Summary
Colletotrichum graminicola, the causal agent of maize anthracnose, is a hemibiotrophic fungus that initially infects living host cells via primary hyphae surrounded by a membrane. A nonpathogenic mutant disrupted in a gene encoding a component of the signal peptidase complex, and believed to be deficient in protein processing and secretion, regained pathogenicity when it was inoculated onto maize leaf sheaths close to the wild‐type fungus. Evidence is presented suggesting that the wild‐type produces a diffusible factor(s) that induces the localized susceptibility of host cells at the borders of expanding colonies, causing them to become receptive to biotrophic invasion. The induced susceptibility effect is limited to a distance of approximately eight cells from the edge of the wild‐type colony, is dosage dependent and is specific to C. graminicola.
Introduction
The fungal genus Colletotrichum includes at least 100 species infecting numerous plant hosts (Farr and Rossmann, 2013). Colletotrichum graminicola (Ces.) Wils., the cause of maize anthracnose, is among the most economically important species, contributing to yield losses of up to $1 billion in the USA in 2011 (Bergstrom and Nicholson, 1999; Frey et al., 2011; Warren and Nicholson, 1975). Colletotrichum graminicola is an intracellular hemibiotroph that begins the infection process as a biotroph, with primary invasive hyphae separated from the living host cytoplasm by a membrane. It then switches to necrotrophy, marked by host cell collapse, the production of secondary invasive hyphae no longer enclosed by a membrane and the development of anthracnose symptoms (Bergstrom and Nicholson, 1999; Mims and Vaillancourt, 2002; O'Connell et al., 2012; Venard and Vaillancourt, 2007a).
The mechanisms involved in the establishment of compatibility and progression to necrotrophy in the C. graminicola–maize interaction are poorly understood. Obligately biotrophic plant pathogens reprogramme living host cells, suppressing cell death and defence responses (Doehlemann et al., 2008, 2009; Eichmann et al., 2004). In contrast, many necrotrophs take advantage of plant defence responses to enhance pathogenicity, and induce host cell death by secreting phytotoxic secondary metabolites (SMs) (Amselem et al., 2011; Cessna et al., 2000; Govrin and Levine, 2002; Rolke et al., 2004). Evidence in the literature suggests that hemibiotrophic Colletotrichum fungi do both, first suppressing, then later inducing, host cell death (Kleemann et al., 2012; O'Connell et al., 2012; Stephenson et al., 2000; Yoshino et al., 2012). Both processes appear to be regulated by secreted compounds, the production of which is tightly regulated to provide the correct function at the appropriate time and place (Gan et al., 2013; Kleemann et al., 2012; O'Connell et al., 2012).
Restriction enzyme‐mediated insertional (REMI) mutagenesis has been utilized previously to identify a C. graminicola gene (Cpr1) required to establish a compatible interaction with maize (Thon et al., 2000). The predicted CPR1 protein is similar to Spc3p from Saccharomyces cerevisiae (E = 1e−22), one of two essential components of the signal peptidase complex (SPC) (Thon et al., 2002). The SPC processes signal peptides as polypeptides cross the endoplasmic reticulum membrane (Fang et al., 1997; Meyer and Hartmann, 1997), the first step in protein transport and secretion (Zimmermann et al., 2006). Thus, CPR1 may have a role in protein transport and secretion in C. graminicola. Restriction enzyme‐mediated insertion occurred in the 3′ untranslated region (UTR) of the Cpr1 gene, resulting in a leaky mutation that reduced significantly, but did not eliminate, expression in culture (Thon et al., 2002). The cpr1 mutant was normal, other than a slight reduction in growth rate, when compared with the wild‐type (WT) in culture (Thon et al., 2002). Moreover, there were no apparent differences between the mutant and WT up to 48 h post‐inoculation (hpi) in maize leaves (Mims and Vaillancourt, 2002). However, by 72 hpi, WT had entered necrotrophy, characterized by the presence of thin, secondary hyphae, the collapse of maize cells and the appearance of lesions. In contrast, hyphae of the mutant remained confined to a few cells, and there was no widespread tissue collapse or symptom development (Mims and Vaillancourt, 2002). It was hypothesized that the mutant was altered in the secretion of compounds involved in biotrophic colonization or the switch to necrotrophy, but not required for initial penetration or for growth in culture (Mims and Vaillancourt, 2002; Thon et al., 2002).
In this study, co‐inoculation assays in detached maize leaf sheaths were used to test two alternative hypotheses: that the mutant is altered in the production of compounds that: (i) suppress defence responses and promote compatibility (like a biotroph); or (ii) induce host defence responses and cell death (like a necrotroph). The challenge of a plant with a compatible pathogen can compromise resistance and lead to infection by a normally incompatible pathogen (Tsuchiya and Hirata, 1973). This phenomenon, which has been observed with powdery mildew and rust fungi, is known as induced susceptibility (Kunoh et al., 1990; Lyngkjær and Carver, 1999; Olesen et al., 2003; Ouchi et al., 1974b). In other pathosystems, inoculation with an incompatible strain can generate resistance against a normally pathogenic strain (Freeman and Rodriguez, 1993; Kunoh et al., 1990; Ouchi et al., 1976b), a phenomenon known as localized induced resistance. If the cpr1 mutant fails to secrete factors normally involved in the suppression of maize defences and cell death during the establishment of biotrophy, co‐inoculation with the WT strain should allow the mutant to grow (induced susceptibility). However, if the cpr1 mutant inappropriately produces inducers of defence responses and cell death, co‐inoculation with the mutant should prevent WT from colonizing (localized induced resistance).
Results
The cpr1 mutant strain germinated and penetrated maize sheath epidermal cells as efficiently as WT, but penetration was delayed
The fungal strains used for these experiments are described in Table 1. They included the WT and mutant tagged with autofluorescent proteins (AFPs) to facilitate visualization in sheath tissues. An AFP‐tagged strain of C. sublineola, which causes sorghum anthracnose, was also included. This strain is nonpathogenic to healthy maize, but will colonize and sporulate on maize tissues that are dead or dying. It was used as a control for maize tissue integrity. A complemented cpr1 strain containing an ectopic copy of the full‐length Cpr1 gene (Thon et al., 2002) was a control for nontarget transformation effects.
Table 1.
Fungal strains used in this study
| Strain | Parental strain | Description | Relevant citation |
|---|---|---|---|
| Wild‐type (WT) | — | Laboratory strain M1.001, aka. M2. Pathogenic to maize | Forgey et al. (1978) |
| WT‐mRFP | M1.001 | Transformed to express red fluorescent protein (RFP) in planta. Pathogenicity normal | This study |
| cpr1 mutant | M1.001 | Nonpathogenic to maize. Obtained by restriction enzyme‐mediated insertional (REMI) mutagenesis. Mutation in 3′ untranslated region (UTR) of the Spc3 orthologous gene, encodes component of signal peptidase | Thon et al. (2000) |
| cpr1‐Zsgreen | cpr1 mutant | cpr1 mutant transformed to express ZsGreen fluorescent protein. Nonpathogenic to maize | Venard and Vaillancourt (2007a, b) |
| Cpr1‐C | cpr1 mutant | cpr1 mutant, complemented by integration of 3.6 kb of genomic DNA containing the WT Cpr1 gene. Pathogenicity normal | Thon et al. (2002) |
| CgSl1‐GFP1 | CgSl1 | Pathogenic to sorghum but nonpathogenic to maize | Venard and Vaillancourt (2007a, b) |
| M1502 | M1.001 | Melanin‐deficient, as a result of UV‐induced mutation in scytalone dehydrogenase gene | Rasmussen and Hanau (1989) |
| M1201 | M1001 | Pyrimidine biosynthetic mutant, as a result of a spontaneous mutation in orotate phosphoribosyl transferase gene. Nonpathogenic to maize. Spores do not germinate or adhere well on maize sheaths | Rasmussen et al. (1992) |
| 90‐23 | M1.001 | Nonpathogenic to maize. Obtained by REMI mutagenesis. Specific mutation unknown | Thon et al. (2000) |
| 9‐4 | M1.001 | Reduced in pathogenicity to maize. Obtained by REMI mutagenesis. Specific mutation unknown | Thon et al. (2000) |
| 80‐37 | M1.001 | Nonpathogenic to maize. Obtained by REMI mutagenesis. Specific mutation unknown | Thon et al. (2000) |
| 83‐45 | M1.001 | Reduced in pathogenicity to maize. Obtained by REMI mutagenesis. Specific mutation unknown | Thon et al. (2000) |
| 84‐14 | M1.001 | Reduced in pathogenicity to maize. Obtained by REMI mutagenesis. Specific mutation unknown | Thon et al. (2000) |
| 84‐6 | M1.001 | Nonpathogenic to maize. Obtained by REMI mutagenesis. Specific mutation unknown | Thon et al. (2000) |
The percentage of spores that germinated to produce appressoria and primary infection hyphae in detached maize sheaths was compared for the mutant and WT strains (Figs 1 and S1, see Supporting Information). Tagging with AFP did not affect pathogenicity (Fig. S1). At 12 hpi, the cpr1 mutant strain showed a slight delay in germination compared with WT (Fig. 1). However, germination of the mutant was not reduced in comparison with the tagged WT‐modified red‐fluorescent protein (mRFP) or the complemented Cpr1‐C strains (Fig. S1), and, by 24 hpi, germination rates were equivalent for the mutant and WT (Figs 1 and S1). At 24 hpi, 41% of WT appressoria had produced invasive primary infection hyphae. At the same time point, 93% of the cpr1 mutant spores had developed appressoria, but less than 5% of these had produced visible infection hyphae (Fig. 1). At 48 hpi, 36% of the cpr1 mutant infection sites had progressed to the production of invasive primary hyphae, which was not statistically significantly different from the rate of colonization achieved by WT 24 h earlier (Fig. 1). It could not be determined whether WT had initiated additional infections between 24 and 48 hpi, as a result of extensive tissue colonization from the original penetration sites. The AFP‐tagged strain of C. sublineola [CgSl1‐green fluorescent protein (GFP)] produced abundant appressoria on detached maize leaf sheaths by 24 hpi, but these only rarely (<1%) produced primary infection hyphae, none of which grew beyond the initially infected cell, even at 96 hpi.
Figure 1.
