Summary
The ascomycete Hymenoscyphus pseudoalbidus (anamorph Chalara fraxinea) causes a lethal disease known as ash dieback on Fraxinus excelsior and Fraxinus angustifolia in Europe. The pathogen was probably introduced from East Asia and the disease emerged in Poland in the early 1990s; the subsequent epidemic is spreading to the entire native distribution range of the host trees. This pathogen profile represents a comprehensive review of the state of research from the discovery of the pathogen and points out knowledge gaps and research needs.
Taxonomy
Members of the genus Hymenoscyphus (Helotiales, Leotiomycetidae, Leotiomycetes, Ascomycota) are small discomycetes which form their ascomata on dead plant material. A phylogeny based on the internal transcribed spacers (ITSs) of the rDNA indicated the avirulent Hymenoscyphus albidus, a species native to Europe, as the closest relative of H. pseudoalbidus.
Symptoms
Hymenoscyphus pseudoalbidus causes necrotic lesions on leaves, twigs and stems, eventually leading to wilting and dieback of girdled shoots. Bark lesions are characterized by a typical dark‐ to cinnamon‐brown discoloration.
Life cycle
Hymenoscyphus pseudoalbidus is heterothallic and reproduces sexually on ash petioles in the litter once a year. Ascospores are wind dispersed and infect ash leaves during the summer. The asexual spores only serve as spermatia.
Tools and techniques
The most important techniques for fungal handling, such as detection, isolation, culturing, storage, crossing and ascocarp production, are briefly described.
Management
Once the disease is established, management is hardly possible. The occurrence of a small fraction of partially tolerant trees constitutes hope for resistance breeding in the future. Healthy‐looking trees should be preserved.
Introduction
At the beginning of the 1990s, severe dieback symptoms on European ash (Fraxinus excelsior L.) were observed for the first time in north‐eastern Poland. Currently, a large part of the native distribution area of F. excelsior is affected by this potentially lethal disease (Pautasso et al., 2013). The causal agent was detected by Kowalski (2001) and later described as a new mitosporic ascomycete, Chalara fraxinea (Kowalski, 2006). The sexual stage was initially morphologically assigned to Hymenoscyphus albidus, a widespread discomycete indigenous to Europe (Kowalski and Holdenrieder, 2009b). However, based on molecular data, Queloz et al. (2011) recognized the pathogen as a new cryptic species and named its teleomorph Hymenoscyphus pseudoalbidus. Recently, morphological and genetic studies have revealed that H. pseudoalbidus is a pathogen introduced to Europe and most probably originates from East Asia (Zhao et al., 2012).
Before the current epidemic, dieback symptoms on mature ash trees had occasionally been noted in Europe for a long time, but they were interpreted as abiotic damage (caused by frost or drought), as a consequence of excessive fructification, maturation feeding of bark beetles (Leperisinus varius and Hylesinus spp.) or combinations of abiotic and biotic factors (Boudier, 1994; Hulden, 1941; Hull and Gibbs, 1991). Similarly, drought and late frost were suspected as inciting factors during the initial stage of the epidemic in Poland, and the presence of numerous more or less pathogenic fungi on affected trees fuelled discussions concerning their role in disease aetiology (Cech, 2006; Przybył, 2002; Pukacki and Przybył, 2005). Although it is sometimes rather difficult to isolate H. pseudoalbidus from symptomatic plant tissues, it is now beyond dispute that H. pseudoalbidus is the causal agent that threatens European ash (F. excelsior) and narrow‐leaved ash (F. angustifolia) in Europe.
The main aim of this review is to summarize the current state of knowledge concerning the emerging ash pathogen H. pseudoalbidus (anamorph: Chalara fraxinea). The review provides information on the following topics: (i) pathogen morphology and taxonomy; (ii) population genetics; (iii) life cycle and reproductive mode; (iv) disease symptoms and host range; (v) host–pathogen interaction; (vi) epidemiology; (vii) ecology; (viii) techniques for pathogen detection and handling; and finally (ix) knowledge gaps and research needs based on implications from other pathosystems. The literature review incorporates, to the best of our knowledge, most relevant knowledge concerning H. pseudoalbidus published until May 2013.
Morphology and Taxonomy
Hymenoscyphus pseudoalbidus is a discomycete that forms numerous white‐stalked apothecia (mostly up to 3 mm in diameter, rarely 8 mm) on the leaf litter of the previous year (Fig. 1a,b). The hymenium is composed of cylindrical paraphyses (1.8–2.4 μm thick, enlarged to 3 μm at the apex) and cylindrical‐clavate asci (80–107 × 8–10 μm), showing a positive iodine reaction and forming hyaline single‐celled ascospores (13–21 × 3.5–5.0 μm) (Fig. 1h). During germination, the ascospores become melanized and single septate in most cases, and appressoria and/or germ tubes are formed (Fig. 1i–l). The asexual stage (C. fraxinea) is characterized by brown phialides (16–24 × 4–5 μm) (Fig. 1s–v), which form short cylindrical hyaline single‐celled conidia (3.2–4.0 × 2.0–2.5 μm) in slimy spore droplets and, occasionally, also in chains. Only the first‐formed conidium is clavate and 6–7 μm long (Kowalski, 2001, 2006) (Fig. 1v). The substrate colonized by H. pseudoalbidus (leaf debris in the litter, occasionally also small stems) becomes blackened by a conspicuous pseudosclerotial layer (Fig. 1a–g) on which the apothecia develop during the summer (Gross and Holdenrieder, 2013; Kowalski and Holdenrieder, 2009b).
Figure 1.

Morphology of Hymenoscyphus pseudoalbidus: (a) mature apothecia on an ash petiole, top view; (b) ascomata, side view; (c) long‐stalked apothecia formed in a moist chamber; see also the small ascomata initials on the surface of the pseudosclerotium; (d) immature apothecia on a small ash stem; (e) apothecia formed on pseudosclerotial leaflet veins; (f) surface view of a pseudosclerotial plate; (g) several pseudosclerotia, separated from each other by demarcation zones, on an ash petiole; (h) freshly ejected ascospores (in water); (i–l) germinating ascospores on malt extract agar, forming germ tubes (gt) and appressoria (ap); (m) ascospores on a cover glass forming appressorial rings (ar); (n) ascospore germinating on cellophane on malt extract agar (MEA) with ash leaves; note two types of exudate: a hyaline mucilage (h) surrounding the spore, and a melanin‐like exudate (l) surrounding the appressorium; (o, p) germinating ascospores on the upper side of a leaflet; note the formation of appressoria (ap), appressorial rings (ar) and surface hyphae (sh); (q, r) an ascospore that germinated on cellophane on water agar, forming two appressoria (ap), entering the substrate and spreading within the cellophane by flattened hyphae (fh) (surface view and deeper level of the same object, in lactophenol blue); (s–v) anamorphic state (Chalara fraxinea): (s,t) groups of phialides formed on the lower side of a necrotic leaflet; (u) phialides formed in culture on MEA; (v) spore droplet on the top of a phialide. Unless otherwise specified, the specimens for microscopy were mounted in lactic acid.
When Kowalski and Holdenrieder (2009b) recognized the teleomorph of C. fraxinea, they tentatively identified it as H. albidus morphologically. However, H. albidus was described in 1850 as Peziza albida Roberge ex Desm. (Rehm, 1896), is considered to be widespread in Europe and has never been reported to be pathogenic. Queloz et al. (2011) molecularly identified the pathogen as a separate cryptic species and named it H. pseudoalbidus (see also the corrigendum, Queloz et al., 2012). The authors collected apothecia from diseased and healthy ash stands and compared them in terms of three genetic sequence markers [internal transcribed spacer (ITS), elongation factor‐1‐α (EF1‐α), calmodulin (CAL)] and by genetic fingerprinting (inter‐simple sequence repeat‐polymerase chain reaction, ISSR‐PCR). Phylogenetic analysis allowed the distinction of two groups, with specimens collected in diseased stands always grouping together and specimens from healthy stands almost exclusively belonging to the other group. Using similar methods, other authors independently confirmed this finding (Feau et al., 2011; Husson et al., 2011). Later, Bengtsson et al. (2012) and Gross et al. (2012b) were able to separate the two cryptic species based on microsatellite (MS) markers. Furthermore, Gross et al. (2012c) showed that the two closely related species exhibit different mating systems (see below). Recently, the presence of croziers at the ascus base of H. pseudoalbidus has been described as an unequivocal morphological distinguishing feature between the two sister species H. pseudoalbidus and H. albidus (Zhao et al., 2012).
The genus Hymenoscyphus belongs to the family Helotiaceae (Helotiales, Leotiomycetidae, Leotiomycetes, Pezizomycotina, Ascomycota). This genus includes more than 150 species worldwide (Kirk et al., 2008) and new species continue to be discovered (Han and Shin, 2008; Zheng and Zhuang, 2013). A phylogeny based on the ITS regions of the rDNA indicated the avirulent H. albidus, a species native to Europe, as the closest known relative of H. pseudoalbidus (V. Queloz, unpublished data). Most Hymenoscyphus species are regarded as saprotrophic decomposers of plant material and, before the emergence of ash dieback, none of the described Hymenoscyphus species was known to cause plant diseases (Wang et al., 2006). However, some forest pathogens within the Helotiaceae are known, e.g. Gremmeniella abietina, causes Brunchorstia disease in conifers, Cenangium ferruginosum causes Cenangium dieback of pines and Crumenulopsis sororia causes bark cankers on pines. Important tree pathogens are also found in other helotialean families, e.g. Lachnellula willkommii (Hyaloscyphaceae), which causes cankers on European larch (Larix decidua), and Phacidium infestans (Phacidiaceae), a snow mould on Swiss stone pine (Pinus cembra) (Butin, 2011). In addition, many species within the Helotiales are endophytic and tend to shift to a pathogenic lifestyle if the host is under stress (Sieber, 2007).
Diversity, Population Differentiation and Distribution of H. pseudoalbidus Populations
Hymenoscyphus albidus, the indigenous cryptic sister species of the pathogen, is mainly known from Europe (http://data.gbif.org/species/2582400/). However, Hosoya et al. (1993) reported Lambertella albida (a synonym of H. albidus) from petioles of decaying leaves of Fraxinus mandshurica var. japonica, as new to Japan. Recently, Zhao et al. (2012) sequenced four genomic DNA markers [ITS, the D1/D2 region of the large subunit (LSU) rDNA gene (28S rDNA), the CAL gene and the translation factor EF1‐α] of six specimens of Japanese L. albida, including the three specimens originally identified by Hosoya et al. (1993). Combining the dataset of Queloz et al. (2011), who also used ITS, CAL and EF1‐α for species delimitation, with the DNA sequences of Japanese specimens, Zhao et al. (2012) showed that L. albida from Japan is actually conspecific with H. pseudoalbidus, and that the sequence diversity among Japanese specimens is considerably higher than that among European ones. A subsequent study incorporating large Japanese and European H. pseudoalbidus populations confirmed the finding of a greater genetic diversity in Japan (Gross et al., 2012a). This led to the conclusion that the fungus has been introduced to Europe from eastern Asia.
Although more detailed knowledge about Asian H. pseudoalbidus population genetics is yet to be obtained, some data are already available for Europe. Using ISSR‐PCR fingerprinting, Rytkönen et al. (2011) distinguished 14 haplotypes among 32 isolates that originated from Finland (20), Estonia (11) and Latvia (1). No measures of population differentiation were provided, but the observed high genotypic diversity indicated sexual reproduction. Kraj et al. (2012) also found a high genotypic diversity within 159 Polish isolates originating from five different regions, which were screened with five different ISSR‐PCR primers. The authors further detected a significant distinction between highland and lowland populations. This remains the only study that has found evidence for a genetic structure among H. pseudoalbidus populations in Europe. Other analyses assessed the population biology of the pathogen using MS markers. These markers are easily reproducible, co‐dominant and evolve rapidly. They facilitate the study of allelic frequencies and are thus more powerful than ISSR‐PCR fingerprinting techniques (Schlotterer, 2004; Sunnucks, 2000). Bengtsson et al. (2012) used a combination of arbitrarily primed PCR (AP‐PCR) and eight MS markers to characterize a broad range of isolates derived from 10 different European countries, and found high genotypic diversity, very low allelic richness and near‐zero pairwise population differentiation (F ST ≤ 0.028). Similar results were obtained by Gross et al. (2012b), who used a set of 18 polymorphic MS markers to screen two larger populations of H. pseudoalbidus originating from Germany. Notably, as in the study of Bengtsson et al. (2012), populations revealed near‐zero differentiation in pairwise comparisons (F ST = 0.022) and the majority of MS loci revealed a bi‐allelic nature. Taken together, the population genetic data point to an outbreeding mating system and a dispersal through ascospores (high genotypic diversity), virtually no population structure (very low F ST values; but see Kraj et al., 2012) and signs of a founder effect (low allelic richness).
