SUMMARY
Plant class IV chitinases have a small amino‐terminal chitin‐binding domain and a larger chitinase domain, and are involved in plant defence against fungal infection. Our previous work on the chitinases ChitA and ChitB from the model monocotyledon Zea mays showed that the chitin‐binding domain is removed by secreted fungal proteases called fungalysins. In this article, we extend this work to dicotyledons. The effects of fungalysin‐like proteases on four class IV chitinases from the model dicotyledon Arabidopsis thaliana were analysed. Four Arabidopsis chitinases were heterologously expressed in Pichia pastoris, purified and shown to have chitinase activity against a chitohexaose (dp6) substrate. The incubation of these four chitinases with Fv‐cmp, a fungalysin protease secreted by Fusarium verticillioides, resulted in the truncation of AtchitIV3 and AtchitIV5. Moreover, incubation with secreted proteins from Alternaria brassicae, a pathogen of A. thaliana and brassica crops, also led to a similar truncation of AtchitIV3 and AtchitIV4. Our finding that class IV chitinases from both dicotyledons (A. thaliana) and monocotyledons (Z. mays) are truncated by proteases secreted by specialized pathogens of each plant suggests that this may be a general mechanism of plant–fungal pathogenicity.
Chitinases (EC 3.2.1.14) are enzymes that catalyse the degradation of chitin, a linear polymer of N‐acetyl‐d‐glucosamine (GlcNAc). Plants produce many types of chitinases. On the basis of sequence, they are grouped into five classes (Neuhaus et al., 1996). Classes I, II and IV have chitinase domains that belong to family 19 of the glycosyl hydrolase classification system, whereas classes III and V belong to family 18 (Henrissat, 1991). In addition to their family 19 chitinase domains, classes I and IV also have amino‐terminal chitin‐binding domains. Class IV chitinases evolved from class I through a series of four deletions (Collinge et al., 1993). One deletion removed a vacuole‐targeting sequence and, as a result, class IV chitinases are secreted to the apoplast rather than being stored in vacuoles. The remaining three deletions shortened the catalytic domain, resulting in a protein with a shorter, wider catalytic cleft and fewer GlcNAc binding sites (Price and Naumann, 2011; Ubhayasekera et al., 2009).
Plant class IV chitinases have been implicated in many biological processes, including defence against fungal pathogens. As fungal hyphae contain chitin, class IV chitinases may either inhibit the growth of fungal hyphae (Huynh et al., 1992; Schlumbaum et al., 1986) or cleave chitin fragments from hyphae to produce signalling molecules that induce plant defences (Kaku et al., 2006). However, evidence that some class IV chitinases are involved in other processes—response to abiotic stress (Gerhardt et al., 2004), defence against bacterial pathogens (Gerhardt et al., 1997), embryogenesis (De Jong et al., 1992; Wiweger et al., 2003)—has also been reported. It is therefore likely that biochemical and functional studies of class IV chitinases will reveal the existence of subclasses with distinct functions.
ChitA and ChitB are class IV chitinases that are abundant in Zea mays kernels. They are truncated during ear rot caused by Bipolaris zeicola (Naumann et al., 2009) or Stenocarpella maydis (Naumann and Wicklow, 2010). This truncation results from direct proteolysis by secreted fungal proteases, termed chitinase‐modifying proteins (cmps). This cmp activity is also present in secreted protein extracts from diverse members from the genus Fusarium, and the protease from F. verticillioides (Fv‐cmp) has been identified as a member of the fungalysin family of proteases (Naumann et al., 2011). Fv‐cmp has been found to be an endoprotease that cleaves in a region of the protein that is conserved in class IV plant chitinases, suggesting that it may be capable of truncating chitinases found in other plants.
