Summary
Resistance genes against Phytophthora infestans (Rpi genes), the most important potato pathogen, are still highly valued in the breeding of Solanum spp. for enhanced resistance. The Rpi genes hitherto explored are localized most often in clusters, which are similar between the diverse Solanum genomes. Their distribution is not independent of late maturity traits. This review provides a summary of the most recent important revelations on the genomic position and cloning of Rpi genes, and the structure, associations, mode of action and activity spectrum of Rpi and corresponding avirulence (Avr) proteins. Practical implications for research into and application of Rpi genes are deduced and combined with an outlook on approaches to address remaining issues and interesting questions. It is evident that the potential of Rpi genes has not been exploited fully.
Introduction
The cultivation of potato and other solanaceous species, such as tomato, still suffers from quantitative and qualitative losses caused by the late blight pathogen Phytophthora infestans, incurring enormous costs for disease prevention. Among the various taxonomic, climatic and geographical variables, the taxonomic level of the host species, and thus the genetic composition of the host, has been identified as the best predictor for the late blight resistance of Solanum spp. (Spooner et al., 2009). The improvement of commercial potato and tomato cultivars by resistance breeding is considered to represent a meaningful contribution to the reduction of yield losses caused by late blight. Potato breeding is complicated by polyploidy, heterozygosity, crossing barriers, linkage drag and high‐quality trait demands (Gebhardt, 2004; Jacobsen and Schouten, 2007; van der Vossen et al., 2003). Although quantitative resistance, as found in S. tuberosum ssp. andigenum, S. berthaultii and S. vernei (Andrivon et al., 2003), S. verrucosum (Rivera‐Peña, 1990), S. microdontum (Sandbrink et al., 2000) and S. paucissectum (Villamon et al., 2005), appears to be more durable (Colon et al., 1995a, b), combining quantitative trait loci (QTLs) for resistance with other desirable traits is more demanding and time consuming in comparison with introducing monogenic resistance. In addition, strong association of partial late blight resistance with late foliage maturity is well documented (Collins et al., 1999; Salaman, 1910; Toxopeus, 1958). Resistance genes against Phytophthora infestans (Rpi genes) are easier to introduce than QTLs, and those that have either been mapped in or cloned from Solanum spp. are listed in Table 1. However, the Rpi gene content of numerous wild Solanum spp. still remains untested (Jacobs et al., 2010), and their resistance has been clarified only partially. Sources of resistance have been reported predominantly in the tuber‐bearing species of the section Petota, and mainly originate from North, Central and South America.
Table 1.
Mapped and/or cloned Solanum spp. Rpi (resistance genes against Phytophthora infestans) genes
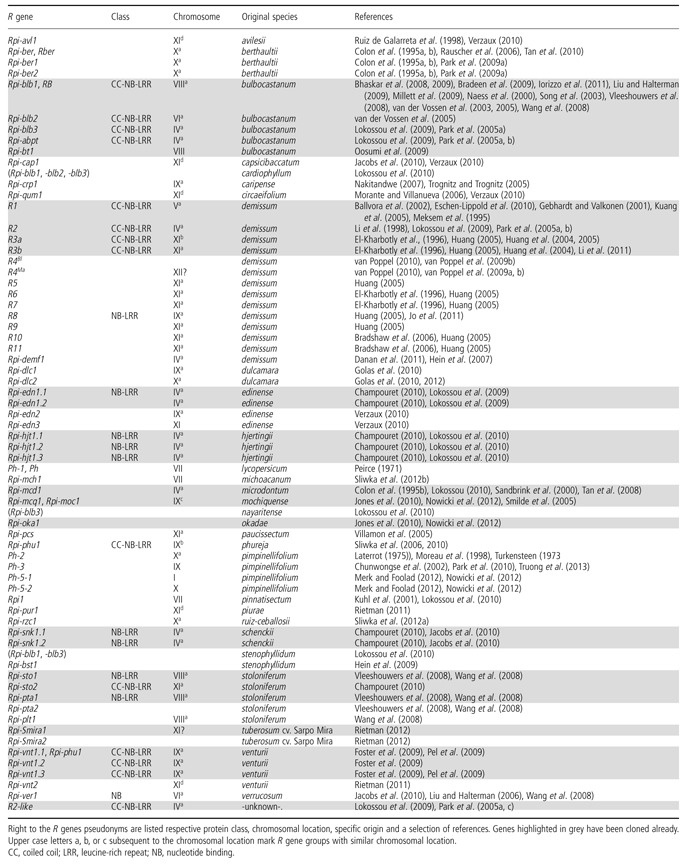
Although R1–R4 were described in 1953 as the first Rpi genes (Black et al., 1953) and numerous other Rpi genes have been discovered since, the investigation of their mechanistic mode of action and their exploitation in resistance breeding have only made significant progress since the 2000s. R1, R3, R2, R4 and R10 of S. demissum have been introgressed into cultivated potato stepwise (van der Lee et al., 2001; Park et al., 2009b; van der Vossen et al., 2005), but were quickly overcome in the field (Müller, 1951; Toxopeus, 1956). Phytophthora infestans isolates collected before its second worldwide migration, starting from Mexico in 1984 (Goodwin and Drenth, 1997), are genetically highly similar (Drenth et al., 1993, 1994; Fry et al., 1992). In contrast, the complex race structure of most P. infestans populations today (Rauscher et al., 2006, Swiezyński et al., 2000) and the prevalence of both A1 and A2 mating types (GILB, 1999) limit the benefit of the introduction of single race‐specific major resistance genes (Drenth et al., 1994). Defeated R genes, however, may still be conducive to late blight resistance (Pedersen and Leath, 1988; Stewart et al., 2003), a possible result of fitness costs arising from the maintenance of virulence factors by the pathogen (Montarry et al., 2010). Currently, a major strategy is to search for and integrate genes that confer broad‐spectrum resistance to late blight (Park et al., 2009a, b), such as Rpi‐blb1 (allelic to RB), Rpi‐blb2 and Rpi‐blb3 (Park et al., 2005a; Song et al., 2003; van der Vossen et al., 2003, 2005) from the Mexican wild species S. bulbocastanum Dunal. (2n = 2x = 24) (Hermsen and Boer, 1971), and Rpi‐blb1 homologues Rpi‐sto1 and Rpi‐pta1 from the tetraploid Central American species S. stoloniferum (Vleeshouwers et al., 2008; Wang et al., 2008). In contrast with other Rpi genes, Rpi‐blb1 and Rpi‐blb2 conferred stable resistance in diverse trials over several years, as demonstrated by slowed lesion development and reduced sporulation (Lozoya‐Saldana et al., 2005; Millett and Bradeen, 2007; Song et al., 2003; van der Vossen et al., 2003, 2005). Given that IpiO1 and IpiO2 presumably cause Avr‐blb1 activity in the majority of European and North American P. infestans isolates analysed to date (Vleeshouwers et al., 2008), the introduction of Rpi‐blb1, Rpi‐sto1 or Rpi‐pta1 could result in broad late blight resistance. The germplasm of S. bulbocastanum (Park et al., 2005a; Ramanna and Hermsen, 1971), S. stoloniferum (Hutten and van Berloo, 2001, referred to as sto or CPC 2093) and S. microdontum has been exploited in the breeding of numerous cultivars. Solanum microdontum Rpi‐mcd1 confers broad‐spectrum late blight resistance of foliage, delaying infection in the field for 3–11 days on average (Tan et al., 2008, 2010), and evidence indicates that the resistance extends to the tuber (Park et al., 2005d). As only one isolate of a larger collection sampled in Poland between 1999 and 2008 was capable of overcoming S. phureja Rpi‐phu1, it has been assumed that this gene could also contribute significantly to durable late blight resistance when introgressed into commercial cultivars together with other Rpi genes (Foster et al., 2009). As an alternative to classical breeding, molecular cloning and the transfer of resistance genes, such as Rpi‐blb1 and Rpi‐blb2, into cultivar Fortuna (Dixelius et al., 2012) is less time‐consuming, avoids linkage drag and overcomes crossing barriers (Park et al., 2009b). However, genetic engineering of crops currently suffers from a considerable lack of appreciation by consumers in several countries (http://www.gmo‐compass.org) and is an expensive process.
Classically, pathogen recognition receptors (PRRs), which monitor conserved pathogen‐associated molecular patterns (PAMPs) to initiate PAMP‐triggered immunity (PTI), have been discerned from R‐gene products, which monitor rather specific effectors to initiate effector‐triggered immunity (ETI) (reviews by Göhre and Robatzek, 2008; Hein et al., 2009; Jones and Dangl, 2006; Zipfel 2008, 2009). A continuum between PTI and ETI, instead of clear dichotomy, has however been suggested, as some PAMPs show little evolutionary conservation, contribute to virulence and/or elicit a strong hypersensitive response (HR) (Thomma et al., 2011). In addition, there is evidence that many mechanisms and molecular components of resistance are shared among PRR‐ and R‐gene‐mediated resistance in plants (reviews by Dangl and Jones, 2001; Deslandes and Rivas, 2011; Ingle et al., 2006; Nürnberger et al., 2004). The gene‐for‐gene hypothesis implies that the product of an R gene recognizes a specific avirulence (Avr) gene product of the pathogen (Flor, 1971; Keen, 1990). This interaction model has, however, been refined subsequently, as direct physical interaction has been observed only in rare experimental settings, e.g. for dicot nucleotide‐binding leucine‐rich repeat (NB‐LRR) protein–Avr protein combinations of flax protein L and AvrL567 from Melampsora lini (Dodds et al., 2006), Arabidopsis RRS1‐R and PopP2 from Ralstonia solanacearum (Deslandes et al., 2003), tobacco N and the p50 helicase domain of Tobacco mosaic virus (TMV) (Ueda et al., 2006), and potato RB and IPI‐O1/IPI‐O4 from P. infestans (Chen et al., 2012). Two advanced models have been developed according to which the R protein perceives modifications of an additional host factor. According to the ‘guard model’, pathogen effectors target and modify functional guardees (van der Biezen and Jones, 1998b; Dangl and Jones, 2001), whereas the ‘decoy model’ hypothesizes the targeting of decoy proteins that act exclusively in effector recognition (van der Hoorn and Kamoun, 2008).
This review summarizes current knowledge on Solanum Rpi genes (SRpigs), Rpi proteins (SRpips), their Avr counterparts and molecular interactions, and intends to support further research and its application by highlighting aspects not yet elucidated and by listing methodical advice. We present a ‘developmental bottleneck’ model, which may explain the decrease in late blight resistance at late developmental stages, even occurring in cultivars harbouring Rpi genes.
Effectors and Avr Proteins—Structure and Function
Effectors may be defined as molecules which are secreted by plant‐associated organisms and alter host cell structure and function (Hogenhout et al., 2009). Avr genes, which are present in plant‐pathogenic viruses, bacteria, fungi, oomycetes, nematodes and insects, encode effectors which are recognized and cause ETI of the host, in contrast with virulence genes (Luderer and Joosten, 2001; Skamnioti and Ridout, 2005; White et al., 2000). Depending on whether their site of action is extracellular or within the host symplast, apoplastic and cytoplasmic effectors are discerned. During infection, P. infestans induces and secretes both apoplastic and cytoplasmic effectors (Damasceno et al., 2008; Haas et al., 2009; Tian et al., 2007; Whisson et al., 2007). Apoplastic effectors may either protect the pathogen against host defences or mediate its invasion (Wawra et al., 2012a). Some apoplastic effectors of P. infestans inhibit host proteases (Song et al., 2009; Tian et al., 2004, 2005, 2007) or glucanases (Damasceno et al., 2008), whereas others putatively hydrolyse glycosylates (McLeod et al., 2003) or disrupt cell wall–plasma membrane adhesion by association with lectin receptor kinases (Gouget et al., 2006; Senchou et al., 2004). Many genes encoding cytoplasmic effectors have been found within the genome of P. infestans strain T30‐4, including 563 genes belonging to the RXLR and 196 genes belonging to the Crinkler (CRN) family (Haas et al., 2009). Both of these consist of modular proteins comprising an N‐terminal signal peptide, an N‐terminal and a C‐terminal domain, which, in several cases, are functional in secretion, translocation into host cells and effector activity, respectively (Haas et al., 2009, Morgan and Kamoun, 2007; Schornack et al., 2010, Fig. 1).
Figure 1.
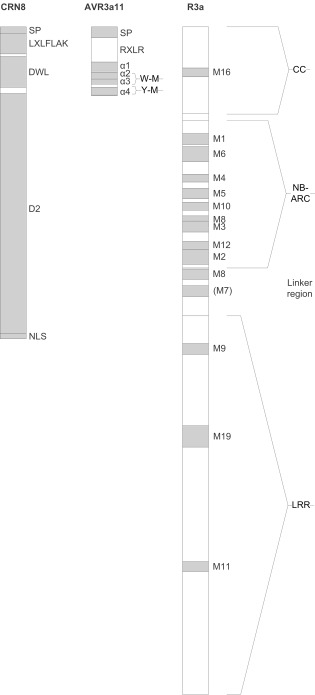
Modular structure of effectors: Phytophthora infestans CRN8 and P. capsici Avr3a11, and Solanum demissum R3a. Structural elements: SP, signal peptide; LXLFLAK, LXLFLAK domain; DWL, DWL domain; D2, D2 domain; NLS, nuclear localization signal; RXLR, RXLR domain; α1–α4, helices α1–α4; W‐M, W‐M motif; Y‐M, Y‐M motif; CC, coiled coil domain; NB‐ARC, NB‐ARC domain; linker region; LRR, LRR domain; M1–M19, motifs 1–19, corresponding to: M16, EDxxD; M1, P loop; M6, RNBS‐A; M4, Kin‐2; M5, RNBS‐B; M10, RNBS‐C; M2, RNBS‐D, M9, LDL; In contrast with the other motifs, M7 did not align well to R3a (only 19% compared with, otherwise, at least 34% amino acid identity). References: Boutemy et al. (2011), van Damme et al. (2012), Huang et al. (2005) and Jupe et al. (2012).
To date, all experimentally verified P. infestans Avr genes encode RXLR effectors (Boyd et al., 2012; Jiang et al., 2008). However, the putative transcription factor pi3.4 has also been suggested as an origin of incompatibility to R3b and R10 (Qutob et al., 2006), in addition to RXLR effectors (Rietman, 2011). The large, highly diverse RXLR superfamily is named after the first of two conserved tetrameric sequence motifs in the N‐terminal half, following the signal peptide (Haas et al., 2009, Jiang et al., 2008; Rehmany et al., 2005; Whisson et al., 2007). The RXLR domain of Avr3a has been shown to mediate homodimerization (Bos et al., 2010). As several RXLR effectors comprise nuclear localization signals (NLSs), it has been suggested that they may partially manipulate host gene expression (Morgan and Kamoun, 2007). Within the C‐terminal half or effector domain, four more motifs have been identified: the K motif (Dou et al., 2008a) and motifs W, Y and L (Jiang et al., 2008). Stretches similar to the 49‐residue WY domain motif, consisting of a W motif, a loop and a Y motif, have been found in 44% of annotated RXLR effectors from P. infestans, P. ramorum and P. sojae (Boutemy et al., 2011). There has been a controversial debate about the occurrence and molecular site of phosphatidylinositol‐3‐phosphate (PI(3)P) binding and its role for effector entry, both in general and for Avr1b in particular. Diverse recent findings have indicated a multitude of specific PI(3)P‐binding affinities for different subgroups of effectors from filamentous pathogens: some effectors bind PI(3)P with the RXLR(‐like) domain (Bhattacharjee et al., 2012a, b; Kale et al., 2010; Plett et al., 2011), others with their C‐terminal part (Gan et al., 2010; Wawra et al., 2012b; Yaeno et al., 2011), or with both domains (Sun et al., 2013), or do not bind to PI(3)P at all (Gan et al., 2010; Yaeno et al., 2011). NMR analysis of AVR3a4 and structure modelling revealed a conserved, positively charged patch within the effector domain, which probably mediates PI(3)P binding of 1b3a subfamily effectors (Sun et al., 2013; Yaeno et al., 2011). Tertiary structure analysis revealed an α‐helical fold conserved in 44% of annotated Phytophthora RXLR effectors (Boutemy et al., 2011), and will be key to the understanding of further structure–function relationships within other effector subfamilies.
Rpi Gene‐Encoded Proteins—Structure and Function
R genes, which account for approximately 1%–3% of the genome of potato, rice and poplar (Andolfo et al., 2013; Kohler et al., 2008; Potato Genome Sequencing Consortium, 2011), have been assigned to four classes (van Ooijen et al., 2007; Table 2).
Table 2.
R‐gene classes of solanaceous plants
| Solanum lycopersicum | Solanum tuberosum | |
|---|---|---|
| CNL | 93 (0.268%) | 163 (0.466%) |
| TNL | 18 (0.052%) | 43 (0.123%) |
| RLP | 176 (0.507%) | 403 (1.151%) |
| RLK | 261 (0.752%) | 301 (0.86%) |
| Others | 198 (0.57%) | 421 (1.203%) |
CC, coiled coil; CNL, CC‐NB‐LRR; LRR, leucine‐rich repeat; NB, nucleotide binding; RLK, receptor‐like kinase; RLP, receptor‐like protein; TIR, Drosophila Toll and mammalian interleukin‐1 receptor; TNL, TIR‐NB‐LRR.
