Summary
Most plant–pathogen interactions do not result in pathogenesis because of pre‐formed defensive plant barriers or pathogen‐triggered activation of effective plant immune responses. The mounting of defence reactions is accompanied by a profound modulation of plant metabolism. Common metabolic changes are the repression of photosynthesis, the increase in heterotrophic metabolism and the synthesis of secondary metabolites. This enhanced metabolic activity is accompanied by the reduced export of sucrose or enhanced import of hexoses at the site of infection, which is mediated by an induced activity of cell‐wall invertase (Cw‐Inv). Cw‐Inv cleaves sucrose, the major transport sugar in plants, irreversibly yielding glucose and fructose, which can be taken up by plant cells via hexose transporters. These hexose sugars not only function in metabolism, but also act as signalling molecules. The picture of Cw‐Inv regulation in plant–pathogen interactions has recently been broadened and is discussed in this review. An interesting emerging feature is the link between Cw‐Inv and the circadian clock and new modes of Cw‐Inv regulation at the post‐translational level.
Keywords: cell‐wall invertase, circadian clock, defence, metabolism, sugar signalling
Introduction
Plants are exposed to a range of different pathogens, including fungi, bacteria, oomycetes and viruses. Plant pathogens have evolved different strategies to interact with their hosts. Generally, plant–microbe interactions can be classified as biotrophic, with the pathogen feeding from living tissue, or necrotrophic, with the pathogen feeding from dead host tissue, which has been actively killed by the pathogen. There is a wide range of trophic lifestyles of pathogens, e.g. hemibiotrophic pathogens show an initial biotrophic phase and a switch to a necrotrophic lifestyle later (Glazebrook, 2005). Plants are resistant to most micro‐organisms they encounter as a result of pre‐formed or activated defence mechanisms. In basal resistance, pathogen‐associated molecular patterns (PAMPs), such as fungal chitin or bacterial flagellin, are recognized by corresponding receptors, leading to the activation of signalling cascades, regulation of gene expression, production of reactive oxygen species and cell‐wall modifications (Schwessinger and Ronald, 2012). Successful pathogens have evolved strategies to bypass plant defence reactions. Virulence is established by effector molecules which enable the pathogen to interfere with its recognition, or the downstream activation of plant defence. In a more specific line of defence, called effector‐triggered immunity, the plant recognizes pathogen‐derived effectors or effector activities, leading to the activation of strong defence reactions, including the hypersensitive cell death reaction (Jones and Dangl, 2006). Plant defence is accompanied by a profound and dynamic modulation of host metabolism. Common metabolic changes are the repression of photosynthesis, sink induction and the production of secondary metabolites (Berger et al., 2007; Biemelt and Sonnewald, 2006). In this response, cell‐wall invertase (Cw‐Inv) plays a pivotal role in metabolic reprogramming and sugar sensing. Here, we summarize the role of Cw‐Inv in defence reactions and extend the model to recent advances, such as a possible interconnection of Cw‐Inv and the circadian clock and new modes of Cw‐Inv regulation.
Cw‐Invs, Key Enzymes in Source–Sink Transitions
Plant tissues can be divided into two major categories: net exporters of sugars (carbon sources) and net importers of sugars (carbon sinks). Source tissues are typically photosynthetically active, CO2‐fixing mesophyll cells of mature leaves. However, there are different types of sink tissue that rely on an external supply of sugars, such as roots, flowers or fruits. The phloem transports sucrose, the main transport sugar in plants, from source to sink tissues. Phloem unloading can be mediated symplastically via plasmodesmata, or via sugar transporters. In the latter case, the extracellular sucrose is either imported into the sink cell via sucrose importers, or sucrose is cleaved irreversibly by Cw‐Inv, yielding glucose and fructose, which are taken up by hexose transporters (Büttner and Sauer, 2000; Koch, 2004). In addition to Cw‐Inv, sucrose cleavage is mediated by cytosolic sucrose synthase, which catalyses the reversible cleavage of sucrose, yielding UDP‐glucose and fructose (Koch, 2004). Symplastic phloem unloading is characteristic for tissues with a high demand for sugars, such as tubers. Cw‐Invs are considered to be key enzymes in apoplastic phloem unloading and sink induction (Sturm and Tang, 1999). The functional coupling of hexose transporters and Cw‐Inv is supported by a coordinated induction after cytokinin application, as shown in Chenopodium rubrum suspension cultures (Ehneß and Roitsch, 1997), and the co‐expression of extracellular invertase and hexose transporters in the endosperm transfer cell layer of barley seeds (Weschke et al., 2003). Cw‐Invs are organized into small gene families with different isoenzymes characterized by tissue‐, organelle‐ and development‐specific expression patterns (Cho et al., 2005; Sherson et al., 2003). Specific functions have been shown for different isoenzymes, particularly for flower, fruit and pollen development, a fact which reflects the diversity of invertase functions in assimilate partitioning.