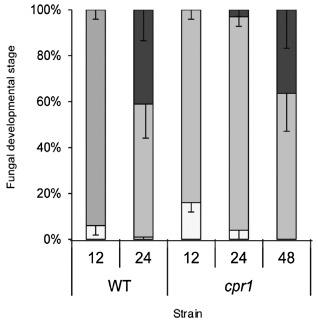
Development of wild‐type (WT) and cpr1 mutant strains on maize leaf sheaths. Percentage of ungerminated spores (white bar), appressoria (light grey bar) and invasive primary hyphae (dark grey bar) at 12, 24 and 48 h post‐inoculation (hpi).
The mutant did not establish a normal biotrophic infection, or progress to necrotrophy in leaf sheaths
Inoculation of maize leaf sheaths with the WT, WT‐mRFP or Cpr1‐C strains resulted in water‐soaked lesions at the inoculation sites within 72 hpi (Fig. 2a). In contrast, inoculation with the cpr1 mutant, cpr1‐Zsgreen or CgSl1‐GFP did not result in the production of visible lesions, even up to 96 hpi (Fig. 2a).
Figure 2.
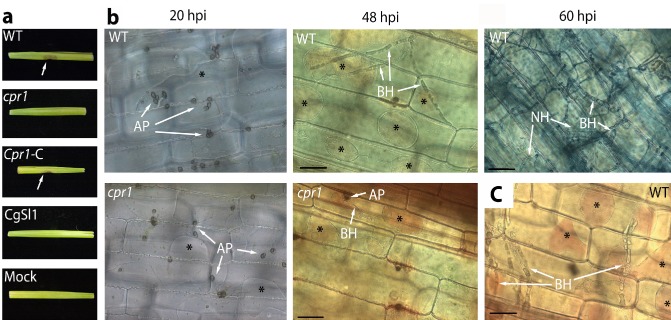
(a) Symptom development in inoculated and mock‐inoculated maize leaf sheaths 96 h post‐inoculation (hpi). (b) Phenotypes of the wild‐type (WT) and cpr1 mutant on maize leaf sheaths at 20, 48 and 60 hpi (for WT only). (c) Penetration of living cells at the edges of a necrotrophic colony. Plasmolysis and neutral red uptake were used to determine host cell viability. Cells that had not yet been colonized or that had just been invaded by biotrophic hyphae usually remained alive, indicated by the presence of plasmolysis, or plasmolysis combined with neutral red staining (asterisks). AP, appressoria; BH, biotrophic primary infection hyphae; NH, necrotrophic hyphae. Scale bars, 50 μm.
The viability stain neutral red, and/or sucrose plasmolysis, was used to determine host cell status during pathogen infection. By 20 hpi, mature melanized appressoria of the WT and cpr1 mutant strains had formed on the inoculated tissue (Fig. 2b). Cells beneath and around these appressoria plasmolysed, indicating that they were alive (Fig. 2b). By 48 hpi, most WT infections had entered and grown beyond one host cell as broad hyphae, narrowing as they crossed apparently intact cell walls (Fig. 2b). Nearly one‐half of these successful infections (45.7%) had colonized at least three cells beyond the point of infection, and nearly 5% had already colonized five cells beyond the infection point (Table 2). On average, 3.8 cells were colonized by WT at 48 hpi. Thirty‐six per cent of the cpr1 mutant appressoria had also entered host cells and produced primary infection hyphae by 48 hpi (Figs 1 and 2b). However, even at 72 hpi, 96% of these mutant infections remained confined to a single cell (Table 2). The maximum number of cells colonized by the mutant strains at 72 hpi was three, and less than 1% of the infection sites had progressed that far (Table 2). The average number of cells colonized was only 0.6.
Table 2.
Colonization of maize leaf sheaths by the wild‐type (WT) (48 h post‐inoculation) versus the cpr1 mutant (72 h post‐inoculation)
| Percentage of infection sites colonizing a maximum of: | |||||
|---|---|---|---|---|---|
| 1 cell | 2 cells | 3 cells | 4 cells | 5 cells | |
| WT | 3.8 ± 4.1 | 30.1 ± 7.1 | 45.7 ± 7.4 | 15.4 ± 5.4 | 4.7 ± 4.6 |
| cpr1 | 95.9 ± 3.7 | 3.5 ± 3.2 | 0.95 ± 2.0 | 0 | 0 |
A combination of neutral red staining and plasmolysis confirmed that some host cells were alive at the time of invasion by either the WT or mutant strains (Fig. 2b). Furthermore, many surrounding cells were also alive. At 60 hpi, WT colony centres switched to necrotrophy, indicated by the appearance of thin, secondary hyphae, obvious tissue maceration and a lack of plasmolysis or staining of host cells (Fig. 2b). Colony edges, however, remained biotrophic, indicated by the continued ability of newly invaded cells and cells beyond the colony borders to plasmolyse and take up neutral red (Fig. 2c). Leaf sheaths were routinely monitored up to 96 hpi and, on several occasions, were retained for up to 6 days, but the cpr1 mutant did not progress further, or produce symptoms. The nonpathogenic CgSl1‐GFP strain produced appressoria on maize sheaths, but only rarely produced infection hyphae. Epidermal cells beneath and surrounding the appressoria appeared to be alive (see Fig. 4n), whereas cells containing rare infection hyphae no longer plasmolysed (not shown).
Figure 4.
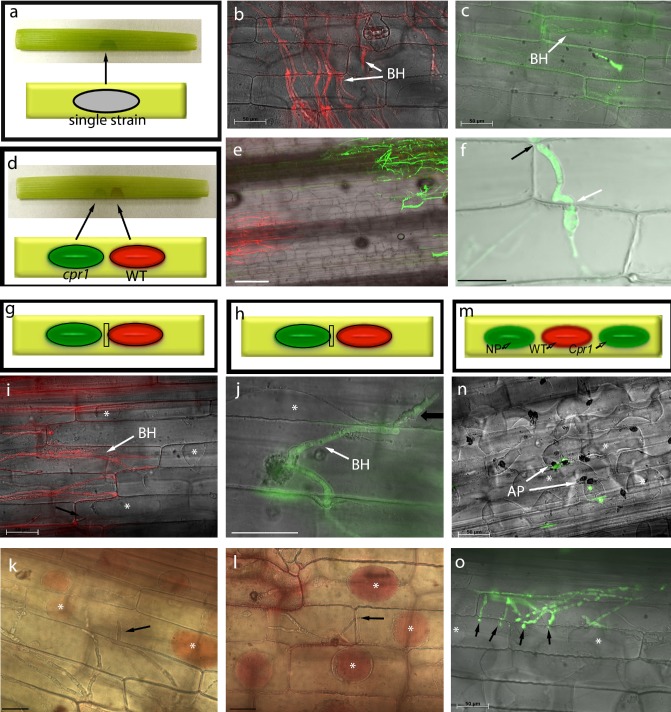
Diagrams of leaf sheath inoculations (a) and co‐inoculations (d, g, h, m). Colonization by WT‐mRFP (b) and cpr1‐Zsgreen (c), at 72 h post‐inoculation (hpi), on maize leaf sheaths. (e) cpr1‐Zsgreen colonization in co‐inoculations with WT‐mRFP. (f) Confocal image showing cpr1‐Zsgreen crossing intact plant cell walls (arrows) in co‐inoculations. Viability of newly invaded plant cells (arrows) and of cells surrounding wild‐type (WT) (i, k) and cpr1 (j, l) colonies in co‐inoculations, demonstrated by plasmolysis and neutral red staining (asterisks), at 60 hpi. (m–o) Triple inoculations with WT‐mRFP, cpr1‐Zsgreen and nonpathogen (NP) CgSl1‐GFP. (n) Nonpathogen failed to penetrate maize tissue, and cells underneath appressoria continued to plasmolyse (asterisks). (o) cpr1‐Zsgreen invading adjacent cells (arrows) that are still alive (asterisks). Scale bars (except f), 50 μm; (f) 20 μm. AP, appressoria; BH, biotrophic primary hyphae.
The pattern of reactive oxygen species (ROS) accumulation differed in mutant interactions
Maize responds to C. graminicola by accumulating ROS, especially H2O2 (Vargas et al., 2012). To detect ROS production in sheaths, 3,3′‐diaminobenzidine (DAB) staining was used. DAB precipitates were observed by 12 hpi in sheaths inoculated with the mutant or WT, but the intensity of staining was noticeably stronger in WT inoculations (Fig. 3a,d). There was an obvious increase in the amount of DAB staining with both strains by 24 hpi. Interestingly, a distinctive halo often formed around penetration sites of the WT strain, but not the mutant strain, where a more diffuse accumulation of DAB‐stained vesicles was typical (Fig. 3b,e). By 48 hpi, the halo pattern had disappeared, and very few precipitates could be detected in WT‐inoculated sheaths (Fig. 3c). In contrast, intense accumulation of DAB precipitates, especially in the cell walls of penetrated cells, and within vesicles beneath penetration sites, was typically observed in cpr1 mutant‐inoculated tissue (Fig. 3f).
Figure 3.
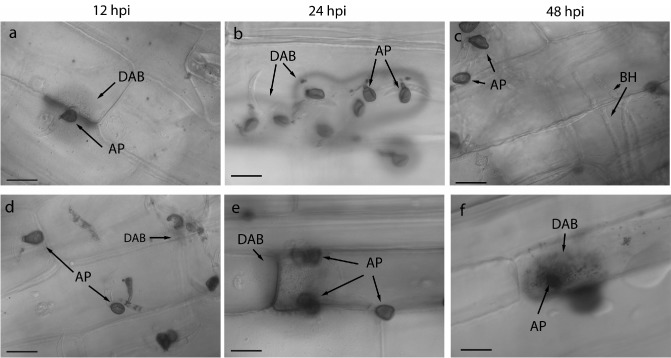
Pattern of reactive oxygen species (ROS) accumulation in maize leaf sheaths inoculated with the wild‐type (WT) (a–c) or cpr1 mutant (d–f), indicated by 3,3′‐diaminobenzidine (DAB) staining. AP, appressoria; BH, biotrophic primary infection hyphae; hpi, hours post‐inoculation. Scale bars, 20 μm.
The mutant strain and the nonpathogenic C. sublineola strain could complete their life cycles on killed maize sheath tissue
To test the role of host cell viability, freeze‐killed and paraquat‐treated leaf sheaths were inoculated. Freezing induced localized tissue damage, whereas paraquat induced systemic damage. Both treatments resulted in a loss of the ability of affected host cells to plasmolyse (not shown). Both treatments also allowed colonization by the mutant strain and the nonpathogen C. sublineola. Colonization by these strains did not differ noticeably from the WT strain. Colonization of damaged sheath tissues was dramatically accelerated, when compared with the colonization of initially healthy tissues, with all three strains progressing through multiple sheath cells within just 24 hpi (not shown). By 60 hpi, individual hyphae could no longer be easily distinguished for any of the strains, and freeze‐killed tissue supported the formation of acervuli containing conidia and setae by all three strains (not shown).
