Summary
Hexanoic acid‐induced resistance (Hx‐IR) is effective against several pathogens in tomato plants. Our study of the mechanisms implicated in Hx‐IR against Pseudomonas syringae pv. tomato DC3000 suggests that hexanoic acid (Hx) treatment counteracts the negative effect of coronatine (COR) and jasmonyl‐isoleucine (JA‐Ile) on the salicylic acid (SA) pathway. In Hx‐treated plants, an increase in the expression of jasmonic acid carboxyl methyltransferase (JMT) and the SA marker genes PR1 and PR5 indicates a boost in this signalling pathway at the expense of a decrease in JA‐Ile. Moreover, Hx treatment potentiates 12‐oxo‐phytodienoic acid accumulation, which suggests that this molecule might play a role per se in Hx‐IR. These results support a positive relationship between the SA and JA pathways in Hx‐primed plants. Furthermore, one of the mechanisms of virulence mediated by COR is stomatal re‐opening on infection with P. syringae. In this work, we observed that Hx seems to inhibit stomatal opening in planta in the presence of COR, which suggests that, on infection in tomato, this treatment suppresses effector action to prevent bacterial entry into the mesophyll.
Introduction
In response to a wide variety of microbial pathogens and insect herbivores, plants have developed numerous defence mechanisms, and many of these defences are induced by pathogen attack. The appropriate regulation of defence responses is important for plant fitness, as the activation of defence responses has deleterious effects on plant growth. Plants partly protect themselves against pathogen challenges by means of constitutive preformed barriers (constitutive resistance). When these barriers fail to prevent the entry of pathogens, plants generally activate another chain of defence responses (pathogen‐induced resistance), such as reinforced barriers and stomatal closure control. These systems are activated when plants detect microbial elicitors, named pathogen‐ or microbe‐associated molecular patterns (PAMPs or MAMPs). Moreover, many plant hormones are involved in these responses, including jasmonates, salicylates and ethylene (ET). Generally, salicylic acid (SA) is part of the defence responses against biotrophic pathogens, whereas jasmonic acid (JA) participates in defence responses against necrotrophs and insects (Beckers and Spoel, 2006). SA induces the expression of a set of defence‐related genes, such as genes encoding some of the pathogenesis‐related (PR) proteins. Although SA has been shown to play an important role in signal transduction, other molecules are also involved directly in plant defence. For example, methyl salicylate (MeSA) has recently been identified as a mobile signal for systemic acquired resistance (SAR) in Tobacco mosaic virus (TMV)‐infected tobacco (Nicotiana tabacum) (Park et al., 2007).
Furthermore, several molecules from the JA synthesis pathway may be involved in plant defence processes. Jasmonates are synthesized in plants via the octadecanoid pathway. JA is regarded as the primary intracellular transducer of this pathway because of its similarity to prostaglandin of animals (Cheong and Choi, 2003; Kazan and Manners, 2008). However, depending on the biological system studied, a number of biosynthetic intermediates, isomers, derivatives and metabolites of the octadecanoid pathway have been observed to be powerful cellular regulators. For instance, the octadecanoid precursors of JA, including linolenic acid, 13(S)‐hydroperoxylinolenic acid and 12‐oxo‐phytodienoic acid (OPDA), have known biological activities in the jasmonate‐regulated responses of potato plants against Pseudomonas syringae pv. maculicola. These activities suggest that OPDA is important in the SAR of potato plants (Avanci et al., 2010; Landgraf et al., 2002) The Arabidopsis opr3 mutant does not produce an isoform of the enzyme 12‐oxophytodienoate reductase (12‐OPR), which is essential for JA biosynthesis (Stintzi and Browse, 2000), but displays increased resistance to the insect Bombus impatiens and the fungus Alternaria brassicicola. The analysis of the transcriptional profile of opr3 indicates that genes formerly characterized as exhibiting JA‐dependent expression after wounding are also expressed in this mutant, which suggests that cyclopentenone jasmonate derivatives can fulfil some JA tasks in vivo (Stintzi et al., 2001).
JA, a hormone, can also be catabolized to form its volatile counterpart methyl jasmonate (MeJA) and numerous conjugates, such as jasmonyl‐leucine (JA‐Leu) and jasmonyl‐isoleucine (JA‐Ile). Amino acid conjugation is necessary for JA activation, and (−)‐JA‐l‐Ile has been demonstrated recently to be the bioactive form of JA (Staswick, 2008).
Previous studies have suggested that MeJA formation is one of the important control points for jasmonate‐regulated plant responses. MeJA may be a candidate for intra‐ and intercellular signal transducers that mediate jasmonate‐responsive plant responses because it can diffuse through membranes. One important aspect of this molecule in plant defences against P. syringae is that MeJA affects plant transpiration (Wang, 1999) by promoting stomatal closure (Gehring et al., 1997; Suhita et al., 2003), which induces alkalinization in the cytosol of guard cells before the production of reactive oxygen species (Suhita et al., 2004). This aspect might affect the plant–bacterium interaction by inhibiting the bacterial suppression of stomatal closure, which was observed in a Pseudomonas–plant interaction.
Pathogens have also evolved complex mechanisms to evade plant defences. Pseudomonas syringae pv. tomato is a hemibiotrophic microorganism, which causes bacterial speck disease in tomato, Arabidopsis thaliana and Brassica spp. One mechanism of P. syringae pv. tomato pathogenesis is to produce the effector coronatine (COR), a JA‐Ile mimic that activates JA‐Ile synthesis. Both molecules suppress SA‐mediated host responses and therefore increase bacterial virulence (Brooks et al., 2005; Katsir et al., 2008). COR also facilitates the entry of P. syringae into A. thaliana tissue by suppressing the closure of stomata, a basal defence response induced on microbial attack (Melotto et al., 2006). Moreover, COR is also required for the growth and persistence of this pathogen in plant tissue (Brooks et al., 2004; Elizabeth and Bender, 2007).
In addition to pathogen attack, several types of induced resistance, such as SAR and induced systemic resistance (ISR), can be produced by other microorganisms or by treatment with certain natural or synthetic compounds (Beckers and Conrath, 2007). During induced resistance, the sensitization of stress responsiveness, also called priming, is not only observed in plants, but also in animals (Jung et al., 2009; Pastor et al., 2012; Pham et al., 2007; Prime‐A‐Plant Group et al., 2006). Priming is the phenomenon that enables cells to respond to low levels of a stimulus in a more rapid and robust manner than is found in nonprimed cells (Conrath, 2011; Conrath et al., 2002). Thus, when plants primed by treatments inducing resistance are subsequently challenged by pathogens or abiotic stresses, these plants show a faster and/or stronger activation of defence responses (Prime‐A‐Plant Group et al., 2006). One chemical inducer of priming, β‐aminobutyric acid (BABA), is a potent inducer of resistance against a wide range of pathogens, and uses a signalling mechanism that differs from SAR and ISR (Ton and Mauch‐Mani, 2004).
Recently, we have demonstrated that hexanoic acid (Hx) induces resistance in Solanum lycopersicum and A. thaliana plants against Botrytis cinerea (Kravchuk et al., 2011; Vicedo et al., 2009). This natural compound acts as an inducer of plant defences by means of a priming mechanism. On infection, the oxylipin OPDA and the bioactive molecule JA‐Ile were significantly induced in treated plants. In addition, abscisic acid (ABA) acted as a positive regulator of Hx‐induced resistance (Hx‐IR) by enhancing callose accumulation (Vicedo et al., 2009).
In this study, we performed assays to determine both the mode of action and the effectiveness of Hx as an inducer of resistance against the hemibiotrophic pathogen P. syringae. We present evidence that Hx primes SA signalling through the inhibition of COR‐mediated bacterial manipulation of the SA pathway. We also show that Hx alters the oxylipin pathway by reducing the accumulation of the active molecule JA‐Ile and by increasing OPDA accumulation. OPDA could participate in the defence against this pathogen, although the involvement of other JA derivatives in the mechanism of Hx‐IR cannot be excluded. These results reveal a positive interaction between the JA and SA pathways in Hx‐primed plants.
