Summary
Sharka disease, caused by Plum pox virus (PPV), is the most important viral disease affecting Prunus species. A major PPV resistance locus (PPVres) has been mapped to the upper part of apricot (Prunus armeniaca) linkage group 1. In this study, a physical map of the PPVres locus in the PPV‐resistant cultivar ‘Goldrich’ was constructed. Bacterial artificial chromosome (BAC) clones belonging to the resistant haplotype contig were sequenced using 454/GS‐FLX Titanium technology. Concurrently, the whole genome of seven apricot varieties (three PPV‐resistant and four PPV‐susceptible) and two PPV‐susceptible apricot relatives (P. sibirica var. davidiana and P. mume) were obtained using the Illumina‐HiSeq2000 platform. Single nucleotide polymorphisms (SNPs) within the mapped interval, recorded from alignments against the peach genome, allowed us to narrow down the PPVres locus to a region of ∼196 kb. Searches for polymorphisms linked in coupling with the resistance led to the identification of 68 variants within 23 predicted transcripts according to peach genome annotation. Candidate resistance genes were ranked combining data from variant calling and predicted functions inferred from sequence homology. Together, the results suggest that members of a cluster of meprin and TRAF‐C homology domain (MATHd)‐containing proteins are the most likely candidate genes for PPV resistance in apricot. Interestingly, MATHd proteins are hypothesized to control long‐distance movement (LDM) of potyviruses in Arabidopsis, and restriction for LDM is also a major component of PPV resistance in apricot. Although the PPV resistance gene(s) remains to be unambiguously identified, these results pave the way to the determination of the underlying mechanism and to the development of more accurate breeding strategies.
Introduction
Sharka disease, caused by Plum pox virus (PPV), was described for the first time infecting plums (Prunus domestica L.) in Bulgaria around 1917 (Atanasoff, 1932). Since then, it has spread into most temperate fruit crop‐growing areas (Capote et al., 2006), and currently is the most important viral disease affecting Prunus species (Scholthof et al., 2011). The global cost of PPV worldwide in the last 30 years has been estimated as 10 000 million euros (Cambra et al., 2006b). PPV is transmitted by aphids in a nonpersistent manner, and therefore chemical treatments are not effective in preventing plant infection. Control measures are primarily focused on the use of certified healthy plants and the eradication of infected trees. However, this latter measure is inefficient because of the time lapse between inoculation and the appearance of symptoms, allowing the persistence of virus reservoirs, especially in endemic areas (Martínez‐Gómez et al., 2000). Moreover, PPV can also infect ornamental and wild Prunus species that serve as potential reservoirs and sources of new inoculum, thereby hindering PPV eradication programmes (James and Thompson, 2006). In this context, although epidemiological studies (Cambra et al., 2006a; Labonne and Dallot, 2006) and improved PPV detection methods (Olmos et al., 2006) have contributed to better management of the disease, the growth of PPV‐resistant varieties would be the ideal long‐term solution.
At the beginning of the 1990s in those European countries severely affected by the disease, apricot (Prunus armeniaca L.) breeding programmes initiated efforts to introgress PPV resistance (reviewed by Badenes and Llácer, 2006; Bassi, 2006; Bassi and Audergon, 2006; Karayiannis, 2006; Rubio et al., 2004). After wide screening of Prunus germplasm, some resistant sources were identified in a handful of North American apricot cultivars (Brooks and Olmo, 1997). These have subsequently been used as PPV resistance donors, even though they are not well adapted to the various growth conditions of different European regions (Martínez‐Gómez et al., 2000). With regard to other Prunus species, some genotypes showing tolerance or a hypersensitive response to PPV infection have been found in plum (Hartmann and Neumüller, 2006), but no resistance sources have yet been found in peach (Prunus persica L. Batsch) (Escalettes et al., 1998). In apricot, the introgression of PPV resistance into commercial cultivars has been accomplished, but breeding progress is still hampered by difficulties inherent to both fruit tree management and PPV resistance phenotyping (Egea et al., 2009; Martínez‐Calvo et al., 2010).
The genetic control of PPV resistance in apricot has remained controversial; however, most inheritance studies agree on the importance of the involvement of one major dominant locus (the PPVres locus) located in the upper part of apricot linkage group 1 (Dondini et al., 2011; Lalli et al., 2008; Lambert et al., 2007; Marandel et al., 2009; Pilarova et al., 2010; Soriano et al., 2008). More recently, Vera‐Ruiz et al. (2011) developed high‐density simple sequence repeat (SSR) linkage maps and narrowed down the PPVres support intervals to 7.3 and 5.9 cM in ‘Lito’ and ‘Goldrich’ maps, respectively, with an estimated size according to the peach genome sequence of 2.16 Mb (Arús et al., 2012; Jung et al., 2004). From this point, Soriano et al. (2012) further refined the PPVres locus to 1.2‐cM and 0.9‐cM intervals in ‘Lito’ and ‘Goldrich’ maps, respectively, corresponding to an interval of ∼270 kb in the peach genome. Moreover, Soriano et al. (2012) developed new markers tightly linked to PPV resistance, providing an efficient tool for marker‐assisted selection in Prunus resistance breeding, and paving the way for future positional cloning by sequencing the PPVres locus. Similar strategies have been employed to identify resistance genes to different pathogens in other tree species. For instance, three clustered genes were identified as candidates for the Rvi15 apple scab resistance gene after sequencing a bacterial artificial chromosome (BAC) clone spanning the resistance locus (Galli et al., 2010). Similarly, Parravicini et al. (2011) discovered in silico two genes as the most probable fire blight resistance genes in apple after sequencing a BAC clone of 189 kb.
In this article, overgo probes, designed from the SSRs spanning the PPVres locus defined by Soriano et al. (2012), have been hybridized against an apricot BAC library in order to construct a physical map for the PPVres region in the cultivar ‘Goldrich’. BAC clones covering the resistant haplotype, as well as nine complete apricot genomes, were sequenced and analysed for polymorphisms within the mapped interval. The identification of segregating markers allowed us to narrow down the PPVres locus to ∼196 kb according to the peach genome syntenic region. Polymorphisms linked in coupling with the resistance were interrogated, and putative functions for all transcripts comprised in this interval were inferred from sequence homology. On the basis of these analyses, candidate resistance genes were ranked and their possible role in apricot PPV resistance is discussed.
Results
Construction of the BAC contig spanning the PPVres locus
The six high‐density filters containing 101 376 clones of the ‘Goldrich’ BAC library were screened in two hybridization rounds. The first round was performed with a pool of six digoxigenin‐labelled overgo probes designed from SSR markers: PGS1.20, PGS1.21, PGS1.22, PGS1.23, PGS1.24 and PGS1.252 (Soriano et al., 2012). Thirteen positive BAC clones were identified and assigned to the corresponding resistant or susceptible haplotype according to the SSR alleles determined by polymerase chain reaction (PCR) typing. Four clones were in coupling phase with the resistance, six in repulsion phase and three were considered as false positives because they could not be confirmed by PCR. In the second round of hybridization, 20 new overgo probes derived from the BAC‐end sequences (BES) of the 10 previously confirmed positive clones were used, identifying 39 additional BACs. After PCR genotyping using BES and SSR primers, 14 clones amplified SSR alleles in coupling with the resistance, six were in repulsion phase and 19 were found to be false positives.
Resistant (R) and susceptible (S) BAC contigs were constructed (Fig. 1) covering the region of ∼270 kb between PGS1.20 and PGS1.252 defined by Soriano et al. (2012) according to the peach genome syntenic region. The R‐contig comprises a region of ∼282 kb fully covered by six BAC clones ranging from ∼24 to ∼119 kb in size. The S‐contig is covered by six BAC clones ranging from ∼25 to ∼80 kb in size and comprises a region of ∼331 kb with an internal gap of ∼58 kb.
Figure 1.
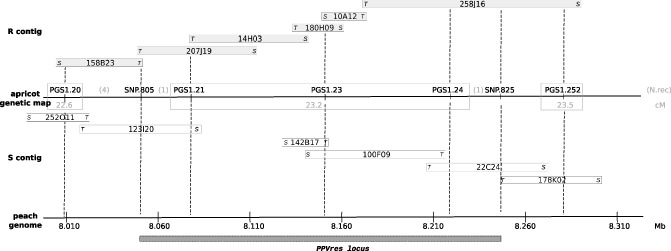
Resistant (R) and susceptible (S) bacterial artificial chromosome (BAC) contigs spanning the PPVres locus in the apricot resistant cultivar ‘Goldrich’. R‐contig BACs (grey boxes) were in coupling phase with Plum pox virus (PPV) resistance and S‐contig BACs (white boxes) were in repulsion phase. BAC‐ends SP6 (S) and T7 (T) are indicated. A line in between these two contigs represents the ‘Goldrich’ genetic map for the PPVres locus. Markers and numbers of recombinants (N.rec) are indicated above the line, and distances in centimorgans (cM) are shown below the line. Markers, anchored into the ‘Goldrich’ genetic map, are connected by broken lines to their syntenic peach genomic positions in megabases (Mb) according to blastn results.
Next‐generation sequencing (NGS) data processing and mapping against the peach genome
Pyrosequencing of the six BACs from the R‐contig produced a total of 127 802 reads (Table 1) ranging from 40 to 641 bp in length and averaging 348 bp, as expected from the 454/GS‐FLX sequencing platform. The sequencing of apricot genomes with Illumina technology produced a total of 1 445 495 513 reads (Table 1), ranging from 91 443 246 for ‘Harlayne’ to 373 801 518 for ‘Canino’. After removing low‐quality regions, as well as vector and adaptor contaminants from all NGS data, 1 391 686 236 trimmed reads (96.27% of raw sequences), with an average length of 95.19 bp for Illumina and 344.79 bp for 454 sequences, were used for subsequent analyses.
Table 1.
