Summary
Plant‐parasitic cyst nematodes induce the formation of a multinucleated feeding site in the infected root, termed the syncytium. Recent studies point to key roles of the phytohormone auxin in the regulation of gene expression and establishment of the syncytium. Nevertheless, information about the spatiotemporal expression patterns of the transcription factors that mediate auxin transcriptional responses during syncytium formation is limited. Here, we provide a gene expression map of 22 auxin response factors (ARFs) during the initiation, formation and maintenance stages of the syncytium induced by the cyst nematode Heterodera schachtii in Arabidopsis. We observed distinct and overlapping expression patterns of ARFs throughout syncytium development phases. We identified a set of ARFs whose expression is predominantly located inside the developing syncytium, whereas others are expressed in the neighbouring cells, presumably to initiate specific transcriptional programmes required for their incorporation within the developing syncytium. Our analyses also point to a role of certain ARFs in determining the maximum size of the syncytium. In addition, several ARFs were found to be highly expressed in fully developed syncytia, suggesting a role in maintaining the functional phenotype of mature syncytia. The dynamic distribution and overlapping expression patterns of various ARFs seem to be essential characteristics of ARF activity during syncytium development.
Keywords: Arabidopsis, auxin response factors, cyst nematodes, gene expression, Heterodera schachtii, syncytium
The phytohormone auxin is a key signalling molecule regulating a wide range of growth and developmental processes. These crucial processes include, for example, apical dominance, root development, vascular differentiation, shoot elongation and embryo patterning (Benjamins and Scheres, 2008; Zhao, 2010). In addition, auxin regulates various cellular processes that are associated with the plant response to biotic stresses (Kazan and Manners, 2009). Consistent with its crucial role in cell differentiation and morphogenesis, it is widely believed that auxin plays key roles in the initiation and formation of the syncytium induced by plant‐parasitic cyst nematodes (for reviews, see Gheysen and Mitchum, 2011 and Grunewald et al., 2009b). Syncytium formation is a very sophisticated process that involves proteins from both nematode (effectors) and host cells that co‐operatively transform normal root cells into the syncytium cell fate type (Hewezi and Baum, 2013). The nematode first initiates contact with a single competent cell, known as an initial feeding cell (IFC). This contact is translated into dramatic cellular modifications, including endoreduplication, cell wall modification and dissolution, disappearance of large vacuoles, and an increased number of organelles and metabolic activity (Golinowski et al., 1996). Remarkably, these modifications are also stimulated in neighbouring cells, which gradually fuse with each other and with the IFC. The successive cell‐to‐cell fusion of several hundred cells surrounding the IFC results in the formation of one large multinucleated syncytium as a novel plant structure. Several lines of evidence point to fundamental roles of auxin in syncytium induction and formation. For example, the synthetic auxin response promoter DR5 has been found to be activated in the developing syncytia on cyst nematode infection (Absmanner et al., 2013; Grunewald et al., 2009a; Karczmarek et al., 2004), demonstrating a rapid increase in auxin concentration. In addition, several auxin response or auxin transport mutants show aberrant feeding cell morphogenesis and structure (Goverse et al., 2000; Grunewald et al., 2009a). Furthermore, a role of auxin in the reprogramming of root cells adjacent to the IFC prior to their merging with the developing syncytium has also been suggested (Goverse et al., 2000; Grunewald et al., 2009a; Karczmarek et al., 2004). Although a local increase in auxin concentration in the developing syncytium and neighbouring cells is expected to activate auxin‐dependent transcriptional programmes, little is known about the auxin signalling that mediates auxin transcriptional responses during syncytium induction and development. Three main components of the auxin signalling pathway have been identified through forward and reverse genetics approaches. These include transport inhibitor response1/auxin‐binding F‐box proteins (TIR1/AFB) auxin receptors, auxin/indole‐3‐acetic acid protein (Aux/IAA) inhibitors and auxin response factors (ARFs) cis‐acting transcription factors (reviewed by Chapman and Estelle, 2009). In Arabidopsis, ARFs are encoded by a large gene family containing 22 members and one truncated gene (ARF23), which contains a stop codon in its DNA‐binding domain and is most probably a pseudogene (Guilfoyle and Hagen, 2007; Remington et al., 2005). These transcription factors bind specifically to auxin‐responsive cis‐acting elements that are frequently found in the promoters of early auxin‐responsive genes, thereby regulating their expression. Although a set of ARFs has been identified as differentially expressed in the syncytium induced by the cyst nematode Heterodera schachtii in Arabidopsis roots (Szakasits et al., 2009), information about the spatiotemporal expression patterns of ARFs during syncytium formation is limited. Here, we describe the spatial expression patterns of all functional ARFs during the initiation, formation and maintenance stages of the syncytium induced by the sugar beet cyst nematode H. schachtii in Arabidopsis thaliana.
