SUMMARY
Lipopolysaccharide (LPS) is an important virulence factor of Xanthomonas citri ssp. citri, the causative agent of citrus canker disease. In this research, a novel gene, designated as nlxA (novel LPS cluster gene of X. citri ssp. citri), in the LPS cluster of X. citri ssp. citri 306, was characterized. Our results indicate that nlxA is required for O‐polysaccharide biosynthesis by encoding a putative rhamnosyltransferase. This is supported by several lines of evidence: (i) NlxA shares 40.14% identity with WsaF, which acts as a rhamnosyltransferase; (ii) sodium dodecylsulphate‐polyacrylamide gel electrophoresis analysis showed that four bands of the O‐antigen part of LPS were missing in the LPS production of the nlxA mutant; this is also consistent with a previous report that the O‐antigen moiety of LPS of X. citri ssp. citri is composed of a rhamnose homo‐oligosaccharide; (iii) mutation of nlxA resulted in a significant reduction in the resistance of X. citri ssp. citri to different stresses, including sodium dodecylsulphate, polymyxin B, H2O2, phenol, CuSO4 and ZnSO4. In addition, our results indicate that nlxA plays an important role in extracellular polysaccharide production, biofilm formation, stress resistance, motility on semi‐solid plates, virulence and in planta growth in the host plant grapefruit.
INTRODUCTION
Xanthomonas citri ssp. citri (syn. Xanthomonas axonopodis pv. citri, Xanthomonas campestris pv. citri or Xanthomonas citri) is the causative agent of citrus canker disease, one of the most serious diseases of citrus worldwide (Civerolo, 1984; Graham et al., 2004). Under natural conditions, X. citri ssp. citri can survive as an epiphyte on host plant surfaces and invade the leaf, stem and fruit through natural openings, including stomata and wounds (Brunings and Gabriel, 2003; Vojnov et al., 2010). The diseased citrus plant forms canker symptoms that are characterized by distinctive raised, necrotic lesions surrounded by oily, water‐soaked margins and yellow chlorotic rings. Severe invasion by the pathogen eventually leads to defoliation, dieback and fruit drop, and the infected fruits are less valuable commercially and/or completely unmarketable (Gottwald et al., 2002).
Lipopolysaccharide (LPS) is an important virulence factor that has been shown to be involved in multiple steps of the disease process for many Gram‐negative bacterial pathogens, including X. citri ssp. citri (Li and Wang, 2011; Newman et al., 2001). LPS is a key component of the outer membrane of Gram‐negative bacteria and protects bacterial cells from the unfavourable conditions of the plant environment (Dow et al., 1995). In plant–pathogen interactions, LPS can function as a pathogen‐associated molecular pattern (PAMP) by eliciting or potentiating plant defence‐related responses (Zeidler et al., 2004). It is a tripartite amphipathic molecule composed of a lipid A moiety, a core oligosaccharide and a polysaccharide chain, termed the O‐antigen or O‐side chain (Raetz and Whitfield, 2002; Vorholter et al., 2001). Recently, the LPS structure of X. citri ssp. citri has been successfully defined (Casabuono et al., 2011). It has been shown that X. citri ssp. citri LPSs are composed mainly of a penta‐ or tetra‐acylated diglucosamine backbone attached to either two pyrophosphorylethanolamine (PP‐EtNH2) groups or to one PP‐EtNH2 group and one phosphorylethanolamine group. The core region consists of a branched oligosaccharide and two phosphate groups, whereas the O‐antigen is composed of a rhamnose homo‐oligosaccharide (Casabuono et al., 2011).
Despite the tremendous progress made in the investigation of LPS, the understanding of the genetic pathway for the LPS biosynthesis of X. citri ssp. citri remains limited as the current understanding is mainly based on sequence similarities with related bacteria. Whole genome sequencing has revealed that X. citri ssp. citri 306 contains a cluster of 16 genes residing between the conserved etfA (XAC3587) and metB (XAC3602), which have been proposed to be involved in LPS biosynthesis (da Silva et al., 2002). However, only a few genes, including wxacO (XAC3596), rfbC (XAC3598) and wzt (XAC3600), have been experimentally characterized in the biosynthesis of LPS and the involvement in plant–pathogen interactions (Casabuono et al., 2011; Li and Wang, 2011). In addition, it has been reported that X. citri ssp. citri LPS shows remarkable differences from other bacterial LPS or Xanthomonas LPS, including the presence of two PP‐EtNH2 groups in the mainly penta‐acylated lipid A moiety, the existence of two 3‐deoxy‐d‐manno‐octulosonic acid (Kdo) units linking lipid A to the core region and the presence of a 3N‐acetylfucosamine (Fuc3NAc) unit in the core (Casabuono et al., 2011). Sugar analysis has revealed that X. citri ssp. citri processes a rhamnose homo‐oligosaccharide as the O‐antigen moiety (Casabuono et al., 2011), which is similar to X. campestris pv. campestris B100 (Braun et al., 2005). However, the configuration and types of sugar linkage of the X. citri ssp. citri O‐polysaccharide have not been determined (Casabuono et al., 2011). Moreover, only rhamnose was detected in the sugar analysis of the LPS O‐antigen of X. citri ssp. citri (Casabuono et al., 2011), which is different from many other Xanthomonas species, in which various sugars [rhamnose and fucose (1999, 2003), rhamnose and xylose (Shashkov et al., 1999), rhamnose and Fuc3NAc (Molinaro et al., 2002)] were identified in the LPS O‐antigen. Thus, it is necessary to obtain a further understanding of the genetic determinants of LPS biosynthesis of X. citri ssp. citri.
In our recent study, nine different mutants of LPS cluster genes were identified by screening the EZ‐Tn5 mutant library X. citri ssp. citri (Yan and Wang, 2011b). Among them, two mutants (406G8 and 415H9) were found to have a transposon located in the intergenic region between rfbC (XAC3598) and a hypothetical gene XAC3597. The rfbC gene putatively encodes a truncated O‐antigen biosynthesis protein and is involved in LPS production (Li and Wang, 2011). XAC3597 encodes a hypothetical protein which is specific to strain X. citri ssp. citri 306 and is absent from other Xanthomonas spp., except X. campestris pv. musacearum NCPPB 4381. Interestingly, a new hypothetical gene (ORF5) was reannotated in the intergenic region between rfbC and XAC3597 where the transposon was located in the 406G8 and 415H9 mutants (Patil et al., 2007). However, this reannotated hypothetical gene has not been characterized experimentally. In this study, we present evidence that the new hypothetical gene, designated as nlxA (novel LPS cluster gene of X. citri ssp. citri), is involved in the biosynthesis of not only LPS, but also extracellular polysaccharide (EPS), and plays a role in biofilm formation, stress resistance, motility and pathogenesis of the citrus canker pathogen X. citri ssp. citri.
RESULTS
A novel gene involved in the LPS biosynthesis of X. citri ssp. citri
Similar phenotypes were obtained for the two mutants 406G8 and 415H9; only the data for one mutant (406G8) are presented. The EZ‐Tn5 location in the chromosome of X. citri ssp. citri 306 is indicated in Fig. 1. A growth curve assay was performed by culturing the wild‐type and the mutant 406G8 in nutrient broth (NB) medium. The mutant grew in a similar manner to the wild‐type (Fig. S1, see Supporting Information). For LPS assay, bacterial cultures were collected at 24 h post‐inoculation (hpi). LPS was extracted from the same volume of bacterial culture for both the wild‐type and the mutant to avoid growth variation between the tested strains. LPS produced by an X. citri ssp. citri wzt mutant, with wzt encoding an ATP‐binding protein of an ABC transporter system for the export of the O‐antigen of LPS, was also included. As illustrated in Fig. 2, the four bands of the O‐antigen part of LPS were missing in the nlxA mutant, which showed a similar LPS pattern to the wzt mutant.
Figure 1.