To determine whether chitinases from dicotyledons are targets for fungal cmps, we constructed five heterologous strains of the methylotrophic yeast Pichia pastoris to produce recombinant class IV chitinases from cDNAs from the model dicotyledon Arabidopsis thaliana. Aided by The Arabidopsis Information Resource (TAIR) (Swarbreck et al., 2008), and a published analysis of chitinases in the genome of A. thaliana (Passarinho and De Vries, 2002), plasmids with complete cDNAs for five of the nine predicted class IV chitinases in the A. thaliana genome were obtained (Fig. 1A). For simplicity, the five chitinases were named in order according to their genome location: AtchitIV1, AtchitIV2, AtchitIV3, AtchitIV4, AtchitIV5. AtchitIV5 has been described previously as AtchitIV (Gerhardt et al., 1997) and AtEP3 (Passarinho et al., 2001). Each of the cDNAs was polymerase chain reaction (PCR) amplified and used to create heterologous strains of P. pastoris that secrete recombinant class IV chitinases. For each of the five strains, cultures were grown to density, and protein expression was induced. After 2 days, cell‐free medium was analysed (Fig. 1B), which indicated that four of the five strains secreted recombinant chitinase. Each of these chitinases was purified from cell‐free medium (Appendix S1, see Supporting Information). AtchitIV2 did not produce a detectable amount of recombinant protein and was not studied further.
Figure 1.
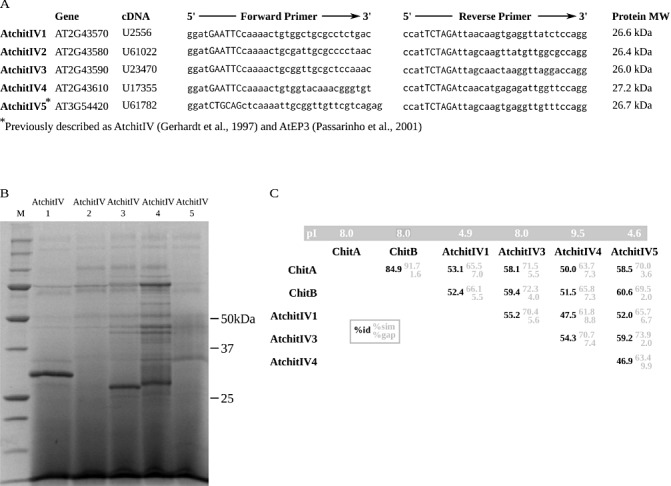
Heterologous expression of class IV chitinases from Arabidopsis thaliana. (A) cDNAs that encode five different class IV plant chitinases showing forward and reverse primers designed for polymerase chain reaction (PCR) cloning. Uppercase letters in the primer sequences indicate restriction sites. Each cDNA was amplified by PCR and cloned into EcoRI/XbaI‐treated pPICZαA, except for AtchitIV5, which was cloned into PstI/XbaI‐treated pPICZαB (Invitrogen, Carlsbad, CA, USA). (B) Sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) analysis of protein expression. Heterologous strains of Pichia pastoris were induced and protein expression was analysed by SDS‐PAGE and Coomassie staining. M, molecular weight marker. (C) Summary of pairwise sequence alignments of ChitA and ChitB from Zea mays and the four expressed A. thaliana class IV chitinases. Percentage identity is in black, and percentage similarity and percentage gap are in grey. Isoelectric points (pI) are shown above each chitinase.
The amino acid sequences of each of the four expressed A. thaliana chitinases and Z. mays ChitA and ChitB were compared by a series of pairwise global alignments (Fig. 1C) (Needleman and Wunsch, 1970). Sequences of the mature recombinant proteins were used. Although Z. mays ChitA and ChitB are 84.9% identical, none of the A. thaliana chitinases share more than 59.2% identity with each other. Both AtchitIV3 and AtchitIV4, like ChitA and ChitB, are basic proteins with high isoelectric points (pI), whereas AtchitIV1 and AtchitIV5 are acidic. The highest identity between A. thaliana and Z. mays chitinases was 60.6% (ChitB and AtchitIV5).
