gene CaMBL1 is required to regulate cell death and defense responses to microbial pathogens. Plant Physiol. 155, 447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, S.Y. , Ma, Z. and Ramachandran, S. (2010) Evolutionary history and stress regulation of the lectin superfamily in higher plants. BMC Evol. Biol. 10, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi, M. , Inzé, D. and Depicker, A. (2002) GATEWAY vectors for Agrobacterium‐ mediated plant transformation. Trends Plant Sci. 7, 193–195. [DOI] [PubMed] [Google Scholar]
- Lannoo, N. , Peumans, W.J. and Van Damme, E.J.M. (2006) The presence of jasmonate‐inducible lectin genes in some but not all Nicotiana species explains a marked intragenus difference in plant responses to hormone treatment. J. Exp. Bot. 57, 3145–3155. [DOI] [PubMed] [Google Scholar]
- Lopez‐Gomez, R. and Gomez‐Lim, M.A. (1992) A method for extracting intact RNA from fruits rich in polysaccharides using ripe mango mesocarp. Hort. Sci. 27, 440–442. [Google Scholar]
- Lyou, S.H. , Park, H.J. , Jung, C. , Sohn, H.B. , Lee, G. , Kim, C.H. , Kim, M. , Choi, Y.D. and Cheong, J.J. (2009) The Arabidopsis AtLEC gene encoding a lectin‐like protein is up‐regulated by multiple stimuli including developmental signal, wounding, jasmonate, ethylene, and chitin elicitor. Mol. Cells, 27, 75–81. [DOI] [PubMed] [Google Scholar]
- Mirelman, D.E. , Galun, E. , Sharon, N. and Lotan, R. (1975) Inhibition of fungal growth by wheat germ agglutinin. Nature, 256, 414–416. [DOI] [PubMed] [Google Scholar]
- Mithöfer, A. , Fliegmann, J. , Neuhaus‐Url, G. , Schwarz, H. and Ebel, J. (2000) The hepta‐β‐glucoside elicitor‐binding proteins from legumes represent a putative receptor family. Biol. Chem. 381, 705–713. [DOI] [PubMed] [Google Scholar]
- Ngai, P.H.K. and Ng, T.B. (2007) A lectin with antifungal and mitogenic activities from red cluster pepper (Capsicum frutescens) seeds. Appl. Microbiol. Biotechnol. 74, 366–371. [DOI] [PubMed] [Google Scholar]
- O'Connel, R.J. , Nash, C. and Bailey, J.A. (1992) Lectin cytochemistry: a new approach to understanding cell differentiation, pathogenesis and taxonomy in Colletotrichum In: Colletotrichum: Biology, Pathology and Control (Bailey J.A. and Jeger M.J., eds), pp. 67–87. Wallingford, Oxfordshire: CAB International. [Google Scholar]
- Pain, N.A. , Green, J.R. , Jones, G.L. and O'Connel, R.J. (1996) Composition and organisation of extracellular matrices around germ tubes and appressoria of Colletotrichum lindemuthianum . Protoplasma, 190, 119–130. [Google Scholar]
- Peumans, W.J. and Van Damme, E.J.M. (1995) Lectins as plant defense proteins. Plant Physiol. 109, 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky, D. (1996) Pathogen quiescence in postharvest diseases. Annu. Rev. Phytopathol. 34, 413–434. [DOI] [PubMed] [Google Scholar]
- Spoalore, S. , Trainotti, L. and Casadoro, G. (2001) A simple protocol for transient gene expression in ripe fleshy fruit mediated by Agrobacterium . J. Exp. Bot. 52, 845–850. [DOI] [PubMed] [Google Scholar]
- Sudmoon, R. , Sattayasai, N. , Bunyatratchata, W. , Chaveerach, A. and Nuchadomrong, S. (2008) Thermostable mannose‐binding lectin from Dendrobium findleyanum with activities dependent on sulfhydryl content. Acta Biochim. Biophys. Sin. 40, 811–818. [PubMed] [Google Scholar]
- Sugui, J.A. , Leite, B. and Nicholson, R.L. (1998) Partial characterization of the extracellular matrix released onto hydrophobic surfaces by conidia and conidial germlings of Colletotrichum graminicola . Physiol. Mol. Plant Pathol. 52, 411–425. [Google Scholar]
- Van Damme, E.J.M. , Allen, A.K. and Peumans, W.J. (1987) Isolation and characterization of lectin with exclusive specificity towards mannose from snow drop (Galanthus nivalis) bulbs. FEBS Lett. 215, 140–144. [Google Scholar]
- Van Damme, E.J.M. , Smeets, K. and Peumans, W.J. (1995) The mannose‐binding monocot lectins and their genes In: Lectins: Biomedical Perspectives (Pusztai A. and Bardocz S., eds), pp. 44–66. London: Taylor and Francis. [Google Scholar]
- Van Damme, E.J.M. , Peumans, W.J. , Barre, A. and Rougé, P. (1998) Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 17, 575–692. [Google Scholar]
- Van Damme, E.J.M. , Barre, A. , Rougé, P. and Peumans, W.J. (2004) Cytoplasmic/nuclear plant lectins: a new story. Trends Plant Sci. 9, 484–489. [DOI] [PubMed] [Google Scholar]
- Van Damme, E.J.M. , Nakamura‐Tsuruta, S. , Smith, D.F. , Ongenaert, M. , Winter, H.C. , Rougé, P. , Goldstein, I.J. , Mo, H. , Kominami, J. , Culerrier, R. , Barre, A. , Hirabayashi, J. and Peumans, W. (2007) Phylogenetic and specificity studies of two‐domain GNA‐related lectins: generation of multispecificity through domain duplication and divergent evolution. Biochem. J. 404, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme, E.J.M. , Lannoo, N. and Peumans, W.J. (2008) Plant lectins. Adv. Bot. Res. 48, 108–209. [Google Scholar]
- Vijayan, M. and Chandra, N. (1999) Lectins. Curr. Opin. Struct. Biol. 9, 707–714. [DOI] [PubMed] [Google Scholar]
- Wang, X.C. , Bauw, G. , Van Damme, E.J.M. , Peumans, W.J. , Chen, Z.L. , Van Montagu, M. , Angenon, G. and Dillen, W. (2001) Gastrodianin‐like mannose‐binding proteins: a novel class of plant proteins with antifungal properties. Plant J. 25, 651–661. [DOI] [PubMed] [Google Scholar]
- Xu, Q. , Liu, Y. , Wang, X. , Gu, H. and Chen, Z. (1998) Purification and characterization of a novel anti‐fungal protein from Gastrodia elata . Plant Physiol. Biochem. 36, 899–905. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Multiple sequence alignment of FaMBL1 and mannose‐binding lectins (MBLs) from other species. FaMBL1 protein sequence (KF962716) was aligned with woodland strawberry (Fragaria vesca) epidermis‐specific secreted glycoprotein (XP_004294119), cacao (Theobroma cacao) MBL (EOY13258), sugar beet (Beta vulgaris) SIEP1L protein (CAA61158), pepper (Capsicum annuum) CaMBL1 (ADG04234), carrot (Daucus carota) cell attachment protein (BAD24818) and Arabidopsis curculin‐like (mannose‐binding) lectin protein (NP_178007). The mannose‐binding domain is boxed.


