SUMMARY
The effect of abiotic stress responses on Potato virus A (PVA; genus Potyvirus) infection was studied. Salt, osmotic and wounding stress all increased PVA gene expression in infected Nicotiana benthamiana leaves. According to the literature, an early response to these stresses is an elevation in cytosolic Ca2+ concentration. The infiltration of 0.1 m CaCl2 into the infected leaf area enhanced the translation of PVA RNA, and this Ca2+‐induced effect was more profound than that induced solely by osmotic stress. The inhibition of voltage‐gated Ca2+ channels within the plasma membrane abolished the Ca2+ effect, suggesting that Ca2+ had to be transported into the cytosol to affect viral gene expression. This was also supported by a reduced wounding effect in the presence of the Ca2+‐chelating agent ethylene glycol tetraacetic acid (EGTA). In the absence of viral replication, the intense synthesis of viral proteins in response to Ca2+ was transient. However, a Ca2+ pulse administered at the onset of wild‐type PVA infection enhanced the progress of infection within the locally infected leaf, and the virus appeared earlier in the systemic leaves than in the control plants. This suggests that the cellular environment was thoroughly modified by the Ca2+ pulse to support viral infection. One message of this study is that the sensing of abiotic stress, which leads to cellular responses, probably via Ca2+ signalling, associated with enhanced virus infection, may lead to higher field crop losses. Therefore, the effect of abiotic stress on plant viral infection warrants further analysis.
INTRODUCTION
Cultivated plants face a variety of environmental stress factors, including abiotic stress factors, such as drought and salt, as well as elevated temperatures and [CO2], and biotic stress factors, such as plant pathogens and wounding. The plant's response to various stress conditions proceeds via plant hormones, signalling molecules and small RNA pathways, and results in complex changes in the expression pattern of several hundred genes (reviewed in Ahuja et al., 2010). Often, partially overlapping sets of genes are activated in different stress responses and several points of crosstalk exist between abiotic and biotic stress responses (reviewed in Fujita et al., 2006). Potyviruses infect monocotyledonous and dicotyledonous plant species in most climatic conditions worldwide, and essentially all types of important crop species are infected by at least one species of potyvirus. The infected plants can be symptomless but, usually, growth reduction and developmental defects occur. Potyvirus infection is associated with many changes in the host transcriptome. For instance, among the genes that respond to Tobacco etch virus (TEV; genus Potyvirus) infection are those that participate in systemic acquired resistance and hypersensitive response pathways, as well as those also induced by drought, salinity, temperature and wounding (Agudelo‐Romero et al., 2008).
Soil salinity is a limiting factor for plant growth. A high concentration of Na+ causes both ionic and osmotic stress for cells (Tester and Davenport, 2003). Osmotic, salt and wounding stress all elevate the cytosolic Ca2+ concentration (Boudsocq et al., 2010; Braam, 2005; Kader and Lindberg, 2010; Kiegle et al., 2000; Knight et al., 1993; Song et al., 2008). Plants release Ca2+ from both intracellular and extracellular stores in response to NaCl and osmotic stress (Cessna et al., 2007; Knight et al., 1997; Leon et al., 1998; Sun et al., 2006). After wounding, Ca2+ can move into a localized region of a cell from the apoplasmic space or the cell wall, which contains approximately 10 000 times more Ca2+ than the cytosol (Reddy and Reddy, 2004). Elevation of the cytosolic Ca2+ concentration activates various signal cascades (Boudsocq et al., 2010; Kader and Lindberg, 2010). For example, cytosolic Ca2+ elevations function as alerting signals in plant innate immunity (Lecourieux et al., 2006). These tightly controlled Ca2+ signatures are responsible for the specificity of various cellular responses to environmentally induced stresses.
Viruses from different viral families perturb cellular Ca2+ homeostasis in various ways as they modify the cellular environment to meet their demands. A role for Ca2+ has been demonstrated in viral entry and replication, as well as in virion formation, maturation and stability (reviewed in Zhou et al., 2009). Viral proteins modulate the Ca2+ flux through pumps and/or channels on the plasma membrane, and also trigger Ca2+ release from internal stores (reviewed in Zhou et al., 2009). A variety of viral proteins interact with cellular Ca2+ sensors and activate Ca2+‐responsive transcription. Among the genes that exhibit altered transcript abundance in response to Potato virus Y (PVY; genus Potyvirus) infection in potato are genes involved in the processes of photosynthesis, perception, signalling and defence responses (Baebler et al., 2009). Genes involved in Ca2+ signalling are up‐regulated at the onset of infection in susceptible potato, indicating that Ca2+ may play an essential role in the establishment of potyvirus infection. When transcriptomic changes caused by Pepper yellow mosaic virus (PepYMV; genus Potyvirus) infection were analysed, 4.9% of the up‐regulated genes were found to be involved in Ca2+‐mediated signal transduction (Alfenas‐Zerbini et al., 2009).
Under field conditions, crop plants are exposed to both viruses and various types of abiotic stress. In order to limit yield losses, it is necessary that we develop an understanding of the molecular programmes involved in these combined effects. An infection assay allowing the quantification of Potato virus A (PVA; genus Potyvirus) gene expression with the reporter enzyme Renilla luciferase (RLUC) has been developed recently (Eskelin et al., 2010). In this study, viral gene expression was found to benefit from both salt/osmotic and wounding stress, as well as from exogenous application of Ca2+ at the onset of virus infection. After the transient translational advantage initiated by all stress conditions was over, the effect initiated by either wounding or CaCl2 continued and led to an accelerated infection process. This study reveals that the sensing of abiotic stress and the subsequent cellular responses increase virus load in the host plant. A combination of abiotic stress and virus infection may therefore lead to higher field crop losses, which is a matter that requires further study.
RESULTS
Influence of salt/osmotic stress on viral gene expression
The influence of salt and osmotic stress on viral gene expression was studied. The viral constructs used in the Agrobacterium‐mediated infection experiments are presented schematically in Fig. 1. When rice was grown in a 50 mm NaCl solution, the concentration of Na+ was found to be 0.6 m in the leaf apoplast (Flowers et al., 1991). Serious bleaching and wilting, as well as enhanced cell death, were observed in Nicotiana benthamiana leaves when they were treated with 0.2 m NaCl for 3 days (Chen et al., 2011). We decided to challenge the leaves with high, moderate and low salt concentrations. The leaves were co‐infiltrated with Agrobacterium carrying the PVAWT and firefly luciferase (FLUC) constructs. Stress conditions were induced 1 day post‐Agrobacterium infiltration (DPI) by infiltrating the abaxial surfaces of N. benthamiana leaves, between leaf cells, with solutions containing 0.7, 0.1 or 0.005 m NaCl. The timing of NaCl application was chosen to coincide with the onset of viral gene expression, as T‐DNA transfer and production of the transcript have been shown to require approximately 1 day (Narasimhulu et al., 1996). Under the severe salt stress induced by 0.7 m NaCl, some of the leaves became dehydrated and died. These leaves were not sampled. The effect of salt/osmotic stress on PVA‐derived RLUC and FLUC gene expression was quantified. In our experiments, FLUC serves as an internal control to report for Agrobacterium‐mediated and virus‐independent gene expression (Eskelin et al., 2010).
Figure 1.