The catalytic activity of each A. thaliana chitinase was confirmed using short polymers of GlcNAc with degree of polymerization 6 (dp6) (Price and Naumann, 2011). Each chitinase was incubated with substrate, and reactions were analysed by matrix‐assisted laser desorption/ionization (MALDI) and detected with a time‐of‐flight (TOF) mass analyser (Fig. 2). All chitinases converted dp6 to smaller products. The products were a mixture of dp5, dp4, dp3 and dp2. The dp5 product was not detected in AtchitIV4 reactions at the 5‐min time point, but was present at the 10‐min time point (not shown). Despite the presence of dp5, dp1 was not detected in any reaction at any time point. Although not conclusive, this result suggests the possible synthesis of dp5 by transglycosylation of dp2 and dp3 products (Taira et al., 2010; Zakariassen et al., 2011). Further experiments, however, are needed to confirm this activity.
Figure 2.
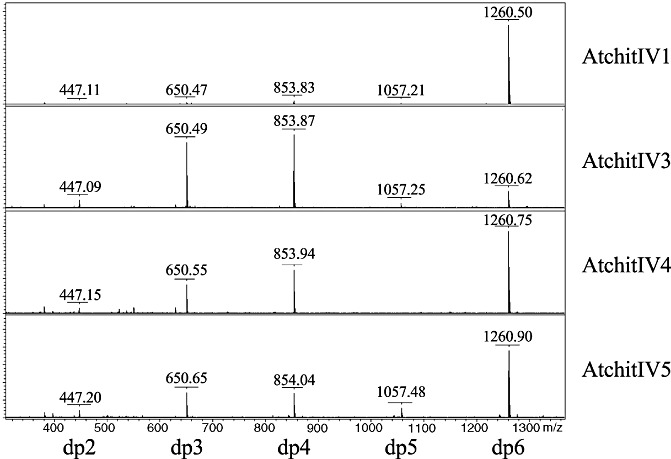
Chitinase activity. Arabidopsis thaliana chitinases were incubated with N‐acetyl‐d‐glucosamine (GlcNAc) oligomers with degree of polymerization 6 (dp6). Reactions consisted of 1 mm hexa‐N‐acetylchitohexaose (dp6) (Seikagaku, Tokyo, Japan) in sodium acetate buffer (10 mm, pH 5.2). Chitinase (0.01 mg protein/mL) was added and reactions were stopped by adding an equal volume of matrix (saturated 2,5‐dihydroxybenzoic acid in acetonitrile). Conversion of dp6 to a mixture of dp5, dp4, dp3 and dp2 was visualized by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS) on a Bruker Daltonic OmniFlex instrument (Billerica, MA, USA).
The purified, active A. thaliana chitinases were tested as substrates to determine whether they could be truncated by Fv‐cmp, a fungalysin protease that truncates Z. mays ChitA and ChitB (Fig. 3A). Each chitinase was incubated in the absence (left lane) or presence (right lane) of Fv‐cmp, and analysed by sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and protein staining. For AtchitIV5, two different fractions from the final step of purification (acetone precipitation) were tested. AtchitIV5, which precipitated at higher acetone concentration (AtchitIV5α), consisted of two major substrate bands (s1, s2). AtchitIV5, which precipitated at lower acetone concentration (AtchitIV5β), consisted of three (s1, s2, s3). Fv‐cmp truncated both AtchitIV3 and AtchitIV5. AtchitIV3, like ChitA and ChitB, was truncated to a specific product. In the AtchitIV5 reactions, the three substrates were converted to two products (p1, p2).
Figure 3.