Life Cycle
To date, most research on ash dieback has focused on the pathosystem F. excelsior–H. pseudoalbidus. If not otherwise specified, we hereafter only refer to this pathosystem.
The entire life cycle of H. pseudoalbidus is completed on Fraxinus leaves. Apothecia are produced during the summer on leaf debris of the previous year (Kirisits and Cech, 2009; Kowalski and Holdenrieder, 2009b; Timmermann et al., 2011). Most apothecia are formed on petioles and rachises (hereafter called petioles) but, occasionally, apothecia are also found on small stems lying on the ground (Kirisits and Cech, 2009; Kirisits et al., 2012; Kowalski and Holdenrieder, 2009b) (Fig. 1d). The main sporulation period is from June to early September, but, under favourable conditions, sporulation can start earlier and last until October (Hietala et al., 2013; Kirisits and Cech, 2009; Kowalski and Holdenrieder, 2009b; Timmermann et al., 2011).
The wind‐dispersed ascospores (Kowalski and Holdenrieder, 2009b; Timmermann et al., 2011) adhere to the leaf surface via a secreted mucilage, which is more or less hyaline around the spore (Fig. 1m,n) and conspicuously melanized around appressoria formed on cellophane on ash leaf agar (Fig. 1n). However, on cellophane laid over water agar, no mucilage is visible (Fig. 1q). Ascospores penetrate the ash leaf cuticle via appressoria (Fig. 1k,o) (Cleary et al., 2013). However, in most cases, the appressorium is reduced to a melanized appressorial ring at the ventral side of the spore (Fig. 1m,p; O. Holdenrieder, unpublished data). In moist chambers, initial lesions on the leaves of ash seedlings occurred 2 weeks after inoculation (Cleary et al., 2013). The further colonization of the leaf has yet to be studied in detail. After massive ascospore inoculation on the upper leaf surface under field conditions (using small Petri dishes containing sporulating apothecia, which were positioned on individual leaflets lying on a support pad), tiny necrotic lesions (approximately 1–3 mm in diameter) developed within 12 days and the inoculated leaflets were shed a few days later without inducing rachis infection (O. Holdenrieder, unpublished data). Leaf shedding was also observed after wound inoculation of the rachis (Kräutler and Kirisits, 2012). On detached leaflets incubated in a moist chamber, necrotic lesions developed within 10 days after inoculation of the adaxial side (O. Holdenrieder, unpublished data). The necrotic lesions on the leaflet lamina frequently spread along the leaf veins and rachis in a proximal direction (Fig. 2b,d) and, occasionally, the pathogen crossed the junction between the petiole and stem, thereby initiating a necrotic lesion on the stem (Kirisits et al., 2009). Stem infection is normally a dead‐end in the life cycle of the pathogen, because fructification occurs only very rarely on dead stems (see above).
Figure 2.

Symptoms of ash dieback: (a) initial stage of leaf infection with many small necrotic spots; from one leaflet, the infection has already spread to the rachis and triggered wilting of distal leaf parts (arrow); (b) necrotic lesion extending along the main leaflet vein in proximal direction (arrow); (c) isolation of fungal mycelia from a surface‐disinfected leaflet fragment containing a necrotic vein as shown in (b); the mycelium of Hymenoscyphus pseudoalbidus is outgrowing only from the vein cuts (arrows); the other mycelia belong to endophytic fungi; (d) initial necrotic lesion on a rachis after infection via the petiolule; the scar left by the shed leaflet is marked by an arrow; (e) necrotic bark lesion on a young stem; (f) necrotic bark lesion on an older stem; (g) wilting shoot in spring; (h) withered shoot distal to a necrotic lesion during summer; (i) withered shoot with attached petioles (and rachises) during winter; (j) internal xylem necrosis initiating a cambial necrosis (arrow); (k) central necrosis still surrounded by healthy peripheral xylem tissues; (l) mature tree dying off and forming epicormic branches.
After leaf fall in the autumn, the fungus starts to produce a characteristic black pseudosclerotial plate on the petioles, occasionally also on leaflet veins and small stems (Fig. 1d–g; Gross and Holdenrieder, 2013; Kirisits et al., 2009; Kowalski and Holdenrieder, 2009b). The pseudosclerotial plate is formed a few cell layers below the substrate surface and the peripheral host tissues are degraded by other organisms until the spring, so that the melanized wall of the pseudosclerotium becomes exposed (Fig. 1a–g). In the following vegetation period, during the second or even the third year after leaf fall, new apothecia develop on the pseudosclerotia and the cycle is closed (Gross and Holdenrieder, 2013; O. Holdenrieder, unpublished data).
The asexual stage occurs during the autumn and winter on ash petioles in the litter, preferentially near developing pseudosclerotial plates (Kowalski and Bartnik, 2010; O. Holdenrieder, unpublished data). Occasionally, phialides can also be found on necrotic leaves (Fig. 1s,t), on wound‐inoculated stems (Kowalski and Holdenrieder, 2009a) and on incubated wood discs (Husson et al., 2012). In vitro, asexual sporulation can be induced on malt extract agar (MEA) by prolonged incubation (1–2 months) at low (5–15 °C) temperatures (Hosoya et al., 1993; Kowalski, 2006; Kowalski and Bartnik, 2010) or on MEA amended with ash leaves (Kirisits et al., 2013). The minute size of the phialoconidia and their inability to germinate on various substrates led to the hypothesis that they act as spermatia (Hosoya et al., 1993; Kirisits et al., 2009). Indirect proof of spermatial function was provided by a parenthood analysis of different apothecia on a single petiole, which showed that not all paternal (fertilizing) strains are found on the same petiole. Consequently, fertilization must occur by the transfer of spermatia from strains on other petioles (Gross et al., 2012c). We do not know during which time of the year fertilization occurs. However, pseudosclerotial petioles collected in January 2013 in Zurich and incubated at 20 °C under 12 h near‐UV light/12 h darkness developed numerous fertile apothecia within 4 weeks (O. Holdenrieder, unpublished data).
Reproductive Mode
Taking into account the high genotypic diversity of H. pseudoalbidus, Bengtsson et al. (2012) suggested a heterothallic mating system. However, outcrossing also occurs in homothallic fungi (Billiard et al., 2012; Pérez et al., 2010; Pontecorvo et al., 1953) and heterothallic fungi are sometimes able to fertilize themselves (Marra et al., 2004). Therefore, the high genotypic diversity of a fungus per se is only an indication of an outbreeding reproductive mode, but no proof of a heterothallic mating system. The molecular characterization of the mating type locus of H. pseudoalbidus and its confirmation in vivo by crossing experiments provided the final proof of the heterothallic mating system of H. pseudoalbidus (Gross et al., 2012c). Moreover, the even distribution of both mating types in two populations originating from the current epidemic periphery (Switzerland) and two populations from the centre (Poland) convincingly demonstrated random mating of all populations and ruled out the hypothesis of an initial clonal distribution (Gross et al., 2012c). In contrast, the avirulent species, H. albidus, has a homothallic mating system (Gross et al., 2012c), does not form an anamorph (Kirisits et al., 2013) and shows a lower genetic diversity (Queloz et al., 2011).
Disease Symptoms and Host Range
Ash dieback is essentially a lethal disease, affecting all age classes from saplings to mature trees (Fig. 2). Symptom development on susceptible hosts starts in late summer with the formation of necrotic lesions on the leaflets (Fig. 2a). The lesions expand preferentially along veins in the proximal direction (Fig. 2b) and spread into the rachis. Thereby, the tissues surrounding the base of the petiolule sometimes remain green at the beginning and the lesion appears first at a short distance from the leaflet or the leaflet scar (Fig. 2d). As soon as the rachis is girdled, the distal part of the leaf wilts and desiccates (Bakys et al., 2009a) (Fig. 2a). Occasionally, direct infection of the rachis might also occur, which is visible as small necrotic lesions without contact to leaflets (Cleary et al., 2013; T. Kowalski, University of Agriculture in Krakow, Krakow, Poland, unpublished data).
The incubation period following ascospore inoculation lasts 10–14 days, but excessive infection pressure leads to premature leaf abscission (see above). Similarly, wound‐infected leaves of ash trees potted outdoors showed initial symptoms after 2 weeks. However, the few necroses developing on the stem originated from natural infections and none developed from the strains used for leaf inoculation (Kräutler and Kirisits, 2012).
In nature, most infected leaves are shed before the pathogen reaches the stem. However, in some cases, the mycelium of the pathogen grows from the petiole into the stem and spreads in the axial direction in the inner shoot tissues (Schumacher et al., 2010). Hyphae preferentially colonize the medullary sheath and the ray parenchyma, but also occur in other tissues ( Fig. 3). The broad hyphae, up to 7 μm wide, grow intracellularly and occasionally form intrahyphal hyphae. At least during the initial stage of infection, no disintegration of the plasmalemma and cell wall of the host cells was observed (Dal Maso et al., 2012; Schumacher et al., 2010).
Figure 3.
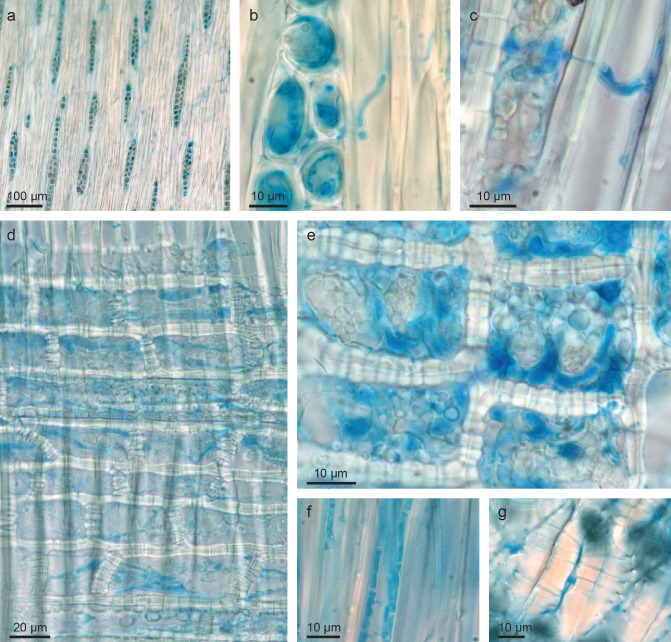
Mycelium of Hymenoscyphus pseudoalbidus in Fraxinus excelsior tissues (stained with lactophenol blue): (a, b) hyphae concentrated in xylem rays (tangential section); (c) hypha growing through a cell wall separating a ray cell from a fibre cell; (d, e) hyphae in xylem ray cells (radial section); (f) hyphae growing in a xylem fibre in an axial direction; (g) hyphae growing within the lumen and pit channels of a bark sclereid.
The xylem infection spreads in the axial direction preferentially within the central tissues and is frequently associated with a brownish discoloration (Fig. 2j,k).
The infection is not systemic, but fungal DNA was occasionally detected up to 10 cm ahead of visible necrotic lesions in apparently healthy inner bark tissue (McKinney et al., 2012b). The radial expansion of the infection leads to the death of bark tissue (Fig. 2j), which is associated with initially dark necrotic lesions that subsequently become characteristically cinnamon brown (Fig. 2e). Bark death is preceded by cambial necrosis (Fig. 2j) (Kowalski, 2001; Kowalski and Holdenrieder, 2008). The lesions on the bark mostly become visible during late autumn and winter, but sometimes not until the spring. This constitutes a risk of pathogen dispersal by facilitating the trade of latently infected plants (Husson et al., 2012; Kirisits et al., 2009). On the necrotic bark, opportunistic fungi (sensu Kirk et al., 2008) develop quickly and complicate diagnosis (see below). As soon as the shoot becomes girdled, the distal part dies. If girdling occurs during late summer, leaflets desiccate, disintegrate and the petioles remain attached during the following winter (Fig. 2i) and are easy to recognize against the background of snow. If girdling occurs during the winter, the buds distal to the infection do not sprout during the following spring. Girdling during the spring or early summer triggers wilting (Fig. 2g). The pathogen continues to spread in the proximal direction and also affects shoot segments older than 1 year. The bark necrosis can cease at the shoot base or a node, but if the stem is split axially, it can be frequently observed that the xylem necrosis expands beyond that point. Even the main stem can become infected via a side branch (Fig. 2e,f). Severely affected trees respond to branch dieback by the formation of epicormic shoots (Fig. 2l) (Engesser et al., 2009; Kowalski and Holdenrieder, 2008; Skovsgaard et al., 2010). If such shoots develop at the stem base, they are perfect entry points for new infections and the tree can die rapidly (Engesser and Meier, 2012; Husson et al., 2012; Metzler and Witzel, 2012). It has been suspected that, under moist conditions at the stem base, lenticels or small wounds can also act as entry points (Husson et al., 2012), but definite proof for this is lacking. Inoculation experiments with colonized wood blocks attached with Parafilm on unwounded annual shoots in summer yielded no infections (O. Holdenrieder, unpublished data).