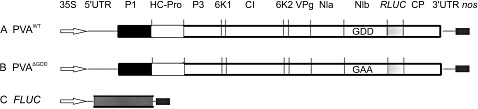
Schematic diagrams of the different Potato virus A (PVA) constructs used in this work. All constructs are under the control of the Cauliflower mosaic virus 35S promoter. (A) PVAWT is an infectious cDNA copy of wild‐type PVA RNA in which the Renilla luciferase (RLUC) gene is inserted between the NIb and coat protein (CP) cistrons. (B) PVAΔGDD is a cDNA copy of replication‐deficient PVA RNA. In the replication‐deficient mutant, the catalytically active GDD motif of the RNA polymerase NIb is substituted with GAA. (C) The expression construct for monocistronic firefly luciferase (FLUC).
Treatment with 0.7 m NaCl reduced FLUC activity to 30% when compared with that in untreated leaves at 2 DPI, whereas 0.1 and 0.005 m NaCl had no significant effect (Fig. 2A, B). FLUC expression in leaves subjected to severe stress did not recover to the control level, even at 3 DPI. Salt stress (both 0.7 and 0.1 m NaCl) enhanced viral gene expression (Fig. 2C, D). The relative levels of RLUC and FLUC activity in comparison with the nontreated control at 2 and 3 DPI are given in Fig. 2E, F. The enhancement caused by 0.1 m salt varied between 1.7‐ and 3.7‐fold, with an average of 2.7‐fold (n= 3 experiments).
Figure 2.
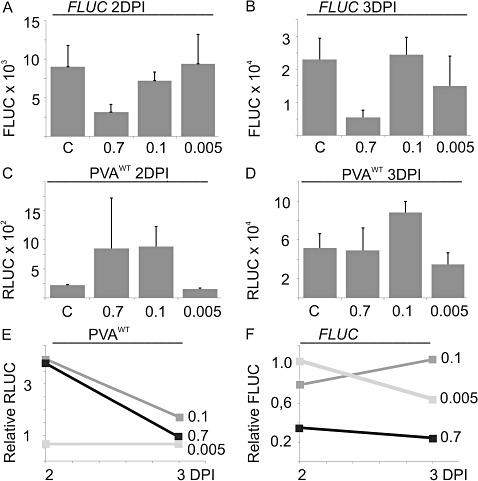
The effect of salt/osmotic stress on viral gene expression. Nicotiana benthamiana plants were co‐infiltrated with Agrobacterium carrying PVAWT and firefly luciferase (FLUC). Salt and osmotic stress was induced by a second infiltration of 0.7, 0.1 or 0.005 m NaCl at 1 day post‐infiltration (DPI). The control (C) did not receive a second infiltration. Samples were collected at 2 and 3 DPI. FLUC (A, B) and normalized PVAWT‐derived Renilla luciferase (RLUC) (C, D) activities. Normalization of the RLUC values is described in the Experimental procedures section. Each column represents the average of 12 parallel plant samples. The error bars report the standard deviation of the mean. (E, F) Fold change of the RLUC and FLUC levels above the nontreated control, which was assigned a relative value of unity.
Influence of wounding on PVA gene expression
The effect of wounding at the onset of PVA infection was studied next. Wounding is tightly connected with the initiation of a plant virus infection, as both mechanical inoculation and transmission by insects cause damage to plant cells. Wounding stress was provided by rubbing carborundum powder on the leaf area that had been infiltrated 1 day earlier with Agrobacterium harbouring PVAWT and FLUC constructs. Carborundum is generally used to boost mechanical inoculation. FLUC and viral RLUC activities were measured at 2 and 3 DPI, and the averages and standard deviations of the activities were calculated. The results from one representative experiment are given in Fig. 3A–D. The relative FLUC activities were slightly higher than those in the control (nontreated sample) (Fig. 3E) but, in five parallel experiments, the effect of wounding on the FLUC activity level varied between 0.3‐ and 2.1‐fold, the average being 0.8, indicating that wounding had no significant effect on FLUC gene expression. In Fig 3F, the viral gene expression was boosted by 4.7‐ and 18.5‐fold relative to the control at 2 and 3 DPI, and, in eight parallel experiments, the effect of wounding on PVAWT gene expression varied between 6.1‐ and 18.5‐fold, the average being 10.3, at 3 DPI. The timing of wounding was found to be important to achieve maximal enhancement in viral gene expression. When wounding was performed at 1.5 DPI, the level of enhancement was lower than when it was performed at 1 DPI (data not shown).
Figure 3.
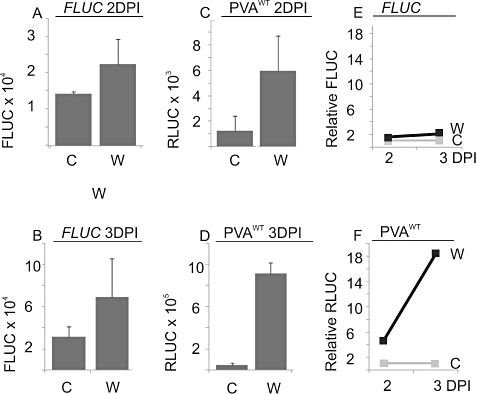
The effect of mechanical wounding on viral gene expression. Nicotiana benthamiana plants were co‐infiltrated with Agrobacterium carrying PVAWT and firefly luciferase (FLUC). Agrobacterium‐infiltrated leaves were either dusted with carborundum beneath the leaves and wounded by rubbing at 1 day post‐infiltration (DPI) (15 plants) (A) or left without this treatment (15 plants) (B). Samples were collected at 2 and 3 DPI, and Renilla luciferase (RLUC) and FLUC activities were measured. The columns represent the average FLUC activity (A, B) and the normalized PVAWT‐derived RLUC activity (C, D). The error bars represent the standard deviation of the mean. The relative level of wounding‐induced enhancement in FLUC (E) and RLUC (F) gene expression is also shown. The untreated control was assigned the relative value of unity.
Among the earliest cellular responses of plant cells to stress stimuli, such as salt, wounding and pathogen infection, is a change in the cytosolic calcium concentration (Boudsocq et al., 2010; Braam, 2005; Kader and Lindberg, 2010; Kiegle et al., 2000; Knight et al., 1993; Song et al., 2008). This could be a factor linked to the enhanced viral gene expression in response to NaCl and wounding treatments. To test the role of Ca2+ in the enhancement of viral gene expression, ethylene glycol tetraacetic acid (EGTA) was infiltrated into the leaves prior to wounding. EGTA is a Ca2+‐selective chelating agent. The hypothesis was that this would reduce the influx of Ca2+ to the cytosol of wounded cells and the resulting elevation in the intracellular Ca2+ concentration. EGTA (10 mm) was infiltrated into the infected leaf area 1 h before wounding. In a representative experiment, wounding enhanced viral gene expression on a relative scale 12.3‐fold over the nonwounded control, whereas the corresponding effect in EGTA‐treated leaves was 4.7‐fold when measured at 3 DPI (Fig. 4). The relative fold of enhancement varied between 3.2 and 4.7 in the presence of EGTA, the average being 4.0 (n= 3 experiments), whereas, in the absence of EGTA, the effect of wounding was, on average, 10.3‐fold (n= 8). Thus, this result suggests that the influx of Ca2+ may be involved in the regulation of viral gene expression after the integrity of the plasma membrane has been violated.
Figure 4.