Seeds from 44 independent homozygous T3 Arabidopsis lines expressing promoter–green fluorescent protein (GFP) constructs of 22 ARFs (ARF1–22) in the Col‐0 background (Rademacher et al., 2011) were planted, and 10‐day‐old seedlings were inoculated with approximately 200 surface‐sterilized, second‐stage juvenile (J2) nematodes per plant, as described previously by Hewezi et al. (2012). The specific promoter activity of these 44 transgenic lines (two lines per ARF construct) was visualized by GFP fluorescence at different time points after H. schachtii infection. All microscopic observations were carried out with a 10× objective on an AxioObserver Z1 (Zeiss, Carl Zeiss Imaging Solutions GmbH, Gottingen, Germany) with a GFP filter set for fluorescent observation (filter set 38 HE, Zeiss). Images were captured with a digital CCD camera (ORCA‐ER, Hamamatsu, Japan) controlled by Openlab software (Improvision, Perkin‐Elmer, Waltham, MA, USA). The time points were carefully chosen to reflect different phases of syncytium development (i.e. initiation, formation and maintenance) (Hewezi et al., 2012). At 2–3 days post‐infection (dpi), the developing syncytia induced by the parasitic J2 stage were structurally distinguishable, wherein several cells surrounding the nematode head were interconnected through cell‐to‐cell fusion. Mapping the expression of the 22 ARFs during the early stage of syncytium formation revealed a high sensitivity of this gene family of transcription factors to nematode infection. With the exception of ARF8, which showed little response to nematode infection, all other ARFs exhibited distinct and overlapping expression patterns. One interesting expression pattern observed was the strong expression of 10 ARFs (ARF3, 6, 10–12, 14, 15 and 20–22) inside the young syncytium, but not in the adjacent cells that would be incorporated into the syncytium at a later stage (Fig. 1). The enhanced activity of these ARFs inside the young syncytia apparently indicates a significant increase in the local accumulation of auxin on nematode infection. These results are consistent with previous studies, in which an increase in auxin accumulation in young syncytia was demonstrated using the synthetic auxin responsive promoter DR5 (Absmanner et al., 2013; Grunewald et al., 2009a; Karczmarek et al., 2004). Because the expression of these ARFs is limited to syncytium, their activities may be involved in the transcriptional regulation of genes involved in specific developmental programmes that are restricted to the syncytial cells. Local accumulation of auxin in developing syncytium that mediates the activation of these ARFs could be a result of the simultaneous activation of the auxin import machinery and inhibition of the auxin export machinery. In support of this suggestion, AUX1‐mediated auxin import was found to be highly up‐regulated in young syncytia (Mazarei et al., 2003), whereas PIN1‐mediated auxin export was down‐regulated (Grunewald et al., 2009a).
Figure 1.
