Summary
Ethylene and jasmonate (JA) have powerful effects when plants are challenged by pathogens. The inducible promoter‐regulated expression of the Arabidopsis ethylene receptor mutant ethylene‐insensitive1‐1 (etr1‐1) causes ethylene insensitivity in petunia. To investigate the molecular mechanisms involved in transgenic petunia responses to Botrytis cinerea related to the ethylene and JA pathways, etr1‐1‐expressing petunia plants were inoculated with Botrytis cinerea. The induced expression of etr1‐1 by a chemical inducer dexamethasone resulted in retarded senescence and reduced disease symptoms on detached leaves and flowers or intact plants. The extent of decreased disease symptoms correlated positively with etr1‐1 expression. The JA pathway, independent of the ethylene pathway, activated petunia ethylene response factor (PhERF) expression and consequent defence‐related gene expression. These results demonstrate that ethylene induced by biotic stress influences senescence, and that JA in combination with delayed senescence by etr1‐1 expression alters tolerance to pathogens.
Introduction
The death of cells can be a programmed event that occurs when plants are attacked by pathogens (Greenberg, 1997). Botrytis cinerea, a model necrotrophic pathogen, triggers the host cell death response because it produces toxins. A hypersensitive reaction (HR) occurs at the site of contact. In Arabidopsis, the HR facilitates further infection with B. cinerea (Govrin and Levine, 2000). For the full pathogenicity of B. cinerea in tobacco, an HR is required (Dickman et al., 2001). Furthermore, delayed or reduced cell death Arabidopsis mutants are more resistant than wild‐type plants to B. cinerea (van Baarlen et al., 2007).
Ethylene, a gaseous hormone, regulates different processes during the lifetime of the plant. Ethylene is involved in the regulation of programmed cell death (PCD) during plant–pathogen interactions (Greenberg, 1997). Ethylene participates in cell death processes (Lam et al., 1999), as shown in the Arabidopsis lesion mimic mutant vad1‐1 affecting ethylene‐insensitive4 (EIN4) (Bouchez et al., 2007). Ethylene perception and signalling transduction have significant roles in mediating the effects of ethylene during senescence. However, the five Arabidopsis ethylene receptors have different roles during plant responses to the toxin fumonisin B1, and the mechanisms of each pathway may differ (Plett et al., 2009).
Ethylene also participates in plant defence responses to pathogens. Ethylene perception and signalling mutants have different disease symptoms when challenged by pathogens; some responses lead to resistance or susceptibility (Knoester et al., 1998), but the response can also be the same as in wild‐type plants (Hoffman et al., 1999; Thomma et al., 1999). For example, the ethylene‐insensitive tomato mutant Never ripe (Nr) has the same visible symptoms as wild‐type plants infected by B. cinerea (Díza et al., 2002), but is more resistant to Fusarium oxysporum (Lund et al., 1998). However, detached Nr leaves have also been reported to be more susceptible to B. cinerea (Audenaer et al., 1999). When infected by the bacterial pathogen Clavibacter michiganensis ssp. michiganensis, which causes plant wilt and canker symptoms, Nr shows delayed symptoms (Balaji et al., 2008). Regardless of the differences in plant responses to a pathogen, exposure to and perception of ethylene are known to activate plant defence‐related proteins, such as the pathogenesis‐related (PR) proteins (van Kan et al., 1995) PR1b and PR5 (osmotin) (Xu et al., 1994), PR3 (Lorrain et al., 2004) and plant defensin1.2 (PDF1.2). These proteins are also known to be activated by the jasmonate (JA) pathway of plant responses (Kunkel and Brooks, 2002; Penninckx et al., 1998). JAs are involved in the mediation of plant responses to pathogens (Farmer and Ryan, 1992; Glazebrook, 2005), and can act synergistically with (Penninckx et al., 1998; Thomma et al., 1999; Xu et al., 1994) or independently of (Díaz et al., 2002) ethylene in different plants, especially when attacked by necrotrophic pathogens (Glazebrook, 2005).
Ethylene‐insensitive1‐1 (etr1‐1)‐expressing plants are insensitive to ethylene (Bleecker et al., 1988; Hall et al., 1999; Hua et al., 1998). Here, we used a reproducible and comprehensive B. cinerea infection system on petunia plants in which etr1‐1 was inducibly expressed. We investigated the disease symptoms of intact plants, detached flowers and leaves from two transgenic lines with different levels of etr1‐1 expression. The analysis measured the proportion of expanding lesions, the lesion growth rate (Díaz et al., 2002), disease severity and disease incidence. We also compared the effects of B. cinerea infection on the PCD response in these lines.
Our objectives were to determine the role of induced etr1‐1 expression on senescence induced by biotic stress, i.e. B. cinerea infection, and the roles of ethylene and JA during petunia responses to B. cinerea.
Results
Fungal growth and disease progress
For coordinated and reproducible B. cinerea infection of petunia plants, we inoculated the detached leaves and flowers and intact plants of two lines, E7H and E9G, in which etr1‐1 expression was induced with dexamethasone (DEX). The infection of detached petunia leaves was essentially the same as the infection of tomato leaves as described by Benito et al. (1998) and Díaz et al. (2002). Primary lesions formed, followed by a quiescent period, and subsequently by expanding lesions. As shown in Fig. 1a, the primary necrotic lesion formed at 1–2 days post‐inoculation (dpi). Primary lesions were observed at 80% of the infected sites of leaves treated with dimethylsulphoxide (DMSO); primary lesions formed on 20% of the sites of leaves treated with DEX. The expansion of lesions on DEX‐induced leaves was slower than on DMSO‐treated leaves at 2 dpi (Fig. 1a). The disease incidence in leaves treated with DMSO was approximately 100%, whereas more than 50% of DEX‐treated leaves still appeared healthy (Fig. 2a). The disease severity of DEX‐treated leaves from the E9G and E7H (Fig. S1) lines was reduced at 3–4 dpi, although not significantly, relative to that of DMSO‐treated leaves (Fig. 2b).
Figure 1.

Representative phenotypes of dexamethasone (DEX) and dimethylsulphoxide (DMSO) treatment on E9G inoculated with Botrytis cinerea. (a) Disease symptoms on the fourth leaves from the top in vitro. (b) Disease phenotype of fully opened flowers before anther dehiscence in vitro. (c) Disease phenotype of 8‐week‐old plantlets. 9XB, DEX treatment–B. cinerea inoculation on E9G; 9DB, DMSO treatment–B. cinerea inoculation on E9G; 9XM, DEX treatment–mock inoculation on E9G; 9DM, DMSO treatment–mock inoculation on E9G. dpi, days post‐inoculation.
Figure 2.
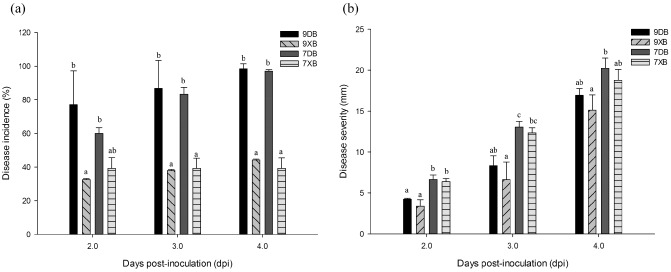
Susceptibility of E9G and E7H to Botrytis cinerea with dexamethasone (DEX) or dimethylsulphoxide (DMSO) treatment. (a) Disease incidence (percentage of inoculation sites with expanding lesions) of the fourth leaves from the top on E7H and E9G lines in vitro. (b) Disease severity (diameter of expanding lesions) of the fourth leaves from the top on E7H and E9G lines in vitro. Mean values are shown from three independent bioreplicates [error bars, ± standard error (SE)] containing at least 40 leaves (80 droplets) for every experiment. Different letters indicate significant differences between treatments of the two lines at a given time point (P ≤ 0.05). 9XB, DEX treatment–B. cinerea inoculation on E9G; 9DB, DMSO treatment–B. cinerea inoculation on E9G; 7XB, DEX treatment–B. cinerea inoculation on E7H; 7DB, DMSO treatment–B. cinerea inoculation on E7H.
To examine other tissues for their responses to B. cinerea, we also inoculated detached flowers (Fig. 1b) and intact plantlets (Fig. 1c). Similar to the disease symptoms on detached leaves, the proportion of expanding lesions and the lesion growth rate were reduced in DEX‐treated plantlets of the two lines relative to the DMSO‐treated control (Table 1). At 5 dpi, 28.33% and 46.77% of the E7H and E9G plantlets, respectively, were healthy. At 8 dpi, the infection was completely subdued in plantlets treated with DEX, whereas, in control plants, saturating infections occurred at 5 dpi (Fig. 1c). The delayed disease progress was also reflected by a statistically significantly decreased number of expanding lesions per flower, as well as a reduced growth rate of the expanding lesions on petunia flowers treated with DEX, when compared with DMSO‐treated flowers (Table 2).
Table 1.