Results
Hx enhances tomato resistance against P. syringae infection
To evaluate and confirm the efficacy of Hx against Pseudomonas in tomato plants, Hx treatments were applied in hydroponic culture, as described in Vicedo et al. (2009), and by soil drench. Four‐week‐old tomato plants (cv. Ailsa Craig) were treated with different Hx concentrations for 48 h under hydroponic conditions prior to P. syringae pv. tomato DC3000 (Pst) inoculation. Statistically significant reductions in disease symptoms (Fig. 1A) and in the size of the bacterial population (Fig. 1B) were observed at 72 h post‐inoculation (hpi), which demonstrated that the inducer effect is concentration dependent. We also checked the efficacy of Hx treatment when Hx was applied by soil drench. In these experiments, a treatment with 0.6 mm Hx at 72 h before inoculation also protected tomato plants by reducing significantly both the symptoms of disease (by approximately 50%) and the bacterial population (Fig. 1C,D).
Figure 1.
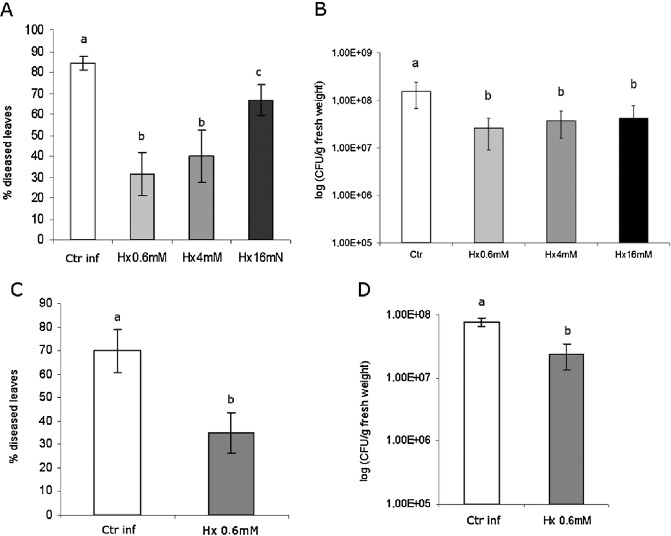
Hexanoic acid (Hx)‐induced resistance against Pseudomonas syringae. Four‐week‐old tomato plants were treated with Hx under hydroponic conditions (A,B) and after soil drench treatment (C,D). After 72 h of inoculation, the disease rating was scored by measuring the percentage of infected leaves in relation to the total number of analysed leaves (A,C) and by recounting of bacterial populations by plating in agar–King's B medium (B,D). Data show average values ± standard error (n = 20). Different letters represent statistically significant differences (P < 0.05; least‐significant difference test). Ctr, untreated and uninoculated plants; Ctr inf, untreated and inoculated plants.
In this work, the analyses of the inducer effect were performed by treating plants in hydroponic conditions with 0.6 mm Hx. This treatment reduced the symptoms by 50%, and the observed decrease in the bacterial population was similar to that described previously by other authors (Jung et al., 2009).
Hx‐IR does not alter callose deposition
We analysed callose deposition, which is a commonly used marker of basal defence activation. Four‐week‐old tomato plants (cv. Ailsa Craig) were treated with Hx as described previously, and callose deposition was analysed by cytological observations at the infection sites at two time points (48 and 72 hpi). As expected, untreated plants with an advanced infection state accumulated callose, but the more resistant Hx‐treated plants presented a similar callose accumulation on infection (Fig. S1, see Supporting Information).
Hx‐IR against P. syringae alters the hormonal profile of tomato plants
To determine whether Hx‐IR involves signalling pathways, the main hormones and metabolites were analysed simultaneously in the leaves of control and Hx‐treated tomato plants at 24, 48 and 72 hpi. On infection with P. syringae, no changes in the SA content were observed in treated plants during the experiment (Fig. 2A). ABA levels were increased slightly at 48 hpi in treated plants compared with untreated plants (Fig. 2B). Interesting results were obtained with regard to the components of the JA pathway. A significant increase in the oxylipin OPDA, a precursor of JA, was found only in Hx‐treated plants at 48 hpi, whereas no changes were found in the JA levels of treated plants relative to the levels of control plants (Fig. 2C,D). Moreover, the peak of the bioactive JA conjugate, JA‐Ile, was observed at 24 hpi and was not produced in Hx‐treated plants. This finding supports a priming effect of the inducer that interferes with the plant response to P. syringae (Fig. 2E).
Figure 2.
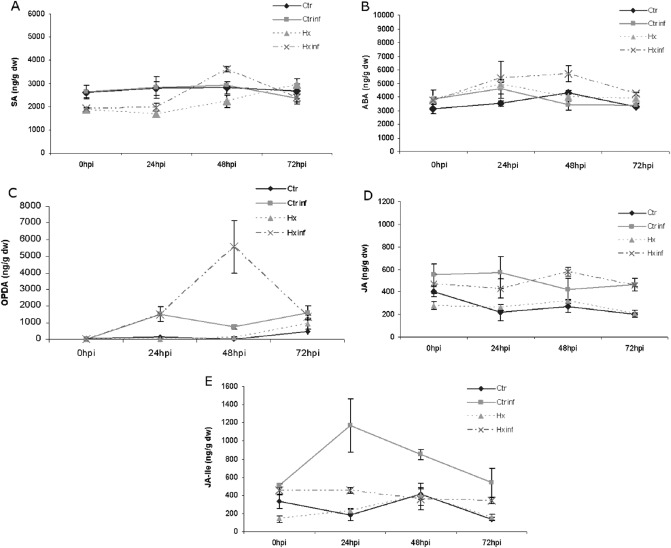
Hormone levels in water‐ and hexanoic acid (Hx)‐treated tomato plants (cv. Ailsa Craig) on Pseudomonas syringae infection. Leaves were collected at various time points and salicylic acid (SA) (A), abscisic acid (ABA) (B), 12‐oxo‐phytodienoic acid (OPDA) (C), jasmonic acid (JA) (D) and jasmonyl‐isoleucine (JA‐Ile) (E) levels were determined by high‐performance liquid chromatography (HPLC)‐mass spectrometry. Data show a representative experiment that was repeated three times; each point is the average of a pool of 10 plants ± standard error. Ctr, untreated and uninoculated plants; Ctr inf, untreated and inoculated plants; hpi, hours post‐inoculation; Hx, treated plants; Hx inf, treated and inoculated plants.
Hx‐IR involves SA and JA signalling pathways
Because the inducer changed the hormonal balance, we next analysed the expression pattern of the marker genes for SA (PR‐1, PR‐5 and SAMT), JA (LoxD, OPR3, JMT, JAR, PIN2 and JAZ1), ET (AccOx) and ABA (Asr1) signalling pathways in leaf samples harvested from treated and untreated plants at 0, 24, 48 and 72 hpi.
Although Hx did not increase the SA levels on infection, as described previously, it potentiated the early expression of the SA‐inducible marker genes PR1 (Fig. 3A), PR5 (Fig. S2A, see Supporting Information) and SAMT, which acts downstream of the SA synthetic pathway (Fig. 3B). No differences in the expression levels of the ABA‐ and ET‐related genes were observed in treated relative to untreated plants on infection (Fig. S2B,C). However, consistent with the hormonal analysis, Hx treatment induced transcript accumulation at 48 hpi of LoxD (Fig. 3C) and OPR3 (Fig. 3D), which are involved in the biosynthesis of OPDA and jasmonates. Interestingly, Hx increased the expression level of JMT (Fig. 3E) and reduced the levels of JAR transcripts (Fig. 3F), which might indicate the formation of MeJA (Fig. 4). To test this hypothesis, we further monitored the expression of the MeJA‐response gene, PIN2 (Fig. 3G). We observed elevated mRNA levels of PIN2 in treated plants within 24 h of treatment.