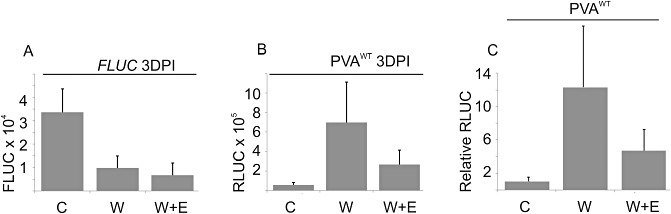
The effect of mechanical wounding on viral gene expression in the presence of the Ca2+ chelator ethylene glycol tetraacetic acid (EGTA). Nicotiana benthamiana plants were co‐infiltrated with Agrobacterium carrying PVAWT and firefly luciferase (FLUC). The Agrobacterium‐infiltrated leaves were left without treatment (C; four plants), dusted with carborundum beneath the leaves and wounded by rubbing at 1 day post‐infiltration (DPI) (W; 4 plants) or pre‐infiltrated with 10 mm EGTA 1 h prior to wounding (W + E; four plants). Samples were collected and Renilla luciferase (RLUC) and FLUC activities were determined at 3 DPI. The average FLUC (A) and average normalized PVAWT‐derived RLUC (B) activities are given. The error bar represents the standard deviation of the mean. (C) The relative levels of wounding‐induced enhancement in RLUC gene expression in the presence (W + E) and absence (W) of EGTA. The average value from the nontreated control plants was assigned the relative value of unity.
Influence of exogenous application of CaCl2 on PVA gene expression and infection
It has been shown that the exogenous application of CaCl2 causes transient changes in the cytosolic Ca2+ concentration (Cessna et al., 1998). The effect of infiltrated Ca2+ on viral gene expression was studied next. CaCl2 solutions (0.005–0.1 m) were infiltrated into leaf areas that had been infiltrated 1 day earlier with Agrobacterium harbouring PVAWT and FLUC. The administration of 0.1 m CaCl2 led to a reduction in FLUC activity (Fig. 5A). The average relative FLUC activity in Ca2+‐treated samples at 3 DPI was 0.35 ± 0.13‐fold that of the nontreated samples (n= 5 experiments). Viral RLUC activity was not affected significantly by either the hypotonic shock caused by the infiltration of plain distilled H2O (dH2O) or by the treatment with 0.005 m CaCl2 when measured at 3 DPI. Treatment with 0.1 m CaCl2 clearly enhanced viral gene expression (Fig. 5B). After infiltration with 0.1 m CaCl2, the relative enhancement in PVAWT‐derived RLUC varied between 6.6‐ and 25.1‐fold, with an average of 14.2‐fold, over the nontreated control (n= 8 experiments), and between 3.5‐ and 13.2‐fold, with an average of 8.5‐fold (n= 7 experiments), over the samples treated with 0.005 m CaCl2.
Figure 5.
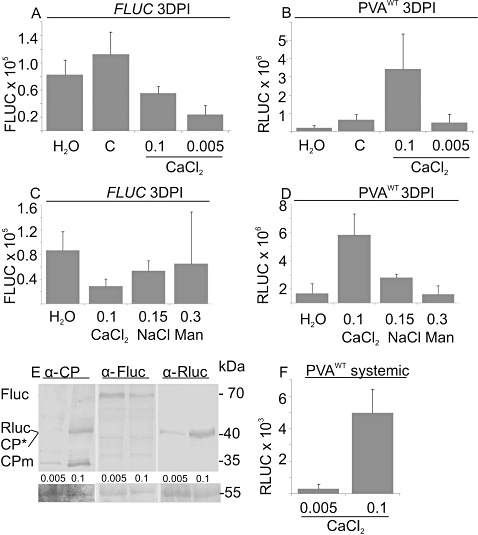
The effect of CaCl2 infiltration on viral gene expression. Nicotiana benthamiana plants were co‐infiltrated with Agrobacterium carrying PVAWT and firefly luciferase (FLUC). A second infiltration of H2O, 0.1 m CaCl2, 0.005 m CaCl2, 0.15 m NaCl or 0.3 m mannitol (Man) was performed at 1 day post‐infiltration (DPI). No second infiltration was performed for the control (C) plants. Samples were collected at 3 DPI, and FLUC and Renilla luciferase (RLUC) activities were determined. Average FLUC activities (A, C) and normalized average PVAWT‐derived RLUC activities (B, D). Each column represents an average measured from four plants. The error bars represent the standard deviation of the mean. (E) Total protein samples prepared from PVAWT‐infected and 0.005 or 0.1 m CaCl2‐treated leaves were separated on a 12% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS PAGE) gel and blotted onto a poly(vinylidene difluoride) (PVDF) membrane. Immunoblots were probed with anti‐Potato virus A coat protein (PVA CP), anti‐FLUC and anti‐RLUC immunoglobulin Gs (IgGs). The molecular weight standard is on the right and the proteins detected are indicated on the left. The Ponceau‐S‐stained immunoblot membrane serves as a loading control. (F) The third leaf up from the point of infiltration was sampled at 4 DPI and analysed for RLUC activity. Each column represents the average from five sample pools. The error bars represent the standard deviation of the mean.
Next, we controlled for the osmotic effects of CaCl2 to determine whether Ca2+ had a specific response and not only that caused by the osmotic effect. It has been shown that iso‐osmotic solutions of salt and sorbitol induce equal internal Ca2+ elevations that are similar in amplitude and duration in tobacco suspension cells (Cessna et al., 2007). Iso‐osmolar solutions (0.1 m CaCl2, 0.15 m NaCl and 0.3 m mannitol) were infiltrated into leaf areas that had been infiltrated 1 day earlier with Agrobacterium harbouring PVAWT and FLUC constructs. CaCl2 treatment reduced FLUC activity (Fig. 5C), as did treatment with NaCl, when compared with the untreated control. Mannitol (0.3 m) caused severe stress in infiltrated leaves. A portion of the samples wilted and died as a result of its application, and these leaves were not sampled. In general, mannitol caused longer lasting osmotic stress than did the iso‐osmolar salts, leaving the leaves looking wet and loose even at 3 DPI. Thus, the application of mannitol induced much more variable responses, which can be seen, for example, by the increased standard deviation of the mean of the FLUC values. In a representative experiment, viral RLUC activities were up‐regulated by CaCl2 treatment, reaching a value seven‐fold greater that that of the untreated control and 2.7‐fold greater than that obtained with iso‐osmolar NaCl treatment at 3 DPI (Fig. 5D). In three parallel experiments, the influence of iso‐osmotic NaCl and mannitol on the relative viral gene expression varied between 1.6‐ and 2.9‐fold (average, 2.4) and 0.9‐ and 3.1‐fold (average, 2.2), respectively, in comparison with the untreated control. We concluded that both salt and osmotic stress enhanced viral gene expression to some extent, but neither of these stresses caused as efficient a response as exogenously applied CaCl2.
To verify the enhanced RLUC and viral protein production in response to CaCl2, an immunoblot analysis was performed (Fig. 5E). In the absence of CaCl2 treatment, the mature coat protein (CPm, 34 kDa) was only faintly detectable with the anti‐CP immunoglobulin Gs (IgGs). As found in an earlier study (Hafren et al., 2010), an additional form of CP (CP*, 39 kDa) was also detected. The modification responsible for the mobility shift is not yet known. The immunoblot analysis revealed an up‐regulation in CP production and a reduction in the amount of FLUC under the 0.1 m CaCl2 treatment. The amount of RLUC increased in response to the 0.1 m CaCl2 treatment, which is in agreement with the increased RLUC enzyme activities detected within the corresponding samples.