Next‐generation sequencing (NGS) data statistics. The total number of raw, cleaned and mapped sequences used from each sample is indicated. Coverage indicates the average number of aligned reads against the reference
| Sample | Phenotype | DNA source | NGS platform | No. raw sequences | Cleaned sequences | Mapped sequences | |||
|---|---|---|---|---|---|---|---|---|---|
| No, | Av. length (bp) | No. | % | Coverage | |||||
| ‘Goldrich’ | R | BACs | 454 | 127802 | 109335 | 344.79 | 43605 | 39.88 | |
| BES, markers | Sanger | 33 | 33 | 1033.88 | 21 | 63.64 | |||
| gDNA | Illumina | 137954275 | 131437990 | 95.18 | 105893 | 0.08 | 221.75 | ||
| ‘Harlayne’ | R | gDNA | Illumina | 91443246 | 87739924 | 95.1 | 70326 | 0.08 | 143.12 |
| ‘Stark Early Orange’ | R | gDNA | Illumina | 156657196 | 150085821 | 95.07 | 114894 | 0.08 | 224.79 |
| ‘Canino’ | S | gDNA | Illumina | 373801518 | 365657436 | 96.40 | 462907 | 0.13 | 1163.02 |
| ‘Krasnoshchekii’ | S | gDNA | Illumina | 142966212 | 137511644 | 95.48 | 114002 | 0.08 | 230.50 |
| ‘Reale d'Imola’ | S | gDNA | Illumina | 161083342 | 152255001 | 95.37 | 121293 | 0.08 | 246.47 |
| ‘Shalakh’ | S | gDNA | Illumina | 97418198 | 93361180 | 93.09 | 66927 | 0.07 | 143.41 |
| Prunus mume | S | gDNA | Illumina | 128834518 | 123740463 | 94.07 | 131072 | 0.11 | 132.95 |
| Prunus sibirica | S | gDNA | Illumina | 155337008 | 149787409 | 96.96 | 133216 | 0.09 | 141.23 |
| Total | 1445623348 | 1381686236 | 1364156 | 0.1 | 294.14 | ||||
BAC, bacterial artificial chromosome; BES, BAC‐end sequence.
Cleaned sequences were aligned onto the peach genome syntenic region from scaffold_1 corresponding to the interval between positions 7 986 205 and 8 281 900, encompassing the interval between PGS1.20 and PGS1.252 positions. As a result, 1 364 156 sequences were successfully mapped, representing 0.1% of the trimmed sequences, and the average read depth for all the bases was 294.14x (Table 1).
Narrowing down the PPVres locus
A selection of single nucleotide polymorphisms (SNPs) identified from the NGS alignment against peach and annotated between positions corresponding to markers PGS1.20–PGS1.21 and PGS1.24–PGS1.252 (Fig. 1) were screened. The identification of segregating SNPs (Table S1, Supporting Information) allowed us to redefine the PPVres locus by introducing them into the ‘Goldrich’ genetic map (Fig. 1). Taking into account the new recombination breakpoints (Fig. 2), the PPVres locus was shrunk to the interval flanked by SNP‐805 (peach genome position: scaffold_1: 8 050 804) and SNP‐825 (peach genome position: scaffold_1: 8 247 059) with three SSR markers co‐segregating with the resistance (PGS1.21, PGS1.23 and PGS1.24) (Fig. 1). As a result, the PPVres locus was predicted to comprise ∼196 kb according to the peach genome syntenic region.
Figure 2.
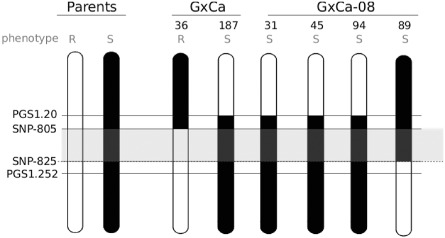
Graphical genotyping of recombinant hybrids belonging to ‘Goldrich' × ‘Canino’ populations (‘G× Ca’ and ‘G×Ca‐08’) at the PPVres locus. Black vertical bars represent susceptible (S) and white bars represent resistant (R) chromosomal regions. Grey box represents the position of the PPVres locus. Recombinant hybrids are numbered at the top.
Screening for candidate resistance genes in the PPVres locus
The annotation of the peach genome sequence by the International Peach Genome Initiative (IPGI), available from the Genome Database for Rosaceae (GDR), showed 31 predicted transcripts in the ∼196‐kb region (Table 2). Three corresponded to alternative transcripts of the same locus (ppa006833m, ppa006841m and ppa006846m). Moreover, three other transcripts (ppb020867m, ppa008960m and ppa017827m), comprised in a ∼22‐kb region (between positions 8 173 840 and 8 196 599), seemed to be partially or completely lost in apricot genomes. Only ppa008960m was present in P. mume and P. sibirica, but showed a low alignment coverage. Variant calling to detect SNPs or deletion/insertion polymorphisms (DIPs) (Tables S2 and S3, see Supporting Information) was performed using the alignment of the NGS data against the peach genome. As a result, between 8022 and 11 272 putative SNPs per genotype and between 2990 and 3242 putative DIPs per genotype were discovered (Table 3). Among the different genic compartments, introns showed greater variant frequency (average: 1 SNP/24 bp and 1 DIP/78 bp) than exons (average: 1 synonymous SNP (sSNP)/140 bp, 1 nonsynonymous SNP (nsSNP)/168 bp and 1 DIP/630 bp) (Table 3). Overall, around 44%–47% of exonic SNPs were nonsynonymous.
Table 2.
Gene content of the PPVres locus in the peach syntenic region. Position and length of the transcripts annotated by the International Peach Genome Initiative (IPGI), as well as the first blastp match on The Arabidopsis Information Resource (TAIR) database, are shown. Overlap length (amino acids), percentage id and E value are indicated for each Prunus/Arabidopsis gene pair. Reciprocal blastp results are also detailed for those pairs being best reciprocal hits (At position in bold). Positions of simple sequence repeat (SSR) and single nucleotide polymorphism (SNP) markers are included as reference. Peach genes not present in apricot genomes are indicated with a grey background
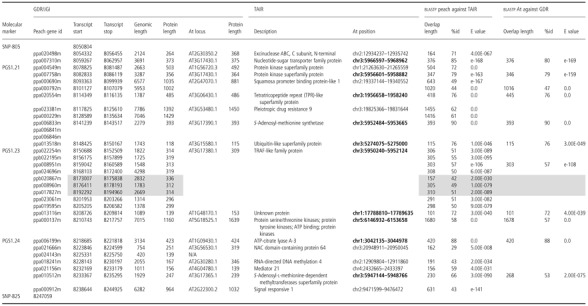
Table 3.
Summary of variants recorded from the alignment of the nine apricot genomes and the bacterial artificial chromosome (BAC) contig sequences against the 196‐kb region of the peach genome containing the PPVres locus. Distribution in different genic compartments is shown for each sample and on average
| ‘Goldrich’ | 8022 | 30 | 1618 | 530/459 | 69 | 5316 | 2990 | 3 | 592 | 139 | 20 | 2236 |
| ‘Harlayne’ | 11070 | 38 | 1937 | 743/584 | 70 | 7698 | 3081 | 5 | 555 | 148 | 19 | 2354 |
| ‘Stark Early Orange’ | 10949 | 30 | 1957 | 727/587 | 72 | 7576 | 3037 | 3 | 577 | 167 | 18 | 2272 |
| ‘Canino’ | 8187 | 31 | 1609 | 501/436 | 70 | 5540 | 3098 | 6 | 571 | 136 | 25 | 2360 |
| ‘Krasnoshchekii’ | 10826 | 31 | 1960 | 732/627 | 73 | 7403 | 3057 | 5 | 605 | 148 | 19 | 2280 |
| ‘Reale d'Imola’ | 10754 | 33 | 1925 | 725/578 | 71 | 7422 | 3050 | 5 | 570 | 163 | 19 | 2293 |
| ‘Shalakh’ | 10678 | 40 | 1942 | 728/583 | 68 | 7317 | 3065 | 5 | 580 | 166 | 16 | 2288 |
| Prunus mume | 11272 | 45 | 1944 | 755/666 | 72 | 7790 | 3242 | 4 | 565 | 154 | 16 | 2503 |
| Prunus sibirica | 11225 | 52 | 2014 | 779/674 | 74 | 7632 | 3075 | 9 | 616 | 162 | 21 | 2267 |
| Average | 10331.44 | 36.7 | 1878.4 | 691/577 | 71.0 | 7077.1 | 3077.2 | 5.0 | 581.2 | 153.7 | 19.2 | 2317.0 |
| % | 0.4 | 18.2 | 6.7/5.6 | 0.7 | 68.5 | 0.2 | 18.9 | 5.0 | 0.6 | 75.3 | ||
| Length region | 196255 | 806 | 45303 | 96860 | 2552 | 50734 | 196255 | 806 | 45303 | 96860 | 2552 | 50734 |
| Frequency (1 variant/X bp) | 19.0 | 22.0 | 24.1 | 140.2/167.9 | 35.9 | 7.2 | 63.8 | 161.2 | 77.9 | 630.3 | 132.8 | 21.9 |
In the screening for candidate resistance genes within the PPVres locus, three premises based on prior knowledge were taken into account. First, PPV resistance is generally accepted to be controlled by a major dominant locus (Dondini et al., 2011; Lalli et al., 2008; Lambert et al., 2007; Marandel et al., 2009; Pilarova et al., 2010; Soriano et al., 2008). Second, the allelic composition of the PPVres locus suggests a common PPV‐resistant ancestor for the resistant cultivars used in this study (‘Goldrich’, ‘Harlayne’ and ‘Stark Early Orange’) (Pilarova et al., 2010; Soriano et al., 2012; Zhebentyayeva et al., 2008). Third, segregation ratios recorded from different apricot intraspecific populations confirm that these resistant varieties are heterozygous for PPV resistance (Karayiannis et al., 2008; Soriano et al., 2012).