Co‐inoculation of leaf sheaths with the WT strain induced susceptibility to the cpr1 mutant
The effect of the co‐inoculation of pathogenic and nonpathogenic strains on living maize leaf sheaths was tested. In order that they could be distinguished within the host tissue, AFP‐tagged strains were used for co‐inoculations and all following experiments, unless stated otherwise. The behaviour of these strains when inoculated alone on leaf sheaths was identical to the nontagged parental strains (Fig. S1). Seventy‐two hours after inoculation separately, the WT‐mRFP strain had colonized more than six cells, whereas cpr1‐ZsGreen remained confined to the first invaded epidermal cell (Fig. 4a–c).
When spores of WT‐mRFP and cpr1‐ZsGreen were mixed in the same inoculation drop, the latter was routinely observed growing beyond the first invaded cell, colonizing up to three cells by 60 hpi (not shown). In more than 100 co‐inoculations, the cpr1‐ZsGreen strain only entered these additional cells when they were also colonized by WT‐mRFP, and no longer plasmolysed. Co‐inoculation with the mutant strain did not prevent WT from colonizing maize normally (e.g. there was no evidence for localized induced resistance).
To test interactions at a distance, mutant and WT strains were co‐inoculated on the same leaf sheath, but with the drops of inoculum separated. Drops were placed as close together as possible without having them drawn together by water tension, a distance of approximately 2.5 mm (Fig. 4d). Co‐inoculation at a distance induced susceptibility to the cpr1 mutant. By 60 hpi, about one‐third of the infections had progressed beyond the initially colonized cell to include the colonization of one or more additional cells (Figs 4e,f and S2, see Supporting Information). In control sheaths in which cpr1‐ZsGreen spore drops were paired with drops of water, growth was similar to that observed in the absence of co‐inoculation (Fig. S2). Similar results were obtained with the untagged strains and the Cpr1‐C strain (not shown). Plasmolysis (Fig. 4i,j) and vital staining (Fig. 4k,l) revealed that many of the newly colonized cells, as well as the uncolonized host cells surrounding the colonies, were alive in the co‐inoculations (Fig. 4g–l). A localized freeze injury 2.5 mm from the inoculation drop did not induce susceptibility to the mutant (not shown).
Co‐inoculations at a distance did not induce susceptibility to the nonpathogen
When spores from CgSl1‐GFP and WT‐mRFP were mixed in the same inoculation drop, CgSl1‐GFP routinely penetrated and colonized the maize cells (not shown). However, as with the cpr1‐Zsgreen strain, this only occurred when the cells had also been colonized by WT‐mRFP and did not plasmolyse. When spores from the nonpathogen and cpr1‐Zsgreen were mixed, both strains germinated and formed appressoria, but neither strain colonized the tissue to a greater extent than the controls.
To test interactions at a distance, triple‐inoculation experiments were conducted, with spore suspensions of the nonpathogen, mutant, and WT strains inoculated at separate locations along the same leaf sheath (Fig. 4m–o). At 60 hpi, growth of CgSl1‐GFP was limited and indistinguishable from the controls (Figs 4n and S3, see Supporting Information). Rarely, the nonpathogen produced a primary infection hypha, but in no case (n = 50 sheaths) was it observed to grow beyond the initially colonized cell (Fig. S3). In contrast, susceptibility to the cpr1‐Zsgreen strains was induced in the triple inoculations, similar to that observed in double inoculations at a distance (Figs 4o and S3). Co‐inoculation of cpr1‐ZsGreen and CgSl1‐GFP1 at a distance, in the absence of WT, did not result in induced susceptibility to either strain (not shown).
Induced susceptibility was dependent on distance, spore concentration and timing of inoculation
The induced susceptibility effect disappeared when the distance between inoculum drops was increased (Table 3). When the WT and mutant were separated by 2.5 mm, 33.4% of the infection sites grew beyond the second colonized cell, compared with only 4% in the control. However, when the distance between the drops was doubled, growth of the mutant no longer differed from the control.
Table 3.
Effect of distance on the growth of the cpr1 mutant in co‐inoculations. Treatments in different classes differ from each other with a significance of P < 0.05
| Treatment | Number of host cells between colonies | Distance between colonies (mm) | Percentage of infection sites progressing beyond one cell | Class |
|---|---|---|---|---|
| 1 | 8.5 ± 1.5 | 2.6 ± 0.46 | 33.5 ± 15 | a |
| 2 | 13.3 ± 2 | 4.1 ± 0.62 | 8.2 ± 14 | b |
| 3 | 22.8 ± 4.9 | 7 ± 0.12 | 9.9 ± 13 | b |
| Control | N/A | N/A | 4.6 ± 4.2 | b |
The induced susceptibility effect was dependent on the initial spore concentration of both strains. A five‐fold reduction in the WT inoculum concentration, or a ten‐fold reduction in either strain, reduced significantly the growth of the cpr1 mutant in co‐inoculations (Table 4).
Table 4.
Effect of inoculum concentration on growth of cpr1 in co‐inoculations. Percentage of infection sites in which hyphae of the cpr1 mutant colonized beyond one cell. Treatments with asterisks (*) differed from the control, with a significance of P < 0.05
| Spore concentration | Wild‐type inoculum | |||
|---|---|---|---|---|
| 5 × 105 | 1 × 105 | 5 × 104 | ||
| cpr1 inoculum | 5 × 105 | 30.33* | 20.5* | 7.33 |
| 1 × 105 | 13.17 | 6.17 | 4.17 | |
| 5 × 104 | 14.5 | 7.71 | 3 | |
Induced susceptibility was also affected by the amount of time elapsed between application of the mutant and WT inocula. The degree of colonization was decreased significantly when the mutant remained alone for 12 h before the WT inoculum was added, and induced susceptibility was no longer observed when the time between inoculations was increased to 24 or 36 h (Fig. 5). Control sheaths indicated that, even at 96 hpi, the cpr1 mutant inoculated alone only rarely (<5%) grew beyond the initially colonized cell. Colonization by WT was not visibly affected by co‐inoculation with the mutant at any time point.
Figure 5.
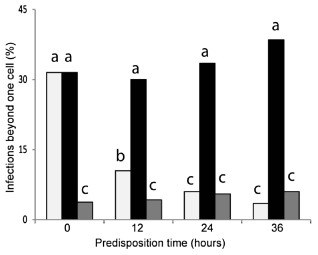
The effect of timing of co‐inoculations (‘predisposition time’) on induced susceptibility. Wild‐type (WT) inoculum was added 0, 12, 24 or 36 h after the mutant inoculum (light gray bars). In the controls, cpr1 and the WT (black bars), or cpr1 and water (grey bars), were applied to the tissue simultaneously, 0, 12, 24 and 36 h after the sheaths had been detached. All treatments were evaluated 60 h after the addition of WT. Treatments with different letters are significantly different from each other (P < 0.05).
The induced susceptibility effect was specific to the cpr1 mutant
Several additional mutants of C. graminicola were tested for their effects in co‐inoculations at a distance. These included a pyrimidine auxotrophic mutant (Rasmussen et al., 1992), a melanin‐deficient mutant (Rasmussen and Hanau, 1989) and several REMI pathogenicity mutants with unknown genetic defects (Thon et al., 2000). All of these mutants were chosen because, like the cpr1 mutant, they were visibly diminished in their ability to colonize maize leaf sheaths. By 60 hpi, spores of the pyrimidine‐deficient mutant M1.201 failed to germinate on leaf sheaths, whereas REMI mutants 90‐23 and 84‐6 produced melanized appressoria, but failed to penetrate the tissue. REMI mutant 80‐37 developed very short invasive hyphae, whereas mutants 83‐45 and 84‐14 produced primary infection hyphae of a normal size which, however, remained confined to the first invaded cell. The melanin‐deficient mutant M1.502 produced nonmelanized appressoria and was delayed in penetration, but colonized up to three cells by 60 hpi. The REMI mutant 9‐4 was similarly delayed in penetration, and also colonized up to three cells by 60 hpi. None of these mutants induced susceptibility to the cpr1 mutant in co‐inoculations at a distance, and WT did not induce an increase in susceptibility to any of these mutants (not shown).
Heat‐killed spores did not induce susceptibility
Previous studies have demonstrated that heat‐killed or attenuated spores can affect the outcome of subsequent pathogen inoculations (Bell and Presley, 1969). However, heat‐killed or heat‐inactivated WT spores did not differ from the water controls when used in co‐inoculation experiments with the cpr1 mutant (not shown).
Discussion
Secreted compounds play important roles in fungal development and in the successful establishment of fungal interactions with plants (Condon et al., 2013; Djamei et al., 2011; Koeck et al., 2011). In this study, detached maize sheath assays were used to investigate the nonpathogenic C. graminicola cpr1 mutant, hypothesized to be deficient in protein transport and secretion (Thon et al., 2002). Initially developed by Sakamoto (1950), detached sheath assays facilitate detailed microscopic observations of living host and pathogen tissues, interacting in unfixed samples (Koga, 1994). Optically clear leaf sheaths allowed unprecedented observation of infection and colonization of sorghum by C. sublineola (Wharton and Julian, 1996; Wharton et al., 2001) and of rice by Magnaporthe oryzae, and led to the recent development of novel concepts in M. oryzae effector biology (Kankanala et al., 2007; Khang et al., 2010; Koga et al., 2004; Mosquera et al., 2009; Valent and Khang, 2010). Fungal penetration and colonization events are reportedly more synchronous in leaf sheaths than blades (Berruyer et al., 2006; Koga, 1994; Mosquera et al., 2009).