Modes of Cw‐Inv Regulation
For a long time, the regulation of Cw‐Inv activity appeared to take place mainly on the transcriptional level. Diverse stimuli, such as hormones, sugars, abiotic and biotic stress stimuli, regulate the transcript levels of Cw‐Inv (Roitsch et al., 2003; and references therein). However, recent studies have highlighted Cw‐Inv regulation on the post‐translational level. As a result of their glycan decoration, Cw‐Invs are rather stable; hence, post‐translational regulation is important to control their activity (Rausch and Greiner, 2004). Post‐translational regulation of Cw‐Inv activity is mediated by proteinaceous invertase inhibitors. These inhibitors target directly the active site of Cw‐Inv in a pH‐dependent manner and compete with sucrose for the same binding site. This suggests that Cw‐Inv activity is regulated by the extracellular sucrose level and pH (Hothorn et al., 2010). Bonfig et al. (2010) have shown that proteinaceous invertase inhibitors regulate Cw‐Inv activity after pathogen challenge. They demonstrated a de‐repression of invertase activity via the down‐regulation of an invertase inhibitor, thus realizing a local sink induction at the site of the defence reaction, in the interaction of Arabidopsis with the hemibiotrophic bacterial pathogen Pseudomonas syringae. Moreover, reduction of Cw‐Inv activity by the overexpression of an invertase inhibitor in roots was able to reduce clubroot gall development in Arabidopsis (Siemens et al., 2011). An interesting novel mode of Cw‐Inv regulation has been proposed by Le Roy et al. (2013). The authors analysed a tobacco Cw‐Inv, which lacks sucrose‐hydrolysing capacity because of the absence of certain essential amino acids responsible for stable substrate binding. In vitro experiments on pollen cell walls suggested that the inactive Cw‐Inv Nin88 counteracts the binding of another, sucrolytically active, Cw‐Inv (cwINV1) to pollen cell walls, thereby leading to higher activities of cwINV1, but also more efficient binding with the corresponding Cw‐Inv inhibitor. Defective Cw‐Invs have been described in several plant species. Therefore, it would be interesting to determine whether such interactions between defective/active Cw‐Invs play a role in the defence response. Further modes of Cw‐Inv regulation are exon skipping (Bournay et al., 1996) and differential transcript formation (Cheng et al., 1999). The former study showed that the characteristic 9‐bp mini‐exon‐II of Cw‐Inv, which encodes three amino acids of the highly characteristic and conserved NDPNG/A‐motif, was lost during cold stress. The latter study reported that the maize Cw‐Inv gene Incw1 encodes two different transcripts because of the different lengths of the 3′‐untranslated region, whereas the 5′‐untranslated region and the coding sequence remain unaffected. Interestingly, it was sufficient to add the metabolizable sugars, sucrose and glucose, to heterotrophic suspension cultures to increase the abundance of the smaller transcript, resulting in increased levels of INCW1 protein/activity. Non‐metabolizable sugars increased the abundance of the larger transcript, but did not result in altered levels of INCW1 protein/activity. The authors speculated that the 3′‐untranslated region of Incw1 may contribute to different RNA processing/translation according to the presence/absence of the corresponding sugars.
The Role of Cw‐Invs in Plant–Pathogen Interactions
In addition to growth‐ and development‐related functions, Cw‐Invs play an important role during the course of plant–pathogen interactions. Numerous studies have shown an increase in Cw‐Inv expression/activity on pathogen challenge in different plant species and corresponding compatible and incompatible pathogen interactions (Table 1). During the course of the incompatible interaction of photoautotrophic tobacco leaves challenged with Phytophthora nicotianae, a metabolic shift from photosynthesis/assimilatory metabolism to a non‐assimilatory metabolism was observed. This was accompanied by an increase in Cw‐Inv activity, reduced sucrose export and an increase in respiration at the infection site. Thereby, the reduced sucrose export from infected source leaves is probably a consequence of increased sucrose cleavage by Cw‐Inv at the infection site (Scharte et al., 2005). An induction of Cw‐Inv is also observed in non‐photosynthetic tissue, as described for the interaction of tomato roots with Fusarium oxysporum. Thereby, the induction of Cw‐Inv proceeds more rapidly in incompatible plants relative to compatible plants. This induction of Cw‐Inv may be crucial to the establishment of an enhanced flow of sucrose to the infection site to feed an increased metabolic activity when defence reactions are mounted (Benhamou et al., 1991). The identification of monosaccharide transporters that are co‐regulated with Cw‐Inv by pathogen infection suggests a close relationship between apoplastic sucrose hydrolysis and hexose uptake during defence responses. Such a co‐regulation was observed in the interaction of Arabidopsis with Erysiphe cichoracearum (Fotopoulos et al., 2003), wheat with powdery mildew (Sutton et al., 2007) and grapevine with powdery and downy mildew (Hayes et al., 2010). Elevated glucose levels serve as sugar signal and regulate gene expression, as outlined below. Voegele et al. (2001) characterized a hexose transporter, HXT1, from the biotrophic rust fungus Uromyces fabae, and showed a localization of HXT1 within the haustorial complex. Moreover, during the course of the Vicia faba–U. fabae interaction, an increase in pathogen invertase gene expression in the periphery of haustoria and of host Cw‐Inv in infected leaves was observed (Voegele et al., 2006). However, the role of the pathogen‐derived Cw‐Inv and hexose transporter is not yet clear; a possible functional link to support nutrient uptake of the pathogen is still under debate. Another possibility is that the U. fabae Cw‐Inv conditions the host for source–sink transition (Voegele et al., 2006).
Table 1.