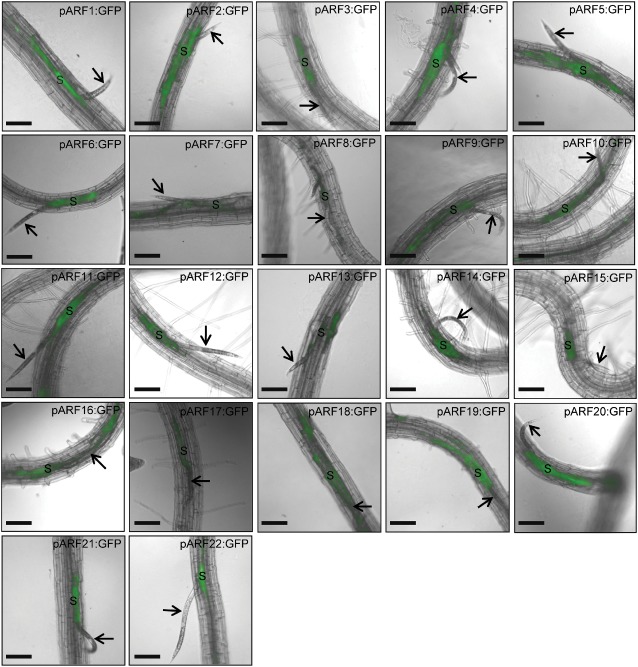
Promoter activity of auxin response factor (ARF) genes visualized by green fluorescent protein (GFP) fluorescence in transgenic Arabidopsis roots at the sedentary second juvenile life stage, 2–3 days after Heterodera schachtii infection. The spatial expression patterns of 22 ARFs were analysed and compared during the syncytium initiation phase. ARF3, 6, 10–12, 14, 15 and 20–22 are expressed inside the developing syncytium. ARF1, 2, 4, 5, 9, 18 and 19 are active in both the syncytial cells and neighbouring cells. ARF7 and 17 are mainly expressed at the edge of the syncytial and neighbouring cells. ARF8 and 16 show a weak response to H. schachtii infection. Arrows point to nematode, and ‘S’ indicates syncytium. Bars, 130 μm.
It has been suggested frequently that cells surrounding the expanding syncytium also experience a gradual and transient increase in auxin concentration (Goverse et al., 2000; Grunewald et al., 2009b). However, ARFs mediating gene expression changes in response to the gradual increase in auxin concentration in neighbouring cells are completely unknown. Here, we found that ARF1, 2, 4, 5, 9, 18 and 19 were strongly activated in both syncytial cells and adjacent cells, which are about to be incorporated into the developing syncytium at a later stage. Notably, the expression of some of these ARFs was mainly localized towards the cell edges, as in the case of ARF4, 5 and 19 (Fig. 1). Interestingly, ARF7 and 17 showed a very similar expression pattern in which the GFP signals were restricted to the edge of the syncytial and neighbouring cells (Fig. 1). These spatial expression patterns may point to the functional localization of ARF‐regulated genes, and are consistent with the finding that several nematode‐regulated genes that function in cell wall modification contain auxin response elements (reviewed by Goverse and Bird, 2011). In addition, the auxin influx transporter LAX3, which activates several cell wall‐modifying enzymes (Swarup et al., 2008), was also found to be induced in the cells surrounding the growing feeding sites (Lee et al., 2011). Therefore, it is tempting to speculate that LAX3‐dependent auxin signalling, which functions in remodelling the cell walls of neighbouring cells, could involve the activity of ARF1, 2, 4, 5, 9, 18 and 19 in general, and ARF7 and 17 in particular. Two lines of evidence support this speculation. First, mutations in two of the above‐mentioned ARFs (ARF7 and 19) completely abolished the induction of LAX3 mRNA after auxin treatment (Swarup et al., 2008). Second, of the six cell wall‐remodelling genes that have been reported to be regulated by LAX3 (Swarup et al., 2008), we were able to identify AuxRE in the promoters of three genes, including pectate lyase (PLA2), xyloglucan:xyloglucosyl transferase (XTR6) and glycosyl hydrolase (GLH17). Careful examination of the expression profiles of ARFs at the 2–3‐dpi time points revealed redundant and non‐redundant expression patterns of ARFs (Table S1, see Supporting Information). For example, ARF5 and 7 are both expressed on the edge of neighbouring cells, but only ARF5 is expressed inside the IFCs (Fig. 1). Interestingly, ARF5 and 7 were found to function redundantly in oriented cell differentiation and jointly in the control of auxin‐responsive cell expansion (Hardtke et al., 2004). We tentatively conclude that specific and overlapping responses of ARFs to local increases in auxin concentration at the early stage of syncytium formation correlate with the role of auxin in shaping the identity of syncytial cells as a new cell type by directing auxin‐responsive genes towards new developmental signalling pathways. In addition, the activation of several ARFs in the cells adjacent to the initial feeding site as early as 2 dpi (Fig. S1, see Supporting Information) suggests that auxin‐dependent gene expression changes take place early on in these cells before they are recruited as part of the syncytium.