When plant tissues are detached, they begin to senesce, and this may affect the normal disease interaction (Audenaert et al., 2002; Benito et al., 1998; Berruyer et al., 2006; Liu et al., 2007). In the current experiments, detached sheath tissues continued to plasmolyse, and did not become susceptible to the cpr1 mutant or to the nonpathogen C. sublineola for up to 6 days. This differs from reports of detached leaf assays in Arabidopsis, where nonpathogens readily infected the detached leaves (Liu et al., 2007). Most experiments in the current study were completed within 72 h. Colletotrichum graminicola infection and colonization of detached leaf sheaths closely resembled previous descriptions of the same isolate infecting intact leaf blades (Mims and Vaillancourt, 2002; Vargas et al., 2012). However, the detached sheath assays had the advantage of allowing detailed observation of infections in living host tissues, demonstrating that primary hyphae colonized adjacent cells at a rate of approximately one every 8 h, usually biotrophically, evidenced by plasmolysis and vital staining assays. The biotrophic phase was brief; colonized cells lost their ability to plasmolyse before the hyphae entered the next cell. The appearance of thin, secondary hyphae, marking the switch to necrotrophy, occurred at approximately 60 hpi, prior to obvious tissue collapse and lesion development, which occurred by 72 hpi. It has been suggested that the necrotrophic switch in C. graminicola occurs synchronously at approximately 72 hpi (Horbach et al., 2009; Sugui and Deising, 2002; Vargas et al., 2012). However, the observations reported here suggest that the growth of C. graminicola resembles the colonization of sorghum by C. sublineola, with host cell death and wall degradation localized to the centre of the colony, whereas the colony margins continue to expand biotrophically (Wharton and Julian, 1996; Wharton et al., 2001).
As reported in earlier studies using maize leaf blades and stalks (Mims and Vaillancourt, 2002; Thon et al., 2000, 2002; Venard and Vaillancourt, 2007a, b), the timing and efficiency of spore germination and appressorial production were similar in the mutant and WT. The first major difference was a delay of approximately 24 h in the penetration and production of primary hyphae by the mutant. The delay could result from an inability to secrete compounds required during the early stages of colonization. In powdery mildew, suppressors of host defences are released at or before appressorial maturity (Komura et al., 1990). Colletotrichum higginsianum appressoria secrete effector proteins at the pore, before penetration (Kleemann et al., 2008, 2012). Although the mutant was delayed, its penetration efficiency (∼40%) ultimately was similar to WT. However, WT colonized up to five cells beyond the infection point within 24 h of entering, whereas the mutant only rarely escaped from the initially infected cell (<5%), and never caused symptoms on leaf sheaths. Thus, the mutant appears to be defective in the induction of host cell accessibility.
Host cells perceive fungal signals before penetration (Kobayashi et al., 1990), and these signals trigger plant defence responses, including the accumulation of ROS and activation of pathogenicity‐related (PR) genes (Kunoh et al., 1990; Veneault‐Fourrey et al., 2005; Yamaoka et al., 1994). Perception of defence elicitors causes host cells to become inaccessible to nonpathogenic fungi (Bradley et al., 1992; Cervone et al., 1989; Chappell and Hahlbrock, 1984; Ouchi et al., 1974a, 1976b). Suppressors produced by a compatible pathogen block inaccessibility, and co‐inoculation with a compatible pathogen can enable colonization by a normally incompatible one, a phenomenon known as induced susceptibility (Carver et al., 1999; Kobayashi et al., 1995; Komura et al., 1990; Kunoh et al., 1988, 1989; Lyngkjaer and Carver, 1999, 2001; Lyngkjaer et al., 2001; Olesen et al., 2003; Ouchi et al., 1974b, 1976a, b; Tsuchiya and Hirata, 1973; Yamaoka et al., 1994). Induced susceptibility is reported to be local, limited to the initially penetrated cell and up to three cells distant from the initial penetration site. The substances responsible for induced susceptibility are unknown.
In the current study, co‐inoculation experiments were designed to test two possible explanations for the behaviour of the cpr1 mutant. The first was that the WT C. graminicola secretes effectors to establish a compatible interaction with maize, and the mutant is unable to secrete these effectors. If this were true, co‐inoculations of the mutant and WT should allow the mutant to grow. The alternative hypothesis was that the mutant inappropriately secretes elicitors of defence responses, which cause neighbouring cells to become inaccessible. If this were true, co‐inoculations of the mutant and WT should prevent the growth of WT.
When the WT and cpr1 mutant were co‐inoculated in the same location, both strains grew. This indicated that the mutant did not induce inaccessibility to the WT strain. The cpr1 mutant only entered cells that had also been colonized by WT and failed to plasmolyse. Other experiments established that the mutant could complete its life cycle on dead maize tissue. Thus, the possibility that the mutant was entering killed cells, rather than living cells rendered accessible by WT effectors, could not be eliminated.
When the WT and mutant were co‐inoculated on maize sheaths at a distance of 2.5 mm (8.5 ± 1.5 epidermal cells) apart, there was a significant increase in the growth of the cpr1 mutant at 60 hpi. Vital staining and plasmolysis assays indicated that both strains invaded cells biotrophically, and that the cells surrounding the two colonies were alive. This argued against the possibility that WT produced a diffusible toxin that killed host cells in advance and allowed the mutant to grow. Further evidence against a toxin was the observation that the nonpathogenic C. sublineola strain did not grow under similar conditions on the same leaf sheaths. These experiments suggested that WT produced or elicited one or more diffusible substances that induced susceptibility of the host cells to the mutant.
Induced susceptibility was observed consistently when the inoculum drops were separated by 2.5 mm. When the distance between the drops was doubled, the effect disappeared. Thus, the inducing substance(s) had a limited ability for diffusion. Nevertheless, the effective distance observed here (8.5 cells) is, to our knowledge, the largest reported for an induced susceptibility study. Experiments in which inoculum concentrations were varied suggested that induced susceptibility was dosage dependent. Reductions in WT or mutant spore concentrations resulted in a decrease in induced susceptibility. These results were reinforced by experiments with various other mutants of C. graminicola that all showed reductions, to varying degrees, in fungal biomass within the host tissue. These mutants were not complemented by WT, demonstrating that the effect was related to the specific defect in the cpr1 mutant. Because decreases in mutant inoculum also reduced the effect, this suggested that the mutant may be producing limited amounts of the inducing factor(s).
Once a fungus is recognized by a plant cell, it becomes irreversibly reprogrammed towards accessibility or inaccessibility, and this cannot be altered by successive inoculations (Kunoh et al., 1988, 1989; Ouchi et al., 1976a, b). Application of WT up to 12 h after inoculation with the mutant still increased significantly the growth of the mutant. However, longer intervals failed to induce susceptibility, suggesting that the host had become irreversibly programmed for inaccessibility. This indicates that the mutant is producing elicitors of defence, although possibly at reduced levels. DAB staining results, which showed strong responses at mutant inoculation sites, supported this idea. The staining results suggested that the mutant was slower to elicit responses than WT, which may explain why full inaccessibility was not induced until 24 h after the mutant had been applied. An alternative possibility is that the mutant was unable to survive for more than 24 h without penetrating. This seems less likely, as it is known that Colletotrichum appressoria can survive for extended periods (Binyamini and Schiffmann‐Nadel, 1972; Muirhead and Deverall, 1981; Zaitlin et al., 2000).
Suppression of defence responses by rusts and powdery mildews can induce local susceptibility to nonpathogens (Kobayashi et al., 1990, 1995; Komura et al., 1990; Kunoh et al., 1985, 1988, 1989, 1990; Yamaoka et al., 1994). Co‐inoculation with C. graminicola at a distance did not induce susceptibility to the nonpathogen C. sublineola, suggesting that the diffusible inducing substance(s) does(do) not override the normal detection and defence response to nonpathogens in maize.
The evidence supports the hypothesis that WT produces a diffusible factor(s) that induces the accessibility of neighbouring host cells to biotrophic colonization, and that the mutant is deficient in the production of these factor(s). The inducer could be a plant signal or a secreted fungal product. Numerous secreted proteins (Bhadauria et al., 2011, 2012; Kleemann et al., 2012; Perfect et al., 1998; Stephenson et al., 2000; Yoshino et al., 2012) and SMs (Horbach et al., 2009; O'Connell et al., 2012; Rasmussen and Hanau, 1989; Takano et al., 1995) are produced during Colletotrichum disease interactions, including during the interaction between maize and C. graminicola. Laser capture microdissection (Tang et al., 2006), yeast signal sequence trapping (Krijger et al., 2008) and suppressive subtractive hybridization (Vargas et al., 2012) have identified approximately 160 C. graminicola genes expressed during the establishment of biotrophy and the switch to necrotrophy, many of which are predicted to encode secreted proteins and SM genes. SMs, many of which are secreted via membrane‐bound transporters, have been implicated directly in C. graminicola pathogenicity. Deletion of Ppt1, a major activator of polyketide synthases and nonribosomal peptide synthetases in C. graminicola, resulted in the loss of pathogenicity (Horbach et al., 2009).
Biotrophic and hemibiotrophic fungi engineer host susceptibility by secreting a variety of small protein effectors, many of which enter the host cell cytoplasm (reviewed by Donofrio and Ramen, 2012; de Jonge et al., 2011; Panstruga and Dodds, 2009; Rafiqi et al., 2012). By mechanisms that are still mostly mysterious, effectors reprogramme host metabolic and defence pathways, induce the production of the enclosing membrane, and block host cell death (de Jonge et al., 2011). Although unrelated mutualists and pathogens target some of the same host pathways to establish compatibility (Donofrio and Ramen, 2012; O'Connell and Panstruga, 2006; Parniske, 2000), most seem to produce a unique set of effectors, with little overlap (Donofrio and Ramen, 2012). Effectors are also highly redundant; mutagenesis studies have identified few that are individually essential (Donofrio and Ramen, 2012). Thus, the targeting of specific effectors is unlikely to result in broad‐spectrum disease control. In bacteria, the type III system is specifically involved in the secretion of effectors (reviewed by Büttner, 2012). Targeting of the type III machinery is being explored for the generalized control of bacterial pathogens (Holmes et al., 2012; Ur‐Rehman et al., 2012). A specialized secretion system for effectors in fungi is unknown, although, in the hemibiotroph M. oryzae, secretion of effectors that entered the host cytoplasm appeared to proceed by a mechanism distinct from the normal cellular secretion pathway (Giraldo et al., 2013). Some of these cytoplasmic effectors migrated several cells beyond the infection site (Khang et al., 2010). The induction of host susceptibility by the cpr1 mutant is compromised, and the ability of WT to complement the mutant at a distance suggests that the mutant cannot secrete necessary diffusible effectors. A homologue of Cpr1 in Medicago truncatula was implicated in the production of a specific subset of effectors necessary for the production of the peribacteroid membrane, and bacteroid development, during nodulation (Van de Velde et al., 2010; Wang et al., 2010). This homologue was expressed specifically in nodules, and differed slightly from the version utilized in the general secretory pathway. Although C. graminicola has only a single Cpr1 gene, post‐transcriptional or post‐translational regulatory mechanisms may alter its activity, so that it serves a similar specialized purpose during biotrophy, regulating the secretion of a set of effectors into the host cytoplasm to programme accommodation. Further characterization of the cpr1 mutant and of the role of the highly conserved CPR1 protein could lead to the identification of targets that might be generally useful for the development of sustainable disease control strategies for biotrophic and hemibiotrophic pathogens.