The pathogen can also colonize parts of the root system (Kowalski, 2001; Kowalski and Lukomska, 2005; Schumacher, 2011; Schumacher et al., 2010), but the roots of severely infected trees are prone to attack by opportunistic fungi (e.g. Armillaria spp.), which accelerates tree death (Bakys et al., 2009b, 2011; Enderle et al., 2013; Husson et al., 2012; Lygis et al., 2006; Skovsgaard et al., 2010). Predisposed trees are also attacked above ground by bark beetles and numerous opportunistic fungi (see below).
Host Range
The natural (original) hosts of H. pseudoalbidus appear to be F. mandshurica and F. chinensis, as these ash species are the only hosts reported from Asia (Hosoya et al., 1993; Zhao et al., 2012). The fungus is not known as a pathogen in Japan (Zhao et al., 2012). In Europe, the known hosts showing disease symptoms include the European ash species F. excelsior (Kowalski, 2006) and F. angustifolia (Kirisits et al., 2010; Schumacher et al., 2007), the North American F. nigra, F. pennsylvanica and F. americana and the Asian F. mandshurica (Drenkhan and Hanso, 2010). Although F. nigra, F. excelsior and F. angustifolia are heavily affected by the disease, F. pennsylvanica is only moderately susceptible, followed by F. mandshurica and F. americana, which appear to be largely tolerant in Estonia. The flowering ash, F. ornus, apparently does not develop the disease under field conditions (Kirisits et al., 2009; see also various contributions in FRAXBACK, 2012), but fructification on naturally infected petioles has been observed (O. Holdenrieder, unpublished data) and F. ornus has been shown to be moderately susceptible in artificial leaf and stem inoculation experiments (Kirisits et al., 2009; Kräutler and Kirisits, 2012). The Asian F. chinensis and F. bungeana, as well as the American F. latifolia, F. pennsylvanica and F. velutina, developed no lesions after wound inoculation on twigs of mature trees in Germany (G. Aas and O. Holdenrieder, unpublished data). However, nursery stock of F. pennsylvanica from Germany was infected naturally and stem inoculation experiments on the remaining healthy plants showed that they were also susceptible (A. Gross, ETH Zurich, Zurich, Switzerland, unpublished data). Unfortunately, data concerning the susceptibility of exotic ash species are very scarce. Moreover, the determination of ash species is difficult and misidentifications frequently occur (Koch et al., 2012). Stem inoculation experiments on other Oleaceae (Forsythia sp., Ligustrum vulgare, Olea europaea, Syringa vulgaris) revealed no necrotic lesions (O. Holdenrieder, unpublished data). The same applies to Acer pseudoplatanus and Sambucus nigra (Kowalski and Holdenrieder, 2009a).
Host–Pathogen Interactions
Inoculation experiments revealed that H. albidus, which is native to Europe, does not cause necrotic lesions on European ash (F. excelsior), whereas the introduced H. pseudoalbidus is pathogenic (Husson et al., 2011; A. Gross and O. Holdenrieder, unpublished data). However, little attention has been paid to virulence differences of H. pseudoalbidus strains so far. Artificial stem inoculations demonstrated the pathogenicity of H. pseudoalbidus in different studies, where all tested isolates were capable of inducing necrotic lesions (Bakys et al., 2009a; Husson et al., 2011; Kowalski and Holdenrieder, 2009a; McKinney et al., 2011; Ogris et al., 2009; Szabo, 2009). Variations in lesion length were observed among strains, but were not significantly different (Bakys et al., 2009a; Husson et al., 2011; Kowalski and Holdenrieder, 2009a). There is a need for virulence assays that incorporate carefully genotyped H. pseudoalbidus isolates and ash clones to balance the factor of host genotypic variability.
Recent studies have shown the presence of partially resistant European ash genotypes in Germany (Enderle et al., 2013; Metzler et al., 2012a), Denmark (Kjær et al., 2012; McKinney et al., 2011), Sweden (Stener, 2012), Lithuania (Pliūra et al., 2011) and Poland (Kowalski et al., 2012). Notably, early leaf senescence correlated significantly with low susceptibility of ash trees in two independent studies (McKinney et al., 2011; Stener, 2012; but see also Kirisits and Freinschlag, 2012). Early leaf shedding might prevent the fungus from growing into the stem. Moreover, a significantly slower necrosis development was observed on early‐senescing ash trees relative to late‐senescing ones (McKinney et al., 2012b), pointing to an additional genetically determined resistance factor. The potential involvement of early leaf shedding in resistance suggests that the speed of growth of H. pseudoalbidus might be a potentially crucial virulence trait. Different isolates of H. pseudoalbidus show a great variability in growth rates on MEA (Kowalski, 2006; Kowalski and Bartnik, 2010). However, these experiments were carried out with virulent isolates, as all were isolated from necrotic lesions.
Many plant pathogens use toxins to kill host cells (Horbach et al., 2011). In H. pseudoalbidus, the phytotoxin viridiol and several viridiol‐related secondary metabolites have been discovered (Andersson et al., 2010, 2012, 2013; Grad et al., 2009). Viridiol was previously only known from Trichoderma spp. (Jones and Hancock, 1987; Wipf and Kerekes, 2003). The compound has been proven to have phytotoxic effects on ash seedlings (Andersson et al., 2010, 2013; Grad et al., 2009). However, the nonpathogenic H. albidus had a similar phytotoxic effect as the pathogenic H. pseudoalbidus on sterile Lemna minor plants cultured in close proximity to the fungus on MEA in Petri dishes (O. Holdenrieder, unpublished data), and viridiol production was very high in a Swiss H. albidus strain (Junker et al., 2013). Therefore, the production of viridiol is probably not the critical virulence factor. Phytotoxic volatile lactones might also be involved in pathogenicity (Metzler et al., 2012b). Recently, using matrix‐assisted laser desorption ionization‐time of flight (MALDI‐TOF) mass spectrometry, the so‐called chalarafraxinines were detected, which represent a group of as yet uncharacterized secondary metabolites produced by the pathogen (Pham et al., 2013). In culture, the mycelium of H. pseudoalbidus shows a positive Bavendamm reaction, indicating the presence of extracellular polyphenoloxidases (Metzler et al., 2012b; Schumacher, 2011; Schumacher et al., 2010), which are involved in the infection process, in defence against competing microorganisms and in lignin degradation (Baldrian, 2006; Mayer and Staples, 2002). However, the same is true for the nonpathogenic H. albidus (O. Holdenrieder, unpublished data). Shotgun pyrosequencing of the H. pseudoalbidus genome revealed numerous genes encoding cell wall‐degrading enzymes (V. Queloz & M. Schlegel, unpublished data), which are typical of necrotrophic fungi (Kämper et al., 2006). However, in its native range, H. pseudoalbidus does not induce symptoms on F. mandshurica. Apparently, on susceptible exotic hosts, the necrotrophic behaviour is accentuated, whereas, on the native host, the hemibiotrophic lifestyle prevails. The mechanism driving this shift is still unknown.
Epidemiology
An epidemic requires: (i) an accumulation of susceptible host individuals; (ii) an aggressive pathogen with a high reproduction rate; and (iii) environmental conditions suitable for the pathogen. It definitely appears that these conditions are fulfilled in Europe over entire regions. European ash is the most widespread Fraxinus species in Europe (FRAXIGEN, 2005), but the standing tree volume is estimated to be low in most European countries (except for the UK with 15%; DEFRA, 2013). Nevertheless, F. excelsior is the second or third most frequent deciduous tree species after European beech (Fagus sylvatica) in central European countries (Kirisits and Cech, 2010; Speich and Brändli, 2011). Moreover, young trees are often abundant, but do not appear in forest inventories, i.e. the number of individual ash trees is much higher than suggested by inventories. European ash is highly susceptible to the pathogen, as can be deduced from the high rate of spread and destructiveness of the ongoing epidemic and the results of infection experiments. The pathogen is highly virulent because it did not co‐evolve with European ash (Zhao et al., 2012). However, a small fraction of ash genotypes are less susceptible to the disease ( Enderle et al., 2013; Kjær et al., 2012; Kowalski et al., 2012; McKinney et al., 2011; Metzler et al., 2012a; Pliūra et al., 2011; Stener, 2012). The tolerance is heritable, giving reason for hope for the recovery of F. excelsior (Pliūra et al., 2013). In Poland, two resistance‐associated molecular markers have been detected (Kowalski et al., 2012).
The disease front of ash dieback moved from eastern Poland to Switzerland (i.e. over more than 1200 km) within 16 years (1992–2008), showing a mean dispersal rate of 75 km per year. Similar estimates have been obtained on the basis of detailed disease progression maps of France (B. Marçais, INRA, Nancy Université, Champenoux, France, personal communication). This is more than twice as fast as the spread of chestnut blight, another introduced disease, in North America (37 km per year) or Italy (29 km per year) (Roane et al., 1986; Shain, 1982), but similar to the dispersal rate of cereal mildew (Limpert et al., 1999). Ash dieback dispersed somewhat less rapidly at the northern and southern limits of the distribution area of F. excelsior. Dispersal rates were 30 km per year in Norway and 50–60 km per year in north‐eastern Italy (Luchi et al., 2012; Solheim et al., 2011). Ascospores are produced in abundance during several months in late spring and summer, i.e. the infectious period is rather long (Kirisits et al., 2009; Timmermann et al., 2011). Ascospores of H. pseudoalbidus are drought sensitive and freshly ejected spores usually have a germination capacity of 90%–100%. The spores died within 24 h in a desiccator, but 62% survived for 24 h at room conditions (approximately 20 °C and 30% relative humidity). For spores deposited on a cellophane sheet and exposed to outdoor conditions in July during a rainy period (but protected from rain and direct sunlight), the germination rate declined to 6%–10% within 5–6 days (O. Holdenrieder, unpublished data). The conspicuous melanization of the ascospores after ejection and the attachment to the host surface (see above) indicate that the spores can probably withstand adverse conditions at this stage rather well (Henson et al., 1999).
Pathogen dispersal can also occur through long‐distance transport of latently infected plants (see above). This probably occurred in Ireland [Department of Agriculture Food and the Marine (Ireland), 2012]. Moreover, pseudosclerotial leaf fragments can be easily dispersed as contaminants of trade goods. The initiation of an epidemic through infected substrate requires the presence of both mating types. This can be achieved by multiple introductions, but also by joint dispersal of several genotypes within a single substrate or by transportation of a single, already fertilized genotype (Gross et al., 2012c). Even ash seeds have been suspected as a potential inoculum source, because the fungus can be detected on them by molecular means (Cleary et al., 2012). However, despite extensive ash seed transfer from Europe to the USA, the pathogen has not yet been reported from North America.
Both disease incidence and severity increased rapidly in most countries after the first observation of the disease (Engesser and Meier, 2012; Metzler, 2011; Rytkönen et al., 2011; Solheim et al., 2012a; Thomsen and Skovgaard, 2012). Disease severity in most ash clones monitored by McKinney et al. (2011) increased steeply from 36% loss of foliage in 2008 to 56% in 2009, but a few clones maintained a low level of damage. In Austria, disease severity (percentage of crown volume affected) increased only moderately from 11% to 16% within 2 years and very few trees died (Kessler et al., 2012). On mature trees, disease progress is slower than on seedlings or young trees (Kirisits and Freinschlag, 2012; Kowalski and Holdenrieder, 2008). This suggests that infection pressure is lower and leaf quality and/or microclimatic conditions are less suitable for infection in the crown.
So far, the epidemic in Europe has progressed according to a sigmoid function. The lag phase lasted almost 10 years, whereas the exponential (log) phase, during which the disease spread over most of northern and central Europe, was of a comparatively short duration (Fig. 4). The northern limits of the distribution area of European ash have already been reached in Scandinavia, whereas there are still some disease‐free areas in eastern, southern and western Europe. Westwards dispersal was probably delayed because of the prevalence of westerly winds. Dispersal to the south, i.e. central Italy, southern France and the Pyrenees, might be hampered by unsuitable climatic conditions (Hauptman et al., 2013). The situation at the epidemic front in eastern Europe is less well known. At least the Baltic States are severely affected (EPPO, 2013), and first infections have been observed in the Ukraine (Davydenko et al., 2012) and Russia near St. Petersburg (Selikhovkin et al., 2013).
Figure 4.