Susceptibility of E7H and E9G plantlets with dexamethasone (DEX) or dimethylsulphoxide (DMSO) treatment to Botrytis cinerea. Data were pooled from two independent experiments. Each plant was considered as a replicate. Different letters indicate significant differences between the treatment of two lines at a given time point (P ≤ 0.05, ± SD, n = 10)
| Treatment | Healthy plants* (%) | Expanding lesion per plant* (%) | Lesion expansion rate† (mm/day) | |||
|---|---|---|---|---|---|---|
| E7H | E9G | E7H | E9G | E7H | E9G | |
| DMSO‐B | 0 | 0 | 6.40 ± 2.01b | 3.50 ± 2.4a | 5.23 ± 1.23b | 3.91 ± 2.23b |
| DEX‐B | 28.33 | 46.77 | 3.90 ± 0.99a | 2.30 ± 1.49a | 4.04 ± 1.47a | 1.87 ± 1.05a |
| DMSO‐M | 100 | 100 | 0 | 0 | 0 | 0 |
| DEX‐M | 100 | 100 | 0 | 0 | 0 | 0 |
B, Botrytis; M, mock; SD, standard deviation.
*At 5 days post‐inoculation (dpi).
†Calculated over the period from 5 to 6 dpi.
Table 2.
Susceptibility of E7H and E9G flowers with dexamethasone (DEX) or dimethylsulphoxide (DMSO) treatment to Botrytis cinerea. Data were pooled from two independent experiments, with eight flowers per treatment. Different letters indicate significant differences between the treatment of two lines at a given time point (P ≤ 0.05, ± SE, n = 8)
| Treatment | Healthy flowers* (%) | Expanding lesion per flower* (%) | Lesion expansion rate† (mm/day) | |||
|---|---|---|---|---|---|---|
| E7H | E9G | E7H | E9G | E7H | E9G | |
| DMSO‐B | 0 | 0 | 100 ± 0.00c | 100 ± 0.00c | 4.58 ± 1.33d | 2.68 ± 0.82c |
| DEX‐B | 65 | 60 | 75 ± 0.89b | 60 ± 0.67a | 2.26 ± 0.63b | 1.43 ± 0.22a |
| DMSO‐M | 100 | 100 | 0 | 0 | 0 | 0 |
| DEX‐M | 100 | 100 | 0 | 0 | 0 | 0 |
B, Botrytis; M, mock; SE, standard error.
*At 5 days post‐inoculation (dpi).
†Calculated over the period from 5 to 6 dpi.
We analysed the expression of etr1‐1 produced by DEX induction by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) (Fig. 3a). As expected, the expression level of etr1‐1 increased in the leaves of DEX‐treated intact plants after mock infection as well as after B. cinerea inoculation. The expression level of etr1‐1 reached a maximum at 3 days and decreased at 4 days after mock infection. However, the expression only increased slightly in B. cinerea‐infected plants induced with DEX.
Figure 3.
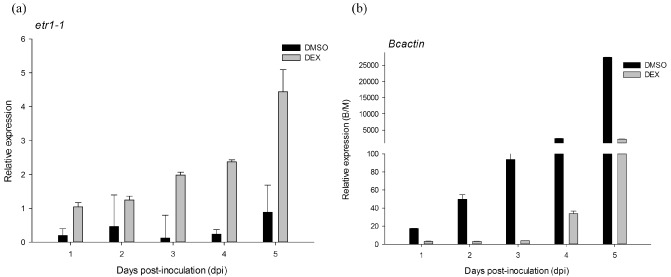
Gene expression patterns in E9G plantlets infected with Botrytis cinerea with dexamethasone (DEX) or dimethylsulphoxide (DMSO) treatment. Eight‐week‐old E9G plantlets, which were irrigated for 4 days with 30 μm DEX or 0.18% DMSO (v/v), were sprayed with 300 μL of a suspension of B. cinerea with 10 mm glucose and 6.7 mm potassium phosphate (pH 6.0) or mock sterile H2O containing the incubation buffer. Total RNA was extracted from leaf samples collected at every day post‐inoculation (dpi). cDNA analysis was performed by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) amplification with specific primers designed by Primer 3 (Table S1). Transcript levels were normalized to 26S rRNA. Data represent two independent replicates [± standard error (SE), n = 10]. B/M, Botrytis/mock.
To determine whether the decrease in disease severity and expanding lesions was consistent with a decrease in fungal biomass, gene expression of B. cinerea actin (Bcactin), a marker for actively growing B. cinerea (Benito et al., 1998; Díaz et al., 2002), was assessed (Fig. 3b). Disease severity‐associated gene expression was found (Figs 1c and 3b). A 25‐fold increase in Bcactin expression was observed in DMSO‐treated infected tissue at 4–5 dpi compared with control plants treated with DEX, a result consistent with the visually accelerated and increased disease symptoms (Fig. 1c and Table 1).
Progressive senescence in infected GVG::etr1‐1 transgenic petunias
Botrytis cinerea kills host cells by producing phytotoxins, such as botrydial, which induces chlorosis and cell collapse, thereby facilitating penetration and colonization (Choquer et al., 2007). This necrotrophic pathogen thrives on dead cells (Plett et al., 2009). Chlorophyll loss is known to be an indicator of senescence (Mach et al., 2001; Plett et al., 2009) and has been used to quantify differences in cell death (Pegadaraju et al., 2005). To determine the degree of cell death induced by B. cinerea and the role of ethylene in cell death, the leaves of E7H and E9G plants treated with DEX or DMSO were investigated for chlorophyll loss. As shown in Fig. 4, infected leaves lost chlorophyll. The chlorophyll loss rate in DEX‐induced tissues (7.77% and 14.27% on intact and detached leaves, respectively) was less than half than (14.92% and 28.21%) the DMSO treatment (Fig. 4a, b).
Figure 4.
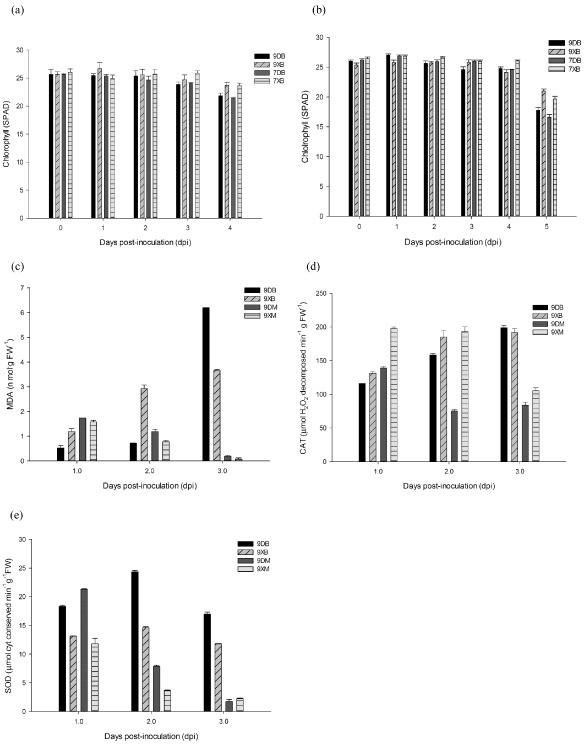
Physiological markers of senescence in petunias. The fourth leaf from the top of 8‐week‐old plantlets was collected and painted with 30 μm dexamethasone (DEX) or 0.18% dimethylsulphoxide (DMSO) (v/v) in 0.6% agar medium. After 2 days, leaves were inoculated with two 10‐μL droplets using a spore suspension of Botrytis cinerea or mock solution. (a) The chlorophyll content in E9G plantlets infected with B. cinerea. Data represent two independent replicates [± standard error (SE), n = 10]. The chlorophyll content of detached leaves (b), malondialdehyde (MDA) level of detached leaves (c), catalase (CAT) level of detached leaves (d) and superoxide dismutase (SOD) level of detached leaves (e) at the indicated time points in vitro. Data represent three independent experiments (± SE, n = 120). Data were subjected to one‐way analysis of variance (ANOVA). Significant differences are indicated by letters (P < 0.05). 9XB, DEX treatment–B. cinerea inoculation on E9G; 9DB, DMSO treatment–B. cinerea inoculation on E9G; 7XB, DEX treatment–B. cinerea inoculation on E7H; 7DB, DMSO treatment–B. cinerea inoculation on E7H; 9XM, DEX treatment–mock inoculation on E9G; 9DM, DMSO treatment–mock inoculation on E9G; FW, fresh weight.