Experimental Procedures
Fungal culture and transformation
The fungal strains used in this study are listed in Table 1. Colletotrichum graminicola strain M1.001 (a.k.a. M2) was the WT (Forgey et al., 1978). A strain of M1.001 expressing mRFP was obtained using a polyethylene glycol‐mediated transformation protocol (Thon et al., 2000). M1.001 protoplasts were transformed with 3 μg of EcoRI‐linearized pCA56, a plasmid containing the mRFP1 gene under the control of the TOXA promoter from Pyrenophora tritici‐repentis, and the hygromycin B phosphotransferase gene from Escherichia coli as a selectable marker (Andrie et al., 2005). Five transformants were recovered and tested for pathogenicity. All behaved similarly in planta, and the strain with the strongest and most consistent fluorescence was chosen for subsequent analysis. The cpr1 mutant strain, cpr1‐Zsgreen, the cpr1 complemented strain and CgSl1‐GFP have been described elsewhere (Thon et al., 2000; Venard and Vaillancourt, 2007a, b). All strains were routinely grown on potato dextrose agar (PDA, Difco Laboratories, Detroit, MI, USA) and maintained at 23 °C under continuous illumination. For plant inoculations, falcate spores were harvested and prepared as described previously (Venard and Vaillancourt, 2007b). Spore suspensions were adjusted to a final concentration of 5 × 105 spores/mL, unless stated otherwise. For experiments involving heat‐killed spores, spore suspensions were boiled in 1.5‐mL Eppendorf tubes for 5 min, washed once by centrifugation and resuspended in fresh sterile water. Heat‐inactivated spores were produced by incubating the spore suspensions at 50 °C for 10 min, and rinsed as described above (Bell and Presley, 1969).
Leaf sheath inoculations
Maize inbred Mo940 was used for this study (Nicholson and Warren, 1976; Thon et al., 2002; Warren and Nicholson, 1975; Warren et al., 1975). Details of plant growth can be found in Appendix S1 (see Supporting Information). Leaf sheaths from the first true leaf in V3 seedlings were used. Plants were cut at the soil line and the tissue was processed and inoculated immediately, using the protocol described by Kankanala et al. (2007), with the following modifications. Sheath pieces were cut into 5‐cm segments, unrolled gently to expose the inner epidermal layer and inoculated with 20 μL of a spore suspension. The inoculation drop was placed on the epidermis directly above the midrib. Inoculated sheaths were suspended horizontally in a Petri plate containing moistened filter paper (Whatman No. 1, Whatman, Hillsboro, OR, USA) and incubated at 23 °C with continuous illumination. For co‐inoculation experiments using mixed spore suspensions, equivalent amounts of each individual suspension were combined prior to inoculation, and 20 μL of the mixture were inoculated as described previously. For co‐inoculation experiments in which the spore drops were separated, 10‐μL drops of each spore suspension were inoculated on the leaf sheath at a distance of approximately 2.5 mm apart. The average length of a maize epidermal cell was 309 ± 20 μm, determined using the measure function in AxioVision software (V4.8, AxioVision, Carl Zeiss Inc., Thornwood, NY, USA ) to measure 11 epidermal cells on each of 15 leaf sheaths (165 cells). This average was multiplied by the number of cells between the inoculation drops on 20 sheaths at 24 hpi to determine the average distance between the drops. Individual inoculated leaf sheaths were prepared and observed under the microscope at various time points up to 72 hpi, as described below.
Paraquat and freeze injury experiments
The herbicide paraquat (1,1′‐dimethyl‐4,4′‐bipyridinium ion) was used to induce systemic cell death in maize leaf sheaths. Equivalent volumes of a paraquat stock solution (10 mm) and a spore suspension were either combined before inoculation or co‐inoculated as separate drops approximately 2.5 mm apart. For freeze injury experiments, a metallic rod (diameter, 3 mm) dipped in liquid nitrogen was used to produce a localized injury in the centre of the unfolded sheath, and a 10‐μL drop of inoculum was immediately applied, either to the same location or to a location approximately 2.5 mm from the freeze‐treated spot.
Timed experiments
Ten‐microlitre drops of mutant spore suspensions were inoculated on maize leaf sheaths. At 0, 12, 24 or 36 hpi, a 10‐μL drop of a WT spore suspension (or a drop of water as a control) was added at a distance of approximately 2.5 mm from the mutant inoculum drop. Control sheaths that were detached at time zero were co‐inoculated at 0, 12, 24 or 36 h after detachment simultaneously with the mutant and WT (or water controls).
Spore concentration experiments
Three different inoculum concentrations (5 × 105, 1 × 105 and 5 × 104 spores/mL) of the WT and mutant strains were co‐inoculated on maize leaf sheaths in all possible combinations. Fungal colonization was evaluated at 60 hpi.
Light microscopy and staining
Leaf sheaths were rinsed gently with deionized water to remove superficial growth, and trimmed before observation, using the method described by Kankanala et al. (2007).
To detect the presence of H2O2, DAB staining was performed (Orozco‐Cardenas and Ryan, 1999). Nontrimmed leaf sheaths were stained for 8 h in a DAB solution (1 mg/mL), pH 3.8, at 25 °C under constant light. Sheaths where cleared in 96% boiling ethanol for 5 min and then transferred to fresh 96% ethanol for 4 h.
Plant cell viability was evaluated by plasmolysis with a solution of 0.75 m sucrose (Kankanala et al., 2007), or by using the viability dye neutral red. Sheaths were stained for 1 h in a neutral red solution (0.01%, 0.85 m KNO3), pH 7.5 (Stadelmann and Kinzel, 1972). Metabolically active cells plasmolysed and accumulated neutral red inside the vacuole (Wharton and Julian, 1996). Fungal hyphae inside leaf sheaths were stained with lactophenol–trypan blue (Yi et al., 2009).
Epifluorescence and confocal laser scanning microscopy
Epifluorescence microscopy was conducted using an Axioplan2 microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY, USA) equipped with Chroma filter sets for GFP and DsRed (Chroma Technology Corp., Rockingham, VT, USA). Micrographs were obtained using an Axiocam MR monochromatic digital camera employing the parameters described by Tsai et al. (2005).
Confocal laser scanning micrographs were acquired on a TCS SP2‐AOBS microscope (Leica Microsystems, Bannockburn, IL, USA). Emission conditions and filters were the same as described in detail by Goodin et al. (2002).
Assessment of fungal growth and development in planta
The growth and development of fungal strains in maize leaf sheaths was routinely assessed at 20, 48 and 60 hpi, unless noted otherwise. More than 1000 infected leaf sheaths were observed for this study. For statistical comparisons of the developmental timelines for the various strains in planta, 100 individual infection sites were evaluated on each of 10 individual sheaths for each strain at 18, 24 and 48 hpi. To statistically compare the relative degree of colonization by the strains, the number of host cells colonized from each of 20 successful penetration sites was measured for 10 individual leaf sheaths at 48 hpi for the M1.001, WT‐mRFP and Cpr1‐C strains, and at 60 hpi for the cpr1 mutant and cpr1‐Zsgreen strains. To quantify colonization by the cpr1 mutant in the co‐inoculation experiments, the number of host cells colonized from each of 20 successful penetration sites was determined on 10 individual leaf sheaths at 60 hpi. All experiments for statistical analyses were repeated at least twice.
Statistical analysis
Differences among treatments were assessed using analysis of variance with the SAS statistical package version 9.3 (SAS Institute Inc., Cary, NC, USA). One‐way analysis of variance was used and, if significant effects were detected, multiple comparisons of means were performed using Tukey's test and least‐significant difference (LSD) methods. Results were expressed as means with their corresponding standard deviations, and differences among or between means were considered to be significant if P ≤ 0.05.
Supporting information
Fig. S1 Development of fungal strains on maize leaf sheaths over time. Percentage of ungerminated, adhered spores (white bar), appressoria (grey bar) and invasive primary hyphae (dark grey bar) 12, 24 and 48 h post‐inoculation (hpi).
Fig. S2 Percentage of penetration sites in which the cpr1 mutant colonized two or more cells when co‐inoculated at a distance from the wild‐type (WT) (left) or water (right). Treatments with different letters differ from each other (P < 0.05).
Fig. S3 Average number of cells colonized by the cpr1 mutant and the nonpathogen in triple inoculations. Treatments with different letters differ from each other (P < 0.05).
Appendix S1 Plant growth. Plants were grown to the V3 stage under 14 h of light in a glasshouse in 3.8 × 21‐cm2 containers (Super SC‐10 UV stabilized; Stuewe & Sons, Inc., Tangent, OR, USA) in a mixture of three parts Pro‐Mix BX (Premiere Horticulture, Ltd., Rivière du Loup, QC, Canada) and two parts sterile topsoil. Seedlings were watered daily to saturation and fertilized two to three times per week with a solution of 150 ppm of Peters 20‐10‐20 (Scotts‐Sierra Horticultural Product Co., Marysville, OH, USA), beginning 1 week after seedling emergence.
Acknowledgements
The authors thank Etta Nuckles and Doug Brown for excellent technical support. This work was funded by the US Department of Agriculture‐Cooperative State Research, Education, and Extension Service (USDA‐CSREES) grant 2009‐34457‐20125 (L.V.).