We next profiled the spatial activity of ARFs during the early third‐stage juvenile (J3) stage of infection (5–6 dpi) to determine whether the spatial distribution and activity patterns of ARFs are extended to include new cells or merely to maintain the expression patterns observed during the syncytium initiation phase (2–3 dpi). We found that ARF3, 11 and 20–22 maintained their spatial expression pattern observed in the syncytium at 2–3 dpi, which is restricted to the IFCs. One remarkable difference was that GFP signals became stronger relative to the earlier time point. This suggests that these ARFs are associated with transcriptional programmes that are selectively related to the central syncytial cells. Interestingly, five ARFs (ARF6, 10, 12, 14 and 15), which primarily showed activity only in the IFCs, displayed an extended expression pattern to include both initial and recently incorporated syncytium cells (Fig. 2). These five ARFs seem to be required to mediate auxin‐dependent gene expression changes only in the cells that are part of the syncytium.
Figure 2.
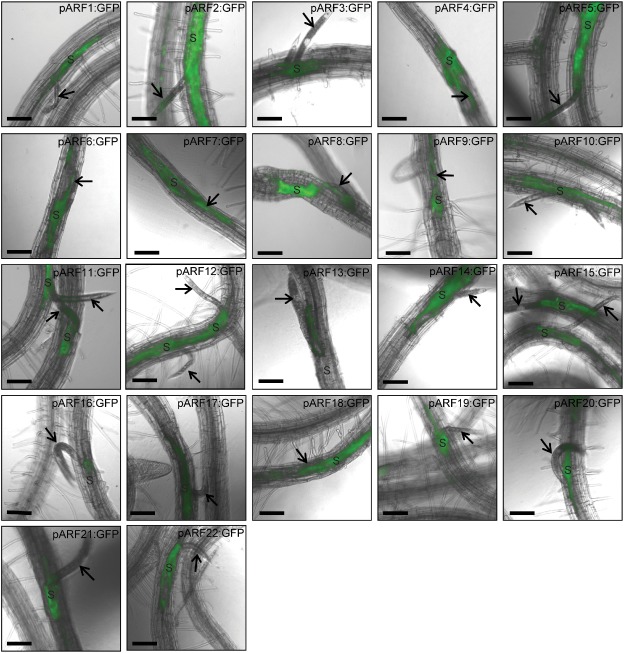
Promoter activity of auxin response factor (ARF) genes visualized by green fluorescent protein (GFP) fluorescence in transgenic Arabidopsis roots at the early third juvenile life stage, 5–6 days after Heterodera schachtii infection. The spatial expression patterns of 22 ARFs were analysed and compared during the syncytium formation phase. The expression of ARF3, 11 and 20–22 is restricted to the initial feeding cells and not extended to include recently incorporated syncytium cells. ARF4, 6, 9, 10, 12, 14, 15 and 19 are expressed in the developing syncytium. ARF1, 2, 5 and 18 are continually expressed in the neighbouring cells. ARF7 and 17 show a remarkable increase in their activity and are mainly expressed at the edge of the syncytial and neighbouring cells. ARF8, 13 and 16 are expressed mostly in the syncytial cells around the nematode head. Arrows point to nematode, and ‘S’ indicates syncytium. Bars, 130 μm.