Number and percentage of candidate resistance genes (Andolfo et al., 2013).
Intracellular NB‐LRR proteins constitute two of these classes: TIR‐NB‐LRR (TNL) proteins, which are rare in monocots (Tarr and Alexander, 2009), but represent a major fraction in dicots (Bakker et al., 2011; Kohler et al., 2008), have an N‐terminal conserved domain that resembles the Drosophila Toll and mammalian interleukin (IL)‐1 receptor (TIR) domain. Proteins of the second class, CC‐NB‐LRR (CNL), often contain an N‐terminal coiled‐coil (CC) structure, occasionally in the form of a leucine zipper region (Hammond‐Kosack and Jones, 1997; Martin et al., 2003; Pan et al., 2000). Recently, the first crystal structure of the CC domain of a CNL revealed a helix–loop–helix structure (Maekawa et al., 2011). This domain supported dimerization to tightly packed rod‐shaped homodimers and presumably supports interaction with other proteins (Lupas, 1996; Maekawa et al., 2011). As opposed to short CC domains, extended CC domains which contain the Solanaceae domain (SD) have exclusively been reported to date for Solanaceae (Lukasik‐Shreepaathy et al., 2012, Mucyn et al., 2006). Additional predicted CC domains within the N‐terminus (Hwang and Williamson, 2003; van der Vossen et al., 2005) and highly conserved tryptophan residues within both block II and block III of the NB site (Pan et al., 2000) have been recognized as further sequence characteristics specific to these CNL proteins with an extended CC domain. Many plant CNLs also contain a non‐TIR (nT) motif of the form EDxxD, which presumably is involved in intramolecular interaction (van Ooijen et al., 2007). To date, all determined SRpigs encode proteins of the CNL class (Table 1).
As NB‐LRRs commonly lack predicted transmembrane segments or signal peptides, they probably reside and perceive Avr products in the cytoplasm (Boyes et al., 1998; van der Vossen et al., 2003). As a result of the prediction of four myristoylation and 43 phosphorylation sites for R1, its putative anchoring in the plasma membrane and participation in signal transduction by (de‐)phosphorylation steps have been suggested (Ballvora et al., 2002).
Several reports suggest that CNLs and TNLs to some extent require different downstream signalling components, indicating involvement of the N‐terminus in downstream signalling: In different plant families, TNL based resistance is strongly dependent on enhanced disease susceptibility 1 (EDS 1) (Gassmann et al., 1999; Liu et al., 2002a; Parker et al., 1996; Shirano et al., 2002), whereas a large fraction of CNLs studied to date seem to mainly depend on nonrace‐specific disease resistance (NDR1) (Aarts et al., 1998; Tornero et al., 2002, review by Martin et al., 2003).
The NB domain occurring in APAF‐1, certain R‐gene products and CED‐4 (NB‐ARC domain; van der Biezen and Jones, 1998a) binds and hydrolyses ATP, but is also important for overall functionality of the R‐gene product (Tameling et al., 2002; Ueda et al., 2006; Walker et al., 1982). In plants, the NB‐ARC domain consists of the three subdomains NB, ARC1 and ARC2 (reviewed by Albrecht and Takken, 2006). In analogy with ADP‐bound APAF‐1, these could form a compact globular NB pocket consisting of a five‐stranded parallel β sheet surrounded by seven α‐helices, a helix bundle or a winged helix fold (van Ooijen et al., 2007; Riedl et al., 2005; Takken and Goverse, 2012). It is assumed that the NB domain functions as a molecular switch, inducing a conformational change by NTP hydrolysis to regulate signal transduction (Leipe et al., 2004). The NB‐ARC domain contains eight highly conserved motifs, of which motifs RNBS‐A and RNBS‐D differ substantially in TIR‐ and non‐TIR‐NB‐LRRs (Jupe et al., 2012; Meyers et al., 2003), and the P loop of the NB site is assumed to be involved in resistance signalling through interaction with the CC or TIR domains (Belkhadir et al., 2004; Moffett et al., 2002).
LRR domains are involved in protein–protein interaction and ligand recognition in all domains of life and also in viruses (Enkhbayar et al., 2004). For CNL, steric structures of LRR domains have been proposed based on related proteins (Takken and Goverse, 2012). LRR domains comprise 2–42 repeats, which, in plants, are formed by 24–28 residues including a 14‐residue core of the pattern LxxLxxLxLxxC/Nxx adopting a β‐sheet structure and adjacent loop regions (van Ooijen et al., 2007). In addition to a conserved VLDL motif in the third repeat, which could possibly function as a nuclear export signal (La Cour et al., 2004), LRRs contain two subdomains common to both TNLs and CNLs (Jupe et al., 2012, Fig. 1). In plant R proteins, the LRR domain is considered to be a main determinant in pathogen recognition specificity (Dodds et al., 2001; Ellis et al., 1999; Rairdan and Moffett, 2006), and to function directly (Ellis et al., 2007; Jia et al., 2000; Ueda et al., 2006) or indirectly (Burch‐Smith et al., 2007; Lokossou, 2010, review by Innes, 2004) in the binding of Avr gene products. Intramolecular interaction of the LRR domain with other regions has been shown for tobacco N (Ueda et al., 2006) and for bell pepper Bs2 in vitro (Leister et al., 2005), and has been suggested for R2 (Lokossou, 2010). The LRR domain generally seems to interact with the ARC1 subdomain, whereas the ARC2 subdomain has been suggested to relay pathogen recognition at the LRR domain into conformational changes, leading to downstream signalling. It is assumed that the entire LRR domain is necessary for activation on interaction with the CC‐NB‐ARC part (Rairdan and Moffett, 2006). Mutations disrupting such inter‐ or intramolecular interactions functioning in the negative regulation of defence responses have been shown to result in the constitutive activation of defence responses and lethality (Bendahmane et al., 2002; Hwang and Williamson, 2003; Shirano et al., 2002; Zhang et al., 2003). The LRR region is the most variable segment in closely related NB‐LRR proteins and its β‐strand/β‐turn motif has been shown to be under divergent selection in Solanum spp. (Parniske et al., 1997; van der Vossen et al., 2000) and other species (Ellis et al., 1999; McDowell et al., 1998; Meyers et al., 1998). In contrast with NB‐LRR proteins, R proteins of the remaining two classes are predicted to contain an N‐terminal extracellular LRR (eLRR) domain. Attached to the eLRR domain by a transmembrane domain, their cytoplasmic part either contains a protein kinase domain (receptor‐like kinases or RLKs) or does not (receptor‐like proteins or RLPs) (van Ooijen et al., 2007). Several R proteins and many candidates do not fit into any of these four classes, and therefore were occasionally assigned to distinct further R‐protein classes (Martin et al., 2003). For evolutionary aspects, we refer to Andolfo et al. (2013), Couch et al. (2006), Kuang et al. (2005) and Wang et al. (2008).
Genomic Location of Rpi Genes
Nonrandom, uneven distribution within specific genomes of the Solanaceae and other plant families (Young, 2000) has been shown for R genes (Gebhardt and Valkonen, 2001; Jacobs et al., 2010) and resistance gene analogues (RGAs) (Andolfo et al., 2013; Bakker et al., 2003, 2011), giving rise to clusters and hot spots of R genes that confer resistance to unrelated, but also similar, antagonists. Within the potato genome draft sequence, 728 RGAs have been mapped to 47 different loci (15.5 RGA/region on average; Bakker et al., 2011). Currently, 60 of the 68 known SRpigs have been mapped, and are dispersed on 16 regions on 10 chromosomes (3.75 Rpi/region; Table 1).
R‐gene clustering may enhance the shuffling of sequence polymorphism through unequal inter‐ and intragenic meiotic recombination, leading to duplication, partial deletion or the reassembly of genes, thereby generating new R‐gene specificities, partially from pseudogenes (Hulbert et al., 2001). The genomic distribution of R genes in the solanaceous species tomato, potato and pepper is not independent, but often corresponds to each other (Andolfo et al., 2013; Grube et al., 2000), enabling the homology‐based identification of Rpi genes (Huang et al., 2005). Some authors have suggested that quantitative resistance to late blight could be a side‐effect of maturity traits with a possible contribution of residual effects of succumbed R genes (Tan et al., 2008; Allefs et al., 2005). Indeed, a potato QTL meta‐analysis revealed the overlapping and juxtaposition of many meta‐QTLs for late blight resistance and maturity, but also clearly distinct QTLs and meta‐QTLs, indicating that pleiotropic or closely linked genes could exist in addition to unlinked genes underlying these two traits (Danan et al., 2011).
The distribution of Solanum Rpi genes and RGAs has been found to be significantly independent of the distribution of late blight resistance meta‐QTLs. Artificial separation as a result of as yet undetected QTLs, or artificial clustering caused by insufficient resolution, however, cannot be excluded.
Interestingly, the genomic location of Solanum RGAs and Rpi genes does not seem to be independent of the location of maturity traits. The number of RGAs and SRpigs located in maturity meta‐QTLs is significantly higher than expected (Danan et al., 2011). Furthermore, the analysis of maturity classes of potato cultivars (http://www.europotato.org) and information on the presence of SRpigs (http://www.euroblight.net) revealed a significant delay in the maturity of 205 cultivars which harbour SRpigs, compared with 547 cultivars lacking SRpigs (J. Rodewald & B. Trognitz, unpublished results; Table 4).
Table 4.
Online resources for potato and late blight (Phytophthora infestans) information
| Description | URL |
|---|---|
| Solanaceae genomic DB and online tools | http://solanaceae.plantbiology.msu.edu/ |
| Solanaceae genomic, genetic, phenotypic and taxonomic DB | http://solgenomics.net/ |
| Gateway to several DB and tools, e.g. SolEST | http://www.eu‐sol.net/ |
| Tomato functional genomics DB | http://ted.bti.cornell.edu/ |
| Potato chromosomal map DB including details such as sequence, gene function and links | http://www.gabipd.org/projects/Pomamo/ |
| Solanum R gene DB including phenotypic, genetic, phylogenetic data, germplasm access | http://www.plantbreeding.wur.nl/SolRgenes/ |
| Field data on isolate abundance, cultivar resistance, fungicide efficiency | http://www.euroblight.net |
| European cultivated potato DB | http://www.europotato.org |
| Potato pedigree DB | http://www.plantbreeding.wur.nl/potatopedigree/ |
DB, database.
Interestingly, pathogen infection may also result in epigenomic and genomic alterations at R clusters, e.g. hypomethylation and rearrangement, as has been shown for Nicotiana tabacum (Boyko et al., 2007).
Rpi Gene Expression
Comparatively few studies on SRpigs have addressed the expression of functional R genes. To fulfil a role as cellular sentinels, R proteins must be present in plant cells prior to attack, and therefore R genes are assumed to be constitutively expressed (Iorizzo et al., 2011). The transcription of R3a (Huang et al., 2005), RB (Song et al., 2003) and Hero (Poch et al., 2006) has indeed been observed in unchallenged leaves of S. tuberosum, S. bulbocastanum and S. lycopersicum, respectively. Although some R genes, such as tomato Bs4, tomato Mi‐1.2 and potato R3a, seem to remain transcribed at steady‐state levels (Goggin et al., 2004; Huang et al., 2005; Schornack et al., 2005), it has been shown that some Solanum resistance gene homologue (RGH) or Rpi genes, such as RB, are induced on late blight infection in Solanum spp. (Henriquez and Daayf, 2010; Kramer et al., 2009; J. Rodewald & B. Trognitz, unpublished results), as is the case for the N. tabacum R gene N following TMV infection (Levy et al., 2004). The presence of some SRpig‐related expressed sequence tags only in pathogen‐challenged libraries (Ronning et al., 2003) could also result from pathogen‐triggered induction of at least some RGHs. There is also evidence for tissue‐specific transcript levels of R genes or RGHs (Brugmans et al., 2008). Some RGAs are referred to as pseudogenes owing to premature stop codons, frameshift indels or large deletions, but are nevertheless expressed (Paal et al., 2004). RB transcript levels were roughly consistent throughout the developmental stages of pre‐flowering, post‐flowering and near‐senescence in RB‐transformed S. tuberosum cv. Dark Red Norland, and therefore it is unlikely that the decrease in late blight resistance observed at increasing physiological age of potato plants is caused by changes in the expression of the RB transgene (Millett et al., 2009). Similar transcript levels of RB within two RB transgenic potato lines at flowering, 2 weeks and 4 weeks after flowering, irrespective of the environmental temperature (10, 20 or 30 °C), further indicates little dependence of transcriptional activity on plant age and temperature (Iorizzo et al., 2011). In RB‐transformed potato cultivars, however, transgene copy number and transcript levels were positively correlated with late blight resistance (Bradeen et al., 2009; Millett et al., 2009), and RB transcript levels during the first 24 h post‐inoculation (hpi) were a major determinant of RB‐mediated late blight resistance level (Kramer et al., 2009). Plant age and environmental temperature hardly influenced RB transcript levels. However, changes in molecules affecting the stability of Rpi transcripts or proteins, or changes in translation potential, could modify Rpi protein levels, and thus the degree of resistance. In our own unpublished trials with the Rpi gene‐harbouring potato cultivar MF‐II, late blight inoculation was followed by a decline in transcripts associated with ribosome biosynthesis, translation and glycolysis, which was specific to short‐day‐conditioned and thus ripening plants. Speculating that the transcription of SRpigs is relatively independent of plant age, we propose the hypothesis depicted in Fig. 2. In the post‐flowering, near‐senescent or senescent stage, translation of SRpig transcripts could be reduced, which would negatively affect resistance. However, there are also alternative causes possible. If the decrease in resistance is really caused by a decline in ETI, it could also result from a decrease in components active in signalling or defence. In this context, the question emerges as to whether day‐length‐conditioned physiological age influences transcript and protein levels of other components of SRpig‐based resistance, the concentration of cellular energy equivalents and the membrane potential.
Figure 2.
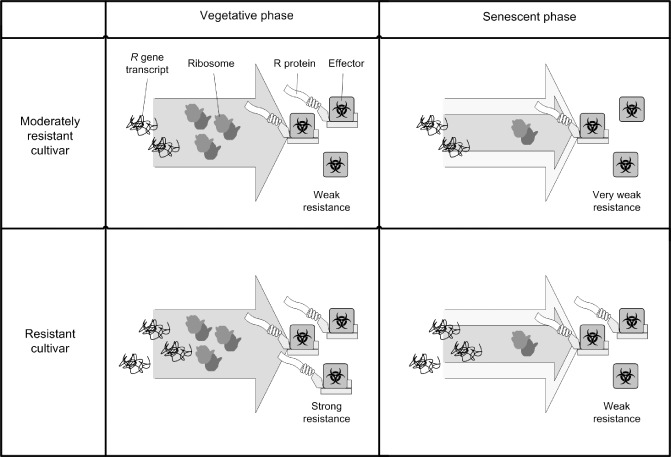
The ‘developmental bottleneck’ model. During the vegetative phase (left column) and the senescent phase (right column), transcript levels of resistance genes against Phytophthora infestans (Rpi) may remain similar; however, they may be lower in moderately resistant cultivars (top row) compared with resistant cultivars (bottom row). As the plant senesces, an assumed decrease in translational capacity could possibly reduce Rpi protein levels. Consequently, susceptibility increases in both genotypes.
Rpi–Avr Interaction and Response—Molecular Functional Mechanisms
Recently, molecular interactions of the four P. infestans RXLR effectors, Avr2, Avr3a, Avr‐blb1 and Avr‐blb2, have been partially elucidated, indicating heterogeneity among CNLs with regard to localization and response mechanisms.
Avr2 is localized inside the host nucleus and cytoplasm, but mainly at the perihaustorial plasma membrane, associated with and mediating the interaction of the putative plant phosphatase BSU‐LIKE PROTEIN1 (BSL1) with R2 (Saunders et al., 2012). Avr‐blb2 accumulates at the perihaustorial plasma membrane, where it binds to and inhibits the secretion of the host papain‐like cysteine protease (PLCP) C14 (Bozkurt et al., 2011). IPI‐O1, which has Avr‐blb1 activity (Chen et al., 2012), binds to an Arabidopsis thaliana lectin receptor kinase, thereby disrupting cell wall–plasma membrane adhesion (Senchou et al., 2004; Gouget et al., 2006). Avr3a inhibits infestin 4‐triggered cell death during the biotrophic phase via the stabilization of the host ubiquitin E3 ligase CMPG1 (Bos et al., 2010).
Several findings have suggested a role for certain non‐Rpi R proteins in defence gene expression. Nuclear location is essential to the function of N (Burch‐Smith et al., 2007). Furthermore, in the presence of the corresponding effector AvrA10, nuclear Hordeum vulgare resistance protein MLA10 associates with the HvWRKY2 transcription factor, which represses genes involved in basal resistance (Shen et al., 2007).