Another means by which B. cinerea may cause host cell death is through the oxidative burst and the release of reactive oxygen species (ROS); these events occur during penetration (Tenberge et al., 2002; Tenberge, 2004) and primary lesion formation (Choquer et al., 2007; van Kan, 2006). The HR, a type of PCD thought to promote B. cinerea growth, is required for the rapid production of ROS (van Kan, 2006). We measured malondialdehyde (MDA) (Fig. 4c), an aldehyde product of lipid peroxidation, considered to be a reliable marker of plant senescence, and the enzymatic antioxidants catalase (CAT) (Fig. 4d) and superoxide dismutase (SOD) (Fig. 4e), which help to mitigate the toxic effects of active oxygen species; the expression of these markers changed in infected and treated tissues. Detached leaves were selected to measure MDA and antioxidant enzymes, because the three colonization phases of B. cinerea were easier to recognize in this tissue. A significant increase in the MDA content of infected leaves was found, regardless of DEX treatment (Fig. 4c), and an increase in MDA content was not observed in mock‐treated leaves; therefore, this response was B. cinerea dependent. The greatest increase in the MDA concentration was observed in leaves that were more severely infected. CAT activity was increased significantly in infected leaves; however, SOD activity increased at 2 dpi, and then declined at 3 dpi (Fig. 4e). In contrast with these results in infected leaves, MDA and CAT levels were reduced significantly in mock‐treated leaves. This demonstrates that detached mock‐treated leaves are not in a stage of senescence, consistent with the visible phenotype (Fig. 1a) and chlorophyll content (Fig. 4b).
In order to determine whether B. cinerea induces senescence in GVG::etr1‐1 transgenic petunias after treatment with DEX or DMSO, as in Arabidopsis and tomato plants (Swartzberg et al., 2008), and whether ethylene regulates the progression of senescence induced by biotic stress, we monitored the expression of nine petunia cysteine protease (PhCP) genes. Increased expression of CPs has been detected during petunia flower senescence (Jones et al., 2005) and PhCPs have been reported to be regulated during senescence by different types of stress (Jones et al., 2005). As shown in Fig. 5, the transcriptional accumulation of the tested PhCPs was activated by B. cinerea infection, with the exception of PhCP2 and PhCP4, which displayed much lower expression levels during the entire monitoring period. The expression level of PhCP8 was highest. The peak of expression of most PhCPs was at 2 dpi, except for PhCP9, which peaked at 4 dpi.
Figure 5.
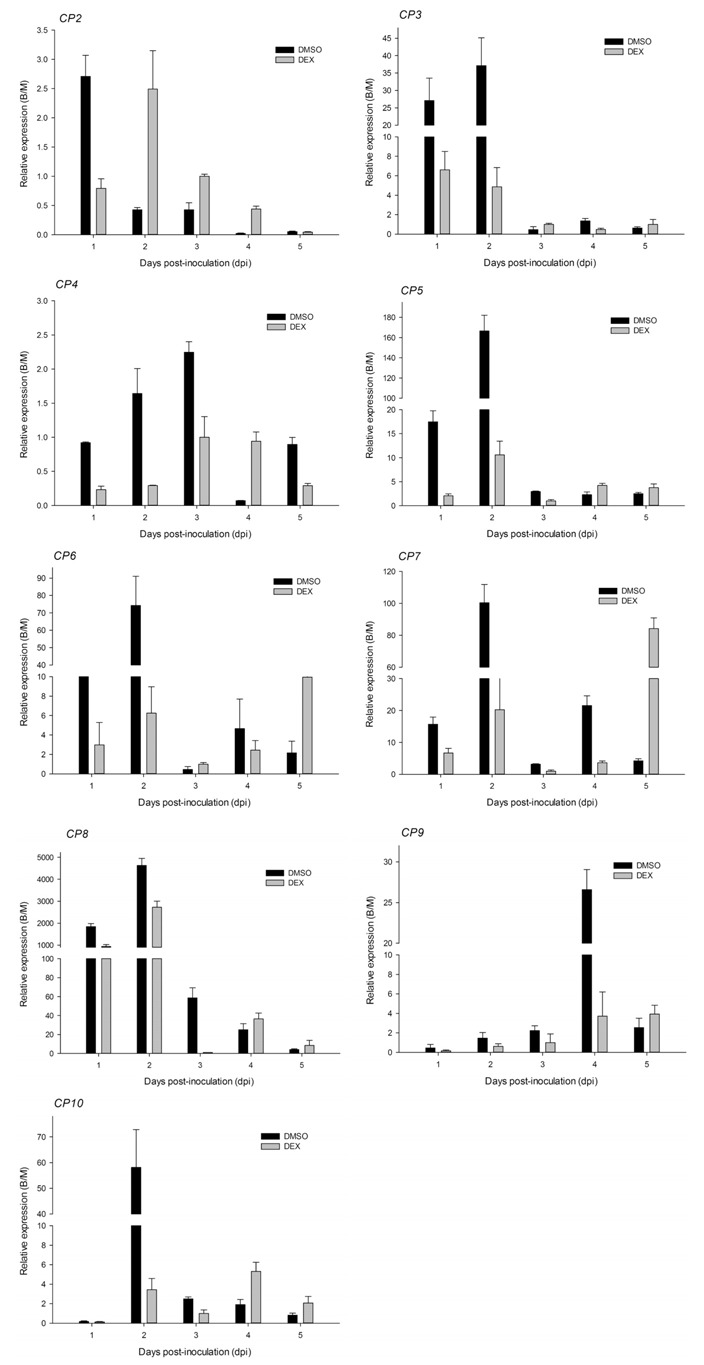
Gene expression patterns of nine cysteine proteases (CPs) in E9G plantlets infected with Botrytis cinerea. B/M, Botrytis/mock.
JA action on infected GVG::etr1‐1 transgenic petunias
To acquire information on ethylene activity during plant–pathogen interactions, we monitored the expression of the genes involved in the ethylene biosynthesis and signalling pathways. 1‐Aminocyclopropane‐1‐carboxylic acid oxidase (ACO) converts 1‐aminocyclopropane‐1‐carboxylic acid (ACC) to ethylene (Kende, 1993), is a critical enzyme in the ethylene biosynthesis pathway (Yang and Hoffman, 1984) and is an appropriate indicator of ethylene production (Balague et al., 1993; Barry et al., 1996; Hamilton et al., 1990; Holdsworth et al., 1987; Liu et al., 1985). As shown in Fig. 6, the PhACO gene expression level was increased by B. cinerea in infected leaves, regardless of DEX treatment. The expression levels of four genes of the ethylene pathway, ETR2 (ethylene receptor 2), ERS1 (ethylene response sensor 1), EIN2 (ethylene‐insensitive 2) and EIL1 (EIN3‐like1), were repressed during the entire inoculation period, apart from at 1 dpi when maximum transcript abundance was observed on DEX‐treated leaves. However, in B. cinerea‐inoculated DMSO‐treated plants, the abundance of transcripts of ETR2, ERS1, EIN2 and EIL1 was increased and reached a maximum at 3 or 4 dpi.
Figure 6.
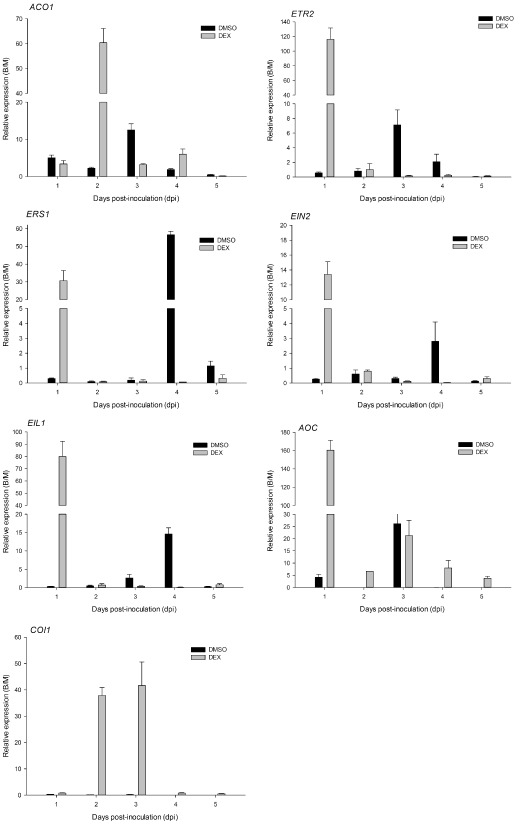
Expression patterns of ethylene and jasmonate (JA) pathway genes in Botrytis cinerea‐inoculated plantlets relative to expression in mock‐inoculated E9G. B/M, Botrytis/mock. See text for gene definitions.
To gain insight into whether the JA pathway was activated when the ethylene pathway was blocked during this plant–pathogen interaction, we compared the expression of allene oxide cyclase (AOC) (Ahkami et al., 2009) and CORONATINE INSENSITIVE 1 (COI1) (Breuillin et al., 2010). AOC and COI1 are important for JA biosynthesis and signalling transduction (Feys et al., 1994; Wasternack and Hause, 2002; Xie et al., 1998). The expression of AOC and COI1 was activated by B. cinerea, reaching a peak at 1 and 3 dpi in DEX‐treated leaves (Fig. 6). The expression of PhCOI1 was inhibited, although PhAOC expression was increased, in DMSO‐treated infected leaves (Fig. 6).