Examples of pathosystems characterized by the modulation of cell‐wall invertase (Cw‐Inv) expression/protein amount/activity during the course of infection
| Plant | Pathogen | Trophic style | Interactiona | Cw‐Inv modulationb | Reference |
|---|---|---|---|---|---|
| Barley, leaf (WT/mlo/Mla12) | Blumeria graminis f.sp. hordei | Biotrophic | C, I | A‐Ind | Swarbrick et al. (2006) |
| Wheat, leaf | Blumeria graminis f.sp. tritici | Biotrophic | C | T‐Ind, A‐Ind | Sutton et al. (2007) |
| Arabidopsis, leaf | Albugo candida | Biotrophic | C | T‐Ind, A‐Ind | Chou et al. (2000) |
| Arabidopsis, leaf | Erysiphe cichoracearum | Biotrophic | C | T‐Ind, A‐Ind | Fotopoulos et al. (2003) |
| Arabidopsis, leaf | Pseudomonas syringae pv. tomato | Hemibiotrophic | C | T‐Ind, A‐Ind | Bonfig et al. (2010) |
| Arabidopsis, root | Plasmodiophora brassicae | Biotrophic | C | T‐Ind | Siemens et al. (2011) |
| Arabidopsis, root | Heterodera schachtii | Biotrophic | C | T‐Rep/A‐Rep | Cabello et al. (2014) |
| Tobacco, leaf | Potato virus Y | C | T‐Ind/A‐Ind | Herbers et al. (2000) | |
| Tobacco, leaf | Phytophthora nicotianae | Hemibiotrophic | I | T‐Ind/A‐Ind | Scharte et al. (2005) |
| Tobacco, leaf | Phytophthora nicotianae | Hemibiotrophic | I | A‐Ind | Essmann et al. (2008) |
| Tomato, leaf | Xanthomonas campestris | Hemibiotrophic | C | A‐Ind | Kocal et al. (2008) |
| Tomato, leaf/fruit | Botrytis cinerea | Necrotrophic | C | A‐Ind/T‐Ind | Hyun et al. (2011) |
| Tomato, root | Fusarium oxysporum f.sp. radicis‐lycopersici | Hemibiotrophic | C, I | P‐Ind | Benhamou et al. (1991) |
| Tomato, root | Glomus intraradices | Symbiotic | S | T‐Ind/A‐Ind | Schaarschmidt et al. (2006) |
| Grapevine, leaf | Erysiphe necator/Plasmopora viticola | Biotrophic | C | T‐Ind, A‐Ind | Hayes et al. (2010) |
| Vicia faba, leaf | Uromyces fabae | Biotrophic | C |
T‐Ind (host) T‐Ind/P‐Ind (U. fabae) |
Voegele et al. (2006) |
| Carrot, root/leaf | Pectobacterium carotovorum | Necrotrophic | C | T‐Ind | Sturm and Chrispeels (1990) |
C, compatible interaction; I, incompatible interaction; S, symbiotic interaction.
A‐Ind, activity induction; A‐Rep, activity repression; P‐Ind, protein amount induction; T‐Ind, transcriptional induction; T‐Rep, transcriptional repression.
RNA interference (RNAi)‐mediated repression of Cw‐Inv activity in tobacco resulted in an impaired defence response following P. nicotianae infection. During the course of infection, the accumulation of apoplastic sugars was reduced/delayed in RNAi plants, the expression of pathogen‐related genes was reduced and the formation of hydrogen peroxide was weak (Essmann et al., 2008). By contrast, overexpression of a rice Cw‐Inv gene, Grain Incomplete Filling 1 (GIF1), resulted in constitutive activation of defence‐related genes, higher resistance to the bacterial pathogen Xanthomonas oryzae pv. oryzae and the fungal pathogen Magnaporthe oryzae, and higher levels of callose deposition during pathogen infection. Moreover, cell walls were thicker at the infection sites of GIF1‐overexpressing lines relative to the wild‐type (Sun et al., 2014).
A detailed analysis of invertase and sucrose synthase gene expression/activities during the interaction of Arabidopsis and the cyst nematode Heterodera schachtii revealed that several sucrose synthase isogenes were induced and cytosolic, vacuolar and Cw‐Inv genes were repressed in the infected roots. This resulted in lower cytosolic, vacuolar and Cw‐Inv activities in syncytia of the infected root, but higher levels of all three classes of invertase activity in the systemic leaf of root‐infected plants relative to non‐infected controls. This indicates a local and systemic modulation of sucrose‐cleaving enzymes following nematode infection (Cabello et al., 2014). Cw‐Inv is important in gall development during clubroot disease in the interaction of the biotrophic protist Plasmodiophora brassicae and Arabidopsis. This was demonstrated by the overexpression of invertase inhibitors in Arabidopsis roots, which resulted in reduced Cw‐Inv activity and reduced development of clubroot symptoms (Siemens et al., 2011). Transcriptional induction of a tomato Cw‐Inv was also observed in a symbiotic plant–microbe interaction during the course of the mycorrhization of tomato roots (Schaarschmidt et al., 2006).
Recently, a pepper Cw‐Inv was shown to be a target of the Xanthomonas campestris effector XopB. Cw‐Inv repression by XopB could interfere with sugar‐mediated defence responses during infection (Sonnewald et al., 2012). The fact that pathogens evolved effectors targeting the host Cw‐Inv underlines the importance of Cw‐Inv regulation in the defence response.