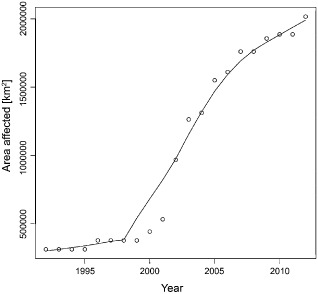
Progress of ash dieback in Europe. The whole area of a country was added to the area affected by the disease in the year of the first report of the disease in that country. The year of the first report was according to fig. 1 in Timmermann et al. (2011). Curve was fitted using LOWESS (f = 0.4) (Cleveland, 1981).
Ecological Considerations
The large‐scale occurrence of the pathogen shows that it tolerates a wide range of environmental conditions. However, the effects of only a few environmental factors have been studied. The mycelial growth rate of H. pseudoalbidus in pure cultures is highest at about 20 °C and ceases at about 30 °C (Kowalski and Bartnik, 2010; Pham et al., 2013). In ash tissues, however, the fungus is more heat sensitive and survival depends not only on temperature, but also on exposure time. In addition, host tissues tolerate a slightly higher temperature than the fungus. Therefore, the development of curative hot water treatments for small plants appears to be possible (Hauptman et al., 2013). The mycelium of H. pseudoalbidus (from cultures on MEA) is rather resistant to frost and drought: it tolerates freezing at −20 °C for at least 2 months and survives even at −70 °C at least for 1 month, showing normal growth after recovery. Mycelia dried at room temperature survived for 7 weeks, but showed a reduced growth rate after transplantation to fresh medium (O. Holdenrieder, unpublished data).
The climate requirements of F. mandshurica in north‐eastern Asia are similar to those of F. excelsior in Europe, except that F. mandshurica tolerates lower minimum temperatures during the winter and is less late frost resistant than F. excelsior (Schenck, 1939; Wang and Dai, 1997). Thus, the climate in most of the range of F. excelsior is suitable for the pathogen.
Disease resistance or tolerance in F. excelsior is a rare phenomenon. Ash dieback will thus dramatically reduce the size of its host populations in Europe, thereby threatening not just ash, but also the organisms depending on F. excelsior (Pautasso et al., 2013). This also applies to microorganisms, as shown by the example of H. albidus (McKinney et al., 2012a), and is proposed for various lichens (Ellis et al., 2012; Jönsson and Thor, 2012). In host tissues weakened or killed by the pathogen, endophytic and opportunistic fungi develop very quickly. On aerial plant parts, the most frequently detected genera are Alternaria, Cladosporium, Diplodia, Fusarium, Phoma and Phomopsis. Some of these can apparently replace H. pseudoalbidus, thereby preventing its expansion. However, such opportunistic fungi can also be pathogenic on their own and might contribute to lesion enlargement (Bakys et al., 2009a, b; Jankovsky and Holdenrieder, 2009; Kowalski and Holdenrieder, 2009a, b; Kowalski and Lukomska, 2005; Lygis et al., 2006; Przybył, 2002; Pukacki and Przybył, 2005; Schumacher et al., 2007). Among these, Botryosphaeria stevensii (anamorph Diplodia mutila) is of special interest with respect to climate warming, because this fungus is known as an opportunistic pathogen from warm regions (Ragazzi et al., 1999; Sánchez et al., 2003; Schumacher et al., 2007, 2010). The root system of affected trees is also attacked by opportunistic fungi, e.g. Armillaria spp., which considerably accelerate ash tree death (Bakys et al., 2011; Husson et al., 2012; Lygis et al., 2006; Skovsgaard et al., 2010).
The control of established ash dieback in the forest is practically impossible and silvicultural recommendations are limited to avoiding the loss of the value of mature ash stands (for a review, see Metzler et al., 2013). Only if the pathogen has been introduced recently through infected nursery stock and there are no infections in the surrounding ecosystems is removal of infected plants recommended (DEFRA, 2013). In principle, ash leaf litter might be removed from nurseries and parks as a prophylactic measure, but this would make sense only in the absence of extraneous inoculum. Chemical control might be possible for small plants, but is counterproductive, as it makes no sense to preserve susceptible plants, which would fuel the epidemic after outplanting. In addition, sublethal fungicide treatments might prevent symptom development and thereby facilitate long‐distance dispersal with latently infected plants. However, there is some hope for the evolution of resistance (see above), which can be facilitated by breeding and appropriate forest management.
Techniques for Pathogen Detection and Handling
Isolation of H. pseudoalbidus from necrotic lesions on the bark is reported to be straightforward (Kowalski, 2006; Queloz et al., 2011), although very low isolation rates have sometimes been found (Bakys et al., 2009a; Jankovsky and Holdenrieder, 2009). A small tissue sample is aseptically taken from the xylem close to the pith, preferentially from the necrotic zone close to healthy tissues. The samples are incubated on an agar‐based culture medium. However, isolation success greatly depends on the season and the age of the necrotic lesion. Fresh necrotic bark lesions usually appear in the autumn and winter, and isolation from the xylem usually yields good results (approximately 75% from late October to April). During the winter months, the isolation success from fresh lesions remains high, but slowly decreases during the spring until it drops to near zero between July and September (A. Gross, unpublished data). Isolation from pseudosclerotia produces good results (90%–100%), regardless of the season. Pseudosclerotial petioles are air dried for a few hours and surface sterilized using absolute ethanol, which evaporates quickly and does not soak into the pseudosclerotium. Once the petioles are dry, the pseudosclerotial plate (Fig. 1f,g) is removed tangentially using a sterile scalpel and small tissue samples are taken and incubated on culture medium (Gross and Holdenrieder, 2013).
The isolation of the pathogen from necrotic leaflets is difficult, because of the presence of numerous fast‐growing fungi (Fig. 2c). Nevertheless, timely transfer of mycelia emerging from the vein cut can yield pure cultures. Hymenoscyphus pseudoalbidus was only very rarely isolated from green leaflets in the autumn (U. Oggenfuss, ETH Zurich, Zurich, Switzerland, unpublished data), but frequently from asymptomatic leaf stalks collected in September (Bakys et al., 2009a).
Methods to isolate single fungal spores have been discussed elsewhere (see, for example, Booth, 1971). Apothecia can be readily generated in the laboratory by incubating pseudosclerotia collected in nature. Gross et al. (2012c) induced ascocarp formation using an incubator with near‐UV light at around 20 °C, as described by Leach (1971). Pseudosclerotia were placed into polystyrol Petri dishes on moist paper tissues. No difference in the viability of spores produced under UV was detected in comparison with field‐grown spores.
Controlled crosses are possible with strains of opposite mating types on sterilized petioles (Gross et al., 2012c). For mating type determination, a multiplex PCR is available (Gross et al., 2012c).
Hymenoscyphus pseudoalbidus grows slowly on standard culture media, such as MEA (mean radial growth rate of 0.52 mm/day; Kirisits et al., 2013) or potato dextrose agar (PDA), and shows great variability on MEA (Fig. 5d). The growth rate of the mycelium is considerably enhanced by adding fresh or frozen ash leaves to MEA (Fig. 5a,b) (Gross et al., 2012c; Kirisits et al., 2013). Hymenoscyphus pseudoalbidus and H. albidus cultures MEA supplemented with ash leaves reveal a characteristic dark‐reddish brown discoloration of the medium and show less variation in colony morphology compared with growth on MEA (Fig. 5b,e). During the winter, frozen leaves can be used instead of fresh leaves, but the growth‐stimulating effect on H. pseudoalbidus is lost slowly with increasing freezing time of the ash leaves (A. Gross, unpublished data). Moreover, germination of H. pseudoalbidus spores was inhibited on a medium in which long‐frozen (>5 months) ash leaves were used, and we recommend the use of MEA for spore viability tests. On cellophane sheets overlaid on agar medium, in addition to superficial mycelium, lobed, flattened irregular hyphae grow within the cellophane (Fig. 5f). However, in plant tissues, such hyphae could not be found (O. Holdenrieder, unpublished data). As for other fungi (Borman et al., 2006), long‐term storage of H. pseudoalbidus in water is possible for at least 4 years (O. Holdenrieder, unpublished data). Ascocarps of H. pseudoalbidus can be differentiated from H. albidus by the presence of a crozier at the ascus base (Zhao et al., 2012), which, however, is difficult to observe. A secure differentiation is achieved by sequencing the ITS region (Queloz et al., 2011). Real‐time PCR assays for the in planta detection of H. pseudoalbidus have been developed by Chandelier et al. (2010) and Ioos et al. (2009). Alternatively, a species‐specific ITS PCR for H. pseudoalbidus detection in tissues of F. excelsior is available (Johansson et al., 2010). These methods were developed prior to the separation of H. albidus and H. pseudoalbidus, and the specificity was not tested for these cryptic species at that time. However, the specificity of the real‐time PCR assays was shown subsequently (A. Chandelier, Walloon Agricultural Research Centre, Gembloux, Belgium, personal communication; Husson et al., 2011) and a specific cleaved amplified polymorphism (CAP) marker was developed, based on the ITS PCR of Johansson et al. (2010). For the detection of infections on the basis of secondary metabolites, MALDI‐TOF mass spectrometry has been suggested (Pham et al., 2013).
Figure 5.
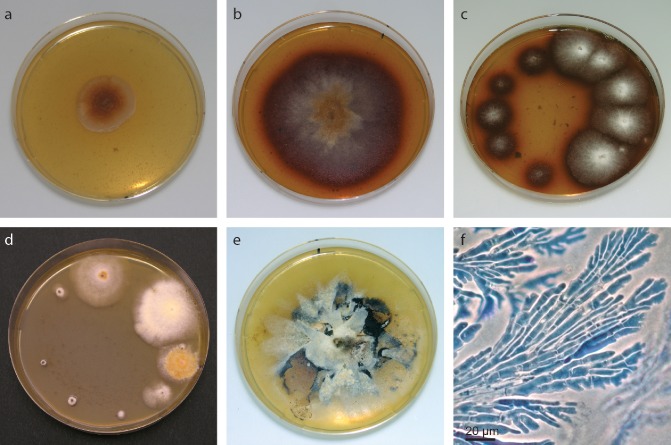
Morphology of Hymenoscyphus pseudoalbidus in culture: (a) colony of the type strain (CBS 122504) growing on malt extract agar (MEA) for 3 weeks at 20 °C (diameter of Petri dish, 9 cm); (b) colony of the type strain growing on ash leaf agar for 3 weeks at 20 °C; (c) isolation from a necrotic shoot (incubated for 3 weeks at 20 °C); the smaller colonies on the left originated from pith tissue, the faster growing colonies on the right originated from central xylem tissue; (d) single spore cultures on MEA (36 days old), showing the variability in growth rate and colony morphology; (e) old culture (stored for several months at 5 °C) on MEA; (f) flattened hyphae growing within a cellophane sheet on ash leaf agar (stained with lactophenol blue).
MS markers for H. pseudoalbidus genotyping and population genetics have been developed by Gross et al. (2012b) and Bengtsson et al. (2012).
The virulence of H. pseudoalbidus can be determined by inoculation of F. excelsior shoots and stems (Kowalski and Holdenrieder, 2009a) or by leaf rachis infections (Kräutler and Kirisits, 2012). Ascospore inoculation (Cleary et al., 2013) can be used to study the natural infection process of H. pseudoalbidus. However, high spore concentrations led to early leaf shedding and no stem necroses were produced following artificial inoculation with ascospores (see above).
Significant insights into pathogenesis and host response can be expected from genomic, transcriptomic, proteomic and metabolomic approaches in the near future, and various genomic projects have been launched (Solheim et al., 2012b; Stenlid et al., 2012). Illumina sequence data have been made public without prior analysis on a website at oadb.tsl.ac.uk as well as a public GitHub repository (https://github.com/ash‐dieback‐crowdsource), comprising assembled transcriptomic and genomic data of a single H. pseudoalbidus isolate, the ‘interaction transcriptome’ of four twig lesions and the transcriptome of an uninfected ash tree (MacLean et al., 2013). Initial analyses presented on the website identified: (i) a full‐length Nep1‐like protein (NLP) homologue (Dong and Kamoun, 2012), which is a putative virulence factor (Gijzen and Nürnberger, 2006); (ii) a secreted protein with four LysM domains belonging to a family of fungal effectors (de Jonge and Thomma, 2009); (iii) 3778 unique pfam domains, including 71 domains linked to copper oxidases and transporters (Crossman, 2012); and (iv) signatures of compositionally nonhomogeneous domains (CNHDs) (MacLean, 2013) frequently associated with effector genes in filamentous fungi (Raffaele and Kamoun, 2012). In addition, the full‐length mating type 1 sequence containing a gene with similarity to a DNA polymerase zeta catalytic subunit has been identified (Saunders, 2013). Research concerning ash dieback in Europe is currently coordinated by the FRAXBACK action (http://www.fraxback.eu; see also FRAXBACK, 2012), funded by the European Cooperation in Science and Technology (COST). An initial ash genome assembly (454 technology) is available for blast searches at http://www.ashgenome.org.