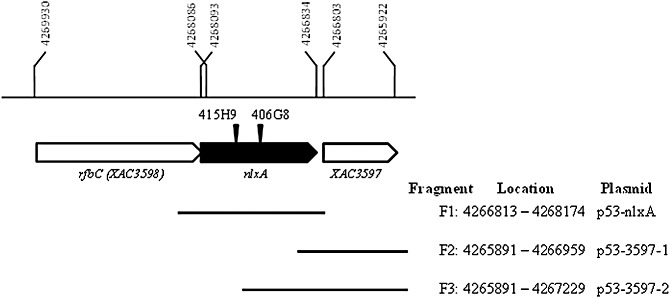
Schematic diagram of the genetic organization of the nlxA and neighbouring genes in the lipopolysaccharide (LPS) gene cluster of Xanthomonas citri ssp. citri 306. The single‐headed arrows represent the locations and orientations of the genes. The nlxA gene is indicated by a filled block arrow. The location of each gene in the chromosome is indicated by numbers. The EZ‐Tn5 insertions in the mutants are indicated by inverted triangles. F1, F2 and F3 represent the DNA fragments used to construct the complementary plasmids. The locations of the three fragments in the chromosome are also given.
Figure 2.
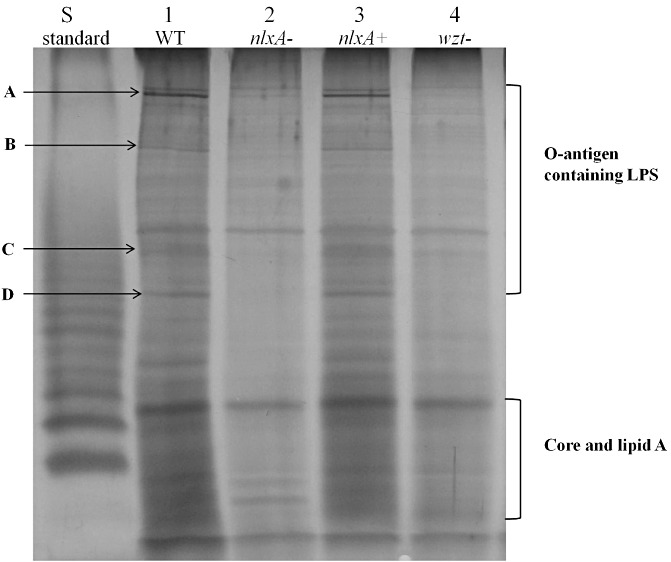
Sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) analysis of the lipopolysaccharide (LPS) produced by the wild‐type Xanthomonas citri ssp. citri 306 and its derivatives. Lanes: 1, wild‐type (WT) containing an empty vector pUFR053; 2, nlxA mutant (406G8) containing an empty vector pUFR053; 3, complementary strain, nlxA mutant (406G8) containing the vector p53‐nlxA; 4, wzt mutant (330D3) containing an empty vector pUFR053; S, LPS standard from Salmonella enterica serovar Typhimurium (10 µg, Sigma). Four bands that were missing in the O‐antigen containing LPS of the nlxA mutant were indicated as A, B, C and D. The experiment was repeated three times with similar results, and the result of only one experiment is presented.
For the complementation experiment, the wild‐type XAC3597 gene was first used to complement the 406G8 mutant, as we originally reasoned that the phenotype of 406G8 resulted from the transposon insertion in the promoter region of XAC3597 (Fig. 1). This was based on the genomic information for X. citri ssp. citri from da Silva et al. (2002) prior to the publication of the reannotation of the LPS gene cluster (Patil et al., 2007). However, attempts to complement the 406G8 mutant using intact XAC3597 plus 155 or 425 bp of upstream fragments did not restore the LPS production and pathogenicity of the mutant (data not shown). Thus, we hypothesized that another gene might exist in the insertion locus of the transposon between XAC3598 and XAC3597. Interestingly, a new hypothetical gene (ORF5) was reannotated in the intergenic region between XAC3598 and XAC3597 by Patil et al. (2007), which further supports our hypothesis. This hypothetical gene contains 1260 nucleotides and is located between nucleotides 4 266 834 and 4 268 093 of the chromosome of X. citri ssp. citri 306 (Fig. 1). LPS production of the 406G8 mutant was restored to the wild‐type level with a fragment containing the intact hypothetical gene (Fig. 2). This experiment indicates that this reannotated gene is a biologically functional gene and plays a role in the biosynthesis of LPS. This hypothetical gene was designated as nlxA (novel LPS cluster gene of X. citri ssp. citri).
The nlxA gene encodes a putative glycosyltransferase (GT)
blast analysis revealed that NlxA shares sequence identity (up to 95.02%) with different homologue proteins from more than 10 organisms, including both Gram‐positive and Gram‐negative bacteria (Table 1). NlxA shares 95.02% and 59.86% identity with only two hypothetical proteins of X. campestris pv. musacearum and X. oryzae pv. oryzae, respectively. NlxA shares an identity of 40.14% with WsaF, which was identified as a rhamnosyltransferase in the synthesis of the S‐layer glycan of the Gram‐positive bacterium Geobacillus stearothermophilus NRS 2004/3a (Steiner et al., 2010). In addition, NlxA shares an identity of 32.55% with WcrW, which was annotated as a putative GT of Streptococcus pneumonia 6803 (Bentley et al., 2006). GTs are a ubiquitous group of enzymes that catalyse the transfer of a sugar moiety from an activated sugar donor onto saccharide or nonsaccharide acceptors, and are involved in the biosynthesis of glycoproteins, glycolipids, oligo‐ and polysaccharides (Coutinho et al., 2003). Large numbers of GTs have been found and are currently classified into 94 families in the CAZy database (http://www.cazy.org). Both WsaF of G. stearothermophilus and WcrW of S. pneumonia belong to the GT4 family, members of which catalyse retaining glycosyl transfer reactions with nucleotide‐sugar, phospho‐sugar and lipid‐phospho‐sugar as donors (Coutinho et al., 2003; Martinez‐Fleites et al., 2006). Protein sequence alignment also revealed that the putative protein of NlxA contains a motif ‘PHPSYPPLE’ which is conserved in all seven proteins of the blast result (Fig. 3). The ‘PHPSYPPLE’ motif has been indicated to be involved in the recognition of deoxythymidinediphosphate (dTDP)‐β‐l‐rhamnose by WsaF to catalyse the glycosyl transfer reaction in G. stearothermophilus (Steiner et al., 2010).
Table 1.
Sequence analysis of NlxA and its homologues.
| Protein | Accession number | Source organism | Annotated function | Length (aa) | Identity (%) |
|---|---|---|---|---|---|
| NlxA | Xanthomonas citri ssp. citri 306 | 419 | 100 | ||
| XcampmN_07905 | ZP_06489485 | Xanthomonas campestris pv. musacearum NCPPB 4381 | Unknown | 422 | 95.02 |
| ORF5 | ABI93189 | Xanthomonas oryzae pv. oryzae BXO8 | Unknown | 417 | 59.86 |
| WsaF | AAR99609 | Geobacillus stearothermophilus NRS 2004/3a | Rhamnosyltransferase of GT4 | 413 | 40.14 |
| PM8797T_20259 | EDL58917 | Planctomyces maris DSM 8797 | Unknown | 430 | 36.70 |
| Roseina2194_02598 | ZP_03754177 | Roseburia inulinivorans DSM 16841 | Unknown | 448* | 34.42 |
| Met49242DRAFT_0030 | ZP_08070643 | Methylocystis sp. ATCC 49242 | Rhamnan synthesis F | 406† | 32.63 |
| WcrW | CAI34499 | Streptococcus pneumonia 6803 | Putative GT4 | 414 | 32.55 |
The C terminal 448 amino acids (aa) of the complete 1030‐aa protein were used for homologue analysis as only the C terminal of the protein showed similarity with NlxA.
Similarly, the C terminal 406 aa of the complete 812‐aa protein were used for homologue analysis with NlxA.
Figure 3.
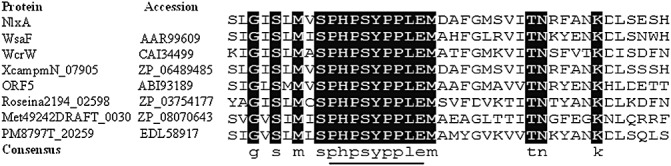
Alignment analysis of NlxA and its homologues. Multiple alignments of NlxA with its homologues indicate the presence of a conserved motif specific for rhamnosyltransferase in NlxA. Only part of the alignment is shown. The names and GenBank accession numbers of the proteins are given. The nine amino acid residues which were identified by Steiner et al. (2010) as a binding signature of the substrate of deoxythymidinediphosphate‐β‐l‐rhamnose of WsaF are underlined.