Figure 3.
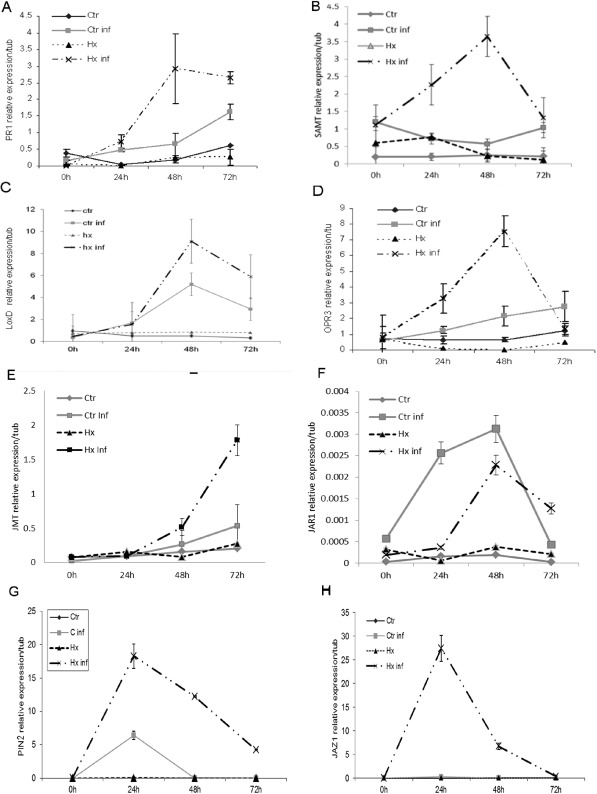
Effect of hexanoic acid (Hx) on the gene expression levels of tomato plants on Pseudomonas syringae infection. Tomato plants were grown, treated and inoculated as described in Experimental procedures. The expression of the PR1 (A), SAMT (B), LoxD (C), OPR3 (D), JMT (E), JAR (F), PIN2 (G) and JAZ1 (H) genes was analysed in cDNA from untreated and uninoculated plants (Ctr), untreated and inoculated plants (Ctr inf), treated plants (Hx) and treated and inoculated plants (Hx inf) at different time points (0, 24, 48, 72 h post‐inoculation). Results show average values of three independent experiments with similar results ± standard error (n = 3).
Figure 4.
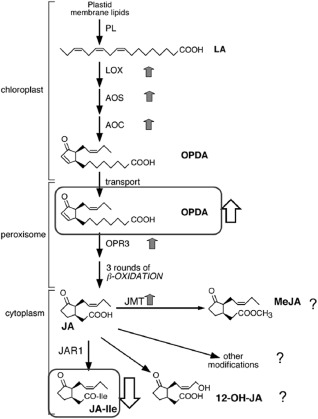
Induction of the oxylipin biosynthethic pathway in hexanoic acid (Hx)‐treated and infected plants. Shaded arrows indicate changes in gene expression: AOC, allene oxide cyclase; AOS, allene oxide synthase; LOX, lipoxygenase; JMT, jasmonic acid carboxyl methyltransferase; OPR3, oxo‐phytodienoic acid reductase. White arrows show the metabolites analysed by liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). JA, jasmonic acid; JA‐Ile, jasmonyl‐isoleucine; JAR1, jasmonate resistant 1; MeJA, methyl jasmonate; LA, linoleic acid; 12‐OH‐JA, 12‐hydroxy jasmonic acid; OPDA, 12‐oxo‐phytodienoic acid; PL, phospholipids.
We extended our analysis to JAZ1 (Fig. 3H), a repressor of the JA signalling pathway, as its SCFCOI1‐dependent ubiquitination is required for the activation of JA‐responsive gene expression (Chini et al., 2007). Notably, JAZ1 expression was induced only in treated and infected plants, which might prevent MYC2 activation of JA‐inducible genes. Taken together, these results suggest that Hx treatment prevents the JAZ1 increase in JA‐Ile levels, the physical interaction between JAZ1 and SlCOI1, which is dependent on the presence of (−)‐JA‐l‐Ile (Fonseca et al., 2009), and the further ubiquitination and proteosomal degradation of JAZ, which would subsequently release MYC2 and activate transcription. These changes were only observed in treated plants on infection, which supports a priming effect for the inducer Hx.
We also analysed tomato mutants impaired in the SA, ABA or JA pathways. Hx treatment did not protect transgenic NahG plants against infection because reduced SA levels were observed (Fig. 5A,B); this indicates a requirement for this hormonal pathway in Hx‐IR against P. syringae.
Figure 5.
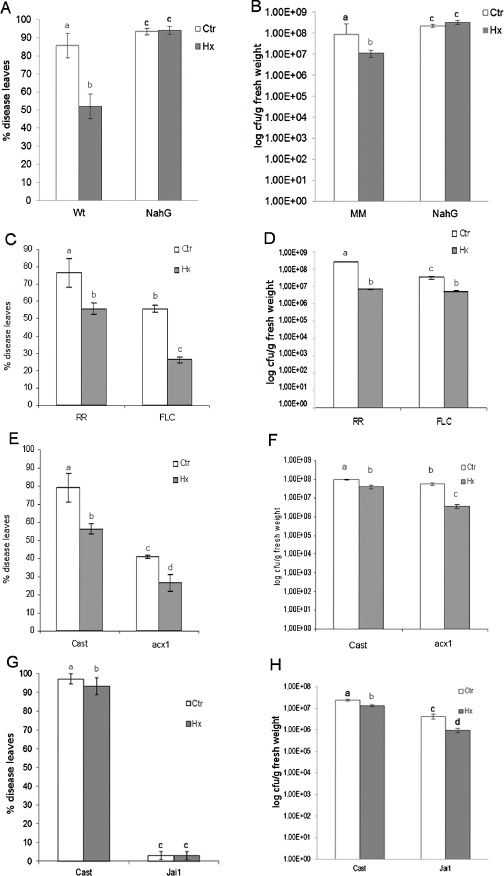
Influence of salicylic acid (SA), abscisic acid (ABA) and jasmonic acid (JA) signalling pathways on hexanoic acid (Hx)‐induced resistance against Pseudomonas syringae. After 72 h of inoculation, the disease rating was scored for wild‐type tomato plants of cv. Moneymaker (MM), Rheinlands Ruhm (RR) and Castlemart (Cast) and their respective SA‐impaired mutant NahG (A,B), ABA‐impaired mutant flacca (C,D) and JA‐impaired mutants acx1 (E,F) and jai1 (G,H). Data show the average values ± standard error (n = 20). Different letters represent statistically significant differences (P < 0.05; least‐significant difference test).
When we tested the ABA‐deficient mutant flacca, an enhanced basal resistance to P. syringae was observed (Fig. 5C,D). This mutant displayed intact Hx‐IR, which supports the notion that ABA does not play an important role in Hx‐IR. The mutant acx1, which is deficient in JA biosynthesis (Li et al., 2005) and acts downstream of OPDA formation, showed an enhanced basal resistance to P. syringae (Fig. 5E,F) and a functional Hx‐IR. This result points to a possible role for OPDA in the protection mediated by the inducer Hx.
When the response of the jai1 mutant, a jasmonic‐insensitive coi1 homologue (Li et al., 2004), to Hx treatment was analysed, both treated and untreated plants exhibited similar phenotypes with few detectable disease symptoms (Fig. 5G). This result might be caused by the high basal resistance of jai1 to P. syringae infection (Zhao et al., 2003). However, when bacterial growth was monitored (Fig. 5H), Hx‐IR seemed to be unaffected. This reduction in bacterial multiplication might indicate that, in addition to plant defence responses, changes in the plant caused by Hx treatment also have an effect on bacterial behaviour. Further studies need to be performed to investigate this possibility.