Because the developing syncytia at 5–6 days after H. schachtii infection (i.e. the early J3 stage) undergo extensive cell‐to‐cell fusion with the distantly reprogrammed neighbouring cells, it was of interest to investigate whether the ARF genes, which displayed activity in the neighbouring cells (ARF1, 2, 4, 5, 9, 18 and 19), preserve this spatial expression pattern. Notably, ARF1, 2, 5 and 18 not only maintained this expression pattern, but there was a gradual increase in the GFP signals in the cells adjacent to the developing syncytia (Fig. 2). In contrast, the expression level of the three remaining ARFs (ARF4, 9 and 19) was strongly diminished in the neighbouring cells, and intense GFP fluorescence was observed only in the developing syncytium, suggesting that the activity of these ARFs became more specific to the syncytium (Fig. 2). ARF7 and 17 maintained their expression patterns observed at the 2–3‐dpi time point, but with a substantial increase in their activities (Fig. 2). ARF8, 13 and 16 showed very limited expression, primarily around the nematode head (Fig. 2). It may be important to mention that the neighbouring cells, which showed GFP expression at the 2–3‐dpi time point, were incorporated into the expanding feeding sites, supporting a role for ARFs in reprogramming neighbouring cells before fusion with the expanding syncytium. The expression of several ARFs in neighbouring cells suggests that, once auxin is accumulated in the IFCs, an auxin gradient is established along the syncytium axis, in which a role for the active auxin transport machinery in regulating these ARFs can be postulated. It has been suggested recently that PIN3 facilitates local auxin transport from the feeding sites towards surrounding cells to bring about syncytium expansion (Grunewald et al., 2009a). In addition, when the auxin transport machinery was chemically or genetically repressed, both feeding site formation and nematode development were largely affected (Goverse et al., 2000; Grunewald et al., 2009a). In this perspective, mutant combinations between PIN3 and the above‐mentioned ARFs will elucidate how PIN3‐mediated ARF regulation influences syncytium development and size.
The 9–10‐dpi time point corresponds to nematode development at the late J3 stage when syncytia reach their maximum size and no additional cells are incorporated. Thus, profiling of the spatial activity of ARFs revealed to what extent auxin distribution patterns were associated with the syncytium maintenance phase. It was not surprising to find that the majority of ARFs show low expression at this time, with only a detectable expression in the syncytium around the nematode head (Fig. 3). Interestingly, eight ARFs (ARF1–3, 7, 17 and 20–22) showed noticeable expression levels in the mature syncytium (Fig. 3), suggesting a role of these transcription factors in the transcriptional control of gene expression that maintains the functional phenotype of the mature syncytium. Similarly, other auxin‐responsive genes, such as LAX1 (Lee et al., 2011) and AUX1 (Mazarei et al., 2003), showed detectable expression levels at and after the J3 stage. However, these results are inconsistent with previous studies in which fully differentiated syncytium did not exhibit an auxin response (Absmanner et al., 2013; Karczmarek et al., 2004). This discrepancy is mainly a result of the fact that these studies used the auxin‐responsive DR5 promoter to visualize the auxin response, and that the DR5 promoter activity reflects only the auxin response, but not the auxin concentration. Therefore, the DR5 promoter does not seem to be sufficiently sensitive to determine the auxin gradient in the fully developed syncytium. It has been suggested that an auxin‐responsive promoter that is about ten times more sensitive than DR5 is needed to detect an auxin gradient that could be produced under specific circumstances (Yamamoto, 2003).
Figure 3.
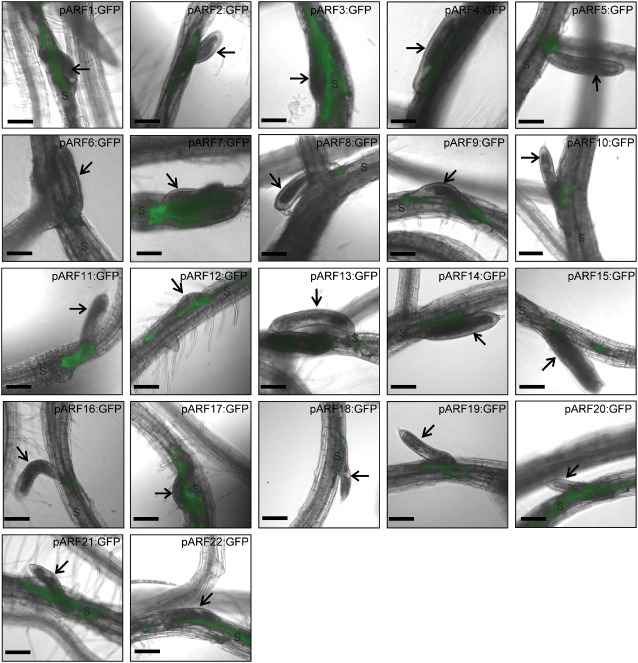
Promoter activity of auxin response factor (ARF) genes visualized by green fluorescent protein (GFP) fluorescence in transgenic Arabidopsis roots at the late third juvenile life stage, 9–10 days after Heterodera schachtii infection. The spatial expression patterns of 22 ARFs were analysed and compared during the syncytium maintenance phase. ARF1–3, 7, 17 and 20–22 are expressed in fully developed syncytium, whereas the expression of the other ARFs is restricted to the syncytial cells around the nematode head. Arrows point to nematode, and ‘S’ indicates syncytium. Bars, 130 μm.