References
- Amselem, J. , Cuomo, C.A. , van Kan, J.A.L. , Viaud, M. , Benito, E.P. , Couloux, A. , Coutinho, P.M. , de Vriesm, R.P. , Dyer, P.S. , Fillinger, S. , Fournier, E. , Gout, L. , Hahn, M. , Kohn, L. , Lapalu, N. , Plummer, K.M. , Pradier, J.M. , Quévillon, E. , Sharon, A. , Simon, A. , ten Have, A. , Tudzynski, B. , Tudzynski, P. , Wincker, P. , Andrew, M. , Anthouard, V. , Beever, R.E. , Beffa, R. , Benoit, I. , Bouzid, O. , Brault, B. , Chen, Z. , Choquer, M. , Collémare, J. , Cotton, P. , Danchin, E.G. , Da Silva, C. , Gautier, A. , Giraud, C. , Giraud, T. , Gonzalez, C. , Grossetete, S. , Güldener, U. , Henrissat, B. , Howlett, B.J. , Kodira, C. , Kretschmer, M. , Lappartient, A. , Leroch, M. , Levis, C. , Mauceli, E. , Neuvéglise, C. , Oeser, B. , Pearson, M. , Poulain, J. , Poussereau, N. , Quesneville, H. , Rascle, C. , Schumacher, J. , Ségurens, B. , Sexton, A. , Silva, E. , Sirven, C. , Soanes, D.M. , Talbot, N.J. , Templeton, M. , Yandava, C. , Yarden, O. , Zeng, Q. , Rollins, J.A. , Lebrun, M.H. and Dickman, M. (2011) Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea . PLoS Genet. 7, e1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrie, R.M. , Martinez, J.P. and Ciuffetti, L.M. (2005) Development of ToxA and ToxB promoter‐driven fluorescent protein expression vectors for use in filamentous ascomycetes. Mycologia, 97, 1152–1161. [DOI] [PubMed] [Google Scholar]
- Audenaert, K. , De Meyer, G.B. and Höfte, M.M. (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid‐dependent signaling mechanisms. Plant Physiol. 128, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, A. and Presley, J. (1969) Heat‐inhibited or heat‐killed conidia of Verticillium albo‐atrum induce disease resistance and phytoalexin synthesis in cotton. Phytopathology, 59, 1147–1151. [Google Scholar]
- Benito, E.P. , ten Have, A. , van't Klooster, J.W. and van Kan, J.A. (1998) Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea . Eur. J. Plant Pathol. 104, 207–220. [Google Scholar]
- Bergstrom, G.C. and Nicholson, R.L. (1999) The biology of corn anthracnose: knowledge to exploit for improved management. Plant Dis. 83, 596–608. [DOI] [PubMed] [Google Scholar]
- Berruyer, R. , Poussier, S. , Kankanala, P. , Mosquera, G. and Valent, B. (2006) Quantitative and qualitative influence of inoculation methods on in planta growth of rice blast fungus. Phytopathology, 96, 346–355. [DOI] [PubMed] [Google Scholar]
- Bhadauria, V. , Banniza, S. , Vandenberg, A. , Selvaraj, G. and Wei, Y. (2011) EST mining identifies proteins putatively secreted by the anthracnose pathogen Colletotrichum truncatum . BMC Genomics, 12, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadauria, V. , Banniza, S. , Vandenberg, A. , Selvaraj, G. and Wei, Y. (2012) Overexpression of a novel biotrophy‐specific Colletotrichum truncatum effector CtNUDIX in hemibiotrophic fungal phytopathogens causes incompatibility with their host plants. Eukaryot. Cell, 12, 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binyamini, N. and Schiffmann‐Nadel, M. (1972) Latent infection in avocado fruit due to Colletotrichum gloeosporioides . Phytopathology, 62, 592–594. [PubMed] [Google Scholar]
- Bradley, D.J. , Kjellbom, P. and Lamb, C.J. (1992) Elicitor‐ and wound‐induced oxidative cross‐linking of a proline‐rich plant cell wall protein: a novel, rapid defense response. Cell, 70, 21–30. [DOI] [PubMed] [Google Scholar]
- Büttner, D. (2012) Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant‐ and animal‐pathogenic bacteria. Microbiol. Mol. Biol. Rev. 76, 262–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver, T. , Lyngkjær, M. , Neyron, L. and Strudwicke, C. (1999) Induction of cellular accessibility and inaccessibility and suppression and potentiation of cell death in oat attacked by Blumeria graminis f. sp. avenae . Physiol. Mol. Plant Pathol. 55, 183–196. [Google Scholar]
- Cervone, F. , Hahn, M.G. , De Lorenzo, G. , Darvill, A. and Albersheim, P. (1989) Host–pathogen interactions: XXXIII. A plant protein converts a fungal pathogenesis factor into an elicitor of plant defense responses. Plant Physiol. 90, 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cessna, S.G. , Sears, V.E. , Dickman, M.B. and Low, P.S. (2000) Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell, 12, 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell, J. and Hahlbrock, K. (1984) Transcription of plant defence genes in response to UV light or fungal elicitor. Nature, 311, 76–78. [Google Scholar]
- Condon, B.J. , Leng, Y. , Wu, D. , Bushley, K.E. , Ohm, R.A. , Otillar, R. , Martin, J. , Schackwitz, W. , Grimwood, J. , MohdZainudin, N. , Xue, C. , Wang, R. , Manning, V.A. , Dhillon, B. , Tu, Z.J. , Steffenson, B.J. , Salamov, A. , Sun, H. , Lowry, S. , LaButti, K. , Han, J. , Copeland, A. , Lindquist, E. , Barry, K. , Schmutz, J. , Baker, S.E. , Ciuffetti, L.M. , Grigoriev, I.V. , Zhong, S. and Turgeon, B.G. (2013) Comparative genome structure, secondary metabolite, and effector coding capacity across Cochliobolus pathogens. PLoS Genet. 9, e1003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamei, A. , Schipper, K. , Rabe, F. , Ghosh, A. , Vincon, V. , Kahnt, J. , Osorio, S. , Tohge, T. , Fernie, A.R. , Feussner, I. , Feussner, K. , Meinicke, P. , Stierhof, Y.D. , Schwarz, H. , Macek, B. , Mann, M. and Kahmann, R. (2011) Metabolic priming by a secreted fungal effector. Nature, 478, 395–398. [DOI] [PubMed] [Google Scholar]
- Doehlemann, G. , Wahl, R. , Horst, R.J. , Voll, L.M. , Usadel, B. , Poree, F. , Stitt, M. , Pons‐Kühnemann, J. , Sonnewald, U. , Kahmann, R. and Kämper, J. (2008) Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis . Plant J. 56, 181–195. [DOI] [PubMed] [Google Scholar]
- Doehlemann, G. , van der Linde, K. , Aβmann, D. , Schwammbach, D. , Hof, A. , Mohanty, A. , Jackson, D. and Kahmann, R. (2009) Pep1, a secreted effector protein of Ustilago maydis, is required for successful invasion of plant cells. PLoS Pathog. 5, e1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donofrio, N.M. and Ramen, V. (2012) Roles and delivery mechanisms of fungal effectors during infection development: common threads and new directions. Curr. Opin. Microbiol. 15, 692–698. [DOI] [PubMed] [Google Scholar]
- Eichmann, R. , Schultheiss, H. , Kogel, K.‐H. and Hückelhoven, R. (2004) The barley apoptosis suppressor homologue BAX inhibitor‐1 compromises nonhost penetration resistance of barley to the inappropriate pathogen Blumeria graminis f. sp. tritici . Mol. Plant–Microbe Interact. 17, 484–490. [DOI] [PubMed] [Google Scholar]
- Fang, H. , Mullins, C. and Green, N. (1997) In addition to SEC11, a newly identified gene, SPC3, is essential for signal peptidase activity in the yeast endoplasmic reticulum. J. Biol. Chem. 272, 13 152–13 158. [DOI] [PubMed] [Google Scholar]
- Farr, D.F. and Rossmann, Z.Y. (2013) Fungal Databases, Systemic Mycology and Microbiology Laboratory, ARS, USDA. Available at: http://nt.ars‐grin.gov/fungaldatabases/ [accessed on May 30, 2013].
- Forgey, W.M. , Blanco, M.H. and Loegering, W.Q. (1978) Differences in pathological capabilities and host specificity of Colletotrichum graminicola on Zea mays . Plant Dis. Rep. 62, 573–576. [Google Scholar]
- Freeman, S. and Rodriguez, R.J. (1993) Genetic conversion of a fungal plant pathogen to a nonpathogenic, endophytic mutualist. Science, 260, 75–78. [DOI] [PubMed] [Google Scholar]
- Frey, T.J. , Weldekidan, T. , Colbert, T. , Wolters, P.J.C.C. and Hawk, J.A. (2011) Fitness evaluation of Rcg1, a locus that confers resistance to Colletotrichum graminicola (Ces.) G.W. Wils. Using near‐isogenic maize hybrids. Crop Sci. 51, 1551–1563. [Google Scholar]
- Gan, P. , Ikeda, K. , Irieda, H. , Narusaka, M. , O'Connell, R.J. , Narusaka, Y. , Takano, Y. , Kubo, Y. and Shirasu, K. (2013) Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 197, 1236–1249. [DOI] [PubMed] [Google Scholar]
- Giraldo, M.C. , Dagdas, Y.F. , Gupta, Y.K. , Mentlak, T.A. , Yi, M. , Martinez‐Rocha, A.L. , Saitoh, H. , Terauchi, R. , Talbot, N.J. and Valent, B. (2013) Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae . Nat. Commun. 4, 1996. doi: 10.1038/ncomms2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin, M.M. , Dietzgen, R.G. , Schichnes, D. , Ruzin, S. and Jackson, A.O. (2002) pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 31, 375–383. [DOI] [PubMed] [Google Scholar]
- Govrin, E.M. and Levine, A. (2002) Infection of Arabidopsis with a necrotrophic pathogen, Botrytis cinerea, elicits various defense responses but does not induce systemic acquired resistance (SAR). Plant Mol. Biol. 48, 267–276. [DOI] [PubMed] [Google Scholar]
- Holmes, T.C. , May, A.E. , Zaleta‐Rivera, K. , Ruby, J.G. , Skewes‐Cox, P. , Fischbach, M.A. , DeRisi, J.L. , Iwatsuki, M. , Omura, S. and Khosla, C. (2012) Molecular insights into the biosynthesis of guadinomine: a type III secretion system inhibitor. J. Am. Chem. Soc. 134, 17 797–17 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbach, R. , Graf, A. , Weihmann, F. , Antelo, L. , Mathea, S. , Liermann, J.C. , Opatz, T. , Thines, E. , Aguirre, J. and Deising, H.B. (2009) Sfp‐type 4′‐phosphopantetheinyl transferase is indispensable for fungal pathogenicity. Plant Cell Online, 21, 3379–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge, R. , Bolton, M.D. and Thomma, B.P.H.J. (2011) How filamentous pathogens co‐opt plants: the ins and outs of fungal effectors. Curr. Opin. Plant Biol. 14, 400–406. [DOI] [PubMed] [Google Scholar]