Mutation of nlxA affects EPS production
Both LPS and EPS are polysaccharides and share certain common genetic determinants in polysaccharide biosynthesis. To test whether the mutation of nlxA affects EPS production in X. citri ssp. citri, the EPS assay was performed for the wild‐type, mutant and complementary strains after culturing for 24 h in NB containing 2% glucose. No significant difference in cell populations was observed among the wild‐type (1.90 ± 0.03), mutant (1.86 ± 0.06) and complementary strain (1.91 ± 0.02) by measuring the optical density at 600 nm (OD600) of the bacterial cultures at the time point of sampling. The amount of EPS produced by the nlxA mutant was 7.13 ± 0.23 mg/mL, which was significantly lower than that of the wild‐type (12.53 ± 1.93 mg/mL) (P < 0.05). EPS biosynthesis by the nlxA mutant was restored to the wild‐type level (13.93 ± 1.08 mg/mL) with p53‐nlxA, which contains the intact nlxA gene.
A capsule assay was conducted to test whether mutation of nlxA affects the capsule. No significant difference was observed between the wild‐type and the nlxA mutant in the capsule staining (data not shown).
nlxA is involved in biofilm formation both in vitro and in vivo
Biofilm formation is an important bacterial trait associated with virulence and has been related to LPS and/or EPS production in many bacteria. To test whether nlxA is involved in biofilm formation of X. citri ssp. citri, biofilm formation was examined in both a glass tube and on citrus leaves. A significant reduction in biofilm formation was detected in the nlxA mutant compared with the wild‐type by culturing the bacteria in a glass tube (Fig. 4A). The complementary strain showed similar biofilm formation to the wild‐type. The nlxA mutant showed earlier biofilm formation than the wild‐type and complementary strains at 10 h after inoculation on the citrus leaf surface (Fig. 4B). However, less biofilm formation was observed for the nlxA mutant than for the wild‐type and complementary strains when the bacteria were incubated for 24 h to fully develop the biofilm on the leaves.
Figure 4.
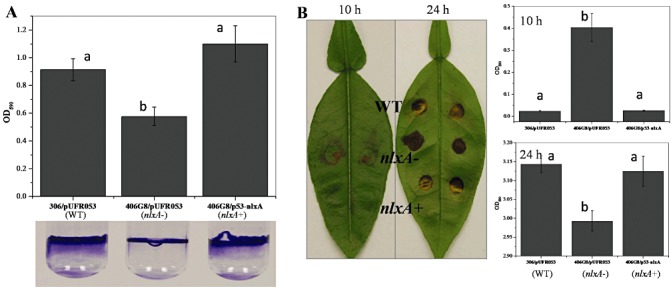
The nlxA gene is involved in biofilm formation. (A) One millilitre of bacterial solution [108 colony‐forming units (CFU)/mL in nutrient broth (NB)] were incubated at 28 °C for 24 h in a glass tube without shaking. (B) Twenty microlitres of bacterial solution (108 CFU/mL in NB) were inoculated onto the citrus abaxial leaf surface and incubated at 28 °C for 10 and 24 h in a humid chamber. Biofilm formation was visualized by crystal violet staining. The stained cells were dissolved in 97% ethanol and quantified by measuring the optical density at 590 nm. Values in the same figure that are significantly different (P < 0.05) are indicated by different letters (a, b). The biofilm assays were repeated three times with similar results with four replicates each time. Data from one representative experiment are shown. Vertical bars represent the standard errors of the means.
nlxA is involved in the resistance of X. citri ssp. citri to various environmental and chemical stresses
To investigate whether nlxA affects the resistance of X. citri ssp. citri to environmental and chemical stresses, the bacterial cells were challenged with different stresses. As shown in Table 2, mutation of nlxA resulted in a significant reduction in pathogen resistance against various compounds, including hydrogen peroxide, polymyxin B, sodium dodecylsulphate (SDS), phenol, CuSO4 and ZnSO4. The resistance of the nlxA mutant to these stresses was restored using the plasmid p53‐nlxA containing the intact nlxA gene.
Table 2.
The nlxA gene is involved in stress resistance against H2O2, polymyxin B, sodium dodecylsulphate (SDS), phenol, CuSO4 and ZnSO4.
| Strains | Bacterial concentration before challenge (108 CFU/mL)*, † | Survival percentage (%) after challenge* | ||||||
|---|---|---|---|---|---|---|---|---|
| Name | Genotype | H2O2 | Polymyxin B | SDS | Phenol | CuSO4 | ZnSO4 | |
| 306/pUFR053 | WT | 9.33 ± 0.58a | 5.76 ± 1.19a | 14.81 ± 1.28a | 0.57 ± 0.03a | 93.51 ± 5.78a | 1.02 ± 0.42a | 1.42 ± 0.32a |
| 406G8/pUFR053 | nlxA− | 10.33 ± 0.85a | 0.85 ± 0.04b | 3.84 ± 1.61b | 0.07 ± 0.03b | 41.76 ± 12.61b | 0.18 ± 0.10b | 0.37 ± 0.08b |
| 406G8/p53‐nlxA | nlxA+ | 10.67 ± 1.53a | 10.48 ± 4.62a | 14.21 ± 2.42a | 0.42 ± 0.13a | 95.83 ± 7.22a | 1.00 ± 0.57a | 1.47 ± 0.19a |
All the experiments were repeated three times independently with four replications each time. Similar results were observed and only one representative result is presented.
For significant difference analysis, the survival percentages of four replications of each strain were calculated independently and then subjected to Student's t‐test. Data with different letters (a, b) denote significant difference (P < 0.01) between strains in the same treatment. Means and standard errors from one representative result are shown.
All strains were cultured for 20 h in nutrient broth (NB). Initial cell concentrations [colony‐forming units (CFU)/mL] were measured by diluting the bacterial cultures appropriately and plating on nutrient agar (NA) plates before challenge with different stresses. For each strain tested, similar cell concentrations were observed in different stress assays. Only one set of the representative data is shown.
nlxA is involved in X. citri ssp. citri motility on semi‐solid plates
The effects of mutation of nlxA on the swimming and swarming motility of X. citri ssp. citri were tested on semi‐solid nutrient agar (NA) plates containing 0.3% and 0.7% agar, respectively. The movement of the wild‐type on both swimming and swarming plates was very slow in the first 2 days post‐inoculation (dpi), but was more rapid after the second day (Fig. 5A,C) (which is similar to other Xanthomonas bacteria and indicates that the movement is inducible in X. citri ssp. citri). Although the mutant was slightly motile on the semi‐solid plates, the swimming and swarming motilities of the nlxA mutant were reduced significantly compared with those of the wild‐type (Fig. 5B,D). The deficiency of the mutant in motility could be complemented by the intact nlxA gene.
Figure 5.
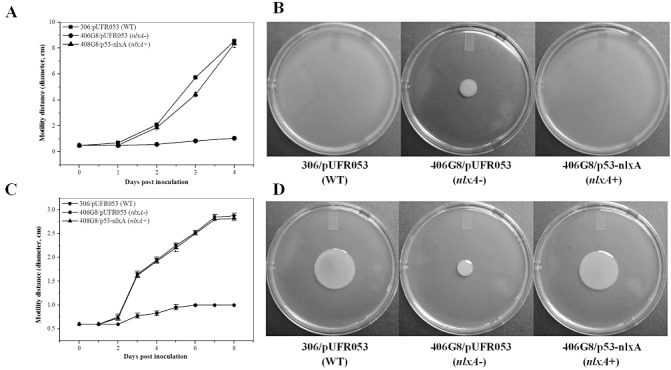
The nlxA gene is involved in swimming and swarming motility. Two microlitres of bacterial solution [108 colony‐forming units (CFU)/mL in nutrient broth (NB)] were inoculated on the swimming assay (0.3% agar) (A, B) and swarming assay (0.7% agar) (C, D) plates. The bacterial motility was measured at different time points as indicated. The movement of bacterial cells was photographed at 5 and 8 days post‐inoculation (dpi) on the swimming and swarming plates, respectively. The experiment was repeated three times with three replicates each time. Means and standard errors of three replicates from one representative result are shown. Vertical bars represent the standard errors of the means.