Implications from Other Pathosystems and Outlook
Although the nature of the F. excelsior–H. pseudoalbidus pathosystem appears to be unique, comparisons with related pathosystems might provide useful insights into the understanding and control of ash dieback. Pathosystems can be related to ash dieback because: (i) the pathogen is phylogenetically related to H. pseudoalbidus; or (ii) the disease is functionally related to ash dieback.
Phylogenetically related host–parasite systems occur within the Helotiales, a group of fungi including a number of pathogens and endophytic fungi. Hymenoscyphus pseudoalbidus is not known to show a pathogenic behaviour in its native range. The behavioural switch to a pathogenic lifestyle in Europe represents the key to understanding its pathogenicity. An example of an endophyte in the Helotiales, which occasionally becomes pathogenic, is Cenangium ferruginosum. This fungus can cause pine dieback when conditions become favourable to the fungus or unfavourable to the host (Jurc et al., 2000), and is a frequently isolated endophyte of symptomless needles of Pinus spp. (Helander et al., 1994). Studies of environmental factors associated with needle colonization by C. ferruginosum have been performed, showing, for example, that the fungus was more frequent on the lower part of a mountain ridge (Sieber et al., 1999). The pine canker pathogen Crumenulopsis sororia shows a versatile behaviour depending on site conditions, such as elevation, exposure or slope (Hayes et al., 1981), and the age of host tissues (Ennos and McConnell, 2003). A review of studies on the variation in infectivity and aggressiveness of pathogens in wild systems concludes that such variation is ubiquitous in space and time (Tack et al., 2012). Investigations along landscape gradients are needed for H. pseudoalbidus to assess environmental variation in ash dieback severity (see Holdenrieder et al., 2004). From studies on environmental variation associated with various pine endophytes (Terhonen et al., 2011), it can be hypothesized that any gradients in ash dieback severity might be related to variation in the ash endophytic community counteracting H. pseudoalbidus. The role of endophytes in preventing tree disease has also been emphasized for Gremmeniella abietina on pines (Ranta et al., 1995; Santamaría et al., 2007).
Many functionally related diseases are caused by introduced pathogens just as selective and lethal as H. pseudoalbidus. For example, Dutch elm disease (DED) has involved two consecutive pandemics across the Northern Hemisphere caused by the ascomycetes Ophiostoma ulmi and Ophiostoma novo‐ulmi, both probably introduced from east Asia (Brasier, 2000). The disease has killed the majority of mature elms. As they have spread, both pathogens have been through remarkable evolutionary events involving horizontal gene transfer (Brasier et al., 2004). Many different management approaches have been used, but because of the explosive intensity of the disease and the high cost of the management applications, wide‐scale eradication and control attempts were rapidly abandoned internationally and have been replaced by longer‐term strategies such as breeding and conservation programmes (Dunn, 2000; Potter et al., 2011). The DED pathogens reproduce asexually as well as sexually, are transmitted by vector bark beetles (Webber, 2004) and cause a systemic infection. Some elm species can reproduce freely from root suckers and some can flower at a relatively early age. In the aftermath of the heavy losses of mature elms caused by O. novo‐ulmi, millions of small recruitment elms have grown up from suckers and seeds across Europe and North America. A new ‘balance’ has been established between host, pathogen and vector, with abundant small elms now under constant attack by the pathogen, but part of the population usually surviving long enough to reproduce (Brasier, 1986). In contrast, F. excelsior usually does not form root suckers and only a few saplings survive the epidemic. Moreover, the environment seems to influence disease expression distinctly (Enderle et al., 2013).
Butternut canker and dogwood anthracnose, caused by the ascomycetes Ophiognomonia clavigignenti‐juglandacearum and Discula destructiva, respectively, show symptoms in North America similar to European ash dieback (Broders et al., 2012; Holzmueller et al., 2010). However, reproduction of these pathogens is predominantly asexual, and therefore the evolutionary potential of these pathogens is much lower than that of H. pseudoalbidus (McDonald and Linde, 2002).
Chestnut canker, which is caused by the introduced ascomycete Cryphonectria parasitica, eradicated its new host Castanea dentata in North America across much of its range. However, in Europe, co‐existence between pathogen and host (Castanea sativa) became possible because of virus‐mediated hypovirulence (Robin and Heiniger, 2001). Therefore, a search for such viruses in H. pseudoalbidus has been initiated (Pliūra et al., 2013).
Other novel and host‐specific tree pathosystems are the emerald ash borer (Agrilus planipennis), an insect that seriously threatens all North American Fraxinus species, and the pine wood nematode (PWN, Bursaphelenchus xylophilus), which kills Pinus spp. in Asia and Western Europe. The emerald ash borer was introduced into North America from East Asia. It was also recently detected in the Moscow region (Selikhovkin et al., 2013) and stimulated research on comparative transcriptomics and secondary metabolites of American and Asian Fraxinus spp., as well as on resistance breeding of ash (e.g. Rebek et al., 2008; Rivera‐Vega et al., 2012; Whitehill et al., 2012). For the PWN pathosystem, significant advances in understanding have been achieved through the application of modern molecular techniques (Shinya et al., 2013), and much can be learned from such approaches concerning ash dieback research.
A lesson from these examples is the importance of avoiding further introductions of pathogen strains, so as to reduce the chances of pathogen recombination and the emergence of even more virulent strains. Therefore, even if H. pseudoalbidus has already been dispersed across much of Europe, it still makes sense to prove its origin more precisely and to stop importing infected plant material from the natural distribution area of F. mandshurica. In addition, it is important to study the biology and ecology of the pathogen in its native range.
The examples mentioned above also demonstrate that not much can be done to prevent the spread of invasive tree diseases, once introduction and establishment have occurred and in the presence of overwhelming inoculum production and a general absence of host resistance. This requires the avoidance of new pathogen introductions, interdisciplinary research on emerging diseases and creative strategies for the mitigation of the impacts of plant diseases also in near‐natural ecosystems (Desprez‐Loustau et al., 2010; Pautasso et al., 2010; Santini et al., 2013).
Acknowledgements
Many thanks to G. Aas, H. O. Baral, M. Beaud, R. Berndt, C. M. Brasier, J. Coffin, R. Engesser, P. Erickson, C. R. Grünig, J.‐G. Han, T. Hosoya, B. Jaroszewicz, M. Jeger, T. Koike, T. Kowalski, E. Landolt, N. Luschka, B. Marçais, B. A. McDonald, T. Nojima, L. Paul, K. Ruprecht, M. Schlegel, T. Wey, P. Zaffarano and Y. Zhao for insights, discussion and help, and to two anonymous reviewers for their in‐depth reviews and valuable suggestions for improvement of the manuscript. The review was partially funded by a grant from the ETH Zurich (ETH‐04 10‐1).
References
- Andersson, P.F. , Johansson, S.B.K. , Stenlid, J. and Broberg, A. (2010) Isolation, identification and necrotic activity of viridiol from Chalara fraxinea, the fungus responsible for dieback of ash. For. Pathol. 40, 43–46. [Google Scholar]
- Andersson, P.F. , Bengtsson, S. , Stenlid, J. and Broberg, A. (2012) B‐norsteroids from Hymenoscyphus pseudoalbidus . Molecules, 17, 7769–7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, P.F. , Bengtsson, S. , Cleary, M. , Stenlid, J. and Broberg, A. (2013) Viridin‐like steroids from Hymenoscyphus pseudoalbidus . Phytochemistry, 86, 195–200. [DOI] [PubMed] [Google Scholar]
- Bakys, R. , Vasaitis, R. , Barklund, P. , Ihrmark, K. and Stenlid, J. (2009a) Investigations concerning the role of Chalara fraxinea in declining Fraxinus excelsior . Plant Pathol. 58, 284–292. [Google Scholar]
- Bakys, R. , Vasaitis, R. , Barklund, P. , Thomsen, I. and Stenlid, J. (2009b) Occurrence and pathogenicity of fungi in necrotic and non‐symptomatic shoots of declining common ash (Fraxinus excelsior) in Sweden. Eur. J. For. Res. 128, 51–60. [Google Scholar]
- Bakys, R. , Vasiliauskas, A. , Ihrmark, K. , Stenlid, J. , Menkis, A. and Vasaitis, R. (2011) Root rot, associated fungi and their impact on health condition of declining Fraxinus excelsior stands in Lithuania. Scand. J. For. Res. 26, 128–135. [Google Scholar]
- Baldrian, P. (2006) Fungal laccases—occurrence and properties. FEMS Microbiol. Rev. 30, 215–242. [DOI] [PubMed] [Google Scholar]
- Bengtsson, S.B.K. , Vasaitis, R. , Kirisits, T. , Solheim, H. and Stenlid, J. (2012) Population structure of Hymenoscyphus pseudoalbidus and its genetic relationship to Hymenoscyphus albidus . Fungal Ecol. 5, 147–153. [Google Scholar]
- Billiard, S. , López‐Villavicencio, M. , Hood, M.E. and Giraud, T. (2012) Sex, outcrossing and mating types: unsolved questions in fungi and beyond. J. Evol. Biol. 25, 1020–1038. [DOI] [PubMed] [Google Scholar]
- Booth, C. (1971) Introduction to general methods In: Methods in Microbiology (Booth C., ed.), pp. 1–47. London: Academic Press. [Google Scholar]
- Borman, A. , Szekely, A. , Campbell, C. and Johnson, E. (2006) Evaluation of the viability of pathogenic filamentous fungi after prolonged storage in sterile water and review of recent published studies on storage methods. Mycopathologia, 161, 361–368. [DOI] [PubMed] [Google Scholar]
- Boudier, B. (1994) Le chancre des jeunes plants de frênes, une maladie grave certaines années mais des perspectives de lutte. Phytoma, 461, 35–36. [Google Scholar]
- Brasier, C.M. (1986) The population biology of Dutch elm disease: its principal features and some implications for other host‐pathogen systems. Adv. Plant Pathol. 5, 55–118. [Google Scholar]
- Brasier, C.M. (2000) Intercontinental spread and continuing evolution of the Dutch elm disease pathogens In: The Elms: Breeding, Conservation and Disease Management (Dunne C.P., ed.), pp. 61–72. Boston: Kluwer Academic Publishers. [Google Scholar]
- Brasier, C.M. , Buck, K.W. , Paoletti, M. , Crawford, L. and Kirk, S.A. (2004) Molecular analysis of evolutionary changes in populations of Ophiostoma novo‐ulmi . Forest Systems, 13, 93–103. [Google Scholar]
- Broders, K.D. , Boraks, A. , Sanchez, A.M. and Boland, G.J. (2012) Population structure of the butternut canker fungus, Ophiognomonia clavigignenti‐juglandacearum, in North American forests. Ecol. Evol. 2, 2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butin, H. (2011) Krankheiten der Wald‐ und Parkbäume: Diagnose—Biologie—Bekämpfung. Stuttgart: Ulmer. [Google Scholar]
- Cech, T. (2006) Auffallende Schadfaktoren an Waldbäumen im Jahr 2005. Forstschutz Aktuell, 35, 6–7. [Google Scholar]
- Chandelier, A. , André, F. and Laurent, F. (2010) Detection of Chalara fraxinea in common ash (Fraxinus excelsior) using real time PCR. For. Pathol. 40, 87–95. [Google Scholar]
- Cleary, M.R. , Arhipova, N. , Gaitnieks, T. , Stenlid, J. and Vasaitis, R. (2012) Natural infection of Fraxinus excelsior seeds by Chalara fraxinea . For. Pathol. 43, 83–85. [Google Scholar]
- Cleary, M.R. , Daniel, G. and Stenlid, J. (2013) Light and scanning electron microscopy studies of the early infection stages of Hymenoscyphus pseudoalbidus on Fraxinus excelsior . Plant Pathol. (in press). doi: 10.1111/ppa.12048 [DOI] [Google Scholar]
- Cleveland, W.S. (1981) LOWESS: a program for smoothing scatterplots by robust locally weighted regression. Am. Stat. 35, 54. [Google Scholar]
- Crossman, L. (2012) Comments on predicted proteins Pfam domains and map to GO, KOG, PRK and EC numbers. Available at http://www.sequenceanalysis.co.uk/CrowdSourcing/2012/12/18/ash‐dieback [accessed on July 12, 2013].