- Kankanala, P. , Czymmek, K. and Valent, B. (2007) Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell Online, 19, 706–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khang, C.H. , Berruyer, R. , Giraldo, M.C. , Kankanala, P. , Park, S.Y. , Czymmek, K. , Kang, S. and Valent, B. (2010) Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell‐to‐cell movement. Plant Cell Online, 22, 1388–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann, J. , Takahara, H. , Stüber, K. and O'Connell, R. (2008) Identification of soluble secreted proteins from appressoria of Colletotrichum higginsianum by analysis of expressed sequence tags. Microbiology, 154, 1204–1217. [DOI] [PubMed] [Google Scholar]
- Kleemann, J. , Rincon‐Rivera, L.J. , Takahara, H. , Neumann, U. , van Themaat, E.V.L. , van der Does, H.C. , Hacquard, S. , Stüber, K. , Will, I. , Schmalenbach, W. , Schmelzer, E. and O′Connell, R.J. (2012) Sequential delivery of host‐induced virulence effectors by appressoria and intracellular hyphae of the phytopathogen Colletotrichum higginsianum . PLoS Pathog. 8, e1002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, I. , Komura, T. , Sakamoto, Y. , Yamaoka, N. and Kunoh, H. (1990) Recognition of a pathogen and a nonpathogen by barley coleoptile cells (I). Cytoplasmic responses to the nonpathogen, Erysiphe pisi, prior to its penetration. Physiol. Mol. Plant Pathol. 37, 479–490. [Google Scholar]
- Kobayashi, I. , Watanabe, H. and Kunoh, H. (1995) Induced accessibility and enhanced inaccessibility at the cellular level in barley coleoptiles. XIV. Evidence for elicitor(s) and suppressor(s) of host inaccessibility from Erysiphe graminis . Physiol. Mol. Plant Pathol. 46, 445–456. [Google Scholar]
- Koeck, M. , Hardham, A.R. and Dodds, P.N. (2011) The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell Microbiol. 13, 1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga, H. (1994) Hypersensitive death, autofluorescence, and ultrastructural changes in cells of leaf sheaths of susceptible and resistant near‐isogenic lines of rice (Pi‐zt) in relation to penetration and growth of Pyricularia oryzae . Can. J. Bot 72, 1463–1477. [Google Scholar]
- Koga, H. , Dohi, K. , Nakayachi, O. and Mori, M. (2004) A novel inoculation method of Magnaporthe grisea for cytological observation of the infection process using intact leaf sheaths of rice plants. Physiol. Mol. Plant Pathol. 64, 67–72. [Google Scholar]
- Komura, T. , Kobayashi, I. , Yamaoka, N. and Kunoh, H. (1990) Induced accessibility and enhanced inaccessibility at the cellular level in barley coleoptiles. VIII. Cytological evidence for suppressor(s) of host inaccessibility from Erysiphe graminis . Physiol. Mol. Plant Pathol. 37, 409–416. [Google Scholar]
- Krijger, J.‐J. , Horbach, R. , Behr, M. , Schweizer, P. , Deising, H.B. and Wirsel, S.G.R. (2008) The yeast signal sequence trap identifies secreted proteins of the hemibiotrophic corn pathogen Colletotrichum graminicola . Mol. Plant–Microbe Interact. 21, 1325–1336. [DOI] [PubMed] [Google Scholar]
- Kunoh, H. , Hayashimoto, A. , Harui, M. and Ishizaki, H. (1985) Induced susceptibility and enhanced resistance at the cellular level in barley coleoptiles. I. The significance of timing of fungal invasion. Physiol. Plant Pathol. 27, 43–54. [Google Scholar]
- Kunoh, H. , Katsuragawa, N. , Yamaoka, N. and Hayashimoto, A. (1988) Induced accessibility and enhanced inaccessibility at the cellular level in barley coleoptiles. III. Timing and localization of enhanced inaccessibility in a single coleoptile cell and its transfer to an adjacent cell. Physiol. Mol. Plant Pathol. 33, 81–93. [Google Scholar]
- Kunoh, H. , Toyoda, K. , Yamaoka, N. and Kobayashi, I. (1989) Induced accessibility and enhanced inaccessibility at the cellular level in barley coleoptiles. V. Duration of stimulus by a non‐pathogen in relation to enhanced inaccessibility. Physiol. Mol. Plant Pathol. 35, 507–518. [Google Scholar]
- Kunoh, H. , Komura, T. , Kobayashi, I. and Yamaoka, N. (1990) Induced accessibility and enhanced inaccessibility at the cellular level in barley coleoptiles. VII. Enhancement of inaccessibility at the prepenetration stage of a nonpathogen. Physiol. Mol. Plant Pathol. 37, 399–407. [Google Scholar]
- Liu, G. , Kennedy, R. , Greenshields, D.L. , Peng, G. , Forseille, L. , Selvaraj, G. and Wei, Y. (2007) Detached and attached Arabidopsis leaf assays reveal distinctive defense responses against hemibiotrophic Colletotrichum spp. Mol. Plant–Microbe Interact. 20, 1308–1319. [DOI] [PubMed] [Google Scholar]
- Lyngkjaer, M. and Carver, T. (1999) Induced accessibility and inaccessibility to Blumeria graminis f. sp. hordei in barley epidermal cells attacked by a compatible isolate. Physiol. Mol. Plant Pathol. 55, 151–162. [Google Scholar]
- Lyngkjær, M.F. and Carver, T. (2001) Conditioning of cellular defence responses to powdery mildew in cereal leaves by prior attack. Mol. Plant Pathol. 1, 41–49. [DOI] [PubMed] [Google Scholar]
- Lyngkjær, M.F. and Carver, T.L.W. (1999) Induced accessibility and inaccessibility to Blumeria graminis f.sp. hordei in barley epidermal cells attacked by a compatible isolate. Physiol. Mol. Plant Pathol. 55, 151–162. [Google Scholar]
- Lyngkjaer, M.F. , Carver, T.L.W. and Zeyen, R. (2001) Virulent Blumeria graminis infection induces penetration susceptibility and suppresses race‐specific hypersensitive resistance against avirulent attack in Mla1‐barley. Physiol. Mol. Plant Pathol. 59, 243–256. [Google Scholar]
- Meyer, H.‐A. and Hartmann, E. (1997) The yeast SPC22/23 homolog Spc3p is essential for signal peptidase activity. J. Biol. Chem. 272, 13 159–13 164. [DOI] [PubMed] [Google Scholar]
- Mims, C.W. and Vaillancourt, L.J. (2002) Ultrastructural characterization of infection and colonization of maize leaves by Colletotrichum graminicola, and by a C. graminicola pathogenicity mutant. Phytopathology, 92, 803–812. [DOI] [PubMed] [Google Scholar]
- Mosquera, G. , Giraldo, M.C. , Khang, C.H. , Coughlan, S. and Valent, B. (2009) Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1–4 as biotrophy‐associated secreted proteins in rice blast disease. Plant Cell Online, 21, 1273–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead, I. and Deverall, B. (1981) Role of appressoria in latent infection of banana fruits by Colletotrichum musae . Physiol. Plant Pathol. 19, 77–84. [Google Scholar]
- Nicholson, R. and Warren, H. (1976) Criteria for evaluation of resistance to maize anthracnose. Phytopathology, 66, 86–90. [Google Scholar]
- O'Connell, R.J. and Panstruga, R. (2006) Tête à tête inside a plant cell: establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytologist, 171, 699–718. [DOI] [PubMed] [Google Scholar]
- O'Connell, R.J. , Thon, M.R. , Hacquard, S. , Amyotte, S.G. , Kleemann, J. , Torres, M.F. , Damm, U. , Buiate, E.A. , Epstein, L. , Alkan, N. , Altmüller, J. , Alvarado‐Balderrama, L. , Bauser, C.A. , Becker, C. , Birren, B.W. , Chen, Z. , Choi, J. , Crouch, J.A. , Duvick, J.P. , Farman, M.A. , Gan, P. , Heiman, D. , Henrissat, B. , Howard, R.J. , Kabbage, M. , Koch, C. , Kracher, B. , Kubo, Y. , Law, A.D. , Lebrun, M.H. , Lee, Y.H. , Miyara, I. , Moore, N. , Neumann, U. , Nordström, K. , Panaccione, D.G. , Panstruga, R. , Place, M. , Proctor, R.H. , Prusky, D. , Rech, G. , Reinhardt, R. , Rollins, J.A. , Rounsley, S. , Schardl, C.L. , Schwartz, D.C. , Shenoy, N. , Shirasu, K. , Sikhakolli, U.R. , Stüber, K. , Sukno, S.A. , Sweigard, J.A. , Takano, Y. , Takahara, H. , Trail, F. , van der Does, H.C. , Voll, L.M. , Will, I. , Young, S. , Zeng, Q. , Zhang, J. , Zhou, S. , Dickman, M.B. , Schulze‐Lefert, P. , Ver Loren van Themaat, E. , Ma, L.J. and Vaillancourt, L.J. (2012) Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 44, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen, K. , Carver, T.L.W. and Lyngkjær, M.F. (2003) Fungal suppression of resistance against inappropriate Blumeria graminis formae speciales in barley, oat and wheat. Physiol. Mol. Plant Pathol. 62, 37–50. [Google Scholar]
- Orozco‐Cardenas, M. and Ryan, C.A. (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Natl. Acad. Sci. USA, 96, 6553–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi, S. , Oku, H. , Hibino, C. and Akiyama, I. (1974a) Induction of accessibility and resistance in leaves of barley by some races of Erysiphe graminis . J. Phytopathol. 79, 24–34. [Google Scholar]
- Ouchi, S. , Oku, H. , Hibino, C. and Akiyama, I. (1974b) Induction of accessibility to a nonpathogen by preliminary inoculation with a pathogen. J. Phytopathol. 79, 142–154. [Google Scholar]
- Ouchi, S. , Hibino, C. and Oku, H. (1976a) Effect of earlier inoculation on the establishment of a subsequent fungus as demonstrated in powdery mildew of barley by a triple inoculation procedure. Physiol. Plant Pathol. 9, 25–32. [Google Scholar]
- Ouchi, S. , Oku, H. and Hibino, C. (1976b) Localization of induced resistance and susceptibility in barley leaves inoculated with the powdery mildew fungus. Phytopathology, 66, 901–905. [Google Scholar]
- Panstruga, R. and Dodds, P.N. (2009) Terrific protein traffic: the mystery of effector protein delivery by filamentous plant pathogens. Science, 324, 748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske, M. (2000) Intracellular accommodation of microbes by plants: a common developmental program for symbiosis and disease? Curr. Opin. Plant Biol. 3, 320–328. [DOI] [PubMed] [Google Scholar]