ERF expression profiles in infected GVG::etr1‐1 transgenic petunias
The expression of ethylene response factors (ERFs), downstream components of the ethylene signalling pathway, was analysed (Fig. 7). ERF transcripts accumulated when tissues were inoculated with B. cinerea, regardless of DEX or DMSO treatment, and reached peaks at various time points post‐inoculation. The maximum expression of certain ERF genes, such as ERF2, ERF3, ERF4 and ERF7, occurred at 3 dpi in DEX‐treated leaves; however, ERF8, ERF4, ERF6, ERF8 and ERF13 were maximal at 4 dpi in DMSO‐treated tissues.
Figure 7.
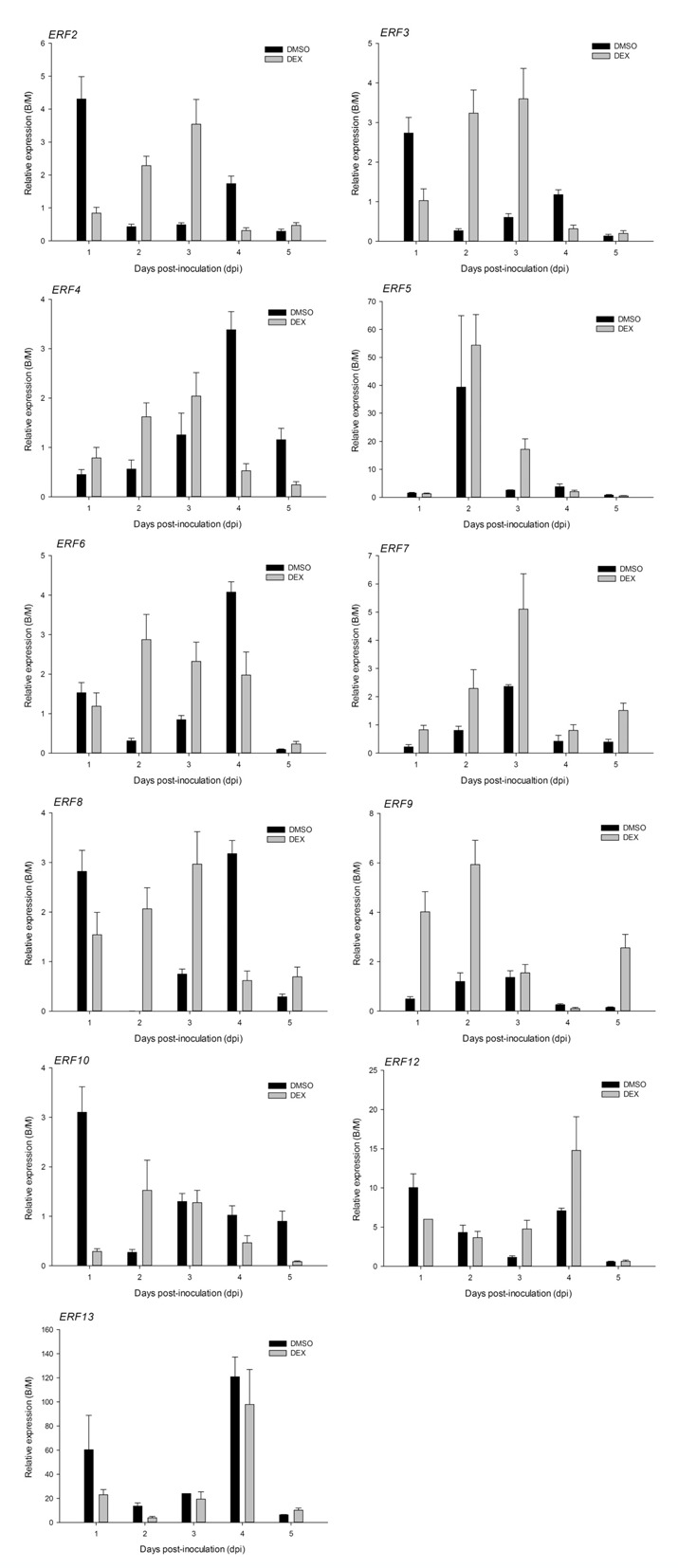
Expression patterns of petunia ethylene response factor (PhERF) genes in Botrytis cinerea‐inoculated plantlets relative to expression in mock‐inoculated E9G. B/M, Botrytis/mock.
Defence‐related gene expression profile in infected GVG::etr1‐1 transgenic petunias
RT‐PCR amplification was used to evaluate whether the expression of defence‐related genes was increased following infection with B. cinerea on DEX‐ and DMSO‐treated tissues (Fig. 8). As shown in Fig. 8, the expression of genes encoding for petunia defence‐related proteins was activated after B. cinerea inoculation of plants with or without DEX induction. The maximum transcript accumulation of PR6, OSM (osmotin; PR5) and Prxs1 (peroxidase1) was higher in DEX‐treated leaves than in DMSO‐treated leaves; by contrast, the expression levels of EX‐CHI (extracellular chitinase) and AC‐CHI (acidic chitinase) were lower in DEX‐treated leaves than DMSO‐treated leaves. The maximum expression level of all tested defence‐related genes, with the exception of D1 (defence1), was seen at 3 dpi on DEX‐induced samples (Fig. 8a). However, the peak of gene transcript accumulation of the tested defence‐related proteins occurred at 4 dpi, except for Prxs1 (at 1 dpi), in DMSO‐treated plants (Fig. 8b).
Figure 8.
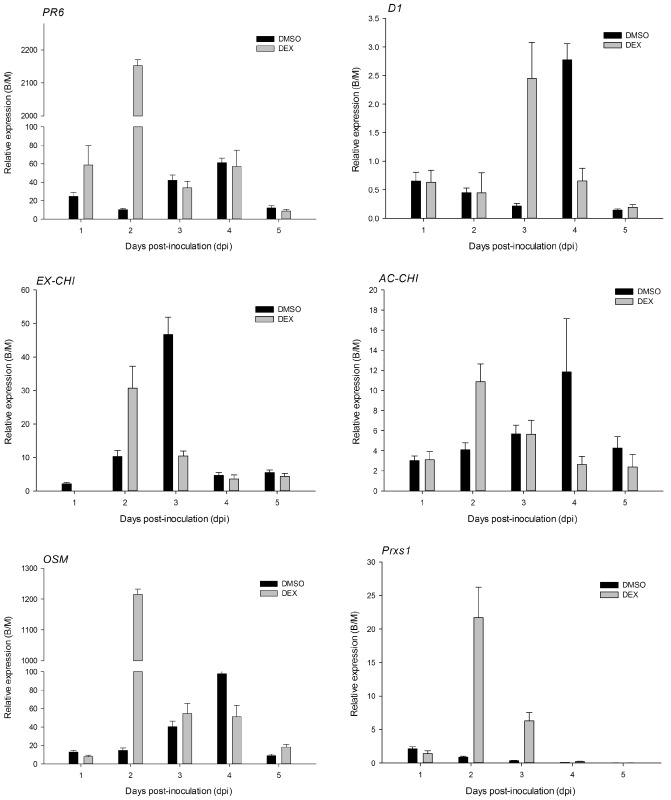
Expression patterns of defence‐related genes in Botrytis cinerea‐inoculated plantlets relative to expression in mock‐inoculated E9G. B/M, Botrytis/mock. See text for gene definitions.
Discussion
The extent of delayed disease symptoms is related to the level of etr1‐1 induction
The ethylene pathway, including ethylene biosynthesis and perception, is known to be involved in the response of plants to abiotic and biotic stresses (Abeles et al., 1992; Reid, 1995). Previous research has demonstrated that ethylene‐insensitive tobacco displays disease symptoms when infected with soil‐borne fungi (Knoester et al., 1998). However, another report has concluded that infection of ethylene‐insensitive soybeans with various pathogens has the opposite outcome (Hoffman et al., 1999). In our experiments, intact plantlets or detached leaves and flowers from inducible ethylene‐insensitive petunia mutants showed delayed and reduced disease symptoms. Obviously, the promotion or suppressive role of ethylene on disease symptoms during a plant–pathogen interaction depends on the plant variety and tissues, as well as the nature of the pathogen. This conclusion was also supported by Boller (1991) and Lund et al. (1998). Another considered reason is that the inducible promoter system can regulate the expression of etr1‐1 at will by the addition of the inducer. As ethylene signalling is involved in multiple aspects of plant development, the mutation of the ethylene receptor can lead to other undesirable physiological effects and defective growth (Knoester et al., 1998). The state of the induced etr1‐1 expression in such a short time in petunia, i.e. 8 days on intact plantlets and 3–4 days on detached leaves or flowers, determines the normal phenotype, and may make petunia more tolerant to grey mould development than the other model plants with constitutive expression of etr1‐1.