The expression profiles of the ARFs during syncytium formation and development are very specific and, in non‐infected plants, we did not observe an expression similar to that associated with nematode infection. The spatiotemporal expression patterns of the ARFs are simplified in Fig. 4 and summarized in Table S1. During the initiation phase of the syncytium, auxin accumulates at a high level in the IFCs, which undergo extensive signalling events mediated by ARFs to guide these cells into a cell type‐specific transcriptional programme. A set of ARFs (ARF3, 6, 10–12, 14, 15 and 20–22) seems to have overlapping and cooperative transcriptional functions that contribute to the initiation phase of the syncytium. Once the syncytium is initiated, the neighbouring cells should receive auxin, as an instructive signal, from the IFCs in order to precondition these cells to a new cell fate. The commitment of the neighbouring cells to a syncytial fate may require the transcriptional activity of ARF1, 2, 4, 5, 9, 18 and 19 initially, and ARF1, 2, 5 and 18 until syncytium formation is complete. The maximum size of the expanding syncytium presumably needs to be marked before major transcriptional alterations occur. ARF2, 5 and 18 could be involved in the labelling of syncytial cell boundaries because of their expanding expression patterns at the early stage of infection (i.e. 2–3 dpi). After the syncytia have reached their maximum size, eight ARFs (ARF1–3, 7, 17 and 20–22), which are expressed in fully developed feeding sites, could have a role in maintaining the functional phenotype of mature syncytia. The active distribution and overlapping expression patterns of various sets of ARFs seem to be essential characteristics of ARF activity during various phases of syncytium development. Finally, the transcriptional activity of these ARFs might not reflect the exact patterns of mRNA accumulation, as some ARFs are known to be post‐transcriptionally regulated by microRNAs (miRNAs). ARF 10, 16 and 17 are targeted by miR160 (Mallory et al., 2005; Wang et al., 2005), whereas ARF6 and 8 are targeted by miR167 (Wu et al., 2006), and both miRNAs are regulated by H. schachtii infection (Hewezi et al., 2008).
Figure 4.
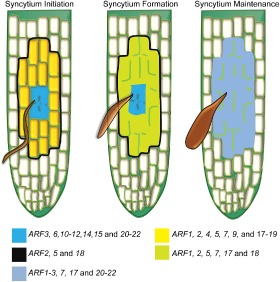
Model illustrating the spatiotemporal expression patterns of the auxin response factors (ARFs) during various stages of syncytium development. During the syncytium initiation phase, ARF3, 6, 10–12, 14, 15 and 20–22 are predominantly expressed in the initial feeding cells, whereas ARF1, 2, 4, 5, 7, 9 and 17–19 are strongly activated in both the syncytial cells and in neighbouring cells. ARF2, 5 and 18 have an expanded expression pattern, which most probably marks the axis of expanding syncytium. During the syncytium formation phase, ARF1, 2, 5, 7, 17 and 18 continue to exhibit high expression in the developing syncytium. During the syncytium maintenance phase, only ARF1–3, 7, 17 and 20–22 are expressed in fully developed syncytium.
Supporting information
Fig. S1 Comparison of gene expression patterns between auxin response factors (ARFs) that are exclusively expressed inside the developing syncytium and those expressed in the neighbouring cells before their fusion with the expanding syncytium. Certain ARFs, such as ARF14, are predominantly expressed inside the developing syncytium (a), whereas others, such as ARF2 and 5, are expressed in the neighbouring cells before their fusion with the expanding syncytium (b, c). Arrows point to nematode, and developing syncytium is outlined. Bars, 130 μm.
Table S1 Summarized expression patterns of auxin response factors (ARFs) during syncytium formation and development.