To investigate whether nlxA plays a role in the production of flagella, the flagella were observed under transmission electron microscopy (TEM). TEM analysis showed that both the wild‐type and the nlxA mutant formed a single polar flagellum on the cell surface (data not shown).
nlxA and other genes in the LPS gene cluster were induced in a surface hardness‐specific manner
It is known that LPS plays a critical role during bacterial swarming, probably by wetting the surface or acting as a surfactant (Toguchi et al., 2000). The expression of genes involved in LPS synthesis of Salmonella typhimurium was elevated in a surface hardness‐specific manner just before the initiation of swarming, and also maximally induced during late exponential growth coincident with maximum swarming activity (Wang et al., 2004). To test whether this also happens in X. citri ssp. citri, the expression of genes in the LPS gene cluster, including nlxA, XAC3588, XAC3590, wxacO, rfbC and wzm in NB and on semi‐solid NA plates (NB + 0.5% agar), was compared. Bacterial cells were collected at 24 hpi, the time point at which the wild‐type starts to show notable movement on motility assay plates (Fig. 5A,C). The expression of nlxA was significantly induced (P < 0.05) on 0.5% agar plates relative to NB (Table 3). Consistently, the expression of other LPS cluster genes, including XAC3588, XAC3590, wxacO, rfbC and wzm, was also significantly induced on semi‐solid NA plates (Table 3).
Table 3.
The expression of nlxA and other lipopolysaccharide (LPS) biosynthesis genes was induced on semi‐solid agar plates.
| Target gene† | Locus tag† | Annotated function | Primer sequence (5' to 3') | Fold change ± SE* | P value (Student's t‐test) |
|---|---|---|---|---|---|
| nlxA | NA | F: AGCCATGCAAACATCGTGTCGTTG | 2.611095 ± 0.705173 | 0.0304023 | |
| R: AAACGTAGGCATCGTCTAAGGCCA | |||||
| NA | XAC3588 | Integral membrane protein | F: TCGGAATTTCTACTGCAGACGCCA | 2.694485 ± 0.200239 | 0.0005489 |
| R: CGAATTCTGCAGCAAGCATGGACA | |||||
| NA | XAC3590 | Oxidoreductase | F: TCCTTCGGGCTTTCTGCAGTATCA | 3.367230 ± 0.324939 | 0.001889 |
| R: TGTTGCCGAATTCCTTCAACACGG | |||||
| wxacO | XAC3596 | Putative transmembrane protein | F: TCTTTATGCGCTCTTGGCCGTAGT | 5.199567 ± 0.647125 | 0.0128718 |
| R: ACATCGGTCATCTGGCGGACATAA | |||||
| rfbC | XAC3598 | Truncated O‐antigen biosynthesis protein | F: ATCCATCACCAGCACCTGTTCGTA | 2.772365 ± 0.842772 | 0.0052181 |
| R: GAATCCGCCAATGGCATCGAAGTT | |||||
| wzm | XAC3601 | ABC transporter permease | F: TTGAAATGGTCCCGATGCTGCTTG | 2.393728 ± 0.488077 | 0.0213009 |
| R: AAAGCAGAACGCTGCTCAGAATGC |
F, forward primer; R, reverse primer.
The wild‐type was cultured in nutrient broth (NB) for 20 h or on a semi‐solid plate for 24 h; the fold change was calculated using gene expression of the wild‐type after culture in NB for 20 h as calibrator according to the formula 2–ΔΔ CT (Livak and Schmittgen, 2001). SE, standard error.
Target genes and the corresponding ID are listed. NA denotes a gene name or ID that has not yet been assigned.
nlxA is involved in the virulence and bacterial survival in planta
As described previously (Yan and Wang, 2011b), the 406G8 mutant was identified by its deficient virulence in grapefruit. In this study, further investigations, including in planta bacterial growth assay and complementation experiments, were performed. In addition, a spraying inoculation method, which mimics natural invasion conditions, was also conducted to determine the role of nlxA in the virulence of the pathogen. The bacterial population of the nlxA mutant in planta was significantly lower than that of the wild‐type by two orders of magnitude at 6 dpi and by more than one order of magnitude at 8, 10 and 14 dpi. Co‐inoculation with the wild‐type strain could not rescue the growth of the mutant in the host plant, as indicated by the similar growth curve to that of the nlxA mutant alone (Fig. 6D). The growth deficiency of the mutant was successfully complemented by the intact nlxA gene. Consistently, the complementary strain caused similar symptoms to the wild‐type on citrus leaves (Fig. 6A).
Figure 6.
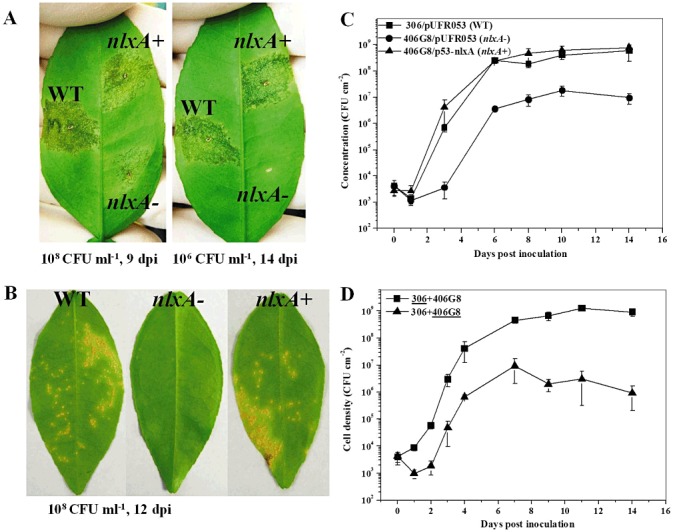
The nlxA gene is important for the virulence and growth in planta of Xanthomonas citri ssp. citri. Grapefruit (Citrus paradise Macf. cv. Duncan) was used for virulence and growth assay. For virulence assay, the wild‐type, nlxA mutant and complementary strain were inoculated into the intercellular spaces (A) or sprayed onto the leaf surfaces (B) of grapefruit. The inoculation concentration of the cell suspensions and the incubation time for photography are shown. The experiments were repeated more than three times with similar results. Only one representative leaf was photographed and presented. For in planta growth assay (C), the wild‐type, nlxA mutant and complementary strain were inoculated into the intercellular spaces of the leaves with a cell suspension of 5 × 105 colony‐forming units (CFU)/mL. For co‐inoculation growth assay (D), cell suspensions (106 CFU/mL) of the wild‐type and nlxA mutant were mixed equally and inoculated into the leaves following the same method. The growth curves of the wild‐type and the nlxA mutant in the co‐inoculation experiment are indicated by underlining. Bacterial cells from the inoculated leaves were recovered at different time points. The in planta growth was measured in quadruplicate and the assays were repeated three times independently. Means and standard errors of four replicates from one representative result are shown. Vertical bars represent the standard errors of the means.
To mimic the natural invasion conditions under which the canker pathogen is spread by rain splash, the bacterial strains were inoculated onto the leaf surfaces by a spraying method. The wild‐type caused typical canker symptoms on citrus leaves at 16 dpi (Fig. 6B). However, no obvious symptoms were observed on the leaves inoculated by the nlxA mutant. The virulence deficiency of the mutant could be complemented by the intact nlxA gene.
DISCUSSION
In this research, we characterized a novel gene nlxA in the LPS cluster of X. citri ssp. citri 306. Our results indicate that nlxA is required for O‐polysaccharide biosynthesis by encoding a putative rhamnosyltransferase. This is supported by several lines of evidence.
-
1
NlxA shares 40.14% identity with WsaF (Table 1), which acts as a rhamnosyltransferase (GT4) in the synthesis of S‐layer glycan and transfers rhamnose sugars from the substrate of dTDP‐β‐l‐rhamnose for the extension of the polysaccharide chain in the Gram‐positive bacterium G. stearothermophilus (Steiner et al., 2010). Structural similarities between S‐layer glycan and LPS O‐antigen have been observed (Schaffer et al., 1996), and the assembly of both types of molecule may follow similar pathways (Steiner et al., 2007; Valvano, 2003). Further sequence analysis revealed that NlxA contains a conserved motif ‘PHPSYPPLE’ (Fig. 3), which is identical to the signature sequence of WsaF for the binding of the rhamnose substrate (Steiner et al., 2010).