The absence of COR compromises Hx‐IR
After we had demonstrated a priming effect of Hx on plant defences, we tested the relevance of the virulence factor COR, a JA‐Ile mimic, on Hx‐IR. Using Pst as a control, Hx‐treated plants (cv. Ailsa Craig) were infected with the P. syringae strain cmaA, which lacks COR (Uppalapati et al., 2007). A reduction in bacterial growth was observed in plants infected with the mutant strain, as reported by Brooks et al. (2004). Hx treatment did not confer resistance to the mutant bacteria (Fig. 6), which supports the hypothesis that the inducer counteracts the effects of COR.
Figure 6.
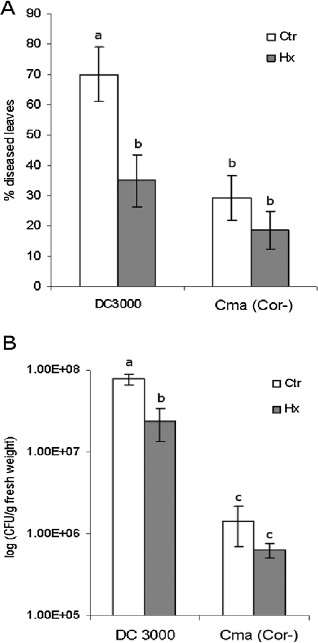
Hexanoic acid priming is absent in tomato plants infected with the coronatineless strain of Pseudomonas syringae (CmaA). Tomato plants were grown and treated as described in Experimental procedures and infected with Pseudomonas syringae pv. tomato DC3000 and with the coronatineless strain of Pseudomonas syringae (CmaA). The percentage of infected leaves (A) and bacterial growth (B) were evaluated 4 days after bacterial dip inoculation. Data show average values ± standard error (n = 20). Different letters represent statistically significant differences (P < 0.05; least‐significant difference test). Ctr, untreated and uninoculated plants; Hx, treated plants.
Hx inhibits stomatal opening mediated by COR
In our previous studies, we observed that, when Hx‐treated plants were inoculated by infiltration, no effect of the treatment was observed (data not shown). The fact that treatment with Hx is effective when P. syringae is inoculated by dipping might indicate that this treatment affects the early stages of infection, such as bacterial entry into the mesophyll. On infection with P. syringae, one of the mechanisms of virulence controlled by COR is stomatal re‐opening (Melotto et al., 2006). To study the possible effect of Hx treatment on this process, we developed an in situ method to measure stomatal opening. Hx‐ and water‐treated tomato plants were sprayed with COR, and the effect on stomata was determined in leaves. Under our experimental conditions, COR induced stomatal opening 1 h after application, which is probably a result of its direct application to the leaf mesophyll and the absence of PAMPs/MAMPs. Interestingly, in plants treated with Hx, the COR‐mediated opening of stomata was prevented (Fig. 7). This effect supports the interference of the inducer with the bacterial virulence mechanisms because, even in the absence of COR, Hx treatment caused stomatal opening. This finding confirmed our previous results showing that Hx enhanced the photosynthetic rate, transpiration rate and stomatal conductance, which were obtained using a portable, open‐system infrared gas analyser LCpro+ (ADC Bioscientific Ltd, Hoddesdon, Hertfordshire, UK) for the analysis of physiological parameters (data not shown).
Figure 7.
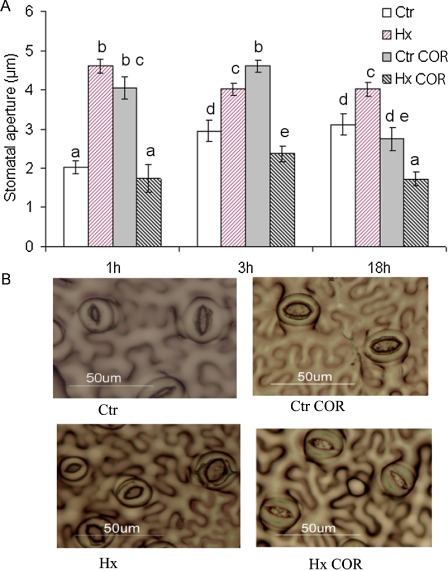
Hexanoic acid inhibits stomatal opening after coronatine (COR) treatment. (A) Stomatal apertures were analysed ‘in situ’ in leaflets of water (Ctr)‐ and hexanoic acid (Hx)‐treated tomato plants at 1, 3 and 18 h after COR treatment. (B) Representative photographs of stomatal aperture taken after COR treatment. Results are means ± standard error (SE) (n > 50 stomata). Different letters represent statistically significant differences (P < 0.05; least‐significant difference test).
The expression patterns of PR1, LoxD and JMT were analysed under these conditions, and demonstrated that COR application altered plant responses. We also observed that Hx induced PR1 expression and JMT induced mRNA accumulation after COR treatment. However, after COR application, no differences in the transcript levels of LoxD were observed between water‐ and Hx‐treated plants (Fig. 8).
Figure 8.
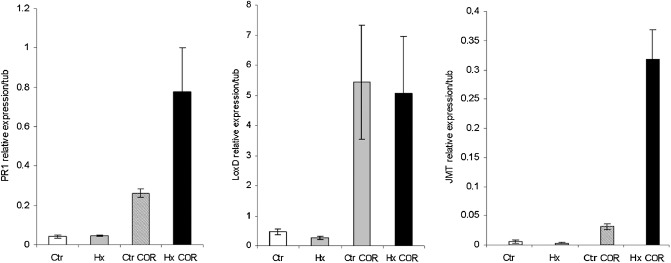
Hexanoic acid treatment represses coronatine (COR)‐mediated inhibition of pathogenesis‐related (PR) protein synthesis. Real‐time quantitative reverse transcriptase‐polymerase chain reaction (qRT‐PCR) was performed for PR1, LoxD and JMT genes. RNAs were extracted from leaves of 4‐week‐old tomato plants, 18 h after treatment with 0.5 μm purified COR. Error bars are the standard deviation (SD) (n = 3).
These results suggest that Hx treatment represses the COR‐mediated stomatal aperture, the inhibition of PR protein synthesis and the alteration of the JA pathway.
Discussion
In this work, we observed a putative interaction between the JA and SA signalling pathways in the priming mechanism of Hx against P. syringae in tomato plants. Although the antagonistic action between JA and SA in plant defence responses is well documented (Koornneef et al., 2008; Li et al., 2004), synergistic effects have also been observed in response to pathogens (Halim et al., 2009; Mur et al., 2006) and fungal elicitors (Xui et al., 2009).
Previous investigations by our research group have shown that Hx protects S. lycopersicum plants against the necrotroph B. cinerea, a pathogen that is difficult to control (Leyva et al., 2008; Vicedo et al., 2009). Furthermore, its efficacy was demonstrated in A. thaliana (Kravchuk et al., 2011). We also observed an approximately 50% reduction in disease symptoms in tomato plants in hydroponic culture, which indicated that Hx induced resistance against P. syringae (Vicedo et al., 2009)
Here, we have demonstrated that Hx treatment protects tomato plants in a concentration‐dependent manner in hydroponic conditions in all the tomato cultivars analysed, and when Hx is applied by soil drench. Taken together, these data demonstrate the versatility of the inducer treatment and the future practical applications in different culture conditions to protect tomato plants against P. syringae.
Although we have demonstrated previously that Hx treatment results in enhanced callose deposition after B. cinerea inoculation (Vicedo et al., 2009), here we have shows that tomato plants display Hx‐IR on bacterial infection without enhanced callose deposition. This result suggests that the mechanism involved in Hx‐IR varies depending on the pathogen. This finding is further confirmed by the results obtained in the analysis of gene expression and hormonal content.