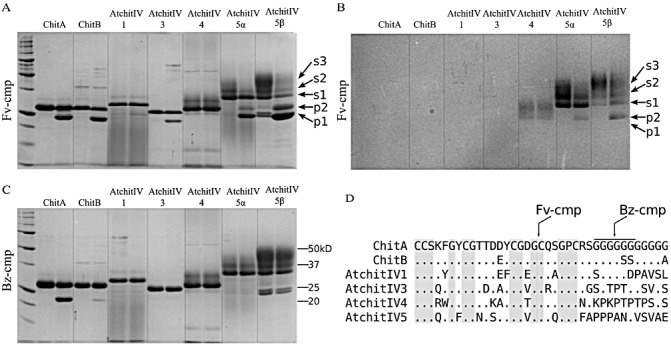
Proteolysis of plant chitinases by fungal chitinase‐modifying proteins (cmps). Each of the Zea mays chitinases (ChitA and ChitB) and the four Arabidopsis thaliana chitinases were incubated alone (left lane) or with purified cmp (right lane). Two different fractions from the final step of AtchitIV5 purification were used: AtchitIV5α, which precipitated at high acetone concentration, and AtchitIV5β, which precipitated at low acetone concentration. Reactions consisted of 10 ng cmp and 20 µg chitinase; reactions were incubated for 2 h at 25 °C. The three substrate bands (s1, s2, s3) and two product bands (p1, p2) refer to the AtchitIV5 reactions. (A) Fv‐cmp (cmp from Fusarium verticillioides) activity analysed by sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) with Coomassie staining. (B) Fv‐cmp activity analysed by SDS‐PAGE with glycoprotein staining (GelCode glycoprotein staining kit; Thermo scientific, Rockford, IL, USA). (C) Bz‐cmp (cmp from Bipolaris zeicola) activity analysed by SDS‐PAGE with Coomassie staining. (D) Peptide sequence alignment in cmp‐targeting region. Dots indicate identity. Grey shading indicates conserved amino acids. Black bar above glycines indicates region of ChitA that is cleaved by Bz‐cmp.
To further examine the AtchitIV5 substrates and products, reactions were analysed by SDS‐PAGE followed by glycoprotein staining (Fig. 3B). Both AtchitIV4 substrate bands and the three AtchitIV5 substrate bands were positively stained, indicating that these proteins are glycosylated. For AtchitIV5, the minor product (p2) was also glycosylated, whereas the major product (p1) was not. Further analysis of AtchitIV5 demonstrated that its glycosylation was asparagine linked (Fig. S1, see Supporting Information). Therefore, the majority of glycosylation occurs on the asparagine residue that is on the amino‐terminal side of the Fv‐cmp cleavage site.
The purified A. thaliana chitinases were then tested as substrates to determine whether they could be truncated by Bz‐cmp, a fungal protease secreted by Bipolaris zeicola that also truncates Z. mays ChitA (Fig. 3C). Bz‐cmp truncated ChitA and, to a reduced amount, ChitB. However, none of the A. thaliana chitinases were cleaved by Bz‐cmp. The cleavage site of Bz‐cmp is in the linker region of ChitA, which contains 11 consecutive glycines (Fig 3D) (Naumann, 2011). The ChitB linker is glycine rich, but is shorter and interrupted by two serines. AtchitIV1 has four consecutive glycines, but was not cleaved by Bz‐cmp. The remaining A. thaliana chitinase linkers lack consecutive glycines and are not cleaved by Bz‐cmp. In contrast, the Fv‐cmp cleavage site is in a region which contains residues that are conserved in all six chitinases.
Secreted proteins from Alternaria brassicae (Berk.) Sacc., the causal agent of grey leaf spot, and a specialized pathogen of plants from the family Brassicaceae (crucifer family), were tested for cmp activity (Parada et al., 2008; Pedras et al., 2001). Two isolates were tested. Each was grown on autoclaved maize seed followed by the extraction of secreted proteins. Fungal secreted proteins were incubated with each of the six class IV chitinase substrates, and the activity was analysed by SDS‐PAGE and peptide staining (Fig. 4). Both fungal isolates gave equivalent results, showing that the Z. mays chitinases were truncated, with preference for ChitB over ChitA. Of the A. thaliana chitinases, AtchitIV3 and, to a lesser extent, AtchitIV4 were truncated, whereas AtchitIV1 and AtchitIV5 were not. The best substrates were Z. mays ChitB and A. thaliana AtchitIV3.
Figure 4.