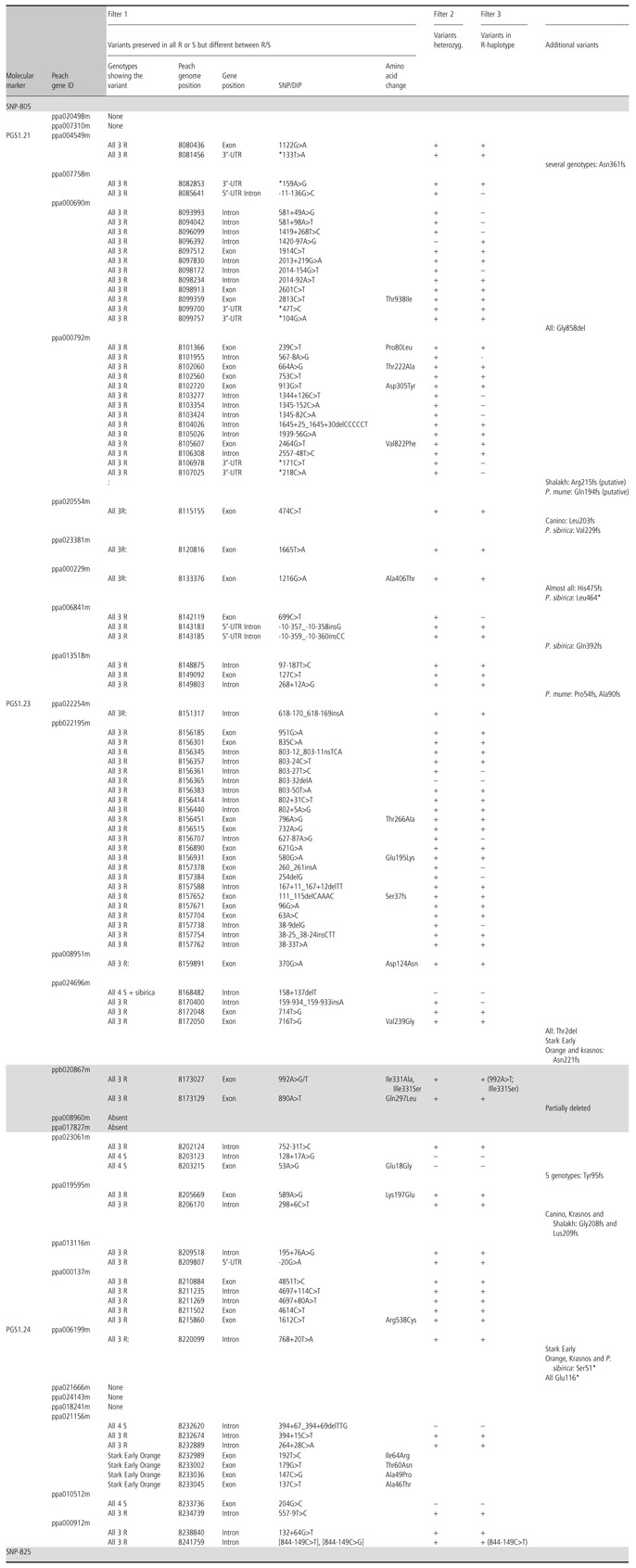
According to these premises, three filters were sequentially applied (Table 4) to discriminate SNPs/DIPs associated with resistance from all detected variants after mapping against the susceptible peach (Table S2). In the first, variants preserved in all resistant or all susceptible varieties were screened. In addition, variants preserved in both ‘Goldrich’ and ‘Harlayne’, or only in ‘Stark Early Orange’, but not in any susceptible variety, were also screened in order to detect mutations affecting the same responsible gene that could be different in ‘Stark Early Orange’ and the other two accessions. As a result, 87 variants within 23 predicted transcripts were detected, and therefore five transcripts (ppa020498m, ppa007310m, ppa021666m, ppa024143m and ppa018241m) were discarded (Table 4). The second filter selected heterozygous variants present in resistant varieties, discarding two of the original 87 (Table 4). Lastly, the third filter selected variants in coupling with the resistance by checking their presence in the BAC R‐contig sequences (Table S3), and reduced the number of variants to 68 located within 23 predicted transcripts, ranging from 1 to 17 per transcript (Table 4). Twelve of these 23 transcripts had additional variants predicted to produce early stop codons, resulting in putative truncated proteins in one or more PPV‐susceptible varieties (Table 4). These 68 variants can be roughly classified into nonsynonymous (12 lead to amino acid changes and one produces a frameshift) and synonymous [16 in exons, 28 in intronic regions and 11 in untranslated regions (UTRs)] (Table 4). Two transcripts show only a single nonsynonymous mutation, 14 show only synonymous mutations (ranging from one to three each) and seven have both (ranging from one to four nonsynonymous and 1–14 synonymous in each). A total of 33 variants are located within just three transcripts.
Table 4.
Filtering process of variants potentially associated with Plum pox virus (PPV) resistance. Filter 1 selected variants conserved in all resistant or all susceptible varieties indicating their type, peach genome position and genic compartments. Filter 2 identified variants present in heterozygosity. Filter 3 found variants present in the R‐haplotype of ‘Goldrich’. Variants putatively leading to frameshift mutations in some genotypes are indicated in the last column. Boundaries of the PPVres locus and peach genes not present in apricot genomes are indicated with a grey background
For most of the transcripts present in the PPVres locus, highly similar Arabidopsis proteins were found, but a few showed moderate/low similarity values with Arabidopsis peptides (Table 2). This was the case for ppb020867, ppa021666m and ppa018241m, all having id < 50%, overlap length < 55% and E values > 1e‐34. Others, such as ppa000690, ppa000792 and ppa008960, showed id < 50%, or an overlap length < 50%, such as ppa020498. In addition, ppa013116 showed homology with an ‘unknown protein’ according to The Arabidopsis Information Resource (TAIR) database (Table 2). Significant similarity degrees were also found between some annotated transcripts in peach. Three were alternative transcripts of the same locus (ppa006841m, ppa006833m and ppa006846m) sharing three variants, but differing in the beginning of the intronic region within the 5′‐UTR. Another two were adjacent transcripts, ppa000690m and ppa000792m, which showed a high similarity (id = 81.4%). Finally, six meprin and TRAF‐C homology domain (MATHd)‐containing genes clustered together in apricot, between positions 8 150 688 and 8 206 582, whereas, in the peach genome, nine MATHd genes were present in the cluster. All six MATHd genes had variants linked in coupling with the resistance, but ppa022254m and ppa023061 had only a 1‐bp insertion within the fourth and fifth introns, respectively. Therefore, there is a low probability that they have any impact on protein function. The other four transcripts showed variants that might have stronger effects. ppa008951m had one nsSNP within the first MATH domain; ppa024696m and ppa019595m had one nsSNP within the second MATH domain and also additional frameshift mutations in some varieties; ppb022195m had a 5‐bp deletion in the three resistant varieties within the second exon that should produce a truncated protein having just 42 amino acids, leading to a loss of both MATH domains.
The 23 transcripts selected during the filtering process can be roughly classified into two groups according to their homology with proteins involved in virus resistance (see Discussion section and Table 2). The first group comprises six transcripts (ppa023381m, ppa000229m, ppa006199m, ppa013518m, ppa020554m, ppa013116m) putatively coding for proteins unrelated to pathogen resistance according to the accumulated evidence. In the second group, eight transcripts (ppa006833m, ppa006841m, ppa006846m, ppa021156m, ppa010512m, ppa00912m, ppa000690, ppa000792m) are somehow related to biotic stress resistance (and, particularly, in some cases with virus resistance), but not to cell‐to‐cell or long‐distance viral movement. In addition, nine transcripts (three putatively coding for serine/threonine kinases and six for MATHd proteins) seem to be closely associated with potyvirus resistance and, particularly, with PPV resistance in Arabidopsis regarding MATHd proteins.
Microsynteny of peach vs. Arabidopsis
The sha3 locus has been reported recently to control PPV infection in Arabidopsis thaliana (Cosson et al., 2010; Pagny et al., 2012). In order to identify putative orthologous genes between Prunus and A. thaliana, blastp analysis of the 31 predicted proteins within the PPVres locus was performed against the TAIR database (E value cut‐off < 1e‐6) (Table 2 and Fig. S1, see Supporting Information). Thus, Prunus/Arabidopsis gene pairs that are blastp best‐reciprocal hits (BRHs) were considered as putative orthologues. Under this criterion, seven peach transcripts were found to be putative orthologues of Arabidopsis transcripts located in the upper part of chromosome 3 (ppa020554m at position ∼1.96 Mb and the rest between ∼5.27–5.97 Mb), two were located in chromosome 1, one at the distal end of chromosome 2 and another on the upper part of chromosome 5.
Putative orthologues were also supported by the conserved gene order between both species along the defined chromosome region (Nozawa and Nei, 2007; Zheng et al., 2005). Interestingly, five of the seven Arabidopsis transcripts located in chromosome 3 preserved the same order, but inverted relative to their putative peach orthologues (Table 2). In addition, all nine peach transcripts putatively coding for TRAF‐like family proteins showed high homology with one Arabidopsis transcript (At3g17380), but ppa008951m was the only blastp BRH with a 55.41% identity along 88.15% of the QS length and an E value of 5e‐95. blasting At3g17380.1 (located in scaffold_3: 5950200–5952235) against the peach genome predicted peptides showed high‐similarity E values (ranging from 7e‐108 to 1e‐80) with all nine TRAF‐like genes present in the cluster.
In addition, blastp analysis of the restricted Tobacco etch virus (TEV) movement 3 (RTM3) protein (coded by the At3g58350.1 transcript located within the sha3 locus in scaffold_3: 21 591 452–21 592 962) against the peach predicted peptides showed a 29.76% similarity along 96% of the QS length (with an E value of 5e‐23) with ppa024552m, a ubiquitin carboxyl‐terminal hydrolase 12 protein (containing one MATH domain) located in the peach scaffold_2: 4 372 558–4 374 144.
Discussion
Physical mapping and variant calling from NGS data
Fine mapping of the PPVres locus in apricot was hampered for years by the difficulties inherent to fruit tree management, the lack of large segregating populations and the limited efficiency of phenotypic resistance assays (Llácer et al., 2007). Recently, Soriano et al. (2012) restricted the PPVres locus to a short interval of ∼270 kb by combining data from two PPV resistance sources (‘Goldrich’ and ‘Stark Early Orange’). Here, we have constructed a detailed physical map of this region by assembling ‘Goldrich’ BAC clones into two separate haplotype contigs and by performing deep sequencing of both the R‐haplotype contig and the entire genome of nine apricot accessions. Segregating SNPs found in the NGS data alignment against the peach genome were used to narrow down the PPVres locus to ∼196 kb. In agreement with the high degree of map collinearity found among Prunus spp. (Dirlewanger et al., 2004; Dondini et al., 2007; Lambert et al., 2004; Olmstead et al., 2008), over 93% of similarity was detected in the PPVres locus between peach and P. armeniaca, P. mume and P. sibirica. However, discrepancies were also found: several misalignments (gaps) were detected (data not shown), and three peach MATH domain‐containing transcripts were found to be partially or completely lost in apricot. Moreover, the presence of additional genes in apricot could not be fully discarded.