We next investigated whether systemic PVAWT infection was enhanced by 0.1 m CaCl2. Leaves treated 1 day earlier with Agrobacterium carrying the PVAWT construct were infiltrated with CaCl2. The 0.005 m CaCl2 treatment, which mimics the average Ca2+ concentration in the cell wall (1–10 mm; Reddy and Reddy, 2004), was included as a control. The first indications of systemic infection appeared, as one of the five 0.1 m CaCl2‐treated samples (each sample being a pool of six leaf discs) had a low but detectable RLUC activity (1 × 102 photons/µL s) in the third leaf up from the site of local infection at 3 DPI. At 4 DPI, sample pools from 0.1 m CaCl2‐treated plants showed RLUC activity that was 16‐fold greater than the average RLUC activity of the samples treated with 0.005 m CaCl2 in the systemic leaves (Fig. 5F). For a comparison, at the same time, the activity in the samples treated with 0.005 m CaCl2 was 11‐fold less than in the samples treated with 0.1 m CaCl2 in the infiltrated leaves (data not shown). In conclusion, enhanced local activity caused by 0.1 m Ca2+ treatment, followed by the rapid development of systemic infection in these plants, was observed.
Timing of CaCl2 application affects the level of enhancement of viral gene expression
When the effect of wounding on PVA gene expression was studied, it was found that the timing of carborundum treatment was an important factor in determining the level of enhancement (data not shown). In the next experiment, PVAWT infection and FLUC expression were initiated with Agrobacterium co‐infiltration as performed previously, but 0.1 m CaCl2 was applied at different time points in relation to the Agrobacterium infiltration. Samples for RLUC and FLUC activity measurements were collected at 3 DPI. When CaCl2 was included in the Agrobacterium infiltration buffer, PVA gene expression was lower than in the untreated control sample (see Fig. 6A, column 0 DPI). The addition of CaCl2 had the strongest effect on viral gene expression when applied 1–1.5 DPI after Agrobacterium infiltration (Fig. 6A; columns 1 and 1.5 DPI). When calcium was applied at 2 DPI, no enhancement in RLUC expression was detected at 3 DPI (Fig. 6A, column 2 DPI). A second addition of 0.1 m CaCl2 at 2 DPI, after the first application at 1 DPI, led to a lower level of RLUC accumulation relative to the samples that had undergone only one CaCl2 treatment at 1 DPI (Fig. 6A, column 1 + 2 DPI).
Figure 6.
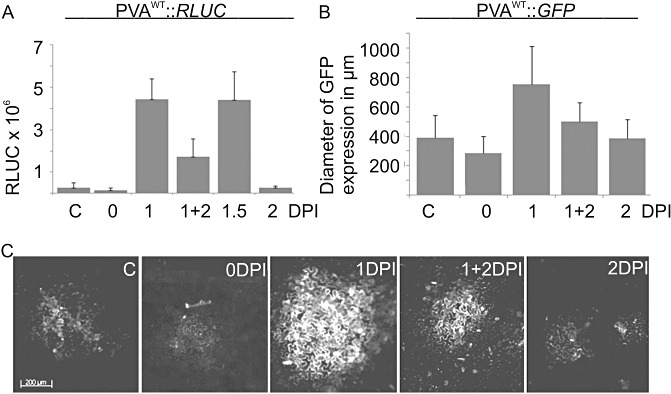
The effect of time of CaCl2 application on viral gene expression. (A) PVAWT infection and firefly luciferase (FLUC) expression were initiated with Agrobacterium co‐infiltration. In the control (C), there was no subsequent infiltration. In the other samples, CaCl2 (0.1 m) was applied by infiltration either simultaneously with Agrobacterium[0 days post‐infiltration (DPI)] or at 1, 1 + 2, 1.5 and 2 DPI. (A) Each column represents the normalized average PVAWT Renilla luciferase (RLUC) activity at 3 DPI and the error bars represent the standard deviation of the mean. (B) Statistical analysis of the size of the PVAWT infection foci. The diameters of the infection foci were measured with the aid of virus‐derived green fluorescent protein (GFP) fluorescence from samples treated as in (A). The diameter of GFP expression was measured from 259 (C), 116 (0 DPI), 188 (1 DPI), 174 (1 + 2 DPI) and 209 (2 DPI) infection foci. The error bar represents the standard deviation of the mean. (C) Representative infection foci from each sample type visualized by fluorescence microscopy.
To better understand the role of timing in this Ca2+‐induced phenomenon, the experiment was repeated in the presence of PVAWT tagged with green fluorescent protein (GFP). The addition of Ca2+ simultaneously with Agrobacterium had an adverse effect on viral gene expression (Fig. 6A; 0 DPI). Only faintly visible and small GFP fluorescence foci (Fig. 6B, C; foci = 286 ± 113 µm; n= 116) were detected at 3 DPI in the leaves that had been simultaneously infiltrated with Agrobacterium carrying PVAWT::GFP and 0.1 m CaCl2, suggesting that the transfer of T‐DNA was hampered by the presence of extra Ca2+. The diameters of the fluorescent infection foci were 390 ± 152 µm and 385 ± 132 µm at 3 DPI in plants with no subsequent treatment after Agrobacterium infiltration (n= 259) and in those treated with 0.1 m CaCl2 at 2 DPI (n= 209), respectively. Interestingly, a clear increase in the size of the infection foci (755 ± 259 µm) was observed when the plants were treated with 0.1 m CaCl2 at 1 DPI (n= 188) (Fig. 6B, C). A double calcium treatment, with calcium being applied at 1 and 2 DPI, did not allow viral infection to proceed as efficiently as with a single Ca2+ treatment. The infection foci in the case of the double calcium treatment were 500 ± 127 µm in diameter (n= 174).
Inhibition of Ca2+ pumps at the plasma membrane by LaCl3 prevents the Ca2+ effect on viral gene expression
Next, we tested whether externally applied CaCl2 is transported into the cytosol to affect the expression of PVAWT. Several studies have shown that LaCl3 is a specific inhibitor of Ca2+ channels (Gould et al., 1982; Kawano et al., 2004; Knight et al., 1997). La3+ can be used to block Ca2+ channels located at the plasma membrane, and thereby prevent the first spike in Ca2+ oscillation in the cytosol (Lachaud et al., 2010). Eighteen hours post‐Agrobacterium infiltration of PVAWT and FLUC, the same leaf area was infiltrated with a 1 mm LaCl3 solution or H2O. Six hours thereafter, half of the treated leaves were infiltrated for the third time, with either H2O or 0.1 m CaCl2 solution. Four types of differently treated samples were analysed: H2O + H2O, H2O + CaCl2, LaCl3+ H2O and LaCl3+ CaCl2. La3+ (1 mm) did not affect the translation initiation of capped FLUC mRNA, as FLUC activity was similar in La3+‐ and H2O‐treated samples (Fig. 7A). Viral RLUC expression increased two‐fold in response to 1 mm La3+ treatment in the absence of Ca2+, whereas, in the presence of Ca2+, La3+ treatment blocked CaCl2‐induced enhancement of viral gene expression (Fig. 7B). Thus, these results suggest that Ca2+ applied exogenously needs to be transported into the cytosol through voltage‐gated Ca2+ channels of the plasma membrane to affect viral gene expression.
Figure 7.