-
2
Sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) showed that four bands of the O‐antigen part of LPS were missing in the LPS production of the nlxA mutant (Fig. 2), indicating that nlxA is required for LPS O‐antigen biosynthesis. This result is also consistent with the recent report that the O‐antigen moiety of X. citri ssp. citri LPS is composed of a rhamnose homo‐oligosaccharide (Casabuono et al., 2011). In addition, the LPS pattern of the nlxA mutant is similar to that of the wzt mutant (Fig. 2). The wzt gene encodes an ATP‐binding protein of an ABC transporter system and is required for O‐antigen export.
-
3
Mutation of nlxA resulted in a significant reduction in resistance of X. citri ssp. citri to different stresses, including SDS, polymyxin B, H2O2, phenol, CuSO4 and ZnSO4 (Table 2). LPS is known to be a key component of the outer membrane of Gram‐negative bacteria and a protective barrier against harsh environmental conditions (Dow et al., 1995). Therefore, NlxA probably functions as a rhamnosyltransferase in the O‐antigen biosynthesis of X. citri ssp. citri.
In the sequenced Xanthomonas spp., the homologues of nlxA were only found in X. campestris pv. musacearum NCPPB 4381, a causative agent of banana wild disease (Studholme et al., 2010), X. oryzae pv. oryzae BXO8, a causative agent of rice blight disease (Patil et al., 2007), and X. citri ssp. citri 306, a causative agent of citrus canker disease. This indicates that the nlxA gene characterized in this work is a unique LPS biosynthesis gene to only a small and diverse group of Xanthomonas. Great variations have been observed among LPS biosynthesis gene clusters of both animal pathogenic (Reeves and Wang, 2002) and plant pathogenic (Patil et al., 2007) bacteria. These variations have been proposed to reflect the highly immunogenic nature of LPS and a need for the pathogen to vary LPS to evade detection by the host (Patil et al., 2007). The presence of nlxA in only a few pathogenic bacteria of Xanthomonas might suggest a special role played by nlxA in the LPS assembly in these strains. However, the LPS structures of X. campestris pv. musacearum NCPPB 4381 and X. oryzae pv. oryzae BXO8 have not been characterized, making it difficult to analyse the structural similarities or differences between them and X. citri ssp. citri. The genetic evidence presented here demonstrates that nlxA is important for the LPS biosynthesis and virulence of X. citri ssp. citri (2, 6). It would be interesting to investigate the role of this unique gene in the configuration of the LPS O‐antigen, and whether it is involved in host recognition.
The effect of NlxA on the pathogenicity of X. citri ssp. citri probably results from its function in both LPS and EPS biosynthesis. The EPS composition of X. citri ssp. citri remains unknown. The EPS structure of X. campestris consists of a β‐1,4‐linked d‐glucose backbone with trisaccharide side chains composed of mannose‐(β‐1,4)‐glucuronic acid‐(β‐1,2)‐mannose attached to alternate glucose residues in the backbone by α‐1,3 linkages, and does not contain rhamnose (Jansson et al., 1975). However, a recent study has indicated that the EPSs produced by different Xanthomonas spp. vary. The EPS synthesized by X. campestris pv. pruni contains glucose, rhamnose, mannose and glucuronic acid in its chemical composition, different from the known xanthan composition (Borges and Vendruscolo, 2007). Thus, NlxA might contribute to EPS biosynthesis by transferring rhamnose. In addition, both LPS and EPS are known virulence factors and protect cells from the harsh in planta environment. Mutations in LPS and gum genes result in reduced virulence of Xanthomonas spp. A mutant of gumB is defective in gum production, and forms reduced disease symptoms in lemon (Citrus limon) (Rigano et al., 2007). Mutation in the LPS biosynthesis genes wxacO and rfbC result in virulence deficiency in grapefruit (Li and Wang, 2011). The reduced pathogenicity (Fig. 6A,B) and bacterial population (Fig. 6C) of the nlxA mutant are consistent with its reduced tolerance to H2O2 and phenol (Table 2). H2O2 is the primary reactive oxygen species (ROS) generated during the oxidative burst by plants in response to microbial pathogen attack in both compatible and incompatible interactions (Bolwell and Wojtaszek, 1997; Bolwell et al., 2002). Peak concentrations of H2O2 were observed at 24 h and between 8 and 10 days after inoculation in sweet orange ‘Hamlin’ infected by X. citri ssp. citri (Kumar et al., 2011). Mutations in LPS‐ and EPS‐related genes resulted in reduced tolerance of Xanthomonas spp. against H2O2 (Kemp et al., 2004; Li and Wang, 2011). In addition, phenolic compounds are another large class of secondary metabolites produced by plants to defend themselves against pathogens (Freeman and Beattie, 2008). Large amounts of phenolic compounds were found in the peel of 13 commercial citrus species, with a range of 104.2 to 223.2 mg gallic acid equivalent/g of extract power (Ghasemi et al., 2009). The deficiency in phenol tolerance of the nlxA mutant (Table 2) is consistent with a previous report indicating that phenol and phenolic compounds cause an injury of membrane permeability of Escherichia coli cells (Heipieper et al., 1991). Thus, our results further support the involvement of NlxA in LPS and EPS production.
In conclusion, a novel gene, designated as nlxA, in the LPS gene cluster of X. citri ssp. citri was characterized. Our results indicate that the nlxA gene might function as a rhamnosyltransferase to transfer rhamnose in the LPS O‐antigen biosynthesis. In addition, our results indicate that nlxA plays an important role in EPS production, biofilm formation, stress resistance, motility on semi‐solid plates, virulence and in planta growth in the host plant grapefruit.
EXPERIMENTAL PROCEDURES
Bacterial strains, growth conditions, plasmids
The bacterial strains and plasmids used in this study are listed in Table 4. The wild‐type strain of X. citri ssp. citri 306 and its derivatives were cultured at 28 °C in NB (Difco, Detroit, IL, USA) and on NA (Difco) plates. Escherichia coli strain was cultured at 37 °C in Luria–Bertani (LB) medium. When required, the growth media were supplemented with ampicillin (50 µg/mL), chloramphenicol (20 µg/mL), gentamycin (5 µg/mL), kanamycin (50 µg/mL) and rifamycin (50 µg/mL).
Table 4.
Bacterial strains, plasmids and primers for complementation.
| Strains or plasmids | Relevant characteristic* | Reference or source |
|---|---|---|
| Escherichia coli strain | ||
| EC100D pir+ | F‐ mcrAΔ(mrr‐hsdRMS‐mcrBC) φ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139Δ(ara‐leu)7697 galU galKλ ‐ rpsL nupG pir+ DHFR | Epicentre |
| Xanthomonas citri ssp. citri strains | ||
| 306 | Wild‐type; Rifr | DPI |
| 406G8 | nlxA::EZ‐Tn5 derivative of strain 306; Rifr Kmr | Yan and Wang (2011b) |
| 415H9 | nlxA::EZ‐Tn5 derivative of strain 306; Rifr Kmr | Yan and Wang (2011b) |
| 330D3 | wzt::EZ‐Tn5 derivative of strain 306; Rifr Kmr | Yan and Wang (2011b) |
| Plasmids | ||
| pUFR053 | Shuttle vector, IncW Mob+ mob(P) lacZ+Par+ Gmr, | El Yacoubi et al. (2007) |
| p53‐nlxA | A 1362 bp EcoRI‐HindIII fragment containing wild‐type nlxA gene was ligated into pUFR053. Gmr, | This study |
| p53‐3597‐1 | A 1069 bp SacI‐HindIII fragment containing wild‐type XAC3597 gene was ligated into pUFR053. Gmr, | This study |
| p53‐3597‐2 | A 1339 bp SacI‐HindIII fragment containing wild‐type XAC3597 was ligated into pUFR053. Gmr, | This study |
| Primers | ||
| nlxAF1 | 5'‐TAGAATTCATCGTGAATCCGGAGATCCGTTC (EcoRI) | This study |
| nlxAR1 | 5'‐ATAAGCTTAACAACAAGGACTACAGCTGG (HindIII) | This study |
| 3597F1 | 5'‐TCGAGCTCGAATCAAGGCATATGCTCTCG (SacI) | This study |
| 3507F2 | 5'‐TTGAGCTCGATCTCGGGCCGTTCGTCCTG (SacI) | This study |
| 3597R1 | 5'‐TTCAAGCTTACGCGAGCCACTTACCAC (HindIII) | This study |
DPI, Division of Plant Industry of the Florida Department of Agriculture and Consumer Services, Gainesville, FL, USA.