The association of Avr2, BSL1 and R2 corresponds to a three‐molecule interaction as described by the guard model and the decoy model. Corroborating evidence for the guard and decoy hypothesis has also been obtained for interactions of Solanaceae spp. with other pathogens, i.e. in the guard/bait/Avr systems Prf/Pto/AvrPto (tomato; Mucyn et al., 2006), Cf‐2/Rcr3/Avr2 (tomato; Rooney et al., 2005) and N/NIP1/p50 (tobacco; van Ooijen et al., 2007). Interestingly, binding of the guardee Rcr3pim by the Cladosporium fulvum Avr2 protein is detected by Cf2 in tomato, whereas its association with P. infestans effectors EPIC1 or EPIC2B does not trigger innate immunity (Song et al., 2009). Direct Avr–R protein binding supposedly involves the LRR domain, which could subsequently release the R protein from autoinhibition. This is expected to result in a conformational change of the NB‐ARC domain and the exchange of ADP for ATP (Takken and Goverse, 2012)
Figure 3 depicts known interactions of ETI components. The components of ETI are listed in Table 3 .
Figure 3.
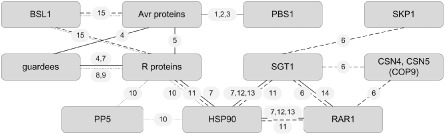
Molecular interactions of effector‐triggered immunity (ETI) components in different pathosystems. Full lines depict interactions in the host Arabidopsis thaliana, broken lines in Nicotiana benthamiana and dotted lines in Solanum lycopersicum. Avr, avirulence; BSL, BSU‐LIKE PROTEIN; COP, CONSTITUTIVE PHOTOMORPHOGENIC; CSN, COP9 signalosome; HSP, heat shock protein; PBS, avrPphB susceptible; PP, protein phosphatase; RAR, required for Mla12 resistance; SGT, suppressor of the G2 allele of skp1; SKP, S phase kinase‐associated protein. References: 1, Warren et al. (1999; 2, Swiderski & Innes (2001; 3, Shao et al. (2003; 4, Mackey et al. (2003; 5, Deslandes et al. (2003; 6, Liu et al. (2002b); 7, Hubert et al. (2003; 8, Rathjen et al. (1999; 9, Mucyn et al. (2006; 10, de la Fuente van Bentem et al. (2005); 11, Liu et al. (2004; 12, Hubert et al. (2009; 13, Boter et al. (2007; 14, Azevedo et al. (2002; 15, Saunders et al. (2012.
Table 3.
Further components of effector‐triggered immunity (ETI) in different pathosystems
| ETI component | Species | Reference(s) |
|---|---|---|
| COl1 | Solanum lycopersicum | Ekengren et al. (2003) |
| L19 (ribosomal protein) | S. lycopersicum | Gabriels et al. (2006) |
| MAPKKs (MEK1, MEK2), MAPKs | S. lycopersicum | Ekengren et al. (2003) |
| NPR1 | S. lycopersicum | Ekengren et al. (2003) |
| NTF6 | S. lycopersicum | Ekengren et al. (2003) |
| RCR3 | S. lycopersicum | Dixon et al. (2000) |
| TGA1a, TGA2.2 | S. lycopersicum | Ekengren et al. (2003) |
| WIPK | S. lycopersicum | Ekengren et al. (2003) |
| BSL1 | S. lycopersicum, Nicotiana benthamiana | Saunders et al. (2012) |
| EDS1 | N. benthamiana | Peart et al. (2002) |
| L30 (ribosomal protein) | N. benthamiana | Lu et al. (2003) |
| MAPKK (MEK2) | N. benthamiana | Jin et al. (2003) |
| NRG1 | N. benthamiana | Peart et al. (2005) |
| SIPK | N. benthamiana | Jin et al. (2002, 2003) |
| snRNA associated proteins | N. benthamiana | Lu et al. (2003) |
| WIPK | N. benthamiana | Jin et al. (2002, 2003) |
| RAR2 | Hordeum vulgare | Jørgensen (1988, 1996), Freialdenhoven et al. (1994) |
| WRKY1, WRKY2 | H. vulgare | Shen et al. (2007) |
| MPK6 | Arabidopsis thaliana | Menke et al. (2004) |
| NIM1 | A. thaliana | Delaney et al. (1995) |
| PBS2, PBS3 | A. thaliana | Warren et al. (1999), Swiderski & Innes (2001) |
| EDS1 | A. thaliana | Aarts et al. (1998), Falk et al. (1999), Parker et al. (1996) |
| NDR1 | A. thaliana | Aarts et al. (1998), Hubert et al. (2003) |
| NPR1 | A. thaliana | Parker et al. (1996) |
| PAD4 | A. thaliana | Feys & Parker (2000), Parker et al. (2000), Austin et al. (2002) |
| Salicylic acid | A. thaliana | Delaney et al. (1994), Mauch‐Mani & Slusarenko (1996) |
Calcium influx and apoplastic alkalinization (Piedras et al., 1998), the activation of mitogen‐activated protein kinases (MAPKs; Ligterink et al., 1997; Romeis et al., 1999), the production of reactive oxygen species (ROS; Piedras et al., 1998) and transcriptional reprogramming within 30 min (Durrant et al., 2000) have been postulated as the earliest events in R‐gene‐based resistance in plants. Suppression of the host HR by RXLR effectors is presumably crucial during the early biotrophic stage of infection by hemibiotrophs (Tyler, 2009).
Several studies have shed light on Rpi‐blb1‐mediated late blight defence mechanisms (Song et al., 2003; Vleeshouwers et al., 2008). Although the level of transcripts encoding PR‐1b, PR‐2a, PR‐5 and the HR‐associated Hin1 increased moderately in susceptible and RB‐harbouring partially resistant plants for only roughly 48 hpi and then remained elevated, an increase in transcript levels of the same genes was observed for 96 hpi in the resistant cultivar carrying R9 (Chen and Halterman, 2011). As the timing of HR induction and the onset of PR gene expression were similar in partially resistant RB plants and immune R9 plants, the same authors proposed that partial resistance genes, such as RB, could trigger molecular mechanisms similar to SRpigs, which confer immunity, but may differ in timing and/or intensity of the elicited defence responses.
Potato plants of the cultivar Katahdin transformed with the RB gene differed from untransformed Katahdin plants by a consistent, instead of a decreasing, protein level of ribulose bisphosphate carboxylase small chain 2A, by the lack of the oxygen‐evolving enhancer protein 1, by an increase, instead of constant, ascorbate peroxidase (APX) levels, and by larger amounts of Qor‐like protein after inoculation with P. infestans (Liu and Halterman, 2009).
The R‐gene‐based resistance may result from rapid post‐infectional biosynthesis of antimicrobial phytoalexins (Ingham, 1973; Müller and Börger, 1940), which, in the Solanaceae family, include polyacetylenes, coumarins, stilbenoids, isoflavans, isoflavones and sesquiterpenoids (Harborne, 1999; Pedras and Ahiahonu, 2005), such as capsidiol (Shibata et al., 2010).
Although StCathB transcript levels peaked at 15 hpi in the incompatible potato–late blight interaction, they slowly increased during 72 hpi in the compatible interaction. Although the induction of components of the 9‐lipoxygenase (9‐LOX) pathway, which produces several oxylipins toxic to P. infestans (Prost et al., 2005), has been shown (Kolomiets et al., 2000), R1‐based resistance of S. tuberosum to P. infestans remained unaffected by RNAi‐mediated down‐regulation of key enzymes of the 9‐ and 13‐LOX‐derived oxylipin pathways, namely 9‐LOX, 9‐divinyl ether synthase, allene oxide cyclase, 12‐oxophytodienoic acid reductase 3 and coronatine‐insensitive 1, suggesting that neither 9‐LOX‐derived oxylipins nor jasmonic acid are essential for R1‐based resistance of potato (Eschen‐Lippold et al., 2010).
Detection Specificity
High sequence similarity between R genes or R proteins does not necessarily imply close taxonomic specificity. In the Solanum spp.–P. infestans system, as well as in other plant–pathogen pathosystems (Grube et al., 2000), identical or slightly altered taxonomic specificity may result from minor changes in R‐gene sequence (Champouret, 2010; Li et al., 2011; Lokossou et al., 2009, 2010; Pel et al., 2009; Vleeshouwers et al., 2008), as well as from larger ones, as in the case of R3a and R3b, sharing only 65%, and Rpi‐blb1 and Rpi‐bt1, sharing only 78%, amino acid identity (Oosumi et al., 2009). However, Rpi‐blb2 and Mi‐1 share 82% amino acid identity and confer resistance to such different organisms as P. infestans and nematodes, aphids and white fly (van der Vossen et al., 2005), and Rx1 and Gpa2 of potato share 88% identity, conferring resistance to either Potato virus X (PVX) or Globodera pallida (van der Vossen et al., 2000). In remarkable contrast with the idea that broad‐spectrum resistance results from an R protein guarding the target of multiple effectors (Nombela et al., 2003; Vos et al., 1998), the broad‐spectrum SRpip RB interacts directly with IPI‐O1 and IPI‐O4, whereas narrow‐spectrum R2‐based resistance requires association with a third protein, BSL1.
Some SRpips recognize multiple Avr proteins (van Poppel et al., 2009a), and some Avr proteins elicit responses by several different SRpips (Lokossou et al., 2009, 2010; Vleeshouwers et al., 2008). Both phenomena may possibly result from ancestral effectors or R genes after taxonomic diversification events. Mechanistically, the detection of multiple effectors could theoretically arise from: (i) the structural similarity of effectors; (ii) a common bait of these effectors; or (iii) several different baits being associated with the same R protein. The modern discipline of effectoromics examines and instrumentalizes R–Avr interaction specificities to screen for new R genes (Vleeshouwers et al., 2008); hitherto explored activating (incompatible) R–Avr pairs were listed recently by Champouret (2010), and a more detailed and comprehensive overview can be found elsewhere (Halterman et al., 2010; Morgan and Kamoun, 2007; Oh et al., 2009, 2010).
Molecular Basis of Avirulence and Virulence of Effectors
The RXLR domain is not required for elicitor activity of PiAvr4 (van Poppel et al., 2008) or Avr3a (Bos et al., 2006). The C‐terminal half of effectors and its W motifs obviously play a prominent role in (a)virulence activity. A proline at amino acid 129, which is located within the W motif, has been demonstrated to be a determinant of virulence for IPI‐O1 and IPI‐O4 (Chen et al., 2012) and, within a putative recombination between an IPI‐O4 and another IPI‐O family member (Halterman et al., 2010), it is assumed to increase the aggressiveness of the Guatemalan isolate 68 (Chen et al., 2012). The dependence of virulence on the W motif has also been shown for both Avr3a and Avr4.
With position 103 placed within the single W motif (Dou et al., 2008a), a two‐amino‐acid change from K80I103 to E80M103 reduced the avirulence activity of Avr3a (Armstrong et al., 2005; Bos et al., 2010), the relocalization of Avr3a and R3a to late endosomes (Engelhardt et al., 2012) and the suppression of INF1‐triggered cell death (Bos et al., 2010). PiAvr4 contains the three W motifs W1–W3, of which W2, in combination with either W1 or W3, is required to trigger an R4‐based HR, therefore determining virulence (van Poppel et al., 2009a). The virulence of P. infestans isolates towards potatoes harbouring R4, however, is also caused by frameshift mutations and truncations, also indicating major changes, but not the 27 single amino acid changes in Avr4, as the divergence of P. infestans and P. mirabilis served to remove PiAvr4 activity (van Poppel et al., 2008, 2009a, b). Likewise, the C‐terminal region determines the virulence or avirulence activity of RXLR‐dEER effector Avrblb2 from P. infestans, and Avr1b from P. sojae (Bos et al., 2006; Oh et al., 2009): Mutations from Val69, Ala69 or Ile69 to Phe69 in Avrblb2 caused a lack of avirulence activity (Oh et al., 2009).
Variations in 10 amino acids within the C‐terminal region, as well as truncations affecting the WD40 domain, also caused avirulence of the putative transcription factor pi3.4 (Qutob et al., 2006).
Conclusions
The draft genomes of several Phytophthora spp. have enabled the identification and examination of numerous effector candidates (Haas et al., 2009; Jiang et al., 2008; Raffaele et al., 2010; Wang et al., 2008). Recently, the first crystal structure and solution structure models of Phytophthora effectors have been published (Boutemy et al., 2011; Sun et al., 2013; Yaeno et al., 2011), and remote homology modelling has enabled the prognosis of the tertiary structure of homologous effectors. Based on the first models (Takken and Goverse, 2012), the tertiary structure of SRpips from the comprehensive candidate lists for several genomes (Andolfo et al., 2013; Jupe et al., 2012; Tomato Genome Consortium, 2012) may be estimated. The continued structural classification of effectors and R proteins holds the potential to increase the efficiency of effector screens. These will be required for precise and rapid verification of effector and SRpig candidates. The identification of only 68 functional SRpigs to date, compared with the large number of candidates and the existence of roughly 1500 Solanum spp., indicates the presumably widely unexploited potential of SRpig‐mediated resistance. For the identification of further interaction partners in ETI, yeast two‐hybrid screens with SRpips, effectors, ligands such as BSL1, PI(3)P, chaperones, and combinations thereof, may be helpful. This will possibly also lead to the recognition of further components, such as SGT1 and RAR1. The roles of predicted glycosylation, myristoylation and phosphorylation sites (Ballvora et al., 2002; van der Vossen et al., 2003) remain to be elucidated. A major challenge will be the analysis of the steric interaction of effectors and SRpips; the analysis of combinations with presumably direct interaction (e.g. Rpi‐blb1, Avr‐blb1) could precede the analysis of increasingly complex interactions of several molecules (e.g. R2, BSL1, PiAvr2). Supplemented by functional analyses with targeted mutations, structures essential for effector reception and activity will become more apparent. The combination of targeted mutation‐based findings with interaction models will also enable better insight into the functions of domains and conserved motifs, such as the K motif, which is frequently found in the 1b3a family (Sun et al., 2013).
The availability of completely sequenced genomes (Potato Genome Sequencing Consortium, 2011; Tomato Genome Consortium, 2012) has enabled further approaches. Jupe et al. (2012) classified the potato CNL candidates into nine subgroups, several of which do not contain any functionally characterized SRpigs. Do SRpigs exist which, in contrast with the hitherto functionally analysed SRpigs, do not belong to subgroups 1, 4, 5, 6 or 8? Given the hitherto limited structural discernibility of SRpigs and other CNLs, a comparison of SRpigs with non‐SRpig R genes may possibly reveal further structural characteristics typical for SRpigs. Do SRpips exist, which belong to other classes, such as the TNL, RLK or RLP class? Further examination of determined resistance loci and cloning of SRpigs, such as Rpi‐cap1, Rpi‐qum1, Rpi‐avl1 and R4, which share a locus with the TNL class gene N on chromosome XI, will possibly answer this question.
Furthermore, the sequenced genomes will provide further insight into the evolution of R genes, and therefore allow conclusions to be drawn on which evolutionary processes have proved to be advantageous.
SRpigs are not distributed evenly, but cluster together at genomic positions well conserved between Solanum spp., enabling approaches, such as synteny‐based gene localization and isolation, to be employed. Genomic locations of SRpigs and RGAs are not independent from, but associated with, late maturity, as is the case for quantitative resistance. However, the fact that very early cultivars harbouring R1 or R3 exist suggests the existence of Rpi loci without linkage to maturity. Do other genes involved in ETI signalling or defence possibly co‐localize to R‐gene clusters?
According to the guard model and the decoy model, the recognition specificity would not necessarily depend solely on R proteins. Instead, secondary molecules functioning as baits could adapt the switch feature of R proteins to diverse effector topologies. Theoretically, recognition capacity might therefore be multiplied, limited by the number and affinity of the bait molecules. Conversely, several R genes could associate with the same bait, which possibly could be the case for BSL1 and PiAvr2. PiAvr2 is recognized by R2, R2‐like, Rpi‐abpt, Rpi‐blb3, Rpi‐edn1.1, Rpi‐snk1.1, Rpi‐snk1.2 and Rpi‐hjt1.1–Rpi‐hjt1.3. These receptors partially share only 92.1% amino acid identity (Champouret, 2010). For Rpi‐blb1 and IPI‐O, however, direct interaction has been observed in vitro (Chen et al., 2012). In contrast with the receptors detecting PiAvr2, these receptors (Rpi‐blb1, Rpi‐sto1, Rpi‐pta1) share at least 99.6% amino acid identity. Similarly, receptors detecting Avr3a (R3a, Rpi‐sto2) and Avr‐vnt1 (Rpi‐vnt1.1–Rpi‐vnt1.3) share at least 99.7% and 98.2% amino acid identity, respectively. This could possibly be an indication for a more direct interaction between SRpigs and effectors in comparison with the case of PiAvr2. However, initial experiments did not reveal a direct interaction of R3a and Avr3a (Engelhardt et al., 2012)
Interaction sites of numerous effectors which suppress both PTI and ETI (Wang et al., 2011) remain to be determined. Depending on the oomycete–host pathosystem, PRRs are selectively absent from the extrahaustorial matrix (Koh et al., 2005; Lu et al., 2012; Micali et al., 2011), evoking the question of whether some effectors (Oh et al., 2009; Wang et al., 2011) might possibly interfere with PRR synthesis and/or localization.