We further investigated whether the main signalling pathways are involved in Hx‐IR. It is widely accepted that the SA pathway is induced against biotrophs and hemibiotrophs, such as P. syringae. Plants activate SA‐dependent defences that are effective against this pathogen and are able to neutralize some of the immune‐suppressive effects, such as the effector‐induced auxin response described by Wang et al. (2007). However, some pathogens are also able to suppress SA‐dependent defences by injecting different virulence effectors into the host cell via a type III protein secretion system. For instance, the virulence factor HopI1 is able to suppress SA accumulation. In concrete terms, P. syringae produces the phytotoxin COR and promotes the synthesis of JA‐Ile, the bioactive molecule that inhibits the expression of SA‐responsive genes (Pieterse et al., 2009). COR functions as a JA‐Ile mimic by suppressing SA‐dependent defences (Brooks et al., 2005; Uppalapati et al., 2007), which thereby promotes plant susceptibility to this pathogen. An intact COR molecule is required for both the suppression of SA‐mediated defence responses and full disease symptom development in tomato (Uppalapati et al., 2007).
The efficacy of Hx suggests that it might activate the SA pathway. Indeed, on infection of Hx‐treated plants, Hx treatment primed the induction of PR1 and PR5 and potentiated the expression of SAMT without changes in SA levels. These results may reflect control of this pathway which is downstream of SA production. Considering that the conversion of SA into MeSA is required for SAR in tobacco and that the silencing of SAMT leads to the loss of SAR (Park et al., 2007), it is possible that MeSA is involved in the SAR of tomato plants. Although the role of SA/MeSA in local defence seems to be conserved between Arabidopsis and tomato (Amet et al., 2010), it is currently unclear whether this role also extends to systemic responses, because, in Arabidopsis, MeSA production was not found to be essential for SAR; instead, the mobile metabolite azelaic acid seems to be involved (Attaran et al., 2009; Jung et al., 2009).
The role of the SA pathway in Hx‐IR was confirmed in SA‐deficient NahG plants. These results confirm that the SA pathway is required for Hx‐IR and support the hypothesis that SA levels present in treated plants on infection before its conversion to MeSA are sufficient for the early activation of PR proteins involved in defence.
Interestingly, Hx also induced the JA pathway. In treated plants, a faster and stronger accumulation of OPDA was produced 48 h after P. syringae infection. However, this accumulation was not accompanied by an increase in JA and JA‐Ile, which was observed in water‐treated plants on infection. These results suggest that Hx treatment prevents the increase in JA‐Ile associated with bacterial infection in the pathogenesis system (Pieterse et al., 2009). However, we cannot discard the possibility that OPDA or other JA derivatives are involved in the mechanism of Hx‐IR. The oxylipin OPDA, which is a regulator of plant defences and is also active against microorganisms, including B. cinerea (Prost et al., 2005; Vellosillo et al., 2007), might play a role per se in Hx‐IR or may remain an inactive conjugate in chloroplasts.
At the transcriptional level, the expression of genes in the JA pathway supports the previously described results. Interestingly, Hx induced the expression of JMT after infection, whereas the synthesis of JA‐Ile decreased. This result may indicate that MeJA is released into the environment to diminish intracellular JA levels and may prevent the synthesis of the bioactive signal JA‐Ile. Furthermore, the increase in PIN2 and JAZ1 transcripts in treated plants on infection reinforces this idea. The low levels of JA‐Ile in these plants may prevent the formation of JAZ–COI1 complexes; thus, JAZ proteins may not be ubiquitinated and degraded, and MYC2 may not be released to activate the transcription of JA‐inducible genes.
The Hx‐IR against B. cinerea (Vicedo et al., 2009) also involves the JA‐dependent defence pathway, but, in this case, it primes the accumulation of JA‐Ile.
Here, we have shown that Hx counteracts the action of COR in tomato plants. Previous studies have demonstrated that COR facilitates the entry of P. syringae into A. thaliana tissue by suppressing the closure of stomata, a basal defence response that is induced on microbial attack (Melotto et al., 2006). To confirm that Hx inhibits the response of tomato plants to COR, we analysed the stomatal aperture in treated and untreated plants after the application of COR by spraying. We confirmed that Hx inhibited the COR‐dependent stomatal aperture. In our experimental conditions, COR did not induce stomatal closure 1 h after spraying, as reported previously (Melotto et al., 2006; Tsai et al., 2011). This result may be a result of the direct application of COR on leaf mesophyll and the direct observation of the stomatal aperture on the surface of intact leaves. In these conditions, we observed that COR opens the stomata. However, Hx seems to inhibit stomatal opening in the presence of COR, which suggests that it represses the action of COR in infected tomato plants by preventing the entry of bacteria into the mesophyll. This finding may indicate that bacteria enter the mesophyll of treated plants more slowly than in untreated plants and that quorum sensing (QS) may not take place. Many species of bacteria use QS to coordinate gene expression according to the density of their local population (Miller and Bassler, 2001). Many traits, including the production of virulence factors, are regulated by QS and presumably play important and context‐dependent roles in the lifestyles of microbes that utilize QS. The attainment of a bacterial concentration needed for QS establishment may be prevented by Hx treatment and, with a faster induction of plant defences, Hx treatment may block disease development. However, a larger amount of PR1 in Hx‐treated relative to untreated plants after COR application suggests that the inducer treatment alters the negative effect of COR on the SA pathway. It is tempting to speculate that priming in these circumstances is a consequence of the inhibition of COR action on stomatal opening and on the inactivation of the SA pathway, which are both important aspects in the pathogenesis of P. syringae.
In conclusion, we have shown that Hx‐IR alters the content of several phytohormones, which correlates with changes in the expression of the genes involved in their biosynthesis and the responses they regulate. This study suggests that Hx primes the SA signalling pathway through the inhibition of COR‐mediated bacterial manipulation of this pathway. Hx treatment also increases the accumulation of OPDA in infected plants and prevents the accumulation of JA‐Ile promoted by bacterial infection. Notably, the Hx priming of the JA biosynthesis pathway does not inhibit the SA‐dependent signalling pathway or the expression of SA‐responsive genes on P. syringae infection. This unusual complementary relationship between the JA and SA pathways supports previous observations suggesting that a synergistic interaction exists between these pathways. Moreover, we have observed that changes in bacterial populations are not correlated with changes in disease development; this observation might point to a response of the bacterium to new conditions encountered in plants.
Experimental Procedures
Microbial strains, growth conditions and plant material
Pst was grown in King's B medium (KB) (King et al., 1954) at 28 °C. Rifampicin was added to KB at 50 μg/mL. The CmaA mutant (Brooks et al., 2004) was grown in KB with rifampicin (50 μg/mL) and kanamycin (25 μg/mL).
The following tomato (S. lycopersicum Mill) genotypes were used in our studies: wild‐type Ailsa Craig, Rheinlands Ruhm, Moneymaker and Castlemart. We are grateful to Jonathan Jones (John Innes Centre, Norwich, Norfolk, UK) for seeds of the SA‐deficient NahG tomato plant in the background Moneymaker, and to G. Howe (Michigan State University, East Lansing, MI, USA) for the JA pathway mutants, acx1 and jai1, in the background Castlemart. The jai1‐1 homozygous plants were selected from an F2 population, as described by Li et al. (2004).
The ABA pathway mutant used was the ABA‐deficient mutant flacca in the background Ailsa (LA3613), which was provided by the Tomato Genetics Resource Center (TGRC), University of California, Davis, CA, USA.
Seeds were germinated in either vermiculite (for hydroponic culture) or jiffy peat pellets. Plants were grown at day and night temperatures of 24 °C and 18 °C, respectively, with 16 h of light and 8 h of darkness, and 60% relative humidity (RH).