Finally, pathogen‐derived extracellular invertases and sugar metabolism add to the complexity of metabolic regulation in plant–pathogen interactions. An increase in host and pathogen invertase gene expression was observed in the V. faba–U. fabae interaction (Voegele et al., 2006). Moreover, a fungal invertase plays a role in the grapevine–Botrytis cinerea (Ruiz and Ruffner, 2002) and sunflower–Sclerotinia sclerotiorum (Jobic et al., 2007) interactions. A model illustrating a possible function and regulation of Cw‐Inv in plant–pathogen interactions is shown in Fig. 1.
Figure 1.
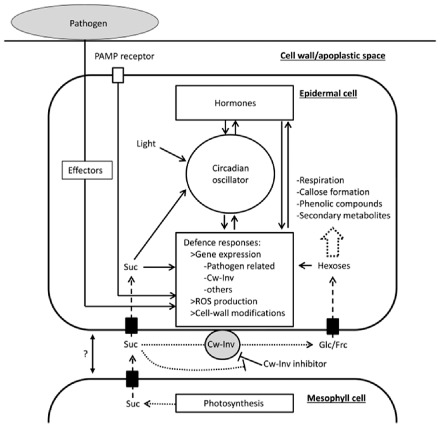
Function and regulation of cell‐wall invertase (Cw‐Inv) in plant–pathogen interactions (exemplified for fungal/bacterial leaf pathogens). Cw‐Inv cleaves sucrose (Suc), the major transport sugar, yielding glucose (Glc) and fructose (Frc). Pathogen challenge leads to an induction of Cw‐Inv expression or de‐repression of Cw‐Inv activity, mediated by the down‐regulation of proteinaceous Cw‐Inv inhibitors, resulting in enhanced sucrose cleavage. After uptake, hexoses are further metabolized to support respiration or the synthesis of secondary metabolites, notably the formation of callose or phenolic compounds. In addition, sucrose and hexoses act as signalling molecules. Therefore, Cw‐Inv locates at an integration point of hormonal, sugar, defence and diurnal/circadian regulation. The latter is a newly emerging aspect of Cw‐Inv regulation and could be crucial to the coordination of diurnal growth patterns and defence reactions. Moreover, Cw‐Inv is a target of pathogen effectors, underlining the importance of Cw‐Inv in the defence response. Black boxes represent sugar transporters. Dotted arrows depict metabolic reactions, broken arrows sugar transport and full arrows signalling interactions. PAMP, pathogen‐associated molecular pattern; ROS, reactive oxygen species.
The Role of Cw‐Inv in the Integration of Sugar and Defence Signalling
Soluble sugars, mostly hexoses and sucrose, not only provide energy and carbon resources, but also act as signalling molecules (Jang and Sheen, 1997; Koch, 1996; Rolland et al., 2006; Wind et al., 2010). The role of hexokinase in plant sugar signalling is well established (Jang and Sheen, 1997; Xiao et al., 2000). In addition to hexokinase, further sugar‐sensing pathways have been described: glucose sensing at the plasma membrane via a G‐protein‐coupled pathway (Grigston et al., 2008), sugar sensing via Snf‐related protein kinases (Baena‐González et al., 2007) and the possibility of hexose sensing in the secretory pathway (Herbers et al., 1996). Sucrose signalling is poorly understood; yet, a recent study describes a sucrose‐induced receptor kinase in Arabidopsis (Wu et al., 2013). There is evidence for sucrose acting as a signalling molecule in plant defence (Gómez‐Ariza et al., 2007). Sugar‐mediated transcriptional regulation of Cw‐Inv is widely observed and, in most reports, Cw‐Inv expression is induced by sugars. Glucose and sucrose increase the level of a Cw‐Inv of pea (Zhang et al., 1997), Chenopodium rubrum (Roitsch et al., 1995) and tomato (Proels and Roitsch, 2009). Sugar‐induced expression of Cw‐Inv is of particular interest, as this mechanism allows for the maintenance or amplification of the initial induction of invertase via feed‐forward regulation (Roitsch et al., 2000). Moreover, sucrose cleavage by Cw‐Inv alters the ratio of sucrose to hexoses in the apoplast, and can therefore actively modulate sugar signalling. Sugar partitioning can be altered by SWEET‐type sugar transporters, which are targeted by bacterial effectors (Chen et al., 2010). SWEETs may not only be relevant to support pathogen growth, but may also interfere with sugar signalling. Increasing glucose levels result in a repression of photosynthetic gene expression and an induced expression of pathogenesis‐related (PR) genes (Berger et al., 2007; Biemelt and Sonnewald, 2006). The down‐regulation of photosynthesis gene expression, most probably via hexose signalling, is a central feature following pathogen attack (Bischof et al., 2011; Bonfig et al., 2006; Chou et al., 2000; Swarbrick et al., 2006). The impaired photosynthetic activity in infected tissue amplifies metabolic source–sink transitions. A number of defence‐related genes have been found to be induced by soluble sugars (Gómez‐Ariza et al., 2007; Herbers et al., 1996). Overexpression of a yeast invertase in the vacuole or apoplast of tobacco results in the constitutive induction of defence‐related genes, elevated callose content and peroxidase activities (Herbers et al., 1996). Infection of tobacco plants with Potato virus Y leads to the inhibition of sugar export, induction of defence responses and a higher resistance towards viral attack (Herbers et al., 2000). Based on these findings, Herbers et al. (2000) proposed a model of sugar‐enhanced plant defence.