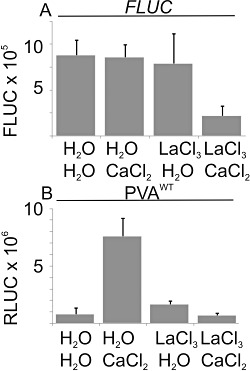
Inhibition of Ca2+ pumps located at the plasma membrane by LaCl3 prevents the Ca2+‐induced effect on viral gene expression. Nicotiana benthamiana plants were co‐infiltrated with Agrobacterium carrying PVAWT and firefly luciferase (FLUC). Eighteen hours post‐Agrobacterium infiltration, the plants were infiltrated with either distilled H2O (dH2O) or 1 mm LaCl3, followed by a third infiltration with either dH2O or 0.1 m CaCl2 within 1 h. Four types of PVAWT‐infected samples were collected: dH2O + dH2O, dH2O + CaCl2, LaCl3+ dH2O and LaCl3+ CaCl2 at 3 days post‐infiltration (DPI). The FLUC and Renilla luciferase (RLUC) activities were determined. Each column represents the average FLUC (A) or the normalized average PVAWT‐derived RLUC (B) from four parallel samples, and the error bars represent the standard deviation of the mean.
Effect of CaCl2 on gene expression of PVAWT and a replication‐deficient PVAΔGDD
Next, the effects of Ca2+ on viral gene expression of replicating (PVAWT) and replication‐deficient (PVAΔGDD) RNA were compared. The infection assay was carried out by infiltrating either PVAWT or PVAΔGDD combined with FLUC construct. CaCl2 was infiltrated at 1 DPI and samples were collected at 2, 3 and 4 DPI. As earlier, FLUC values were generally reduced in re‐infiltrated samples relative to the untreated control (Fig. 8A–C, G–H). In a representative PVA infection assay performed with the replication‐deficient PVAΔGDD mutant, where expression is derived from the translation of capped viral RNA, a seven‐fold increase in expression was detected at 2 DPI with 0.1 m CaCl2 (Fig. 8I). In five individual experiments, the relative Ca2+‐derived enhancement in PVAΔGDD RLUC activity varied in the range 5–15‐fold, the average being eight‐fold at 2 DPI. Wounding enhanced gene expression from the PVAΔGDD virus six‐fold, and no enhancement was detected at 3 days post‐wounding (data not shown). Similar to the wounding experiment, in this Ca2+ experiment, the RLUC values from the replication‐deficient mutant were transiently elevated, and the effect had disappeared by 3 DPI (Fig. 8J, L). After infiltration with 0.1 m CaCl2, PVAWT expression was 15‐ and 23‐fold higher than that of the untreated control at 2 and 3 DPI, respectively (Fig. 8K), but, by 4 DPI, the relative activity difference between these two samples had declined to 11‐fold. The actual RLUC activities in PVAWT‐infected samples in one representative experiment are shown in Fig. 8D–F. Thus, when the translational enhancement of CaCl2 in PVAΔGDD expression was over, the boost in PVAWT expression still continued.
Figure 8.
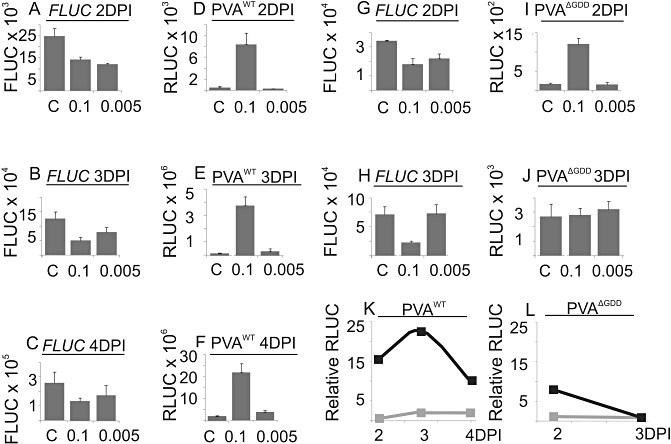
The effect of CaCl2 on the expression of PVAWT and replication‐deficient PVAΔGDD. Nicotiana benthamiana plants were co‐infiltrated with Agrobacterium carrying either PVAWT or PVAΔGDD and firefly luciferase (FLUC). A second infiltration of either 0.1 or 0.005 m CaCl2 was performed at 1 day post‐infiltration (DPI). The control (C) was not infiltrated for the second time. Samples were collected at 2, 3 and 4 DPI, and the Renilla luciferase (RLUC) and FLUC activities were determined. FLUC activity when co‐infiltrated with PVAWT (A–C) and with PVAΔGDD (G,H). Normalized average PVAWT‐derived (12 plants) (D–F) and PVAΔGDD‐derived (12 plants) (I,J) RLUC activities. The error bars represent the standard deviation of the mean. (K,L) Relative level of enhancement of PVA WT‐ and PVAΔGDD‐derived RLUC activities, respectively, induced by 0.1 or 0.005 m CaCl2. The values from the nontreated control plants were assigned the relative value of unity.
DISCUSSION
Translational control of gene expression is one of the major means by which plants adapt to a changing environment. This study sought to determine whether cellular stress conditions affect the gene expression of a potyvirus. Viral gene expression was quantified by monitoring the RLUC activity derived from the PVA genome (Eskelin et al., 2010). Virus‐derived RLUC production was enhanced under all stress conditions used in this study, suggesting that there is a general benefit for viral translation under stress. The strong translational effect in response to 0.1 m NaCl treatment ceased within 2 days. After that, the transient enhancement in viral gene expression in the infiltrated leaves boosted the subsequent infection only slightly. Wounding stress promoted viral gene expression in the infiltrated leaves for a longer period than did salt stress. Reduction of the pool of free Ca2+ ions by EGTA in the extracellular milieu reduced the response of viral gene expression to wounding, suggesting that Ca2+ has a role in promoting viral gene expression.
After the infiltration of hyperosmotic solutions into the apoplasmic space, water diffusion from cells to the apoplast is most probably sensed by membrane‐ or cell wall‐embedded turgor receptor kinases that may initiate osmotic signalling cascades (Seifert and Blaukopf, 2010; Urao et al., 1999) and lead to the activation of ion channels located on the plasma membrane. The externally derived influx of Ca2+ into the cytosol also plays an essential role in signalling to salt stress (Cessna et al., 2007). Ca2+ applied to the apoplasmic space led to enhanced PVA gene expression in N. benthamiana leaves. CaCl2‐induced strong enhancement was transient for nonreplicating PVAΔGDD, whereas the expression of replicating PVAWT continued to rise, although the initial translational advantage was over. Over time, the difference slowly started to level off in the infiltrated leaf, probably because the local viral accumulation became saturated, whereas virus accumulation in the control leaves still continued. Although the osmotic and salt effects contribute to the enhanced viral gene expression level when exogenous Ca2+ is applied, Ca2+ treatment leads to a more profound response than seen with iso‐osmolar salt and mannitol treatments. This indicates that there is, in addition, a specific Ca2+‐derived effect. The Ca2+ effect was blocked in the presence of a voltage‐gated channel blocker, La3+, which suggests a role for the influx of Ca2+ from the extracellular space to the cytosol and cytosolic Ca2+ relays in the enrichment of viral gene expression. This assumption is supported by the less pronounced effect to wounding in the presence of a Ca2+ chelator EGTA than in its absence. Both cell‐to‐cell and systemic spread of PVA infection occurred more rapidly in Ca2+‐treated plants than in control plants. We have shown that PVA spread begins later than 2 days post‐Agrobacterium infiltration (Eskelin et al., 2010). As Ca2+ treatment had an adverse effect on the progress of viral infection when administered at the time at which viral spread was expected to start, it seems that the viral transport mechanism per se may not benefit from Ca2+ treatment. It is probable that Ca2+‐derived alterations in the cellular environment accelerate and strengthen the progress of infection within individual cells, which consequently speeds up the spread of the infection.