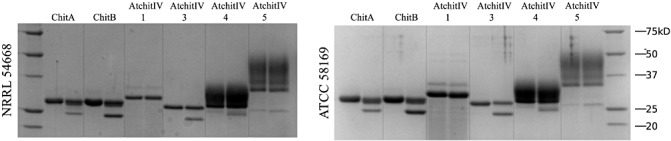
Secreted protein extracts of Alternaria brassicae. Secreted proteins were extracted from solid‐substrate fermentation cultures of two isolates of A. brassicae: QM‐1733 and ATCC 58169. Each of the six chitinase substrates were incubated either alone (left lane) or with secreted proteins (right lane). After incubation, samples were analysed by sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and peptide staining. AtchitIV5 substrate was an equal mixture of both AtchitIV5α and AtchitIV5β. Isolate QM‐1733 (=NRRL 54668, =CBS 102.24) was originally isolated from a leaf spot of Cochlearia officinalis by P. C. Bolle (1924). Isolate ATCC 58169 was isolated from Brassica tournefortii and deposited by P. J. Cotty. Both isolates were grown as potato dextrose agar slant cultures and on autoclaved maize as described previously for other plant pathogens (Naumann and Wicklow, 2010).
In conclusion, we found that some class IV chitinases from A. thaliana are susceptible to cleavage by proteases secreted by fungal pathogens, whereas others are resistant. In our experiments, Fv‐cmp, a fungalysin purified from the maize pathogen F. verticillioides, efficiently truncated AtchitIV3 and AtchitIV5, but not AtchitIV1 or AtchitIV4 (Fig 3A). Additional experiments also showed that a fungal pathogen of crucifers, A. brassicae, secretes cmp activity. When protein extracts were incubated with purified chitinases, truncation of ChitA and ChitB from Z. mays and AtchitIV3 and AtchitIV4 from A. thaliana was observed (Fig. 4). These results provide direct experimental evidence that the truncation of plant class IV chitinases by secreted fugal proteases—first observed in diseased ears of Z. mays—may represent a broad pathogenic mechanism utilized by diverse fungi to promote plant disease (Soanes et al., 2007).
Of the four dicotyledon chitinases tested, only AtchitIV3 was truncated by both Fv‐cmp and the A. brassicae extracts. Despite sharing less than 60% amino acid identity with ChitA and ChitB (Fig. 1D), AtchitIV3 is similar to these maize chitinase in a number of ways. Like ChitA and ChitB, it binds insoluble chitin at pH 8, and was purified by the same method (Appendix S1, see Supporting Information). The three chitinases are basic, nonglycosylated and have the same triad of aromatic amino acids in the chitin‐binding domain (F–Y–Y)—residues predicted to be important in carbohydrate binding (Aboitiz et al., 2004). We speculate that these similarities might be shared by a subclass within plant class IV chitinases, a subclass of chitinases that are susceptible to truncation by fungal cmps.
Supporting information
Fig. S1 Glycosylation analysis of AtchitIV5. (A) Peptide‐N‐glycosidase F (PNGase F) treatment of AtchitIV5 converted glycosylated chitinase into a single major product (⋆) with a predicted mass of 26.7 kDa. (B) AtchitIV5 contains three asparagine residues with a minimal N‐linked glycosylation motif of N–X–(T/S) (inverted sequence). (C) Model of Fv‐cmp (chitinase‐modifying protein from Fusarium verticillioides) truncation of glycosylated AtchitIV5. Fv‐cmp cleaves AtchitIV5, releasing a small, amino‐terminal glycoprotein product and the large, carboxy‐terminal product p1 (2.37 kDa).
Appendix S1 Chitinase purification.