Possible Roles of Cw‐Invs as Targets and Modulators of Circadian Regulation
Plants must anticipate light/dark cycles for optimal photosynthetic performance. To this end, they possess a sophisticated molecular machinery, the circadian clock, representing an internal timing mechanism consisting of autoregulatory interlocked transcriptional feedback loops (Carré and Veflingstad, 2013). The circadian clock can anticipate the 24‐h periodicity of the day length and regulate physiological responses, such as plant growth and development. Circadian regulation interplays with other stimuli, such as hormones and sugars. Interestingly, among the sugar signals, it is mostly sucrose which affects the circadian clock (Dalchau et al., 2011). Moreover, Haydon et al. (2013) have shown that endogenous sugars derived from photosynthesis can entrain the circadian clock. An overview of the interconnection of light and sugar signals, the clock and immune responses has been provided by Roden and Ingle (2009) and Bolouri Moghaddam and Van den Ende (2013). Here, we briefly state the role of the circadian clock in regulating defence responses and discuss a possible connection of the circadian clock and Cw‐Inv. Arabidopsis plants are least susceptible to Pseudomonas syringae pv. tomato DC3000 infection in the subjective morning, and PAMP receptors and PAMP‐triggered callose deposition is higher at this time of the day. In arrhythmic plants, no such temporal differences were observed (Bhardwaj et al., 2011). Wang et al. (2011) demonstrated that CCA1 controls resistance (R)‐gene‐mediated resistance in Arabidopsis against downy mildew. Thereby, the circadian control of R genes allows for the anticipation of infection when pathogen challenge is highest—in this pathosystem, as a result of a diurnal pattern of spore dispersion. Leaf sucrose levels and invertase expression have been shown to follow a diurnal or circadian expression profile (Bläsing et al., 2005; Ruts et al., 2012). A tomato Cw‐Inv, Lin6, has been reported to follow a diurnal cycle in photoautotrophic cell cultures. Furthermore, in a heterologous expression system, it has been shown that CCA1 and LHY, transcription factors acting in the core of the Arabidopsis circadian clock, activate the Lin6 promoter (Proels and Roitsch, 2009). With regard to the central role of Cw‐Invs in the defence response, a circadian/diurnal regulation of Cw‐Inv could be crucial to coordinate source–sink transitions during diurnal growth patterns and defence reactions. Thereby, Cw‐Inv‐mediated changes in the sucrose to hexose level might modulate sugar signalling.
Concluding Remarks
Cw‐Invs play a central role in the carbohydrate supply of sink tissues and the regulation of source–sink transitions. This places Cw‐Inv in the centre of metabolic regulations during the course of plant–pathogen interactions, which are characterized by a profound reorganization of metabolism to meet an increased metabolic activity. Cw‐Invs are regulated by sugar‐, hormone‐ and stress‐related signals and do not only serve as metabolic enzymes, but can generate sugar‐based signals themselves via the modulation of sucrose/hexose ratios. Hence, several signals converge at the site of Cw‐Inv regulation. Cw‐Inv‐generated sugar signals, in turn, can modulate the gene expression of pathogen‐related or metabolic genes. Therefore, Cw‐Inv acts at an integration point of hormonal, sugar, defence and diurnal/circadian regulation. The latter is a newly emerging aspect of Cw‐Inv regulation and could be crucial to the coordination of diurnal growth patterns and defence reactions. Epidermal cells, which often come into contact with pathogens first and mount the initial layer of defence (Fig. 1), are characterized, in most plants, by an absence of chloroplasts, and thus depend on sucrose import from neighbouring mesophyll cells. Hence, there should be communication and carbon flux between epidermal and mesophyll cells to allow for an appropriate defence response. In most studies on plant–pathogen interactions, a dissection of epidermal and mesophyll cells has not been addressed. For a comprehensive understanding of metabolic reprogramming in defence reactions, it would be interesting to dissect epidermal and adjacent mesophyll cells and analyse underlying signalling events after pathogen attack.
Acknowledgements
Experimental work in the laboratory of R.H. is supported by the German Research Foundation, the Federal Ministry of Education and Research and the Helmholtz Society portfolio topic ‘Sustainable Bioeconomy’.