Comparison of resistant/susceptible genotypes allowed us to screen for differential variants at the PPVres locus by considering the genetic evidence supporting the proposed model for PPV resistance in apricot (Soriano et al., 2008; Vera‐Ruiz et al., 2011). Thus, only those variants in coupling with the resistance and present in heterozygosity in all the resistant varieties or, alternatively, variants in repulsion with the resistance and present in homozygosity in all the susceptible varieties, were considered to be relevant. A total of 68 variants within 23 putative transcripts were found to fulfil these requirements, but none belonged to the second type. From these, 23% were nonsynonymous variants, leading to amino acid or frameshift changes.
It is generally accepted that most nonsilent mutations are not effectively neutral, leading to functional changes in the affected protein in hominids, Drosophila and enteric bacteria (Eyre‐Walker and Keightley, 2007). However, a recent survey across human SNP–disease associations showed that, although DNA variants pursued for functional validation are usually those predicted to lead to significant amino acid changes, sSNPs show a similar likelihood and effect size for disease association (Chen et al., 2010). Interestingly, variants causing a premature stop codon are most likely to be associated with disease and have a higher effect size than other SNP types (Chen et al., 2010). As a whole, none of the 23 transcripts can be fully rejected according to the reported variants. In this study, we have combined information derived from the type of mutation detected in every transcript and their putative function inferred from sequence homology to classify them with regard to their likelihood of being candidate genes.
Ranking candidate genes within the PPVres locus
In recent years, a number of dominant and recessive virus resistance genes have been identified and characterized in plants (Maule et al., 2007). Dominant resistance is generally associated with so‐called R genes that confer resistance to bacteria, fungi, nematodes and viruses (Soosaar et al., 2005). In particular, R genes conferring dominant virus resistance mostly fall into the nucleotide‐binding site‐leucine‐rich repeat class (Maule et al., 2007). However, there are a few exceptions, such as the RTM resistance genes. Interestingly, the first three RTM genes were initially found to restrict long‐distance movement (LDM) of TEV in A. thaliana, but recent evidence has allowed their effects to be extended to other potyviruses, such as PPV (Decroocq et al., 2006). Recently, two new RTM loci have been genetically identified in A. thaliana (Cosson et al., 2012), indicating that at least five proteins are involved in the complex RTM resistance process. However, recessive resistance has been commonly related to mutations of components of the eukaryotic translation initiation complex, mostly affecting the eukaryotic initiation factor 4E necessary for virus replication (Robaglia and Caranta, 2006). Their implication in PPV resistance has not been fully demonstrated, but a striking correlation was found by Marandel et al. (2009) in Prunus davidiana (Carrière) Franch. Nevertheless, in spite of these significant advances, no factor involved in the PPV resistance mechanism has been identified to date in its natural host, Prunus spp.
Fine mapping and NGS variant analysis led us to restrict the number of candidate genes for PPV resistance in apricot to 23. However, implication in PPV resistance of 12 of these seems quite unlikely: six have little or no direct connection to genes known to confer resistance to pathogens, and the other six have been linked to virus resistance, but the reported phenotypes differ substantially from that which has been observed for PPV resistance in apricot. Within this latter group, ppa006833m, ppa006841m and ppa006846m represent alternative transcripts of a unique gene sharing as BRH an Arabidopsis S‐adenosyl‐methionine (AdoMet) synthetase. Recently, Użarowska et al. (2009) identified an AdoMet synthetase as a candidate gene for resistance to two potyviruses in maize. However, other candidate genes were also proposed by these authors, and therefore a direct correlation with this phenotype should be further confirmed. Moreover, the role of the apricot transcript does not seem plausible as two DIPs within the intronic region of the 5′‐UTR should explain the PPV resistance phenotype. Three additional transcripts putatively encode for peptides highly similar to transcriptional regulatory proteins. ppa021156m is similar to the MED21 subunit (At4g04780) of the mediator multiprotein complex, a coactivator required by DNA‐binding transcription factors for transcriptional activation of polymerase II‐transcribed genes (Sato et al., 2004). Reduced expression of Arabidopsis MED21 has been associated with disease susceptibility and embryo‐lethal phenotypes (Dhawana et al., 2009). The first intron has been suggested to play an important regulatory role in many genes, most probably involved in transcriptional control (Majewski and Ott, 2012). This apricot transcript has one SNP within the first intron in the resistant varieties, but its location at the beginning of the intron could imply a low plausibility of its effect (Chen et al., 2010). ppa010512m is the BRH of the At3g17365 protein, an AdoMet‐dependent methyltransferase. Recently, an AdoMet methyltransferase was described as an innate defence protein against potexvirus accumulation in Nicotiana benthamiana (Cheng et al., 2009). However, the implication of AdoMet methyltransferases has not been reported for potyvirus infection. Moreover, resistant varieties have just one SNP within the seventh intron, and therefore a role for this gene in PPV resistance seems unlikely. Lastly, ppa000912m is partially similar to the Arabidopsis signal responsive 1 (SR1) protein, which has been described as a negative regulator of plant defences (Qiu et al., 2012). SR1 acts as a negative regulator of salicylic acid (SA)‐mediated plant immunity by repressing the transcription of two SA signaling‐positive regulators (Du et al., 2009; Nie et al., 2012). ppa000912m has two SNPs predicted within an exonic splicing enhancer of the first intron and within the fifth intron, respectively. Moreover, dominant loss‐of‐function SR1 mutants have been shown to display enhanced resistance to fungal and bacterial pathogens (Nie et al., 2012). As a whole, this evidence would support a possible implication of this gene in the apricot PPV resistance. However, the type of constitutive disease resistance (including the hypersensitive response) associated with SR1 mutants (Du et al., 2009) does not fit with the observed symptoms during PPV infection in apricot.
Candidate genes for PPV resistance in apricot
The compiled evidence suggests that PPV resistance in apricot is more closely linked to the restriction of the virus LDM than to specific host resistance responses (Dicenta et al., 2003; Ion‐Nagy et al., 2006). Thus, the most compelling candidate genes within the PPVres locus include three types of genes encoding proteins with known functions associated with the control of virus movement. In the first type, ppa000690m and ppa000792m have a plant‐specific DNA‐binding domain and also ankyrin repeats. Both transcripts are homologous to the squamosa promoter‐binding‐like protein 1 belonging to a family of DNA‐binding proteins and putative transcription factors (Cardon et al., 1999). Ankyrin repeats have been related to cell‐to‐cell tobamovirus movement (Ueki et al., 2010); however, no similarity between the apricot transcripts and the ankyrin repeat‐containing protein described by Ueki et al. (2010) was observed (data not shown). Moreover, the ankyrin repeat has been found in many proteins, spanning a wide range of functions, typically involved in the mediation of specific protein–protein interactions (Mosavi et al., 2004). Altogether, the implication of these two proteins in PPV resistance in apricot seems quite unlikely.
The second class includes three transcripts showing similarity to serine/threonine protein kinases (STKs), which have been reported to be important factors for defence signal transduction (Xu and Deng, 2010). Moreover, glycosylation and phosphorylation have been suggested to play, in a broad sense, crucial roles in the regulation of potyviral capsid protein (CP) functions (Fernández‐Fernández et al., 2002), among which is involvement in the movement of infectious viral RNA from cell to cell and over long distances (Dolja et al., 1994). ppa004549m shows high similarity to three Arabidopsis STK proteins (At1g56720, At5g18500 and At2g42960). Its R‐haplotype has one SNP within the 3′‐UTR that could have an important effect on gene expression regulation, because it is located within a predicted hsa‐miR‐223 target site. Interaction between microRNA (miRNA) and its target mRNA appears to result in translational repression or, in some cases, cleavage of cognate mRNAs, causing partial or full silencing of the respective protein‐coding genes (Laios et al., 2008). We could hypothesize that the expression of this STK protein could not be repressed in apricot‐resistant varieties, therefore having an active phosphorylation function, and, conversely, phosphorylation of viral movement proteins has been implicated in plant virus movement (Ivanov et al., 2003). ppa000137m is the putative orthologue of the Arabidopsis At5g18525, a DDB1‐CUL4‐associated factor 1 (DCAF1) protein which could be a substrate receptor for the CUL4 RING ubiquitin ligase complex (Lee et al., 2008). Human DCAF1 acts by providing human immunodeficiency virus type 1 with the equipment for the degradation of specific host proteins and by counteracting its proteasome targeting by another cellular E3 ubiquitin ligase (Le Rouzic et al., 2008). However, in plants, mutant analyses have shown that DCAF1 has an essential role in plant embryogenesis and is involved in multiple developmental pathways (Zhang et al., 2008). ppa007758m, a putative orthologue of the Arabidopsis At3g17410 STK protein, is highly similar to the Solanum lycopersicum L. Pto kinase interactor 1 (Pti1) (id = 87%). It has one SNP within a predicted selenocysteine insertion sequence element in the 3′‐UTR, a region thought to be implicated in protein–protein interactions (Martin et al., 1998). Moreover, At3g17410 expression was induced by infections of PPV and other positive‐sense RNA viruses (Babu et al., 2008). However, Pti has been reported to be involved in the Pto‐mediated signalling pathway, leading to the hypersensitive response against fungi (Zhou et al., 1995), a type of response that does not fit with the observed phenotype in the case of PPV resistance in apricot.