- Perfect, S.E. , O'Connell, R.J. , Green, E.F. , Doering‐Saad, C. and Green, J.R. (1998) Expression cloning of a fungal proline‐rich glycoprotein specific to the biotrophic interface formed in the Colletotrichum–bean interaction. Plant J. 15, 273–279. [DOI] [PubMed] [Google Scholar]
- Rafiqi, M. , Ellis, J.G. , Ludowici, V.A. , Hardham, A.R. and Dodds, P.N. (2012) Challenges and progress towards understanding the role of effectors in plant–fungal interactions. Curr. Opin. Plant Biol. 15, 477–482. [DOI] [PubMed] [Google Scholar]
- Rasmussen, J. and Hanau, R. (1989) Exogenous scytalone restores appressorial melanization and pathogenicity in albino mutants of Colletotrichum graminicola . Can. J. Plant Pathol. 11, 349–352. [Google Scholar]
- Rasmussen, J.B. , Panaccione, D.G. , Fang, G.‐C. and Hanau, R.M. (1992) The PYR1 gene of the plant pathogenic fungus Colletotrichum graminicola: selection by intraspecific complementation and sequence analysis. Mol. Gen. Genet. 235, 74–80. [DOI] [PubMed] [Google Scholar]
- Rolke, Y. , Liu, S. , Quidde, T. , Williamson, B. , Schouten, A. , Weltring, K.‐M. , Siewers, V. , Tenberge, K.B. , Tudzynski, B. and Tudzynski, P. (2004) Functional analysis of H2O2‐generating systems in Botrytis cinerea: the major Cu–Zn‐superoxide dismutase (BCSOD1) contributes to virulence on French bean, whereas a glucose oxidase (BCGOD1) is dispensable. Mol. Plant Pathol. 5, 17–27. [DOI] [PubMed] [Google Scholar]
- Sakamoto, M. (1950) On the new method of inoculation on rice plants with the blast fungus. Rep. Inst. Agric. Res. Tohoku Univ. 1, 15–23. [Google Scholar]
- Stadelmann, E.J. and Kinzel, H. (1972) Vital staining of plant cells. Methods Cell Physiol. 5, 325–405. [Google Scholar]
- Stephenson, S.A. , Hatfield, J. , Rusu, A.G. , Maclean, D.J. and Manners, J.M. (2000) CgDN3: an essential pathogenicity gene of Colletotrichum gloeosporioides necessary to avert a hypersensitive‐like response in the host Stylosanthes guianensis . Mol. Plant–Microbe Interact. 13, 929–941. [DOI] [PubMed] [Google Scholar]
- Sugui, J.A. and Deising, H.B. (2002) Isolation of infection‐specific sequence tags expressed during early stages of maize anthracnose disease development. Mol. Plant Pathol. 3, 197–203. [DOI] [PubMed] [Google Scholar]
- Takano, Y. , Kubo, Y. , Shimizu, K. , Mise, K. , Okuno, T. and Furusawa, I. (1995) Structural analysis of PKS1, a polyketide synthase gene involved in melanin biosynthesis in Colletotrichum lagenarium . Mol. Gen. Genet. 249, 162–167. [DOI] [PubMed] [Google Scholar]
- Tang, W. , Coughlan, S. , Crane, E. , Beatty, M. and Duvick, J. (2006) The application of laser microdissection to in planta gene expression profiling of the maize anthracnose stalk rot fungus Colletotrichum graminicola . Mol. Plant–Microbe Interact. 19, 1240–1250. [DOI] [PubMed] [Google Scholar]
- Thon, M. , Nuckles, E. and Vaillancourt, L. (2000) Restriction enzyme‐mediated integration used to produce pathogenicity mutants of Colletotrichum graminicola . Mol. Plant–Microbe Interact. 13, 1356–1365. [DOI] [PubMed] [Google Scholar]
- Thon, M.R. , Nuckles, E.M. , Takach, J.E. and Vaillancourt, L.J. (2002) CPR1: a gene encoding a putative signal peptidase that functions in pathogenicity of Colletotrichum graminicola to maize. Mol. Plant–Microbe Interact. 15, 120–128. [DOI] [PubMed] [Google Scholar]
- Tsai, C.‐W. , Redinbaugh, M.G. , Willie, K.J. , Reed, S. , Goodin, M. and Hogenhout, S.A. (2005) Complete genome sequence and in planta subcellular localization of maize fine streak virus proteins. J. Virol. 79, 5304–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya, K. and Hirata, K. (1973) Growth of various powdery mildew fungi on the barley leaves infected preliminarily with the barley powdery mildew fungus. Ann. Phytopathol Soc. Jpn. 39, 396–403. [Google Scholar]
- Ur‐Rehman, T. , Slepenkin, A. , Chu, H. , Blomgren, A. , Dahlgren, M.K. , Zetterström, C.E. , Peterson, E.M. , Elofsson, M. and Gylfe, A. (2012) Pre‐clinical pharmacokinetics and anti‐chlamydial activity of salicylidene acylhydrazide inhibitors of bacterial type III secretion. J. Antibiot. 65, 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent, B. and Khang, C.H. (2010) Recent advances in rice blast effector research. Curr. Opin. Plant Biol. 13, 434–441. [DOI] [PubMed] [Google Scholar]
- Van de Velde, W. , Zehirov, G. , Szatmari, A. , Debreczeny, M. , Ishihara, H. , Kevei, Z. , Farkas, A. , Mikulass, K. , Nagy, A. , Tiricz, H. , Satiat‐Jeunemaître, B. , Alunni, B. , Bourge, M. , Kucho, K. , Abe, M. , Kereszt, A. , Maroti, G. , Uchiumi, T. , Kondorosi, E. and Mergaert, P. (2010) Plant peptides govern terminal differentiation of bacteria in symbiosis. Science, 327, 1122–1126. [DOI] [PubMed] [Google Scholar]
- Vargas, W.A. , Martín, J.M.S. , Rech, G.E. , Rivera, L.P. , Benito, E.P. , Díaz‐Mínguez, J.M. , Thon, M.R. and Sukno, S.A. (2012) Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotricum graminicola in maize. Plant Physiol. 158, 1342–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venard, C. and Vaillancourt, L. (2007a) Colonization of fiber cells by Colletotrichum graminicola in wounded maize stalks. Phytopathology, 97, 438–447. [DOI] [PubMed] [Google Scholar]
- Venard, C. and Vaillancourt, L. (2007b) Penetration and colonization of unwounded maize tissues by the maize anthracnose pathogen Colletotrichum graminicola and the related nonpathogen C. sublineolum . Mycologia, 99, 368–377. [DOI] [PubMed] [Google Scholar]
- Veneault‐Fourrey, C. , Laugé, R. and Langin, T. (2005) Nonpathogenic strains of Colletotrichum lindemuthianum trigger progressive bean defense responses during appressorium‐mediated penetration. Appl. Environ. Microbiol. 71, 4761–4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Griffitts, J. , Starker, C. , Federova, E. , Limpens, E. , Ivanov, S. , Bisseling, T. and Long, S. (2010) A nodule‐specific protein secretory pathway required for nitrogen‐fixing symbiosis. Science, 327, 1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, H. and Nicholson, R. (1975) Kernel infection, seedling blight, and wilt of maize caused by Colletotrichum graminicola . Phytopathology, 65, 620–623. [Google Scholar]
- Warren, H. , Nicholson, R. and Turner, M. (1975) Field reaction of corn inbreds to Colletotrichum graminicola [Fungus diseases]. Plant Dis. Rep. 59, 767–769. [Google Scholar]
- Wharton, P. and Julian, A. (1996) A cytological study of compatible and incompatible interactions between Sorghum bicolor and Colletotrichum sublineolum . New Phytol. 134, 25–34. [Google Scholar]
- Wharton, P. , Julian, A. and O'Connell, R. (2001) Ultrastructure of the infection of Sorghum bicolor by Colletotrichum sublineolum . Phytopathology, 91, 149–158. [DOI] [PubMed] [Google Scholar]
- Yamaoka, N. , Toyoda, K. , Kobayashi, I. and Kunoh, H. (1994) Induced accessibility and enhanced inaccessibility at the cellular level in barley coleoptiles. XIII. Significance of haustorium formation by the pathogen Erysiphe graminis for induced accessibility to the non‐pathogen E. pisi as assessed by nutritional manipulations. Physiol. Mol. Plant Pathol. 44, 217–225. [Google Scholar]
- Yi, M. , Chi, M.–H. , Khang, C.H. , Park S.–Y., Kang, S. , Valent, B. and Lee, Y.–H. (2009) The ER chaperone LHS1 involved in asexual development and rice infection by the blast fungus Magnaporthe oryzae. The Plant Cell Online, 21, 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino, K. , Irieda, H. , Sugimoto, F. , Yoshioka, H. , Okuno, T. and Takano, Y. (2012) Cell death of Nicotiana benthamiana is induced by secreted protein NIS1 of Colletotrichum orbiculare and is suppressed by a homologue of CgDN3. Mol. Plant–Microbe Interact. 25, 625–636. [DOI] [PubMed] [Google Scholar]
- Zaitlin, B. , Zehr, E.I. and Dean, R.A. (2000) Latent infection of peach caused by Colletotrichum gloeosporioides and Colletotrichum acutatum . Can. J. Plant Pathol. 22, 224–228. [Google Scholar]
- Zimmermann, R. , Müller, L. and Wullich, B. (2006) Protein transport into the endoplasmic reticulum: mechanisms and pathologies. Trends Mol. Med. 12, 567–573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Development of fungal strains on maize leaf sheaths over time. Percentage of ungerminated, adhered spores (white bar), appressoria (grey bar) and invasive primary hyphae (dark grey bar) 12, 24 and 48 h post‐inoculation (hpi).
Fig. S2 Percentage of penetration sites in which the cpr1 mutant colonized two or more cells when co‐inoculated at a distance from the wild‐type (WT) (left) or water (right). Treatments with different letters differ from each other (P < 0.05).
Fig. S3 Average number of cells colonized by the cpr1 mutant and the nonpathogen in triple inoculations. Treatments with different letters differ from each other (P < 0.05).
Appendix S1 Plant growth. Plants were grown to the V3 stage under 14 h of light in a glasshouse in 3.8 × 21‐cm2 containers (Super SC‐10 UV stabilized; Stuewe & Sons, Inc., Tangent, OR, USA) in a mixture of three parts Pro‐Mix BX (Premiere Horticulture, Ltd., Rivière du Loup, QC, Canada) and two parts sterile topsoil. Seedlings were watered daily to saturation and fertilized two to three times per week with a solution of 150 ppm of Peters 20‐10‐20 (Scotts‐Sierra Horticultural Product Co., Marysville, OH, USA), beginning 1 week after seedling emergence.