Acknowledgements
We thank Drs Neal Stewart and Mitra Mazarei for critical reading of this paper and Dr Andreas Nebenfuhr for providing access to his microscopy facility. This work was supported by Hewezi Laboratory startup funds from the University of Tennessee and a University of Tennessee AgResearch Innovation Grant (2013). The authors declare that no conflicts of interest exist.
Accession numbers: ARF1 (At1g59750), ARF2 (AT5G62000), ARF3 (At2g33860), ARF4 (At5g60450), ARF5 (At1g19850), ARF6 (AT1G30330), ARF7 (AT5G20730), ARF8 (AT5G37020), ARF9 (At4g23980), ARF10 (At2g28350), ARF11 (AT2G46530), ARF12 (T1G34310), ARF13 (AT1G34170), ARF14 (AT1G35540, ARF15 (AT1G35520), ARF16 (AT4G30080), ARF17 (At1g77850), ARF18 (At3g61830), ARF19 (AT1G19220), ARF20 (AT1G35240), ARF21 (AT1G34410) and ARF22 (AT1G34390).
References
- Absmanner, B. , Stadler, R. and Hammes, U.Z. (2013) Phloem development in nematode‐induced feeding sites: the implications of auxin and cytokinin. Front. Plant Sci. 4, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins, R. and Scheres, B. (2008) Auxin: the looping star in plant development. Annu. Rev. Plant Biol. 59, 443–465. [DOI] [PubMed] [Google Scholar]
- Chapman, E.J. and Estelle, M. (2009) Mechanism of auxin‐regulated gene expression in plants. Annu. Rev. Genet. 43, 265–285. [DOI] [PubMed] [Google Scholar]
- Gheysen, G. and Mitchum, M.G. (2011) How nematodes manipulate plant development pathways for infection. Curr. Opin. Plant Biol. 14, 415–421. [DOI] [PubMed] [Google Scholar]
- Golinowski, W. , Grundler, F.M.W. and Sobczak, M. (1996) Changes in the structure of Arabidopsis thaliana during female development of the plant‐parasitic nematode Heterodera schachtii . Protoplasma, 194, 103–116. [Google Scholar]
- Goverse, A. and Bird, D. (2011) The role of plant hormones in nematode feeding cell formation In: Genomics and Molecular Genetics of Plant–Nematode Interactions (Jones J., Gheysen G. and Fenoll C., eds), pp. 325–347. Heidelberg: Springer. [Google Scholar]
- Goverse, A. , Overmars, H. , Engelbertink, J. , Schots, A. , Bakker, J. and Helder, J. (2000) Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol. Plant–Microbe Interact. 13, 1121–1129. [DOI] [PubMed] [Google Scholar]
- Grunewald, W. , Cannoot, B. , Friml, J. and Gheysen, G. (2009a) Parasitic nematodes modulate PIN‐mediated auxin transport to facilitate infection. PLoS Pathog. 5, e1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald, W. , van Noorden, G. , van Isterdael, G. , Beeckman, T. , Gheysen, G. and Mathesius, U. (2009b) Manipulation of auxin transport in plant roots during rhizobium symbiosis and nematode parasitism. Plant Cell, 21, 2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle, T.J. and Hagen, G. (2007) Auxin response factors. Curr. Opin. Plant Biol. 10, 453–460. [DOI] [PubMed] [Google Scholar]
- Hardtke, C.S. , Ckurshumova, W. , Vidaurre, D.P. , Singh, S.A. , Stamatiou, G. , Tiwari, S.B. , Hagen, G. , Guilfoyle, T.J. and Berleth, T. (2004) Overlapping and non‐redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development, 131, 1089–1100. [DOI] [PubMed] [Google Scholar]
- Hewezi, T. and Baum, T.J. (2013) Manipulation of plant cells by cyst and root‐knot nematode effectors. Mol. Plant–Microbe Interact. 26, 9–16. [DOI] [PubMed] [Google Scholar]
- Hewezi, T. , Howe, P. , Maier, T.R. and Baum, T.J. (2008) Arabidopsis small RNAs and their targets during cyst nematode parasitism. Mol. Plant–Microbe Interact. 21, 1622–1634. [DOI] [PubMed] [Google Scholar]
- Hewezi, T. , Maier, T.R. , Nettleton, D. and Baum, T.J. (2012) The Arabidopsis microRNA396‐GRF1/GRF3 regulatory module acts as a developmental regulator in the reprogramming of root cells during cyst nematode infection. Plant Physiol. 159, 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczmarek, A. , Overmars, H. , Helder, J. and Goverse, A. (2004) Feeding cell development by cyst and root‐knot nematodes involves a similar early, local and transient activation of a specific auxin‐inducible promoter element. Mol. Plant Pathol. 5, 343–346. [DOI] [PubMed] [Google Scholar]