Pseudomonas syringae bioassays
Four‐week‐old tomato plants of S. lycopersicum Mill. cv. Ailsa Craig were treated with Hx using two different methods: application in the nutritive solution of hydroponic culture or in a soil drench application.
For experiments using Hx application in the nutritive solution of hydroponic culture, 3‐week‐old plants grown in vermiculite were transferred 1 week prior to treatment into hydroponic conditions in tanks containing Hoagland solution. Plants were treated with water or Hx to a nonbactericide concentration (0.6 mm) at pH 6 in the nutrient solution 48 h before inoculation (Leyva et al., 2008). The disease rate was scored at 72 hpi by determining the percentage of dark‐brown spots on the leaf surface. At least three samples for colony counting and 20 samples for disease rate scoring were taken for each treatment over a 3‐day period. For molecular and hormonal analyses, the samples were taken at 0, 24, 48 and 72 hpi. At least three samples for colony counting and 20 samples for disease rate scoring were taken for each treatment over a 3‐day period. Each experiment was independently conducted at least three times.
For Hx treatments applied by soil drench, Hx was added to 4‐week‐old plants grown in jiffy peat pellets at the same concentration as described previously, but 72 h before bacterial inoculation. The disease rate and colony counting were performed as described previously.
In all experiments, plants were then maintained at 24 °C with 16 h of light and 8 h of darkness and 100% RH.
For inoculation, Pst was grown in KB at 28 °C for 24 h. Bacterial suspensions were adjusted to 5 × 105 colony‐forming units (CFU)/mL in sterile MgSO4 (10 mm) containing 0.01% of the surfactant Silwet L‐77 (Osi Specialties, Danbury, CT, USA), as described previously (Katagiri et al., 2002). Pathogen inoculation was performed by dipping the third and fourth leaves into the bacterial suspension.
Each experiment was independently conducted at least three times. When Hx was applied in the nutritive solution of hydroponic culture or by soil drench, similar results were obtained.
Quantification of callose deposition
Samples of 10 leaves were collected for callose staining at 48 and 72 hpi, and callose deposition was determined as described by Flors et al. (2007).
Analysis of gene expression by quantitative reverse transcriptase‐polymerase chain reaction (qRT‐PCR)
RNA was extracted from tomato leaves with the Total Quick RNA Cells and Tissues Kit (Talent, Trieste, Italy) at the specified time points post‐inoculation. Leaf tissue was collected from five treated and five untreated plants. Samples for qRT‐PCR were obtained from the experiments described previously. To assess differential gene expression, the differences in the cycle numbers during the linear amplification phase between samples containing cDNA from infected and uninfected plants were used.
The expression detected from the tomato actin and tubulin genes was used as an internal reference. The primers used for actin, PR1 and LoxD have been described by Flors et al. (2007); the primers Letub and LeACCOx have been described by Vicedo et al. (2009); the primers LeAOC and LeOPR3 have been described by Uppalapati et al. (2005); LeAOS1 has been described by López‐Ráez et al. (2009). In addition, in this work, we used the following primers: LeAsr1 forward (fw) primer 5′‐ACACCACCACCACCTGT‐3′ and LeAsr1 reverse (rev) primer 5′‐GTGTTTGTGTGCATGTTGTGGA‐3′; LeJMT fw primer 5′‐GGTTCAAAGTGCATGAGAGCT‐3′ and LeJMT rev primer 5′‐TACACCACACACTGAAGGAAA‐3′; LePR5 fw primer 5′‐GAGGTTCATGCCAAACTGGTC‐3′ and LePR5 rev primer 5′‐CCGTCAACCAAAGAAATGTCC‐3′; LeSAMT fw primer 5′‐TCAATATACACCATCACAAGGAGAAG‐3′ and LeSAMT rev primer 5′‐GCTCTCATGCACTTTGACACATTG‐3′; LePIN2 fw primer 5′‐CGTTCACAAGGAAAATCGTTAAT‐3′ and LePIN2 rev primer 5′‐CTTGGGTTCATCACTCTCTCCTT‐3′; and LeJAZ1 fw primer 5′‐CGTCCGTTGAAACAAATCCT‐3′ and LeJAZ1 rev primer 5′‐GGGGTTCTGTTTGTTGGCTA‐3′. Triplicate analyses were performed on all reactions using the cDNA samples derived from three independent experiments.
Stomatal aperture analysis in planta
Tomato plants were maintained in the same culture conditions and treated as described for the P. syringae bioassays (24 °C with 16 h of light and 8 h of darkness and 100% RH). The third and fourth leaves were sprayed with water or 0.5 μm of purified COR. Leaflets were placed on glass slides with the adaxial epidermis in contact with dental resin (Delgado et al., 2011; Geisler et al., 2000). For stomatal aperture analysis, images of random regions were taken using a Leica IRB microscope equipped with a Leica DC300F camera (Leica Microsystems CMS GmbH, Wetzlar, Germany). The stomatal aperture was analysed using the Eclipse‐Net software of the Laboratory imaging program (http://www.laboratory‐imaging.com). Approximately 50 stomata from each leaflet were measured.
Chromatographic analysis
For hormonal analysis, fresh material was frozen in liquid nitrogen, ground and freeze dried. Dry tissue (0.05 g) was immediately homogenized in 2.5 mL of ultrapure water, and a mixture of internal standards was added prior to extraction (100 ng of [2H6]‐ABA, 100 ng of prostaglandin B1 (Pinfield‐Wells et al., 2005), dihydrojasmonic acid (Flors et al., 2007) and propylparaben). After extraction, a 20‐μL aliquot was injected directly into the high‐performance liquid chromatography (HPLC) system. Analyses were carried out using a Waters Alliance 2690 HPLC system (Milford, MA, USA) with a nucleosil ODS reverse‐phase column (100 mm × 2 mm i.d.; 5 μm) (Scharlab, Barcelona, Spain). The chromatographic system was interfaced to a Quatro liquid chromatography (LC) (quadrupole–hexapolequadrupole) mass spectrometer (Micromass, Manchester, UK).
Statistical analysis
Statistical analysis was carried out using a one‐way analysis of variance in the Statgraphics‐plus software of Windows V.5 (Statistical Graphics Corp., Rockville, MD, USA). The means were expressed with standard errors and compared using a Fisher's least‐significant difference test at the 95% confidence interval. All the experiments were repeated at least three times.
Supporting information
Fig. S1 Callose deposition in untreated (Ctr) and hexanoic acid‐treated (Hx) plants on infection with Pseudomonas syringae. Leaves of tomato plants were sampled, stained with calcofluor/aniline blue and analysed by epifluorescence microscopy. Quantification was performed by determining the number of yellow pixels/million corresponding to pathogen‐induced callose on digital photographs of infected leaves. Data show average valuesn ± standard error (SE) (n = 10). Different letters represent statistically significant differences (P < 0.05; least‐significant difference test). Higher magnification views are shown of callose deposition in tomato leaves infected with P. syringae visualized with bright light and UV.
Fig. S2 Expression analyses of marker genes PR5, Asr1 and AccOx for salicylic acid (SA), abscisic acid (ABA) and ethylene (ET) signalling pathways, respectively, in priming by hexanoic acid against Pseudomonas syringae. Tomato plants were grown, treated and inoculated as described in Experimental procedures. Genes were analysed in cDNA from untreated and uninoculated plants (Ctr), untreated and inoculated plants (Ctr inf), treated plants (Hx), and treated and inoculated plants (Hx inf) at different time points. Results show average values of three independent experiments with similar results ± standard error (SE) (n = 3).