In our previous research, we observed that the etr1‐1 gene was more strongly expressed in the E9 line relative to the E7 line (Wang et al., unpublished data). Gallie (2010) demonstrated that the extent of ethylene insensitivity was caused by the level of etr1‐1 expression. Reduced or delayed disease symptoms in plantlets, leaves and flowers in the E9 line relative to the E7 line supports the conclusion that the reduced disease phenotype is related to a higher level of the etr1‐1 expression.
A notable point is that about 50% of the inoculated samples did not become infected when etr1‐1 expression was induced in either line. Although the lack of a wound may have contributed to the reduction in infection, as B. cinerea infects through wounds or natural openings on diverse plant organs (Holz et al., 2004), the consistent incidence of disease in the two lines suggests that the extent of pathogen resistance of the detached leaves of etr1‐1‐expressing plants is important for the response of petunia to B. cinerea.
Ethylene regulates PCD induced by the pathogen
During the colonization of host tissue, the lower chlorophyll loss rate and increased MDA levels resulting from B. cinerea infection of DEX‐ rather than DMSO‐treated leaves demonstrates that ethylene responses play a role in delaying necrosis induced by B. cinerea.
Botrytis cinerea boosts senescence through the production of ethylene (Cristescu et al., 2002), which promotes leaf senescence, together with age‐related factors (Grbić and Bleecker, 1995), as well as fruit ripening and flower senescence (Klee and Clark, 2010). Apart from ethylene, B. cinerea is known to produce abscisic acid (ABA) (Sharon et al., 2007), a known inducer of senescence (Lim et al., 2007). Infection with B. cinerea induced the senescence‐associated gene SAG12 (Swartzberg et al., 2008), a member of a group of genes with senescence‐specific patterns of transcript accumulation (Lohman et al., 1994; Thomma et al., 1999). In our experiments, it is worth mentioning that the expression of PhCP2 and PhCP4 was not related to the senescence induced by B. cinerea, although PhCP2 and PhCP4 have been included in the group of genes up‐regulated during senescence by phylogenetic analysis (Jones et al., 2005). Other PhCPs, for example PhCP3, which was grouped with genes up‐regulated by drought and wounding (Jones et al., 2005), or PhCP6 and PhCP7, genes up‐regulated by wounding, infection or PCD (Asp et al., 2004; Lidgett et al., 1995; Schmid et al., 1999), did not exhibit senescence‐associated expression in response to B. cinerea. Higher expression of PhCP10, which is very similar to SAG12 (Jones et al., 2005), and PhCP8 was found at 2 and 3 dpi on DMSO‐ rather than DEX‐treated plantlets. Together with the visible senescence symptoms, our results show that PhCP8 and PhCP10 are senescence‐associated genes and induced ethylene insensitivity in petunia retards the senescence caused by B. cinerea.
The independent roles of ethylene and JA on the plant response to pathogen
Were other ethylene receptors involved in the reduction in disease symptoms in etr1‐1‐expressing petunias? The expression of ETR2, ERS1, EIN2 and EIL1 was inhibited, together with the induced expression of etr1‐1, after infection. Typically, plants produce high levels of ethylene when challenged by pathogens, especially necrotrophs, such as B. cinerea (Broekaert et al., 2006; van Loon et al., 2006; Tsuchisaka et al., 2009). In addition, microorganisms, such as B. cinerea, can produce ethylene (Cristescu et al., 2002). The gene expression of ACO was observed on both DEX‐ and DMSO‐treated leaves, which supports the conclusion that the ethylene levels produced by either the petunia or the pathogen during the interaction are not sufficient to affect the expression and function of ethylene receptors by a feedback mechanism. These results show that the induced expression of etr1‐1 using the GVG promoter totally prevents the ethylene signalling pathway from participating in the plant defence response to B. cinerea.
More interestingly, the expression of PhCOI1 was almost entirely suppressed when the ethylene signalling pathway was activated, although the biosynthesis of JA was predicted because of the expression of PhAOC. However, transcriptional accumulation of PhCOI1 was detected when the ethylene pathway was blocked. The PhERFs showing maximum expression levels at 3 dpi were PhERF2, PhERF3, PhERF4, PhERF7 and PhERF8; this was the same expression pattern as for PhCOI1 in DEX‐treated leaves. The coincidence of expression patterns between these PhERFs and PhCOI1 suggests that JA‐independent ethylene induces or regulates the expression of these ERF genes. In addition, the expression of ETR2, ERS1, EIN2 and EIL1 was activated without DEX treatment after inoculation. The maximum expression levels of EIN2 and EIL1, which play a central role in the transduction of the ethylene signal to downstream targets (Shibuya et al., 2004), corresponded to the peak levels of PhERF4, PhERF6, PhERF8 and PhERF13 at 4 dpi; this led us to conclude that these four ERF genes were activated through the ethylene signalling pathway during the interaction between petunia and B. cinerea. Consequently, we propose that: (i) when petunia was assailed by B. cinerea, ethylene and JA independently established signal transduction; and (ii) PhERF4 and PhERF8 were activated through either the JA or ethylene pathway. These two genes appeared to perform a different function from Arabidopsis ERF1 and ORA59, which are integrators of the JA and ethylene signalling pathways (Leon‐Reyes et al., 2010; Lorenzo et al., 2003; Pré et al., 2008). The peak of expression of these two genes occurred 1 day earlier in the JA pathway than in the ethylene pathway. This result might be related to the delayed senescence induced by the expression of etr1‐1. This provides a research direction for the future to investigate the detailed functions of PhERF4 and PhERF8 in JA and ethylene mutants.
The expression of plant genes encoding for PR proteins was assessed during the petunia–B. cinerea interaction. In contrast with other species, for example, tomato infected by bacterial wilt and canker pathogen, no significant differences in the expression of defence‐related genes were found in comparison with the ethylene‐insensitive mutant Nr and wild‐type plants (Balaji et al., 2008). In our experiments, the expression of PR6, OSM and Prxs1 was increased when etr1‐1‐expressing plants encountered B. cinerea. The JA‐dependent expression of PR6, OSM (PR5) and Prxs1 genes has been reported previously (Buzi et al., 2004; Hondo et al., 2007; Xu et al., 1994; Zahn et al., 2005); 35‐, 12‐ and 11‐fold greater expression of these three genes, respectively, were seen in DEX‐treated relative to DMSO‐treated leaves. These results further suggest that the JA pathway was active during the petunia response to B. cinerea infection when etr1‐1 was induced. The peak expression levels of these genes were seen at 3 dpi, followed by the expression of PhCOI1 and PhERF2, PhERF3, PhERF4, PhERF7 and PhERF8. The coincidence of the gene expression profiles of PhCOI1, PhERFs and JA‐associated defence‐related genes suggests that the JA signalling pathway is responsible for the delayed disease symptoms and decreased infection by B. cinerea. However, in DMSO‐treated plantlets, the peak expression of all of the tested defence‐related genes, with the exception of Prxs1, was 4 dpi, which is consistent with the peak level of PhEIL1 and other PhERFs (PhERF4, PhERF6, PhERF8 and PhERF13). These results suggest that ethylene is involved in regulating the petunia basal adaptation response to B. cinerea. This conclusion is supported by the higher expression levels of EX‐CHI and AC‐CHI, both of which are ethylene response genes (Rakwal et al., 2004), in DMSO‐treated leaves relative to DEX‐treated leaves.
In conclusion, our findings provide a model of ethylene/JA‐independent responses when petunia plants are challenged by exposure to a necrotrophic pathogen, B. cinerea (Fig. 9). Petunia plants exposed to biotic stresses launch signalling events, including the biosynthesis and signalling transduction of ethylene, completely independent of the JA pathway, which activates the downstream expression of PhERF4, PhERF6, PhERF8 and PhERF13. These pathways then promote plant basal defence responses, although, finally, plant death occurs. When the ethylene signalling pathway is blocked, the biosynthesis and signalling transduction pathways of JA are activated, which trigger the expression of PhERF2, PhERF3, PhERF4, PhERF7 and PhERF8, thereby increasing the expression of defence‐related genes. The specific characteristics of ethylene insensitivity and the defence response activated by the JA pathway are responsible for petunia tolerance to this pathogen.
Figure 9.
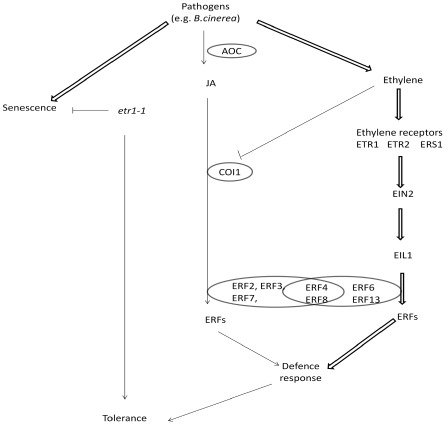
Model of ethylene/jasmonate (JA) independent pathways of petunia response to pathogens. When petunia encounters pathogen, the biosynthesis and activation signalling of the ethylene pathway are induced (wide arrows). Subsequently, some petunia ethylene response factor (PhERF) genes, such as PhERF4, PhERF6, PhERF8 and PhERF13, are activated in order to induce plants to launch the basal defence response, although finally programmed cell death and senescence occur. Otherwise, the ethylene signalling pathway is completely blocked by induced ethylene‐insensitive1‐1 (etr1‐1) expression, and the biosynthesis and consequent signalling of the JA pathway are induced by B. cinerea infection (narrow arrows). Some PhERFs, such as PhERF2, PhERF3, PhERF4, PhERF7 and PhERF8, are activated at the transcriptional level. In turn, the transcriptional accumulation of defence‐related genes, together with delayed senescence (narrow arrows) makes ethylene‐insensitive petunia tolerant to the pathogen to some extent (narrow arrows). See text for gene definitions.