The third class of PPVres locus candidate genes is formed by a cluster of six MATHd‐containing genes, two of which have synonymous variants with putatively low impact, three of which have at least one amino acid change within a MATH domain, and one of which has a 5‐bp deletion that should produce a truncated protein lacking both MATH domains. Taking into account that the mutated allele is dominant and shows resistance in heterozygosity, the implication of these apricot transcripts could be more plausibly explained by a gain‐of‐function or a dominant negative mutation, where either the mutant protein inhibits directly the activity of the wild‐type protein through dimerization, or competes with the wild‐type protein for another protein that is required for normal function (Fay and Spencer, 2005).
Interestingly, other MATHd‐containing proteins have been described to be involved in virus LDM. The RTM3 gene, which encodes a MATHd‐only protein (Cosson et al., 2010), is one of the dominant RTM genes underlying potyvirus resistance previously described in Arabidopsis (Chisholm et al., 2001; Decroocq et al., 2006; Mahajan et al., 1998). However, a mutation in any one of the three first identified RTM genes completely abolishes the resistance (Cosson et al., 2010). Although compelling, the genetics of RTM resistance seems to be different from that of PPV in apricot, as the mutated protein is predicted to confer PPV resistance in heterozygosity. Interestingly, the sha3 locus, which encompasses the RTM3 gene and includes another seven RTM3‐like TRAF domain‐containing genes, has been described recently to restrict PPV LDM in Arabidopsis in a recessive fashion (Pagny et al., 2012). However, apricot MATH cluster genes show highest similarity to At3g17380 in Arabidopsis, which is located on the upper part of chromosome 3, whereas the sha3 locus maps at the bottom of the same chromosome. It has been suggested that most of the MATH genes of the MATHd‐only protein family are organized in clusters in the Arabidopsis genome (Cosson et al., 2010). Similarly, in the apricot PPVres locus and in the syntenic peach region, clusters of six and nine TRAF‐like genes, respectively, were observed. However, their putative Arabidopsis orthologous gene (At3g17380) seems to be a single‐copy gene, suggesting that duplication events probably occurred after the diversification of these species. Finally, the two new genetically identified RTM loci are not in PPVres locus syntenic locations, as RTM4 maps in Arabidopsis chromosome 1 and RTM5 in chromosome 2 (Cosson et al., 2012).
In conclusion, this is the first report in which whole‐genome sequencing (WGS) (seven apricot varieties and two wild relatives) and the physical map of the PPVres locus have been combined. Our data support member(s) of a cluster of MATHd‐containing genes as the most promising candidate gene(s) for PPV resistance in apricot. The functions of the MATHd‐like proteins are not well known, although currently available evidence suggests that they might link specific protein substrates to ubiquitin ligase complexes (Zapata et al., 2007). Functional analyses are currently in progress in Arabidopsis and plum, taking advantage of the high transformation efficiency of the latter species (Petri et al., 2008), in order to elucidate the role of TRAF‐like proteins in PPV resistance. The results presented here will contribute to the implementation of new strategies to improve breeding for resistance, including the introgression of resistance monitored by marker‐assisted selection. They will also contribute to increase the knowledge of the natural resistance to PPV in the main plant–pathogen systems of PPV.
Experimental Procedures
Plant material
Nine apricot genotypes were selected for WGS, representing three PPV‐resistant cultivars (‘Goldrich’, ‘Stark Early Orange’ and ‘Harlayne’), four PPV‐susceptible cultivars [‘Canino’, ‘Shalakh’, ‘Krasnoshchekii (syn. ‘Hungarian Best’) and ‘Reale d'Imola'] and two PPV‐susceptible nondomesticated species: Prunus mume (Sieb.) Sieb & Zucc. and Prunus sibirica L. var. davidiana (Carrière) (Table 5). For WGS, all cultivars and species, except ‘Canino’, came from the repository at Nikita Botanical Garden, Crimea, Ukraine, and have been genotyped previously in Zhebentyayeva et al. (2008). The Spanish genotype ‘Canino’ is maintained as part of the germplasm collection at IVIA, Valencia, Spain.
Table 5.
Apricot cultivars used in this study. Geographical origin, pedigree and Plum pox virus (PPV) resistance phenotype are indicated
| Cultivar | Geographical area | Country of origin | Pedigree | PPV resistance | Reference |
|---|---|---|---|---|---|
| ‘Goldrich’ | North America | USA | Sunglo × Perfection* | R | Dosba et al. (1992) |
| ‘Harlayne’ | North America | Canada | [(Reliable × o.p.) × o.p.] × Sunglo* | R | Dosba et al. (1992) |
| ‘Stark Early Orange’ | North America | USA | Unknown* | R | Syrgiannidis (1980) |
| ‘Canino’ | Western Europe | Spain | Unknown† | S | Avinent et al. (1993) |
| ‘Krasnoshchekii’ | Eastern Europe | Ukraine | Unknown‡ | S | Evaluated at IVIA |
| ‘Reale d'Imola’ | Western Europe | Italy | Unknown† | S | Tradafirescu and Topor (1999) |
| ‘Shalakh’ (syn. Erevan) | Irano‐Caucasian | Armenia | Unknown‡ | S | Karayiannis (1989) |
| Prunus mume | Japan and China§ | S | James and Thompson (2006) | ||
| Prunus sibirica | Eastern Siberia, Manchuria and northern China§ | S | James and Thompson (2006) |
DNA extraction
Genomic DNA from ‘G×Ca’ and ‘G×Ca‐08’ recombinant hybrids and their parents (‘Goldrich’ and ‘Canino’) was isolated from young leaves using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's protocol, and stored at −20 °C until use. Genomic DNA from the Nikita Botanical Garden samples was extracted and stored at −20 °C according to the previously published protocol by Zhebentyayeva et al. (2008). DNA integrity was checked on agarose gel and quantification was performed using a Nanodrop ND‐1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA).
Construction of the BAC contig spanning the PPVres locus
BAC clone identification was carried out using digoxigenin‐labelled overgo probes hybridized in pools (Madishetty et al., 2007) against an apricot BAC library developed from the PPV‐resistant cultivar ‘Goldrich’ and printed on six high‐density filters (Vilanova et al., 2003). Overgo probes were designed using the Overgo 1.02i software (http://www.mouse‐genome.bcm.tmc.edu/webovergo/OvergoInput.asp), prepared as described by Hilario et al. (2007) and hybridized following the manufacturer's instructions (Roche, Basle, Switzerland). In a first hybridization round, six overgo probes were designed using, as templates, the flanking sequences of the SSR markers developed for the fine mapping of the PPVres locus by Soriano et al. (2012). Positive BAC clones were assigned to their corresponding R‐ or S‐haplotype by PCR screening. BES were obtained from all positive clones, and primer pairs were designed using Primer3 software (Rozen and Skaletsky, 2000) to verify overlapping BACs by PCR. Twenty additional overgo probes were designed from BES for a second hybridization round to cover those gaps in which clones did not overlap. BES were blasted against the peach v1.0 genome sequence (IPGI, http://www.rosaceae.org/species/prunus_persica/genome_v1.0) using a stand‐alone version of blast (Altschul et al., 1990) in order to indicate the orientation of the BAC clones and to provide an indirect estimation of their size.
Next‐generation sequencing
BAC clones of the R‐haplotype contig cv. ‘Goldrich’ were massively parallel pyrosequenced as a pool using 454 GS‐FLX Titanium NGS technology (Roche), commercially conducted by Macrogen Inc. (Seoul, South Korea). WGS of the nine apricot genomes was conducted on an Illumina HiSeq2000 platform, using 100‐bp paired‐end reads. Sequencing of cv. ‘Canino’ was commercially conducted by Macrogen Inc. The remaining six cultivars and two nondomesticated species were sequenced at genomic facilities at DHMRI (David H. Murdock Research Institution, Kannapolis, NC, USA; http://www.dhmri.org).
NGS data analysis
All raw reads were processed using the ngs_backbone pipeline (Blanca et al., 2011) with the configuration file ‘backbone.conf’ (File S1, see Supporting Information). After removing the low‐quality regions as well as vector and adaptor contaminants, cleaned reads were aligned to the peach genome syntenic region using CLC Genomics Workbench software v. 5.1 (Aarhus, Denmark). The FASTA sequence of the syntenic peach genome region was downloaded from the GDR website (http://www.rosaceae.org) (Jung et al., 2004) and used as reference. Variant calling to detect SNPs or DIPs was performed with CLC Genomics Workbench software v. 5.1 using the following parameters: DIPs: minimum (min) coverage, 4; min variant frequency, 35%; maximum (max) expected variation, 2; SNPs: window length, 11; max number of gaps and mismatches, 3; min average of surrounding bases, 15; min quality of central base, 20; min coverage, 4; min variant frequency, 35%; max expected variation, 2.
SNP linkage map
A selection of SNPs identified from the NGS data and annotated between positions corresponding to markers PGS1.20–PGS1.21 and PGS1.24–PGS1.252 were screened in ‘Goldrich’, ‘Canino’ and the six recombinant hybrids described by Soriano et al. (2012) in order to narrow down the PPVres locus. Primers (Table S1) were developed using Primer3 software (Rozen and Skaletsky, 2000). PCR amplifications were performed in a GeneAmp® PCR System 9700 thermal cycler (Perkin–Elmer, Freemont, CA, USA) in a final volume of 20 μL, containing 75 mm Tris‐HCl, pH 8.8, 20 mm (NH4)2SO4, 1.5 mm MgCl2, 0.1 mm of each deoxynucleoside triphosphate (dNTP), 20 ng of genomic DNA and 1 U of Taq polymerase (Invitrogen, Carlsbad, CA, USA). The temperature profile was: 10 min at 95 °C, followed by 40 cycles of 20 s at 94 °C, 45 s at 52 °C and 1 min at 72 °C, finishing with 10 min at 72 °C. After amplification, PCR products were purified following the manufacturer's instructions for the High Pure PCR Product Purification Kit (Roche). DNA sequencing was performed using an ABI Prism 3130 genetic analyser, following the manufacturer's instructions for the BigDye terminator v.3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA). Each PCR fragment was sequenced using both forward and reverse primers. Sequences were edited and assembled using the PREGAP4 and GAP4 modules of the Staden software package (Staden et al., 2000).
Filtering of SNPs and DIPs in coupling with PPV resistance
Peach genome annotation data available from the GDR were used as a reference to identify associated polymorphisms within the predicted transcripts.