Finally, there is the need to examine the relevance and effects of the various putative influences on transcript and protein levels (Cooke et al., 2012). Very recently, evidence has been found that host‐ and pathogen‐owned RNAi mechanisms could affect both R genes (Tomato Genome Consortium, 2012) and effectors (Vetukuri et al., 2012), and that some effectors may suppress the host‐encoded RNA silencing machinery (Qiao et al., 2013). Our knowledge on SRpigs and RXLR effectors has increased tremendously during the last decade. However, many issues of R‐protein–effector interaction, signalling and defence remain to be elucidated. The role of CRN and other non‐RXLR effectors remains largely unexplored, and Rpi gene‐based resistance remains an important and exciting field of research.
References
- Aarts, N. , Metz, M. , Holub, E. , Staskawicz, B.J. , Daniels, M.J. and Parker, J.E. (1998) Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene‐mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA, 95, 10 306–10 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht, M. and Takken, F.L.W. (2006) Update on the domain architectures of NLRs and R proteins. Biochem. Biophys. Res. Commun. 339, 459–462. [DOI] [PubMed] [Google Scholar]
- Allefs, J.J.H. , Muskens, M.W.M. and van der Vossen, E.A.G. (2005) Breeding for foliage late blight resistance in the genomics era In: Potato in Progress. Science Meets Practice (Haverkort A.J. and Struik P.C., eds), pp. 255–267. Wageningen: Wageningen Academic Publishers. [Google Scholar]
- Andolfo, G. , Sanseverino, W. , Rombauts, S. , van de Peer, Y. , Bradeen, J.M. , Carputo, D. , Frusciante, L. and Ercolano, M.R. (2013) Overview of tomato (Solanum lycopersicum) candidate pathogen recognition genes reveals important Solanum R locus dynamics. New Phytol. 197, 223–237. [DOI] [PubMed] [Google Scholar]
- Andrivon, D. , Corbière, R. , Lucas, J.‐M. , Pasco, C. , Gravoueille, J.‐M. , Pellé, R. , Dantec, J. and P. and Ellissèche, D. (2003) Resistance to late blight and soft rot in six potato progenies and glycoalkaloid contents in the tubers. Am. J. Potato Res. 80, 125–134. [Google Scholar]
- Armstrong, M.R. , Whisson, S.C. , Pritchard, L. , Bos, J.I. , Venter, E. , Avrova, A.O. , Rehmany, A.P. , Böhme, U. , Brooks, K. , Cherevach, I. , Hamlin, N. , White, B. , Fraser, A. , Lord, A. , Quail, M.A. , Churcher, C. , Hall, N. , Berriman, M. , Huang, S. , Kamoun, S. , Beynon, J.L. and Birch, P.R.J. (2005) An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proc. Natl. Acad. Sci. USA, 102, 7766–7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin, M.J. , Muskett, P. , Kahn, K. , Feys, B.J. , Jones, J.D.G. and Parker, J.E. (2002) Regulatory role of SGT1 in early R gene‐mediated plant defenses. Science, 295, 2077–2080. [DOI] [PubMed] [Google Scholar]
- Azevedo, C. , Sadanandom, A. , Kitagawa, K. , Freialdenhoven, A. , Shirasu, K. and Schulze‐Lefert, P. (2002) The RAR1 interactor SGT1, an essential component of R gene‐triggered disease resistance. Science, 295, 2073–2076. [DOI] [PubMed] [Google Scholar]
- Bakker, E. , Butterbach, P. , Rouppe van der Voort, J. , van der Vossen, E. , van Vliet, J. , Bakker, J. and Goverse, A. (2003) Genetic and physical mapping of homologues of the virus resistance gene Rx1 and the cyst nematode resistance gene Gpa2 in potato. Theor. Appl. Genet. 106, 1524–1531. [DOI] [PubMed] [Google Scholar]
- Bakker, E. , Borm, T. , Prins, P. , van der Vossen, E. , Uenk, G. , Arens, M. , de Boer, J. , van Eck, H. , Muskens, M. , Vossen, J. , van der Linden, G. , van Ham, R. , Klein‐Lankhorst, R. , Visser, R. , Smant, G. , Bakker, J. and Goverse, A. (2011) A genome‐wide genetic map of NB‐LRR disease resistance loci in potato. Theor. Appl. Genet. 123, 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballvora, A. , Ercolano, M.R. , Weiss, J. , Meksem, K. , Bormann, C.A. , Oberhagemann, P. , Salamini, F. and Gebhardt, C. (2002) The R1 gene for potato resistance to late blight (Phytophthora infestans) belongs to the leucine zipper/NBS/LRR class of plant resistance genes. Plant J. 30, 361–371. [DOI] [PubMed] [Google Scholar]
- Belkhadir, Y. , Subramaniam, R. and Dangl, J.L. (2004) Plant disease resistance protein signaling: NBS‐LRR proteins and their partners. Curr. Opin. Plant Biol. 7, 391–399. [DOI] [PubMed] [Google Scholar]
- Bendahmane, A. , Farnham, G. , Moffett, P. and Baulcombe, D.C. (2002) Constitutive gain‐of‐function mutants in a nucleotide binding site‐leucine rich repeat protein encoded at the Rx locus of potato. Plant J. 32, 195–204. [DOI] [PubMed] [Google Scholar]
- Bhaskar, P.B. , Raasch, J.A. , Kramer, L.C. , Neumann, P. , Wielgus, S.M. , Austin‐Phillips, S. and Jiang, J. (2008) Sgt1, but not Rar1, is essential for the RB‐mediated broad‐spectrum resistance to potato late blight. BMC Plant Biol. 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar, P.B. , Venkateshwaran, M. , Wu, L. , Ane, J.M. and Jiang, J. (2009) Agrobacterium‐mediated transient gene expression and silencing: a rapid tool for functional gene assay in potato. PLoS ONE, 4, e5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee, S. , Stahelin, R.V. , Speicher, K.D. , Speicher, D.W. and Haldar, K. (2012a) Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell. Cell, 148, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee, S. , Stahelin, R.V. and Haldar, K. (2012b) Host targeting of virulence determinants and phosphoinositides in blood stage malaria parasites. Trends Parasitol. 28, 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Biezen, E.A. and Jones, J.D.G. (1998a) The NB‐ARC domain: a novel signalling motif shared by plant resistance gene products and regulators of cell death in animals. Curr. Biol. 8, R226–R228. [DOI] [PubMed] [Google Scholar]
- van der Biezen, E.A. and Jones, J.D.G. (1998b) Plant disease‐resistance proteins and the gene‐for‐gene concept. Trends Biochem. Sci. 23, 454–456. [DOI] [PubMed] [Google Scholar]
- Black, W. , Mastenbroek, C. , Mills, W.R. and Peterson, L.C. (1953) A proposal for an international nomenclature of races of Phytophthora infestans and of genes controlling immunity in Solanum demissum derivatives. Euphytica, 2, 173–179. [Google Scholar]
- Bos, J.I. , Kanneganti, T.D. , Young, C. , Cakir, C. , Huitema, E. , Win, J. , Armstrong, M.R. , Birch, P.R. and Kamoun, S. (2006) The C‐terminal half of Phytophthora infestans RXLR effector AVR3a is sufficient to trigger R3a‐mediated hypersensitivity and suppress INF1‐induced cell death in Nicotiana benthamiana . Plant J. 48, 165–176. [DOI] [PubMed] [Google Scholar]
- Bos, J.I.B. , Armstrong, M.R. , Gilroy, E.M. , Boevink, P.C. , Hein, I. , Rosalind, M.T. , Zhendong, T. , Engelhardt, S. , Vetukuri, R.R. , Harrower, B. , Dixelius, C. , Bryan, G. , Sadanandom, A. , Whisson, S.C. , Kamoun, S. and Birch, P.R.J. (2010) Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc. Natl. Acad. Sci. USA, 107, 9909–9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boter, M. , Amigues, B. , Peart, J. , Breuer, C. , Kadota, Y. , Casais, C. , Moore, G. , Kleanthous, C. , Ochsenbein, F. , Shirasu, K. and Guerois, R. (2007) Structural and functional analysis of SGT1 reveals that its interaction with HSP90 is required for the accumulation of Rx, an R protein involved in plant immunity. Plant Cell, 19, 3791–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutemy, L.S. , King, S.R. , Win, J. , Hughes, R.K. , Clarke, T.A. , Blumenschein, T.M. , Kamoun, S. and Banfield, M.J. (2011) Structures of Phytophthora RXLR effector proteins: a conserved but adaptable fold underpins functional diversity. J. Biol. Chem. 286, 35 834–35 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, L.A. , Ridout, C. , O'Sullivan, D.M. , Leach, J.E. and Leung, H. (2012) Plant–pathogen interactions: disease resistance in modern agriculture. Trends Genet. 29, 233–240. [DOI] [PubMed] [Google Scholar]
- Boyes, D.C. , Nam, J. and Dangl, J.L. (1998) The Arabidopsis thaliana RPM1 disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response. Proc. Natl. Acad. Sci. USA, 95, 15 849–15 854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko, A. , Kathiria, P. , Zemp, F.J. , Yao, Y. , Pogribny, I. and Kovalchuk, I. (2007) Transgenerational changes in the genome stability and methylation in pathogen‐infected plants: (virus‐induced plant genome instability). Nucleic Acids Res. 35, 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt, T.O. , Schornack, S. , Win, J. , Shindo, T. , Ilyas, M. , Oliva, R. , Cano, L.M. , Jones, A.M.E. , Huitema, E. , van der Hoorn, R.A.L. and Kamoun, S. (2011) Phytophthora infestans effector AVRblb2 prevents secretion of a plant immune protease at the haustorial interface. Proc. Natl. Acad. Sci. USA, 108, 20 832–20 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradeen, J.M. , Iorizzo, M. , Mollov, D.S. , Raasch, J. , Kramer, L.C. , Millett, B.P. , Austin‐Phillips, S. , Jiang, J. and Carputo, D. (2009) Higher copy numbers of the potato RB transgene correspond to enhanced transcript and late blight resistance levels. Mol. Plant–Microbe Interact. 22, 437–446. [DOI] [PubMed] [Google Scholar]
- Bradshaw, J.E. , Bryan, G.J. , Lees, A.K. , McLean, K. and Solomon‐Blackburn, R.M. (2006) Mapping the R10 and R11 genes for resistance to late blight (Phytophthora infestans) present in the potato (Solanum tuberosum) R‐gene differentials of Black. Theor. Appl. Genet. 112, 744–751. [DOI] [PubMed] [Google Scholar]
- Brugmans, B. , Wouters, D. , van Os, H. , Hutten, R. , van der Linden, G. , Visser, R.G.F. , van Eck, H.J. and van der Vossen, E.A.G. (2008) Genetic mapping and transcription analyses of resistance gene loci in potato using NBS profiling. Theor. Appl. Genet. 117, 1379–1388. [DOI] [PubMed] [Google Scholar]
- Burch‐Smith, T.M. , Schiff, M. , Caplan, J.L. , Tsao, J. , Czymmek, K. and Dinesh‐Kumar, S.P. (2007) A novel role for the TIR domain in association with pathogen‐derived elicitors. PLoS Biol. 5, e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champouret, N. (2010) Functional genomics of Phytophthora infestans effectors and Solanum resistance genes. PhD Thesis, Wageningen University, Wageningen.
- Chen, Y. and Halterman, D.A. (2011) Phenotypic characterization of potato late blight resistance mediated by the broad‐spectrum resistance gene RB . Phytopathology, 101, 263–270. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Liu, Z. and Halterman, D.A. (2012) Molecular determinants of resistance activation and suppression by Phytophthora infestans effector IPI‐O. PLoS Pathog. 8, e1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunwongse, J. , Chunwongse, C. , Black, L. and Hanson, P. (2002) Molecular mapping of the Ph‐3 gene for late blight resistance in tomato. J. Hort. Sci. Biotechnol. 77, 281–286. [Google Scholar]
- Collins, A. , Milbourne, D. , Ramsay, L. , Meyer, R. , Chatot‐Balandras, C. , Oberhagemann, P. , De Jong, W. , Gebhardt, C. , Bonnel, E. and Waugh, R. (1999) QTL for field resistance to late blight in potato are strongly correlated with maturity and vigour. Mol. Breed. 5, 387–398. [Google Scholar]
- Colon, L.T. , Budding, D.J. , Keizer, L.C.P. and Pieters, M.M.J. (1995a) Components of resistance to late blight (Phytophthora infestans) in eight South American Solanum species. Eur. J. Plant Pathol. 101, 441–456. [Google Scholar]
- Colon, L.T. , Jansen, R.C. and Budding, D.J. (1995b) Partial resistance to late blight (Phytophthora infestans) in hybrid progenies of four South American Solanum species crossed with diploid S. tuberosum . Theor. Appl. Genet. 90, 691–698. [DOI] [PubMed] [Google Scholar]
- Cooke, D.E. , Cano, L.M. , Raffaele, S. , Bain, R.A. , Cooke, L.R. , Etherington, G.J. , Deahl, K.L. , Farrer, R.A. , Gilroy, E.M. , Goss, E.M. , Grünwald, N.J. , Hein, I. , MacLean, D. , McNicol, J.W. , Randall, E. , Oliva, R.F. , Pel, M.A. , Shaw, D.S. , Squires, J.N. , Taylor, M.C. , Vleeshouwers, V.G. , Birch, P.R. , Lees, A.K. and Kamoun, S. (2012) Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLoS Pathog. 8, e1002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch, B.C. , Spangler, R. , Ramos, C. and May, G. (2006) Pervasive purifying selection characterizes the evolution of I2 homologs. Mol. Plant–Microbe Interact. 19, 288–303. [DOI] [PubMed] [Google Scholar]
- la Cour, T. , Kiemer, L. , Mølgaard, A. , Gupta, R. , Skriver, K. and Brunak, S. (2004) Analysis and prediction of leucine‐rich nuclear export signals. Protein Eng. Des. Sel. 17, 527–536. [DOI] [PubMed] [Google Scholar]
- Damasceno, C.M.B. , Bishop, J.G. , Ripoll, D.R. , Win, J. , Kamoun, S. and Rose, J.K.C. (2008) Structure of the Glucanase Inhibitor Protein (GIP) family from Phytophthora species suggests coevolution with plant endo‐β‐1,3‐glucanases. Mol. Plant–Microbe Interact. 21, 820–830. [DOI] [PubMed] [Google Scholar]
- van Damme, M. , Bozkurt, T.O. , Cakir, C. , Schornack, S. , Sklenar, J. , Jones, A.M. and Kamoun, S. (2012) The Irish potato famine pathogen Phytophthora infestans translocates the CRN8 kinase into host plant cells. PLoS Pathog. 8, e1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danan, S. , Veyrieras, J.‐B. and Lefebvre, V. (2011) Construction of a potato consensus map and QTL meta‐analysis offer new insights into the genetic architecture of late blight resistance and plant maturity traits. BMC Plant Biol. 11, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl, J.L. and Jones, J.D.G. (2001) Plant pathogens and integrated defence responses to infection. Nature, 411, 826–833. [DOI] [PubMed] [Google Scholar]
- Delaney, T.P. , Uknes, S. , Vernooij, B. , Friedrich, L. , Weymann, K. , Negrotto, D. , Gaffney, T. , Gut‐Rella, M. , Kessmann, H. , Ward, E. and Ryals, J. (1994) A central role of salicylic acid in plant disease resistance. Science, 266, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Delaney, T.P. , Friedrich, L. and Ryals, J. (1995) Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc. Natl. Acad. Sci. USA, 92, 6602–6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes, L. and Rivas, S. (2011) The plant cell nucleus: a true arena for the fight between plants and pathogens. Plant Signal Behav. 6, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes, L. , Olivier, J. , Peeters, N. , Feng, D.X. , Khounlotham, M. , Boucher, C. , Somssich, I. , Genin, S. and Marco, Y. (2003) Physical interaction between RRS1‐R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc. Natl. Acad. Sci. USA, 100, 8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixelius, C. , Fagerström, T. and Sundström, J.F. (2012) European agricultural policy goes down the tubers. Nature Biotechnol. 30, 492–493. [DOI] [PubMed] [Google Scholar]
- Dixon, M.S. , Golstein, C. , Thomas, C.M. , van Der Biezen, E.A. and Jones, J.D. (2000) Genetic complexity of pathogen perception by plants: the example of Rcr3, a tomato gene required specifically by Cf‐2. Proc. Natl. Acad. Sci. USA, 97, 8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds, P.N. , Lawrence, G.J. and Ellis, J.G. (2001) Six amino acid changes confined to the leucine‐rich repeat beta‐strand/beta‐turn motif determine the difference between the P and P2 rust resistance specificities in flax. Plant Cell, 13, 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds, P.N. , Lawrence, G.J. , Catanzariti, A.M. , Teh, T. , Wang, C.‐I.A. , Ayliffe, M.A. , Kobe, B. and Ellis, J.G. (2006) Direct protein interaction underlies gene‐for‐gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl. Acad. Sci. USA, 103, 8888–8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou, D. , Kale, S.D. , Wang, X. , Chen, Y. , Wang, Q. , Jiang, R.H.Y. , Arredondo, F.D. , Anderson, R.G. , Thakur, P.B. , McDowell, J.M. , Wang, Y. and Tyler, B. (2008a) Conserved C‐terminal motifs required for avirulence and suppression of cell death by Phytophthora sojae effector Avr1b. Plant Cell, 20, 1118–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth, A. , Goodwin, S.B. , Fry, W.E. and Davidse, L.C. (1993) Genotypic diversity of Phytophthora infestans in The Netherlands revealed by DNA polymorphisms. Phytopathology, 83, 1087–1092. [Google Scholar]