The initiation of viral infection via Agrobacterium does not reflect a natural infection process. When an Agrobacterium‐mediated infection assay is used, viral RNA enters the cytoplasm as a capped transcript, which has undergone splicing. When PVA enters the cell during the natural infection process, the viral RNA released from particles contains viral protein genome‐linked (VPg) at its 5′ end (Oruetxebarria et al., 2001) and the infection is initiated from a noncapped transcript in the cytoplasm. However, after replication has taken place, the infection initiated by Agrobacterium proceeds similarly to the natural infection. The method used for the quantification of gene expression made it possible to measure simultaneously the expression of monocistronic FLUC mRNA. Although viral gene expression was boosted, stress conditions reduced FLUC translation. The difference in the translational response between viral and reporter gene expression under the stress conditions indicated that these two types of RNA were expressed by different mechanisms. In spite of the presence of a cap on the viral RNA transcripts derived from the nucleus, it is probable that the monocistronic reporter transcript utilized a cap‐dependent translational pathway, whereas RLUC production from viral RNA proceeded via a cap‐independent translational pathway.
In general, reprogramming of translation as a result of cellular stress compromises cap‐dependent translation and allows internal ribosome entry site (IRES)‐mediated translation to predominate (reviewed in Spriggs et al., 2008). This is largely caused by phosphorylation of the α‐subunit of eukaryotic initiation factor‐2 (eIF2) (reviewed in Holick and Sonenberg, 2005), which could explain why, in most cases, stress treatment led to a reduction in cap‐dependent FLUC expression. In addition, viral infection may contribute to the phosphorylation of the α‐subunit of eIF2. Double‐stranded viral replication intermediates activate dsRNA‐activated kinase (PKR)‐like kinase in plants (Crum et al., 1988). PKR is responsible for eIF2 α‐subunit phosphorylation. An inhibitor of PKR kinase, P58IPK, is needed for both virulence and to prevent eIF2 α‐subunit phosphorylation‐mediated cell death during tobamo‐ and potyvirus infections in N. benthamiana (Bilgin et al., 2003). Another kinase, GCN2, with a function in eIF2 α‐subunit phosphorylation, has been identified in Arabidopsis (Zhang et al., 2003) and rice (Halford, 2006). GCN2 affects eIF2 phosphorylation under various stress conditions (Lageix et al., 2008), but not after potyvirus infection (Zhang et al., 2008). Phosphorylation of the eIF2 α‐subunit via e.g. these two kinases under conditions induced by both virus infection and abiotic stress could be responsible for the observed reduction in FLUC production.
Ca2+ modifies the cellular environment to promote PVA infection beyond translation. A transient local increase in Ca2+ concentration can induce several different types of cellular response, depending on the concentration, timing and oscillation of the Ca2+ signal, and on the types of Ca2+ receptor that perceive the signal (reviewed by Boudsocq et al., 2010; Das and Pandey, 2010; Dodd et al., 2010; Kader and Lindberg, 2010; Kudla et al., 2010). About 3% of the open reading frames in plants are regulated by Ca2+ (Finkler et al., 2007). Calmodulin (CaM) and CaM‐like protein, which act either indirectly by regulating transcription, or directly as enzyme effectors, have been found to be induced as an early response to potyvirus infection in potato (Baebler et al., 2009). TEV HC‐Pro has been shown to bind to a CaM‐related protein, rgs‐CaM, which has three EF‐hand calcium‐binding motifs in its C‐terminal domain (Anandalakshmi et al., 2000). The expression of rgs‐CaM is up‐regulated during TEV infection. Papaya ringspot virus (PRSV; genus Potyvirus) HC‐Pro interacts with calreticulin, which is significantly up‐regulated in the early stage of PRSV infection (Shen et al., 2010). These data suggest that the interaction between potyviral HC‐Pro and Ca2+‐binding proteins may interfere with plant calcium signalling pathways and affect viral infection or host defence. These proteins may thus have an important role in the establishment of the infection. It remains of interest to study further the molecular basis of the Ca2+ effect in PVA infection.
One conclusion of this study is that the sensing of abiotic stress, which probably leads to signalling via elevated cytoplasmic Ca2+, increases virus load and may therefore lead to higher crop losses in fields. Therefore, the effect of abiotic stress on plant viral infection warrants further analysis. Another interesting hypothesis is that the establishment of infection via mechanical inoculation or aphid transmission probably causes a local spike in Ca2+ in newly infected cells. When probing the phloem cells with a stylet, aphids damage the cell wall and the plasma membrane of epidermal cells, which are the sites for the initiation of viral infection. Therefore, the first cells to receive a viral particle from the stylet are wounded cells. As shown in this study, this is beneficial for the initial rounds of translation of viral RNA and for subsequent replication, and may be essential for the establishment of infection when only a few copies of viral RNA are present.
EXPERIMENTAL PROCEDURES
Gene constructs
The viral constructs used in the Agrobacterium‐mediated infection experiments (cloning described in Eskelin et al., 2010) were based on the full‐length infectious cDNA copies of PVA‐B11 (Puurand et al., 1996; GenBank accession no. AJ296311) tagged with an intron containing the RLUC gene (35S‐PVA::RLUC; Gabrenaite‐Verkhovskaya et al., 2008) under the control of the Cauliflower mosaic virus (CaMV) 35S promoter. This cDNA construct is referred to throughout this article as PVAWT (Fig. 1A). A corresponding viral construct in which a GFP gene (cloned as in Kelloniemi et al., 2006) replaced the RLUC gene was used to follow the overall process of viral infection by fluorescence microscopy. The replication‐deficient PVA (PVAΔGDD; Fig. 1B) used in this study is described in Eskelin et al. (2010). It was constructed by replacing the aspartic acid residues of the catalytically active GDD motif (Kamer and Argos, 1984) of PVA NIb with alanine residues (Fig 1B). Cloning of the monocistronic FLUC construct, which was used as an internal control, was performed as in Eskelin et al. (2010) (Fig. 1C).
Agroinfiltration procedure and sampling of plants
The PVA infection assay was conducted essentially as described in Eskelin et al. (2010). Briefly, 10 mL of Agrobacterium culture grown in the presence of acetosyringone (20 µm) was centrifuged (5 min, 3000 g); the cells were washed with dH2O, and were resuspended in induction buffer [10 mm 2‐(N‐morpholino)ethanesulfonic acid (MES) (pH 6.3), 10 mm MgCl2 and 150 µm acetosyringone]. The optical density at 600 nm (OD600) was adjusted to 0.05 or 0.1 for Agrobacterium carrying the PVA constructs (the specific value is given for each experiment) and to 0.001 for Agrobacterium carrying a monocistronic FLUC construct, which was used as an internal control. Nicotiana benthamiana plants grown in a 16 h, 22 °C/8 h, 18 °C light/dark cycle were infiltrated at the four‐ to six‐leaf stage with a mixture of Agrobacterium carrying PVA and FLUC. At 18–48 h post‐agroinfiltration (the timing is specified for each experiment), the chemical compound to be analysed (CaCl2, 0.005–0.2 m; NaCl, 0.005–0.7 m; mannitol, 0.3 m; EGTA, 10 mm; LaCl3, 1 mm; Sigma‐Aldrich, Steinheim Germany) was infiltrated into the region previously infiltrated with Agrobacterium. Wounding stress was induced by rubbing the lower leaves with carborundum abrasive. Four to 15 plants received the same treatment per experiment, and each experiment was repeated at least twice. Sampling was carried out at certain time points by cutting 5‐mm discs with a cork borer from the infiltrated leaves. Three to six samples, each consisting of a pool of several discs, were collected for each sample type. The discs were immediately frozen in liquid nitrogen and stored at −80 °C. The spread of virus was followed in some experiments by the visualization of GFP fluorescence derived from 35S‐PVA::GFP. Nicotiana benthamiana leaves were infiltrated with Agrobacterium suspension at OD600= 0.001, and GFP fluorescence was tracked in the infiltrated leaves until 4 DPI.