In order to discriminate the SNPs/DIPs linked in coupling with the resistance from all the variants found against peach, three filters were sequentially applied: (i) selected variants must be conserved within the group of resistant or susceptible varieties, being different between groups; (ii) resistant cultivars must be heterozygous for the variant; (iii) variants must be present in the resistant haplotype and therefore in the 454 BAC contig sequences. Complete transcripts with variants fulfilling these three requirements were interrogated in order to detect additional changes that might discard them as candidate genes for PPV resistance. All the routine of filtering and mathematical/statistical calculations was performed using OpenOffice Calc 3.0.1.
Microsynteny analysis between peach and A. thaliana
blastp analysis of the 31 proteins encoded by the predicted transcripts comprised within the peach syntenic region was performed against the TAIR database (version TAIR10 proteins), using the TAIR blast 2.2.8 tool (http://www.arabidopsis.org/Blast/index.jsp), with an E value cut‐off < 1e‐6, in order to predict gene functions based on homology (Table 2). This table also indicates those Prunus/Arabidopsis gene pairs that are blastp BRHs (blasting Arabidopsis proteins against the peach predicted peptides annotated by IPGI) identifying putative orthologues. The same procedure was used to predict putative orthologues in Prunus of some A. thaliana genes present in the sha3 locus (Pagny et al., 2012).
Supporting information
Fig. S1 Comparative analysis of the Prunus PPVres locus with the Arabidopsis genome. Peach transcripts, annotated by the International Peach Genome Initiative (IPGI), are represented by black boxes. Full lines connect Prunus/Arabidopsis gene pairs that are best‐reciprocal blastp hits (E value cut‐off < 1e‐6). Broken lines correspond to the best unidirectional blastp hit when no best‐reciprocal hit was obtained. Distances in kilobases are shown on the left of the peach physical map and in megabases on the right of the Arabidopsis chromosomes. Markers defining the PPVres locus are represented on the left of the peach map as a reference.
File S1 Configuration parameters for cleaning sequence reads with ngs_backbone pipeline (backbone.conf).
Table S1 Overgo and polymerase chain reaction (PCR) primers employed.
Table S2 Variants [single nucleotide polymorphisms (SNPs)/deletion/insertion polymorphisms (DIPs)] recorded from the alignment of the nine apricot genomes and bacterial artificial chromosome (BAC) contig sequences against the peach genome.
Table S3 Variants [single nucleotide polymorphisms (SNPs)/deletion/insertion polymorphisms (DIPs)] recorded from the alignment of the bacterial artificial chromosome (BAC) contig sequences from the R‐haplotype of ‘Goldrich’ against the peach genome.
Acknowledgements
This research was supported by grants from the Ministerio de Educación y Ciencia of Spain (Research Projects AGL2007‐60709 and AGL2010‐20595), the European Union Seventh Framework Programme (FP7/2007–2013, grant agreement: 204429), the European Regional Development Fund (ERDF), a grant from the US Department of Agriculture (USDA) Foreign Agricultural Service (FAS) Technical Assistance for Specialty Crops (TASC) program and a cooperative agreement between USDA‐ARS/Clemson University.
We acknowledge Dr Albert Glenn Abbott for his comments on the manuscript.
References
- Altschul, S.F. , Gish, W. , Miller, W. , Myers, E.W. and Lipman, D.J. (1990) Basic local alignment search tool. J. Mol. Biol. 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Arús, P. , Verde, I. , Sosinski, B. , Zhebentyayeva, T. and Abbott, A. (2012) The peach genome. Tree Genet. Genomes, 8, 531–547. [Google Scholar]
- Atanasoff, D. (1932) Plum Pox. A New Virus Disease. Bulgaria: Yearbook University of Sofia 1932/33, Faculty of Agronomy. [Google Scholar]
- Avinent, L. , Hermoso de Mendoza, A. , Llácer, G. and García, S. (1993) Transmisión del virus de la sharka y sensibilidad varietal en albaricoquero In: Actas del II Congreso Ibérico de Ciencias Hortícolas, Zaragoza, Spain (Acta nº 9), pp. 200–206. Madrid: Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria. [Google Scholar]
- Babu, M. , Griffiths, J.S. , Huang, T.S. and Wang, A. (2008) Altered gene expression changes in Arabidopsis leaf tissues and protoplasts in response to Plum pox virus infection. BMC Genomics, 9, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenes, M.L. and Llácer, G. (2006) Breeding for resistance: breeding for Plum pox virus resistant apricots (Prunus armeniaca L.) in Spain. EPPO Bull. 36, 323–326. [Google Scholar]
- Bassi, D. (2006) Breeding for resistance: breeding for resistance to Plum pox virus in Italy. EPPO Bull. 36, 327–329. [Google Scholar]
- Bassi, D. and Audergon, J.M. (2006) Apricot breeding, update and perspectives. Acta Hort. 701, 279–294. [Google Scholar]
- Blanca, J.M. , Pascual, L. , Ziarsolo, P. , Nuez, F. and Cañizares, J. (2011) Ngs_backbone: a pipeline for read cleaning, mapping and SNP calling using Next Generation Sequence. BMC Genomics, 12, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, R.M. and Olmo, H.P. (1997) The Brooks and Olmo Register of Fruit and Nut Varieties, 3rd edn. Alexandria: ASHS Press. [Google Scholar]
- Cambra, M. , Capote, N. , Cambra, M.A. , Llácer, G. , Botella, P. and López‐Quílez, A. (2006a) Epidemiology of sharka disease in Spain. EPPO Bull. 36, 271–275. [Google Scholar]
- Cambra, M. , Capote, N. , Myrta, A. and Llácer, G. (2006b) Plum pox virus and the estimated costs associated with sharka disease. EPPO Bull. 36, 202–204. [Google Scholar]
- Capote, N. , Cambra, M. , Llácer, G. , Petter, F. , Platts, L.G. , Roy, A.S. and Smith, I.M. (2006) Current status of Plum pox virus and sharka disease worldwide. EPPO Bull. 36, 205–218. [Google Scholar]
- Cardon, G. , Höhmann, S. , Klein, J. , Nettesheim, K. , Saedler, H. and Huijser, P. (1999) Molecular characterisation of the Arabidopsis SBP‐box genes. Gene, 237, 91–104. [DOI] [PubMed] [Google Scholar]
- Chen, R. , Davydov, E.V. , Sirota, M. and Butte, A.J. (2010) Non‐synonymous and synonymous coding SNPs show similar likelihood and effect size of human disease association. PLoS ONE, 5, e13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, C.W. , Hsiao, Y.Y. , Wu, H.C. , Chuang, C.M. , Chen, J.S. , Tsai, C.H. , Hsu, Y.H. , Wu, Y.C. , Lee, C.C. and Meng, M. (2009) Suppression of bamboo mosaic virus accumulation by a putative methyltransferase in Nicotiana benthamiana . J. Virol. 83, 5796–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm, S.T. , Parra, M.A. , Anderberg, R.J. and Carrington, J.C. (2001) Arabidopsis RTM1 and RTM2 genes function in phloem to restrict long‐distance movement of Tobacco Etch Virus. Plant Physiol. 127, 1667–1675. [PMC free article] [PubMed] [Google Scholar]
- Cosson, P. , Sofer, L. , Le, H. , Leger, V. , Schurdi‐Levraud, V. , Whitham, S.A. , Yamamoto, M.K. , Gopalan, S. , Le Gall, O. , Candresse, T. , Carrington, J.C. and Revers, F. (2010) RTM3 which controls long distance movement of potyviruses is a member of a new plant gene family encoding a meprin and TRAF homology (MATH) domain‐containing protein. Plant Physiol. 154, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson, P. , Schurdi‐Levraud, V. , Le, Q.H. , Sicard, O. , Caballero, M. , Roux, F. , Le Gall, O. , Candresse, T. and Revers, F. (2012) The RTM resistance to potyviruses in Arabidopsis thaliana: natural variation of the RTM genes and evidence for the implication of additional genes. PLoS ONE, 7, e39169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroocq, V. , Sicard, O. , Alamillo, J.M. , Lansac, M. , Eyquard, J.P. , García, J.A. , Candresse, T. , Le Gall, O. and Revers, F. (2006) Multiple resistance traits control Plum pox virus infection in Arabidopsis thaliana . Mol. Plant–Microbe Interact. 19, 541–549. [DOI] [PubMed] [Google Scholar]
- Della Strada, G. , Pennone, F. , Fideghelli, C. , Monastra, F. and Cobianchi, D. (1989) Monografía di cultivar di albicocco. Ministerio dell'Agricoltura e delle Foreste. Direzione Generale della Produzione Agricola. Rome: Instituto Sperimentale per la Frutticolture. [Google Scholar]
- Dhawana, R. , Luoa, H. , Foersterb, A.M. , AbuQamara, S. , Duc, H.N. , Briggsc, S.D. , Scheidb, O.M. and Mengistea, T. (2009) HISTONE MONOUBIQUITINATION1 interacts with a subunit of the mediator complex and regulates defense against necrotrophic fungal pathogens in Arabidopsis. Plant Cell, 21, 1000–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicenta, F. , Martínez‐Gómez, P. , Rubio, M. and Audergon, J.M. (2003) Localisation and movement of Plum pox virus in apricot stem tissues. Ann. Appl. Biol. 142, 99–105. [Google Scholar]