Supporting info item
Supporting info item
ACKNOWLEDGEMENTS
We thank Kurt Sollenberger and Trina Hartman for technical assistance. Mention of any trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
REFERENCES
- Aboitiz, N. , Vila‐Perello, M. , Groves, P. , Asensio, J.L. , Andreu, D. , Canada, F.J. and Jimenez‐Barbero, J. (2004) NMR and modeling studies of protein–carbohydrate interactions: synthesis, three‐dimensional structure, and recognition properties of a minimum hevein domain with binding affinity for chitooligosaccharides. Chembiochem 5, 1245–1255. [DOI] [PubMed] [Google Scholar]
- Collinge, D.B. , Kragh, K.M. , Mikkelsen, J.D. , Nielsen, K.K. , Rasmussen, U. and Vad, K. (1993) Plant chitinases. Plant J. 3, 31–340. [DOI] [PubMed] [Google Scholar]
- De Jong, A.J. , Cordewener, J. , Lo Schiavo, F. , Terzi, M. , Bandekerchkhove, J. , Van Kammen, A. and De Vries, S.C. (1992) A carrot somatic embryo mutant is rescued by chitinase. Plant Cell, 4, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt, L.B.A. , Sachetto‐Martins, G. , Contarini, M.G. , Sandroni, M. , de P Ferreira, R. , de Lima, V.M. , Cordeiro, M.C. , de Oliveira, D.E. and Margis‐Pinheiro, M. (1997) Arabidopsis thaliana class IV chitinase is early induced during the interaction with Xanthomonas campestris . FEBS Lett. 419, 69–75. [DOI] [PubMed] [Google Scholar]
- Gerhardt, L.B.A. , Magioli, C. , Perez, A.B.U.C.M. , Margis, R. , Sachetto‐Martins, G. and Margis‐Pinheiro, M. (2004) AtchitIV gene expression is stimulated under abiotic stresses and is spatially and temporally regulated during embryo development. Genet. Mol. Biol. 27, 118–123. [Google Scholar]
- Henrissat, B. (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 280, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh, Q.K. , Hironaka, C.M. , Levine, E.B. , Smith, C.E. , Borgmeyer, J.R. and Shah, D.M. (1992) Antifungal proteins from plants. Purification, molecular cloning, and antifungal properties of chitinases from maize seed. J. Biol. Chem. 267, 6635–6640. [PubMed] [Google Scholar]
- Kaku, H. , Nishizawa, Y. , Ishii‐Minami, N. , Akimoto‐Tomiyama, C. , Dohmae, N. , Takio, K. , Minami, E. and Shibuya, N. (2006) Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. USA, 103, 11 086–11 091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann, T.A. (2011) Modification of recombinant maize ChitA chitinase by fungal chitinase‐modifying proteins. Mol. Plant Pathol. 12, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann, T.A. and Wicklow, D.T. (2010) Allozyme‐specific modification of a maize seed chitinase by a protein secreted by the fungal pathogen Stenocarpella maydis . Phytopathology, 100, 645–654. [DOI] [PubMed] [Google Scholar]
- Naumann, T.A. , Wicklow, D.T. and Kendra, D.F. (2009) Maize seed chitinase is modified by a protein secreted by Bipolaris zeicola . Physiol. Mol. Plant Pathol. 74, 134–141. [Google Scholar]
- Naumann, T.A. , Wicklow, D.T. and Price, N.P.J. (2011) Identification of a chitinase modifying protein from Fusarium verticillioides: truncation of a host resistance protein by a fungalysin metalloprotease. J. Biol. Chem. 286, 35 358–35 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman, S.B. and Wunsch, C.D. (1970) A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 48, 443–453. [DOI] [PubMed] [Google Scholar]
- Neuhaus, J. , Fritig, B. , Linthorst, H.J.M. , Meins Jr, R. , Mikkelsen, J.D. and Ryals, J. (1996) A revised nomenclature for chitinase genes. Plant Mol. Biol. Rep. 14, 102–104. [Google Scholar]
- Parada, R.Y. , Sakuno, E. , Mori, N. , Oka, K. , Egusa, M. , Kodama, M. and Otani, H. (2008) Alternaria brassicae produces a host‐specific protein toxin from germinating spores on host leaves. Phytopathology, 98, 458–463. [DOI] [PubMed] [Google Scholar]