References
- Baena‐González, E. , Rolland, F. , Thevelein, J.M. and Sheen, J. (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature, 448, 938–942. [DOI] [PubMed] [Google Scholar]
- Benhamou, N. , Grenier, J. and Chrispeels, M.J. (1991) Accumulation of β‐fructosidase in the cell walls of tomato roots following infection by a fungal wilt pathogen. Plant Physiol. 97, 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, S. , Sinha, A.K. and Roitsch, T. (2007) Plant physiology meets phytopathology: plant primary metabolism and plant‐pathogen interactions. J. Exp. Bot. 58, 4019–4026. [DOI] [PubMed] [Google Scholar]
- Bhardwaj, V. , Meier, S. , Petersen, L.N. , Ingle, R.A. and Roden, L.C. (2011) Defence responses of Arabidopsis thaliana to infection by Pseudomonas syringae are regulated by the circadian clock. PLoS ONE, 6, e26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemelt, S. and Sonnewald, U. (2006) Plant–microbe interactions to probe regulation of plant carbon metabolism. J. Plant Physiol. 163, 307–318. [DOI] [PubMed] [Google Scholar]
- Bischof, M. , Eichmann, R. and Hückelhoven, R. (2011) Pathogenesis‐associated transcriptional patterns in Triticeae. J. Plant Physiol. 168, 9–19. [DOI] [PubMed] [Google Scholar]
- Bläsing, O.E. , Gibon, Y. , Günther, M. , Höhne, M. , Morcuende, R. , Osuna, D. , Thimm, O. , Usadel, B. , Scheible, W.‐R. and Stitt, M. (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis . Plant Cell, 17, 3257–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri Moghaddam, M.R. and Van den Ende, W. (2013) Sweet immunity in the plant circadian regulatory network. J. Exp. Bot. 64, 1439–1449. [DOI] [PubMed] [Google Scholar]
- Bonfig, K.B. , Schreiber, U. , Gabler, A. , Roitsch, T. and Berger, S. (2006) Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta, 225, 1–12. [DOI] [PubMed] [Google Scholar]
- Bonfig, K.B. , Gabler, A. , Simon, U.K. , Luschin‐Ebengreuth, N. , Hatz, M. , Berger, S. , Muhammad, N. , Zeier, J. , Sinha, A.K. and Roitsch, T. (2010) Post‐translational derepression of invertase activity in source leaves via down‐regulation of invertase inhibitor expression is part of the plant defense response. Mol. Plant, 3, 1037–1048. [DOI] [PubMed] [Google Scholar]
- Bournay, A.‐S. , Hedley, P.E. , Maddison, A. , Waugh, R. and Machray, G.C. (1996) Exon skipping induced by cold stress in a potato invertase gene transcript. Nucleic Acid Res. 24, 2347–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner, M. and Sauer, N. (2000) Monosaccharide transporters in plants: structure, function and physiology. Biochim. Biophys. Acta, 1465, 263–274. [DOI] [PubMed] [Google Scholar]
- Cabello, S. , Lorenz, C. , Crespo, S. , Cabrera, J. , Ludwig, R. , Escobar, C. and Hofmann, J. (2014) Altered sucrose synthase and invertase expression affects the local and systemic sugar metabolism of nematode‐infected Arabidopsis thaliana plants. J. Exp. Bot. 65, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré, I. and Veflingstad, S.R. (2013) Emerging design principles in the Arabidopsis circadian clock. Semin. Cell Dev. Biol. 24, 393–398. [DOI] [PubMed] [Google Scholar]
- Chen, L.‐Q. , Hou, B.‐H. , Lalonde, S. , Takanaga, H. , Hartung, M.L. , Qu, X.‐Q. , Guo, W.‐J. , Kim, J.‐G. , Underwood, W. , Chaudhuri, B. , Chermak, D. , Antony, G. , White, F.F. , Somerville, S.C. , Mudgett, M.B. and Frommer, W.B. (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature, 468, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, W.‐H. , Taliercio, E.W. and Chourey, P.S. (1999) Sugars modulate an unusual mode of control of the cell‐wall invertase gene (Incw1) through its 3′ untranslated region in a cell suspension culture of maize. Proc. Natl. Acad. Sci. USA, 96, 10 512–10 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, J.‐I. , Lee, S.‐K. , Ko, S. , Kim, H.‐K. , Jun, S.‐H. , Lee, Y.‐H. , Bhoo, S.H. , Lee, K.‐W. , An, G. , Hahn, T.‐R. and Jeon, J.‐S. (2005) Molecular cloning and expression analysis of the cell‐wall invertase gene family in rice (Oryza sativa L.). Plant Cell Rep. 24, 225–236. [DOI] [PubMed] [Google Scholar]
- Chou, H.‐M. , Bundock, N. , Rolfe, S.A. and Scholes, J.D. (2000) Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol. Plant Pathol. 1, 99–113. [DOI] [PubMed] [Google Scholar]
- Dalchau, N. , Baek, S.J. , Briggs, H.M. , Robertson, F.C. , Dodd, A.N. , Gardner, M.J. , Stancombe, M.A. , Haydon, M.J. , Stan, G.‐B. , Gonçalves, J.M. and Webb, A.A.R. (2011) The circadian oscillator gene GIGANTEA mediates a long‐term response of the Arabidopsis thaliana circadian clock to sucrose. Proc. Natl. Acad. Sci. USA, 108, 5104–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehneß, R. and Roitsch, T. (1997) Co‐ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J. 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Essmann, J. , Schmitz‐Thom, I. , Schön, H. , Sonnewald, S. , Weis, E. and Scharte, J. (2008) RNA interference‐mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol. 147, 1288–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulos, V. , Gilbert, M.J. , Pittman, J.K. , Marvier, A.C. , Buchanan, A.J. , Sauer, N. , Hall, J.L. and Williams, L.E. (2003) The monosaccharide transporter gene, AtSTP4, and the cell‐wall invertase, Atβfruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum . Plant Physiol. 132, 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook, J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Gómez‐Ariza, J. , Campo, S. , Rufat, M. , Estopà, M. , Messeguer, J. , San Segundo, B. and Coca, M. (2007) Sucrose‐mediated priming of plant defence responses and broad‐spectrum disease resistance by overexpression of the maize pathogenesis‐related PRms protein in rice plants. Mol. Plant–Microbe Interact. 20, 832–842. [DOI] [PubMed] [Google Scholar]