Experimental Procedures
Plant material
Petunia seedlings were obtained by germinating seeds in Murashige and Skoog (MS) medium. The T2 seeds of transgenic Petunia × hybrida cv. Mitchell Diploid (MD) with GVG:etr1‐1 of E7 and E9 lines, which showed different expression levels of etr1‐1 after DEX induction in our previous research, were first surface sterilized in 15% bleach for 20 min and then in 70% ethanol for 45 s, followed by three washes with sterile distilled water. After placing the seeds (20 seeds/plate), the plates were kept at room temperature under continuous low fluorescence light (∼40 μmol/m2/s) for 21 days. The young seedlings were then carefully removed from the MS medium and placed in 10‐cm pots. Plants were grown in a growth chamber with a 16‐h photoperiod, at 25 °C in the light period and 22 °C in the dark period. Plants were fertilized once a week with nitrogen at 300 mg/L from 15N‐5P‐15K Cal Mag (Peters soluble fertilizer, The Scotts Co., Marysville, OH, USA).
Plant treatments
DEX (Sigma‐Aldrich, St. Louis, MO, USA) was stored as a 10 mm solution in 60% (v/v) dimethylsulphoxide (DMSO) (Sigma‐Aldrich) at −20 °C. DEX was added to vase solution (Chrysal, Miami, FL, USA) to achieve induction. Unless otherwise stated, 30 μm DEX was used (Craft et al., 2005).
Intact plants, detached leaves and flowers were subjected to different treatments with 30 μm DEX or without the chemical inducer, i.e. 0.18% (v/v) DMSO, as a control. Eight‐week‐old plants were irrigated with the chemicals for 4 days before inoculation. The irrigation solution was replaced every 4 days after inoculation according to plant requirements and previous reports (Wielopolska et al., 2005). The fourth leaves from the top were collected and placed in boxes containing 0.6% agar medium. The inducers were painted 2 days before inoculation and painting was maintained during the inoculation period when needed. Flowers from T3 plants of selected lines were harvested when fully open, but before anther dehiscence. Flowers were placed in 2‐mL tubes containing chemicals 2 days before inoculation.
Inoculation assays
Botrytis cinerea Pers.:Fr. strain B05.10 was grown on potato dextrose agar plates (Sigma‐Aldrich). The conidial suspension was filtered through a cloth mesh (Cantu et al., 2008). After removal of the supernatant and resuspension of the conidia in sterile distilled water, an inoculation suspension was prepared containing 1.0 × 106 conidia/mL with 10 mm glucose and 6.7 mm potassium phosphate (pH 6.0) (Audenaert et al., 2002; Díaz et al., 2002). Conidia were pregerminated without shaking for 2 h in the inoculation suspension at 22 °C (Asselbergh et al., 2007; Díaz et al., 2002).
For the leaf disc and flower assays, two 10‐μL droplets were used to inoculate each petunia leaf, which was incubated at 25 °C under dark conditions. Disease severity and disease incidence were evaluated and analysed every day after inoculation. At least 80 inoculation drops were evaluated for each treatment. Detached flowers were inoculated under the same induction conditions as the detached leaves. The numbers of healthy flowers, expanding lesions per flower and lesion expansion were investigated after 3 days and during the period from 2 to 3 days after inoculation. At least 16 droplets were analysed for each treatment.
For intact plant assays, 300 μL of the conidial suspension were sprayed on the leaves. Inoculated plants were grown in a sealed light transmittance box with a pinhole foil in a 16 h/8 h (∼40 μmol/m2/s) photoperiod growth chamber, at 25 °C in the light period and 22 °C in the dark period. The same indices as detached flowers were performed on the fifth day and in the period between 5 and 6 days after inoculation. At least 10 plants were assessed in each treatment.
Chlorophyll measurements
Chlorophyll in intact plant leaves and detached leaves was measurement by SPAD (Konica, Osaka, Japan) every day after inoculation (Coste et al., 2010). Three spots per leaf were selected to read. At least 45 and 40 leaves from the in vivo and in vitro investigations, respectively, were evaluated for each treatment.
Lipid peroxidation and antioxidant enzyme analysis
MDA activity was assayed to assess lipid peroxidation in approximately 0.2 g (fresh weight, FW) of detached petunia leaves using a modified thiobarbituric acid (TBA)‐MDA assay (Hodges et al., 1999). Approximately 0.5 g FW of detached leaves were homogenized in a prechilled mortar and pestle in liquid nitrogen with 0.5 g of polyvinylpolypyrrolidone, and incubated in enzyme extract buffer containing potassium phosphate buffer (pH 7.5) for 0.5 h at 4 °C. The extract buffer containing ground tissue was then centrifuged (Eppendorf, Hamburg, Germany) at 13 362 × g for 20 min at 4 °C. The supernatant was divided into two identical aliquots. Each 0.1‐mL supernatant was supplemented with the reaction buffer for SOD or CAT analysis, according to Hodges and Forney (2000). All enzymes were assayed spectrophotometrically at 25 °C with UV‐VISO 2450 (Shimadzu, Kyoto, Japan).
RNA extraction and qRT‐PCR analysis
Total RNA from separate, duplicate petunia leaves in vivo was isolated using Trizol (TaKaRa, Dalian, China). One microgram of total RNA was reverse transcribed using the PrimeScript RT reagent with the gDNA Eraser Kit (TaKaRA), according to the manufacturer's instructions. In brief, 1 μg of total RNA was cleared of DNA with 2 μL of 5 × gDNA Eraser Buffer and 1 μL of gDNA Eraser for 2 min at 42 °C. The 10‐μL reaction volume was reverse transcribed at 37 °C for 15 min in a 20‐μL reaction volume containing 4 μL of 5 × PrimeScript Buffer2, 1 μL of PrimeScript RT Enzyme Mix I and 1 μL of RT Primer Mix. The reaction was stopped by incubation at 85 °C for 5 s. One microlitre of diluted 5 × cDNA was used as the template for PCR amplification using SYBR GREEN Taq (TaKaRa). Specific primers were designed by the Primer 3 program and are listed in Table S1 (see Supporting Information). Each gene was tested in a volume of 20 μL. Amplifications were performed using an Applied Biosystems 7300 system (Applied Biosystems, Shanghai, China) with the following procedure: 2 min at 95 °C, followed by 40 cycles of 45 s at 95 °C, 30 s at 60 °C and 45 s at 72 °C. Fluorescence was measured at the end of each cycle. Analysis of the melting curves provided evidence for the absence of nonspecific products and primer dimers. For data analysis, average threshold cycle (C T) values were calculated for each gene of interest, on the basis of three independent biological samples as indicated (Balaji et al., 2008), and were normalized and used to calculate the relative transcript levels as described elsewhere (Pfaffl, 2001). 26SrRNA was used as an internal standard for normalization (Balaji et al., 2008).
Statistical analyses
All statistical analyses were performed using the SPSS package (Version 16.0; SPSS Inc., Chicago, IL, USA). One‐way analysis of variance (ANOVA) was performed for experiments with one independent variable. Duncan's test was used as the post hoc test if significant differences were found.
Supporting information
Fig. S1 The representative phenotype of dexamethasone (DEX)‐ and dimethylsulphoxide (DMSO)‐treated E7H petunias inoculated by Botrytis cinerea.
Table S1 Gene‐specific primers for real‐time quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR).
Acknowledgements
This work was supported by the National 863 Projects of China (No. 2011AA100204).