Gmr, Kmr and Rifr indicate resistance to gentamycin, kanamycin and rifamycin, respectively; for primers, specific restriction sites of the primers are shown in italic and the names of the restriction enzymes are given.
Preparation of LPS and SDS‐PAGE analysis
For the extraction of LPS, X. citri ssp. citri strains were cultured for 24 h at 28 °C in NB medium with shaking (200 rpm). The cell populations of the wild‐type, mutant and complementary strains were tested by measuring OD600 values of the bacterial cultures at the time point of sampling. No significant differences were observed in the OD600 values among the wild‐type, mutant and complementary strains (Fig. S1). Ten millilitres of the cultures were collected and centrifuged for 40 min at 3202 g. LPS from the harvested bacterial cells was extracted with a 50% phenol–water solution and treated with proteinase K following a previous report (Nesper et al., 2000). Ten microlitres of LPS sample were separated by SDS‐PAGE and visualized using silver staining following the manufacturer's instructions (Bio‐Rad Laboratories, Inc., Hercules, CA, USA). Standard LPS from Salmonella enterica serovar Typhimurium was obtained from Sigma (St. Louis, MO, USA). The experiment was repeated three times with similar results, and the result of only one experiment is presented.
Construction of complementary vectors
To complement the 406G8 and 415H9 mutants, three different DNA fragments were cloned into the shuttle vector pUFR053. The primers used for the amplification of different fragments are listed in Table 4. As shown in Fig. 1, fragment 1 (F1) was amplified using primers nlxAF1/nlxAR1 and only contains the nlxA gene. F2 and F3, containing only XAC3597 with different sizes of the putative promoter region, were amplified using primers 3597F1/3597R1 and 3597F2/3597R1, respectively. The PCR products were then cloned into the shuttle vector pUFR053 using the restriction enzyme sites given in Table 4. The resulting plasmids p53‐nlxA, p53‐3597‐1 and p53‐3597‐2 (Fig. 1), respectively, were transformed into the mutants.
EPS and capsule assays
EPS assay was performed as described previously (Vojnov et al., 1998). Briefly, X. citri ssp. citri strains were grown for 24 h in NB containing 2% glucose at 28 °C with shaking at 200 rpm. The cell populations of the wild‐type, mutant and complementary strains were tested by measuring OD600 values of the bacterial cultures at the time point of sampling. No significant differences in the cell populations were observed among the wild‐type, mutant and complementary strains. The cells were removed from 10‐mL portions of the cultures by centrifugation (3202 g for 40 min). The EPS in the supernatant was precipitated by centrifugation after the addition of three volumes of 99% ethyl alcohol, and the EPS pellet was weighed after drying. The experiments were repeated three times independently with four replicates each time.
A capsule‐staining kit (Eng Scientific Inc., Clifton, NJ, USA) was used for capsule assay as described previously (Guo et al., 2010).
Biofilm formation assay
Biofilm formation in glass tubes and on leaf surfaces was performed as described previously (Yan and Wang, 2011a). Briefly, fresh bacterial cells were collected from NA plates and re‐inoculated into sterilized NB to obtain a final concentration of 108 colony‐forming units (CFU)/mL. Rifamycin (50 µg/mL) was added to the bacterial suspensions to avoid potential contamination. For biofilm formation assay in glass tubes, 1 mL of the cell suspension was added to sterilized borosilicate glass tubes. The tubes were kept for 24 h in a humidified chamber at 28 °C without shaking. The cultures were then discarded and the tubes were washed three times using tap water. The biofilm formation on the tubes was visualized by staining with 0.1% crystal violet. For biofilm formation assay on leaf surfaces, 20 µL of the cell suspension were dropped onto the citrus leaf surfaces. The leaves were kept for 10 or 24 h in a humidified chamber at 28 °C without shaking. The biofilm formed on the leaf surfaces was stained with 0.1% crystal violet and rinsed three times using tap water. The stain remaining in cells in glass tubes or on leaf surfaces was dissolved in 95% ethanol and quantified by measuring the optical density at 590 nm using an Agilent 8453 UV–visible spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA, USA). The average of four replicates was used for quantitative measurement. The biofilm assays were repeated three times with similar results with four replicates each time.
Stress tolerance assay
The wild‐type and its derivatives were cultured in NB at 28 °C with shaking at 200 rpm. The early stationary stage (20 hpi) cells were collected and challenged with different toxic compounds. Before challenging by different chemicals, the cell populations of the wild‐type, mutant and complementary strains were tested by measuring the living cells in the samples. No significant differences in the cell populations were observed among the wild‐type, mutant and complementary strains (Table 2). For H2O2, the bacteria were incubated for 40 min with a final concentration of H2O2 of 20 mm. For polymyxin B, the bacteria were incubated for 90 min with a final concentration of polymyxin B of 15 µg/mL. For SDS, the bacteria were incubated for 10 min with a final concentration of SDS of 0.1% (w/v). For phenol, the bacteria were incubated for 15 min with a final concentration of phenol of 10 mm. For CuSO4 and ZnSO4, the bacteria were incubated for 8 min with a final concentration of 10 mm for both CuSO4 and ZnSO4. The living cells were counted by serial dilution on NA plates containing the corresponding antibiotics. The experiments were repeated three times independently with four replicates each time.
Motility assays
Swimming and swarming motilities were examined as described previously (Li and Wang, 2011) on NA plates containing 0.3% (w/v) agar (Difco, Franklin Lakes, NJ, USA) and 0.7% (w/v) agar, respectively. The experiment was repeated three times with similar results.
TEM analysis of flagella
The wild‐type and its derivatives were cultured overnight on semi‐solid NA medium (NB + 0.5% agar) in a glass tube at 28 °C. Cells were resuspended by adding 1 mL of sterilized distilled water to the tube and incubating for 15 min without shaking. The bacterial suspension was loaded onto copper grids. The grids were stained with 1% (v/v) uranyl acetate for 1 min and washed three times with water. Samples were examined using a Philips FEI Morgagni 268 transmission electron microscope (FEI Company, Eindhoven, the Netherlands) operating at 60 kV.
Pathogenicity and in planta growth assays
The pathogenicity assay was performed using two different methods as described elsewhere (Yan and Wang, 2011a) in a quarantine glasshouse facility at the Citrus Research and Education Center, Lake Alfred, FL, USA. Briefly, fully expanded, immature leaves of grapefruit (Citrus paradise Macf. Cv. ‘Duncan’) were used. The X. citri ssp. citri strains were cultured for 2 days on NA plates at 28 °C and were resuspended in sterile tap water. For the pathogenicity assays, a bacterial suspension (108 CFU/mL) was sprayed onto the leaf surface, and two different bacterial suspensions (108 and 106 CFU/mL) were injected into the intercellular spaces of leaves with a needleless syringe. All the tests were repeated three times independently.
For the in planta growth assay, fresh bacterial cells were collected from NA plates and resuspended in sterile tap water at a concentration of 5 × 105 CFU/mL and infiltrated into the leaves of cv. Duncan grapefruit with a needleless syringe. For co‐inoculation experiments, equal volumes of cell suspensions (106 CFU/mL) of wild‐type and mutant were mixed and infiltrated into the leaves using the same method. Leaf discs (leaf area, 1 cm2) from inoculated leaves were excised with a cork borer and ground in 1 mL of sterile tap water. The samples were serially diluted and plated onto NA plates with appropriate antibiotics. The bacterial colonies were counted after incubation at 28 °C for 3 days. The in planta growth was measured in quadruplicate and the assays were repeated three times independently.