- Grigston, J.C. , Osuna, D. , Scheible, W.‐R. , Liu, C. , Stitt, M. and Jones, A.M. (2008) d‐glucose sensing by a plasma membrane regulator of G signaling protein, AtRGS1. FEBS Lett. 582, 3577–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon, M.J. , Mielczarek, O. , Robertson, F.C. , Hubbard, K.E. and Webb, A.A.R. (2013) Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature, 502, 689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, M.A. , Feechan, A. and Dry, I.B. (2010) Involvement of abscisic acid in the coordinated regulation of a stress‐inducible hexose transporter (VvHT5) and a cell wall invertase in grapevine in response to biotrophic fungal infection. Plant Physiol. 153, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers, K. , Meuwly, P. , Frommer, W.B. , Métraux, J.‐P. and Sonnewald, U. (1996) Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell, 8, 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers, K. , Takahata, Y. , Melzer, M. , Mock, H.‐P. , Hajirezaei, M. and Sonnewald, U. (2000) Regulation of carbohydrate partitioning during the interaction of potato virus Y with tobacco. Mol. Plant Pathol. 1, 51–59. [DOI] [PubMed] [Google Scholar]
- Hothorn, M. , Van den Ende, W. , Lammens, W. , Rybin, V. and Scheffzek, K. (2010) Structural insights into the pH‐controlled targeting of plant cell‐wall invertase by a specific inhibitor protein. Proc. Natl. Acad. Sci. USA, 107, 17 427–17 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun, T.K. , Eom, S.H. , Rim, Y. and Kim, J.‐S. (2011) Alteration of the expression and activation of tomato invertases during Botrytis cinerea infection. Plant Omics J. 4, 413–417. [Google Scholar]
- Jang, J.‐C. and Sheen, J. (1997) Sugar sensing in higher plants. Trends Plant Sci. 2, 208–214. [Google Scholar]
- Jobic, C. , Boisson, A.‐M. , Gout, E. , Rascle, C. , Fèvre, M. , Cotton, P. and Bligny, R. (2007) Metabolic processes and carbon nutrient exchanges between host and pathogen sustain the disease development during sunflower infection by Sclerotinia sclerotiorum . Planta, 226, 251–265. [DOI] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kocal, N. , Sonnewald, U. and Sonnewald, S. (2008) Cell wall‐bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv vesicatoria . Plant Physiol. 148, 1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, K. (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 7, 235–246. [DOI] [PubMed] [Google Scholar]
- Koch, K.E. (1996) Carbohydrate‐modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 509–540. [DOI] [PubMed] [Google Scholar]
- Le Roy, K. , Vergauwen, R. , Struyf, T. , Yuan, S. , Lammens, W. , Mátrai, J. , De Maeyer, M. and Van den Ende, W. (2013) Understanding the role of defective invertases in plants: tobacco Nin88 fails to degrade sucrose. Plant Physiol. 161, 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proels, R.K. and Roitsch, T. (2009) Extracellular invertase LIN6 of tomato: a pivotal enzyme for the integration of metabolic, hormonal, and stress signals is regulated by a diurnal rhythm. J. Exp. Bot. 60, 1555–1567. [DOI] [PubMed] [Google Scholar]
- Rausch, T. and Greiner, S. (2004) Plant protein inhibitors of invertases. Biochim. Biophys. Acta, 1696, 253–261. [DOI] [PubMed] [Google Scholar]
- Roden, L.C. and Ingle, R.A. (2009) Lights, Rhythms, Infection: the role of light and the circadian clock in determining the outcome of plant–pathogen interactions. Plant Cell, 21, 2546–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch, T. , Bittner, M. and Godt, D.E. (1995) Induction of apoplastic invertase of Chenopodium rubrum by d‐glucose and a glucose analogue and tissue‐specific expression suggest a role in sink–source regulation. Plant Physiol. 108, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch, T. , Ehneß, R. , Goetz, M. , Hause, B. , Hofmann, M. and Sinha, A.K. (2000) Regulation and function of extracellular invertase from higher plants in relation to assimilate partitioning, stress responses and sugar signalling. Aust. J. Plant Physiol. 27, 815–825. [Google Scholar]
- Roitsch, T. , Balibrea, M.E. , Hofmann, M. , Proels, R. and Sinha, A.K. (2003) Extracellular invertase: key metabolic enzyme and PR protein. J. Exp. Bot. 54, 513–524. [DOI] [PubMed] [Google Scholar]