References
- Abeles, F.B. , Morgan, P.W. and Saltveit, M.E. (1992) Ethylene in Plant Biology, 2nd edn. San Diego, CA: Academic Press. [Google Scholar]
- Ahkami, A.H. , Lischewski, S. , Haensch, K‐T. , Porfirova, S. , Hofmann, J. , Rolletschek, H. , Melzer, M. , Franken, P. , Hause, B. , Druege, U. and Hajirezaei, M.R. (2009) Molecular physiology of adventitious root formation in Petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytol. 181, 613–625. [DOI] [PubMed] [Google Scholar]
- Asp, T. , Bowra, S. , Borg, S. and Holm, P.B. (2004) Molecular cloning, functional expression in Escherichia coli and enzymatic characterisation of a cysteine protease from white clover (Trifolium repens). Biochim. Biophys. Acta, 1699, 111–122. [DOI] [PubMed] [Google Scholar]
- Asselbergh, B. , Curvers, K. , França, S.C. , Audenaert, K. , Vuylsteke, M. , Van Breusegem, F. and Höfte, M. (2007) Resistance to Botrytis cinerea in sitiens, an abscisic acid‐deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol. 144, 1863–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert, K. , De Meyer, G. and Höfte, M. (1999) Pathways involved in control of B. cinerea via induced resistance. Med. Fac. Landbouwwet. Rijksuniv. Gent. 64, 477–488. [Google Scholar]
- Audenaert, K. , De Meyer, G.B. and Hofte, M.M. (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid‐dependent signaling mechanisms. Plant Physiol. 128, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baarlen, P. , Woltering, E.J. , van Staats, M. and Kan, J.A.L. (2007) Histochemical and genetic analysis of host and non‐host interactions of Arabidopsis with three Botrytis species: an important role for cell death control. Mol. Plant Pathol. 8, 41–54. [DOI] [PubMed] [Google Scholar]
- Balague, C. , Watson, C.F. , Turner, A.J. , Rouge, P. , Picton, S. , Pech, J.C. and Grierson, D. (1993) Isolation of a ripening and wound‐induced cDNA from Cucumis melo L. encoding a protein with homology to the ethylene‐forming enzyme. Eur. J. Biochem. 212, 27–34. [DOI] [PubMed] [Google Scholar]
- Balaji, V. , Mayrose, M. , Sherf, O. , Jacob‐Hirsch, J. , Eichenlaub, R. , Iraki, N. , Manulis‐Sasson, S. , Rechavi, G. , Barash, I. and Sessa, G. (2008) Tomato transcriptional changes in response to Clavibacter michiganensis subsp. michiganensis reveal a role for ethylene in disease development. Plant Physiol. 146, 1797–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, C.S. , Blume, B. , Bouzayen, M. , Cooper, W. , Hamilton, A.J. and Grierson, D. (1996) Differential expression of the 1‐aminocyclopropane‐1‐carboxylate oxidase gene family of tomato. Plant J. 9, 525–535. [DOI] [PubMed] [Google Scholar]
- Benito, E.P. , ten Have, A. , van‘t Klooster, J.W. and van Kan, J.A.L. (1998) Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea . Eur. J. Plant Pathol. 104, 207–220. [Google Scholar]
- Bleecker, A.B. , Estelle, M.A. , Somerville, C. and Kende, H. (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana . Science, 241, 1086–1089. [DOI] [PubMed] [Google Scholar]
- Boller, T. (1991) Ethylene in pathogenesis and disease resistance In: The Plant Hormone Ethylene (Mattoo A.K. and Suttle J.C., eds), pp. 293–314. Boca Raton, FL: CRC Press. [Google Scholar]
- Bouchez, O. , Huard, C. , Lorrain, S. , Roby, D. and Balague, C. (2007) Ethylene is one of the key elements for cell death and defense response control in the Arabidopsis lesion mimic mutant vad1. Plant Physiol. 145, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuillin, F. , Schramm, J. , Hajirezaei, M. , Ahkami, A. , Favre, P. , Druege, U. , Hause, B. , Bucher, M. , Kretzschmar, T. , Bossolini, E. , Kuhlemeier, C. , Martinoia, E. , Franken, P. , Scholz, U. and Reinhardt, D. (2010) Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J. 64, 1002–1017. [DOI] [PubMed] [Google Scholar]
- Broekaert, W.F. , Delaure, S.L. , De Bolle, M.F. and Cammue, B.P. (2006) The role of ethylene in host–pathogen interactions. Annu. Rev. Phytopathol. 44, 393–416. [DOI] [PubMed] [Google Scholar]
- Buzi, A. , Chilosi, G. and Magro, P. (2004) Induction of resistance in melon seedlings against soil‐borne fungal pathogens by gaseous treatments with methyl jasmonate and ethylene. J. Phytopathol. 152, 491–497. [Google Scholar]
- Cantu, D. , Vicente, A.R. , Greve, L.C. , Dewey, F.M. , Bennett, A.B. , Labavitch, J.M. and Powell, A.L.T. (2008) The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea . Proc. Natl. Acad. Sci. USA, 105, 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquer, M. , Fournier, E. , Kunz, C. , Levis, C. , Pradier, J.M. , Simon, A. and Viaud, M. (2007) Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 277, 1–10. [DOI] [PubMed] [Google Scholar]
- Coste, S. , Baraloto, C. , Leroy, C. , Marcon, É. , Renaud, A. , Richardson, A. , Roggy, J‐C. , Schimann, H. , Uddling, J. and Hérault, B. (2010) Assessing foliar chlorophyll contents with the SPAD‐502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann. Forest Sci. 67, 607. [Google Scholar]
- Craft, J. , Samalova, M. , Baroux, C. , Townley, H. , Martinez, A. , Jepson, I. , Tsiantis, M. and Moore, I. (2005) New pOp/LhG4 vectors for stringent glucocorticoid‐dependent transgene expression in Arabidopsis . Plant J. 41, 899–918. [DOI] [PubMed] [Google Scholar]
- Cristescu, S.M. , De Martinis, D. , Te Lintel Hekkert, S. , Parker, D.H. and Harren, F.J. (2002) Ethylene production by Botrytis cinerea in vitro and in tomatoes. Appl. Environ. Microbiol. 68, 5342–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz, J. , ten Have, A. and van Kan, J.A.L. (2002) The role of ethylene and wound signaling in resistance of tomato to Botrytis cinerea . Plant Physiol. 129, 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman, M.B. , Park, Y.K. , Oltersdorf, T. , Li, W. , Clemente, T. and French, R. (2001) Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc. Natl. Acad. Sci. USA, 98, 6957–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díza, J. , Have, A.T. and Kan, J.A.L.V. (2002) The role of ethylene and wound signaling in resistance of tomato to Botrytis cinerea . Plant Physiol. 129, 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, E.E. and Ryan, C.A. (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound‐inducible proteinase inhibitors. Plant Cell, 4, 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys, B. , Benedetti, C.E. , Penfold, C.N. and Turner, J.G. (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell, 6, 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie, D.R. (2010) Regulated ethylene insensitivity through the inducible expression of the Arabidopsis etr1‐1 mutant ethylene receptor in tomato. Plant Physiol. 152, 1928–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook, J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Govrin, E.M. and Levine, A. (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea . Curr. Biol. 10, 751–757. [DOI] [PubMed] [Google Scholar]
- Grbić, V. and Bleecker, A. (1995) Ethylene regulates the timing of leaf senescence in Arabidopsis . Plant J. 8, 595–602. [Google Scholar]
- Greenberg, J.T. (1997) Programmed cell death in plant–pathogen interactions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 525–545. [DOI] [PubMed] [Google Scholar]
- Hall, A.E. , Grace Chen, Q. , Findell, J.L. , Eric Schaller, G. and Bleecker, A.B. (1999) The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol. 121, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, A.J. , Lycett, G.W. and Grierson, D. (1990) Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 346, 284–287. [Google Scholar]
- Hodges, D.M. and Forney, C.F. (2000) The effects of ethylene, depressed oxygen and elevated carbon dioxide on antioxidant profiles of senescing spinach leaves. J. Exp. Bot. 51, 645–655. [DOI] [PubMed] [Google Scholar]
- Hodges, D.M. , DeLong, J.M. , Forney, C.F. and Prange, R.K. (1999) Improving the thiobarbituric acid‐reactive‐substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 207, 604–611. [DOI] [PubMed] [Google Scholar]
- Hoffman, T. , Schmidt, J.S. , Zheng, X. and Bent, A.F. (1999) Isolation of ethylene‐insensitive soybean mutants that are altered in pathogen susceptibility and gene for gene disease resistance. Plant Physiol. 119, 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth, M.J. , Bird, C.R. , Ray, J. , Schuch, W. and Grierson, D. (1987) Structure and expression of an ethylene‐related mRNA from tomato. Nucleic Acids. Res. 15, 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz, G. , Coertze, S. and Williamson, B. (2004) The ecology of Botrytis on plant surfaces In: Botrytis: Biology, Pathology and Control (Elad Y., Williamson B., Tudzynski P. and Delen N., eds), pp. 9–27. Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- Hondo, D. , Hase S, Kanayama, Y. , Yoshikawa, N. , Takenaka, S. and Takahashi, H. (2007) The LeATL6‐associated ubiquitin/proteasome system may contribute to fungal elicitor‐activated defense response via the jasmonic acid‐dependent signaling pathway in tomato. Mol. Plant Microbe Interact. 20, 72–81. [DOI] [PubMed] [Google Scholar]