- Drenth, A. , Tas, I.C.Q. and Govers, F. (1994) DNA fingerprinting uncovers a new sexually reproducing population of Phytophthora infestans in the Netherlands. Eur. J. Plant Pathol. 100, 97–107. [Google Scholar]
- Durrant, W.E. , Rowland, O. , Piedras, P. , Hammond‐Kosack, K.E. and Jones, J.D.G. (2000) cDNA‐AFLP reveals a striking overlap in race‐specific resistance and wound response gene expression profiles. Plant Cell, 12, 963–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekengren, S.K. , Liu, Y. , Schiff, M. , Dinesh‐Kumar, S.P. and Martin, G.B. (2003) Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto‐mediated disease resistance in tomato. Plant J. 36, 905–917. [DOI] [PubMed] [Google Scholar]
- El‐Kharbotly, A. , Palomino‐Sánchez, C. , Salamini, F. , Jacobsen, E. and Gebhardt, C. (1996) R6 and R7 alleles of potato conferring race‐specific resistance to Phytophthora infestans (Mont.) de Bary identified genetic loci clustering with the R3 locus on chromosome XI. Theor. Appl. Genet. 92, 880–884. [DOI] [PubMed] [Google Scholar]
- Ellis, J.G. , Lawrence, G.J. , Luck, J.E. and Dodds, P.N. (1999) Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene‐for‐gene specificity. Plant Cell, 11, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, J.G. , Dodds, P.N. and Lawrence, G.J. (2007) Flax rust resistance gene specificity is based on direct resistance–avirulence protein interactions. Annu. Rev. Phytopathol. 45, 289–306. [DOI] [PubMed] [Google Scholar]
- Engelhardt, S. , Boevink, P.C. , Armstrong, M.R. , Ramos, M.B. , Hein, I. and Birch, P.R. (2012) Relocalization of late blight resistance protein R3a to endosomal compartments is associated with effector recognition and required for the immune response. Plant Cell, 24, 5142–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkhbayar, P. , Kamiya, M. , Osaki, M. , Matsumoto, T. and Matsushima, N. (2004) Structural principles of leucine‐rich repeat (LRR) proteins. Proteins, 54, 394–403. [DOI] [PubMed] [Google Scholar]
- Eschen‐Lippold, L. , Altmann, S. , Gebhardt, C. , Göbel, C. , Feussner, I. and Rosahl, S. (2010) Oxylipins are not required for R gene‐mediated resistance in potato. Eur. J. Plant Pathol. 127, 437–442. [Google Scholar]
- Falk, A. , Feys, B.J. , Frost, L.N. , Jones, J.D.G. , Daniels, M.J. and Parker, J.E. (1999) EDS1, an essential component of R gene‐mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad. Sci. USA, 96, 3292–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys, B.J. and Parker, J.E. (2000) Interplay of signaling pathways in plant disease resistance. Trends Genet. 16, 449–455. [DOI] [PubMed] [Google Scholar]
- Flor, H.H. (1971) Current status of the gene‐for‐gene concept. Annu. Rev. Phytopathol. 71, 275–296. [Google Scholar]
- Foster, S.J. , Park, T.H. , Pel, M. , Brigneti, G. , Sliwka, J. , Jagger, L. , van der Vossen, E. and Jones, J.D. (2009) Rpi‐vnt1.1, a Tm‐22 homolog from Solanum venturii, confers resistance to potato late blight. Mol. Plant–Microbe Interact. 22, 589–600. [DOI] [PubMed] [Google Scholar]
- Freialdenhoven, A. , Scherag, B. , Hollricher, K. , Collinge, D.B. , Thordal‐Christensen, H. and Schulze‐Lefert, P. (1994) Nar‐1 and Nar‐2, two loci required for Mla12‐specified race‐specific resistance to powdery mildew in barley. Plant Cell, 6, 983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry, W.E. , Goodwin, S.B. , Matuszak, J.M. , Spielman, L.J. , Milgroom, M.G. and Drenth, A. (1992) Population genetics and intercontinental migrations of Phytophthora infestans . Annu. Rev. Phytopathol. 30, 107–129. [Google Scholar]
- de la Fuente van Bentem, S. , Vossen, J.H. , de Vries, K.J. , van Wees, S. , Tameling, W.I.L. , Dekker, H.L. , de Koster, C.G. , Haring, M.A. , Takken, F.L.W. and Cornelissen, B.J.C. (2005) Heat shock protein 90 and its co‐chaperone protein phosphatase 5 interact with distinct regions of the tomato I‐2 disease resistance protein. Plant J. 43, 284–298. [DOI] [PubMed] [Google Scholar]
- Gabriels, S.H.E. , Takken, F.L.W. , Vossen, J.H. , de Jong, C.F. , Liu, Q. , Turk, S.C.H.J. , Wachowski, L.K. , Peters, J. , Witsenboer, H.M.A. , de Wit, P.J.G.M. and Joosten, M.H.A.J. (2006) cDNA‐AFLP combined with functional analysis reveals novel genes involved in the hypersensitive response. Mol. Plant–Microbe Interact. 19, 567–576. [DOI] [PubMed] [Google Scholar]
- Gan, P.H. , Rafiqi, M. , Ellis, J.G. , Jones, D.A. , Hardham, A.R. and Dodds, P.N. (2010) Lipid binding activities of flax rust AvrM and AvrL567 effectors. Plant Signal Behav. 5, 1272–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann, W. , Hinsch, M.E. and Staskawicz, B.J. (1999) The Arabidopsis RPS4 bacterial‐resistance gene is a member of the TIR‐NBS‐LRR family of disease‐resistance genes. Plant J. 20, 265–277. [DOI] [PubMed] [Google Scholar]
- Gebhardt, C. (2004) Potato genetics: molecular maps and more in biotechnology in agriculture and forestry In: Molecular Marker Systems, Vol. 55 (Nagata T., Lörz H., Widholm J.M., eds), pp. 215–227. Berlin, Heidelberg: Springer‐Verlag. [Google Scholar]
- Gebhardt, C. and Valkonen, J.P. (2001) Organization of genes controlling disease resistance in the potato genome. Annu. Rev. Phytopathol. 39, 79–102. [DOI] [PubMed] [Google Scholar]
- GILB (1999) Late blight: a threat to global food security. In: Proceedings of the Global Initiative on Late Blight Conference, March 16–19, 1999, Quito, Ecuador .
- Goggin, F.L. , Shah, G. , Williamson, V.M. and Ullman, D.E. (2004) Developmental regulation of Mi‐mediated aphid resistance is independent of Mi‐1.2 transcript levels. Mol. Plant–Microbe Interact. 17, 532–536. [DOI] [PubMed] [Google Scholar]
- Göhre, V. and Robatzek, S. (2008) Breaking the barriers: microbial effector molecules subvert plant immunity. Annu. Rev. Phytopathol. 46, 189–215. [DOI] [PubMed] [Google Scholar]
- Golas, T.M. , Sikkema, A. , Gros, J. , Feron, R.M.C. , van den Berg, R.G. , van der Weerden, G.M. , Mariani, C. and Allefs, J.J.H.M. (2010) Identification of a resistance gene Rpi‐dlc1 to Phytophthora infestans in European accessions of Solanum dulcamara . Theor. Appl. Genet. 120, 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golas, T.M. , van de Geest, H. , Gros, J. , Sikkema, A. , D′Agostino, N. , Nap, J.P. , Mariani, C. , Allefs, J.J. and Rieu, I. (2012) Comparative next‐generation mapping of the Phytophthora infestans resistance gene Rpi‐dlc2 in a European accession of Solanum dulcamara . Theor. Appl. Genet. 126, 59–68. [DOI] [PubMed] [Google Scholar]
- Goodwin, S.B. and Drenth, A. (1997) Origin of the A2 mating type of Phytophthora infestans outside Mexico. Phytopathology, 87, 992–999. [DOI] [PubMed] [Google Scholar]
- Gouget, A. , Senchou, V. , Govers, F. , Sanson, A. , Barre, A. , Rougé, P. , Pont‐Lezica, R. and Canut, H. (2006) Lectin receptor kinases participate in protein–protein interactions to mediate plasma membrane–cell wall adhesions in Arabidopsis . Plant Physiol. 140, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube, R.C. , Radwanski, E.R. and Jahn, M. (2000) Comparative genetics of disease resistance within the Solanaceae. Genetics, 155, 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, B.J. , Kamoun, S. , Zody, M.C. , Jiang, R.H.Y. , Handsaker, R.E. , Cano, L.M. , Grabherr, M. , Kodira, C.D. , Raffaele, S. , Torto‐Alalibo, T. , Bozkurt, T.O. , Ah‐Fong, A.M.V. , Alvarado, L. , Anderson, V.L. , Armstrong, M.R. , Avrova, A. , Baxter, L. , Beynon, J. , Boevink, P.C. , Bollmann, S.R. , Bos, J.I.B. , Bulone, V. , Cai, G. , Cakir, C. , Carrington, J.C. , Chawner, M. , Conti, L. , Costanzo, S. , Ewan, R. , Fahlgren, N. , Fischbach, M.A. , Fugelstad, J. , Gilroy, E.M. , Gnerre, S. , Green, P.J. , Grenville‐Briggs, L.J. , Griffith, J. , Grünwald, N.J. , Horn, K. , Horner, N.R. , Hu, C.‐H. , Huitema, E. , Jeong, D.‐H. , Jones, A.M.E. , Jones, J.D.G. , Jones, R.W. , Karlsson, E.K. , Kunjeti, S.G. , Lamour, K. , Liu, Z. , Ma, L. , MacLean, D. , Chibucos, M.C. , McDonald, H. , McWalters, J. , Meijer, H.J.G. , Morgan, W. , Morris, P.F. , Munro, C.A. , O'Neill, K. , Ospina‐Giraldo, M. , Pinzón, A. , Pritchard, L. , Ramsahoye, B. , Ren, Q. , Restrepo, S. , Roy, S. , Sadanandom, A. , Savidor, A. , Schornack, S. , Schwartz, D.C. , Schumann, U.D. , Schwessinger, B. , Seyer, L. , Sharpe, T. , Silvar, C. , Song, J. , Studholme, D.J. , Sykes, S. , Thines, M. , van de Vondervoort, P.J.I. , Phuntumart, V. , Wawra, S. , Weide, R. , Win, J. , Young, C. , Zhou, S. , Fry, W. , Meyers, B.C. , van West, P. , Ristaino, J. , Govers, F. , Birch, P.R.J. , Whisson, S.C. , Judelson, H.S. and Nusbaum, C. (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans . Nature, 461, 393–398. [DOI] [PubMed] [Google Scholar]
- Halterman, D.A. , Chen, Y. , Sopee, J. , Berduo‐Sandoval, J. and Sánchez‐Pérez, A. (2010) Competition between Phytophthora infestans effectors leads to increased aggressiveness on plants containing broad‐spectrum late blight resistance. PLoS ONE, 5, e10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond‐Kosack, K.E. and Jones, J.D.G. (1997) Plant disease resistance genes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 575–607. [DOI] [PubMed] [Google Scholar]
- Harborne, J.B. (1999) The comparative biochemistry of phytoalexin induction in plants. Biochem. Syst. Ecol. 27, 335–367. [Google Scholar]
- Hein, I. , McLean, K. , Chalhoub, B. and Bryan, G.J. (2007) Generation and screening of a BAC library from a diploid potato clone to unravel durable late blight resistance on linkage group IV. Int. J. Plant Genomics, 2007, 51421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein, I. , Gilroy, E.M. , Armstrong, M.R. and Birch, P.R.J. (2009) The zig‐zag‐zig in oomycete–plant interactions. Mol. Plant Pathol. 10, 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez, M.A. and Daayf, F. (2010) Identification and cloning of differentially expressed genes involved in the interaction between potato and Phytophthora infestans using a subtractive hybridization and cDNA‐AFLP combinational approach. J. Integr. Plant Biol. 52, 453–467. [DOI] [PubMed] [Google Scholar]
- Hermsen, J.G.T. and Boer, A.J.E. (1971) The effect of colchicine treatment on Solanum acaule and S. bulbocastanum. A complete analysis of ploidy chimeras in S. bulbocastanum . Euphytica, 20, 171–180. [Google Scholar]
- Hogenhout, S.A. , van der Hoorn, R.A. , Terauchi, R. and Kamoun, S. (2009) Emerging concepts in effector biology of plant‐associated organisms. Mol. Plant–Microbe Interact. 22, 115–122. [DOI] [PubMed] [Google Scholar]
- van der Hoorn, R.A. and Kamoun, S. (2008) From Guard to Decoy: a new model for perception of plant pathogen effectors. Plant Cell, 20, 2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. (2005) The discovery and characterization of the major late blight resistance complex in potato: genomic structure, functional diversity and implications. Chapter 5: high allelic diversity of the potato R3 complex locus suggests a novel strategy for late blight control. PhD Thesis, Wageningen University, Wageningen.
- Huang, S. , Vleeshouwers, V.G.A. , Werij, J.S. , Hutten, R.C.B. , van Eck, H.J. , Visser, R.G.F. and Jacobsen, E. (2004) The R3 resistance to Phytophthora infestans in potato is conferred by two closely linked R genes with distinct specificities. Mol. Plant–Microbe Interact. 17, 428–435. [DOI] [PubMed] [Google Scholar]
- Huang, S. , van der Vossen, E.A.G. , Kuang, H. , Vleeshouwers, V.G.A.A. , Zhang, N. , Borm, T.J.A. , van Eck, H.J. , Baker, B. , Jacobsen, E. and Visser, R. (2005) Comparative genomics enabled the isolation of the R3a late blight resistance gene in potato. Plant J. 42, 251–261. [DOI] [PubMed] [Google Scholar]
- Hubert, D.A. , Tornero, P. , Belkhadir, Y. , Krishna, P. , Takahashi, A. , Shirasu, K. and Dangl, J.L. (2003) Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J. 22, 5679–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert, D.A. , He, Y. , McNulty, B.C. , Tornero, P. and Dangl, J.L. (2009) Specific Arabidopsis HSP90.2 alleles recapitulate RAR1 cochaperone function in plant NB‐LRR disease resistance protein regulation. Proc. Natl. Acad. Sci. USA, 106, 9556–9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert, S.H. , Webb, C.A. , Smith, S.M. and Sun, Q. (2001) Resistance gene complexes: evolution and utilization. Annu. Rev. Phytopathol. 39, 285–312. [DOI] [PubMed] [Google Scholar]
- Hutten, R.C.B. and van Berloo, R. (2001) An online potato pedigree database. URL: http://www.plantbreeding.wur.nl/potatopedigree/ [accessed on May 11, 2013].
- Hwang, C.‐F. and Williamson, V.M. (2003) Leucine‐rich repeat‐mediated intramolecular interactions in nematode recognition and cell death signaling by the tomato resistance protein Mi. Plant J. 34, 585–593. [DOI] [PubMed] [Google Scholar]
- Ingham, J.L. (1973) Disease resistance in higher plants: the concept of pre‐infectional and post‐infectional resistance. J. Phytopathol. 78, 314–335. [Google Scholar]
- Ingle, R.A. , Carstens, M. and Denby, K.J. (2006) PAMP recognition and the plant–pathogen arms race. BioEssays, 28, 880–889. [DOI] [PubMed] [Google Scholar]
- Innes, R.W. (2004) Guarding the goods. New insights into the central alarm system of plants. Plant Physiol. 135, 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorizzo, M. , Mollov, D.S. , Carputo, D. and Bradeen, J.M. (2011) Disease resistance gene transcription in transgenic potato is unaltered by temperature extremes and plant physiological age. Eur. J. Plant Pathol. 130, 469–476. [Google Scholar]
- Jacobs, M.M.J. , Vosman, B. , Vleeshouwers, V.G.A.A. , Visser, R.G.F. , Henken, B. and van den Berg, R.G. (2010) A novel approach to locate Phytophthora infestans resistance genes on the potato genetic map. Theor. Appl. Genet. 120, 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen, E. and Schouten, H.J. (2007) Cisgenesis strongly improves introgression breeding and induced translocation breeding of plants. Trends Biotechnol. 25, 219–223. [DOI] [PubMed] [Google Scholar]
- Jia, Y. , McAdams, S.A. , Bryan, G.T. , Hershey, H.P. and Valent, B. (2000) Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 19, 4004–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, R.H. , Tripathy, S. , Govers, F. and Tyler, B.M. (2008) RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members. Proc. Natl. Acad. Sci. USA, 105, 4874–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, H. , Axtell, M.J. , Dahlbeck, D. , Ekwenna, O. , Zhang, S. , Staskawicz, B. and Baker, B. (2002) NPK1, an MEKK1‐like mitogen‐activated protein kinase kinase kinase, regulates innate immunity and development in plants. Dev. Cell, 3, 291–297. [DOI] [PubMed] [Google Scholar]
- Jin, H. , Liu, Y. , Yang, K.‐Y. , Kim, C.Y. , Baker, B. and Zhang, S. (2003) Function of a mitogen‐activated protein kinase pathway in N gene‐mediated resistance in tobacco. Plant J. 33, 719–731. [DOI] [PubMed] [Google Scholar]
- Jo, K.‐R. , Arens, M. , Kim, T.‐Y. , Jongsma, M.A. , Visser, R.G.F. , Jacobsen, E. and Vossen, J.H. (2011) Mapping of the S. demissum late blight resistance gene R8 to a new locus on chromosome IX. Theor. Appl. Genet. 123, 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J. , Foster, S.J. , Chu, Z. , Park, T.‐H. , van Der, V.E.A. , Pel, M.A. and Visser, R.G.F. (2010) Late blight resistance genes and methods. United States Patent Publication; Application number 12/669,871.