Luciferase activity determination
FLUC and RLUC activities were determined using the Dual Luciferase Assay Kit (Promega, Madison, WI, USA), according to the supplier's instructions. The luciferase activities (photons/s) were measured with a Luminoscan TL Plus Device (Thermo Labsystems, Helsinki, Finland). The delay and measurement times were adjusted to 1 and 5 s, respectively. The mean values for RLUC and FLUC activities were calculated from parallel samples. The average FLUC value of a given sample set derived from one particular condition was calculated and used to normalize RLUC replicate samples of that particular condition. RLUC normalization was carried out using the formula [(FLUCaverage/FLUCsample) × RLUC]. The error bars represent the standard deviation of the mean.
Immunoblotting
Samples were extracted using Dual‐Luciferase Extraction Buffer (Promega) and the soluble protein amounts were estimated by measuring A 280 nm. Equal amounts of total protein were separated in a 12% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) gel. Immunodetection was performed as described previously (Hafren and Mäkinen, 2008) using FLUC‐polyclonal antibody (1 : 2000 dilution; Sigma L0159 IgG), RLUC‐monoclonal antibody (1 : 1000 dilution; Millipore, Temeculla, CA, USA) or CP‐polyclonal antibody (Merits et al., 1999).
ACKNOWLEDGEMENTS
Sini Lindström and Taru Rautavesi are acknowledged for their technical assistance in the glasshouse and laboratory. Financial support from the Academy of Finland (grant nos. 121622 and 1138329) is acknowledged.
REFERENCES
- Agudelo‐Romero, P. , Carbonell, P. , Iglesia, F. , Carrera, J. , Rodrigo, G. , Jaramillo, A. , Pérez‐Amador, M.A. and Elena, S.F. (2008) Changes in the gene expression profile of Arabidopsis thaliana after infection with tobacco etch virus. Virol. J. 5, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja, I. , de Vos, R.C.H. , Bones, A.M. and Hall, R.D. (2010) Plant molecular stress responses face climate change. Trends Plant Sci. 15, 664–674. [DOI] [PubMed] [Google Scholar]
- Alfenas‐Zerbini, P. , Maia, I.G. , Fávaro, R.G. , Cascardo, J.C.M. , Brommonschenkel, S.H. and Zerbini, F.M. (2009) Genome‐wide analysis of differentially expressed genes during the early stages of tomato infection by a potyvirus. Mol. Plant–Microbe Interact. 22, 352–361. [DOI] [PubMed] [Google Scholar]
- Anandalakshmi, R. , Marathe, R. , Gel, X. , Herr, J.M. Jr , Mau, C. , Mallory, A. , Pruss, G. , Bowman, L. and Vance, V.B. (2000) A calmodulin‐related protein that suppresses posttranscriptional gene silencing in plants. Science, 290, 142–144. [DOI] [PubMed] [Google Scholar]
- Baebler, S. , Krecic‐Stres, H. , Rotter, A. , Kogovsek, P. , Cankar, K. , Kok, E.J. , Gruden, K. , Kovac, M. , Zel, J. , Pompe‐Novak, M. and Ravnikar, M. (2009) PVYNTN elicits a diverse gene expression response in different potato genotypes in the first 12 h after inoculation. Mol. Plant Pathol. 10, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgin, D.D. , Liu, Y. , Schiff, M. and Dinesh‐Kumar, S.P. (2003) P58(IPK), a plant ortholog of double‐stranded RNA‐dependent protein kinase PKR inhibitor, functions in viral pathogenesis. Dev. Cell, 4, 651–661. [DOI] [PubMed] [Google Scholar]
- Boudsocq, M. , Willmann, M.R. , McCormack, M. , Lee, H. , Shan, L. , He, P. , Bush, J. , Cheng, S.H. and Sheen, J. (2010) Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature, 464, 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam, J. (2005) In touch: plant responses to mechanical stimuli. New Phytol. 165, 373–389. [DOI] [PubMed] [Google Scholar]
- Cessna, S.G. , Chandra, S. and Low, P.S. (1998) Hypo‐osmotic shock of tobacco cells stimulates Ca2+ fluxes deriving first from external and then internal Ca2+ stores. J. Biol. Chem. 273, 27 286–27 291. [DOI] [PubMed] [Google Scholar]
- Cessna, S.G. , Matsumoto, T.K. , Lamb, G.N. , Rice, S.J. and Wenger Hochstedler, W. (2007) The externally derived portion of the hyperosmotic shock‐activated cytosolic calcium pulse mediates adaptation to ionic stress in suspension‐cultured tobacco cells. J. Plant Physiol. 164, 815–823. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Han, H. , Jiang, P. , Nie, L. , Bao, H. , Fan, P. , Lv, S. , Feng, J. and Li, Y. (2011) Transformation of β‐lycopene cyclase genes from Salicornia europaea and Arabidopsis conferred salt tolerance in Arabidopsis and tobacco. Plant Cell Physiol. 52, 909–921. [DOI] [PubMed] [Google Scholar]
- Crum, C.J. , Hu, J. , Hiddinga, H.J. and Roth, D.A. (1988) Tobacco mosaic virus infection stimulates the phosphorylation of a plant protein associated with double‐stranded RNA‐dependent protein kinase activity. J. Biol. Chem. 263, 13 440–13 443. [PubMed] [Google Scholar]
- Das, R. and Pandey, G.K. (2010) Expression analysis and role of calcium regulated kinases in abiotic stress signaling. Curr. Genomics, 11, 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd, A.N. , Kudla, J. and Sanders, D. (2010) The language of calcium signalling. Annu. Rev. Plant Biol. 2, 593–620. [DOI] [PubMed] [Google Scholar]
- Eskelin, K. , Suntio, T. , Hyvärinen, S. , Hafren, A. and Mäkinen, K. (2010) Renilla luciferase‐based quantification of early Potato virus A infection initiated with Agrobacterium infiltration in N. benthamiana leaves. J. Virol. Methods, 164, 101–110. [DOI] [PubMed] [Google Scholar]
- Finkler, A. , Kaplan, B. and Fromm, H. (2007) Ca‐responsive cis‐elements in plants. Plant Signal. Behav. 2, 17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers, T.J. , Hajibagherb, M.A. and Yeo, A.R. (1991) Ion accumulation in the cell walls of rice plants growing under saline conditions: evidence for the Oertli hypothesis. Plant Cell Environ. 14, 319–325. [Google Scholar]
- Fujita, M. , Fujita, Y. , Noutoshi, Y. , Takahashi, F. , Narusaka, Y. , Yamaguchi‐Shinozaki, K. and Shinozaki, K. (2006) Crosstalk between abiotic and biotic stress response: a current view from the point of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 9, 436–442. [DOI] [PubMed] [Google Scholar]
- Gabrenaite‐Verkhovskaya, R. , Andreev, A.A. , Kalinina, N.O. , Torrance, L. , Taliansky, M.E. and Mäkinen, K. (2008) The cylindrical inclusion protein of potato virus A is associated with a subpopulation of particles isolated from infected plants. J. Gen. Virol. 89, 829–838. [DOI] [PubMed] [Google Scholar]
- Gould, R.J. , Murphy, K.M. and Snyder, S.H. (1982) [3H]Nitrendipine‐labeled calcium channels discriminate inorganic calcium agonists and antagonists. Proc. Natl. Acad. Sci. USA, 79, 3656–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafren, A. and Mäkinen, K. (2008) Purification of viral genome‐linked protein VPg from Potato virus A infected plants reveals several posttranslationally modified forms of the protein. J. Gen. Virol. 89, 1509–1518. [DOI] [PubMed] [Google Scholar]