- Hua, J. , Sakai, H. , Nourizadeh, S. , Chen, Q.G. , Bleecker, A.B. , Ecker, J.R. and Meyerowitz, E.M. (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis . Plant Cell, 10, 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M.L. , Chaffin, G.S. , Eason, J.R. and Clark, D.G. (2005) Ethylene‐sensitivity regulates proteolytic activity and cysteine protease gene expression in petunia corollas. J. Exp. Bot. 56, 2733–2744. [DOI] [PubMed] [Google Scholar]
- van Kan, J.A.L. (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 11, 247–253. [DOI] [PubMed] [Google Scholar]
- van Kan, J.A.L. , Cozijnsen, T. , Danhash, N. and De Wit, P.J. (1995) Induction of tomato stress protein mRNAs by ethephon, 2,6‐dichloroisonicotinic acid and salicylate. Plant Mol. Biol. 27, 1205–1213. [DOI] [PubMed] [Google Scholar]
- Kende, H. (1993) Ethylene biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 283–307. [Google Scholar]
- Klee, H.J. and Clark, D.G. (2010) Ethylene signal transduction in fruits and flowers In: Plant Hormones (Davies P.J., ed.), pp. 377–398. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Knoester, M. , Loon, L.C.V. , Heuvel, J.V.D. , Hennig, J. , Bol, J.F. and Linthorst, H.J.M. (1998) Ethylene‐insensitive tobacco lacks nonhost resistance against soil‐borne fungi. Proc. Natl. Acad. Sci. USA, 95, 1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel, B.N. and Brooks, D.M. (2002) Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 5, 325–331. [DOI] [PubMed] [Google Scholar]
- Lam, E. , Pontier, D. and Pozo, O. (1999) Die and let live—programmed cell death in plants. Curr. Opin. Plant Biol. 2, 502–507. [DOI] [PubMed] [Google Scholar]
- Leon‐Reyes, A. , Du, Y. , Koornneef, A. , Proietti, S. , Korbes, A.P. , Memelink, J. , Pieterse, C.M. and Ritsema, T. (2010) Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic Acid. Mol. Plant–Microbe Interact. 23, 187–197. [DOI] [PubMed] [Google Scholar]
- Lidgett, A.J. , Moran, M. , Wong, K.A. , Furze, J. , Rhodes, M.J. and Hamill, J.D. (1995) Isolation and expression pattern of a cDNA encoding a cathepsin B‐like protease from Nicotiana rustica . Plant Mol. Biol. 29, 379–384. [DOI] [PubMed] [Google Scholar]
- Lim, P.O. , Kim, H.J. and Gil Nam, H. (2007) Leaf senescence. Annu. Rev. Plant Biol. 58, 115–136. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Hoffman, N.E. and Yang, S.F. (1985) Promotion by ethylene of the capability to convert 1‐aminocyclopropane‐1‐carboxylic acid to ethylene in preclimacteric tomato and cantaloupe Fruits. Plant Physiol. 77, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman, K.N. , Gan, S. , John, M.C. and Amasino, R.M. (1994) Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiol. Plant. 92, 322–328. [Google Scholar]
- van Loon, L.C. , Geraats, B. and Linthorst, H.J. (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 11, 184–191. [DOI] [PubMed] [Google Scholar]
- Lorenzo, O. , Piqueras, R. , Sanchez‐Serrano, J.J. and Solano, R. (2003) ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell, 15, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain, S. , Lin, B. , Auriac, M.C. , Kroj, T. , Saindrenan, P. , Nicole, M. , Balagué, C. and Roby, D. (2004) Vascular associated death1, a novel GRAM domain‐containing protein, is a regulator of cell death and defense responses in vascular tissues. Plant Cell, 16, 2217–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, S.T. , Stall, R.E. and Klee, H.J. (1998) Ethylene regulates the susceptible response to pathogen infection on tomato. Plant Cell, 10, 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach, J.M. , Castillo, A.R. , Hoogstraten, R. and Greenberg, J.T. (2001) The Arabidopsis‐accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc. Natl. Acad. Sci. USA, 98, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegadaraju, V. , Knepper, C. , Reese, J. and Shah, J. (2005) Premature leaf senescence modulated by the Arabidopsis PHYTOALEXIN DEFICIENT4 gene is associated with defense against the phloem‐feeding green peach aphid. Plant Physiol. 139, 1927–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx, I.A.M.A. , Thomma, B.P.H.J. , Buchala, A. , Métraux, J.P. and Broekaerta, W.F. (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell, 10, 2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M.W. (2001) A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plett, J.M. , Cvetkovska, M. , Makenson, P. , Xing, T. and Regan, S. (2009) Arabidopsis ethylene receptors have different roles in Fumonisin B‐1‐induced cell death. Physiol. Mol. Plant Pathol. 74, 18–26. [Google Scholar]
- Pré, M. , Atallah, M. , Champion, A. , De Vos, M. , Pieterse, C.M.J. and Memelink, J. (2008) The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 147, 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakwal, R. , Yang, G. and Komatsu, S. (2004) Chitinase induced by jasmonic acid, methyl jasmonate, ethylene and protein phosphatase inhibitors in rice. Mol. Biol. Rep. 31, 113–119. [DOI] [PubMed] [Google Scholar]
- Reid, M.S. (1995) Ethylene in plant growth, development, and senescence In Plant Hormones: Physiology, Biochemistry and Molecular Biology, 2nd edn (Davies P.J., ed.), pp. 486–508. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Schmid, M. , Simpson, D. and Gietl, C. (1999) Programmed cell death in castor bean endosperm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proc. Natl. Acad. Sci. USA, 96, 14 159–14 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon, A. , Elad, Y. , Barakat, R. and Tudzynski, P. (2007) Phytohormones in Botrytis–plant interactions In: Botrytis: Biology, Pathology and Control (Elad Y., Williamson B., Tudzynski P. and Delen N., eds), pp. 163–179. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Shibuya, K. , Barry, K.G. , Ciardi, J.A. , Loucas, H.M. , Underwood, B.A. , Nourizadeh, S. , Ecker, J.R. , Klee, H.J. and Clark, D.G. (2004) The central role of PhEIN2 in ethylene responses throughout plant development in petunia. Plant Physiol. 136, 2900–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzberg, D. , Kirshner, B. , Rav‐David, D. , Elad, Y. and Granot, D. (2008) Botrytis cinerea induces senescence and is inhibited by autoregulated expression of the IPT gene. Eur. J. Plant Pathol. 120, 289–297. [Google Scholar]
- Tenberge, K.B. (2004) Morphology and cellular organisation in Botrytis interactions with plants In: Botrytis: Biology, Pathology and Control (Elad Y., Williamson B., Tudzynski P. and Delen N., eds), pp. 67–84. Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- Tenberge, K.B. , Beckedorf, M. , Hoppe, B. , Schouten, A. , von den Solf, M. and Driesch, M. (2002) In situ localization of AOS in host–pathogen interactions. Microsc. Microanal. 8, 250–251. [Google Scholar]
- Thomma, B.P.H.J. , Eggermont, K. , Tierens, K.F.M. and Broekaert, W.F. (1999) Requirement of functional ethylene‐insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea . Plant Physiol. 121, 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka, A. , Yu, G.X. , Jin, H.L. , Alonso, J.M. , Ecker, J.R. , Zhang, X.M. , Gao, S. and Theologis, A. (2009) A combinatorial interplay among the 1‐aminocyclopropane‐1‐carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana . Genetics, 183, 979–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack, C. and Hause, B. (2002) Jasmonates and octadecanoids: signals in plant stress responses and development. Prog. Nucleic Acid Res. Mol. Biol. 72, 165–221. [DOI] [PubMed] [Google Scholar]
- Wielopolska, A. , Townley, H. , Moore, I. , Waterhouse, P. and Helliwell, C. (2005) A high‐throughput inducible RNAi vector for plants. Plant Biotechnol. J. 3, 583–590. [DOI] [PubMed] [Google Scholar]
- Xie, D.X. , Feys, B.F. , James, S. , Nieto‐Rostro, M. and Turner, J.G. (1998) COI1: an Arabidopsis gene required for jasmonate‐regulated defense and fertility. Science, 280, 1091–1094. [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Chang, P.‐F.L. , Liu, D. , Narasimhan, M.L. , Raghothama, K.G. , Hasegawa, P.M. and Bressan, R.A. (1994) Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell, 6, 1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S.F. and Hoffman, N.E. (1984) Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 35, 155–189. [Google Scholar]
- Zahn, M. , Wimalasekara, R. , Göbel, C. , Feussner, I. , Holk, A. and Scherer, G.F.E. (2005) Expression of Arabidopis phospholipase A genes in Petunia × hybrida. Increased hypersensitive‐like response after infection with Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000 demonstrates a function for phospholipase A in pathogen defence. Physiol. Mol. Plant Pathol. 67, 2–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 The representative phenotype of dexamethasone (DEX)‐ and dimethylsulphoxide (DMSO)‐treated E7H petunias inoculated by Botrytis cinerea.
Table S1 Gene‐specific primers for real‐time quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR).