- Dal Maso, E. , Fanchin, G. , Mutto Accordi, S. , Scattolin, L. and Montecchio, L. (2012) Ultrastructural modifications in common ash tissues colonised by Chalara fraxinea . Phytopathol. Mediterr. 51, 599–606. [Google Scholar]
- Davydenko, K. , Stenlid, J. and Vasaitis, R. (2012) Situation with ash in Eastern Ukraine: stand characteristics, health condition, ongoing work and research needs In: Interim Report from Chalara fraxinea, FRAXBACK Meeting in Vilnius, 13–14 November 2012 (Mainprize N., Hendry S. and Weir J., eds), p. 44 Bristol: Forestry Commission. [Google Scholar]
- DEFRA (2013) Chalara Management Plan (Department for Environment Food & Rural Affairs , ed.). London: Department for Environment Food & Rural Affairs. [Google Scholar]
- Department of Agriculture Food and the Marine [Ireland] (2012) Ash Dieback (Chalara). Department of Agriculture, Food and the Marine. Available at http://www.agriculture.gov.ie/forestservice/ashdiebackchalara [accessed on May 15, 2013].
- Desprez‐Loustau, M.‐L. , Courtecuisse, R. , Robin, C. , Husson, C. , Moreau, P.‐A. , Blancard, D. , Selosse, M.‐A. , Lung‐Escarmant, B. , Piou, D. and Sache, I. (2010) Species diversity and drivers of spread of alien fungi (sensu lato) in Europe with a particular focus on France. Biol. Invasions, 12, 157–172. [Google Scholar]
- Dong, S. and Kamoun, S. (2012) Nep1‐like proteins (NLPs) identified in AT1 assembled transcripts using tblastn. Available at http://oadb.tsl.ac.uk/?p=235 [accessed on May 10, 2013].
- Drenkhan, R. and Hanso, M. (2010) New host species for Chalara fraxinea . New Dis. Rep. 22, 16. [Google Scholar]
- Dunn C.P. (ed.) (2000) The Elms: Breeding, Conservation and Disease Management. Boston: Kluwer Academic Publishers. [Google Scholar]
- Ellis, C.J. , Coppins, B.J. and Hollingsworth, P.M. (2012) Tree fungus: lichens under threat from ash dieback. Nature, 491, 672. [DOI] [PubMed] [Google Scholar]
- Enderle, R. , Peters, F. , Nakou, A. and Metzler, B. (2013) Temporal development of ash dieback symptoms and spatial distribution of collar rots in a provenance trial of Fraxinus excelsior . Eur. J. Forest Res. doi: 10.1007 [Google Scholar]
- Engesser, R. and Meier, F. (2012) Eschenwelke wird noch bedrohlicher: Aktuelle Verbreitung und neuer Infektionsweg. Wald u. Holz, 12, 35–39. [Google Scholar]
- Engesser, R. , Queloz, V. , Meier, R. , Kowalski, T. and Holdenrieder, O. (2009) Das Triebsterben der Esche in der Schweiz. Wald u. Holz, 6, 24–27. [Google Scholar]
- Ennos, R.A. and McConnell, K.C. (2003) Variation in host resistance and pathogen selective value in the interaction between Pinus sylvestris and the fungus Crumenulopsis sororia . Heredity, 91, 193–201. [DOI] [PubMed] [Google Scholar]
- EPPO (2013) EPPO alert list: Chalara fraxinea—ash dieback. Available at http://www.eppo.int/QUARANTINE/Alert_List/fungi/Chalara_fraxinea.htm [accessed on April 14, 2013].
- Feau, N. , Decourcelle, T. , Husson, C. , Desprez‐Loustau, M.‐L. and Dutech, C. (2011) Finding single copy genes out of sequenced genomes for multilocus phylogenetics in non‐model fungi. PLoS ONE, 6, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAXBACK (2012) Interim Report from Chalara fraxinea, FRAXBACK Meeting in Vilnius, 13–14 November 2012. Bristol: Forestry Commission; Available at http://www.bentley.onesuffolk.net/assets/Uploads/Bentley‐pdf/ASH‐Dieback/C‐fraxinea‐FRAXBACK‐MEETING‐13‐14‐November‐2012.pdf [accessed on May 15, 2013]. [Google Scholar]
- FRAXIGEN (2005) Ash Species in Europe: Biological Characteristics and Practical Guidelines for Sustainable Use. Oxford: Oxford Forestry Institute. [Google Scholar]
- Gijzen, M. and Nürnberger, T. (2006) Nep1‐like proteins from plant pathogens: recruitment and diversification of the NPP1 domain across taxa. Phytochemistry, 67, 1800–1807. [DOI] [PubMed] [Google Scholar]
- Grad, B. , Kowalski, T. and Kraj, W. (2009) Studies on secondary metabolite produced by Chalara fraxinea and its phytotoxic influence on Fraxinus excelsior . Phytopathologia, 54, 61–69. [Google Scholar]
- Gross, A. and Holdenrieder, O. (2013) On the longevity of Hymenoscyphus pseudoalbidus in petioles of Fraxinus excelsior . For. Pathol. 43, 168–170. [Google Scholar]
- Gross, A. , Dobbertin, M. and Rigling, D. (2012a) Situation with ash in Switzerland In: Interim Report from Chalara fraxinea, FRAXBACK Meeting in Vilnius, 13–14 November 2012 (Mainprize N., Hendry S. and Weir J., eds), pp. 41–42. Bristol: Forestry Commission. [Google Scholar]
- Gross, A. , Grünig, C.R. , Queloz, V. and Holdenrieder, O. (2012b) A molecular toolkit for population genetic investigations of the ash dieback pathogen Hymenoscyphus pseudoalbidus . For. Pathol. 42, 252–264. [Google Scholar]
- Gross, A. , Zaffarano, P.L. , Duo, A. and Grünig, C.R. (2012c) Reproductive mode and life cycle of the ash dieback pathogen Hymenoscyphus pseudoalbidus . Fungal Genet. Biol. 49, 977–986. [DOI] [PubMed] [Google Scholar]
- Han, J.‐G. and Shin, H.‐D. (2008) Hymenoscyphus ginkgonis sp.nov. growing on leaves of Ginkgo biloba . Mycotaxon, 103, 189–195. [Google Scholar]
- Hauptman, T. , Piškur, B. , de Groot, M. , Ogris, N. , Ferlan, M. and Jurc, D. (2013) Temperature effect on Chalara fraxinea: heat treatment of saplings as a possible disease control method. For. Pathol. (in press). doi: 10.1111/efp.12038 [DOI] [Google Scholar]
- Hayes, A.J. , Newton, N.G. , Jolly, G.M. , Wood, J. and Anderson, M.H. (1981) The prediction of Crumenulopsis sororia (karst) groves—incidence on lodgepole pine (Pinus contorta Dougl.) using multiple regression techniques. Eur. J. For. Pathol. 11, 396–411. [Google Scholar]
- Helander, M.L. , Sieber, T.N. , Petrini, O. and Neuvonen, S. (1994) Endophytic fungi in Scots pine needles: spatial variation and consequences of simulated acid rain. Can. J. Bot. 72, 1108–1113. [Google Scholar]
- Henson, J.M. , Butler, M.J. and Day, A.W. (1999) The dark side of the mycelium: melanins of phytopathogenic fungi. Annu. Rev. Phytopathol. 37, 447–471. [DOI] [PubMed] [Google Scholar]
- Hietala, A.M. , Timmermann, V. and Børja, I. (2013) The invasive ash dieback pathogen Hymenoscyphus pseudoalbidus exerts maximal infection pressure prior to the onset of host leaf senescence. Fungal Ecol. 6, 302–308. [Google Scholar]
- Holdenrieder, O. , Pautasso, M. , Weisberg, P.J. and Lonsdale, D. (2004) Tree diseases and landscape processes: the challenge of landscape pathology. Trends Ecol. Evol. 19, 446–452. [DOI] [PubMed] [Google Scholar]
- Holzmueller, E.J. , Jose, S. and Jenkins, M.A. (2010) Ecological consequences of an exotic fungal disease in eastern U.S. hardwood forests. For. Ecol. Manage. 259, 1347–1353. [Google Scholar]
- Horbach, R. , Navarro‐Quesada, A.R. , Knogge, W. and Deising, H.B. (2011) When and how to kill a plant cell: infection strategies of plant pathogenic fungi. J. Plant Physiol. 168, 51–62. [DOI] [PubMed] [Google Scholar]
- Hosoya, T. , Otani, Y. and Furuya, K. (1993) Materials for the fungus flora of Japan (46). T. Mycol. Soc. Jpn. 34, 429–432. [Google Scholar]
- Hulden, E. (1941) Studien über Fraxinus excelsior L. Acta Bot. Fenn. 28, 1–234. [Google Scholar]
- Hull, S.K. and Gibbs, J.N. (1991) Ash dieback: a survey of non‐woodland trees. For. Comm. Bull. 93, 1–32. [Google Scholar]
- Husson, C. , Scala, B. , Cael, O. , Frey, P. , Feau, N. , Ioos, R. and Marcais, B. (2011) Chalara fraxinea is an invasive pathogen in France. Eur. J. Plant Pathol. 130, 311–324. [Google Scholar]
- Husson, C. , Caël, O. , Grandjean, J.P. , Nageleisen, L.M. and Marçais, B. (2012) Occurrence of Hymenoscyphus pseudoalbidus on infected ash logs. Plant Pathol. 61, 889–895. [Google Scholar]
- Ioos, R. , Kowalski, T. , Husson, C. and Holdenrieder, O. (2009) Rapid in planta detection of Chalara fraxinea by a real‐time PCR assay using a dual‐labelled probe. Eur. J. Plant Pathol. 125, 329–335. [Google Scholar]
- Jankovsky, L. and Holdenrieder, O. (2009) Chalara fraxinea—ash dieback in the Czech Republic. Plant Prot. Sci. 45, 74–78. [Google Scholar]
- Johansson, S.B.K. , Vasaitis, R. , Ihrmark, K. , Barklund, P. and Stenlid, J. (2010) Detection of Chalara fraxinea from tissue of Fraxinus excelsior using species‐specific ITS primers. For. Pathol. 40, 111–115. [Google Scholar]
- Jones, R.W. and Hancock, J.G. (1987) Conversion of viridin to viridiol by viridin‐producing fungi. Can. J. Microbiol. 33, 963–966. [DOI] [PubMed] [Google Scholar]
- de Jonge, R. and Thomma, B.P.H.J. (2009) Fungal LysM effectors: extinguishers of host immunity? Trends Microbiol. 17, 151–157. [DOI] [PubMed] [Google Scholar]
- Jönsson, M.T. and Thor, G. (2012) Estimating coextinction risks from epidemic tree death: affiliate lichen communities among diseased host tree populations of Fraxinus excelsior . PLoS ONE, 7, e45701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker, C. , Mandey, F. , Pais, A. , Ebel, R. and Schulz, B. (2013) Hymenoscyphus pseudoalbidus and Hymenoscyphus albidus: viridiol concentration and virulence do not correlate. For. Pathol. (in press). doi: 10.1111/efp.12066 [DOI] [Google Scholar]
- Jurc, D. , Jurc, M. , Sieber, T.N. and Bojovic, S. (2000) Endophytic Cenangium ferruginosum (Ascomycota) as a reservoir for an epidemic of cenangium dieback in Austrian pine. Phyton, 40, 103–108. [Google Scholar]
- Kämper, J. , Kahmann, R. , Bolker, M. , Ma, L.‐J. , Brefort, T. , Saville, B.J. , Banuett, F. , Kronstad, J.W. , Gold, S.E. , Muller, O. , Perlin, M.H. , Wosten, H.A.B. , de Vries, R. , Ruiz‐Herrera, J. , Reynaga‐Pena, C.G. , Snetselaar, K. , McCann, M. , Perez‐Martin, J. , Feldbrugge, M. , Basse, C.W. , Steinberg, G. , Ibeas, J.I. , Holloman, W. , Guzman, P. , Farman, M. , Stajich, J.E. , Sentandreu, R. , Gonzalez‐Prieto, J.M. , Kennell, J.C. , Molina, L. , Schirawski, J. , Mendoza‐Mendoza, A. , Greilinger, D. , Munch, K. , Rossel, N. , Scherer, M. , Vranes, M. , Ladendorf, O. , Vincon, V. , Fuchs, U. , Sandrock, B. , Meng, S. , Ho, E.C.H. , Cahill, M.J. , Boyce, K.J. , Klose, J. , Klosterman, S.J. , Deelstra, H.J. , Ortiz‐Castellanos, L. , Li, W. , Sanchez‐Alonso, P. , Schreier, P.H. , Hauser‐Hahn, I. , Vaupel, M. , Koopmann, E. , Friedrich, G. , Voss, H. , Schluter, T. , Margolis, J. , Platt, D. , Swimmer, C. , Gnirke, A. , Chen, F. , Vysotskaia, V. , Mannhaupt, G. , Guldener, U. , Munsterkotter, M. , Haase, D. , Oesterheld, M. , Mewes, H.‐W. , Mauceli, E.W. , DeCaprio, D. , Wade, C.M. , Butler, J. , Young, S. , Jaffe, D.B. , Calvo, S. , Nusbaum, C. , Galagan, J. and Birren, B.W. (2006) Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis . Nature, 444, 97–101. [DOI] [PubMed] [Google Scholar]