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Jørgensen, J.H. (1996) Effect of three suppressors on the expression of powdery mildew resistance genes in barley. Genome, 39, 492–498. [DOI] [PubMed] [Google Scholar]
- Jørgensen, J.H. (1988) Genetic analysis of barley mutants with modifications of powdery mildew resistance gene Mla12 . Genome, 30, 129–132. [Google Scholar]
- Jupe, F. , Pritchard, L. , Etherington, G.J. , Mackenzie, K. , Cock, P.J. , Wright, F. , Sharma, S.K. , Bolser, D. , Bryan, G.J. , Jones, J.D. and Hein, I. (2012) Identification and localisation of the NB‐LRR gene family within the potato genome. BMC Genomics, 13, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale, S.D. , Gu, B. , Capelluto, D.G.S. , Dou, D. , Feldman, E. , Rumore, A. , Arredondo, F.D. , Hanlon, R. , Fudal, I. , Rouxel, T. , Lawrence, C.B. , Shan, W. and Tyler, B. (2010) External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell, 142, 284–295. [DOI] [PubMed] [Google Scholar]
- Keen, N.T. (1990) Gene‐for‐gene complementarity in plant–pathogen interactions. Annu. Rev. Genet. 24, 447–463. [DOI] [PubMed] [Google Scholar]
- Koh, S. , André, A. , Edwards, H. , Ehrhardt, D. and Somerville, S. (2005) Arabidopsis thaliana subcellular responses to compatible Erysiphe cichoracearum infections. Plant J. 44, 516–529. [DOI] [PubMed] [Google Scholar]
- Kohler, A. , Rinaldi, C. , Duplessis, S. , Baucher, M. , Geelen, D. , Duchaussoy, F. , Meyers, B.C. , Boerjan, W. and Martin, F. (2008) Genome‐wide identification of NBS resistance genes in Populus trichocarpa . Plant Mol. Biol. 66, 619–636. [DOI] [PubMed] [Google Scholar]
- Kolomiets, M.V. , Chen, H. , Gladon, R.J. , Braun, E.J. and Hannapel, D.J. (2000) A leaf lipoxygenase of potato induced specifically by pathogen infection. Plant Physiol. 124, 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, L.C. , Choudoir, M.J. , Wielgus, S.M. , Bhaskar, P.B. and Jiang, J. (2009) Correlation between transcript abundance of the RB gene and the level of the RB‐mediated late blight resistance in potato. Mol. Plant–Microbe Interact. 22, 447–455. [DOI] [PubMed] [Google Scholar]
- Kuang, H. , Wei, F. , Marano, M.R. , Wirtz, U. , Wang, X. , Liu, J. , Shum, W.P. , Zaborsky, J. , Tallon, L.J. , Rensink, W. , Lobst, S. , Zhang, P. , Tornqvist, C.E. , Tek, A. , Bamberg, J. , Helgeson, J. , Fry, W. , You, F. , Luo, M.C. , Jiang, J. , Buell, C.R. and Baker, B. (2005) The R1 resistance gene cluster contains three groups of independently evolving, type I R1 homologues and shows substantial structural variation among haplotypes of Solanum demissum . Plant J. 44, 37–51. [DOI] [PubMed] [Google Scholar]
- Kuhl, J.C. , Hanneman, R.E. and Havey, M.J. (2001) Characterization and mapping of Rpi1, a late‐blight resistance locus from diploid (1EBN) Mexican Solanum pinnatisectum . Mol. Genet. Genomics, 265, 977–985. [DOI] [PubMed] [Google Scholar]
- Laterrot, H. (1975) Sélection pour la résistance au Mildiou, Phytophthora infestans Mont., de Bary chez la Tomate. Ann. Amelior. Plant. 25, 129–149. [Google Scholar]
- van der Lee, T. , Testa, A. , van't Klooster, J. , van den Berg‐Velthuis, G. and Govers, F. (2001) Chromosomal deletion in isolates of Phytophthora infestans correlates with virulence on R3, R10, and R11 potato lines. Mol. Plant–Microbe Interact. 14, 1444–1452. [DOI] [PubMed] [Google Scholar]
- Leipe, D.D. , Koonin, E.V. and Aravind, L. (2004) STAND, a class of P‐loop NTPases including animal and plant regulators of programmed cell death: multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. J. Mol. Biol. 343, 1–28. [DOI] [PubMed] [Google Scholar]
- Leister, R.T. , Dahlbeck, D. , Day, B. , Li, Y. , Chesnokova, O. and Staskawicz, B.J. (2005) Molecular genetic evidence for the role of SGT1 in the intramolecular complementation of Bs2 protein activity in Nicotiana benthamiana . Plant Cell, 17, 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, M. , Edelbaum, O. and Sela, I. (2004) Tobacco mosaic virus regulates the expression of its own resistance gene N . Plant Physiol. 135, 2392–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , Huang, S. , Guo, X. , Li, Y. , Yang, Y. , Guo, Z. , Kuang, H. , Rietman, H. , Bergervoet, M. , Vleeshouwers, V.G.G.A. , van der Vossen, E.A.G. , Qu, D. , Visser, R.G.F. , Jacobsen, E. and Vossen, J.H. (2011) Cloning and characterization of R3b; members of the R3 superfamily of late blight resistance genes show sequence and functional divergence. Mol. Plant–Microbe Interact. 24, 1132–1142. [DOI] [PubMed] [Google Scholar]
- Li, X. , van Eck, H.J. , Rouppe van der Voort, J.N.A.M. , Huigen, D.‐J. , Stam, P. and Jacobsen, E. (1998) Autotetraploids and genetic mapping using common AFLP markers: the R2 allele conferring resistance to Phytophthora infestans mapped on potato chromosome 4. Theor. Appl. Genet. 96, 1121–1128. [Google Scholar]
- Ligterink, W. , Kroj, T. , zur Nieden, U. , Hirt, H. and Scheel, D. (1997) Receptor‐mediated activation of a MAP kinase in pathogen defense of plants. Science, 276, 2054–2057. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Schiff, M. , Marathe, R. and Dinesh‐Kumar, S.P. (2002a) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N‐mediated resistance to tobacco mosaic virus. Plant J. 30, 415–429. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Schiff, M. , Serino, G. , Deng, X.‐W. and Dinesh‐Kumar, S.P. (2002b) Role of SCF ubiquitin‐ligase and the COP9 signalosome in the N gene‐mediated resistance response to Tobacco mosaic virus . Plant Cell, 14, 1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Burch‐Smith, T. , Schiff, M. , Feng, S. and Dinesh‐Kumar, S.P. (2004) Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J. Biol. Chem. 279, 2101–2108. [DOI] [PubMed] [Google Scholar]
- Liu, Z. and Halterman, D. (2006) Identification and characterization of RB‐orthologous genes from the late blight resistant wild potato species Solanum verrucosum . Physiol. Mol. Plant Pathol. 69, 230–239. [Google Scholar]
- Liu, Z. and Halterman, D. (2009) Analysis of proteins differentially accumulated during potato late blight resistance mediated by the RB resistance gene. Physiol. Mol. Plant Pathol. 74, 151–160. [Google Scholar]
- Lokossou, A.A. (2010) Dissection of the major late blight resistance cluster on potato linkage group IV. PhD Thesis. Wageningen University, Wageningen.
- Lokossou, A.A. , Park, T.‐H. , van Arkel, G. , Arens, M. , Ruyter‐Spira, C. , Morales, J. , Whisson, S.C. , Birch, P.R.J. , Visser, R.G.F. , Jacobsen, E. and van der Vossen, E.A.G. (2009) Exploiting knowledge of R/Avr genes to rapidly clone a new LZ‐NBS‐LRR family of late blight resistance genes from potato linkage group IV. Mol. Plant–Microbe Interact. 22, 630–641. [DOI] [PubMed] [Google Scholar]
- Lokossou, A.A. , Rietman, H. , Wang, M. , Krenek, P. , van der Schoot, H. , Henken, B. , Hoekstra, R. , Vleeshouwers, V.G.A. , van der Vossen, E.A.G. , Visser, R.G.F. , Jacobsen, E. and Vosman, B. (2010) Diversity, distribution, and evolution of Solanum bulbocastanum late blight resistance genes. Mol. Plant–Microbe Interact. 23, 1206–1216. [DOI] [PubMed] [Google Scholar]
- Lozoya‐Saldana, H. , Belmar‐Diaz, C. , Bradeen, J.M. and Helgeson, J.P. (2005) Characterization of Phytophthora infestans isolates infecting transgenic and somatic hybrid potatoes resistant to the pathogen in the Toluca Valley, Mexico. Am. J. Potato Res. 82, 79. [Google Scholar]
- Lu, R. , Malcuit, I. , Moffett, P. , Ruiz, M.T. , Peart, J. , Wu, A.‐J. , Rathjen, J.P. , Bendahmane, A. , Day, L. and Baulcombe, D.C. (2003) High throughput virus‐induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J. 22, 5690–5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y.J. , Schornack, S. , Spallek, T. , Geldner, N. , Chory, J. , Schellmann, S. , Schumacher, K. , Kamoun, S. and Robatzek, S. (2012) Patterns of plant subcellular responses to successful oomycete infections reveal differences in host cell reprogramming and endocytic trafficking. Cell Microbiol. 14, 682–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luderer, R. and Joosten, M.H.A. (2001) Avirulence proteins of plant pathogens: determinants of victory and defeat. Mol. Plant Pathol. 2, 355–364. [DOI] [PubMed] [Google Scholar]
- Lukasik‐Shreepaathy, E. , Slootweg, E. , Richter, H. , Goverse, A. , Cornelissen, B.J. and Takken, F.L. (2012) Dual regulatory roles of the extended N terminus for activation of the tomato MI‐1.2 resistance protein. Mol. Plant‐Microbe Interact. 25, 1045–1057. [DOI] [PubMed] [Google Scholar]
- Lupas, A. (1996) Coiled coils: new structures and new functions. Trends Biochem. Sci. 21, 375–382. [PubMed] [Google Scholar]
- Mackey, D. , Belkhadir, Y. , Alonso, J.M. , Ecker, J.R. and Dangl, J.L. (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2‐mediated resistance. Cell, 112, 379–389. [DOI] [PubMed] [Google Scholar]
- Maekawa, T. , Cheng, W. , Spiridon, L.N. , Töller, A. , Lukasik, E. , Saijo, Y. , Liu, P. , Shen, Q.H. , Micluta, M.A. , Somssich, I.E. , Takken, F.L. , Petrescu, A.J. , Chai, J. and Schulze‐Lefert, P. (2011) Coiled‐coil domain‐dependent homodimerization of intracellular barley immune receptors defines a minimal functional module for triggering cell death. Cell Host Microbe, 9, 187–199. [DOI] [PubMed] [Google Scholar]
- Martin, G.B. , Bogdanove, A.J. and Sessa, G. (2003) Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 54, 23–61. [DOI] [PubMed] [Google Scholar]
- Mauch‐Mani, B. and Slusarenko, A.J. (1996) Production of salicylic acid precursors is a major function of phenylalanine ammonia‐lyase in the resistance of Arabidopsis to Peronospora parasitica . Plant Cell, 8, 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell, J.M. , Dhandaydham, M. , Long, T.A. , Aarts, M.G. , Goff, S. , Holub, E.B. and Dangl, J.L. (1998) Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of Arabidopsis. Plant Cell, 10, 1861–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod, A. , Smart, C.D. and Fry, W.E. (2003) Characterization of 1,3‐beta‐glucanase and 1,3;1,4‐beta‐glucanase genes from Phytophthora infestans . Fungal Genet. Biol. 38, 250–263. [DOI] [PubMed] [Google Scholar]
- Meksem, K. , Leister, D. , Peleman, J. , Zabeau, M. , Salamini, F. and Gebhardt, C. (1995) A high‐resolution map of the vicinity of the R1 locus on chromosome V of potato based on RFLP and AFLP markers. Mol. Gen. Genet. 249, 74–81. [DOI] [PubMed] [Google Scholar]
- Menke, F.L.H. , van Pelt, J.A. , Pieterse, C.M.J. and Klessig, D.F. (2004) Silencing of the mitogen‐activated protein kinase MPK6 compromises disease resistance in Arabidopsis. Plant Cell, 16, 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk, H.L. and Foolad, M.R. (2012) Parent–offspring correlation estimate of heritability for late blight resistance conferred by an accession of the tomato wild species Solanum pimpinellifolium . Plant Breed. 131, 203–210. [Google Scholar]
- Meyers, B.C. , Shen, K.A. , Rohani, P. , Gaut, B.S. and Michelmore, R.W. (1998) Receptor‐like genes in the major resistance locus of lettuce are subject to divergent selection. Plant Cell, 10, 1833–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers, B.C. , Kozik, A. , Griego, A. , Kuang, H. and Michelmore, R.W. (2003) Genome‐wide analysis of NBS‐LRR‐encoding genes in Arabidopsis. Plant Cell, 15, 809–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micali, C.O. , Neumann, U. , Grunewald, D. , Panstruga, R. , and O′Connell, R. (2011) Biogenesis of a specialized plant‐fungal interface during host cell internalization of Golovinomyces orontii haustoria. Cell Microbiol. 13, 210–226. [DOI] [PubMed] [Google Scholar]
- Millett, B.P. and Bradeen, J.M. (2007) Development of allele‐specific PCR and RT–PCR assays for clustered resistance genes using a potato late blight resistance transgene as a model. Theor. Appl. Genet. 114, 501–513. [DOI] [PubMed] [Google Scholar]
- Millett, B.P. , Mollov, D.S. , Iorizzo, M. , Carputo, D. and Bradeen, J.M. (2009) Changes in disease resistance phenotypes associated with plant physiological age are not caused by variation in R gene transcript abundance. Mol. Plant–Microbe Interact. 22, 362–368. [DOI] [PubMed] [Google Scholar]
- Moffett, P. , Farnham, G. , Peart, J. and Baulcombe, D.C. (2002) Interaction between domains of a plant NBS‐LRR protein in disease resistance‐related cell death. EMBO J. 21, 4511–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarry, J. , Hamelin, F.M. , Glais, I. , Corbière, R. and Andrivon, D. (2010) Fitness costs associated with unnecessary virulence factors and life history traits: evolutionary insights from the potato late blight pathogen Phytophthora infestans . BMC Evol. Biol. 10, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morante, M.C. and Villanueva, N.M. (2006) Short communication. Resistance to Phytophthora infestans in populations of wild potato species in the Sorata microcentre of genetic diversity, La Paz, Bolivia. Spanish J. Agric. Res. 4, 156–160. [Google Scholar]
- Moreau, P. , Thoquet, P. , Olivier, J. , Laterrot, H. and Grimsley, N. (1998) Genetic mapping of Ph‐2, a single locus controlling partial resistance to Phytophthora infestans in tomato. Mol. Plant‐Microbe Interact. 11, 259–269. [Google Scholar]
- Morgan, W. and Kamoun, S. (2007) RXLR effectors of plant pathogenic oomycetes. Curr. Opin. Microbiol. 10, 332–338. [DOI] [PubMed] [Google Scholar]
- Mucyn, T.S. , Clemente, A. , Andriotis, V.M.E. , Balmuth, A.L. , Oldroyd, G.E.D. , Staskawicz, B.J. and Rathjen, J.P. (2006) The tomato NB‐ARC‐LRR protein Prf interacts with Pto kinase in vivo to regulate specific plant immunity. Plant Cell, 18, 2792–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, K.O. (1951) Über die Herkunft der W‐Sorten, ihre Entwicklungsgeschichte und ihre bisherige Nutzung in der praktischen Kartoffelzüchtung. Z. Pflanzenzüchtg. 29, 366–387. [Google Scholar]
- Müller, K.O. and Börger, H. (1940) Experimentelle Untersuchungen über die Phytophthora‐Resistenz der Kartoffel. Arb. Biol. Reichsanst. Land. Forstwirtsch. 23, 189–231. [Google Scholar]
- Naess, S.K. , Bradeen, J.M. , Wielgus, S.M. , Haberlach, G.T. , McGrath, J.M. and Helgeson, J.P. (2000) Resistance to late blight in Solanum bulbocastanum is mapped to chromosome 8. Theor. Appl. Genet. 101, 697–704. [Google Scholar]
- Nakitandwe, J. (2007) Molecular characterisation of Phytophthora infestans resistance genes in Solanum caripense and development of molecular markers for genetic mapping. Chapter 4: a major gene for resistance to Phytophthora infestans mapped on chromosome IX of Solanum caripense . PhD Thesis, University of Natural Resources and Life Sciences, Vienna.