RNA extraction and quantitative reverse transcription‐polymerase chain reaction (QRT‐PCR)
Bacterial cells for RNA extraction were cultured in liquid or on plates. For experiments in liquid, the wild‐type strain was inoculated into sterilized NB with a start concentration of 107 CFU/mL and incubated for 20 h at 28 °C with shaking at 200 rpm. For experiments on plates, 10 mL of bacterial suspension (108 CFU/mL) were spread onto the surface of a semi‐solid plate (NB with 0.5% agar) and the excess liquid was removed with Kim wipes. The plates were allowed to dry with the lid off for 10 min on the bench top and then shifted to 28 °C for 24 h. The bacterial cells were harvested by centrifugation (3202 g for 20 min) and treated with RNA Protect Bacterial Reagent (Qiagen, Valencia, CA, USA). Bacterial total RNA was extracted using an RNeasy Mini Kit (Qiagen). Contaminated genomic DNA was removed by treatment with a TURBO DNA‐free kit (Ambion, Austin, TX, USA). The concentration of RNA was determined with a NanoDrop ND‐1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and adjusted to 50 ng/µL.
The RNA obtained was subjected to one‐step QRT‐PCR assay with a 7500 fast real‐time PCR system (Applied Biosystems, Foster City, CA, USA) using a QuantiTect SYBR green RT‐PCR kit (Qiagen). The gene‐specific primers listed in Table 3 were designed to generate products of 100–250 bp based on the genome sequence of 306. The 16S rRNA (forward primer, CGCTTTCGTGCCTCAGTGTCAGTGTTGG; reverse primer, GGCGTAAAGCGTGCGTAGGTGGTGGTT) was used as an endogenous control. The relative fold change of target gene expression was calculated using the formula 2–ΔΔCT (Livak and Schmittgen, 2001). Three biological replicates were used for each strain. QRT‐PCR was repeated once with another three independent biological replicates.
Supporting information
Fig. S1 Growth curve of the 406G8 mutant and the wild‐type strain 306 in Nutrient Broth (NB). Fresh bacterial cells were collected from nutrient agar (NA) plates and resuspended in a flask containing 30 mL sterilized NB with a start concentration of OD600 0.03. The flasks were kept at 28°C with shaking at 200 rpm. Bacterial population (OD600) was monitored at different time points as indicated. This experiment was repeated three times with three replicates each time. Similar results were observed at each time and the result of only one experiment was present. Vertical bars represent the standard errors of the mean.
Supporting info item
ACKNOWLEDGEMENTS
This work was supported by the Citrus Research and Development Foundation, Lake Alfred, FL, USA.
REFERENCES
- Bentley, S.D. , Aanensen, D.M. , Mavroidi, A. , Saunders, D. , Rabbinowitsch, E. , Collins, M. , Donohoe, K. , Harris, D. , Murphy, L. and Quail, M.A. (2006) Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2, e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell, G. and Wojtaszek, P. (1997) Mechanisms for the generation of reactive oxygen species in plant defence—a broad perspective. Physiol. Mol. Plant Pathol. 51, 347–366. [Google Scholar]
- Bolwell, G.P. , Bindschedler, L.V. , Blee, K.A. , Butt, V.S. , Davies, D.R. , Gardner, S.L. , Gerrish, C. and Minibayeva, F. (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three‐component system. J. Exp. Bot. 53, 1367–1376. [PubMed] [Google Scholar]
- Borges, D. and Vendruscolo, C.T. (2007) Xanthan synthesized by strains of Xanthomonas campestris pv. pruni: production, viscosity and chemical composition. Biosci. J. 23, 67–73. [Google Scholar]
- Braun, S.G. , Meyer, A. , Holst, O. , Pühler, A. and Niehaus, K. (2005) Characterization of the Xanthomonas campestris pv. campestris lipopolysaccharide substructures essential for elicitation of an oxidative burst in tobacco cells. Mol. Plant–Microbe Interact. 18, 674–681. [DOI] [PubMed] [Google Scholar]
- Brunings, A. and Gabriel, D. (2003) Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 4, 141–157. [DOI] [PubMed] [Google Scholar]
- Casabuono, A. , Petrocelli, S. , Ottado, J. , Orellano, E.G. and Couto, A.S. (2011) Structural analysis and involvement in plant innate immunity of Xanthomonas axonopodis pv. citri lipopolysaccharide. J. Biol. Chem. 286, 25 628–25 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civerolo, E. (1984) Bacterial canker disease of citrus. J. Rio Grande Val. Hortic. Soc. 37, 127–146. [Google Scholar]
- Coutinho, P.M. , Deleury, E. , Davies, G.J. and Henrissat, B. (2003) An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328, 307–317. [DOI] [PubMed] [Google Scholar]
- Dow, J. , Osbourn, A. , Wilson, T. and Daniels, M. (1995) A locus determining pathogenicity of Xanthomonas campestris is involved in lipopolysaccharide biosynthesis. Mol. Plant–Microbe Interact. 8, 768–777. [DOI] [PubMed] [Google Scholar]
- El Yacoubi, B. , Brunings, A. , Yuan, Q. , Shankar, S. and Gabriel, D. (2007) In planta horizontal transfer of a major pathogenicity effector gene. Appl. Environ. Microbiol. 73, 1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, B.C. and Beattie, G.A. (2008) An overview of plant defenses against pathogens and herbivores. Plant Health Instr. DOI: 10.1094/PHI‐I‐2008‐0226‐01. [Google Scholar]
- Ghasemi, K. , Ghasemi, Y. and Ebrahimzadeh, M. (2009) Antioxidant activity, phenol and flavonoid contents of 13 Citrus species peels and tissues. Pak. J. Pharm. Sci. 22, 277–281. [PubMed] [Google Scholar]
- Gottwald, T.R. , Graham, J.H. and Schubert, T.S. (2002) Citrus canker: the pathogen and its impact. Plant Health Prog. DOI: 10.1094/PHP‐2002‐0812‐1001‐RV. Available at http://www.plantmanagementnetwork.org/php/. [Google Scholar]
- Graham, J. , Gottwald, T. , Cubero, J. and Achor, D. (2004) Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 5, 1–15. [DOI] [PubMed] [Google Scholar]
- Guo, Y. , Sagaram, U.S. , Kim, J.‐S. and Wang, N. (2010) Requirement of the galU gene for polysaccharide production by and pathogenicity and growth in planta of Xanthomonas citri subsp. citri . Appl. Environ. Microbiol. 76, 2234–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heipieper, H.J. , Keweloh, H. and Rehm, H.J. (1991) Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli . Appl. Environ. Microbiol. 57, 1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson, P. , Kenne, L. and Lindberg, B. (1975) Structure of the extracellular polysaccharide from Xanthomonas campestris . Carbohydr. Res. 45, 275–282. [DOI] [PubMed] [Google Scholar]
- Kemp, B.P. , Horne, J. , Bryant, A. and Cooper, R.M. (2004) Xanthomonas axonopodis pv. manihotis gumD gene is essential for EPS production and pathogenicity and enhances epiphytic survival on cassava (Manihot esculenta). Physiol. Mol. Plant Pathol. 64, 209–218. [Google Scholar]
- Kumar, N. , Ebel, R.C. and Roberts, P.D. (2011) H2O2 metabolism during sweet orange (Citrus sinensis L. Osb.) ‘Hamlin’Xanthomonas axonopodis pv. citri interaction. Sci. Hortic. 128, 465–472. [Google Scholar]
- Li, J. and Wang, N. (2011) The wxacO gene of Xanthomonas citri subsp. citri encodes a protein with a role in lipopolysaccharide biosynthesis, biofilm formation, stress tolerance and virulence. Mol. Plant Pathol. 12, 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K. and Schmittgen, T. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2–DeltaDeltaCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Martinez‐Fleites, C. , Proctor, M. , Roberts, S. , Bolam, D.N. , Gilbert, H.J. and Davies, G.J. (2006) Insights into the synthesis of lipopolysaccharide and antibiotics through the structures of two retaining glycosyltransferases from family GT4. Chem. Biol. 13, 1143–1152. [DOI] [PubMed] [Google Scholar]