- Dirlewanger, E. , Graziano, E. , Joobeur, T. , Garriga‐Calderé, F. , Cosson, P. , Howad, W. and Arús, P. (2004) Comparative mapping and marker‐assisted selection in Rosaceae fruit crops. Proc. Natl. Acad. Sci. USA, 101, 9891–9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolja, V.V. , Haldeman, R. , Robertson, N.L. , Dougherty, W.G. and Carrington, J.C. (1994) Distinct functions of capsid protein in assembly and movement of tobacco etch potyvirus in plants. EMBO J. 13, 1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondini, L. , Lain, O. , Geuna, F. , Banfi, R. , Gaiotti, F. , Tartarini, S. , Bassi, D. and Testolin, R. (2007) Development of a new SSR‐based linkage map in apricot and analysis of synteny with existing Prunus maps. Tree Genet. Genomes, 3, 239–249. [Google Scholar]
- Dondini, L. , Lain, O. , Vendramin, V. , Rizzo, M. , Vivoli, D. , Adami, M. , Guidarelli, M. , Gaiotti, F. , Palmisano, F. , Bazzoni, A. , Boscia, D. , Geuna, F. , Tartarini, S. , Negri, P. , Castellano, M. , Savino, V. , Bassi, D. and Testolin, R. (2011) Identification of QTL for resistance to plum pox virus strains M and D in Lito and Harcot apricot cultivars. Mol. Breed. 27, 289–299. [Google Scholar]
- Dosba, F. , Orliac, S. , Dutranoy, F. , Maison, P. , Massonie, G. and Audergon, J.M. (1992) Evaluation of resistance to plum pox virus in apricot trees. Acta Hort. 309, 211–219. [Google Scholar]
- Du, L. , Ali, G.S. , Simons, K.A. , Hou, J. , Yang, T. , Reddy, A.S. and Poovaiah, B.W. (2009) Ca2+/calmodulin regulates salicylic‐acid‐mediated plant immunity. Nature, 457, 1154–1158. [DOI] [PubMed] [Google Scholar]
- Egea, J. , Campoy, J. , Dicenta, F. , Burgos, L. , Patino, J.L. and Ruiz, D. (2009) ‘Estrella’ and ‘Sublime’ apricot Cultivars. Hortscience, 44, 469–470. [Google Scholar]
- Escalettes, V. , Dosba, F. , Lansac, M. and Eyquard, J.P. (1998) Genetic resistance to Plum pox potyvirus in peaches. Acta Hort. 465, 689–698. [Google Scholar]
- Eyre‐Walker, A. and Keightley, P.D. (2007) The distribution of fitness effects of new mutations. Nat. Rev. Genet. 8, 610–618. [DOI] [PubMed] [Google Scholar]
- Fay, D. and Spencer, A. (2005) Genetic mapping and manipulation: Chapter 8—dominant mutations in WormBook In: WormBook (The C. elegans Research Community , eds.). doi: 10.1895/wormbook.1.7.1. http://www.wormbook.org [accessed on March 28, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Fernández, M.R. , Camafeita, E. , Bonay, P. , Méndez, E. , Albar, J.P. and García, J.A. (2002) The capsid protein of a plant single‐stranded RNA virus is modified by O‐linked N‐acetylglucosamine. J. Biol. Chem. 277, 135–140. [DOI] [PubMed] [Google Scholar]
- Galli, P. , Patocchi, A. , Broggini, G.A.L. and Gessler, C. (2010) The Rvi15 (Vr2) apple scab resistance locus contains three TIR‐NBS‐LRR genes. Mol. Plant–Microbe Interact. 23, 608–617. [DOI] [PubMed] [Google Scholar]
- Hartmann, W. and Neumüller, M. (2006) Breeding for resistance: breeding for Plum pox virus resistant plums (Prunus domestica L.) in Germany. EPPO Bull. 36, 332–336. [Google Scholar]
- Hilario, E. , Bennell, T.F. and Rikkerink, E. (2007) Screening a BAC library with nonradioactive overlapping oligonucleotide (overgo) probes. Methods Mol. Biol. 353, 79–91. [DOI] [PubMed] [Google Scholar]
- Ion‐Nagy, L. , Lansac, M. , Eyquard, J.P. , Salvador, B. , Garcia, J.A. , Le Gall, O. , Hernould, M. , Schurdi‐Levraud, V. and Decroocq, V. (2006) PPV long‐distance movement is occasionally permitted in resistant apricot hosts. Virus Res. 120, 70–78. [DOI] [PubMed] [Google Scholar]
- Ivanov, K.I. , Puustinen, P. , Gabrenaite, R. , Vihinen, H. , Rönnstrand, L. , Valmu, L. , Kalkkinen, N. and Mäkinen, K. (2003) Phosphorylation of the potyvirus capsid protein by protein kinase CK2 and its relevance for virus infection. Plant Cell, 15, 2124–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, D. and Thompson, D. (2006) Hosts and symptoms of Plum pox virus: ornamental and wild Prunus species. EPPO Bull. 36, 222–224. [Google Scholar]
- Jung, S. , Jesudurai, C. , Staton, M. , Du, Z. , Ficklin, S. , Cho, I. , Abbott, A. , Tomkins, J. and Main, D. (2004) GDR (genome database for Rosaceae): integrated web resources for Rosaceae genomics and genetics research. BMC Bioinformatics, 5, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiannis, I. (1989) Susceptibility of apricot cultivars to plum pox virus in Greece. Acta Hort. 235, 271–274. [Google Scholar]
- Karayiannis, I. (2006) Breeding for resistance: conventional breeding for Plum pox virus resistant apricots (Prunus armeniaca L.) in Greece. EPPO Bull. 36, 319–322. [Google Scholar]
- Karayiannis, I. , Thomidis, T. and Tsaftaris, A. (2008) Inheritance of resistance to Plum pox virus in apricot (Prunus armeniaca L.). Tree Genet. Genomes, 4, 143–148. [Google Scholar]
- Labonne, G. and Dallot, S. (2006) Epidemiology of sharka disease in France. EPPO Bull. 36, 267–270. [Google Scholar]
- Laios, A. , O'Toole, S. , Flavin, R. , Martin, C. , Kelly, L. , Ring, M. , Finn, S.P. , Barrett, C. , Loda, M. , Gleeson, N. , D'Arcy, T. , McGuinness, E. , Sheils, O. , Sheppard, B. and O'Leary, J. (2008) Potential role of miR‐9 and miR‐223 in recurrent ovarian cancer. Mol. Cancer, 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli, D.A. , Abbott, A.G. , Zhebentyayeva, T.N. , Badenes, M.L. , Damsteegt, V. , Polák, J. , Krška, B. and Salava, J. (2008) A genetic linkage map for an apricot (Prunus armeniaca L.) BC1 population mapping plum pox virus resistance. Tree Genet. Genomes, 4, 481–493. [Google Scholar]
- Lambert, P. , Hagen, L.S. , Arús, P. and Audergon, J.M. (2004) Genetic linkage maps of two apricot cultivars (Prunus armeniaca L.) compared with the almond Texas × peach Earlygold reference map for Prunus. Theor. Appl. Genet. 108, 1120–1130. [DOI] [PubMed] [Google Scholar]
- Lambert, P. , Dicenta, F. , Rubio, M. and Audergon, J.M. (2007) QTL analysis of resistance to sharka disease in the apricot (Prunus armeniaca L.) ‘Polonais’ × ‘Stark Early Orange’ F1 progeny. Tree Genet. Genomes, 3, 299–309. [Google Scholar]
- Layne, R.E.C. , Bailey, C.H. and Hough, L.F. (1996) Apricots In: Fruit Breeding. Vol. II. Tree and Tropical Fruits (Janick J. and Moore J.N., eds), pp. 79–111. New York, NY: John Wiley & Sons. [Google Scholar]
- Le Rouzic, E. , Morel, M. , Ayinde, D. , Belaïdouni, N. , Letienne, J. , Transy, C. and Margottin‐Goguet, F. (2008) Assembly with the Cul4A‐DDB1DCAF1 ubiquitin ligase protects HIV‐1 Vpr from proteasomal degradation. J. Biol. Chem. 283, 21 686–21 692. [DOI] [PubMed] [Google Scholar]
- Lee, J.H. , Terzaghi, W. , Gusmaroli, G. , Charron, J.B. , Yoon, H.J. , Chen, H. , He, Y.J. , Xiong, Y. and Deng, X.W. (2008) Characterization of Arabidopsis and rice DWD proteins and their roles as substrate receptors for CUL4‐RING E3 ubiquitin ligases. Plant Cell, 20, 152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llácer, G. , Badenes, M.L. and Romero, C. (2007) Problems in the determination of inheritance of plum pox virus resistance in apricot. Acta Hortic. 781, 263–267. [Google Scholar]
- Madishetty, K. , Condamine, P. , Svensson, J.T. , Rodriguez, E. and Close, T.J. (2007) An improved method to identify BAC clones using pooled overgos. Nucleic Acids Res. 35, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan, S.K. , Chisholm, S.T. , Whitham, S.A. and Carrington, J.C. (1998) Identification and characterization of a locus (RTM1) that restricts long‐distance movement of tobacco etch virus in Arabidopsis thaliana . Plant J. 14, 177–186. [DOI] [PubMed] [Google Scholar]
- Majewski, J. and Ott, J. (2012) Distribution and characterization of regulatory elements in the human genome. Genome Res. 12, 1827–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marandel, G. , Salava, J. , Abbott, A. , Candresse, T. and Decroocq, V. (2009) Quantitative trait loci meta‐analysis of Plum pox virus resistance in apricot (Prunus armeniaca L.): new insights on the organization and the identification of genomic resistance factors. Mol. Plant Pathol. 10, 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, G.W., III , Harney, J.W. and Berry, M.J. (1998) Functionality of mutations at conserved nucleotides in eukaryotic SECIS elements is determined by the identity of a single nonconserved nucleotide. RNA, 4, 65–73. [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Calvo, J. , Llácer, G. and Badenes, M.L. (2010) Rafel and Xelva, two apricot varieties resistant to sharka. HortScience, 45, 1904–1905. [Google Scholar]
- Martínez‐Gómez, P. , Dicenta, F. and Audergon, J.M. (2000) Behaviour of apricot (Prunus armeniaca L.) cultivars in the presence of sharka (Plum pox potyvirus): a review. Agronomie, 20, 407–422. [Google Scholar]
- Maule, A.J. , Caranta, C. and Boulton, M.I. (2007) Sources of natural resistance to plant viruses: status and prospects. Mol. Plant Pathol. 8, 223–231. [DOI] [PubMed] [Google Scholar]