- Nombela, G. , Williamson, V.M. and Muniz, M. (2003) The root‐knot nematode resistance gene Mi‐1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci . Mol. Plant–Microbe Interact. 16, 645–649. [DOI] [PubMed] [Google Scholar]
- Nowicki, M. , Foolad, M.R. , Nowakowska, M. and Kozik, E.U. (2012) Potato and tomato late blight caused by Phytophthora infestans: an overview of pathology and resistance breeding. Plant Dis. 96, 4–17. [DOI] [PubMed] [Google Scholar]
- Nürnberger, T. , Brunner, F. , Kemmerling, B. and Piater, L. (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunol. Rev. 198, 249–266. [DOI] [PubMed] [Google Scholar]
- Oh, H.‐S. , Park, D.H. and Collmer, A. (2010) Components of the Pseudomonas syringae type III secretion system can suppress and may elicit plant innate immunity. Mol. Plant–Microbe Interact. 23, 727–739. [DOI] [PubMed] [Google Scholar]
- Oh, S.‐K. , Young, C. , Lee, M. , Oliva, R. , Bozkurt, T.O. , Cano, L.M. , Win, J. , Bos, J.I.B. , Liu, H.‐Y. , van Damme, M. , Morgan, W. , Choi, D. , Van der Vossen, E.A.G. , Vleeshouwers, V.G.A.A. and Kamoun, S. (2009) In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi‐blb2. Plant Cell, 21, 2928–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooijen, G. , van den Burg, H.A. , Cornelissen, B.J. and Takken, F.L. (2007) Structure and function of resistance proteins in solanaceous plants. Annu. Rev. Phytopathol. 45, 43–72. [DOI] [PubMed] [Google Scholar]
- Oosumi, T. , Rockhold, D.R. , Maccree, M.M. , Deahl, K.L. , McCue, K.F. and Belknap, W.R. (2009) Gene Rpi‐bt1 from Solanum bulbocastanum confers resistance to late blight in transgenic potatoes. Am. J. Potato Res. 86, 456–465. [Google Scholar]
- Paal, J. , Henselewski, H. , Muth, J. , Meksem, K. , Menéndez, C.M. , Salamini, F. , Ballvora, A. and Gebhardt, C. (2004) Molecular cloning of the potato Gro1‐4 gene conferring resistance to pathotype Ro1 of the root cyst nematode Globodera rostochiensis, based on a candidate gene approach. Plant J. 38, 285–297. [DOI] [PubMed] [Google Scholar]
- Pan, Q. , Wendel, J. and Fluhr, R. (2000) Divergent evolution of plant NBS‐LRR resistance gene homologues in dicot and cereal genomes. J. Mol. Evol. 50, 203–213. [DOI] [PubMed] [Google Scholar]
- Park, P.H. , Chae, Y. , Kim, H.‐R. , Chung, K.‐H. , Oh, D.‐G. and Kim, K.‐T. (2010) Development of a SCAR marker linked to Ph‐3 in Solanum ssp. Korean J. Breed. Sci. 42, 139–143. [Google Scholar]
- Park, T.‐H. , Gros, J. , Sikkema, A. , Vleeshouwers, V.G.A. , Muskens, M. , Allefs, S. , Jacobsen, E. , Visser, R.G.F. and van der Vossen, E.A.G. (2005a) The late blight resistance locus Rpi‐blb3 from Solanum bulbocastanum belongs to a major late blight R gene cluster on chromosome 4 of potato. Mol. Plant–Microbe Interact. 18, 722–729. [DOI] [PubMed] [Google Scholar]
- Park, T.‐H. , Vleeshouwers, V.G.A. , Hutten, R.C.B. , van Eck, H.J. , van der Vossen, E. , Jacobsen, E. and Visser, R.G.F. (2005b) High‐resolution mapping and analysis of the resistance locus Rpi‐abpt against Phytophthora infestans in potato. Mol. Breed. 16, 33–43. [Google Scholar]
- Park, T.H. , Vleeshouwers, V.G.A. , Huigen, D.J. , van der Vossen, E.A.G. , van Eck, H.J. and Visser, R.G.F. (2005c) Characterization and high‐resolution mapping of a late blight resistance locus similar to R2 in potato. Theor. Appl. Genet. 111, 591–597. [DOI] [PubMed] [Google Scholar]
- Park, T.‐H. , Vleeshouwers, V.G.A. , Kim, J.‐B. , Hutten, R.C.B. and Visser, R.G.F. (2005d) Dissection of foliage and tuber late blight resistance in mapping populations of potato. Euphytica, 143, 75–83. [Google Scholar]
- Park, T.‐H. , Foster, S. , Brigneti, G. and Jones, J.D.G. (2009a) Two distinct potato late blight resistance genes from Solanum berthaultii are located on chromosome 10. Euphytica, 165, 269–278. [Google Scholar]
- Park, T.‐H. , Vleeshouwers, V.G.A. , Jacobsen, E. , van der Vossen, E. and Visser, R.G.F. (2009b) Molecular breeding for resistance to Phytophthora infestans (Mont.) de Bary in potato (Solanum tuberosum L.): a perspective of cisgenesis. Plant Breed. 128, 109–117. [Google Scholar]
- Parker, J.E. , Holub, E.B. , Frost, L.N. , Falk, A. , Gunn, N.D. and Daniels, M.J. (1996) Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell, 8, 2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, J.E. , Feys, B.J. , van der Biezen, E.A. , Noel, L. , Aarts, N. , Austin, M.J. , Botella, M.A. , Frost, L.N. , Daniels, M.J. and Jones, J.D.G. (2000) Unravelling R gene‐mediated disease resistance pathways in Arabidopsis . Mol. Plant Pathol. 1, 17–24. [DOI] [PubMed] [Google Scholar]
- Parniske, M. , Hammond‐Kosack, K.E. , Golstein, C. , Thomas, C.M. , Jones, D.A. , Harrison, K. , Wulff, B.B.H. and Jones, J.D.G. (1997) Novel disease resistance specificities result from sequence exchange between tandemly repeated genes at the Cf‐4/9 locus of tomato. Cell, 91, 821–832. [DOI] [PubMed] [Google Scholar]
- Peart, J.R. , Cook, G. , Feys, B.J. , Parker, J.E. and Baulcombe, D.C. (2002) An EDS1 orthologue is required for N‐mediated resistance against tobacco mosaic virus. Plant J. 29, 569–579. [DOI] [PubMed] [Google Scholar]
- Peart, J.R. , Mestre, P. , Lu, R. , Malcuit, I. and Baulcombe, D.C. (2005) NRG1, a CC‐NB‐LRR protein, together with N, a TIR‐NB‐LRR protein, mediates resistance against tobacco mosaic virus. Curr. Biol. 15, 968–973. [DOI] [PubMed] [Google Scholar]
- Pedersen, W.L. and Leath, S. (1988) Pyramiding major genes for resistance to maintain residual effects. Annu. Rev. Phytopathol. 26, 369–378. [Google Scholar]
- Pedras, M.S.C. and Ahiahonu, P.W.K. (2005) Metabolism and detoxification of phytoalexins and analogs by phytopathogenic fungi. Phytochemistry, 66, 391–411. [DOI] [PubMed] [Google Scholar]
- Peirce, L.C. (1971) Linkage tests with Ph conditioning resistance to race 0, Phytophthora infestans . Rep. Tomato Genet. Coop. 21, 30. [Google Scholar]
- Pel, M.A. , Foster, S.J. , Park, T.‐H. , Rietman, H. , van Arkel, G. , Jones, J.D.G. , Van Eck, H.J. , Jacobsen, E. , Visser, R.G.F. and Van der Vossen, E.A.G. (2009) Mapping and cloning of late blight resistance genes from Solanum venturii using an interspecific candidate gene approach. Mol. Plant–Microbe Interact. 22, 601–615. [DOI] [PubMed] [Google Scholar]
- Piedras, P. , Hammond‐Kosack, K.E. , Harrison, K. and Jones, J.D.G. (1998) Rapid, Cf‐9‐ and Avr9‐dependent production of active oxygen species in tobacco suspension cultures. Mol. Plant–Microbe Interact. 11, 1155–1166. [Google Scholar]
- Plett, J.M. , Kemppainen, M. , Kale, S.D. , Kohler, A. , Legué, V. , Brun, A. , Tyler, B.M. , Pardo, A.G. and Martin, F. (2011) A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr. Biol. 21, 1197–1203. [DOI] [PubMed] [Google Scholar]
- Poch, H.L.C. , Lopez, R.H.M. and Kanyuka, K. (2006) Functionality of resistance gene Hero, which controls plant root‐infecting potato cyst nematodes, in leaves of tomato. Plant Cell Environ. 29, 1372–1378. [DOI] [PubMed] [Google Scholar]
- van Poppel, P.M.J. (2010) The Phytophthora infestans avirulence gene Avr4 and its potato counterpart R4 . PhD Thesis. Wageningen University, Wageningen.
- van Poppel, P.M.J. , Guo, J. , van de Vondervoort, P.J.I. , Jung, M.W.M. , Birch, P.R.J. , Whisson, S.C. and Govers, F. (2008) The Phytophthora infestans avirulence gene Avr4 encodes an RXLR‐dEER effector. Mol. Plant–Microbe Interact. 21, 1460–1470. [DOI] [PubMed] [Google Scholar]
- van Poppel, P.M.J. , Jiang, R.H.Y. , Sliwka, J. and Govers, F. (2009a) Recognition of Phytophthora infestans Avr4 by potato R4 is triggered by C‐terminal domains comprising W motifs. Mol. Plant Pathol. 10, 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Poppel, P.M.J. , Huigen, D.J. and Govers, F. (2009b) Differential recognition of Phytophthora infestans races in potato R4 breeding lines. Phytopathology, 99, 1150–1155. [DOI] [PubMed] [Google Scholar]
- Potato Genome Sequencing Consortium (2011) Genome sequence and analysis of the tuber crop potato. Nature, 475, 189–195. [DOI] [PubMed] [Google Scholar]
- Prost, I. , Dhondt, S. , Rothe, G. , Vicente, J. , Rodriguez, M.J. , Kift, N. , Carbonne, F. , Griffiths, G. , Esquerré‐Tugayé, M.T. , Rosahl, S. , Castresana, C. , Hamberg, M. and Fournier, J. (2005) Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 139, 1902–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, Y. , Liu, L. , Xiong, Q. , Flores, C. , Wong, J. , Shi, J. , Wang, X. , Liu, X. , Xiang, Q. , Jiang, S. , Zhang, F. , Wang, Y. , Judelson, H.S. , Chen, X. and Ma, W. (2013) Oomycete pathogens encode RNA silencing suppressors. Nat Genet. 45, 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qutob, D. , Tedman‐Jones, J. and Gijzen, M. (2006) Effector‐triggered immunity by the plant pathogen Phytophthora . Trends Microbiol. 14, 470–473. [DOI] [PubMed] [Google Scholar]
- Raffaele, S. , Win, J. , Cano, L.M. and Kamoun, S. (2010) Analyses of genome architecture and gene expression reveal novel candidate virulence factors in the secretome of Phytophthora infestans . BMC Genomics, 11, 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rairdan, G.J. and Moffett, P. (2006) Distinct domains in the ARC region of the potato resistance protein Rx mediate LRR binding and inhibition of activation. Plant Cell, 18, 2082–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanna, M.S. and Hermsen, J.G.T. (1971) Somatic chromosome elimination and meiotic chromosome pairing in the triple hybrid 6x‐(Solanum acaule × S. bulbocastanum) × 2 × S. phureja . Euphytica, 20, 470–481. [Google Scholar]
- Rathjen, J.P. , Chang, J.H. , Staskawicz, B.J. and Michelmore, R.W. (1999) Constitutively active Pto induces a Prf‐dependent hypersensitive response in the absence of avrPto. EMBO J. 18, 3232–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher, G.M. , Smart, C.D. , Simko, I. , Bonierbale, M. , Mayton, H. , Greenland, A. and Fry, W.E. (2006) Characterization and mapping of RPi‐ber, a novel potato late blight resistance gene from Solanum berthaultii . Theor. Appl. Genet. 112, 674–687. [DOI] [PubMed] [Google Scholar]
- Rehmany, A.P. , Gordon, A. , Rose, L.E. , Allen, R.L. , Armstrong, M.R. , Whisson, S.C. , Kamoun, S. , Tyler, B.M. , Birch, P.R.J. and Beynon, J.L. (2005) Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines. Plant Cell, 17, 1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl, S.J. , Li, W. , Chao, Y. , Schwarzenbacher, R. and Shi, Y. (2005) Structure of the apoptotic protease‐activating factor 1 bound to ADP. Nature, 434, 926–933. [DOI] [PubMed] [Google Scholar]
- Rietman, H. (2011) Putting the Phytophthora infestans genome sequence at work; multiple novel avirulence and potato resistance gene candidates revealed. PhD Thesis, Wageningen University, Wageningen.