Acknowledgements
This work was supported by the National R&D Plan (AGL2010‐22300‐C03‐01 and AGL2010‐22300‐C03‐02). Loredana Scalschi is the recipient of a PhD fellowship from the Ministerio de Educación (Grant AP2008‐01064). The authors are grateful to the Serveis Centrals d'Instrumentació Científica (SCIC) from Universitat Jaume I (UJI, Castellón, Spain). We thank Montaña Mena and Alberto de Marcos‐Serrano (Universidad de Castilla‐La Mancha, Spain) for their help with the stomatal study, Victor Flors (UJI) for his corrections and suggestions, and Brigitte Mauch‐Mani (University of Neuchätel, Switzerland) for the JA‐Ile standard. This article is dedicated to Purificacion Escribano and Agustí Flors. We have missed them since 2011.
References
- Amet, K. , Krasikov, V. , Allmann, S. , Rep, M. , Takken, F.L.W. and Schuurink, R.C. (2010) Methyl salicylate production in tomato affects biotic interactions. Plant J. 62, 124–134. [DOI] [PubMed] [Google Scholar]
- Attaran, E. , Zeier, T.E. , Griebel, T. and Zeier, J. (2009) Methyl salicylate production and jasmonate signalling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell, 21, 954–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanci, N.C. , Luche, A.A. , Godman, G.H. and Godman, M.H.S. (2010) Jasmonates are phytohormones with multiple functions, inducing plant defense and reproduction. Genet. Mol. Res. 9, 484–505. [DOI] [PubMed] [Google Scholar]
- Beckers, G.J.M. and Conrath, U. (2007) Priming for stress resistance: from the lab to the field. Curr. Opin. Plant Biol. 10, 425–431. [DOI] [PubMed] [Google Scholar]
- Beckers, G.J.M. and Spoel, S.H. (2006) Fine‐tuning plant defence signalling: salicylate versus jasmonate. Plant Biol. 8, 1–10. [DOI] [PubMed] [Google Scholar]
- Brooks, D.M. , Hernández‐Guzmán, G. , Kloek, A.P. , Alarcón‐Chaidez, F. , Sreedharan, A. , Rangaswamy, V. , Peñaloza‐Vázquez, A. , Bender, C.L. and Kunkel, B.N. (2004) Identification and characterization of a well‐defined series of coronatine biosynthetic mutants of Pseudomonas syringae pv. tomato DC3000. Mol. Plant–Microbe Interact. 17, 162–174. [DOI] [PubMed] [Google Scholar]
- Brooks, D.M. , Bender, C.L. and Kunkel, B.N. (2005) The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid‐dependent defences in Arabidopsis thaliana . Mol. Plant Pathol. 6, 629–640. [DOI] [PubMed] [Google Scholar]
- Cheong, J.J. and Choi, Y.D. (2003) Methyl jasmonate as a vital substance in plants. Trends Genet. 19, 409–413. [DOI] [PubMed] [Google Scholar]
- Chini, A. , Fonseca, S. , Fernández, G. , Adie, B. , Chico, J.M. , Lorenzo, O. , García‐Casado, G. , López‐Vidriero, I. , Lozano, F.M. , Ponce, M.R. , Micol, J.L. and Solano, R. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature, 448, 666–671. [DOI] [PubMed] [Google Scholar]
- Conrath, U. (2011) Molecular aspects of defence priming. Trends Plant Sci. 16, 524–531. [DOI] [PubMed] [Google Scholar]
- Conrath, U. , Pieterse, C.M. and Mauch‐Mani, B. (2002) Priming in plant–pathogen interactions. Trends Plant Sci. 7, 210–216. [DOI] [PubMed] [Google Scholar]
- Delgado, D. , Alonso‐Blanco, C. , Fenoll, C. and Mena, M. (2011) Natural variation in stomatal abundance of Arabidopsis thaliana includes cryptic diversity for different developmental processes. Ann. Bot. 107, 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizabeth, S.V. and Bender, C.L. (2007) The phytotoxin coronatine from Pseudomonas syringae pv. tomato DC3000 functions as a virulence factor and influences defense pathways in edible Brassicas. Mol. Plant Pathol. 8, 83–92. [DOI] [PubMed] [Google Scholar]
- Flors, V. , Leyva, M.O. , Vicedo, B. , Finiti, I. , Real, M.D. , García‐Agustín, P. , Bennett, A.B. and González‐Bosch, C. (2007) Absence of the endo‐α‐1,4‐glucanases Cel1 and Cel2 reduces susceptibility to Botrytis cinerea in tomato. Plant J. 52, 1027–1040. [DOI] [PubMed] [Google Scholar]
- Fonseca, S. , Chico, J.M. and Solano, R. (2009) The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr. Opin. Plant Biol. 12, 539–547. [DOI] [PubMed] [Google Scholar]
- Gehring, C.A. , Irving, H.R. , McConchie, R. and Parish, R.W. (1997) Jasmonates induce intracellular alkalization and closure of Paphiopedilum guard cell. Ann. Bot. 80, 485–489. [Google Scholar]
- Geisler, M. , Nadeau, J. and Sack, F.D. (2000) Oriented asymmetric divisions that generate the stomatal spacing pattern in Arabidopsis are disrupted by the too many mouths mutation. Plant Cell, 12, 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim, V.A. , Altmann, S. , Ellinger, D. , Eschen‐Lippold, L. , Miersch, O. , Scheel, D. and Rosahl, S. (2009) PAMP‐induced defense responses in potato require both salicylic acid and jasmonic acid. Plant J. 57, 230–242. [DOI] [PubMed] [Google Scholar]
- Jung, H.W. , Tschaplinski, T.J. , Wang, L. , Glazebrook, J. and Greenberg, J.T. (2009) Priming in systemic plant immunity. Science, 324, 89–91. [DOI] [PubMed] [Google Scholar]
- Katagiri, F. , Thilmony, R. and He, S.Y. (2002) The Arabidopsis thaliana–Pseudomonas syringae interaction In: The Arabidopsis Book (Somerville C.R. and Meyerowitz E.M., eds), available at 10.1199/tab.0039. Rockville, MD: American Society of Plant Biologists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsir, L. , Schilmiller, A.L. , Staswick, P.E. , He, S.Y. and Howe, G.A. (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. USA, 105, 7100–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazan, K. and Manners, J.M. (2008) Jasmonate signaling: toward an integrated view. Plant Physiol. 146, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, E.O. , Ward, M.K. and Raney, D.E. (1954) Two simple media for the demonstration of phycocyanin and fluorescein. J. Lab. Clin. Med. 44, 301–307. [PubMed] [Google Scholar]
- Koornneef, A. , Leon‐Reyes, A. , Ritsema, T. , Verhage, A. , DenOtter, F.C. , Van Loon, L.C. and Pieterse, C.M. (2008) Kinetics of salicylate‐mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol. 147, 1358–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchuk, Z. , Vicedo, B. , Flors, V. , Camañes, G. , González‐Bosch, C. and García‐Agustín, P. (2011) Priming for JA‐dependent defenses using hexanoic acid is an effective mechanism to protect Arabidopsis against B. cinerea . J. Plant Physiol. 168, 359–366. [DOI] [PubMed] [Google Scholar]
- Landgraf, P. , Feussner, I. , Hunger, A. , Scheel, D. and Rosahl, S. (2002) Systemic accumulation of 12‐oxo‐phytodienoic acid in SAR‐induced potato plants. Eur. J. Plant Pathol. 108, 279–283. [Google Scholar]
- Leyva, M.O. , Vicedo, B. , Finiti, I. , Flors, V. , Del Amo, G. , Real, M.D. , García‐Agustín, P. and González‐Bosch, C. (2008) Preventive and postinfection control of Botrytis cinerea in tomato plants by hexanoic acid. Plant Pathol. 57, 1038–1046. [Google Scholar]