- Kessler, M. , Cech, T.L. , Brandstetter, M. and Kirisits, T. (2012) Dieback of ash (Fraxinus excelsior and Fraxinus angustifolia) in Eastern Austria: disease development on monitoring plots from 2007 to 2010. J. Agric. Ext. Rural Dev. 4, 223–226. [Google Scholar]
- Kirisits, T. and Cech, T. (2009) Beobachtungen zum sexuellen Stadium des Eschentriebsterben‐Erregers Chalara fraxinea in Österreich. Forstschutz Aktuell, 48, 21–25. [Google Scholar]
- Kirisits, T. and Cech, T. (2010) Massnahmen gegen das Eschentriebsterben. Kärntner Forstverein Information 62, 31–33. [Google Scholar]
- Kirisits, T. and Freinschlag, C. (2012) Ash dieback caused by Hymenoscyphus pseudoalbidus in a seed plantation of Fraxinus excelsior in Austria. J. Agric. Ext. Rural Dev. 4, 184–191. [Google Scholar]
- Kirisits, T. , Matlakova, M. , Mottinger‐Kroupa, S. , Cech, T. and Halmschlager, E. (2009) The current situation of ash dieback caused by Chalara fraxinea in Austria. In: Proceedings of the IUFRO Working Party 7.02.02 Meeting in Eğirdir, Turkey, 11–16 May 2009 (Doğmuş‐Lehtijärvi T., ed.). , p. 21 Isparta, Turkey: Süleyman Demirel University, Faculty of Forestry. [Google Scholar]
- Kirisits, T. , Matlakova, M. , Mottinger‐Kroupa, S. , Halmschlager, E. and Lakatos, F. (2010) Chalara fraxinea associated with dieback of narrow‐leafed ash (Fraxinus angustifolia). Plant Pathol. 59, 411. [Google Scholar]
- Kirisits, T. , Kritsch, P. , Kräutler, K. , Matlakova, M. and Erhard, H. (2012) Ash dieback associated with Hymenoscyphus pseudoalbidus in forest nurseries in Austria. J. Agric. Ext. Rural Dev. 4, 223–226. [Google Scholar]
- Kirisits, T. , Dämpfle, L. and Kräutler, K. (2013) Hymenoscyphus albidus is not associated with an anamorphic stage and displays slower growth than Hymenoscyphus pseudoalbidus on agar media. For. Pathol. (in press). doi: 10.1111/efp.12042 [DOI] [Google Scholar]
- Kirk, P.M. , Cannon, P.F. , Minter, D.W. and Stalpers, J.A. (2008) Ainsworth and Bisby's Dictionary of the Fungi. Wallingford: CAB International. [Google Scholar]
- Kjær, E.D. , McKinney, L.V. , Nielsen, L.R. , Hansen, L.N. and Hansen, J.K. (2012) Adaptive potential of ash (Fraxinus excelsior) populations against the novel emerging pathogen Hymenoscyphus pseudoalbidus . Evol. Appl. 5, 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, J.L. , Carey, D.W. , Knight, K.S. , Poland, T. , Herms, D.A. and Mason, M.E. (2012) Breeding strategies for the development of emerald ash borer‐resistant North American ash. In: Proceedings of the 4th International Workshop on the Genetics of Host–Parasite Interactions in Forestry: Disease and Insect Resistance in Forest Trees, Eugene, OR, 31 July–5 August 2011 (Sniezko R.A., Yanchuk A.D., Kliejunas J.T., Palmieri K.M., Alexander J.M. and Frankel S.J., eds), pp. 235–239. Gen. Tech. Rep. PSW‐GTR‐240. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture. [Google Scholar]
- Kowalski, T. (2001) O zamieraniu jesionów. Trybuna Leśnika 4, 6–7. [Google Scholar]
- Kowalski, T. (2006) Chalara fraxinea sp.nov. associated with dieback of ash (Fraxinus excelsior) in Poland. For. Pathol. 36, 264–270. [Google Scholar]
- Kowalski, T. and Bartnik, C. (2010) Morphologial variation in colonies of Chalara fraxinea isolated from ash (Fraxinus excelsior L.) stems with symptoms of dieback and effects of temperature on colony growth and structure. Acta Agrobot. 63, 99–106. [Google Scholar]
- Kowalski, T. and Holdenrieder, O. (2008) A new fungal diesease of ash in Europe (in German). Schweiz. Z. Forstwes. 159, 45–50. [Google Scholar]
- Kowalski, T. and Holdenrieder, O. (2009a) Pathogenicity of Chalara fraxinea . For. Pathol. 39, 1–7. [Google Scholar]
- Kowalski, T. and Holdenrieder, O. (2009b) The teleomorph of Chalara fraxinea, the causal agent of ash dieback. For. Pathol. 39, 304–308. [Google Scholar]
- Kowalski, T. and Lukomska, A. (2005) The studies on ash dying (Fraxinus excelsior L.) in the Włoszczowa Forest Unit stands [Poland] (in Polish). Acta Agrobot. 58, 429–439. [Google Scholar]
- Kowalski, T. , Kraj, W. and Szeszycki, T. (2012) Badania nad zamieraniem jesionu w drzewostanach nadleśnictwa rokita. Acta Agraria e Silvestria L, 3–22. [Google Scholar]
- Kraj, W. , Zarek, M. and Kowalski, T. (2012) Genetic variability of Chalara fraxinea, dieback cause of European ash (Fraxinus excelsior L.). Mycol. Prog. 11, 37–45. [Google Scholar]
- Kräutler, K. and Kirisits, T. (2012) The ash dieback pathogen Hymenoscyphus pseudoalbidus is associated with leaf symptoms on ash species (Fraxinus spp.). J. Agric. Ext. Rural Dev. 4, 261–265. [Google Scholar]
- Leach, C.M. (1971) A practical guide to the effects of visible and ultraviolet light on fungi In: Methods in Microbiology (Booth C., ed.), pp. 609–664. New York: Academic Press. [Google Scholar]
- Limpert, E. , Godet, F. and Müller, K. (1999) Dispersal of cereal mildews across Europe. Agr. Forest. Meteorol. 97, 293–308. [Google Scholar]
- Luchi, N. , Montecchio, L. and Santini, A. (2012) Situation with ash in Italy: stand characteristics, health condition, ongoing work and research needs In: Interim Report from Chalara fraxinea, FRAXBACK Meeting in Vilnius, 13–14 November 2012 (Mainprize N., Hendry S. and Weir J., eds), pp. 25–26. Bristol: Forestry Commission. [Google Scholar]
- Lygis, V. , Vasiliauskas, R. , Larsson, K.‐H. and Stenlid, J. (2006) Wood‐inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes . Scand. J. For. Res. 20, 337–346. [Google Scholar]
- MacLean, D. (2013) Signatures of compositionally non‐homogeneous domains (CNHD) in draft genome. Available at http://oadb.tsl.ac.uk/?p=521 [accessed on June 25, 2013].
- MacLean, D. , Yoshida, K. , Edwards, A. , Crossman, L. , Clavijo, B. , Clark, M. , Swarbreck, D. , Bashton, M. , Chapman, P. , Gijzen, M. , Caccamo, M. , Downie, A. , Kamoun, S. and Saunders, D. (2013) Crowdsourcing genomic analyses of ash and ash dieback—power to the people. GigaScience, 2, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra, R.E. , Cortesi, P. , Bissegger, M. and Milgroom, M.G. (2004) Mixed mating in natural populations of the chestnut blight fungus, Cryphonectria parasitica . Heredity, 93, 189–195. [DOI] [PubMed] [Google Scholar]
- Mayer, A.M. and Staples, R.C. (2002) Laccase: new functions for an old enzyme. Phytochemistry, 60, 551–565. [DOI] [PubMed] [Google Scholar]
- McDonald, B.A. and Linde, C. (2002) Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 40, 349–379. [DOI] [PubMed] [Google Scholar]
- McKinney, L.V. , Nielsen, L.R. , Hansen, J.K. and Kjaer, E.D. (2011) Presence of natural genetic resistance in Fraxinus excelsior (Oleaceae) to Chalara fraxinea (Ascomycota): an emerging infectious disease. Heredity, 106, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney, L.V. , Thomsen, I.M. , Kjær, E.D. , Bengtsson, S.B.K. and Nielsen, L.R. (2012a) Rapid invasion by an aggressive pathogenic fungus (Hymenoscyphus pseudoalbidus) replaces a native decomposer (Hymenoscyphus albidus): a case of local cryptic extinction? Fungal Ecol. 5, 663–669. [Google Scholar]
- McKinney, L.V. , Thomsen, I.M. , Kjaer, E.D. and Nielsen, L.R. (2012b) Genetic resistance to Hymenoscyphus pseudoalbidus limits fungal growth and symptom occurrence in Fraxinus excelsior . For. Pathol. 42, 69–74. [Google Scholar]
- Metzler, B. (2011) Eschentriebsterben weiter zunehmend. FVA Waldschutz‐INFO, 2, 1–4. [Google Scholar]
- Metzler, B. and Witzel, G.M. (2012) Eschentriebsterben: Schadensintensivierung durch Stammfußnekrosen. FVA Waldschutz‐INFO, 3, 1–4. [Google Scholar]
- Metzler, B. , Enderle, R. , Karopka, M. , Toepfner, K. and Aldinger, E. (2012a) Development of ash dieback in a provenance trial on different sites in southern Germany. Allg. Forst‐ Jagdztg. 183, 168–180. [Google Scholar]
- Metzler, B. , Junker, C. , Lenz, H. , Enderle, R. and Schulz, B. (2012b) Ash dieback: situation and research in Germany In: Interim Report from Chalara fraxinea, FRAXBACK Meeting in Vilnius, 13–14 November 2012 (Mainprize N., Hendry S. and Weir J., eds), pp. 22–23. Bristol: Forestry Commission. [Google Scholar]
- Metzler, B. , Baumann, M. , Baier, U. , Heydeck, P. , Bressem, U. and Lenz, H. (2013) Bundesweite Zusammenstellung: Handlungsempfehlungen beim Eschentriebsterben. AFZ‐DerWald, 5, 17–20. [Google Scholar]
- Ogris, N. , Hauptman, T. and Jurc, D. (2009) Chalara fraxinea causing common ash dieback newly reported in Slovenia. Plant Pathol. 58, 1173. [Google Scholar]
- Pautasso, M. , Dehnen‐Schmutz, K. , Holdenrieder, O. , Pietravalle, S. , Salama, N. , Jeger, M.J. , Lange, E. and Hehl‐Lange, S. (2010) Plant health and global change—some implications for landscape management. Biol. Rev. 85, 729–755. [DOI] [PubMed] [Google Scholar]
- Pautasso, M. , Aas, G. , Queloz, V. and Holdenrieder, O. (2013) European ash (Fraxinus excelsior) dieback—a conservation biology challenge. Biol. Conserv. 158, 37–49. [Google Scholar]
- Pérez, G. , Slippers, B. , Wingfield, B.D. , Hunter, G.C. and Wingfield, M.J. (2010) Micro‐ and macrospatial scale analyses illustrate mixed mating strategies and extensive geneflow in populations of an invasive haploid pathogen. Mol. Ecol. 19, 1801–1813. [DOI] [PubMed] [Google Scholar]
- Pham, T. , Zaspel, I. , Schuemann, M. , Stephanowitz, H. and Krause, E. (2013) Rapid in‐vivo and in‐vitro detection of Chalara fraxinea by means of mass spectrometric techniques. Am. J. Plant Sci. 4, 444–453. [Google Scholar]
- Pliūra, A. , Lygis, V. , Suchockas, V. and Bartkevicius, E. (2011) Performance of twenty four European Fraxinus excelsior populations in three Lithuanian progeny trials with a special emphasis on resistance to Chalara fraxinea . Balt. For. 17, 17–34. [Google Scholar]
- Pliūra, A. , Lygis, V. , Gustiene, A. , Marčiulyniene, D. and Suchockas, V. (2013) Situation with ash in Lithuania: stand characteristics, health condition, ongoing work and research needs In: Interim Report from Chalara fraxinea, FRAXBACK Meeting in Vilnius, 13–14 November 2012 (Mainprize N., Hendry S. and Weir J., eds), pp. 27–29. Bristol: Forestry Commission. [Google Scholar]
- Pontecorvo, G. , Roper, J.A. , Hemmons, L.M. , Macdonald, K.D. and Bufton, A.W.J. (1953) The genetics of Aspergillus nidulans . Adv. Genet. 5, 141–238. [DOI] [PubMed] [Google Scholar]