- Hafren, A. , Hofius, D. , Rönnholm, G. , Sonnewald, U. and Mäkinen, K. (2010) HSP70 and its cochaperone CPIP promote potyvirus infection in Nicotiana benthamiana by regulating coat protein functions. Plant Cell, 22, 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford, N.G. (2006) Regulation of carbon and amino acid metabolism, roles of sucrose nonfermenting‐1‐related protein kinase‐1 and general control nonderepressible‐2‐related protein kinase. Adv. Bot. Res. 43, 93–142. [Google Scholar]
- Holick, M. and Sonenberg, N. (2005) Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 6, 318–327. [DOI] [PubMed] [Google Scholar]
- Kader, M.A. and Lindberg, S. (2010) Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal. Behav. 5, 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer, G. and Argos, P. (1984) Primary structural comparison of RNA‐dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 12, 7269–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, T. , Kadono, T. , Fumoto, K. , Lapeyrie, F. , Kuse, M. , Isobe, M. , Furuichi, T. and Muto, S. (2004) Aluminum as a specific inhibitor of plant TPC1 Ca2+ channels. Biochem. Biophys. Res. Commun. 5, 40–45. [DOI] [PubMed] [Google Scholar]
- Kelloniemi, J. , Mäkinen, K. and Valkonen, J. (2006) S‐COMT and GFP, unlike sorcin, are successfully expressed from a potyvirus‐based gene vector in plants. Biochemie, 88, 505–513. [DOI] [PubMed] [Google Scholar]
- Kiegle, E. , Moore, C.A. , Haseloff, J. , Tester, M.A. and Knight, M.R. (2000) Cell‐type‐specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J. 23, 267–278. [DOI] [PubMed] [Google Scholar]
- Knight, H. , Trewavas, A.J. and Knight, M.R. (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 12, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Knight, M.R. , Read, N.D. , Campbell, A.K. and Trewavas, A.J. (1993) Imaging calcium dynamics in living plants using semi‐synthetic recombinant aequorins. J. Cell Biol. 121, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla, J. , Batistic, O. and Hashimoto, K. (2010) Calcium signals: the lead currency of plant information processing. Plant Cell, 22, 541–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaud, C. , Da Silva, D. , Cotelle, V. , Thuleau, P. , Xiong, T.C. , Jauneau, A. , Brière, C. , Graziana, A. , Bellec, Y. , Faure, J.D. , Ranjeva, R. and Mazars, C. (2010) Nuclear calcium controls the apoptotic‐like cell death induced by d‐erythro‐sphinganine in tobacco cells. Cell Calcium, 47, 92–100. [DOI] [PubMed] [Google Scholar]
- Lageix, S. , Lanet, E. , Pouch‐Pélissier, M.N. , Espagnol, M.C. , Robaglia, C. , Deragon, J.M. and Pélissier, T. (2008) Arabidopsis eIF2alpha kinase GCN2 is essential for growth in stress conditions and is activated by wounding. BMC Plant Biol. 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourieux, D. , Ranjeva, R. and Pugin, A. (2006) Calcium in plant defence‐signalling pathways. New Phytol. 171, 249–269. [DOI] [PubMed] [Google Scholar]
- Leon, J. , Rojo, E. , Titarenko, E. and Sanchez‐Serrano, J.J. (1998) Jasmonic acid‐dependent and ‐independent wound signal transduction pathways are differentially regulated by Ca2+/calmodulin in Arabidopsis thaliana . Mol. Gen. Genet. 258, 412–419. [DOI] [PubMed] [Google Scholar]
- Merits, A. , Guo, D. , Järvekülg, L. and Saarma, M. (1999) Biochemical and genetic evidence for interactions between potato A potyvirus encoded proteins P1 and P3 and proteins of the putative replication complex. Virology, 263, 15–22. [DOI] [PubMed] [Google Scholar]
- Narasimhulu, S.B. , Deng, X.‐B. , Sarria, R. and Gelvin, S.B. (1996) Early transcription of Agrobacterium T‐DNA genes in tobacco and maize. Plant Cell, 8, 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oruetxebarria, I. , Guo, D. , Merits, A. , Mäkinen, K. , Saarma, M. and Valkonen, J.P.T. (2001) Identification of the genome‐linked protein in virions of Potato virus A, with comparison to other members in genus Potyvirus. Virus Res. 73, 103–112. [DOI] [PubMed] [Google Scholar]
- Puurand, Ü. , Valkonen, J.P.T. , Mäkinen, K. , Rabenstein, F. and Saarma, M. (1996) Infectious in vitro transcripts from cloned cDNA of the potato A potyvirus. Virus Res. 40, 135–140. [DOI] [PubMed] [Google Scholar]
- Reddy, V.S. and Reddy, A.S.N. (2004) Proteomics of calcium‐signaling components in plants. Phytochemistry, 65, 1745–1776. [DOI] [PubMed] [Google Scholar]
- Seifert, G.J. and Blaukopf, C. (2010) Irritable walls: the plant extracellular matrix and signalling. Plant Physiol. 153, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, W. , Yan, P. , Gao, L. , Pan, X. , Wu, J. and Zhou, P. (2010) Helper component‐proteinase (HC‐Pro) protein of Papaya ringspot virus interacts with papaya calreticulin. Mol. Plant Pathol. 11, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.Y. , Zhang, Z.B. , Shao, H.B. , Guo, X.L. , Cao, H.X. , Zhao, H.B. , Fu, Z.Y. and Hu, X.J. (2008) Relationship between calcium decoding elements and plant abiotic‐stress resistance. Int. J. Biol. Sci. 26, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs, K.A. , Stoneley, M. , Bushell, M. and Willis, A.E. (2008) Re‐programming of translation following cell stress allows IRES‐mediated translation to predominate. Biol. Cell, 100, 27–38. [DOI] [PubMed] [Google Scholar]
- Sun, Q.P. , Guo, Y. , Sun, Y. , Sun, D.Y. and Wang, X.J. (2006) Influx of extracellular Ca2+ involved in jasmonic‐acid‐induced elevation of [Ca2+]cyt and JR1 expression in Arabidopsis thaliana . J. Plant Res. 119, 343–350. [DOI] [PubMed] [Google Scholar]
- Tester, M. and Davenport, R. (2003) Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 91, 503–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao, T. , Yakubov, B. , Satoh, R. , Yamaguchi‐Shinozaki, K. , Seki, M. , Hirayama, T. and Shinozaki, K. (1999) A transmembrane hybrid‐type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell, 11, 1743–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Dickinson, J.R. , Paul, M.J. and Halford, N.G. (2003) Molecular cloning of an Arabidopsis homologue of GCN2, a protein kinase involved in co‐ordinated response to amino acid starvation. Planta, 217, 668–675. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Wang, Y. , Kanyuka, K. , Parry, M.A.J. , Powers, S.J. and Halford, N.G. (2008) GCN2‐dependent phosphorylation of eukaryotic translation initiation factor 2α in Arabidopsis. J. Exp. Bot. 59, 3131–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Frey, T.K. and Yang, J.J. (2009) Viral calciomics: interplays between Ca2+ and virus. Cell Calcium, 46, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