- Molinaro, A. , Lanzetta, R. , Evidente, A. , Parrilli, M. and Holst, O. (1999) Isolation and characterisation of the lipopolysaccharide from Xanthomonas hortorum pv. vitians . FEMS Microbiol. Lett. 181, 49–53. [DOI] [PubMed] [Google Scholar]
- Molinaro, A. , De Castro, C. , Lanzetta, R. , Parrilli, M. , Petersen, B.O. , Broberg, A. and Duus, J.Ø. (2002) NMR and MS evidences for a random assembled O‐specific chain structure in the LPS of the bacterium Xanthomonas campestris pv. vitians . Eur. J. Biochem. 269, 4185–4193. [DOI] [PubMed] [Google Scholar]
- Molinaro, A. , Silipo, A. , Lanzetta, R. , Newman, M.A. , Dow, J.M. and Parrilli, M. (2003) Structural elucidation of the O‐chain of the lipopolysaccharide from Xanthomonas campestris strain 8004. Carbohydr. Res. 338, 277–281. [DOI] [PubMed] [Google Scholar]
- Nesper, J. , Kapfhammer, D. , Klose, K. , Merkert, H. and Reidl, J. (2000) Characterization of Vibrio cholerae O1 antigen as the bacteriophage K139 receptor and identification of IS1004 insertions aborting O1 antigen biosynthesis. J. Bacteriol. 182, 5097–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, M.A. , Dow, J. and Daniels, M. (2001) Bacterial lipopolysaccharides and plant–pathogen interactions. Eur. J. Plant Pathol. 107, 95–102. [Google Scholar]
- Patil, P.B. , Bogdanove, A.J. and Sonti, R.V. (2007) The role of horizontal transfer in the evolution of a highly variable lipopolysaccharide biosynthesis locus in xanthomonads that infect rice, citrus and crucifers. BMC Evol. Biol. 7, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz, C.R.H. and Whitfield, C. (2002) Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves, P. and Wang, L. (2002) Genomic organization of LPS‐specific loci. Curr. Top. Microbiol. Immunol. 264, 109–126. [PubMed] [Google Scholar]
- Rigano, L. , Siciliano, F. , Enrique, R. , Sendín, L. , Filippone, P. , Torres, P. , Qüesta, J. , Dow, J. , Castagnaro, A. and Vojnov, A. (2007) Biofilm formation, epiphytic fitness, and canker development in Xanthomonas axonopodis pv. citri . Mol. Plant–Microbe Interact. 20, 1222–1230. [DOI] [PubMed] [Google Scholar]
- Schaffer, C. , Wugeditsch, T. , Neuninger, C. and Messner, P. (1996) Are S‐layer glycoproteins and lipopolysaccharides related? Microb. Drug Resist. 2, 17–23. [DOI] [PubMed] [Google Scholar]
- Shashkov, A.S. , Senchenkova, S.N. , Laux, P. , Ahohuendo, B.C. , Kecskés, M.L. , Rudolph, K. and Knirel, Y.A. (1999) Structure of the O‐chain polysaccharide of the lipopolysaccharide of Xanthomonas campestris pv. manihotis GSPB 2755 and GSPB 2364. Carbohydr. Res. 323, 235–239. [DOI] [PubMed] [Google Scholar]
- da Silva, A.C. , Ferro, J.A. , Reinach, F.C. , Farah, C.S. , Furlan, L.R. , Quaggio, R.B. , Monteiro‐Vitorello, C.B. , Van Sluys, M.A. , Almeida, N.F. , Alves, L.M. , do Amaral, A.M. , Bertolini, M.C. , Camargo, L.E. , Camarotte, G. , Cannavan, F. , Cardozo, J. , Chambergo, F. , Ciapina, L.P. , Cicarelli, R.M. , Coutinho, L.L. , Cursino‐Santos, J.R. , El‐Dorry, H. , Faria, J.B. , Ferreira, A.J. , Ferreira, R.C. , Ferro, M.I. , Formighieri, E.F. , Franco, M.C. , Greggio, C.C. , Gruber, A. , Katsuyama, A.M. , Kishi, L.T. , Leite, R.P. , Lemos, E.G. , Lemos, M.V. , Locali, E.C. , Machado, M.A. , Madeira, A.M. , Martinez‐Rossi, N.M. , Martins, E.C. , Meidanis, J. , Menck, C.F. , Miyaki, C.Y. , Moon, D.H. , Moreira, L.M. , Novo, M.T. , Okura, V.K. , Oliveira, M.C. , Oliveira, V.R. , Pereira, H.A. , Rossi, A. , Sena, J.A. , Silva, C. , de Souza, R.F. , Spinola, L.A. , Takita, M.A. , Tamura, R.E. , Teixeira, E.C. , Tezza, R.I. , Trindade dos Santos, M. , Truffi, D. , Tsai, S.M. , White, F.F. , Setubal, J.C. and Kitajima, J.P. (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature, 417, 459–463. [DOI] [PubMed] [Google Scholar]
- Steiner, K. , Novotny, R. , Patel, K. , Vinogradov, E. , Whitfield, C. , Valvano, M.A. , Messner, P. and Schaffer, C. (2007) Functional characterization of the initiation enzyme of S‐layer glycoprotein glycan biosynthesis in Geobacillus stearothermophilus NRS 2004/3a. J. Bacteriol. 189, 2590–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, K. , Hagelueken, G. , Messner, P. , Schäffer, C. and Naismith, J.H. (2010) Structural basis of substrate binding in WsaF, a rhamnosyltransferase from Geobacillus stearothermophilus . J. Mol. Biol. 397, 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme, D.J. , Kemen, E. , MacLean, D. , Schornack, S. , Aritua, V. , Thwaites, R. , Grant, M. , Smith, J. and Jones, J.D.G. (2010) Genome‐wide sequencing data reveal virulence factors implicated in banana Xanthomonas wilt. FEMS Microbiol. Lett. 310, 182–192. [DOI] [PubMed] [Google Scholar]
- Toguchi, A. , Siano, M. , Burkart, M. and Harshey, R.M. (2000) Genetics of swarming motility in Salmonella enterica serovar Typhimurium: critical role for lipopolysaccharide. J. Bacteriol. 182, 6308–6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvano, M.A. (2003) Export of O‐specific lipopolysaccharide. Front. Biosci. 8, S452–S471. [DOI] [PubMed] [Google Scholar]
- Vojnov, A. , Zorreguieta, A. , Dow, J. , Daniels, M. and Dankert, M. (1998) Evidence for a role for the gumB and gumC gene products in the formation of xanthan from its pentasaccharide repeating unit by Xanthomonas campestris . Microbiology, 144, 1487–1493. [DOI] [PubMed] [Google Scholar]
- Vojnov, A.A. , Morais do Amaral, A. , Dow, J.M. , Castagnaro, A.P. and Marano, M.R. (2010) Bacteria causing important diseases of citrus utilise distinct modes of pathogenesis to attack a common host. Appl. Microbiol. Biotechnol. 87, 467–477. [DOI] [PubMed] [Google Scholar]
- Vorholter, F. , Niehaus, K. and Pühler, A. (2001) Lipopolysaccharide biosynthesis in Xanthomonas campestris pv. campestris: a cluster of 15 genes is involved in the biosynthesis of the LPS O‐antigen and the LPS core. Mol. Genet. Genomics, 266, 79–95. [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Frye, J.G. , McClelland, M. and Harshey, R.M. (2004) Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol. Microbiol. 52, 169–187. [DOI] [PubMed] [Google Scholar]
- Yan, Q. and Wang, N. (2011a) The ColR/ColS two‐component system plays multiple roles in the pathogenicity of the citrus canker pathogen Xanthomonas citri subsp. citri . J. Bacteriol. 193, 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Q. and Wang, N. (2011b) High‐throughput screening and analysis of genes of Xanthomonas citri subsp. citri involved in citrus canker symptom development. Mol. Plant–Microbe Interact. 25, 69–84. [DOI] [PubMed] [Google Scholar]
- Zeidler, D. , Zähringer, U. , Gerber, I. , Dubery, I. , Hartung, T. , Bors, W. , Hutzler, P. and Durner, J. (2004) Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc. Natl. Acad. Sci. USA, 101, 15 811–15 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Growth curve of the 406G8 mutant and the wild‐type strain 306 in Nutrient Broth (NB). Fresh bacterial cells were collected from nutrient agar (NA) plates and resuspended in a flask containing 30 mL sterilized NB with a start concentration of OD600 0.03. The flasks were kept at 28°C with shaking at 200 rpm. Bacterial population (OD600) was monitored at different time points as indicated. This experiment was repeated three times with three replicates each time. Similar results were observed at each time and the result of only one experiment was present. Vertical bars represent the standard errors of the mean.
Supporting info item