- Potter, C. , Harwood, T. , Knight, J. and Tomlinson, I. (2011) Learning from history, predicting the future: the UK Dutch elm disease outbreak in relation to contemporary tree disease threats. Philos. Trans. R. Soc. B, 366, 1966–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybył, K. (2002) Fungi associated with necrotic apical parts of Fraxinus excelsior shoots. For. Pathol. 32, 387–394. [Google Scholar]
- Pukacki, P.M. and Przybył, K. (2005) Frost injury as a possible inciting factor in bud and shoot necroses of Fraxinus excelsior L. J. Phytopathol. 153, 512–516. [Google Scholar]
- Queloz, V. , Grünig, C.R. , Berndt, R. , Kowalski, T. , Sieber, T.N. and Holdenrieder, O. (2011) Cryptic speciation in Hymenoscyphus albidus . For. Pathol. 41, 133–142. [Google Scholar]
- Queloz, V. , Grunig, C.R. , Berndt, R. , Kowalski, T. , Sieber, T.N. and Holdenrieder, O. (2012) Corrigendum. For. Pathol. 42, 352. [Google Scholar]
- Raffaele, S. and Kamoun, S. (2012) Genome evolution in filamentous plant pathogens: why bigger can be better. Nat. Rev. Microbiol. 10, 417–430. [DOI] [PubMed] [Google Scholar]
- Ragazzi, A. , Moricca, S. and Dellavalle, I. (1999) Interactions between Quercus spp. and Diplodia mutila under water stress conditions. Z. Pflanzenkrankh. Pflanzenschutz—J. Plant Dis. Protect. 106, 495–500. [Google Scholar]
- Ranta, H. , Neuvonen, S. and Ylimartimo, A. (1995) Interactions of Gremmeniella abietina and endophytic fungi in shoots of Scots pine trees treated with simulated acid rain. J. Appl. Ecol. 32, 67–75. [Google Scholar]
- Rebek, E.J. , Herms, D.A. and Smitley, D.R. (2008) Interspecific variation in resistance to emerald ash borer (Coleoptera: Buprestidae) among North American and Asian ash (Fraxinus spp.). Environ. Entomol. 37, 242–246. [DOI] [PubMed] [Google Scholar]
- Rehm, H. (1896) Ascomyceten: Hysteriaceen und Discomyceten In: Kryptogramen‐Flora von Deutschland, Österreich und der Schweiz (Rabenhorst L., ed.), p. 1275 Leipzig: Eduard Kummer. [Google Scholar]
- Rivera‐Vega, L. , Mamidala, P. , Koch, J.L. , Mason, M.E. and Mittapalli, O. (2012) Evaluation of reference genes for expression studies in ash (Fraxinus spp.). Plant Mol. Biol. Rep. 30, 242–245. [Google Scholar]
- Roane, M.K. , Griffin, G.J. and Elkins, J.R. (1986) Chestnut Blight, Other Endothial Diseases, and the Genus Endothia. St. Paul, MN: American Phytopathological Society (APS). [Google Scholar]
- Robin, C. and Heiniger, U. (2001) Chestnut blight in Europe: diversity of Cryphonectria parasitica, hypovirulence and biocontrol. For. Snow Landsc. Res. 76, 361–367. [Google Scholar]
- Rytkönen, A. , Lilja, A. , Drenkhan, R. , Gaitnieks, T. and Hantula, J. (2011) First record of Chalara fraxinea in Finland and genetic variation among isolates sampled from Åland, mainland Finland, Estonia and Latvia. For. Pathol. 41, 169–174. [Google Scholar]
- Sánchez, M.E. , Venegas, J. , Romero, M.A. , Phillips, A.J.L. and Trapero, A. (2003) Botryosphaeria and related taxa causing oak canker in southwestern Spain. Plant Dis. 87, 1515–1521. [DOI] [PubMed] [Google Scholar]
- Santamaría, O. , Tejerina, L. , Pajares, J.A. and Diez, J.J. (2007) Effects of associated fungi Sclerophoma pythiophila and Cenangium ferruginosum on Gremmeniella abietina dieback in Spain. For. Pathol. 37, 121–128. [Google Scholar]
- Santini, A. , Ghelardini, L. , De Pace, C. , Desprez‐Loustau, M.L. , Capretti, P. , Chandelier, A. , Cech, T. , Chira, D. , Diamandis, S. , Gaitniekis, T. , Hantula, J. , Holdenrieder, O. , Jankovsky, L. , Jung, T. , Jurc, D. , Kirisits, T. , Kunca, A. , Lygis, V. , Malecka, M. , Marcais, B. , Schmitz, S. , Schumacher, J. , Solheim, H. , Solla, A. , Szabò, I. , Tsopelas, P. , Vannini, A. , Vettraino, A.M. , Webber, J. , Woodward, S. and Stenlid, J. (2013) Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 197, 238–250. [DOI] [PubMed] [Google Scholar]
- Saunders, D. (2013) Analysis of the MAT1 locus of C. fraxinea KW1 isolate. Available at http://oadb.tsl.ac.uk/?p=329 [accessed on May 3, 2013].
- Schenck, C.A. (1939) Fremdländische Wald‐ und Parkbäume. III. Band: Laubhölzer. Berlin: Verlag von Paul Parey. [Google Scholar]
- Schlotterer, C. (2004) The evolution of molecular markers—just a matter of fashion? Nat. Rev. Genet. 5, 63–69. [DOI] [PubMed] [Google Scholar]
- Schumacher, J. (2011) The general situation regarding ash dieback in Germany and investigations concerning the invasion and distribution strategies of Chalara fraxinea in woody tissue. EPPO Bull. 41, 7–10. [Google Scholar]
- Schumacher, J. , Wulf, A. and Leonhard, S. (2007) First record of Chalara fraxinea T. Kowalski sp.nov. in Germany—the causal agent of novel damages on ash trees (in German). Nachrichtenbl. Deut. Pflanzenschutz 59, 121–123. [Google Scholar]
- Schumacher, J. , Kehr, R. and Leonhard, S. (2010) Mycological and histological investigations of Fraxinus excelsior nursery saplings naturally infected by Chalara fraxinea . For. Pathol. 40, 419–429. [Google Scholar]
- Selikhovkin, A.V. , Musolin, D.L. and Lukmazova, E.A. (2013) Situation with ash in Russian Federation: stand characteristics, health condition, ongoing work and research needs In: Interim Report from Chalara fraxinea, FRAXBACK Meeting in Vilnius, 13–14 November 2012 (Mainprize N., Hendry S. and Weir J., eds), pp. 34–35. Bristol: Forestry Commission. [Google Scholar]
- Shain, L. (1982) Strategies for enhancing dissemination of hypovirulence in Endothia parasitica: state of the art In: Proceedings of the US Forestry Service American Chestnut Cooperators' Meeting in Morgantown, West Virginia, (5–7 January 1982 Smith H.C. and MacDonald W.C., eds), pp. 175–183. Morgantown, WV: West Virginia University Books. [Google Scholar]
- Shinya, R. , Morisaka, H. , Takeuchi, Y. , Futai, K. and Ueda, M. (2013) Making headway in understanding pine wilt disease: what do we perceive in the postgenomic era? J. Biosci. Bioeng. 116, 1–8. [DOI] [PubMed] [Google Scholar]
- Sieber, T.N. (2007) Endophytic fungi in forest trees: are they mutualists? Fungal Biol. Rev. 21, 75–89. [Google Scholar]
- Sieber, T.N. , Rys, J. and Holdenrieder, O. (1999) Mycobiota in symptomless needles of Pinus mugo ssp. uncinata . Mycol. Res. 103, 306–310. [Google Scholar]
- Skovsgaard, J.P. , Thomsen, I.M. , Skovgaard, I.M. and Martinussen, T. (2010) Associations among symptoms of dieback in even‐aged stands of ash (Fraxinus excelsior L.). For. Pathol. 40, 7–18. [Google Scholar]
- Solheim, H. , Timmermann, V. , Børja, I. and Hietala, A.M. (2011) Yggdrasils helsetilstand—Askeskuddsjuke er på frammarsj. Skogeieren, 96, 34–36. [Google Scholar]
- Solheim, H. , Timmermann, V. , Talgø, V. and Røsberg, I. (2012a) Ash dieback in Norway. Forstschutz Aktuell, 55, 49–51. [Google Scholar]
- Solheim, H. , Tollefsrud, M.M. , Hietala, A.M. , Nagy, Z.A. , Timmermann, V. , Bøja, I. , Myking, T. , Sønstebø, J.H. and Smith, A.V. (2012b) Situation with ash and ash dieback in Norway—current situation and ongoing research In: Interim Report from Chalara fraxinea, FRAXBACK Meeting in Vilnius, 13–14 November 2012 (Mainprize N., Hendry S. and Weir J., eds), pp. 31–32. Bristol: Forestry Commission. [Google Scholar]
- Speich, S. and Brändli, U.‐B. (2011) Drittes Schweizerisches Landesforstinventar—Ergebnistabellen im Internet. Zweite stark erweiterte Ausgabe. Available at http://www.lfi.ch/resultate/ [accessed on May 6, 2013]. Birmensdorf, Eidg. Forschungsanstalt WSL.
- Stener, L.‐G. (2012) Clonal differences in susceptibility to the dieback of Fraxinus excelsior in southern Sweden. Scand. J. For. Res. 28, 205–216. [Google Scholar]
- Stenlid, J. , Bengtsson, S.B.K. , Cleary, M.R. , Andersson, P.F. , Broberg, A. , Stener, L.‐G. and Vasaitis, R. (2012) Research on Chalara dieback in Sweden In: Interim Report from Chalara fraxinea, FRAXBACK Meeting in Vilnius, 13–14 November 2012 (Mainprize N., Hendry S. and Weir J., eds), p. 41 Bristol: Forestry Commission. [Google Scholar]
- Sunnucks, P. (2000) Efficient genetic markers for population biology. Trends Ecol. Evol. 15, 199–203. [DOI] [PubMed] [Google Scholar]
- Szabo, I. (2009) First report of Chalara fraxinea affecting common ash in Hungary. Plant Pathol. 58, 797. [Google Scholar]
- Tack, A.J.M. , Thrall, P.H. , Barrett, L.G. , Burdon, J.J. and Laine, A.L. (2012) Variation in infectivity and aggressiveness in space and time in wild host–pathogen systems: causes and consequences. J. Evol. Biol. 25, 1918–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhonen, E. , Marco, T. , Sun, H. , Jalkanen, R. , Kasanen, R. , Vuorinen, M. and Asiegbu, F. (2011) The effect of latitude, season and needle‐age on the mycota of Scots pine (Pinus sylvestris) in Finland. Silva Fenn. 45, 301–317. [Google Scholar]
- Thomsen, I.M. and Skovgaard, J.P. (2012) Silvicultural strategies for forest stands with ash dieback. Forstschutz Aktuell, 55, 18–20. [Google Scholar]
- Timmermann, V. , Børja, I. , Hietala, A.M. , Kirisits, T. and Solheim, H. (2011) Ash dieback: pathogen spread and diurnal patterns of ascospore dispersal, with special emphasis on Norway. EPPO Bull. 41, 14–20. [Google Scholar]
- Wang, Q. and Dai, L. (1997) Fraxinus mandshurica Rupr., 1857. Enzyklopädie der Holzgewächse III‐2, 1–6. [Google Scholar]
- Wang, Z. , Binder, M. , Schoch, C.L. , Johnston, P.R. , Spatafora, J.W. and Hibbett, D.S. (2006) Evolution of helotialean fungi (Leotiomycetes, Pezizomycotina): a nuclear rDNA phylogeny. Mol. Phylogenet. Evol. 41, 295–312. [DOI] [PubMed] [Google Scholar]
- Webber, J.F. (2004) Experimental studies on factors influencing the transmission of Dutch elm disease. Forest Systems, 13, 197–205. [Google Scholar]
- Whitehill, J.A. , Opiyo, S. , Koch, J. , Herms, D. , Cipollini, D. and Bonello, P. (2012) Interspecific comparison of constitutive ash phloem phenolic chemistry reveals compounds unique to manchurian ash, a species resistant to emerald ash borer. J. Chem. Ecol. 38, 499–511. [DOI] [PubMed] [Google Scholar]
- Wipf, P. and Kerekes, A.D. (2003) Structure reassignment of the fungal metabolite TAEMC161 as the phytotoxin viridiol. J. Nat. Prod. 66, 716–718. [DOI] [PubMed] [Google Scholar]
- Zhao, Y.‐J. , Hosoya, T. , Baral, H.‐O. , Hosaka, K. and Kakishima, M. (2012) Hymenoscyphus pseudoalbidus, the correct name for Lambertella albida reported from Japan. Mycotaxon, 122, 25–41. [Google Scholar]
- Zheng, H. and Zhuang, W. (2013) Four new species of the genus Hymenoscyphus (fungi) based on morphology and molecular data. Sci. Chin. Life Sci. 56, 90–100. [DOI] [PubMed] [Google Scholar]