- Kazan, K. and Manners, J.M. (2009) Linking development to defense: auxin in plant–pathogen interactions. Trends Plant Sci. 14, 373–382. [DOI] [PubMed] [Google Scholar]
- Lee, C. , Chronis, D. , Kenning, C. , Peret, B. , Hewezi, T. , Davis, E.L. , Baum, T.J. , Hussey, R.S. , Bennett, M. and Mitchum, M.G. (2011) The novel cyst nematode effector protein 19C07 interacts with the Arabidopsis auxin influx transporter LAX3 to control feeding site development. Plant Physiol. 155, 866–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory, A.C. , Bartel, D.P. and Bartel, B. (2005) MicroRNA‐directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell, 17, 1360–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarei, M. , Lennon, K.A. , Puthoff, D.P. , Rodermel, S.R. and Baum, T.J. (2003) Expression of an Arabidopsis phosphoglycerate mutase homologue is localized to apical meristems, regulated by hormones, and induced by sedentary plant‐parasitic nematodes. Plant Mol. Biol. 53, 513–530. [DOI] [PubMed] [Google Scholar]
- Rademacher, E.H. , Moller, B. , Lokerse, A.S. , Llavata‐Peris, C.I. , van den Berg, W. and Weijers, D. (2011) A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family. Plant J. 68, 597–606. [DOI] [PubMed] [Google Scholar]
- Remington, D.L. , Vision, T.J. , Guilfoyle, T.J. and Reed, J.W. (2005) Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Physiol. 135, 1738–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup, K. , Benková, E. , Swarup, R. , Casimiro, I. , Péret, B. , Yang, Y. , Parry, G. , Nielsen, E. , De Smet, I. , Vanneste, S. , Levesque. M.P. , Carrier, D. , James, N. , Calvo, V. , Ljung, K. , Kramer, E. , Roberts, R. , Graham, N. , Marillonnet, S. , Patel, K. , Jones, J.D. , Taylor, C.G. , Schachtman, D.P. , May, S. , Sandberg, G. , Benfey, P. , Friml, J. , Kerr, I. , Beeckman, T. , Laplaze, L. and Bennett, M.J. (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 10, 946–954. [DOI] [PubMed] [Google Scholar]
- Szakasits, D. , Heinen, P. , Wieczorek, K. , Hofmann, J. , Wagner, F. , Kreil, D.P. , Sykacek, P. , Grundler, F.M.W. and Bohlmann, H. (2009) The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant J. 57, 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.W. , Wang, L.J. , Mao, Y.B. , Cai, W.J. , Xue, H.W. and Chen, X.Y. (2005) Control of root cap formation by microRNA‐targeted auxin response factors in Arabidopsis. Plant Cell, 17, 2204–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M.‐F. , Tian, Q. and Reed, J.W. (2006) Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development, 133, 4211–4218. [DOI] [PubMed] [Google Scholar]
- Yamamoto, K.T. (2003) Happy end in sight after 70 years of controversy. Trends Plant Sci. 8, 359–360. [DOI] [PubMed] [Google Scholar]
- Zhao, Y.D. (2010) Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 61, 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Comparison of gene expression patterns between auxin response factors (ARFs) that are exclusively expressed inside the developing syncytium and those expressed in the neighbouring cells before their fusion with the expanding syncytium. Certain ARFs, such as ARF14, are predominantly expressed inside the developing syncytium (a), whereas others, such as ARF2 and 5, are expressed in the neighbouring cells before their fusion with the expanding syncytium (b, c). Arrows point to nematode, and developing syncytium is outlined. Bars, 130 μm.
Table S1 Summarized expression patterns of auxin response factors (ARFs) during syncytium formation and development.