- Rietman, H. , Bijsterbosch, G. , Cano, L.M. , Lee, H.R. , Vossen, J.H. , Jacobsen, E. , Visser, R.G. , Kamoun, S. and Vleeshouwers, V.G. (2012) Qualitative and quantitative late blight resistance in the potato cultivar Sarpo Mira is determined by the perception of five distinct RXLR effectors. Mol. Plant‐Microbe Interact. 25, 910–909. [DOI] [PubMed] [Google Scholar]
- Rivera‐Peña, A. (1990) Wild tuber‐bearing species of Solanum and incidence of Phytophthora infestans (Mont.) de Bary on the western slopes of the volcano Nevado de Toluca. 5. Type of resistance to P. infestans . Potato Res. 33, 479–486. [Google Scholar]
- Romeis, T. , Piedras, P. , Zhang, S. , Klessig, D.F. , Hirt, H. and Jones, J.D. (1999) Rapid Avr9‐ and Cf‐9‐dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell, 11, 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronning, C.M. , Stegalkina, S.S. , Ascenzi, R.A. , Bougri, O. , Hart, A.L. , Utterbach, T.R. , Vanaken, S.E. , Riedmuller, S.B. , White, J.A. , Cho, J. , Pertea, G.M. , Lee, Y. , Karamycheva, S. , Sultana, R. , Tsai, J. , Quackenbush, J. , Griffiths, H.M. , Restrepo, S. , Smart, C.D. , Fry, W.E. , Van Der Hoeven, R. , Tanksley, S. , Zhang, P. , Jin, H. , Yamamoto, M.L. , Baker, B.J. and Buell, C.R. (2003) Comparative analyses of potato expressed sequence tag libraries. Plant Physiol. 131, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney, H.C.E. , van't Klooster, J.W. , van der Hoorn, R.A.L. , Joosten, M.H.A.J. , Jones, J.D.G. and de Wit, P.J.G.M. (2005) Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf‐2‐dependent disease resistance. Science, 308, 1783–1786. [DOI] [PubMed] [Google Scholar]
- Ruiz de Galarreta, J.l. , Carrasco, A. , Salazar, A. , Barrena, I. , Iturritxa, E. , Marquinez, R. , Legorburu, F.J. and Ritter, E. (1998) Wild Solanum species as resistance sources against different pathogens of potato. Potato Res. 41, 57–68. [Google Scholar]
- Salaman, R.N. (1910) The inheritance of colour and other characters in the potato. J. Genet. 1, 7–46. [Google Scholar]
- Sandbrink, J.M. , Colon, L.T. , Wolters, P.J.C. and Stiekema, W.J. (2000) Two related genotypes of Solanum microdontum carry different segregating alleles for field resistance to Phytophthora infestans . Mol. Breed. 6, 215–225. [Google Scholar]
- Saunders, D.G.O. , Breen, S. , Win, J. , Schornack, S. , Hein, I. , Bozkurt, T.O. , Champouret, N. , Vleeshouwers, V.G.A.A. , Birch, P.R.J. , Gilroy, E.M. and Kamoun, S. (2012) Host protein BSL1 associates with Phytophthora infestans RXLR effector AVR2 and the Solanum demissum immune receptor R2 to mediate disease resistance. Plant Cell, 24, 3420–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornack, S. , Peter, K. , Bonas, U. and Lahaye, T. (2005) Expression levels of avrBs3‐like genes affect recognition specificity in tomato Bs4‐ but not in pepper Bs3‐mediated perception. Mol. Plant–Microbe Interact. 18, 1215–1225. [DOI] [PubMed] [Google Scholar]
- Schornack, S. , van Damme, M. , Bozkurt, T.O. , Cano, L.M. , Smoker, M. , Thines, M. , Gaulin, E. , Kamoun, S. and Huitema, E. (2010) Ancient class of translocated oomycete effectors targets the host nucleus. Proc. Natl. Acad. Sci. USA, 107, 17 421–17 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senchou, V. , Weide, R. , Carrasco, A. , Bouyssou, H. , Pont‐Lezica, R. , Govers, F. and Canut, H. (2004) High affinity recognition of a Phytophthora protein by Arabidopsis via an RGD motif. Cell. Mol. Life Sci. 61, 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, F. , Golstein, C. , Ade, J. , Stoutemyer, M. , Dixon, J.E. and Innes, R.W. (2003) Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science, 301, 1230–1233. [DOI] [PubMed] [Google Scholar]
- Shen, Q.‐H. , Saijo, Y. , Mauch, S. , Biskup, C. , Bieri, S. , Keller, B. , Seki, H. , Ülker, B. , Somssich, I.E. and Schulze‐Lefert, P. (2007) Nuclear activity of MLA immune receptors links isolate‐specific and basal disease‐resistance responses. Science, 315, 1098–1103. [DOI] [PubMed] [Google Scholar]
- Shibata, Y. , Kawakita, K. and Takemoto, D. (2010) Age‐related resistance of Nicotiana benthamiana against hemibiotrophic pathogen Phytophthora infestans requires both ethylene‐ and salicylic acid‐mediated signaling pathways. Mol. Plant–Microbe Interact. 23, 1130–1142. [DOI] [PubMed] [Google Scholar]
- Shirano, Y. , Kachroo, P. , Shah, J. and Klessig, D.F. (2002) A gain‐of‐function mutation in an Arabidopsis Toll Interleukin1 receptor‐nucleotide binding site‐leucine‐rich repeat type R gene triggers defense responses and results in enhanced disease resistance. Plant Cell, 14, 3149–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamnioti, P. and Ridout, C.J. (2005) Microbial avirulence determinants: guided missiles or antigenic flak? Mol. Plant Pathol. 6, 551–559. [DOI] [PubMed] [Google Scholar]
- Sliwka, J. , Jakuczun, H. , Lebecka, R. , Marczewski, W. , Gebhardt, C. and Zimnoch‐Guzowska, E. (2006) The novel, major locus Rpi‐phu1 for late blight resistance maps to potato chromosome IX and is not correlated with long vegetation period. Theor. Appl. Genet. 113, 685–695. [DOI] [PubMed] [Google Scholar]
- Sliwka, J. , Jakuczun, H. , Kaminski, P. and Zimnoch‐Guzowska, E. (2010) Marker‐assisted selection of diploid and tetraploid potatoes carrying Rpi‐phu1, a major gene for resistance to Phytophthora infestans . J. Appl. Genet. 51, 133–140. [DOI] [PubMed] [Google Scholar]
- Sliwka, J. , Jakuczun, H. , Chmielarz, M. , Hara‐Skrzypiec, A. , Tomczyńska, I. , Kilian, A. , and Zimnoch‐Guzowska, E. (2012a) Late blight resistance gene from Solanum ruiz‐ceballosii is located on potato chromosome X and linked to violet flower colour. BMC Genet. 13, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwka, J. , Jakuczun, H. , Chmielarz, M. , Hara‐Skrzypiec, A. , Tomczyńska, I. , Kilian, A. and Zimnoch‐Guzowska, E. (2012b) A resistance gene against potato late blight originating from Solanum × michoacanum maps to potato chromosome VII. Theor. Appl. Genet. 124, 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilde, W.D. , Brigneti, G. , Jagger, L. , Perkins, S. and Jones, J.D. (2005) Solanum mochiquense chromosome IX carries a novel late blight resistance gene Rpi‐moc1 . Theor. Appl. Genet. 110, 252–258. [DOI] [PubMed] [Google Scholar]
- Song, J. , Bradeen, J.M. , Naess, S.K. , Raasch, J.A. , Wielgus, S.M. , Haberlach, G.T. , Liu, J. , Kuang, H. , Austin‐Phillips, S. , Buell, C.R. , Helgeson, J.P. and Jiang, J. (2003) Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proc. Natl. Acad. Sci. USA, 100, 9128–9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J. , Win, J. , Tian, M. , Schornack, S. , Kaschani, F. , Ilyas, M. , van der Hoorn, R.A. and Kamoun, S. (2009) Apoplastic effectors secreted by two unrelated eukaryotic plant pathogens target the tomato defense protease Rcr3. Proc. Natl. Acad. Sci. USA, 106, 1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner, D.M. , Jansky, S.H. and Simon, R. (2009) Tests of taxonomic and biogeographic predictivity: resistance to disease and insect pests in wild relatives of cultivated potato. Crop Sci. 49, 1367–1376. [Google Scholar]
- Stewart, H.E. , Bradshaw, J.E. and Pande, B. (2003) The effect of the presence of R‐genes for resistance to late blight (Phytophthora infestans) of potato (Solanum tuberosum) on the underlying level of field resistance. Plant Pathol. 52, 193–198. [Google Scholar]
- Sun, F. , Kale, S.D. , Azurmendi, H.F. , Li, D. , Tyler, B.M. and Capelluto, D.G. (2013) Structural basis for interactions of the Phytophthora sojae RxLR effector Avh5 with phosphatidylinositol 3‐phosphate and for host cell entry. Mol. Plant–Microbe Interact. 26, 330–344. [DOI] [PubMed] [Google Scholar]
- Swiderski, M.R. and Innes, R.W. (2001) The Arabidopsis PBS1 resistance gene encodes a member of a novel protein kinase subfamily. Plant J. 26, 101–112. [DOI] [PubMed] [Google Scholar]
- Swiezyński, K.M. , Domański, L. , Zarzycka, H. and Zimnoch‐Guzowska, E. (2000) The reaction of potato differentials to Phytophthora infestans isolates collected in nature. Plant Breed. 119, 119–126. [Google Scholar]
- Takken, F.L. and Goverse, A. (2012) How to build a pathogen detector: structural basis of NB‐LRR function. Curr. Opin. Plant Biol. 15, 375–384. [DOI] [PubMed] [Google Scholar]
- Tameling, W.I. , Elzinga, S.D. , Darmin, P.S. , Vossen, J.H. , Takken, F.L. , Haring, M.A. and Cornelissen, B.J. (2002) The tomato R gene products I‐2 and MI‐1 are functional ATP binding proteins with ATPase activity. Plant Cell, 14, 2929–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, M.Y. , Hutten, R.C.B. , Celis, C. , Park, T.‐H. , Niks, R.E. , Visser, R.G.F. and van Eck, H.J. (2008) The RPi‐mcd1 locus from Solanum microdontum involved in resistance to Phytophthora infestans, causing a delay in infection, maps on potato chromosome 4 in a cluster of NBS‐LRR genes. Mol. Plant–Microbe Interact. 21, 909–918. [DOI] [PubMed] [Google Scholar]
- Tan, M.Y. , Hutten, R.C.B. , Visser, R.G.F. and van Eck, H.J. (2010) The effect of pyramiding Phytophthora infestans resistance genes RPi‐mcd1 and RPi‐ber in potato. Theor. Appl. Genet. 121, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr, D.E. and Alexander, H.M. (2009) TIR‐NBS‐LRR genes are rare in monocots: evidence from diverse monocot orders. BMC Res. Notes, 2, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma, B.P.H. , Nürnberger, T. and Joosten, M.H.A.J. (2011) Of PAMPs and effectors: the blurred PTI–ETI dichotomy. Plant Cell, 23, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, M. , Huitema, E. , Da Cunha, L. , Torto‐Alalibo, T. and Kamoun, S. (2004) A Kazal‐like extracellular serine protease inhibitor from Phytophthora infestans targets the tomato pathogenesis‐related protease P69B. J. Biol. Chem. 279, 26 370–26 377. [DOI] [PubMed] [Google Scholar]
- Tian, M. , Benedetti, B. and Kamoun, S. (2005) A second Kazal‐like protease inhibitor from Phytophthora infestans inhibits and interacts with the apoplastic pathogenesis‐related protease P69B of tomato. Plant Physiol. 138, 1785–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, M. , Win, J. , Song, J. , van der Hoorn, R. , van der Knaap, E. and Kamoun, S. (2007) A Phytophthora infestans cystatin‐like protein targets a novel tomato papain‐like apoplastic protease. Plant Physiol. 143, 364–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomato Genome Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature, 485, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero, P. , Merritt, P. , Sadanandom, A. , Shirasu, K. , Innes, R.W. and Dangl, J.L. (2002) RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell, 14, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toxopeus, H.J. (1956) Reflections on the origin of new physiologic races in Phytophthora infestans and the breeding for resistance in potatoes. Euphytica, 5, 221–237. [Google Scholar]
- Toxopeus, H.J. (1958) Some notes on the relations between field resistance to Phytophthora infestans in leaves and tubers and ripening time in Solanum tuberosum subsp. tuberosum . Euphytica, 7, 123–130. [Google Scholar]
- Trognitz, F. and Trognitz, B.R. (2005) Survey of resistance gene analogs in Solanum caripense, a relative of potato and tomato, and update on R gene genealogy. Mol. Genet. Genomics, 274, 595–605. [DOI] [PubMed] [Google Scholar]
- Truong, H.T.H , Tran, H.N. , Choi, H.S. , Park, P.H. and Lee, H.E. (2013) Development of a co‐dominant SCAR marker linked to the Ph‐3 gene for Phytophthora infestans resistance in tomato (Solanum lycopersicum). Eur. J. Plant Pathol. 136, 237–245. [Google Scholar]
- Turkensteen, L.J. (1973) Partial resistance of tomatoes against Phytophthora infestans, the late blight fungus. PhD Thesis, Wageningen University, Wageningen. [Google Scholar]
- Tyler, B.M. (2009) Entering and breaking: virulence effector proteins of oomycete plant pathogens. Cell. Microbiol. 11, 13–20. [DOI] [PubMed] [Google Scholar]
- Ueda, H. , Yamaguchi, Y. and Sano, H. (2006) Direct interaction between the tobacco mosaic virus helicase domain and the ATP‐bound resistance protein, N factor during the hypersensitive response in tobacco plants. Plant Mol. Biol. 61, 31–45. [DOI] [PubMed] [Google Scholar]
- Verzaux, E. (2010) Resistance and susceptibility to late blight in Solanum: gene mapping, cloning and stacking. PhD Thesis. Wageningen University, Wageningen.
- Vetukuri, R.R. , Åsman, A.K. , Tellgren‐Roth, C. , Jahan, S.N. , Reimegård, J. , Fogelqvist, J. , Savenkov, E. , Söderbom, F. , Avrova, A.O. , Whisson, S.C. and Dixelius, C. (2012) Evidence for small RNAs homologous to effector‐encoding genes and transposable elements in the oomycete Phytophthora infestans . PLoS One, 7, e51399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamon, F.G. , Spooner, D.M. , Orrillo, M. , Mihovilovich, E. , Perez, W. and Bonierbale, M. (2005) Late blight resistance linkages in a novel cross of the wild potato species Solanum paucissectum (series Piurana). Theor. Appl. Genet. 111, 1201–1214. [DOI] [PubMed] [Google Scholar]
- Vleeshouwers, V.G.A. , Rietman, H. , Krenek, P. , Champouret, N. , Young, C. , Oh, S.‐K. , Wang, M. , Bouwmeester, K. , Vosman, B. , Visser, R.G.F. , Jacobsen, E. , Govers, F. , Kamoun, S. and Van der Vossen, E.A.G. (2008) Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes. PLoS ONE, 3, e2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, P. , Simons, G. , Jesse, T. , Wijbrandi, J. , Heinen, L. , Hogers, R. , Frijters, A. , Groenendijk, J. , Diergaarde, P. , Reijans, M. , Fierens‐Onstenk, J. , de Both, M. , Peleman, J. , Liharska, T. , Hontelez, J. and Zabeau, M. (1998) The tomato Mi‐1 gene confers resistance to both root‐knot nematodes and potato aphids. Nat. Biotechnol. 16, 1365–1369. [DOI] [PubMed] [Google Scholar]
- van der Vossen, E. , Sikkema, A. , Hekkert, B.L. , Gros, J. , Stevens, P. , Muskens, M. , Wouters, D. , Pereira, A. , Stiekema, W. and Allefs, S. (2003) An ancient R gene from the wild potato species Solanum bulbocastanum confers broad‐spectrum resistance to Phytophthora infestans in cultivated potato and tomato. Plant J. 36, 867–882. [DOI] [PubMed] [Google Scholar]
- van der Vossen, E.A.G. , van der Voort, J.N.A.M. , Kanyuka, K. , Bendahmane, A. , Sandbrink, H. , Baulcombe, D.C. , Bakker, J. , Stiekema, W.J. and Klein‐Lankhorst, R.M. (2000) Homologues of a single resistance‐gene cluster in potato confer resistance to distinct pathogens: a virus and a nematode. Plant J. 23, 567–576. [DOI] [PubMed] [Google Scholar]
- van der Vossen, E.A.G. , Gros, J. , Sikkema, A. , Muskens, M. , Wouters, D. , Wolters, P. , Pereira, A. and Allefs, S. (2005) The Rpi‐blb2 gene from Solanum bulbocastanum is an Mi‐1 gene homolog conferring broad‐spectrum late blight resistance in potato. Plant J. 44, 208–222. [DOI] [PubMed] [Google Scholar]
- Walker, J.E. , Saraste, M. , Runswick, M.J. and Gay, N.J. (1982) Distantly related sequences in the alpha‐ and beta‐subunits of ATP synthase, myosin, kinases and other ATP‐requiring enzymes and a common nucleotide binding fold. EMBO J. 1, 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Allefs, S. , van den Berg, R.G. , Vleeshouwers, V.G.A. , van der Vossen, E.A.G. and Vosman, B. (2008) Allele mining in Solanum: conserved homologues of Rpi‐blb1 are identified in Solanum stoloniferum . Theor. Appl. Genet. 116, 933–943. [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Han, C. , Ferreira, A.O. , Yu, X. , Ye, W. , Tripathy, S. , Kale, S.D. , Gu, B. , Sheng, Y. , Sui, Y. , Wang, X. , Zhang, Z. , Cheng, B. , Dong, S. , Shan, W. , Zheng, X. , Dou, D. , Tyler, B.M. and Wang, Y. (2011) Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. Plant Cell, 23, 2064–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, R.F. , Merritt, P.M. , Holub, E. and Innes, R.W. (1999) Identification of three putative signal transduction genes involved in R gene‐specified disease resistance in Arabidopsis. Genetics, 152, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawra, S. , Belmonte, R. , Löbach, L. , Saraiva, M. , Willems, A. and van West, P. (2012a) Secretion, delivery and function of oomycete effector proteins. Curr. Opin. Microbiol. 15, 685–691. [DOI] [PubMed] [Google Scholar]
- Wawra, S. , Agacan, M. , Boddey, J.A. , Davidson, I. , Gachon, C.M. , Zanda, M. , Grouffaud, S. , Whisson, S.C. , Birch, P.R. , Porter, A.J. and van West, P. (2012b) Avirulence protein 3a (AVR3a) from the potato pathogen Phytophthora infestans forms homodimers through its predicted translocation region and does not specifically bind phospholipids. J. Biol. Chem. 287, 38 101–38 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisson, S.C. , Boevink, P.C. , Moleleki, L. , Avrova, A.O. , Morales, J.G. , Gilroy, E.M. , Armstrong, M.R. , Grouffaud, S. , van West, P. , Chapman, S. , Hein, I. , Toth, I.K. , Pritchard, L. and Birch, P. (2007) A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature, 450, 115–118. [DOI] [PubMed] [Google Scholar]
- White, F.F. , Yang, B. and Johnson, L.B. (2000) Prospects for understanding avirulence gene function. Curr. Opin. Plant Biol. 3, 291–298. [DOI] [PubMed] [Google Scholar]
- Yaeno, T. , Li, H. , Chaparro‐Garcia, A. , Schornack, S. , Koshiba, S. , Watanabe, S. , Kigawa, T. , Kamoun, S. and Shirasu, K. (2011) Phosphatidylinositol monophosphate‐binding interface in the oomycete RXLR effector AVR3a is required for its stability in host cells to modulate plant immunity. Proc. Natl. Acad. Sci. USA, 108, 14 682–14 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, N.D. (2000) The genetic architecture of resistance. Curr. Opin. Plant Biol. 3, 285–290. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Goritschnig, S. , Dong, X. and Li, X. (2003) A gain‐of‐function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1‐1, constitutive 1 . Plant Cell, 15, 2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel, C. (2008) Pattern‐recognition receptors in plant innate immunity. Curr. Opin. Immunol. 20, 10–16. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. (2009) Early molecular events in PAMP‐triggered immunity. Curr. Opin. Plant Biol. 12, 414–420. [DOI] [PubMed] [Google Scholar]