- Passarinho, P.A. and De Vries, S.C. (2002) Arabidopsis chitinases: a genomic survey In: The Arabidopsis Book (Somerville C.R. and Meyerowitz E.M., eds), pp. 1–25. Rockville, MD: American Society of Plant Biologists; Available at http://www.aspb.org/publications/arabidopsis/. [accessed on Sept 1, 2010]. [Google Scholar]
- Passarinho, P.A. , Van Hengel, A.J. , Fransz, P.F. and De Vries, S.C. (2001) Expression pattern of the Arabidopsis thaliana AtEP3/AtchitIV endochitinase gene. Planta, 212, 556–567. [DOI] [PubMed] [Google Scholar]
- Pedras, M.S.C. , Zaharia, I.L. , Gai, Y. , Zhou, Y. and Ward, D.E. (2001) In planta sequential hydroxylation and glycosylation of a fungal phytotoxin: avoiding cell death and overcoming the fungal invader. Proc. Natl. Acad. Sci. USA, 98, 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, N.P.J. and Naumann, T.A. (2011) A high‐throughput matrix‐assisted laser desorption/ionization‐time‐of‐flight mass spectrometry‐based assay of chitinase activity. Anal. Biochem. 411, 94–99. [DOI] [PubMed] [Google Scholar]
- Schlumbaum, A. , Mauch, F. , Vogeli, U. and Boller, T. (1986) Plant chitinases are potent inhibitors of fungal growth. Nature, 324, 365–367. [Google Scholar]
- Soanes, D.M. , Richards, T.A. and Talbot, N.J. (2007) Insights from sequencing fungal and oomycete genomes: what can we learn about plant disease and the evolution of pathogenicity? Plant Cell, 19, 3318–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbreck, D. , Wilks, C. , Lamesch, P. , Berardini, T.Z. , Garcia‐Hernandez, M. , Foerster, H. , Li, D. , Meyer, T. , Muller, R. , Ploetz, L. , Radenbaugh, A. , Singh, S. , Swing, V. , Tissier, C. , Zhang, P. and Huala, E. (2008) The Arabidopsis Information Resource (TAIR): gene structure and function annotation. Nucleic Acids Res. 36, D1009–D1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira, T. , Fujiwara, M. , Dennhart, N. , Hayashi, H. , Onaga, S. , Ohnuma, T. , Letzel, T. , Sakuda, S. and Fukamizo, T. (2010) Transglycosylation reaction catalyzed by a class V chitinase from cycad, Cycas revoluta: a study involving site‐directed mutagenesis, HPLC, and real‐time ESI‐MS. Biochim. Biophys. Acta, 1804, 668–675. [DOI] [PubMed] [Google Scholar]
- Ubhayasekera, W. , Rawat, R. , Ho, S.W. , Wiweger, M. , Von Arnold, S. , Chye, M.L. and Mowbray, S.L. (2009) The first crystal structures of a family 19 class IV chitinase: the enzyme from Norway spruce. Plant Mol. Biol. 71, 277–280. [DOI] [PubMed] [Google Scholar]
- Wiweger, M. , Farbos, I. , Ingouff, M. , Lagercrantz, U. and Von Arnold, S. (2003) Expression of Chia4‐Pa chitinase genes during somatic and zygotic embryo development in Norway spruce (Picea abies): similarities and differences between gymnosperm and angiosperm class IV chitinases. J. Exp. Bot. 54, 2691–2699. [DOI] [PubMed] [Google Scholar]
- Zakariassen, H. , Hansen, M.C. , Joranli, M. , Eijsink, V.G. and Sorlie, M. (2011) Mutational effects on transglycosylating activity of family 18 chitinases and construction of a hypertransglycosylating mutant. Biochemistry, 50, 5693–5703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Glycosylation analysis of AtchitIV5. (A) Peptide‐N‐glycosidase F (PNGase F) treatment of AtchitIV5 converted glycosylated chitinase into a single major product (⋆) with a predicted mass of 26.7 kDa. (B) AtchitIV5 contains three asparagine residues with a minimal N‐linked glycosylation motif of N–X–(T/S) (inverted sequence). (C) Model of Fv‐cmp (chitinase‐modifying protein from Fusarium verticillioides) truncation of glycosylated AtchitIV5. Fv‐cmp cleaves AtchitIV5, releasing a small, amino‐terminal glycoprotein product and the large, carboxy‐terminal product p1 (2.37 kDa).
Appendix S1 Chitinase purification.
Supporting info item
Supporting info item