- Rolland, F. , Baena‐Gonzalez, E. and Sheen, J. (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu. Rev. Plant Biol. 57, 675–709. [DOI] [PubMed] [Google Scholar]
- Ruiz, E. and Ruffner, H.P. (2002) Immunodetection of Botrytis‐specific invertase in infected grapes. J. Phytopathol. 150, 76–85. [Google Scholar]
- Ruts, T. , Matsubara, S. , Wiese‐Klinkenberg, A. and Walter, A. (2012) Diel patterns of leaf and root growth: endogenous rhythmicity or environmental response? J. Exp. Bot. 63, 3339–3351. [DOI] [PubMed] [Google Scholar]
- Schaarschmidt, S. , Roitsch, T. and Hause, B. (2006) Arbuscular mycorrhiza induces gene expression of the apoplastic invertase LIN6 in tomato (Lycopersicon esculentum) roots. J. Exp. Bot. 57, 4015–4023. [DOI] [PubMed] [Google Scholar]
- Scharte, J. , Schön, H. and Weis, E. (2005) Photosynthesis and carbohydrate metabolism in tobacco leaves during an incompatible interaction with Phytophthora nicotianae . Plant Cell Environ. 28, 1421–1435. [Google Scholar]
- Schwessinger, B. and Ronald, P.C. (2012) Plant innate immunity: perception of conserved microbial signatures. Annu. Rev. Plant Biol. 63, 451–482. [DOI] [PubMed] [Google Scholar]
- Sherson, S.M. , Alford, H.L. , Forbes, S.M. , Wallace, G. and Smith, S.M. (2003) Roles of cell‐wall invertases and monosaccharide transporters in the growth and development of Arabidopsis . J. Exp. Bot. 54, 525–531. [DOI] [PubMed] [Google Scholar]
- Siemens, J. , Gonzalez, M.‐C. , Wolf, S. , Hofmann, C. , Greiner, S. , Du, Y. , Rausch, T. , Roitsch, T. and Ludwig‐Müller, J. (2011) Extracellular invertase is involved in the regulation of clubroot disease in Arabidopsis thaliana . Mol. Plant Pathol. 12, 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald, S. , Priller, J.P.R. , Schuster, J. , Glickmann, E. , Hajirezaei, M.‐R. , Siebig, S. , Mudgett, M.B. and Sonnewald, U. (2012) Regulation of cell wall‐bound invertase in pepper leaves by Xanthomonas campestris pv. vesicatoria type three effectors. PLoS ONE, 7, e51763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm, A. and Chrispeels, M.J. (1990) cDNA cloning of carrot extracellular β‐fructosidase and its expression in response to wounding and bacterial infection. Plant Cell, 2, 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm, A. and Tang, G.‐Q. (1999) The sucrose‐cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci. 4, 401–407. [DOI] [PubMed] [Google Scholar]
- Sun, L. , Yang, D.‐L. , Kong, Y. , Chen, Y. , Li, X.‐Z. , Zeng, L.‐J. , Li, Q. , Wang, E.‐T. and He, Z.‐H. (2014) Sugar homeostasis mediated by cell wall invertase Grain Incomplete Filling 1 (GIF1) plays a role in pre‐existing and induced defence in rice. Mol. Plant Pathol. 15, 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, P.N. , Gilbert, M.J. , Williams, L.E. and Hall, J.L. (2007) Powdery mildew infection of wheat leaves changes host solute transport and invertase activity. Physiol. Plant. 129, 787–795. [Google Scholar]
- Swarbrick, P.J. , Schulze‐Lefert, P. and Scholes, J.D. (2006) Metabolic consequences of susceptibility and resistance (race‐specific and broad‐spectrum) in barley leaves challenged with powdery mildew. Plant Cell Environ. 29, 1061–1076. [DOI] [PubMed] [Google Scholar]
- Voegele, R.T. , Struck, C. , Hahn, M. and Mendgen, K. (2001) The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae . Proc. Natl. Acad. Sci. USA, 98, 8133–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegele, R.T. , Wirsel, S. , Möll, U. , Lechner, M. and Mendgen, K. (2006) Cloning and characterization of a novel invertase from the obligate biotroph Uromyces fabae and analysis of expression patterns of host and pathogen invertases in the course of infection. Mol. Plant–Microbe Interact. 19, 625–634. [DOI] [PubMed] [Google Scholar]
- Wang, W. , Barnaby, J.Y. , Tada, Y. , Li, H. , Tör, M. , Caldelari, D. , Lee, D.‐U. , Fu, X.‐D. and Dong, X. (2011) Timing of plant immune responses by a central circadian regulator. Nature, 470, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschke, W. , Panitz, R. , Gubatz, S. , Wang, Q. , Radchuk, R. , Weber, H. and Wobus, U. (2003) The role of invertases and hexose transporters in controlling sugar ratios in maternal and filial tissues of barley caryopses during early development. Plant J. 33, 395–411. [DOI] [PubMed] [Google Scholar]
- Wind, J. , Smeekens, S. and Hanson, J. (2010) Sucrose: metabolite and signalling molecule. Phytochemistry, 71, 1610–1614. [DOI] [PubMed] [Google Scholar]
- Wu, X.N. , Rodriguez, C.S. , Pertl‐Obermeyer, H. , Obermeyer, G. and Schulze, W.X. (2013) Sucrose‐induced receptor kinase SIRK1 regulates a plasma membrane aquaporin in Arabidopsis. Mol. Cell. Proteomics, 12, 2856–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, W. , Sheen, J. and Jang, J.‐C. (2000) The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol. Biol. 44, 451–461. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Cohn, N.S. and Mitchell, J.P. (1997) A pea cell‐wall invertase gene (PsInv‐1) with tissue‐specific expression. Plant Physiol. Biochem. 35, 751–760. [Google Scholar]