- Li, C. , Schilmiller, A.L. , Liu, G. , Lee, G.I. , Jayanty, S. , Sageman, C. , Vrebalov, J. , Giovannoni, J.J. , Yagi, K. , Kobayashi, Y. and Howe, G.A. (2005) Role of b‐oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell, 17, 971–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Brader, G. and Palva, E.T. (2004) The WRKY70 transcription factor: a node of convergence for jasmonate‐mediated and salicylate‐mediated signals in plant defense. Plant Cell, 16, 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Ráez, J.A. , Verhage, A. , Fernandez, I. , Garcıa, J.M. , Azcon‐Aguilar, C. , Flors, V. and Pozo, M.J. (2009) Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J. Exp. Bot. 61, 2589–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto, M. , Underwood, W. , Koczan, J. , Nomura, K. and Yang, H.S. (2006) Plant stomata function in innate immunity against bacterial invasion. Cell, 126, 969–980. [DOI] [PubMed] [Google Scholar]
- Miller, M.B. and Bassler, B.L. (2001) Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199. [DOI] [PubMed] [Google Scholar]
- Mur, L.A.J. , Kenton, P. , Atzorn, R. , Miersch, O. and Wasternack, C. (2006) The outcomes of concentration‐specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 140, 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.W. , Kaimoyo, E. , Kumar, D. , Mosher, S. and Klessig, D.F. (2007) Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science, 318, 113–116. [DOI] [PubMed] [Google Scholar]
- Pastor, V. , Luna, E. , Mauch‐Mani, B. , Ton, J. and Flors, V. (2012) Primed plants do not forget. Environ. Exp. Bot. Available at doi: 10.1016/j.envexpbot.2012.02.013. [DOI] [Google Scholar]
- Pham, L.N. , Dionne, M.S. , Shirasu‐Hiza, M. and Schneider, D.S. (2007) A specific primed immune response in Drosophila is dependent on phagocytes. Plos Pathog. 3, e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse, C.M.J. , Leon‐Reyes, A. , Van der Ent, S. and Van Wees, S.C.M. (2009) Networking by small‐molecule hormones in plant immunity. Nature, 5, 308–316. [DOI] [PubMed] [Google Scholar]
- Pinfield‐Wells, H. , Rylott, E.L. , Gilday, A.D. , Graham, S. , Job, K. , Larson, T.R. and Graham, I.A. (2005) Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J. 43, 861–872. [DOI] [PubMed] [Google Scholar]
- Prime‐A‐Plant Group , Conrath, U. , Beckers, G.J.M. , Flors, V. , García‐Agustín, P. , Jakab, G. , Mauch, F. , Newman, M.A. , Pieterse, C.M.J. , Poinssot, B. , Pozo, M.J. , Pugin, A. , Schaffrath, U. , Jurriaan Ton, J. , Wendehenne, D. , Laurent Zimmerli, L. and Mauch‐Mani, B. (2006) Priming: getting ready for battle. Mol. Plant–Microbe Interact. 19, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Prost, I. , Dhondt, S. , Rothe, G. , Vicente, J. , Rodriguez, M.J. , Kift, N. , Carbonne, F. , Griffiths, G. , Esquerré‐Tugayé, M.T. , Rosahl, S. , Castresana, C. , Hamberg, M. and Joëlle Fournier, J. (2005) Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 139, 1902–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick, P.E. (2008) JAZing up jasmonate signaling. Trends Plant Sci. 13, 66–71. [DOI] [PubMed] [Google Scholar]
- Stintzi, A. and Browse, J. (2000) The Arabidopsis male‐sterile mutant, opr3, lacks the 12‐oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA, 97, 10 625–10 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi, A. , Weber, H. , Reymond, P. , Browse, J. and Farmer, E.E. (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc. Natl. Acad. Sci. USA, 98, 12 837–12 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita, D. , Kolla, V.A. , Vavasseur, A. and Raghavendra, A.S. (2003) Different signaling pathways involved during the suppression of stomatal opening by methyl jasmonate or abscisic acid. Plant Sci. 164, 481–488. [Google Scholar]
- Suhita, D. , Saghavendra, A.S. , Kwak, J.M. and Vavasseur, A. (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate‐ and abscisic acid‐induced stomatal closure. Plant Physiol. 134, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton, J. and Mauch‐Mani, B. (2004) Beta‐amino‐butyric acid‐induced resistance against necrotrophic pathogens is based on ABA‐dependent priming for callose. Plant J. 38, 119–130. [DOI] [PubMed] [Google Scholar]
- Tsai, C. , Singh, P. , Chen, C. , Thomas, J. , Weber, J. , Mauch‐Mani, B. and Zimmerli, L. (2011) Priming for enhanced defence responses by specific inhibition of the Arabidopsis response to coronatine. Plant J. 65, 469–479. [DOI] [PubMed] [Google Scholar]
- Uppalapati, S.R. , Ayoubi, P. , Weng, H. , Palmer, D.A. , Mitchell, R.E. , Jones, W. and Bender, C.L. (2005) The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 42, 201–217. [DOI] [PubMed] [Google Scholar]
- Uppalapati, S.R. , Ishiga, Y. , Wangdi, T. , Kunkel, B.N. , Anand, A. , Mysore, K.S. and Bender, C.L. (2007) The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol. Plant–Microbe Interact. 20, 955–965. [DOI] [PubMed] [Google Scholar]
- Vellosillo, T. , Martínez, M. , López, M.A. , Vicente, J. , Cascón, D.L. , Hamberg, M. and Castresana, C. (2007) Oxylipins produced by the 9‐lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signalling cascade. Plant Cell, 19, 831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicedo, B. , Flors, V. , Leyva, M.D. , Finiti, I. , Kravchuk, Z. , Real, M.D. , García‐Agustín, P. and González‐Bosch, C. (2009) Hexanoic acid‐induced resistance against Botrytis cinerea in tomato plants. Mol. Plant–Microbe Interact. 22, 1455–1465. [DOI] [PubMed] [Google Scholar]
- Wang, D.K. , Pei, K.M. , Fu, Y.P. , Sun, Z.X. , Li, S.J. , Liu, H.Q. , Tang, K. , Han, B. and Tao, Y.Z. (2007) Genome‐wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene, 394, 13–24. [DOI] [PubMed] [Google Scholar]
- Wang, S.Y. (1999) Methyl jasmonate reduces water stress in strawberry. J. Plant Growth Regul. 18, 127–134. [DOI] [PubMed] [Google Scholar]
- Xui, M. , Dong, J. , Wang, H. and Huang, L. (2009) Complementary action of jasmonic acid on salicylic acid in mediating fungal elicitor‐induced flavonol glycoside accumulation of Ginkgo biloba cells. Plant Cell Environ. 32, 960–967. [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Thilmony, R. , Bender, C.L. , Schaller, A. , He, S.Y. and Howe, G.A. (2003) Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant, 36, 485–499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Callose deposition in untreated (Ctr) and hexanoic acid‐treated (Hx) plants on infection with Pseudomonas syringae. Leaves of tomato plants were sampled, stained with calcofluor/aniline blue and analysed by epifluorescence microscopy. Quantification was performed by determining the number of yellow pixels/million corresponding to pathogen‐induced callose on digital photographs of infected leaves. Data show average valuesn ± standard error (SE) (n = 10). Different letters represent statistically significant differences (P < 0.05; least‐significant difference test). Higher magnification views are shown of callose deposition in tomato leaves infected with P. syringae visualized with bright light and UV.
Fig. S2 Expression analyses of marker genes PR5, Asr1 and AccOx for salicylic acid (SA), abscisic acid (ABA) and ethylene (ET) signalling pathways, respectively, in priming by hexanoic acid against Pseudomonas syringae. Tomato plants were grown, treated and inoculated as described in Experimental procedures. Genes were analysed in cDNA from untreated and uninoculated plants (Ctr), untreated and inoculated plants (Ctr inf), treated plants (Hx), and treated and inoculated plants (Hx inf) at different time points. Results show average values of three independent experiments with similar results ± standard error (SE) (n = 3).