- Mosavi, L.K. , Cammett, T.J. , Desrosiers, D.C. and Peng, Z.‐Y. (2004) The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 13, 1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie, H. , Zhao, C. , Wu, G. , Wu, Y. , Chen, Y. and Tang, D. (2012) SR1, a calmodulin‐binding transcription factor, modulates plant defense and ethylene‐induced senescence by directly regulating NDR1 and EIN3. Plant Physiol. 158, 1847–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa, M. and Nei, M. (2007) Evolutionary dynamics of olfactory receptor genes in Drosophila species. Proc. Natl. Acad. Sci. USA, 104, 7122–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos, A. , Capote, N. and Candresse, T. (2006) Detection and characterization of Plum pox virus: molecular methods. EPPO Bull. 36, 262–266. [Google Scholar]
- Olmstead, J.W. , Sebolt, A.M. , Cabrera, A. , Sooriyapathirana, S.S. , Hammar, S. , Iriarte, G. , Wang, D. , Chen, C.Y. , van der Knaap, E. and Iezzoni, A.F. (2008) Construction of an intra‐specific sweet cherry (Prunus avium L.) genetic linkage map and synteny analysis with the Prunus reference map. Tree Genet. Genomes, 4, 897–910. [Google Scholar]
- Pagny, G. , Paulstephenraj, P.S. , Poque, S. , Sicard, O. , Cosson, P. , Eyquard, J.‐P. , Caballero, M. , Chague, A. , Gourdon, G. , Negrel, L. , Candresse, T. , Mariette, S. and Decroocq, V. (2012) Family‐based linkage and association mapping reveals novel genes affecting Plum pox virus infection in Arabidopsis thaliana . New Phytol. 196, 873–886. [DOI] [PubMed] [Google Scholar]
- Parravicini, G. , Gessler, C. , Denancé, C. , Lasserre‐Zuber, P. , Vergne, E. , Brisset, M.N. , Patocchi, A. , Durel, C.E. and Broggini, G.A.L. (2011) Identification of serine/threonine kinase and nucleotide‐binding site–leucine‐rich repeat (NBS‐LRR) genes in the fire blight resistance quantitative trait locus of apple cultivar ‘Evereste’. Mol. Plant Pathol. 12, 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri, C. , Webb, K. , Hily, J.M. , Dardick, C. and Scorza, R. (2008) High transformation efficiency in plum (Prunus domestica L.): a new tool for functional genomics studies in Prunus spp. Mol. Breed. 22, 581–591. [Google Scholar]
- Pilarova, P. , Marandel, G. , Decroocq, V. , Salava, J. , Krka, B. and Abbott, A.G. (2010) Quantitative trait analysis of resistance to Plum pox virus in the apricot F1 progeny ‘Harlayne' × ‘Vestar’. Tree Genet. Genomes, 6, 467–475. [Google Scholar]
- Qiu, Y. , Xi, J. , Du, L. , Suttle, J.C. and Poovaiah, B.W. (2012) Coupling calcium/calmodulin‐mediated signaling and herbivore‐induced plant response through calmodulin‐binding transcription factor AtSR1/CAMTA3. Plant Mol. Biol. 79, 89–99. [DOI] [PubMed] [Google Scholar]
- Robaglia, C. and Caranta, C. (2006) Translation initiation factors: a weak link in plant RNA virus infection. Trends Plant Sci. 11, 40–45. [DOI] [PubMed] [Google Scholar]
- Rozen, S. and Skaletsky, H.J. (2000) Primer3 on the WWW for general users and for biologist programmers In: Bioinformatics Methods and Protocols (Misener S. and Krawetz S.A., eds), pp. 365–386. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- Rubio, M. , Martinez‐Gomez, P. , Dicenta, F. and Audergon, J.M. (2004) Testing of genetic control hypothesis for Plum pox resistance in apricot. Acta Hort. 663, 265–267. [Google Scholar]
- Sato, S. , Tomomori‐Sato, C. , Parmely, T.J. , Florens, L. , Zybailov, B. , Swanson, S.K. , Banks, C.A. , Jin, J. , Cai, Y. , Washburn, M.P. , Conaway, J.W. and Conaway, R.C. (2004) A set of consensus mammalian mediator subunits identified by multidimensional protein identification technology. Mol. Cell, 14, 685–691. [DOI] [PubMed] [Google Scholar]
- Scholthof, K.‐B.G. , Adkins, S. , Czosnek, H. , Palukaitis, P. , Jacquot, E. , Hohn, T. , Hohn, B. , Saunders, K. , Candresse, T. , Ahlquist, P. , Hemenway, C. and Foster, G. (2011) Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 12, 938–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soosaar, J.L.M. , Burch‐Smith, T.M. and Dinesh‐Kumar, S.P. (2005) Mechanisms of plant resistance to viruses. Nat. Rev. Microbiol. 3, 789–798. [DOI] [PubMed] [Google Scholar]
- Soriano, J.M. , Vera‐Ruiz, E.M. , Vilanova, S. , Martinez‐Calvo, J. , Llácer, G. , Badenes, M.L. and Romero, C. (2008) Identification and mapping of a locus conferring plum pox virus resistance in two apricot improved linkage maps. Tree Genet. Genomes, 4, 391–402. [Google Scholar]
- Soriano, J.M. , Domingo, M.L. , Zurigaga, E. , Romero, C. , Zhebentyayeva, T. , Abbott, A.G. and Badenes, M.L. (2012) Identification of SSR markers tightly associated with PPV resistance in apricot. Mol. Breed. 30, 1017–1026. [Google Scholar]
- Staden, R. , Beal, K.F. and Bonfield, J.K. (2000) The Staden Package, 1998. Computer methods in molecular biology In: Bioinformatics Methods and Protocols (Misener S. and Krawetz S.A., eds), pp. 115–130. Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- Syrgiannidis, G. (1980) Selection of two apricot varieties resistant to sharka virus. Acta Phytopathol. Sci. Hung. 15, 85–87. [Google Scholar]
- Tradafirescu, M. and Topor, E. (1999) Investigation on the susceptibility of some apricot to plum pox (Sharka) virus. Acta Hort. 488, 787–791. [Google Scholar]
- Ueki, S. , Spektor, R. , Natale, D.M. and Citovsky, V. (2010) ANK, a host cytoplasmic receptor for the Tobacco mosaic virus cell‐to‐cell movement protein, facilitates intercellular transport through plasmodesmata. PLoS Pathog. 6, e1001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Użarowska, A. , Dionisio, G. , Sarholz, B. , Piepho, H.P. , Xu, M. , Ingvardsen, C.R. , Wenzel, G. and Lübberstedt, T. (2009) Validation of candidate genes putatively associated with resistance to SCMV and MDMV in maize (Zea mays L.) by expression profiling. BMC Plant Biol. 9, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera‐Ruiz, E.M. , Soriano, J.M. , Romero, C. , Zhebentyayeva, T. , Terol, J. , Zuriaga, E. , Llácer, G. , Abbott, A.G. and Badenes, M.L. (2011) Narrowing down the apricot Plum pox virus resistance locus and comparative analysis with the peach genome syntenic region. Mol. Plant Pathol. 12, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilanova, S. , Romero, C. , Abernathy, D. , Abbott, A.G. , Burgos, L. , Llácer, G. and Badenes, M.L. (2003) Construction and application of a bacterial artificial chromosome (BAC) library of Prunus armeniaca L. for the identification of clones linked to the self‐incompatibility locus. Mol. Gen. Genet. 269, 685–691. [DOI] [PubMed] [Google Scholar]
- Xu, Q. and Deng, X. (2010) Cloning and phylogenetic analyses of serine/threonine kinase class defense‐related genes in a wild fruit crop ‘chestnut rose’. BMC Res. Notes, 3, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata, J.M. , Martínez‐García, V. and Lefebvre, S. (2007) Phylogeny of the TRAF/MATH domain. Adv. Exp. Med. Biol. 597, 1–24. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Feng, S. , Chen, F. , Chen, H. , Wang, J. , McCall, C. , Xiong, Y. and Deng, X.W. (2008) Arabidopsis DDB1‐CUL4 ASSOCIATED FACTOR1 forms a nuclear E3 ubiquitin ligase with DDB1 and CUL4 that is involved in multiple plant developmental processes. Plant Cell, 20, 1437–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhebentyayeva, T.N. , Reighard, G.L. , Lalli, D. , Gorina, V.M. , Krška, B. and Abbott, A.G. (2008) Origin of resistance to plum pox virus in apricot: what new AFLP and targeted SSR data analyses tell. Tree Genet. Genomes, 4, 403–417. [Google Scholar]
- Zheng, X.H. , Lu, F. , Wang, Z.‐Y. , Zhong, F. , Hoover, J. and Mural, R. (2005) Using shared genomic synteny and shared protein functions to enhance the identification of orthologous gene pairs. Bioinformatics, 21, 703–710. [DOI] [PubMed] [Google Scholar]
- Zhou, J. , Loh, Y.T. , Bressan, R.A. and Martin, G.B. (1995) The tomato gene Pti1 encodes a serine/threonine kinase that is phosphorylated by Pto and is involved in the hypersensitive response. Cell, 83, 925–935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Comparative analysis of the Prunus PPVres locus with the Arabidopsis genome. Peach transcripts, annotated by the International Peach Genome Initiative (IPGI), are represented by black boxes. Full lines connect Prunus/Arabidopsis gene pairs that are best‐reciprocal blastp hits (E value cut‐off < 1e‐6). Broken lines correspond to the best unidirectional blastp hit when no best‐reciprocal hit was obtained. Distances in kilobases are shown on the left of the peach physical map and in megabases on the right of the Arabidopsis chromosomes. Markers defining the PPVres locus are represented on the left of the peach map as a reference.
File S1 Configuration parameters for cleaning sequence reads with ngs_backbone pipeline (backbone.conf).
Table S1 Overgo and polymerase chain reaction (PCR) primers employed.
Table S2 Variants [single nucleotide polymorphisms (SNPs)/deletion/insertion polymorphisms (DIPs)] recorded from the alignment of the nine apricot genomes and bacterial artificial chromosome (BAC) contig sequences against the peach genome.
Table S3 Variants [single nucleotide polymorphisms (SNPs)/deletion/insertion polymorphisms (DIPs)] recorded from the alignment of the bacterial artificial chromosome (BAC) contig sequences from the R‐haplotype of ‘Goldrich’ against the peach genome.


